Abstract
Alcohol use disorder (AUD) and cannabis use disorder (CUD) are associated with brain alterations particularly involving fronto‐cerebellar and meso‐cortico‐limbic circuitry. However, such abnormalities have additionally been reported in other psychiatric conditions, and until recently there has been few large‐scale investigations to compare such findings. The current study uses the Enhancing Neuroimaging Genetics through Meta‐Analysis (ENIGMA) consortium method of standardising structural brain measures to quantify case–control differences and to compare brain‐correlates of substance use disorders with those published in relation to other psychiatric disorders. Using the ENIGMA protocols, we report effect sizes derived from a meta‐analysis of alcohol (seven studies, N = 798, 54% are cases) and cannabis (seven studies, N = 447, 45% are cases) dependent cases and age‐ and sex‐matched controls. We conduct linear analyses using harmonised methods to process and parcellate brain data identical to those reported in the literature for ENIGMA case–control studies of major depression disorder (MDD), schizophrenia (SCZ) and bipolar disorder so that effect sizes are optimally comparable across disorders. R elationships between substance use disorder diagnosis and subcortical grey matter volumes and cortical thickness were assessed with intracranial volume, age and sex as co‐variates . After correcting for multiple comparisons, AUD case–control meta‐analysis of subcortical regions indicated significant differences in the thalamus, hippocampus, amygdala and accumbens, with effect sizes (0.23) generally equivalent to, or larger than |0.23| those previously reported for other psychiatric disorders (except for the pallidum and putamen). On measures of cortical thickness, AUD was associated with significant differences bilaterally in the fusiform gyrus, inferior temporal gyrus, temporal pole, superior frontal gyrus, and rostral and caudal anterior cingulate gyri. Meta‐analysis of CUD case–control studies indicated reliable reductions in amygdala, accumbens and hippocampus volumes, with the former effect size comparable to, and the latter effect size around half of that reported for alcohol and SCZ. CUD was associated with lower cortical thickness in the frontal regions, particularly the medial orbitofrontal region, but this effect was not significant after correcting for multiple testing. This study allowed for an unbiased cross‐disorder comparison of brain correlates of substance use disorders and showed alcohol‐related brain anomalies equivalent in effect size to that found in SCZ in several subcortical and cortical regions and significantly greater alterations than those found in MDD in several subcortical and cortical regions. Although modest, CUD results overlapped with findings reported for AUD and other psychiatric conditions, but appear to be most robustly related to reduce thickness of the medial orbitofrontal cortex.
Keywords: addiction, alcohol, cannabis, structural neuroimaging
We compared the impact of alcohol and cannabis use disorders to other major psychiatric conditions using standardised objectively brain derived metrics. Through a meta‐analytic cross‐disorder framework, comparable and unique volumetric variations were observed in participants with addiction.
1. INTRODUCTION
The Global Burden of Disease Studies (Ezzati, Lopez, Rodgers, & Murray, 2004) have been critical in developing methods to study health outcomes and have proven invaluable when advocating for health equity, both cross‐nationally and across diseases. The 2010 update (Lozano, Naghavi, Foreman, et al., 2012) systematically quantified prevalence of 1,160 sequelae of 289 diseases and injuries across 21 geographical regions. Results for specific diseases and impairments have highlighted the high rates of disability from mental disorders (particularly depression) and substance use disorders (Whiteford et al., 2013). Alcohol and cannabis are among the most widely abused substances globally, and second only to tobacco use in terms of frequency of use (Degenhardt et al., 2013; Ritchie & Roser, 2019).
Harmful alcohol use and dependence have long been associated with cognitive impairments in multiple neuropsychological domains, including evidence from the very earliest case–control studies employing standardised test batteries, such as the Luri‐Nebraska Battery or the Wechsler Adult Intelligence Scale (Chmielewski & Golden, 1980; Miller & Orr, 1980). However, a long‐standing challenge in these and successive studies is the multifactorial aetiology of such impairments, including pre‐existing variation, foetal alcohol effects, and both direct alcohol‐induced excitotoxicity in the brain and indirect toxicity related to factors such as impaired nutrition (e.g. thiamine deficiency; Joyce, 1994), head injury, liver disease, psychiatric comorbidity, and complex interactions between these factors (Tarter & Alterman, 1984). Cognitive deficits have also been recognised in social drinkers (Parsons & Nixon, 1998), with the suggestion that there is a continuum of deficits related to intensity of alcohol use, including impairments in verbal and non‐verbal performance, learning, memory, abstract reasoning, and speed of information processing and efficiency (Parsons, 1998). Finally, recent studies have focused on more selective and aetiologically relevant impairments in emotion and reward processing (Kornreich et al., 2001; Townshend & Duka, 2003).
Multiple structural imaging studies have shown generalised cortical atrophy particularly for measures of grey (Fein et al., 2002) and white matter integrity (Gallucci et al., 1989) in alcohol dependence (Sullivan & Pfefferbaum, 2005). Other reports of brain‐related abnormalities include the disruption of white matter tracts (Pfefferbaum & Sullivan, 2005) and abnormal functional activity (Rosenbloom, Sullivan, & Pfefferbaum, 2003). Functional imaging studies in alcohol dependence have identified lower cerebral metabolism in frontal brain regions that show a correlation with executive neuropsychological deficits (Wang et al., 1993). Studies in the alcohol‐related Wernicke–Korsakoff Syndrome have shown prominent fronto‐striatal impairment (Reed et al., 2003).
Recent attempts to reconcile inconsistent findings across neuroimaging studies with alcohol dependent patients have led to the proposal that, despite alcohol's widespread acute effects on brain function, brain deficits due to chronic and severe alcohol consumption might be limited to fronto‐cerebellar and meso‐cortico‐limbic circuitry, while other brain circuits might be spared or might even undergo compensatory changes (Chanraud, Pitel, Müller‐Oehring, Pfefferbaum, & Sullivan, 2012). However, prior studies may simply not have been sufficiently powered to reliably detect brain‐related impairments and compensatory processes due to small sample sizes, variability across neuroimaging methods, and failure to distinguish disease‐specific neurological abnormalities. Standard meta‐analysis across neuroimaging studies has proven difficult, mainly due to heterogeneity in the methods used to collect, quality control and parcellate brain data. These limitations also apply to any potential comparisons with other psychiatric conditions. Structural brain abnormalities have also been observed in adults with heavy or problematic cannabis use (Batalla, Bhattacharyya, Yuecel, et al., 2013). Heavy cannabis users have been shown to have lower grey matter density in the right parahippocampus and greater grey matter density in the precentral gyrus and right thalamus (Matochik, Eldreth, Cadet, & Bolla, 2005), and anterior cerebellum (Cousijn et al., 2012). Other studies reported cannabis use and misuse associated with bilateral volumetric reductions in the hippocampus (Ashtari et al., 2011; Matochik et al., 2005; Yücel et al., 2008) and amygdala (Cousijn et al., 2012; Yücel et al., 2008). However, findings have not always been replicated, so further studies are needed to confirm these observations (Nader & Sanchez, 2018).
One approach used by researchers to see beyond inconsistent findings in human substance dependence is the meta‐analysis. Recent meta‐analyses on alcohol use disorder (AUD) observed grey matter abnormalities in corticostriatal‐limbic circuits, such as prefrontal cortical regions, thalamus, striatum and hippocampus (Klaming et al., 2019; Xiao et al., 2015; Yang, Tian, Zhang, et al., 2016). Even if several meta‐analyses were performed to determine general impacts of a single drug on the adult brain, to our knowledge no meta‐analyses compared two widely consumed drugs to other major psychiatric conditions to evaluate the relative volumetric variations observed between cases and controls among psychiatric disorders. Similarly, a recent meta‐analysis observed that the hippocampus and the orbitofrontal cortex were most consistently identified as having structural alterations in regular cannabis users (Lorenzetti, Chye, Silva, Solowij, & Roberts, 2019a).
1.1. Quantifying brain anomalies across disorders
There is a recent trend in psychiatric epidemiology to create standardised metrics for the purpose of cross‐jurisdiction and cross‐disorder comparisons (Murray, Barber, Foreman, et al., 2015; Whiteford et al., 2013) However, until recently, such harmonised approaches have not been used in the field of psychiatric neuroimaging. The development of such methods would help to quantify the subtle and specific brain‐related correlates of major psychiatric conditions, which may contribute to, or reflect, an individual's specific symptom profile, general quality of life, and disability. More objective measures of brain impairment may help to identify disorder‐specific processes and quantify brain‐related impairment and may also help to reduce the effect of social stigma when evaluating disease‐related impairment, or when addressing gaps in access to services. In the case of substance use disorders, brain structural measures have been linked to the duration and severity of the disorder, as well as likelihood of relapse (Zahr, 2014). However, research on other psychiatric conditions, such as mood disorders, psychosis, and attention‐deficit/hyperactivity disorder (ADHD) has reported abnormalities in similar brain structures (Hoogman et al., 2017; Schmaal, Hibar, Sämann, et al., 2017; van Erp, Hibar, Rasmussen, et al., 2016). Despite potential similarities in brain‐related outcomes, public health policies on how these conditions are managed dramatically differ across cultures and across disorders. AUDs are detrimentally under‐treated in most parts of the world, relative to other psychiatric conditions (Kohn, Saxena, Levav, & Saraceno, 2004). However, it is not clear if the treatment gaps observed across disorders can be justified based on objective indicators of impairment.
The current study aims to capitalise on the methods developed in the Enhancing Neuroimaging Genetics through Meta‐Analysis (ENIGMA) consortium to use standardised protocols for quantifying and comparing the brain‐related correlates of various psychiatric conditions for the purpose of promoting health equity. Using standardised protocols to harmonise neuroimaging data and conduct meta‐analyses for cross‐disorder comparisons (Hibar, Stein, Renteria, et al., 2015; Stein, Medland, Vasquez, et al., 2012; Thompson, Stein, Medland, et al., 2014), we report, for the first time, effect sizes derived from meta‐analysis involving alcohol dependent and cannabis dependent cases and age‐ and sex‐matched controls worldwide. Results of this analysis will be compared to effects found in large‐scale international studies of major depressive disorder (MDD), schizophrenia (SCZ), bipolar disorder (BD) and ADHD using identical brain parcellation methods and co‐variates (Hoogman et al., 2017; Schmaal et al., 2017; van Erp et al., 2016).
The ENIGMA network (Thompson et al., 2014) was formed to address the need for replicability and increased sample sizes and to increase power for genome‐wide association studies of brain measures. With the development of standard anatomical templates and coordinate‐based reference systems, researchers worldwide can now relate their new findings to previous results in a consistent way. The pooling of datasets across sites and clinical samples now allows us to study uncommon or complex phenomena and compare findings across disorders. The most commonly used statistical approach in ENIGMA is a meta‐analysis, in which evidence for association is combined using effect sizes for each separate site, which are weighted to adjust for each site's sample size and error variance.
Several disease‐specific working groups have been formed, focusing on performing meta‐analysis of case–control disease differences of measures extracted using the ENIGMA protocols. This approach also allows for the unification of both case–control and cohort datasets with a standard protocol and allows for the largest imaging studies of the human brain to be performed while focusing on a particular disease process. To date, ENIGMA working groups such as MDD, BD and SCZ have reported small to moderate effect sizes in terms of brain‐related abnormalities (Hoogman et al., 2017; Schmaal et al., 2017; van Erp et al., 2016), such as alterations in hippocampus (except for ADHD), amygdala (except for MDD) and thalamus (except for ADHD and MDD).
The combined datasets contain participants with structural brain data and addiction phenotyping and involves both cohort and case–control designs, representing individuals with ages ranging from 12–60 years and a number of different addiction phenotypes. For the purpose of the current study, we omit cohort studies and focus on case–control samples representing AUD and cannabis use disorder (CUD) since these substances are among the most consumed worldwide (Degenhardt et al., 2013; Peacock, Leung, Larney, et al., 2018; Ritchie & Roser, 2019). To be able to compare effect sizes for addiction subgroups to published results from other ENIGMA disease groups, we focused our first set of analyses on structural measures of subcortical and cortical brain regions (Hoogman et al., 2017; Schmaal et al., 2017; van Erp et al., 2016).
ENIGMA provides quality control procedures for harmonising neuroimaging and genetic data, available online through http://enigma.loni.ucla.edu/ongoing/gwasma‐of‐subcortical‐structures. While many working groups within ENIGMA are now moving towards the ‘mega‐analysis’ strategy, where all phenotypic and genotypic data are sent to a centralised site for pooling and analysis, the first ENIGMA studies used a ‘meta‐analysis’ approach. Meta‐analyses circumvent barriers associated with data sharing across sites and countries and allow sites to maintain responsibility for their data and its integrity. This approach also has advantages when the purpose of the study is to make cross‐disorder comparisons. ENIGMA‐Addiction recently evaluated the subject‐specific volumetric variations in drug‐specific groups using innovative methods (Chye, Mackey, Gutman, et al., 2019; Mackey et al., 2018). These approaches observed subcortical and cortical variations in alcohol‐dependent participants but not in cannabis‐dependent participants, which is interesting given the fact that a recent meta‐analysis observed an association between cannabis use and reduced volumes in subcortical regions and thinning of cortical thickness in the orbitofrontal cortex (Lorenzetti, Chye, Silva, Solowij, & Roberts, 2019b). Similar variations were observed in ADHD, MDD, SCZ and BD but have never been compared to drug‐specific variations using common metrics.
2. SUBJECTS AND METHODS
2.1. Samples
The ENIGMA‐Addiction Working Group includes international samples with neuroimaging and clinical data from substance dependent patients and healthy controls. This is an ongoing study and new research groups continue to join the consortium regularly. Inclusion criteria for study enrolment were that sites must agree to their data being processed using the ENIGMA scripts and basic information on dependence criteria and patterns of early use are available for cases and controls. Demographic details for seven international samples of alcohol‐dependent subgroups and seven international samples of cannabis use are presented in Table 1. The number of participants included vary from the sample of the mega‐analysis performed by Mackey et al. (2018) because sites that did not include a control group could not derive a comparable effect size. Most participants were classified as having substance use disorder, or not, using validated structured diagnostic interviews that conform to Diagnostic and Statistical Manual of Mental Disorders‐Fourth‐TR Edition (American Psychiatric Association, 1994) criteria. One site included chronic cannabis users for whom DSM criteria could not be confirmed (Hester, Nestor, & Garavan, 2009). Similarly to the mega‐analysis of the ENIGMA Addiction working group, subjects with a lifetime history of neurological disease and/or a current DSM‐IV axis I diagnosis (other than depressive and anxiety disorders) were excluded from the analyses. AUD and CUD cases were mostly lifetime dependence cases (Mackey et al., 2018). Participants with a co‐occurring substance use disorder were removed from the analyses. All control participants were confirmed with similar interviews to be free of any substance use disorder. All participating sites obtained approval from local institutional review boards and ethics committees. All study participants provided written informed consent at their local institution for the local study. CHU Ste Justine provided ethical approval for this meta‐analysis. This study includes 435 cases with primary AUD and 363 matched healthy controls; and 200 cases of CUD and 247 controls.
TABLE 1.
Demographic details for each site
| Substance of dependence | Number of studies | Groups | N | Female | Age |
|---|---|---|---|---|---|
| All | 14 | Case | 635 | 188 | 28.12 |
| Control | 610 | 208 | 29.23 | ||
| Alcohol | 7 | Case | 435 | 127 | 32.43 |
| Control | 363 | 136 | 33.58 | ||
| IRC (Sinha & Li, 2007, Li et al., 2009, Seo et al., 2011) | Case | 43 | 11 | 28.05 | |
| Control | 84 | 21 | 37.49 | ||
| Effects of heavy alcohol abuse on adolescent brain structure and function (Fein et al., 2013) | Case | 60 | 34 | 14.81 | |
| Control | 56 | 31 | 14.94 | ||
| NIAAA (Senatorov et al., 2015, Grodin et al., 2013, Momenan et al., 2012) | Case | 212 | 57 | 31.11 | |
| Control | 140 | 67 | 38.48 | ||
| Neuro‐ADAPT (Korucuoglu et al., 2017) | Case | 18 | 6 | 19.35 | |
| Control | 23 | 11 | 18.72 | ||
| NESDA‐AD (Sjoerds et al., 2014) | Case | 42 | 19 | 48.6 | |
| Control | 20 | 6 | 48.29 | ||
| ADPG study (Jansen et al., 2015, van Holst et al., ,2014) ) | Case | 28 | 0 | 43.43 | |
| Control | 24 | 0 | 37.17 | ||
| TrIP study (Schmaal et al., 2014) | Case | 32 | 0 | 41.69 | |
| Control | 16 | 0 | 39.94 | ||
| Cannabis | 7 | Case | 200 | 61 | 23.80 |
| Control | 247 | 72 | 24.88 | ||
| Trinity‐THC (Hester et al., 2009) | Case | 15 | 2 | 23.27 | |
| Control | 15 | 4 | 22.4 | ||
| Orr (Orr et al., 2013) | Case | 13 | 1 | 16.00 | |
| Control | 14 | 1 | 16.77 | ||
| Cannabis prospective (Cousijn et al., 2012, 2013, 2014) | Case | 38 | 12 | 21.85 | |
| Control | 40 | 15 | 21.39 | ||
| ADS | Case | 7 | 6 | 18.96 | |
| Control | 93 | 44 | 19.00 | ||
| Chronic cannabis users (Barcelona) (Batalla et al., 2014, Blanco‐Hinojoet al., 2017, Pujol et al., 2014, ) | Case | 16 | 1 | 35.00 | |
| Control | 18 | 2 | 38.98 | ||
| Chronic cannabis (Yücelet al., 2008, Solowij et al., 2011, 2013, Lorenzetti et al., 2015) | Case | 81 | 39 | 30.47 | |
| Control | 38 | 6 | 33.21 | ||
| Chronic cannabis‐memory (Zalesky et al., 2012, Harding et al., 2012, Jakabek et al., 2016, Yücel et al., 2016) | Case | 30 | 0 | 21.03 | |
| Control | 29 | 0 | 22.41 |
2.2. Image processing and analysis
Structural T1‐weighted magnetic resonance imaging brain scans were acquired at each site. Using the fully automated and validated segmentation software FreeSurfer (Fischl et al., 2002), the segmentations of seven subcortical grey matter regions (nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen and thalamus), 34 cortical regions, lateral ventricles, and total intracranial volume (ICV) were derived following standardised protocols designed to facilitate harmonised image analysis across multiple sites (http://enigma.ini.usc.edu/protocols/imaging-protocols). Image acquisition parameters and software descriptions for each sample are similar to those in previous ENIGMA studies (Hoogman et al., 2017; Schmaal et al., 2017; van Erp et al., 2016), to facilitate the between‐disorder comparison. A majority of the datasets were prepared using CBRAIN, a network of high‐performance computing facilities in Canada (Sherif, Rioux, Rousseau, et al., 2014). Sites followed the ENIGMA protocols for quality control http://enigma.ini.usc.edu/protocols/imaging-protocols/. The detection of outliers and visual inspection were performed in a series of standard planes to avoid the inclusion of poorly segmented and mislabelled structures. Quality control procedures at each site were conducted according to standardised protocols to minimise potential site effects. Additional visual inspection was performed on a randomly selected sub‐sample of participants centrally at the University of Vermont to ensure uniformity of quality control across sites.
2.3. Statistical framework of meta‐analysis
Consistent with other ENIGMA working groups, multiple linear regression analyses derived case versus control group differences within each site‐specific sample using the mean volume of bilateral subcortical region of interests (ROIs) ([left + right]/2) along with left and right thickness and surface area for each cortical ROI as the outcome measures and control/case as the binary grouping independent variable. In order to make results comparable with those of yielded from the SCZ, MDD, BD, and ADHD working groups, models for subcortical ROIs covary for age, sex and total ICV, and models for cortical ROIs covary for age and sex. In order to be consistent with the reported findings of the other working groups, we did not covary for past 30‐day alcohol and nicotine use in the analyses. Furthermore, because past 30‐day substance use is so highly correlated with dependence scores, co‐varying for this variable in meta‐analysis would likely lead to underestimation of main differences between cases and controls. Regression models were fit for each site separately and t‐statistics were used to estimate effect sizes. A Cohen's d‐effect size estimate was obtained using an inverse variance‐weighted random‐effect meta‐analysis model in R (metafor package; Viechtbauer, 2010). Uncorrected and false discovery rate (FDR) corrected p values are reported and, are indicated as significant effect sizes by an asterisk in figures below. I‐square indices were calculated to provide a measure of heterogeneity. Significance level was determined with a p FDR < .05 for all regions of interest. Differences between effect sizes were considered significant if confidence intervals (CIs; or SEs) did not overlap, which is appropriate when Cohen's d is derived based on sample sizes above 20 (Lakens, 2013). We performed a post hoc sensitivity analysis on the meta‐analytic results for the alcohol and cannabis subgroups excluding the two adolescent sites using a leave‐one‐out approach (Viechtbauer, 2010). The inclusion of adolescent sites in the AUD and CUD analyses might lead to greater variability and inconsistent findings due to the volumetric variations that occur during the adolescence (Lenroot, Gogtay, Greenstein, et al., 2007). Because the CIs for the adolescent sites overlap with the adult‐only sites for all regions of interest, it did not significantly differ from the rest of the sample. The Results section includes significant variations when the adolescent sites are included since structural effect sizes remained consistent for subcortical volumes and cortical thickness (Supporting Information Figures 1–4). The Cohen's d values were obtained after adjustment for age, the adolescent sites were included in final AUD and CUD sample sizes in order to increase the sample size.
3. RESULTS
Figure 1 shows the forest plot with effect sizes and 95% CIs for subcortical volumes when comparing AUD cases to their matched controls: FDR corrected significant differences are shown in the thalamus (d = −0.23, CI = [−0.42, −0.04]), putamen (d = −0.27, CI = [−0.45, −0.08]), hippocampus (d = −0.50, CI = [−0.76, −0.24]), amygdala (d = −0.39, CI = [−0.63, −0.16]) and the accumbens (d = −0.30, CI = [−0.49, −0.12]). The caudate (d = −0.04, CI = [−0.22, 0.15]) and the pallidum (d = −0.10, CI = [−0.24, 0.04]) were not significant following the FDR correction.
FIGURE 1.
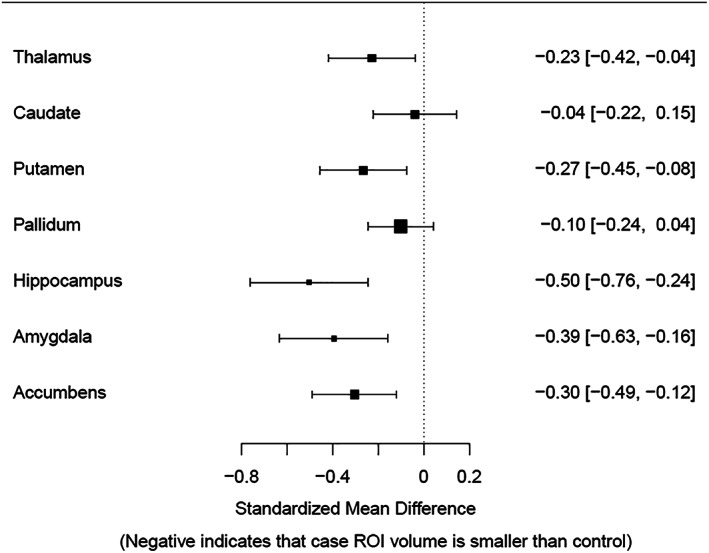
Forest plot with effect sizes and confidence intervals for bilateral subcortical volume for the alcohol use disorder versus controls comparison controlling for age, sex when females were included, and intracranial volume. Error bars represent 95% confidence intervals. The caudate and the pallidum were not significant following the false discovery rate correction. ROI, region of interest
Figure 2 presents the case control comparisons for CUD, showing non‐significant case–control differences after correcting for multiple comparisons.
FIGURE 2.
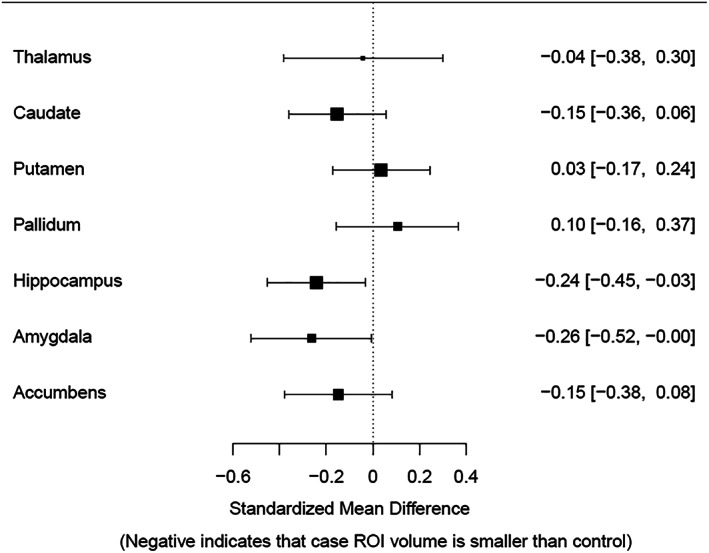
Forest plot with effect sizes and confidence intervals for bilateral subcortical volume for the cannabis use disorder versus controls comparison controlling for age, sex when females were included, and intracranial volume. Error bars represent 95% confidence intervals. All results are non‐significant following false discovery rate correction. ROI, region of interest
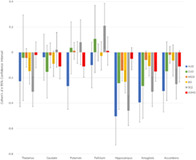
Figure 3 presents a comparison of subcortical results for AUD and CUD and previously published ENIGMA meta‐analyses on MDD, SCZ, BD and ADHD. If CIs overlap between groups for a ROI, no significant difference was observed. The magnitude of effect sizes for case–control AUD comparisons appear larger than effect sizes reported for MDD, BD, and ADHD except for the caudate and the pallidum. However, these effect sizes do not differ significantly from the other psychiatric conditions since the CIs overlap. In comparison to effects reported for SCZ, CIs overlapped between AUD and SCZ on all subcortical ROIs. When comparing other subcortical regions across AUD and MDD, CIs did not overlap for the putamen, hippocampus, amygdala and accumbens, with AUD associated with significantly smaller volumes in these regions. When comparing AUD and BD, CIs overlap for all regions but the amygdala, with significantly greater differences evident in AUD case control comparison (AUD associated with smaller volume). The effects reported for CUD in the amygdala and accumbens appear comparable to those reported for AUD and SCZ, but due to large CIs, these effects were not shown to significantly differ from non‐effect line and the other disorders. CUD observations also overlapped with AUD and SCZ findings. These CUD results suggest considerable heterogeneity or variability in the CUD studies (Supporting Information Figure 5).
FIGURE 3.
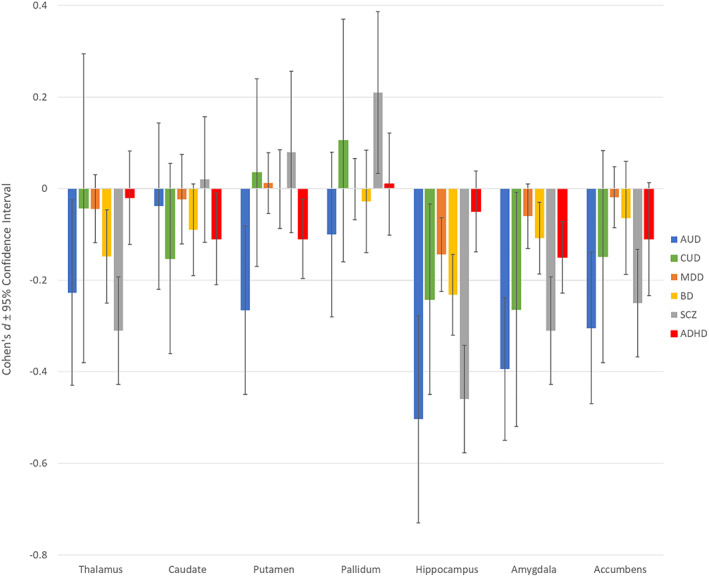
Comparison between bilateral subcortical results for alcohol use disorder (AUD), cannabis use disorder (CUD), depression (MDD), psychotic disorder (SCZ), bipolar disorder (BPD) and attention‐deficit/hyperactivity disorder (ADHD). Error bars represent 95% confidence intervals. Significant volumetric variations when compared to age‐, sex‐ and disorder‐matched controls was observed when the confidence intervals did not overlap with non‐effect line at 0 and survived false discovery rate correction. While significant reductions are observed in the ADHD for the putamen, amygdala and the caudate, none of these results remained in ADHD adult‐specific analyses
Analysis of cortical thickness by AUD case–control comparisons shows FDR‐corrected significant bilateral differences in the caudal anterior cingulate, fusiform, inferior temporal, parahippocampal, posterior cingulate, superior frontal and temporal pole. Table 2 presents effect sizes and CIs for cortical thickness in each ROI for AUD. Table 3 presents effect sizes and CIs for cortical thickness for CUD in each ROI. None of the CUD‐control comparisons on cortical thickness were shown to survive FDR correction for multiple testing across all ROIs, but marginal, FDR‐corrected effects were revealed for the medial orbitofrontal cortex (p FDR < .1), caudal middle frontal (p FDR = .1), precentral gyrus (p FDR = .1) and insula (p FDR = .1).
TABLE 2.
Full meta‐analytic results for volume and thickness of each bilateral structure for the alcohol use disorder versus controls comparison controlling for age, sex and intracranial volume (for subcortical regions only)
| ROI | ES | SE | 95% CI.LB | 95% CI.UB | I 2 | p | p FDR | Controls | Cases |
|---|---|---|---|---|---|---|---|---|---|
| Thalamus | −0.2272 | 0.0976 | −0.4184 | −0.0359 | 28.84 | .0199 | .0279 | 359 | 345 |
| Caudate | −0.0382 | 0.094 | −0.2225 | 0.1461 | 28.12 | .6844 | .6844 | 361 | 437 |
| Putamen | −0.2656 | 0.0966 | −0.455 | −0.0762 | 30.53 | .006 | .0105 | 361 | 436 |
| Pallidum | −0.1002 | 0.0727 | −0.2427 | 0.0423 | 0.01 | .1681 | .1961 | 365 | 437 |
| Hippocampus | −0.5037 | 0.1325 | −0.7634 | −0.244 | 60.85 | .0001 | .0010 | 362 | 437 |
| Amygdala | −0.3942 | 0.1215 | −0.6323 | −0.156 | 53.99 | .0012 | .0031 | 364 | 437 |
| Accumbens | −0.3044 | 0.0948 | −0.4901 | −0.1187 | 28.27 | .0013 | .0031 | 362 | 436 |
| Bankssts | −0.15 | 0.147 | −0.43 | 0.14 | 68.609 | .319 | .5784 | 360 | 436 |
| Caudalanteriorcingulate | −0.24 | 0.073 | −0.38 | −0.09 | 0.00 | .001 | .0067 | 365 | 437 |
| Caudalmiddlefrontal | −0.1 | 0.154 | −0.4 | 0.2 | 71.767 | .517 | .7563 | 365 | 437 |
| Cuneus | −0.03 | 0.142 | −0.31 | 0.25 | 66.9 | .817 | .9496 | 364 | 437 |
| Entorhinal | −0.22 | 0.137 | −0.48 | 0.05 | 62.843 | .115 | .3001 | 362 | 431 |
| Fusiform | −0.47 | 0.084 | −0.64 | −0.31 | 14.048 | .00 | .0001 | 365 | 436 |
| Inferior parietal | −0.01 | 0.149 | −0.3 | 0.29 | 70.21 | .965 | .9647 | 365 | 437 |
| Inferior temporal | −0.36 | 0.081 | −0.52 | −0.21 | 10.367 | .00 | .0001 | 365 | 437 |
| Isthmus cingulate | −0.22 | 0.103 | −0.43 | −0.02 | 37.955 | .029 | .0997 | 364 | 436 |
| Lateral occipital | −0.16 | 0.119 | −0.39 | 0.08 | 52.71 | .188 | .4576 | 365 | 437 |
| Lateral orbitofrontal | −0.29 | 0.119 | −0.53 | −0.06 | 52.526 | .014 | .0531 | 365 | 436 |
| Lingual | −0.01 | 0.143 | −0.29 | 0.27 | 67.39 | .942 | .9647 | 365 | 437 |
| Medial orbitofrontal | −0.13 | 0.116 | −0.36 | 0.1 | 49.999 | .265 | .5630 | 364 | 436 |
| Middle temporal | −0.23 | 0.137 | −0.49 | 0.04 | 63.939 | .1 | .2833 | 361 | 437 |
| Parahippocampal | −0.25 | 0.073 | −0.39 | −0.1 | 0.003 | .001 | .0053 | 364 | 437 |
| Paracentral | −0.19 | 0.196 | −0.58 | 0.19 | 82.825 | .323 | .5784 | 365 | 437 |
| Pars opercularis | −0.01 | 0.131 | −0.27 | 0.24 | 61.005 | .921 | .9647 | 365 | 437 |
| Pars orbitalis | −0.06 | 0.109 | −0.28 | 0.15 | 43.834 | .556 | .7563 | 365 | 437 |
| Pars triangularis | −0.03 | 0.167 | −0.36 | 0.29 | 76.22 | .839 | .9496 | 365 | 437 |
| Pericalcarine | 0.05 | 0.166 | −0.28 | 0.37 | 75.831 | .781 | .9496 | 365 | 436 |
| Postcentral | 0.05 | 0.203 | −0.35 | 0.45 | 84.059 | .809 | .9496 | 365 | 437 |
| Posterior cingulate | −0.29 | 0.115 | −0.51 | −0.06 | 49.129 | .012 | .0498 | 365 | 437 |
| Precentral | −0.13 | 0.189 | −0.5 | 0.24 | 81.542 | .503 | .7563 | 365 | 437 |
| Precuneus | −0.15 | 0.14 | −0.43 | 0.12 | 66.082 | .284 | .5686 | 365 | 437 |
| Rostralanteriorcingulate | −0.31 | 0.073 | −0.45 | −0.17 | 0.001 | .000 | .0002 | 365 | 437 |
| Rostral middle frontal | −0.03 | 0.178 | −0.38 | 0.32 | 79.1 | .866 | .9496 | 365 | 437 |
| Superior frontal | −0.18 | 0.073 | −0.33 | −0.04 | 0.002 | .011 | .0498 | 365 | 436 |
| Superior parietal | −0.11 | 0.186 | −0.47 | 0.25 | 80.83 | .549 | .7563 | 365 | 436 |
| Superior temporal | −0.21 | 0.182 | −0.57 | 0.14 | 79.566 | .242 | .5483 | 360 | 435 |
| Supramarginal | −0.14 | 0.148 | −0.43 | 0.15 | 69.05 | .344 | .5853 | 361 | 436 |
| Frontal pole | 0.06 | 0.073 | −0.08 | 0.2 | 0.00 | .408 | .6611 | 365 | 437 |
| Temporal pole | −0.32 | 0.073 | −0.46 | −0.17 | 0.00 | .00. | .0002 | 365 | 437 |
| Transverse temporal | 0.05 | 0.152 | −0.25 | 0.34 | 71.255 | .76 | .9496 | 365 | 437 |
| Insula | −0.21 | 0.12 | −0.45 | 0.03 | 53.324 | .08 | .2471 | 363 | 436 |
Abbreviations: Bankssts, banks of the superior temporal sulcus; CI.LB, confidence interval lower bound; CI.UB, confidence interval upper bound; ES, effect size; p FDR, adjusted p‐value for the seven sites following a BH correction; ROI, region of interest.
PFDR less than 0.05 are in bold.
TABLE 3.
Full meta‐analytic results for volume and thickness of each bilateral structure for the cannabis use disorder versus controls comparison controlling for age, sex and intracranial volume (for subcortical regions only)
| ROI | ES | SE | 95% CI.LB | 95% CI.UB | I 2 | p | p FDR | Controls | Cases |
|---|---|---|---|---|---|---|---|---|---|
| Thalamus | −0.0425 | 0.1744 | −0.3844 | 0.0821 | 58.47 | .8074 | .8074 | 249 | 200 |
| Caudate | −0.1526 | 0.1062 | −0.3608 | 0.0556 | 0.00 | .1508 | .3519 | 250 | 200 |
| Putamen | 0.0349 | 0.1062 | −0.1732 | 0.2431 | 0.00 | .7421 | .8074 | 250 | 199 |
| Pallidum | 0.1049 | 0.1333 | −0.1563 | 0.3662 | 30.98 | .4312 | .6037 | 250 | 200 |
| Hippocampus | −0.2416 | 0.1071 | −0.4515 | −0.0318 | 0.00 | .024 | .1608 | 249 | 196 |
| Amygdala | −0.2638 | 0.1322 | −0.5229 | −0.0048 | 29.6 | .0459 | .1608 | 249 | 200 |
| Accumbens | −0.1483 | 0.1175 | −0.3787 | 0.0821 | 14.28 | .207 | .3623 | 250 | 200 |
| Bankssts | −0.12 | 0.11 | −0.34 | 0.09 | 0.00 | .259 | .4797 | 229 | 186 |
| Caudalanteriorcingulate | 0.01 | 0.107 | −0.2 | 0.22 | 0.005 | .952 | .9783 | 247 | 200 |
| Caudalmiddlefrontal | −0.26 | 0.106 | −0.47 | −0.05 | 0.00 | .015 | .1031 | 248 | 200 |
| Cuneus | −0.14 | 0.143 | −0.42 | 0.14 | 39.02 | .325 | .5017 | 248 | 200 |
| Entorhinal | −0.1 | 0.154 | −0.4 | 0.2 | 41.499 | .512 | .6217 | 230 | 175 |
| Fusiform | −0.14 | 0.127 | −0.39 | 0.11 | 25.048 | .277 | .4797 | 250 | 200 |
| Inferior parietal | −0.17 | 0.123 | −0.41 | 0.07 | 20.127 | .173 | .3667 | 246 | 199 |
| Inferior temporal | −0.07 | 0.133 | −0.33 | 0.19 | 30.497 | .609 | .6681 | 248 | 200 |
| Isthmus cingulate | 0.00 | 0.128 | −0.25 | 0.25 | 25.557 | .978 | .9783 | 246 | 200 |
| Lateral occipital | −0.11 | 0.134 | −0.37 | 0.16 | 31.126 | .43 | .5727 | 247 | 199 |
| Lateral orbitofrontal | −0.2 | 0.128 | −0.45 | 0.05 | 25.254 | .12 | .3667 | 250 | 200 |
| Lingual | −0.16 | 0.118 | −0.4 | 0.07 | 14.98 | .165 | .3667 | 248 | 200 |
| Medial orbitofrontal | −0.33 | 0.107 | −0.54 | −0.12 | 0.00 | .002 | .0672 | 248 | 199 |
| Middle temporal | −0.01 | 0.108 | −0.22 | 0.2 | 0.00 | .918 | .9749 | 239 | 192 |
| Parahippocampal | −0.19 | 0.141 | −0.47 | 0.09 | 37.631 | .18 | .3667 | 250 | 200 |
| Paracentral | −0.23 | 0.109 | −0.44 | −0.01 | 3.011 | .037 | .2075 | 249 | 200 |
| Pars opercularis | −0.06 | 0.108 | −0.28 | 0.15 | 0.00 | .549 | .6436 | 243 | 197 |
| Pars orbitalis | −0.09 | 0.107 | −0.3 | 0.12 | 0.00 | .382 | .5414 | 249 | 199 |
| Pars triangularis | −0.18 | 0.107 | −0.39 | 0.03 | 0.00 | .093 | .3221 | 249 | 197 |
| Pericalcarine | −0.29 | 0.144 | −0.57 | −0.01 | 39.396 | .043 | .2076 | 248 | 200 |
| Postcentral | −0.23 | 0.161 | −0.55 | 0.08 | 51.628 | .15 | .3667 | 249 | 200 |
| Posterior cingulate | −0.12 | 0.138 | −0.39 | 0.15 | 35.024 | .382 | .5414 | 248 | 200 |
| Precentral | −0.3 | 0.121 | −0.54 | −0.06 | 17.566 | .013 | .1031 | 244 | 200 |
| Precuneus | −0.16 | 0.123 | −0.41 | 0.08 | 20.083 | .183 | .3667 | 246 | 200 |
| Rostralanteriorcingulate | −0.08 | 0.107 | −0.29 | 0.13 | 0.00 | .456 | .5738 | 246 | 200 |
| Rostral middle frontal | −0.06 | 0.107 | −0.27 | 0.15 | 0.00 | .594 | .6681 | 249 | 199 |
| Superior frontal | −0.16 | 0.106 | −0.37 | 0.05 | 0.00 | .129 | .3667 | 248 | 200 |
| Superior parietal | −0.21 | 0.126 | −0.46 | 0.04 | 23.49 | .095 | .3221 | 250 | 200 |
| Superior temporal | −0.15 | 0.145 | −0.43 | 0.14 | 38.979 | .312 | .5017 | 239 | 193 |
| Supramarginal | −0.11 | 0.137 | −0.37 | 0.16 | 32.748 | .438 | .5727 | 244 | 200 |
| Frontal pole | −0.11 | 0.106 | −0.32 | 0.09 | 0.007 | .282 | .4797 | 250 | 200 |
| Temporal pole | −0.3 | 0.107 | −0.5 | −0.09 | 0.00 | .006 | .0955 | 250 | 200 |
| Transverse temporal | −0.2 | 0.106 | −0.41 | 0.01 | 0.00 | .061 | .2580 | 249 | 200 |
| Insula | −0.29 | 0.119 | −0.53 | −0.06 | 15.824 | .014 | .1031 | 250 | 200 |
Abbreviations: Bankssts, banks of the superior temporal sulcus; CI.LB, confidence interval lower bound; CI.UB, confidence interval upper bound; ES, effect size; p FDR, adjusted p‐value for the seven sites following a BH correction; ROI, region of interest.PFDR less than 0.05 are bolded
Figure 4 presents the comparison between bilateral cortical thickness results for AUD, CUD and previously published ENIGMA meta‐analysis comparing MDD cases to controls on comparable cortical thickness measures. ROIs reported in Figure 4 were significantly different between cases and controls for MDD, AUD or both of them. There is an overlap in confidence for most ROIs except temporal pole, where AUD and CUD showed greater reductions in cortical thickness when compared to MDD. However, the temporal pole for CUD did not survive FDR correction, so it is not a reliable finding. Relative to MDD effects, greater AUD‐related reductions are observed in the fusiform since the CIs do not overlap. Even if reduced cortical thickness in the medial orbitofrontal cortex for CUD is greater than in MDD since the intervals do not overlap, the large CIs for CUD suggest using caution interpreting this finding.
FIGURE 4.
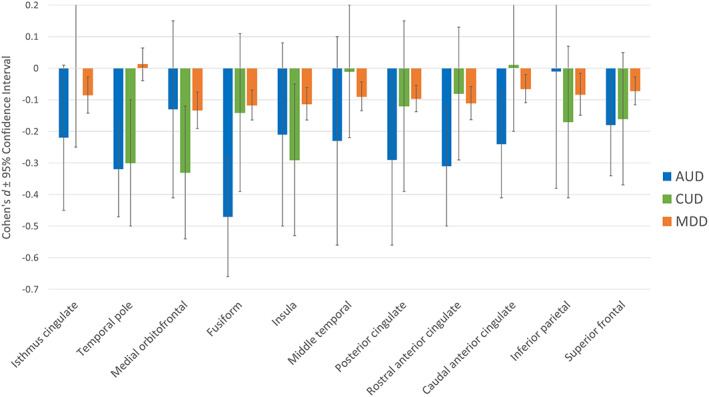
Comparison between bilateral cortical thickness results for alcohol (AUD), cannabis use disorder (CUD) and depression (MDD) on region of interests. Bilateral effects represent mean unilateral effect for each region. Error bars represent 95% confidence intervals. Significant volumetric variations when compared to age‐, sex‐ and disorder‐matched controls was observed when the confidence intervals did not overlap with non‐effect line at 0 and survived false discovery rate correction
Figure 5 compares AUD and CUD effects to the results reported for BD (Hibar, Westlye, Doan, et al., 2018; Hibar, Westlye, van Erp, et al., 2016) and SCZ (van Erp et al., 2016; Van Erp, Walton, Hibar, et al., 2018) . Most regions identified in the AUD cortical thickness analyses were also reported to be significant for BD and SCZ, with the exception of the rostral anterior cingulate and the temporal pole, which were not reported as significant for BD but were significant for SCZ. However, most of the effect sizes showed overlapping CIs, suggesting no discernible differences. The CIs do not overlap for temporal pole between AUD and BD, with greater case–control differences (smaller volumes) in AUD. Of interest, the four regions that marginally discriminated CUD from controls showed overlap with AUD, BD and SCZ findings (medial orbitofrontal, caudal middle frontal, precentral gyrus and insula). This figure also shows that relative to other disorders, there was considerable heterogeneity in AUD and CUD findings (large CIs) for cortical thickness measures.
FIGURE 5.
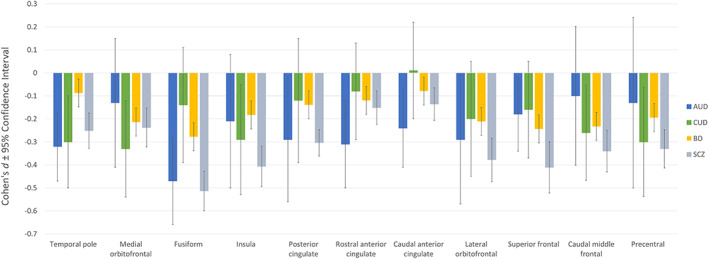
Comparison between bilateral cortical thickness results for alcohol use disorder (AUD), cannabis use disorder (CUD), bipolar disorder (BD) and schizophrenia (SCZ) on region of interests. Bilateral effects represent mean unilateral effect for each region. Significant volumetric variations when compared to age‐, sex‐ and disorder‐matched controls was observed when the confidence intervals did not overlap with non‐effect line at 0 and survived false discovery rate correction. Error bars represent 95% confidence intervals
4. CONCLUSION AND CONTEXT
Health outcomes of various neurological and psychiatric conditions are currently compared using models such as global burden of disease, focusing on years of life lost, or severe indicators of disability. However, less developed are the models focusing on the quantification of brain‐related impairment. This study represents a first step in developing and validating a method for comparing brain health/impairment for which the next steps will necessarily require association with quality of life or disability measures. We observed reduced bilateral volumes in the thalamus, putamen, hippocampus, amygdala in alcohol‐dependent participants. Additionally, AUD subjects showed reduced cortical thickness in the rostral and caudal anterior cingulate gyri, fusiform, inferior temporal, parahippocampal, posterior cingulate, superior frontal and temporal pole. No significant subcortical volume or cortical thickness variation was observed in cannabis‐dependent subjects. The AUD variations mentioned above are consistent with previous meta‐analytic findings (Xiao et al., 2015; Yang et al., 2016), which support the proposal that alcohol dependence consistently impacts specific subcortical and cortical regions in the adult brain. The altered subcortical and cortical regions in AUD reported could give some insight into the pathophysiology of alcohol dependence. The amygdala, accumbens, hippocampus and cingulate regions showed reduced volumes in AUD. These observations are interesting since these regions are part of the meso‐cortico‐limbic system, which has been shown to be central to addictive behaviours in pre‐clinical studies (Baler & Volkow, 2006). This meta‐analysis of human neuroimaging studies confirms reliable alcohol‐related brain impairment in this brain system.
The current findings suggest that AUD is associated with significant alterations in subcortical brain structures and at a magnitude that is comparable to or larger than other major psychiatric disorders. Furthermore, results for alcohol were comparable to those previously reported for patients with psychosis, at least within the thalamus, hippocampus, amygdala and accumbens and were significantly larger than MDD in the hippocampus, amygdala and accumbens. When comparing AUD findings to MDD findings on cortical thickness across all ROIs of the brain, AUD was generally associated with reductions in cortical thickness across many of the same brain regions that were associated with MDD, but alcohol produced larger effect sizes, with two regions showing significantly smaller volumes in AUD participants compared to MDD participants: fusiform gyrus and temporal pole. Compared to SCZ and BD, AUD‐related differences in brain volume overlapped with those reported for these other disorders, but there was evidence that AUD might be particularly linked to abnormalities in the cingulate.
The absence of significant variations that survived FDR correction in CUD participants is interesting given the debate on the potential neurotoxic impact of cannabis dependence on the hippocampus and the medial orbitofrontal cortex (Lorenzetti et al., 2019b; Rocchetti et al., 2013). This could be partly explained by the substantial heterogeneity observed within the included studies (Supporting Information Figure 5). Even if the reduced thickness observed in the medial orbitofrontal and the insula cortices is consistent with previous findings in long‐term users (Battistella et al., 2014; Lorenzetti et al., 2019b), further studies are required to determine if these observations are part of the structural signature of the CUD pathophysiology.
The current findings suggest that AUD is associated with significant alterations in subcortical brain structures and at a magnitude that is comparable to or larger than other major psychiatric disorders. Furthermore, results for alcohol were comparable to those previously reported for patients with psychosis, at least within the thalamus, hippocampus, amygdala and accumbens and were significantly larger than MDD in the hippocampus, amygdala and accumbens. When comparing AUD findings to MDD findings on cortical thickness across all ROIs of the brain, AUD was generally associated with reductions in cortical thickness across many of the same brain regions that were associated with MDD, but alcohol produced larger effect sizes, with two regions showing significantly smaller volumes in AUD participants compared to MDD participants: fusiform gyrus and temporal pole. Compared to SCZ and BD, AUD‐related differences in brain volume overlapped with those reported for these other disorders, but there was evidence that AUD might be particularly linked to abnormalities in the cingulate. By contrast, cortical thickness analyses on CUD case–control studies suggest convergent findings with a previous study from the ENIGMA‐Addiction working group (Mackey et al., 2018). The regions that most distinguished CUD cases from controls (though only achieving marginal statistical significance) also significantly discriminated BD and SCZ cases from controls. Hibar et al. and Van Erp et al. both previously reported reduced cortical thickness in medial orbitofrontal, insula, caudal middle frontal and precentral gyrus in BD and SCZ, which were not observed for AUD in the current study but appear marginally significant for CUD in the current study (Hibar et al., 2018; Van Erp et al., 2018). Recognising that these findings require further investigation due to the non‐significance revealed for cannabis after FDR correction, they are worth pursuing considering that cannabis and psychosis show a strong relationship in clinical and epidemiologic studies (reviewed by Gage, Hickman, & Zammit, 2016). This relationship has been shown to be independent of other substance use and other mental health symptoms (Bourque, Afzali, O'Leary‐Barrett, & Conrod, 2017). These effects should also be further investigated to determine if they reflect a potential unexamined effect of substance misuse in the BD and SCZ studies, or a potential common underlying vulnerability towards cannabis use and psychotic disorders.
Using this preliminary study as a proof‐of‐concept, future meta‐analyses focusing on pooling across disease groups will help to identify brain attributes that are common to mental disorders and those that are more disease‐specific, using multi‐level analytic strategies. These findings could then guide more focused, region of interest analyses incorporating behavioural, genetic, or brain diffusion/connectivity measures. There might also be some value in linking these findings to other global measures of disability, for example, Global Assessment of Functioning measures, to help identify the brain regions that most affect disability across disorders and within specific disorders.
The current findings of smaller volumes and cortical alteration in the hippocampus, amygdala, accumbens, temporal pole, fusiform of AUD patients relative to patients with MDD (and comparable to patients with SCZ or BD are consistent with the observation that alcohol dependence is associated with specific impairment in memory encoding and recall (Chanraud et al., 2012). However, effect sizes for all subcortical regions (including pallidum and putamen) along with alterations in the anterior cingulate, indicate that alcohol dependence is potentially associated with impairment in brain regions implicated in affect regulation, mood, attention/concentration, motivation, processing speed, behavioural control and executive functions (Tanabe, Regner, Sakai, Martinez, & Gowin, 2019). These functions are not typically measured on brief tests of general intellectual functioning or memory and require more elaborate neuropsychological and repeated behavioural assessments in order to be detected. The current findings suggest a need for investment in cognitive rehabilitative services for patients with AUD that are comparable in terms of specificity and intensity to those available to patients with SCZ, and focusing on more subcortically mediated functions.
Disability measures can be confounded by factors such as social desirability, stigma, education and socioeconomic status, and might explain the inequities in treatment gap observed across the mental and neurologic disorders. The current findings, using an objectively derived brain metric, allow for a cross‐disorder comparison that is free of culturally influenced biases and places AUD in the same class as SCZ, and far above MDD, in terms of brain‐related impairment.
Substance use disorders are detrimentally under‐treated in society, despite their demonstrated social and health costs (Rehm et al., 2007). For example, one community‐based psychiatric epidemiology study conducted by the Global Burden of Disease consortium examined standardised diagnostic instruments and statistics on percentage of individuals receiving care across various WHO regions. This study found that the treatment gap was not equitable across mental disorders and that the largest treatment gap was reported for AUDs: 92% of alcohol‐dependent individuals in Europe were recognised as not having received services, while only 17% of individuals with SCZ were not receiving services (Kohn et al., 2004). The findings from the current study provide some context for understanding the level of brain‐related impairment that alcohol‐dependent patients experience relative to people suffering from other mental disorders and could eventually be used to inform policy to promote health equity in addition to brain‐based rehabilitative services.
The available data from CUD case–control comparisons suggests that there is significant heterogeneity in these studies, limiting the confidence around possible case–control differences. While effect sizes in some subcortical and cortical regions were similar in magnitude to those revealed for alcohol and other psychiatric conditions, the CIs around these effect sizes were so large that no conclusions could be drawn regarding whether such differences were statistically significant. This variation could be partly explained by the methodological differences among the included sites for assessing cannabis abuse.
Another limitation was related to the historical variation in diagnostic criteria for SUD, which changed between 2000 and 2014 in order to better capture the nature of addiction to a variety of substances of abuse. Therefore, in the current meta‐analysis, some studies used more conservative inclusion criteria than others, and this is particularly the case for cannabis studies. However, heterogeneity within CUD studies appear just as evident as across CUD studies. More research is required to evaluate the brain alterations in heavy and problematic cannabis use, potentially placing greater attention on severity of CUD or quantity or type of substance abused, in order to reduce variability within and across studies. Recognising that the current study did not control for severity substance use disorder, in order to be optimally comparable with published results on other psychiatric conditions, it is recommended that future ENIGMA studies attempting to compare and contrast brain correlates of substance use and psychiatric conditions derive global (harmonised) severity measures to further reduce variability within and across studies.
Similarly, in order to be optimally comparable with the published results for other psychiatric disorders, the effects of tobacco use were not controlled for (Stoychev, 2019). Considering that psychiatric conditions and substance use disorders have different patterns of co‐occurrence with nicotine dependence, future studies could explore the role of this confounding variable and other co‐variates to begin to explain disorder‐specific and disorder‐general volumetric variations.
Sex‐specific results were also not reported by all working groups, therefore complete comparisons of the sex‐related differences would have been limited. Future studies should consider making sex‐specific results available in a consistent way in order to compare sex‐specific variations across psychiatric disorders. Although the presence of other substance use disorders (other than nicotine dependence) were exclusion criteria for inclusion in each of the AUD and CUD studies, the analyses did not control for concurrent and recreational substance use. Efforts to develop harmonised quantitative measures of quantity and frequency of drug use are underway within the ENIGMA‐Addiction working group and will be investigated in the future.
Finally, the number and sample sizes of substance use disorder studies were generally smaller than those reported for MDD, SCZ and BD, which also likely contributed to the greater variance in effects in the SUD studies (AUD studies also showed high heterogeneity). These findings, suggest the need for further investment in research on brain correlates of substance use disorders, considering their demonstrated role in global health (Ezzati et al., 2004) and the magnitude of the effects reported in this study.
Supporting information
Data S1 Supporting Information
ACKNOWLEDGMENTS
This study was supported by the following project grants: National Institutes of Health (NIH) Grant U54 EB 020403 with funds provided for the trans‐NIH Big Data to Knowledge (BD2K) initiative; R01 MH116147 to P. M. T., N. J., and P. J. C.; NIDA 1R01DA047119‐01, awarded to Hugh Garavan and P. J. C., and a Canada Research Chair, awarded to P. J. C. Data collection: O. K. received support for the Neuro‐ADAPT study from VICI grant no. 453.08.01 from the Netherlands Organization for Scientific Research (NWO) awarded to R. W. D. J. V. received funding for the TrIP study from ZonMW grant no. 31160003 from the Netherlands Organization for Scientific Research (NWO). D. J. V. received funding for the NESDA‐AD study from ZonMW grant no. 31160004 from the Netherlands Organization for Scientific Research (NWO). R. J. v. H. received funding for the ADPG study from ZonMW grant no. 91676084 from the Netherlands Organization for Scientific Research (NWO). D. J. V. received funding for the DABIS study from VIDI grant no. 016.08.322 from the Netherlands Organization for Scientific Research (NWO) awarded to Ingmar H A Franken. J. C. received funding for the Cannabis Prospective study from ZonMW grant no. 31180002 from the Netherlands Organization for Scientific Research (NWO). R. S. received funds from NIH/NIDA: PL30‐1DA024859‐01 and NIH/NCRR: UL1‐RR24925‐01. M. Y. was supported by a National Health and Medical Research Council Fellowship (#1117188) and the David Winston Turner Endowment Fund.
Navarri X, Afzali MH, Lavoie J, et al. How do substance use disorders compare to other psychiatric conditions on structural brain abnormalities? A cross‐disorder meta‐analytic comparison using the ENIGMA consortium findings. Hum Brain Mapp. 2022;43:399–413. 10.1002/hbm.25114
Funding information National Institute on Drug Abuse, Grant/Award Numbers: 1R21DA038381, 1R01DA047119‐01; NIH Clinical Center, Grant/Award Numbers: U54 EB 020403, R01 MH116147; David Winston Turner Endowment Fund; National Health and Medical Research Council Fellowship, Grant/Award Number: 1117188; NIH/NIDA, Grant/Award Numbers: UL1‐RR24925‐01, PL30‐1DA024859‐01; VIDI, Grant/Award Number: 016.08.322; ZonMW, Grant/Award Numbers: 31180002, 91676084, 31160004, 31160003; VICI, Grant/Award Number: 453.08.01
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- American Psychiatric Association . (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Ashtari, M. , Avants, B. , Cyckowski, L. , Cervellione, K. L. , Roofeh, D. , Cook, P. , … Kumra, S. (2011). Medial temporal structures and memory functions in adolescents with heavy cannabis use. Journal of Psychiatric Research, 45(8), 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler, R. D. , & Volkow, N. D. (2006). Drug addiction: The neurobiology of disrupted self‐control. Trends in Molecular Medicine, 12(12), 559–566. [DOI] [PubMed] [Google Scholar]
- Batalla, A. , Bhattacharyya, S. , Yuecel, M. , Fusar‐Poli, P. , Crippa, J. A. , Nogue, S. , … Martin‐Santos, R. (2013). Structural and functional imaging studies in chronic cannabis users: A systematic review of adolescent and adult findings. PLoS One, 8(2), e55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalla, A. , Soriano‐Mas, C. , López‐Solà, M. , Torrens, M. , Crippa, J. A. , Bhattacharyya, S. , … Martín‐Santos, R. (2014). Modulation of brain structure by catechol‐O‐methyltransferaseVal158Metpolymorphism in chronic cannabis users. Addiction Biology, 19(4), 722–732. [DOI] [PubMed] [Google Scholar]
- Battistella, G. , Fornari, E. , Annoni, J.‐M. , Chtioui, H. , Dao, K. , Fabritius, M. , … Giroud, C. (2014). Long‐term effects of cannabis on brain structure. Neuropsychopharmacology, 39(9), 2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco‐Hinojo, L. , Pujol, J. , Harrison, B. J. , Macià, D. , Batalla, A. , Nogué, S. , & Martín‐Santos, R. (2017). Attenuated frontal and sensory inputs to the basal ganglia in cannabis users. Addiction Biology, 22(4), 1036–1047. [DOI] [PubMed] [Google Scholar]
- Bourque, J. , Afzali, M. H. , O'Leary‐Barrett, M. , & Conrod, P. (2017). Cannabis use and psychotic‐like experiences trajectories during early adolescence: The coevolution and potential mediators. Journal of Child Psychology and Psychiatry, 58(12), 1360–1369. [DOI] [PubMed] [Google Scholar]
- Chanraud, S. , Pitel, A.‐L. , Müller‐Oehring, E. M. , Pfefferbaum, A. , & Sullivan, E. V. (2012). Remapping the brain to compensate for impairment in recovering alcoholics. Cerebral Cortex, 23(1), 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski, C. , & Golden, C. J. (1980). Alcoholism and brain damage: An investigation using the Luria‐Nebraska Neuropsychological Battery. International Journal of Neuroscience, 10(2–3), 99–105. [DOI] [PubMed] [Google Scholar]
- Chye, Y. , Mackey, S. , Gutman, B. A. , Ching, C. R. K. , Batalla, A. , Blaine, S. , … Garavan, H. (2019). Subcortical surface morphometry in substance dependence: An ENIGMA addiction working group study. Addiction Biology, e12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn, J. , Wiers, R. W. , Ridderinkhof, K. R. , van den Brink, W. , Veltman, D. J. , & Goudriaan, A. E. (2012). Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. NeuroImage, 59(4), 3845–3851. [DOI] [PubMed] [Google Scholar]
- Cousijn, J. , Goudriaan, A. E. , Ridderinkhof, K. R. , van den Brink, W. , Veltman, D. J. , & Wiers, R. W. (2013). Neural responses associated with cue‐reactivity in frequent cannabis users. Addiction Biology, 18(3), 570–580. [DOI] [PubMed] [Google Scholar]
- Cousijn, J. , Wiers, R. W. , Ridderinkhof, K. R. , van den Brink, W. , Veltman, D. J. , & Goudriaan, A. E. (2014). Effect of baseline cannabis use and working‐memory network function on changes in cannabis use in heavy cannabis users: A prospective fMRI study. Human Brain Mapping, 35(5), 2470–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt, L. , Ferrari, A. J. , Calabria, B. , Hall, W. D. , Norman, R. E. , McGrath, J. , … Vos, T. (2013). The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: Results from the GBD 2010 study. PLoS One, 8(10), e76635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati, M. , Lopez, A. , Rodgers, A. , & Murray, C. (2004). Comparative quantification of health risks: Global and regional burden of disease attributable to selected major risk factors. Geneva, Switzerland: WHO. [Google Scholar]
- Fein, G. , Greenstein, D. , Cardenas, V. A. , Cuzen, N. L. , Fouche, J.‐P. , Ferrett, H. , … Stein, D. J. (2013). Cortical and subcortical volumes in adolescents with alcohol dependence but without substance or psychiatric comorbidities. Psychiatry Research: Neuroimaging, 214(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein, G. , Sclafani, V. , Cardenas, V. A. , Goldmann, H. , Tolou‐Shams, M. , & Meyerhoff, D. J. (2002). Cortical gray matter loss in treatment‐naive alcohol dependent individuals. Alcoholism: Clinical and Experimental Research, 26(4), 558–564. [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , … Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Gage, S. H. , Hickman, M. , & Zammit, S. (2016). Association between cannabis and psychosis: Epidemiologic evidence. Biological Psychiatry, 79(7), 549–556. [DOI] [PubMed] [Google Scholar]
- Gallucci, M. , Amicarelli, I. , Rossi, A. , Stratta, P. , Masciocchi, C. , Zobel, B. B. , … Passariello, R. (1989). MR imaging of white matter lesions in uncomplicated chronic alcoholism. Journal of Computer Assisted Tomography, 13(3), 395–398. [DOI] [PubMed] [Google Scholar]
- Grodin, E. N. , Lin, H. , Durkee, C. A. , Hommer, D. W. , & Momenan, R. (2013). Deficits in cortical, diencephalic and midbrain gray matter in alcoholism measured by VBM: Effects of co‐morbid substance abuse. NeuroImage: Clinical, 2, 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, I. H. , Solowij, N. , Harrison, B. J. , Takagi, M. , Lorenzetti, V. , Lubman, D. I. , & Yücel, M. (2012). Functional Connectivity in Brain Networks Underlying Cognitive Control in Chronic Cannabis Users. Neuropsychopharmacology, 37(8), 1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester, R. , Nestor, L. , & Garavan, H. (2009). Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology, 34(11), 2450–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Stein, J. L. , Renteria, M. E. , Arias‐Vasquez, A. , Desrivières, S. , Jahanshad, N. , … Medland, S. E. (2015). Common genetic variants influence human subcortical brain structures. Nature, 520(7546), 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Westlye, L. T. , Doan, N. T. , Jahanshad, N. , Cheung, J. W. , Ching, C. R. K. , … Andreassen, O. A. (2018). Cortical abnormalities in bipolar disorder: An MRI analysis of 6503 individuals from the ENIGMA bipolar disorder working group. Molecular Psychiatry, 23(4), 932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Westlye, L. T. , van Erp, T. G. M. , Rasmussen, J. , Leonardo, C. D. , Faskowitz, J. , … Andreassen, O. A. (2016). Subcortical volumetric abnormalities in bipolar disorder. Molecular Psychiatry, 21(12), 1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman, M. , Bralten, J. , Hibar, D. P. , Mennes, M. , Zwiers, M. P. , Schweren, L. S. J. , … Franke, B. (2017). Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross‐sectional mega‐analysis. The Lancet Psychiatry, 4(4), 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakabek, D. , Yücel, M. , Lorenzetti, V. , & Solowij, N. (2016). An MRI study of white matter tract integrity in regular cannabis users: effects of cannabis use and age. Psychopharmacology, 233(19‐20), 3627–3637. [DOI] [PubMed] [Google Scholar]
- Jansen, J. M. , van Holst, R. J. , van den Brink, W. , Veltman, D. J. , Caan, M.W. A. , & Goudriaan, A. E. (2015). Brain function during cognitive flexibility and white matter integrity in alcohol‐dependent patients, problematic drinkers and healthy controls. Addiction Biology, 20(5), 979–989. [DOI] [PubMed] [Google Scholar]
- Joyce, E. M. (1994). Aetiology of alcoholic brain damage: Alcoholic neurotoxicity or thiamine malnutrition? British Medical Bulletin, 50(1), 99–114. [DOI] [PubMed] [Google Scholar]
- Klaming, R. , Harlé, K. M. , Infante, M. A. , Bomyea, J. , Kim, C. , & Spadoni, A. D. (2019). Shared gray matter reductions across alcohol use disorder and posttraumatic stress disorder in the anterior cingulate cortex: A dual meta‐analysis. Neurobiology of Stress, 10, 100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, R. , Saxena, S. , Levav, I. , & Saraceno, B. (2004). The treatment gap in mental health care. Bulletin of the World Health Organization, 82, 858–866. [PMC free article] [PubMed] [Google Scholar]
- Kornreich, C. , Blairy, S. , Philippot, P. , Hess, U. , Noël, X. , Streel, E. , … Verbanck, P. (2001). Deficits in recognition of emotional facial expression are still present in alcoholics after mid‐ to long‐term abstinence. Journal of Studies on Alcohol, 62(4), 533–542. [DOI] [PubMed] [Google Scholar]
- Korucuoglu, O. , Gladwin, T. E. , Baas, F. , Mocking, R. J.T. , Ruhé, H. G. , Groot, P.F.C. , & Wiers, R. W. (2017). Neural response to alcohol taste cues in youth: effects of the OPRM1 gene. Addiction Biology, 22(6), 1562–1575. [DOI] [PubMed] [Google Scholar]
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t‐tests and ANOVAs. Frontiers in Psychology, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot, R. K. , Gogtay, N. , Greenstein, D. K. , Molloy Wells, E. , Wallace, G. L. , Clasena, L. S. , … Giedd, J. N. (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage, 36(4), 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. R. , Luo, X. , Yan, P. , Bergquist, K. , & Sinha, R. (2009). Altered Impulse Control in Alcohol Dependence: Neural Measures of Stop Signal Performance. Alcoholism: Clinical and Experimental Research, 33(4), 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti, V. , Chye, Y. , Silva, P. , Solowij, N. , & Roberts, C. A. (2019a). Neuroscience c. does regular cannabis use affect neuroanatomy? An updated systematic review and meta‐analysis of structural neuroimaging studies. European Archives of Psychiatry and Clinical Neuroscience, 269(1), 59–71. [DOI] [PubMed] [Google Scholar]
- Lorenzetti, V. , Chye, Y. , Silva, P. , Solowij, N. , & Roberts, C. A. (2019b). Does regular cannabis use affect neuroanatomy? An updated systematic review and meta‐analysis of structural neuroimaging studies. European Archives of Psychiatry and Clinical Neuroscience, 269(1), 59–71. [DOI] [PubMed] [Google Scholar]
- Lorenzetti, V. , Solowij, N. , Whittle, S. , Fornito, A. , Lubman, D. I. , Pantelis, C. , & Yücel, M. (2015). Gross morphological brain changes with chronic, heavy cannabis use. British Journal of Psychiatry, 206(1), 77–78. [DOI] [PubMed] [Google Scholar]
- Lozano, R. , Naghavi, M. , Foreman, K. , Lim, S. , Shibuya, K. , Aboyans, V. , … Murray, C. G. L. (2012). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet, 380(9859), 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey, S. , Allgaier, N. , Chaarani, B. , Spechler, P. , Orr, C. , Bunn, J. , … Garavan, H. (2018). Mega‐analysis of gray matter volume in substance dependence: General and substance‐specific regional effects. American Journal of Psychiatry, 176(2), 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik, J. A. , Eldreth, D. A. , Cadet, J.‐L. , & Bolla, K. I. (2005). Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence, 77(1), 23–30. [DOI] [PubMed] [Google Scholar]
- Miller, W. R. , & Orr, J. (1980). Nature and sequence of neuropsychological deficits in alcoholics. Journal of Studies on Alcohol, 41(3), 325–337. [DOI] [PubMed] [Google Scholar]
- Momenan, R. , Steckler, L. E. , Saad, Z. S. , van Rafelghem, S. , Kerich, M. J. , & Hommer, D. W. (2012). Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Research: Neuroimaging, 204(2‐3), 101–111. [DOI] [PubMed] [Google Scholar]
- Murray, C. J. L. , Barber, R. M. , Foreman, K. J. , Abbasoglu Ozgoren, A. , Abd‐Allah, F. , Abera, S. F. , … Vos, T. (2015). Global, regional, and national disability‐adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. The Lancet, 386(10009), 2145–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader, D. A. , & Sanchez, Z. M. (2018). Effects of regular cannabis use on neurocognition, brain structure, and function: A systematic review of findings in adults. The American Journal of Drug and Alcohol Abuse, 44(1), 4–18. [DOI] [PubMed] [Google Scholar]
- Orr, C. , Morioka, R. , Behan, B. , Datwani, S. , Doucet, M. , Ivanovic, J. , … Garavan, H. (2013). Altered resting‐state connectivity in adolescent cannabis users. The American Journal of Drug and Alcohol Abuse, 39(6), 372–381. [DOI] [PubMed] [Google Scholar]
- Parsons, O. A. (1998). Neurocognitive deficits in alcoholics and social drinkers: A continuum? Alcoholism, Clinical and Experimental Research, 22(4), 954–961. [PubMed] [Google Scholar]
- Parsons, O. A. , & Nixon, S. J. (1998). Cognitive functioning in sober social drinkers: A review of the research since 1986. Journal of Studies on Alcohol, 59(2), 180–190. [DOI] [PubMed] [Google Scholar]
- Peacock, A. , Leung, J. , Larney, S. , Colledge, S. , Hickman, M. , Rehm, J. , … Degenhardt, L. (2018). Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction, 113(10), 1905–1926. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum, A. , & Sullivan, E. V. (2005). Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: Evidence from diffusion tensor imaging. Neuropsychopharmacology, 30(2), 423–432. [DOI] [PubMed] [Google Scholar]
- Pujol, J. , Blanco‐Hinojo, L. , Batalla, A. , López‐Solà, M. , Harrison, B. J. , Soriano‐Mas, C. , … Martín‐Santos, R. (2014). Functional connectivity alterations in brain networks relevant to self‐awareness in chronic cannabis users. Journal of Psychiatric Research, 51, 68–78. [DOI] [PubMed] [Google Scholar]
- Reed, L. J. , Lasserson, D. , Marsden, P. , Stanhope, N. , Stevens, T. , Bello, F. , … Kopelman, M. D. (2003). FDG‐PET findings in the Wernicke–Korsakoff syndrome. Cortex, 39(4–5), 1027–1045. [DOI] [PubMed] [Google Scholar]
- Rehm, J. , Gnam, W. , Popova, S. , Baliunas, D. , Brochu, S. , Fischer, B. , … Taylor, B. (2007). The costs of alcohol, illegal drugs, and tobacco in Canada, 2002. Journal of Studies on Alcohol and Drugs, 68(6), 886–895. [DOI] [PubMed] [Google Scholar]
- Ritchie, H. , & Roser, M. (2019) Alcohol consumption. Retrieved from https://ourworldindata.org/alcohol-consumption.
- Rocchetti, M. , Crescini, A. , Borgwardt, S. , Caverzasi, E. , Politi, P. , Atakan, Z. , & Fusar‐Poli, P. (2013). Is cannabis neurotoxic for the healthy brain? A meta‐analytical review of structural brain alterations in non‐psychotic users. Psychiatry and Clinical Neurosciences, 67(7), 483–492. [DOI] [PubMed] [Google Scholar]
- Rosenbloom, M. , Sullivan, E. V. , & Pfefferbaum, A. (2003). Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol Research and Health, 27(2), 146–152. [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Goudriaan, A. E. , Joos, L. , Dom, G. , Pattij, T. , van den Brink, W. , & Veltman, D. J. (2014). Neural substrates of impulsive decision making modulated by modafinil in alcohol‐dependent patients. Psychological Medicine, 44(13), 2787–2798. [DOI] [PubMed] [Google Scholar]
- Schmaal, L. , Hibar, D. P. , Sämann, P. G. , Hall, G. B. , Baune, B. T. , Jahanshad, N. , … Veltman, D. J. (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Molecular Psychiatry, 22(6), 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatorov, V. V. , Damadzic, R. , Mann, C. L. , Schwandt, M. L. , George, D. T. , Hommer, D. W. , … Momenan, R. (2015). Reduced anterior insula, enlarged amygdala in alcoholism and associated depleted von Economo neurons. Brain, 138(1), 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, D. , Jia, Z. , Lacadie, C. M. , Tsou, K. A. , Bergquist, K. , & Sinha, R. (2011). Sex differences in neural responses to stress and alcohol context cues. Human Brain Mapping, 32(11), 1998–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherif, T. , Rioux, P. , Rousseau, M.‐E. , Kassis, N. , Beck, N. , Reza Adalat, R. , … Evans, A. C. (2014). CBRAIN: A web‐based, distributed computing platform for collaborative neuroimaging research. Frontiers in Neuroinformatics, 8, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, R. , & Li, C. S. R. (2007). Imaging stress‐ and cue‐induced drug and alcohol craving: association with relapse and clinical implications. Drug and Alcohol Review, 26(1), 25–31. [DOI] [PubMed] [Google Scholar]
- Sjoerds, Z. , van den Brink, W. , Beekman, A. T. F. , Penninx, B. W. J. H. , Veltman, D. J. (2014). Response inhibition in alcohol‐dependent patients and patients with depression/anxiety: a functional magnetic resonance imaging study. Psychological Medicine, 44(8), 1713–1725. [DOI] [PubMed] [Google Scholar]
- Solowij, N. , Yücel, M. , Respondek, C. , Whittle, S. , Lindsay, E. , Pantelis, C. , & Lubman, D. I. (2011). Cerebellar white‐matter changes in cannabis users with and without schizophrenia. Psychological Medicine, 41(11), 2349–2359. [DOI] [PubMed] [Google Scholar]
- Solowij, N. , Walterfang, M. , Lubman, D. I. , Whittle, S. , Lorenzetti, V. , Styner, M. , & Yücel, M. (2013). Alteration to hippocampal shape in cannabis users with and without schizophrenia. Schizophrenia Research, 143(1), 179–184. [DOI] [PubMed] [Google Scholar]
- Stein, J. L. , Medland, S. E. , Vasquez, A. A. , Hibar, D. P. , Senstad, R. E. , Winkler, A. M. , … Thompson, P. T. , & Enhancing Neuro Imaging Genetics through Meta‐Analysis (ENIGMA) Consortium (2012). Identification of common variants associated with human hippocampal and intracranial volumes. Nature Genetics, 44(5), 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoychev, K. R. (2019). Neuroimaging studies in patients with mental disorder and co‐occurring substance use disorder: Summary of findings. Frontiers in Psychiatry, 10, 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, E. V. , & Pfefferbaum, A. (2005). Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology, 180(4), 583–594. [DOI] [PubMed] [Google Scholar]
- Tanabe, J. , Regner, M. , Sakai, J. , Martinez, D. , & Gowin, J. (2019). Neuroimaging reward, craving, learning, and cognitive control in substance use disorders: Review and implications for treatment. The British Journal of Radiology, 92(1101), 20180942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter, R. E. , & Alterman, A. I. (1984). Neuropsychological deficits in alcoholics: Etiological considerations. Journal of Studies on Alcohol, 45(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Thompson, P. M. , Stein, J. L. , Hibar, D. P. , Arias Vasquez, A. , Renteria, M. E. , … Crespo‐Facorro, B. (2014). The ENIGMA consortium: Large‐scale collaborative analyses of neuroimaging and genetic data. Brain Imaging and Behavior, 8(2), 153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townshend, J. M. , & Duka, T. (2003). Mixed emotions: Alcoholics' impairments in the recognition of specific emotional facial expressions. Neuropsychologia, 41(7), 773–782. [DOI] [PubMed] [Google Scholar]
- van Erp, T. G. M. , Hibar, D. P. , Rasmussen, J. M. , Glahn, D. C. , Pearlson, G. D. , Andreassen, O. A. , … Turner, J. A. , & ENIGMA Schizophrenia Working Group (2016). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Molecular Psychiatry, 21(4), 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Erp, T. G. M. , Walton, E. , Hibar, D. P. , Schmaal, L. , Jiang, W. , Glahn, D. C. , … Turner, J. A. (2018). Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biological Psychiatry, 84(9), 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst, R. J. , Clark, L. , Veltman, D. J. , van den Brink, W. , & Goudriaan, A. E. (2014). Enhanced striatal responses during expectancy coding in alcohol dependence. Drug and Alcohol Dependence, 142, 204–208. [DOI] [PubMed] [Google Scholar]
- van Holst, R. J. , de Ruiter, M. B. , van den Brink, W. , Veltman, D. J. , & Goudriaan, A. E. (2012). A voxel‐based morphometry study comparing problem gamblers, alcohol abusers, and healthy controls. Drug and Alcohol Dependence, 124(1‐2), 142–148. [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. [Google Scholar]
- Wang, G. J. , Volkow, N. D. , Roque, C. T. , Cestaro, V. L. , Hitzemann, R. J. , Cantos, E. L. , … Dhawan, A. P. (1993). Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology, 186(1), 59–65. [DOI] [PubMed] [Google Scholar]
- Whiteford, H. A. , Degenhardt, L. , Rehm, J. , Baxter, A. J. , Ferrari, A. J. , Erskine, H. E. , … Vos, T. (2013). Global burden of disease attributable to mental and substance use disorders: Findings from the global burden of disease study 2010. The Lancet, 382(9904), 1575–1586. [DOI] [PubMed] [Google Scholar]
- Xiao, P. , Dai, Z. , Zhong, J. , Zhu, Y. , Shi, H. , & Pan, P. J. D. (2015). Regional gray matter deficits in alcohol dependence: A meta‐analysis of voxel‐based morphometry studies. Drug and Alcohol Dependence, 153, 22–28. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Tian, F. , Zhang, H. , Zeng, J. , Chen, T. , Wang, S. , … Gong, Q. (2016). Cortical and subcortical gray matter shrinkage in alcohol‐use disorders: A voxel‐based meta‐analysis. Neuroscience & Biobehavioral Reviews, 66, 92–103. [DOI] [PubMed] [Google Scholar]
- Yücel, M. , Lorenzetti, V. , Suo, C. , Zalesky, A. , Fornito, A. , Takagi, M. J. , … Solowij, N. (2016). Hippocampal harms, protection and recovery following regular cannabis use. Translational Psychiatry, 6(1), e710–e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel, M. , Solowij, N. , Respondek, C. , Whittle, S. , Fornito, A. , Pantelis, C. , & Lubman, D. I. (2008). Regional brain abnormalities associated with long‐term heavy cannabis use. Archives of General Psychiatry, 65(6), 694–701. [DOI] [PubMed] [Google Scholar]
- Yücel, M. , Solowij, N. , Respondek, C. , Whittle, S. , Fornito, A. , Pantelis, C. , & Lubman, D. I. (2008). Regional Brain Abnormalities Associated With Long‐term Heavy Cannabis Use. Archives of General Psychiatry, 65(6), 694. [DOI] [PubMed] [Google Scholar]
- Zahr, N. M. (2014). Structural and microstructral imaging of the brain in alcohol use disorders. In Handbook of clinical neurology (Vol. 125, pp. 275–290). Amsterdam, The Netherlands: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky, A. , Solowij, N. , Yucel, M. , Lubman, D. I. , Takagi, M. , Harding, I. H. , & Seal, M. (2012). Effect of long‐term cannabis use on axonal fibre connectivity. Brain, 135(7), 2245–2255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


