Abstract
Reproducibility is one of the most important issues for generalizing the results of clinical research; however, low reproducibility in neuroimaging studies is well known. To overcome this problem, the Enhancing Neuroimaging Genetics through Meta‐Analysis (ENIGMA) consortium, an international neuroimaging consortium, established standard protocols for imaging analysis and employs either meta‐ and mega‐analyses of psychiatric disorders with large sample sizes. The Cognitive Genetics Collaborative Research Organization (COCORO) in Japan promotes neurobiological studies in psychiatry and has successfully replicated and extended works of ENIGMA especially for neuroimaging studies. For example, (a) the ENIGMA consortium showed subcortical regional volume alterations in patients with schizophrenia (n = 2,028) compared to controls (n = 2,540) across 15 cohorts using meta‐analysis. COCORO replicated the volumetric changes in patients with schizophrenia (n = 884) compared to controls (n = 1,680) using the ENIGMA imaging analysis protocol and mega‐analysis. Furthermore, a schizophrenia‐specific leftward asymmetry for the pallidum volume was demonstrated; and (b) the ENIGMA consortium identified white matter microstructural alterations in patients with schizophrenia (n = 1,963) compared to controls (n = 2,359) across 29 cohorts. Using the ENIGMA protocol, a study from COCORO showed similar results in patients with schizophrenia (n = 696) compared to controls (n = 1,506) from 12 sites using mega‐analysis. Moreover, the COCORO study found that schizophrenia, bipolar disorder (n = 211) and autism spectrum disorder (n = 126), but not major depressive disorder (n = 398), share similar white matter microstructural alterations, compared to controls. Further replication and harmonization of the ENIGMA consortium and COCORO will contribute to the generalization of their research findings.
Keywords: magnetic resonance imaging (MRI), mega‐analysis, meta‐analysis, neuroimaging, psychiatric disorders, reproducibility, schizophrenia
The Cognitive Genetics Collaborative Research Organization (COCORO), which is a Japanese consortium established to elucidate psychiatric disorders and brain functions, replicated the results of the ENIGMA consortium, such as subcortical volume abnormalities and white matter microstructural abnormalities in schizophrenia, using the mega‐analysis method. These replication studies strengthened the reliability and generalization of the results of the ENIGMA consortium based on common and different methods in both consortiums. Further replication and harmonization of large consortia will contribute to the generalization of their research findings.
1. INTRODUCTION
The reproducibility of research findings is facing a crisis in the field of science. Nature's survey of 1,500 researchers revealed that more than half have failed to reproduce their own experiments and that 70% of researchers have tried and failed to reproduce another scientist's experiments (Baker, 2016). In the field of psychology, only 39 key findings out of 100 from published studies could be reproduced (Baker, 2015). This increasing concern has also been found in neuroimaging genetics as false positive results in the literature (Carter et al., 2017).
The Enhancing Neuroimaging Genetics through Meta‐Analysis (ENIGMA) consortium, which is an international neuroimaging consortium aimed at clarifying brain structure and function, was established to overcome this crisis using meta‐ (and recently partially mega‐) analysis. Meta‐analysis integrates the results of several independent studies using effect sizes, standard errors, and confidence intervals (Egger, Smith, & Phillips, 1997). The ENIGMA consortium has shown the potential of large‐scale neuroimaging initiatives with data pooling across facilities all over the world (Thompson et al., 2020). The efforts of this consortium have succeeded, as evidenced by the large body of literature (Thompson et al., 2017); however, reproducibility is still a concern. The Cognitive Genetics Collaborative Research Organization (COCORO) consists of 39 institutes in Japan and runs projects in several fields, including neuroimaging, neurophysiology, neurocognition, genetics, and neurobiology. This review introduces and discusses mainly neuroimaging studies within COCORO aiming to replicate and extend works of ENIGMA. First, we will (a) mention meta‐ and mega‐analyses employed in neuroimaging studies of the ENIGMA consortium and in those of COCORO, next, we will (b) introduce COCORO, including studies other than those focusing solely on neuroimaging, and finally, we will (c) introduce the neuroimaging studies developed within COCORO aiming to replicate and extend the work of ENIGMA.
2. META‐ANALYSIS AND MEGA‐ANALYSIS
Meta‐analyses employed in the ENIGMA consortium have an advantage in that they use common protocols for imaging analysis and check group‐level analyzed data at central (Table 1). In COCORO, replication studies have been carried out using mega‐analysis, taking advantage of the characteristics of a single country. In collaboration with COCORO, the meta‐analysis findings of the ENIGMA consortium, such as subcortical volume and white matter microstructural abnormalities in schizophrenia (Kelly et al., 2018; van Erp et al., 2016), have been successfully replicated using mega‐analyses (Koshiyama et al., 2020; Okada et al., 2016). As its name implies, ENIGMA has employed a meta‐analysis approach (although recently they have also started employing some mega‐analyses as described below). Although meta‐analysis is the most conventional approach and an inexpensive way of pooling data using standard deviations and number of subjects across studies, one issue needs to be considered from a neuroimaging point of view, which is a lack of imaging analysis harmonization. To overcome this issue, the ENIGMA consortium established standard protocols for imaging analysis and successfully provided higher harmonization across cohorts. As a further step, COCORO has employed a mega‐analysis approach from the beginning. Mega‐analysis pools individual data from multiple sites and analyzes the main effects in one single analysis. The ENIGMA consortium recently published a mega‐analysis of their data (Boedhoe et al., 2019; Hoogman et al., 2017; Kong et al., in press; Mackey et al., 2019; Postema et al., 2019; Tozzi et al., 2019). One of the reasons why COCORO has employed mega‐analysis from the beginning is that COCORO aims to replicate and reconfirm the results of the ENIGMA consortium (especially ENIGMA‐Schizophrenia) with a different strategy.
TABLE 1.
Comparison of imaging methodologies employed in the Enhancing Neuroimaging Genetics through Meta‐Analysis (ENIGMA) consortium and in the Cognitive Genetics Collaborative Research Organization (COCORO)
| General meta‐analysis study | ENIGMA consortium | COCORO | |
|---|---|---|---|
| Method | Meta‐analysis | Meta‐analysis/mega‐analysis | Mega‐analysis |
| Ethics | Individual institute | Individual institute | Central |
| Sample size | Medium | Large | Medium |
| Nationality | — | Multiple (worldwide) | Single (Japanese) |
| Quality assurance/analysis | |||
| T1‐weighted MRI/DTI check | Individual institute | Individual institute | Central |
| Converting from DICOM to NIFTI a | Individual institute | Individual institute | Central/individual institute |
| Imaging analysis protocol | Separate protocol | Common protocol | Common protocol |
| Execution of imaging analysis b | Individual institute | Individual institute | Central |
| Analyzed individual data check | Individual institute | Individual institute/central | Central |
| Analyzed group level data check c | Individual institute | Central | Central |
| Available disorder data | — | Schizophrenia, bipolar disorder, major depressive disorder, autism spectrum disorder (ASD), attention‐deficit/hyperactivity disorder (ADHD), epilepsy, human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), Parkinson's disease, addiction, post‐traumatic stress disorder (PTSD), stroke recovery, obsessive‐compulsive disorder (OCD), 22q11.2 deletion syndrome, eating disorder | Schizophrenia, bipolar disorder, major depressive disorder, autism spectrum disorder (ASD) |
Abbreviations: DTI, diffusion tensor imaging; MRI, magnetic resonance imaging.
In COCORO, most data was converted from DICOM to NIFTI at central; however, some data was converted at individual institutes.
Imaging analysis of FreeSurfer for structural MRI and DTI analysis was done using ENIGMA standard protocols; the same hardware was used for the execution of imaging analysis throughout each project in COCORO.
Data was checked using for example, histogram.
Setting up a mega‐analysis is like walking on a thorny path, and COCORO researchers have faced many obstacles. The first challenge was ethical data sharing. The ENIGMA consortium has recently been promoting the collection of individual data from all sites to conduct mega‐analyses for almost all projects. However, for example, the ENIGMA consortium's ethical rules make it difficult to access the data on Japanese sites. Therefore, the ENIGMA consortium has a limitation in that mega‐analyses can only be partially achieved. In COCORO, a data repository was established to pool data among sites. Before establishing the repository, each site needed to obtain approval from their institutional review boards (IRB) every time a new site joined the consortium. Establishing the repository enabled easy data pooling and sharing among sites. The COCORO headquarters is like a hub, and each site is a node linked to the hub.
COCORO also faced two time‐consuming and large challenges: quality assurance of data and data analysis in different environments. Compared to the large sample sizes employed by the ENIGMA consortium, the sample sizes in COCORO are relatively moderate. Therefore, we had two strategies for increasing statistical power: (a) we performed stricter quality assurance and (b) we eliminated hardware differences in FreeSurfer results by using a single imaging analysis hardware. Quality assurance of T1‐weighted MRI consisted of several procedures. The first step was a visual check of all original images by two different researchers at central. Almost all DICOM images were converted to NIFTI at central, except for those of one site. The data was analyzed using the ENIGMA standard protocols. Then, the data was visually checked again after imaging analysis, since corrupt parcellation or segmentation could occur for each individual data. At the group level, we checked the histograms. For diffusion data, the DICOM tags were also checked at central to assure that the same parameters were applied to each image for a site. The DICOM was converted to NIFTI at central, partially at individual sites (4 among 12 sites). Then, FA images were calculated according to the ENIGMA standard protocols by using one imaging analysis hardware, and the FA images were checked visually as well as checking histograms at central.
Another challenge COCORO had to overcome was the importance of using different environments to process data. As previous research indicates, the researchers had to face the fact that different operating systems and hardware result in different results with the same data even when the same version of FreeSurfer was used (Gronenschild et al., 2012). Considering the small effect size of psychiatric disease on neuroimaging data, analysis environment differences might obscure the truth. From this experience, the researchers carefully made sure that imaging analysis was done in a single environment at central.
3. COGNITIVE GENETICS COLLABORATIVE RESEARCH ORGANIZATION (COCORO)
COCORO is the largest collaborative effort in biological psychiatry in Japan. The purpose of COCORO is to elucidate the mechanisms of psychiatric disorders and brain function. COCORO started with 24 institutes in 2013 and now consists of 39 institutes in Japan (Figure 1). When we intended to expand COCORO globally after accomplishing several studies, we learned about the ENIGMA consortium, and decided to collaborate with ENIGMA rather than expanding COCORO globally. Researchers in various fields such as neuroscience, molecular biology, genome science, psychiatry, neuroimaging, cognitive science, neurophysiology, psychology and pharmacology have a meeting every 6 months, exchange views, and pioneer and promote new research fields. Several projects and topics are currently being investigated in COCORO, such as ethical issues for large collaborations, standard cognitive batteries, standard protocols for brain magnetic resonance imaging (MRI), tools for measurement of cognitive decline in schizophrenia, the development of auxiliary diagnostic methods using deficits of eye movement and brain local volume abnormalities in schizophrenia, a mega‐analysis of neuroimaging in psychiatric disorders, the genetics and neurobiology of psychiatric disorders, and translational research between clinical and basic research. COCORO started using a database of the Human Brain Phenotype Consortium (HBPC), the largest research resource database of human brain phenotypes in Japan; the various types of data and material, including genomic deoxyribonucleic acid (DNA), blood ribonucleic acid (RNA), serum, plasma, lymphoblastoid cells, induced pluripotent stem (iPS) cells, neuropsychological data, neuroimaging data, neurophysiological data and personality traits, are collected and shared among the researchers participating in COCORO (Table 2). At present, the data from approximately 8,760 subjects, involving 4,300 healthy controls, 2,500 subjects with schizophrenia, 1,300 subjects with mood disorders, 360 subjects with autism spectrum disorder and 300 subjects with other disorders, are available in COCORO, and this database is gradually growing. Many studies have been conducted using the COCORO database so far. This review article provides a brief overview of these studies in the research categories of (a) neurocognition, (b) neurophysiology, (c) genetics and neurobiology, and (d) neuroimaging.
FIGURE 1.
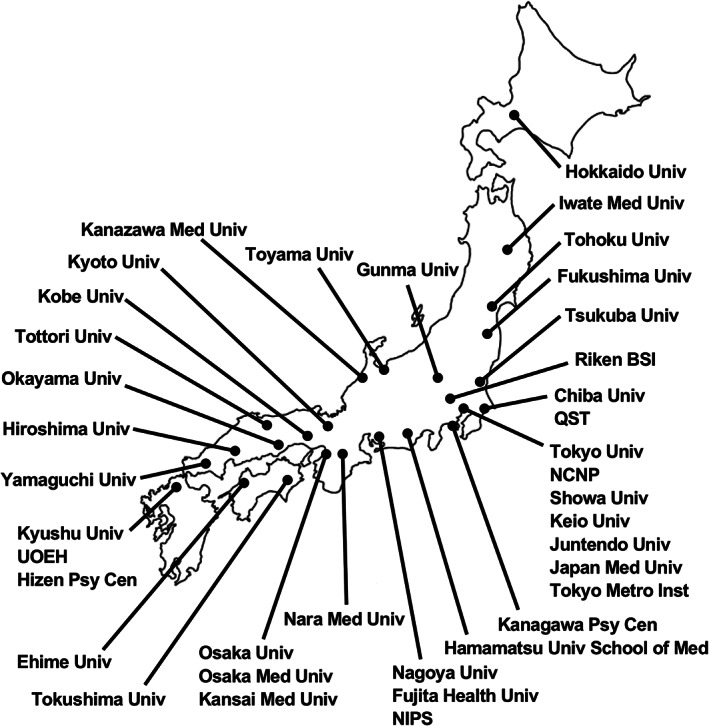
Institutions of the Cognitive Genetics Collaborative Research Organization (COCORO). The Cognitive Genetics Collaborative Research Organization (COCORO) consists of many psychiatric facilities in Japan. COCORO means “Mind” in Japanese. There are 39 institutes participating in COCORO. The purpose of COCORO is to elucidate the mechanisms of psychiatric disorders and brain functions. Abbreviations: Med, Medical; NCNP, National Center of Neurology and Psychiatry; NIPS, National Institute for Physiological Sciences; Psy Cen, Psychiatric Center; QST, National Institutes for Quantum and Radiological Science and Technology; Riken BSI, Riken Brain Science Institute; Tokyo Metro Inst, Tokyo Metropolitan Institute of Medical Science; Univ, University; UOEH, University of Occupational and Environmental Health
TABLE 2.
Available research resource database of human brain phenotypes in the Human Brain Phenotype Consortium (HBPC)
| Categories | Contents |
|---|---|
| Research resource | Deoxyribonucleic acid (DNA), plasma, blood ribonucleic acid (RNA), Lymphoblastoid cell line, induced pluripotent stem (iPS) cells |
| Neurocognitive battery | Intelligence quotient (IQ), working memory, verbal memory, visual memory, executive function, verbal fluency, vigilance, facial cognition, social cognition |
| Neuroimaging | Three‐dimensional brain morphology, diffusion tensor imaging (DTI), resting‐state functional magnetic resonance imaging (MRI) |
| Neurophysiology | Eye movement, prepulse inhibition (PPI), electroencephalography (EEG) |
| Personality | Temperament and character inventory (TCI), schizotypal personality questionnaire (SPQ), the autism‐spectrum quotient (AQ) |
3.1. Neurocognition
Studies on neurocognition have been performed to identify disease‐associated changes in the cognitive and social functions of psychiatric patients using the database of neurocognitive batteries (Fujino et al., 2014, 2016; Ohi et al., 2019; Sumiyoshi et al., 2018). A representative achievement is related to the works on cognitive decline of patients with schizophrenia. Fujino et al. (2017) demonstrated cognitive decline associated with schizophrenia and estimated the differences between premorbid intellectual functioning measured using the Japanese Adult Reading Test‐25 and the current full‐scale intelligence quotient (IQ) measured by WAIS‐III in patients with schizophrenia. The proportion of patients who scored minus 20 points or less was 39.7%. A discriminant analysis demonstrated that the difference score accurately discriminated the patients from healthy controls with 81.6% accuracy for 446 patients with schizophrenia and 686 healthy controls. Ohi et al. (2017) noted that there is a subgroup of patients with schizophrenia (approximately 30%) who have mild or minimal intellectual deficits (cognitive decline) following the onset of the disorder. These findings indicate that a careful assessment of intellectual deficits is important in identifying appropriate interventions, including medication, cognitive remediation and social/community services.
Another work involved the development of a WAIS‐III short form to evaluate intellectual status in patients with schizophrenia, maintaining the predictability for the full‐scale IQ test and representativeness for the IQ structure, consistency of subtests across versions, sensitivity to functional outcome measures and conciseness in administration time (Sumiyoshi et al., 2016). Sumiyoshi, Fujino, Yamamori, et al. (2018) found that cognitive decline, in addition to impaired social function and psychiatric symptoms, plays a key role in work disturbances in patients with schizophrenia. The research paradigms developed within schizophrenia studies have been extended to examinations of other mental illnesses, such as autism spectrum disorder (Kaneko et al., in press).
3.2. Neurophysiology
Eye movement abnormalities are often associated with mental illness, including schizophrenia (Kikuchi et al., 2018; Miura et al., 2014; Morita et al., 2017, 2018, 2019a, 2019b, 2019c; Shiino et al., 2019). One of the studies in COCORO involved the development of an integrated eye movement score as a neurophysiological marker of schizophrenia with relatively small data samples (patients with schizophrenia, n = 40; healthy controls, n = 69; Miura et al., 2014). Morita et al. (2017) subsequently replicated the initial findings with a larger sample (patients with schizophrenia, n = 85; healthy controls, n = 252) and proposed a more practical score that can be obtained from only a few examinations taking less than 15 min. A discriminant analysis using three eye movement measures, the scanpath length during the free viewing test, the horizontal position gain during the smooth pursuit test, and the duration of fixations during the fixation stability test, distinguished patients with schizophrenia from healthy controls with 82% accuracy. Shiino et al. (2019) suggested that these eye movement examinations also help distinguish subjects with autism spectrum disorder and those with schizophrenia. Positive correlations between eye movement performance and social/cognitive functions have been elucidated in patients with schizophrenia (Morita et al., 2018, 2019a). Efforts seeking biological mechanisms underlying eye movement abnormalities in schizophrenia have revealed genetic associations with the performance of smooth pursuit eye movements (Kikuchi et al., 2018) and a positive correlation between cortical thickness of the pars opercularis and free‐viewing performance (Morita et al., 2019b). Additional previous and current eye movement research in COCORO are described in a previous review article (Morita et al., 2019c).
3.3. Genetics and neurobiology
One of the advantages of COCORO is that it has ample experience in genetic and neurobiological studies, suggesting that it could be developed to include imaging genetics research. Several genome‐wide association studies and copy number variation (CNV) analyses with psychiatric diseases and related phenotypes such as schizophrenia, bipolar disorder, autism spectrum disorder, treatment response and cognitive impairment have been reported (Higashiyama et al., 2016; Ikeda et al., 2015, 2018, 2019; Kushima et al., 2017; Ohi et al., 2015, 2016, 2018).
Basic and clinical studies have effectively interacted in the genetics and neurobiology categories in COCORO. De novo CNVs and single‐nucleotide variants (SNVs) were reported in autism spectrum disorder and schizophrenia, which was followed by a neurobiological study using animal/cellular models to provide insight into the disease‐causative molecular and cellular pathology and provide useful information for the development of new effective treatment strategies for both disorders (Baba et al., 2019; Hashimoto et al., 2016; Kushima et al., 2018; Matsumura et al., 2016, 2019).
Other achievements involve studies on DNA methylation (Inoshita et al., 2015; Kinoshita et al., 2014, 2017), telomeres (Zhang et al., 2018), plasma (Kudo et al., 2018; Setoyama et al., 2016; Yamamori et al., 2014, 2016), gene functions (Nishi et al., 2014; Shirai et al., 2015; Tomioka et al., 2018; Toriumi et al., 2014; Uno et al., 2015), tumor necrosis factor (TNF)‐α from microglia (Ohgidani et al., 2017) and iPS cells (Nakazawa et al., 2017). Therefore, plenty of genetic and neurobiological studies within COCORO have potential for further imaging genetics studies in COCORO, and some studies have already been published as described in the following sections.
3.4. Neuroimaging
The neuroimaging studies in COCORO include research on imaging genetics, showing genetic risk variants of schizophrenia associated with left superior temporal gyrus volume and common variants at 1p36 associated with superior frontal gyrus volume (Hashimoto et al., 2014, 2015; Ohi et al., 2014). Nakazawa et al. (2016) demonstrated a newly discovered brain‐enriched sorting nexin in mice and that the sorting nexin in humans is associated with gray matter volumes of the temporal and frontal gyri of patients with schizophrenia, suggesting a new molecular pathology of neuropsychiatric disorders. Kudo et al. (2018) showed that increased plasma levels of soluble tumor necrosis factor receptor 2 (sTNFR2) are associated with smaller hippocampal volume and cognitive impairment in patients with schizophrenia, suggesting a possible biological mechanism of schizophrenia. Toriumi et al. (2014) demonstrated a single nucleotide polymorphism (SNP) in the human NAT8L gene related to reward dependence, a personality trait, and gray matter volume in the caudate nucleus in healthy subjects, suggesting that NAT8L might affect human personality.
Another study demonstrated altered orbitofrontal cortex sulcogyral pattern distributions in the left hemisphere in patients with schizophrenia (Isomura et al., 2017). Recently, Nemoto et al. (2019) successfully discriminated schizophrenia cases from control data based on brain structures as measured with MRI, regardless of differences in MRI scanners, in their multicenter study in COCORO. Furthermore, Yasuda et al. (2020) have shown functional brain abnormalities such as hyperconnectivity between the thalamus and a broad range of brain regions in cognitively deteriorated patients with schizophrenia compared to healthy controls using resting‐state functional MRI; they also found hyperconnectivity between the nucleus accumbens and the superior and middle frontal gyri in cognitively preserved patients with schizophrenia compared with deteriorated patients. As described above, we have been investigating genetic and molecular mechanism underlying abnormal findings of neuroimaging studies and neural networks underlying cognitive dysfunction and developing diagnostic assistance tools. Further details of the neuroimaging studies are described in the following sections.
4. NEUROIMAGING STUDIES DEVELOPED WITHIN COCORO AIMING TO REPLICATE AND EXTEND THE WORK OF ENIGMA
The techniques of imaging analysis protocols developed in ENIGMA were introduced into COCORO; the first study to utilize these techniques was from Okada et al. (2016), in which they successfully replicated findings by ENIGMA‐SZ (Schizophrenia; van Erp et al., 2016) and additionally provided novel findings on abnormal asymmetries in the pallidum volume in patients with schizophrenia. Recently, diffusion tensor imaging (DTI) studies replicated the findings from the ENIGMA Schizophrenia DTI Working Group (Kelly et al., 2018) and further demonstrated the association between structural connectivity and impaired social function in schizophrenia (Koshiyama et al., 2018a) and different white matter microstructures associated with different psychiatric disorders (Koshiyama et al., 2020). COCORO has been closely collaborating with ENIGMA by providing Japanese cohort data and participating in more than 10 projects from ENIGMA involving case–control, imaging genetics, and clinical implication studies (Adams et al., 2016; Franke et al., 2016; Guadalupe et al., 2017; Hibar et al., 2015, 2017, 2018a; Kelly et al., 2018; Sonderby et al., 2018; Thompson et al., 2017; van Erp et al., 2018; Walton et al., 2017, 2018; Wong et al., 2019; Writing Committee for the ENIGMA‐CNV Working Group et al., 2019). Future collaboration between the ENIGMA consortium and COCORO for replication and harmonization is expected.
4.1. Subcortical volume alterations in schizophrenia and clinical implications
Prior studies have reported volumetric alterations in subcortical regions in schizophrenia, but the reported results are sometimes heterogeneous. In this context, van Erp et al. (2016) meta‐analyzed subcortical regional volume differences between patients with schizophrenia (n = 2,028) and controls (n = 2,540) across 15 cohorts in ENIGMA‐SZ (Figure 2). Patients with schizophrenia showed smaller‐than‐normal volumes in the hippocampus, amygdala, thalamus and accumbens and larger‐than‐normal volumes in the pallidum. In a research project by COCORO, a multisite large‐scale cross‐sectional investigation of subcortical regional volumetric differences between patients with schizophrenia (n = 884) and healthy subjects (n = 1,680) using the same imaging analysis protocols as those of the van Erp et al. (2016) study was conducted using mega‐analytic methods (Okada et al., 2016). COCORO successfully replicated the rank order of effect sizes for subcortical volumetric changes in schizophrenia reported by van Erp et al. (2016). Furthermore, a schizophrenia‐specific leftward asymmetry for the pallidum volume was demonstrated. Next, the effect of the duration of illness and antipsychotics on subcortical volumes in schizophrenia was examined (Hashimoto et al., 2018), revealing a positive association between daily antipsychotic dose and left pallidum volume as well as a leftward laterality of the pallidum. The duration of illness was positively associated with bilateral pallidum volumes.
FIGURE 2.
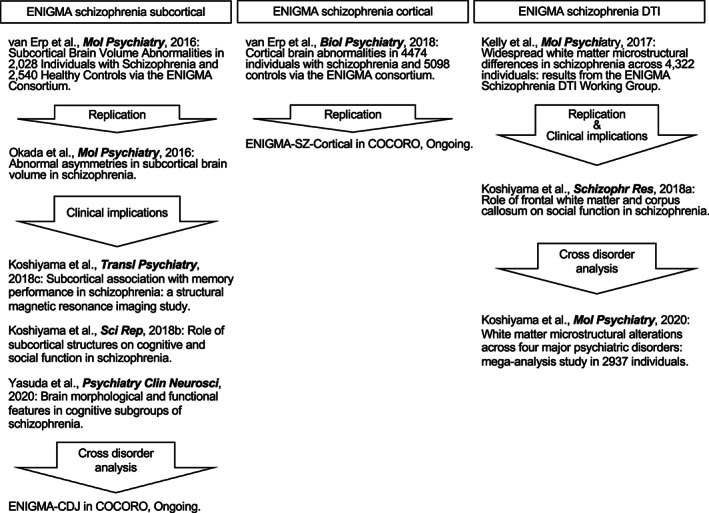
Development of the Enhancing Neuroimaging Genetics through Meta‐Analysis (ENIGMA) consortium studies in the Cognitive Genetics Collaborative Research Organization (COCORO)
In the COCORO study, the HBPC database was employed to show the clinical implications of these subcortical volume reductions. Using this database, Koshiyama et al. (2018c) not only replicated a hippocampal volume and memory association similar to that from previous findings (Guo et al., 2014; Herold et al., 2015; Knochel et al., 2014), but also found that the volume of the nucleus accumbens is associated with memory performance in 174 patients with schizophrenia. Additionally, Koshiyama et al. (2018b) found a correlation between the nucleus accumbens volume and the digit symbol coding score, a known, distinctively characteristic index of cognitive deficits in schizophrenia, as well as a correlation between the thalamic volume and social function scores in 163 patients with schizophrenia. Taken together, these results show that cortical–subcortical circuitry, which consists of subcortical nuclei such as the nucleus accumbens and thalamus, may play important roles in cognitive and social functions in schizophrenia. The next step will be to investigate the causal relationship between the neural circuitry and cognitive/social consequences by translating between animal and human studies with an aim toward ultimately developing circuit‐based intervention strategies in the treatment of schizophrenia.
4.2. Cortical thickness and surface alterations in schizophrenia
The profile of cortical volume abnormalities in schizophrenia is not fully understood, despite many published structural brain imaging studies. In this context, van Erp et al. (2018) compared 4,474 patients with schizophrenia and 5,098 control subjects via the ENIGMA consortium (Figure 2). They revealed that individuals with schizophrenia had a widespread thinner cortex and smaller surface area compared to healthy subjects, with the largest effect sizes in both the frontal and temporal lobe regions. In addition, in a multimodal study from COCORO, an association between eye‐movement characteristics and cortical thickness in schizophrenia was reported (Morita et al., 2019b). Furthermore, there is an ongoing research plan in COCORO to examine whether the results of van Erp et al. (2018) can be replicated in the Japanese dataset.
4.3. White matter microstructural alterations in schizophrenia
Kelly et al. (2018) performed a large‐scale meta‐analysis (2,359 healthy controls and 1,963 patients with schizophrenia from 29 independent international studies; Figure 2) using the established ENIGMA‐Diffusion Tensor Imaging (ENIGMA‐DTI) Working Group protocol, which harmonized the processing of diffusion data from multiple sites (http://enigma.usc.edu/ongoing/dti-working-group/; Jahanshad et al., 2013; Kochunov et al., 2014, 2015) and identified white matter microstructural alterations along with atlas‐defined regions of interest in patients with schizophrenia in the ENIGMA Schizophrenia DTI Working Group. This study identified widespread microstructural alterations, including the anterior corona radiata, corpus callosum, cingulum, fornix and uncinate fasciculus, in schizophrenia. Using the ENIGMA‐DTI imaging analysis protocol, a study from COCORO on patients with schizophrenia (Koshiyama et al., 2020) showed results similar to those of the study from Kelly et al. (2018). The study showed alterations of white matter regions such as the anterior corona radiata, corpus callosum, fornix and uncinate fasciculus in patients with schizophrenia (n = 696) compared to healthy controls (n = 1,506; Koshiyama et al., 2020). Furthermore, relevant to the clinical implications of white matter microstructural alterations, Koshiyama et al. (2018a) demonstrated an association between social function and structural connectivity in the anterior corona radiata and corpus callosum in patients with schizophrenia (n = 149). These findings are in line with the findings of Kochunov et al. (2017), who demonstrated the role of the anterior corona radiata and corpus callosum on working memory and processing speed in patients with schizophrenia (n = 166) using the ENIGMA‐DTI protocol.
4.4. Psychiatric disorders other than schizophrenia
The ENIGMA consortium reported subcortical volumetric characteristics in various other psychiatric disorders, such as bipolar disorder (Hibar et al., 2016), major depressive disorder (Schmaal et al., 2016), and autism spectrum disorder (van Rooij et al., 2018). However, similarities and differences in the subcortical volumes across these psychiatric disorders have not yet been made clear. These concerns are expected to be addressed soon in more detail by using a Japanese dataset deposited in COCORO.
The ENIGMA consortium recently reported cortical volumetric abnormalities in bipolar disorder (Hibar et al., 2018b), major depressive disorder (Schmaal et al., 2017), and autism spectrum disorder (van Rooij et al., 2018). However, cortical volumetric characteristics that are common and specific across these psychiatric disorders have not yet been made clear. An ongoing research project to address this matter by using a large‐scale Japanese dataset that has been collected though COCORO might elucidate the commonality and differences among psychiatric diseases.
Recently, several studies in the ENIGMA consortium presented white matter microstructural alterations in patients with psychiatric disorders other than schizophrenia. Favre et al. (2019) examined individual tensor‐derived regional metrics from 26 cohorts, leading to a sample size of n = 3,033 (1,482 patients with bipolar disorder and 1,551 healthy controls) within the ENIGMA Bipolar Disorder Working Group; they demonstrated that altered white matter connectivity within the corpus callosum, the cingulum and the fornix are strongly associated with bipolar disorder. van Velzen et al. (in press) examined white matter anisotropy and diffusivity in 1,305 major depressive disorder patients and 1,602 healthy controls from 20 samples worldwide within the Major Depressive Disorder Working Group of the ENIGMA consortium; they found the largest alterations in the corpus callosum and corona radiata in patients compared to healthy controls.
In a COCORO study, Koshiyama et al. (2020) examined white matter microstructural alterations across four major psychiatric disorders (patients with schizophrenia [n = 696], bipolar disorder [n = 211], autism spectrum disorder [n = 126], major depressive disorder [n = 398] and healthy comparison subjects [n = 1,506; total n = 2,937 from 12 sites in Japan]) using mega‐analysis. In comparison with healthy subjects, they found that schizophrenia, bipolar disorder and autism spectrum disorder share similar white matter microstructural differences in the body of the corpus callosum and that schizophrenia and bipolar disorder featured comparable changes in the limbic system, such as the fornix and cingulum. By comparison, alterations in tracts connecting neocortical areas, such as the uncinate fasciculus, were observed only in schizophrenia. In contrast, the COCORO study failed to show a significant difference in major depressive disorder compared to healthy subjects, while van Velzen et al. (in press) showed alterations in the anterior corona radiata and corpus callosum in patients with major depressive disorder compared to healthy controls. These inconsistencies may be due to the difference in sample size; Cohen's d effect size of −0.25 for fractional anisotropy (FA) in the anterior corona radiata in major depressive disorder (n = 921) compared to healthy controls (n = 1,265) in the study of van Velzen et al. (in press) was similar to the −0.19 for FA in Koshiyama et al. (2020). In a direct comparison between schizophrenia and bipolar disorder in the study of Koshiyama et al. (2020), there were no significant differences. Thus, based on the findings from Favre et al. (2019) and Koshiyama et al. (2020), white matter alterations in the corpus callosum and the limbic system, such as the fornix and cingulum, may be common in patients with schizophrenia and those with bipolar disorder. Schizophrenia and bipolar disorder may have similar pathological characteristics. In addition, interestingly, the regional white matter alterations are similar to those of 22q11.2 deletion carriers (Villalon‐Reina et al., in press). The 22q11 Deletion Syndrome Working Group of the ENIGMA consortium identified widespread white matter alterations in the corona radiata, corpus callosum, superior longitudinal fasciculus, posterior thalamic radiations, and sagittal stratum. White matter alterations, especially in the corpus callosum, may be widely common in individuals with psychiatric disorders and 22q11.2 deletion carriers.
5. LIMITATIONS
There are several limitations in neuroimaging studies within COCORO. First, sample sizes employed in COCORO are relatively moderate compared to the large sample sizes of the ENIGMA consortium. Studies with small sample sizes generally have limited statistical power and increased false‐positive rates. However, replication studies were necessary to generalize original results from the ENIMGA consortium and the results were replicated in the COCORO studies despite the smaller sample sizes compared to those of the ENIGMA consortium. All facilities participating in COCORO were in a single country, in which penetration rate of MRI is high; therefore, we were able to collect moderate size cohorts and perform replication studies. Although the sample sizes were limited, we could successfully replicate and reconfirm the results of ENIGMA consortium with high quality data and contribute to the generalization of their research findings (Thompson et al., 2020). Second, among the facilities participating in COCORO, Osaka university participated in the diffusion imaging study of patients with schizophrenia by the ENIGMA Schizophrenia DTI Working Group (Kelly et al., 2018; patients with schizophrenia, n = 71; healthy controls, n = 249). Therefore, the participants of patients with schizophrenia and healthy comparison subjects in the diffusion imaging study by COCORO (Koshiyama et al., 2020) overlapped with the study by the ENIGMA consortium (Kelly et al., 2018). Although careful interpretation of the results needs to be considered, the effect may be limited because of the relatively small sample size (7.4%) of the cohort relative to whole sample size of the ENIGMA study. Participants did not overlap between the study of subcortical volume in schizophrenia by ENIGMA‐SZ (van Erp et al., 2016) and that by COCORO (Okada et al., 2016).
6. CONCLUSION
Scientific progress relies on reproducible and reliable research. However, the fact that many basic and clinical studies lack reproducibility has become a challenge in the scientific community. The lack of reproducibility of research findings may hinder clinical application and waste valuable clinical resources. The ENIGMA consortium and COCORO take advantage of each other's methodological characteristics to complementarily examine reproducibility and increase scientific reliability. Further harmonized and corporative research among international consortiums is warranted in basic and clinical research.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors would like to thank all the COCORO members. This work was supported by Grants‐in‐Aid for Scientific Research (KAKENHI; Grant Number JP18K18164) from the Ministry of Education, Culture, Sports, Science and Technology‐Japan (MEXT) and Japan Society for the Promotion of Science (JSPS); Brain/MINDS & Beyond studies (Grant Number JP19dm0307002) from the Japan Agency for Medical Research and Development (AMED). The funder had no role in this manuscript.
Koshiyama D, Miura K, Nemoto K, et al. Neuroimaging studies within Cognitive Genetics Collaborative Research Organization aiming to replicate and extend works of ENIGMA. Hum Brain Mapp. 2022;43:182–193. 10.1002/hbm.25040
Funding information Japan Agency for Medical Research and Development, Grant/Award Number: JP19dm0307002; Grants‐in‐Aid for Scientific Research, Grant/Award Number: JP18K18164
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Adams, H. H. , Hibar, D. P. , Chouraki, V. , Stein, J. L. , Nyquist, P. A. , Renteria, M. E. , … Thompson, P. M. (2016). Novel genetic loci underlying human intracranial volume identified through genome‐wide association. Nature Neuroscience, 19(12), 1569–1582. 10.1038/nn.4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba, M. , Yokoyama, K. , Seiriki, K. , Naka, Y. , Matsumura, K. , Kondo, M. , … Nakazawa, T. (2019). Psychiatric‐disorder‐related behavioral phenotypes and cortical hyperactivity in a mouse model of 3q29 deletion syndrome. Neuropsychopharmacology, 44(12), 2125–2135. 10.1038/s41386-019-0441-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, M. (2015). First results from psychology's largest reproducibility test. Nature. 10.1038/nature.2015.17433 [DOI] [Google Scholar]
- Baker, M. (2016). 1,500 scientists lift the lid on reproducibility. Nature, 533(7604), 452–454. 10.1038/533452a [DOI] [PubMed] [Google Scholar]
- Boedhoe, P. S. W. , Heymans, M. W. , Schmaal, L. , Abe, Y. , Alonso, P. , Ameis, S. H. , … Twisk, J. W. R. (2019). An empirical comparison of meta‐ and mega‐analysis with data from the ENIGMA obsessive‐compulsive disorder working group. Frontiers in Neuroinformatics, 12, 102. 10.3389/fninf.2018.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, C. S. , Bearden, C. E. , Bullmore, E. T. , Geschwind, D. H. , Glahn, D. C. , Gur, R. E. , … Weinberger, D. R. (2017). Enhancing the Informativeness and Replicability of imaging genomics studies. Biological Psychiatry, 82(3), 157–164. 10.1016/j.biopsych.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger, M. , Smith, G. D. , & Phillips, A. N. (1997). Meta‐analysis: Principles and procedures. BMJ, 315(7121), 1533–1537. 10.1136/bmj.315.7121.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre, P. , Pauling, M. , Stout, J. , Hozer, F. , Sarrazin, S. , Abe, C. , … Houenou, J. (2019). Widespread white matter microstructural abnormalities in bipolar disorder: Evidence from mega‐ and meta‐analyses across 3033 individuals. Neuropsychopharmacology, 44(13), 2285–2293. 10.1038/s41386-019-0485-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke, B. , Stein, J. L. , Ripke, S. , Anttila, V. , Hibar, D. P. , van Hulzen, K. J. E. , … Sullivan, P. F. (2016). Genetic influences on schizophrenia and subcortical brain volumes: Large‐scale proof of concept. Nature Neuroscience, 19(3), 420–431. 10.1038/nn.4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino, H. , Sumiyoshi, C. , Sumiyoshi, T. , Yasuda, Y. , Yamamori, H. , Ohi, K. , … Imura, O. (2016). Predicting employment status and subjective quality of life in patients with schizophrenia. Schizophrenia Research: Cognition, 3, 20–25. 10.1016/j.scog.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino, H. , Sumiyoshi, C. , Sumiyoshi, T. , Yasuda, Y. , Yamamori, H. , Ohi, K. , … Imura, O. (2014). Performance on the Wechsler adult intelligence scale‐III in Japanese patients with schizophrenia. Psychiatry and Clinical Neurosciences, 68(7), 534–541. 10.1111/pcn.12165 [DOI] [PubMed] [Google Scholar]
- Fujino, H. , Sumiyoshi, C. , Yasuda, Y. , Yamamori, H. , Fujimoto, M. , Fukunaga, M. , … for COCORO . (2017). Estimated cognitive decline in patients with schizophrenia: A multicenter study. Psychiatry and Clinical Neurosciences, 71(5), 294–300. 10.1111/pcn.12474 [DOI] [PubMed] [Google Scholar]
- Gronenschild, E. H. , Habets, P. , Jacobs, H. I. , Mengelers, R. , Rozendaal, N. , van Os, J. , & Marcelis, M. (2012). The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One, 7(6), e38234. 10.1371/journal.pone.0038234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe, T. , Mathias, S. R. , van Erp, T. G. M. , Whelan, C. D. , Zwiers, M. P. , Abe, Y. , … Francks, C. (2017). Human subcortical brain asymmetries in 15,847 people worldwide reveal effects of age and sex. Brain Imaging and Behavior, 11(5), 1497–1514. 10.1007/s11682-016-9629-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, X. , Li, J. , Wang, J. , Fan, X. , Hu, M. , Shen, Y. , … Zhao, J. (2014). Hippocampal and orbital inferior frontal gray matter volume abnormalities and cognitive deficit in treatment‐naive, first‐episode patients with schizophrenia. Schizophrenia Research, 152(2–3), 339–343. 10.1016/j.schres.2013.12.015 [DOI] [PubMed] [Google Scholar]
- Hashimoto, N. , Ito, Y. M. , Okada, N. , Yamamori, H. , Yasuda, Y. , Fujimoto, M. , … COCORO . (2018). The effect of duration of illness and antipsychotics on subcortical volumes in schizophrenia: Analysis of 778 subjects. NeuroImage: Clinical, 17, 563–569. 10.1016/j.nicl.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, R. , Ikeda, M. , Yamashita, F. , Ohi, K. , Yamamori, H. , Yasuda, Y. , … Ozaki, N. (2014). Common variants at 1p36 are associated with superior frontal gyrus volume. Translational Psychiatry, 4, e472. 10.1038/tp.2014.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, R. , Nakazawa, T. , Tsurusaki, Y. , Yasuda, Y. , Nagayasu, K. , Matsumura, K. , … Hashimoto, H. (2016). Whole‐exome sequencing and neurite outgrowth analysis in autism spectrum disorder. Journal of Human Genetics, 61(3), 199–206. 10.1038/jhg.2015.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, R. , Ohi, K. , Yamamori, H. , Yasuda, Y. , Fujimoto, M. , Umeda‐Yano, S. , … Takeda, M. (2015). Imaging genetics and psychiatric disorders. Current Molecular Medicine, 15(2), 168–175. 10.2174/1566524015666150303104159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold, C. J. , Lasser, M. M. , Schmid, L. A. , Seidl, U. , Kong, L. , Fellhauer, I. , … Schroder, J. (2015). Neuropsychology, autobiographical memory, and hippocampal volume in “younger” and “older” patients with chronic schizophrenia. Frontiers in Psychiatry, 6, 53. 10.3389/fpsyt.2015.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Adams, H. H. H. , Jahanshad, N. , Chauhan, G. , Stein, J. L. , Hofer, E. , … Ikram, M. A. (2017). Novel genetic loci associated with hippocampal volume. Nature Communications, 8, 13624. 10.1038/ncomms13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Cheung, J. W. , Medland, S. E. , Mufford, M. S. , Jahanshad, N. , Dalvie, S. , … International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF‐GC) . (2018a). Significant concordance of genetic variation that increases both the risk for obsessive‐compulsive disorder and the volumes of the nucleus accumbens and putamen. The British Journal of Psychiatry, 213(1), 430–436. 10.1192/bjp.2018.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Stein, J. L. , Renteria, M. E. , Arias‐Vasquez, A. , Desrivieres, S. , Jahanshad, N. , … Medland, S. E. (2015). Common genetic variants influence human subcortical brain structures. Nature, 520(7546), 224–229. 10.1038/nature14101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Westlye, L. T. , Doan, N. T. , Jahanshad, N. , Cheung, J. W. , Ching, C. R. K. , … Andreassen, O. A. (2018b). Cortical abnormalities in bipolar disorder: An MRI analysis of 6503 individuals from the ENIGMA bipolar disorder working group. Molecular Psychiatry, 23(4), 932–942. 10.1038/mp.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Westlye, L. T. , van Erp, T. G. , Rasmussen, J. , Leonardo, C. D. , Faskowitz, J. , … Andreassen, O. A. (2016). Subcortical volumetric abnormalities in bipolar disorder. Molecular Psychiatry, 21(12), 1710–1716. 10.1038/mp.2015.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama, R. , Ohnuma, T. , Takebayashi, Y. , Hanzawa, R. , Shibata, N. , Yamamori, H. , … Arai, H. (2016). Association of copy number polymorphisms at the promoter and translated region of COMT with Japanese patients with schizophrenia. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 171B(3), 447–457. 10.1002/ajmg.b.32426 [DOI] [PubMed] [Google Scholar]
- Hoogman, M. , Bralten, J. , Hibar, D. P. , Mennes, M. , Zwiers, M. P. , Schweren, L. S. J. , … Franke, B. (2017). Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross‐sectional mega‐analysis. Lancet Psychiatry, 4(4), 310–319. 10.1016/s2215-0366(17)30049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, M. , Takahashi, A. , Kamatani, Y. , Momozawa, Y. , Saito, T. , Kondo, K. , … Iwata, N. (2019). Genome‐wide association study detected novel susceptibility genes for schizophrenia and shared trans‐populations/diseases genetic effect. Schizophrenia Bulletin, 45(4), 824–834. 10.1093/schbul/sby140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, M. , Takahashi, A. , Kamatani, Y. , Okahisa, Y. , Kunugi, H. , Mori, N. , … Iwata, N. (2018). A genome‐wide association study identifies two novel susceptibility loci and trans population polygenicity associated with bipolar disorder. Molecular Psychiatry, 23(3), 639–647. 10.1038/mp.2016.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, M. , Yoshimura, R. , Hashimoto, R. , Kondo, K. , Saito, T. , Shimasaki, A. , … Iwata, N. (2015). Genetic overlap between antipsychotic response and susceptibility to schizophrenia. Journal of Clinical Psychopharmacology, 35(1), 85–88. 10.1097/JCP.0000000000000268 [DOI] [PubMed] [Google Scholar]
- Inoshita, M. , Numata, S. , Tajima, A. , Kinoshita, M. , Umehara, H. , Yamamori, H. , … Ohmori, T. (2015). Sex differences of leukocytes DNA methylation adjusted for estimated cellular proportions. Biology of Sex Differences, 6, 11. 10.1186/s13293-015-0029-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura, S. , Hashimoto, R. , Nakamura, M. , Hirano, Y. , Yamashita, F. , Jimbo, S. , … Onitsuka, T. (2017). Altered sulcogyral patterns of orbitofrontal cortex in a large cohort of patients with schizophrenia. NPJ Schizophrenia, 3, 3. 10.1038/s41537-016-0008-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad, N. , Kochunov, P. V. , Sprooten, E. , Mandl, R. C. , Nichols, T. E. , Almasy, L. , … Glahn, D. C. (2013). Multi‐site genetic analysis of diffusion images and voxelwise heritability analysis: A pilot project of the ENIGMA‐DTI working group. NeuroImage, 81, 455–469. 10.1016/j.neuroimage.2013.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko, S. , Kato, T. A. , Makinodan, M. , Komori, T. , Ishida, R. , Kishimoto, N. , … Kishimoto, T. (in press). The self‐construal scale: A potential tool for predicting subjective well‐being of individuals with autism spectrum disorder. Autism Research. 10.1002/aur.2242 [DOI] [PubMed] [Google Scholar]
- Kelly, S. , Jahanshad, N. , Zalesky, A. , Kochunov, P. , Agartz, I. , Alloza, C. , … Donohoe, G. (2018). Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA schizophrenia DTI working group. Molecular Psychiatry, 23(5), 1261–1269. 10.1038/mp.2017.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, M. , Miura, K. , Morita, K. , Yamamori, H. , Fujimoto, M. , Ikeda, M. , … Hashimoto, R. (2018). Genome‐wide association analysis of eye movement dysfunction in schizophrenia. Scientific Reports, 8(1), 12347. 10.1038/s41598-018-30646-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, M. , Numata, S. , Tajima, A. , Ohi, K. , Hashimoto, R. , Shimodera, S. , … Ohmori, T. (2014). Aberrant DNA methylation of blood in schizophrenia by adjusting for estimated cellular proportions. Neuromolecular Medicine, 16(4), 697–703. 10.1007/s12017-014-8319-5 [DOI] [PubMed] [Google Scholar]
- Kinoshita, M. , Numata, S. , Tajima, A. , Yamamori, H. , Yasuda, Y. , Fujimoto, M. , … Ohmori, T. (2017). Effect of clozapine on DNA methylation in peripheral leukocytes from patients with treatment‐resistant schizophrenia. International Journal of Molecular Sciences, 18(3), 632. 10.3390/ijms18030632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knochel, C. , Stablein, M. , Storchak, H. , Reinke, B. , Jurcoane, A. , Prvulovic, D. , … Oertel‐Knochel, V. (2014). Multimodal assessments of the hippocampal formation in schizophrenia and bipolar disorder: Evidences from neurobehavioral measures and functional and structural MRI. Neuroimage: Clinical, 6, 134–144. 10.1016/j.nicl.2014.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov, P. , Coyle, T. R. , Rowland, L. M. , Jahanshad, N. , Thompson, P. M. , Kelly, S. , … Hong, L. E. (2017). Association of White Matter with Core Cognitive Deficits in patients with schizophrenia. JAMA Psychiatry, 74(9), 958–966. 10.1001/jamapsychiatry.2017.2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov, P. , Jahanshad, N. , Marcus, D. , Winkler, A. , Sprooten, E. , Nichols, T. E. , … van Essen, D. C. (2015). Heritability of fractional anisotropy in human white matter: A comparison of human Connectome project and ENIGMA‐DTI data. NeuroImage, 111, 300–311. 10.1016/j.neuroimage.2015.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov, P. , Jahanshad, N. , Sprooten, E. , Nichols, T. E. , Mandl, R. C. , Almasy, L. , … Glahn, D. C. (2014). Multi‐site study of additive genetic effects on fractional anisotropy of cerebral white matter: Comparing meta and megaanalytical approaches for data pooling. NeuroImage, 95, 136–150. 10.1016/j.neuroimage.2014.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, X. Z. , Boedhoe, P. S. W. , Abe, Y. , Alonso, P. , Ameis, S. H. , Arnold, P. D. , … Francks, C. (in press). Mapping cortical and subcortical asymmetry in obsessive‐compulsive disorder: Findings from the ENIGMA consortium. Biological Psychiatry. 10.1016/j.biopsych.2019.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama, D. , Fukunaga, M. , Okada, N. , Morita, K. , Nemoto, K. , Usui, K. , … COCORO . (2020). White matter microstructural alterations across four major psychiatric disorders: Mega‐analysis study in 2937 individuals. Molecular Psychiatry, 25, 883–895. 10.1038/s41380-019-0553-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama, D. , Fukunaga, M. , Okada, N. , Morita, K. , Nemoto, K. , Yamashita, F. , … Hashimoto, R. (2018a). Role of frontal white matter and corpus callosum on social function in schizophrenia. Schizophrenia Research, 202, 180–187. 10.1016/j.schres.2018.07.009 [DOI] [PubMed] [Google Scholar]
- Koshiyama, D. , Fukunaga, M. , Okada, N. , Yamashita, F. , Yamamori, H. , Yasuda, Y. , … Hashimoto, R. (2018b). Role of subcortical structures on cognitive and social function in schizophrenia. Scientific Reports, 8(1), 1183. 10.1038/s41598-017-18950-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama, D. , Fukunaga, M. , Okada, N. , Yamashita, F. , Yamamori, H. , Yasuda, Y. , … Hashimoto, R. (2018c). Subcortical association with memory performance in schizophrenia: A structural magnetic resonance imaging study. Translational Psychiatry, 8(1), 20. 10.1038/s41398-017-0069-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo, N. , Yamamori, H. , Ishima, T. , Nemoto, K. , Yasuda, Y. , Fujimoto, M. , … Hashimoto, R. (2018). Plasma levels of soluble tumor necrosis factor receptor 2 (sTNFR2) are associated with hippocampal volume and cognitive performance in patients with schizophrenia. The International Journal of Neuropsychopharmacology, 21(7), 631–639. 10.1093/ijnp/pyy013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushima, I. , Aleksic, B. , Nakatochi, M. , Shimamura, T. , Okada, T. , Uno, Y. , … Ozaki, N. (2018). Comparative analyses of copy‐number variation in autism spectrum disorder and schizophrenia reveal etiological overlap and biological insights. Cell Reports, 24(11), 2838–2856. 10.1016/j.celrep.2018.08.022 [DOI] [PubMed] [Google Scholar]
- Kushima, I. , Aleksic, B. , Nakatochi, M. , Shimamura, T. , Shiino, T. , Yoshimi, A. , … Ozaki, N. (2017). High‐resolution copy number variation analysis of schizophrenia in Japan. Molecular Psychiatry, 22(3), 430–440. 10.1038/mp.2016.88 [DOI] [PubMed] [Google Scholar]
- Mackey, S. , Allgaier, N. , Chaarani, B. , Spechler, P. , Orr, C. , Bunn, J. , … Garavan, H. (2019). Mega‐analysis of gray matter volume in substance dependence: General and substance‐specific regional effects. The American Journal of Psychiatry, 176(2), 119–128. 10.1176/appi.ajp.2018.17040415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura, K. , Baba, M. , Nagayasu, K. , Yamamoto, K. , Kondo, M. , Kitagawa, K. , … Nakazawa, T. (2019). Autism‐associated protein kinase D2 regulates embryonic cortical neuron development. Biochemical and Biophysical Research Communications, 519(3), 626–632. 10.1016/j.bbrc.2019.09.048 [DOI] [PubMed] [Google Scholar]
- Matsumura, K. , Nakazawa, T. , Nagayasu, K. , Gotoda‐Nishimura, N. , Kasai, A. , Hayata‐Takano, A. , … Hashimoto, H. (2016). De novo POGZ mutations in sporadic autism disrupt the DNA‐binding activity of POGZ. Journal of Molecular Psychiatry, 4, 1. 10.1186/s40303-016-0016-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, K. , Hashimoto, R. , Fujimoto, M. , Yamamori, H. , Yasuda, Y. , Ohi, K. , … Takeda, M. (2014). An integrated eye movement score as a neurophysiological marker of schizophrenia. Schizophrenia Research, 160(1–3), 228–229. 10.1016/j.schres.2014.10.023 [DOI] [PubMed] [Google Scholar]
- Morita, K. , Miura, K. , Fujimoto, M. , Shishido, E. , Shiino, T. , Takahashi, J. , … Hashimoto, R. (2018). Abnormalities of eye movement are associated with work hours in schizophrenia. Schizophrenia Research, 202, 420–422. 10.1016/j.schres.2018.06.064 [DOI] [PubMed] [Google Scholar]
- Morita, K. , Miura, K. , Fujimoto, M. , Yamamori, H. , Yasuda, Y. , Iwase, M. , … Hashimoto, R. (2017). Eye movement as a biomarker of schizophrenia: Using an integrated eye movement score. Psychiatry and Clinical Neurosciences, 71(2), 104–114. 10.1111/pcn.12460 [DOI] [PubMed] [Google Scholar]
- Morita, K. , Miura, K. , Fujimoto, M. , Yamamori, H. , Yasuda, Y. , Kudo, N. , … Hashimoto, R. (2019a). Eye movement abnormalities and their association with cognitive impairments in schizophrenia. Schizophrenia Research, 209, 255–262. 10.1016/j.schres.2018.12.051 [DOI] [PubMed] [Google Scholar]
- Morita, K. , Miura, K. , Fujimoto, M. , Yamamori, H. , Yasuda, Y. , Kudo, N. , … Hashimoto, R. (2019b). Eye‐movement characteristics of schizophrenia and their association with cortical thickness. Psychiatry and Clinical Neurosciences, 73(8), 508–509. 10.1111/pcn.12865 [DOI] [PubMed] [Google Scholar]
- Morita, K. , Miura, K. , Kasai, K. , & Hashimoto, R. (2019c). Eye movement characteristics in schizophrenia: A recent update with clinical implications. Neuropsychopharmacology Reports, 40, 2–9. 10.1002/npr2.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa, T. , Hashimoto, R. , Sakoori, K. , Sugaya, Y. , Tanimura, A. , Hashimotodani, Y. , … Kano, M. (2016). Emerging roles of ARHGAP33 in intracellular trafficking of TrkB and pathophysiology of neuropsychiatric disorders. Nature Communications, 7, 10594. 10.1038/ncomms10594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa, T. , Kikuchi, M. , Ishikawa, M. , Yamamori, H. , Nagayasu, K. , Matsumoto, T. , … Hashimoto, R. (2017). Differential gene expression profiles in neurons generated from lymphoblastoid B‐cell line‐derived iPS cells from monozygotic twin cases with treatment‐resistant schizophrenia and discordant responses to clozapine. Schizophrenia Research, 181, 75–82. 10.1016/j.schres.2016.10.012 [DOI] [PubMed] [Google Scholar]
- Nemoto, K. , Shimokawa, T. , Fukunaga, M. , Yamashita, F. , Tamura, M. , Yamamori, H. , … Arai, T. (2019). Differentiation of schizophrenia using structural MRI with consideration of scanner differences: A real‐world multisite study. Psychiatry and Clinical Neurosciences, 74, 56–63. 10.1111/pcn.12934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi, A. , Numata, S. , Tajima, A. , Kinoshita, M. , Kikuchi, K. , Shimodera, S. , … Ohmori, T. (2014). Meta‐analyses of blood homocysteine levels for gender and genetic association studies of the MTHFR C677T polymorphism in schizophrenia. Schizophrenia Bulletin, 40(5), 1154–1163. 10.1093/schbul/sbt154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgidani, M. , Kato, T. A. , Hosoi, M. , Tsuda, M. , Hayakawa, K. , Hayaki, C. , … Kanba, S. (2017). Fibromyalgia and microglial TNF‐alpha: Translational research using human blood induced microglia‐like cells. Scientific Reports, 7(1), 11882. 10.1038/s41598-017-11506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi, K. , Hashimoto, R. , Ikeda, M. , Yamamori, H. , Yasuda, Y. , Fujimoto, M. , … Takeda, M. (2015). Glutamate networks implicate cognitive impairments in schizophrenia: Genome‐wide association studies of 52 cognitive phenotypes. Schizophrenia Bulletin, 41(4), 909–918. 10.1093/schbul/sbu171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi, K. , Hashimoto, R. , Ikeda, M. , Yamashita, F. , Fukunaga, M. , Nemoto, K. , … Takeda, M. (2014). Genetic risk variants of schizophrenia associated with left superior temporal gyrus volume. Cortex, 58, 23–26. 10.1016/j.cortex.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Ohi, K. , Kikuchi, M. , Ikeda, M. , Yamamori, H. , Yasuda, Y. , Fujimoto, M. , … Hashimoto, R. (2016). Polygenetic components for schizophrenia, bipolar disorder and rheumatoid arthritis predict risk of schizophrenia. Schizophrenia Research, 175(1–3), 226–229. 10.1016/j.schres.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Ohi, K. , Sumiyoshi, C. , Fujino, H. , Yasuda, Y. , Yamamori, H. , Fujimoto, M. , … Hashimoto, R. (2018). Genetic overlap between general cognitive function and schizophrenia: A review of cognitive GWASs. International Journal of Molecular Sciences, 19(12), 3822. 10.3390/ijms19123822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi, K. , Sumiyoshi, C. , Fujino, H. , Yasuda, Y. , Yamamori, H. , Fujimoto, M. , … Hashimoto, R. (2017). A brief assessment of intelligence decline in schizophrenia as represented by the difference between current and premorbid intellectual quotient. Frontiers in Psychiatry, 8, 293. 10.3389/fpsyt.2017.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi, K. , Sumiyoshi, C. , Fujino, H. , Yasuda, Y. , Yamamori, H. , Fujimoto, M. , … Hashimoto, R. (2019). A 1.5‐year longitudinal study of social activity in patients with schizophrenia. Frontiers in Psychiatry, 10, 567. 10.3389/fpsyt.2019.00567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, N. , Fukunaga, M. , Yamashita, F. , Koshiyama, D. , Yamamori, H. , Ohi, K. , … Hashimoto, R. (2016). Abnormal asymmetries in subcortical brain volume in schizophrenia. Molecular Psychiatry, 21(10), 1460–1466. 10.1038/mp.2015.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postema, M. C. , van Rooij, D. , Anagnostou, E. , Arango, C. , Auzias, G. , Behrmann, M. , … Francks, C. (2019). Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nature Communications, 10(1), 4958. 10.1038/s41467-019-13005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Hibar, D. P. , Samann, P. G. , Hall, G. B. , Baune, B. T. , Jahanshad, N. , … Veltman, D. J. (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Molecular Psychiatry, 22(6), 900–909. 10.1038/mp.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Veltman, D. J. , van Erp, T. G. , Samann, P. G. , Frodl, T. , Jahanshad, N. , … Hibar, D. P. (2016). Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Molecular Psychiatry, 21(6), 806–812. 10.1038/mp.2015.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoyama, D. , Kato, T. A. , Hashimoto, R. , Kunugi, H. , Hattori, K. , Hayakawa, K. , … Kanba, S. (2016). Plasma metabolites predict severity of depression and suicidal ideation in psychiatric patients‐a multicenter pilot analysis. PLoS One, 11(12), e0165267. 10.1371/journal.pone.0165267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiino, T. , Miura, K. , Fujimoto, M. , Kudo, N. , Yamamori, H. , Yasuda, Y. , … Hashimoto, R. (2019). Comparison of eye movements in schizophrenia and autism spectrum disorder. Neuropsychopharmacol Reports, 40, 92–95. 10.1002/npr2.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai, Y. , Fujita, Y. , Hashimoto, R. , Ohi, K. , Yamamori, H. , Yasuda, Y. , … Hashimoto, K. (2015). Dietary intake of Sulforaphane‐rich broccoli sprout extracts during juvenile and adolescence can prevent phencyclidine‐induced cognitive deficits at adulthood. PLoS One, 10(6), e0127244. 10.1371/journal.pone.0127244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderby, I. E. , Gustafsson, O. , Doan, N. T. , Hibar, D. P. , Martin‐Brevet, S. , Abdellaoui, A. , … 16p11.2 European Consortium, for the ENIGMA‐CNV working group . (2018). Dose response of the 16p11.2 distal copy number variant on intracranial volume and basal ganglia. Molecular Psychiatry, 25, 584–602. 10.1038/s41380-018-0118-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi, C. , Fujino, H. , Sumiyoshi, T. , Yasuda, Y. , Yamamori, H. , Fujimoto, M. , & Hashimoto, R. (2018). Semantic memory Organization in Japanese Patients with Schizophrenia Examined with Category Fluency. Frontiers in Psychiatry, 9, 87. 10.3389/fpsyt.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi, C. , Fujino, H. , Sumiyoshi, T. , Yasuda, Y. , Yamamori, H. , Ohi, K. , … Hashimoto, R. (2016). Usefulness of the Wechsler intelligence scale short form for assessing functional outcomes in patients with schizophrenia. Psychiatry Research, 245, 371–378. 10.1016/j.psychres.2016.08.018 [DOI] [PubMed] [Google Scholar]
- Sumiyoshi, C. , Fujino, H. , Yamamori, H. , Kudo, N. , Azechi, H. , Fujimoto, M. , … Hashimoto, R. (2018). Predicting work outcome in patients with schizophrenia: Influence of IQ decline. Schizophrenia Research, 201, 172–179. 10.1016/j.schres.2018.05.042 [DOI] [PubMed] [Google Scholar]
- Thompson, P. M. , Andreassen, O. A. , Arias‐Vasquez, A. , Bearden, C. E. , Boedhoe, P. S. , Brouwer, R. M. , … ENIGMA Consortium . (2017). ENIGMA and the individual: Predicting factors that affect the brain in 35 countries worldwide. Neuroimage, 145(Pt B), 389–408. 10.1016/j.neuroimage.2015.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, P. M. , Jahanshad, N. , Ching, C. R. K. , Salminen, L. E. , Thomopoulos, S. I. , Bright, J. , … ENIGMA Consortium . (2020). ENIGMA and Global Neuroscience: A decade of large‐scale studies of the brain in health and disease across more than 40 countries. Translational Psychiatry, 10(1), 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka, Y. , Numata, S. , Kinoshita, M. , Umehara, H. , Watanabe, S. Y. , Nakataki, M. , … Ohmori, T. (2018). Decreased serum pyridoxal levels in schizophrenia: Meta‐analysis and Mendelian randomization analysis. Journal of Psychiatry & Neuroscience, 43(3), 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriumi, K. , Kondo, M. , Nagai, T. , Hashimoto, R. , Ohi, K. , Song, Z. , … Nabeshima, T. (2014). Deletion of SHATI/NAT8L increases dopamine D1 receptor on the cell surface in the nucleus accumbens, accelerating methamphetamine dependence. The International Journal of Neuropsychopharmacology, 17(3), 443–453. 10.1017/S1461145713001302 [DOI] [PubMed] [Google Scholar]
- Tozzi, L. , Garczarek, L. , Janowitz, D. , Stein, D. J. , Wittfeld, K. , Dobrowolny, H. , … Frodl, T. (2019). Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: Mega‐analytic findings from a large multi‐site cohort. Psychological Medicine, 50, 1–12. 10.1017/s003329171900093x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno, K. , Nishizawa, D. , Seo, S. , Takayama, K. , Matsumura, S. , Sakai, N. , … Nitta, A. (2015). The piccolo Intronic single nucleotide polymorphism rs13438494 regulates dopamine and serotonin uptake and shows associations with dependence‐like behavior in genomic association study. Current Molecular Medicine, 15(3), 265–274. 10.2174/1566524015666150330145722 [DOI] [PubMed] [Google Scholar]
- van Erp, T. G. , Hibar, D. P. , Rasmussen, J. M. , Glahn, D. C. , Pearlson, G. D. , Andreassen, O. A. , … Turner, J. A. (2016). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Molecular Psychiatry, 21(4), 547–553. 10.1038/mp.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp, T. G. M. , Walton, E. , Hibar, D. P. , Schmaal, L. , Jiang, W. , Glahn, D. C. , … Turner, J. A. (2018). Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing Neuro imaging genetics through meta analysis (ENIGMA) consortium. Biological Psychiatry, 84(9), 644–654. 10.1016/j.biopsych.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij, D. , Anagnostou, E. , Arango, C. , Auzias, G. , Behrmann, M. , Busatto, G. F. , … Buitelaar, J. K. (2018). Cortical and subcortical brain Morphometry differences between patients with autism Spectrum disorder and healthy individuals across the lifespan: Results from the ENIGMA ASD working group. The American Journal of Psychiatry, 175(4), 359–369. 10.1176/appi.ajp.2017.17010100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen, L. S. , Kelly, S. , Isaev, D. , Aleman, A. , Aftanas, L. I. , Bauer, J. , … Schmaal, L. (in press). White matter disturbances in major depressive disorder: A coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Molecular Psychiatry. 10.1038/s41380-019-0477-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon‐Reina, J. E. , Martinez, K. , Qu, X. , Ching, C. R. K. , Nir, T. M. , Kothapalli, D. , … Bearden, C. E. (in press). Altered white matter microstructure in 22q11.2 deletion syndrome: A multisite diffusion tensor imaging study. Molecular Psychiatry. 10.1038/s41380-019-0450-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton, E. , Hibar, D. P. , van Erp, T. G. , Potkin, S. G. , Roiz‐Santianez, R. , Crespo‐Facorro, B. , … Ehrlich, S. (2017). Positive symptoms associate with cortical thinning in the superior temporal gyrus via the ENIGMA schizophrenia consortium. Acta Psychiatrica Scandinavica, 135(5), 439–447. 10.1111/acps.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton, E. , Hibar, D. P. , van Erp, T. G. M. , Potkin, S. G. , Roiz‐Santianez, R. , Crespo‐Facorro, B. , … Ehrlich, S. (2018). Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychological Medicine, 48(1), 82–94. 10.1017/S0033291717001283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, T. Y. , Radua, J. , Pomarol‐Clotet, E. , Salvador, R. , Albajes‐Eizagirre, A. , Solanes, A. , … Nickl‐Jockschat, T. (2019). An overlapping pattern of cerebral cortical thinning is associated with both positive symptoms and aggression in schizophrenia via the ENIGMA consortium. Psychological Medicine, 16, 1–12. 10.1017/S0033291719002149 [DOI] [PubMed] [Google Scholar]
- Writing Committee for the ENIGMA‐CNV Working Group , van der Meer, D. , Sonderby, I. E. , Kaufmann, T. , Walters, G. B. , Abdellaoui, A. , … Andreassen, O. A. (2019). Association of copy number variation of the 15q11.2 BP1‐BP2 region with cortical and subcortical morphology and cognition. JAMA Psychiatry, 77(4), 1–11. 10.1001/jamapsychiatry.2019.3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori, H. , Hashimoto, R. , Fujita, Y. , Numata, S. , Yasuda, Y. , Fujimoto, M. , … Takeda, M. (2014). Changes in plasma D‐serine, L‐serine, and glycine levels in treatment‐resistant schizophrenia before and after clozapine treatment. Neuroscience Letters, 582, 93–98. 10.1016/j.neulet.2014.08.052 [DOI] [PubMed] [Google Scholar]
- Yamamori, H. , Ishima, T. , Yasuda, Y. , Fujimoto, M. , Kudo, N. , Ohi, K. , … Hashimoto, R. (2016). Assessment of a multi‐assay biological diagnostic test for mood disorders in a Japanese population. Neuroscience Letters, 612, 167–171. 10.1016/j.neulet.2015.12.019 [DOI] [PubMed] [Google Scholar]
- Yasuda, Y. , Okada, N. , Nemoto, K. , Fukunaga, M. , Yamamori, H. , Ohi, K. , … Hashimoto, R. (2020). Brain morphological and functional features in cognitive subgroups of schizophrenia. Psychiatry and Clinical Neurosciences, 74, 191–203. 10.1111/pcn.12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Hishimoto, A. , Otsuka, I. , Watanabe, Y. , Numata, S. , Yamamori, H. , … Sora, I. (2018). Longer telomeres in elderly schizophrenia are associated with long‐term hospitalization in the Japanese population. Journal of Psychiatric Research, 103, 161–166. 10.1016/j.jpsychires.2018.05.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


