Abstract
MRI‐derived brain measures offer a link between genes, the environment and behavior and have been widely studied in bipolar disorder (BD). However, many neuroimaging studies of BD have been underpowered, leading to varied results and uncertainty regarding effects. The Enhancing Neuro Imaging Genetics through Meta‐Analysis (ENIGMA) Bipolar Disorder Working Group was formed in 2012 to empower discoveries, generate consensus findings and inform future hypothesis‐driven studies of BD. Through this effort, over 150 researchers from 20 countries and 55 institutions pool data and resources to produce the largest neuroimaging studies of BD ever conducted. The ENIGMA Bipolar Disorder Working Group applies standardized processing and analysis techniques to empower large‐scale meta‐ and mega‐analyses of multimodal brain MRI and improve the replicability of studies relating brain variation to clinical and genetic data. Initial BD Working Group studies reveal widespread patterns of lower cortical thickness, subcortical volume and disrupted white matter integrity associated with BD. Findings also include mapping brain alterations of common medications like lithium, symptom patterns and clinical risk profiles and have provided further insights into the pathophysiological mechanisms of BD. Here we discuss key findings from the BD working group, its ongoing projects and future directions for large‐scale, collaborative studies of mental illness.
Keywords: bipolar disorder, cortical surface area, cortical thickness, ENIGMA, mega‐analysis, meta‐analysis, MRI, neuroimaging, psychiatry, volume
This review discusses the major challenges facing neuroimaging research of bipolar disorder and highlights the major accomplishments, ongoing challenges and future goals of the ENIGMA Bipolar Disorder Working Group.
1. INTRODUCTION
1.1. Overview
Bipolar disorder (BD) is a severe mental disorder characterized by episodic alterations in mood and activity levels including depression, hypomania and mania. It is a leading cause of disability and affects ~1% of the world's population (Merikangas et al., 2011; Vieta et al., 2018). As a chronic illness, BD can lead to long‐term functional impairments and reduced quality of life for both patients and caregivers (Oldis et al., 2016; Perlick, Rosenheck, Clarkin, Raue, & Sirey, 2001; Vigo, Thornicroft, & Atun, 2016), which confer significant societal costs (Ekman, Granstrom, Omerov, Jacob, & Landen, 2013). Challenges remain in identifying robust and reproducible biomarkers to better understand the neurobiology, nosology, diagnosis and targeted treatments that are needed to improve patient outcomes in BD. Mental health professionals continue to rely on a phenomenology‐based diagnostic system as opposed to validated biological markers 1 to diagnose and treat BD. This is reflected in current diagnostic classification systems, namely the DSM‐5 (APA, 2013) and ICD‐10 (WHO, 1993; https://apps.who.int/iris/handle/10665/246208), which use the number and profile of symptoms to delineate the unique and overlapping clinical features of mental disorders, and allow individuals to be categorized based on threshold criteria (BD diagnosis criteria provided in Supplemental Materials). While such approaches have improved the reliability of diagnosing BD, misdiagnosis remains common, and hence biomarkers such as those recently accepted for other disorders such as Alzheimer's disease (Jack Jr. et al., 2018) are urgently needed for BD. To date, there are still no valid biomarkers for the diagnosis of any mental disorder, including BD (Quevedo & Yatham, 2018; Vieta et al., 2018; Vieta & Phillips, 2007).
Non‐invasive, in vivo measures of brain structure and function derived from magnetic resonance imaging (MRI) have shed light on the underlying brain alterations associated with BD. However, factors such as clinical heterogeneity, high costs of data acquisition, variable processing and analysis protocols, underpowered and generally cross‐sectional research designs, post analysis hypothesizing and publication bias have resulted in variable findings and hindered the discovery of validated biomarkers (Figure 1).
FIGURE 1.
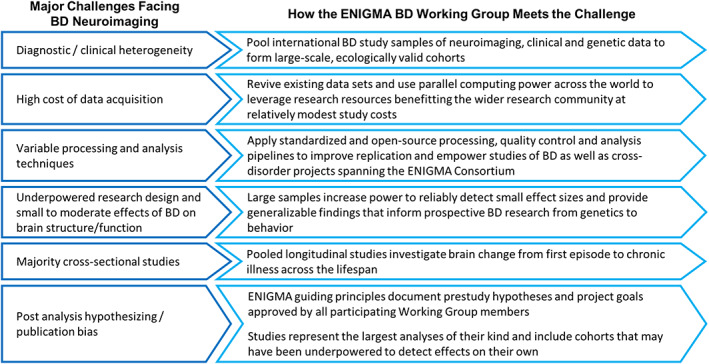
Major challenges facing neuroimaging studies of BD and how the ENIGMA BD Working Group meets these challenges
1.2. Key challenges facing the use of neuroimaging to study bipolar disorder
Neuroimaging has been used as a powerful, non‐invasive tool to study mental disorders such as BD for several decades, ever since early studies revealing enlarged ventricles in patients with schizophrenia (Johnstone, Crow, Frith, Husband, & Kreel, 1976). A key goal of psychiatric neuroimaging research is to use MRI measures of brain structure and function as a link between phenomenology, course, outcome, treatment response, genetics and behavior. Unlike behavioral outcome measures, MRI‐derived brain measures have been proposed to serve as an “endophenotype” – or a marker more closely related to the underlying biology of a disorder. Such markers may have a simpler genetic architecture or be more tractable to study compared to behavioral phenotypes. There is mounting evidence that these in vivo brain endophenotypes may help define the causal pathways between heterogeneous behavioral alterations and genetic factors associated with a given mental disorder (Bigos & Weinberger, 2010; Glahn, Thompson, & Blangero, 2007; Gottesman & Shields, 1973, 1976; Holland et al., 2020; Le & Stein, 2019).
A large body of both structural and functional neuroimaging research has associated BD with abnormalities in the neural circuitry of emotion and reward processing (Phillips & Swartz, 2014). Structural studies have shown BD‐related variation in cortical regions including prefrontal, anterior temporal and insula cortices (Ganzola & Duchesne, 2017; Hajek et al., 2013; Rimol et al., 2012) as well as alterations in subcortical structures such as the hippocampus, thalamus and amygdala in individuals with BD compared to healthy controls (HC) (Hajek et al., 2009; Hajek, Kopecek, Hoschl, & Alda, 2012; Rimol et al., 2010). Studies of white matter in individuals with BD using diffusion MRI (dMRI) have found disruptions in fronto‐limbic white matter (WM) regions important to emotion regulation and reward processing (Phillips & Swartz, 2014). Functional neuroimaging studies have tied altered prefrontal, amygdala, temporal and ventral striatum activity during emotion and reward tasks to BD (Phillips & Swartz, 2014; Sepede et al., 2012, 2015, 2020).
However, while prior meta‐analyses have found relative consensus across studies (Ganzola & Duchesne, 2017), conflicting results are not uncommon in neuroimaging studies of BD. For example, reports of both larger and smaller structural volumes in participants with BD versus HC are widely cited in the literature (Phillips & Swartz, 2014). Such discrepancies can be traced to a core set of challenges facing neuroimaging studies of BD (Figure 1; see Supplemental Materials for further details on these challenges).
First, BD is a broad diagnostic category with varied clinical characteristics likely including related underlying subtypes (i.e., biotypes that subdivide or stratify conventional diagnostic categories). These subtypes likely vary in underlying genetic and environmental risk, treatment response, psychiatric/medical comorbidity, clinical course, prognosis and pathophysiology (Duffy, Vandeleur, Heffer, & Preisig, 2017). Even the most carefully designed studies of BD will likely include participant heterogeneity, as we do not fully understand the underlying neurobiological stratification. We discuss the challenges of linking current categorical diagnostic constructs of BD to underlying biological measures in the Supplemental Materials. Study participant heterogeneity in key variables affecting brain measures (e.g., diagnostic method, age at scan, age of onset, illness duration, medication status, etc.) adds noise to the already complicated task of detecting the subtle effects of the disorder on MRI‐derived brain metrics. Second, the high cost of MRI data collection can restrict study sample sizes (most studies collect data from fewer than 100 participants), which may limit the statistical power to detect neurobiological effects or model complex factors modulating BD symptoms. Third, variable processing and analysis techniques for MRI make results hard to compare across studies (Botvinik‐Nezer et al., 2020). The power of traditional literature‐based meta‐analyses in BD neuroimaging studies is somewhat limited by a lack of such standardized techniques (see the following section for more detail). Fourth, most research studies are cross‐sectional, which limits analyses to static brain traits as opposed to potential brain alterations over the course of illness in BD. Fifth, underpowered and heterogeneous study samples may yield false positives or overestimate effects – a situation known as the “winner's curse” (Button et al., 2013). Lastly, many studies suffer from post analysis hypothesizing or publication bias. All these challenges degrade the reliability of findings, mandating the design of more replicable and generalizable studies.
Under current diagnostic criteria, BD patient heterogeneity (Charney et al., 2017; Stahl et al., 2019; Wolfers et al., 2018) can affect study power depending on sample size (Button et al., 2013). In smaller samples, patient heterogeneity can dilute the overall effect of interest, either making it harder to detect subtle BD alterations or overestimating true BD effects. In larger consortium studies with increased statistical power, patient heterogeneity can lead to a more “ecologically valid” sample that represents a larger fraction of the patient space. While this heterogeneity in large samples can lead to more replicable findings that apply more widely to BD in the general population, subtle biological effects relevant to particular patient subgroups may be obscured, requiring data‐driven, post‐hoc stratification. Smaller, well‐controlled studies that are able to implement deeper phenotyping and novel analysis methods may be equipped to isolate the underlying pathophysiology associated with particular patient subgroups or symptom profiles, as long as the sample sizes are still adequately powered to detect the effects.
Current structural and functional neuroimaging measures may only account for a limited proportion of the overall variance in a complex phenotypic trait such as BD diagnosis (Paulus & Thompson, 2019). Larger research samples may overcome the power limitations of smaller studies and improve sensitivity to subtle brain signatures (Westlye, Alnaes, van der Meer, Kaufmann, & Andreassen, 2019). They may also offer a greater opportunity to model factors that contribute to complex phenotypes such as BD. For example, some medications commonly used to treat BD have shown morphometric effects on MRI‐derived brain metrics (Abe et al., 2015; Abramovic et al., 2016; Hajek et al., 2014; Haukvik et al., 2020; Lyoo et al., 2010; Moore et al., 2009; Moore, Bebchuk, Wilds, Chen, & Manji, 2000; Sarrazin et al., 2019), including gray matter increases with lithium and atrophic effects of anticonvulsants (Hibar et al., 2018). These medication‐related brain changes may be critical in the treatment of BD, but they may also make brain alterations in the disorder harder to detect, as most are highly confounded with illness status or severity. The modeling of complex factors, such as medication history, can be greatly improved in larger, pooled study samples.
These core challenges have contributed to the “replication crisis” affecting much of biomedical research (Button et al., 2013; Dumas‐Mallet, Button, Boraud, Gonon, & Munafo, 2017; Ioannidis, 2008, 2011, 2017). To address these challenges, and to contribute to a recent shift in psychiatry toward large consortium efforts, the ENIGMA Bipolar Disorder Working Group was formed to pool data, expertise and computational resources to discover factors that reliably affect brain structure and function in BD.
Importantly, clinical symptoms (either categorical or dimensional) remain the most useful metrics available to clinicians treating patients with BD. While some of the research tools presented in this article may one day improve BD care, these tools and methods must be rigorously validated and standardized if they are ever to be truly useful in a clinical setting. In the following sections, we discuss how large‐scale efforts provide a path forward, and serve as an example of the power of team science in pooling existing data and expertise to tackle the core challenges facing BD research and treatment.
1.3. The ENIGMA consortium: Large‐scale, collaborative studies of brain structure and function
An increasing number of common variants throughout the human genome have been associated with risk for BD. The Psychiatric Genomics Consortium (PGC) spearheaded a global effort to discover these risk loci by coordinating large‐scale psychiatric genetics studies (https://www.med.unc.edu/pgc/). Many early genetic studies prior to the PGC found associations between polymorphisms in candidate genes and brain measures that later failed to replicate in larger independent samples (Farrell et al., 2015). Genome‐wide analyses have now revealed hundreds of common genetic variants that are reliably associated with psychiatric disorders (Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2018; Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014; Stahl et al., 2019; Wray et al., 2018), as well as discovered significant genetic overlap between major disorders (Brainstorm et al., 2018; Gandal et al., 2018).
As the PGC has done for genetics, large‐scale, collaborative neuroimaging studies offer the power to answer new questions and address prior inconsistencies in the literature. The ENIGMA Consortium, launched in 2009, aimed to identify genetic variants that are consistently associated with brain structure and function by performing genome‐wide association studies (GWAS) on measures from brain MRI. To overcome the statistical power limitations of GWAS (as most genetic polymorphisms associated with brain measures account for less than 1% of the overall variance in any given brain measure), the initial ENIGMA projects recruited samples with both MRI and genetic data and implemented standardized processing and analysis methods. These protocols enabled highly powered, prospective meta‐analyses on a scale not previously possible (i.e., 99% power to detect genetic loci explaining at least 1% of the variance in a given brain trait). These initial studies identified new genetic variants associated with variability in brain volumes such as the hippocampus (Hibar et al., 2015; Stein et al., 2012). More recently, collaborations between ENIGMA and other large‐scale international consortia, such as CHARGE, were able to replicate initial findings in larger independent samples and provided new insights into the genetic mechanisms influencing brain structure (Adams et al., 2016; Grasby et al., 2020; Hibar et al., 2017; Satizabal et al., 2019), function (Smit et al., 2018), and development (Brouwer et al., 2017).
The success of the initial ENIGMA multi‐site GWAS led to the formation of over 50 collaborative ENIGMA Working Groups developing or using standardized protocols and studying a wide range of neurodegenerative, neurodevelopmental and psychiatric disorders. Working in parallel, and sharing standardized tools, the ENIGMA clinical working groups published the largest neuroimaging studies of BD (Hibar et al., 2016; Hibar et al., 2018), MDD (Schmaal et al., 2016; Schmaal et al., 2017), schizophrenia (SCZ) (van Erp et al., 2016; van Erp et al., 2018), obsessive compulsive disorder (OCD) (Boedhoe et al., 2017; Boedhoe et al., 2018), attention deficit hyperactivity disorder (ADHD) (Hoogman et al., 2017, 2019), autism spectrum disorder (ASD) (van Rooij et al., 2018), epilepsy (Whelan et al., 2018), substance use disorders (Mackey et al., 2019), PTSD (Dennis et al., 2019; Logue et al., 2018) and 22q11.2 deletion syndrome (Ching et al., 2020; Sun et al., 2018; Villalon‐Reina et al., 2019). A more thorough review of efforts across the ENIGMA Consortium may be found in several recent articles (Bearden & Thompson, 2017; Thompson et al., 2014; Thompson et al., 2020).
The ENIGMA Consortium offers a number of advantages compared to previous smaller‐scale neuroimaging studies. ENIGMA takes advantage of existing data sets, reviving smaller samples that have often concluded data collection and primary publications. To date, the ENIGMA consortium has incorporated over 70,000 scans from a range of disorders and healthy individuals. ENIGMA currently includes over 2,000 scientists from over 340 institutions, spanning 45 countries. By taking advantage of existing data and using parallel computing power across the world, ENIGMA has leveraged research resources that benefit the wider research community at a relatively modest study cost.
In the conventional, retrospective, literature‐based meta‐analysis, summary statistics including effect sizes, confidence intervals and standard errors are extracted from published studies (which have often used different processing and analysis pipelines). These are then combined mathematically to estimate an overall effect (e.g., regional brain volume differences between BD and HC). A key strength of the ENIGMA approach over more traditional meta‐analyses is the implementation of standardized processing and analysis protocols to perform coordinated, prospective meta‐ and mega‐analyses. These protocols, which are publicly available (http://enigma.ini.usc.edu/protocols), serve several purposes: 1) ENIGMA‐standardized protocols make it possible to efficiently and consistently extract measures from MRI data and to perform robust quality assessment and statistical modeling across tens to hundreds of international research centers with varying neuroimaging expertise, 2) standardized processing and analysis leads to more unbiased investigations of brain measures not possible via traditional meta‐analyses that combine published effect sizes derived from varied processing and analysis protocols (e.g., regions of interest combined with whole brain analyses, mass‐univariate combined with multivariate analyses, etc.), 3) pooling data can overcome publication bias and boost statistical power by including cohorts that may have been underpowered on their own to detect effects on particular brain measures or associations with symptom constructs, 4) harmonized protocols using standardized and publicly available pipelines promote “open science” by increasing transparency and encouraging replication, 5) it makes large‐scale, post‐hoc subgroup explorations possible and lastly, 6) it allows for the direct comparison of large‐scale, standardized data across ENIGMA Working Groups to better understand common and distinct patterns that span traditional diagnostic boundaries.
Importantly, and as previously mentioned, large‐scale ENIGMA BD Working Group efforts do not replace well‐designed, smaller‐scale neuroimaging studies, but rather complement one another. While the initial ENIGMA studies were well powered to capture generalizable effects across study samples, they can miss subtle, subtype‐specific effects that may be better captured by smaller well‐controlled studies focusing on particular subtypes or symptom patterns in BD, or with targeted deeper phenotyping not economically feasible in large‐scale studies. While the large samples pooled through ENIGMA increase sensitivity to smaller effect sizes, and are arguably more generalizable to the wider population, potential lurking confounds or artifacts (e.g., head motion see Pardoe, Hiess, & Kuzniecky, 2016; Yao et al., 2017) that may span independently collected study samples must be carefully considered and modeled.
Lastly, the international coordination of ENIGMA studies requires consensus and cooperation across a large number of centers worldwide. In our experience, one of the more important steps to starting a successful ENIGMA Working Group is to be pragmatic about the initial studies carried out by the group. Focusing early efforts on a core set of available variables and feasibly derived brain measures lowers the bar for participation and helps to incorporate the greatest number of Working Group members. These early studies, while not necessarily applying the most novel of brain metrics (e.g., volumes) or analysis models (e.g., general linear models) are more feasible to accomplish while simultaneously providing important consensus building findings in the literature. The trust and infrastructure formed through these initial pragmatic studies and publications helps build momentum toward more ambitious projects using more advanced metrics (e.g., vertex‐wise measures, connectivity measures using graph theory, etc.) and more advanced analyses (e.g., multivariate analysis, machine learning, structural covariance analysis, etc.).
2. THE ENIGMA BIPOLAR DISORDER WORKING GROUP
The ENIGMA Bipolar Disorder Working Group was formed in 2012 to address some of the core limitations in BD research and foster collaborative discoveries using the wider ENIGMA Consortium research model. The Working Group consists of an international team of over 150 clinicians, neuroscientists, bioengineers, and geneticists who pool research resources from 20 countries and 55 institutions to conduct large‐scale neuroimaging studies of BD. The group has combined multimodal neuroimaging data from 46 study cohorts which include ~3,500 BD participants and ~ 8,500 healthy controls, making it the largest neuroimaging consortium effort to study BD (Figure 2 ). In the following sections we discuss the Bipolar Disorder Working Group and its contributions to understanding brain alterations in BD, including guiding principles, current findings, ongoing projects and future directions that aim to advance neurobiological research of BD and other mental disorders.
FIGURE 2.
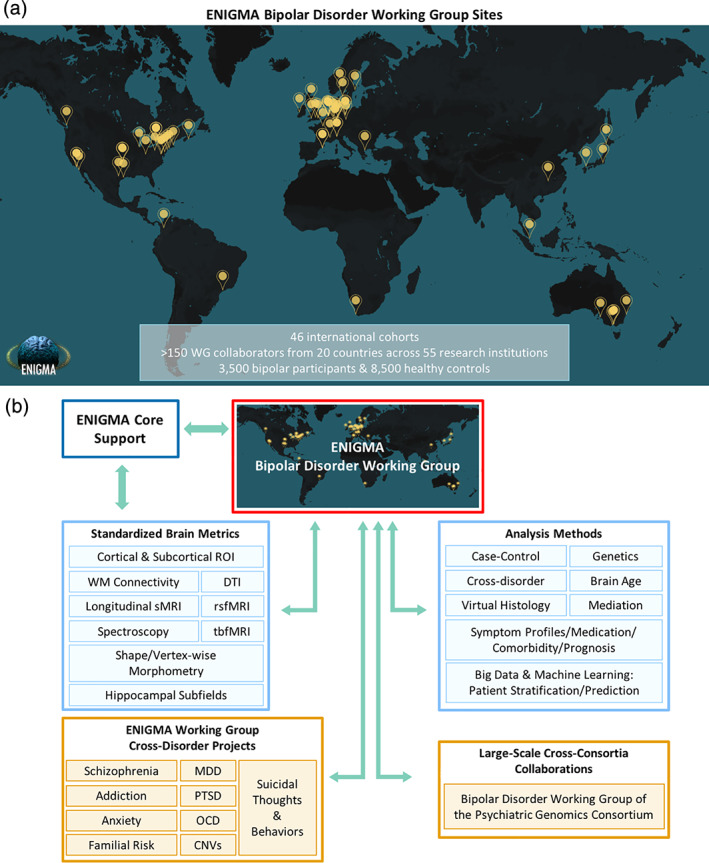
(a) ENIGMA Bipolar Disorder Working Group sites across the world including over 150 researchers from 20 countries and 55 institutions. (b) Schematic of ENIGMA Bipolar Disorder Working Group as it fits into the larger ENIGMA Consortium network. rsfMRI, resting‐state functional MRI; tbfMRI, task‐based functional MRI; WM, white matter; DTI, diffusion tensor imaging; MDD, major depressive disorder; PTSD, post‐traumatic stress disorder; OCD, obsessive–compulsive disorder; CNVs, copy number variants; Familial Risk, relatives of individuals with psychiatric illness (including bipolar disorder and schizophrenia)
2.1. Goals and guiding principles
The primary research goals of the Bipolar Disorder Working Group are to:
Make reliable discoveries that improve our understanding of the pathophysiological mechanisms of BD.
Provide clinically relevant information to help improve BD nosology, diagnosis, mechanistically‐directed interventions and treatment outcomes.
To accomplish these goals, the Bipolar Disorder Working Group implemented ENIGMA‐standardized protocols using open‐source and widely available processing platforms such as FreeSurfer (Fischl et al., 2002) and FSL (Smith et al., 2006), as well as tools specifically developed for large‐scale, multisite projects (Figure 2 ).
The BD Working Group operates under a set of simple guiding principles (Figure 3). First, primary data sharing is not required to participate in the working group; meta‐analysis is always the encouraged/default analysis framework. This helps garner the greatest number of participating samples and mitigates many data sharing challenges (Zugman et al., 2020). Working Group projects operate on an “opt‐in” principle, where members retain full ownership and control over their data and are free to decide which project proposals they wish to participate in. Furthermore, members can withdraw their data or resources from a project at any time prior to publication.
FIGURE 3.
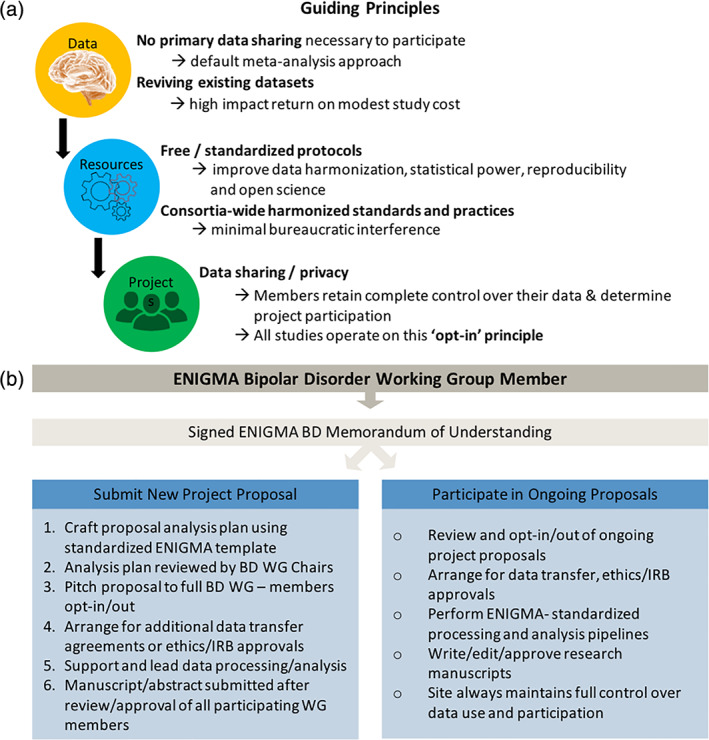
(a) Outline of the ENIGMA BD Working Group guiding principles. (b) Flow diagram showing working group logistics including memorandum of understanding, participation in and development of new research proposals, data sharing, etc. Ethics/IRB: The ENIGMA BD Working group is experienced with navigating international research ethics and institutional review boards, which may require additional approval depending on project specifics. More information on the ENIGMA BD Working group including the Memorandum of Understanding can be found online (http://enigma.ini.usc.edu/ongoing/enigma‐bipolar‐working‐group/)
New Working Group members are asked to sign a Memorandum of Understanding (MOU) that has been standardized across the ENIGMA Working Groups and sets a basic framework to protect participating sites' data privacy, facilitate data sharing, encourage academic productivity, ensure appropriate publication credit and authorship. It also provides a system to track and archive data, analyses and publications related to the BD Working Group (http://enigma.ini.usc.edu/ongoing/enigma-bipolar-working-group/ Figure 3). The Working Group's scientific initiatives and basic policies are overseen by two Chairs, Dr. Ole A. Andreassen and Dr. Christopher R. K. Ching, who are responsible for ensuring that working group studies are carried out in accordance with appropriate ethical guidelines.
Working group members are provided an equal opportunity to propose a project analysis plan. Analysis plans are developed and submitted in a standardized format with the help of the Working Group Chairs and then presented to the full Working Group membership via email and conference calls. Project leaders pitch their analysis plans to the Working Group and each member reviews and decides whether to contribute their data sample to the proposed projects (opt‐in). Most analysis plans earn widespread participation from the BD Working Group members, resulting in large‐scale studies that could not have been accomplished independently.
The BD Working Group's initial goal was to demonstrate the power and feasibility of large‐scale, collaborative neuroimaging analyses by establishing minimal bureaucratic barriers for participation and using standardized and publicly available processing and analysis protocols (Figures 2 and 3 ). To date, the BD Working Group has published five peer‐reviewed studies and currently coordinates over 18 active and ongoing analysis projects led by Working Group members from around the world.
3. f PUBLISHED STUDIES
3.1. Overview
The ENIGMA Bipolar Disorder Working Group has published five peer‐reviewed studies, each representing the largest neuroimaging studies of their kind and helping to answer the question of which brain structures are reliably associated with BD, its subtypes, and other clinical measures such as illness duration, severity, genetic risk, and common medications. Overall, these studies point to a diffuse pattern of brain alterations including smaller subcortical volumes, lower cortical thickness and altered white matter integrity in groups of individuals with BD compared to healthy controls. Small to moderate effect sizes are observed for ENIGMA‐standardized brain measures, in line with prior reports from the BD literature. Common medications such as lithium appear to have a normalizing effect on gray and white matter structures, whereas other treatments such as anticonvulsants appear to have the opposite effect. Standardized, ROI‐based cortical and subcortical brain measures were useful in parsing differences between patients and non‐affected relatives as well as providing above chance predictive accuracy in classifying BD individuals from controls using common machine learning techniques.
3.2. Alterations to subcortical volumes and associations with common pharmacological treatments
The ENIGMA BD Working Group's first study was a meta‐analysis of subcortical gray matter brain volumes in 1,710 BD participants and 2,594 HC from 20 international sites, to identify effects of the disorder on regional morphometry, and rank structural brain metrics for case–control differences. On average, higher bilateral ventricular volumes and lower hippocampal, amygdala and thalamic volumes were detected in BD versus HC (Figure 4a ) (Hibar et al., 2016). Importantly, all case–control effect sizes were small to moderate and varied to some degree across the 20 study samples. When combining the effects across sites meta‐analytically, clearer patterns emerged (Figure 4b). The group differences may reflect either accelerated atrophy in BD with potential disease‐related neuroprogression, chronic effects of the illness, or medication. Alternatively, smaller volumes may represent a potential risk factor for BD arising in early stages of development. Importantly, prior meta‐analyses were either unable to detect case–control differences in amygdala volume or reported variable effects (Altshuler et al., 2000; Chang et al., 2005).
FIGURE 4.
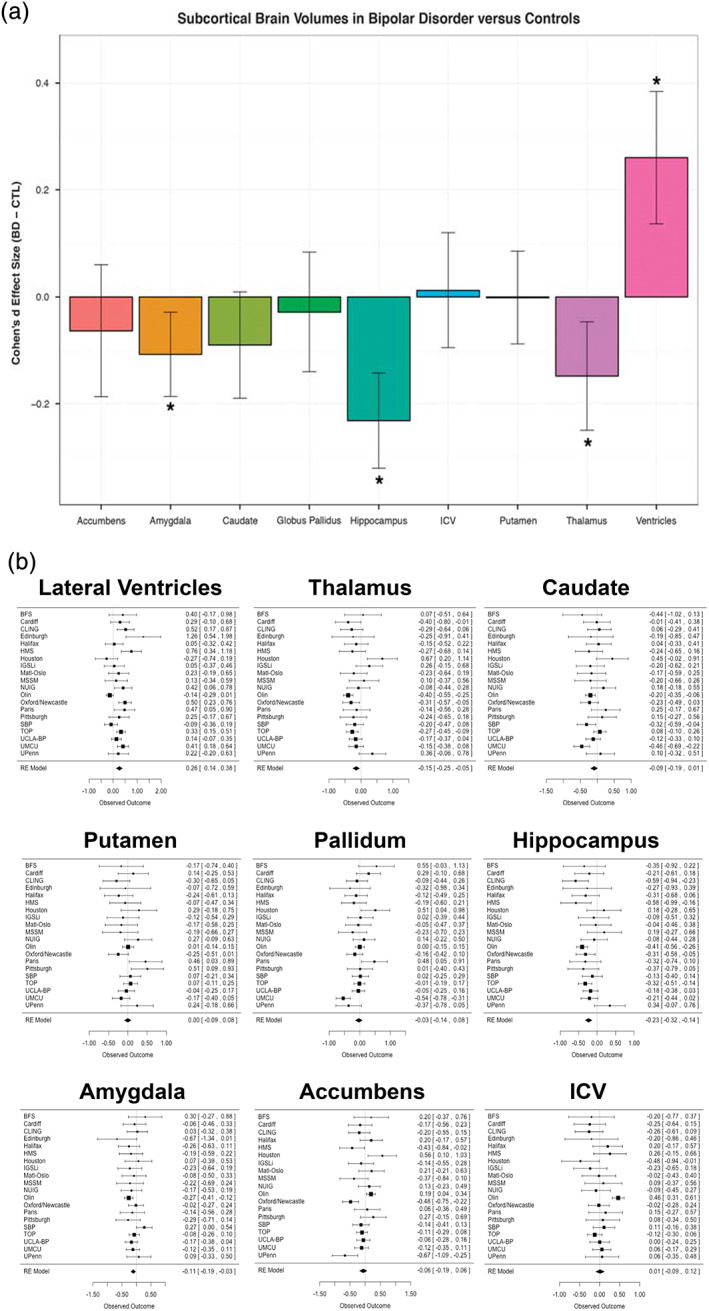
Findings from Subcortical volumetric abnormalities in bipolar disorder (Hibar et al., 2016). (a) Cohen's d effect size estimates for subcortical differences between individuals with BD versus healthy controls (HC) using ENIGMA‐standardized FreeSurfer volumes. Statistical model accounts for age, sex, and intracranial volume. Error bars indicate mean effect size ± standard error of the mean. Results passing study‐wide significance threshold are indicated by (*) including the amygdala which showed a trending effect. (b) Forest plots displaying the effect size estimates (adjusted Cohen's d) for each of the 20 study sites in the comparison of individuals with BD versus HC at each subcortical structure along with the overall inverse variance‐weighted random‐effects meta‐analysis results (RE Model)
No structural brain differences were detected between BD subtypes (BD‐I, BD‐II and BD‐NOS). Lithium treatment was associated with larger thalamic volumes compared to non‐treated individuals with BD. Compared to HC, individuals with BD not on lithium treatment had smaller hippocampal and thalamic volumes, on average, and larger lateral ventricles. Participants with BD taking anticonvulsants had smaller hippocampal volumes compared to non‐treated participants with BD. As discussed in the published study, these cross‐sectional results should be interpreted cautiously as medication status is confounded to some extent with illness characteristics (such as symptom severity). Furthermore, a simple binary coding (prescribed/not prescribed) was used to determine medication status at the time of scan. Current studies are investigating such medication factors as treatment history, dose, and serum level, to better model interactions between different pharmacological agents – polypharmacy is common in BD – and their associated effects on brain structure (see Ongoing and Future Studies section below and Supplemental Materials).
3.3. Widespread cortical thickness alterations in BD and associations with pharmacological treatment
Prior meta‐analyses reported lower cortical thickness in the anterior cingulate, paracingulate, superior temporal gyrus and prefrontal regions associated with BD (Hanford, Nazarov, Hall, & Sassi, 2016; Phillips & Swartz, 2014). Surface area findings have been more variable – some larger studies detected no differences between BD and HC (Rimol et al., 2010 ). In our second study, again the largest of its kind, we focused on cortical structure (2,447 BD and 4,056 HC), examining ENIGMA‐standardized measures of cortical thickness and surface area in an expanded ENIGMA BD sample including 28 international sites (Hibar et al., 2018). Compared to controls, individuals with BD exhibited a widespread pattern of thinner cortex (Figure 5a ). Interestingly, and in agreement with previous large sample studies, no case–control differences were detected for cortical surface area. Longer illness duration was associated with a pattern of lower cortical thickness but not with surface area alterations. As in the subcortical study, no significant differences were detected between BD clinical subtypes.
FIGURE 5.
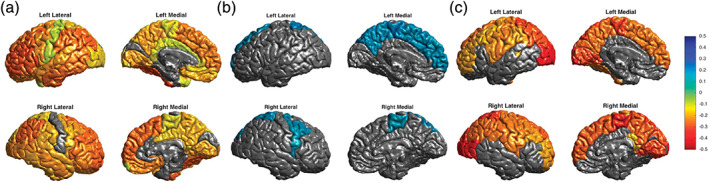
Findings from Cortical abnormalities in bipolar disorder: an MRI analysis of 6,503 individuals from the ENIGMA Bipolar Disorder Working Group (Hibar et al., 2018). (a) A widespread pattern of thinner cortex in adult individuals with BD versus HC. Cohen's d effect sizes plotted in regions passing correction for multiple comparisons. (b) Thicker cortex in adult individuals with BD taking lithium medication at time of scan. (c) Thinner cortex in adult individuals with BD associated with anticonvulsant treatment at time of scan
With regard to medication status, we found significantly higher cortical thickness in participants with BD also taking lithium at the time of scan, with the largest effects in the left paracentral gyrus (Figure 5b ). Anticonvulsant treatment was associated with lower cortical thickness, with the greatest effects in bilateral occipital gyri (Figure 5C ). Atypical “second‐generation” antipsychotics were associated with lower cortical surface area in the rostral middle frontal gyrus, whereas typical “first‐generation” antipsychotics were associated with higher surface area in the left inferior parietal gyrus.
The cortical findings were largely in line with prior reports of thinner frontal and temporal cortices in BD. Notably, regions with the largest BD versus HC differences included the ventrolateral prefrontal cortex, an area long implicated in BD pathophysiology. Important new contributions include the observation of lower thickness in inferior parietal, fusiform, and inferior temporal regions in adults with BD. Structural deficits in these regions have been tied to disruptions in sensorimotor integration (Caspers, Zilles, Laird, & Eickhoff, 2010) and language (Vigneau et al., 2006), and may relate to altered emotion perception and rapid mood changes in BD. Further studies are needed to probe the functional relevance of regional cortical differences, and how these effects may be interrelated (e.g., using structural covariance analysis).
3.4. Multi‐site machine learning using brain MRI to identify bipolar disorder
Differential diagnosis of BD remains a challenge, with mis‐diagnosis leading to delays in effective treatments. In an effort to improve diagnostic accuracy and, ultimately, personalize treatments, machine learning can be used to find complex patterns in neuroimaging data that predict diagnostic categories, or prognosis. In our first such study, the Bipolar Working Group evaluated the capacity of a linear support vector machine classifier to predict the diagnosis of BD using standardized cortical and subcortical ROI‐based brain features from 853 individuals with BD and 2,167 HC acquired from 13 international sites (Nunes et al., 2018). Under appropriate cross‐validation procedures, diagnosis of BD was classified with a sensitivity of 0.66 (95% CI [0.63, 0.69]), a specificity of 0.65 (0.62, 0.67) and an area under the receiver operating characteristic (ROC) curve of 0.71 (0.69, 0.74) (Figure 6 ). Informative features were in agreement with previous findings, including the importance of hippocampus, amygdala (Hajek et al., 2009, 2012), and cortical regions such as inferior frontal and precentral gyri (Hibar et al., 2018).
FIGURE 6.
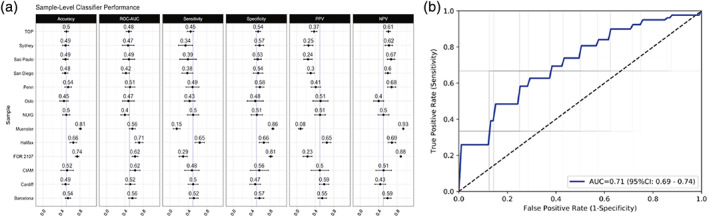
Findings from Using structural MRI to identify bipolar disorders ‐ 13 site machine learning study in 3020 individuals from the ENIGMA Bipolar Disorders Working Group (Nunes et al., 2018). (a) Support vector machine (SVM) classifier performance trained on each site independently, including mean and 95% confidence intervals for accuracy, area under the receiver operating curve (ROC‐AUC), sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). (b) Receiver operating curves from aggregate individual‐level analysis with dashed line indicating chance performance, blue line indicating mean ROC and gray lines indicating ROC curves from individual folds
When interpreting these results, we considered several issues. BD and its clinical subtypes are difficult to diagnose leading to noise in the target labels (i.e., derived brain measures). In addition, the illness shows marked clinical and neurobiological heterogeneity. Many brain alterations in BD are likely secondary to illness burden, presence or absence of comorbid conditions (Hajek et al., 2014; Hajek, McIntyre, & Alda, 2016), or treatments (Hibar et al., 2018; Van Gestel et al., 2019). Since these secondary changes are not found in all participants and reflect factors beyond the diagnosis, they cannot be used diagnostically. Last but not least, we worked with regional brain measures, not raw/voxelwise data. This approach involves information loss in the feature engineering process and using raw data could improve classification accuracy. While clinician judgment for BD diagnosis and treatment continues to outperform robust machine learning methods such as those studied here, the accuracy observed in this study provides a realistic and fair estimate of classification performance, which can be achieved in a large, ecologically valid, multisite sample of individuals with BD based on regional brain structure measures.
Our study provided further clues about the impact of data handling on classification performance. Performing the machine learning analyses on data pooled across the sites yielded a much better performance than meta‐analysis of site level results – the typical analytic method in multisite collaborations. Thus, future multisite brain‐imaging machine learning studies should attempt to move toward sharing of individual, raw data, not only site‐level results. Developing an ethico‐legal framework to facilitate safe sharing of raw data is a key and critical component of advancing medical machine learning (Passos et al., 2019).
3.5. Diffuse white matter alterations challenging the existing models of bipolar disorder
Diffusion tensor imaging (DTI) studies of BD implicate widespread white matter (WM) alterations within and beyond the fronto‐limbic regions that appear to precede emotional instability (Phillips & Swartz, 2014). Limbic and non‐limbic tract disruptions have been reported (Canales‐Rodriguez et al., 2014) but inconsistencies exist in the literature and are likely due to the aforementioned research challenges. In our first DTI project, we aimed to identify generalizable WM microstructural alterations with a standardized processing and analysis framework (Jahanshad et al., 2013; Smith et al., 2006) to study case–control differences, as well as associations with clinical characteristics in BD. The project pooled data from 1,482 individuals with BD and 1,551 HC from 26 research samples across 12 countries. We used both meta‐ and mega‐analyses to form the largest multicenter DTI study of BD to date. Fractional anisotropy (FA) – a measure of white matter directionality, coherence and integrity – was lower, on average, in participants with BD across 29 out of 44 WM regions of interest, with strongest effects in the corpus callosum and cingulum (Figure 7 ). Higher FA was associated with later disorder onset and shorter illness duration. Lithium treatment was associated with higher FA, both globally and in a number of regions of interest including the corona radiata, posterior thalamic radiation and internal capsule (Favre et al., 2019). No significant FA alterations were detected between BD subtypes (BD‐I and BD‐II diagnosis) or associated with antidepressant use, illness severity (measured by number of mood episodes/duration of illness) or history of psychotic symptoms.
FIGURE 7.
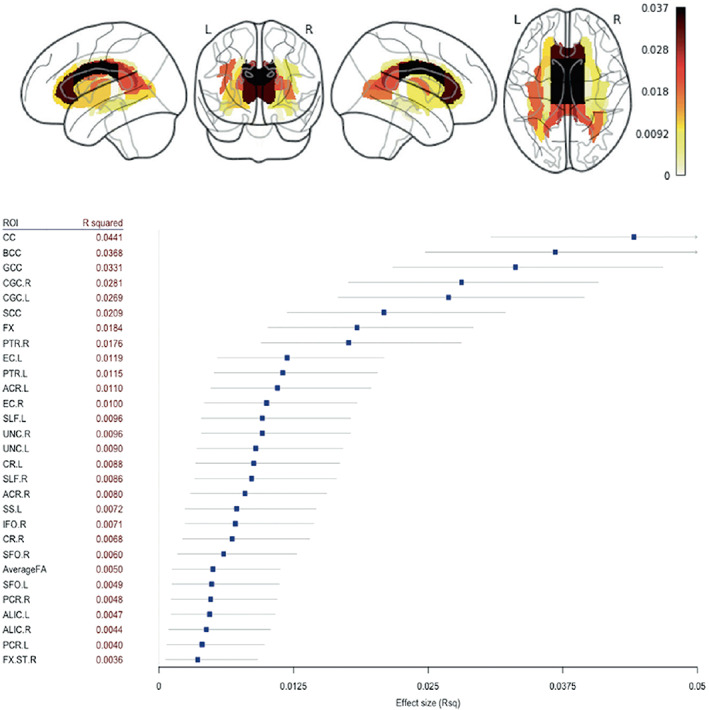
Findings from Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega‐ and meta‐analyses across 3,033 individuals (Favre et al., 2019). Mega‐analysis fractional anisotropy (FA) differences between BD and HC across 43 white matter (WM) tracts and the whole‐brain skeleton with R squared effect sizes and confidence intervals ranked by increasing order of magnitude for the regions showing significant group differences. R, right; .L, left; CC, corpus callosum; BCC, body of the corpus callosum; GCC, genu of the corpus callosum; CGC, cingulum; SCC, splenium of corpus callosum; FX, fornix; PTR, posterior thalamic radiation; EC, external capsule; ACR, anterior corona radiata; SLF, superior longitudinal fasciculus; UNC, uncinate fasciculus; CR, corona radiata; SS, sagittal stratum; IFO, inferior fronto‐occipital fasciculus, SFO, superior fronto‐occipital fasciculus; Average FA, average FA across full skeleton; PCR, posterior corona radiata; ALIC, anterior limb of the internal capsule; FXST, fornix (cres) / stria terminalis
Unlike prior studies, we reported widespread FA alterations in BD – a pattern similar to results from the ENIGMA Schizophrenia Working Group DTI study (Kelly et al., 2018). This suggests that a global pattern of microstructural abnormalities may span both disorders. Altered WM microstructure in the cingulum, a major limbic pathway, is in agreement with prior reports of disrupted fronto‐limbic connectivity in BD (Mahon, Burdick, & Szeszko, 2010; Phillips & Swartz, 2014). However, the role of the corpus callosum in BD is not clear. WM alterations in individuals with BD with psychotic symptoms have been reported (Sarrazin et al., 2014), but the role of the corpus callosum in emotion processing or mood switching is not fully understood (Linke et al., 2013; Wang et al., 2008). Lower corpus callosum FA was reported in the ENIGMA DTI meta‐analysis of SCZ (Kelly et al., 2018) and MDD (van Velzen et al., 2019), suggesting overlapping pathophysiology in psychosis and affective disorders (Koshiyama et al., 2019). Further studies are needed to evaluate the extent to which the corpus callosum might be differentially affected in these related disorders. Preliminary data suggest that disruption of interhemispheric connectivity is a disease marker rather than a vulnerability marker to BD (Chepenik et al., 2010; Linke et al., 2013). Nonetheless, we identified extensive FA‐related WM abnormalities, which challenges current pathophysiological models of BD. Future models should not be limited to fronto‐limbic networks, and should consider interhemispheric dysconnectivity as a feature of interest in BD.
An important limitations of the study was the focus on the most commonly used DTI measure, FA, as opposed to including other scalar measures such as mean, axial, and radial diffusivity derived from the tensor model. The tensor, the most common method of modeling diffusion MRI, while robust and widely used, has well documented limitations such as inability to resolve crossing fibers – which are present in a large proportion of brain white matter – or to disentangle the intra‐ and extra‐axonal compartments. As detailed below, ongoing studies from the ENIGMA BD Working Group are currently applying more advanced analysis techniques to model white matter. Furthermore, future BD research will likely benefit from new diffusion MRI methods to model white matter tissue microstructure such as Neurite Orientation Dispersion and Density Imaging (NODDI) (Zhang, Schneider, Wheeler‐Kingshott, & Alexander, 2012), which has been used to highlight the impact of lithium on neurite density (Sarrazin et al., 2019).
3.6. Mapping familial risk to brain structure across bipolar disorder and schizophrenia
Most neuroimaging studies to date have compared individuals with BD to HC. The interpretation of case–control findings is complicated by many of the aforementioned study limitations. An alternative approach is to study first‐degree family members (i.e., offspring, siblings, parents or co‐twins), as they are at higher risk for the disorder but are otherwise healthy and unmedicated. As first‐degree relatives share, on average, half of the genes with their ill relative (except for monozygotic co‐twins who share all their genes), a family design provides a unique angle from which to study the effect of BD risk on brain structure and function.
The ENIGMA‐Relatives Working Group has taken a cross‐disorder approach to the study of BD and SCZ relatives, motivated by overlapping clinical symptoms, including delusions, hallucinations, mania, depression and anxiety, as well as shared genetic and epidemiological risk (Cross‐Disorder Group of the Psychiatric Genomics Consortium, 2013; Kempf, Hussain, & Potash, 2005; Pearlson, 2015). While BD and SCZ do show distinct symptom patterns and clinical course (Bora, 2015; Correll, Penzner, Frederickson, et al., 2007; Murray & Sham, 2004), it remains unclear whether they represent discrete entities shaped by distinct etiology and pathogenesis, or if they represent a spectrum of mood‐psychosis disorders. The ENIGMA‐Relatives Working Group has aimed to identify overlapping and distinct features of SZ and BD in first‐degree relatives of patients with these disorders.
In the largest study to date, standardized subcortical and global brain measures were meta‐analyzed across healthy first‐degree relatives of individuals with either BD (FDRs‐BD) or SZ (FDRs‐SZ) (de Zwarte, Brouwer, Agartz, et al., 2019). A total of 6,008 participants from 34 family cohorts, including 1,228 FDRs‐SZ, 852 FDRs‐BD, 2,246 HC, 1,016 participants with SZ and 666 with BD were included. The main findings included: 1) FDRs‐BD had larger average intracranial volumes (ICV), whereas FDRs‐SZ showed smaller thalamic volumes compared with HC, 2) in FDRs‐BD, ICV explained the larger brain volumes in other regions, whereas in FDRs‐SZ, brain volumes and thickness effect sizes became significantly smaller compared to HC after statistical correction for ICV, 3) brain alterations differed between the relative types, but no clear pattern was detected, and 4) findings were not confounded by other psychiatric diagnoses in the relatives (Figure 8 ).
FIGURE 8.
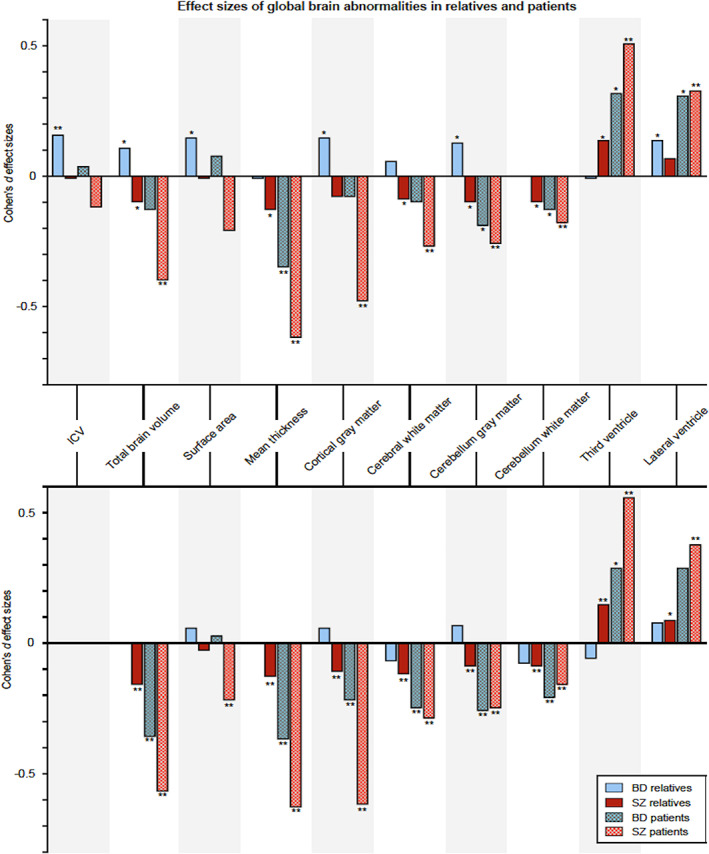
Findings from The association between familial risk and brain abnormalities is disease‐specific: an ENIGMA–Relatives study of schizophrenia and bipolar disorder (de Zwarte et al., 2019). Top: Cohen's d effect sizes comparing BD and SCZ relatives and healthy controls across global brain measures. Bottom: global effect sizes adjusted for total intracranial volume (ICV). *Nominally significant (p < .05 uncorrected); **q < .05 corrected for multiple comparisons
When considering the finding of larger ICV in FDRs‐BD but not FDRs‐SZ, as well as prior reports indicating a diverging pattern of ICV volume for individuals with BD and SCZ (i.e., significantly smaller ICV in SZ but not BD) (Hibar et al., 2016; van Erp et al., 2016), the ENIGMA‐Relatives Working Group recently investigated whether differences in Intelligence Quotient (IQ) or educational attainment could explain the findings in SZ and BD relatives. These most recent findings are detailed in de Zwarte et al. from this issue.
4. ONGOING AND FUTURE STUDIES
Building on these initial studies, the ENIGMA BD Working Group has a growing list of ongoing projects to: (a) more finely map the initial ROI‐based findings of altered cortical/subcortical gray matter and WM alterations in BD by using advanced, high‐definition brain morphometric features including vertex‐ or voxel‐wise analysis, (b) improve classification and individual‐level prediction using data driven clustering/biotyping with more advanced machine learning techniques, (c) investigate the impact of polygenic risk and gene expression markers on large‐scale standardized brain measures, (d) empower future mega‐analyses that allow more sophisticated designs than meta‐analyses, and (e) study how individual symptoms, functional domains and risk factors map onto measures of brain structure and function that cross classic diagnostic boundaries, revealing shared and distinct brain markers of mental illness. Further details of the ongoing Bipolar Disorder Working Group projects may be found in the Supplemental Materials.
4.1. Finer mapping of BD‐related brain variation
The ENIGMA Bipolar Working Group is currently applying advanced techniques that model subcortical shape morphometry, hippocampal subfield volumes, WM connectivity, neurometabolites, longitudinal brain change and brain aging across thousands of individuals with BD and HC. These methods, now standardized for multisite studies, aim to better characterize the spatial distribution of BD‐related alterations across the brain, track the longitudinal trajectory of such brain changes across the course of illness, and determine the extent to which those changes may interact with normal brain aging processes.
4.2. Machine learning for better classification and individual‐level prediction
Most ENIGMA projects to date have applied relatively simple mass univariate analysis methods when studying brain features. Combining multiple imaging modalities will likely improve predictions of diagnosis and prognosis. In addition to classical multivariate approaches, machine learning models are being applied to neuroimaging data across a variety of ENIGMA BD projects to predict diagnostic groups, treatment response, and to characterize subtypes or clusters within groups of individuals diagnosed with BD. Our initial efforts in the diagnostic classification of BD showed promise (Nunes et al., 2018), but accuracy is likely to improve with the addition of functional neuroimaging measures (Han, De Berardis, Fornaro, & Kim, 2019; Phillips & Vieta, 2007), as well as techniques that merge multimodal structural, functional, vertex‐wise metrics and in‐depth clinical information. The BD Working Group is tackling new challenges that arise when using higher‐dimensional data and when fitting complex models to provide improved individual‐level predictions.
4.3. Large‐scale direct comparisons of brain measures across psychiatric disorders
A key question in psychiatric neuroimaging is the extent to which brain variations are shared or differentiate major psychiatric disorders. Many mental illnesses overlap in symptomatology, response to medication and underlying genetic risk. Examples include known intersections between BD and MDD and SCZ (Pearlson, 2015; Rink, Pagel, Franklin, & Baethge, 2016). Whether shared clinical features reflect similar underlying brain structure and function is poorly understood. As many clinically‐focused ENIGMA Working Groups have completed studies of cortical and subcortical structure, direct comparison of brain measures for tens of thousands of participants is now possible. The resulting data set represents the largest collection of psychiatric neuroimaging data ever amassed using standardized processing techniques, and the largest samples of BD, MDD, and SCZ neuroimaging data ever analyzed.
The initial case–control studies from the ENIGMA clinical working groups (Figure 9 ) suggest overlapping and distinct patterns of brain alterations across disorders. Notably, ENIGMA SCZ case–control cortical effects were more widespread and greater in magnitude than those found in BD and MDD. In line with prior hypotheses regarding underlying biological correlates of BD versus MDD, frontal lobe systems showed greater deficits in ENIGMA BD cases, whereas limbic regions tended to show greater deficits in ENIGMA MDD cases. Smaller hippocampal volume is another common finding across published ENIGMA psychiatric studies. Preliminary analyses, correlating effect sizes across published ENIGMA psychiatric working group studies, indicate significant correlations between cortical and subcortical MRI alterations across disorders, which may be partially explained by common underlying genetic markers (Ching et al., 2020).
FIGURE 9.
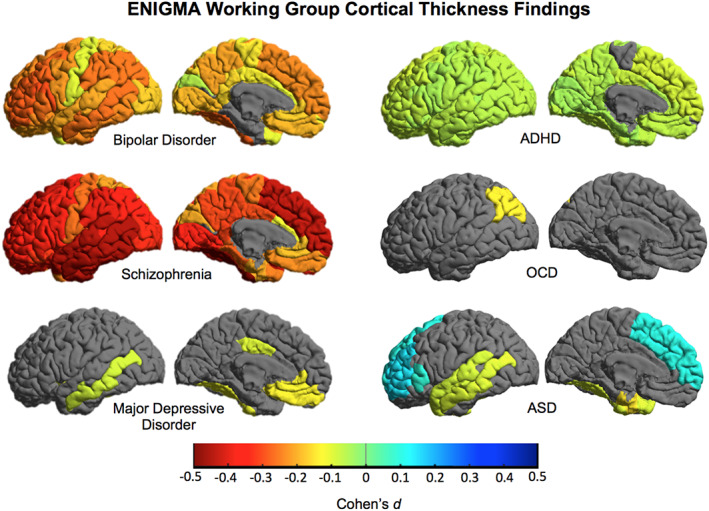
Cortical thickness differences across ENIGMA working groups. Cohen's d effect sizes comparing cases versus healthy controls (HC) plotted across 34 bilateral cortical ROIs from ENIGMA‐standardized FreeSurfer protocol (http://enigma.ini.usc.edu/protocols/). Warmer colors indicate lower thickness in cases/patients, whereas cooler colors indicate greater thickness in cases/patients versus HC. Results derived from published ENIGMA studies: bipolar disorder (N = 4,419, 28 sites, Hibar et al., 2018), major depressive disorder (N = 10,105, 15 sites, Schmaal et al., 2017), schizophrenia (N = 9,572, 39 sites, van Erp et al., 2018), attention deficit hyperactivity disorder (ADHD N = 4,180, 36 sites, Hoogman et al., 2019), obsessive–compulsive disorder (OCD N = 3,665, 27 sites, Boedhoe et al., 2018) and autism spectrum disorder (ASD N = 3,222, 49 sites, van Rooij et al., 2018)
For pragmatic reasons, and to help incorporate the greatest number of Working Group sites in initial analyses, the first ENIGMA BD Working Group studies tended to include a limited number of essential variables such as age, sex, diagnosis, age of illness onset, binary (yes/no) medication status at the time of scan, and simple severity measures. The next phase of ENIGMA cross‐disorder analyses is tackling the challenges of deeper phenotyping and the heterogeneity across samples. The ENIGMA BD Working Group is collecting in‐depth clinical and demographic information in a harmonized effort with the ENIGMA SCZ and MDD Working Groups from over 200 data sets. Metrics of interest include education, socioeconomic status, IQ, body mass index, number of psychiatric hospitalizations, number of episodes (depressive, manic, psychotic), substance use disorder, comorbid psychiatric disorders, current/lifetime medication treatment, medication dose, medication serum level, and detailed behavioral/symptom information. These measures will provide the basis for comparisons not dependent on classic diagnostic categorizations. Work is underway to find overlapping scales and measures and to resolve clinical/behavioral measurement harmonization and empower analyses more in line with the Research Domain Criteria (RDoC) framework (Insel, 2014). Clinical information will also allow for analyses such as network modeling of individual symptoms (Borsboom & Cramer, 2013) and thus a more detailed characterization than traditional diagnostic categories. Such modeling can also be done in combination with brain and other biological measures, as in recent studies of MDD (Fried et al., 2019; Hilland et al., 2019).
Advanced supervised and unsupervised machine learning techniques are being applied to examine the extent to which BD, MDD and SCZ can be discriminated on the basis of ENIGMA‐standardized brain measures. Of particular interest is whether multivariate, machine learning techniques can discriminate between alternative patient groupings and cluster individuals according to duration of illness, symptom severity, presence of depressive and/or psychotic symptoms, substance use, treatment response and other characteristics that might improve individual‐level predictive accuracy.
4.4. Linking genetic risk and gene expression to brain alterations across psychiatric disorders
The ENIGMA bipolar disorder working group is uniquely positioned to study how genetic risk loci affect brain structure and function. In collaboration with the Psychiatric Genomics Consortium Working Group on Bipolar Disorders (PGC‐BD), we are deriving polygenic risk scores (PRS) across participating ENIGMA BD Working Group sites using recent discoveries from the PGC‐BD group (Bipolar Disorder and Schizophrenia Working Group of the PGC, 2018; Stahl et al., 2019). The ENIGMA PRS protocol is freely available online (http://enigma.ini.usc.edu/protocols/) and is empowering projects to map BD‐PRS vulnerability across brain structures, as well as genetic risk for other psychiatric disorders. This effort aims to both identify brain regions at risk in BD as well as construct overlapping genotype–phenotype risk maps across common psychiatric disorders, which may reveal more mechanistic models of shared and unique disease processes.
A key challenge in neuroimaging is bridging the gap between in vivo MRI and ex vivo histology (Paus, 2018). To address this, several ENIGMA working groups applied a “virtual histology” approach, where profiles of structural brain metrics, namely group differences in cortical thickness, are related to gradients in cell‐specific gene expression using data from the Allen Human Brain Atlas (Hawrylycz et al., 2012; Patel et al., 2018; Shin et al., 2017). Inter‐regional profiles of group differences in cortical thickness have been generated meta‐analytically across over 12,000 cases and 15,000 HC from the ENIGMA BD, ASD, ADHD, OCD, MDD and SCZ Working Groups. Associations of a given cell type with a profile of group differences in cortical thickness are correlated with cell‐specific gene expression to better characterize shared and unique gene expression potentially driving cortical alterations across these major neuropsychiatric disorders.
5. FUTURE PERSPECTIVES: REPLICATION, BIG DATA AND SHIFTING THE PARADIGM IN PSYCHIATRIC RESEARCH
BD is a complex illness where individual factors confer a small proportion of the overall risk. The ENIGMA Bipolar Disorder Working Group is focused on applying innovative, big data approaches to provide reliable new discoveries on the biological underpinnings of BD, and to generate clinically relevant findings to improve diagnosis and treatment.
The current psychiatric research paradigm, which seeks markers that link high‐level, behavioral‐based diagnostic labels to underlying biology, is slowly changing. Neuroimaging may not provide the larger BD‐related effect sizes once proposed by the “endophenotypes” hypothesis, but the combination of large‐scale neuroimaging data sets has led to a better understanding of the brain regions that mediate the link between genetic risk and the behavioral manifestations of BD.
Several limitations to the ENIGMA Bipolar Disorder Working Group approach must be discussed. First, Working Group sites collected data independently and used a range of tools and methods that can differ with respect to MRI scanner (hardware and software), biospecimen collection, behavioral assessment, study inclusion/exclusion criteria and overall data quality. While ENIGMA studies deploy standardized processing and analysis techniques that reduce the variance across site measurements, true data harmonization is only possible through prospective data collection. Second, the ENIGMA Bipolar Working Group consists of fairly Eurocentric data samples. Future efforts must be made to incorporate additional data sets from all corners of the world to garner an even more ecologically valid sample of BD phenotypes. Third, early ENIGMA studies initially deploy pragmatic analysis plans, using only the most common measures across Working Group sites (e.g., binary medication status at time of scan, primary diagnosis, etc.) and applying mass univariate analysis methods (e.g., BD case vs. HC differences assessed by multiple linear regression). While this approach has led to the incorporation of many standardized data sets and has helped to clarify basic brain structure alterations in BD, group‐level results only allow for broad generalizations about individuals with BD and have limited clinical applications when assessing and treating individual patients. Fourth, all of the published studies from the Bipolar Disorder Working Group have so far used a cross‐sectional design. There is increasing evidence that BD is a neuroprogressive disorder – our own results indicate associations between duration of illness and brain structure (Favre et al., 2019; Hibar et al., 2018) – which requires a longitudinal design that may inform better clinical staging and treatment of the illness (Salagre et al., 2018). Longitudinal studies that include participants with varying ages of onset may also help to address whether brain alterations are indeed inherent markers of BD or are a consequence of factors associated with illness duration. Longitudinal studies in the context of therapeutic trials will also clarify the effect of interventions on the brain, although there are many challenges in integrating such data across sites.
With these limitations in mind, the ENIGMA Bipolar Disorder Working Group has several future research goals:
Incorporate advanced and standardized multimodal measures. Combining multimodal biological measures that encompass genetic, neurochemical, neuroimaging and behavioral factors will likely lead to more clinically relevant biomarkers for improved patient‐level predictions and a better understanding of BD pathophysiology. Innovative standardized protocols now being applied in the ENIGMA Bipolar Disorder Working Group include white matter connectivity, resting and task‐based fMRI, spectroscopy, vertex‐wise shape morphometry, longitudinal brain change and deeper clinical and behavioral phenotyping.
Advance novel, big data techniques. We are currently applying advanced machine learning models to predict classic diagnostic groups, as well as potential patient subgroup stratification along behavioral and treatment measures. New multivariate GWAS methods are helping to reveal more of the hidden genetic risk for BD (e.g., the MOSTest, see van der Meer et al., 2019) and are being applied through our collaboration with the PGC. These genetic findings further empower the ENIGMA Bipolar Working Group's ability to map genetic risk to brain structure and function.
Cross‐disorder studies. Large‐scale, transdiagnostic efforts such as those being carried out between the ENIGMA BD, MDD, and SCZ working groups will help us understand the common and unique neurobiological factors underlying mental illnesses.
Hypothesis‐driven prospective analyses. Our international collaborations are driving future prospective studies with more harmonized data collection. Lessons learned from ongoing gross neuroimaging analyses inform future micro‐ and ultrascale studies of cellular morphometry, distribution, and synaptic structure and function, which may provide a more mechanistic link between genes and behavior in BD (Le & Stein, 2019). A centrally coordinated, long‐term study of a large cohort of individuals with BD across the lifespan is desperately needed. Such a study, collecting neuroimaging, cellular, molecular and other deeper phenotyping measures akin to studies such as UK Biobank and other longitudinal cohorts (McInnis & Greden, 2016; Miller et al., 2016; Yatham, Kauer‐Sant'Anna, Bond, Lam, & Torres, 2009), and integrated with pharmacological and behavioral treatment interventions would provide an ambitious path forward to addressing many of the key challenges facing BD research.
The ENIGMA BD Working Group has demonstrated that large‐scale, international collaborations can empower replicable and generalizable studies of BD. To the goal of more open and reproducible science, the ENIGMA Consortium is building an Organic Data Science (ODS) tool to facilitate complex and dynamic working group activities via a systematic information system. Based on Semantic MediaWiki, ENIGMA‐ODS provides cross‐working group data queries and project tracking to improve study reproducibility and help to overcome barriers to efficiency that are inherent in large‐scale projects (Jahanshad et al., 2015). Furthermore, the use of publicly available and standardized processing and analysis protocols may empower future “living studies” or continuously updated research findings (e.g., associations with subcortical volume and psychotic symptoms), with semi‐automated addition of future cohorts to an ever‐increasing ENIGMA‐standardized research sample.
Engaging patients is vital to ensure that biomedical research, and the subsequent interventions and tools, meet the needs of individuals living with BD. The Milken Institute and the Depression and Bipolar Support Alliance recently surveyed over 6,000 individuals living with MDD and BD to better understand research priorities from individuals living with these illnesses (Altimus, 2019). Survey respondents identified the ability to be independent or act according to one's own will as the top wellness priority, while only 20% of individuals identified the lack of symptoms of acute MDD or BD as a measure of wellness. Furthermore, 54% of respondents reported experiencing both MDD and BD symptoms, contrary to a discrete diagnosis given by clinicians. These discrepancies in both wellness priorities and manifestation of the disorder demonstrate that the most pressing needs of individuals with BD may not align with the goals of researchers. Moving forward, the ENIGMA BD working group aims to actively engage user groups to help focus our research goals and engage new participants in future studies.
The ENIGMA BD Working Group is actively recruiting new research collaborators and the infrastructure allows for new groups to be efficiently incorporated into ongoing and future analyses. Groups interested in joining, contributing data, and starting their own research proposal using the largest neuroimaging data set in BD research are encouraged to contact the Working Group Chairs.
The ENIGMA Bipolar Working Group will continue to be guided by the collective expertise of a strong network of neuroscientists, psychiatrists, data scientists, bioengineers and geneticists (Guglielmi, 2018). Big data consortia efforts offer the opportunity to work cohesively on related research questions, bringing diverse information to bear on neuroscientific problems, and will continue to provide valuable discoveries, revealing consensus findings and informing future hypothesis‐driven studies of BD and other neuropsychiatric disorders.
All images taken from original publications are approved for reprint under creative commons licensing.
CONFLICT OF INTEREST
O. A. A. received Speaker's honorarium from Lundbeck and is a consultant for HealthLytix. M. B. was supported by an unrestricted grant from AstraZeneca. A. C. B. is a full‐time employee of P1vital Ltd. C. R. K. C. and P. M. T. have received partial research support from Biogen, Inc. (Boston, USA) for work unrelated to the topic of this manuscript. T. E. has received a speaker's fee from Lundbeck. G. M. G. is a NIHR Emeritus Senior Investigator, holds shares in P1vital and P1Vital products and has served as consultant, advisor or C. M. E. speaker in the last 3 years for Allergan, Angelini, Compass pathways, MSD, Janssen, Lundbeck (/Otsuka or /Takeda), Medscape, Minerva, P1Vital, Pfizer, Sage, Servier, Shire, Sun Pharma. D. P. H. is a full‐time employee of Genentech, Inc. A. M. M. has received research support from the Eli Lilly, Janssen and The Sackler Trust. J. C. S. has participated in research funded by Forest, Merck, BMS, and GSK and has been a speaker for Pfizer and Abbott. Marsal Sanches has received research grants from Janssen. All other authors from this site report no conflicts of interest to declare. D. J. S. has received research grants and/or consultancy honoraria from Lundbeck and Sun. E. V. has received grants and served as consultant, advisor or CME speaker for the following entities (work unrelated to the topic of this manuscript): AB‐Biotics, Abbott, Allergan, Angelini, Dainippon Sumitomo Pharma, Galenica, Janssen, Lundbeck, Novartis, Otsuka, Sage, Sanofi‐Aventis, and Takeda.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
Core funding for ENIGMA was provided by the NIH Big Data to Knowledge (BD2K) program under consortium grant U54 EB020403, by the ENIGMA World Aging Center (R56 AG058854), and by the ENIGMA Sex Differences Initiative (R01 MH116147). M. A. was supported by Canadian Institutes of Health Research. D. A. is funded by the South‐Eastern Norway Regional Health Authority (2019107). O. A. A. was supported by Supported by Research Council of Norway, South East Norway Health Authority, EU CoMorMent grant, and the NIH. The BIPOLIFE Bipolar Network Consortium funded by BMBF (Germany). C. E. B. was supported by R01MH075007, R01MH095454, K23MH074644‐01 (to C. E. B.), Colciencias and Codi‐University of Antioquia (to C. L.‐J.), K08MH086786 (to S. C. F.), R03MH105808 (S. C. F./C. E. B.). MBel was supported by the Italian Ministry of Health with the grant: GR‐2010‐2319022 Immune gene expression and white matter pathology in first‐manic patients before and after treatment. A multimodal imaging genetic study”. F. B. was supported by Italian Ministry of Health RF‐2011‐02350980, RF‐2011‐02349921, European Union EU‐FP7‐HEALTH‐F2‐2008‐222963 “MOODINFLAME”. MBer is supported by a National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (APP1059660 and APP1156072). This project was supported by an unrestricted grant from AstraZeneca. H. P. B. was supported by National Institute of Mental Health, Internal Bipolar Disorder Foundation, Brain and Behavior Research Foundation. E. B. and T. E. are supported by the Research Council of Norway, the South‐Eastern Norway Regional Health Authority, Oslo University Hospital and a research grant from Mrs. Throne‐Holst. C. B., E. V. and J. M. G. were supported by the Spanish Ministry of Science, Innovation and Universities/Economy and Competitiveness/Instituto de Salud Carlos III (PI15/00283), co‐financed by Fondo Europeo de Desarrollo Regional (FEDER) Funds from the European Commission (“A Way of Making Europe”), CIBERSAM, and the Departament de Salut de la Generalitat de Catalunya (2017SGR1365, SLT002/16/00331, SLT006/17/00357), CERCA Programme/Generalitat de Catalunya. P. B. was partially supported by grants from the Italian Ministry of Health (RF‐2016‐02364582). This work was supported by the Generalitat de Catalunya (2017SGR01271) and several grants funded by Instituto de Salud Carlos III co‐funded by the European Regional Development Fund/European Social Fund “Investing in your future”: Miguel Servet Research Contract (CPII16/00018 to E. P.‐C.), Sara Borrell Research Contract (CD16/00264 to M. F.‐V. and CD18/00029 to E. J. C.‐R.), and Research Projects (PI15/00277 to E. J. C.‐R., PI18/00810 to E. P.‐C. and PI18/00877 to R. S.). The funding organizations played no role in the study design, data collection and analysis, or manuscript approval. This research was funded by the Health Research Board (HRB‐HRA‐POR‐324) awarded to DMC and the Irish Research Council (IRC) Postgraduate Scholarships awarded to LN and Dr Genevieve McPhilemy. Thank you to the participants, the Welcome‐Trust HRB Clinical Research Facility and the Centre for Advanced Medical Imaging, St. James Hospital, Dublin, Ireland. TMCA was supported by NARSAD. C. R. K. C. was supported by NIA T32AG058507; NIH/NIMH 5T32MH073526; NIH grant U54EB020403 from the Big Data to Knowledge (BD2K) Program. This work was funded by the German Research Foundation (DFG, grant FOR2107 DA1151/5‐1 and DA1151/5‐2 to U. D.; SFB‐TRR58, Projects C09 and Z02 to U. D.) and the Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster (grant Dan3/012/17 to U. D.). A. M. D.‐Z. was supported by PRISMA U.T Colciencias and CODI‐UdeA. E. D. was supported by Bipli ANR. L. T. E. was supported by Desert‐Pacific MIRECC, NIH MH083968. P. F. was supported by the grant Infrastructure d'avenir en Biologie Santé ‐ ANR‐11‐INBS‐0006 and the Agence Nationale pour la Recherche (ANR‐11‐IDEX‐0004 Labex BioPsy, ANR‐10‐COHO‐10‐01 psyCOH), Fondation pour la Recherche Médicale (Bioinformatique pour la biologie 2014) and the Fondation de l'Avenir (Recherche Médicale Appliquée 2014). The UNSW group contribution was supported by funding from the Australian National Medical and Health Research Council (NHMRC) Program Grant 1037196 and Project Grants 1066177 and 1063960, and the Lansdowne Foundation. J. M. F. was also supported by the Janette Mary O'Neil Research Fellowship. D. C. G. was supported by R01 MH080912, R01 MH096957 and R01 MH106324. B. I. G. was supported by CIHR, Ontario Mental Health Foundation. G. M. G. notes that the views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health. M. J. G. was supported by NHMRC Project APP630471. The CliNG database was established in Göttingen, Germany between 2008–2015. Recruitment for the CliNG study sample was partially supported by the Deutsche Forschungsgemeinschaft (DFG) via the Clinical Research Group 241 “Genotype‐phenotype relationships and neurobiology of the longitudinal course of psychosis”, TP2 (PI Gruber; http://www.kfo241.de; grant numbers GR 1950/5‐1 and GR 1950/10‐1). T. P. G. was supported by The Research Council of Norway (#223273); South‐Eastern Norway Regional Health Authority (#2017112). B. C. M. H. was supported by EU‐FP7‐HEALTH‐222963 “MOODINFLAME” and EU‐FP7‐PEOPLE‐ 286334 “PSYCHAID”. T. H., received funding for these studies from Canadian Institutes of Health Research (grant #142255), Brain and Behavior Research Foundation (Independent Investigator Award # 23412), the Ministry of Health of the Czech Republic (grants number 16–32791A, 16‐32696A). N. H. was supported by KAKENHI:17 K10287. U. K. H.‐A. was supported by the South‐Eastern Norway Health Authorities (grant number 2012–132); Norwegian Research Council (grant number 223273). J. H. was supported by Agence Nationale pour la Recherche (ANR‐11‐IDEX‐0004 Labex BioPsy, ANR‐10‐COHO‐10‐01 psyCOH), Fondation pour la Recherche Médicale (Bioinformatique pour la biologie 2014). F. M. H. acknowledges support from University of Cape Town's Research Committee and South African funding bodies the Medical Research Council and National Research Foundation. A. J. was supported by grants by the Deutsche Forschungsgemeinschaft, DFG, Grant Nos. JA 1890/7‐1 and JA 1890/7‐2) and the German Federal Ministry of Education and Research (Grant No. 01EE1404F). M. J. K. is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. This work was supported by FOR 2107 DFG grant numbers to: T. T. J. K. (KI 588/14‐1, KI 588/14‐2), U. D. (DA 1151/5‐1, DA 1151/5‐2), A. K. (KR 3822/5‐1, KR 3822/7‐2), I. N. (NE 2254/1‐2). H. K. was supported by the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and development, AMED (16dm0107100h0001). R. K. was supported by The William K Warren Foundation. Part of this study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. M. L. was supported by Swedish Research Council (2018‐02653), the Swedish foundation for Strategic Research (KF10‐0039), the Swedish Brain foundation, and the Swedish Federal Government under the LUA/ALF agreement (ALF 20170019, ALFGBG‐716801). This research was supported by the German Ministry for Education and Research (BMBF) grants NGFNplus MooDS 01GS08148, e:Med program O1ZX1314B and O1ZX1314G as well as Forschungsnetz AERIAL 01EE1406A and 01EE1406B. This work is supported by a NARSAD Distinguished Investigator Grant to H. W. and DFG grant Wa 1539/11‐1, ER 724/4‐1. C. L.‐J. was supported by PRISMA U.T Colciencias and CODI‐UdeA. B. J. M. received funding from the Canadian Institutes of Health Research and a NARSAD Independent Investigator Award from the Brain and Behavior Research Foundation. CEM was supported by the NIHR Oxford Health Biomedical Research Centre and Wellcome Centre for Integrative Neuroimaging. K. M. was supported by a Grant‐in‐Aid for “Integrated research on neuropsychiatric disorders” and “Understanding of molecular and environmental bases for brain health” carried out under the Strategic Research Program for Brain Sciences (SRPBS) by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), an Intramural Research Grant (24‐11) for Neurological and Psychiatric Disorders of NCNP, and KAKENHI from the Japan Society for the Promotion of Science (grant numbers 24591716 and 15 K09832). C. M. was supported by Studies supported by the Health Research Board (HRA_POR/2011/100). A. M. M. was supported by the Wellcome Trust (104036/Z/14/Z, 216767/Z/19/Z) and UKRI MRC (MC_PC_17209, MR/S035818/1). P. B. M. was supported by Australian National Health and Medical Research Council. A. C. N. was supported by Funded by the NIMH Intramural Research Program. A. N. was supported by Nova Scotia Health Research Foundation and the Killam Trust. R. A. O. was supported by 5R01MH090553. B. J. O. was supported by The Australian cohort collection was supported by the Australian National Health and Medical Research Council Program Grants (Grant No. 510135 and Grant No. 1037196) and Project Grants (Grant No. 1063960 and Grant No. 1066177), the Lansdowne Foundation and the Janette Mary O'Neil Research Fellowship. Y. P. was supported by NSERC (CGS) scholarship. M. E. P. was supported by Fondation pour la Recherche Médicale. T. P. was supported by the Canadian Institute of Health Research, Natural Sciences and Engineering Research Council. M. L. P. was supported by R37MH100041, R01MH059929, R01MH060952, R01MH076971, and the Pittsburgh Foundation. J. A. P.‐Z. was supported by the Research Group in Psychiatry, Department of Psychiatry, Faculty of Medicine, Universidad de Antioquia, Medellín, Antioquia, Colombia. M. P. was supported by DRCI CHU Grenoble and Sante et Societe Federative Research Structure. E. P.‐C. was supported by Generalitat de Catalunya (2017SGR01271); Instituto de Salud Carlos III (CPII16/00018 to E. P.‐C. and PI18/00810). Y. Q. was supported by the Australian National Health and Medical Research Council of Australia (NHMRC, APP630471 and APP1081603), and the Macquarie University's ARC Centre of Excellence in Cognition and its Disorders (CE110001021). J. R. was supported by the Spanish Ministry of Science, Innovation and Universities / Economy and Competitiveness/Instituto de Salud Carlos III (CPII19/00009), co‐financed by ERDF Funds from the European Commission (“A Way of Making Europe”) and CIBERSAM. M. M. R. was supported by Netherlands Organization for Health Research and Development (ZonMw), program Mental Health, education of investigators in mental health (OOG; #100‐002‐034). G. R. was supported by the Australian National Medical and Health Research Council (Program Grant 1037196), and the Lansdowne Foundation. The DIADE study is funded through ZonMW Geestkracht OOG 2007 (#100002034). H. G. R. is supported by a NWO/ZonMW VENI grant (#016.126.059). T. D. S. was supported by R01MH107703, K23MH098130, NARSAD. J. S. was supported by R21MH113871; P20GM121312. A. H. S. was supported by ZonMw, Hersenstichting, AMC Amsterdam. K. S. was supported by by funding sources NHG (SIG/12004) and SBIC (RP C‐009). J. C. S. was supported by Jair C. Soares was supported in part by NIMH grant R01 MH085667, the Dunn Foundation and the Pat Rutherford, Jr. Endowed Chair in Psychiatry. M. S. was supported by MRI was performed at the Frankfurt Brain Imaging Centre, supported by the German Research Council (DFG) and the German Ministry for Education and Research (BMBF; Brain Imaging Center Frankfurt/Main, DLR 01GO0203). D. J. S. is supported by the SA MRC. C. K. T. was supported by Research Council of Norway #223273, Research Council of Norway #288083, Research Council of Norway #230345, Southern and Eastern Norway Regional Health Authority #2019069. H. S. T. was supported by National Research Foundation of South Africa, University of Cape Town. G. V. T. was supported by Professor EC Aifantis, As. Professor A. Konstantinidis (Thessaloniki, Greece). G. T. was supported by a postgraduate scholarship from the Irish Research Council (GOIPG/2018/2464). A. V. was supported by MH059929 and MH110420. M. W. was supported by the German Research Foundation (DFG) under grant numbers CRC 636/C6 and We3638/5‐1. L. T. W. was supported by The European Research Council under the European Union's Horizon 2020 research and Innovation program (ERC StG, Grant 802998); The Research Council of Norway (249795). H. C. W. was supported by Wellcome Trust. DHW was supported by NIH grants K23MH085096, R01MH101111.
The São Paulo (Brazil) studies have been supported by grants from FAPESP‐Brazil (#2009/14891‐9, 2010/18672‐7, 2012/23796‐2 & 2013/03905‐4), CNPq‐Brazil (#478466/2009 & 480370/2009), the Wellcome Trust (UK) and the Brain & Behavior Research Foundation (2010 NARSAD Independent Investigator Award granted to Geraldo F. Busatto). C. A. Z. was supported by Intramural Research Program, National Institute of Mental Health. L.‐L. Z. was supported by National Natural Science Foundation of China (61722313), Fok Ying Tung Education Foundation (161057), and Science & Technology Innovation Program of Hunan Province (2018RS3080). Lastly, we would like to thank all of the ENIGMA Consortium Working Group Members for all their efforts in helping this international consortium effort thrive.
APPENDIX A. MEMBERS OF ENIGMA‐BIPOLAR DISORDER WORKING GROUP
Geraldo F. Busatto (Laboratory of Psychiatric Neuroimaging (LIM‐21), Departamento e Instituto de Psiquiatria, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil)
Tiffany M. Chaim‐Avancini (Laboratory of Psychiatric Neuroimaging (LIM‐21), Departamento e Instituto de Psiquiatria, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil)
Sara Dallaspezia (Vita‐Salute San Raffaele University, Milan, Italy; Division of Neuroscience, Psychiatry and Psychobiology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy)
Benjamin I. Goldstein (Centre for Youth Bipolar Disorder, Sunnybrook, Toronto, Ontario, Canada)
Guy M. Goodwin (Department of Psychiatry, University of Oxford, Oxford, England)
Pedro G. P. Rosa (Laboratory of Psychiatric Neuroimaging (LIM‐21), Departamento e Instituto de Psiquiatria, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, SP, Brazil)
Naoki Hashimoto (Department of Psychiatry, Hokkaido University Graduate School of Medicine, Sapporo, Japan)
Matthew J. Kempton (Institute of Psychiatry, Psychology & Neuroscience, King's College London, London, England)
Axel Krug (Department of Psychiatry and Psychotherapy, Philipps‐University Marburg, Marburg, Germany; Department of Psychiatry and Psychtherapy, University of Bonn, Germany)
Hiroshi Kunugi (Department of Mental Disorder Research, National Institute of Neuroscience, National Center of Neurology and Psychiatry, Tokyo, Japan)
Rayus Kuplicki (Laureate Institute for Brain Research, Tulsa, Oklahoma)
Clare E. Mackay (Department of Psychiatry, University of Oxford, Oxford, England)
Urlik F. Malt (Institute of Clinical Medicine, University of Oslo, Oslo, Norway)
Koji Matsuo (Department of Psychiatry, Faculty of Medicine, Saitama Medical University, Moroyama, Iruma, Japan)
Andrew M. McIntosh (Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, England)
Sean R. McWhinney (Department of Psychiatry, Dalhousie University, Halifax, Nova Scotia, Canada)
Hisashi Narita (Department of Psychiatry, Hokkaido University Graduate School of Medicine, Sapporo, Japan)
Jonathan Savitz (Laureate Institute for Brain Research, Tulsa, Oklahoma, USA; Oxley College of Health Sciences, The University of Tulsa, Tulsa, Oklahoma)
Henk S. Temmingh (Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa; Valkenberg Hospital, Western Cape Department of Health, Observatory, South Africa)
Sarah Trost (Department of Psychiatry and Psychotherapy, University Medical Center Goettingen, Goettingen, Germany)
Holly B. Van Gestel (Department of Psychiatry, Dalhousie University, Halifax, Nova Scotia, Canada)
Amelia Versace (University of Pittsburgh, Pittsburgh, Pennsylvania)
Marcus V. Zanetti (Laboratory of Psychiatric Neuroimaging (LIM‐21), Departamento e Instituto de Psiquiatria, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil; Instituto de Ensino e Pesquisa, Hospital Sírio‐Libanês, São Paulo, SP, Brazil)
Laboratory of Psychiatric Neuroimaging (LIM‐21), Departamento e Instituto de Psiquiatria, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil
Ching CRK, Hibar DP, Gurholt TP, et al. What we learn about bipolar disorder from large‐scale neuroimaging: Findings and future directions from the ENIGMA Bipolar Disorder Working Group. Hum Brain Mapp. 2022;43:56–82. 10.1002/hbm.25098
The members of the collaboration are provided in Appendix A.
ENDNOTE
Contributor Information
Christopher R. K. Ching, Email: cching47@gmail.com.
Bradley J. MacIntosh, Email: bmac@sri.utoronto.ca.
DATA AVAILABILITY STATEMENT
This is a review article of the ENIGMA Bipolar Disorder Working Group activities and includes no primary data. Those that wish to become members of the ENIGMA Bipolar Disorder Working Group are encouraged to contact the Working Group Chairs.
REFERENCES
- Abe, C. , Ekman, C. J. , Sellgren, C. , Petrovic, P. , Ingvar, M. , & Landen, M. (2015). Manic episodes are related to changes in frontal cortex: A longitudinal neuroimaging study of bipolar disorder 1. Brain, 138(Pt 11), 3440–3448. 10.1093/brain/awv266 [DOI] [PubMed] [Google Scholar]
- Abramovic, L. , Boks, M. P. , Vreeker, A. , Bouter, D. C. , Kruiper, C. , Verkooijen, S. , … van Haren, N. E. (2016). The association of antipsychotic medication and lithium with brain measures in patients with bipolar disorder. European Neuropsychopharmacology, 26(11), 1741–1751. 10.1016/j.euroneuro.2016.09.371 [DOI] [PubMed] [Google Scholar]
- Adams, H. H. , Hibar, D. P. , Chouraki, V. , Stein, J. L. , Nyquist, P. A. , Renteria, M. E. , … Thompson, P. M. (2016). Novel genetic loci underlying human intracranial volume identified through genome‐wide association. Nature Neuroscience, 19(12), 1569–1582. 10.1038/nn.4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimus, C. (2019) Supporting wellness: A survey of lived experience. [PDF file]. Retrieved from: http://milkeninstitute.org/sites/default/files/reports-pdf/Supporting%20Wellness%20-%20Survey%20of%20Lived%20Experience%20with%20Depression%20and%20Bipolar%20Updated.pdf
- Altshuler, L. L. , Bartzokis, G. , Grieder, T. , Curran, J. , Jimenez, T. , Leight, K. , … Mintz, J. (2000). An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological Psychiatry, 48(2), 147–162. 10.1016/s0006-3223(00)00836-2 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association & American Psychiatric Association. DSM‐5 Task Force . (2013). Diagnostic and statistical manual of mental disorders : DSM‐5 (5th ed.). Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Atkinson, A. J. , Colburn, W. A. , DeGruttola, V. G. , DeMets, D. L. , Downing, G. J. , Hoth, D. F. , … Grp, B. D. W. (2001). Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clinical Pharmacology & Therapeutics, 69(3), 89–95. 10.1067/mcp.2000.113989 [DOI] [PubMed] [Google Scholar]
- Bearden, C. E. , & Thompson, P. M. (2017). Emerging global initiatives in Neurogenetics: The enhancing neuroimaging genetics through meta‐analysis (ENIGMA) consortium. Neuron, 94(2), 232–236. 10.1016/j.neuron.2017.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos, K. L. , & Weinberger, D. R. (2010). Imaging genetics‐‐days of future past. NeuroImage, 53(3), 804–809. 10.1016/j.neuroimage.2010.01.035 [DOI] [PubMed] [Google Scholar]
- Bipolar Disorder & Schizophrenia Working Group of the Psychiatric Genomics, C . (2018). Genomic dissection of Bipolar disorder and schizophrenia, including 28 subphenotypes. Cell, 173(7), 1705–1715. 10.1016/j.cell.2018.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedhoe, P. S. , Schmaal, L. , Abe, Y. , Ameis, S. H. , Arnold, P. D. , Batistuzzo, M. C. , … van den Heuvel, O. A. (2017). Distinct subcortical volume alterations in pediatric and adult OCD: A worldwide meta‐ and mega‐analysis. The American Journal of Psychiatry, 174(1), 60–69. 10.1176/appi.ajp.2016.1602020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedhoe, P. S. W. , Schmaal, L. , Abe, Y. , Alonso, P. , Ameis, S. H. , Anticevic, A. , … van den Heuvel, O. A. (2018). Cortical abnormalities associated with pediatric and adult obsessive‐compulsive disorder: Findings from the ENIGMA obsessive‐compulsive disorder working group. The American Journal of Psychiatry, 175(5), 453–462. 10.1176/appi.ajp.2017.17050485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora, E. (2015). Developmental trajectory of cognitive impairment in bipolar disorder: Comparison with schizophrenia. European Neuropsychopharmacology, 25(2), 158–168. 10.1016/j.euroneuro.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Borsboom, D. , & Cramer, A. O. (2013). Network analysis: An integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology, 9, 91–121. 10.1146/annurev-clinpsy-050212-185608 [DOI] [PubMed] [Google Scholar]
- Botvinik‐Nezer, R. , Holzmeister, F. , Camerer, C. F. , Dreber, A. , Huber, J. , Johannesson, M. , … Schonberg, T. (2020). Variability in the analysis of a single neuroimaging dataset by many teams. Nature, 582, 84–88. 10.1038/s41586-020-2314-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainstorm, C. , Anttila, V. , Bulik‐Sullivan, B. , Finucane, H. K. , Walters, R. K. , Bras, J. , … Murray, R. (2018). Analysis of shared heritability in common disorders of the brain. Science, 360(6395), eaap8757. 10.1126/science.aap8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer, R. M. , Panizzon, M. S. , Glahn, D. C. , Hibar, D. P. , Hua, X. , Jahanshad, N. , … Hulshoff Pol, H. E. (2017). Genetic influences on individual differences in longitudinal changes in global and subcortical brain volumes: Results of the ENIGMA plasticity working group. Human Brain Mapping, 38(9), 4444–4458. 10.1002/hbm.23672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button, K. S. , Ioannidis, J. P. , Mokrysz, C. , Nosek, B. A. , Flint, J. , Robinson, E. S., & Munafo, M. R. (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews. Neuroscience, 14(5), 365–376. doi: 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Canales‐Rodriguez, E. J. , Pomarol‐Clotet, E. , Radua, J. , Sarro, S. , Alonso‐Lana, S. , Del Mar Bonnin, C. , … Salvador, R. (2014). Structural abnormalities in bipolar euthymia: A multicontrast molecular diffusion imaging study. Biological Psychiatry, 76(3), 239–248. 10.1016/j.biopsych.2013.09.027 [DOI] [PubMed] [Google Scholar]
- Caspers, S. , Zilles, K. , Laird, A. R. , & Eickhoff, S. B. (2010). ALE meta‐analysis of action observation and imitation in the human brain. NeuroImage, 50(3), 1148–1167. 10.1016/j.neuroimage.2009.12.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, K. , Karchemskiy, A. , Barnea‐Goraly, N. , Garrett, A. , Simeonova, D. I. , & Reiss, A. (2005). Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 44(6), 565–573. 10.1097/01.chi.0000159948.75136.0d [DOI] [PubMed] [Google Scholar]
- Charney, A. W. , Ruderfer, D. M. , Stahl, E. A. , Moran, J. L. , Chambert, K. , Belliveau, R. A. , … Sklar, P. (2017). Evidence for genetic heterogeneity between clinical subtypes of bipolar disorder. Translational Psychiatry, 7(1), e993. 10.1038/tp.2016.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepenik, L. G. , Raffo, M. , Hampson, M. , Lacadie, C. , Wang, F. , Jones, M. M. , … Blumberg, H. P. (2010). Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Research, 182(3), 207–210. 10.1016/j.pscychresns.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching, C. R. K. , Gutman, B. A. , Sun, D. , Villalon Reina, J. , Ragothaman, A. , Isaev, D. , … Bearden, C. E. (2020). Mapping subcortical brain alterations in 22q11.2 deletion syndrome: Effects of deletion size and convergence with idiopathic neuropsychiatric illness. The American Journal of Psychiatry, 177(7), 589–600. 10.1176/appi.ajp.2019.19060583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll, C. U. , Penzner, J. B. , Frederickson, A. M. , Richter, J. J., Auther, A. M., Smith, C. W., … Cornblatt, B. A. (2007). Differentiation in the preonset phases of schizophrenia and mood disorders: Evidence in support of a bipolar mania prodrome. Schizophrenia Bulletin, 3(3), 703–714. 10.1093/schbul/sbm028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross‐Disorder Group of the Psychiatric Genomics Consortium . (2013). Identification of risk loci with shared effects on five major psychiatric disorders: A genome‐wide analysis. Lancet, 381(9875), 1371–1379. 10.1016/S0140-6736(12)62129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zwarte, S. M. C. , Brouwer, R. M. , Agartz, I. , Alda, M., Aleman, A., Alpert, K. I., … van Haren, N. E. M. (2019). The association between familial risk and brain abnormalities is disease‐specific: an ENIGMA–Relatives study of schizophrenia and bipolar disorder. Biological Psychiatry, 86(7), 545–556. 10.1016/j.biopsych.2019.03.985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis, E. L. , Disner, S. G. , Fani, N. , Salminen, L. E. , Logue, M. , Clarke, E. K. , … Morey, R. A. (2019). Altered white matter microstructural organization in posttraumatic stress disorder across 3047 adults: Results from the PGC‐ENIGMA PTSD consortium. Molecular Psychiatry. 10.1038/s41380-019-0631-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, A. , Vandeleur, C. , Heffer, N. , & Preisig, M. (2017). The clinical trajectory of emerging bipolar disorder among the high‐risk offspring of bipolar parents: Current understanding and future considerations. International Journal of Bipolar Disorders, 5(1), 37. 10.1186/s40345-017-0106-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas‐Mallet, E. , Button, K. S. , Boraud, T. , Gonon, F. , & Munafo, M. R. (2017). Low statistical power in biomedical science: A review of three human research domains. Royal Society Open Science, 4(2), 160254. 10.1098/rsos.160254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman, M. , Granstrom, O. , Omerov, S. , Jacob, J. , & Landen, M. (2013). The societal cost of bipolar disorder in Sweden. Social Psychiatry and Psychiatric Epidemiology, 48(10), 1601–1610. 10.1007/s00127-013-0724-9 [DOI] [PubMed] [Google Scholar]
- Farrell, M. S. , Werge, T. , Sklar, P. , Owen, M. J. , Ophoff, R. A. , O'Donovan, M. C. , … Sullivan, P. F. (2015). Evaluating historical candidate genes for schizophrenia. Molecular Psychiatry, 20(5), 555–562. 10.1038/mp.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre, P. , Pauling, M. , Stout, J. , Hozer, F. , Sarrazin, S. , Abé, C. , … Houenou, J. (2019). Widespread white matter microstructural abnormalities in bipolar disorder: Evidence from mega‐ and meta‐analyses across 3033 individuals. Neuropsychopharmacology, 44, 2285–2293. 10.1038/s41386-019-0485-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , … Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. 10.1016/s0896-6273(02)00569-x [DOI] [PubMed] [Google Scholar]
- Fried, E. I. , von Stockert, S. , Haslbeck, J. M. B. , Lamers, F. , Schoevers, R. A. , & Penninx, B. (2019). Using network analysis to examine links between individual depressive symptoms, inflammatory markers, and covariates. Psychological Medicine, 1–9. 10.1017/S0033291719002770 [DOI] [PubMed] [Google Scholar]
- Gandal, M. J. , Haney, J. R. , Parikshak, N. N. , Leppa, V. , Ramaswami, G. , Hartl, C. , … Geschwind, D. H. (2018). Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science, 359(6376), 693–697. 10.1126/science.aad6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzola, R. , & Duchesne, S. (2017). Voxel‐based morphometry meta‐analysis of gray and white matter finds significant areas of differences in bipolar patients from healthy controls. Bipolar Disorders, 19(2), 74–83. 10.1111/bdi.12488 [DOI] [PubMed] [Google Scholar]
- Glahn, D. C. , Thompson, P. M. , & Blangero, J. (2007). Neuroimaging endophenotypes: Strategies for finding genes influencing brain structure and function. Human Brain Mapping, 28(6), 488–501. 10.1002/hbm.20401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman, I. I. , & Shields, J. (1973). Genetic theorizing and schizophrenia. The British Journal of Psychiatry, 122(566), 15–30. 10.1192/bjp.122.1.15 [DOI] [PubMed] [Google Scholar]
- Gottesman, I. I. , & Shields, J. (1976). A critical review of recent adoption, twin, and family studies of schizophrenia: Behavioral genetics perspectives. Schizophrenia Bulletin, 2(3), 360–401. 10.1093/schbul/2.3.360 [DOI] [PubMed] [Google Scholar]
- Grasby, K. L. , Jahanshad, N. , Painter, J. N. , Colodro‐Conde, L. , Bralten, J. , Hibar, D. P. , … Medland, S. (2020). Enhancing NeuroImaging Genetics through Meta‐Analysis Consortium‐Genetics working group. The genetic architecture of the human cerebral cortex. Science, 367(6484). 10.1126/science.aay6690 [DOI] [PMC free article] [PubMed]
- Guglielmi, G. (2018). The world's largest set of brain scans are helping reveal the workings of the mind and how diseases ravage the brain. Science. 10.1126/science.aat0994 [DOI] [Google Scholar]
- Hajek, T. , Bauer, M. , Simhandl, C. , Rybakowski, J. , O'Donovan, C. , Pfennig, A. , … Alda, M. (2014). Neuroprotective effect of lithium on hippocampal volumes in bipolar disorder independent of long‐term treatment response. Psychological Medicine, 44(3), 507–517. 10.1017/S0033291713001165 [DOI] [PubMed] [Google Scholar]
- Hajek, T. , Calkin, C. , Blagdon, R. , Slaney, C. , Uher, R. , & Alda, M. (2014). Insulin resistance, diabetes mellitus, and brain structure in bipolar disorders. Neuropsychopharmacology, 39(12), 2910–2918. 10.1038/npp.2014.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek, T. , Cullis, J. , Novak, T. , Kopecek, M. , Blagdon, R. , Propper, L. , … Alda, M. (2013). Brain structural signature of familial predisposition for bipolar disorder: Replicable evidence for involvement of the right inferior frontal gyrus. Biological Psychiatry, 73(2), 144–152. 10.1016/j.biopsych.2012.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek, T. , Kopecek, M. , Hoschl, C. , & Alda, M. (2012). Smaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: A meta‐analysis. Journal of Psychiatry & Neuroscience, 37(5), 333–343. 10.1503/jpn.110143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek, T. , Kopecek, M. , Kozeny, J. , Gunde, E. , Alda, M. , & Hoschl, C. (2009). Amygdala volumes in mood disorders‐‐meta‐analysis of magnetic resonance volumetry studies. Journal of Affective Disorders, 115(3), 395–410. 10.1016/j.jad.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Hajek, T. , McIntyre, R. , & Alda, M. (2016). Bipolar disorders, type 2 diabetes mellitus, and the brain. Current Opinion in Psychiatry, 29(1), 1–6. 10.1097/YCO.0000000000000215 [DOI] [PubMed] [Google Scholar]
- Han, K. M. , De Berardis, D. , Fornaro, M. , & Kim, Y. K. (2019). Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 91, 20–27. 10.1016/j.pnpbp.2018.03.022 [DOI] [PubMed] [Google Scholar]
- Hanford, L. C. , Nazarov, A. , Hall, G. B. , & Sassi, R. B. (2016). Cortical thickness in bipolar disorder: A systematic review. Bipolar Disorders, 18(1), 4–18. 10.1111/bdi.12362 [DOI] [PubMed] [Google Scholar]
- Haukvik, U. K. , Gurholt, T. P. , Nerland, S. , Elvsåshagen, T. , Akudjedu, T. N. , Alda, M. , … for the ENIGMA Bipolar Disorder Working group . (2020). In vivo hippocampal subfield volumes in bipolar disorder – A mega‐analysis from the ENIGMA consortium. In submission Human Brain Mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz, M. J. , Lein, E. S. , Guillozet‐Bongaarts, A. L. , Shen, E. H. , Ng, L. , Miller, J. A. , … Abajian, C. (2012). An anatomically comprehensive atlas of the adult human brain transcriptome. Nature, 489(7416), 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Adams, H. H. H. , Jahanshad, N. , Chauhan, G. , Stein, J. L. , Hofer, E. , … Ikram, M. A. (2017). Novel genetic loci associated with hippocampal volume. Nature Communications, 8, 13624. 10.1038/ncomms13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Stein, J. L. , Renteria, M. E. , Arias‐Vasquez, A. , Desrivieres, S. , Jahanshad, N. , … Medland, S. E. (2015). Common genetic variants influence human subcortical brain structures. Nature, 520(7546), 224–229. 10.1038/nature14101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Westlye, L. T. , Doan, N. T. , Jahanshad, N. , Cheung, J. W. , Ching, C. R. K. , … Andreassen, O. A. (2018). Cortical abnormalities in bipolar disorder: An MRI analysis of 6503 individuals from the ENIGMA Bipolar disorder working group. Molecular Psychiatry, 23(4), 932–942. 10.1038/mp.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Westlye, L. T. , van Erp, T. G. , Rasmussen, J. , Leonardo, C. D. , Faskowitz, J. , … Andreassen, O. A. (2016). Subcortical volumetric abnormalities in bipolar disorder. Molecular Psychiatry, 21(12), 1710–1716. 10.1038/mp.2015.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilland, E. , Landro, N. I. , Kraft, B. , Tamnes, C. K. , Fried, E. I. , Maglanoc, L. A. , & Jonassen, R. (2019). Exploring the links between specific depression symptoms and brain structure: A network study. In Exploring the links between specific depression symptoms and brain. Structure: A Network Study. Psychiatry Clin Neurosci. 10.1111/pcn.12969 [DOI] [PubMed] [Google Scholar]
- Holland, D. , Frei, O. , Desikan, R. , Fan, C. C. , Shadrin, A. A. , Smeland, O. B. , … Dale, A. M. (2020). Beyond SNP heritability: Polygenicity and discoverability of phenotypes estimated with a univariate Gaussian mixture model. PLoS Genetics, 16(5), e1008612. 10.1371/journal.pgen.1008612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman, M. , Bralten, J. , Hibar, D. P. , Mennes, M. , Zwiers, M. P. , Schweren, L. S. J. , … Franke, B. (2017). Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross‐sectional mega‐analysis. Lancet Psychiatry, 4(4), 310–319. 10.1016/S2215-0366(17)30049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman, M. , Muetzel, R. , Guimaraes, J. P. , Shumskaya, E. , Mennes, M. , Zwiers, M. P. , … Franke, B. (2019). Brain imaging of the cortex in ADHD: A coordinated analysis of large‐scale clinical and population‐based samples. The American Journal of Psychiatry, 176(7), 531–542. 10.1176/appi.ajp.2019.18091033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel, T. R. (2014). The NIMH research domain criteria (RDoC) project: Precision medicine for psychiatry. The American Journal of Psychiatry, 171(4), 395–397. 10.1176/appi.ajp.2014.14020138 [DOI] [PubMed] [Google Scholar]
- Ioannidis, J. P. (2008). Why most discovered true associations are inflated. Epidemiology, 19(5), 640–648. 10.1097/EDE.0b013e31818131e7 [DOI] [PubMed] [Google Scholar]
- Ioannidis, J. P. (2011). Excess significance bias in the literature on brain volume abnormalities. Archives of General Psychiatry, 68(8), 773–780. 10.1001/archgenpsychiatry.2011.28 [DOI] [PubMed] [Google Scholar]
- Ioannidis, J. P. (2017). Acknowledging and overcoming nonreproducibility in basic and preclinical research. JAMA, 317(10), 1019–1020. 10.1001/jama.2017.0549 [DOI] [PubMed] [Google Scholar]
- Jack, C. R., Jr. , Bennett, D. A. , Blennow, K. , Carrillo, M. C. , Dunn, B. , Haeberlein, S. B. , … Contributors . (2018). NIA‐AA research framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement, 14(4), 535–562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad, N. , Kochunov, P. V. , Sprooten, E. , Mandl, R. C. , Nichols, T. E. , Almasy, L. , … Glahn, D. C. (2013). Multi‐site genetic analysis of diffusion images and voxelwise heritability analysis: A pilot project of the ENIGMA‐DTI working group. NeuroImage, 81, 455–469. 10.1016/j.neuroimage.2013.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad, N. , Madsen, S. , Azatian, Y. , Hibar, D. P. , Thompson, P. M. , Gil, Y. (2015) Supporting the consortium for enhancing NeuroImaging genetics through meta‐analysis (ENIGMA) through the organic data science framework. Presented at the Conference on Science of Team Science (SciTS), Bethesda, MD, June 2015.
- Johnstone, E. C. , Crow, T. J. , Frith, C. D. , Husband, J. , & Kreel, L. (1976). Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet, 2(7992), 924–926. 10.1016/s0140-6736(76)90890-4 [DOI] [PubMed] [Google Scholar]
- Kelly, S. , Jahanshad, N. , Zalesky, A. , Kochunov, P. , Agartz, I. , Alloza, C. , … Donohoe, G. (2018). Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA schizophrenia DTI working group. Molecular Psychiatry, 23(5), 1261–1269. 10.1038/mp.2017.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf, L. , Hussain, N. , & Potash, J. B. (2005). Mood disorder with psychotic features, schizoaffective disorder, and schizophrenia with mood features: Trouble at the borders. International Review of Psychiatry, 17(1), 9–19. 10.1080/09540260500064959 [DOI] [PubMed] [Google Scholar]
- Koshiyama, D. , Fukunaga, M. , Okada, N. , Morita, K. , Nemoto, K. , Usui, K. , … COCORO . (2019). White matter microstructural alterations across four major psychiatric disorders: Mega‐analysis study in 2937 individuals. Molecular Psychiatry, 25, 883–895. 10.1038/s41380-019-0553-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, B. D. , & Stein, J. L. (2019). Mapping causal pathways from genetics to neuropsychiatric disorders using genome‐wide imaging genetics: Current status and future directions. Psychiatry and Clinical Neurosciences, 73(7), 357–369. 10.1111/pcn.12839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke, J. , King, A. V. , Poupon, C. , Hennerici, M. G. , Gass, A. , & Wessa, M. (2013). Impaired anatomical connectivity and related executive functions: Differentiating vulnerability and disease marker in bipolar disorder. Biological Psychiatry, 74(12), 908–916. 10.1016/j.biopsych.2013.04.010 [DOI] [PubMed] [Google Scholar]
- Logue, M. W. , van Rooij, S. J. H. , Dennis, E. L. , Davis, S. L. , Hayes, J. P. , Stevens, J. S. , … Morey, R. A. (2018). Smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA‐PGC study: Subcortical Volumetry results from posttraumatic stress disorder consortia. Biological Psychiatry, 83(3), 244–253. 10.1016/j.biopsych.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo, I. K. , Dager, S. R. , Kim, J. E. , Yoon, S. J. , Friedman, S. D. , Dunner, D. L. , & Renshaw, P. F. (2010). Lithium‐induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: A longitudinal brain imaging study. Neuropsychopharmacology, 35(8), 1743–1750. 10.1038/npp.2010.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey, S. , Allgaier, N. , Chaarani, B. , Spechler, P. , Orr, C., Bunn, J. , … Garavan H. ENIGMA Addiction Working Group . (2019). Mega‐analysis of gray matter volume in substance dependence: General and substance‐specific regional effects. The American Journal of Psychiatry, 176(2), 119–128. doi: 10.1176/appi.ajp.2018.17040415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon, K. , Burdick, K. E. , & Szeszko, P. R. (2010). A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neuroscience and Biobehavioral Reviews, 34(4), 533–554. 10.1016/j.neubiorev.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnis, M. G. , & Greden, J. F. (2016). Longitudinal studies: An essential component for complex psychiatric disorders. Neuroscience Research, 102, 4–12. 10.1016/j.neures.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Merikangas, K. R. , Jin, R. , He, J. P. , Kessler, R. C. , Lee, S. , Sampson, N. A. , … Zarkov, Z. (2011). Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Archives of General Psychiatry, 68(3), 241–251. 10.1001/archgenpsychiatry.2011.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, K. L. , Alfaro‐Almagro, F. , Bangerter, N. K. , Thomas, D. L. , Yacoub, E. , Xu, J. , … Smith, S. M. (2016). Multimodal population brain imaging in the UKbiobank prospective epidemiological study. Nature Neuroscience, 19(11), 1523–1536. 10.1038/nn.4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, G. J. , Bebchuk, J. M. , Wilds, I. B. , Chen, G. , & Manji, H. K. (2000). Lithium‐induced increase in human brain grey matter. Lancet, 356(9237), 1241–1242. 10.1016/s0140-6736(00)02793-8 [DOI] [PubMed] [Google Scholar]
- Moore, G. J. , Cortese, B. M. , Glitz, D. A. , Zajac‐Benitez, C. , Quiroz, J. A. , Uhde, T. W. , … Manji, H. K. (2009). A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment‐responsive bipolar disorder patients. The Journal of Clinical Psychiatry, 70(5), 699–705. 10.4088/JCP.07m03745 [DOI] [PubMed] [Google Scholar]
- Murray, R. M. , & Sham, P. (2004). van OJ, et al. a developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophrenia Research, 71(2–3), 405–416. 10.1016/j.schres.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Nunes, A. , Schnack, H. G. , Ching, C. R. K. , Agartz, I. , Akudjedu, T. N. , Alda, M. , … Group, E. B. D. W . (2018). Using structural MRI to identify bipolar disorders – 13 site machine learning study in 3020 individuals from the ENIGMA Bipolar disorders working group. Molecular Psychiatry. 10.1038/s41380-018-0228-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldis, M. , Murray, G. , Macneil, C. A. , Hasty, M. K. , Daglas, R. , Berk, M. , … Cotton, S. M. (2016). Trajectory and predictors of quality of life in first episode psychotic mania. Journal of Affective Disorders, 195, 148–155. 10.1016/j.jad.2016.02.018 [DOI] [PubMed] [Google Scholar]
- Pardoe, H. R. , Hiess, R. K. , & Kuzniecky, R. (2016). Motion and morphometry in clinical and nonclinical populations. NeuroImage, 135, 177–185. 10.1016/j.neuroimage.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Passos, I. C. , Ballester, P. L. , Barros, R. C. , Librenza‐Garcia, D. , Mwangi, B. , Birmaher, B. , … Kapczinski, F. (2019). Machine learning and big data analytics in bipolar disorder: A position paper from the International Society for Bipolar Disorders big Data Task Force. Bipolar Disorders, 21(7), 582–594. 10.1111/bdi.12828 [DOI] [PubMed] [Google Scholar]
- Patel, Y. , Shin, J. , Gowland, P. A. , Pausova, Z. , Paus, T. , & IMAGEN consortium . (2018). Maturation of the human cerebral cortex during adolescence: Myelin or dendritic arbor? Cerebral Cortex, 29(8), 3351–3362. 10.1093/cercor/bhy204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus, M. P. , & Thompson, W. K. (2019). The challenges and opportunities of small effects: The new Normal in academic psychiatry. JAMA Psychiatry, 76(4), 353–354. 10.1001/jamapsychiatry.2018.4540 [DOI] [PubMed] [Google Scholar]
- Paus, T. (2018). Imaging microstructure in the living human brain: A viewpoint. NeuroImage, 182, 3–7. 10.1016/j.neuroimage.2017.10.013 [DOI] [PubMed] [Google Scholar]
- Pearlson, G. D. (2015). Etiologic, phenomenologic, and endophenotypic overlap of schizophrenia and bipolar disorder. Annual Review of Clinical Psychology, 11, 251–281. 10.1146/annurev-clinpsy-032814-112915 [DOI] [PubMed] [Google Scholar]
- Perlick, D. A. , Rosenheck, R. R. , Clarkin, J. F. , Raue, P. , & Sirey, J. (2001). Impact of family burden and patient symptom status on clinical outcome in bipolar affective disorder. The Journal of Nervous and Mental Disease, 189(1), 31–37. 10.1097/00005053-200101000-00006 [DOI] [PubMed] [Google Scholar]
- Phillips, M. L. , & Swartz, H. A. (2014). A critical appraisal of neuroimaging studies of bipolar disorder: Toward a new conceptualization of underlying neural circuitry and a road map for future research. The American Journal of Psychiatry, 171(8), 829–843. 10.1176/appi.ajp.2014.13081008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. L. , & Vieta, E. (2007). Identifying functional neuroimaging biomarkers of bipolar disorder: Toward DSM‐V. Schizophrenia Bulletin, 33(4), 893–904. 10.1093/schbul/sbm060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo, J. , & Yatham, L. N. (2018). Biomarkers in mood disorders: Are we there yet? Journal of Affective Disorders, 233, 1–2. 10.1016/j.jad.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Rimol, L. M. , Hartberg, C. B. , Nesvag, R. , Fennema‐Notestine, C. , Hagler, D. J., Jr. , Pung, C. J. , … Agartz, I. (2010). Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biological Psychiatry, 68(1), 41–50. 10.1016/j.biopsych.2010.03.036 [DOI] [PubMed] [Google Scholar]
- Rimol, L. M. , Nesvag, R. , Hagler, D. J., Jr. , Bergmann, O. , Fennema‐Notestine, C. , Hartberg, C. B. , … Dale, A. M. (2012). Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biological Psychiatry, 71(6), 552–560. 10.1016/j.biopsych.2011.11.026 [DOI] [PubMed] [Google Scholar]
- Rink, L. , Pagel, T. , Franklin, J. , & Baethge, C. (2016). Characteristics and heterogeneity of schizoaffective disorder compared with unipolar depression and schizophrenia ‐ a systematic literature review and meta‐analysis. Journal of Affective Disorders, 191, 8–14. 10.1016/j.jad.2015.10.045 [DOI] [PubMed] [Google Scholar]
- Salagre, E. , Dodd, S. , Aedo, A. , Rosa, A. , Amoretti, S. , Pinzon, J. , … Grande, I. (2018). Toward precision psychiatry in Bipolar disorder: Staging 2.0. Front. Psychiatry, 9, 641. 10.3389/fpsyt.2018.00641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin, S. , Poupon, C. , Linke, J. , Wessa, M. , Phillips, M. , Delavest, M. , … Houenou, J. (2014). A multicenter tractography study of deep white matter tracts in bipolar I disorder: Psychotic features and interhemispheric disconnectivity. JAMA Psychiatry, 71(4), 388–396. 10.1001/jamapsychiatry.2013.4513 [DOI] [PubMed] [Google Scholar]
- Sarrazin, S. , Poupon, C. , Teillac, A. , Mangin, J. F. , Polosan, M. , Favre, P. , … Houenou, J. (2019). Higher in vivo cortical intracellular volume fraction associated with lithium therapy in Bipolar disorder: A multicenter NODDI study. Psychotherapy and Psychosomatics, 88(3), 171–176. 10.1159/000498854 [DOI] [PubMed] [Google Scholar]
- Satizabal, C. L. , Adams, H. H. H. , Hibar, D. P. , White, C. C. , Knol, M. J. , Stein, J. L. , … Ikram, M. A. (2019). Genetic architecture of subcortical brain structures in 38,851 individuals. Nature Genetics, 51(11), 1624–1636. 10.1038/s41588-019-0511-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C . (2014). Biological insights from 108 schizophrenia‐associated genetic loci. Nature, 511(7510), 421–427. 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Hibar, D. P. , Samann, P. G. , Hall, G. B. , Baune, B. T. , Jahanshad, N. , … Veltman, D. J. (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Molecular Psychiatry, 22(6), 900–909. 10.1038/mp.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Veltman, D. J. , van Erp, T. G. , Samann, P. G. , Frodl, T. , Jahanshad, N. , … Hibar, D. P. (2016). Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Molecular Psychiatry, 21(6), 806–812. 10.1038/mp.2015.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepede, G. , Chiacchiaretta, P. , Gambi, F. , Di Iorio, G. , De Berardis, D. , Ferretti, A. , … Di Giannantonio, M. (2020). Bipolar disorder with and without a history of psychotic features: fMRI correlates of sustained attention. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 98, 109817. 10.1016/j.pnpbp.2019.109817 [DOI] [PubMed] [Google Scholar]
- Sepede, G. , De Berardis, D. , Campanella, D. , Perrucci, M. G. , Ferretti, A. , Salerno, R. M. , … Gambi, F. (2015). Neural correlates of negative emotion processing in bipolar disorder. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 60, 1–10. 10.1016/j.pnpbp.2015.01.016 [DOI] [PubMed] [Google Scholar]
- Sepede, G. , De Berardis, D. , Campanella, D. , Perrucci, M. G. , Ferretti, A. , Serroni, N. , … Gambi, F. (2012). Impaired sustained attention in euthymic bipolar disorder patients and non‐affected relatives: An fMRI study. Bipolar Disorders, 14(7), 764–779. 10.1111/bdi.12007 [DOI] [PubMed] [Google Scholar]
- Shin, J. , French, L. , Xu, T. , Leonard, G. , Perron, M. , Pike, G. B. , … Paus, T. (2017). Cell‐specific gene‐expression profiles and cortical thickness in the human brain. Cerebral Cortex, 28(9), 3267–3277. 10.1093/cercor/bhx197 [DOI] [PubMed] [Google Scholar]
- Smit, D. J. A. , Wright, M. J. , Meyers, J. L. , Martin, N. G. , Ho, Y. Y. W. , Malone, S. M. , … Boomsma, D. I. (2018). Genome‐wide association analysis links multiple psychiatric liability genes to oscillatory brain activity. Human Brain Mapping, 39(11), 4183–4195. 10.1002/hbm.24238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Johansen‐Berg, H. , Rueckert, D. , Nichols, T. E. , Mackay, C. E. , … Behrens, T. E. (2006). Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage, 31(4), 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Stahl, E. A. , Breen, G. , Forstner, A. J. , McQuillin, A. , Ripke, S. , Trubetskoy, V. , … Bipolar Disorder Working Group of the Psychiatric Genomics Consortium . (2019). Genome‐wide association study identifies 30 loci associated with bipolar disorder. Nature Genetics, 51(5), 793–803. 10.1038/s41588-019-0397-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, J. L. , Medland, S. E. , Vasquez, A. A. , Hibar, D. P. , Senstad, R. E. , Winkler, A. M. , … Enhancing Neuro Imaging Genetics through Meta‐Analysis, C . (2012). Identification of common variants associated with human hippocampal and intracranial volumes. Nature Genetics, 44(5), 552–561. 10.1038/ng.2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, D. , Ching, C. R. K. , Lin, A. , Forsyth, J. K. , Kushan, L. , Vajdi, A. , … Bearden, C. E. (2018). Large‐scale mapping of cortical alterations in 22q11.2 deletion syndrome: Convergence with idiopathic psychosis and effects of deletion size. Mol psychiatry. doi: 10.1038/s41380-018-0078-5 [DOI] [PMC free article] [PubMed]
- Thompson, P. M. , Jahanshad, N. , Ching, C. R. K. , Salminen, L. E. , Thomopoulos, S. , … The ENIGMA Consortium . (2020). ENIGMA and global neuroscience: A decade of large‐scale studies of the brain in health and disease across more than 40 countries as a neural correlate of treatment. Translational Psychiatry, 10, 100. 10.1038/s41398-020-0705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, P. M. , Stein, J. L. , Medland, S. E. , Hibar, D. P. , Vasquez, A. A. , Renteria, M. E. , … Saguenay Youth Study Group . (2014). The ENIGMA consortium: Large‐scale collaborative analyses of neuroimaging and genetic data. Brain Imaging and Behavior, 8(2), 153–182. 10.1007/s11682-013-9269-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer, D. , Frei, O. , Kaufmann, T. , Shandrin, A. A. , Devor, A. , Smeland, O. A. , … Dale, A. M. (2019). Making the MOSTest of imaging genetics. BioRxiv. 10.1101/767905 [DOI] [Google Scholar]
- van Erp, T. G. , Hibar, D. P. , Rasmussen, J. M. , Glahn, D. C. , Pearlson, G. D. , Andreassen, O. A. , … Turner, J. A. (2016). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Molecular Psychiatry, 21(4), 585. 10.1038/mp.2015.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp, T. G. M. , Walton, E. , Hibar, D. P. , Schmaal, L. , Jiang, W. , Glahn, D. C. , … Turner, J. A. (2018). Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing Neuro imaging genetics through meta analysis (ENIGMA) consortium. Biological Psychiatry, 84(9), 644–654. 10.1016/j.biopsych.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gestel, H. , Franke, K. , Petite, J. , Slaney, C. , Garnham, J. , Helmick, C. , … Hajek, T. (2019). Brain age in bipolar disorders: Effects of lithium treatment. The Australian and New Zealand Journal of Psychiatry, 53(12), 1179–1188. 10.1177/0004867419857814 [DOI] [PubMed] [Google Scholar]
- van Rooij, D. , Anagnostou, E. , Arango, C. , Auzias, G. , Behrmann, M. , Busatto, G. F. , … Buitelaar, J. K. (2018). Cortical and subcortical brain Morphometry differences between patients with autism Spectrum disorder and healthy individuals across the lifespan: Results from the ENIGMA ASD working group. The American Journal of Psychiatry, 175(4), 359–369. 10.1176/appi.ajp.2017.17010100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen, L. S. , Kelly, S. , Isaev, D. , Aleman, A. , Aftanas, L. I. , Bauer, J. , … Schmaal, L. (2019). White matter disturbances in major depressive disorder: A coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Molecular Psychiatry, 25(7), 1511–1525. 10.1038/s41380-019-0477-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian, G. , & Keshavan, M. S. (2016). Biomarkers in psychiatry ‐ a critique. Annals of Neurosciences, 23(1), 3–5. 10.1159/000443549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieta, E. , Berk, M. , Schulze, T. G. , Carvalho, A. F. , Suppes, T. , Calabrese, J. R. , … Grande, I. (2018). Bipolar disorders. Nature Reviews. Disease Primers, 4, 18008. 10.1038/nrdp.2018.8 [DOI] [PubMed] [Google Scholar]
- Vieta, E. , & Phillips, M. L. (2007). Deconstructing bipolar disorder: A critical review of its diagnostic validity and a proposal for DSM‐V and ICD‐11. Schizophrenia Bulletin, 33(4), 886–892. 10.1093/schbul/sbm057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau, M. , Beaucousin, V. , Herve, P. Y. , Duffau, H. , Crivello, F. , Houde, O. , … Tzourio‐Mazoyer, N. (2006). Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage, 30(4), 1414–1432. 10.1016/j.neuroimage.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Vigo, D. , Thornicroft, G. , & Atun, R. (2016). Estimating the true global burden of mental illness. Lancet Psychiatry, 3(2), 171–178. 10.1016/S2215-0366(15)00505-2 [DOI] [PubMed] [Google Scholar]
- Villalon‐Reina, J. E. , Martinez, K. , Qu, X. , Ching, C. R. K. , Nir, T. M. , Kothapalli, D. , … Bearden, C. E. (2019). Altered white matter microstructure in 22q11.2 deletion syndrome: A multisite diffusion tensor imaging study. Mol psychiatry. doi: 10.1038/s41380-019-0450-0 [DOI] [PMC free article] [PubMed]
- Wang, F. , Kalmar, J. H. , Edmiston, E. , Chepenik, L. G. , Bhagwagar, Z. , Spencer, L. , … Blumberg, H. P. (2008). Abnormal corpus callosum integrity in bipolar disorder: A diffusion tensor imaging study. Biological Psychiatry, 64(8), 730–733. 10.1016/j.biopsych.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye, L. T. , Alnaes, D. , van der Meer, D. , Kaufmann, T. , & Andreassen, O. A. (2019). Population‐based mapping of polygenic risk for schizophrenia on the human brain: New opportunities to capture the dimensional aspects of severe mental disorders. Biological Psychiatry, 86(7), 499–501. 10.1016/j.biopsych.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Whelan, C. D. , Altmann, A. , Botia, J. A. , Jahanshad, N. , Hibar, D. P. , Absil, J. , … Sisodiya, S. M. (2018). Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain, 141(2), 391–408. 10.1093/brain/awx341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers, T. , Doan, N. T. , Kaufmann, T. , Alnaes, D. , Moberget, T. , Agartz, I. , … Marquand, A. F. (2018). Mapping the heterogeneous phenotype of schizophrenia and Bipolar disorder using normative models. JAMA Psychiatry, 75(11), 1146–1155. 10.1001/jamapsychiatry.2018.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (1993). The ICD‐10 classification of mental and behavioural disorders : Diagnostic criteria for research. Geneva: World Health Organization. [Google Scholar]
- Wray, N. R. , Ripke, S. , Mattheisen, M. , Trzaskowski, M. , Byrne, E. M. , Abdellaoui, A. , … Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . (2018). Genome‐wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nature Genetics, 50(5), 668–681. 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, N. L. , Winkler, A. M. , Barrett, J. , Book, G. A. , Beetham, T. , Horseman, R. , … Glahn, D. C. (2017). Inferring pathobiology from structural MRI in schizophrenia and bipolar disorder: Modeling head motion and neuroanatomical specificity. Human Brain Mapping, 38(8), 3757–3770. 10.1002/hbm.23612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatham, L. N. , Kauer‐Sant'Anna, M. , Bond, D. J. , Lam, R. W. , & Torres, I. (2009). Course and outcome after the first manic episode in patients with bipolar disorder: Prospective 12‐month data from the systematic treatment optimization program for early mania project. Canadian Journal of Psychiatry, 54(2), 105–112. 10.1177/070674370905400208 [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Schneider, T. , Wheeler‐Kingshott, C. A. , & Alexander, D. C. (2012). NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage, 61(4), 1000–1016. 10.1016/j.neuroimage.2012.03.07 [DOI] [PubMed] [Google Scholar]
- Zugman, A. , Harrewijn, A. , Cardinale, E. M. , Zwiebel, H. , Freitag, G. F. , Werwath, K. E. , … Winkler, A. M. , for the ENIGMA‐Anxiety Working Group (2020). Mega‐analysis methods in ENIGMA: The experience of the generalized anxiety disorder working group. PsyArXiv Preprint DOI 10.31234/osf.io/n62vc. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
This is a review article of the ENIGMA Bipolar Disorder Working Group activities and includes no primary data. Those that wish to become members of the ENIGMA Bipolar Disorder Working Group are encouraged to contact the Working Group Chairs.


