Abstract
Nanomaterials, recently have found burgeoning attention in the field of agriculture, owing to the positive correlation between nanoparticle (NP) application and the enhanced nutritional status of the applied plants. A wide range of NPs, namely carbon‐based NPs, titanium dioxide NPs, silica NPs etc. has been found to influence plants in a positive way by increasing their nutrient uptake ratio, nutrient usage efficiency, among others. All these attributes have paved the way for possible improvement in plant growth, development, vigour etc. through the use of these NPs, mainly as nanofertiliser. In view of all these, it can also be concluded that in the global scenario of increased demand of food production and supply in the coming years, nanotechnology promises to play a critical role. In this review, an attempt has been made to consolidate all the positive trends with respect to application of NPs on plants, along with their probable mechanism of action, which may provide a comprehensive insight for researchers working in this field.
Inspec keywords: reviews, nanoparticles, agriculture, nanotechnology, titanium compounds, crops, nanofabrication, fertilisers, food products, nanobiotechnology
Other keywords: nanotechnological interventions, plant growth, positive correlation, nanoparticle application, enhanced nutritional status, applied plants, carbon‐based NPs, titanium dioxide NPs, silica NPs, influence plants, nutrient uptake ratio, nutrient usage efficiency, positive trends
1 Introduction
The word ‘nano’ refers to the Greek word which means ‘dwarf’ [1], was first mentioned in 1959 by the Noble laureate physicist Richard Phillips Feynman [2, 3], while ‘nanotechnology’ was first defined by Norio Taniguchi in 1974 [4]. Technically, ‘nano’ means one billionth, i.e. 10−9 which has found synonymous usage with the word ‘nanoparticle’ (NP) having size dimension between 1 and 100 nm. Substances having size in this range in all three dimensions are called NPs [5, 6]. Although the term is comparatively new to human knowledge, its existence in nature is as old as nature itself, be it in the volcanic dusts, lunar dusts, forest fires, mineral composites, or even changing colours of butterfly wings [7, 8], they had always been present in nature (Fig. 1)!
Fig. 1.
Nanoparticles are present in various forms in natural phenomena
Recently, research in the field of nanotechnology has gained tremendous attention due to remarkable differences in the properties of NPs and their corresponding bulk materials. Nanoparticles are characterised by their relative larger surface area, unique patterns of distribution on surface, surface charge ratio etc. which are mainly responsible for their altered optical and chemical behaviour. Their enhanced reactivity has also been attributed to their randomness of distribution in the atomic level which increases entropy, and thereby Gibbs free energy is also increased. The unique properties of NPs have been schematically represented in Fig. 2. Comparison of the effects of nanomaterials versus their ‘bulk’ counterparts in a number of research articles have revealed that NPs have a more pronounced effect on the growth, development and vigour of plants, and the reason may well rest on the particle size differences [9, 10].
Fig. 2.
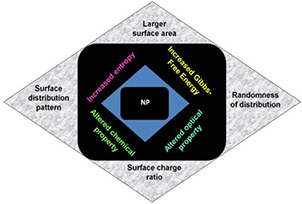
Unique physicochemical properties of NPs, mostly attributed of their nano‐scale size
These have been exploited to develop nanofertilisers, micronutrient solutions and such as agrochemical solutions, which are now used to improve plant growth and vigour. Nanofertilisers increase the bioavailability of nutrients in the rhizosphere [11]. The nano‐encapsulated fertilisers have been characterised with slow and steady release of active chemicals [12], that is, effective through a prolonged time duration within the plant system. Recently, nanoporous zeolite‐based nanofertilisers have been reported to increase the effectiveness of nitrogen uptake [13]. Natural zeolite modified with aminopropyltrimethoxy silane has more affinity to bind to the nitrogen of urea owing to the presence of amine group on its surface. Thus, uptake of urea is increased and the adsorption ensures controlled release of the fertiliser [14].
In cereals, application of nanomaterials specially nanocomposites having compositions of nitrogen, potassium, phosphorous, other micronutrients, amino acids, mannose etc. have been reported to increase uptake ratio and utilisation of those nutrients. [15].
Current literature has substantiated positive correlation between application of NPs on plants and improvement of plant growth and vigour [16, 17, 18, 19]. These reports have been specifically obtained for NPs, namely titanium dioxide (TiO2) NPs, silica (SiO2) NPs, zinc oxide (ZnO) NPs and all carbon‐based NPs. The degree of manifestation depends on the dose, concentration and frequency of application of the NP and also on the type of NP used, and it varies largely from species to species. The mode of action of each NP is also different, and hence a broad picture is presented here with respect to the plant species used, concentration of NP, effect observed and the probable mechanism of action within the system (Table 1). Even seed priming has come up as an alternative solution for a synchronised and increased germination percentage, improved nutritional content, improved seed vigour, especially under stressful condition [38, 39].
Table 1.
Summarises the growth‐related effects of different NPs on several plant species
| NPs | Effective concentration | Plant/plant part applied with | Effects observed | Probable mechanism | Reference (s) |
|---|---|---|---|---|---|
| AuNPs | 10 ppm | B. juncea seedlings | (i) increased number of leaves, plant length, stem thickness, number of branches per plant; (ii) increased chlorophyll content by 29%; and (iii) accelerated rate of seed germination | (i) antagonistic effect on ethylene; increased effect of gibberellic acid; (ii) increased water and molecular oxygen uptake by seed capsule | Arora et al. [20] |
| MWCNTs | 2 g/l | rye grass tomato whole plant barley, soybean, corn seeds tobacco cell culture | enhanced seedling root growth greater plant biomass, more flowers and fruits | increased Ca and Fe nutrient uptake altered gene expression, enhanced water absorption by aquaporins. Genes such as NNtPIP1 (aquaporin) responsible for water transport, CycB responsible for cell division, NtLRX1 responsible for cell wall formation are upregulated | Villagarcia et al. [21]; Tiwari 2014 [22]; Lin and Xing [23]; Khodakovskaya et al. [24]; Lahiani et al. [25] |
| citric acid‐coated CNTs | 6 g/l | C. arietinum | increased water uptake and growth | nanotubes form an ‘aligned network’ inside vascular tissue | Tripathi et al. [26] |
| wsCNTs | 6 µg/ml | C. arietinum | enhanced root and shoot length, increased branching | better water retention and absorption | Tripathy 2011 [26] |
| TiO2 NPs | — | spinach seeds, leaves | increased and accelerated growth | increases the activity of several enzymes and promotes the adsorption of nitrate | Zheng et al. 2005 [27]; Hong et al. [28]; Yang et al. [29]; Gao et al. [30] |
| TiO2 | 0.5 g/l | L. minor | plant elongation, increased fresh weight. | increased photosynthesis, enzyme activity, increased chemical fixation of atmospheric oxygen | Yang et al. [29]; Gao et al. [30]; Su et al. [31]; Song et al. [32] |
| TiO2 | 20 g/l | T. aestivum; foliar application | stem elongation, increased yield, flowering, biomass, ear mass, seed number, improved starch and gluten content | modified activities of different nitrogen metabolising enzymes, namely nitrate reductase, glutamate dehydrogenase, glutamine synthase and glutamic‐pyruvic transaminase | Jaberzadeh et al. [33]; Yang et al. [34]; Mishra 2014 [35] |
| CuO NP | 1 mg/l | Elodea densa plant | photosynthesis rate increased to 130–140% of control | increased lipid peroxidation, increased catalase and SOD activities | Nekrasov et al. [36] |
| ZnNP | 10 mg/l | P. americanum, mixed with soil | increased accumulation of soluble leaf proteins (19.9%); chlorophyll (18.4%); enhanced root area (18.4%); shoot length (10.8%); increased grain yield (29.5%); and dry biomass (12.0%) | enhanced activity of different enzymes, namely phytase, alkaline phosphatase, acid phosphatase, dehydrogenase | Tarafdar et al. [37] |
2 Plant growth and development
A wide range of NPs is effective in improving plant growth, development and vigour. Our understanding of the different NPs and their mode of action mainly rests on the available literature. These include single‐walled and multiwalled carbon nanotubes (SWCNT/MWCNT), magnetised iron (Fe) NPs, aluminium, copper (Cu), gold (Au), silver (Ag), Si, ZnNPs, ZnO, TiO2, cerium oxide etc.
2.1 Carbon‐based NPs
Nanoparticles have larger surface area to volume ratio; this particularly allows them to retain nutrients for a longer period within the plant system. Thus, nanofertilisers and nano‐encapsulated materials are more stable [40]. Carbon‐based NMs including fullerols, C60/70 fullerenes, carbon NPs and CNTs, both SWCNTs and MWCNTs have been found to enhance plant growth [41]. Both SWCNTs and MWCNTs have been detected in tomato fruits, leaves and roots of treated plants, using photo‐thermal and photo‐acoustic mapping techniques, where they have been found to enhance plant biomass [42]. Changes in water channel proteins or aquaporins and significant alteration of total gene expression in the exposed tomatoes have also been established by the same authors. In a separate set of experiments, MWCNTs, have been found to bring about a 55–64% increase in growth in cultured tobacco cells in comparison with controls [43] (Fig. 3).
Fig. 3.
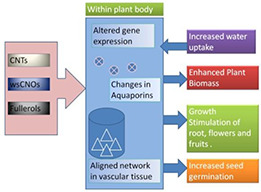
Carbon‐based NPs, namely CNTs, wsCNOs and fullerols have been reported to increase water uptake in plants post‐treatment followed by enhanced plant biomass, growth and germination in tomato plants and tobacco cells. These NPs bring about changes in aquaporin water channels and form an ‘aligned network’ within the vascular bundle of the plants, both of which facilitate water uptake efficiency. Mechanism of action by alteration in gene expression has also been reported
Citric acid‐coated CNTs (at a concentration of 6 g l−1) treatment of Cicer arietinum for 10 days has shown stimulation of growth and intercellular uptake of NPs [26]. According to the authors, the water uptake efficiency of the treated plants increases due to the presence of ‘aligned network’ in the vascular bundles, which have been formed from the applied nanotubes. Even the number of fruits and flowers has been found to be twice that of control sets when tomato plants have been grown in CNT supplemented soil [42]. Similarly, water‐soluble carbon nano‐onions (wsCNOs) (at a concentration of 10–30 mg l−1) and water‐soluble carbon nanodots (at a concentration of 150 mg l−1) have been shown to exhibit growth enhancement effect on C. arietinum [44] and wheat root [45], respectively.
In a separate study with bitter melon, Momordica charantia, water‐soluble fullerene, or fullerol [C60 (OH)20] exposed plants showed increased biomass and phytomedicinal content [46].
SWCNTs have been found to enhance the root growth of germinated onion (Allium cepa) and cucumber seeds (Cucumis sativus) at test duration of 48 h when supplemented with concentrations 0.16, 0.9 and 5 g l−1 [47]. In contrast, negligible effects have been observed on seeds of cabbage (Brassica oleracea) and carrot (Daucus carota) and growth inhibitory effects have been reported in case of tomato seeds [21, 25, 42]. However, fruit yield in tomato has been reported to increase by more than twofolds (nine fruits per plant) with 50 mg l−1 of CNTs application as against control sets involving water application (four fruits per plant) and activated carbon application (three per plant) [42].
2.2 Titanium dioxide NPs
TiO2 application can improve plant growth as they have been found to be involved in chemical fixation of N2 of air, thereby providing more nitrogen and phosphorous nutrients to the plants. TiO2 have also been found to improve the enzyme activities and photosynthetic function of the plant in the presence of sunlight [29, 30, 31]. In a 7‐day growth chamber study, 0.5 g l−1 of Ti NPs have been reported to increase fresh weight (two times) and stem elongation (>2.5 times) in Lemna minor (duckweed) in comparison with bulk TiO2 solution [32]. However, higher concentration (>0.5 g l−1) showed negative impacts [32]. Owing to their property of effective photocatalyst under ultraviolet (UV) radiation, TiO2 NPs have been extensively studied for photocatalytic applications in industries. The various arenas include solar energy mediated decomposition of organic compounds and subsequent evolution of H2 as a fuel [48]. All these fields of application pave the way for new possibilities of improving plant photosynthesis and related physiological activities with leaf spray of Ti NPs at optimum concentrations, thereby improving plant growth parameters. This has been particularly tested in spinach by separate and independent research groups in two ways – leaf spray and seed treatment, and in both the types of administrations. TiO2 NPs have been found to have positive results [27, 28, 29, 30]. Seed treatment of naturally aged spinach seeds with both bulk and nano‐sized TiO2 showed that plants produced from nano‐treated aged seeds had more dry weight (73%), photosynthetic rate (three times more) and chlorophyll content (45% more) compared with control over germination period of 30 days. The growth rate of spinach seeds was inversely proportional to the material size indicating that smaller the nanomaterials, the better the germination. It has been speculated that TiO2 NPs augments photo‐sterilisation and photo‐generation of ‘active oxygen such as superoxide and hydroxide anions’ which, in turn, improves the seeds’ stress tolerance ability as also the water and oxygen intake efficiency. The net result is enhanced germination. The authors concurred that the nano‐size of TiO2 might have increased the absorption of inorganic nutrients, accelerated the breakdown of organic substances, and also caused quenching of oxygen free radicals formed during the photosynthetic process, hence increasing the photosynthetic rate. Furthermore, 2.5% rutile TiO2 NPs solution treatment of spinach (Spinacea oleracea) resulted in increased RubisCO activity, overall photosynthetic rate and more specifically, increased chlorophyll formation (by 23%) and growth (by 63–76%) [27]. In a separate study in the same plant, with anatase TiO2 NPs, yielded increase in fresh mass (58.2%), dry mass (69.8%), chlorophyll content (19.0%), photosynthetic rate (29.9%) and RubisCO activity (250%) [29]. According to Lang et al., under nitrogen deficiency condition, anatase TiO2 NPs can directly induce reduction of atmospheric nitrogen to ammonia of soil, in the presence of sunlight, and hence the growth promoting effect [29]. Similar growth enhancement effects have been observed in wheat (Triticum aestivum), where a 20 g l−1 application of Ti NPs promoted increase in biomass, ear mass, flowering, stem elongation and seed number [33]. Fig. 4 summarises the probable mechanism of action and the resulting morphological effects on plants on TiO2 treatment.
Fig. 4.
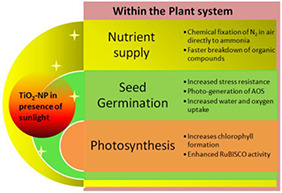
TiO2 NPs, in the presence of UV rays of sunlight acts as effective photocatalyst promoting seed germination as well as growth
However, further research is needed to understand how the reactions about plant growth improvement are coordinated in the presence of TiO2 NPs and not interfered by the said NPs. With a mixture of 4 g l−1 rutile and anatase forms of TiO2 NPs negative impacts on seed germination have also been reported.
2.3 SiO2 NPs
SiO2 NPs have been found to exhibit growth‐enhancing properties in plants. The two broadways mechanisms by which that has been done are increases in gas exchange and improvement of chlorophyll fluorescence parameters. The direct consequences of these are increases in effective and actual photochemical efficiency, electron transport rate, photochemical quench, Photosystem (PS) II potential activity, stomatal conductance and transpiration rate [49, 50]. Fig. 5 shows the various mechanisms by which SiO2 can improve plant growth parameters. Now, the various changes that NPs can bring about within plants largely depends on the properties of particular NPs used, chemical composition, surface covering and dose of application [51]. Effect of engineered NPs too depends on these mentioned properties along with the plant species [52].
Fig. 5.
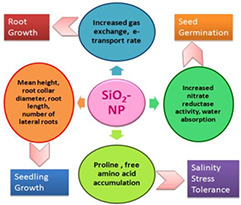
SiO2 NPs typically bring about increased growth parameters in root, seedling, seed germination and also salinity stress tolerance
Rice plants treated with Si‐coated quantum dots (QDs) showed marked increase in root growth as against rice plants treated with QDs and without QDs.
2.4 Zinc oxide NPs
In comparison to bulk zinc sulphate, ZnO NPs at concentrations of 1000 mg l−1, showed increased growth, germination rate, chlorophyll content and pod yield in peanut (Arachis hypogea), though, at higher doses (1000 mg l−1), phytotoxicity was observed [53]. Treatment of cluster bean leaves (Cyamopsis tetragonoloba L.), with biosynthesised ZnO NPs from extracellular secretions of Aspergillus fumigatus, also showed considerable increase in total protein (17.2%), rhizospheric microbial population (13.6%), among other attributes such as shoot elongation, root elongation and chlorophyll content [54]. In a separate set of experiments, the researchers obtained similar results with 10 mg l−1 foliar spray of ZnNPs, biosynthesised from Rhizoctonia bataticola, on soilgrown pearl millet (Pennisetum americanum), where ZnNPs treatment increased activities of different enzymes such as phytase (72.7%), alkaline phosphatase (22.58%), acid phosphatase (14.18%), dehydrogenase (9.22%) along with other parameters such as shoot length (10.8%), chlorophyll content (18.4%), root area (18.4%), dry biomass (12.0%), grain yield (29.5%) and soluble leaf proteins (19.9%), in contrast to bulk treated plants [37]. Positive results have also been obtained in chickpea (C. arietinum L var. HC‐1) treated with 1.5 mg l−1 ZnO NPs solution as against bulk treatment; however, higher dose of the NP (10 mg l−1) have been shown to become toxic [55].
2.5 Iron NPs
Alidoust and Isoda exposed soybean to iron(III) oxide (Fe2 O3) NP, citrate‐coated Fe2 O3 NPs, bulk Fe2 O3 and citrate‐coated bulk Fe2 O3 and observed increased root length and photosynthesis in the Fe2 O3 ‐NP treated plants; however, the enhancement was less pronounced when NP was introduced through soil supplementation rather than foliar spray. The authors have further speculated that the reason for such differences in results may be due to extensive precipitation of Fe ions in soil [56]. A four‐fold increase in plant growth and biomass was obtained in hydroponically grown spinach treated with phosphate‐sorbed zero‐valent Fe NPs, along with 11–21 times increase in roots, stem and leaves [57], though, no such physiological change was observed in Spathyphyllum (an ornamental species) on similar treatment [58].
2.6 Manganese (Mn) NPs
Interesting results have been obtained in hydroponically grown mung bean (Vigna radiata) treated with MnNP or manganese(II) sulphate (concentrations up to 1 mg l−1), where the bulk treatment showed toxic effects at higher doses, and the NP treatment brought about a 10–100% increase in bean shoot, root, fresh weight, dry weight and rootlet number, at more moderate doses. The authors have further explained that the toxicity may have arisen due to a large initial burst of Mn ions released from the salt form; on the other hand, the NP form facilitated a very slow and controlled release of the nano‐metal. NP treatment also increased carotene phosphorylation, oxygen evolution, nitrogen metabolism and chlorophyll content [59, 60].
2.7 nano‐hydroxyapatite
Liu and Lal reported that nano‐hydroxyapatite (nHA) increased growth rate by 32.6% and seed yield by 20.4%, in Glycine max (soybean) in greenhouse conditions, as against application of soluble phosphorous fertilisers [Ca(H2 PO4)2]. According to the authors, properties such as higher stability and viscosity of the nHA, imparted longer retention time of the NP in the porous medium, which enhanced the growth parameters [61].
Thus, it is evident from the available literature that several factors, namely particle concentration, plant part exposed, mode of exposure and even the plant species concerned, are important attributes in designing and controlling NP‐mediated strategies in agriculture.
3 Seed germination
Raskar and Laware used different concentrations of ZnO NPs (1, 20, 30, 40 mg l−1) to study their effects on onion seeds with respect to seed growth, seedling development and seed germination rate. At lower concentrations germination rate enhancement was more pronounced but the impact was negative when higher concentrations of these NPs were used. These results also conformed to increasing chromosomal abnormalities with higher concentrations of ZnO NP [62]. Positive effects of ZnO NPs, on seed germination, when applied in lower concentrations, have also been found by different researchers in soybean, peanut and wheat [54, 62, 63]. Among different crop species, namely cucumber, tomato and alfalfa, only cucumber seeds showed enhanced germination properties when treated with different concentrations of ZnO NPs [64].
Shah and Belozerova studied the germination of lettuce seeds under the influence of different NPs over 15 days after addition of the NPs in the soil. The authors reported that at low concentrations, Au and palladium NPs; Si and CuNPs at higher concentrations; moreover, a combination of Au and CuNPs significantly influences plant growth after 15 days of incubation, improving the shoot/root ratio. The researchers further speculated that the enhanced effects were due to some indirect mechanism since they were observed only after 15 days of incubating the NPs in soil [65].
Germination and growth of naturally aged spinach seeds treated with nano‐sized rutile TiO2 and non‐nano‐TiO2 were studied in aged spinach seeds, in terms of seed germination rate, index and vigour index of germinated seedlings [27]. At 0.25–4% nano‐TiO2 concentration, all these attributes were found to increase along with plant dry weight, RubisCO activity, chlorophyll formation and rate of photosynthesis. A 2.5% concentration of TiO2 NP application showed maximum improvement, while with bulk TiO2, the impact was insignificant. Thus, it may be inferred that the effect was inversely proportional to the size of NP used. However, to reveal the exact mechanism by which TiO2 NP improves the growth of spinach seeds further study is needed. It has been found that nitrate reductase activity increased in soybean seeds by external application of SiO2 NP and TiO2 NPs, thereby enhancing seed germination [66]. In another study, it has been shown that seed germination can be increased by the exogenous application of SiO2 NPs and TiO2 NPs by increasing the seeds’ ability to absorb and utilise mineral nutrients and water [27]. In Canola sp., seed germination was found to increase by TiO2 NP treatment as well as seedlings exhibited higher radical and plumule growth [67].
In tomato, seed germination has been shown to improve by lower concentrations of tomato [68]. It has been suggested that increased seed germination rate in maize on SiO2 application is due to improved nutrient supply to the seeds and also the conducive pH and conductivity that is imparted into the growing medium [69]. SiO2 NPs have also been found to improve seedling growth and quality in Changbai larch (Larix olgensis). The improved parameters include mean height, root collar diameter, main root length, number of lateral roots and chlorophyll content [70]. SiO2 NPs have also been found to be effective under abiotic salt stress. It has been reported that SiO2 NP augments seed germination in tomato and squash as also stimulating the antioxidant system in salinity stress [71, 72]. Application of Si, palladium, Au and CuNPs all have been found to have positive impact on lettuce seeds [65].
AuNPs have been reported to improve seed germination in lettuce and cucumber, Brassica juncea, Boswellia ovalifoliolata and in Gloriosa superba [73, 74, 75]. Arora have demonstrated enhanced growth and seed yield in B. juncea when treated with AuNPs at different concentrations of 10, 25, 50 and 100 ppm. AuNPs were found to reduce the time taken for seed germination when applied at 25 ppm concentration, and the number of leaves per plant also increased at 10 ppm of AuNP.
This phenomenon has been explained as a result of slowed down activity of ethylene, thereby decreasing leaf abscission in the presence of AuNPs. However, the average leaf area did not increase in treated seedlings [20]. AuNPs have been reported to augment seed germination and antioxidant system in Arabidopsis thaliana. AuNPs have also been reported to play a key role in altering miRNA levels that modulate different physiological and metabolic processes in plants [76] (Fig. 6).
Fig. 6.
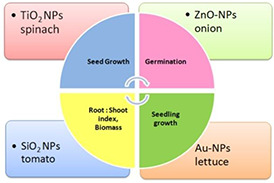
Several NPs, namely AuNPs, ZnO NPs, TiO2 NPs and SiO2 NPs have been shown to augment seed growth, germination rate and percentage, seedling growth, root: shoot indices and seedling biomass in many crops such as spinach, onion, tomato, lettuce, cucumber, peanut, alfalfa, soybean, canola, wheat etc.
4 Negative impact of NPs on plants
Amidst all the positive influences of NPs on plant growth and development, a few literatures have recorded their growth inhibitory effects on plants, mostly when treated with higher concentrations of the NPs. Different groups of NPs have been reported to impart different degrees of toxicity to a different spectrum of plant species. The effect varies mainly with the composition of the NP – the core component present, its form, shape and size as well as the concentration used and the dosage of application. For instance, studies with onions showed inhibitory effects on treatment with AgNPs [77], ZnO NPs [78] and CuNPs [78, 79]. ZnO NPs have been reported to cause disruption of cellular metabolism and cell division stages [78]. On the other hand, rice seeds soaked in ZnO NP solution exhibited ill developed roots and stunted root lengths of seedlings. The effects were more pronounced when seeds were soaked for more days [80]. Negative effects of ZnO NPs were also found in wheat, where decrease in plant biomass was observed. Similar results were also obtained with TiO2 NPs in wheat [81]. TiO2 NPs have been reported to block cytoplasmic connections of plasmodesmata in maize seedlings, thus inhibiting leaf growth and transpiration [73]. Although CNTs increased seed germination and seedling growth in tomato plants, they caused decreased root growth in tomato [24]. Bacillus thuringiensis cotton (Bt transgenic cotton), when treated with SiO2 NPs, also showed some degree of growth inhibition [82].
5 Conclusion
Thus, it is quite evident that different plant species respond differently toward NP treatment and same plant species may also show varied tolerance limits to different NPs. Thus, the degree of tolerance, or in other words, the extent of inhibition varies with the type of NP used, its concentration, dosage of application and the spectrum of plant species tested. This becomes important as many of the reports have shown NP accumulation in plant cells, since plants form the basis of ecosystem, their accumulation of NPs marks NP entry into food chain. The aftermath includes their fate in the ecosystem as well as effect to other consumers including human. Another important fact is that NP amendment in the soil results in their dissolution – this again becomes relevant while considering the effects on soil microbial consortium. Now, the question arises how much of it can be tolerated in the ecosystem, at high concentrations NPs indeed have been found to be toxic, both cytotoxic and genotoxic. Thus. to assess the optimum usable concentration of a particular NP, a lot of research has to be carried out against a broad spectrum of plant species to improve their growth conditions and yield, maximally. This has to also include research with the particular form of the NP that can be used such as elemental form, oxide form, or any other material in combination with other coating material(s) along with their concentration and frequency of application or dosage. Correlation and comparison of data obtained from a wide range of studies have to be accumulated for an integrated study for the purpose.
6 Future prospects
Nanoparticles interact with plants bringing about changes in the plant body, both morphologically and physiologically. Nevertheless, efficiency of NP application depends on the type, chemical composition, size, surface charge, reactivity, dose and frequency of application of the NP [24], as well as, the plant species and the plant part on which they are applied [52]. In this context mentioned may be made of nanoparticulate‐plant‐delivery systems – application of nanotechnology is now extended to the extent of delivering engineered DNA into plant cells, thus expression of desired features can be brought about. At the cellular level, DNA can be manipulated using NP‐based DNA delivery systems that can control the expression of altered genes, thus reducing the time required for transferring the desired genes into a foreign organism. Seeds can be rearranged for specific properties such as colour, yield and even season of growth [83, 84, 85]. Thus, not only external application of nanomaterials can bring about magnanimous output in crops, but also internally, expression patterns can be manipulated faster with the help of nano‐plant‐delivery systems. Indeed, use of nanotechnology can be exploited to create a ‘second’ green revolution in the arena of global food production. However, further research in the relevant fields is necessary to reach the far‐sighted outcomes.
7 References
- 1. Gong P. Li H. He X. et al.: ‘Preparation and antibacterial activity of Fe3 O4 and Ag nanoparticles’, Nanotechnology, 2007, 18, pp. 604 –611 [Google Scholar]
- 2. Lyons K.: ‘Nanotechnology: transforming food and the environment’, Food First Backgrounder, 2010, 16, pp. 1 –4 [Google Scholar]
- 3. Drexler E.: ‘There's plenty of room at the bottom (Richard Feynman, Pasadena, 29 December 1959), metamodern’, Trajectory Technol., 2009, 12, 29. (1), pp. 1 –4 [Google Scholar]
- 4. Taniguchi N.: ‘On the basic concept of nanotechnology’. Proc. Int. Conf. Production Engineering, Tokyo, Japan, 26–29 August 1974, pp. 18 –23, Japan Society of Precision Engineering, 1974 [Google Scholar]
- 5. Klaine S.J. Alvarez P.J.J. Batley G.E. et al.: ‘Nanomaterials in the environment: behavior, fate, bioavailability and effects’, Environ. Toxicol. Chem., 2008, 27, pp. 1825 –1851. 10.1897/08-090.1 [DOI] [PubMed] [Google Scholar]
- 6. Ball P.: ‘Natural strategies for the molecular engineer’, Nanotechnology, 2002, 13, p. 15e28 [Google Scholar]
- 7. Tam H.L. Cheah K.W. Goh D.T.P. et al.: ‘Iridescence and nano‐structure differences in Papilio butterflies’, Opt. Mater. Express, 2013, 3, (8), pp. 1087 –1092 [Google Scholar]
- 8. Monica R.C. Cremonini R.: ‘Nanoparticles and higher plants’, Caryologia, 2009, 62, p. 161e165 [Google Scholar]
- 9. Mochizuki H. Gautam P.K. Sinha S. et al.: ‘Increasing fertilizer and pesticide use efficiency by nanotechnology in desert afforestation, arid agriculture’, J. Arid Land Stud., 2009, 19, (1), pp. 129 –132 [Google Scholar]
- 10. Fahlman B.D.: ‘Materials chemistry’ (Springer, Dordrecht, Netherlands, 2007) [Google Scholar]
- 11. Raliya R. Tarafdar J.C. Gulecha K. et al.: ‘Review article: scope of nanoscience and nanotechnology in agriculture’, J. Appl. Biol. Biotechnol., 2013, 1, (3), pp. 041 –044 [Google Scholar]
- 12. De La Rosa M.C. Monreal C. Schnitzer M. et al.: ‘Nanotechnology in fertilizers’, Nat. Nanotechnol. J., 2010, 5, (2), pp. 91 –96 [DOI] [PubMed] [Google Scholar]
- 13. Manikandan A. Subramanian K.S.: ‘Fabrication and characterisation of nanoporous zeolite‐based N fertilizer’, Afr. J. Agric. Res., 2014, 9, (2), pp. 276 –284 [Google Scholar]
- 14. Hidayat R. Fadillah G. Chasanah U. et al.: ‘Effectiveness of urea nanofertilizer based aminopropyltrimethoxysilane (APTMS)‐zeolite as slow release fertilizer system’, Afr. J. Agric. Res., 2015, 10, (14), pp. 1785 –1788 [Google Scholar]
- 15. Chinnamuthu C.R. Boopathi P.M.: ‘Nanotechnology and agroecosystem’, Madras Agric. J., 2009, 96, (1–6), pp. 17 –31 [Google Scholar]
- 16. Judy J.D. Unrine J.M. Rao W. et al.: ‘Bioavailability of gold nanomaterials to plants: importance of particle size and surface coating’, Environ. Sci. Technol., 2012, 46, pp. 8467 –8474 [DOI] [PubMed] [Google Scholar]
- 17. Kurepa J. Paunesku T. Vogt S. et al.: ‘Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana ’, Nano Lett., 2010, 10, pp. 2296 –2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raliya R. Tarafdar J.D. Biswas P.: ‘Enhancing the mobilization of native phosphorus in the mung bean rhizosphere using ZnO nanoparticles synthesized by soil fungi’, J. Agric. Food Chem., 2016, 64, pp. 3111 –3118 [DOI] [PubMed] [Google Scholar]
- 19. Wang Z. Xie X. Zhao J. et al.: ‘Xylem‐ and phloem‐based transport of CuO nanoparticles in maize (Zea mays L.)’, Environ. Sci. Technol., 2012, 46, pp. 4434 –4441 [DOI] [PubMed] [Google Scholar]
- 20. Arora S. Sharma P. Kumar S. et al.: ‘Gold‐nanoparticle induced enhancement in growth and seed yield of Brassica juncea ’, Plant Growth Regul., 2012, 66, pp. 303 –310, DOI 10.1007/s10725‐011‐9649‐z [Google Scholar]
- 21. Villagarcia H. Dervishi E. de Silva K. et al.: ‘Surface chemistry of carbon nanotubes impacts the growth and expression of water channel protein in tomato plants’, Small, 2012, 8, pp. 2328 –2334 [DOI] [PubMed] [Google Scholar]
- 22. Tiwari D.K. Dasgupta‐Schubert N. Villaseñor Cendejas L.M. et al.: ‘Inter facing carbon nanotubes (CNT) with plants: enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nano agriculture’, Appl. Nanosci., 2014, 4, pp. 577 –591. doi:10.1007/s13204‐013‐0236‐7 [Google Scholar]
- 23. Lin D. Xing B.: ‘Phytotoxicity of nanoparticles: inhibition of seed germination and root growth’, Environ. Pollut., 2007, 150, pp. 243 –250. doi: 10.1016/j.envpol.2007.01.016 [DOI] [PubMed] [Google Scholar]
- 24. Khodakovskaya M.V. DeSilva K. Nedosekin D.A. et al.: ‘Complex genetic, photothermal, and photoacoustic analysis of nanoparticle‐plant interactions’, Proc. Natl. Acad. Sci. USA, 2011, 108, pp. 1028 –1033. doi:10.1073/pnas.1008856108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lahiani M.H. Dervishi E. Chen J. et al.: ‘Impact of carbon nanotube exposure to seeds of valuable crops’, ACS Appl. Mater. Interfaces, 2013, 5, pp. 7965 –7973. doi:10.1021/am402052x [DOI] [PubMed] [Google Scholar]
- 26. Tripathi S. Sonkar S.K. Sarkar S.: ‘Growth stimulation of gram (Cicer arietinum) plant by water‐soluble carbon nanotubes’, Nanoscale, 2011, 3, pp. 1176 –1181. doi:10.1039/C0NR00722F [DOI] [PubMed] [Google Scholar]
- 27. Zheng L. Hong F. Lu S. et al.: ‘Effects of nano‐TiO2 on strength of naturally aged seeds and growth of spinach’, Biol. Trace Elem. Res., 2005, 104, pp. 83 –92 [DOI] [PubMed] [Google Scholar]
- 28. Hong X.T. Wang Z.P. Cai W.M. et al.: ‘Visible‐light‐activated nanoparticle photocatalyst of iodine‐doped titanium dioxide’, Chem. Matter., 2005, 17, pp. 1548 –1552 [Google Scholar]
- 29. Yang F. Liu C. Gao F. et al.: ‘The improvement of spinach growth by nano‐anatase TiO2 treatment is related to nitrogen photoreduction’, Biol. Trace Elem. Res., 2007, 119, pp. 77 –88 [DOI] [PubMed] [Google Scholar]
- 30. Gao F. Liu C. Qu C. et al.: ‘Was improvement of spinach growth by nano‐TiO2 treatment related to the changes of RubisCO Activase?’, Biometals, 2008, 21, pp. 211 –217 [DOI] [PubMed] [Google Scholar]
- 31. Su M. Liu H. Liu C. et al.: ‘Promotion of nano‐anatase TiO2 on the spectral responses and photochemical activities of D1/D2/Cyt b559 complex of spinach’, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 2009, 72, pp. 1112 –1116 [DOI] [PubMed] [Google Scholar]
- 32. Song G.L. Gao Y. Wu H. et al.: ‘Physiological effect of anatase TiO2 nanoparticles on Lemna minor ’, Environ. Toxicol. Chem., 2012, 31, pp. 2147 –2152 [DOI] [PubMed] [Google Scholar]
- 33. Jaberzadeh A. Moaveni P. Tohidi Moghadam H.R. et al.: ‘Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress’, Not Bot Horti Agrobot, 2013, 41, (1), pp. 201 –207 [Google Scholar]
- 34. Yang F. Hong F. You W. et al.: ‘Influences of nano‐anatase TiO2 on the nitrogen metabolism of growing spinach’, Biol. Trace Elem. Res., 2006, 110, pp. 179 –190 [DOI] [PubMed] [Google Scholar]
- 35. Mishra V. Mishra R.K. Dikshit A. et al.: ‘Interactions of nanoparticles with plants: an emerging prospective in the agriculture industry’, in Ahmad P. Rasool S. (Eds.): ‘Emerging technologies and management of crop stress tolerance: biological techniques’, vol. 1, Academic Press, Elsevier, CA, 2014, pp. 159 –180 [Google Scholar]
- 36. Nekrasov G.F. Ushakova O.S. Ermakov A.E. et al.: ‘Effects of copper(II) ions and copper oxide nanoparticles on Elodea densa planch.’, Russ. J. Ecol., 2011, 42, (6), pp. 458 –463 [Google Scholar]
- 37. Tarafdar J.C. Raliya R. Mahawar H. et al.: ‘Development of zinc nanofertilizer to enhance to enhance crop production in pearl millet (Pennisetum americanum)’, Agric. Res., 2014, 3, (3), pp. 257 –262 [Google Scholar]
- 38. Joshi A. Kaur S. Dharamvir K. et al.: ‘Multiwalled carbon nanotubes applied through seed‐priming influence early germination, root hair, growth and yield of bread wheat (Triticum aestivum L.)’, J. Sci. Food Agri., 2017, 8, pp. 3148 –3160 [DOI] [PubMed] [Google Scholar]
- 39. Sundaria N. Singh M. Upreti P. et al.: ‘Seed priming with iron oxide nanoparticles triggers iron acquisition and biofortification in wheat (Triticum aestivum L.) grains’, J. Plant Growth Regul., 2018, 10, pp. 122 –131, 1007/s00344‐018‐9818‐7 [Google Scholar]
- 40. Navarro E. Baun A. Behra R. et al.: ‘Environmental behaviour and ecotoxicity of engineered nanoparticles to algae, plants and fungi’, Ecotoxicology, 2008, 17, pp. 372 –386 [DOI] [PubMed] [Google Scholar]
- 41. Khot L.R. Sankaran S. Maja J.M. et al.: ‘Applications of nanomaterials in agricultural production and crop protection: a review’, Crop Prot., 2012, 35, pp. 64 –70. doi:10.1016/j.cropro.2012. 01.007 [Google Scholar]
- 42. Khodakovskaya M.V. Kim B.S. Kim J.N. et al.: ‘Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community’, Small, 2013, 9, (1), pp. 115 –123 [DOI] [PubMed] [Google Scholar]
- 43. Khodakovskaya M.V. DeSilva K. Biris A.S. et al.: ‘Carbon nanotubes induce growth enhancement of tobacco cells’, ACS Nano, 2012, 6, pp. 2128 –2135. doi:10.1021/nn204643 g [DOI] [PubMed] [Google Scholar]
- 44. Sonkar S.K. Roy M. Babar D.G. et al.: ‘Water soluble carbon nano‐onions from wood wool as growth promoters for gram plants’, Nanoscale, 2012, 4, (24), pp. 7670 –7767 [DOI] [PubMed] [Google Scholar]
- 45. Tripathi S. Sarkar S.: ‘Influence of water‐soluble carbon dots on the growth of wheat plant’, Appl. Nanosci., 2014, 5, pp. 609 –616. doi:10.1007/s13204‐014‐ 0355‐9 [Google Scholar]
- 46. Kole C. Kole P. Randunu K.M. et al.: ‘Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia)’, BMC Biotechnol., 2013, 13, p. 37. doi:10.1186/1472‐ 6750‐13‐37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Canas J.E. Long M. Nations S. et al.: ‘Effects of functionalized and non‐functionalized single‐walled carbon nanotubes on root elongation of select crop species’, Environ. Toxicol. Chem., 2008, 27, pp. 1922 –1931 [DOI] [PubMed] [Google Scholar]
- 48. Gupta S.M. Tripathi M.: ‘A review of TiO2 nanoparticles’, Chin. Sci. Bull., 2011, 56, pp. 1639 –1657 [Google Scholar]
- 49. Siddiqui M.H. Al‐Whaibi M.H. Firoz M. et al.: ‘Role of nanoparticles in plants, nanotechnology and plant sciences’ (Springer, Cham, 2015), pp. 19 –35. http://dx.doi. org/10.1007/978‐3‐319‐14502‐0_2 [Google Scholar]
- 50. Xie Y. He Y. Irwin P.L. et al.: ‘Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni ’, Appl. Environ. Microbiol., 2011, 77, pp. 325 –331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Castiglione R.M. Giorgetti L. Geri C. et al.: ‘The effects of nano‐TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L.’, J. Nanopart. Res., 2011, 13, pp. 2443 –2449 [Google Scholar]
- 52. Ma X.M. Geiser‐Lee J. Deng Y. et al.: ‘Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation’, Sci. Total Environ., 2010, 408, (16), pp. 3053 –3061 [DOI] [PubMed] [Google Scholar]
- 53. Prasad T.N.V.K.V. Sudhakar P. Sreenivasulu Y. et al.: ‘Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut’, J. Plant Nutr., 2012, 35, (6), pp. 905 –927 [Google Scholar]
- 54. Raliya R. Tarafdar J.C.: ‘ZnO nanoparticle biosynthesis and its effect on phosphorous‐mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsis tetragonoloba L.)’, Agric. Res., 2013, 2, pp. 48 –57 [Google Scholar]
- 55. Burman U. Saini M. Praveen‐Kumar: ‘Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings’, Toxicol. Environ. Chem., 2013, 95, (4), pp. 605 –612 [Google Scholar]
- 56. Alidoust D. Isoda A.: ‘Effect of gamma Fe2 O3 nanoparticles on photosynthetic characteristic of soybean [Glycine max (L.) Merr.]: foliar spray versus soil amendment’, Acta Physiol. Plant., 2013, 35, (12), pp. 3365 –3375 [Google Scholar]
- 57. Almeelbi T. Bezbaruah A.: ‘Nanoparticle‐sorbed phosphate: iron and phosphate bioavailability studies with Spinacia oleracea and Selenastrum capricornutum ’, ACS Sustain. Chem. Eng., 2014, 2, (7), pp. 1625 –1632 [Google Scholar]
- 58. Mohamadipoor R. Sedaghathoor S. Khomami A.M.: ‘Effect of application of iron fertilizers in two methods ‘foliar and soil application’ on growth characteristics of Spathyphyllum illusion ’, Eur. J. Exp. Biol., 2013, 3, (1), pp. 232 –239 [Google Scholar]
- 59. Pradhan S. Patra P. Das S. et al.: ‘Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: a detailed molecular, biochemical, and biophysical study’, Environ. Sci. Technol., 2013, 47, (22), pp. 13122 –13131 [DOI] [PubMed] [Google Scholar]
- 60. Pradhan S. Patra P. Mitra S. et al.: ‘Manganese nanoparticles: impact on non‐modulated plant as a potent enhancer in nitrogen metabolism and toxicity study both in vivo and in vitro’, J. Agric. Food Chem., 2014, 62, (35), pp. 8777 –8785 [DOI] [PubMed] [Google Scholar]
- 61. Liu R. La L.R.: ‘Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max)’, Sci. Rep., 2014, 4, (5686), pp. 1 –6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Raskar S.V. Laware S.L.: ‘Effect of zinc oxide nanoparticles on cytology and seed germination in onion’, Int. J. Curr. Microbiol. Appl. Sci., 2014, 3, pp. 467 –473 [Google Scholar]
- 63. Ramesh M. Palanisamy K. Babu K. et al.: ‘Effects of bulk & nano‐titanium dioxide and zinc oxide on physio‐morphological changes in Triticum aestivum Linn.’, J. Glob. Biosci., 2014, 3, pp. 415 –422 [Google Scholar]
- 64. De la Rosa G. Lopez‐Moreno M.L. De Haro D. et al.: ‘Effects of ZnO nanoparticles in alfalfa, tomato, and cucumber at the germination stage: root development and X‐ray absorption spectroscopy studies’, Pure Appl. Chem., 2013, 85, (12), pp. 2161 –2174 [Google Scholar]
- 65. Shah V. Belozerova I.: ‘Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds’, Water Air Soil Pollut., 2009, 197, pp. 143 –148 [Google Scholar]
- 66. Lu C.M. Zhang C.Y. Wen J.Q. et al.: ‘Research of the effect of nanometre materials on germination and growth enhancement of Glycine max and its mechanism’, Soybean Sci., 2002, 21, pp. 168 –172 [Google Scholar]
- 67. Mahmoodzadeh H. Nabavi M. Kashefi H.: ‘Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus)’, J. Ornamental Hortic Plants, 2013, 3, pp. 25 –32 [Google Scholar]
- 68. Siddiqui M.H. Al‐Whaibi M.H.: ‘Role of nano‐SiO2 in germination of tomato (Lycopersicum esculentum seeds mill.)’, Saudi J. Biol. Sci., 2014, 21, pp. 13 –17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Suriyaprabha R. Karunakaran G. Yuvak Kumar R. et al.: ‘Silica nanoparticles for increased silica availability in maize (Zea mays L) seeds under hydroponic conditions’, Curr. Nanosci., 2012, 8, pp. 902 –908 [Google Scholar]
- 70. Bao‐shan L. Shao‐qi D. Chun‐hui L. et al.: ‘Effect of TMS (nanostructured silicon dioxide) on growth of Changbai Larch seedlings’, J. Forest Res., 2004, 15, pp. 138 –140 [Google Scholar]
- 71. Haghighi M. Afifipour Z. Mozafarian M.: ‘The effect of N–Si on tomato seed germination under salinity levels’, J. Biol. Environ. Sci., 2012, 6, pp. 87 –90 [Google Scholar]
- 72. Siddiqui M.H. Al‐whaibi M. Faisal M. et al.: ‘Nano‐silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L’, Environ. Toxicol. Chem., 2014, 33, pp. 2429 –37, 10.1002/etc.2697 [DOI] [PubMed] [Google Scholar]
- 73. Barrena R. Casals E. Colón J. et al.: ‘Evaluation of the ecotoxicity of model nanoparticles’, Chemosphere, 2009, 75, (7), pp. 850 –857 [DOI] [PubMed] [Google Scholar]
- 74. Savithramma N. Ankanna S. Bhumi G.: ‘Effect of nanoparticles on seed germination and seedling growth of Boswellia ovalifoliolata – an endemic and endangered medicinal tree taxon’, Nano Vis., 2012, 2, pp. 61 –68 [Google Scholar]
- 75. Gopinath K. Gowri S. Karthika V. et al.: ‘Green synthesis of gold nanoparticles from fruit extract of Terminalia arjuna, for the enhanced seed germination activity of Gloriosa superba ’, J. Nanostruct. Chem., 2014, 4, pp. 1 –11 [Google Scholar]
- 76. Kumar V. Guleria P. Kumar V. et al.: ‘Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana ’, Sci. Total Environ., 2013, 461, pp. 462 –468. doi: 10.1016/j.scitotenv.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 77. Kumari M. Gole A.M. Chandrasekaran N.: ‘Genotoxicity of silver nanoparticles in Allium cepa ’, Sci. Tot. Environ., 2009, 407, pp. 5243 –5246 [DOI] [PubMed] [Google Scholar]
- 78. Ghodake G. Seo Y.D. Park D. et al.: ‘Phytotoxicity of carbon nanotubes assesses by Brassica juncea and Phaseolus mungo ’, J. Nanoelectron. Optoelectron., 2010, 5, (2), pp. 157 –160 (4). DOI 10.1166/jno.2010.1084 [Google Scholar]
- 79. Lee W.M. Any J. Yoon H. et al.: ‘Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water‐insoluble nanoparticles’, Environ. Toxicol. Chem., 2008, 27, pp. 1915 –1921 [DOI] [PubMed] [Google Scholar]
- 80. Boonyanitipong P. Kostsup B. Kumar P. et al.: ‘Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oriza sativa L.’, Int. J. Biosci. Biochem. Bioinf., 2011, 1, (4), pp. 282 –285 [Google Scholar]
- 81. Du W. Sun Y. Ji R. et al.: ‘Tio2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil’, J. Environ. Monit., 2011, 13, pp. 822 –828 [DOI] [PubMed] [Google Scholar]
- 82. Le V.N. Rui Y. Gui X. et al.: ‘Uptake, transport, distribution and bio‐effects of SiO2 nanoparticles in Bt‐Transgenic cotton’, J. Nanobiotechnol., 2014, 12, (50), doi:10.1186/s12951‐014‐0050‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Miller G. Kinnear S.: ‘Nanotechnology the new threat to food’, Clean Food Org., 2007, 4, pp. 1 –7 [Google Scholar]
- 84. Nair R. Varghese S.H. Nair B.G. et al.: ‘Nanoparticulate material delivery to plants’, Plant Sci., 2010, 179, pp. 154 –163 [Google Scholar]
- 85. Gutiérrez F.J. Mussons M.L. Gatón P. et al.: ‘Nanotechnology and food industry’, in Benjamin Valdez (Ed.): ‘Scientific, health and social aspects of the food industry’ ISBN: 978‐953‐307‐916‐5, InTech., (InTech., Croatia, 2011), pp. 95 –128 [Google Scholar]


