Abstract
Development of a green chemistry process for the synthesis of silver nanoparticles (AgNPs) has become a focus of interest. Characteristics of AgNPs were determined using techniques, such as ultraviolet–visible spectroscopy (UV–vis), Fourier transform infrared (FTIR) analysis, scanning electron microscopy (SEM), energy‐dispersive X‐ray spectroscopy and X‐ray diffraction (XRD). The synthesised AgNPs using Thymus kotschyanus had the most growth inhibition against gram‐positive bacteria such as Staphylococcus aureus and Bacillus subtilise, while the growth inhibition of AgNPs at 1000–500 µg/ml occurred against Klebsiella pneumonia and at 1000–250 µg/ml of AgNPs was observed against E. coli. The UV–vis absorption spectra confirmed the formation of the AgNPs with the characteristic peak at 415 nm and SEM micrograph acknowledged spherical particles in a nanosize range. FTIR measured the possible biomolecules that are responsible for stabilisation of AgNPs. XRD analysis exhibited the crystalline nature of AgNPs and showed face‐centred cubic structure. The synthesised AgNPs revealed significant antibacterial activity against gram‐positive bacteria.
Inspec keywords: visible spectra, microorganisms, ultraviolet spectra, biomedical materials, nanofabrication, nanoparticles, X‐ray diffraction, scanning electron microscopy, molecular biophysics, X‐ray chemical analysis, nanomedicine, silver, antibacterial activity, Fourier transform infrared spectra
Other keywords: green chemistry process, ultraviolet–visible spectroscopy, gram‐positive bacteria, silver nanoparticles, Thymus kotschyanus aqueous extract, UV–vis spectroscopy, Fourier transform infrared spectroscopy, FTIR analysis, scanning electron microscopy, energy‐dispersive X‐ray spectroscopy, SEM micrograph, X‐ray diffraction, XRD, Staphylococcus aureus, Bacillus subtilise, Klebsiella pneumonia, E. coli, UV–vis absorption spectra, face‐centred cubic structure, antibacterial activity, antimicrobial activity, wavelength 415.0 nm, Ag
1 Introduction
Nanoparticles (NPs) are the unique form of materials, which are included 1–100 nm dimensions or less. The therapeutic applications of silver NPs (AgNPs) are currently being increased due to their potential of the silver ions in the nanoscale range [1]. In medicine, silver incorporated compounds such as silver sulphadiazine have been widely used as an antimicrobial agent for the microbial elimination of surface tissues or external wounds. Another application of silver is as an antiseptic coating in medical devices such as wound dressing wounds, urinary catheters, and endotracheal breathing tubes. Actually, the antimicrobial potential of inorganic AgNPs is dramatically stronger than those of the corresponding bulk structures [2, 3].
Generally, metallic NPs are synthesised from inorganic metals by several chemical methods including gas condensation, attrition, precipitation, implantation, pyrolysis and hydrothermal treatment [1, 4]. These methods employ many chemical reagents or consume excessive energy to progress the NP synthesis reactions by a multi‐step process. Chemical precursors and toxic solvents in the reaction pots are ultimately considered as hazardous wastes, threatening the living environment and human health [5]. Currently, an alternative method has developed based on the usage of biological reagents, which have more advantages over the chemical synthesis. Despite the chemical synthesis protocols, biologically‐based methods provide eco‐friendly and easily‐handleable condition using low‐cost materials [6, 7, 8]. These methods are known as green synthesis in which a living system such as bacteria, fungi, algae and plant extracts are often responsible for the reduction of metals for NPs formation [9]. Besides, synthesis of various types of metal NPs using plant extracts is found to be more efficient, easily controllable due to the existence of various metabolites like emulsifying, capping and stabilising agents [10].
In traditional medicine, Thymus genus is employed for treating spasms, inflammation, and bloat in the ruminant digestive system [11]. Amongst current known 300–400 species, Thymus kotschyanus (T. kotschyanus) (common thyme) is widely used in Iranian traditional medicine [12]. Thyme is an Iranian flora, naturally distributed in the mountainous semi‐grazing areas and grows as the perennial subshrubs [13]. In the current years, the therapeutic potential of thyme extract was approved by many studies such as the treatment of retinal neovascularisation, immunodeficiency syndrome, infection and cancer [14, 15, 16].
AgNPs have a wide usage in the biological and chemical process, due to increasing absorption capacity to the various ligands such as toxins, enzymes, virus, and chemicals [17]. Considering that AgNPs have exhibited a great antimicrobial activity against the most resistant pathogens, this research focused on the green synthesis of these NPs using T. kotschyanus as reactive agents in the aqueous phase. Furthermore, biosynthesis of AgNPs by T. kotschyanus extract is a first study to develop a cost–benefit method for making AgNPs. Therefore, our study highlights the green synthesis of metal NPs by thyme extract with their antimicrobial bioactivity as a different formulation compared with the traditional application.
2 Experimental section
2.1 Preparation of plant extract
A wild‐grown species of T. kotschyanus were collected from mountainous areas around Khorramabad city, Lorestan province, Iran. The plant leaves were washed with distilled water, air‐dried and ground into fine pieces. To prepare the extract, 10 g of leaf powder was transferred in 100 ml of sterile distilled water and boiled for 45 min in 70°C. The mixture was then filtered by filtrate paper and the extract was kept for NPs synthesis.
2.2 Biosynthesis of AgNPs
For the synthesis of AgNPs, 40 ml of T. kotschyanus extract was mixed with 40 ml of AgNO3 solution and stirred continuously for 30 min. The formation of NPs resulting from AgNO3 reduction was monitored by observation of the colour change in the mixture after the overnight incubation at the room temperature. Lastly, separation of prepared NPs was performed by centrifugation of the reaction mixture at 8000 rpm for 10 min and the NPs were then washed and dried at 60°C.
2.3 Characterisation of NPs
The NPs powder, AgNO3 and crude extract of T. kotschyanus were subjected for analysing possible structural evidence involved in the reducing AgNO3 using spectroscopy instruments such as ultraviolet‐visible (UV–vis), Fourier transform infrared (FTIR), and energy‐dispersive X‐ray (EDX) as well as scanning electron microscopy (SEM). The UV–vis spectrums were drawn using a UV–vis spectrophotometer instrument (Jenway 6100, Dunmow, Essex, UK) in the range of 300–700 nm. FTIR spectroscopy was carried out for predicting responsible groups for NPs synthesis and stabilisation. The FTIR spectra were recorded for the T. kotschyanus extract and AgNPs in the range of 4000–400 cm−1. An X‐ray diffractometer (Philips X'PERT PROMPD) with Cu Kα radiation (1.5409 Å) was used to study the size and crystalline structure of the particles. The dried AgNPs were also analysed by SEM and EDX spectroscopy (Mira3).
2.4 Evaluation of antimicrobial activity
The antibacterial activity of the synthesised AgNPs was studied by disc diffusion method. Some standard bacterial strains including Staphylococcus aureus (PTCC 1112), Bacillus cereus (PTCC 1556), Escherichia coli (PTCC 1330) and Klebsiella pneumonia (PTCC 1053) were applied. The discs were impregnated with about 35 μl of the synthesised AgNPs in various concentrations (100–1000 μg/ml). A loopful of bacterial cells was spread on Mueller‐Hinton agar plates, AgNPs‐impregnated discs were then placed over the surface of plates and incubated at 37°C for 18–24 h [18]. The zone of inhibition around the discs was measured and compared with amikacin standard disc as a positive control.
3 Results and discussion
3.1 Biosynthesis of AgNPs
The biosynthesis of AgNPs was detected through a visual colour change in the solution. At first, the AgNO3 and thyme extract solutions were clear and relatively light red, which followed by AgNPs formation, the solution colour turned to dark brown (Fig. 1). Some chemical compounds in the T. kotschyanus extract such as polyphenols, alkaloids, flavonoids, saponins, and steroids are responsible for reducing various metal ions to the corresponding NPs.
Fig. 1.

Colour changes in the reaction solution
(a) Aqueous extract of thyme leaf, (b) Aqueous solution of 1 mM AgNO3, (c) Reaction mixture (plant extract + AgNO3 solution) after AgNPs formation
3.2 UV–vis spectroscopy
Fig. 2 presents the UV–vis spectral profile of AgNO3, plant extract solution and respective reaction mixture. The difference of spectra curves could be attributed to excitation of surface plasmon vibrations originated from the presence of AgNPs in the reaction mixture [18]. The UV–vis spectrum of colloidal solutions of AgNPs synthesised from T. kotschyanus extract had an absorbance peak at 415 nm. The frequency and width of the surface plasmon absorption depend on the size and the shape of the metal NPs as well as on the dielectric constant of the metal itself and the surrounding medium [19, 20]. Besides, the shift of the absorption wavelength observed in the spectra could be correlated with the colour change of the colloidal AgNPs solution.
Fig. 2.
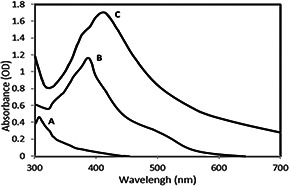
UV–vis spectra of AgNPs
(a) AgNO3 solution, (b) T. kotschyanus extract, (c) AgNPs solution
3.3 SEM and EDX
The AgNPs synthesised by aqueous T. kotschyanus extract was morphologically characterised using SEM. Analysis of the SEM micrograph showed that the most of AgNPs were spherical along with few aggregates in some points (Fig. 3). As shown in Fig. 3, spherical NPs are ranged from nanoscale particle size to large aggregates. There are unknown causes that could affect monodispersity and the stability of NPs in a complex reaction mixture like plant extracts. It is found that the shape of metal NPs can be affected by pH, temperature, electrostatic force and other physiochemical factors. Thus, according to the findings of Markus et al. [21] aggregation occurred due to the interaction of concentrated AgNPs with plant‐derived organic materials like proteins and polysaccharides. Therefore, in the preparation of the AgNPs sample for SEM, NP aggregation and nucleation could occur for AgNPs. EDS analysis of NPs showed that the strongest peak appeared at 3 keV is associated with aggregated AgNPs nanocrystals. On the other hand, EDS revealed Ag element formed no Ag oxide in the crystallisation process.
Fig. 3.
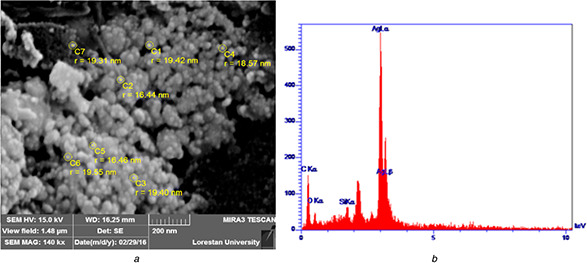
SEM‐EDX profile obtained from
(a) AgNPs synthesised from T. kotschyanus, (b) EDX spectrum recorded from the AgNPs
3.4 FTIR spectroscopy study
The FTIR analysis displayed functional groups of biomolecules that have a possibly critical role in the formation and stabilisation of NP crystals. As shown in Fig. 4 a, which presents the FTIR spectrum of crude extract, a broad signal was observed about 3377 cm−1 can be attributed to O–H or C–N stretching vibration modes in primary amines of aromatic compounds [22]. The others peaks, 2941.54, 1452.09 and 669.32 cm−1 observed can be associated with C–H bond in alcohols and phenols, carbonyl present in the plant extract.
Fig. 4.
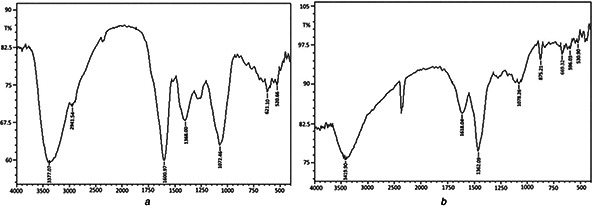
FTIR spectra of vacuum dried powder of
(a) T. kotschyanus extract, (b) AgNPs
Fig. 4 b presents the FTIR spectrum of the AgNPs that exhibits chemical shifts in a number of adsorption peaks compared with the plant extract. The results suggest that plant‐derived bioactive compounds could provide a satisfying condition for the synthesising of the AgNPs, capping of their crystals and maintaining without the using chemical catalysts or other stabilisers. Correspondence of the role of biological compounds, proteins, and exopolysaccharides, polyphenols and alkaloid is well known that can bind to AgNPs through numerous functional groups and therefore caused the stabilisation of AgNPs [23].
3.5 XRD analysis
The crystalline properties of AgNPs were studied by the XRD analysis (Fig. 5). The XRD pattern of AgNPs synthesised showed four distinct diffraction peaks at 2θ value ranging from 10 to 80 of 38.08°, 44.27°, 64.42° and 77.36°, corresponding to the crystalline planes (111), (200), (220) and (311) of face‐centred cubic (fcc) silver. The clear and narrow peaks of the XRD profile evidenced that the AgNPs were crystalline form in nature. The average crystalline size of the synthesised AgNPs was determined using Debye–Scherrer equation, which the estimated mean size of the particle was 22 nm. The XRD pattern of AgNPs synthesised in this study was in agreement with the other results as reported by Moteriya and Chanda [24] and Saravanakumar et al. [8].
Fig. 5.
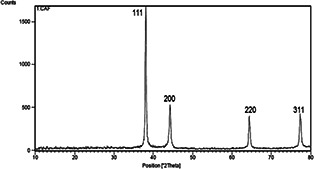
XRD pattern of the AgNPs
3.6 Antimicrobial activity study
Antimicrobial experiment for studying inhibitory effects of AgNPs was performed against four bacterial strains as the candidates of current pathogens. Inhibition zone obtained from the disc diffusion method revealed that AgNPs synthesised by T. kotschyanus had a favourable antimicrobial impact in the laboratory condition over the agar plates (Fig. 6). The results indicated the best efficacy of AgNPs against the growth of S. aureus and B. cereus in 1000 and 500 μg/ml, respectively. However, the green synthesised AgNPs in our study had greater growth inhibition against gram‐positive bacteria, while a lower activity was observed against K. pneumonia and E. coli in the high concentrations of AgNPs. The diameter of the inhibition zone for all tested concentrations of AgNPs achieved for bacterial strains is presented in Table 1.
Fig. 6.
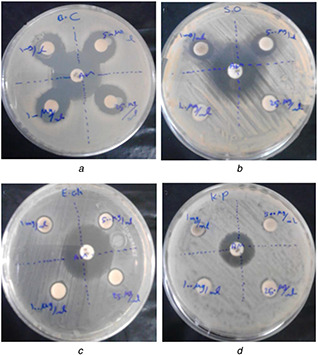
Antibacterial effect of the AgNPs against bacteria
(a) B. cereus S., (b) S. aureus, (c) E. coli, (d) K. pneumonia
Table 1.
Bacterial growth inhibition biosynthesised AgNPs based on disc diffusion method
| AgNPs, µg/ml | Zone of inhibition, mm | |||
|---|---|---|---|---|
| S. aureus | B. cereus | K. pneumonia | E. coli | |
| 1000 | 10.33 ± 1.2 | 20.33 ± 3.25 | 5 ± 1.63 | 7.33 ± 1.38 |
| 500 | 9.67 ± 1.65 | 20 ± 2.58 | 2.67 ± 0.41 | 7 ± 1.06 |
| 250 | 8.33 ± 1.47 | 19 ± 2.14 | — | 2.33 ± 0.06 |
| 100 | 8 ± 1.07 | 18.67 ± 2.28 | — | — |
Our findings were in agreement with the other similar reports that had previously studied on antimicrobial properties of AgNPs synthesised by the different plant extracts. Accordingly, Arokiyaraj et al. (2016) reported that AgNPs synthesized by aqueous extract of Rheum palmatum had a strongest antibacterial activity against S. aureus and P. aeruginosa [25]. On the other hand, Ciobanu et al. [26] showed strongly antimicrobial and antibiofilm potential of silver‐doped hydroxyapatite NPs (Ag: Hap‐NPs) against both gram‐positive and gram‐negative bacteria. As such, Bonilla et al. [27] showed a potent antifungal activity of AgNPs against fluconazole‐resistant Candida species. As a result of our study, AgNPs synthesised by T. kotschyanus considerably affected the growth of gram‐positive bacteria, while no study was performed on its antifungal or antibiofilm activity.
4 Conclusion
Plant‐based synthesis of AgNPs has increasingly been developed for the different applications. The formation of metallic NPs practically carried out under the optimised condition by the various catalytic mechanisms. Plant‐based bioactive compounds as the suitable catalysts are considered the safest and feasible for the promotion of NP synthesis. The number of the organic compounds is found thus far in the plant extracts which can act as reducing agents and also stabilise the nanostructures in the reaction condition. Hence, T. kotschyanus is well known as one of the most important herbs with different uses in traditional medicine, our success for biosynthesis AgNPs by this plant extract can be valuable from the view of both safety and economic development.
5 Acknowledgments
The authors acknowledge the Centre of the Laboratory in Lorestan University for providing support in carrying out SEM‐EDX analysis. This work was supported by the Deputy of Research, Medical Science of Lorestan University, Khorramabad, Iran.
6 References
- 1. Heydari R.: ‘Biological applications of biosynthesized silver nanoparticles through the utilization of plant extracts’, Herb. Med. J., 2017, 2, (2), pp. 87 –95 [Google Scholar]
- 2. Ragaa A.F.H. Mahmoud A.M. Kamel F.E.: ‘Antibacterial activity of silver nanoparticles using ulva fasciata extracts as reducing agent and sodium dodecyl sulfate as stabilizer’, Int. J. Pharmacol., 2018, 14, (3), pp. 359 –368 [Google Scholar]
- 3. Gomaa E.Z.: ‘Silver nanoparticles as an antimicrobial agent: a case study on Staphylococcus aureus and Escherichia coli as models for gram‐positive and gram‐negative bacteria’, J. Gen. Appl. Microbiol., 2017, 63, pp. 36 –43 [DOI] [PubMed] [Google Scholar]
- 4. Rashidipour M. Heydari R.: ‘Biosynthesis of silver nanoparticles using extract of olive leaf: synthesis and in vitro cytotoxic effect on MCF‐7 cells’, J. Nanostruct. Chem., 2014, 4, (3), p. 112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heydari R. Rashidipour M.: ‘Green synthesis of silver nanoparticles using extract of oak fruit hull (jaft): synthesis and in vitro cytotoxic effect on MCF‐7 cells’, Int. J. Breast Cancer., 2015, 2015, p. 846743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jagathesan G. Rajiv P.: ‘Biosynthesis and characterization of iron oxide nanoparticles using Eichhornia crassipes leaf extract and assessing their antibacterial activity’, Biocatal. Agric. Biotechnol., 2018, 13, pp. 90 –94 [Google Scholar]
- 7. Samant S. Naik M. Parulekar K. et al.: ‘Selenium reducing Citrobacter fruendii strain KP6 from Mandovi estuary and its potential application in selenium nanoparticle synthesis’, Proc. Indian Natl. Sci. Acad, India Sect. B Biol. Sci., 2016, 88, (2), pp. 1 –8 [Google Scholar]
- 8. Saravanakumar A. Peng M.M. Ganesh M. et al.: ‘Low‐cost and eco‐friendly green synthesis of silver nanoparticles using Prunus japonica (Rosaceae) leaf extract and their antibacterial, antioxidant properties’, Artif. Cells Nanomed. Biotechnol., 2016, 45, (6), pp. 1 –7 [DOI] [PubMed] [Google Scholar]
- 9. Faramarzi M.A. Sadighi A.: ‘Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures’, Adv. Colloid Interface Sci., 2013, 189, pp. 1 –20 [DOI] [PubMed] [Google Scholar]
- 10. Ahmed S. Ahmad M. Swami B.L. et al.: ‘A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise’, J. Adv. Res., 2016, 7, (1), pp. 17 –28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Afonso A.F. Pereira O.R. Neto R.T. et al.: ‘Health‐promoting effects of Thymus herba‐barona, Thymus pseudolanuginosus, and Thymus caespititius decoctions’, Int. J. Mol. Sci., 2017, 18, (9), p. 1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stahl‐Biskup E. Sáez F.: ‘Thyme: the genus Thymus’ (CRC Press, London, 2003, 1st edn.) [Google Scholar]
- 13. Nickavar B. Mojab F. Dolat‐Abadi R.: ‘Analysis of the essential oils of two Thymus species from Iran’, Food Chem., 2005, 90, (4), pp. 609 –611 [Google Scholar]
- 14. Ocaña A. Reglero G.: ‘Effects of thyme extract oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on cytokine production and gene expression of ox LDL‐stimulated THP‐1‐macrophages’, J. Obes., 2012, 2012, p. 104706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramachandran L. Nair C.K.K.: ‘Therapeutic potentials of silver nanoparticle complex of α‐lipoic acid’, Nanomat. Nanotechnol., 2011, 1, p. 14 [Google Scholar]
- 16. Tohidi B. Rahimmalek M. Arzani A.: ‘Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran’, Food. Chem., 2017, 220, pp. 153 –161 [DOI] [PubMed] [Google Scholar]
- 17. Chen Z. Li Z. Chen G. et al.: ‘In situ formation of AgNPs on S. cerevisiae surface as bionanocomposites for bacteria killing and heavy metal removal’, Int. J. Environ. Sci. Technol., 2017, 14, (8), pp. 1635 –1642 [Google Scholar]
- 18. Thirumurugan A. Tomy N. Ganesh R.J. et al.: ‘Biological reduction of silver nanoparticles using plant leaf extracts and its effect on increased antimicrobial activity against clinically isolated organism’, Der Pharm. Chem., 2010, 2, (6), pp. 279 –284 [Google Scholar]
- 19. Ahmad N. Sharma S. Singh V. et al.: ‘Biosynthesis of silver nanoparticles from Desmodium triflorum: a novel approach towards weed utilization’, Biotechnol. Res. Int., 2011, 2011, p. 454090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mukherjee P. Ahmad A. Mandal D. et al.: ‘Bioreduction of AuCl4− ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed’, Angew. Chem. Int. Ed., 2001, 40, (19), pp. 3585 –3588 [DOI] [PubMed] [Google Scholar]
- 21. Markus J. Wang D. Kim Y.‐J. et al.: ‘Biosynthesis, characterization, and bioactivities evaluation of silver and gold nanoparticles mediated by the roots of Chinese herbal Angelica pubescens maxim’, Nanoscale. Res. Lett., 2017, 12, p. 46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marzban A. Ebrahimipour G. Danesh A.: ‘Bioactivity of a novel glycolipid produced by a halophilic Buttiauxella sp. and improving submerged fermentation using a response surface method’, Molecules., 2016, 21, (2), p. 1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D. Markus J. Wang C. et al.: ‘Green synthesis of gold and silver nanoparticles using aqueous extract of Cibotium barometz root’, Artif. Cells Nanomed. Biotechnol., 2017, 45, (8), pp. 1548 –1555 [DOI] [PubMed] [Google Scholar]
- 24. Moteriya P. Chanda S.: ‘Synthesis and characterization of silver nanoparticles using Caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities’, Artif. Cells Nanomed. Biotechnol., 2017, 45, (8), pp. 1556 –1567 [DOI] [PubMed] [Google Scholar]
- 25. Arokiyaraj S. Vincent S. Saravanan M. et al.: ‘Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa’, Artif. Cells Nanomed. Biotechnol., 2016, 45, (2), pp. 372 –379 [DOI] [PubMed] [Google Scholar]
- 26. Ciobanu C.S. Iconaru S.L. Le Coustumer P. et al.: ‘Vibrational investigations of silver‐doped hydroxyapatite with antibacterial properties’, J. Spectrosc., 2013, 2013, p. 471061 [Google Scholar]
- 27. Bonilla J.J.A. Guerrero D.J.P. Suárez C.I.S. et al.: ‘In vitro antifungal activity of silver nanoparticles against fluconazole‐resistant Candida species’, World J. Microbiol. Biotechnol., 2015, 31, (11), pp. 1801 –1809 [DOI] [PubMed] [Google Scholar]


