Abstract
Current research trends on iron nanoparticles (FeNPs) are extensively focused because of their unique magnetic and electrical properties mostly applicable in essential medical devices. However, their fungicidal property against plant pathogens is very less known until date. Present study demonstrates a green technique for blending of FeNPs by utilising aqueous extract of neem leaf (Azadirachta indica A. Juss.) as reducing agent. Various characterisation techniques such as ultraviolet (UV)–visible spectroscopy, Fourier transform infrared spectroscopy transmission electron microscopy, scanning electron microscopy and X‐ray diffraction were performed for FeNPs. The authors’ results demonstrate the more cluster formation of FeNPs with size distribution of 20–80 nm. The bio‐fabricated FeNPs showed enhanced biocidal activity against economically important phytopathogens of apple such as Alternaria mali, Botryosphaeria dothidea and Diplodia seriata. From the obtained results, it can be suggested that further delve into green synthesis of FeNPs can address future biotechnology concerns to limit the synthesis of FeNPs by conventional methods. Furthermore, the field study on pathogenic fungi can be an effective step to verify their agricultural applications.
Inspec keywords: biotechnology, iron, nanoparticles, microorganisms, electric properties, agrochemicals, ultraviolet spectra, visible spectra, Fourier transform infrared spectra, transmission electron microscopy, scanning electron microscopy, X‐ray diffraction, spectrochemical analysis, nanofabrication
Other keywords: biofabrication, iron nanoparticles, fungicidal property, apple orchard phytopathogens, magnetic properties, electrical properties, medical devices, plant pathogens, green technique, neem leaf, Azadirachta indica A Juss, ultraviolet‐visible spectroscopy, Fourier transform infrared spectroscopy, transmission electron microscopy, scanning electron microscopy, X‐ray diffraction, cluster formation, size distribution, biocidal activity, Alternaria mali, Botryosphaeria dothidea, Diplodia seriata, green synthesis, biotechnology, pathogenic fungi, size 20 nm to 80 nm
1 Introduction
Iron nanoparticles (FeNPs) are applicable in magnetic storage devices, ferro‐fluids, magnetic refrigeration systems [1, 2, 3] and some essential medical techniques such as bio‐separation, magnetic resonance imaging, enzyme linked immunosorbent assay, drug delivery and hyperthermia [4]. These essential applications of FeNPs are essentially associated with their small size, high magnetism, low toxicity and microwave absorption properties [5, 6]. Numerous chemical methods have been employed for FeNP synthesis. Chemical co‐precipitation technique of iron salts [7], electrospray syntheses [8], microemulsions [9], sonochemical reactions [10], hydrolysis and thermolysis of precursors [11], hydrothermal reactions [12], sol–gel synthesis [13] are some of the common methods used for FeNP synthesis. Owing to need for development of an efficient synthesis of bioactive compounds using nanoparticles in an ecologically and economically favourable way, various researchers have put steps for green synthesis of Fe3 O4 nanoparticles [14, 15, 16, 17, 18]. Moreover, synthesis of iron oxide nanoparticles using phytochemicals is a simple, cost effective, less toxic and ecofriendly method which has been used for generation of other heavy metal nanoparticles as well.
The metal oxide powders of ZnO, MgO and CaO have been quantitatively evaluated for their antimicrobial activities in culture media against Staphylococcus aureus, Escherichia coli or fungi [19, 20]. The active oxygen species generated by these metal oxide particles could be considered as the main components behind the mechanism of their antibacterial activity. In this view, nanoparticles of corresponding metal oxides can be considered as potential antimicrobial agents [21]. However, only a little is known about the effects of nanoparticles on fungal pathogens and least work has been conducted to access the antimicrobial property of FeNPs against plant pathogenic fungi as per our literature survey. Present paper aims to demonstrate the toxicity of biologically synthesised FeNPs in comparison with their bulk materials against plant pathogens of apple orchards under laboratory conditions. The phytopathogen species chosen here count for great ecological importance and the risk assessment associated with them needs attention to practical applications. The study was conducted to synthesise FeNPs using leaves of A. indica, a medicinally important plant. We propose a non‐toxic, facile, rapid and green synthesis route to prepare magnetite nanoparticles using water solvent. The work was successfully conducted by the support of previous literatures. Microscopic and non‐microscopic characterisation was done to elucidate the synthesis of nanoparticles. To the best of our knowledge, this is the first comparative toxicity evaluation study of biologically synthesised FeNPs against fungal pathogens of apple orchards.
2 Methods
2.1 Plant extract preparation
The disease free and fresh leaves of Azadirachta indica were collected and shade dried after vigorously washing with double distilled water (DDW). Further, sterilisation was employed with 0.1% mercuric chloride for 20 s followed by washing with DDW. The plant material was shade dried and then crushed into powder form. About 15 g of this plant material was now added to 200 ml CO2 free DDW and kept in shaker at 80°C and 150 rpm for 6 h. After shakeup, the extract was separated by regular centrifugation at 10,000 rpm followed by filtration using Whatman No. 1 filter paper. The final volume of 100 ml of supernatant was collected as pure extract and stored at 4°C for further use.
2.2 Synthesis of iron oxide nanoparticles using plant extract
FeNPs were synthesised by using procedures followed previously [22, 23]. About 0.1M FeCl2. 6H2 O was treated with 10% plant extract in the ratio of 1:2. The mixture was then shaked well at 100°C until greenish colour turned completely to dark black solution. The solution was then allowed to stand (in petriplates) inside oven at 60°C for 72 h. Finally, the blackish dry mass was collected from petriplates and was given for characterisation study using various techniques.
3 Characterisation of nanoparticles
3.1 Detection of FeNPs by a ultraviolet–visible spectrophotometer
Primary detection of absorption spectrum of FeNPs employed by using UV–vis spectrophotometer (UV‐260 Shimadzu) with 1 cm quartz cuvettes observable in the range of 200–700 nm. The measurements were conducted with highly diluted solutions.
4 Characterisation of FeNPs by different methods
Infrared spectrophotometer (Bruker VERTEX‐70) was used to determine the different functional groups present in the extracts and FeNPs by conventional method using a very thin pellet with KBr within the range of 400–4000 cm−1. Scanning electron microscopy (SEM) studies were performed on scanning electron microscope (JEOL JSM 5600) to detect the morphology and shape of FeNPs. The 200 kV ultra high‐resolution transmission electron microscope (Tecnai G2 20) was used for transmission electron microscopy (TEM) studies to visualise the size and shape of FeNPs. Nanoparticles (well dispersed) were dropped onto the carbon‐coated 200 mesh copper grid, and dried at ambient temperature, before loading into the microscope. X‐ray diffraction (XRD) measurements were conducted with an XRD (Bruker D8) operated at a voltage of 40 kV and 30 mA with Cu K∝ radiation in 2θ configuration. The crystallite domain size was calculated from the width of the XRD peaks by assuming that they were free from non‐uniform strains.
5 Antifungal activity of FeNPs
5.1 Fungal isolation and identification
Three economically important phytopathogenic fungi of apple orchards: namely, Alternaria mali, Botryosphaeria dothidea and Diplodia seriata were selected for study (Table 1). These fungi cause heavy loss in vegetables, fruits and other crop varieties especially apple orchards. Disease surveys were carried out in apple plantations (Malus domestica) in March 2013–September 2013 in the North Kashmir and southern parts of Srinagar city located in the western Himalayas. Segments of symptomatic parts such as leaves, stem, bark and branches were collected and incubated in moist chambers for 2–3 days to induce development of fruiting structures in case of stem and bark cankers (Table 1). The infected parts along with healthy portions were transferred to potato dextrose agar (PDA) and incubated at 25°C. The three isolates of each pathogen were then inoculated onto the surface of water agar (2% agar) in Petri dishes for 2–3 weeks at 25°C to induce sporulation. Conidial masses from fruiting structures were spread on the surface of PDA in sterile drops of water. Single germinating conidia were isolated after 12–16 h and then transferred and maintained at 22°C on clean PDA plates to achieve single spore isolation. Furthermore, the morphological identification was carried out by experts at Division of Plant Pathology, S.K. University of Science and Technology‐K. Isolates were characterised based on culture morphology on PDA media. Conidia from each of these cultures were mounted on lactophenol and examined using a light microscope. These fungi were grown on PDA media for further experimentation.
Table 1.
List of identified fungi and their pathogen infection on apple trees (M. domestica Borkh.)
| Scientific name | Common name | Main symptoms |
|---|---|---|
| D. seriata | branch canker | black smoky round or oval areas of dead, sunken bark |
| B. dothidea | stem canker | round or oval areas of dead, sunken bark on stems |
| A. mali | Alternaria leaf blotch | irregular necrotic patches on leaves |
6 Antifungal assay of FeNPs nanoparticles
At the centre of petriplates loaded with PDA media, 5 days old test fungi were transferred and incubated (25 ± 2)°C by using inoculum disc of 4 mm in diameter on opposite sides with upside below [24]. The antifungal assay of FeNPs carried out by using food poisoning method as described by some authors [25]. Test samples including neem extract, FeNPs, Fe+2 ion solution and sample prepared by equal combination of neem extract and FeNP were considered for comparative studies. Each fraction was brought at an initial concentration of 1000 ppm and different working concentrations of (i.e. 10, 25, 50 and 100 ppm) by diluting the original stock solution with DDW. About 5 ml of each sample was poured into growth media prior to plating in a Petri dish (90 × 15 mm2). Untreated petriplates were used as negative control throughout the observations. The media was then incubated at room temperature to check for any contamination. After 48 h of incubation, agar plugs of uniform size (diameter, 8 mm) harvested from the edge of 7 day old culture were inoculated simultaneously at the centre of each Petri dish containing samples, followed by incubation at 28 ± 2°C for more than 5 days. Growth inhibition was calculated when growth of mycelia in the control plate reached the edge of the Petri dish.
7 Data analysis
Radial growth of fungal mycelium was recorded for four replicates as mean±SD against control. The following formula was used for calculation of the inhibition rate (percentage):
where R is the radial growth of fungal mycelia on the control plate and r is the radial growth of fungal mycelia on the plate treated with sample isolates.
8 Results and discussion
8.1 Characterisation of FeNPs
The surface plasmon resonance (SPR) absorption transition is the most characteristic part of nanoparticles. After 24 h of incubation in the dark room condition, yellow colour reaction mixture was turned into dark brown due to reduction of Fe+2 ions to Fe0 (Figs. 1 a –c). It may be due to the excitation of SPR for the synthesised nanoparticles [26]. Fig. 2 shows strong peak at 272 nm exhibited by iron chloride and neem extract reaction mixture, which confirms the formation of FeNPs. Our measurements are in agreement with the previous reports, where the FeNPs were prepared using green tea extract [22] and Passiflora tripartite [27] mediated synthesis of FeNPs.
Fig. 1.

Synthesis of FeNPs using A. indica leaf extract where
(a) 10% Neem extract solution, (b) 0.1 M FeCl2 6H2 O solution, (c) Formation of dark brown FeNPs due to reduction of Fe+2 ions to Fe0
Fig. 2.
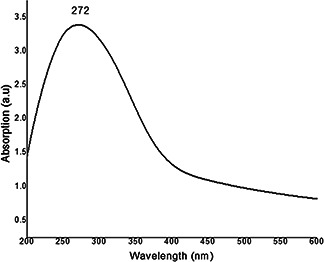
UV–vis spectroscopy of phyto‐synthesised FeNPs using A. indica leaf extract
The Fourier transform IR spectroscopy (FTIR) report of neem extract and FeNPs is given in Fig. 3. A broad band around 3000 cm−1 centred at 3325 cm−1 is because of N–H stretching and bending vibration of amine group NH2 and O–H the overlapping of the stretching vibration of aqueous neem leaf extract molecules. Peaks at 2916 and 2850 cm−1 indicate methyl C–H stretch, and the peak at 1728 cm−1 indicate –CHO group of neem extract. Sharp peak at 1725 cm−1 indicates the presence of ester bonds while as the peaks at 1522 and 1453 cm−1 can be assigned to stretching vibrations of aromatic nitro compounds and carbonate ions, respectively, present in neem extracts. The band around 600 cm−1 indicated the Fe–O stretching of FeNPs, as reported earlier [28], which indicate the formation of iron oxide nanoparticles. The overall FTIR image shows that neem extract has a best reducing capability for FeNPs as confirmed by other studies [29].
Fig. 3.
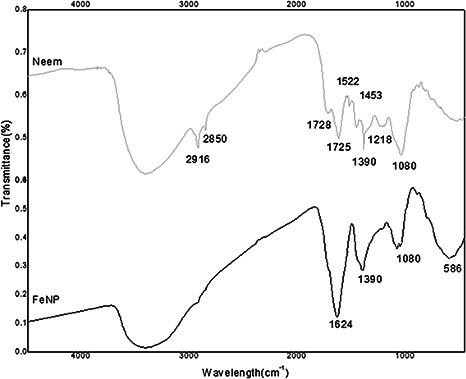
IR spectra of phyto‐synthesised FeNPs and A. indica leaf extract
The SEM micrograph of synthesised FeNPs is given in Fig. 4. Owing to the presence of plant debris, the FeNPs are shown in clustered form embedded in the plant materials. Figs. 5 (a –d) show the TEM images of FeNPs with different configurations. The average size of nanoparticles was detected to be in the range of 20–80 nm as shown in Figs. 5 a, b and d. The selected area electron diffraction (SAED) also demonstrates the less crystalline nature of FeNPs as shown in Fig. 5 c. Bio‐coatings were also visible along with the FeNPs (Fig. 5 b) which corroborate the ability of neem extract to act as coating material for FeNPs. The XRD report (Fig. 6) shows the dense descending slope with no sharp peaks in FeNPs. The FeNPs did not show sharp diffraction peaks related to extended crystalline structure. Instead, a broad band is seen to appear which is typical for amorphous materials and for ultra‐small crystalline materials where diffraction peaks cannot be well‐resolved [30]. Such observations have been seen in the previous reports with plant extract oriented synthesis of FeNPs [22, 27, 30, 31, 32]. The XRD report is in support to our microscopy results as discussed earlier.
Fig. 4.
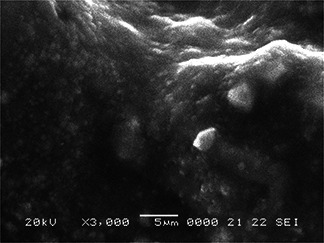
SEM images of FeNPs fabricated using A. indica leaf extract
Fig. 5.
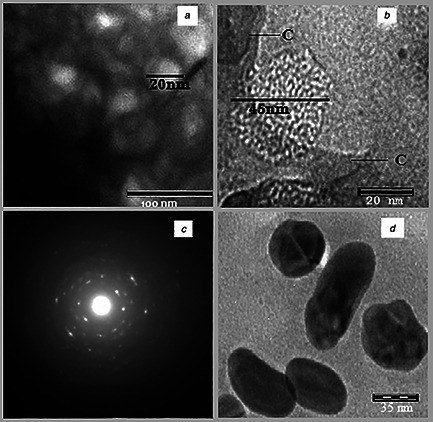
TEM images of phyto‐synthesised FeNPs using A. indica leaf extract at:
a 100 nm, (b) 20 nm, (c) SAED image of FeNPs, (d) 35 nm
Fig. 6.
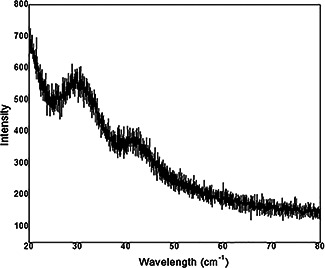
XRD pattern of FeNPs synthesised using A. indica leaf extract
9 Antifungal study of FeNPs against phytopathogenic fungi
The percentage of growth inhibition by FeNPs and other fractions is given in Table 2. A. indica leaf extract was effective against all the test fungi. The inhibition rate was dose dependent. However, D. seriata mycelium was 10% more inhibited than other fungi by A. indica. Present study confirms the previous reports of fungicidal property of A. indica extract. A. indica extract was seen to show antimicrobial activity against phytopathogens such as Alternaria alternaria [29], anthracnose of pepper [30], early blight of tomato [31], powdery mildew of pea [32], sheath blight of rice [33], rice tungro virus [34], bacterial blight of rice [35], Fusarium oxysporum, A. niger and A. flavus [36] and other protozoan and bacteria [37]. Azadirachtin, a terpenoid present in A. indica is a liable antimicrobial component in it [38].
Table 2.
Inhibitory rate (%) of FeNPs synthesised using Azadirachta indica leaf extract against various plants pathogenic fungi at different concentrations in vitro
| Fungi | Inhibition rates (%)A | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ai a | Feb | FeNP Ai | FeNP Ai + Ai | |||||||||||||
| 10 ppm | 25 ppm | 50 ppm | 100 ppm | 10 ppm | 25 ppm | 50 ppm | 100 ppm | 10 ppm | 25 ppm | 50 ppm | 100 ppm | 10 ppm | 25 ppm | 50 ppm | 100 ppm | |
| B. dothidea | 27.1 ± 0.5 | 38.1 ± 2.1 | 52.4 ± 1.3 | 67 ± 0.2 | 15 ± 1.0 | 32 ± 2.1 | 40 ± 1.2 | 53 ± 2.2 | 30 ± 1.2 | 45 ± 1.3 | 63 ± 2.1 | 83 ± 1.4 | 29.1 ± 1.2 | 44.2 ± 3.1 | 68.1 ± 0.6 | 82.5 ± 1.2 |
| D. seriata | 21.1 ± 2.1 | 40.2 ± 0.3 | 63.8 ± 2.1 | 75 ± 0.5 | 11 ± 2.1 | 18 ± 2.1 | 29 ± 2.1 | 44 ± 1.6 | 28 ± 2.1 | 41 ± 3.5 | 58 ± 3.1 | 79 ± 1.3 | 35.1 ± 0.4 | 50.1 ± 1.5 | 68.2 ± 3.1 | 80.3 ± 1.5 |
| A. mali | 33.1 ± 1.0 | 48.2 ± 0.5 | 55.2 ± 0.3 | 68.7 ± 3.1 | 1.3 ± 2.1 | 10 ± 2 | 16 ± 2.0 | 30 ± 0.5 | 27 ± 2.0 | 37 ± 1.0 | 53 ± 2.0 | 78.5 ± 1.0 | 25.3 ± 0.9 | 40.1 ± 0.2 | 59.2 ± 2.5 | 79.2 ± 1.3 |
A Inhibition rate = .
Colony diameters were measured at 7 days post‐inoculation: inhibition rates were determined based on four replicates of each experiment represented as means ± SD: inhibition rate of control for each fungus = 0%.
a Ai = A. indica leaf extract solution.
b Fe = Fe2+ ion solution.
FeNP Ai : solution of FeNP synthesised by using A. indica leaf extract.
The mycelial growth of all test fungi was also inhibited by bulk Fe that was dose dependent. A. mali showed more resistance toward Fe followed by D. seriata. On the other hand, FeNPs had a considerable inhibitory effect against all test fungi. At 100 ppm, FeNPs showed above 80% inhibition rate against all the test fungi with highest inhibition rate against B. dothidea (84%) which was followed by D. seriata and A. mali (80%) as shown in Table 2. There are reports of antimicrobial activity of different metal nanoparticles particularly copper and silver nanoparticles. Cioffi et al. reported the antifungal activity of polymer‐based copper nanocomposite against plant pathogenic fungi [39]. Park et al. studied the efficacy of nano‐sized silica–silver (silica–silver nanoparticles) in the control of plant pathogenic fungi: namely, Botrytis cinerea, Rhizoctonia solani, Colletotrichum gloeosporioides, Magnaporthe grisea and Pythium ultimum [40]. Sangeetha et al. also determined the antimicrobial efficacy of bulk Zn, green and chemically synthesised ZnO nanoparticle against various bacterial and fungal pathogens [41]. They concluded that the green ZnO nanoparticles show more enhanced biocidal activity against various pathogens when compared with bulk Zn. However, present study is the first indication of antifungal property of FeNPs against pathogenic test fungi of apple orchards. To manipulate the synergetic effect of FeNPs and their pioneer neem extract on mycelial growth of pathogenic fungi, a treatment of combination sample created by mixing 50% each of the plant extract and FeNP correspondingly for all the concentrations was evaluated. The results (Table 2) demonstrate considerable effect of this combination against all test fungi. These treatments were rather more efficient than other fractions. Thus, the combination of neem extract and FeNPs added a value to their corresponding inhibition rates. On the basis of our observations, it can be concluded that the FeNPs synthesised biologically are the effective antimicrobial agents against pathogenic microorganisms than iron bulk material and this activity can be enhanced when combined with plant extract inducing the antimicrobial activity.
10 Conclusion
This paper presents a green, facile and one‐step preparation of economically important FeNPs. Neem (A. indica) leaf extract was utilised as bio‐agent for FeNP formation. Aqueous extract of neem leaf broth successfully produced nano‐sized FeNPs of varied shape and morphology as depicted and clarified from our microscopic and non‐microscopic characterisation techniques. The phytochemicals present in neem extract are responsible for reduction of FeNPs as predicted from our FTIR study. The study further confirms their antifungal activity against highly concerned phytopathogenic fungi of apple orchards. The tests carried out with combination of plant extract and nanoparticles determined a many fold enhancement in fungicidal activity. Nano‐sized particles penetrate the plasma membrane easily to cause cell death. To our best knowledge, this is the first comparative evaluation study of biologically synthesised FeNPs against fungal pathogens of apple orchards. However, the work needs some further approval by applying FeNPs in field studies. Finally, we affirm that the present study can open up an idea for carrying out antifungal study of FeNPs in order to make them applicable in agriculture science. In addition, the study necessitates about the research concerns over morphology controlling factors of FeNP synthesis.
11 References
- 1. Fertman V.E.: ‘Magnetic fluids guidebook: Properties and applications’ (Hemisphere Publishing Co., New York, 1990) [Google Scholar]
- 2. Berkovsky B. Medvedev V. Krakov M.: ‘Magnetic fluids: engineering applications’ (Oxford University Press, Oxford, 1993) [Google Scholar]
- 3. Ziolo E. Giannelis B. Weinstein P. et al.: ‘Matrix‐mediated synthesis of nanocrystalline γ ‐Fe2 O3: a new optically transparent magnetic material’, Science, 1992, 257, pp. 219 –223 [DOI] [PubMed] [Google Scholar]
- 4. Sophie L. Delphine F. Marc P. et al.: ‘Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications’, Chem. Rev., 2008, 108, pp. 2064 –2110 [DOI] [PubMed] [Google Scholar]
- 5. Chang R. Yu J. Ma X. et al.: ‘Polysaccharides as stabilizers for the synthesis of magnetic nanoparticles’, Carbohydr. Polym., 2011, 83, (2), pp. 640 –644 [Google Scholar]
- 6. Jia K. Zhao R. Zhong J. et al.: ‘Preparation and microwave absorption properties of loose nanoscale Fe3 O4 spheres’, J. Magn. Magn. Mater., 2010, 322, pp. 2167 –2171 [Google Scholar]
- 7. Martinez I. Espinosa M. Perez R. et al.: ‘Synthesis of magnetite (Fe3 O4) nanoparticles without surfactants at room temperature’, J. Mater. Lett., 2007, 61, pp. 4447 –4451 [Google Scholar]
- 8. Basak S. Chen D. Biswas P.: ‘Electrospray of ionic precursor solutions to synthesize iron oxide nanoparticles: modified scaling law’, Chem. Eng. Sci., 2007, 64, (4), pp. 1263 –1268 [Google Scholar]
- 9. Chin A. Yaacob I.: ‘Synthesis and characterization of magnetic iron oxide nanoparticles via w/o microemulsion and Massart's procedure’, J. Mater. Process. Technol., 2007, 191, pp. 235 –237 [Google Scholar]
- 10. Kim E. Lee H. Kwak B. et al.: ‘Synthesis of ferro fluid with magnetic nanoparticles by sonochemical method for MRI contrast agent’, J. Magn. Magn. Mater., 2005, 289, pp. 328 –330 [Google Scholar]
- 11. Kimata M. Nakagawa D. Hasegawa M.: ‘Preparation of monodisperse magnetic particles by hydrolysis of iron alkoxide’, Powder Technol., 2003, 132, pp. 112 –118 [Google Scholar]
- 12. Wan J. Chen X. Wang Z. et al.: ‘A soft‐template‐assisted hydrothermal approach to single‐crystal Fe3 O4 nanorods’, J. Cryst. Growth, 2005, 276, pp. 571 –576 [Google Scholar]
- 13. Albornoz C. Jacobo S.: ‘Preparation of a biocompatible magnetic film from an aqueous ferrofluid’, Magn. Magn. Mater., 2006, 305, pp. 12 –10 [Google Scholar]
- 14. Cai Y. Shen Y. Xie A. et al.: ‘Green synthesis of soya bean sprouts – mediated superparamagnetic Fe3 O4 nanoparticles’, J. Magn. Magn. Mater., 2010, 322, pp. 2938 –2943 [Google Scholar]
- 15. Lu W. Shen Y. Xie A. et al.: ‘Green synthesis and characterization of superparamagnetic Fe3 O4 nanoparticles’, J. Magn. Magn. Mater., 2010, 322, pp. 1828 –1833 [Google Scholar]
- 16. Chrysochoou M. Johnston C. Dahal G.: ‘A comparative evaluation of hexavalent chromium treatment in contaminated soil by calcium polysulfide and green – tea nanoscale zerovalent iron’, J. Hazard. Mater., 2012, 201, (202), pp. 33 –42 [DOI] [PubMed] [Google Scholar]
- 17. Venkateswarlu S. Rao Y. Balaji T. et al.: ‘Biogenic synthesis of Fe3 O4 magnetic nanoparticles using plantain peel extract’, Mater. Lett., 2013, 100, pp. 241 –244 [Google Scholar]
- 18. Hoag G. Collings J. Holcomb J. et al.: ‘Degradation of bromothymol blue by ‘greener’ nanoscale zero‐valent iron synthesized using tea polyphenols’, J. Mater. Chem., 2009, 19, pp. 8671 –8677 [Google Scholar]
- 19. Sawai J.: ‘Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay’, J. Microbiol. Methods, 2003, 54, pp. 177 –182 [DOI] [PubMed] [Google Scholar]
- 20. Sawai J. Yoshikawa T.: ‘Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay’, J. Appl. Microbiol., 2004, 96, pp. 803 –809 [DOI] [PubMed] [Google Scholar]
- 21. Ales P.C. Milan K. Renata V. et al.: ‘Antifungal activity of silver nanoparticles against Candida spp.’, Biomaterials, 2009, 30, pp. 6333 –6340 [DOI] [PubMed] [Google Scholar]
- 22. Xiulan W. Lanlan H. Zuliang C. et al.: ‘Synthesis of iron‐based nanoparticles by green tea extract and their degradation of malachite’, Ind. Crops Prod., 2013, 51, pp. 342 –347 [Google Scholar]
- 23. Valentin V. M. Svetlana S. M. Andrew J. L. et al.: ‘Biosynthesis of stable iron oxide nanoparticles in aqueous extracts of Hordeum vulgare and Rumex acetosa plants’, Langmuir, 2014, 30, (20), pp. 5982 –5988 [DOI] [PubMed] [Google Scholar]
- 24. Uddin N. Rahman A. Nazim Uddin A. et al.: ‘Antioxidant, cytotoxic and antimicrobial properties of ethanol extract’, Int. J. Biol. Med. Res., 2010, 1, (4), pp. 341 –346 [Google Scholar]
- 25. Sang W.K. Jin H.J. Kabir L. et al.: ‘Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi’, Mycobiology, 2012, 40, (1), pp. 53 –58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gopinatha V. Mubarak A. Priyadarshini S. et al.: ‘Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach’, Colloids Surf. B, Biointerfaces, 2012, 96, pp. 69 –74 [DOI] [PubMed] [Google Scholar]
- 27. Brajesh K. Kumari S. Luis C. et al.: ‘Biogenic synthesis of iron oxide nanoparticles for 2‐arylbenzimidazole fabrication’, J. Saudi Chem. Soc., 2014, 18, pp. 364 –369 [Google Scholar]
- 28. Gotic M. Jurkin T. Music S.: ‘From iron (III) precursor to magnetite and vice versa’, Mater. Res. Bull., 2009, 44, pp. 2014 –2021 [Google Scholar]
- 29. John C.: ‘Interpretation of infrared spectra’, in: Meyers R.A. (ED.) ‘A practical approach encyclopedia of analytical chemistry’. (Coates Consulting, John Wiley & Sons Ltd, Newtown, USA, Chichester, 2000), pp. 10815 –11083 [Google Scholar]
- 30. Swadeshmukul S. Rovelyn T. Nikoleta T. et al.: ‘Synthesis and characterization of silica‐coated iron oxide nanoparticles in microemulsion: the effect of nonionic surfactants’, Langmuir, 2001, 17, pp. 2900 –2906 [Google Scholar]
- 31. Mahnaz M. Farideh N. Mansor B.A. et al.: ‘Green biosynthesis and characterization of magnetic iron oxide (Fe3 O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract’, Molecules, 2013, 18, pp. 5954 –5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Monalisa P. Nayak P.L.: ‘Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Azadirachta indica ( Neem)’, World J. Nano Sci. Technol., 2013, 2, (1), pp. 06 –09 [Google Scholar]
- 33. Dharam V. Sharma R.K.: ‘Efficacy of fungicides XXIX studies on the fungicidal properties of neem oil’, Indian J. Plant Pathol., 1985, 3, pp. 241 –242 [Google Scholar]
- 34. Nduagu C. Ekefan E.J. Nwankiti A.O.: ‘Effect of some crude plant extracts on growth of Colletotrichum capsici (Synd) Butler & Bisby, causal agent of pepper anthracnose’, J. Appl. Biosci., 2008, 6, pp. 184 –190 [Google Scholar]
- 35. Patil M.J. Ukey U.P. Raut B.T.: ‘Evaluation of fungicides and botanicals for the management of early blight (Alternaria solani) of tomato’, PKV Res. J., 2001, 25, pp. 49 –51 [Google Scholar]
- 36. Surwase A.G. Badgire D.R. Suryawanshi A.P.: ‘Management of pea powdery mildew by fungicides, botanicals and bio‐agents’, Ann. Plant Prot. Sci., 2009, 17, pp. 384 –388 [Google Scholar]
- 37. Kandhari J.: ‘Management of sheath blight of rice through fungicides and botanicals’, Indian Phytopathol., 2007, 60, pp. 214 –217 [Google Scholar]
- 38. Muthamilan M. Revathy N.: ‘Management of tungro virus disease of rice with antagonists and botanicals’, J. Ecobiol., 2007, 19, pp. 81 –88 [Google Scholar]
- 39. Sunder S. Singh R. Dodan D.S.: ‘Management of bacterial blight of rice with botanical extracts and non‐conventional chemicals’, Plant Dis. Res., 2005, 20, pp. 12 –17 [Google Scholar]
- 40. Sitara U. Naseem J. Sultana N.: ‘Antifungal effect of essential oils on in vitro growth of pathogenic fungi’, Pak. J. Bot., 2008, 40, pp. 409 –414 [Google Scholar]
- 41. Atawodi S.E. Atawodi J.C.: ‘ Azadirachta indica (neem) a plant of microbial and pharmacological activity’, Phytochem. Rev., 2009, 8, pp. 601 –620 [Google Scholar]
- 42. Malkhan S.G. Shahid A. Masood A. et al.: ‘Efficacy of plant extracts in plant disease management’, 2012, 3, (3), pp. 425 –433 [Google Scholar]


