Abstract
Originally identified as an antagonist of Ras action, Rap1 exhibits many Ras-independent effects, including a role in signaling pathways initiated by cyclic AMP (cAMP). Since cAMP is a critical mediator of the effects of thyrotropin (TSH) on cell proliferation and differentiation, we examined the regulation of Rap1 by TSH in a continuous line of rat thyroid-like cells. Both cAMP and protein kinase A (PKA) contribute to the regulation of Rap1 activity and signaling by TSH. TSH activates Rap1 through a cAMP-mediated and PKA-independent mechanism. TSH phosphorylates Rap1 in a PKA-dependent manner. Interference with PKA activity blocked phosphorylation but not the activation of Rap1. Rather, PKA inhibitors prolonged Rap1 activation, as did expression of a Rap1A mutant lacking a PKA phosphorylation site. These results indicate that PKA elicits negative feedback regulation on cAMP-stimulated Rap1 activity in some cells. The dual regulation of Rap1 by cAMP and PKA extends to downstream effectors. The ability of TSH to stimulate Akt phosphorylation was markedly enhanced by the expression of activated Rap1A and was repressed in cells expressing a putative dominant-negative Rap1A mutant. Although the expression of activated Rap1A was sufficient to stimulate wortmannin-sensitive Akt phosphorylation, TSH further increased Akt phosphorylation in a phosphatidylinositol 3-kinase- and PKA-dependent manner. The ability of TSH to phosphorylate Akt was impaired in cells expressing a Rap1A mutant that could be activated but not phosphorylated. These findings indicate that dual signals, Rap1 activation and phosphorylation, contribute to TSH-stimulated Akt phosphorylation. Rap1 plays an essential role in cAMP-regulated differentiation. TSH effects on thyroid-specific gene expression, but not its effects on proliferation, were markedly enhanced in cells expressing activated Rap1A and repressed in cells expressing a dominant-negative Rap1A mutant. These findings reveal complex regulation of Rap1 by cAMP including PKA-independent activation and PKA-dependent negative feedback regulation. Both signals appear to be required for TSH signaling to Akt.
A role for Rap1 in cell signaling was first suggested by the demonstration of forskolin-stimulated Rap1 activity (1). It is now evident that Rap1 can be activated by a diverse array of second messengers, including calcium, diacylglycerol, and cyclic AMP (cAMP) (reviewed in reference 52). The ability of cAMP to activate Rap1, coupled with the recent discovery of cAMP-regulated Rap1 guanine nucleotide exchange factors (GEFs), argues for an important role for Rap1 in cAMP-mediated signaling (12, 13, 25). This is further supported by the findings that Rap1 proteins are substrates for protein kinase A (PKA), although the significance of phosphorylation remains unclear. In vitro phosphorylation of Rap1B was reported to enhance guanine nucleotide exchange and inhibit membrane binding, suggesting that PKA may regulate Rap1 activation and/or localization (26). Indeed, the treatment of intact platelets with PGE1 or cAMP analogs induced translocation of Rap1B from the membrane to the cytosol (21). More recently, cAMP was found to have no effect on the partitioning of Rap1A between aqueous and detergent phases in HL60 cells (33). Acting via PKA, cAMP phosphorylated Rap1A in human neutrophils, although in this case in vitro phosphorylation had no effect on guanine nucleotide binding, exchange, or GTPase activity (41). Two intriguing findings suggest that phosphorylation regulates Rap1 association with other proteins. PKA-phosphorylated Rap1A was shown to be impaired in its ability to associate with cytochrome b558 (4) and Raf-1 (23). In the latter case, Rap1 phosphorylation also impaired its ability to inhibit Ras-mediated activation of Raf-1. Our findings suggest additional roles for PKA-mediated phosphorylation: negative feedback regulation of Rap1 activity and phosphatidylinositol 3-kinase (PI3K)-dependent signaling to Akt. These findings reveal further complexity in cAMP effects on Rap1, which appear to involve PKA-independent, as well as PKA-dependent, pathways.
Because cAMP is an essential mediator of hormone effects on cell proliferation and differentiation, we investigated the role of Rap1 in Wistar rat thyroid (WRT) cells where TSH stimulates proliferation and differentiated gene expression through cAMP (30). We report that TSH activates Rap1 through a PKA-independent mechanism in WRT cells, similar to reports in other cell types (13, 15, 33). Stable expression of an activated Rap1A mutant in WRT cells enhanced hormone-mediated differentiation but not hormone-stimulated proliferation. The effects of Rap1 in WRT cells directly oppose those of Ras, where constitutive activation promotes proliferation at the expense of differentiation (17, 31, 32, 36). These data suggest that the balance in the activity of Ras- and Rap1-mediated signals may be an important determinant of the highly specialized ability of hormones to regulate proliferation and differentiation in their target cells.
MATERIALS AND METHODS
Reagents.
TSH, 8BrcAMP, forskolin, insulin, and Coon's modified Ham's F-12 medium were from Sigma (St. Louis, Mo.), and calf serum was from Gibco-BRL (Gaithersburg, Md.). PKA inhibitors were from Calbiochem (La Jolla, Calif.), LY294002 and wortmannin were from BioMol (Plymouth Meeting, Pa.), and PD98059 was from Cell Signaling Technology (Beverly, Mass.). Rap1, p21, ERK2, and horseradish peroxidase-conjugated antibodies were from Santa Cruz (Santa Cruz, Calif.), phospho-specific (S473) Akt and Akt1 antibodies were from Cell Signaling Technology, the thyroglobulin antibody was from Dako Corporation (Carpinteria, Calif.), and the bromodeoxyuridine (BrdU) antibody was from Amersham (Piscataway, N.J.). The EE, NIS, and HA antibodies were generous gifts from G. Walter (Department of Medicine, University of California, San Diego), N. Carrasco (Albert Einstein College of Medicine), and J. Field (Department of Pharmacology, University of Pennsylvania), respectively. Phospho-specific S6 antibody was kindly provided by M. Birnbaum (Howard Hughes Medical Institute, University of Pennsylvania).
Cell culture.
Thyroid cells were propagated at 37°C in 5% CO2 in 3H medium (Coon's modified Ham's F-12 medium containing crude bovine TSH (1 mU/ml), insulin (10 μg/ml), transferrin (5 μg/ml), and 5% calf serum. Incubation in hormone-, growth factor-, and serum-free conditions was performed for 48 h in basal medium (Coon's modified Ham's F-12 medium) for all experiments with the exception of DNA synthesis, where insulin was included in the basal medium. Stimulations were performed using TSH at 1 mU/ml, forskolin at 10 μM, and 8BrcAMP at 1 mM. H89 (10 μM), LY294002 (30 μM), wortmannin (75 nM), and PD98059 (50 μM) were added 60 min prior to stimulation. EE-tagged Rap1A63E and Rap1A17N expression vectors have been described previously (34). The Rap1AS180A mutant was made by PCR, sequenced, and subcloned into the hygromycin B-resistant mammalian expression vector pCGN-hygro (50). Thyroid cells stably expressing wild-type and mutant forms of Rap1A were generated by Lipofectin-mediated transfection (10 μg of DNA/2 × 106 cells), followed by selection in 3H medium containing Geneticin (300 μg/ml; for Rap1A63E and Rap1A17N) or hygromycin (150 μg/ml; for Rap1AS180A). Multiple clones expressing each construct were isolated and maintained in Geneticin (150 μg/ml)- or hygromycin (75 μg/ml)-supplemented 3H medium.
Rap1 activation.
A glutathione S-transferase (GST) fusion of the Rap binding domain (RBD) of RalGDS was expressed and purified as described previously (35). Rap1 activation was assessed essentially as described earlier (11) with minor modifications. Cells were grown to 80% confluence, starved in basal medium for 48 h, and stimulated with cAMP elevating agents and analogs or vehicle for the indicated times. Following lysis in modified RIPA buffer (50 mM Tris-HCl, pH 8.0; 150 mM NaCl; 0.5% deoxycholate; 1% NP-40; 0.1% sodium dodecyl sulfate [SDS]; 10 mM NaF; 2 mM Na3VO4; aprotinin, leupeptin, and Pefabloc [each at 10 μg/ml]) containing 50 μg of GST-RalGDS RBD per ml, the protein concentrations were determined using a DC Protein Assay (Bio-Rad Laboratories, Hercules, Calif.). Cell proteins (400 μg) were incubated with glutathione-Sepharose beads for 1 h at 4°C. Following three washes in lysis buffer, bound proteins were eluted in sample buffer, heated for 5 min at 95°C, separated by SDS-polyacrylamide gel electrophoresis (PAGE) on a 12% polyacrylamide gel, and subjected to immunoblotting. In parallel, total cell lysates (20 μg) were analyzed by Western blot with a polyclonal Rap1-specific antibody.
Immunoblotting.
Equal amounts of whole-cell lysates (20 to 30 μg) were separated on 8% (for NaI symporter [NIS]), 6% (for thyroglobulin [Tg]) 10% (for mitogen-activated protein kinase) gels by SDS-PAGE and then subjected to Western blotting with the appropriate antibodies (7). p21 expression was analyzed in total cell extracts (20 μg) separated by SDS–12% PAGE. Akt and S6 phosphorylation were analyzed as described earlier (8). Primary antibodies (0.25 μg/ml) were incubated overnight at 4°C and secondary antibodies (1:3,000) for 0.5 h at room temperature. Bound antibodies were detected using enhanced chemiluminescence (Amersham, Piscataway, N.J.). Densitometric analyses were performed using ScanMaker 4 (Microtek) and NIH image software. Statistical analyses were made by using the paired Student's t test.
DNA synthesis and growth curves.
For DNA synthesis studies, cells were arrested in insulin (0.5 μg/ml)-supplemented basal medium for 48 h since inclusion of insulin potentiates the effects of TSH on DNA synthesis (6). DNA synthesis was assessed by determining BrdU incorporation as previously described (30). Growth curves were performed by plating 2 × 105 cells overnight in 3H medium. The following day, duplicate plates were harvested and trypsinized, and the cell number that plated was determined by using a hemacytometer. Replicate plates were refed each day, and duplicate plates were analyzed at 24, 48, and 72 h after plating essentially as described previously (36).
RESULTS
PKA-dependent and -independent pathways contribute to cAMP-regulated Rap1 activity.
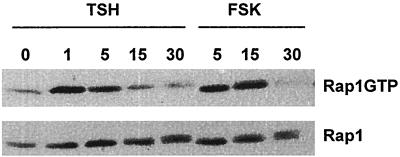
Rap1GTP levels were examined in WRT cells arrested in TSH-, insulin-, and serum-deficient basal medium and following acute treatment with TSH (Fig. 1, Rap1GTP). Low levels of GTP-bound Rap1 were retrieved from cells in basal medium. In the absence of all other growth factors, TSH increased the proportion of GTP-bound Rap1 within 1 min of its addition to cells in basal medium. Rap1GTP levels were maximal at 1 to 15 min and declined to basal levels within 30 min following treatment. The effects of TSH on Rap1 activity were reproduced by forskolin (Fig. 1) and 8BrcAMP (data not shown), indicating that they were cAMP mediated, as are most TSH effects in WRT cells.
FIG. 1.
TSH activates Rap1 through a cAMP-mediated pathway in rat thyroid cells. Cells arrested in basal medium for 48 h were stimulated with TSH or forskolin (FSK) for the times indicated (in minutes). GTP-bound Rap1 (Rap1GTP), assessed by a GST pulldown assay as described in Materials and Methods) and total cellular Rap1 (Rap1) were detected by immunoblotting with a polyclonal Rap1-specific antibody. In control experiments, Rap1 was not detected following incubation of cell lysates with glutathione-Sepharose alone or with GST immobilized on glutathione-Sepharose. The effects of cAMP elevating agents on Rap1 activity were not a consequence of alterations in Rap1 expression, which was similar in starved and cAMP-stimulated cells. Similar results were observed in 10 experiments.
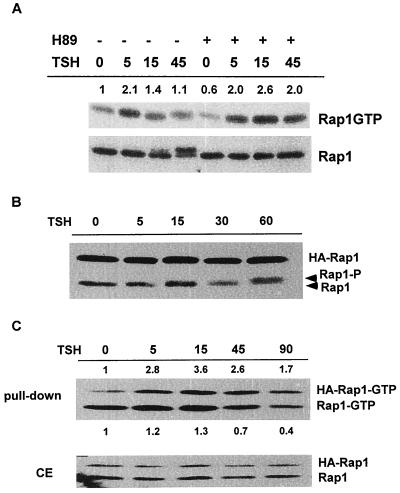
cAMP has been reported to activate Rap1 through direct effects on cAMP-regulated Rap1 GEFs (12, 13), at least one of which is highly expressed in human thyroid cells (25). Inclusion of H89, a highly selective PKA inhibitor (9), did not impair TSH-stimulated Rap1 activation. Treatment with TSH for 5 min stimulated a twofold increase in GTP-bound Rap1 in the absence or presence of H89 (Fig. 2A, Rap1GTP). Although Rap1 proteins are substrates for PKA (26, 41, 46), the role of PKA-mediated phosphorylation has remained enigmatic. TSH stimulated Rap1 phosphorylation, leading to its slower migration on SDS-PAGE (Fig. 2A, Rap1). Phosphorylation was first detected at 15 min and was maximal at 45 min following TSH addition, times much later than the effects on Rap1 activation. As expected, pretreatment with H89 abolished Rap1 phosphorylation, as indicated by the absence of a mobility shift at 15 and 45 min (Fig. 2A). This confirms the activity of H89 in these cells and indicates that Rap1 activation and phosphorylation are mediated through distinct pathways. Pretreatment with RpcAMPS, an independently acting PKA inhibitor (43), also blocked phosphorylation but not activation of Rap1 by TSH (data not shown). RpcAMPS consistently increased basal levels of GTP-bound Rap, suggesting that it may bind and activate cAMP-RapGEFs in addition to its activity as a PKA inhibitor.
FIG. 2.
cAMP activates Rap1 in a PKA-independent manner. (A) Cells arrested in basal medium were pretreated with H89 prior to stimulation with TSH for the indicated times (in minutes). Rap1 activation (Rap1GTP) and expression (Rap1) were examined. TSH stimulated a Rap1 mobility shift that was first detected at 15 min and was maximal at 45 min. Inclusion of H89 abolished the mobility shift (Rap1) but not activation (Rap1-GTP). The numbers shown directly above each lane indicate the fold activation of Rap1 over basal levels as determined by densitometry. Six experiments were performed with similar results. (B) Rap1AS180A-expressing cells were incubated in basal medium for 48 h and stimulated with TSH for the times indicated (in minutes), and cell extracts were prepared and analyzed by Western blotting. TSH induced a mobility shift of endogenous cellular Rap1 (Rap1-P) without affecting the mobility of transfected Rap1AS180A (HA-Rap1). The mobility of Rap1AS180A was not affected by TSH in 10 experiments performed in five independent Rap1AS180A cell lines. (C) Rap1AS180A-expressing cells were arrested in basal medium (for 48 h) and stimulated with TSH as indicated (min), and Rap1 activity was determined (pulldown, upper panel). The numbers above each lane indicate the fold activation over basal levels of transfected Rap1AS180A (HA-Rap1-GTP). Those shown below each lane indicate the fold activation over basal levels of endogenous Rap1 (Rap1-GTP). Total cell extracts (CE) were analyzed in parallel. Similar results were obtained in eight experiments using three Rap1AS180A cell lines.
Unexpectedly, Rap1 activity was prolonged in the presence of H89. In the absence of H89, the proportion of GTP-bound Rap1 decreased to near-basal levels at 15 min (1.4-fold over basal) and 45 min (1.1-fold) following TSH treatment. In contrast, Rap1GTP levels remained elevated at these times (2.6-fold over the basal level at 15 min; 2-fold over the basal level at 45 min) in the presence of H89. Indeed, Rap1GTP levels were increased over basal levels for as long as 90 min following addition of TSH to H89-treated cells ([1.84 ± 0.7]-fold in the presence of H89 and [0.3 ± 0.3]-fold in the absence of H89; P < 0.05, n = 3). These results suggest that PKA activity somehow impairs Rap1 activity stimulated by TSH.
To further explore the role of PKA in the regulation of Rap1 activity, experiments were conducted in cells expressing a Rap1A mutant in which the single PKA phosphorylation site was mutated to alanine (Rap1AS180A). Unlike endogenous Rap1 in the same cells, transfected Rap1AS180A did not exhibit a mobility shift following stimulation with TSH (Fig. 2B, HA-Rap1). Despite the inability of PKA to phosphorylate this mutant Rap1A protein, TSH activated Rap1AS180A (Fig. 2C, top). This confirms the PKA-independent nature of cAMP-stimulated Rap1 activity in WRT cells. Expression of Rap1AS180A increased the proportion of cellular Rap1 that was GTP-bound under basal conditions, thereby obscuring TSH effects on the endogenous Rap1 activity in these cells. These results may be related to decreased expression of Rap1GAPI and/or Rap1GAPII in these cells compared to WRT cells (O. M. Tsygankova, unpublished results). Significantly, activation of Rap1S180A by TSH was prolonged compared to the activation of endogenous Rap1 in parental cells (HA-Rap1-GTP at 90 min, 2.8 ± 1.1; P < 0.005, n = 5, from three independent S180A cell lines). This effect was not seen in cells transfected with cellular Rap1A where cAMP activated both transfected and endogenous Rap1A with kinetics similar to those for endogenous Rap1A activation in parental cells (data not shown). These data further support the idea that Rap1 activity is subject to negative feedback regulation by PKA and highlight the complexity of cAMP effects on Rap1 which appear to involve PKA-independent activation as well as PKA-dependent negative feedback regulation.
Characterization of Rap1A-expressing WRT cells.
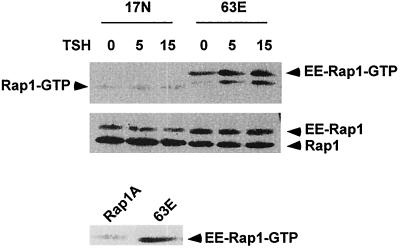
Because WRT cells exhibit very low transfection efficiencies in transient assays, stable cell lines expressing epitope-tagged cellular Rap1A and activated (Rap1A63E) and putative dominant-negative (Rap1A17N) Rap1 mutants were isolated. Rap1A was selected for these studies since WRT cells express detectable levels of Rap1A but not Rap1B (Tsygankova, unpublished). The biochemical characteristics of the Rap1A mutants have been characterized previously (28, 34). The GTPase-deficient phenotype of Rap1A63E was confirmed by assessing the levels of GTP-bound Rap1A63E in cells in basal medium and following treatment with TSH. Rap1A63E-expressing cells exhibited basally elevated levels of GTP-bound Rap1A63E compared to cells expressing similar levels of transfected Rap1A (Fig. 3, lower panel). Treatment with TSH or 8BrcAMP (data not shown) increased the proportion of GTP-bound Rap1A63E in these cells (Fig. 3, EE-Rap1-GTP in upper panel). Similar effects have been reported for Rap1B, where cAMP elevation further increased GTP loading on a Rap1B12V mutant (1). In cells expressing equivalent levels of Rap1A17N, this mutant was not GTP bound under basal conditions, nor did it become GTP-bound following treatment with TSH (Fig. 3). Endogenous cellular Rap1 was not activated in either cell line (the faster-migrating minor band in Rap1A63E cells does not comigrate with endogenous Rap1). In contrast, endogenous Rap1 was activated by TSH in cells expressing transfected Rap1A (data not shown), and endogenous Rap1 was basally activated in cells expressing Rap1AS180A (Fig. 2C). Based on the absence of detectable cellular Rap1 activity, cells expressing Rap1A63E were used to elucidate cellular effects elicited in response to expression of activated Rap1A, and cells expressing Rap1A17N to determine cellular responses that require Rap1A activity.
FIG. 3.
Rap activation in cells stably expressing activated and dominant-negative Rap1A mutants. Cell lines expressing activated (63E) and dominant-negative (17N) Rap1A mutants were arrested in basal medium and stimulated with TSH for the times indicated (in minutes), and the Rap1 activity was assessed. Rap1A63E cells exhibited increased the levels of GTP-bound Rap1 (EE-Rap1-GTP) in basal medium compared to cells expressing equivalent levels of transfected Rap1A (bottom panel). TSH further increased GTP loading on transfected Rap1A63E. Rap1A17N was not basally GTP bound, nor did TSH increase GTP loading on this mutant Rap1A protein. The faster-migrating band observed in Rap1A63E cells did not comigrate with endogenous Rap1, whose migration is indicated by the arrow at the left (Rap1-GTP). Similar results were obtained in five experiments.
Rap1A and cAMP-mediated proliferation and differentiation.
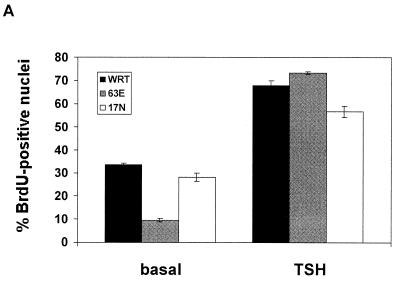
Thyroid cells are one of the few cellular models where cAMP stimulates cell proliferation. Given that cAMP activates Rap1 in these cells, together with a report that ectopic expression of Rap1B enhanced cAMP-stimulated proliferation in Swiss 3T3 cells (2), we determined whether expression of activated Rap1A enhanced cell cycle progression. Cells were incubated in insulin-supplemented basal medium for 2 days, conditions wherein parental cells are quiescent and subsequent TSH treatment stimulates maximal levels of DNA synthesis (6). Basal levels of DNA synthesis were consistently repressed in Rap1A63E-expressing cells compared to parental cells or cells expressing Rap1A17N (Fig. 4A). Addition of TSH or 8BrcAMP (data not shown) stimulated DNA synthesis to similar levels in all cell lines.
FIG. 4.
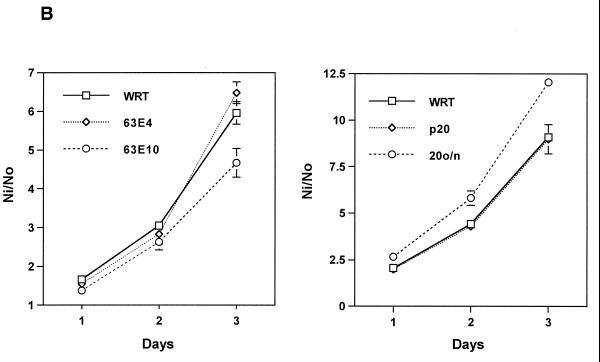
Rap1A and cell proliferation. (A) DNA synthesis was assessed in cells arrested in insulin-supplemented basal medium and following stimulation with TSH for 48 h. Parental cells (WRT) are shown in black bars, Rap1A63E cells in gray bars and Rap1A17N cells in white bars. The bars indicate the mean ± the standard error of the mean from five experiments. Similar results were observed in three Rap1A63E cell lines and in two Rap1A17N cell lines. (B) Growth rates were determined in WRT cells and in two Rap1A63E (clones 4 and 10) and Rap1A17N (p20, 20o/n) cell lines. Rap1A17N pool 20 (p20) was derived from a mass population of transfected cells, while 20o/n is a clonal line. Expression of transfected Rap1A17N in these lines was similar to that of Rap1A63E in clone 4. The bars indicate the mean ± the standard error of the mean from four independent experiments. Ni/No is the ratio of the cell number at each day (Ni) over the number of cells plated at day 0 (No).
To further explore the effects of Rap1A on cell proliferation, the growth rates of Rap1A63E-and Rap1A17N-expressing cells were determined. Cells expressing modest levels of Rap1A63E (clone 4) grew at the same rate as parental cells (Fig. 4B). Cells expressing higher levels of Rap1A63E (clone 10) increased in number more slowly than did the parental cells. These cells also exhibited a higher death rate than parental cells (A. Saavedra, submitted for publication). Cells expressing modest levels of Rap1A17N (equivalent to Rap1A63E expression in clone 4) exhibited a growth rate similar to that of parental cells (Fig. 4B). For both Rap1A63E and Rap1A17N cell lines, proliferation remained TSH dependent (data not shown).
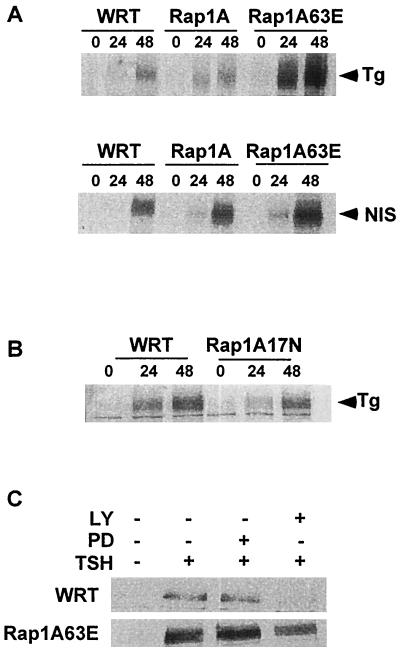
TSH regulates the expression of thyroid-specific genes, including the TSH receptor (reviewed in reference 29), Tg, and the NIS (reviewed in references 16 and 38). To elucidate the effects of Rap1A on thyroid-specific gene expression, the ability of TSH to stimulate Tg and NIS expression in cells expressing various Rap1A mutants was analyzed. Cells were incubated in the absence of TSH for 7 days to abolish preexisting Tg and NIS protein. Under these conditions, Tg and NIS expression were undetectable (Fig. 5A, lane 0). TSH and 8BrcAMP (data not shown) stimulated Tg and NIS expression in parental cells, an effect that was strikingly enhanced in Rap1A63E cells. In contrast, the ability of TSH to stimulate Tg (Fig. 5B) and NIS expression (data not shown) was impaired in Rap1A17N cells. Inclusion of the PI3K inhibitor LY294002 abolished the effects of TSH on Tg expression in parental cells and reduced Tg expression in Rap1A63E cells (Fig. 5C). Taken together, these results indicate that Rap1 and PI3K contribute to the effects of TSH on Tg expression.
FIG. 5.
Rap1A and thyroid-specific gene expression. (A) WRT, Rap1A- and Rap1A63E-expressing cells were arrested in basal medium for 7 days and then stimulated with TSH for the times indicated (in hours), and whole-cell lysates were prepared and subjected to Western blotting with Tg- and NIS-specific antibodies. Five experiments were performed with similar results. Tg and NIS expression were enhanced in three independent Rap1A63E cell lines. (B) TSH-stimulated Tg expression was compared in WRT and in Rap1A17N-expressing cells as described above. In order to detect Tg expression in Rap1A17N cells, the Western blots shown here were exposed for longer times than those in panel A, which is why the Tg expression appears to be higher in WRT cells in this panel. Similar results were obtained with two independent Rap1A17N cell lines in four experiments. (C) WRT and Rap1A63E cells were pretreated with LY294002 or PD98059 before stimulation with TSH for 24 h. Cell extracts were subjected to Western blotting with an anti-Tg antibody. Two experiments were performed with similar results.
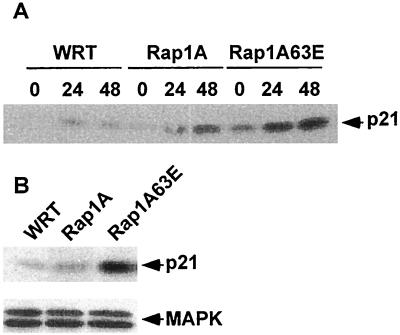
To gain insight into the potential mechanism(s) through which Rap1A regulates thyroid-specific gene expression, the expression of p21WAF1/Cip1 was analyzed since its expression is known to increase during differentiation (19, 20, 24, 37, 47). TSH stimulated a modest increase in p21 expression at 24 to 48 h in parental cells (Fig. 6A), a time that correlates well with its effects on Tg and NIS expression (Fig. 5). The effects of TSH on p21 expression were markedly enhanced in Rap1A63E- and Rap1A-expressing cells. Expression of p21 was also dramatically increased in Rap1A63E cells in the absence of TSH (Fig. 6B), a finding that may be related to the decreased levels of DNA synthesis observed in these cells under these conditions (Fig. 4A). The expression of Rap1A and Rap1A63E had no effect on MAPK expression, indicating that ectopic Rap1A expression does not globally stimulate gene expression. As for Tg expression, TSH stimulated p21 expression through a PI3K-dependent pathway (data not shown).
FIG. 6.
Rap1A enhances p21 expression. (A) WRT and Rap1A-transfected cells were starved and stimulated as described in legend to Fig. 5, and lysates were subjected to Western blotting with a p21-specific antibody. Similar results were obtained in three independent Rap1A and Rap1A63E cell lines in five independent experiments. (B) p21 and MAPK expression in cells arrested for 48 h in basal medium. Similar results were obtained in three independent Rap1A63E cell lines in six experiments.
TSH and Rap1 regulate Akt phosphorylation.
TSH stimulates PI3K-dependent effects on Akt phosphorylation (8). Therefore, the regulation of Akt phosphorylation was examined in cells expressing Rap1A mutants. Under basal conditions, Rap1A63E cells exhibited increased Akt phosphorylation (Fig. 7A). The ability of TSH to stimulate Akt phosphorylation was markedly enhanced in Rap1A63E- and Rap1A-expressing cells, suggesting that Rap1 contributes to the regulation of Akt phosphorylation in thyroid cells. As in parental cells (8), Akt phosphorylation was not detectable until 30 to 45 min following TSH addition (Fig. 7B). This time course was substantially delayed compared to TSH effects on Rap1 activity (Fig. 1) but similar to the time course for Rap1 phosphorylation (Fig. 2A). As PKA phosphorylates Rap1, the role of PKA in the regulation of Akt phosphorylation was examined. Inclusion of 10 μM H89 abolished TSH-stimulated Akt phosphorylation in parental cells and reduced Akt phosphorylation to basal levels in Rap1A63E and Rap1A cells (Fig. 7A). The effects of H89 were specific for PKA in that 10 μM H89 did not impair insulin- or serum-stimulated Akt phosphorylation (data not shown). These data suggest that both Rap1 and PKA contribute to Akt phosphorylation, effects that are mediated through PI3K since wortmannin impaired TSH-stimulated Akt phosphorylation in Rap1A-transfected cells (Fig. 7C).
FIG. 7.
TSH and Rap1 activity regulate Akt phosphorylation. (A) Cells arrested in basal medium for 48 h were pretreated with H89 and then stimulated with TSH for 45 min. Cell lysates were subjected to Western blotting with an antibody to active phosphorylated (Ser473) Akt1 (Akt-P). Similar results were obtained in three Rap1A63E cell lines in six experiments. (B) Lysates prepared from cells arrested as described above and treated with TSH for the times indicated (in minutes) were analyzed by Western blotting with the phospho-specific Akt antibody. The expression of cellular or mutant Rap1A constructs had no effect on the total levels of Akt1 expression (data not shown). Three experiments in three independent Rap1A63E cell lines were performed with similar results. (C) Cells in basal medium were pretreated with wortmannin, stimulated with TSH for 45 min, and subjected to Western blotting with the phospho-specific Akt1 antibody. Equal protein loading in panels A, B, and C was confirmed by Western blotting with an anti-MAPK2 antibody (data not shown). Three experiments using three independent Rap1A and Rap1A63E cell lines were performed with similar results.
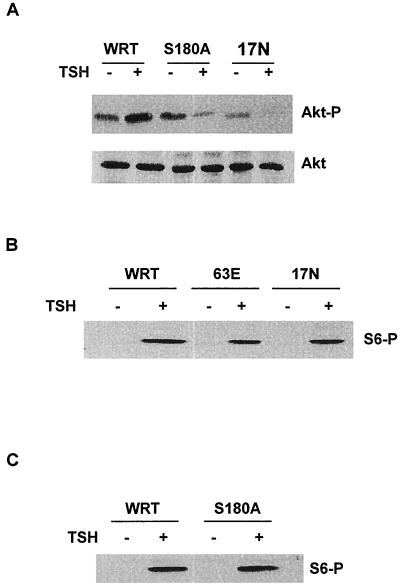
To gain additional support for this idea, Akt phosphorylation was examined in cells expressing Rap1 mutants that cannot be activated (Rap1A17N) or phosphorylated (Rap1AS180A). As shown in Fig. 8A, the ability of TSH to stimulate Akt phosphorylation was impaired in both Rap1AS180A- and Rap1A17N-expressing cells. These effects were specific for Akt since TSH-stimulated S6 phosphorylation, an indicator of p70S6k activity, was not impaired in these cells, nor was it enhanced in cells expressing Rap1A63E (Fig. 8B and C). Collectively, these data support the idea that both Rap1 activity and phosphorylation contribute to the regulation of Akt. Expression of activated Rap1A is sufficient to stimulate a PI3K-dependent increase in Akt phosphorylation in cells in basal medium. Treatment with TSH further increases Akt phosphorylation, an effect that is markedly enhanced in Rap1A63E- and Rap1A-expressing cells and impaired in Rap1AS180A- and Rap1A17N-expressing cells.
FIG. 8.
Rap1A phosphorylation and activation are required for TSH-stimulated Akt phosphorylation. (A) WRT, Rap1AS180A, and Rap1A17N cells were arrested in basal medium for 48 h and stimulated with TSH for 45 min, and cell lysates were subjected to immunoblotting with phospho-specific Akt1 (Akt-P) and Akt1 (Akt) antibodies. Similar results were obtained in five experiments. (B and C) WRT and Rap1A mutant-transfected cells were arrested in basal medium and stimulated with TSH for 45 min, and cell lysates were analyzed for S6 protein phosphorylation (S6-P), an indicator of p70S6K activity, using a phospho-specific S6 antibody. Five experiments using two independent isolates of Rap1A63E, Rap1A17N, and Rap1AS180A cell lines yielded similar results. Equal protein loading was confirmed by Western blotting for MAPK2 (data not shown).
DISCUSSION
Given the important role for cAMP in the regulation of Rap1 activity, we examined cAMP effects on Rap1 in WRT cells, in which hormone-stimulated proliferation and differentiated gene expression proceed through cAMP. TSH rapidly activated Rap1, an effect that was reproduced by cAMP elevating agents, but PKA independent. TSH also phosphorylated Rap1 in a PKA-dependent manner, although with delayed kinetics compared to Rap1 activation. The mechanistic aspects of activation have not been explored; however, it is tempting to speculate that cAMP-regulated GEFs mediate this effect, particularly given their robust expression in human thyroid tissue (25). Although PKA contributes to cAMP-stimulated Rap1 activity in PC12 (51) and IGF-1 receptor-overexpressing NIH 3T3 cells (1), in many instances PKA is not required for cAMP-stimulated Rap1 activity (13, 15, 25). Despite this, Rap1 proteins are PKA substrates, supporting an important, albeit unknown role for phosphorylation. Our findings suggest two novel roles for PKA-mediated phosphorylation: negative feedback regulation of Rap1 activity and the stimulation of Rap1-dependent Akt phosphorylation. Lastly, our data revealed that Rap1 plays an important role in hormone-mediated differentiation, effects that are in direct opposition to those of Ras.
TSH elicited a rapid and transient activation of endogenous Rap1, which we believe to be Rap1A. Rap1 activity was increased within 1 min of stimulation, and decreased to basal levels 15 to 30 min after treatment. The kinetics of Rap1 activation were shared by multiple cAMP elevating agents and analogs and are quite similar to those reported for cAMP-stimulated Rap1 activity in HL60 cells where cGMP regulates differentiation (33). Two findings support a role for PKA-mediated phosphorylation in negative feedback regulation of Rap1 activity. Pretreatment with PKA inhibitors prolonged cAMP-stimulated Rap1 activity. Consistent with these findings, activation of a mutant Rap1A protein lacking a PKA phosphorylation site (Rap1AS180A) by cAMP was substantially prolonged compared to the activation of endogenous Rap1. The ability of Rap1AS180A to be activated provides further evidence that Rap1 activation and phosphorylation are separable in WRT cells. Feedback regulation of Rap1 activity may be particularly important in cells where cAMP is a critical effector of hormone effects on proliferation, differentiation, and survival. Indeed, other examples of negative feedback regulation by TSH have been reported. TSH stimulates not only cAMP accumulation but also PKA-dependent increases in phosphodiesterase activity, thereby ensuring that cAMP elevation is transient (39).
Several lines of evidence indicate that Rap1 mediates TSH effects on Akt phosphorylation. The expression of an activated Rap1A mutant was sufficient to stimulate basal levels of Akt phosphorylation, documenting that Rap1 activation can lead to Akt phosphorylation. In addition, the ability of TSH to stimulate Akt phosphorylation was markedly enhanced by ectopic expression of cellular or activated Rap1A. Importantly, the ability of TSH to stimulate Akt phosphorylation was severely impaired in cells expressing a Rap1A mutant (Rap1A17N) that could not be activated. These findings implicate PI3K as a putative effector for Rap1, a result that is consistent with the inhibitory effects of wortmannin on TSH- and Rap1A-stimulated Akt phosphorylation.
As for Rap1 activity, both cAMP and PKA apparently contribute to the regulation of Akt phosphorylation. Acting through cAMP, TSH activates Rap1, an event sufficient to increase Akt phosphorylation. However, unlike its effects on Rap1 activity, TSH-stimulated Akt phosphorylation requires PKA activity. In addition, the time course for Akt phosphorylation most closely matched that of TSH-stimulated phosphorylation rather than the activation of Rap1. Both Rap1 and Akt phosphorylation were observed at 45 min after TSH treatment, whereas TSH activated Rap1 within 1 min of its addition to cells. Notably, TSH-stimulated Akt phosphorylation was impaired in cells expressing a Rap1AS180A, a mutant lacking a PKA phosphorylation site, just as it was in cells expressing a Rap1A mutant (Rap1A17N) that could not be activated. Collectively, the data argue that cAMP-stimulated Akt phosphorylation is regulated by two Rap1-dependent signals: activation and phosphorylation.
The precedent for PKA-mediated phosphorylation acting as a regulatory step to modulate association of Rap1 with other proteins has been previously established. PKA-mediated phosphorylation was shown to negatively regulate association of Rap1A with cytochrome b (4) and Raf-1 (22, 23). Our findings extend these observations to include positive effects of phosphorylation on Rap1 signaling to Akt. In this regard, cAMP effects on Rap1 may be similar to its effects on Ras, where cAMP impairs signaling to Raf-1 and enhances signaling to other effectors, for example, RalGDS (27). The mechanism through which phosphorylation regulates Rap1 activity and signaling to Akt is under investigation. Phosphorylation may increase the ability of Rap1 to stably interact with PI3K, although we have been unable to detect association between Rap1 and PI3K, studies hampered by our inability to transiently overexpress these molecules. However, Rap1 has been reported to bind to PI3K in vitro (14). Whatever the mechanism, this interaction is temporally correlated with Rap1 inactivation through a mechanism that remains to be identified.
Upon initial inspection, the requirement for PKA in TSH-stimulated Akt phosphorylation was different from our earlier findings, where TSH stimulated Akt phosphorylation in a PKA-independent manner (8). In our previous report, H89 was used at 25 μM, the lowest concentration sufficient to abolish CREB phosphorylation in WRT cells. Since that time we discovered that chronic treatment with H89 stimulates apoptosis when used at this concentration, an effect accompanied by increased levels of Akt phosphorylation (Saavedra, unpublished). Based on these findings, H89 was used at 10 μM in the present studies. At this concentration, H89 failed to stimulate Akt phosphorylation and blocked the effects of TSH on Rap1 and Akt phosphorylation in parental and Rap1A-transfected cells. These results support the idea that PKA activity is required for TSH effects on Akt phosphorylation, an effect that was masked by H89-stimulated Akt phosphorylation in our previous report.
Recent studies indicated that Rap1A17N does not form a stable complex with C3G, suggesting that this mutant may be inactive rather than dominant negative in disrupting C3G signaling (3). Whether Rap1A17N forms a stable complex with cAMP-Rap1GEFs has not been reported. When expressed at modest levels in WRT cells, cAMP elevating agents did not activate Rap1A17N under conditions where GTP loading was observed on an activated Rap1A mutant. This finding, together with the inhibitory effects of Rap1A17N on cAMP-stimulated gene expression (48), PKA-stimulated B-Raf activity (49), superoxide production (18, 34), and several other phenotypes (5, 42) supports the notion that Rap1A17N can impair Rap1-mediated signals stimulated by some agents, including cAMP and PKA.
The physiologic roles of Rap1 are poorly understood. The most striking effect of Rap1A in WRT cells was its marked enhancement of thyroid specific gene expression regulated by cAMP. The expression of activated Rap1A enhanced TSH effects on Tg and NIS expression, markers of thyroid differentiation. In contrast, expression of Rap1A17N impaired the expression of these genes by TSH. These results were obtained in cells overexpressing these mutant Rap1A proteins and therefore must be interpreted with caution. However, the level of ectopic Rap1A expression in these lines was modest and, with the exception of Rap1A63E clone 10, less than endogenous Rap1. These data support the notion that Rap1 plays an essential role in thyroid differentiation. Similar to these findings, Rap1B has been reported to contribute to cAMP-dependent differentiation in PC12 cells (49). A role for appropriately processed Rap1A in cyclic nucleotide-dependent differentiation in HL60 cells has been suggested (33, 44). All of these findings are consistent with those reported in Drosophila, where Rap1 plays a critical role in morphogenic differentiation, including terminal differentiation of eye imaginal disks. The effects of Rap1 on differentiation appear to be cell type selective since inhibitory effects of Rap1 on myogenic differentiation have been reported (40).
We identified p21 as a target of Rap1A in thyroid cells. Intriguingly, genetic analysis in Drosophila identified Dacapo, a protein highly homologous to rat p21, as a target of Rap1 (10). Similarly, a yeast two-hybrid screen identified the putative tumor suppressor Krit1, an ankyrin repeat protein with homology to p16-p15INK4 proteins, as a specific binding partner for Rap1 (45). Bacterially expressed Dacapo inhibited cyclinE-cdk2 kinase activity in vitro and antagonized S-phase entry in imaginal disk cells. These results are not dissimilar from those in WRT cells, where expression of activated Rap1A was accompanied by a marked increase in p21 expression and a significant repression in DNA synthesis under basal conditions. Collectively, our findings indicate that Rap1 activity must be regulated in order for TSH to coordinately stimulate proliferation and differentiation.
In summary, our findings reveal an intricate and complex regulation of Rap1 by cAMP. cAMP elicits dual effects on Rap1 activity: PKA-independent activation followed by PKA-dependent negative feedback regulation. In addition to its negative modulatory role, PKA appears to direct Rap1-mediated signals to discrete effector pathways, at least one of which includes Akt. Such complex regulation of Rap1 activity and signaling may be particularly important in endocrine cells where cAMP is a critical regulator of cell proliferation, differentiation, and survival.
ACKNOWLEDGMENTS
We thank Gregory Prendergast for expert technical assistance.
This work was funded by Public Health Service grants DK45696 and DK02494 from NIDDK to J.L.M. and American Cancer Society grant RPG-00-125-01TBE to L.A.Q. J.F.R. was supported by training grant T32 H07774 from the NHLBI.
REFERENCES
- 1.Altschuler D L, Peterson S N, Ostrowski M C, Lapetina E G. Cyclic AMP-dependent activation of Rap1b. J Biol Chem. 1995;270:10373–10376. doi: 10.1074/jbc.270.18.10373. [DOI] [PubMed] [Google Scholar]
- 2.Altschuler D L, Ribeiro-Neto F. Mitogenic and oncogenic properties of the small G protein Rap1b. Proc Natl Acad Sci USA. 1998;95:7475–7479. doi: 10.1073/pnas.95.13.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berghe N V D, Cool R H, Horn G, Wittinghofer A. Biochemical characterization of C3G: an exchange factor that discriminates between Rap1 and Rap2 and is not inhibited by Rap1A(S17N) Oncogene. 1997;15:845–850. doi: 10.1038/sj.onc.1201407. [DOI] [PubMed] [Google Scholar]
- 4.Bokoch G M, Quilliam L A, Bohl B P, Jesaitis A J, Quinn M T. Inhibition of Rap1A binding to cytochrome b558 of NADPH oxidase by phosphorylation of Rap1A. Science. 1991;254:1794–1796. doi: 10.1126/science.1763330. [DOI] [PubMed] [Google Scholar]
- 5.Caron E, Self A J, Hall A. The GTPase Rap1 controls functional activation of macrophage integrin αMβ2 by LPS and other inflammatory mediators. Curr Biol. 2000;10:974–978. doi: 10.1016/s0960-9822(00)00641-2. [DOI] [PubMed] [Google Scholar]
- 6.Cass L A, Meinkoth J L. Differential effects of cyclic adenosine 3′, 5′-monophosphate on p70 ribosomal S6 kinase. Endocrinology. 1998;139:1991–1998. doi: 10.1210/endo.139.4.5880. [DOI] [PubMed] [Google Scholar]
- 7.Cass L A, Meinkoth J L. Ras signaling through PI3K confers hormone-independent proliferation that is compatible with differentiation. Oncogene. 2000;19:924–932. doi: 10.1038/sj.onc.1203393. [DOI] [PubMed] [Google Scholar]
- 8.Cass L A, Summers S A, Prendergast G V, Backer J M, Birnbaum M J, Meinkoth J L. PKA-dependent and -independent signaling pathways contribute to cAMP-stimulated proliferation. Mol Cell Biol. 1999;19:5882–5891. doi: 10.1128/mcb.19.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inove T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- 10.DeNooij J C, Letendre M A, Hariharan I K. A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell. 1996;87:1237–1247. doi: 10.1016/s0092-8674(00)81819-x. [DOI] [PubMed] [Google Scholar]
- 11.DeRooij J, Bos J L. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 12.DeRooij J, Rehmann H, van Tries M, Cool R H, Wittinghofer A, Bos J L. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275:20829–20836. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- 13.DeRooij J, Zwartkruis F T, Verheijen M H G, Cool R H, Nijman S M B, Wittinghofer A, Bos J L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 14.Downward J. Role of phosphoinositide-3-OH kinase in Ras signaling. Adv Second Messenger Phosphoprotein Res. 1997;31:1–10. doi: 10.1016/s1040-7952(97)80004-3. [DOI] [PubMed] [Google Scholar]
- 15.Dremier S, Vandeput F, Zwartkruis F J T, Bos J L, Dumont J E, Maenhaut C. Activation of the small G protein Rap1 in dog thyroid cells by both cAMP-dependent and -independent pathways. Biochem Biophys Res Commun. 2000;267:7–11. doi: 10.1006/bbrc.1999.1919. [DOI] [PubMed] [Google Scholar]
- 16.Dumont J E, Lamy F, Roger P, Maenhaut C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thryotropin and other factors. Physiol Rev. 1992;72:667–697. doi: 10.1152/physrev.1992.72.3.667. [DOI] [PubMed] [Google Scholar]
- 17.Fusco A, Berlinghieri M T, Fiore P P D, Portella G, Grieco M, Vecchio G. One- and two-step transformation of rat thyroid epithelial cells by retroviral oncogenes. Mol Cell Biol. 1987;7:3365–3370. doi: 10.1128/mcb.7.9.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabig T G, Crean C D, Mantel P L, Rosli R. Function of wild-type or mutant Rac2 and Rap1a GTPases in differentiated HL60 cell NADPH oxidase activation. Blood. 1995;85:804–811. [PubMed] [Google Scholar]
- 19.Guo K, Warg J, Andros V, Smith R C, Walsh K. MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol Cell Biol. 1995;15:3823–3829. doi: 10.1128/mcb.15.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhce J, Hanron G J, Beach D, Lassar A B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 21.Hata Y, Kaibuchi K, Kawamura S, Hiroyoshi M, Shirataki H, Takai Y. Enhancement of the action of smg p21 GTP/GTP exchange protein by the protein kinase A-catalyzed phosphorylation of smg p21. J Biol Chem. 1991;266:6571–6577. [PubMed] [Google Scholar]
- 22.Hu C-D, Kariya K, Kotani G, Shirouzu M, Yokoyama S, Kataoka T. Coassociation of Rap1A and Ha-Ras with Raf-1 N-terminal region interferes with Ras-dependent activation of Raf-1. J Biol Chem. 1997;272:11702–11705. doi: 10.1074/jbc.272.18.11702. [DOI] [PubMed] [Google Scholar]
- 23.Hu C-D, Kariya K, Okada T, Qi X, Song C, Kataoka T. Effect of phosphorylation on activities of Rap1A to interact with Raf-1 and to suppress Ras-dependent Raf-1 activation. J Biol Chem. 1999;274:48–51. doi: 10.1074/jbc.274.1.48. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Lin J, Su Z Z, Collart F R, Huberman E, Fisher P B. Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21 WAF1/CIP1 expression in the absence of p53. Oncogene. 1994;9:3397–3406. [PubMed] [Google Scholar]
- 25.Kawasaki H, Springett G M, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman D E, Graybiel A M. A family of cAMP-binding proteins that directly activate Rapl. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 26.Kawata M, Kikuchi A, Hoshijima M, Yamamoto K, Hashimoto E, Yamamura H, Takai Y. Phosphorylation of smg p21, a ras p21-like GTP-binding protein, by cyclic AMP-dependent protein kinase in a cell-free system and in response to prostaglandin E1 in intact human platelets. J Biol Chem. 1989;264:15688–15695. [PubMed] [Google Scholar]
- 27.Kikuchi A, Williams L T. Regulation of interaction of ras p21 with Ra1GDS and Raf-1 by cyclic AMP-dependent protein kinase. J Biol Chem. 1996;271:588–594. doi: 10.1074/jbc.271.1.588. [DOI] [PubMed] [Google Scholar]
- 28.Kitayama H, Matsuzaki T, Ikawa Y, Noda M. Genetic analysis of the Kirsten-ras-revertant 1 gene: Potentiation of its tumor suppressor activity by specific point mutations. Proc Natl Acad Sci USA. 1990;87:4284–4288. doi: 10.1073/pnas.87.11.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohn L D, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287–384. doi: 10.1016/s0083-6729(08)60658-5. [DOI] [PubMed] [Google Scholar]
- 30.Kupperman E, Wen W, Meinkoth J L. Inhibition of thyrotropin-stimulated DNA synthesis by microinjection of inhibitors of cellular ras and the cyclic AMP dependent protein kinase. Mol Cell Biol. 1993;13:4477–4484. doi: 10.1128/mcb.13.8.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kupperman E, Wofford D, Wen W, Meinkoth J L. Ras inhibits thyroglobulin expression but not cyclic adenosine monophosphate-mediated signaling in Wistar rat thyrocytes. Endocrinology. 1996;137:96–104. doi: 10.1210/endo.137.1.8536648. [DOI] [PubMed] [Google Scholar]
- 32.Lemoine N R, Staddon S, Bond J, Wyllie F S, Shaw J J, Wynford-Thomas D. Partial transformation of human thyroid epithelial cells by mutant Ha-ras oncogene. Oncogene. 1990;5:1833–1837. [PubMed] [Google Scholar]
- 33.Lintig F C V, Pilz R B, Boss G R. Quantitative determination of Rap1 activation in cyclic nucleotide-treated HL-60 cells: lack of Rap1 activation in variant cells. Oncogene. 2000;19:4029–4034. doi: 10.1038/sj.onc.1203741. [DOI] [PubMed] [Google Scholar]
- 34.Maly F E, Quilliam L A, Dorseuil O, Der C J, Bokoch G M. Activated or dominant inhibitory mutants of Rap1A decrease the oxidative burst of Epstein-Barr virus-transformed human B lymphocytes. J Biol Chem. 1994;269:18743–18746. [PubMed] [Google Scholar]
- 35.Miller M J, Prigent S, Kupperman E, Rioux L, Park S-H, Feramisco J R, White M A, Rutkowski J L, Meinkoth J L. RalGDS functions in Ras- and cAMP-mediated growth stimulation. J Biol Chem. 1997;272:5600–5605. doi: 10.1074/jbc.272.9.5600. [DOI] [PubMed] [Google Scholar]
- 36.Miller M J, Rioux L, Prendergast G V, Cannon S, White M A, Meinkoth J L. Differential effects of protein kinase A on ras effector pathways. Mol Cell Biol. 1998;18:3718–3726. doi: 10.1128/mcb.18.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Missero C, Calauti E, Eckner R, Chin J, Tsai L H, Livingston D M, Gotto G P. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc Natl Acad Sci USA. 1995;92:5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Missero C, Cobellis G, DeFelice M, DiLauro R. Molecular events involved in differentiation of thyroid follicular cells. Mol Cell Endocrinol. 1998;140:37–43. doi: 10.1016/s0303-7207(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 39.Oki N, Takahashi S-I, Hidaka H, Conti M. Short-term feedback regulation of cAMP in FRTL-5 thyroid cells. Role of PDE4D3 phosphodiesterase activation. J Biol Chem. 2000;275:10831–10837. doi: 10.1074/jbc.275.15.10831. [DOI] [PubMed] [Google Scholar]
- 40.Pizon V, Mechali F, Baldacci G. Rap1A GTP/GDP cycles determine the intracellular location of the late endocytic compartments and contribute to myogenic differentiation. Exp Cell Res. 1999;246:56–68. doi: 10.1006/excr.1998.4284. [DOI] [PubMed] [Google Scholar]
- 41.Quilliam L A, Mueller H, Bohl B P, Prossnitz V, Sklar L A, Der C J, Bokoch G M. Rap1A is a substrate for cyclic AMP-dependent protein kinase in human neutrophils. J Immunol. 1991;147:1628–1635. [PubMed] [Google Scholar]
- 42.Reedquist K A, Ross E, Koop E A, Wolthuis R M F, Zwartkruis F J T, Kooyk Y v, Salmon M, Buckley C D, Bos J L. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148:1151–1158. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothermel J D, Botelho L H P. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3′, 5′-(cyclic)phosphorothioate with purified cyclic AMP-dependent kinase. Biochem J. 1988;251:757–762. doi: 10.1042/bj2510757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheele J S, Pilz R B, Lintig F C V, Boss G R. Deficient posttranslational processing of Rap1A in variant HL-60 cells. Oncogene. 1998;17:2211–2223. doi: 10.1038/sj.onc.1202137. [DOI] [PubMed] [Google Scholar]
- 45.Serebriiskii I, Estojak J, Sonoda G, Testa J R, Golemis E A. Association of Krev-1/Rap1a with Krit1, a novel ankyrin repeat-containing protein encoded by a gene mapping to 7q21–22. Oncogene. 1997;15:1043–1049. doi: 10.1038/sj.onc.1201268. [DOI] [PubMed] [Google Scholar]
- 46.Siess W, Winegar D A, Lapetina E G. Rap1B is phosphorylated by protein kinase A in intact human platelets. Biochem Biophys Res Commun. 1990;170:944–950. doi: 10.1016/0006-291x(90)92182-y. [DOI] [PubMed] [Google Scholar]
- 47.Steinman R A, Hoffman B, Gullcuf C, Liebermann D A, El-Houseini M E. Induction p21 (WAF-1/CIP1) during differentiation. Oncogene. 1994;9:3389–3396. [PubMed] [Google Scholar]
- 48.Swanson D J, Zellmer E, Lewis E J. AP1 proteins mediate the cAMP response of the dopamine β-hydroxylase gene. J Biol Chem. 1998;273:24065–24074. doi: 10.1074/jbc.273.37.24065. [DOI] [PubMed] [Google Scholar]
- 49.Vossler M R, Yao H, York R D, Pan M-G, Rim C S, Stork P J. cAMP activates MAP kinase and EIk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 50.Westwick J K, Lambert Q T, Clark G J, Symons M, Van Aelst L, Pestell R G, Der C J. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao H, York R D, Misra-Press A, Carr D W, Stork P J S. The cyclic adenosine monophosphate-dependent protein kinase (PKA) is required for the sustained activation of mitogen-activated kinases and gene expression by nerve growth factor. J Biol Chem. 1998;273:8240–8247. doi: 10.1074/jbc.273.14.8240. [DOI] [PubMed] [Google Scholar]
- 52.Zwartkruis F J T, Bos J L. Ras and Rap1: two highly related small GTPases with distinct function. Exp Cell Res. 1999;253:157–165. doi: 10.1006/excr.1999.4695. [DOI] [PubMed] [Google Scholar]