Abstract
Objective:
To assess the association of bipolar disorder (BD) with risk of major adverse cardiac events (MACE) after adjusting for established cardiovascular disease (CVD) risk factors.
Patients and Methods:
We conducted a population-based historical cohort study using the Rochester Epidemiology Project. Patients aged >30 with a clinical encounter from 1998–2000 with no prior MACE, atrial fibrillation, or heart failure, were followed through March 1, 2016. BD diagnosis was validated by chart review. Cox proportional hazards regression models were adjusted for established CVD risk factors, alcohol use disorder (AUD), other substance use disorders (SUD), and major depressive disorder (MDD).
Results:
The cohort included 288 individuals with BD (0.81%) and 35,326 individuals without BD as the reference group (Ref). Median (IQR) follow-up was 16.5 (14.6–17.5) years. 5636 MACE events occurred (BD: 59, Ref: 5577). Survival analysis showed an association between BD and MACE (median event-free-survival rates BD: 0.80, Ref: 0.86; log-rank P=.018). Multivariate regression adjusting for age and sex also yielded an association between BD and MACE (HR: 1.93; 95% CI: 1.43–2.52; P<.001). The association remained significant after further adjusting for smoking, diabetes mellitus, hypertension, HDL cholesterol, and body mass index (HR: 1.66; 95% CI 1.17–2.28; P=.006), and for AUD, SUD, and MDD (HR: 1.56; 95% CI 1.09–2.14; P=.010).
Conclusion:
In this study, BD was associated with an increased risk of MACE which persisted after adjusting for established CVD risk factors, SUDs, and MDD. These results suggest that BD is an independent risk factor for major clinical cardiac disease outcomes.
Keywords: Bipolar disorder, cardiovascular disease, major adverse cardiovascular outcomes, cohort
Introduction
Bipolar disorder (BD) is associated with excess mortality both for natural and unnatural (i.e. suicide) causes of death(1). The largest contributor to excess mortality for patients with BD is cardiovascular disease (CVD)(2, 3).
The link between CVD and serious mental illness including BD is compelling, with some of the earliest associations recorded as early as 1933(4) and with more rigorous examinations through Scandinavian health registries beginning in the late 20th century(5). A recent meta-analysis, including more than 3.2 million patients with severe mental illness, confirmed these earlier observations of significantly higher risk of CVD and CVD mortality(6, 7). However, study results looking at CVD outcomes in cohorts restricted to BD have been inconsistent because of problems with study design and adjustment for cardiac risk factors. Cross-sectional studies have not reliably shown significant association of BD with CVD after adjusting for covariates. Although longitudinal studies have demonstrated increased risk of CVD and mortality in bipolar cohorts both in adjusted and unadjusted models, adjustment for CVD risk factors has been inconsistent and heterogeneous(7, 8). Therefore, it is unclear whether the increased risk of CVD and mortality is primarily mediated by known CVD risk factors or whether BD contributes additional risk via an unknown pathophysiological mechanism. Indeed, patients with BD have been shown to have a higher prevalence of CVD risk factors including obesity, hypertension, dyslipidemia, diabetes, and metabolic syndrome(9–11) and have been shown in some studies to develop CVD risk factors and CVD at a younger age than people without BD(6, 12). Although Goldstein et al. suggest that development of CVD at a younger age is independent of traditional risk factors, their findings were based on survey data and did not include measurements of blood pressure, lipids, blood sugar, or other factors(6). Moreover, a smaller cohort study from our group with risk factor measurements and greater diagnostic certainty did find an increased risk of myocardial infarction (MI) or stroke in patients with BD. However, these associations were no longer significant after adjustment for CVD risk factors(13).
In summary, relatively few cohort studies have investigated the incidence of ischemic heart disease, atherosclerotic CVD events, and mortality in patients with BD, and most of these studies are Scandinavian register-based studies(14). However, such national registry studies are weakened by a lack of clinical validation of BD and limited information regarding CVD risk factors(2, 15). Additionally, many of these studies have focused solely on mortality from CVD and other causes rather than assessing nonfatal cardiovascular events(2, 3, 14–18). Because nonfatal diseases are increasingly responsible for disability(19, 20), there is a critical need to gain a better understanding of the role of BD in contributing to a wide range of fatal and nonfatal major adverse cardiac events (MACE), a clinically meaningful composite outcome encompassing MI, ischemic and hemorrhagic stroke, percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), and total mortality.
The hypothesis of the present study is that patients with BD are at greater risk of MACE compared to the general population after adjusting for known CVD risk factors.
Methods
Design and data sources
We conducted a population-based, historical cohort study using resources from the Rochester Epidemiology Project (REP), a federally-funded medical records-linkage system that indexes medical diagnoses, prescriptions, procedures, and other health-related information from the medical care providers in Olmsted County MN, including Olmsted Medical Center, Mayo Clinic, and several private practices(21, 22).Using the population census in the year 2000, we identified all residents of Olmsted County, MN, older than 30 years of age who sought primary care (community internal medicine and family medicine visits identified electronically with billing and procedural codes from the records-linkage system) between 1998 and 2003 and were followed up through March 1, 2016 (periods where information was available from the REP). We excluded those who had missing laboratory values, did not provide research authorization(23) – all included individuals signed an authorization form for their medical records to be used in a deidentified manner for research, or who had a known history of coronary artery disease (defined as a history of stable or unstable angina, MI or coronary revascularization), stroke, atrial fibrillation, or heart failure at baseline. This approach was implemented to approximate a primary prevention cohort and to better understand how BD might be associated with cardiovascular outcomes. The institutional review boards of both the Mayo Clinic and Olmsted Medical Center reviewed and approved the study protocol.
All clinical information was extracted electronically from the REP. Collected baseline information included participant demographics, clinical diagnosis, laboratory values, blood pressure measurements, and medications, as previously described(21). Individuals’ clinical diagnoses, including CVD, major depressive disorder (MDD), alcohol use disorder (AUD), and other substance use disorders (SUD) were ascertained electronically using codes from the International Classification of Diseases 9th and 10th Edition (ICD-9/10)(24). To decrease false-positive results, 2 occurrences of a code (either the same code or 2 different codes within the code set for a given disease) separated by >30 days and occurring within 5 years before the index date were required for diagnosis, an approach that has been validated in the past(25, 26).
Possible individuals with BD were screened electronically using ICD-9/10 and HICDA billing codes (n=1829). However, because of lack of specificity of some billing codes and because codes were assigned by a wide range of providers from various specialties, we used several approaches to identify individuals with a higher probability of having BD including: (1) cross-matching with the Mayo Clinic Bipolar Biobank Study(27); (2) cross-matching with the results from a previous cohort study(13); (3) identifying patients who were prescribed mood stabilizers including lithium, sodium valproate, carbamazepine and lamotrigine, at baseline or during the cohort follow-up; and (4) identifying patients who had inpatient hospitalizations for BD. The electronic medical records (EMR) of all 627 individuals who were identified by the aforementioned methods were then manually reviewed by MF and to confirm the diagnosis of BD using DSM-IV-TR criteria for manic and hypomanic episodes(28). All of the patients identified by MF were confirmed by repeat chart review by RJM. Disagreements were discussed and a mutual decision was reached. 15 patients initially included in the BD group by MF were excluded after repeat review and discussion.
Outcomes
The primary outcome was the incidence of MACE, defined as the composite outcome of MI, ischemic and hemorrhagic stroke, PCI, CABG, and death in patients with BD compared to the reference group. Secondary outcomes assessed non-violent or cardiovascular related deaths as part of the MACE outcome. Mortality information was obtained from the REP, which records vital status from federal and Minnesota death registries. ICD-9 and ICD-10 were used to define underlying cause of death(29). Cardiovascular deaths were classified with ICD-9 codes 390 to 398, 402, and 404 to 429, and with ICD-10 codes I00 to I09, I11, I13, and I20 to I51. Violent deaths were classified with ICD-9 codes E800-E959 and ICD-10 codes, T36, T71, T80-T86, X71-X83. Each individual in the study sample was passively followed with a thorough review of the EMR in the records-linkage system, from the baseline visit (index date) through the occurrence of MACE or last contact with the REP.
Statistical Analysis
We summarized baseline patient characteristics with frequencies and percentages, means ± standard deviations (SD), or medians and interquartile range (IQR). Patient characteristics were compared with Chi-square test, Fisher exact test, 2-sample t-tests, or Wilcoxon rank-sum test as appropriate. For the primary outcome, the association between BD and MACE was first estimated with the Kaplan-Meier method and tested using log-rank test. Cox proportional hazard models were then estimated and adjusted for age, sex, and factors known to be associated with MACE or thought to mediate the association between BD and MACE. We also performed sensitivity analyses that censored violent deaths (those deemed to be related to homicide, suicide, or other death due to firearm) from the MACE outcome. Post-hoc analyses were performed adjusting for the Charlson Comorbidity Index and including only cardiovascular-specific mortality. Findings were summarized using Kaplan-Meier event-free rates, hazard ratios (HRs), and 95% confidence intervals (CIs). The assumption of proportionality for the Cox proportional hazards regression models was assessed and met. In all analyses, 2-tailed P<0.05 values were considered statistically significant. All analyses were completed using JMP, Version 14.1.0 (SAS Institute Inc, Cary, NC).
Results
During the study period, 39,160 Olmsted County, MN residents sought primary care and 35,614 met eligibility criteria. The baseline characteristics of the 3,546 excluded individuals are shown in Table S1 (Supplemental Digital Content). Of the included individuals, 288 (0.81%) had confirmed BD. The other 35,326 individuals comprised the reference group. At baseline, the BD group was younger (47.7±10.9 vs 49.8±13.3 years, respectively, P=.001) and more racially homogeneous (94.01% vs 92.69% European Ancestry, P=0.014) than the reference group. The BD group also had higher body mass index (BMI) (29.68±7.81 vs 28.02±6.34, P=0.002), lower diastolic blood pressure (78.86±23.94 vs 80.35±11.62 mmHg, P=.037), lower high-density lipoprotein (HDL) cholesterol (51.6±16.52 vs 53.31±15.44, P=.034), and higher rates of hypertension (HTN) (21.53% vs 17.25%, P<.001), diabetes mellitus (DM) (14.58% vs 10.21%, P=.021), chronic kidney disease (CKD) (5.2% vs 4.3%, P=.042), current smoking (31.94% vs 15.18%, P<.001), AUD (22.92% vs 4.36%, P<.001), and SUD (11.81% vs 1.51%, P<.001). Other measured baseline demographic and CVD risk factor characteristics did not differ between the two groups (Table 1).
Table 1.
Baseline characteristics of patients with bipolar disorder and the reference group.
| All n=35,614 n (%) or Mean (SD) |
Reference Group n=35,326 n (%) or Mean (SD) |
Bipolar Disorder n=288 n (%) or Mean (SD) |
Between-Group Comparison P-value |
|
|---|---|---|---|---|
| Age (years) | 49.8 (13.2) | 49.8 (13.3) | 47.7 (10.9) | .001 |
| Male | 16,435(46.1%) | 16,311 (46.1%) | 124 (43.1%) | .29 |
| European Ancestry | 33,013 (92.7%) | 32,742 (92.7%) | 271 (94.1%) | .014 |
| BMI | 28.03 (6.3) | 28.02 (6.3) | 29.7 (7.8) | .002 |
| HTN History | 6,157 (17.3%) | 6,095 (17.3%) | 62 (21.5%) | <.001 |
| Total Cholesterol | 203.8 (39.2) | 203.8 (39.2) | 207.9 (41.7) | .093 |
| HDL | 53.3 (15.5) | 53.3 (15.4) | 51.6 (16.5) | .034 |
| Current Smoking | 5,453 (15.3%) | 5,361 (15.2%) | 92 (31.9%) | <.001 |
| DM History | 3,648 (10.2%) | 3,606 (10.2%) | 42 (14.6%) | .021 |
| COPD History | 1,637(4.6%) | 1,622(4.6%) | 15 (5.2%) | .084 |
| CKD History | 1,517(4.3%) | 1,502(4.3%) | 15 (5.2%) | .042 |
| Creatinine (mg/dL) | 1.05 (0.42) | 1.05 (0.43) | 1.06 (0.32) | .57 |
| AUD | 1,605 (4.5%) | 1,539 (4.4%) | 66 (22.9%) | <.001 |
| SUD | 569 (1.6%) | 535 (1.5%) | 34 (11.8%) | <.001 |
| Charlson Comorbidity Index | 0.30 (0.85) | 0.30 (0.84) | 0.45 (1.09) | <.001 |
N- Number; SD- Standard Deviation; BMI- Body Mass Index; HTN- Hypertension; HDL- High Density Lipoprotein; DM- Diabetes Mellitus; COPD- Chronic Obstructive Pulmonary Disease; CKD- Chronic Kidney Disease; AUD- Alcohol Use Disorder; SUD- Substance Use Disorder
During a median (IQR) follow-up of 16.5 (14.6–17.5) years, there were a total of 5,636 MACE events, including 2,777 deaths. 59 events occurred in the bipolar group and 5,577 in the reference group. A summary of MACE events is detailed in Table 2.
Table 2:
Summary of MACE events in patients with bipolar disorder and reference group.
| All n=35,614 n |
Reference Group n=35,326 n |
Bipolar Disorder n=288 n |
|
|---|---|---|---|
| MI | 1,800 | 1,784 | 16 |
| Stroke | 98 | 96 | 2 |
| Death | 2,777 | 2,745 | 32 |
| PCI | 795 | 787 | 8 |
| CABG | 166 | 165 | 1 |
| Total Events | 5,636 | 5,577 | 59 |
MACE- Major Adverse Cardiac Events, N- Number; MI- Myocardial Infarction; PCI- Percutaneous Coronary Intervention; CABG- Coronary Artery Bypass Grafting. Table presents first recorded event included in the survival analysis.
BD was associated with an increased risk of MACE. The 15-year event-free-survival rates were 0.81 for those with BD vs 0.86 for reference individuals (log-rank P=.018) (Figure 1). Univariate analysis showed increased HR for MACE for all established risk factors (Table 3) and also for AUD (HR=1.59; 95% CI:1.41–1.79; P<0.001), BD (HR=1.45; 95% CI:1.04–1.84; P=.025), and MDD (HR=1.11; 95% CI:1.01–1.21; P=.010). An inverse relationship was found between MACE and HDL (HR=0.98; 95% CI:0.98–0.99; P=.001). Multivariate analysis adjusting for age and sex (Model 1, Table 3) yielded a significant association between BD and MACE (HR=1.93; 95% CI:1.43–2.52; P<.001). After adjustment for smoking, DM, HTN, HDL, and BMI in addition to age and sex (Model 2, Table 3), the association between BD and MACE remained significant (HR=1.66; 95% CI:1.17–2.28; P=0.005). Further adjustment for AUD, SUD, and MDD (Model 3, Table 3) demonstrated a consistent relationship between BD and MACE (HR=1.56; 95% CI:1.09–2.14; P=.010).
Figure 1.
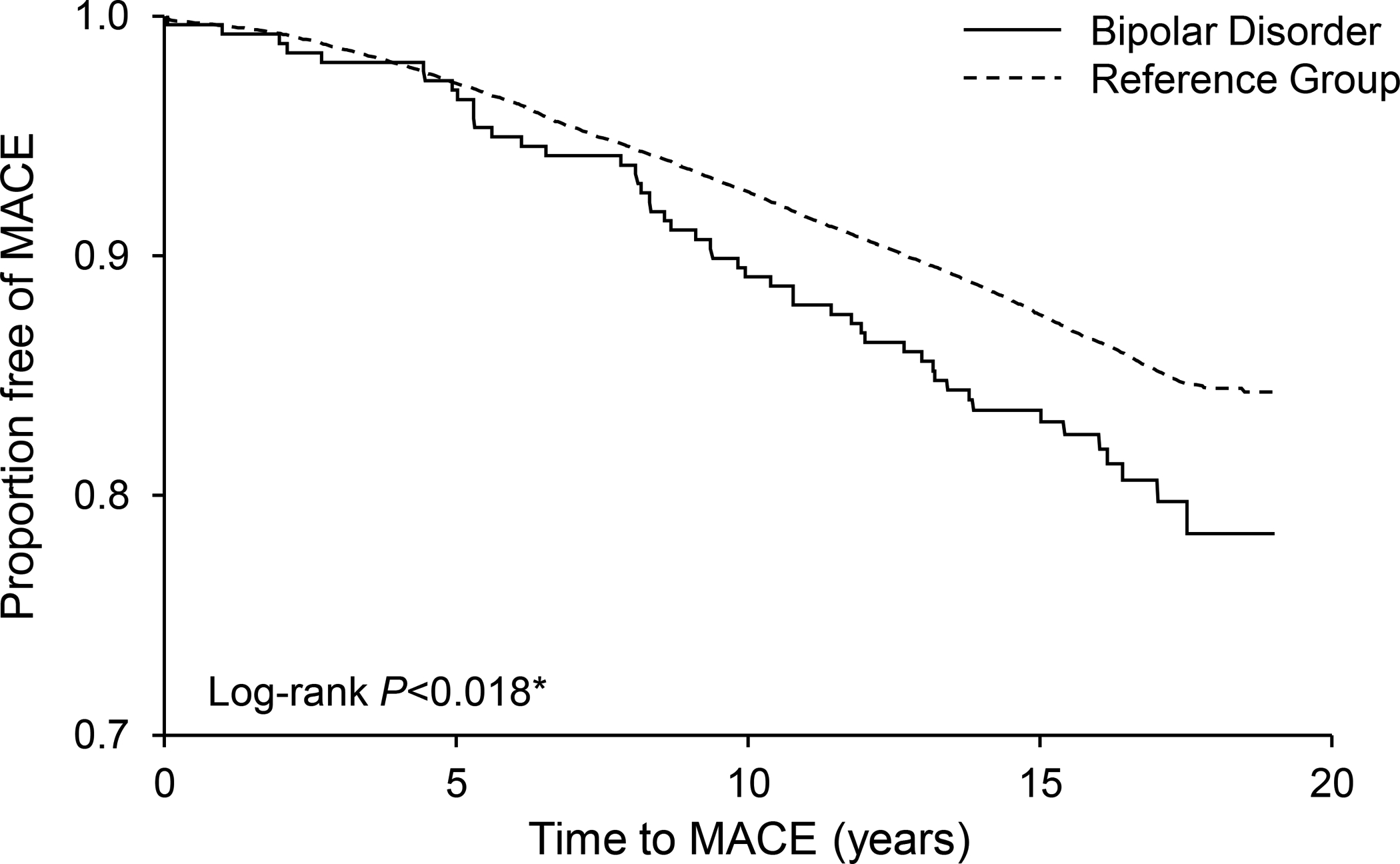
Kaplan-Meier curves showing the association between bipolar disorder and major adverse cardiovascular events (MACE).
Table 3.
Cox proportional hazard models testing the association between bipolar disorder and MACE.
| Univariate analysis | HR | 95% CI | P-value |
|---|---|---|---|
| Bipolar disorder | 1.45 | 1.04–1.84 | .025 |
| Age | 1.08 | 1.08–1.09 | <.001 |
| Male Sex | 1.60 | 1.51–1.70 | <.001 |
| BMI | 0.997 | 0.99–1.00 | .18 |
| HDL | 0.86 | 0.84–0.88 | <.001 |
| HTN History | 3.09 | 2.90–3.29 | <.001 |
| Current Smoking | 1.2 | 1.11–1.30 | <.001 |
| Diabetes History | 2.81 | 2.60–3.02 | <.001 |
| CKD History | 0.98 | 0.84–1.12 | .74 |
| Alcohol Use Disorder | 1.59 | 1.41–1.79 | <.001 |
| Other Substance Use Disorder | 1.15 | 0.91–1.44 | .22 |
| Major Depression at Baseline | 1.11 | 1.01–1.21 | .010 |
| Multivariate analysis | |||
| Model 1 | |||
| Bipolar Disorder | 1.93 | 1.43–2.52 | <.001 |
| Model 2 | |||
| Bipolar Disorder | 1.66 | 1.17–2.28 | .005 |
| Model 3 | |||
| Bipolar Disorder | 1.56 | 1.09–2.14 | .010 |
| Model 4 | |||
| Bipolar Disorder | 1.77 | 1.37–2.28 | <.001 |
The models above adjust for the confounders specified below:
Model 1: Age, sex
Model 2: Age, sex, smoking, diabetes, HTN, BMI, HDL
Model 3: Age, sex, smoking, diabetes, CKD, HTN, alcohol use disorder, BMI, HDL, other substance use disorder, major depression.
Model 4: adjusted for age, sex and the Charlson comorbidity index.
Represents Hazard ratio per each 10 mg/dL increase.
HR- Hazard Ratio; CI- Confidence Interval; BMI- Body Mass Index; HDL- High-Density Lipoprotein; HTN- Hypertension; CKD- Chronic Kidney Disease
Of the 2,777 deaths in the entire cohort, 47 were classified as violent (2 in the BD group and 45 in the reference group). Sensitivity analysis excluding violent deaths did not change the association between BD and MACE (Model 3 HR=1.58; 95% CI: 1.12–2.23; P=.009). Adjusting for the Charlson Comorbidity Index similarly did not change the association (Model 4 HR=1.77; 95% CI: 1.37–2.28; P<.001). Repeat analysis including only deaths due to cardiovascular mortality resulted in similar findings (Table S2, Supplemental Digital Content).
Discussion
This population-based historical cohort study found a significant association between BD and MACE even after adjusting for known CVD risk factors, addiction, and MDD. To our knowledge, this is the first study to interrogate the longstanding association between this clinically important composite outcome and BD after adjusting for baseline conventional CVD risk factors.
The primary finding of this study suggests that BD may convey additional CVD risk to patients beyond the risk generated by baseline CVD risk factors. This is a notable finding because prior longitudinal studies have had methodological drawbacks that make interpretation of their results challenging. The BD-CVD association may have been either overstated or understated previously due to factors such as lack of complete adjustment for baseline CVD risk or inclusion of individuals without valid BD diagnoses. This study, albeit with significant limitations, attempts to address these concerns by adjusting for maximal CVD risk factor data and including only patients with a chart-reviewed diagnosis of BD. Ultimately, our fully adjusted HR of 1.56 in 288 BD patients aligns with the meta-analytic result of a covariate-adjusted HR of 1.57 for BD-CVD risk in the BD subset of greater than 3.2 million patients with serious mental illness published previously(7).
The mechanism by which BD adds to CVD risk remains unknown although a number of theories include: inflammation(30), mitochondrial dysfunction(31), autonomic dysfunction(32), hypothalamic-pituitary-adrenal axis dysfunction(33, 34), oxidative stress(35), sedentary lifestyle(36), and others. The present study does not provide much further clarity in this regard, and much work remains to be done to clarify the biological mechanisms. Lack of access to care has been proposed to explain why patients with mental illness experience such extensive medical comorbidity(37, 38). In our study, both patients with BD and reference individuals were, at least for a period of time between 1998–2000, treatment-seeking. Nevertheless, interactions with the healthcare system were insufficient to negate the disparities in CVD risk in this study. It is possible that the BD patients in our study, despite being treatment-seeking, received different care over the 16.5 years of follow-up compared to the reference group. This difference, in turn, may have contributed both to the observed disparities in known CVD risk factors and to disparities in as-yet-unknown causes of increased risk, resulting in the persistently elevated risk profile seen here despite our adjusting for CVD risk factors.
Another notable aspect of this study population is that Olmsted County, MN is relatively racially homogeneous with a population that is >80% European Ancestry and with a higher than national-average education level and household income(39). Residents of Olmsted County also have access to healthcare facilities providing high-quality care. The increased risk of MACE in patients with BD in this population is all the more remarkable since socioeconomic and racial disparities frequently magnify the increased risk of morbidity and mortality arising from mental illness(40–42). Although we do not know whether patients with BD in our study population were of lower socioeconomic status than the reference individuals, racial homogeneity was higher in the BD group than the reference group. Additionally, regardless of socioeconomic status, our patients with BD had access to clinical care during at least some portion of the study period. Our findings, therefore, underscore the importance of CVD monitoring plus early diagnosis and treatment of heart conditions in patients with BD, regardless of their baseline demographic characteristics.
This study has a number of strengths. First, although BD diagnoses were initially ascertained using administrative codes, final diagnoses were determined via chart review by two investigators, including a board-certified psychiatrist (RJM). Indeed, this resulted in elimination of approximately 84% of the charts with a code due to insufficient evidence to support the BD diagnosis. Although high, this exclusion rate is similar to the 70% of charts that were excluded after psychiatrist review in our prior study of BD(13) and in other non-psychiatric REP studies. For example, the confirmation rate of MI diagnostic codes was only 33% in another REP study(43). The individuals with BD in this study have historically demonstrated symptoms that accurately reflect their underlying diagnosis, thereby enhancing the validity of the present findings. A second strength of this study is the long duration of follow-up. The mean length of follow-up was 16.5 years, representing one of the longest follow-up durations for any BD cohort in the United States(12, 44). Third, this cohort consisted of individuals with a primary care visit during the study period, which ensured that baseline CVD risk factors were available for analysis. All BD patients and reference individuals had baseline levels of total cholesterol, HDL cholesterol, systolic and diastolic blood pressures, diabetes status, smoking status, and hypertension treatment status as inclusion criteria. This requirement is, to our knowledge, more stringent than any prior study of patients with BD(7, 12) and is critical for determining whether known CVD risk factors are the primary contributors to MACE or whether there is a distinct pathophysiological mechanism that increases MACE risk in the BD population. Fourth, the patients in our cohort were not required to have seen a psychiatrist nor to have been psychiatrically or medically hospitalized, potentially making these data more generalizable to community settings than prior studies that have utilized inclusion criteria of hospitalization or presentation to psychiatric clinics(3, 17). Of note, we also did not exclude anyone from the reference group with a history of other psychiatric illnesses including schizophrenia or other serious mental illness that has been demonstrated to increase CVD risk. Fifth, our study endpoint included both fatal and nonfatal cardiac events whereas prior studies have most commonly focused on fatal cardiac outcomes(6, 13, 16, 45–47). Nonfatal cardiac outcomes are significant contributors to morbidity, disability, and healthcare costs(48); therefore including them in addition to mortality is both clinically meaningful and provides potential targets for future intervention.
The inability to track quality of care discussed above is one limitation of this study, but there are other limitations. The increased CVD risk in people with BD seen here could be due to residual confounding by differences in CVD risk factors not accounted for in our analysis. However, it is known that the major CVD risk factors account for the majority of CVD events, making this possibility less plausible. We were unable to adjust adequately for the impact of medications over time due to the complexity of medication reporting in the database and lack of information about patient compliance. Medications used for BD, particularly antipsychotics, can have adverse impacts on known CVD risk factors and can potentially result in adverse cardiovascular events on their own(49–51). Our adjustments for baseline CVD risk factors likely mitigated the risk of confounding from medications but did not entirely eliminate it due to the possible interactions of medications with risk factors over time. Additionally, our cohort was followed during a time period when at least 8 new second-generation antipsychotic medications were FDA-approved for at least one indication. Therefore, some BD patients may have been started on new agents not accounted for in the baseline risk factor measurements. Moreover, because BD diagnosis and antipsychotic medication use are highly correlated, it is quite difficult to interpret an analysis adjusting for antipsychotic exposure (i.e. statistically removing the effect of medication). The strong association between the two factors may inadvertently cancel out the effect of BD on the risk of MACE, thus underestimating the true interaction. Because of our inability to assess medication use over time in the cohort, treatment effects such as the roles of early versus late diagnosis and treatment, consistent treatment versus intermittent treatment, or type of treatment received, were unable to be accounted for. Further research is necessary to determine the potential impacts of these treatment factors on CVD risk in subgroups of patients with BD.
Another limitation of this study is the small sample size which necessitated combining patients with bipolar I disorder, bipolar II disorder, and BD not otherwise specified into a single cohort. Subgroup analyses were not possible due to limited statistical power. The small sample size also resulted in relatively few nonfatal events such as MI, stroke, PCI, or CABG (Table 2). Nevertheless, our primary finding was statistically significant and consistent with that from a large meta-analysis of longitudinal cohort studies of patients with BD(7). Additionally, the proportion of individuals with BD is consistent with prevalence rates in other studies(18, 52). Finally, although the use of a composite endpoint is meaningful clinically and particularly relevant for this population, composite endpoints are more difficult to interpret than singular endpoints and may limit comparability between studies(53). In this study, we believed that the clinical relevance of the composite endpoint and the enhanced power it granted outweighed the negative aspects of its use.
Because BD was associated with increased CVD risk beyond the effects mediated by the established risk factors, CVD risk calculators such as the American College of Cardiology/American Heart Association’s ASCVD risk estimator may underestimate CVD risk in patients with BD. Adjustments to these risk calculators may be necessary to account for the impact of BD. Our findings also underscore the importance of the future development of medical and lifestyle interventions to more effectively address the burden of CVD in patients with BD. Such interventions may need to be tailored to the unique challenges presented in BD and will require interdisciplinary collaborations between psychiatry, psychology, cardiology, physical medicine and rehabilitation, case management, occupational and physical therapy, and likely several other disciplines. This study provides further impetus to develop these programs because it underscores the pernicious and ultimately deadly relationship between BD and CVD.
Supplementary Material
Acknowledgments
Financial support and conflict of interest disclosure: This work was supported in part by the European Regional Development Fund - Project ENOCH (No. CZ.02.1.01/0.0/0.0/16_019/0000868), resources of the Rochester Epidemiology Project which is supported by the National Institute on Aging under award R01AG034676, and by grant CONICYT PFCHA/MAGISTER BECAS CHILE/2012 - 73130844, and by funding from the Mayo Clinic Center for Individualized Medicine (CIM).
Mark A. Frye, M.D. reports the following conflicts of interest: Grant Support : Assurex Health, Mayo Foundation, Medibio; Consultant (Mayo): Actify Neurotherapies, Allergan, Intra-Cellular Therapies, Inc., Janssen, Myriad, Neuralstem Inc., Sanofi, Takeda, Teva Pharmaceuticals; CME/Travel/Honoraria: American Physician Institute, CME Outfitters, Global Academy for Medical Education; Financial Interest / Stock ownership / Royalties: None. All other authors declare no conflicts of interest. Presented in part at the Annual Meeting of the American Psychiatric Association, San Francisco, CA, May 2019.
Abbreviations
- AUD
Alcohol Use Disorder
- BD
Bipolar Disorder
- BMI
Body Mass Index
- CABG
Coronary Artery Bypass Graft
- CI
Confidence Interval
- CKD
Chronic Kidney Disease
- CVD
Cardiovascular Disease
- DALY
Disability-Adjusted Life Years
- DM
Diabetes Mellitus
- HLD
High-Density Lipoprotein
- HR
Hazard Ratio
- HTN
Hypertension
- IQR
Interquartile Range
- MACE
Major Adverse Cardiac Events
- MDD
Major Depressive disorder
- MI
Myocardial Infarction
- MN
Minnesota
- PCI
Percutaneous Coronary Intervention
- REP
Rochester Epidemiologic Project
- SD
Standard Deviation
- SUD
Substance Use Disorder
References
- 1.DALYs GBD, Collaborators H. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ösby U, Brandt L, Correia N, Ekbom A, Sparén P. Excess mortality in bipolar and unipolar disorder in Sweden. Archives of general psychiatry. 2001;58:844–50. [DOI] [PubMed] [Google Scholar]
- 3.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. Journal of affective disorders. 2002;68:167–81. [DOI] [PubMed] [Google Scholar]
- 4.Derby IM. Manic-depressive “Exhaustion” deaths. The Psychiatric Quarterly. 1933;7:436–49. [Google Scholar]
- 5.Anda R, Williamson D, Jones D, Macera C, Eaker E, Glassman A, Marks J. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology. 1993;4:285–94. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein BI, Schaffer A, Wang S, Blanco C. Excessive and premature new-onset cardiovascular disease among adults with bipolar disorder in the US NESARC cohort. The Journal of clinical psychiatry. 2015;76:163–9. [DOI] [PubMed] [Google Scholar]
- 7.Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, Thapa-Chhetri N, Fornaro M, Gallicchio D, Collantoni E. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prieto M, Cuéllar-Barboza A, Bobo WV, Roger VL, Bellivier F, Leboyer M, West CP, Frye MA. Risk of myocardial infarction and stroke in bipolar disorder: a systematic review and exploratory meta-analysis. Acta Psychiatrica Scandinavica. 2014;130:342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, Miller DD, Haynes WG. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Annals of Clinical Psychiatry. 2008;20:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardenas J, Frye MA, Marusak SL, Levander EM, Chirichigno JW, Lewis S, Nakelsky S, Hwang S, Mintz J, Altshuler LL. Modal subcomponents of metabolic syndrome in patients with bipolar disorder. Journal of Affective Disorders. 2008;106:91–7. [DOI] [PubMed] [Google Scholar]
- 11.Johannessen L, Strudsholm U, Foldager L, Munk-Jørgensen P. Increased risk of hypertension in patients with bipolar disorder and patients with anxiety compared to background population and patients with schizophrenia. Journal of affective disorders. 2006;95:13–7. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Miller GE, Raghuveer G, Stoney CM, Wasiak H, McCrindle BW. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease. Circulation. 2015;132:965–86. [DOI] [PubMed] [Google Scholar]
- 13.Prieto ML, Schenck LA, Kruse JL, Klaas JP, Chamberlain AM, Bobo WV, Bellivier F, Leboyer M, Roger VL, Brown RD Jr. Long-term risk of myocardial infarction and stroke in bipolar I disorder: A population-based Cohort Study. Journal of affective disorders. 2016;194:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westman J, Hällgren J, Wahlbeck K, Erlinge D, Alfredsson L, Ösby U. Cardiovascular mortality in bipolar disorder: a population-based cohort study in Sweden. BMJ open. 2013;3:e002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crump C, Sundquist K, Winkleby MA, Sundquist J. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA psychiatry. 2013;70:931–9. [DOI] [PubMed] [Google Scholar]
- 16.Callaghan RC, Khizar A. The incidence of cardiovascular morbidity among patients with bipolar disorder: a population-based longitudinal study in Ontario, Canada. J Affect Disord. 2010;122:118–23. [DOI] [PubMed] [Google Scholar]
- 17.Laursen TM, Munk-Olsen T, Nordentoft M, Mortensen PB. Increased mortality among patients admitted with major psychiatric disorders: a register-based study comparing mortality in unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia. The Journal of clinical psychiatry. 2007;68:899–907. [DOI] [PubMed] [Google Scholar]
- 18.Laursen TM, Wahlbeck K, Hällgren J, Westman J, Ösby U, Alinaghizadeh H, Gissler M, Nordentoft M. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PloS one. 2013;8:e67133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, Detraux J, Gautam S, Moller HJ, Ndetei DM, Newcomer JW, Uwakwe R, Leucht S. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onyeka In MMBAP, Collier Hoegh M M, Naheim Eien Em CM, Nwaru Bi MP, Melle I MDP. Comorbidity of Physical Disorders Among Patients With Severe Mental Illness With and Without Substance Use Disorders: A Systematic Review and Meta-Analysis. J Dual Diagn. 2019;15:192–206. [DOI] [PubMed] [Google Scholar]
- 21.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melton LJ 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. [DOI] [PubMed] [Google Scholar]
- 23.Yawn BP, Yawn RA, Geier GR, Xia Z, Jacobsen SJ. The impact of requiring patient authorization for use of data in medical records research. J Fam Pract. 1998;47:361–5. [PubMed] [Google Scholar]
- 24.H-ICDA, hospital adaptation of ICDA. 2 ed. Activities CoPaH, editor. Ann Arbor, MI1973. [Google Scholar]
- 25.Melton LJ 3rd, Rocca WA, Roger VL. Development of population research at Mayo Clinic. Mayo Clin Proc. 2014;89:e17–20. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain AM, St Sauver JL, Gerber Y, Manemann SM, Boyd CM, Dunlay SM, Rocca WA, Finney Rutten LJ, Jiang R, Weston SA, Roger VL. Multimorbidity in heart failure: a community perspective. The American journal of medicine. 2015;128:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frye MA, McElroy SL, Fuentes M, Sutor B, Schak KM, Galardy CW, Palmer BA, Prieto ML, Kung S, Sola CL. Development of a bipolar disorder biobank: differential phenotyping for subsequent biomarker analyses. International journal of bipolar disorders. 2015;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Psychiatric Association., American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 29.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, Rocca WA. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fries GR, Walss-Bass C, Bauer ME, Teixeira AL. Revisiting inflammation in bipolar disorder. Pharmacol Biochem Behav. 2019;177:12–9. [DOI] [PubMed] [Google Scholar]
- 31.Morris G, Walder K, McGee SL, Dean OM, Tye SJ, Maes M, Berk M. A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav Rev. 2017;74:1–20. [DOI] [PubMed] [Google Scholar]
- 32.Henry BL, Minassian A, Paulus MP, Geyer MA, Perry W. Heart rate variability in bipolar mania and schizophrenia. J Psychiatr Res. 2010;44:168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daban C, Vieta E, Mackin P, Young AH. Hypothalamic-pituitary-adrenal axis and bipolar disorder. Psychiatr Clin North Am. 2005;28:469–80. [DOI] [PubMed] [Google Scholar]
- 34.Fries GR, Vasconcelos-Moreno MP, Gubert C, dos Santos BT, Sartori J, Eisele B, Ferrari P, Fijtman A, Ruegg J, Gassen NC, Kapczinski F, Rein T, Kauer-Sant’Anna M. Hypothalamic-pituitary-adrenal axis dysfunction and illness progression in bipolar disorder. Int J Neuropsychopharmacol. 2014;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatch J, Andreazza A, Olowoyeye O, Rezin GT, Moody A, Goldstein BI. Cardiovascular and psychiatric characteristics associated with oxidative stress markers among adolescents with bipolar disorder. J Psychosom Res. 2015;79:222–7. [DOI] [PubMed] [Google Scholar]
- 36.Vancampfort D, Firth J, Schuch F, Rosenbaum S, De Hert M, Mugisha J, Probst M, Stubbs B. Physical activity and sedentary behavior in people with bipolar disorder: A systematic review and meta-analysis. J Affect Disord. 2016;201:145–52. [DOI] [PubMed] [Google Scholar]
- 37.Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shim RS, Compton MT, Zhang S, Roberts K, Rust G, Druss BG. Predictors of Mental Health Treatment Seeking and Engagement in a Community Mental Health Center. Community Ment Health J. 2017;53:510–4. [DOI] [PubMed] [Google Scholar]
- 39.Cuellar LA, Prieto ED, Cabaleiro LV, Garda HA. Apolipoprotein A-I configuration and cell cholesterol efflux activity of discoidal lipoproteins depend on the reconstitution process. Biochim Biophys Acta. 2014;1841:180–9. [DOI] [PubMed] [Google Scholar]
- 40.Carliner H, Collins PY, Cabassa LJ, McNallen A, Joestl SS, Lewis-Fernandez R. Prevalence of cardiovascular risk factors among racial and ethnic minorities with schizophrenia spectrum and bipolar disorders: a critical literature review. Compr Psychiatry. 2014;55:233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin JL, McLean G, Park J, Martin DJ, Connolly M, Mercer SW, Smith DJ. Impact of socioeconomic deprivation on rate and cause of death in severe mental illness. BMC Psychiatry. 2014;14:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wildes JE, Marcus MD, Fagiolini A. Obesity in patients with bipolar disorder: a biopsychosocial-behavioral model. J Clin Psychiatry. 2006;67:904–15. [DOI] [PubMed] [Google Scholar]
- 43.Roger VL, Jacobsen SJ, Weston SA, Goraya TY, Killian J, Reeder GS, Kottke TE, Yawn BP, Frye RL. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–8. [DOI] [PubMed] [Google Scholar]
- 44.Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder. Ann Clin Psychiatry. 2011;23:40–7. [PMC free article] [PubMed] [Google Scholar]
- 45.Lin HC, Tsai SY, Lee HC. Increased risk of developing stroke among patients with bipolar disorder after an acute mood episode: a six-year follow-up study. J Affect Disord. 2007;100:49–54. [DOI] [PubMed] [Google Scholar]
- 46.Wu HC, Chou FH, Tsai KY, Su CY, Shen SP, Chung TC. The incidence and relative risk of stroke among patients with bipolar disorder: a seven-year follow-up study. PLoS One. 2013;8:e73037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu SI, Chen SC, Liu SI, Sun FJ, Juang JJ, Lee HC, Kao KL, Dewey ME, Prince M, Stewart R. Relative Risk of Acute Myocardial Infarction in People with Schizophrenia and Bipolar Disorder: A Population-Based Cohort Study. PLoS One. 2015;10:e0134763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams MD, Shah ND, Wagie AE, Wood DL, Frye MA. Direct costs of bipolar disorder versus other chronic conditions: an employer-based health plan analysis. Psychiatric Services. 2011;62:1073–8. [DOI] [PubMed] [Google Scholar]
- 49.Polcwiartek C, Kragholm K, Schjerning O, Graff C, Nielsen J. Cardiovascular safety of antipsychotics: a clinical overview. Expert Opin Drug Saf. 2016;15:679–88. [DOI] [PubMed] [Google Scholar]
- 50.Silva A, Ribeiro M, Sousa-Rodrigues CF, Barbosa FT. Association between antipsychotics and cardiovascular adverse events: A systematic review. Rev Assoc Med Bras (1992). 2017;63:261–7. [DOI] [PubMed] [Google Scholar]
- 51.Knoph KN, Morgan RJ 3rd, Palmer BA, Schak KM, Owen AC, Leloux MR, Patel M, Leung JG. Clozapine-induced cardiomyopathy and myocarditis monitoring: A systematic review. Schizophr Res. 2018;199:17–30. [DOI] [PubMed] [Google Scholar]
- 52.Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breitling LP, Mons U, Hahmann H, Koenig W, Rothenbacher D, Brenner H. Composite End Points: Implications of Changing Compositions With Longer Follow-Up. Circ Cardiovasc Qual Outcomes. 2017;10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


