Abstract
Background:
Children are vulnerable to adverse health effects associated with phthalates, and food is one source of exposure. A comprehensive analysis investigating urinary phthalate metabolite concentrations in relation to food type and source has yet to be undertaken.
Objectives:
We use reduced rank regression, a dimension reduction method, to identify dietary patterns associated with urinary phthalate metabolites in children in a large US study.
Methods:
We used data from 2,369 participants 6–19 years old from the 2011—2016 National Health and Nutrition Examination Survey who recalled their diet over the 24 hours prior to urine collection. We used dietary data to estimate intake and source (i.e., prepared at a restaurant vs. purchased from a grocery store) of 136 food groups. We used reduced rank regression to identify dietary patterns explaining variation in overall urinary concentrations of ∑di-2-ethylhexyl phthalate and seven phthalate metabolites. We also examined pairwise associations between food groups and urinary phthalate metabolites.
Results:
We identified eight dietary patterns that cumulatively explained 12.1% of variation in urinary phthalate metabolites, including a dietary pattern characterized by certain starchy vegetables (e.g., plantains and lima beans), quick breads, and citrus juice prepared at a restaurant. A one SD increase in this food pattern score was associated with a 37.2% higher monocarboxyoctyl phthalate (MCOP) concentration (95% CI: 30.3, 44.4). We also observed weak associations between certain food groups and urinary phthalate metabolites (e.g., a one SD increase in intake of certain starchy vegetables prepared at a restaurant was associated with a 1.8% [95% CI: 0.7, 2.8] higher MCOP).
Conclusions:
Children whose diets were characterized by higher consumption of certain starchy vegetables, quick breads, and citrus juices prepared at a restaurant had higher urinary phthalate metabolites. More detailed information on the specific methods of food processing and details on packaging materials is needed.
Keywords: children, diet, dietary pattern, phthalates, National Health, Nutrition Examination Study
Introduction
Phthalate exposure in children is associated with a variety of adverse health effects, including cognitive dysfunction [1], behavioral problems [2], and pulmonary dysfunction [3]. Diet is a primary exposure pathway for high molecular weight phthalate diesters di(2-ethylhexyl) phthalate (DEHP), di-n-octyl phthalate (DnOP), diisononyl phthalate (DiNP), and diisodecyl phthalate (DiDP) [4–6], as well as low molecular weight phthalates dibutyl phthalate (DBP) and diisobutyl phthalate (DiBP) [6, 7].
Phthalates contaminate foods through their widespread use in food processing and packaging materials. For example, DEHP and other high molecular weight phthalates are used to soften polyvinyl chloride, a flexible plastic with applications in commercial milking, gaskets found in the metallic caps of glass jars, and gloves used by food handlers [8]. Because they are not covalently bonded to polymers, phthalates can leach into the foods they contact, particularly at higher temperatures [9–11]. Phthalates have also been detected in assorted food packaging materials, including plastic films [DBP and butyl benzyl phthalate (BBzP)], printing inks (e.g., DBP and DEHP), and adhesives used in cardboard boxes and other paper products (e.g., DBP, DiBP, DEHP, and BBzP) [8]. Some of these phthalate applications (e.g., vinyl gloves used by food handlers and plastic films and other single-serve packaging) are more likely to be used in foods prepared in a restaurant. Finally, environmental contamination can result in uptake of phthalates by crops consumed by humans and livestock [7].
Previous studies have related intake of food groups to urinary phthalate metabolite concentrations without considering heterogeneity within food groups or higher phthalate concentrations in foods prepared at restaurants vs purchased from a grocery store [12–14]. For example, in 6–85 year old participants in the 2003—2004 National Health and Nutrition Examination Survey (NHANES), Colacino et al. reported positive associations of eggs and poultry intake with urinary DEHP metabolites; dairy intake with urinary mono(3-carboxypropyl) phthalate (MCPP); and vegetable and meat intake with urinary monoethyl phthalate (MEP) [15]. Similarly, in 6–19 year old children and adolescents in 2003—2008 NHANES, Trasande et al. found that combined intake of meat, poultry, and fish was positively associated with urinary DEHP metabolites, MCPP, and mono-isobutyl phthalate (MiBP) [16]. These examinations of intake using broad food categories may obscure associations with individual foods and do not account for other aspects of the dietary environment such as food source, providing limited guidance for individuals desiring to reduce their phthalate exposure [17]. Several studies have related overall fast food [14, 18] or cafeteria food [12] consumption to higher concentrations of select urinary phthalate metabolites. One study that jointly examined food and food source reported that associations between a food item and urinary phthalate metabolite concentrations were only present when the food was purchased away from home, but this analysis was limited to a small number of foods [13].
Here, using the highly granular dietary data collected in NHANES, we use reduced rank regression to relate intake of 136 unique food groups in two food settings (foods purchased from a grocery store vs. prepared at a restaurant) to urinary phthalate metabolite concentrations. Classifying foods using dietary patterns provides both a strategy for dimension reduction as well as a way to account for interactions between foods eaten in combination. We use data from 6–10 year old children participating in NHANES.
Materials and Methods
Study population
We combined data from 2011—2012, 2013—2014, and 2015—2016 NHANES, a survey of the civilian, non-institutionalized US population. Participants completed in-person interviews, including a 24-hour dietary recall, and provided urine samples. Of 8,149 2011—2016 NHANES participants with measured urinary phthalate metabolite concentrations, 2,940 were between 6–19 years of age. After excluding participants with missing data on the dietary recall (n=366) or other covariates (n=205), 2,369 participants were included in our analysis. For participants 7–17 years, their parents or guardians provided consent and they provided written assent; participants ≥18 years gave informed consent. The Maine Medical Center Institutional Review Board determined that this study was not human subjects research.
Demographics
NHANES interviewers obtained data on participant age, sex, race/ethnicity, household income, and family size during in-person interviews at the participants’ homes. For participants under 16 years, a proxy (a family member or legal guardian, preferably with the most knowledge of the child) completed the interview; participants ≥16 years were interviewed directly. NHANES analysts calculated income to poverty ratio as the ratio of household income to the poverty threshold, per Department of Health and Human Services Poverty Guidelines. We then categorized this into the following groups: <100%, 100-<200%, or ≥200%.
Dietary assessment
Urinary phthalates have short half-lives (6–24 hours); therefore, we related recent diet to urinary phthalate metabolite concentrations as we considered it to be the most relevant exposure window [19]. NHANES interviewers used the United States Department of Agriculture’s (USDA) Automated Multiple Pass Method to administer a 24-hour dietary recall to NHANES participants at Mobile Examination Centers [20]. Participants reported the types and amounts of food they consumed during the prior 24 hours (midnight to midnight), and used measuring guides (e.g., mugs, bottles, thickness sticks) to help quantify the amount of food or beverage consumed. A proxy answered questions on dietary intake for children 6 to 8 years with the child’s assistance. Older children and adolescents answered questions on dietary intake themselves, with children 9–11 years assisted by a proxy. The USDA’s Food and Nutrient Database for Dietary Studies was used to code the individual food items reported [21].
We categorized food items into food groups using the What We Eat in America database, a classification scheme of approximately 152 food and beverage categories produced for each NHANES cycle. We excluded 15 food groups that were specific to infants (e.g., ‘baby food: cereals’) or contained only alcohol (i.e. ‘beer’) (Table S1). Yogurt was assessed differently across NHANES cycles; therefore, we calculated total yogurt intake as the sum of intake of both yogurt varieties. Because intake of fast food [14] and school cafeteria food [12] has been associated with higher concentrations of urinary phthalate metabolite concentrations, we further categorized each food group as being prepared at a restaurant or purchased from a grocery store, resulting in 272 unique food categories (i.e., 136 categories for food purchased from a grocery store and 136 categories for food prepared at a restaurant). We categorized food as ‘purchased from a grocery store’ if it was obtained from a store or grown or caught by someone the participant knew; we categorized food obtained from any other source (e.g., restaurant, cafeteria, fast food) as ‘prepared at a restaurant’ (Table S2). We excluded any food groups that were consumed by 10 or fewer participants (food groups purchased from a grocery store, n=11; food groups prepared at a restaurant, n=19). For the 242 remaining food groups (food groups purchased from a grocery store, n=125; food groups prepared at a restaurant, n=117), we calculated each participant’s total intake of each food group in grams.
Urinary phthalate metabolite measurements
NHANES technicians collected spot urine samples during the in-person exam at the Mobile Examination Center. Samples were stored at −20°C before being shipped to the National Center for Environmental Health for analysis. Briefly, analytes were quantified using solid-phase extraction coupled with online high-performance liquid chromatography and tandem mass spectrometry [CDC 22]. Prior to public release of the data, NHANES analysts replaced concentrations below the limit of detection with the limit of detection divided by the square root of 2. We a priori decided to include urinary phthalate metabolites for which ≥60% of participants had concentrations above the limit of detection. For 2011—2016 NHANES, this included 11 urinary phthalate metabolites: mono (2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-carboxypentyl phthalate (MECPP), monobenzyl phthalate (MBzP), monobutyl phthalate (MBP), MiBP, MEP, MCPP, monocarboxyoctyl phthalate (MCOP), and monocarboxy-isononyl phthalate (MCNP). We approximated total DEHP exposure using the molar sum of four DEHP metabolites: MEHP, MEHHP, MEOHP, MECPP (μmol/L) [23].
Statistical analysis
We used reduced rank regression (RRR) to identify dietary patterns that explained variation in urinary phthalate metabolite concentrations [24, 25]. We selected this a posteriori method of dimension reduction because it identifies dietary patterns that explain the most variability in urinary phthalate metabolite concentrations. In contrast, principal component analysis, an a priori dimension reduction method, would identify dietary patterns based on the clustering of food groups without respect to urinary phthalate metabolites.
We adjusted RRR models for urinary creatinine concentrations, sociodemographics [age, sex, race/ethnicity, and income to poverty ratio (continuous)], and NHANES subsample weights. We selected confounders based on previously described associations with urinary phthalates [23, 26]. We adjusted for NHANES subsample weights to account for potential bias that be introduced by oversampling a population for a certain exposure (i.e., race/ethnicity) [27]. RRR models cannot be directly adjusted for covariates; therefore, we adjusted for covariates using the residual method [28], consistent with previous studies using RRR [25, 29]. The residual method effectively creates a covariate-adjusted exposure, and is equivalent to adjusting for covariates in the model. To do this, we regressed urinary concentrations of each of the eight urinary phthalate metabolites on urinary creatinine and the sociodemographic variables in eight separate linear regression models and used the residuals as the dependent variable in the RRR model. Because RRR models do not allow for survey analysis procedures to account for the complex survey design, our results are not generalizable to the US population.
We modeled urinary DEHP and the seven urinary phthalate metabolites simultaneously in our model using a matrix, allowing us to identify dietary patterns associated with the overall phthalate mixture. For each participant, we obtained a factor score (i.e., dietary pattern score) that described their adherence to a given dietary pattern, with a higher score indicating greater adherence to the pattern. For each dietary pattern, we identified the food groups with the strongest (≥0.1 or ≤−0.1) model loadings. Next, for each dietary pattern, we used linear regression to quantify the association between each dietary pattern score and individual ln-transformed urinary phthalate metabolites. To aid interpretation, we reported results as the percent difference [% difference = (exp(beta) − 1) × 100] in urinary phthalate metabolite concentrations per standard deviation (SD) increment in dietary pattern score.
Sensitivity analyses
We examined associations between pairwise combinations of food groups and individual ln-transformed urinary phthalate metabolites using linear regression, accounting for multiple comparisons using a Benjamini-Hochberg false discovery rate correction of 0.1 for each set of 242 analyses per individual urinary phthalate metabolite [30].
We conducted RRR using SAS EG, version 7.1 (SAS Institute, Inc.); all other analyses were performed in R (Core Team. 2018. R: A language and environment for statistical computing. Vienna Austria:R Foundation for Statistical Computing).
Results
Participants had a median age of (IQR) 11.0 (8.0) years and were 48.7% female (Table 1). Twenty-eight percent of participants were non-Hispanic white, 25.3% were non-Hispanic Black, and 22.7% were Mexican American. Of the eight measured urinary phthalate metabolites, the median urine concentration was highest for MCOP (15.2 ng/mL), followed by MBP (13.6 ng/mL), and MBzP (8.0 ng/mL) (Table S3).
Table 1.
Unweighteda characteristics of 2,369 6–19 year old participants in the 2011–2016 National Health and Nutrition Examination Survey, overall and by quartile of dietary pattern 1 score.
| Dietary pattern 1 score (dairy foods), quartilesb,c | |||||
|---|---|---|---|---|---|
| Overall | Q1 (n=593) |
Q2 (n=592) |
Q3 (n=592) |
Q4 (n=592) |
|
| Median (IQR) or N(%) | |||||
| Age, years | 11.0 (8.0) | 11.0 (8.0) | 10.0 (7.0) | 11.0 (7.0) | 11.0 (7.0) |
| Female, N (%) | 1,154 (48.7) | 285 (48.1) | 305 (51.4) | 285 (48.1) | 279 (47.1) |
| Race/ethnicity, N (%) | |||||
| White, non-Hispanic | 659 (27.8) | 147 (24.8) | 154 (26.0) | 176 (29.7) | 182 (30.7) |
| Black, non-Hispanic | 600 (25.3) | 174 (29.4) | 167 (28.2) | 121 (20.4) | 138 (23.3) |
| Asian, non-Hispanic | 202 (8.5) | 60 (10.1) | 61 (10.3) | 44 (7.4) | 37 (6.3) |
| Mexican American | 538 (22.7) | 119 (20.1) | 130 (21.9) | 148 (25.0) | 141 (23.8) |
| Other Hispanic | 236 (10.0) | 63 (10.6) | 47 (7.9) | 69 (11.7) | 57 (9.6) |
| Other/multi-racial | 134 (5.7) | 29 (4.9) | 34 (5.7) | 34 (5.7) | 37 (6.3) |
| Income to poverty ratio, N (%) | |||||
| <100% | 808 (34.1) | 202 (34.1) | 211 (35.6) | 205 (34.6) | 190 (32.1) |
| 100–200% | 660 (27.9) | 159 (26.9) | 143 (24.1) | 183 (30.9) | 175 (30.0) |
| ≥200% | 901 (38.0) | 231 (39.0) | 239 (40.3) | 204 (34.5) | 227 (38.3) |
| Urinary phthalate concentrations | |||||
| ∑DEHP, μmol/L | 0.11 (0.14) | 0.10 (0.12) | 0.11 (0.14) | 0.11 (0.14) | 0.12 (0.17) |
| MCOP, ng/mL | 15.2 (30.3) | 13.0 (22.3) | 13.0 (20.9) | 15.1 (29.1) | 23.4 (59.1) |
| MCNP, ng/mL | 2.6 (3.2) | 2.3 (2.8) | 2.4 (3.0) | 2.7 (3.4) | 3.2 (4.4) |
| MCPP, ng/mL | 2.1 (3.4) | 1.9 (2.5) | 1.9 (3.2) | 2.0 (3.3) | 2.9 (6.3) |
| MBzP, ng/mL | 8.0 (15.3) | 7.9 (13.4) | 8.4 (17.9) | 7.0 (16.2) | 8.4 (15.0) |
| MEP, ng/mL | 28.9 (57.7) | 30.4 (63.1) | 30.6 (65.0) | 26.4 (48.6) | 28.9 (56.1) |
| MBP, ng/mL | 13.6 (18.7) | 13.7 (18.4) | 13.5 (17.1) | 13.5 (17.1) | 13.8 (20.8) |
| MiBP, ng/mL | 10.4 (14.8) | 10.8 (14.3) | 10.0 (14.8) | 10.0 (14.8) | 9.7 (15.9) |
Models do not account for the complex sample design, and therefore results are not generalizable to the US population.
The dietary pattern score reflects adherence to the dietary pattern, with a higher score reflecting greater adherence.
Calculated using unweighted survey data adjusted for urinary creatinine, age, sex, income to poverty ratio, race/ethnicity, and NHANES subsample weights.
Abbreviations: DEHP, di-2-ethylhexyl phthalate; IQR, interquartile range; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MCOP, monocarboxyoctyl phthalate; MCNP, monocarboxy-isononyl phthalate; MCPP, mono (3-carboxypropyl) phthalate; MEP, mono-ethyl phthalate; MiBP, mono-isobutyl phthalate
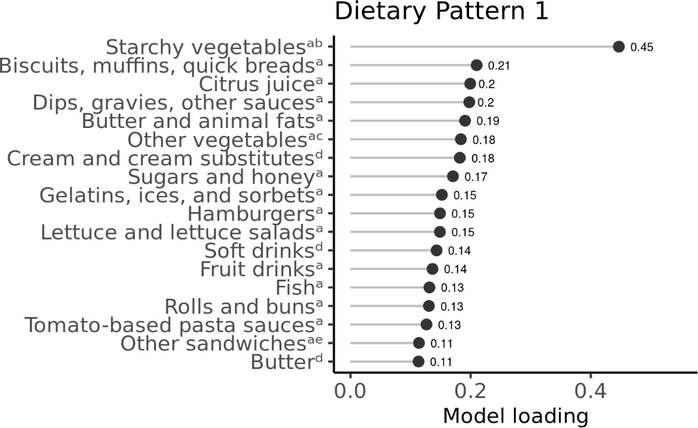
In our RRR model, we calculated eight dietary patterns cumulatively explaining 12.1% of the variability in urinary phthalate metabolite concentrations, and we describe here the three patterns explaining the most variability (combined total of 8.4%). Dietary pattern 1, which explained 4.6% of the total variability in total urinary phthalate metabolites, was characterized by greater intake of starchy vegetables other than potatoes (e.g., plantains, lima beans, and peas); biscuits, muffins, and quick breads; and citrus juice prepared at a restaurant (Figure 1). Dips, gravies, and other sauces prepared at a restaurant; butter prepared at a restaurant or purchased from a grocery store; and cream purchased from a grocery store also had high model loadings onto dietary pattern 1. Dietary pattern 1 was most strongly associated with urinary ∑DEHP, MCOP, MCNP, and MCPP. For example, a one SD increment in the dietary pattern 1 score was associated with a 13.0% higher urinary ∑DEHP (95% CI, 8.6, 17.6), 37.2% higher urinary MCOP (95% CI, 30.3, 44.4), 22.3% higher urinary MCNP (95% CI, 17.6, 27.3), and 32.5% higher urinary MCPP (95% CI, 26.4, 38.8). Correlations between food groups that loaded strongly onto this dietary pattern were generally weak (i.e., all correlations were −0.2 to 0.2) (data not shown). Most foods with high loadings on dietary pattern 1 were consumed by relatively few participants (Table S4). For example, during the 24-hour recall period, 0.6% of participants (n=13) reported consuming starchy vegetables other than potatoes prepared at a restaurant, 4.3% (n=101) reported consuming biscuits, muffins, and quick breads prepared at a restaurant, and 5.3% (n=126) reported consuming citrus juice prepared at a restaurant.
Figure 1.
Food groups with high model loadings (≥0.1 or ≤ −0.1) on dietary pattern 1, 2,369 6–19 year old participants in the 2011–2016 National Health and Nutrition Examination Survey. Model loadings can be interpreted as correlation coefficients between the food group and dietary pattern score.
a Prepared at a restaurant.
b Starchy vegetables other than potatoes such as plantains, lima beans, and peas.
c Vegetables other than dark green vegetables, red/orange vegetables, lettuce, carrots, peas, and tomatoes such as peppers, cabbage, cauliflower, and mushrooms.
d Purchased from a grocery store.
e Sandwiches other than hamburgers and egg/breakfast sandwiches such as ham, roast beef, and fish sandwiches.
Dietary pattern 2, which was characterized by greater intake of citrus juice and sandwich ingredients (e.g., rolls, mayonnaise, and cold cuts) prepared at a restaurant (Figure S1), explained an additional 2.2% of the variability in urinary phthalate metabolites. For each one SD increment in dietary pattern 2 score, urinary MCOP was 13.2% higher (95% CI, 7.4, 19.4), urinary MCPP was 16.5% higher (95% CI, 11.1, 22.3), and urinary MBzP was 7.6% higher (95% CI, 2.2, 13.4) (Table 2).
Table 2.
Percent difference in urinary phthalate metabolite concentrations per standard deviation increment in dietary pattern score among 2,369 6–19 year old participants in the 2011–2016 National Health and Nutrition Examination Survey.
| % difference (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| High molecular weight urinary phthalate metabolites | Low molecular weight urinary phthalate metabolites | |||||||
| ∑DEHP | MCOP | MCNP | MCPP | MBzP | MEP | MBP | MiBP | |
| Dietary pattern 1 | 13.0 (8.6, 17.6) | 37.2 (30.3, 44.4) | 22.3 (17.6, 27.3) | 32.5 (26.4, 38.8) | 2.6 (−2.6, 8.1) | 3.5 (−1.9, 9.2) | 4.1 (−0.6, 9.0) | 1.5 (−2.8, 6.0) |
| Dietary pattern 2 | 1.4 (−2.6, 5.5) | 13.2 (7.4, 19.4) | 0.9 (−3.1, 5.1) | 16.5 (11.1, 22.3) | 7.6 (2.2, 13.4) | −4.2 (−9.2, 1.1) | 6.6 (1.8, 11.6) | 4.1 (−0.3, 8.7) |
| Dietary pattern 3 | 6.1 (1.9, 10.4) | −8.1 (−12.8, −3.1) | −4.0 (−7.8, 0.0) | −1.9 (−6.6, 2.9) | 13.3 (7.6, 19.3) | 13.9 (7.9, 20.1) | 14.3 (9.2, 19.6) | 8.0 (3.4, 12.7) |
Abbreviations: DEHP, di-2-ethylhexyl phthalate; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MCOP, monocarboxyoctyl phthalate; MCNP, monocarboxy-isononyl phthalate; MCPP, mono (3-carboxypropyl) phthalate; MEP, mono-ethyl phthalate; MiBP, mono-isobutyl phthalate
Dietary pattern 3, which was characterized of greater intake of starchy vegetables other than potatoes, nuts and seeds, and cold cuts prepared at a restaurant (Figure S2), explained an additional 1.6% of the total variability in urinary phthalate metabolites. For each one SD increment in dietary pattern 3 score, MEP was 13.9% higher MEP (95% CI, 7.9, 20.1), MBP was 14.3% higher (95% CI, 9.2, 19.6), and MBzP was 13.3% higher (95% CI, 7.6, 19.3) (Table 2).
We show pairwise associations between urinary phthalate metabolites and individual food groups for all food groups with model loadings ≥0.1 or ≤ −0.1 on dietary pattern 1 (Table S5a). Intake of butter prepared at a restaurant was associated with a relatively large, but imprecise, increase in urinary phthalate metabolite concentrations [e.g., per 1 SD increase, 3.9% (95% CI, 0.2, 7.8) higher MCOP]. We also observed an association between a 1 SD increase in consumption of biscuits, muffins, and quick breads (i.e., foods commonly prepared with high-fat dairy) prepared at a restaurant and 0.7% higher MCOP (95% CI, 0.4, 1.1), 0.5% higher MCNP (95% CI, 0.2, 0.7), and 0.6% higher MCPP (95% CI, 0.3, 0.9) (Table S5a). Second, we present pairwise associations for food groups that did not have a high model loading on dietary pattern 1, but were significantly associated with at least one urinary phthalate metabolite (n=18) (Table S5b). Similar to our findings in Table S5a, several dairy products prepared at a restaurant were weakly associated with individual urinary phthalate metabolites [e.g., per 1 SD increment in intake: reduced-fat flavored milk and 0.1% higher urinary MCPP (95% CI, 0.1, 0.2), milkshakes and 0.2% higher urinary MCOP (95% CI, 0.1, 0.3), and low-fat milk and 0.2% higher urinary MBzP (95% CI, 0.1, 0.2). Finally, we observed weak associations between urinary phthalate metabolites and several meat food groups prepared at a restaurant, including ground beef, chicken sandwiches, and mixed poultry and urinary MCOP and chicken sandwiches and bacon and urinary MCNP.
Discussion
Using data from 2011—2016 NHANES, we found that diet explained 12.1% of the variability in certain urinary phthalate metabolites. Children and adolescents with greater adherence to a dietary pattern characterized by intake of starchy vegetables other than potatoes, quick breads, and citrus juice prepared at a restaurant had higher urinary ∑DEHP, MCOP, MCNP, and MCPP concentrations. These foods may be exposure sources of phthalates or reflect lifestyle behaviors correlated with higher phthalate exposure. For example, DINP and DIDP (parent diesters of MCOP and MCNP, respectively) are used in a wide variety of housing materials, including flooring and carpet backing [31]. If children with high consumption of starchy vegetables other than potatoes, quick breads, and citrus juice prepared at a restaurant also spent more time indoors than children with lower intake of these foods, there could be a spurious association between intake of these foods and urinary MCOP and MCNP.
Dietary pattern 1 was, in part, characterized by intake of butter, cream, and foods commonly prepared with butter or other high-fat dairy (i.e., biscuits, muffins, and quick breads and dip, gravies, and other sauces). While dairy food groups prepared at a restaurant had the highest model loadings on dietary pattern 1, cream and butter purchased from a grocery store also had high model loadings, suggesting contamination of the foods themselves. Pairwise effect estimates for butter prepared at a restaurant with MCOP, MCNP, and MCPP were relatively large, but imprecise. Similarly, intake of biscuits, muffins, and quick breads and dips, gravy, and other sauces was associated with urinary MCOP, MCNP, and MCPP, although some associations were no longer statistically significant after adjustment for multiple comparisons. We also observed pairwise associations of urinary MCOP, MBzP, and MCPP with several dairy foods that were not part of dietary pattern 1. Consistent with the fact that high molecular weight phthalates are lipophilic [32], we observed associations with higher-fat dairy foods (e.g., milkshakes and whole-fat and low-fat milk), whereas non-fat milk was not associated with urinary phthalate metabolites.
Our finding of an association between dairy intake and higher urinary phthalate metabolites is somewhat in line with previous NHANES studies. For example, in 2003—2008 NHANES, greater total milk intake was associated with higher concentrations of ∑high molecular weight urinary phthalate metabolites [16], and in 2003—2004 NHANES, total dairy intake was positively associated with urinary MCPP [15]. Surprisingly, we did not observe an association between higher intake of dairy foods and urinary ∑DEHP as has been reported by other studies [16, 33]. This may reflect a trend toward substituting DEHP with other PVC plasticizers, including DiNP (parent diester of MCOP), DiDP (parent diester of MCNP), and DnOP (parent diester of MCPP). Future studies should examine associations between foods and urinary phthalate metabolites using more recent data, as it becomes available, to account for time trends in phthalate applications in food processing and packaging. In light of recommendations for children to consume 2–3 cups of milk/dairy daily to meet calcium requirements [30], regulatory agencies and the dairy industry should consider substituting phthalate-containing milk tubing with silicone or other materials free of phthalates to reduce phthalate exposure in children.
Dietary pattern 1 was also characterized by higher intake of starchy vegetables other than potatoes (e.g., plantains, lima beans, and peas) and other vegetables (e.g., avocado, cabbage, mushrooms). Total vegetable intake was associated with higher urinary MEP and ∑low molecular weight phthalate metabolites in two previous NHANES investigations; however, investigation of individual vegetables was limited [15, 16]. In a UK survey, ‘other vegetables’ had higher concentrations of DEHP, DiBP, and DBP than green vegetables, potatoes, or canned vegetables, but concentrations were low compared to those in dairy and meat [34]. While narrower than those in previous studies, our vegetable food groups were still quite broad, containing vegetables with a wide variety of typical packaging and preparation. Quantification of phthalate concentrations in individual starchy and other vegetables would aid interpretation of our findings.
Dietary patterns 2 and 3 were largely comprised of sandwich ingredients, including meat. Additionally, five meat food groups prepared at a restaurant, including chicken sandwiches; mixed poultry dishes (e.g., chicken stew); burgers; ground beef; and bacon, were associated with at least one urinary phthalate metabolite in pairwise analyses. Previous NHANES studies have reported null [16] or weakly positive [15] associations between total meat (i.e., combined beef, pork, veal, lamb, and game) and urinary phthalate metabolites. By relating finer food groups to urinary phthalate metabolites, we are better able to inform individuals desiring to reduce their phthalate exposure. Our approach also identifies foods that could be targeted in ‘farm to table’ studies measuring phthalate concentrations at various steps along the food processing chain to understand the extent to which phthalates are introduced through various environmental sources (e.g., feed), processing, and/or packaging [10].
A high model loading could indicate that a food group is high in phthalates, but may also be explained by confounding. For example, citrus juice prepared away from home loaded strongly on to dietary patterns 1 and 2, but univariate associations between citrus juice (both prepared at home and purchased from a restaurant) were null. High model loadings could also occur due to correlations between a given food group with other food groups with high model loadings, although correlations between citrus juice and other foods that loaded onto dietary patterns 1 and 2 were weak. Finally, a high model loading could be explained by correlations between a food group with other exposures or behaviors related to phthalate exposure (e.g., time spent indoors, in contact with building materials high in phthalates).
Despite the importance of diet to exposure to some low molecular weight phthalates [6, 7], and measurement of low molecular weight phthalates in certain foods [35–37], we only observed significant associations between diet and high molecular weight urinary phthalate metabolites. One possible explanation for this observation is that food packaging containing low molecular weight phthalates (e.g., plastic films and cardboard boxes) may be used for a wide variety of foods, whereas high molecular weight phthalates are more closely tied to a particular food (e.g., dairy) due to specific uses in food processing. Our ability to further explore this hypothesis would be aided by an updated assessment of phthalate applications in food processing and packaging [8].
Our findings support a growing body of literature that shows the setting in which foods are obtained may be at least as important as the food itself with respect to phthalate exposure. Foods purchased from restaurants can be highly processed and handled, both of which are potential sources of phthalate exposure. Differences in packaging between foods purchased from restaurants and those prepared at home may also result in higher phthalate content of restaurant foods. Foods purchased from cafeterias and fast food restaurants, in particular, are more likely to be packaged and heated in single servings, where there is a high degree of surface area contact between the food and packaging. Few studies to date have examined food source and urinary phthalate metabolite concentrations. Similar to our findings, in 2005—2014 NHANES, children and adolescents who reported recent intake of any cafeteria food (vs. no intake) had cumulative urinary phthalate metabolites that were 15% and 45% higher, respectively, with similar findings observed for intake of foods from fast-food and full-service restaurants [13]. As we also observed, certain foods (sandwiches, pizza, and fried potatoes) were only associated with urinary phthalate metabolites when prepared away from home [13]. Other studies have also reported that individuals who consumed foods from fast food restaurants [14, 18] and school cafeterias [12] had higher urinary phthalate metabolites, particularly DEHP and DiNP [12–14]. Future studies could also test for interactions between food types and sources. We extend these findings by providing a comprehensive assessment of the impact of 136 food groups prepared at a restaurant or purchased from a grocery store. Future studies should continue to investigate sources of phthalate exposure (e.g., use of gloves by food handlers) in foods prepared outside the home.
We found that diet explained 12.1% of the variability in urinary phthalate metabolites. We are unaware of previous studies reporting percentage of variability in urinary phthalate metabolites explained by diet; however, this number is somewhat smaller than our finding of 18.1% for per- and polyfluoroalkyl substances [29]. Our findings should be interpreted in light of the large amount of within-person variability in both spot urine phthalate metabolites and 24-hour dietary recalls. Day-to-day changes in exposure sources (e.g., diet) and metabolism result in within-person variability in spot urine samples [38]. Diet, particularly when measured using a 24-hour recall, is also subject to within-person variability. Due to the short period of time over which diet was recalled, we may have missed associations with less frequently consumed foods and likely identified patterns of foods eaten in combination (vs. eaten by the same person over a longer period). Studies with repeated measures of urinary phthalates and diet would reduce the impact of within-person variation in urinary phthalate metabolites and diet. Finally, phthalates have a short half-life, and while we limited our analysis to foods consumed within the 24 hours prior to urine donation, future studies would benefit from examining the extent to which timing of consumption impacts urinary phthalate metabolite concentrations.
Other limitations of our study include a lack of data on packaging and whether foods purchased from a grocery store were prepared at home or sold ready-to-eat. We combined foods prepared at full-service restaurants, fast food restaurants, and cafeterias into a ‘prepared at a restaurant’ category, but the use of phthalates in food preparation and packaging materials may differ between these settings. Also, food groupings were created based on the nutritional properties of foods rather than phthalate concentrations. Despite this, we believe that many food groups were comprised of foods with a similar phthalate profile, such as food groups that contained only a single food item (e.g., apple juice) or those that contained foods with similar processing and packaging (e.g., beans). An advantage of using USDA food groups is that they can be easily replicated by other investigators. Similar to other studies applying novel methods to NHANES data [39–41], we did not perform survey analysis procedures that accounted for the complex sample design and our findings are thus not generalizable to the US population. By adjusting for NHANES subsample weights as a covariate in our phthalate metabolite linear models, we accounted for potential bias that may be introduced by oversampling (or undersampling) specific populations as part of the NHANES survey methodology and bias from non-response. Finally, while our study used data from a diverse population, and our RRR models adjust for race/ethnicity, it is unknown to what extent our findings would be replicated within specific racial/ethnic populations, which may have population-specific dietary patterns or sources of phthalate exposure.
In children and adolescents participating in 2011—2016 NHANES, we observed higher urinary phthalate metabolites in those who adhered to a dietary pattern characterized by intake of starchy vegetables other than potatoes, quick breads, and citrus juice prepared at a restaurant. Also, several dairy and meat food groups were associated with individual urinary phthalate metabolites. Child dietary behaviors are distinct from those of adults, and high phthalate foods such as dairy may comprise a relatively larger portion of their diet. Our study extends previous reports on diet and urinary phthalate metabolites in NHANES by modeling dietary intake using dietary patterns, which account for interactions between foods eaten in combination and assess the impact of diet on the overall phthalate burden. This approach also allowed us to parsimoniously, yet comprehensively, report associations between dietary intake jointly classified using both 136 unique food groups and food setting (prepared at a restaurant vs. purchased from a grocery store). We confirm previous reports of associations of greater intake of dairy and meat with higher urinary phthalate metabolites. We also report hypothesis-generating associations with newly identified food groups such as starchy vegetables other than potatoes. A novel addition of our study is that we classify food groups by source (i.e., prepared at a restaurant versus purchased at a grocery store). Additional insight into the source of high phthalate-containing foods can inform interventions to reduce food contact with phthalates through substitution of source-specific materials (e.g., gloves or packaging used specifically at restaurants). Future studies investigating how phthalates, particularly non-DEHP plasticizers, are introduced into foods are needed to inform interventions to reduce phthalate exposure in children.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Health [R01ES030101, K23ES024803].
Footnotes
The authors declare they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li N, Papandonatos GD, Calafat AM, Yolton K, Lanphear BP, Chen A, Braun JM: Identifying periods of susceptibility to the impact of phthalates on children’s cognitive abilities. Environ Res 2019, 172:604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniel S, Balalian AA, Insel BJ, Liu X, Whyatt RM, Calafat AM, Rauh VA, Perera FP, Hoepner LA, Herbstman J et al. : Prenatal and early childhood exposure to phthalates and childhood behavior at age 7 years. Environ Int 2020, 143:105894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YM, Kim J, Cheong HK, Jeon BH, Ahn K: Exposure to phthalates aggravates pulmonary function and airway inflammation in asthmatic children. PLoS One 2018, 13(12):e0208553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toxicological profile for di-2-ethylhexylphthalate [https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=684&tid=65]
- 5.Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K: What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal 2006, 26(3):803–824. [DOI] [PubMed] [Google Scholar]
- 6.CHAP (Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives): U.S. Consumer Product Safety Commission, Directorate for Health Sciences, Bethesda, MD. In.; 2014. [Google Scholar]
- 7.National Toxicology Program (NTP) NTP-CERHR Monograph on the potential human reproductive and developmental effects of di-n-butyl phthalate. Alexandria, VA: US Department of Health and Human Services, National Toxicology Program (NTP), Center for the Evaluation of Risks to Human Reproduction (CERHR): 60; 2000. In. [Google Scholar]
- 8.Cao X-L: Phthalate Esters in Foods: Sources, Occurrence, and Analytical Methods. Comprehensive Reviews in Food Science and Food Safety 2010, 9(1):21–43. [DOI] [PubMed] [Google Scholar]
- 9.Tsumura Y, Ishimitsu S, Kaihara A, Yoshii K, Nakamura Y, Tonogai Y: Di(2-ethylhexyl) phthalate contamination of retail packed lunches caused by PVC gloves used in the preparation of foods. Food Addit Contam 2001, 18(6):569–579. [DOI] [PubMed] [Google Scholar]
- 10.Fierens T, Van Holderbeke M, Willems H, De Henauw S, Sioen I: Transfer of eight phthalates through the milk chain--a case study. Environ Int 2013, 51:1–7. [DOI] [PubMed] [Google Scholar]
- 11.Fankhauser-Noti A, Grob K: Migration of plasticizers from PVC gaskets of lids for glass jars into oily foods: Amount of gasket material in food contact, proportion of plasticizer migrating into food and compliance testing by simulation. Trends in Food Science & Technology 2006, 17(3):`105–112. [Google Scholar]
- 12.Munoz I, Colacino JA, Lewis RC, Arthur AE, Meeker JD, Ferguson KK: Associations between school lunch consumption and urinary phthalate metabolite concentrations in US children and adolescents: Results from NHANES 2003–2014. Environ Int 2018, 121(Pt 1):287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varshavsky JR, Morello-Frosch R, Woodruff TJ, Zota AR: Dietary sources of cumulative phthalates exposure among the U.S. general population in NHANES 2005–2014. Environ Int 2018, 115:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zota AR, Phillips CA, Mitro SD: Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003–2010. Environ Health Perspect 2016, 124(10):1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colacino JA, Harris TR, Schecter A: Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ Health Perspect 2010, 118(7):998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trasande L, Sathyanarayana S, Jo Messito M, R SG, Attina TM, Mendelsohn AL: Phthalates and the diets of U.S. children and adolescents. Environ Res 2013, 126:84–90. [DOI] [PubMed] [Google Scholar]
- 17.Pacyga DC, Sathyanarayana S, Strakovsky RS: Dietary Predictors of Phthalate and Bisphenol Exposures in Pregnant Women. Adv Nutr 2019, 10(5):803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins DJ, Eliot M, Sathyanarayana S, Calafat AM, Yolton K, Lanphear BP, Braun JM: Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol 2014, 48(15):8881–8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson WA, Castle L, Scotter MJ, Massey RC, Springall C: A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit Contam 2001, 18(12):1068–1074. [DOI] [PubMed] [Google Scholar]
- 20.USDA Automated Multiple-Pass Method for Dietary Recalls [https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/ampm-usda-automated-multiple-pass-method/]
- 21.Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, Arifeen SE, Huda SN, Vahter M: Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol 2011, 40(6):1593–1604. [DOI] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Phthalate Metabolites Laboratory Procedure Manual. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention [Google Scholar]
- 23.Zota AR, Calafat AM, Woodruff TJ: Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect 2014, 122(3):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann K, Schulze MB, Schienkiewitz A, Nothlings U, Boeing H: Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol 2004, 159(10):935–944. [DOI] [PubMed] [Google Scholar]
- 25.Tabung FK, Wang W, Fung TT, Hu FB, Smith-Warner SA, Chavarro JE, Fuchs CS, Willett WC, Giovannucci EL: Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br J Nutr 2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM: Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 2004, 112(3):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahrens KA, Cole SR, Westreich D, Platt RW, Schisterman EF: A cautionary note about estimating effects of secondary exposures in cohort studies. Am J Epidemiol 2015, 181(3):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett WC: Nutritional epidemiology, third edition. Oxford: Oxford University Press; 2012. [Google Scholar]
- 29.Seshasayee SM, Rifas-Shiman SL, Chavarro JE, Carwile JL, Lin PD, Calafat AM, Sagiv SK, Oken E, Fleisch AF: Dietary patterns and PFAS plasma concentrations in childhood: Project Viva, USA. Environ Int 2021, 151:106415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y: Controlling the fasle discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol) 1995, 57:289–300. [Google Scholar]
- 31.NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Di-isononyl Phthalate (DINP). Ntp cerhr mon 2003(2):i–III90. [PubMed] [Google Scholar]
- 32.Dickerson RN: Di(2-ethylhexyl)phthalate as a plasticizer for intravenous bags and tubing: a toxicological quandary. Nutrition 1997, 13(11–12):1010–1012. [DOI] [PubMed] [Google Scholar]
- 33.Mervish N, McGovern KJ, Teitelbaum SL, Pinney SM, Windham GC, Biro FM, Kushi LH, Silva MJ, Ye X, Calafat AM et al. : Dietary predictors of urinary environmental biomarkers in young girls, BCERP, 2004–7. Environ Res 2014, 133:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradley EL, Burden RA, Bentayeb K, Driffield M, Harmer N, Mortimer DN, Speck DR, Ticha J, Castle L: Exposure to phthalic acid, phthalate diesters and phthalate monoesters from foodstuffs: UK total diet study results. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2013, 30(4):735–742. [DOI] [PubMed] [Google Scholar]
- 35.Zota AR, Yau A, McCray NL, VanNoy BN, Adamkiewicz G: Phthalate and novel plasticizer concentrations in food items from fast food chains. International Society of Environmental Epidemiology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradley EL, Burden RA, Leon I, Mortimer DN, Speck DR, Castle L: Determination of phthalate diesters in foods. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2013, 30(4):722–734. [DOI] [PubMed] [Google Scholar]
- 37.Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, Hommel M, Imran N, Hynan LS, Cheng D et al. : Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect 2013, 121(4):473–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johns LE, Cooper GS, Galizia A, Meeker JD: Exposure assessment issues in epidemiology studies of phthalates. Environ Int 2015, 85:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park SK, Zhao Z, Mukherjee B: Construction of environmental risk score beyond standard linear models using machine learning methods: application to metal mixtures, oxidative stress and cardiovascular disease in NHANES. Environmental health : a global access science source 2017, 16(1):102–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M-C, Mínguez-Alarcón L, Bellavia A, Williams PL, James-Todd T, Hauser R, Chavarro JE, Chiu Y-H: Serum beta-carotene modifies the association between phthalate mixtures and insulin resistance: The National Health and Nutrition Examination Survey 2003–2006. Environ Res 2019, 178:108729–108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu B, Jiang Y, Jin X, He L: Using three statistical methods to analyze the association between exposure to 9 compounds and obesity in children and adolescents: NHANES 2005–2010. Environmental health : a global access science source 2020, 19(1):94–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.