Abstract
Background and aims
Coronary artery calcium (CAC) scores have been shown to be associated with CVD and cancer mortality. The use of CAC scores for overall and lung cancer mortality risk prediction for patients in the Coronary Artery Calcium Consortium was analyzed.
Methods
We included 55,943 patients aged 44-84 years without known heart disease from the CAC Consortium. There were 1,088 cancer deaths, among which 231 were lung cancer, identified by death certificates with a mean follow-up of 12.2 ± 3.9 years. Fine-and-Gray competing-risk regression was used for overall and lung cancer-specific mortality, accounting for the competing risk of CVD death and after adjustment for CVD risk factors. Subdistribution hazard ratios (SHR) were reported.
Results
The mean age of all patients was 57.1 ± 8.6 years, 34.9% were women, and 89.6% were white. Overall, CAC was strongly associated with cancer mortality. Lung cancer mortality increased with increasing CAC scores, with rates per 1000-person years of 0.2 (95% CI: 0.1-0.3) for CAC = 0 and 0.8 (95% CI: 0.6-1.0) for CAC ≥ 400. Compared with CAC = 0, hazards were increased for those with CAC ≥ 400 for lung cancer mortality [SHR: 1.7 (95% CI: 1.2-2.6)], which was driven by women [SHR: 2.3 (95% CI: 1.1-4.8)], but not significantly increased for men. Risks were higher in those with positive smoking history [SHR: 2.2 (95% CI: 1.2-4.2)], with associations driven by women [SHR: 4.0 (95% CI: 1.4-11.5)].
Conclusions
CAC scores were associated with increased risks for lung cancer mortality, with strongest associations for current and former smokers, especially in women. Used in conjunction with other clinical variables, our data pinpoint a potential synergistic use of CAC scanning beyond CVD risk assessment for identification of high-risk lung cancer screening candidates.
Keywords: Coronary arterial calcium, Cancer, Lung cancer, Cardiovascular disease, Risk prediction, Prevention
Graphical Abstract
1. Introduction
Coronary artery calcium (CAC) reflects lifetime exposure to cardiovascular risk factors and can be used for cardiovascular disease (CVD) risk assessment. However, as a marker of biologic age, CAC is also associated with an increased risk for cancer. Lung cancer represents a leading cause of cancer-related mortality worldwide and in the United States 1, 2. The underlying environmental and lifestyle risk factors increasing lung cancer risks closely mirror those of CVD 3. Smoking in particular represents a leading and preventable risk factor that increases lung cancer and CVD mortality 4-6. Although CAC has been shown to be associated with overall cancer mortality in smokers 7, the predictive role of CAC for lung cancer outcomes is not fully understood.
Important randomized trials have shown a beneficial role for annual low-dose chest CT scans in lung cancer screening for high-risk patients 8, 9. This has led to revised guideline recommendations including the recently updated version of the US Preventive Services Task Force (USPSTF) statement that recommends annual screening scans for high-risk smokers aged 50-80 years 10. Previous studies could also confirm a reliable CAC quantification in non-ECG gated lung cancer screening scans as well 11. Of note, CAC was associated with higher rates of cardiovascular mortality in the lung cancer screening populations in the National Lung Cancer Screening Trial (NLST) and other studies 12, 13 pinpointing the potential for a combined risk prediction of both diseases in this high-risk population. Also, smokers with the presence of CAC had significant higher all-cause mortality rates including cancer deaths when compared to non-smokers 7, 14. However, it remains unknown to what extent CAC assessment primarily used for CVD risk assessment can also predict lung cancer outcomes. This might have implications for combined CVD and lung cancer risk assessment and help to optimize preventive efforts in high-risk individuals.
We therefore aimed to determine whether CAC scores derived from routine cardiac computed tomography is also associated with the subsequent development of lung cancer mortality in the well-characterized Coronary Artery Calcium Consortium cohort of asymptomatic patients free of CVD and known lung cancer at the time of scanning.
2. Patients and methods
2.1. Study popidation
We analyzed data from the CAC Consortium, a multicenter study of individuals without known CVD. Patients underwent a physician- ordered CAC scan using a standardized protocol for clinical CVD risk stratification between 1991 and 2010. Full details of the study design and the CAC Consortium cohort have been previously described 15.
Four participating field centers enrolled asymptomatic patients aged 18 years or older, without known coronary heart disease (CHD) at the time of the CAC scan in the CAC Consortium: Cedars-Sinai Medical Center, Los Angeles, California (n=13,972); PrevaHealth Wellness Diagnostic Center, Columbus, Ohio (n=7,042); Harbor-UCLA Medical Center, Torrance, California (n=25,563); and Minneapolis Heart Institute, Minneapolis, Minnesota (n=20,059). To ensure sufficient data availability and in keeping with the 2019 American Heart Association/American College of Cardiology Primary Prevention guideline, we excluded individuals <44 and >84 years of age (n = 10,963), for a total of 55,943 asymptomatic patients aged 44 to 84 years. A total of 4,229 current cigarette smokers were identified in our primary analysis. Data on current and former status was available in a subset of 27,243 provided by Cedars-Sinai Medical Center and Minneapolis Heart Institute, Minneapolis. In this subset 8,528 past smokers were retained. Case numbers for all cancer deaths by cancer entities in the entire CAC consortium and in our primary analysis are summarized in Supplemental Table 1.
Consent was obtained from all study participants at individual centers at the time of CAC scanning, and Institutional Review Board approval for coordinating center activities was obtained at the Johns Hopkins Hospital.
2.2. Baseline characteristics and data extraction
Briefly, each site obtained patient demographics and cardiovascular risk factors from the CAC referral or by means of a semi-structured interview at the time of the CAC scan. Categorical CVD risk factors were documented: hypertension, dyslipidemia, diabetes mellitus, current smoking status, and family history of CHD.
A detailed description of mortality ascertainment can be found elsewhere 16. In brief, death certificates were obtained from the National Death Index, and on the basis of International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) codes, underlying causes of death were classified into the following groups: CVD and cancer (Supplemental Table 2). Further, ICD-9/-10 information was used to identify cancer subtype. CVD mortality was defined in the CAC Consortium as death from coronary heart disease (CHD), stroke, heart failure and any other circulatory disease. For further analyses, the indicated events, CVD- and cancer-related deaths, were defined as competing events. The time to event was defined as the time from baseline (scan date) until date of death or June 1, 2014 (if alive).
2.3. Definitions of CVD risk factors
Patients’ information, including demographic, clinical, and laboratory data, was obtained as part of a routine clinical visit at each site. Ethnicity was self-assessed as White, Asian, Black, or Hispanic at enrollment. Smoking status was defined as current smoker and non-smoker. As stated above, in a subset provided by Cedars-Sinai Medical Center and Minneapolis Heart Institute, additional information on former smoking history was available for 27,243 patients. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg and/or antihypertensive medication use. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dl or use of diabetes medication. Dyslipidemia was defined as the previous diagnosis of primary hyperlipidemia (LDL-C >160 mg/dL), previous diagnosis of dyslipidemia (elevated triglycerides and/or low high-density lipoprotein cholesterol [HDL-C]) or treatment with lipid lowering medications. If concomitant laboratory lipid results were present, dyslipidemia was additionally defined as LDL-C >160 mg/dL, HDL-C <40 mg/dL in men and <50 mg/dL in women, or fasting triglycerides >150 mg/dL. Family history of CHDwas defined as positive if any first-degree relative had any history of myocardial infarction with or without coronary revascularization.
2.4. CAC measurement
Non-contrast cardiac-gated computer tomography (CT) scanning and interpretation were performed as previously described 17. CAC was determined and calculated for all analyses using the Agatston method 18. CAC was assessed using either a cardiac-gated electron-beam CT scanner or a multidetector CT system. Further details on the CAC computed tomographic scanning protocol have been previously described 15. We defined the following CAC groups: CAC = 0, CAC 1-99, CAC 100-399 and CAC ≥ 400.
2.5. Statistical analyses
Baseline characteristics were stratified by CAC group (0, 1-99, 100-399, ≥400). We calculated the overall and cancer-related mortality rates per 1000 person- years’ follow-up within each CAC score group. Fine and Gray competing risk regression models were constructed to assess the relative cancer-type specific mortality risk, modeling CVD death as a competing event. The following adjustment models were defined:
Model 1: adjustment for age and sex (sex adjustment not applicable for sex-specific hazards);
Model 2: adjustment for age, sex, hyperlipidemia, hypertension, diabetes mellitus, current smoking status, and family history.
Thus, a model adjusting for general inter-individual parameters (age and sex status), but without adjustment for conventional CVD risk factors (Model 1) is provided. Model 2, however, is additionally adjusted for conventional CVD risk factors. Moreover, to demonstrate the relative association of CAC groups with specific cancer type risk across age, sub-hazard distributions as a function of age (x-axis) were used with fully adjusted sub-distribution hazard ratios (y-axis). All analyses were performed by Stata version 15 (Stata Inc., TX, USA), and two-tailed p-values ≤ 0.05 were considered significant.
3. Results
There were 55,943 patients with available information on cancer mortality. During a mean follow-up period of 12.2 (±3.9) years, 1,088 cancer-related deaths were reported, of which 231 accounted for lung cancers. Baseline characteristics are summarized in Table 1. The mean age of all cancer-related deaths was 64.5 (±9.1) years and 34.8% were women (Table 1). The distribution of conventional cardiovascular risk factors differed between the reported lung cancer deaths, other deaths and alive patients. Particularly, in those patients with reported lung cancer deaths, hypertension, diabetes, and current smoking occurred more frequently when compared to alive participants (Table 1). However, among those with lung cancer mortality, we observed a two-fold greater number of current smokers, while the distribution of other CVD risk factors was similar to all cancer-related deaths (Table 1).
Table 1.
Baseline characteristics
| Characteristics | Main analyses |
All cancer deaths (n = 1,088) |
Lung cancer deaths |
Other deaths |
No death | p-value |
|---|---|---|---|---|---|---|
| (n = 55,943) | (n = 231) | (n = 2,601) | (n = 53,111) | |||
| Age, mean (SD) in years | 57.1 (8.6) | 64.5 (9.1) | 63.9 (8.9) | 65.8 (9.9) | 53.1 (8.3) | 0.0001 |
| Sex | ||||||
| Men | 65.1 | 65.2 | 66.2 | 66.9 | 65.0 | 0.133 |
| Women | 34.9 | 34.8 | 33.8 | 33,1 | 35.0 | |
| Ethnicity | ||||||
| White | 89.6 | 92.5 | 91.4 | 87.7 | 89.7 | 0.000 |
| Asian | 3.6 | 2.6 | 4.0 | 2.4 | 3.6 | |
| Black | 2.2 | 2.6 | 2.0 | 4.8 | 2.1 | |
| Hispanic | 2.9 | 1.7 | 2.0 | 4.3 | 2.9 | |
| Family history for CHD | 45.2 | 40.2 | 39.8 | 40.9 | 45.4 | 0.000 |
| Hypertension | 33.3 | 40.9 | 40.7 | 48.5 | 32.6 | 0.000 |
| Dyslipidemia | 55.4 | 55.7 | 56.7 | 58.7 | 55.4 | 0.004 |
| Diabetes | 7.4 | 12.3 | 10.4 | 16.0 | 7.0 | 0.000 |
| Body Mass Index, mean (SD) | 27.5 (5.3) | 27.3 (5.2) | 26.5 (4.7) | 27.5 (5.7) | 29.6 (5.2) | 0.055 |
| Current smoker | 9.3 | 13.2 | 24.7 | 12.0 | 9.1 | 0.000 |
| Mean CAC (SD) | 187.7 (506.7) | 411.3 (818.0) | 464.2 (904.1) | 566.8 (970.7) | 168.0 (461.6) | 0.0001 |
| CAC = 0 | 39.3 | 24.7 | 22.9 | 17.4 | 40.4 | 0.002 |
| CAC 1-99 | 32.5 | 28.3 | 24.2 | 25.7 | 32.9 | |
| CAC 100-399 | 15.6 | 21.9 | 24.7 | 21,7 | 15.3 | |
| CAC ≥400 | 12.7 | 25.1 | 28.2 | 35.3 | 11.5 |
Patients aged 44-84 years.
Values are column percentages (%) or as indicated. CAC = coronary artery calcium score. CHD = coronary heart disease. SD = standard deviation.
p-values represent categorial comparisons for lung cancer deaths vs. other deaths vs. no death.
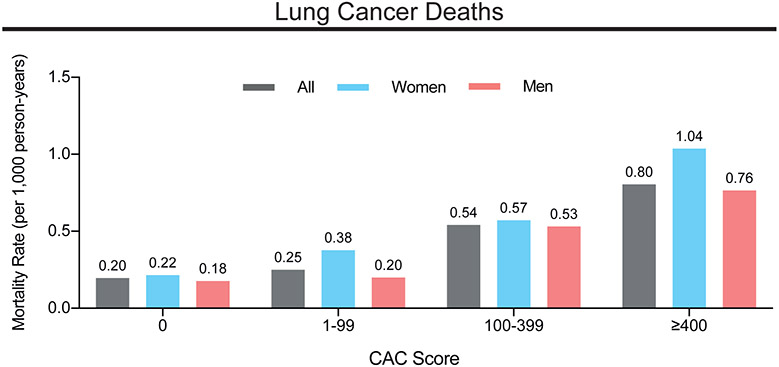
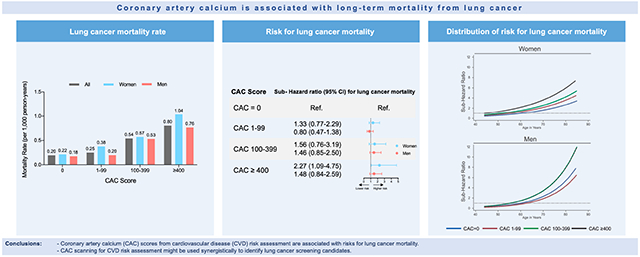
Lung cancer represented the largest proportion of cancer deaths in the CAC Consortium (21.2% of cancer-related deaths). For lung cancer, mortality increased with CAC score groups for all patients, but especially in women, with the highest mortality rate in the CAC ≥400 subgroup (Figure 1). This association clearly remained after adjustment for conventional cardiovascular risk factors. For all cancer-related deaths, we similarly observed increasing mortality rates with higher CAC scores (Supplemental Figure 1).
Figure 1.
Mortality rates for lung cancer deaths by CAC score group and sex.
Mortality rates for lung cancer as rate per 1,000 person-years in all patients, men and women are shown. CAC = coronary artery calcium score.
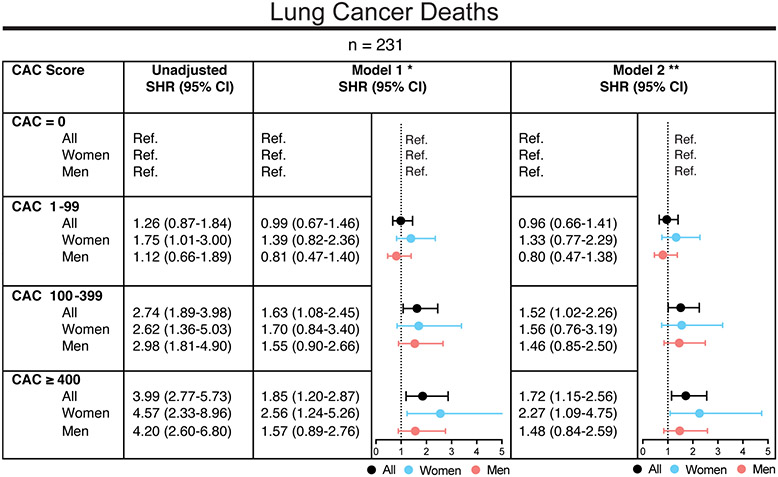
In all analyzed models, CAC was strongly associated with lung cancer death (Figure 2). As shown in Figure 2, for CAC ≥ 400, risks of lung cancer mortality increased 1.85-fold (95% CI: 1.20-2.87) in the age- and sex-adjusted model (Model l) and 1.72-fold (95% CI: 1.15-2.56) in the fully adjusted model for traditional risk factors (Model 2), when compared to CAC = 0. While this association was less strong, but significant for lower CAC scores, starting at CAC ≥ 100, no significant difference was observed for CAC 1-99 when compared to CAC = 0 (Figure 2). Overall, the increased risks for lung cancer mortality with higher CAC scores were largely driven by women (Figure 2). For all cancer-related deaths, multivariable adjusted subdistribution hazard ratios were also highest for CAC ≥ 400 (SHR 1.26, 95% CI: 1.03-1.55) as compared to CAC = 0 (Supplemental Figure 2).
Figure 2.
Risks for lung cancer mortality by CAC score group and sex.
Subdistribution hazard ratios (SHR) for lung cancer mortality with CVD as competing event and with CAC = 0 as a reference group are depicted. The following models are shown stratified by CAC score groups: Unadjusted model; Model 1 (*) was adjusted for age and sex (except for sex-specific SHR); Model 2 (**) was adjusted for age, sex (except for sex-specific SHR), hypertension, hyperlipidemia, smoking, family history, diabetes. CAC = coronary artery calcium score.
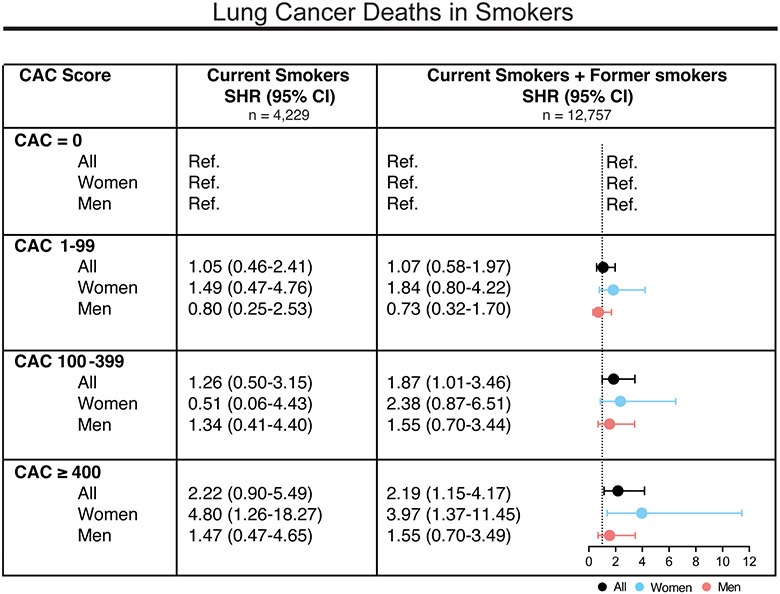
Among the group of smokers, mortality risks increased significantly with CAC when considering all participants with positive smoking history i.e., current smokers and former smokers (Figure 3). Current smokers and former smokers with CAC ≥ 400 were at 2.19-fold (95% CI: 1.15.-4.17) higher risk for lung cancer-related deaths when compared to those with CAC = 0. For current smokers only, we observed a similar trend, while the increased risk for CAC ≥ 400 was solely significant in women (Figure 3). These findings remained consistent when the age- and sex-adjusted model (Model 1), as well as the fully adjusted model (Model 2), was applied to current smokers and former smokers. (Supplemental Figure 3). Additional adjustment for smoking status in all participants with available smoking data could further support these associations (Supplemental Figure 4). Overall, the observed associations between CAC and lung cancer deaths, specifically in those with smoking history, appeared to be largely driven by women.
Figure 3.
Risks for lung cancer mortality in smokers by CAC score group and sex.
Subdistribution hazard ratios (SHR) for lung cancer mortality with CVD as competing event and with CAC = 0 as a reference group are depicted. Grouped as current smokers and those with positive smoking history (current smokers + former smokers). Unadjusted data stratified by CAC score are shown.
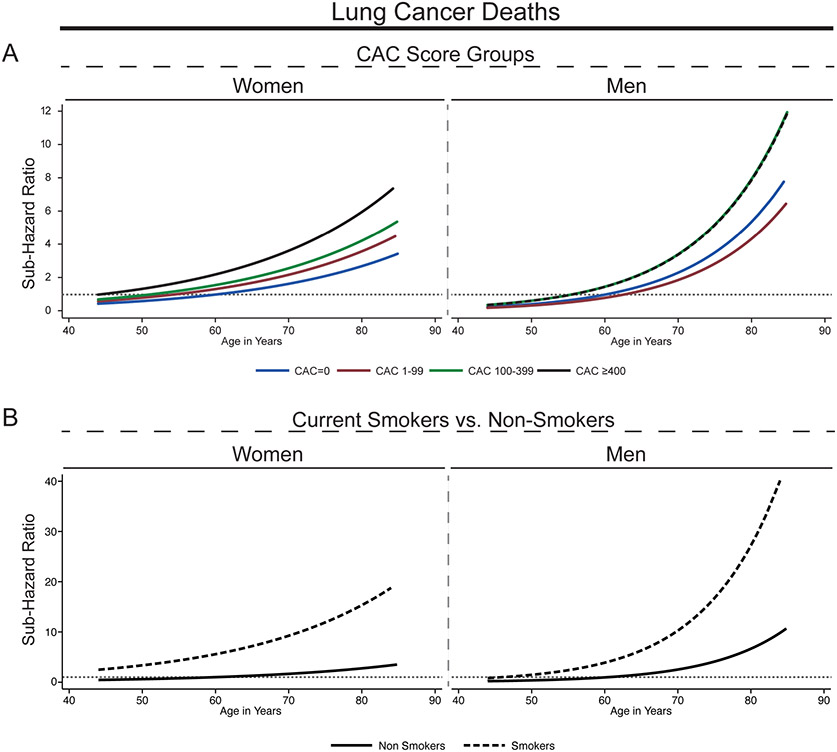
The sex-specific continuous relationships between age and lung cancer-related mortality stratified by CAC score groups and smoking status are shown in Figure 4. The curve slopes steepen with increasing CAC and the steepest increase in sub distribution hazards was observed in men (Figure 4A). Among the group of current smokers, we observed a similar age-dependent increase of risks for lung cancer mortality for both women and men (Figure 4B).
Figure 4.
Subdistribution hazard ratios for lung cancer mortality as a function of age by CAC score group, smoking status and sex.
Graphed subdistribution hazard ratios (SHR) as a function of age. SHR were adjusted for conventional risk factors for age, sex (except for sex-specific SHR), hypertension, hyperlipidemia, smoking, family history, diabetes. SHR for lung cancer mortality by CAC score groups and sex (A) and by smoking status and sex (B). All SHR are referred to the CAC = 0 group at age 60 years. Lung cancer mortality risk increased exponentially with age and showed the steepest increase for smoking men with CAC ≥ 400. Dotted grey line indicates SHR = 1. CAC = coronary artery calcium score.
4. Discussion
Our exploratory analysis demonstrates an independent association between baseline CAC and long-term lung cancer mortality for the CAC Consortium. This association is independent of smoking and underlying traditional risk factors. We additionally show that the detection of CAC can predict higher mortality rates of lung cancer especially in smoking women.
To our knowledge, our study is the first to confirm the predictive value of CAC deriving from intended CVD risk assessment for lung cancer outcomes. Our results, together with prior data from the CAC Consortium, underscore the importance of smoking in both CVD, overall cancer, and lung cancer mortality.19
Large, randomized trials such as the National Lung Screening Trial (NLST) demonstrated the beneficial use of annual CT in heavy smokers, with a reduction of lung cancer and all-cause mortality 9, 20. The predictive value of CAC as assessed in lung cancer screening scans with respect to cardiovascular mortality has been confirmed in these screening cohorts 21, 22. These studies prompted many US expert groups to implement CT-based screening in their recommendations for individuals at high risk due to smoking history, with the most recent USPSTF update form 2021 calling for annual scans in adults aged 50-80 years with at least 20 pack-years 10. Of note, these studies exclusively incorporated high-risk patients with current or former tobacco use who are also at an increased risk for cardiovascular events. While quantitative CAC assessment according to the Agatston method in these usually non-gated low-dose CT lung cancer scans is not validated, recent findings indicate that semiquantitative CAC evaluation in low-dose CT scans represent a comparable approach for prognostic considerations for CVD risks 12. Although CAC is generally thought to be a result of exposure to traditional cardiovascular risk factors including smoking, smoking cessation remains a crucial factor for CVD risk reduction that is independent of CAC 7, 23. Additionally, among those smokers with CAC = 0, 7% are reported to have a CVD event as shown for the Multi-Ethnic Study of Atherosclerosis (MESA) study cohort, which is a population-based study cohort investigating the characteristics of subclinical CVD.23 Recent studies indicate that cancer-driving mechanisms, such as chronic inflammation and proliferative processes including increased cell proliferation / cell cycle progression and inhibition of cell death, can be also found in CVD progression 24, 25. Overall, clear associations between CAC and cancer incidence and mortality have been shown for the MESA and CAC Consortium 26, 27. Thus, the presence of CAC is thought to be an additional risk factor for smokers who are already at increased risk for both lung cancer and CVD mortality. CAC thus might help define further subgroups among smokers with distinctively higher risks for adverse outcomes.
Therefore, the present study adds further details in identifying specific groups with high CAC and increased risk for lung cancer mortality that might inform screening approaches and eventually help target preventive strategies. For example, current models for prediction of lung cancer risk do not include findings from CAC scoring. In fact, the current understanding of lung cancer risks and respective preventive efforts largely relies on the smoking status 8. Conversely, CAC-based CVD risk calculations, particularly in women and smokers, might consider lung cancer as an addition potential outcome for future risk prediction 28.
The efficacy of low-dose CT for lung cancer screening has been broadly shown and is recommended for high-risk populations by U.S. societies 8. Coronary arteries are in the field of view, which offers the opportunity to assess CAC burden and provide maximal clinical value from these studies with regards to refining CVD risk. Combining CT-based risk screening for lung cancer and CAC assessment represents an important opportunity building on the overlapping risk factors of lung cancer and CVD that might offer simultaneous preventive efforts against both diseases 29. However, combined CVD- and cancer-risk prediction efforts are still in their infancy 19, 27, 30. Well-designed clinical trials will be necessary to determine the impact of dual screening on CVD and lung cancer-specific prevention.
Women have been shown to have higher mortality rates from CVD and cancer when compared to men with the same CAC score 26. Although historically lung cancer mortality rates have been higher in men than in women, there is emerging evidence that sex-specific differences in incidence rates have reversed 31. Higher incidence rates among young women when compared to men were reported, which might have contributed to our findings with slightly higher mortality rates in women.
There are some relevant limitations to this study: first, we do not have detailed information on the history of smoking, in particular the number of pack-years was not available. Second, we did not assess variables that may have both promoted CAC abnormality and cancer risk, including the duration of exposure to CVD risk factors (years of diabetes, physical inactivity, poor diet, markers of inflammation). Third, we did not have any information on the clinical methods to diagnose lung cancer. In limited cases, the diagnosis of lung cancer may have been initially detected on the CAC scan, however, such early detection would be expected to have lowered the actual mortality rate, and thus may represent a conservative bias in the assessment of CAC and long-term lung cancer mortality. Ultimately, outcomes are based only on death certificates; however, this is the same method used by the Centers for Disease Control to monitor mortality trends in the United States. Most of the patients in the CAC Consortium are Caucasian and we were therefore not able to examine whether there are ethnicity-based differences in the observed relationship.
The findings of our exploratory study allow several considerations for future risk assessment tools and preventive strategies. Patients with smoking history and higher CAC scores were at a higher risk for lung cancer death, which might prompt for enlarged lung cancer screening in this cohort. Thus, CAC scanning, in conjunction with further clinical variables, might be used to identify candidates for lung cancer screening to ensure detection at a stage of disease.
Supplementary Material
Highlights.
Coronary artery calcium (CAC) scores from cardiovascular disease (CVD) risk assessment are associated with risks for lung cancer mortality.
Associations between CAC and lung cancer mortality were strongest in current or former smokers, especially in women.
CAC scanning for CVD risk assessment might be used synergistically to identify lung cancer screening candidates.
Financial support
Dr. Blaha has received support from National Institutes of Health award L30 HL110027 for this project.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Siegel RL, Miller KD and Jemal A, Cancer statistics, 2020, CA Cancer J Clin, 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Stewart BW and Wild CP, World cancer report 2014, 2014. [Google Scholar]
- [3].Islami F, Goding Sauer A, Miller KD, et al. , Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States, CA Cancer J Clin, 2018;68:31–54. [DOI] [PubMed] [Google Scholar]
- [4].Benjamin EJ, Muntner P, Alonso A, et al. , Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association, Circulation, 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- [5].Jeon J, Holford TR, Levy DT, et al. , Smoking and Lung Cancer Mortality in the United States From 2015 to 2065: A Comparative Modeling Approach, Ann Intern Med, 2018;169:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kamimura D, Cain LR, Mentz RJ, et al. , Cigarette Smoking and Incident Heart Failure: Insights From the Jackson Heart Study, Circulation, 2018;137:2572–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mirbolouk M, Kianoush S, Dardari Z, et al. , The association of coronary artery calcium score and mortality risk among smokers: The coronary artery calcium consortium, Atherosclerosis, 2020;294:33–40. [DOI] [PubMed] [Google Scholar]
- [8].de Koning HJ, van der Aalst CM, de Jong PA, et al. , Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial, N Engl J Med, 2020;382:503–513. [DOI] [PubMed] [Google Scholar]
- [9].National Lung Screening Trial Research, T, Aberle DR, Adams AM, et al. , Reduced lung-cancer mortality with low-dose computed tomographic screening, N Engl J Med, 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Force, USPST, Krist AH, Davidson KW, et al. , Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement, JAMA, 2021;325:962–970. [DOI] [PubMed] [Google Scholar]
- [11].Huang YL, Wu FZ, Wang YC, et al. , Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score, Eur Radiol, 2013;23:1226–1233. [DOI] [PubMed] [Google Scholar]
- [12].Chiles C, Duan F, Gladish GW, et al. , Association of Coronary Artery Calcification and Mortality in the National Lung Screening Trial: A Comparison of Three Scoring Methods, Radiology, 2015;276:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shemesh J, Henschke CI, Shaham D, et al. , Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease, Radiology, 2010;257:541–548. [DOI] [PubMed] [Google Scholar]
- [14].McEvoy JW, Blaha MJ, Rivera JJ, et al. , Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification, JACC Cardiovasc Imaging, 2012;5:1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Blaha MJ, Whelton SP, Al Rifai M, et al. , Rationale and design of the coronary artery calcium consortium: a multicenter cohort study, Journal of cardiovascular computed tomography, 2017;11:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Peng AW, Mirbolouk M, Orimoloye OA, et al. , Long-Term All-Cause and Cause-Specific Mortality in Asymptomatic Patients With CAC ≥1,000: Results From the CAC Consortium, JACC Cardiovasc Imaging, 2020;13:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Carr JJ, Nelson JC, Wong ND, et al. , Calcified Coronary Artery Plaque Measurement with Cardiac CT in Population-based Studies: Standardized Protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study, Radiology, 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- [18].Agatston AS, Janowitz WR, Hildner FJ, et al. , Quantification of coronary artery calcium using ultrafast computed tomography, J Am Coll Cardiol, 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- [19].Botteri E, Iodice S, Bagnardi V, et al. , Smoking and colorectal cancer: a meta-analysis, Jama, 2008;300:2765–2778. [DOI] [PubMed] [Google Scholar]
- [20].Walter JE, Heuvelmans MA, de Jong PA, et al. , Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial, Lancet Oncol, 2016;17:907–916. [DOI] [PubMed] [Google Scholar]
- [21].Chiles C, Duan F, Gladish GW, et al. , Association of Coronary Artery Calcification and Mortality in the National Lung Screening Trial: A Comparison of Three Scoring Methods, Radiology, 2015;276:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jacobs PC, Gondrie MJA, van der Graaf Y, et al. , Coronary Artery Calcium Can Predict All-Cause Mortality and Cardiovascular Events on Low-Dose CT Screening for Lung Cancer, American Journal of Roentgenology, 2012;198:505–511. [DOI] [PubMed] [Google Scholar]
- [23].Leigh A, McEvoy JW, Garg P, et al. , Coronary Artery Calcium Scores and Atherosclerotic Cardiovascular Disease Risk Stratification in Smokers, JACC Cardiovasc Imaging, 2019;12:852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Alfaddagh A, Martin SS, Leucker TM, et al. , Inflammation and cardiovascular disease: From mechanisms to therapeutics, American Journal of Preventive Cardiology, 2020;4:100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Narayan V, Thompson EW, Demissei B, et al. , Mechanistic Biomarkers Informative of Both Cancer and Cardiovascular Disease: JACC State-of-the-Art Review, J Am Coll Cardiol, 2020;75:2726–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dzaye O, Al Rifai M, Dardari Z, et al. , Coronary Artery Calcium as a Synergistic Tool for the Age- and Sex-Specific Risk of Cardiovascular and Cancer Mortality: The Coronary Artery Calcium Consortium, J Am Heart Assoc, 2020;9:e015306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Whelton SP, Al Rifai M, Dardari Z, et al. , Coronary artery calcium and the competing long-term risk of cardiovascular vs. cancer mortality: the CAC Consortium, Eur Heart J Cardiovasc Imaging, 2019;20:389–395. [DOI] [PubMed] [Google Scholar]
- [28].McClelland RL, Jorgensen NW, Budoff M, et al. , 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study), Journal of the American College of Cardiology, 2015;66:1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ruparel M, Quaife SL, Dickson JL, et al. , Evaluation of cardiovascular risk in a lung cancer screening cohort, Thorax, 2019;74:1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mehta LS, Watson KE, Barac A, et al. , Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association, Circulation, 2018;137:e30–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jemal A, Miller KD, Ma J, et al. , Higher Lung Cancer Incidence in Young Women Than Young Men in the United States, N Engl J Med, 2018;378:1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.