Abstract
Circulating urate levels are determined by the balance between urate production and excretion, a homeostasis regulated by the function of urate transporters in key epithelial tissues and cell types. Our understanding of these physiological processes and identification of the genes encoding the urate transporters has advanced significantly, leading to a greater ability to predict risk for urate associated diseases and identify new therapeutics that directly target urate transport. Here we review the identified urate transporters and their organization and function in the renal tubule, the intestinal enterocytes, and other important cell types to provide a fuller understanding of the complicated process of urate homeostasis and its role in human disease. Further, we provide a review of the genetic tools that have provided the unbiased catalyst for transporter identification as well as a discussion of the role of transporters in determining the observed significant sex differences in urate associated disease risk.
Keywords: gout, urate, hyperuricemia, ABCG2, SLC2A9, SLC22A12, URAT1
I. Introduction
Uric acid is a weak organic acid with a pKa of 5.75, and at physiological pH exists primarily in its protonated form, urate. Urate has peak solubility at pH 5.5[1], and is less soluble at more alkaline, including physiological, pH[2]. Urate is the terminal metabolite of purine metabolism in humans and the other great apes, due to accumulation of three mutations in the uric acid oxidase (uricase) gene (UOX) resulting in complete loss of function[3]. The pseudogenization of UOX in humans is the culmination of a diminishing gradient of uricase activity in all primates[3], supporting strong selective pressure for the loss of uricase function and the increase of circulating urate levels[4]. Urate is produced from the degradation of purine nucleotides and amino acids, mediated by xanthine oxidase. Without uricase activity, humans have serum urate (SU) levels 5 to 6 times higher than other mammals[5]. The gradual loss of uricase activity likely permitted adaptation to increased urate load in humans, mitigating many pathophysiological consequences associated with uricase silencing, including the acute urate nephropathy renal failure observed in uricase knock-out mice[6]. The possible benefit of elevated SU is still hotly debated, however the disadvantages for human health are clear: increased SU results in hyperuricemia (>6.8 mg/dL), a condition which increases the risk of precipitation of monosodium urate crystals and gout, as well as a risk factor for cardiometabolic diseases[7].
The number of affected individuals with hyperuricemia in the United States is estimated to be 47.2 million (20%); with 27.9 million individuals (11.9%) having severe hyperuricemia (>7mg/dL), and men (20.2%) are affected five times more often than women (4.2%)[7]. These large numbers of hyperuricemic individuals result in an equally high prevalence of gout of approximately 4% in the United States, Europe, and Southeast Asia[7, 8]. In addition to a causal role in gout, hyperuricemia is independently associated with major drivers in human health including renal diseases, hypertension, cardiovascular disease, and metabolic syndrome[7–10]. Beneath each of these pathologies is a derangement in the careful balance between urate production and excretion with significant biological consequences. This urate homeostasis, predominantly determined by urate excretion, is maintained by epithelial transport systems of the liver, kidney, and intestine[11], but is mirrored by similar systems at the tissue and cell layer in the blood brain barrier, placenta, and in chondrocytes[12, 13]. Here we will review the molecular mechanisms of urate transport in an effort to understand urate homeostasis and the consequences of its disruption, and the role of genetics in determining risk of urate related pathologies.
II. Genome-wide association study evidence
Several approaches have been utilized to attempt to identify urate transporter machinery. Gout risk and SU levels display a strong heritable component, estimated between 40–70%[14, 15], making SU a strong candidate for genomic exploration. Initial studies examined families with pathological SU levels using a labor-intensive comparative and candidate based cloning approach. This led to the identification of urate transporter URAT1, encoded by SLC22A12[16]. Subsequent work proved that other members of the SLC22 gene family (the Organic Anion Transporters or OATs) have affinity for urate[17, 18]; however, physiological relevance of these transporters has been difficult to substantiate[19]. Frustratingly, the candidate approach failed to identify secretory urate transporters or transporters linked to increased disease risk. Fortunately, the advent of genome-wide association studies (GWAS) provided an unbiased approach to identify urate associated genes. GWAS find correlations between a given condition and common single nucleotide polymorphisms (SNPs), which serve as markers for genomic space[20]. After identification of these genomic regions, additional analyses can then be performed to identify those genes most likely to underlie the associated SNP, in some cases identifying novel causal variants that contribute to disease risk.
An abundant number of urate associated GWAS have been conducted with large and diverse populations, greatly expanding our knowledge of the key urate transporters in humans[21, 22]. The three most commonly associated genes are ABCG2, SLC2A9, and SLC22A12[21–27], and variants in these three genes have been shown to contribute to the largest portion of the measured variability in SU levels (~5%)[22]. In addition, a recent targeted study demonstrated the common ABCG2 variant rs2231142 is the only locus strongly associated with early onset gout in both European and Polynesian individuals[28]. Some additional urate associated transporters include SLC16A9[22, 24, 25], SLC17A1–4[21, 22, 24–26], SLC22A6[22], SLC22A7[22, 27], and SLC22A11[24, 25], with some evidence for SLC22A9 in East Asian populations[29], as well as SLC22A8, SLC22A13, and ABCC4 in populations with chronic renal insufficency of either European or African ancestry[30] and in Māori and Pacific Island populations of New Zealand[31]. In addition to genes coding for transport proteins, more than one hundred other loci have associated with either serum urate levels, gout risk, or both, providing an extremely rich genetic understanding of networks of associated genes, including genes that modify transporters. For example, a recent comprehensive trans-ancestry GWAS, Tin et al[22] attempted to identify common variants causal for alterations in urate levels. They identified both well-established urate transporter gene variants in ABCG2[32], as well as in the transcription factor genes HNF1A and HNF4A[22], both of which have been shown to affect expression of several urate transporters[22, 33–38]. Finally, computational approaches can be added to trans-ancestral meta-analyses to improve fine mapping of candidate causal variants to single SNP resolution, and have recently identified causal variants in SLC2A9 for both urate (rs3775948) and gout (rs4697701), and another variant in ABCG2 (rs2622621) in gout along with the previously established rs2231142[39].
A second genetic methodology that has aided in our understanding of the mechanisms of urate transport is whole exon association studies. These use whole exon or whole exome sequencing of patient populations again compared to phenotypes, specifically powered to discover both common and rare causal variants associated with SU or gout risk. Tin et al[40] found a large number of exclusively rare variants in SLC22A12 and demonstrated a select few were loss of function variants, illustrating key functional components of the URAT1 transporter. They also found a large number of both common and rare variants in SLC2A9, helping better understand the structure and function of the SLC2A9 (GLUT9) urate transporter protein[40]. Thus, genetic studies provide valuable information into the identification of various urate associated genes, including urate transporters themselves, variations in allele frequency in populations of different ethnicities, and sex differences. In addition, this work has illuminated, through the identification of functional variants, the structure and function of the key transport proteins providing insights into the unique human handling of urate and road maps to future therpautic targets.
III. Urate handling in the kidney
Urate homeostasis is the balance between urate production and excretion, a dynamic process that requires physiological adaptation and flexibility. Urate excretion occurs primarily through the normal excretion pathways for all metabolites and waste products: the kidney and the gastrointestinal tract. Of these two, the kidneys are responsible for 70% of the total urate excretion (Figure 1), joining the other primary human nitrogenous waste product, urea, expelled in the urine[41, 42]. Like our understanding of urea handling in the human kidney, urate excretion appears more complicated than necessary for simply waste elimination. After urate is freely filtered at the renal glomerulus, transporter proteins within the convoluted S1 segment of the proximal tubule facilitate the reabsorption of urate as part of the initial bulk reabsorption of many organic anions[19, 43, 44] (Figure 1C). This early proximal urate reabsorption may be influenced by either sodium or volume status. Clinical studies showed that increased dietary sodium and the resulting increases in volume, blood pressure, and renin, correlated with decreased serum urate, consistent with decreased tubule reabsorption[45]. In rats, decreases in extracellular fluid volume increases urate reabsorption in the proximal tubule and decreases urate excretion, while increases in extracellular fluid volume have the opposite effect[46], similar to what has been reported in humans[45]. Overall, the majority of the filtered urate (estimated as high as 95%)[19] is reabsorbed, an odd fate for a waste product, but may be a result of efficient but low specificity general anion capture in the early proximal tubule. The next step is avid and energetically costly secretion of urate back into the tubule lumen, as much as 50% of the original filtered load[44]. This may occur in the next 2 segments of the proximal tubule (S2, S3), the segments with the highest expression of secretory transporters (Figure 1D). The process is completed with a second phase of reabsorption in the latter S2 and S3 segments resulting in a dynamic fractional excretion of urate (FEUA) ranging from 5 to 15%[19, 43, 44]. This model of urate handling is based largely on experiments in animal models and in vitro assays and remains somewhat controversial[19]. The recent identification of many key human urate transporters, their functional assessment, and expression along the human nephron, has supported the functionally distinct phases of urate handling along the tubule. However, confirmatory protein localization in human tissue is often missing and so testing this hypothesis remains a driving force in urate research.
Fig. 1. Renal and Intestinal Urate Physiology.
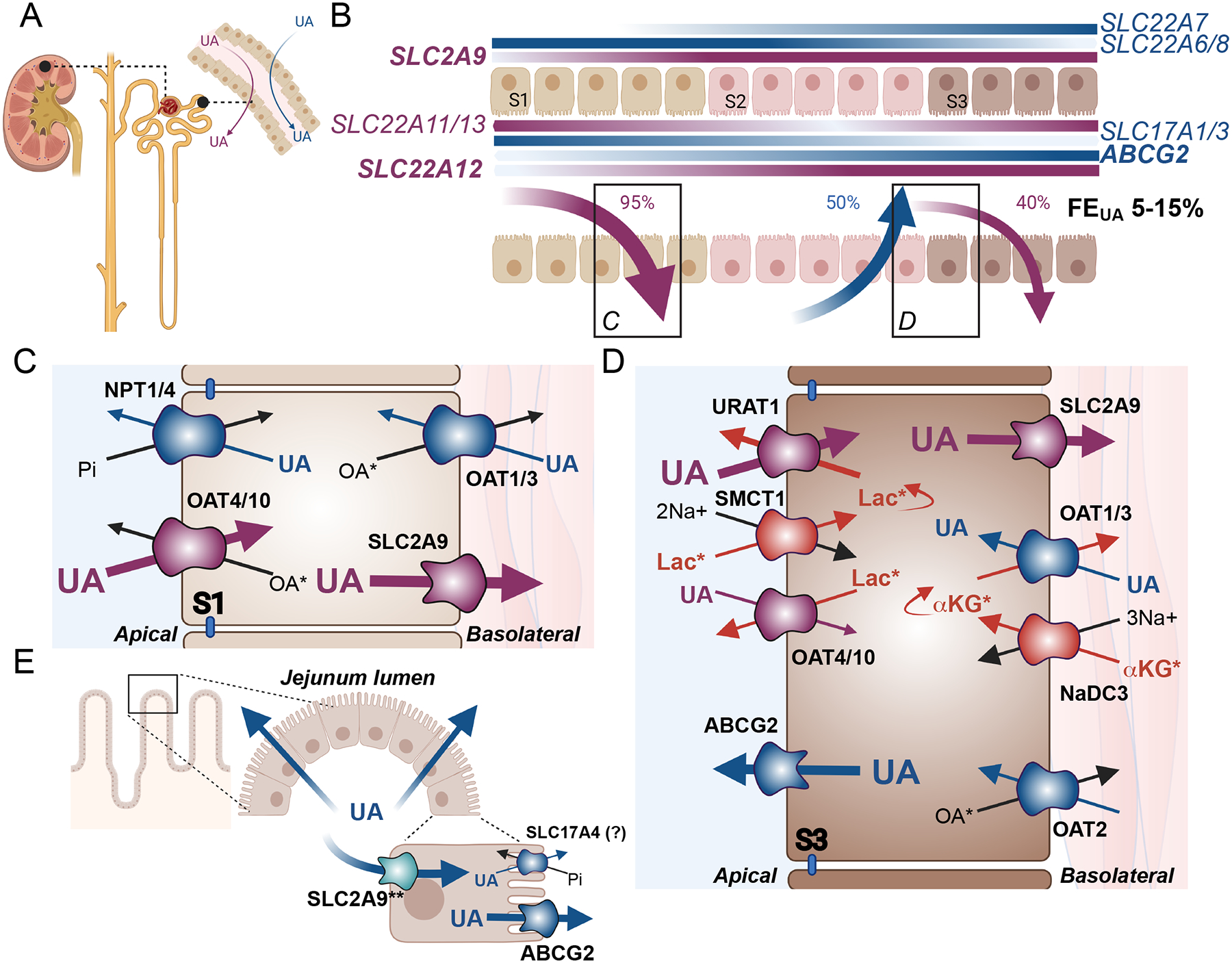
(A) The proximal tubule of the renal nephron is the principal site of urate (UA) handling through both secretion (blue arrow) and reabsorption (purple arrow). (B) Expression patterns of human urate transporter genes. Secretory transporter genes are shown in blue, while reabsorptive transporter genes are shown in purple. Gradients are displayed on the membranes of expression, with SLC22A6/7/8 and SLC2A9 coding for basolateral proteins, and ABCG2, SLC17A1/3, and SLC22A11/12/13 encoding apical transporters. The darker the color, the higher the expression, based on data from the Kidney Interactive Transcriptomics database[62]. Percentages delineate the amount of the original urate filtered load is either reabsorbed (purple arrows) or secreted (blue arrow) leading to a final fraction excretion of urate (FEUA) shown in black. Transporter protein localization and transport patterns are shown for the S1 (C) and S3 (D) segments. (C) Transporters most abundantly expressed in the S1 segments include transporters of the secretory pathway: NPT1 (SLC17A1), NPT4 (SLC17A3), OAT1 (SLC22A6) and OAT3 (SLC22A8), shown in blue, and transporters of the reabsorptive pathway: OAT4 (SLC22A11), OAT10 (SLC22A13), and SLC2A9/GLUT9 (SLC2A9), shown in purple. (D) Transporters most abundantly expressed in the S3 segment include secretory pathway transporters ABCG2/BCRP (ABCG2), OAT1, OAT2 (SLC22A7) and OAT3 shown in blue, and reabsorptive pathway transporters URAT1 (SLC22A12), SLC2A9, OAT4, and OAT10, shown in purple. Additional transporters that are functionally coupled to urate transport include the sodium cotransporters SMCT1 (SLC5A8) and NaDC3 (SLC13A3) shown in red. Blue and purple arrows indicate the direction of urate transport, while black and red arrows indicate transport of counter ions. (E) Urate is also excreted through the jejunum segment of the small intestine, where urate may enter through basolateral SLC2A9 (**well established in mice, requires further confirmation in humans), and is then secreted into the lumen primarily through ABCG2 with some potential contribution from SLC17A4 (SLC14A4).. *Some transporters have many known endogenous substrates, with only the most relevant shown. Lac: lactate; αKG: alpha-ketoglutarate; OA: organic anion; Pi: inorganic phosphate. Endogenous OAs for the OATs may include the following: OAT1: medium chain fatty acids, citrulline, prostaglandin E2 prostaglandin F2, cyclic nucleotides (cAMP, cGMP), folate[47, 50, 53]; OAT2: cAMP, GMP, GDP, GTP, cGMP, glutamate, glutarate, α-ketoglutarate, L-ascorbate, orotic acid, trigonelline, hypoxanthine, prostaglandin E2, prostaglandin F2, estrone-3-sulfate, dehydroepiandrosterone sulphate[50, 67]; OAT3: cAMP, cortisol, glutarate, prostaglandin E2, prostaglandin F2α, dehydroepiandrosterone sulphate, estrone sulphate, estradiol-17β-glucuronide, taurocholate, cholate, indoxyl sulphate [47, 50, 54, 130]; OAT4 estrone-3-sulfate, prostaglandin E2, prostaglandin F2, dehydroepiandrosterone sulphate[50, 67]; OAT10: lactate, nicotinate, glutathione, succinate[50, 92]. An additional endogenous OA for NPT1 and NP4 is inorganic phosphate co-transported in cis with Na+[57, 58]. SMCT1 mediates sodium dependent transport of monocarboxylates including short chain fatty acids, pyruvate and nicotinate[131], while NaDC3 mediates sodium coupled transport of di- and tri-carboxylates including, α-ketoglutarate, glutarate and its derivatives, citrate, succinate, and amino acid N-acetyl-L-aspartate[75].
Functional characterization of several candidate transporters have revealed a number of proteins which have some affinity for urate (Figure 1). Based on renal tubular expression in vivo, the initial bulk reabsorption is likely conducted by apically expressed OAT4 (encoded by SLC22A11), and OAT10 (encoded by SLC22A13), and other still unindentified probable transporters with lower urate affinity, to remove urate from the tubular lumen, then transported basolaterally back into the interstitum via SLC2A9[11, 17, 47–50]. Interestingly, in mice the key urate reabsorption transporter SLC2A9 is not expressed in the proximal tubule[51] and kidney specific knock out of SLC2A9 results in mice with moderate hyperuricosuria, polyuria with no concentration defect, and no change in SU, urine pH, or renal structure[52]. In contrast, humans with loss of function mutations in SLC2A9 experience significant hypouricemia[23] as discussed below, demonstrating differences in the role of SLC2A9 between the two species, and suggesting renal urate handling in humans is an adaptation to the increases in filtered urate load resulting from the loss of uricase function. Urate in the peritubular capillary then re-enters the tubule though basolateral transporters OAT1 (encoded by SLC22A6) and OAT3 (encoded by SLC22A8)[17, 47, 53–55], where it is secreted through the primary secretory transporter, ABCG2 (BCRP), expressed on the apical brush border membrane, with further secretion contributed potentially by apically localized Na+ / phosphate co-transporters, NPT1 (encoded by SLC17A1) and NPT4 (encoded by SLC17A3)[32, 56–58]. Post secretory reabsorption is likely facilitated primarily by URAT1 (encoded by SLC22A12), which is expressed at the apical brush border membrane, coupled with SLC2A9 on the basolateral membrane to transport urate to the peritubular capalaries[16, 23, 49, 59]. SLC16A9 is also preferentially expressed in proximal tubule cells[60], however the precise localization and role in urate transport is not currently understood. Paradoxically, protein localization in human proximal tubules shows ABCG2 mediated secretion and URAT1 mediated reabsorption can occur in the same cell[61], begging the obvious question of why urate is secreted and reabsorbed simultaneously; could the nephron be using urate to perform other physiological work?
Recent advances in single cell RNA-Seq data have revealed that many of the urate associated transporters have different expression patterns along the nephron (Figure 1B). All of these transporters are expressed in the three segments of the proximal tubule, however SLC17A1, SLC17A3, SLC22A6 and SLC22A8 have highest mRNA expression in the S1 segment, ABCG2, SLC2A9, and SLC22A12 have highest mRNA expression in the S3 segment, while SLC22A11 and SLC22A13 have similar mRNA expression levels in both the S1 and S3 segments[62]. Of note, some of these transporter proteins, including ABCG2, may be long lived within the cell[63], rendering mRNA levels deceptively low. Thus, different segments of the proximal tubule may have heterologous expression of a given set of transporters.
Further evidence supporting the involvement of URAT1 and SLC2A9 in urate reabsorption is found in patients with renal hypouricemia. Symptoms of hypouricemia may include hematuria and hypercalciuria, but more often include recurrent episodes of nephrolithiasis acute kidney injury (AKI) usually from dehydration due to intense or frequent exercise or gastroenteritis, or posterior reversible encephalopathy syndrome in patients with exercise associated AKI[64]. Hypouricemia type 1 (OMIM #220150) is the more common condition and associated with homozygous or compound heterozygous loss of function variants in SLC22A12, while hypouricemia type 2 (OMIM #612076) is caused by either heterozygous or homozygous defects in SLC2A9[64, 65]. These conditions have been best characterized in patients of East Asian ancestry, but additional evidence has shown that these variants are also found in a variety of ethnic groups including Arab Israelis, Ashkenazi Jews, Iraqi Jews, as well as individuals of various European ancestries[64]. Approximately 150 variants have been identified in the SLC22A12 gene, and over 100 variants have been described in SLC2A9, some of which have known associations with gout[40, 64]. URAT1 expression and localization appears evolutionarily conserved, however, the human version of the URAT1 protein has a much higher affinity for urate than that of mice and rats. This is due to substitution of a few key amino acids within the primate transporter, which likely took place in the same timeframe as primates loss of uricase function, providing further evidence for a selection advantage to increased urate retention[66].
One possible explanation for the complexity in urate transport is that urate provides crucial driving forces for the movement of other critical anions and electrolytes along the nephron. For example, URAT1 is an exchanger, and thus reabsorbs urate when another counter-ion is secreted in trans. URAT1 has affinity for numerous metabolically active anions, including lactate, ketoglutarate (αKG) and β-hydroxybutyrate(βHB)[16]. OAT1/3 can also transport these citric acid cycle intermediates [47, 50, 54, 67]. Some of these anions are generated within the cell as a product of anaerobic (lactate) or aerobic (αKG and βHB) glucose catabolism, or these molecules can enter the cells through transporters. Lactate is freely filtered at the glomerulus and can be used for gluconeogenesis in the renal cortex [68], and uptake is increased by acidosis [69]. Lactate is primarily reabsorbed by sodium-coupled transporters encoded by SLC5A8 and SLC5A12, whose protein products SMCT1 and SMCT2 are expressed on the apical side of proximal tubular cells [70, 71]. Interestingly, mice that are null for both SLC5A8 and SLC5A12 demonstrate increased urinary excretion of both lactate and urate indicating a functional coupling of lactate and urate transport in vivo [72]. The importance of sodium-dependent urate transport is further supported from data showing that healthy subjects administered the SGLT2 inhibitor phloridzin demonstrated increased FEUA[73] and that mice deficient in SGLT1 demonstrate glucosuria and uricosuria with the addition of SLGT2 inhibitor canagliflozin, evidence that increased luminal glucose may induce uricosuria, and that URAT1 is required for this effect[74]. On the basolateral side, another sodium-dependent transporter NaDC3 (encoded by SLC13A3) also transports metabolic intermediates succinate, αKG, and citrate into the cell[75]. NaDC3 may couple with the basolateral OATs, in order to exchange αKG for urate[11]. Taken together, this data provides evidence for potential roles for urate in energy metabolism and sodium handling. Furthermore, the expression and activity of these transporters in the late proximal tubule seem to be conserved across species; however, further studies are required to elucidate the roles of these transporters in urate handling in humans.
Renal function may also contribute to hyperuricemia and gout susceptibility. Not only has hyperuricemia been reported as an independent risk factor for chronic kidney disease (CKD) [76], but patients with worsening kidney function may also be more likely to develop gout[77]. There is also evidence that mild hyperuricemia may correlate with kidney damage. Increased SU levels may predict development of end stage renal disease (CKD stage 5)[76], and overall prevalence of gout in CKD is 3 to 6 times higher than in the general population[7, 77]. However, urate lowering therapy (ULT) is currently only recommended for CKD patients with gout, and not asymptomatic hyperuricemia, as urate-lowering therapy with allopurinol does not alter progression of CKD in those without gout[78, 79]. Both allopurinol and febuxostat are xanthine oxidase inhibitors[80]. These and other ULT drugs, including uricosuric agents, have complex interactions with urate transporter proteins (reviewed in detail in [81], with selected examples in Table 1). For example, both allopurinol and oxypurinol are substrates of ABCG2 (Figure 1D)[82], and this interaction may cause reduced response to allopurinol urate lowering therapy, especially in patients with the ABCG2-Q141K variant[83, 84]. The primary target for most uricosurics is URAT1, with some drugs also inhibiting other reabsorptive transporters including OAT4 and OAT10 (Table 1a), thereby decreasing reabsorption and increasing urinary urate excretion. Other drugs used to treat kidney disease can also affect urate levels, including diuretics and SGLT2 inhibitors (Table 1b). Diuretics have been shown to increase SU levels, acting as competitive inhibitors for urate on OAT1 – 3[85]. Loop diuretics can also directly inhibit ABCG2[86] and NPT4[58], which leads to a decrease in urate secretion. The uricosuric effect of interactions between SGLT2 inhibitors and urate transporters is much less well characterized, with some evidence that SGLT2 inhibitor luseogliflozin (approved for use in Japan) does not affect reabsorptive urate transport activity of SLC2A9, URAT1, OAT4 or OAT10[87], indicating glucose may be influencing urate levels by some other mechanism, which remains unclear.
Table 1a.
Drugs that Increase Urinary Urate Excretion
| Drug | Drug Action | Interactions with Urate Transporters (Secretion Pathway) | Interactions with Urate Transporters (Reabsorption Pathway) | Effects on Serum Urate |
|---|---|---|---|---|
| Primary Uricosuric Agents | ||||
| Probenecid | Renal tubule reabsorption inhibitor | High affinity inhibitor of OAT1[53] and OAT3[130], lower affinity inhibitor of OAT2[67] and NPT4[58] | Lower affinity inhibitor of URAT1[132] and OAT4[67] | Decreased SU due to decreased urate reabsorption at higher doses Increased SU due to inhibition of secretory transporters |
| Benzbromarone* | Renal tubule reabsorption inhibitor | OAT1 inhibitor[53] | Inhibitor of SLC2A9 [133] and URAT1[134] | Decreased SU due to increased urinary excretion with decreased urate reabsorption |
| Sulfinpyrazone | Renal tubule reabsorption inhibitor | --- | URAT1 inhibitor [132, 135] | |
| Lesinurad | Renal tubule reabsorption inhibitor | Minimal effects on OAT1 and OAT3[136] | Inhibitor of URAT1 and OAT4[137] | |
| Verinurad† | Renal tubule reabsorption inhibitor | --- | URAT1 inhibitor[138] | |
| Dotinurad† | Renal tubule reabsorption inhibitor | --- | URAT1 inhibitor[139] | |
| Arhalofenate† | Renal tubule reabsorption inhibitor | --- | Inhibitor of URAT1 and OAT4[140] | |
| Agents with Secondary Uricosuric Properties | ||||
| Tranilast† | Anti-inflammatory with pleiotropic effects | Moderate inhibition of NPT1, OAT1, and OAT3, with no inhibition of ABCG2[141] | High affinity inhibition of URAT1, SLC2A9, OAT4 and OAT10[141] | Preferential inhibition of urate reabsorption results in decreased SU |
| Losartan | Angiotensin II receptor antagonist (antihypertensive) | ABCG2 inhibitor[86] | Inhibitor of URAT1[134] and SLC2A9[133] | |
| Fenofibrate | PPARα activator (cholesterol lowering) | Inhibitor of ABCG2[86, 142] and OAT3 [143] | Moderate inhibition of URAT1 [144] | |
| Xanthine oxidase inhibitors (with net urinary urate excretion) | ||||
| Allopurinol | Xanthine oxidase inhibitor | Substrate of ABCG2 [83, 84] and OAT2[145] | --- | Decreased urate production with potential decreased urate secretion |
| Febuxostat | Xanthine oxidase inhibitor | ABCG2 inhibitor[86] | --- | |
| Topiroxostat† | Xanthine oxidase inhibitor | Inhibition of ABCG2[86], OAT1 and OAT3[81] | --- | |
SU: serum urate; ---: No known interactions;
clinical trials ongoing, drugs are not currently FDA approved in the US
Benzbromarone has been withdrawn in the US due to concerns with hepatotoxicity [146]
Table 1b.
Drugs that Decrease Urinary Urate Excretion
| Drug | Drug Action | Interactions with Urate Transporters (Secretion Pathway) | Interactions with Urate Transporters (Reabsorption Pathway) | Effects on Serum Urate |
|---|---|---|---|---|
| Loop Diuretics | ||||
| Furosemide | NKCC2 Inhibitor (loop diuretic) | Inhibitor of ABCG2[86] and NPT4[58]; substrate of OAT3[85] | --- | Direct inhibition of urate secretion with competitive inhibition of urate transport, leads to increased SU due to decreased secretion; |
| Bumetanide | NKCC2 Inhibitor (loop diuretic) | NPT4 inhibitor[58]; substrate of OAT1[85], OAT2[67], OAT3[85], | Substrate of OAT4[67] | Competitive inhibition of urate transport, leads to increased SU due to decreased secretion |
| Ethacrynic acid | NKCC2 Inhibitor (loop diuretic) | NPT4 inhibitor[58]; substrate of oAt3[85] | --- | |
| Torasemide | NKCC2 Inhibitor (loop diuretic) | Substrate of OAT1 and OAT3[147] | Substrate of OAT4[147] | Competitive inhibition of urate transport at the basolateral membrane, and increased urate uptake at the tubule lumen lead to increased SU |
| Thiazide Diuretics | ||||
| Bendroflumethiazide | NCC inhibitor (diuretic) | Substrate of OAT1 and OAT3[148] | --- | Competitive inhibition of urate transport, leads to increased SU due to decreased secretion Increased SU due to increased reabsorption |
| Chlorothiazide Cyclothiazide Trichlormethiazide | NCC Inhibitor (diuretic) | Substrate of OAT1[85] | --- | |
| Hydrochlorothiazide | NCC Inhibitor (diuretic) | Substrate of OAT1[85] | Substrate of OAT4[48] | |
| SGLT2 Inhibitors | ||||
| Canagliflozin | SLGT2 inhibitor (glucosuric) | Substrate of ABCG2, but not OAT1 or OAT3 [149] | URAT1 not inhibited but is required for uricosuric effect[74] | Uricosuric effects may be related to increased tubular glucose concentration[74, 87] or increased urate secretion, however the mechanisms are currently unknown |
| Dapagliflozin | SLGT2 inhibitor (glucosuric) | May improve OAT3 function[85] | Increased reduction in SU without influencing urate excretion in combination with febuxostat and verinurad [150] | |
| Empagliflozin | SLGT2 inhibitor (glucosuric) | Substrate of ABCG2[81]; may upregulate ABCG2 expression [151]; some interactions with OAT3 and minimally with OAT1[152] | --- | |
| Ertugliflozin | SLGT2 inhibitor (glucosuric) | Substrate of ABCG2 [81] | --- | |
| Other Drugs | ||||
| Aspirin | NSAID | Inhibition of OAT1 and OAT3 [153] | Substrate of URAT1[153] | Low does can increase SU due to increased reabsorption and decreased secretion High doses can cause inhibition of URAT1, with SU due to decreased reabsorption |
SU: serum urate; NCC: Sodium Chloride Co-transporter; NKCC2: Sodium-Potassium-Chloride Co-transporter; PPARα: peroxisome proliferator-activated receptor alpha; SGLT2: sodium glucose cotransporter 2; NSAID: nonsteroidal anti-inflammatory drug; ---: No known interactions
IV. Urate handling in the gut
Several of the urate transporters expressed in the kidney are also found in intestinal epithelial cells (Reviewed in [88]), including ABCG2, SLC2A9 and others. ABCG2 is localized to the apical compartment of the intestinal enterocytes[89]. SLC2A9 is expressed primarily on the basolateral side of enterocytes in mice[61, 90], poised to provide basolateral entry, coupled with ABCG2 on the apical side (Figure 1E). Disruption of this pathway in mice with the intestine specific knockout of SLC2A9 or the knock-in of the human gout variant ortholog Q140K ABCG2, leads to significantly reduced intestinal urate excretion, moderate hyperuricemia, and metabolic syndrome[61, 90]. However, the role of SLC2A9 in human intestine is less clear. Other urate associated transporter genes expressed in the intestine include SLC17A4[91] expressed on the apical brush border, SLC22A13[92] and SLC16A9[93], but where these proteins localize and how they contribute in intestinal excretion remains to be confirmed.
The most well characterized intestinal urate transporter is ABCG2. Matsuo et al demonstrated that patients with end stage renal disease who had severely reduced renal urate excretion were highly dependent upon ABCG2 mediated secretion in the gut [94]. Thus, patients with loss of function variants in ABCG2 activity displayed a higher degree of hyperuricemia compared with those without. A recent human interventional trial measured urate handling in individuals with the common Q141K variant (rs2231142) of ABCG2 compared to a control cohort. They showed in the control cohort that extrarenal (intestinal) excretion of urate was the primary driver of variation in SU levels during a urate loading test, whereas for Q141K ABCG2 individuals, renal excretion was the primary source of variation in SU, and there was a significant loss of extrarenal excretion. To understand the mechanism, in the same study, Hoque et al created a mouse ortholog model of the Q141K ABCG2 variant. These animals replicated the human phenotype: hyperuricemia, moderate reduction in renal excretion, and significant loss to intestinal urate excretion[61]. ABCG2 is expressed in the brush border of the villi cells in the jejunum and ileum in mice, and mice with the ABCG2 variant showed a complete loss of ABCG2 mediated intestinal excretion correlated with a severe reduction in ABCG2 abundance. Interestingly, the Q141K mouse model also confirmed the role of ABCG2 in renal excretion, but they found that, in contrast to the intestines, the variant showed only a moderate decline in abundance and function. Similar results were observed in ABCG2 whole body knockout mice, which demonstrate increased SU levels and decreased intestinal excretion of urate[95].
V. Urate handling in the liver
Increased urate production is another potential cause of hyperuricemia. The liver is the major site of urate production[19, 96], where urate precursors enter hepatic cells, and are then metabolized to urate through xanthine oxidase. These precursors include endogenous nucleotide purines, including AMP and GMP, as well as dietary purines and fructose[97]. Fructose metabolism rapidly consumes ATP, stimulating AMP deaminase, leading to increased urate production[98]. Increased fructose intake through either sucrose or high fructose corn syrup can not only increase SU levels through increased ATP consumption, but can also increase risk for developing several urate associated co-morbidities including metabolic syndrome, fatty liver disease, insulin resistance, and type 2 diabetes[99]. In vitro analyses of human hepatocytes have shown that increased urate, independent of fructose, can stimulate fructose metabolism through up-regulation of fructokinase (KHK)[100], and that inhibition of urate production blocked fructose-induced hepatocyte triglyceride accumulation in both human HepG2 cells and in male rats fed high fructose treated with allopurinol[100]. Thus, there is evidence that it is the increased urate as a result of higher fructose intake that plays a role in fructose associated co-morbidities.
The liver also expresses several urate transporters, including ABCG2, SLC2A9, SLC22A7 and SLC22A11[49, 89, 101] supporting an important role for urate transport in the liver that has yet to be determined, particularly in humans. SLC2A9 is expressed on the apical surface of hepatocytes in humans[49], as well as mice[102]. Interestingly, liver-specific SLC2A9 knock out mice (LG9KO) demonstrate higher SU than whole body SLC2A9 knock out mice (G9KO), with a lower increase in FEUA (~25%) compared to G9KO mice (~100% for males, ~150% for females), implying SLC2A9 may be essential for urate uptake into murine livers[102]. In addition, ABCG2 is expressed in the liver, specifically at the bile canalicular membrane, oriented to efflux urate into the bile, leading to eventual deposition in the intestine[89]. However, oxonate-treated rats demonstrated only a 0.68% recovery of administered 14[C] uric acid in the bile, compared to 42.58% in the urine and 8.90% in the intestinal lumen[103], supporting a lesser contribution to overall urate excretion from the liver. Human hepatic excretion of urate in the bile has yet to be determined[12], so the effect of common ABCG2 variants in the liver is unclear.
VI. Sex differences in urate handling
Sex differences in human physiology have recently come into focus in a variety of fields, including immunological[104–106] and renal diseases[107–111]. There is also substantial evidence that male sex is a significant risk factor for hyperuricemia and gout[112, 113], with men up to 4 times more likely to be affected than women[8]. This observation has recently been coupled to investigations of sex differences in urate handling in the kidney[61], as well as differential effects of pathogenic variants in urate transporter genes[22, 114]. A recent review[115] argued that even though SU levels tend to be lower in females[7], females with elevated SU levels have increased risk of associated co-morbidities, including hypertension[116–118], chronic kidney disease[117, 119, 120], and type 2 diabetes[117, 121]. GWAS evidence has shown almost 200 loci associated with SU levels[22, 29], but only three of these SNPs have sex specific effects; two in urate transporters ABCG2 and SLC2A9, and the third in gene encoding urate transporter scaffold protein PDZK1[22, 114]. Thus, the observed sex differences are more likely due to intrinsic differences in urate handling in males compared to females, with some evidence that estrogen may play a role in regulating either urate transporters themselves[122–124], or urate associated transcription factors[125, 126]. This is further supported by the fact that the chance of females becoming hyperuricemic increases 5-fold after menopause, and this risk can be mitigated by hormone replacement therapy[127–129]. However, further studies are required to elucidate the precise mechanisms for the differential regulation of urate handling between males and females.
VII. Summary
Urate homeostasis is a complicated process that involves several organ systems. Several urate transport proteins have been identified through genetic associations using GWAS and in vitro studies. Urate transport in the kidney has been the most well characterized; however, the purpose and regulation of this intricate process of reabsorption, secretion, and additional reabsorption has yet to be elucidated. This complex system implies the kidney may be using urate as a counter-ion to perform some other function that remains to be determined. Further study is also required to identify the full complement and subsequent roles of the urate and related transporters in both the intestine and the liver to better understand whole body urate homeostasis. This greater understanding could lead to insights into the mechanisms regarding sex differences in urate handling, as well as potential novel therapeutic targets and treatments to improve quality of life of patients afflicted with hyperuricemia, gout, and other related co-morbidities.
VIII. Practice Points
Serum urate levels are influenced by the urate transport mechanisms of the kidney and intestine excretion pathways.
Pathological disruptions of these key excretory organs, as observed in CKD and intestinal infection, increases serum urate and risk for gout.
Recent genetic analysis has revealed much of the heritability of hyperuricemia and gout risk is due to functional variants in the key urate transporter genes, ABCG2, SLC22A12, and SLC2A9.
Understanding individuals genetic background may inform on risk for urate associated disease and point to patient centered treatment options.
IX. Research Agenda
Additional research into the physiology and identities of urate transporters to better understand the counter intuitive nature of the kidney’s handling of urate
Functional studies examining the role of urate as a counter-ion for some other physiologically important process, such as sodium or water balance
Additional laboratory research examining the role of how urate and glucose each influence renal handling of the other, and mechanistic studies into the contributions of SGLT2
Identification and characterization of additional urate transporters in the intestine and the liver
Functional studies to determine the underlying mechanisms for the sex differences in urate handling
Acknowledgements
The authors would like to acknowledge members of the Woodward lab for their helpful discussions. Figures were prepared with the assistance of BioRender.com.
Funding
This research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, R01DK114091 (O.M.W.) and F32DK124960 (V.L.H.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
In the past three years O.M.W has received research funding from AstraZeneca.
References
- 1.Shimizu T, Nishikawa M, Matsushige H. 139 THE SOLUBILITY OF URIC ACID AND MONOSODIUM URATE IN URINE. Pediatric Research. 1988;24(1):134–. [DOI] [PubMed] [Google Scholar]
- 2.Wilcox WR, Khalaf A, Weinberger A, et al. Solubility of uric acid and monosodium urate. Med Biol Eng. 1972;10(4):522–31. [DOI] [PubMed] [Google Scholar]
- 3.Friedman TB, Polanco GE, Appold JC, et al. On the loss of uricolytic activity during primate evolution--I. Silencing of urate oxidase in a hominoid ancestor. Comp Biochem Physiol B. 1985;81(3):653–9. [DOI] [PubMed] [Google Scholar]
- 4.Oda M, Satta Y, Takenaka O, et al. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19(5):640–53. [DOI] [PubMed] [Google Scholar]
- 5.So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120(6):1791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Wakamiya M, Vaishnav S, et al. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci U S A. 1994;91(2):742–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen-Xu M, Yokose C, Rai SK, et al. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey 2007–2016. Arthritis Rheumatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo CF, Grainge MJ, Zhang W, et al. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–62. [DOI] [PubMed] [Google Scholar]
- 9.Mazzali M, Kanbay M, Segal MS, et al. Uric acid and hypertension: cause or effect? Curr Rheumatol Rep. 2010;12(2):108–17. [DOI] [PubMed] [Google Scholar]
- 10.Dalbeth N, Allan J, Gamble GD, et al. Effect of body mass index on serum urate and renal uric acid handling responses to an oral inosine load: experimental intervention study in healthy volunteers. Arthritis Res Ther. 2020;22(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandal AK, Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. 2015;77:323–45. [DOI] [PubMed] [Google Scholar]
- 12.Woodward OM, Kottgen A, Kottgen M. ABCG transporters and disease. FEBS J. 2011;278(18):3215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang B, Duan M, Long B, et al. Urate transport capacity of glucose transporter 9 and urate transporter 1 in cartilage chondrocytes. Mol Med Rep. 2019;20(2):1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilk JB, Djousse L, Borecki I, et al. Segregation analysis of serum uric acid in the NHLBI Family Heart Study. Hum Genet. 2000;106(3):355–9. [DOI] [PubMed] [Google Scholar]
- 15.Nath SD, Voruganti VS, Arar NH, et al. Genome scan for determinants of serum uric acid variability. J Am Soc Nephrol. 2007;18(12):3156–63. [DOI] [PubMed] [Google Scholar]
- 16.Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417(6887):447–52. [DOI] [PubMed] [Google Scholar]
- 17.Eraly SA, Vallon V, Rieg T, et al. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics. 2008;33(2):180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L, Shi Y, Zhuang S, et al. Recent advances on uric acid transporters. Oncotarget. 2017;8(59):100852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012;19(6):358–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kottgen A. Genome-wide association studies in nephrology research. Am J Kidney Dis. 2010;56(4):743–58. [DOI] [PubMed] [Google Scholar]
- 21.Dehghan A, Kottgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372(9654):1953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tin A, Marten J, Halperin Kuhns VL, et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat Genet. 2019;51(10):1459–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40(4):437–42. [DOI] [PubMed] [Google Scholar]
- 24.Kolz M, Johnson T, Sanna S, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5(6):e1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kottgen A, Albrecht E, Teumer A, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama A, Nakaoka H, Yamamoto K, et al. GWAS of clinically defined gout and subtypes identifies multiple susceptibility loci that include urate transporter genes. Ann Rheum Dis. 2017;76(5):869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakatochi M, Kanai M, Nakayama A, et al. Genome-wide meta-analysis identifies multiple novel loci associated with serum uric acid levels in Japanese individuals. Commun Biol. 2019;2:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaidi F, Narang RK, Phipps-Green A, et al. Systematic genetic analysis of early-onset gout: ABCG2 is the only associated locus. Rheumatology (Oxford). 2020. [DOI] [PubMed] [Google Scholar]
- 29.Boocock J, Leask M, Okada Y, et al. Genomic dissection of 43 serum urate-associated loci provides multiple insights into molecular mechanisms of urate control. Hum Mol Genet. 2020. [DOI] [PubMed] [Google Scholar]
- 30.Bhatnagar V, Richard EL, Wu W, et al. Analysis of ABCG2 and other urate transporters in uric acid homeostasis in chronic kidney disease: potential role of remote sensing and signaling. Clin Kidney J. 2016;9(3):444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanner C, Boocock J, Stahl EA, et al. Population-Specific Resequencing Associates the ATP-Binding Cassette Subfamily C Member 4 Gene With Gout in New Zealand Maori and Pacific Men. Arthritis Rheumatol. 2017;69(7):1461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodward OM, Kottgen A, Coresh J, et al. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106(25):10338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheret C, Doyen A, Yaniv M, et al. Hepatocyte nuclear factor 1 alpha controls renal expression of the Npt1-Npt4 anionic transporter locus. J Mol Biol. 2002;322(5):929–41. [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi R, Kusuhara H, Hattori N, et al. Regulation of the expression of human organic anion transporter 3 by hepatocyte nuclear factor 1alpha/beta and DNA methylation. Mol Pharmacol. 2006;70(3):887–96. [DOI] [PubMed] [Google Scholar]
- 35.Saji T, Kikuchi R, Kusuhara H, et al. Transcriptional regulation of human and mouse organic anion transporter 1 by hepatocyte nuclear factor 1 alpha/beta. J Pharmacol Exp Ther. 2008;324(2):784–90. [DOI] [PubMed] [Google Scholar]
- 36.Jin L, Kikuchi R, Saji T, et al. Regulation of tissue-specific expression of renal organic anion transporters by hepatocyte nuclear factor 1 alpha/beta and DNA methylation. J Pharmacol Exp Ther. 2012;340(3):648–55. [DOI] [PubMed] [Google Scholar]
- 37.Prestin K, Wolf S, Feldtmann R, et al. Transcriptional regulation of urate transportosome member SLC2A9 by nuclear receptor HNF4alpha. Am J Physiol Renal Physiol. 2014;307(9):F1041–51. [DOI] [PubMed] [Google Scholar]
- 38.Prestin K, Hussner J, Ferreira C, et al. Regulation of PDZ domain-containing 1 (PDZK1) expression by hepatocyte nuclear factor-1alpha (HNF1alpha) in human kidney. Am J Physiol Renal Physiol. 2017;313(4):F973–F83. [DOI] [PubMed] [Google Scholar]
- 39.Takei R, Cadzow M, Markie D, et al. Trans-ancestral dissection of urate- and gout-associated major loci SLC2A9 and ABCG2 reveals primate-specific regulatory effects. J Hum Genet. 2021;66(2):161–9. [DOI] [PubMed] [Google Scholar]
- 40.Tin A, Li Y, Brody JA, et al. Large-scale whole-exome sequencing association studies identify rare functional variants influencing serum urate levels. Nat Commun. 2018;9(1):4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esparza Martin N, Garcia Nieto V. Hypouricemia and tubular transport of uric acid. Nefrologia. 2011;31(1):44–50. [DOI] [PubMed] [Google Scholar]
- 42.Roch-Ramel F, Werner D, Guisan B. Urate transport in brush-border membrane of human kidney. Am J Physiol. 1994;266(5 Pt 2):F797–805. [DOI] [PubMed] [Google Scholar]
- 43.Gutman AB, Yu TF. A three-component system for regulation of renal excretion of uric acid in man. Trans Assoc Am Physicians. 1961;74:353–65. [PubMed] [Google Scholar]
- 44.Maesaka JK, Fishbane S. Regulation of renal urate excretion: a critical review. Am J Kidney Dis. 1998;32(6):917–33. [DOI] [PubMed] [Google Scholar]
- 45.Lei L, Wang JG. Dietary Sodium Intake and Serum Uric Acid: A Mini-Review. Pulse (Basel). 2018;6(1–2):124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinman EJ, Eknoyan G, Suki WN. The influence of the extracellular fluid volume on the tubular reabsorption of uric acid. J Clin Invest. 1975;55(2):283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Motohashi H, Sakurai Y, Saito H, et al. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002;13(4):866–74. [DOI] [PubMed] [Google Scholar]
- 48.Hagos Y, Stein D, Ugele B, et al. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol. 2007;18(2):430–9. [DOI] [PubMed] [Google Scholar]
- 49.Caulfield MJ, Munroe PB, O’Neill D, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008;5(10):e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol Aspects Med. 2013;34(2–3):413–35. [DOI] [PubMed] [Google Scholar]
- 51.Chen L, Chou CL, Knepper MA. A Comprehensive Map of mRNAs and Their Isoforms across All 14 Renal Tubule Segments of Mouse. J Am Soc Nephrol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Auberson M, Stadelmann S, Stoudmann C, et al. SLC2A9 (GLUT9) mediates urate reabsorption in the mouse kidney. Pflugers Arch. 2018;470(12):1739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ichida K, Hosoyamada M, Kimura H, et al. Urate transport via human PAH transporter hOAT1 and its gene structure. Kidney Int. 2003;63(1):143–55. [DOI] [PubMed] [Google Scholar]
- 54.Bakhiya A, Bahn A, Burckhardt G, et al. Human organic anion transporter 3 (hOAT3) can operate as an exchanger and mediate secretory urate flux. Cell Physiol Biochem. 2003;13(5):249–56. [DOI] [PubMed] [Google Scholar]
- 55.Motohashi H, Nakao Y, Masuda S, et al. Precise comparison of protein localization among OCT, OAT, and MATE in human kidney. J Pharm Sci. 2013;102(9):3302–8. [DOI] [PubMed] [Google Scholar]
- 56.Huls M, Brown CD, Windass AS, et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73(2):220–5. [DOI] [PubMed] [Google Scholar]
- 57.Iharada M, Miyaji T, Fujimoto T, et al. Type 1 sodium-dependent phosphate transporter (SLC17A1 Protein) is a Cl(−)-dependent urate exporter. J Biol Chem. 2010;285(34):26107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jutabha P, Anzai N, Kitamura K, et al. Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate. J Biol Chem. 2010;285(45):35123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Augustin R, Carayannopoulos MO, Dowd LO, et al. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem. 2004;279(16):16229–36. [DOI] [PubMed] [Google Scholar]
- 60.Habuka M, Fagerberg L, Hallstrom BM, et al. The kidney transcriptome and proteome defined by transcriptomics and antibody-based profiling. PLoS One. 2014;9(12):e116125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoque KM, Dixon EE, Lewis RM, et al. The ABCG2 Q141K hyperuricemia and gout associated variant illuminates the physiology of human urate excretion. Nat Commun. 2020;11(1):2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu H, Uchimura K, Donnelly EL, et al. Comparative Analysis and Refinement of Human PSC-Derived Kidney Organoid Differentiation with Single-Cell Transcriptomics. Cell Stem Cell. 2018;23(6):869–81 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peng H, Qi J, Dong Z, et al. Dynamic vs static ABCG2 inhibitors to sensitize drug resistant cancer cells. PLoS One. 2010;5(12):e15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mancikova A, Krylov V, Hurba O, et al. Functional analysis of novel allelic variants in URAT1 and GLUT9 causing renal hypouricemia type 1 and 2. Clin Exp Nephrol. 2016;20(4):578–84. [DOI] [PubMed] [Google Scholar]
- 65.Dinour D, Gray NK, Ganon L, et al. Two novel homozygous SLC2A9 mutations cause renal hypouricemia type 2. Nephrol Dial Transplant. 2012;27(3):1035–41. [DOI] [PubMed] [Google Scholar]
- 66.Tan PK, Farrar JE, Gaucher EA, et al. Coevolution of URAT1 and Uricase during Primate Evolution: Implications for Serum Urate Homeostasis and Gout. Mol Biol Evol. 2016;33(9):2193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Enomoto A, Takeda M, Shimoda M, et al. Interaction of human organic anion transporters 2 and 4 with organic anion transport inhibitors. J Pharmacol Exp Ther. 2002;301(3):797–802. [DOI] [PubMed] [Google Scholar]
- 68.Stumvoll M, Meyer C, Perriello G, et al. Human kidney and liver gluconeogenesis: evidence for organ substrate selectivity. Am J Physiol. 1998;274(5):E817–26. [DOI] [PubMed] [Google Scholar]
- 69.Bellomo R. Bench-to-bedside review: lactate and the kidney. Crit Care. 2002;6(4):322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coady MJ, Chang MH, Charron FM, et al. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol. 2004;557(Pt 3):719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gopal E, Umapathy NS, Martin PM, et al. Cloning and functional characterization of human SMCT2 (SLC5A12) and expression pattern of the transporter in kidney. Biochim Biophys Acta. 2007;1768(11):2690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thangaraju M, Ananth S, Martin PM, et al. c/ebpdelta Null mouse as a model for the double knock-out of slc5a8 and slc5a12 in kidney. J Biol Chem. 2006;281(37):26769–73. [DOI] [PubMed] [Google Scholar]
- 73.Skeith MD, Healey LA, Cutler RE. Effect of phloridzin on uric acid excretion in man. Am J Physiol. 1970;219(4):1080–2. [DOI] [PubMed] [Google Scholar]
- 74.Novikov A, Fu Y, Huang W, et al. SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am J Physiol Renal Physiol. 2019;316(1):F173–F85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bergeron MJ, Clemencon B, Hediger MA, et al. SLC13 family of Na(+)-coupled di- and tri-carboxylate/sulfate transporters. Mol Aspects Med. 2013;34(2–3):299–312. [DOI] [PubMed] [Google Scholar]
- 76.Borghi C, Agabiti-Rosei E, Johnson RJ, et al. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur J Intern Med. 2020;80:1–11. [DOI] [PubMed] [Google Scholar]
- 77.Mohammed E, Browne LD, Kumar AUA, et al. Prevalence and treatment of gout among patients with chronic kidney disease in the Irish health system: A national study. PLoS One. 2019;14(1):e0210487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Badve SV, Pascoe EM, Tiku A, et al. Effects of Allopurinol on the Progression of Chronic Kidney Disease. N Engl J Med. 2020;382(26):2504–13. [DOI] [PubMed] [Google Scholar]
- 79.Doria A, Galecki AT, Spino C, et al. Serum Urate Lowering with Allopurinol and Kidney Function in Type 1 Diabetes. N Engl J Med. 2020;382(26):2493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res (Hoboken). 2020;72(6):744–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tatrai P, Erdo F, Dornyei G, et al. Modulation of Urate Transport by Drugs. Pharmaceutics. 2021;13(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakamura M, Fujita K, Toyoda Y, et al. Investigation of the transport of xanthine dehydrogenase inhibitors by the urate transporter ABCG2. Drug Metab Pharmacokinet. 2018;33(1):77–81. [DOI] [PubMed] [Google Scholar]
- 83.Roberts RL, Wallace MC, Phipps-Green AJ, et al. ABCG2 loss-of-function polymorphism predicts poor response to allopurinol in patients with gout. Pharmacogenomics J. 2017;17(2):201–3. [DOI] [PubMed] [Google Scholar]
- 84.Wen CC, Yee SW, Liang X, et al. Genome-wide association study identifies ABCG2 (BCRP) as an allopurinol transporter and a determinant of drug response. Clin Pharmacol Ther. 2015;97(5):518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hasannejad H, Takeda M, Taki K, et al. Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther. 2004;308(3):1021–9. [DOI] [PubMed] [Google Scholar]
- 86.Miyata H, Takada T, Toyoda Y, et al. Identification of Febuxostat as a New Strong ABCG2 Inhibitor: Potential Applications and Risks in Clinical Situations. Front Pharmacol. 2016;7:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chino Y, Samukawa Y, Sakai S, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35(7):391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu X, Li C, Zhou P, et al. Uric acid transporters hiding in the intestine. Pharm Biol. 2016;54(12):3151–5. [DOI] [PubMed] [Google Scholar]
- 89.Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61(8):3458–64. [PubMed] [Google Scholar]
- 90.DeBosch BJ, Kluth O, Fujiwara H, et al. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat Commun. 2014;5:4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Togawa N, Miyaji T, Izawa S, et al. A Na+-phosphate cotransporter homologue (SLC17A4 protein) is an intestinal organic anion exporter. Am J Physiol Cell Physiol. 2012;302(11):C1652–60. [DOI] [PubMed] [Google Scholar]
- 92.Bahn A, Hagos Y, Reuter S, et al. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem. 2008;283(24):16332–41. [DOI] [PubMed] [Google Scholar]
- 93.Nakayama A, Matsuo H, Shimizu T, et al. Common missense variant of monocarboxylate transporter 9 (MCT9/SLC16A9) gene is associated with renal overload gout, but not with all gout susceptibility. Hum Cell. 2013;26(4):133–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsuo H, Tsunoda T, Ooyama K, et al. Hyperuricemia in acute gastroenteritis is caused by decreased urate excretion via ABCG2. Sci Rep. 2016;6:31003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ichida K, Matsuo H, Takada T, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun. 2012;3:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hediger MA, Johnson RJ, Miyazaki H, et al. Molecular physiology of urate transport. Physiology (Bethesda). 2005;20:125–33. [DOI] [PubMed] [Google Scholar]
- 97.Maiuolo J, Oppedisano F, Gratteri S, et al. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14. [DOI] [PubMed] [Google Scholar]
- 98.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and - independent fatty liver. J Biol Chem. 2012;287(48):40732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One. 2012;7(10):e47948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hilgendorf C, Ahlin G, Seithel A, et al. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007;35(8):1333–40. [DOI] [PubMed] [Google Scholar]
- 102.Preitner F, Bonny O, Laverriere A, et al. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci U S A. 2009;106(36):15501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hosomi A, Nakanishi T, Fujita T, et al. Extra-renal elimination of uric acid via intestinal efflux transporter BCRP/ABCG2. PLoS One. 2012;7(2):e30456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38. [DOI] [PubMed] [Google Scholar]
- 105.Becerra-Diaz M, Strickland AB, Keselman A, et al. Androgen and Androgen Receptor as Enhancers of M2 Macrophage Polarization in Allergic Lung Inflammation. J Immunol. 2018;201(10):2923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dolsen MR, Crosswell AD, Prather AA. Links Between Stress, Sleep, and Inflammation: Are there Sex Differences? Curr Psychiatry Rep. 2019;21(2):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Costacou T, Fried L, Ellis D, et al. Sex differences in the development of kidney disease in individuals with type 1 diabetes mellitus: a contemporary analysis. Am J Kidney Dis. 2011;58(4):565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bjornstad P, Cherney DZ. Renal Hyperfiltration in Adolescents with Type 2 Diabetes: Physiology, Sex Differences, and Implications for Diabetic Kidney Disease. Curr Diab Rep. 2018;18(5):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Antlanger M, Noordzij M, van de Luijtgaarden M, et al. Sex Differences in Kidney Replacement Therapy Initiation and Maintenance. Clin J Am Soc Nephrol. 2019;14(11):1616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shepard BD. Sex Differences in Diabetes and Kidney Disease: Mechanisms and Consequences. Am J Physiol Renal Physiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Layton AT, Sullivan JC. Recent advances in sex differences in kidney function. Am J Physiol Renal Physiol. 2019;316(2):F328–F31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harrold LR, Etzel CJ, Gibofsky A, et al. Sex differences in gout characteristics: tailoring care for women and men. BMC Musculoskelet Disord. 2017;18(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harrold LR, Yood RA, Mikuls TR, et al. Sex differences in gout epidemiology: evaluation and treatment. Ann Rheum Dis. 2006;65(10):1368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Narang RK, Topless R, Cadzow M, et al. Interactions between serum urate-associated genetic variants and sex on gout risk: analysis of the UK Biobank. Arthritis Res Ther. 2019;21(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Halperin Kuhns VL, Woodward OM. Sex Differences in Urate Handling. Int J Mol Sci. 2020;21(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yoshitomi R, Fukui A, Nakayama M, et al. Sex differences in the association between serum uric acid levels and cardiac hypertrophy in patients with chronic kidney disease. Hypertens Res. 2014;37(3):246–52. [DOI] [PubMed] [Google Scholar]
- 117.Kuwabara M, Niwa K, Hisatome I, et al. Asymptomatic Hyperuricemia Without Comorbidities Predicts Cardiometabolic Diseases: Five-Year Japanese Cohort Study. Hypertension. 2017;69(6):1036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin YK, Lin YP, Lee JT, et al. Sex-specific association of hyperuricemia with cardiometabolic abnormalities in a military cohort: The CHIEF study. Medicine (Baltimore). 2020;99(12):e19535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chang HY, Tung CW, Lee PH, et al. Hyperuricemia as an independent risk factor of chronic kidney disease in middle-aged and elderly population. Am J Med Sci. 2010;339(6):509–15. [DOI] [PubMed] [Google Scholar]
- 120.Redon P, Maloberti A, Facchetti R, et al. Gender-related differences in serum uric acid in treated hypertensive patients from central and east European countries: findings from the Blood Pressure control rate and CArdiovascular Risk profilE study. J Hypertens. 2019;37(2):380–8. [DOI] [PubMed] [Google Scholar]
- 121.Yamada T, Fukatsu M, Suzuki S, et al. Elevated serum uric acid predicts impaired fasting glucose and type 2 diabetes only among Japanese women undergoing health checkups. Diabetes Metab. 2011;37(3):252–8. [DOI] [PubMed] [Google Scholar]
- 122.Ee PL, Kamalakaran S, Tonetti D, et al. Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res. 2004;64(4):1247–51. [DOI] [PubMed] [Google Scholar]
- 123.Imai Y, Ishikawa E, Asada S, et al. Estrogen-mediated post transcriptional down-regulation of breast cancer resistance protein/ABCG2. Cancer Res. 2005;65(2):596–604. [PubMed] [Google Scholar]
- 124.Matsubayashi M, Sakaguchi YM, Sahara Y, et al. 27-Hydroxycholesterol regulates human SLC22A12 gene expression through estrogen receptor action. FASEB J. 2021;35(1):e21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Selva DM, Hogeveen KN, Innis SM, et al. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest. 2007;117(12):3979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weltmeier F, Borlak J. A high resolution genome-wide scan of HNF4alpha recognition sites infers a regulatory gene network in colon cancer. PLoS One. 2011;6(7):e21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hak AE, Curhan GC, Grodstein F, et al. Menopause, postmenopausal hormone use and risk of incident gout. Ann Rheum Dis. 2010;69(7):1305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ioannou GN, Boyko EJ. Effects of menopause and hormone replacement therapy on the associations of hyperuricemia with mortality. Atherosclerosis. 2013;226(1):220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sumino H, Ichikawa S, Kanda T, et al. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet. 1999;354(9179):650. [DOI] [PubMed] [Google Scholar]
- 130.Cha SH, Sekine T, Fukushima JI, et al. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59(5):1277–86. [DOI] [PubMed] [Google Scholar]
- 131.Wright EM. Glucose transport families SLC5 and SLC50. Mol Aspects Med. 2013;34(2–3):183–96. [DOI] [PubMed] [Google Scholar]
- 132.Shin HJ, Takeda M, Enomoto A, et al. Interactions of urate transporter URAT1 in human kidney with uricosuric drugs. Nephrology (Carlton). 2011;16(2):156–62. [DOI] [PubMed] [Google Scholar]
- 133.Anzai N, Ichida K, Jutabha P, et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem. 2008;283(40):26834–8. [DOI] [PubMed] [Google Scholar]
- 134.Nindita Y, Hamada T, Bahrudin U, et al. Effect of losartan and benzbromarone on the level of human urate transporter 1 mRNA. Arzneimittelforschung. 2010;60(4):186–8. [DOI] [PubMed] [Google Scholar]
- 135.Tan PK, Ostertag TM, Miner JN. Mechanism of high affinity inhibition of the human urate transporter URAT1. Sci Rep. 2016;6:34995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shen Z, Yeh LT, Wallach K, et al. In Vitro and In Vivo Interaction Studies Between Lesinurad, a Selective Urate Reabsorption Inhibitor, and Major Liver or Kidney Transporters. Clin Drug Investig. 2016;36(6):443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miner JN, Tan PK, Hyndman D, et al. Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res Ther. 2016;18(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tan PK, Liu S, Gunic E, et al. Discovery and characterization of verinurad, a potent and specific inhibitor of URAT1 for the treatment of hyperuricemia and gout. Sci Rep. 2017;7(1):665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kuriyama S. Dotinurad: a novel selective urate reabsorption inhibitor as a future therapeutic option for hyperuricemia. Clin Exp Nephrol. 2020;24(Suppl 1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Neogi T, Choi HK. Editorial: Pursuit of a Dual-Benefit Antigout Drug: A First Look at Arhalofenate. Arthritis Rheumatol. 2016;68(8):1793–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mandal AK, Mercado A, Foster A, et al. Uricosuric targets of tranilast. Pharmacol Res Perspect. 2017;5(2):e00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Elsby R, Martin P, Surry D, et al. Solitary Inhibition of the Breast Cancer Resistance Protein Efflux Transporter Results in a Clinically Significant Drug-Drug Interaction with Rosuvastatin by Causing up to a 2-Fold Increase in Statin Exposure. Drug Metab Dispos. 2016;44(3):398–408. [DOI] [PubMed] [Google Scholar]
- 143.Chu XY, Bleasby K, Yabut J, et al. Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J Pharmacol Exp Ther. 2007;321(2):673–83. [DOI] [PubMed] [Google Scholar]
- 144.Uetake D, Ohno I, Ichida K, et al. Effect of fenofibrate on uric acid metabolism and urate transporter 1. Intern Med. 2010;49(2):89–94. [DOI] [PubMed] [Google Scholar]
- 145.Kobayashi Y, Ohshiro N, Sakai R, et al. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol. 2005;57(5):573–8. [DOI] [PubMed] [Google Scholar]
- 146.Narang RK, Dalbeth N. Management of complex gout in clinical practice: Update on therapeutic approaches. Best Pract Res Clin Rheumatol. 2018;32(6):813–34. [DOI] [PubMed] [Google Scholar]
- 147.Hagos Y, Bahn A, Vormfelde SV, et al. Torasemide transport by organic anion transporters contributes to hyperuricemia. J Am Soc Nephrol. 2007;18(12):3101–9. [DOI] [PubMed] [Google Scholar]
- 148.Vallon V, Rieg T, Ahn SY, et al. Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol. 2008;294(4):F867–73. [DOI] [PubMed] [Google Scholar]
- 149.Mamidi R, Dallas S, Sensenhauser C, et al. In vitro and physiologically-based pharmacokinetic based assessment of drug-drug interaction potential of canagliflozin. Br J Clin Pharmacol. 2017;83(5):1082–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stack AG, Han D, Goldwater R, et al. Dapagliflozin Added to Verinurad Plus Febuxostat Further Reduces Serum Uric Acid in Hyperuricemia: The QUARTZ Study. J Clin Endocrinol Metab. 2021;106(5):e2347–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu YH, Chang YP, Li T, et al. Empagliflozin Attenuates Hyperuricemia by Upregulation of ABCG2 via AMPK/AKT/CREB Signaling Pathway in Type 2 Diabetic Mice. Int J Biol Sci. 2020;16(3):529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fu Y, Breljak D, Onishi A, et al. Organic anion transporter OAT3 enhances the glucosuric effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol. 2018;315(2):F386–F94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ben Salem C, Slim R, Fathallah N, et al. Drug-induced hyperuricaemia and gout. Rheumatology (Oxford). 2017;56(5):679–88. [DOI] [PubMed] [Google Scholar]


