Abstract
Background:
Conventionally grown fruits and vegetables (FVs) are the main source of general population exposure to pesticide residues.
Objective:
To evaluate the relation of intake of high- and low-pesticide-residue FVs with cancer risk.
Methods:
We followed 150,830 women (Nurses’ Health Study, 1998-2016, and Nurses’ Health Study II, 1999-2017) and 29,486 men (Health Professionals Follow-up Study, 1998-2016) without a history of cancer. We ascertained FV intake via validated food frequency questionnaires and categorized FVs as having high or low pesticide residue levels based on USDA surveillance data. We used Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI) of total and site-specific cancer related to quintiles of high- and low-pesticide-residue FV intake.
Results:
We documented 23,678 incident cancer cases during 2,862,118 person-years of follow-up. In the pooled multivariable analysis, neither high- nor low-pesticide-residue FV intake was associated with cancer. The HRs (95% CI) per 1 serving/day increase in intake were 0.99 (0.97-1.01) for high- and 1.01 (0.99-1.02) for low-pesticide-residue FVs. Additionally, we found no association between high-pesticide-residue FV intake and risk of specific sites, including malignancies previously linked to occupational pesticide exposure ([HR, 95% CI comparing extreme quintiles of intake] lung [1.17 (0.95-1.43)], non-Hodgkin lymphoma [0.89 (0.72-1.09)], prostate [1.31 (0.88-1.93)]) or inversely related to intake of organic foods (breasts [1.03 (0.94-1.31)]).
Conclusions:
These findings suggest that overall exposure to pesticides through FV intake is not related to cancer risk, although they do not rule out associations with specific chemicals or sub-types of specific cancers.
1. INTRODUCTION
Cancer is among the primary causes of mortality worldwide, causing more than 9.5 million deaths in 2018.(1) Moreover, cancer incidence is expected to rise in the coming years due to the steady increase in life expectancy (2-4).
Fruits and vegetables (FVs) are considered an essential part of a healthy diet, and their role in the prevention of chronic diseases is well-known, mostly for their protective effect on cardiovascular disease (CVD) and cancer.(5, 6) Nevertheless, in the general population, conventionally grown FVs are the main route of chronic exposure to pesticides.(7-10) The USDA Pesticide Data Program (PDP), which since 1991 has systematically surveyed pesticide residues in FVs sold in the U.S., reports that more than 50% of conventionally grown FVs in the U.S. contain detectable levels of pesticides, and 31% contain 2 or more pesticides.(11) However, while occupational exposure to some pesticides used in agriculture is known to be carcinogenic,(12) it is unclear if exposure to pesticide residues through diet results in comparable risks or mitigates the health benefits of FV. Although two previous risk-benefit analyses sugges that the benefits of consuming of conventionally grown FV at known contamination levels outweigh potential risks on cancer incidence,(13, 14) we have previously reported that the relationship between FV with CVD differs for high- and low-pesticide-residue FVs (15) and the relation of pesticide residues with cancer risk has not been directly addressed in epidemiologic studies. To address this important question, we classified FV intake based on the corresponding pesticide residue status and examined the association of FV-based pesticide residue exposure with total and site-specific cancer risk in three large prospective cohorts of U.S. health professionals. We hypothesized that intake of high-pesticide-residue FVs would be related to a higher risk of cancer, particularly malignancies previously related to occupational exposure to pesticides: lung, non-Hodgkin lymphoma (NHL), and prostate,(16) whereas intake of low-pesticide residue FVs would have an inverse association.
2. METHODS
2.1. Study population
The study included three large prospective U.S. cohorts with similar designs and methods: the Nurses’ Health Study (NHS), established in 1976 with 121,700 female registered nurses aged 30-55, the Nurses’ Health Study II (NHSII), initiated in 1989, with 116,429 female registered nurses aged 25-42, and the Health Professionals Follow-up Study (HPFS), established in 1986, with 51,529 male health professionals aged 40-75 NHSII.(17) Participants of all three cohorts complete biennial questionnaires to report sociodemographic factors, lifestyle, and health conditions. Every four years, dietary information has been assessed with a validated food frequency questionnaire (FFQ) with 131 food items.(18-20) Of note, to maximize the overlap of available data from the FFQs and the PDP, we set the present analysis baseline to 1998 for NHS and HPFS and 1999 for NHSII. The cohorts have achieved response rates over 90% in most follow-up cycles. We excluded participants with a diagnosis of cancer at baseline, with missing or unreliable values on total energy intake (<500 or >3500 kcal/day for women and <800 or >4200 kcal/day for men), and with missing data in more than half of the FV consumption-related questions. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. Participant informed consent was implied by return of the baseline and follow-up questionnaires.
2.2. Pesticide residue assessment
The Pesticide Residue Burden Score (PRBS) is a scoring system that allows the assessment of pesticide residue content of FVs using surveillance data collected as part of the PDP. This score has been validated against urinary pesticide metabolites in other cohorts.(21, 22) Among 3,679 participants in NHANES, representative of the U.S. population, three scores: the PRBS, the organophosphate pesticide score (OP-PRBS), and the organochlorine pesticide score (OC-PRBS) were associated with measured urinary or serum pesticide biomarkers.(21) In addition, among 90 men presenting to the Massachusetts General Hospital and participating in the EARTH study, two urinary samples for seven biomarkers of OP, pyrethroid, and herbicide 2,4-dichlorophenoxyacetic acid were positively associated with high pesticide residue FV intake and inversely related to low pesticide residue FV intake.(22) We linked follow-up period-specific FV intake data as reported in the FFQ with the corresponding PDP data.(11) FFQ data from 1998 (NHS/HPFS) and 1999 (NHSII) were matched with PDP data from 1996 to 1999; FFQ data from 2002 (NHS/HPFS) and 2003 (NHSII) were matched with PDP data from 2000 to 2003; etc. Each fruit or vegetable was categorized according to 3 PDP estimations: the percentage of samples that presented detectable pesticide residues, the percentage of samples with pesticide residues above the tolerances levels, and, the percentage of samples which contained three or more individual detectable pesticides. For each contamination measure, a score of 0 (lowest), 1, or 2 (highest) was given to each FV based on the tertile distribution of each measure; those component scores were summed to calculate the PRBS, which could thus range from 0 to 6. For each period, FVs with a PRBS ≥4 were considered as high-pesticide-residue FVs, those with a PRBS<4 as low-pesticide-residue FVs, and those without contamination information in a specific period as undetermined-pesticide-residue FVs. Finally, intakes of high-, low-, and undetermined-pesticide-residue FVs were summed for each participant.
2.3. Covariates
Height was reported at enrollment. The biennial follow-up questionnaires included current weight, (which was used to update body mass index (BMI)), physical activity, family history of cancer, physical examination in past 2 years, history of colonoscopy or sigmoidoscopy, smoking in pack-years, current multivitamin use, and regular aspirin use. In the NHS and NHSII, we also obtained data on menopausal status, current hormone therapy use, and mammography in the past 2 years. HPFS participants reported on prostate-specific antigen testing in the past 2 years on each questionnaire. We obtained information on total energy intake, alcohol intake, and the Alternate Healthy Eating Index (AHEI) from the same quadrennial FFQs we used for creating PRBS score.(23, 24)
2.4. Outcome assessment
Participants reported newly diagnosed cancers in biennial questionnaires. Self-reports were confirmed by review of medical and pathology records by study physicians blinded to exposure status. Specifically, data on histology, anatomic location, and stage of the cancer were assessed. Only confirmed cases according to the international classification of diseases, eighth revision and ninth revision (ICD-8 and ICD-9), were counted as events in the analyses. For prostate cancer, only advanced prostate cancer was considered as an endpoint. The primary endpoint of our study was total cancer incidence; we examined incidence of selected specific sites as secondary outcomes. Specifically, we separately examined malignancies previously related to occupational exposure to pesticides (lung, NHL, and advanced prostate),(16) those previously related to organic food intake (NHL and breast),(25) and those with at least 400 incident cases accrued during follow-up (colorectal, kidney, endometrial, ovarian). We also examined an aggregate group of all other sites combined (bladder, pancreas, leukaemia, melanoma, myeloma, brain, stomach, oesophagus, pharynx, larynx, liver, oral, and cervical cancers).
2.5. Statistical analysis
Participants were followed from the date of the return of 1998 or 1999 questionnaires until the earliest among the date of cancer diagnosis (except for non-melanoma skin cancer), death or the end of follow-up (June 2014 for most cancers in HPFS and site-specific cancers in NHS, June 2016 for total cancer in NHS and lung cancer in HPFS, or December 2017 for NHSII). Associations of intakes of high- and low-pesticide-residue FVs with total and site-specific cancers were estimated as hazard ratios (HRs) and 95% confidence intervals (CI) from Cox proportional hazards regression models. We considered baseline and cumulative averaged intakes to characterize diet over the follow-up period. Briefly, analyses based on baseline diet, used only the information reported by participants on the 1998 (NHS/HPFS) and 1999 (NHSII) to characterize exposure over the entire follow-up period. In cumulative averaged intake analyses, the 1998/1999 diet assessments was the assigned exposure status for all cases newly identified through 2002/2003; the average of the 1998/1999 diet and the 2002/2003 diet was assigned to incident cases documented between 2003/2004 and 2006/2007; the average of the 1998/1999, 2002/2003 and 2006/2007 assessments was assigned to new cases documented between 2007/2008 and 2010/2011; and so forth. Baseline and cumulative averaged intakes of high- and low-pesticide-residue FVs were modeled as quintiles of intake, in categories of absolute intake, and as continuous variables using restricted cubic splines to allow for non-linearity.We fit age-adjusted models, as well as multivariable models adjusted for height (cm), BMI (quintiles), ethnicity (white/non-white), physical activity (quintile of metabolic equivalent task [MET]-hours/week), family history of cancer (yes/no), physical examination in the past 2 years (yes/no), history of colonoscopy or sigmoidoscopy (yes/no), smoking in pack-years (never smoker, 1-4.9, 5-19.9, 20-39.9, or ≥40), current multivitamin use (yes/no), regular aspirin use (yes/no), total energy intake (quintiles), alcohol intake (0, 0.1-4.9, 5.0-14.9, 15.0-29.9, or ≥30 g/day), and AHEI score, the latter excluding criteria for intake of FVs and alcohol (quintiles) and included to control for overall diet quality. We further adjusted for postmenopausal hormone use (premenopausal/never/past/current) and mammography in the past 2 years (yes/no) in NHS and NHSII and for prostate-specific antigen testing in the past 2 years in HPFS. Intakes of high-, low- and undetermined-pesticide-residue FVs were simultaneously included in all models. Analyses were performed for each of the three cohorts and then pooled using a fixed-effects model. To test for linear associations, P for linear trend was obtained by modeling quintiles of high- and low-pesticide-residue FV intake as continuous variables. Statistical significance was established at a two-sided P less than 0.05. Analyses were carried out with SAS software version 9.4 for UNIX (SAS Institute, Cary, NC).
3. RESULTS
We identified 23,678 incident cases of cancer during 2,862,118 person-years of follow-up, including 6,842 breast cancers, 1,790 colorectal cancers, 1,357 lung cancers, 1,357 NHLs, 1,316 endometrial cancers, 605 ovarian cancers, 380 advanced prostate cancers, and 4,940 cancers from all other sites combined. At baseline, compared to participants in the lowest quintile of high-pesticide-residue FV intake, those in the highest quintile of intake were more physically active and more likely to be never smokers, consumed more multivitamin supplements and total calories, and had more cancer screenings (Table 1). A similar pattern of baseline participant characteristics was observed when comparing extreme quintiles of low-pesticide-residue FV intake (Table 1). Intakes of low- and high-pesticide-residue FVs were positively related to each other (rSpearman = 0.63 in NHS, 0.70 in NHSII, and 0.62 in HPFS).
Table 1.
Baseline characteristics of participants according to quintiles of high- and low-pesticide-residue fruit and vegetable intake.
| Quintile of High-Pesticide-Residue Fruit and Vegetable Intake |
Quintile of Low-Pesticide-Residue Fruit and Vegetable Intake |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 |
| Nurses’ Health Study (1998) | ||||||
| Number of participants | 14,317 | 14,028 | 13,948 | 14,314 | 14,034 | 14,026 |
| High-pesticide-residue FVs (servings/day) | 0.5(0.2) | 1.3(0.1) | 3.1(1.0) | 0.9(0.7) | 1.5(0.8) | 2.2(1.2) |
| Low-pesticide-residue FVs (servings/day) | 1.4(0.9) | 2.2(1.0) | 3.1(1.4) | 0.8(0.3) | 2.0(0.2) | 4.1(1.1) |
| Total FVs (servings/day) | 2.5(1.2) | 4.5(1.3) | 7.6(2.3) | 2.4(1.1) | 4.5(1.1) | 7.7(2.2) |
| Age (y) | 64.3(7.3) | 64.4(7.0) | 64.2(6.9) | 64.5(7.3) | 64.2(7.1) | 64.1(6.8) |
| Height (cm) | 163.6(6.2) | 163.8(6.1) | 163.9(6.2) | 163.5(6.2) | 163.9(6.1) | 163.9(6.1) |
| BMI (kg/m2) | 26.8(5.5) | 26.8(5.2) | 26.3(5.2) | 26.9(5.4) | 26.7(5.3) | 26.4(5.2) |
| Postmenopause (%) | 94.3 | 93.6 | 94.1 | 94.0 | 93.8 | 94.1 |
| White (%) | 97.3 | 97.7 | 97.4 | 97.1 | 97.7 | 97.3 |
| Physical activity (MET-hours/wk) | 11.8(16.5) | 16.9(19.7) | 24.0(26.4) | 13.0(17.7) | 17.0(22.0) | 22.7(24.9) |
| Family history of cancer (%) | 26.9 | 28.0 | 28.7 | 27.1 | 28.2 | 28.4 |
| Physical examination in the past 2 years (%) | 87.5 | 90.7 | 92.0 | 88.0 | 90.6 | 91.6 |
| History of colonoscopy or sigmoidoscopy (%) | 19.3 | 22.0 | 23.1 | 20.9 | 21.5 | 22.6 |
| Mammography in the past 2 years (%) | 84.3 | 88.4 | 89.5 | 84.3 | 88.2 | 89.2 |
| Never smoker (%) | 41.8 | 46.0 | 49.9 | 42.0 | 46.4 | 49.1 |
| No of pack-years among ever smokers | 18.0 (23.3) | 13.1(19.4) | 10.2 (16.5) | 17.5(23.0) | 12.9(19.4) | 11.1(17.7) |
| Current hormone therapy use (%) | 43.5 | 47.2 | 48.2 | 44.7 | 47.9 | 47.7 |
| Current multivitamin usef (%) | 55.5 | 61.8 | 64.8 | 56.1 | 61.1 | 64.2 |
| Regular aspirin use (%) | 28.1 | 29.1 | 28.3 | 26.8 | 28.4 | 29.7 |
| Total energy intake (kcal/day) | 1,462(481) | 1,711(495) | 2,007(538) | 1,407(458) | 1,706(467) | 2,085(533) |
| Alcohol intake (g/day) | 4.9(9.9) | 5.2(9.2) | 4.7(8.1) | 4.3(9.1) | 5.1(9.2) | 5.3(9.0) |
| Modified AHEI (score)* | 37.9(8.3) | 39.2(8.5) | 40.9(8.7) | 41.4(8.7) | 38.7(8.5) | 37.8(8.0) |
| Nurses’ Health Study II (1999) | ||||||
| Number of participants | 16,396 | 16,418 | 16,202 | 16,236 | 16,218 | 16,209 |
| High-pesticide-residue FVs (servings/day) | 0.4(0.1) | 1.2(0.1) | 2.9(1.2) | 0.7(0.6) | 1.3(0.8) | 2.2(1.3) |
| Low-pesticide-residue FVs (servings/day) | 1.2(0.8) | 1.9(0.9) | 3.0(1.5) | 0.7(0.2) | 1.8(0.1) | 3.9(1.2) |
| Total FVs (servings/day) | 2.0(1.0) | 3.7(1.2) | 7.0(2.4) | 1.9(1.0) | 3.8(1.2) | 7.0(2.4) |
| Age (y) | 44.3(4.7) | 44.6(4.6) | 45.1(4.6) | 44.5(4.7) | 44.7(4.6) | 44.9(4.6) |
| Height (cm) | 164.5(6.6) | 164.8(6.5) | 164.9(6.7) | 164.5(6.7) | 164.9(6.6) | 165.0(6.7) |
| BMI (kg/m2) | 27.2(6.7) | 26.4(6.1) | 25.9(5.8) | 27.0(6.5) | 26.4(6.1) | 26.1(6.0) |
| Postmenopause (%) | 23.0 | 22.2 | 22.6 | 23.4 | 22.3 | 22.5 |
| White (%) | 95.7 | 96.8 | 96.5 | 95.5 | 97.0 | 96.1 |
| Physical activity (MET-hours/wk) | 12.9(7.8) | 18.2(22.5) | 24.7(26.5) | 14.4(19.4) | 17.9(21.8) | 23.2(26.5) |
| Family history of cancer (%) | 18.8 | 20.2 | 20.3 | 19.5 | 19.8 | 19.6 |
| Physical examination in the past 2 years (%) | 84.3 | 87.4 | 88.2 | 84.8 | 87.4 | 87.7 |
| History of colonoscopy or sigmoidoscopy (%) | 8.3 | 8.3 | 8.6 | 8.3 | 8.5 | 8.6 |
| Mammography in the past two years (%) | 75.0 | 77.3 | 77.7 | 75.2 | 77.4 | 78.1 |
| Never smoker (%) | 64.2 | 66.8 | 66.6 | 64.3 | 65.9 | 68.3 |
| No of pack-years among ever smokers | 5.8(10.8) | 4.4(8.6) | 3.9(7.6) | 5.6(10.5) | 4.4(8.7) | 3.8(7.7) |
| Current hormone therapy use (%) | 1.0 | 1.0 | 1.2 | 1.1 | 1.1 | 1.1 |
| Current multivitamin use (%) | 50.5 | 57.5 | 63.4 | 50.8 | 57.9 | 63.4 |
| Regular aspirin use (%) | 6.7 | 5.8 | 6.0 | 6.4 | 6.1 | 6.0 |
| Total energy intake (kcal/day) | 1,545(514) | 1,813(521) | 2,129(556) | 1,471(478) | 1,806(492) | 2,219(544) |
| Alcohol intake (g/day) | 3.5(7.8) | 4.1(7.3) | 4.1(6.8) | 3.3(7.3) | 4.0(7.0) | 4.3(7.1) |
| Modified AHEI (score)* | 36.9(8.9) | 38.4(9.4) | 40.7(9.8) | 39.9(9.2) | 38.5(9.6) | 37.4(9.3) |
| Health Professionals Follow-up Study (1998) | ||||||
| Number of participants | 5,918 | 5,741 | 5,829 | 5,890 | 5,898 | 5,903 |
| High-pesticide-residue FVs (servings/day) | 0.5(0.2) | 1.4(0.1) | 3.4(1.2) | 1.0(0.8) | 1.5(0.9) | 2.4(1.4) |
| Low-pesticide-residue FVs (servings/day) | 1.6(1.0) | 2.4(1.1) | 3.4(1.6) | 0.9(0.3) | 2.2(0.2) | 4.5(1.3) |
| Total FVs (servings/day) | 2.7(1.3) | 4.7(1.4) | 8.3(2.6) | 2.6(1.2) | 4.7(1.2) | 8.3(2.5) |
| Age (y) | 63.3(9.1) | 64.4(9.0) | 66.0(9.0) | 63.8(9.1) | 64.6(9.0) | 64.8(9.1) |
| Height (cm) | 178.1(6.7) | 178.4(6.8) | 178.5(6.9) | 178.2(6.9) | 178.4(6.8) | 178.6(6.7) |
| BMI (kg/m2) | 26.3(3.6) | 26.2(3.6) | 25.7(3.6) | 26.3(3.6) | 26.1(3.6) | 25.9(3.6) |
| White (%) | 90.1 | 91.9 | 91.2 | 90.2 | 91.7 | 90.6 |
| Physical activity (MET-hours/wk) | 25.0(31.6) | 34.5(38.3) | 46.0(46.1) | 27.6(33.7) | 34.2(37.1) | 43.7(45.6) |
| Family history of cancer (%) | 28.7 | 28.8 | 29.6 | 27.8 | 29.5 | 29.2 |
| Physical examination in the past 2 years (%) | 79.9 | 81.7 | 83.7 | 79.5 | 82.8 | 83.0 |
| History of colonoscopy or sigmoidoscopy (%) | 25.1 | 27.2 | 29.5 | 25.5 | 27.8 | 27.9 |
| Prostate specific antigen testing in the past 2 years (%) | 72.4 | 74.4 | 77.7 | 71.8 | 75.9 | 76.5 |
| Never smoker (%) | 47.9 | 52.5 | 57.4 | 49.0 | 51.8 | 56.9 |
| No of pack-years among ever smokers (%) | 15.6(21.2) | 11.9(17.3) | 9.7(15.8) | 15.0(20.7) | 12.1(17.8) | 10.2(16.4) |
| Current multivitamin use (%) | 55.7 | 59.2 | 62.9 | 55.6 | 60.1 | 63.7 |
| Regular aspirin use (%) | 39.0 | 38.8 | 37.0 | 38.3 | 39.4 | 37.7 |
| Total energy intake (kcal/day) | 1,722(557) | 1,991(580) | 2,300(644) | 1,659(520) | 1,976(547) | 2,388(637) |
| Alcohol intake (g/day) | 11.7(16.3) | 11.2(13.9) | 9.6(12.1) | 11.0(15.4) | 11.0(13.8) | 10.4(12.9) |
| Modified AHEI (Score)* | 32.1(8.1) | 34.6(7.9) | 38.2(8.4) | 35.1(8.7) | 34.5(8.3) | 35.4(7.9) |
Values are means (SD) or percentages. All variables except age are age-standardized.
Abbreviations: Modified AHEI, Modified Alternate Healthy Eating Index score; BMI, body mass index; METs: metabolic equivalent tasks; FV: fruits and vegetables;
Modified AHEI: AHEI-2010, but excluding criteria for intakes of fruits and vegetables, and alcohol.
METs are defined as the ratio of calories needed per kilogram of body weight per hour of physical activity divided by the calories needed per kilogram of body weight at rest.
Neither high- nor low-pesticide-residue baseline FV intake in the range of 0 to 5 servings per day was associated with cancer risk (Figure 1). There was no heterogeneity across studies (P for heterogeneity>0.1). The pooled multivariable-adjusted HRs (95% CI) of total cancer associated with a 1 serving/day increase in intake were 1.01 (0.99-1.02) for low-pesticide-residue FVs and 0.99 (0.97-1.01) for high-pesticide residue FVs. These results were similar when high- and low-pesticide-residue FVs were modeled as quintiles of intake (Table 2). The pooled multivariable-adjusted HRs (95% CIs) of total cancer across increasing non-reference quintiles (vs first [reference] quintile) of baseline high-pesticide-residue FV intake were 1.02 (0.97-1.06), 1.00 (0.95-1.04), 0.97 (0.93-1.02), and 1.00 (0.95-1.05), with a P for trend of 0.77. The pooled multivariable-adjusted HRs (95% CI) of total cancer across increasing non-reference quintiles of low-pesticide-residue FV intake were 0.98 (0.94-1-02), 0.98 (0.93-1.02), 1.02 (0.97-1.07), and 0.99 (0.95-1.04), with a P for trend of 0.74 (Table 2).
Figure 1. Baseline fruit and vegetable intake, considering pesticide residue status, and risk of total cancer.
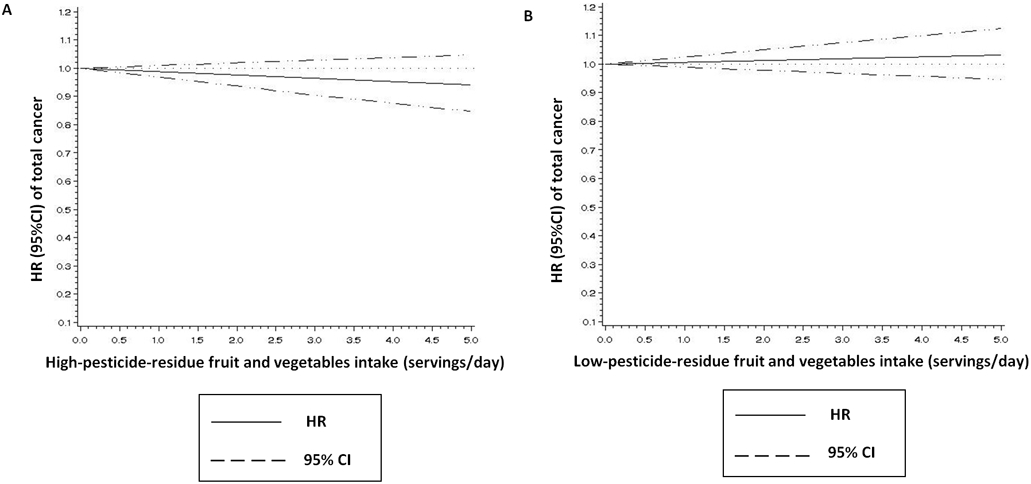
1 Adjusted for age, height (cm), body mass index (BMI) (quintiles), ethnicity (white/non-white), physical activity (quintile of metabolic equivalent task-hours/week), family history of cancer (yes/no), physical examination in the past 2 years (yes/no), history of colonoscopy or sigmoidoscopy (yes/no), mammography in the past 2 years (yes/no, in NHS and NHS II), prostate-specific antigen testing in the past 2 years (yes/no, in HPFS), number of pack-years among ever smokers (never smoker, 1-4.9, 5-19.9, 20-39.9, or ≥40), postmenopausal hormone use (premenopausal/never/past/current, in NHS and NHSII), current multivitamin use (yes/no), regular aspirin use (yes/no), total energy intake (quintiles), alcohol intake (0, 0.1-4.9, 5.0-14.9, 15.0-29.9, or ≥30 g/day), and Alternate Healthy Eating Index score excluding criteria for intake of fruits and vegetables and alcohol (quintiles).
A Additionally adjusted for intakes of low-pesticide-residue fruits and vegetables (servings/day) and other fruits and vegetables with undetermined residues (servings/day).
B Additionally adjusted for intakes of high-pesticide-residue fruits and vegetables (servings/day) and other fruits and vegetables with undetermined residues (servings/day).
Table 2.
Pooled hazard ratios (95% confidence intervals) of total cancer and site-specific cancers according to baseline intakes of high- and low-pesticide-residue fruits and vegetables in the NHS, NHSII, and HPFS.
| Quintiles of High-Pesticide-Residue Fruit and Vegetable Intake | P, trend | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Total cancer (n=23,678) a,b | ||||||
| Person-years | 574,497 | 564,868 | 575,514 | 576,170 | 571,069 | |
| Cases | 4,869 | 4,789 | 4,731 | 4,633 | 4,656 | |
| Age-adjusted | 1 (Ref.) | 0.98( 0.94, 1.02) | 0.95( 0.91, 0.99) | 0.91( 0.88, 0.95) | 0.92 (0.88, 0.96) | <.0001 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 1.02 (0.97, 1.06) | 1.00 (0.95, 1.04) | 0.97 (0.93, 1.02) | 1.00 (0.95, 1.05) | 0.77 |
| Colorectal cancer (n=1790) | ||||||
| Cases | 389 | 389 | 333 | 355 | 324 | |
| Age-adjusted1 | 1 (Ref.) | 0.99 (0.86, 1.14) | 0.82 (0.71, 0.95) | 0.86 (0.74, 0.99) | 0.78 (0.67, 0.91) | <.001 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 1.01 (0.87, 1.17) | 0.85 (0.73, 1.00) | 0.91 (0.77, 1.07) | 0.86 (0.71, 1.03) | 0.06 |
| Lung cancer (n=1,357) | ||||||
| Cases | 326 | 313 | 276 | 198 | 244 | |
| Age-adjusted | 1 (Ref.) | 0.94 (0.80, 1.10) | 0.80 (0.68, 0.94) | 0.56 (0.47, 0.67) | 0.68 (0.58, 0.81) | <.0001 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 1.15 (0.97, 1.35) | 1.09 (0.91, 1.30) | 0.83 (0.68, 1.02) | 1.17 (0.95, 1.43) | 0.76 |
| Non-Hodgkin lymphoma (n=1,357) | ||||||
| Cases | 274 | 251 | 284 | 292 | 256 | |
| Age-adjusted | 1 (Ref.) | 0.93 (0.78, 1.11) | 1.03 (0.88, 1.22) | 1.03 (0.87, 1.21) | 0.91 (0.76, 1.08) | 0.46 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 0.89 (0.75, 1.06) | 0.98 (0.82, 1.18) | 0.97 (0.80, 1.17) | 0.89 (0.72, 1.09) | 0.51 |
| Breast cancer (n=6,842) c | ||||||
| Cases | 1,364 | 1,365 | 1,395 | 1331 | 1,387 | |
| Age-adjusted | 1 (Ref.) | 1.01 (0.94, 1.09) | 1.01 (0.94, 1.09) | 0.96 (0.89, 1.03) | 1.00 (0.93, 1.08) | 0.58 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 1.01 (0.94, 1.09) | 1.01 (0.93, 1.10) | 0.97 (0.86, 1.06) | 1.03 (0.94, 1.13) | 0.70 |
| Endometrial cancer (n=1,316) c | ||||||
| Cases | 231 | 261 | 255 | 277 | 292 | |
| Age-adjusted | 1 (Ref.) | 1.14( 0.95, 1.36) | 1.08 (0.90, 1.29) | 1.16 (0.98, 1.39) | 1.20( 1.01, 1.43) | 0.05 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 1.40 (1.16, 1.68) | 1.05 (0.87, 1.28) | 1.11 (0.91, 1.36) | 1.12 (0.90, 1.39) | 0.42 |
| Ovarian cancer (n=605) c | ||||||
| Cases | 114 | 117 | 124 | 119 | 131 | |
| Age-adjusted | 1 (Ref.) | 1.04 (0.80, 1.34) | 1.07( 0.83, 1.39) | 1.04( 0.80, 1.34) | 1.11( 0.86, 1.43) | 0.43 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 1.03 (0.79, 1.35) | 1.09( 0.83, 1.44) | 1.04( 0.78, 1.39) | 1.17( 0.85, 1.59) | 0.16 |
| Advanced prostate cancer (n=380) c | ||||||
| Cases | 70 | 70 | 80 | 68 | 92 | |
| Age-adjusted | 1 (Ref.) | 0.94 (0.67, 1.32) | 1.07 (0.77, 1.48) | 0.88 (0.63, 1.24) | 1.13 (0.82, 1.56) | 0.46 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 0.97 (0.69, 1.37) | 1.13 (0.80, 1.61) | 0.99 (0.68, 1.44) | 1.31 (0.88, 1.93) | 0.14 |
| Other cancers (n=4,940) d | ||||||
| Cases | 964 | 1,014 | 951 | 1,000 | 1,011 | |
| Age-adjusted | 1 (Ref.) | 1.05 (0.95, 1.15) | 0.99( 0.90, 1.09) | 1.00( 0.91, 1.10) | 1.02 (0.92, 1.12) | 0.66 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 1.05 (0.96, 1.15) | 0.99 (0.90, 1.09) | 1.02 (0.92, 1.13) | 1.06 (0.95, 1.18) | 0.41 |
| Quintiles of Low-Pesticide-Residue Fruit and Vegetable Intake | P, trend | |||||
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Total cancer (n=23,678) a,b | ||||||
| Person-years | 570,828 | 571,324 | 574,020 | 572,398 | 573,548 | |
| Cases | 4,889 | 4,708 | 4,649 | 4,802 | 4,630 | |
| Age-adjusted | 1 (Ref.) | 0.96 (0.92, 0.99) | 0.94 (0.90, 0.98) | 0.96 (0.93, 1.00) | 0.93 (0.89, 0.97) | <0.01 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 0.98 (0.94, 1.02) | 0.98 (0.93, 1.02) | 1.02 (0.97, 1.07) | 0.99 (0.95, 1.04) | 0.74 |
| Colorectal cancer (n=1,790) | ||||||
| Cases | 374 | 362 | 349 | 371 | 334 | |
| Age-adjusted1 | 1 (Ref.) | 0.96 (0.83, 1.11) | 0.90 (0.78, 1.05) | 0.95 (0.83, 1.10) | 0.87 (0.75, 1.01) | 0.08 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 1.02 (0.87, 1.18) | 1.01 (0.86, 1.18) | 1.11 (0.94, 1.31) | 1.06 (0.89, 1.27) | 0.38 |
| Lung cancer (n=1,357) | ||||||
| Cases | 339 | 275 | 262 | 247 | 234 | |
| Age-adjusted | 1 (Ref.) | 0.79 (0.67, 0.93) | 0.75 (0.63, 0.88) | 0.70 (0.59, 0.83) | 0.66 (0.56, 0.78) | <.0001 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 0.89 (0.76, 1.06) | 0.92 (0.77, 1.10) | 0.95 (0.79, 1.15) | 0.95 (0.78, 1.17) | 0.91 |
| Non-Hodgkin lymphoma (n=1,357) | ||||||
| Cases | 251 | 289 | 283 | 288 | 246 | |
| Age-adjusted | 1 (Ref.) | 1.17 (0.98, 1.39) | 1.14 (0.96, 1.35) | 1.12(0.95, 1.34) | 0.92 (0.77, 1.10) | 0.35 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 1.15 (0.97, 1.37) | 1.12 (0.93, 1.35) | 1.10(0.91, 1.34) | 0.98 (0.79, 1.21) | 0.48 |
| Breast cancer (n=6,842) c | ||||||
| Cases | 1,373 | 1,356 | 1,373 | 1,387 | 1,353 | |
| Age-adjusted | 1 (Ref.) | 0.98( 0.91, 1.06) | 0.99 (0.92, 1.06) | 1.00 (0.93, 1.08) | 0.97 (0.90, 1.05) | 0.63 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 0.98 (0.91, 1.06) | 0.99 (0.91, 1.07) | 1.00 (0.92, 1.09) | 0.99 (0.90, 1.08) | 0.92 |
| Endometrial cancer (n=1,316) c | ||||||
| Cases | 244 | 238 | 259 | 280 | 295 | |
| Age-adjusted | 1 (Ref.) | 0.97 (0.81, 1.16) | 1.05 (0.88, 1.25) | 1.12 (0.95, 1.34) | 1.17 0.99, 1.39) | 0.02 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 0.93 (0.77, 1.12) | 0.96 (0.80, 1.17) | 1.06 (0.87, 1.28) | 1.07 (0.87, 1.32) | 0.24 |
| Ovarian cancer (n=605) c | ||||||
| Cases | 125 | 120 | 139 | 99 | 122 | |
| Age-adjusted | 1 (Ref.) | 0.96 (0.74, 1.23) | 1.09( 0.85, 1.39) | 0.78( 0.60, 1.02) | 0.96( 0.75, 1.24) | 0.45 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 0.93 (0.72, 1.21) | 1.04 (0.80, 1.36) | 0.73 (0.54, 0.98) | 0.89 (0.65, 1.21) | 0.26 |
| Advanced prostate cancer (n=380) c | ||||||
| Cases | 68 | 75 | 77 | 81 | 79 | |
| Age-adjusted | 1 (Ref.) | 1.09 (0.78, 1.52) | 1.04 (0.74, 1.45) | 1.15 (0.83, 1.59) | 1.08 (0.77, 1.50) | 0.66 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 1.11 (0.79, 1.56) | 1.05 (0.74, 1.49) | 1.12 (0.78, 1.61) | 1.02 (0.69, 1.51) | 0.99 |
| Other cancers (n=4,940) d | ||||||
| Cases | 1,011 | 967 | 948 | 1,021 | 993 | |
| Age-adjusted | 1 (Ref.) | 0.97 (0.88, 1.06) | 0.93 (0.85, 1.03) | 1.01 (0.92, 1.11) | 0.97 (0.88, 1.06) | 0.87 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 0.95 (0.87, 1.04) | 0.93 (0.84, 1.02) | 1.00 (0.91, 1.11) | 0.98 (0.88, 1.09) | 0.85 |
Abbreviations: NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; HPFS, Health Professionals Follow-up Study.
Adjusted for age, height (cm), body mass index (BMI) (quintiles), ethnicity (white/non-white), physical activity (quintile of metabolic equivalent task-hours/week), family history of cancer (yes/no), physical examination in the past 2 years (yes/no), history of colonoscopy or sigmoidoscopy (yes/no), mammography in the past 2 years (yes/no, in NHS and NHSII), prostate-specific antigen testing in the past 2 years (yes/no, in HPFS), number of pack-years among ever smokers (never smoker, 1-4.9, 5-19.9, 20-39.9, or ≥40), postmenopausal hormone use (premenopausal/never/past/current, in NHS and NHSII), current multivitamin use (yes/no), regular aspirin use (yes/no), total energy intake (quintiles), alcohol intake (0, 0.1-4.9, 5.0-14.9, 15.0-29.9, or ≥30 g/day), and Alternate Healthy Eating Index score excluding criteria for intake of fruits and vegetables and alcohol (quintiles).
Additionally adjusted for intakes of low-pesticide-residue fruits and vegetables (quintiles) and for fruits and vegetables with undetermined residues (quintiles).
Additionally adjusted for intakes of high-pesticide-residue fruits and vegetables (quintiles) and for fruits and vegetables with undetermined residues (quintiles).
Included only advanced prostate cancer in the total cancer endpoint.
Pooled results for women and men.
Sex-specific results for women (breast, endometrial, and ovarian cancers) and men (advanced prostate cancer).
Other cancers include kidney, bladder, pancreatic, leukaemia (in aggregate, excluding lymphoid), melanoma, multiple myeloma, brain, stomach, oesophageal, pharyngeal, laryngeal, liver, oral, and cervical (only in women).
When specific sites were examined, we found no association between intake of high-pesticide-residue FVs or low-pesticide-residue FV and risk of any sites. The HRs (95% CI) comparing individuals in the highest quintile of high-pesticide-residue FV intake to individuals in the lowest quintile were: 1.17 (0.95-1.43) for lung cancer, 0.89 (0.72-1.09) for NHL, 1.03 (0.94-1.13) for advanced prostate cancer, and 1.04 (0.94-1.15) for breast cancer (Table 2). Intake of FVs with undetermined pesticide residue status was also unrelated to cancer risk (Table S1).
Results were consistent when baseline intake was modeled in categories of absolute intake (Table S2), although in these analyses there was a weak positive association of low-pesticide-residue FV with total cancer when intake was modeled as quintiles of cumulative average intake. Participants in the highest quartile of low-pesticide-residue FV consumption had 11% (95CI: 5%-18%) higher total cancer risk than those in the lowest quartile of consumption (Table S3).
4. DISCUSSION
We assessed the association of high- and low-pesticide-residue FV intake with total cancer, 7 individual sites, and an aggregate endpoint comprising 14 additional sites in three large prospective cohorts of U.S. men and women. Contrary to our hypothesis, but in agreement with two previous risk-benefit analyses (13, 14) we found no relation of intakes of high- or low-pesticide-residue FVs with risk of total cancer or specific malignancies, including malignancies previously linked to occupational exposure (lung, NHL, prostate), or sites in which an inverse association with greater intake of organic foods (NHL, breast) was previously reported. These findings suggest that despite the well described carcinogenicity of some agricultural pesticides in occupational settings, exposure to pesticide residues through the diet may not result in a comparable increase in risk. Similarly, the lack of association of low-pesticide-residue FV intake with malignancies previously inversely linked to intake of organic foods suggests that the associations with organic foods might reflect other factors associated with organic food consumption (high educational level, physical activity, no smoking, moderate alcohol consumption)(25, 26) rather than a true biological effect resulting from decreased exposure to pesticides through the diet.
To the best of our knowledge, there is no previous epidemiologic research on the relationship between exposure to pesticide residues through the diet and risk of total cancer or site-specific cancer. In contrast, the relationship between FV intake and cancer risk has been widely studied. A recent meta-analysis of 14 prospective studies found that FV intake was associated with a reduction of total cancer risk of 3% for consumption of up to 600 g/day (6 servings/day), with no further reductions at higher intake levels.(27) In addition, others have previously evaluated the relation between intake of organic foods with cancer risk,(25) in order to examine the same underlying biological hypothesis of our study, namely, that exposure to pesticide residues through the diet is related to cancer risk. In the NutriNet-Santé Prospective Cohort Study, participants in the highest quartile of organic food consumption had 34% (95CI: 5%-55%) lower breast cancer risk and 86% (95CI: 34%-97%) lower NHL risk than those in the lowest quartile of consumption.(25, 28) In the Million Women’s Study, organic food consumption was also related to a lower risk of NHL but was unrelated to total cancer risk and positively related to breast cancer risk.(29) None of those studies directly performed a comparison of overall FVs vs high- and low-pesticide-residue FV intake. Our findings are not in agreement with those of the two studies focused on organic food consumption, a discrepancy which begs explanation . In our study, intakes of high- and low-pesticide-residue FVs were not only positively related to each other but also had very similar relations with baseline characteristics, and in particular with behavioral characteristics, as well as similar nutritional profiles associated with higher intakes of both high- and low-pesticide-residue FVs. Consumption of organic foods, on the other hand, is a complex behavior that may not be entirely captured even with adjustment for multiple behavioral correlates, allowing ample opportunity for residual confounding. The previous findings for organic foods might be the result of unmeasured confounding for behavioral correlates of organic food consumption. For example, in the NutriNet-Santé Prospective Cohort Study, while the association between organic food consumption and cancer risk persists despite adjustments for a variety of social and demographic factors related to organic food consumption, in stratified analyses these associations appear to be restricted to subgroups of individuals more likely to consume organic foods including women, older individuals, and individuals with a family history of cancer,(25) raising concerns about residual confounding, including by well-established risk factors that may not have been entirely accounted for, and unmeasured confounding.
The current study has several strengths. No previous study has evaluated the relationship between exposure to pesticides through FV intake and total cancer risk as well as with risk of cancer as several individual sites. We pooled data from 3 large prospective cohorts with more than 14 years of follow-up – twice as long as the NutriNet-Sante study – and a large number of cases. Analyses were adjusted for many potential confounders and were consistent after modeling dietary exposure in different ways.. Several limitations should also be noted. Firstly, diet was self-reported by participants, and exposure to pesticides was obtained indirectly from PDP data. However, the PRBS score we used has been previously validated. Importantly, pesticide residue exposure from FV intake classified by this method was associated with direct biomarkers of exposure to pesticides in a clinical sample from Boston and in representative sample of the general population of the U.S. as we have previously described in the methods section.(21, 22) Secondly, the 14 years of follow-up may not be enough of a latency for all of the cancer sites despite the similar results observed when assessing diet just at baseline and as cumulative average over the follow-up period. However, insufficient length of follow-up would further strengthen the argument that the previously reported findings for organic food consumption and cancer risk do not represent a biological effect but rather residual and unmeasured confounding by behavioral factors. Thirdly, we could not differentiate fresh from canned or organically versus conventionally produced FVs. At the same time, our classification method prioritizes classification of overall contamination with pesticides rather than contamination with specific chemicals. Thus, the findings reflect uncertain amounts of misclassification and lack of specificity of pesticide exposure.(30, 31) Likewise, we did not have direct biomarker measures of exposure to pesticides, but we have previously reported that the classification method used in this study is associated with biomarkers of exposure to pesticides in a clinical sample from Boston and in samples representative of the general population of the U.S.(21, 22) Also, assessments of exposure to pesticides through other sources, such as at home application of pesticides. However, this would be a concern to the extent to which these additional sources are related to exposure via diet, especially if findings suggested an association. In the face of null findings and uncertainty of how other sources of exposure to pesticides relate to exposure via diet, it difficult to ascertain the impact this information could have added. Another potential limitation is the possible overadjustment for potential confounders for some of the individual cancer sites. Moreover, even though we examined specific sites, associations may still not adequately capture tumor heterogeneity within a single site. For example, the findings for NHL may reflect a mixture of null, positive and/or inverse associations for individual histologic subtypes, the separate examination of which was beyond the scope of this analysis.(32, 33) Finally, participants were all health professionals, which increased the internal validity of the findings but diminished their external validity.
In conclusion, in these three large prospective cohorts of health professionals, overall exposure to pesticide residues through intake of FVs was not associated with cancer risk. This findings stand in contrast to the well documented carcinogenicity of agricultural pesticide exposure in occupational settings. However, our findings do not rule out the possibility of carcinogenicity of dietary exposure to specific chemicals or effects on subtypes of individual cancers based on histological or molecular heterogeneity.
Supplementary Material
Highlights.
Conventionally grown FVs are the main source of exposure to pesticides.
Overall exposure to pesticides through FV intake is not related to cancer risk.
We cannot dismiss other associations with sub-types of specific cancers.
We cannot rule out other relationships with specific chemicals.
Acknowledgement:
We would like to thank the participants and staff of the NHS, NHSII, and HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding information:
Supported by grants U01 HL145386, R24 ES028521, UM1 CA186107, P01 CA87969, U01 CA176726, R01 HL034594, R01 HL088521, and U01 CA 167552 from the National Institutes of Health.
Footnotes
Conflict of interest: The Authors declare that there is no conflict of interest.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Noncommunicable diseases [Internet]. 2018. [cited 2020 June 6]. Available from: https://www-who-int.gate2.inist.fr/en/news-room/fact-sheets/detail/noncommunicable-diseases.
- 3.Global Burden of Disease Cancer C, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The Global Burden of Cancer 2013. JAMA oncology. 2015;1(4):505–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom DE, Cafiero E, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, et al. The Global Economic Burden of Noncommunicable Diseases [Internet]. Program on the Global Demography of Aging; 2012. [cited 2019 Jan 22]. (PGDA Working Papers). Report No.: 8712. Available from: https://ideas.repec.org/p/gdm/wpaper/8712.html. [Google Scholar]
- 5.Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, et al. The 2015 Dietary Guidelines Advisory Committee Scientific Report: Development and Major Conclusions. Advances in nutrition. 2016;7(3):438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63(25 Pt B):2960–84. [DOI] [PubMed] [Google Scholar]
- 7.Xue J, Zartarian V, Tornero-Velez R, Tulve NS. EPA's SHEDS-multimedia model: children's cumulative pyrethroid exposure estimates and evaluation against NHANES biomarker data. Environment international. 2014;73:304–11. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y, Li C, Zhang X, Zhang X, Pang Y, Zhang S, et al. Route-specific daily uptake of organochlorine pesticides in food, dust, and air by Shanghai residents, China. Environment international. 2012;50:31–7. [DOI] [PubMed] [Google Scholar]
- 9.Fortes C, Mastroeni S, Pilla MA, Antonelli G, Lunghini L, Aprea C. The relation between dietary habits and urinary levels of 3-phenoxybenzoic acid, a pyrethroid metabolite. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;52:91–6. [DOI] [PubMed] [Google Scholar]
- 10.Lu C, Barr DB, Pearson MA, Waller LA. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environmental health perspectives. 2008;116(4):537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.USDA AMS. U.S. Department of Agriculture, Pesticide Data Program (PDP), annual summary 2018. Available from: www.ams.usda.gov/datasets/pdp.
- 12.Suratman S, Edwards JW, Babina K. Organophosphate pesticides exposure among farmworkers: pathways and risk of adverse health effects. Reviews on environmental health. 2015;30(1):65–79. [DOI] [PubMed] [Google Scholar]
- 13.Reiss R, Johnston J, Tucker K, DeSesso JM, Keen CL. Estimation of cancer risks and benefits associated with a potential increased consumption of fruits and vegetables. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2012;50(12):4421–7. [DOI] [PubMed] [Google Scholar]
- 14.Valcke M, Bourgault MH, Rochette L, Normandin L, Samuel O, Belleville D, et al. Human health risk assessment on the consumption of fruits and vegetables containing residual pesticides: A cancer and non-cancer risk/benefit perspective. Environment international. 2017;108:63–74. [DOI] [PubMed] [Google Scholar]
- 15.Chiu YH, Sandoval-Insausti H, Ley SH, Bhupathiraju SN, Hauser R, Rimm EB, et al. Association between intake of fruits and vegetables by pesticide residue status and coronary heart disease risk. Environment international. 2019;132:105113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, et al. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. The Lancet Oncology. 2015;16(5):490–1. [DOI] [PubMed] [Google Scholar]
- 17.Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, et al. Origin, Methods, and Evolution of the Three Nurses' Health Studies. American journal of public health. 2016;106(9):1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. American journal of epidemiology. 1992;135(10):1114–26; discussion 27-36. [DOI] [PubMed] [Google Scholar]
- 19.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared With Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am J Epidemiol. 2018;187(5):1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol. 2017;185(7):570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Chiu YH, Hauser R, Chavarro J, Sun Q. Overall and class-specific scores of pesticide residues from fruits and vegetables as a tool to rank intake of pesticide residues in United States: A validation study. Environment international. 2016;92-93:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu YH, Williams PL, Minguez-Alarcon L, Gillman M, Sun Q, Ospina M, et al. Comparison of questionnaire-based estimation of pesticide residue intake from fruits and vegetables with urinary concentrations of pesticide biomarkers. Journal of exposure science & environmental epidemiology. 2018;28(1):31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public health nutrition. 2006;9(1A):152–7. [DOI] [PubMed] [Google Scholar]
- 24.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. The Journal of nutrition. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baudry J, Assmann KE, Touvier M, Alles B, Seconda L, Latino-Martel P, et al. Association of Frequency of Organic Food Consumption With Cancer Risk: Findings From the NutriNet-Sante Prospective Cohort Study. JAMA internal medicine. 2018;178(12):1597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baudry J, Debrauwer L, Durand G, Limon G, Delcambre A, Vidal R, et al. Urinary pesticide concentrations in French adults with low and high organic food consumption: results from the general population-based NutriNet-Sante. Journal of exposure science & environmental epidemiology. 2019;29(3):366–78. [DOI] [PubMed] [Google Scholar]
- 27.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. The American journal of clinical nutrition. 2018;108(5):1069–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seconda L, Baudry J, Alles B, Touvier M, Hercberg S, Pointereau P, et al. Prospective associations between sustainable dietary pattern assessed with the Sustainable Diet Index (SDI) and risk of cancer and cardiovascular diseases in the French NutriNet-Sante cohort. European journal of epidemiology. 2020. [DOI] [PubMed] [Google Scholar]
- 29.Bradbury KE, Balkwill A, Spencer EA, Roddam AW, Reeves GK, Green J, et al. Organic food consumption and the incidence of cancer in a large prospective study of women in the United Kingdom. British journal of cancer. 2014;110(9):2321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.USDA U.S. Department of Agriculture's National Agricultural Statistics Service (NASS). 2016 Certified Organic Survey. September 2017. Available from: www.nass.usda.gov/organics. [Google Scholar]
- 31.Whitacre DM. Reviews of Environmental Contamination and Toxicology: Springer; 2009. [Google Scholar]
- 32.Morton LM, Slager SL, Cerhan JR, Wang SS, Vajdic CM, Skibola CF, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. Journal of the National Cancer Institute Monographs. 2014;2014(48):130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Piler SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. World Health Organization Classification of Tumors. Lyon, International Agency for Research on Cancer (IARC). Ed. 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


