Abstract
Objectives
To give an overview over the associations between self-reported health literacy and medication adherence in older adults.
Design
A systematic literature review of quantitative studies published in English and German.
Data sources
MEDLINE via PubMed, CINAHL, Cochrane Library, Epistemonikos and LIVIVO were searched.
Eligibility criteria
Included studies had to examine the associations between self-reported health literacy and medication adherence in the elderly (samples including ≥66% of ≥60 years old) and had to use a quantitative methodology and had to be written in English or German.
Data extraction and synthesis
All studies were screened for inclusion criteria by two independent reviewers. A narrative synthesis was applied to analyse all included studies thematically. Quality assessment was conducted using the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.
Results
We found 2313 studies, of which nine publications from eight studies were included in this review. Five studies reported a majority of participants with limited health literacy, one study reported a majority of participants with adequate health literacy, and three publications from two studies only reported mean levels of health literacy. Eight publications from seven studies used self-reports to measure medication adherence, while one study used the medication possession ratio. Overall, six publications from five studies reported significantly positive associations between health literacy and medication adherence while two studies reported positive but non-significant associations between both constructs and one study reported mixed results.
Conclusion
In this review, associations between self-reported health literacy and medication adherence are rather consistent, indicating positive associations between both constructs in older adults. However, concepts and measures of health literacy and medication adherence applied in the included studies still show a noteworthy amount of heterogeneity (eg, different use of cutoffs). These results reveal the need for more differentiated research in this area.
PROSPERO registration number
CRD42019141028.
Keywords: public health, geriatric medicine, statistics & research methods
Strengths and limitations of this study.
To our knowledge, this is the first systematic review to specifically give an overview of existing literature on the association between self-reported health literacy and medication adherence in older adults.
The review protocol was registered prospectively, and the review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Overall, the included studies showed a considerable level of heterogeneity, and the quality of the included studies was predominantly fair, which is a limitation of this review.
Health literacy is still commonly assessed with performance-based measures, making literature searches for self-reports in this field challenging.
Introduction
Within the last decades, demographic change and increasing life expectancy have put older adults (≥60 years old as defined by the United Nations)1 in the focus of healthcare research. With increasing age, the risk of chronic diseases and comorbidities rises, resulting in a growing number of necessary treatments (eg, medication), and adherence to these treatments becomes crucial to reduce adverse reactions and ensure safe and effective care. In this context, health literacy (HL), often defined as ‘the degree to which individuals have the capacity to obtain, process and understand basic health information and services needed to make appropriate health decisions’,2 has been identified as a key influencing factor of improving health-related behaviour in the elderly.3 Accordingly, (elderly) people with low levels of HL use healthcare more often and show higher rates of hospitalisation than those with high levels of HL.3 4
Research also confirmed low HL as a predictor of poor health outcomes linking lower HL to higher age,5 6 lower income5 and lower education.3 7 In addition, HL has been repeatedly linked to medication adherence (MA), commonly defined as ‘the extent to which a patient’s behaviour corresponds with the prescribed medication dosing regime, including time, dosing and interval of medication intake’.8 MA has been the focus of this research since the number of medications taken commonly increases with increasing age, making medication the most common form of therapy in the elderly, often resulting in polypharmacy.9 10 Thus, MA still plays a crucial role in the elderly patient’s care. However, research into the associations between HL and MA stays inconclusive.11–16 While multiple studies report (significantly) positive associations between HL and MA,17–21 others report (significantly) negative associations.22 23
Systematic reviews specifically conducted to analyse the relationship between HL and MA in the elderly resulted in mixed findings as they often included studies with a variety of populations and measures of HL.12 16 24 Older adults have commonly been examined as a homogenous group not taking into account possible differences in levels of HL and MA between subgroups of age (eg, 65–70 years old, 71–75 years old, 76–80 years old, 85+ years old).6 25 In addition, reviews and meta-analyses examining the associations between HL and MA in older age commonly included samples with a wide age range only focusing on the mean age of samples. Since these samples often include (undisclosed) proportions of younger adults and subgroups are not reported, results may not adequately reflect the relationship between HL and MA in older adults.24 26 Previous reviews commonly aimed to include a wide selection of validated measures of HL. However, since only a low proportion of relevant studies is measuring HL with self-reports, these reviews often resulted in a focus on the so-called legacy instruments of HL (ie, REALM,27 TOFHLA)12 24 28 and, thus, included different measures and concepts of HL, which may have led to unknown bias.15 26 As recently stated by Nguyen et al,29 these often-deployed legacy tools may measure different aspects of literacy and may not be appropriate to assess HL in older adults. Accordingly, limited HL was found to be strongly associated with older age when measured with the TOFHLA (mainly assessing reading, comprehension and numeracy skills)28 while limited HL had weak associations with older age30 when measured with the REALM (mainly assessing medical vocabulary).27
As of late, these methodological shortcomings in research into HL have been increasingly recognised, leading to a broader discussion about the conceptualisation and measurement of HL. Most recently, researchers started concentrating on self-report measures of HL as new questionnaires from more comprehensive concepts were developed (eg, the European HL Survey Questionnaire; HLS-EU-Q31). Compared with performance-based measures, self-reports of HL commonly offer a fast, easy and inexpensive way to collect data and have a lower risk of stigma.29 Accordingly, self-reports present important advantages when assessing HL in different populations and contexts as they can be applied more effortless. More recently, some studies began to investigate levels of HL in different subgroups of older age, resulting in a renewed call for more differentiated methods and analyses in this population.25 32
Thus, our review aims to systematically review the evidence on self-reported HL and MA in older adults (≥60 years old), including: (1) the levels of self-reported HL and MA (if available, levels of different subgroups), (2) the associations between self-reported HL and MA, (3) how self-reported HL and MA are measured and (if available) (4) moderator and mediator effects of other psychosocial factors.
Methods
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.33 A checklist of PRISMA items can be found in online supplemental file 1. This review was registered with the International Prospective Register of Systematic Reviews (PROSPERO). The protocol is presented in online supplemental file 2.
bmjopen-2021-056307supp001.pdf (49.2KB, pdf)
bmjopen-2021-056307supp002.pdf (42.7KB, pdf)
Eligibility criteria
Population
Studies examining elderly adults aged 60 years and older were included. In case of study samples with a wider age range, only studies with ≥66% of participants 60 years and older were included to ensure only including studies with a majority of older adults.
Intervention
No specific interventions were included in the criteria. Nevertheless, only studies that assessed associations (eg, correlation, effect size) between self-reported HL and MA were deemed eligible. Studies that assessed HL solely with a performance-based test instrument (eg, REALM,27 TOFHLA)28 were excluded from this review.
Outcomes
Studies examining HL with a validated self-report (subjective measure) as well as MA (measured by, eg, questionnaires, refill records) were included.
Study design
Only primary quantitative research (Randomized controlled trials, prospective and retrospective cohort studies and cross-sectional studies) published in English or German was included. In case of multiple time points, only baseline data were included to ensure comparability.
Data sources and search strategy
An electronic search was performed in five electronic databases (MEDLINE via PubMed (1984–2021), CINAHL (1995–2021), Cochrane Library (1997–2021), Epistemonikos (1995–2021), LIVIVO (1966–2021)) between 15 July and 30 July 2019 by the first author and updated again in July 2021. The search was not limited to a specific time frame. A comprehensive search strategy was applied using combinations of the following search terms: ‘Health literacy’, ‘illiteracy’, ‘treatment adherence and compliance’, ‘patient compliance’, ‘compliance’, ‘patient adherence’ ‘adherence’, ‘non-adherence’, ‘nonadherence’, ‘medication adherence’, ‘discontinuation’, ‘non-compliance’, ‘noncompliance’, ‘termination’, ‘refill’, ‘aged’, ‘old’, ‘older’, ‘elderly’, ‘geriatric’, ‘oldest’, ‘elders’. As these databases use partially different search algorithms, the search strategy was adapted using Medical Subject Headings and Boolean operators (‘AND’, ‘OR’) if applicable (online supplemental table S1). Although this systematic review focuses on self-reports of HL, the terms ‘self-report’ or ‘subjective’ were not included for reasons of higher sensitivity.
bmjopen-2021-056307supp003.pdf (99.4KB, pdf)
In addition, reference lists from eligible articles were hand searched accordingly. All references were subsequently imported into Endnote V.X8 reference management software for screening purposes.
Study selection and screening
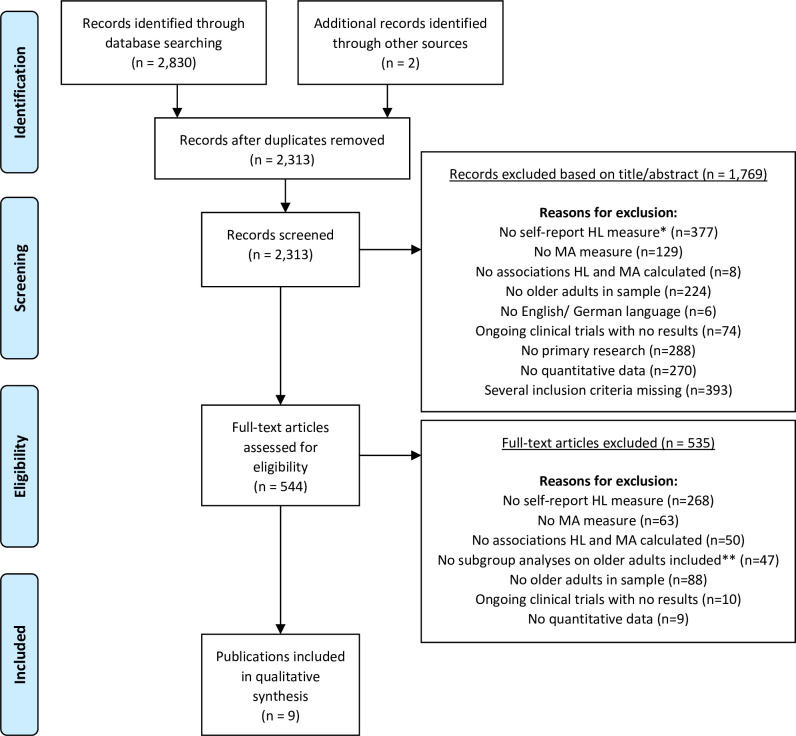
After removal of duplicates, two raters (MSS, SP-H) screened titles and abstracts of all remaining studies for eligibility. A checklist was developed for this purpose, which included a list of inclusion and exclusion criteria, such as type of measure of HL, MA and included sample, to allow for a careful screening process. As many studies include HL only as a secondary outcome and may thus not state it in the study’s title or abstract, a more liberal title/abstract screening was conducted. Accordingly, two raters (MSS, SP-H) assessed the full texts of all previously screened studies independently. Figure 1 shows specific reasons for study exclusion, which included lack of self-report HL measure, lack of MA measure, lack of associations between HL and MA, lack of older adults in sample, lack of English or German language, being an ongoing clinical trial with no results, lack of primary research (eg, book chapter), lack of quantitative data (eg, interview study) or several of these reasons. In case of discrepancies, conflicts were discussed until consensus was reached.
Figure 1.
PRISMA flow diagram. *No HL measure available (n=184); NVS, Newest Vital Sign (n=35); REALM, Rapid Estimate of Adult Literacy in Medicine (n=63); TOFHLA, Test of Functional Health Literacy in Adults (n=90); other performance-based measure (n=5). **Only for samples that not exclusively focus on elders. HL, health literacy; MA, medication adherence; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Quality assessment
The methodological quality of all studies included in this review was assessed using the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (NHLBI, NIH).34 Since only baseline data from quantitative research were included, the NHLBI was deemed appropriate. The NHLBI contains 14 criteria mainly to assess the internal validity of a study. Each item was answered ‘yes’ (if criterion was met), ‘no’ (if criterion was not met) or ‘cannot determine/not applicable/not reported’. As the NHLBI is not meant to assess the study quality by simply summing up its scores, an overall quality rating (‘good’, ‘fair’, ‘poor’) for each study included a comprehensive and critical appraisal of each criterion as well as the study as a whole. This included, for example, the number of participants, the precision of the findings and the risk of bias of the included studies.
Data extraction and synthesis
All relevant data were extracted by the first author with the help of a data extraction checklist that was developed for this purpose and contained the following information about each included study: title, authors, year published, study design and setting, sample and sample size, age subgroups, definition and assessment of HL and MA, moderator and mediator effects (if available), statistical measures to calculate associations between HL and MA (eg, correlation), statistical significance if available.
As the studies showed heterogeneity due to differences in study design, participants, risk of bias and operationalisation of HL and MA (eg, different use of cutoffs and levels of HL), a narrative synthesis was applied to analyse the studies thematically.
Patient and public involvement
Patients or the public were not involved in this study.
Results
Search results
The literature search resulted in a total of 2313 studies after removal of duplicates. After screening for title and abstract, another 1769 studies were excluded based on exclusion criteria (figure 1). Full texts of 544 studies were screened and nine publications from eight studies met all eligibility criteria and were, thus, included in this review (figure 1). The main reason for study exclusion in the screening process was lack of self-reports of HL measure.
Study characteristics
Overall study characteristics are presented in table 1. All included publications were published between 2013 and 2020 with sample sizes between n=116 and n=12 159 (median=293). The proportion of female participants ranged from 33% to 100% (median=53.6%). All studies adopted a cross-sectional design (five survey studies). Three studies (four publications) were conducted in South Korea and one study each in China, USA, Pakistan, Israel and Thailand. Studies were conducted across settings of tertiary care hospitals (n=5), primary healthcare (n=1), private healthcare centres (n=1), community healthcare centres (n=1) and clinics (n=1). All studies examined patients/adults with different types of (chronic) diseases: hypertension (n=2), heart diseases (n=1), atrial fibrillation (n=2), osteoporosis (n=1), several chronic diseases (n=3). Due to eligibility criteria restricting included samples to those with ≥66% of older adults (60 years of age and older), all studies focused on the elderly and only two studies also included patients younger than 60 years (table 1). Five studies included samples with a higher proportion of women.
Table 1.
Overall summary of included studies
| Authors, year | Setting, country | Sample | Methodological quality* | ||||
| N | Age (years), mean (±SD) | % Female | Age subgroups | Disease | |||
| Lee et al, 201335 | Tertiary Care Hospitals, South Korea | n=293 | 65+ M=74.4 (6.3) |
46.8% | NA | Chronic diseases | Fair |
| Lee et al, 201736 | Tertiary Care Hospital, South Korea | n=291 | 65+ M=NA |
53.6% | 65–74 (57.0%) ≥75 (43.0%) |
Chronic diseases | Fair |
| Lu et al, 201941 |
Tertiary Care Hospital, China | n=598 | M=65.8 (9.4) | 33.3% | ≤60 (21.5%) 61–70 (43.0%) 71–80 (29.7%) ≥81 (5.7%) |
Coronary heart disease | Fair |
| Reading et al, 201937 | Private Care Centres, USA | n=12 159 | 21+ 72.7 (64.4–79.9†, adherent patients) 70.1 (59.5–79.1†, nonadherent patients) |
43.0% | <65 (27.2%) 65–74 (30.8%) 75–84 (30.5%) ≥85 (11.5%) |
Atrial fibrillation | Poor |
| Saqlain et al, 201944 | Tertiary Care Centres, Pakistan | n=262 | 65+ M=NA |
64.5% | 65–75 (84.7%) 76–85 (11.1%) >85 (4.2%) |
Hypertension | Fair |
| Seong et al, 201938 | Tertiary General Hospital, South Korea | n=277 | 65+ M=74.2 (7.2) |
40.8% | 65–70 (32.1%) 70–79 (45.5%) ≥80 (22.4%) |
Atrial fibrillation | Fair |
| Shehadeh-Sheeny et al, 201345 | Clinics, Israel | n=303 | 60+ M=71 (6.04) |
100% | 60–65 (21.5%) 66–75 (54.1%) 76–85 (24.4%) |
Osteoporosis | Fair |
| Song and Park, 202039 | Community Health Centre, South Korea | n=116 | 65+ M=72.7 (6.1) |
69.8% | 65–69 (38.8%) 70–79 (43.1%) ≥80 (18.1%) |
Chronic diseases | Fair |
| Wannasirikul et al, 201646 | Primary Care Centre, Thailand | n=600 | 60–70 M=65.3 (NA) |
75.8% | 60–65 (52.7%) 66–70 (47.3%) |
Hypertension | Fair |
*Methodological quality of studies was measured using the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (NHLBI, NIH,34 further details can be found in online supplemental table S2).
†Median (IQR).
NA, not available/not reported.
bmjopen-2021-056307supp004.pdf (273.8KB, pdf)
Quality assessment
Study quality in terms of methodological quality and risk of bias was considered poor for one publication and fair for eight publications (online supplemental table S2). In most cases, low study quality occurred from lack of randomisation, blinding and longitudinal data. Accordingly, results in this review should be interpreted with caution.
HL—key findings
In five publications from four studies,35–39 self-reported HL was measured using a selection of questions from the Brief Health Literacy Screen (BHLS).40 The BHLS employs 3–15 questions (eg, ‘How often do you have someone help you read hospital materials?’) to identify people with inadequate levels of HL. Another study41 used the short version of the HLS-EU-Q, which was designed by the HLS-EU Consortium based on a conceptual framework of HL.31 One study assessed HL with the Single Item Literacy Screener (SILS), which asks ‘How often do you need to have someone help when you read instructions, pamphlets or other written material from your doctor or pharmacy?’.42 Another two studies adopted the Functional, Communicative, and Critical Health Literacy questionnaire (FCCHL) developed by Ishikawa et al,43 a validated questionnaire that assesses three areas of HL: functional HL, communicative HL and critical HL.
Results on the overall levels of HL were mixed, yet a tendency towards limited HL (ie, marginal, low, inadequate) in the elderly was observable. While three publications from two studies35 36 39 only reported mean levels of HL in samples with patients aged 65 years and older, six studies reported different levels of HL (eg, marginal, low or adequate HL). Three of these six studies38 41 44 used cut-offs recommended by the original authors of the assessment instruments, whereas three studies37 45 46 did not report how they calculated HL scores. Five of these six studies38 41 44–46 found that a majority of the respective samples reported limited HL levels (ie, more people had low scores of HL; range from 62.6% to 92.5%, median=74.5%), whereas one study37 found that a majority of the sample reported adequate levels of HL (ie, more people had high scores of HL; 76.9%).
MA—key findings
Four publications from three studies35 36 39 44 employed versions of the Morisky Medication Adherence Scale (MMAS)47 to assess MA. The MMAS consists of four to eight questions asking about different aspects of medication intake behaviour (eg, ‘Do you sometimes forget to take your medication?’).47 One study41 used the Medical Outcomes Study Specific Adherence Scale (MOS-SAS),48 which addresses MA (‘How often have you done each of the following in the past 4 weeks: Took medication as prescribed (on time without skipping dosis)?’) as well as heart-healthy lifestyle behaviour (ie, six preventive behaviours for coronary heart disease, eg, low-salt diet). One study38 used a single-item adopted from Wu et al49 to assess MA (‘In the past week, have you forgotten to take your antithrombotic medication for various reasons?’). Another study37 adopted three questions from the Coronary Artery Risk Development in Young Adults50 to assess MA ((1) ‘In the past month, how often did you take your medications as the doctor prescribed?’, (2) ‘In the past month, how often did you forget to take 1 or more of your prescribed medications?’, (3) ‘In the past month, how often did you decide to skip 1 or more of your prescribed medications?’). MA was also assessed by the medication possession ratio (MPR) in one study.45 The MPR commonly represents the period during which a patient has an adequate amount of supply of his/her medication available over a predefined amount of time (eg, a year). One study assessed MA with the Adherence to Refills and Medication Scale,51 which assesses if a patient can correctly take and refill his or her medication on schedule.
Overall, five publications from four studies35 36 38 44 45 found that a majority of the sample reported low levels of MA (ie, more non-adherers; range from 50.2% to 69.4%, median=59.0%) while three studies,37 41 46 in contrast, found that a majority of the sample reported high levels of MA (ie, more adherers; range from 84.7% to 98.3%, median=93.7%). One study reported a sample mean score of MA only.39
Age subgroups—key findings
Seven studies36–39 41 44 45 included in this review examined age subgroups for differences in HL and/or MA. All of these studies conducted subgroup analyses for differences in MA while only one of these studies41 examined differences in HL between age subgroups (eg, 65–75 years old, 76–85 years old, >85 years old; table 2).
Table 2.
Results of age subgroup analyses on associations between age and health literacy, and age and medication adherence
| Authors, year | Age subgroups reported | Age subgroup analyses |
| Lee et al, 201335 | NA | None conducted. |
| Lee et al, 201736 | 65–74 (57.0%) ≥75 (43.0%) |
No significant differences in MA between age groups (χ²=0.391, p=0.835). |
| Lu et al, 201941 | ≤60 (21.5%) 61–70 (43.0%) 71–80 (29.7%) >81 (5.7%) |
Patients with limited HL were significantly older than those with adequate HL (p<0.05). Age was not a significant predictor for limited HL in ≥81-year-old patients compared with
Age was not a significant predictor for medication nonadherence in ≥81-year-old patients compared with
|
| Reading et al, 201937 | <65 (27.2%) 65–74 (30.8%) 75–84 (30.5%) ≥85 (11.5%) |
Nonadherence to medication significantly differed according to age (p<0.001). Age was a significant predictor for nonadherence to medication in <65-year-old patients compared with
Age was not a significant predictor for nonadherence to medication in <65-year-old patients compared with
|
| Saqlain et al, 201944 | 65–75 (84.7%) 76–85 (11.1%) >85 (4.2%) |
No significant differences in MA between age groups (χ²=1.631, p=0.442). |
| Seong et al, 201938 | 65–70 (32.1%) 70–79 (45.5%) ≥80 (22.4%) |
Adherence to medication significantly differed with respect to age (χ²=15.15, p<0.001). Age was a significant predictor for nonadherence to medication in ≥80-year-old patients (univariate regression) compared with
Age was not a significant predictor for nonadherence to medication in ≥80-year-old patients (multivariate regression) compared with
|
| Shehadeh-Sheeny et al, 201345 | 60–65 (21.5%) 66–75 (54.1%) 76–85 (24.4%) |
No significant differences in MA between age groups (p=0.23). |
| Song and Park, 202039 | 65–69 (38.8%) 70–79 (43.1%) ≥80 (18.1%) |
Adherence to medication significantly differed with respect to age (Z=8.37, p<0.001). Post hoc analysis showed higher MA in 65–69 year-old adults (M=5.1 (2.3)) compared with 70–79 (M=4.0 (2.0)) and ≥80-year-old adults (M=3.0 (1.9)), respectively. |
| Wannasirikul et al 201646 | 60–65 (52.7%) 66–70 (47.3%) |
None conducted. |
AOR, adjusted OR; HL, health literacy; MA, medication adherence; NA, not available/ not reported.
Overall, four studies36 41 44 45 found no significant differences in MA between age subgroups while one study37 reported age as a significant predictor of medication non-adherence as younger patients (<65 years old) were more likely to be non-adherent compared with old/older patients (age groups 65–74 years old and 75–84 years old) but not compared with the oldest (≥85 years old). One study39 reported higher MA in 65–69-year-old adults compared with 70–79-year-old adults and ≥80-year-old adults. Another study38 reported significant differences in adherence levels between age subgroups but did not confirm age as a significant predictor of medication non-adherence in multivariate analyses. Age was significantly associated with HL in one study41 as patients with limited HL were significantly older compared with those with adequate HL. However, regression analyses did not confirm age as a predictor of limited HL (table 2).
Associations between HL and MA
Results of the analyses on associations between HL and MA are depicted in table 3. In addition, an overview of cut-offs and categories used for the measures of HL and MA in the included studies are depicted in online supplemental table S3. All studies conducted analyses on these associations. Overall, six publications from five studies35–37 39 44 46 reported positive and statistically significant associations between HL and MA while two studies41 45 did not find any significant associations, and one study38 reported mixed findings. In detail, one of two publications35 from one study confirmed HL as the strongest predictor for MA in a hierarchical regression analysis while another publication35 from this study found significantly positive associations between HL and MA but reported self-efficacy to be the strongest predictor for HL in their support vector machine model. Another study41 found no significant differences between limited compared with adequate HL in (medication) non-adherent patients with coronary heart disease. However, the study reported that patients with limited HL were more likely to be non-adherent to secondary adherence measures (ie, heart-healthy lifestyle, alcohol intake control, exercise, stress management) and suggested that changing how to take your pills may be easier than changing lifestyle behaviour. In a study among ethnically diverse patients with atrial fibrillation,37 patients with inadequate levels of HL were significantly more likely to be non-adherent to medication than those with adequate levels of HL. In addition, the study found that included patients with self-reported physical inactivity (vs physical activity), alcohol use (vs no alcohol use) and diabetes mellitus were more likely to be non-adherent to medication, whereas patients with diagnosis of hypertension were less likely to be non-adherent to medication. A study on outpatients with hypertension44 found positive and statistically significant associations between HL and MA as well as a higher likelihood of patients with adequate levels of HL to be adherent to medication compared with patients with inadequate levels of HL. In their multivariate logistic regression, the same study found that in addition to adequate HL, self-reported good and moderate subjective health as well as independence in activities of daily living were also independent predictors of MA in the elderly. Another study38 reported significant differences in adherence to antithrombotic medication by levels of HL but did not confirm HL as a significant predictor for MA in older adults. They concluded that a significant association between HL and MA might exist still since, in their univariate regression, the rate of inadequate HL was higher in the group of non-adherent patients compared with adherent patients. However, in their multivariate logistic regression, the authors38 found only cognitive impairment to be a significant predictor of medication non-adherence in older patients with atrial fibrillation. One study45 found no significant association between HL and MA in a population of female osteoporosis patients and found only self-reported income to be a significant predictor of adherence in the conducted multivariate logistic regression. Another study39 found significantly positive associations between HL and MA. In their multiple regression analysis, the authors also found that income, number of chronic diseases, vision problems and HL were significant predictors of MA. One other study46 analysed the relationship between HL, MA and blood pressure levels in primary care patients with hypertension using a Structural Equation Modelling (SEM) approach, which supported the existence of a causal relationship between these factors. Accordingly, HL had a positive but small statistically significant direct effect on MA. Literacy and cognitive ability had the biggest direct effects on both HL and MA. Additionally, HL had the biggest significantly negative direct effect on blood pressure levels (ie, the higher the HL, the lower the blood pressure level). Based on the SEM, the authors of this study46 suggested a mediator effect of HL on MA, even though no analysis was conducted. None of the other studies performed mediator and/or moderator analyses concerning HL and/or MA and other factors.
Table 3.
Detailed analyses of health literacy and medication adherence
| Authors, year | Sample and setting | HL measures | MA measure | Key results | Associations between HL and MA and further outcomes |
| Lee et al, 201335 | n=293, 65+years M=74.4 years (6.3) Patients with chronic diseases from tertiary care hospitals in Cheonan, South Korea |
BHLS three questions |
MMAS-4 | Mean HL was 8.3 (1.9). n=120 (41.0%) patients were adherent to medication. |
Significant associations between HL and MA (p=NA). Self-efficacy was strongest predictor for MA in SVM model. Other factors significantly associated with MA were number of medication types, daily pill counts, duration after diagnosis. |
| Lee et al, 201736 | n=291, 65+years M=NA Patients with chronic diseases from tertiary care hospital in South Korea |
BHLS 15 questions |
MMAS-8 | Mean HL was 46.61 (12.66). n=89 (30.6%) patients were highly adherent with MMAS Score of 8. Mean MA was at a medium level (M=6.32 (1.61)). |
HL positively correlated with MA (r=0.25, p<0.001). HL was strongest predictor of MA in hierarchical linear regression (β=0.190, p<0.001). Other significant predictors of MA in regression were perceived health status (β=0.132, p<0.02), use of magnifying glass (β=0.166, p<0.003), assistance with medication administration (β=0.120, p<0.035). |
| Lu et al, 201941 |
n=598 M=65.8 years (9.4) Patients with coronary heart disease from tertiary hospital in Shanghai, China |
HLS-EU-Q16 | MOS-SAS | HL was limited for n=444 (74.5%) and adequate for n=152 (25.5%) patients. Patients with limited HL were significantly older than those with adequate HL (p=0.003). n=505 (84.7%) patients were adherent to medication. |
No significant associations between HL and MA (χ²=NA, p=0.125). No significant predictive relationship between limited HL and medication nonadherence (AOR (95% CI)=0.66 (0.39–1.11), p=0.113). Patients with limited HL compared with those with adequate HL were more likely to be nonadherent to overall heart-healthy lifestyle behaviour (AOR (95% CI)=1.69 (1.13–2.53), p=0.010), exercise (AOR (95% CI)=1.50 (1.01–2.22), p=0.046), alcohol intake control (AOR (95% CI)=2.19 (1.21–3.96), p=0.010), and stress management (AOR (95% CI)=2.09 (1.32–3.29), p=0.002). |
| Reading et al, 201937 | n=12 159, 21+ years Age median was 72.7 and 70.1 years for adherent and nonadherent patients, respectively Ethnically diverse patients with atrial fibrillation from Northern California, USA |
BHLS three questions |
CARDIA (three questions) |
n=9349 (76,9%) patients had adequate HL. n=771 (6.3%) patients were nonadherent to medication. Significant differences in MA between age subgroups (p<0.001). |
Patients with inadequate HL were more likely to be nonadherent to medication compared with those with adequate HL (AOR (95% CI)=1.32 (1.09–1.60), p<0.01) in multivariate logistic regression model. Patients were more likely to be nonadherent to medication if physically inactive (AOR (95% CI)=1.57 (1.16–2.13), p<0.01), drinking alcohol (AOR (95% CI)=1.91 (1.51–2.43), p<0.001), having diagnosis of diabetes mellitus (AOR (95% CI)=1.22 (1.01–1.48), p<0.05), having 1–7 days of self-reported poor physical health (AOR (95% CI)=1.43 (1.17–1.75), p<0.001). Patients were less likely to be nonadherent to medication if having diagnosis of hypertension (AOR (95% CI)=0.72 (0.60–0.87), p<0.05), age between 65–74 (AOR (95% CI)=0.68 (0.55–0.83), p<0.001) and age between 75–84 (AOR (95% CI)=0.67 (0.53–0.84), p<0.001). |
| Saqlain et al, 201944 | n=262, 65+years M=NA Outpatients with hypertension from tertiary healthcare centres in Islamabad, Pakistan |
SILS | MMAS-4 | n=98 (37.4%) patients had adequate HL. n=102 (38.9%) patients were adherent to medication. |
Positive and statistically significant associations between HL and MA (χ²=24.356, p<0.001). Patients with adequate HL were more likely to be adherent to medication compared with those with inadequate HL (OR (95% CI)=3.37 (1.91–5.96), p<0.001). Other significant predictors of MA were self-reported good (OR (95% CI)=4.25 (1.45–12.44), p<0.008) and moderate (OR (95% CI)=3.54 (1.37–9.16), p<0.009) subjective health and independence in activities of daily living (OR (95% CI)=2.97 (1.15–5.85), p<0.002). |
| Seong et al, 201938 | n=277, 65+years M=74.2 (7.2) Outpatients with atrial fibrillation undergoing antithrombotic therapy in tertiary general hospital in South Korea |
BHLS three questions |
Single item | HL levels (M=7.9 (3.5)) were inadequate, marginal, and adequate for 28.1%, 45.5%, and 26.4% of patients, respectively. n=139 (50.2%) patients were nonadherent to medication. Significant differences in MA between age subgroups (p<0.001). |
Positive and statistically significant associations between HL and MA (χ²=22.00, p<0.001). Significant predictive relationship between marginal/ inadequate HL and medication nonadherence in univariate logistic regression analysis (OR (95% CI)=2.55 (1.29–3.90), p=0.004) but not in multivariate logistic regression analysis (OR (95% CI)=1.45 (0.79–2.64), p=0.232), where only cognitive impairment was significant predictor for medication nonadherence (OR (95% CI)=2.63 (1.42–4.85), p=0.002). |
| Shehadeh-Sheeny et al, 201345 | n=303, 60+years M=71 (6.04) Female Arab patients with osteoporosis from three clinics in Israel |
FCCHL | MPR | n=75 (24.8%) patients had high HL compared with n=164 (54.1%) and n=64 (21.1%) with medium and low HL, respectively. n=125 (41.3%) patients had high MA. |
No significant associations between MA and HL (p=0.44). 46.7% of patients with high HL were more adherent to medication compared with 35.9% of patients with low HL. In multivariate logistic regression only self-reported income was a significant predictor of MA (OR (95% CI)=1.26 (1.01–1.58), p=0.037). |
| Song and Park, 202039 | n=116, 65+years M=72.7 (6.1) Community-dwelling older adults in healthcare centre, South Korea |
BHLS 15 questions |
MMAS-8 | Mean HL was 42.4 (6.6). Mean MA was at a medium level (M=4.3 (2.2)). |
HL positively correlated with MA (r=0.42, p<0.001). In multiple regression analysis HL was significant predictor of MA (β=0.23, p<0.001). Other significant predictors of MA were income (β=0.35, p<0.001), number of chronic diseases (β=−0.33, p<0.001), and vision problems (β=−0.32, p<0.001). |
| Wannasirikul et al, 201646 | n=600, 60–70 years M=65.3 Patients with hypertension from primary healthcare centre in Sa Kaeo Province, Thailand |
FCCHL | ARMS | Mean HL was 40.0 (10.4). HL levels were inadequate, marginal, and adequate for 48.7%, 43.8%, and 7.5% of patients, respectively. MA was good for 98.3% of patients. |
SEM supports causal relationship between HL, MA, and blood pressure. HL had a significantly positive direct effect on MA in SEM (β=0.08, p<0.05). Cognitive ability ((β=0.22, p<0.05) and literacy (β=0.46, p<0.05) had biggest and significantly positive direct effect on MA. Literacy (β=0.15, p<0.05) and cognitive ability (β=0.52, p<0.05) had biggest and significantly positive direct effect on HL. HL had biggest significantly negative direct effect on blood pressure level (β=-0.14, p<0.05). MA had a significantly negative direct effect on blood pressure level (β=-0.02, p<0.05). Results suggest mediator effect of HL on MA. |
AOR, adjusted OR; ARMS, Adherence to Refills and Medications Scale; BHLS, Brief Health Literacy Screen; CARDIA, Coronary Artery Risk Development in Young Adults; FCCHL, Functional, Communicative, and Critical Health Literacy Questionnaire; HL, health literacy; HLS-EU-Q, European HL Survey Questionnaire; MA, medication adherence; MMAS, Morisky Medication Adherence Scale; MOS-SAS, Medical Outcomes Study Specific Adherence Scale; MPR, medication possession ratio; NA, not available/ not reported; SILS, Single Item Literacy Screener; SVM, support vector machine.
bmjopen-2021-056307supp005.pdf (101.2KB, pdf)
Discussion
The aim of this study was to give a systematic overview of the associations between HL and MA in older adults. Although research on HL and MA in older adults has rapidly increased in the last years, mixed results are a common denominator in this area.15 52 Accordingly, previous systematic reviews resulted in a range of conclusions as they included a variety of HL concepts, different (younger) age groups and a range of methodologically different instruments (self-reports as well as performance-based measures) to assess HL.12 16 24 26 52 To our knowledge, this is the first systematic review to focus specifically on self-reported HL while explicitly including studies with samples of older adults. We found that only few validated instruments of self-reported HL are used and that most studies still rely on legacy measures to assess HL even though their use has been criticised repeatedly and self-reports of HL offer a range of advantages.29 Studies included in our review mostly assessed MA in older adults through self-reports, even though a wide range of tools is known.53 54
Based on a rather high level of uncertainty due to low study quality and risk of bias, results in this review appear to be more consistent in contrast to previous reviews15 16 as many included studies reported positive and statistically significant associations between HL and MA. This could be explained by the fact that only older adults (at least 66% of older adults in samples, not based on the samples’ mean age) were examined in the included studies, and associations in this group may be more prominent compared with studies that also include subgroups of younger people. One review,24 for example, aimed to review literature that examined HL and MA in older adults with cardiovascular disease or diabetes. Included studies in the review had to assess HL with legacy instruments only and had to include samples of participants with a ‘[…] mean age [of] at least 50 years or with at least a third of participants aged 50 years or older […]’ and could not confirm an association between HL and MA. As stated earlier, inclusion of younger participants may have resulted in unknown bias from age. Yet another bias may have resulted from the utilisation of legacy measures with different conceptualisations of HL, since the REALM and TOFHLA, two of the most prominent legacy tools of HL, are confirmed to assess different aspects of literacy rather than HL and may, thus, be differently impacted by a person’s intelligence.29 Accordingly, Loke et al stated in their review that functional measures of HL may not be adequate and ‘[n]ew methods of measuring health literacy beyond the functional level are needed […]’.
In another review, Ostini et al16 included studies with samples of all age groups, not disclosing how HL and MA were measured in these studies and suggested the existence of a U-shaped relationship between HL and non-adherence as patients with high levels of HL may intentionally not adhere while those with low HL levels may unintentionally not adhere. Looking at the included studies in their review, only one study used a self-report measure of HL (BHLS) while all other used one of the performance-based legacy instruments. Since legacy measures of HL rather focus on literacy skills and we could not find any indication of a U-shaped relationship in our review, we want to point out that, while we cannot confirm or rule out a U-shaped relationship between literacy skills and MA, our review might suggest that it does not exist between self-reported HL and MA in older adults. While people with low literacy skills may not be able to understand/read labels/instructions and, therefore, not adhere (or rather unintentionally not comply) to their medication more often, people with higher literacy skills might read instructions first and subsequently (intentionally) decide not to take their medications due to, for example, possible side effects they read about. However, this phenomenon is not easily transferrable onto other and in some cases broader theoretical concepts of self-reported HL measures (eg, HLS-EU-Q), since those not only include literacy skills but also other individual skills and situational aspects and may, thus, show another linear or non-linear association with adherence. Since empirical data on possible associations between literacy and self-reported HL are still widely lacking, we need more research to explore and develop comprehensive theories in this area.
Five studies38 41 44–46 included in this review found that a majority of participants in the respective samples reported limited (ie, inadequate, low, marginal) HL. This is consistent with other research that showed that older people commonly reach only low levels of self-reported health literacy3 25 32 even though this research is very scarce. HL was measured by versions of four different self-reports (BHLS,40 55 HLS-EU-Q3, SILS42 and FCCHL.43 This shows that self-reporting HL measures are still rarely used when examining older adults, even though the Health literacy Tool Shed56 lists 29 self-report instruments for HL in English alone (58 without language restrictions).
MA was assessed through self-reports in all but one of the included studies.35–39 41 44 46 Nevertheless, we recommend a more detailed description of operationalisation of MA as many studies still use the concepts of adherence and compliance interchangeably. Interestingly, we had to exclude many studies from this review even though they assessed some form of adherence, because they only included measures of general preventive behaviour (eg, physical activity) and not MA. However, the use of such secondary adherence measures might be a promising approach to get a more comprehensive picture of adherence in older adults.54 Especially, a multimethod approach could be helpful since self-reported adherence may also be affected by cognitive bias and/or social desirability in older adults. As such, the utilisation of both direct (eg, laboratory measures) and indirect (eg, self-reports) measures of adherence54 57 may help to get a better understanding of adherence and its associations with self-reported HL in older adults. A number of studies in this review also included measures of secondary prevention (eg, physical activity, heart-healthy lifestyle behaviour) as well as other factors (eg, income, cognitive ability) providing further knowledge on possible confounders in the mechanisms between HL and MA. Accordingly, several studies confirmed multiple other factors as predictors for MA (eg, health status,36 37 44 income,39 45 physical activity,37 44 cognitive ability)38 46 and/or HL (eg, cognitive ability,46 stress management).41 In a recent systematic review and meta-analysis by Lim et al,58 the authors examined the associations between physical activity and HL and found that older adults with inadequate levels of HL were ‘[…] less likely […] to report engaging in physical activity […]’ than those with adequate HL, showing the importance of also addressing secondary adherence measures in future research in this area. Notably, their review also included younger adults (samples with mean age ≥55 years) and different of HL measures (legacy measures and self-reports).
Even though we also encourage researchers to assess HL with a multimethod approach (eg, subjective and objective instruments), we suggest a more rigorous differentiation in analysis and interpretation when comparing HL measures that are based on different concepts (eg, legacy tools and self-reports). This may also help to clarify further the associations between self-reported HL and literacy as measured by legacy instruments. As stated by Nguyen et al,29 a separation in analyses of objective and subjective measures of HL as well as a closer alignment of HL theory and measurement could help clarify the relationship between HL and MA. This idea was also supported by one of the studies39 included in this review, which aimed at comparing two different measures of HL (self-report vs legacy measure). The authors found that even though both measures were significantly and positively correlated to MA, only the self-report was a significant predictor for MA in older adults suggesting that self-reports may be more fitting to access HL when predicting MA since ‘[…] assessing older adults’ experiences of limited health literacy is more appropriate for catching any decreased medication adherence […]’.
This review additionally confirms that age subgroup analyses are conducted very rarely for self-reported HL but quite often for MA. This may result from the fact that research on MA in the elderly is traditionally older than research on HL in the elderly and with regards to HL, most studies still treat older people as a homogenous group.25 Most studies in this review did not find any significant associations between age and MA and only two studies37 39 reported significant differences in MA between age subgroups. Accordingly, one study37 reported that young/young–old people (21–65 years old) were more likely not to adhere to their medication compared with old/older adults (65–84 years old) but not oldest adults (≥85 years old). A second study39 reported higher MA in 65–69-year-old adults compared with older/oldest adults (70–90 years old). Not surprisingly, only one study conducted analyses on the relationship between age and HL,41 showing that patients with limited HL were significantly older compared with those with adequate HL. Even though generalisability is very limited, these results reveal the necessity for more differentiated analyses (eg, of subgroups) in future HL and MA research on older adults. In context of demographic change and increasing life expectancy, more differentiated analyses could help to understand specific needs and barriers of elderly (patient) populations with different chronic diseases. Importantly, definitions of old age are often inconsistent and include people from ages 60, 65 or 70 years and over. These dissimilarities in the definitions of old age may result from differences in cultural and/or economic standards (eg, USA vs Asia) and often manifest in different demographic changes and/or different life expectancies, thus resulting in a different quality of healthcare in groups of older adults. Consequently, when looking at older adults’ healthcare and health outcomes, it is critical to include contextual aspects such as cultural or economic standards.
Studies in this review show some inconsistencies in the use of cut-offs, use and wording of HL levels. Of all included studies, six studies37 38 41 44–46 reported categories of HL (eg, adequate), of which only three38 41 44 reported cut-offs for these categories. Three publications35 36 39 from two studies reported neither categories nor cut-offs for HL and only five publications35 36 38 39 46 from four studies reported mean values of HL. For example, Shehadeh-Sheeny et al calculated scores for low, medium and high levels of HL while Wannasirikul et al calculated scores for adequate, marginal and inadequate HL levels even though no cutoffs were reported/available by neither the authors nor the FCCHL measure both studies used. The inconsistent use of cut-offs and wording may indicate a lack of certainty and experience in the application of self-reports enhancing the call for more differentiated research and the development of easy-to-use but still valid tools.
Strength and limitations
The strengths of this study include the exhaustive methodology and comprehensive search strategy that were used. As we followed a strict screening procedure, we are confident that we found all eligible studies. Since we excluded all studies that measured HL with performance-based instruments, we aimed to reduce bias, resulting from fundamental differences in constructs and concepts. Although we see this exclusion as a considerable advantage, we cannot eliminate the possibility of bias still resulting from theoretical or practical differences in self-reports as some of them are built on more complex conceptual frameworks than others. Additionally, there are advantages in assessing HL in older adults with self-reports since they reduce the possible bias of performance-based measures, resulting from fear of stigma and/or (time) pressure. Nevertheless, we recognise the inherent limitations of self-reporting tools that may also have biased our results.
Other limitations should be considered. All studies included in this review were cross-sectional, thus we cannot determine any direction of causality. The fair to poor methodological quality of the included studies may also increase the risk of (unknown) bias. Given the heterogeneity of the studies, a meta-analysis (eg, pooled ORs) could not be conducted, thus limiting further understanding of the relationship between HL and MA in older adults. Accordingly, certainty of evidence of these results is low. Additionally, our search strategy in this review limited included studies to English and German, which could bias results due to missing research in other languages. Finally, we were not able to include EMBASE as a database in our search. Even though we are very confident that we did not miss a substantial amount of literature, this must be considered as a limitation of this review.
Conclusions
Based on a rather high level of uncertainty, included literature in this review suggests that self-reported HL and MA in older adults show a somewhat straightforward positive association. While previous research on HL and MA in older adults did not always find clear associations, many studies included in this review reported significantly positive associations between HL and MA. In addition, HL plays an important role as a predictor of MA in older adults as several studies in this review could confirm. However, other factors (eg, cognitive ability) appear equally important in predicting MA in older adults, and future studies should also focus on secondary adherence measures (eg, physical activity) when examining the associations between HL and MA in the elderly. Finally, study heterogeneity and methodological weaknesses reveal a definitive need for more differentiated research regarding different definitions, concepts and measures of HL and MA as well as longitudinal research designs and studies that analyse age subgroups in older adults.
Supplementary Material
Acknowledgments
We would like to thank Dr rer. biol. hum. Laura Inhestern for her advice during the preparation of the search for this review.
Footnotes
Contributors: MSS is the guarantor. All authors were involved in the design and planning of the review. MSS prepared, performed and redefined the searches after consultation with SP-H and CB. MSS and SP-H performed screening and data extraction with the help of CB in case of disagreements or discussion. All authors contributed to the data analysis and interpretation. MSS wrote the first draft which was critically revised by SP-H and CB.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants, as it is a systematic review.
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division . World population ageing 2019: highlights (ST/ESA/SER.A/430) 2019.
- 2.Ratzan SC, Parker RM. Health literacy - identification and response. J Health Commun 2006;11:713–5. 10.1080/10810730601031090 [DOI] [PubMed] [Google Scholar]
- 3.Consortium H-E . Comparative report on health literacy in eight EU member states. The European health literacy project 2009–2012. Vienna: Ludwig Boltzmann Institute for Health Promotion Research 2012.
- 4.Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011;155:97–107. 10.7326/0003-4819-155-2-201107190-00005 [DOI] [PubMed] [Google Scholar]
- 5.Chesser AK, Keene Woods N, Smothers K, et al. Health literacy and older adults: a systematic review. Gerontol Geriatr Med 2016;2:2333721416630492. 10.1177/2333721416630492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogt D, Schaeffer D, Messer M, et al. Health literacy in old age: results of a German cross-sectional study. Health Promot Int 2018;33:739–47. 10.1093/heapro/dax012 [DOI] [PubMed] [Google Scholar]
- 7.Wolf MS, Feinglass J, Thompson J, et al. In search of 'low health literacy': threshold vs. gradient effect of literacy on health status and mortality. Soc Sci Med 2010;70:1335–41. 10.1016/j.socscimed.2009.12.013 [DOI] [PubMed] [Google Scholar]
- 8.Gast A, Mathes T. Medication adherence influencing factors—an (updated) overview of systematic reviews. Syst Rev 2019;8:1–17. 10.1186/s13643-019-1014-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoel RW, Giddings Connolly RM, Takahashi PY. Polypharmacy management in older patients. Mayo Clin Proc 2021;96:242–56. 10.1016/j.mayocp.2020.06.012 [DOI] [PubMed] [Google Scholar]
- 10.Chiatti C, Bustacchini S, Furneri G, et al. The economic burden of inappropriate drug prescribing, lack of adherence and compliance, adverse drug events in older people: a systematic review. Drug Saf 2012;35:73–87. 10.1007/BF03319105 [DOI] [PubMed] [Google Scholar]
- 11.Huang Y-M, Shiyanbola OO, Smith PD. Association of health literacy and medication self-efficacy with medication adherence and diabetes control. Patient Prefer Adherence 2018;12:793–802. 10.2147/PPA.S153312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martins NFF, Abreu DPG, Silva BTD, et al. Functional health literacy and adherence to the medication in older adults: integrative review. Rev Bras Enferm 2017;70:868–74. 10.1590/0034-7167-2016-0625 [DOI] [PubMed] [Google Scholar]
- 13.Park NH, Song MS, Shin SY, et al. The effects of medication adherence and health literacy on health-related quality of life in older people with hypertension. Int J Older People Nurs 2018;13:e12196. 10.1111/opn.12196 [DOI] [PubMed] [Google Scholar]
- 14.Roh YH, Koh YD, Noh JH, et al. Effect of health literacy on adherence to osteoporosis treatment among patients with distal radius fracture. Arch Osteoporos 2017;12:42. 10.1007/s11657-017-0337-0 [DOI] [PubMed] [Google Scholar]
- 15.Zhang NJ, Terry A, McHorney CA. Impact of health literacy on medication adherence: a systematic review and meta-analysis. Ann Pharmacother 2014;48:741–51. 10.1177/1060028014526562 [DOI] [PubMed] [Google Scholar]
- 16.Ostini R, Kairuz T. Investigating the association between health literacy and non-adherence. Int J Clin Pharm 2014;36:36–44. 10.1007/s11096-013-9895-4 [DOI] [PubMed] [Google Scholar]
- 17.Lindquist LA, Go L, Fleisher J, et al. Relationship of health literacy to intentional and unintentional non-adherence of hospital discharge medications. J Gen Intern Med 2012;27:173–8. 10.1007/s11606-011-1886-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf MS, Davis TC, Osborn CY, et al. Literacy, self-efficacy, and HIV medication adherence. Patient Educ Couns 2007;65:253–60. 10.1016/j.pec.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 19.Kripalani S, Gatti ME, Jacobson TA. Association of age, health literacy, and medication management strategies with cardiovascular medication adherence. Patient Educ Couns 2010;81:177–81. 10.1016/j.pec.2010.04.030 [DOI] [PubMed] [Google Scholar]
- 20.Bauer AM, Schillinger D, Parker MM, et al. Health literacy and antidepressant medication adherence among adults with diabetes: the diabetes study of Northern California (DISTANCE). J Gen Intern Med 2013;28:1181–7. 10.1007/s11606-013-2402-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayo-Gamble TL, Mouton C. Examining the association between health literacy and medication adherence among older adults. Health Commun 2018;33:1124–30. 10.1080/10410236.2017.1331311 [DOI] [PubMed] [Google Scholar]
- 22.Mosher HJ, Lund BC, Kripalani S, et al. Association of health literacy with medication knowledge, adherence, and adverse drug events among elderly veterans. J Health Commun 2012;17:241–51. 10.1080/10810730.2012.712611 [DOI] [PubMed] [Google Scholar]
- 23.Fang MC, Machtinger EL, Wang F, et al. Health literacy and anticoagulation-related outcomes among patients taking warfarin. J Gen Intern Med 2006;21:841–6. 10.1111/j.1525-1497.2006.00537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loke YK, Hinz I, Wang X, et al. Systematic review of consistency between adherence to cardiovascular or diabetes medication and health literacy in older adults. Ann Pharmacother 2012;46:863–72. 10.1345/aph.1Q718 [DOI] [PubMed] [Google Scholar]
- 25.Vogt D, Berens EM, Schaeffer D. Gesundheitskompetenz im höheren Lebensalter [Health Literacy in Advanced Age]. Gesundheitswesen 2020;82:407–12. [DOI] [PubMed] [Google Scholar]
- 26.Geboers B, Brainard JS, Loke YK, et al. The association of health literacy with adherence in older adults, and its role in interventions: a systematic meta-review. BMC Public Health 2015;15:903. 10.1186/s12889-015-2251-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med 1993;25:391–5. [PubMed] [Google Scholar]
- 28.Parker RM, Baker DW, Williams MV, et al. The test of functional health literacy in adults: a new instrument for measuring patients' literacy skills. J Gen Intern Med 1995;10:537–41. 10.1007/BF02640361 [DOI] [PubMed] [Google Scholar]
- 29.Nguyen TH, Paasche-Orlow MK, McCormack LA. The state of the science of health literacy measurement. Stud Health Technol Inform 2017;240:189–203. 10.3233/ISU-170827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi LC, Wardle J, Wolf MS, et al. Aging and functional health literacy: a systematic review and meta-analysis. J Gerontol B Psychol Sci Soc Sci 2016;71:445–57. 10.1093/geronb/gbu161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sørensen K, Van den Broucke S, Pelikan JM, et al. Measuring health literacy in populations: illuminating the design and development process of the European Health Literacy Survey Questionnaire (HLS-EU-Q). BMC Public Health 2013;13:948. 10.1186/1471-2458-13-948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berens E-M, Vogt D, Messer M, et al. Health literacy among different age groups in Germany: results of a cross-sectional survey. BMC Public Health 2016;16:1151. 10.1186/s12889-016-3810-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Heart, Lung, and Blood Institute . Quality assessment tool for observational cohort and cross-sectional studies, 2014. Available: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort [Accessed 5 May 2020].
- 35.Lee SK, Kang B-Y, Kim H-G, et al. Predictors of medication adherence in elderly patients with chronic diseases using support vector machine models. Healthc Inform Res 2013;19:33–41. 10.4258/hir.2013.19.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y-M, Yu HY, You M-A, et al. Impact of health literacy on medication adherence in older people with chronic diseases. Collegian 2017;24:11–18. 10.1016/j.colegn.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 37.Reading SR, Black MH, Singer DE, et al. Risk factors for medication non-adherence among atrial fibrillation patients. BMC Cardiovasc Disord 2019;19:38. 10.1186/s12872-019-1019-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seong H-J, Lee K, Kim B-H, et al. Cognitive impairment is independently associated with Non-Adherence to antithrombotic therapy in older patients with atrial fibrillation. Int J Environ Res Public Health 2019;16 10.3390/ijerph16152698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song M-S, Park S. Comparing two health literacy measurements used for assessing older adults' medication adherence. J Clin Nurs 2020;29:4313–20. 10.1111/jocn.15468 [DOI] [PubMed] [Google Scholar]
- 40.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004;36:588–94. [PubMed] [Google Scholar]
- 41.Lu M, Ma J, Lin Y, et al. Relationship between patient’s health literacy and adherence to coronary heart disease secondary prevention measures. J Clin Nurs 2019;28:2833–43. 10.1111/jocn.14865 [DOI] [PubMed] [Google Scholar]
- 42.Morris NS, MacLean CD, Chew LD, et al. The single item literacy screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract 2006;7:21. 10.1186/1471-2296-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishikawa H, Takeuchi T, Yano E. Measuring functional, communicative, and critical health literacy among diabetic patients. Diabetes Care 2008;31:874–9. 10.2337/dc07-1932 [DOI] [PubMed] [Google Scholar]
- 44.Saqlain M, Riaz A, Malik MN, et al. Medication adherence and its association with health literacy and performance in activities of daily Livings among elderly hypertensive patients in Islamabad, Pakistan. Medicina 2019;55 10.3390/medicina55050163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shehadeh-Sheeny A, Eilat-Tsanani S, Bishara E, et al. Knowledge and health literacy are not associated with osteoporotic medication adherence, however income is, in Arab postmenopausal women. Patient Educ Couns 2013;93:282–8. 10.1016/j.pec.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 46.Wannasirikul P, Termsirikulchai L, Sujirarat D, et al. Health literacy, medication adherence, and blood pressure level among hypertensive older adults treated at primary health care centers. Southeast Asian J Trop Med Public Health 2016;47:109–20. [PubMed] [Google Scholar]
- 47.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74. 10.1097/00005650-198601000-00007 [DOI] [PubMed] [Google Scholar]
- 48.Sherbourne CD, Hays RD, Ordway L, et al. Antecedents of adherence to medical recommendations: results from the medical outcomes study. J Behav Med 1992;15:447–68. 10.1007/BF00844941 [DOI] [PubMed] [Google Scholar]
- 49.Wu J-R, DeWalt DA, Baker DW, et al. A single-item self-report medication adherence question predicts hospitalisation and death in patients with heart failure. J Clin Nurs 2014;23:2554–64. 10.1111/jocn.12471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials 1991;12:1S–77. 10.1016/0197-2456(91)90002-4 [DOI] [PubMed] [Google Scholar]
- 51.Kripalani S, Risser J, Gatti ME, et al. Development and evaluation of the adherence to refills and medications scale (ARMS) among low-literacy patients with chronic disease. Value Health 2009;12:118–23. 10.1111/j.1524-4733.2008.00400.x [DOI] [PubMed] [Google Scholar]
- 52.Miller TA. Health literacy and adherence to medical treatment in chronic and acute illness: a meta-analysis. Patient Educ Couns 2016;99:1079–86. 10.1016/j.pec.2016.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int 2015;2015:217047 10.1155/2015/217047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep 2019;92:117–22. 10.15386/mpr-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008;23:561–6. 10.1007/s11606-008-0520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Health literacy tool shed, 2020. Available: https://healthliteracy.bu.edu [Accessed 17 Aug 2020].
- 57.McRae-Clark AL, Baker NL, Sonne SC, et al. Concordance of direct and indirect measures of medication adherence in a treatment trial for cannabis dependence. J Subst Abuse Treat 2015;57:70–4. 10.1016/j.jsat.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim ML, van Schooten KS, Radford KA. Association between health literacy and physical activity in older people: a systematic review and meta-analysis. Health Promot Int 2020:daaa072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-056307supp001.pdf (49.2KB, pdf)
bmjopen-2021-056307supp002.pdf (42.7KB, pdf)
bmjopen-2021-056307supp003.pdf (99.4KB, pdf)
bmjopen-2021-056307supp004.pdf (273.8KB, pdf)
bmjopen-2021-056307supp005.pdf (101.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.