Abstract
Aims/hypothesis
Menstrual cycle dysfunction has been associated with many endocrine-related diseases, but evidence linking menstrual cycle dysfunction with gestational diabetes mellitus (GDM) is scant. The current study investigated the association of pre-pregnancy menstrual cycle regularity and length during adolescence, early adulthood and mid-adulthood with the subsequent risk of GDM.
Methods
Between 1993 and 2009, we followed 10 906 premenopausal women participating in the Nurses' Health Study II who reported menstrual cycle characteristics during adolescence (age 14-17 years), early adulthood (age 18-22 years) and mid-adulthood (age 29-46 years). Incident GDM was ascertained from a self-reported questionnaire regarding physician diagnosis. Log-binomial models with generalised estimating equations were used to estimate the RRs and 95% CI for the associations between menstrual cycle characteristics and GDM.
Results
We documented 578 incident cases of GDM among 14 418 pregnancies over a 16 year follow-up. After adjusting for potential confounders, women reporting always having irregular menstrual cycles during mid-adulthood had a 65% (95% CI 21, 125%) higher risk of GDM than women reporting very regular cycles. GDM risk was also greater among women reporting that their cycles were usually ≥32 days during mid-adulthood, compared with women reporting cycles between 26 and 31 days (RR 1.42 [95% CI 1.15, 1.75]). The risk of GDM was greater for women whose cycles changed from regular early in their reproductive years to irregular or from <32 days to ≥32 days during mid-adulthood, compared with women whose cycles remained <32 days or regular, respectively.
Conclusions/interpretation
Women whose cycles were long or irregular during mid-adulthood, but not in adolescence or young adulthood, were at higher risk of GDM.
Keywords: Epidemiology, Gestational diabetes mellitus, Menstrual cycle, Pregnancy, Public health
Graphical Abstract

Introduction
Gestational diabetes mellitus (GDM), defined as glucose intolerance with onset or first recognition during pregnancy, has become one of the most common pregnancy complications worldwide [1]. The global prevalence of GDM varies from 1.8% to 24.9%, depending on population characteristics, screening methods and diagnostic criteria [2]. In the USA, the prevalence of GDM has increased from 0.3% in 1979–1980 to 7.6% in 2007–2014 [3, 4]. Because GDM is associated with considerable risks to both mothers and the developing fetus [5], it is critical to identify groups with increased susceptibility and develop strategies to promote prevention.
The normal ovulatory menstrual cycle is a vital sign of women’s overall health [6]. However, irregular or long menstrual cycles, reflecting functional disruption of the neuroendocrine hypothalamic-pituitary-ovarian (HPO) axis, are estimated to affect nearly 20% of reproductive age women [7]. Menstrual cycle dysfunction has been associated with many endocrine-related diseases, including insulin resistance and type 2 diabetes [8-10]. However, evidence linking irregular or long menstrual cycles with GDM is scant and inconsistent [11, 12]. Inference from previous studies is hampered by limited sample size, poorly characterised cycle patterns (e.g., regular vs irregular) and a lack of information on key confounders including BMI, diet quality and lifestyle factors [11, 12]. More importantly, no study has assessed whether the same phenotype across different stages of a woman’s reproductive lifespan (e.g., adolescence, early adulthood and mid-adulthood) has a similar association with GDM. To address these important knowledge gaps, we prospectively investigated the association between pre-pregnancy menstrual cycle regularity and length during adolescence (age 14-17 years), early adulthood (age 18-22 years) and mid-adulthood (age 29-46 years) with the risk of GDM among women participating in a large ongoing prospective cohort study.
Methods
Study population
The Nurses’ Health Study (NHS) II is an ongoing prospective cohort that was established in 1989 by recruiting 116 429 reproductive age female nurses (age 25–42 years) in the USA [13]. The cohort is followed biennially using validated questionnaires since inception to update participants’ lifestyle and dietary variables, medical information and incident diseases. The response rate for each follow-up cycle exceeds 90%. The NHS II protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health (Protocol number: 2009-P-002375), and those of participating registries as required. Returning completed questionnaires indicates informed consent.
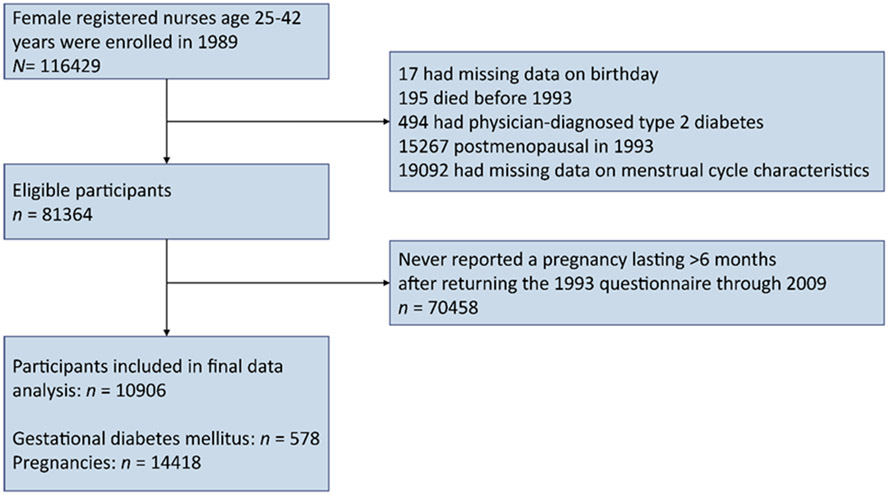
We excluded the women who had missing data on birthday or menstrual cycle characteristics, or those who had received a diagnosis of type 2 diabetes, reached menopause, or had died by 1993 (Fig. 1). Additionally, NHS II participants were eligible for inclusion in the current study if they reported at least one pregnancy lasting >6 months after returning the 1993 questionnaire when cycle characteristics during mid-adulthood were collected. The end of the follow-up was up to the return of the 2009 questionnaire when most participants had passed reproductive age. Finally, 10 906 premenopausal women with 14 418 pregnancies (7832 women had one, 2553 had two, and 521 had three or more pregnancies) were included for our current analysis. Participants’ age-adjusted characteristics in 1993 were similar between included women and those excluded because of incomplete data on menstrual cycle characteristics (electronic supplementary material [ESM] Table 1).
Fig. 1.
Flow chart for study population
Menstrual cycle characteristics
In the 1989 questionnaire, participants reported the usual regularity (age 14-17 and 18-22 years) and length (age 18-22 years) of their menstrual cycles, excluding periods of pregnancy or when using oral contraceptives (OC) [14]. Similarly, in 1993 participants reported their current menstrual cycle regularity and length (then age 29-46 years) [14]. Cycle length was reported as ‘≤21 days’, ‘21-25 days’, ‘26-31 days’, ‘32-39 days’, ‘40-50 days’ or ‘>50 days or too irregular to estimate’. For cycle regularity, questionnaire choices included ‘very regular (±3 days)’, ‘regular’, ‘usually irregular’, ‘always irregular’ and ‘no periods’. Given that OC use affects cycle characteristics, and that OC is often used to treat ovulation disorders [15], we considered women who used OC for more than 2 months per year as a separate exposure group. The reliability of self-reported menstrual cycle questions has been validated previously in other studies and a subgroup of NHS II participants (n=26 421) [7, 16]. Among women who reported regular cycles, the majority of women (84.3%) also reported a normal cycle length of 26-31 days; only 0.6% reported extreme cycle length (< 21 days, ≥40 days, or too irregular to estimate) [8]. Likewise, among women who always had irregular cycles, 62.2% reported an extreme cycle length and only 10.3% reported a normal cycle length [8].
GDM ascertainment
Women reported incident GDM diagnosis through the biennial questionnaires up to 2003. GDM diagnosed between 2004 and 2009 was ascertained through a 2009 pregnancy questionnaire, which collected retrospective information on all previous pregnancies, including the order and years of births and pregnancy complications. During the period of our current analysis, the National Diabetes Data Group criteria were widely used by physicians for GDM diagnosis. In a validation study conducted among 114 participants from this cohort, 94% of the self-reported GDM events were confirmed by medical records [17]. In another random subgroup of parous women in this cohort who were free of GDM (n=100), 100% of responders underwent frequent prenatal urine screenings during pregnancy and 83% underwent a glucose screening test [17], indicating a high degree of GDM surveillance.
Covariates
Participants reported the date of birth, height, body weight at age 18, and ethnicity at recruitment. Current body weight, smoking status, OC use, menopausal status, gravidity, infertility history and family history of diabetes were obtained at baseline and then updated biennially. We calculated BMI (kg/m2) at age 18 and during each follow-up cycle. Physical activity was ascertained in 1997, 2001 and 2005 [18]; we calculated the total hours per week spent on moderate-to-vigorous activities before pregnancy (e.g., brisk walking, bicycling, swimming, racquetball, jogging, running and tennis). Dietary intake and alcohol consumption was ascertained every 4 years using a semi-quantitative food frequency questionnaire [19]. The overall dietary quality before pregnancy was assessed by calculating a summary diet score based on the Alternate Healthy Eating Index (AHEI) [20]. The reliability of self-reported body weight, smoking habit, physical activity and diet in this cohort has been validated in previous studies [18, 21, 22].
Patient and public involvement statement
This research was done without patient involvement. Patients and the public were not invited to comment on the study design and were not consulted to develop patient-relevant outcomes or interpret the results. Patients and the public were not invited to contribute to the writing or editing of this document for readability or accuracy.
Data analysis
Participants’ characteristics at baseline in 1993 were presented according to the categories of menstrual cycle regularity and length determined during mid-adulthood. To account for potential correlations between repeated pregnancies within individuals, multivariable log-binomial models with generalised estimating equations (GEEs) were applied to estimate the RRs and 95% CIs for the associations of cycle regularity and length during adolescence, early adulthood and mid-adulthood with the risk of incident GDM during follow-up. To assess the effect of change in menstrual cycle patterns across the reproductive lifespan, we cross-classified participants according to their menstrual cycle patterns during adolescence or early adulthood and mid-adulthood. Multivariable models were adjusted for current age (continuous), age at menarche (continuous), BMI at age 18 years (continuous), ethnicity (white or others), family history of diabetes (yes or no) and parity (1, 2, 3 or ≥4). In a secondary multivariable model, we further adjusted for time-varying alcohol consumption (0, 0.1-5.0 or ≥5.1 g/day), BMI (<23, 23-24.9, 25-29.9, 30-34.9 or ≥35 kg/m2), physical activity (<150 or ≥150 min/week), smoking status (never, past or current), and AHEI 2010 score (below or above median) during follow-up. Information from the previous biennial cycle was carried forward for missing data (<5% for any covariates); otherwise, a separate missing data category was created.
We evaluated effect modification by performing analyses stratified by BMI at age 18 years and in 1993 (<25 vs ≥25 kg/m2). We also evaluated effect modification by time-varying lifestyle factors (i.e., diet quality, BMI, smoking, physical activity), maternal age, parity and infertility history. Multiplicative interaction was assessed by comparing the multivariable log-binomial models with and without the product term between cycle regularity or length and effect modifiers using the likelihood ratio test [23].
Several sensitivity analyses were conducted to assess the robustness of associations between menstrual cycle characteristics and the risk of GDM. First, we included women in multivariable log-binomial models who provided partial data on menstrual cycle characteristics during adolescence, early adulthood and mid-adulthood to assess the influence of missing data. Second, we reanalysed the associations between menstrual cycle characteristics during adolescence and early adulthood and GDM by including pregnancies reported after the return of the 1989 questionnaire up to the end of 2009 to assess potential selection bias. Third, we excluded women who reported a GDM diagnosis before 1993, as they might have modified their diet and lifestyle in a way that could influence subsequent GDM risk. Fourth, we excluded women older than 40 years in 1993 to reduce the possibility of misclassifying women who experienced early signs of menopause. Fifth, we excluded women reporting ‘no period’ or ‘>50 days or too irregular to estimate’ from the exposure categories to minimise exposure misclassification. Sixth, we excluded women reporting hirsutism, endometriosis or uterine fibroids to test if our findings were driven by polycystic ovary syndrome or other gynaecological conditions [24]. Seventh, we excluded pregnancies with multiple births (twins or higher). Finally, we restricted our analysis to non-Hispanic white women to explore the potential influence of ethnic or racial minority groups. All analyses were performed with SAS 9.3 for UNIX (SAS Institute, Cary, NC, USA). All tests were two-sided and the p-value threshold for a statistical significance was 0.05.
Results
Participants’ age-adjusted characteristics according to menstrual cycle regularity and length in 1993 are presented in Table 1. The maternal mean age at pregnancy was 30.80 ± 5.15 years. Among women who did not use OC (8355), 384 (4.6%) reported that their current menstrual cycles were always irregular or they had no periods; 1851 (22.2%) reported that their current cycle length was ≥32 days or too irregular to estimate (Table 1). Compared with women reporting very regular cycles, women who reported they always experienced irregular menstrual cycles or no periods had apparently higher baseline BMI (26.2±6.9 vs 23.5±4.3 kg/m2) and greater prevalence of family history of diabetes (26.8 vs 19.3%). Similar results were observed among women who reported that their usual cycle length was ≥32 days or too irregular to estimate compared with women reporting a normal cycle length (26-31 days).
Table 1.
Age-standardised baseline characteristics of the study population according to menstrual cycle regularity in mid-adulthood (age 29–46 years) among 10,906 premenopausal women who contributed 14,418 pregnancies from NHS II
| Characteristic | Menstrual cycle regularitya | Menstrual cycle lengtha | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Very regular (n=5400) |
Regular (n=2061) |
Usually irregular (n=510) |
Always irregular or no periods (n=384) |
<26 days (n=738) |
26–31 days (n=5766) |
≥32 days or too irregular to estimate (n=1851) |
|||
| Age at menarche, years | 12.5 (1.4) | 12.7 (1.5) | 12.8 (1.7) | 12.9 (1.8) | 12.4 (1.4) | 12.5 (1.4) | 12.8 (1.6) | ||
| BMI at age 18 years, kg/m2 | 21 (2.9) | 20.9 (2.9) | 21.3 (3.5) | 21.5 (3.6) | 21.1 (3.3) | 20.9 (2.9) | 21.1 (3.1) | ||
| Current age, yearsb | 33.9 (3.1) | 33.6 (3.1) | 33.1 (2.9) | 33.2 (3) | 34.2 (3.2) | 33.8 (3.1) | 33.3 (3) | ||
| Current BMI, kg/m2 | 23.5 (4.3) | 23.6 (4.5) | 24.3 (5.1) | 26.2 (6.9) | 23.7 (4.7) | 23.5 (4.3) | 24.4 (5.3) | ||
| White, % | 93.1 | 92.7 | 87.8 | 93.4 | 91.4 | 93.2 | 91.8 | ||
| Family history of diabetes, % | 19.3 | 21.1 | 23.6 | 26.8 | 19.7 | 20.3 | 21.1 | ||
| Ever or currently married, % | 94.1 | 93.3 | 94.8 | 95.6 | 91.1 | 94.0 | 95.1 | ||
| Parity | 1.3 (1.2) | 1.3 (1.2) | 1.2 (1) | 1.2 (1.2) | 1.1 (1.1) | 1.2 (1.2) | 1.3 (1.2) | ||
| Alcohol consumption, g/day | 3.1 (5.3) | 3 (5.3) | 2.4 (3.7) | 2.7 (5.1) | 3.2 (6) | 3.1 (5.3) | 2.7 (4.6) | ||
| Never smoked, % | 28.7 | 28.8 | 22.7 | 26.5 | 31.4 | 28.7 | 25.5 | ||
| Total energy intake, kJ/day | 7683.9 (2299.5) | 7752.1 (2356.8) | 7591.0 (2198.3) | 7739.1 (2270.6) | 7539.6 (2317.9) | 7703.6 (2317.1) | 7744.6 (2274.0) | ||
| AHEI score | 48.3 (10.7) | 47.5 (11) | 47.5 (10.5) | 47.7 (11.1) | 48.6 (11) | 48.1 (10.8) | 47.5 (10.7) | ||
| Physical activity, h/week | 3 (4.4) | 2.9 (4.5) | 2.5 (4) | 2.7 (5) | 3 (4.6) | 3 (4.5) | 2.7 (4.2) | ||
Values are means (SD) for continuous variables and percentages for categorical variables and are standardised to the age distribution of the study population
Age-standardised characteristics of OC users are not shown (n=2551)
Value is not age adjusted
We documented 578 incident cases of GDM among 14 418 pregnancies during 16 years of follow-up. Menstrual cycle characteristics during adolescence and early adulthood were not associated with GDM. However, women reporting that their menstrual cycles were always irregular during mid-adulthood showed 112% (95% CI 56, 190%) higher risk of GDM than the women reporting very regular cycles (Fig. 2). The association was attenuated but remained after further adjustment for time-varying diet, alcohol consumption, smoking status and physical activity during follow-up (RR 1.65 [95% CI 1.21, 2.25]). The risk of GDM was also greater among women who reported a long cycle length (≥32 days) during mid-adulthood compared with women with a cycle length of 26-31 days (Fig. 2). In the fully adjusted models, women who reported long cycle length had 42% (95% CI 15, 75%) higher risk of GDM, compared with women reporting a normal cycle length. There was no evidence of interaction between cycle length and regularity on the risk of GDM (Table 2).
Fig. 2.

Adjusted RRs (95% CI) for incidence of GDM according to menstrual cycle regularity and length during adolescence (age 14–17 years), early adulthood (age 18–22 years) and mid-adulthood (age 29–46 years) among 10,906 premenopausal women who contributed 14,418 pregnancies from the NHS II (1993–2009). NA: not applicable. aMultivariable model was adjusted for age (continuous), age at menarche (continuous), BMI at age 18 years (continuous), ethnicity (white or others), family history of diabetes (yes or no) and parity (1, 2, 3 or ≥4). bBased on multivariable model with additional adjustment for time-varying alcohol consumption (0, 0.1–5.0 or ≥5.1 g/day), BMI (<23, 23–24.9, 25–29.9, 30–34.9 or ≥35 kg/m2), physical activity (<150 or ≥150 min/week), smoking status (never, past or current) and AHEI 2010 score (below or above median) during follow-up.
Table 2.
Adjusted RRs (95% CI) for incidence of GDM according to joint categories of menstrual cycle regularity and length in early adulthood (age 18–22 years) and mid-adulthood (age 29–46 years) (NHS II, 1993–2009)
| Cycle regularity | Cycle length | GDM/pregnancies | RRs (95% CI) | |||
|---|---|---|---|---|---|---|
| Multivariable adjusteda | Further adjustment for lifestyle factorsb |
|||||
| 18–22 years | ||||||
| OC users | OC users | 313/7383 | 1.18 (0.98, 1.42) | 1.17 (0.97, 1.41) | ||
| Very regular or regular | <32 days | 168/4438 | 1.00 (reference) | 1.00 (reference) | ||
| ≥32 days | 18/657 | 0.72 (0.45, 1.17) | 0.74 (0.46, 1.20) | |||
| Irregular or no cycles | <32 days | 17/553 | 0.80 (0.49, 1.30) | 0.87 (0.53, 1.42) | ||
| ≥32 days | 62/1387 | 1.15 (0.87, 1.53) | 1.14 (0.86, 1.51) | |||
| p for interactionc | 0.06 | 0.13 | ||||
| 29–46 years | ||||||
| OC users | OC users | 151/3521 | 1.23 (1.01, 1.50) | 1.24 (1.02, 1.50) | ||
| Very regular or regular | <32 days | 290/8256 | 1.00 (reference) | 1.00 (reference) | ||
| ≥32 days | 72/1452 | 1.52 (1.18, 1.95) | 1.45 (1.13, 1.87) | |||
| Irregular or no cycles | <32 days | 13/205 | 1.69 (0.98, 2.91) | 1.50 (0.87, 2.57) | ||
| ≥32 days | 52/984 | 1.51 (1.14, 2.02) | 1.30 (0.98, 1.73) | |||
| p for interactionc | 0.12 | 0.11 | ||||
Multivariable model was adjusted for age (continuous), age at menarche (continuous), BMI at age 18 years (continuous), ethnicity (white or others), family history of diabetes (yes or no) and parity (1, 2, 3 or ≥4)
Based on multivariable model with additional adjustment for time-varying alcohol consumption (0, 0.1–5.0 or ≥5.1 g/day), BMI (<23, 23–24.9, 25–29.9, 30–34.9 or ≥35 kg/m2), physical activity (<150 or ≥150 min/week), smoking status (never, past or current) and AHEI 2010 score (below or above median) during follow-up
p for interaction as tested by excluding women reporting OC use
We then cross-classified women according to their menstrual cycle characteristics at different age ranges across their reproductive life (Table 3). Women who reported a usual cycle length shorter than 32 days in early adulthood but longer in mid-adulthood were more than twice as likely to experience GDM than women who maintained short cycle length (RR 1.98 [95% CI 1.34, 2.92]). A similar pattern was observed for women whose cycles changed from regular early in their reproductive years to irregular in mid-adulthood (Table 3).
Table 3.
Adjusted RRs (95% CI) for incidence of GDM according to changes in menstrual cycle characteristics among 10,906 premenopausal women who contributed 14,418 pregnancies from the NHS II (1993–2009)
| Changes in menstrual cycle characteristics | GDM/pregnancies | RRs (95% CI) | |||
|---|---|---|---|---|---|
| Multivariable modelsa | Final models adjusted for lifestyle factorsb |
||||
| Change in regularity from age 14–17 to 29–46 years | |||||
| Maintaining regular | 234/6493 | 1.00 (reference) | 1.00 (reference) | ||
| Regular to irregular | 18/268 | 1.70 (1.07, 2.72) | 1.43 (0.89, 2.29) | ||
| Irregular to regular | 83/2287 | 1.02 (0.79, 1.30) | 1.02 (0.80, 1.31) | ||
| Irregular maintained | 38/787 | 1.36 (0.98, 1.89) | 1.21 (0.87, 1.67) | ||
| OC user | 205/4583 | 1.24 (1.03, 1.49) | 1.21 (1.01, 1.46) | ||
| Change in regularity from age 18–22 to 29–46 years | |||||
| Maintaining regular | 142/3912 | 1.00 (reference) | 1.00 (reference) | ||
| Regular to irregular | 12/145 | 1.96 (1.10, 3.49) | 1.68 (0.93, 3.01) | ||
| Irregular to regular | 37/1104 | 0.92 (0.64, 1.31) | 0.94 (0.66, 1.35) | ||
| Irregular maintained | 22/445 | 1.33 (0.86, 2.04) | 1.20 (0.78, 1.83) | ||
| OC user | 365/8812 | 1.18 (0.98, 1.43) | 1.17 (0.96, 1.42) | ||
| Change in length from age 18–22 to 29–46 years | |||||
| Maintaining <32 days | 123/3558 | 1.00 (reference) | 1.00 (reference) | ||
| <32 to ≥32 days | 28/408 | 2.13 (1.44, 3.16) | 1.98 (1.34, 2.92) | ||
| ≥32 to <32 days | 29/774 | 1.08 (0.73, 1.61) | 1.10 (0.73, 1.63) | ||
| ≥32 days maintained | 33/866 | 1.11 (0.77, 1.62) | 1.04 (0.71, 1.50) | ||
| OC user | 365/8812 | 1.26 (1.03, 1.54) | 1.24 (1.01, 1.52) | ||
Multivariable model was adjusted for age (continuous), age at menarche (continuous), BMI at age 18 years (continuous), ethnicity (white or others), family history of diabetes (yes or no) and parity (1, 2, 3 or ≥4)
Based on multivariable model with additional adjustment for time-varying alcohol consumption (0, 0.1–5.0 or ≥5.1 g/day), BMI (<23, 23–24.9, 25–29.9, 30–34.9 or ≥35 kg/m2), physical activity (<150 or ≥150 min/week), smoking status (never, past or current) and AHEI 2010 score (below or above median) during follow-up
The associations between menstrual cycle characteristics during mid-adulthood and GDM risk across strata of BMI at age 18 years and 1993 are depicted in Table 4. There was no evidence of any significant differences in these relations across strata of BMI. Similarly, we found no evidence that the relations between cycle characteristics and GDM differed according to time-varying BMI, diet quality, smoking, physical activity, age, parity or infertility history (ESM Table 2).
Table 4.
Adjusted RRs (95% CI) for incidence of GDM in relation to irregular and long menstrual cycles in mid-adulthood (age 29–46 years), stratified by BMI at age 18 years and in 1993 (NHS II, 1993–2009)
| BMI at age 18 yearsa (RRs 95% CI) | BMI in 1993b (RRs 95% CI) | |||||
|---|---|---|---|---|---|---|
| <25 kg/m2 (GDM=502) | ≥25 kg/m2 (GDM=76) |
<25 kg/m2 (GDM=305) |
≥25 kg/m2 (GDM=273) |
|||
| Regularity | ||||||
| OC user | 1.09 (0.88, 1.35) | 1.60 (0.93, 2.75) | 1.18 (0.90, 1.54) | 1.20 (0.89, 1.62) | ||
| Very regular | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||
| Regular | 1.01 (0.80, 1.28) | 0.99 (0.47, 2.07) | 0.92 (0.67, 1.26) | 1.09 (0.79, 1.51) | ||
| Usually irregular | 0.83 (0.52, 1.32) | 1.30 (0.51, 3.29) | 0.67 (0.34, 1.32) | 1.12 (0.65, 1.91) | ||
| Always irregular/no period | 1.61 (1.13, 2.30) | 1.97 (1.03, 3.76) | 1.70 (1.01, 2.86) | 1.84 (1.25, 2.70) | ||
| p for interactionc | 0.19 | 0.29 | ||||
| Length (days) | ||||||
| OC users | 1.18 (0.95, 1.46) | 1.85 (1.06, 3.24) | 1.37 (1.05, 1.80) | 1.21 (0.90, 1.63) | ||
| ≤25 | 1.30 (0.94, 1.80) | 1.67 (0.69, 4.06) | 1.52 (1.02, 2.28) | 1.18 (0.75, 1.88) | ||
| 26–31 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||
| ≥32 or too irregular to estimate | 1.37 (1.09, 1.72) | 1.91 (1.08, 3.35) | 1.52 (1.11, 2.07) | 1.36 (1.02, 1.81) | ||
| p for interactionc | 0.41 | 0.86 | ||||
Multivariable model was adjusted for age (continuous), age at menarche (continuous), ethnicity (white or others), family history of diabetes (yes or no) and parity (1, 2, 3 or ≥4), as well as time-varying alcohol consumption (0, 0.1–5.0 or ≥5.1 g/day), BMI (<23, 23–24.9, 25–29.9, 30–34.9 or ≥35 kg/m2), physical activity (<150 or ≥150 min/week), smoking status (never, past or current) and AHEI 2010 score (below or above median) during follow-up
Multivariable model was adjusted for age (continuous), age at menarche (continuous), BMI at age 18 years (continuous), ethnicity (white or others), family history of diabetes (yes or no) and parity (1, 2, 3 or ≥4), as well as time-varying alcohol consumption (0, 0.1–5.0 or ≥5.1 g/day), physical activity (<150 or ≥150 min/week), smoking status (never, past or current) and AHEI 2010 score (below or above median) during follow-up
p for interaction as tested by excluding women reporting OC use
Last, we conducted a series of sensitivity analyses to evaluate the robustness of the associations between menstrual cycle characteristics and the risk of GDM. The findings were similar when we included women who provided partial data on menstrual cycle characteristics during adolescence, early adulthood and mid-adulthood (ESM Table 3), when we included pregnancies reported before 1993 (ESM Table 4), and when we excluded women reporting a GDM diagnosis before 1993 (ESM Table 5). The associations of irregular and long menstrual cycle during mid-adulthood with the risk of GDM also persisted when we excluded women who were older than 40 years in 1993 or those who reported ‘no period’ or ‘>50 days or too irregular to estimate’, when we excluded participants with hirsutism, endometriosis, uterine fibroids or multiple births, and when our analysis was restricted to white women (ESM Table 6).
Discussion
Results from this large prospective cohort revealed that pre-pregnancy irregular and long menstrual cycles during mid-adulthood were associated with a greater risk of GDM, especially for women who converted from short or regular cycles in adolescence or young adulthood to long or irregular patterns in mid-adulthood. These relations were independent of the BMI determined across the reproductive lifespan, as well as other well known risk factors for GDM such as advanced maternal age, greater parity and unhealthy lifestyles. Cycle characteristics in adolescence and early adulthood were not associated with GDM.
Irregular and long menstrual cycles have been associated with a greater risk of insulin resistance and type 2 diabetes [8-10]. Polycystic ovary syndrome, a condition commonly characterised by menstrual dysfunction including long or irregular cycles, was also associated with an increased prevalence of GDM [25]. Evidence linking menstrual cycle characteristics and GDM, however, is scant and inconsistent. In a small case–control study conducted among 170 American women, Haver and colleagues reported that irregular menstrual cycle was more prevalent among women with GDM than the comparison group (24% vs 7%) [11]. In a recent prospective cohort conducted among 3490 Swedish women, Dishi and colleagues reported that irregular cycle was unrelated to GDM [12]; instead, they found a greater risk of GDM among women reporting long menstrual cycles (>36 days) compared with women reporting cycles between 25 and 30 days (OR 1.6 [95% CI 0.98, 2.67]). Inferences of findings from these studies were hindered by several potential methodological limitations. Our study was able to improve upon these studies as a result of our larger sample size, prospective design, finer characterisation of menstrual cycle characteristics at multiple time points, and detailed data on important covariates (e.g., OC use, BMI and lifestyle factors).
Our findings extend and refine the existing evidence in this area. Because OCs affect cycle characteristics and are often used to treat women presenting with menstrual cycle disorders, it is important to eliminate the ‘noise’ of OC use at the time of menstrual cycle characteristic assessment. In this study, we categorised women who used OCs as a separate exposure group, which allowed us to obtain estimates that were independent of OC use. Besides, while it is abundantly clear that obesity, unhealthy lifestyles, advanced age and greater parity are important risk factors for GDM [26], no studies have evaluated whether the association between menstrual cycle dysfunction and GDM was modified by these risk factors. The absence of effect modification by BMI, unhealthy lifestyles, advanced age, greater parity and infertility history suggests that menstrual cycle characteristics might be independent risk factors for GDM. We also noted that the relations of long and irregular menstrual cycles with greater GDM risk persisted when we excluded women with hirsutism, endometriosis or uterine fibroids, indicating that these relations were not solely driven by polycystic ovary syndrome or other common gynaecologic conditions. Finally, in contrast to previous studies that retrospectively assessed menstrual cycle characteristics at one point in time, we collected cycle characteristics at three different time points across women’s reproductive lifespan. Interestingly, we found that the risk of GDM was greater among women who converted from initial short or regular cycles to long or irregular patterns compared with women maintaining short or regular cycles across their reproductive lifespan. These findings suggested that the transition from healthy to unhealthy cycle phenotypes might be a surrogate of metabolic changes (e.g., insulin resistance) that play a critical role in the development of GDM.
Irregular and long menstrual cycles could be indicators of unfavourable hormonal and metabolic phenotypes that have been implicated in the aetiology of GDM. The disrupted hormonal environment is hypothesised to play a critical role in the association between menstrual cycle dysfunction and incident GDM. Irregular and long menstrual cycles are strongly associated with hyperinsulinaemia [27], which can inhibit the production of sex hormone-binding globulin and consequently higher levels of free testosterone [28], both of which are known as risk factors for GDM and type 2 diabetes [29-32]. Besides the disrupted hormonal environment, irregular and long menstrual cycles have been associated with underlying lipid metabolism and metabolic disorders (e.g., insulin resistance) [33-35], which may also be involved in the development of GDM [36]. Previous studies have documented that women with polycystic ovary syndrome, for whom ovarian dysfunction – including long or irregular cycles – and excess androgens are distinctive clinical features, have hyperinsulinism, insulin resistance, and lipid metabolic disorders [37, 38].
The strengths of this study include its large sample size, prospective design with a long-term follow-up, a high response rate of each follow-up cycle, availability of menstrual cycle characteristics across the reproductive lifespan, and comprehensive measurements of important confounding factors. Our study also has some limitations. First, measurement error in self-reported menstrual cycle characteristics is inevitable, though previous studies have documented the validity of self-reported menstrual cycle characteristics [7, 16]. In this case, however, exposure misclassification is suspected to be non-differential with respect to incident GDM, potentially biasing estimations towards the null. Second, incident GDM diagnosis was self-reported through the biennial questionnaires. However, a high degree of accuracy of self-reported GDM against medical record review has been confirmed among a subgroup of participants from this cohort [17]. Further, the overall rate of GDM in this cohort (5.6%) fell within the range of the estimated GDM prevalence in the USA during a similar period (3-6%) [3]. Third, we used the National Diabetes Data Group criteria for GDM diagnosis, which may have resulted in a reduced risk estimation given that more women with mild hyperglycaemia would be diagnosed with GDM based on the Carpenter and Coustan criteria or the International Association of Diabetes and Pregnancy Study Group approach [39]. Fourth, our study participants were mostly white (>95%) and shared a common profession and educational qualification, which may restrict the generalisability of our results. Therefore, further studies involving women of other ethnicity or race with more diverse socioeconomic status are warranted to verify our findings. Finally, although we accounted for various potential confounders (e.g., demographic and reproductive characteristics, BMI at age 18 years and lifestyle factors), residual confounding from unadjusted covariates such as diet quality during pregnancy cannot be fully ruled out.
In conclusion, based on this large prospective cohort, women whose cycles were long (≥32 days) or irregular during mid-adulthood were at an elevated risk of GDM. These relations appeared to be independent of other commonly recognised risk factors for GDM. Our results suggest that menstrual cycle characteristics before pregnancy may serve as early markers for subsequent risk of GDM.
Supplementary Material
Research in context.
What is already known about this subject?
Irregular and long menstrual cycles are common endocrine disorders among women of reproductive age and have been associated with many endocrine-related diseases
However, evidence linking irregular or long menstrual cycles with gestational diabetes mellitus (GDM) is scarce and inconsistent
What is the key question?
Are pre-pregnancy menstrual cycle regularity and length during adolescence, early adulthood and mid-adulthood associated with subsequent risk of GDM?
What are the new findings?
Irregular and long menstrual cycles during mid-adulthood were associated with a greater risk of GDM in later life
These relations were independent of the BMI determined across the reproductive lifespan, as well as other well known risk factors for GDM
How might this impact on clinical practice in the foreseeable future?
Our results suggest that menstrual cycle characteristics before pregnancy may serve as early markers for subsequent risk of GDM
Acknowledgements
We would like to thank the participants and staff of the NHS II for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
Funding
This study was supported by grants U01-HL145386, U01-CA176726, R01-HL034594 and R01-HL088521 from the National Institutes of Health. CLZ is supported by the intramural research programme of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Abbreviations
- AHEI
Alternate Healthy Eating Index
- GDM
Gestational diabetes mellitus
- NHS
Nurses’ Health Study
- OC
Oral contraceptive
Data availability
Data described in the manuscript, code book and analytic code will not be made publicly available. Further information including the procedures for obtaining and accessing data from NHS II is described at https://www.nurseshealthstudy.org/researchers (nhsaccess@channing.harvard.edu). Questionnaires are publicly available at: https://nurseshealthstudy.org/participants/questionnaires.
References
- [1].Buchanan TA, Xiang A, Kjos SL, Watanabe R (2007) What is gestational diabetes? Diabetes Care 30 Suppl 2: S105–111. 10.2337/dc07-s201 [DOI] [PubMed] [Google Scholar]
- [2].Zhu Y, Zhang C (2016) Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Current diabetes reports 16(1): 7. 10.1007/s11892-015-0699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV (2017) Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG 124(5): 804–813. 10.1111/1471-0528.14236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Casagrande SS, Linder B, Cowie CC (2018) Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes research and clinical practice 141: 200–208. 10.1016/j.diabres.2018.05.010 [DOI] [PubMed] [Google Scholar]
- [5].Agha-Jaffar R, Oliver N, Johnston D, Robinson S (2016) Gestational diabetes mellitus: does an effective prevention strategy exist? Nat Rev Endocrinol 12(9): 533–546. 10.1038/nrendo.2016.88 [DOI] [PubMed] [Google Scholar]
- [6].Matteson KA, Zaluski KM (2019) Menstrual Health as a Part of Preventive Health Care. Obstet Gynecol Clin North Am 46(3): 441–453. 10.1016/j.ogc.2019.04.004 [DOI] [PubMed] [Google Scholar]
- [7].Real FG, Svanes C, Omenaas ER, et al. (2007) Menstrual irregularity and asthma and lung function. The Journal of allergy and clinical immunology 120(3): 557–564. 10.1016/j.jaci.2007.04.041 [DOI] [PubMed] [Google Scholar]
- [8].Wang YX, Shan Z, Arvizu M, et al. (2020) Associations of Menstrual Cycle Characteristics Across the Reproductive Life Span and Lifestyle Factors With Risk of Type 2 Diabetes. JAMA Netw Open 3(12): e2027928. 10.1001/jamanetworkopen.2020.27928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Solomon CG, Hu FB, Dunaif A, et al. (2001) Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. Jama 286(19): 2421–2426. 10.1001/jama.286.19.2421 [DOI] [PubMed] [Google Scholar]
- [10].Escobar-Morreale HF (2014) Reproductive endocrinology: Menstrual dysfunction--a proxy for insulin resistance in PCOS? Nature reviews Endocrinology 10(1): 10–11. 10.1038/nrendo.2013.232 [DOI] [PubMed] [Google Scholar]
- [11].Haver MC, Locksmith GJ, Emmet E (2003) Irregular menses: an independent risk factor for gestational diabetes mellitus. American journal of obstetrics and gynecology 188(5): 1189–1191. 10.1067/mob.2003.311 [DOI] [PubMed] [Google Scholar]
- [12].Dishi M, Enquobahrie DA, Abetew DF, Qiu C, Rudra CB, Williams MA (2011) Age at menarche, menstrual cycle characteristics and risk of gestational diabetes. Diabetes research and clinical practice 93(3): 437–442. 10.1016/j.diabres.2011.07.001 [DOI] [PubMed] [Google Scholar]
- [13].Bao Y, Bertoia ML, Lenart EB, et al. (2016) Origin, Methods, and Evolution of the Three Nurses' Health Studies. Am J Public Health 106(9): 1573–1581. 10.2105/AJPH.2016.303338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang YX, Arvizu M, Rich-Edwards JW, et al. (2020) Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. Bmj 371: m3464. 10.1136/bmj.m3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Al Khalifah RA, Florez ID, Dennis B, Thabane L, Bassilious E (2016) Metformin or Oral Contraceptives for Adolescents With Polycystic Ovarian Syndrome: A Meta-analysis. Pediatrics 137(5). 10.1542/peds.2015-4089 [DOI] [PubMed] [Google Scholar]
- [16].Jukic AM, Weinberg CR, Wilcox AJ, McConnaughey DR, Hornsby P, Baird DD (2008) Accuracy of reporting of menstrual cycle length. American journal of epidemiology 167(1): 25–33. 10.1093/aje/kwm265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Solomon CG, Willett WC, Carey VJ, et al. (1997) A prospective study of pregravid determinants of gestational diabetes mellitus. Jama 278(13): 1078–1083 [PubMed] [Google Scholar]
- [18].Wolf AM, Hunter DJ, Colditz GA, et al. (1994) Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 23(5): 991–999. 10.1093/ije/23.5.991 [DOI] [PubMed] [Google Scholar]
- [19].Willett WC, Sampson L, Browne ML, et al. (1988) The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 127(1): 188–199. 10.1093/oxfordjournals.aje.a114780 [DOI] [PubMed] [Google Scholar]
- [20].Chiuve SE, Fung TT, Rimm EB, et al. (2012) Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 142(6): 1009–1018. 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC (1990) Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1(6): 466–473 [DOI] [PubMed] [Google Scholar]
- [22].Yuan C, Spiegelman D, Rimm EB, et al. (2018) Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared With Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am J Epidemiol 187(5): 1051–1063. 10.1093/aje/kwx328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang YX, Arvizu M, Rich-Edwards JW, et al. (2021) Hypertensive Disorders of Pregnancy and Subsequent Risk of Premature Mortality. Journal of the American College of Cardiology 77(10): 1302–1312. 10.1016/j.jacc.2021.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pasch L, He SY, Huddleston H, et al. (2016) Clinician vs Self-ratings of Hirsutism in Patients With Polycystic Ovarian Syndrome: Associations With Quality of Life and Depression. JAMA Dermatol 152(7): 783–788. 10.1001/jamadermatol.2016.0358 [DOI] [PubMed] [Google Scholar]
- [25].Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC (2015) Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 21(5): 575–592. 10.1093/humupd/dmv029 [DOI] [PubMed] [Google Scholar]
- [26].Zhang C, Tobias DK, Chavarro JE, et al. (2014) Adherence to healthy lifestyle and risk of gestational diabetes mellitus: prospective cohort study. BMJ 349: g5450. 10.1136/bmj.g5450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dunaif A (1997) Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18(6): 774–800. 10.1210/edrv.18.6.0318 [DOI] [PubMed] [Google Scholar]
- [28].Plymate SR, Matej LA, Jones RE, Friedl KE (1988) Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 67(3): 460–464. 10.1210/jcem-67-3-460 [DOI] [PubMed] [Google Scholar]
- [29].Rasmussen JJ, Selmer C, Frossing S, et al. (2020) Endogenous Testosterone Levels Are Associated with Risk of Type 2 Diabetes in Women without Established Comorbidity. Journal of the Endocrine Society 4(6): bvaa050. 10.1210/jendso/bvaa050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Muka T, Nano J, Jaspers L, et al. (2017) Associations of Steroid Sex Hormones and Sex Hormone-Binding Globulin With the Risk of Type 2 Diabetes in Women: A Population-Based Cohort Study and Meta-analysis. Diabetes 66(3): 577–586. 10.2337/db16-0473 [DOI] [PubMed] [Google Scholar]
- [31].Thadhani R, Wolf M, Hsu-Blatman K, Sandler L, Nathan D, Ecker JL (2003) First-trimester sex hormone binding globulin and subsequent gestational diabetes mellitus. American journal of obstetrics and gynecology 189(1): 171–176. 10.1067/mob.2003.343 [DOI] [PubMed] [Google Scholar]
- [32].Li MY, Rawal S, Hinkle SN, et al. (2020) Sex Hormone-binding Globulin, Cardiometabolic Biomarkers, and Gestational Diabetes: A Longitudinal Study and Meta-analysis. Maternal-fetal medicine 2(1): 2–9. 10.1097/FM9.0000000000000037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rubba F, Mattiello A, Chiodini P, et al. (2008) Menstrual cycle length, serum lipids and lipoproteins in a cohort of Italian Mediterranean women: findings from Progetto ATENA. Nutr Metab Cardiovasc Dis 18(10): 659–663. 10.1016/j.numecd.2007.12.004 [DOI] [PubMed] [Google Scholar]
- [34].Bouzas IC, Cader SA, Leao L, Kuschnir MC, Braga C (2014) Menstrual cycle alterations during adolescence: early expression of metabolic syndrome and polycystic ovary syndrome. J Pediatr Adolesc Gynecol 27(6): 335–341. 10.1016/j.jpag.2014.01.002 [DOI] [PubMed] [Google Scholar]
- [35].Rostami Dovom M, Ramezani Tehrani F, Djalalinia S, Cheraghi L, Behboudi Gandavani S, Azizi F (2016) Menstrual Cycle Irregularity and Metabolic Disorders: A Population-Based Prospective Study. PLoS One 11(12): e0168402. 10.1371/journal.pone.0168402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF (2015) Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG 122(5): 643–651. 10.1111/1471-0528.13261 [DOI] [PubMed] [Google Scholar]
- [37].Polak K, Czyzyk A, Simoncini T, Meczekalski B (2017) New markers of insulin resistance in polycystic ovary syndrome. Journal of endocrinological investigation 40(1): 1–8. 10.1007/s40618-016-0523-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Amato MC, Vesco R, Vigneri E, Ciresi A, Giordano C (2015) Hyperinsulinism and polycystic ovary syndrome (PCOS): role of insulin clearance. Journal of endocrinological investigation 38(12): 1319–1326. 10.1007/s40618-015-0372-x [DOI] [PubMed] [Google Scholar]
- [39].American Diabetes Association (2013) Diagnosis and classification of diabetes mellitus. Diabetes Care 36 Suppl 1: S67–74. 10.2337/dc13-S067 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book and analytic code will not be made publicly available. Further information including the procedures for obtaining and accessing data from NHS II is described at https://www.nurseshealthstudy.org/researchers (nhsaccess@channing.harvard.edu). Questionnaires are publicly available at: https://nurseshealthstudy.org/participants/questionnaires.