Abstract
Recently, Covid-19 vaccine effectiveness has decreased especially against mild disease due to emergence of the Delta variant and waning protection. In this register-based study among healthcare workers in Finland, the vaccine effectiveness of two-dose mRNA vaccine series against SARS-CoV-2 infection decreased from 82% (95% CI 79–85%) 14–90 days after vaccination to 53% (43–62%) after 6 months. Similar trend was observed for other series. Waning was not observed against Covid-19 hospitalization. These results facilitate decision-making of booster doses for healthcare workers.
Keywords: Vaccine effectiveness, SARS-CoV-2 infection, Waning, Covid-19 hospitalization, Healthcare workers
1. Introduction
Covid-19 vaccines have been highly efficacious [1], [2]. However, their effectiveness has recently decreased, presumably for two reasons. First, the Delta variant, a strain capable of evading vaccine-induced immunity, emerged in spring 2021 [1], [2], [3]. Second, vaccine-induced immunity is waning [2], [3], [4], [5]. Therefore, booster programs have been initiated in many countries [6], [7].
To facilitate decision-making concerning boosters for social and healthcare workers (HCW), we estimated vaccine effectiveness (VE) after the second dose. The objective was to evaluate whether protection declines after two doses of either mRNA or adenovirus vector (AdV) vaccines and after vaccination with first AdV and second mRNA vaccine (heterologous series).
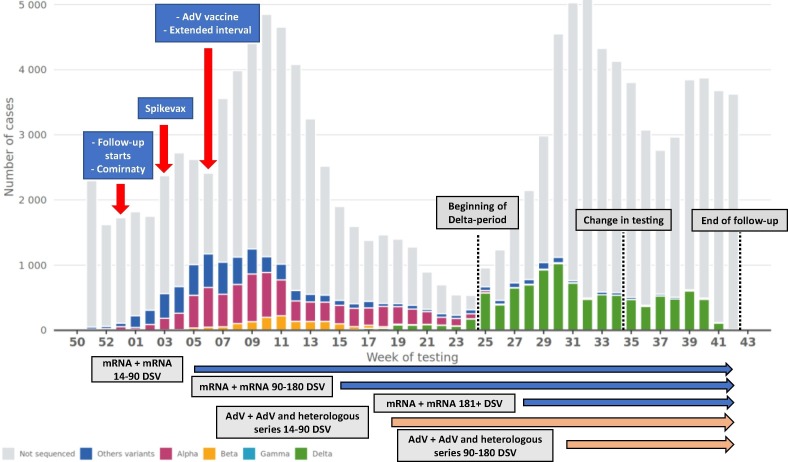
In Finland, the vaccination campaign started 27 December 2020 with vaccination of HCWs caring for Covid-19 patients and of personnel and residents in long-term care units [8]. They were vaccinated with mRNA vaccines (Comirnaty and Spikevax) using standard 3–4 weeks dosing interval through week 5, 2021. Then, simultaneously with introduction of AdV vaccine Vaxzevria, the dosing interval was extended to 12 weeks (Fig. 1 ).
Fig. 1.
Number of laboratory-confirmed SARS-CoV-2 infections by variant if sequenced and calendar week. The horizontal arrows indicate the follow-up periods of two-dose series. Hospital districts ceased testing vaccinated persons with mild Covid-19-related symptoms 26 August 2021. DSV = Days since vaccination, AdV = Adenovirus vector.
2. Material and methods
We conducted a nationwide register-based cohort study to estimate the effectiveness of Covid-19 vaccines among 16–69-year-old HCWs in analogy to our previous study in elderly and chronically ill people [9]. Data of all people licensed to work as HCWs in Finland were extracted from the Social and Healthcare Professionals Register. HCWs included those in non-clinical positions because information on current position was unavailable. HCWs with laboratory-confirmed SARS-CoV-2 infection prior to 27 December 2020 (less than 1% of target population) were excluded.
The exposure was time since latest (first or second) Covid-19 vaccination recorded in the Finnish Vaccination Register. The study outcomes were laboratory-confirmed SARS-CoV-2 infection reported to the National Infectious Diseases Register and Covid-19-related hospitalization reported to the Care Register for Health Care in the week before or the two weeks after laboratory-confirmed SARS-CoV-2 infection [9].
The follow-up started 27 December 2020. Each study subject was considered at risk of each outcome until first occurrence of any of the following events: outcome of interest, death, day 14 after confirmed infection, booster vaccination, or end of study (26 August if outcome was infection, 26 October 2021 otherwise). Using Cox regression, we compared the hazard of each outcome in vaccinated subjects with the corresponding hazard in the unvaccinated. The effect measure was VE, quantified as 1 minus the hazard ratio adjusted for age, sex, presence of medical conditions predisposing to severe Covid-19, and residence in the most affected hospital district Helsinki-Uusimaa (Table 1 ).
Table 1.
Distribution of baseline characteristics and vaccination coverage among healthcare workers (N = 427 905) in Finland, 27 Dec 2020 – 26 Oct 2021. AdV = Adenovirus vector.
|
Number (percentage) of healthcare workers by vaccination status at the end of the study |
||||||||
|---|---|---|---|---|---|---|---|---|
| Age in years | Total | Not vaccinated | AdV vaccine | AdV vaccine + AdV vaccine | AdV vaccine + mRNA vaccine | mRNA vaccine | mRNA vaccine + mRNA vaccine | Other series* |
| 17–32 | 87,238 | 14,746 (17) | 140 (0) | 21 (0) | 3513 (4) | 8297 (10) | 60,486 (69) | 35 (0) |
| 33–41 | 84,638 | 10,937 (13) | 119 (0) | 19 (0) | 3828 (5) | 5515 (7) | 64,182 (76) | 38 (0) |
| 42–50 | 85,861 | 7645 (9) | 107 (0) | 34 (0) | 5087 (6) | 3790 (4) | 69,144 (81) | 54 (0) |
| 51–59 | 86,496 | 5845 (7) | 143 (0) | 40 (0) | 7468 (9) | 2934 (3) | 70,002 (81) | 64 (0) |
| 60–69 | 83,672 | 4276 (5) | 344 (0) | 14,646 (18) | 10,652 (13) | 2071 (2) | 51,599 (62) | 84 (0) |
| Sex | ||||||||
| Male | 59,659 | 5640 (9) | 110 (0) | 1974 (3) | 3996 (7) | 2775 (5) | 45,137 (76) | 27 (0) |
| Female | 368,246 | 37,809 (10) | 743 (0) | 12,786 (3) | 26,552 (7) | 19,832 (5) | 270,276 (73) | 248 (0) |
| Presence of medical conditions predisposing to severe Covid-19** | ||||||||
| No predisposing medical condition | 334,510 | 36,144 (11) | 543 (0) | 8033 (2) | 17,563 (5) | 18,610 (6) | 253,438 (76) | 179 (0) |
| At least one predisposing medical condition | 38,891 | 2638 (7) | 197 (1) | 4236 (11) | 7870 (20) | 1482 (4) | 22,415 (58) | 53 (0) |
| At least one highly predisposing medical condition | 54,504 | 4667 (9) | 113 (0) | 2491 (5) | 5115 (9) | 2515 (5) | 39,560 (73) | 43 (0) |
| In Helsinki-Uusimaa hospital district | ||||||||
| No | 314,689 | 32,089 (10) | 568 (0) | 10,689 (3) | 20,289 (6) | 17,413 (6) | 233,519 (74) | 122 (0) |
| Yes | 113,216 | 11,360 (10) | 285 (0) | 4071 (4) | 10,259 (9) | 5194 (5) | 81,894 (72) | 153 (0) |
| Total cohort | 427,905 | 43,449 (10) | 853 (0) | 14,760 (3) | 30,548 (7) | 22,607 (5) | 315,413 (74) | 275 (0) |
*Series that include at least one vaccination with a Covid-19 vaccine other than Comirnaty, Spikevax and Vaxzevria or with a Covid-19 vaccine that was not properly recorded and could thus not be identified as Comirnaty, Spikevax or Vaxzevria.
**As defined in Supplementary Table 1.
To estimate VE before and after emergence of the Delta variant, we split the follow-up on 21 June 2021 into pre-Delta and Delta period as Delta has accounted for the majority of sequenced cases since then (Fig. 1). We also estimated brand-specific effectiveness of mRNA vaccines excluding those vaccinated first with AdV vaccine.
2.1. Ethical concern
The study was conducted under the Finnish Communicable Disease Act and is part of the Finnish Institute for Health and Welfare surveillance duty to monitor the effectiveness of vaccines [10]. Therefore, the study did not require further ethical review.
3. Results
The cohort included 427,905 HCWs: 291,758 (68%) nurses, 23,886 (6%) physicians, 13,074 (3%) dentists and oral hygienists and 99,187 (23%) other professionals. By the end of follow-up 90% of the HCWs had received at least one vaccination. Two-dose series of mRNA vaccines were received by 315,413 (74%) HCWs. Two-dose AdV vaccine and heterologous series were administered to 14,760 (3%) and 30,548 (7%) HCWs.
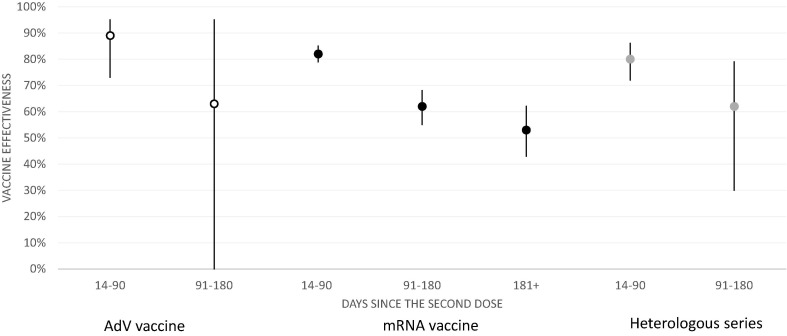
There were 3874 and 1757 laboratory-confirmed SARS-CoV-2 infections in the unvaccinated and vaccinated. VE against infection was 82% (95% confidence interval 79–85%) for mRNA, 89% (73–95%) for AdV and 80% (72–86%) for heterologous vaccine series 14–90 days after the second dose. However, VE appeared to wane over time (Fig. 2 , Supplementary Table 2). VE was 62% (55–68%) for mRNA, 63% (-166–95%) for AdV and 62% (30–79%) for heterologous vaccine series 91–180 days after the second dose.
Fig. 2.
Effectiveness of AdV (white), mRNA (black) and heterologous (grey) vaccine series against laboratory-confirmed SARS-CoV-2 infection among healthcare workers (N = 427 905) in Finland, 27 Dec 2020 – 26 Aug 2021. AdV = Adenovirus vector.
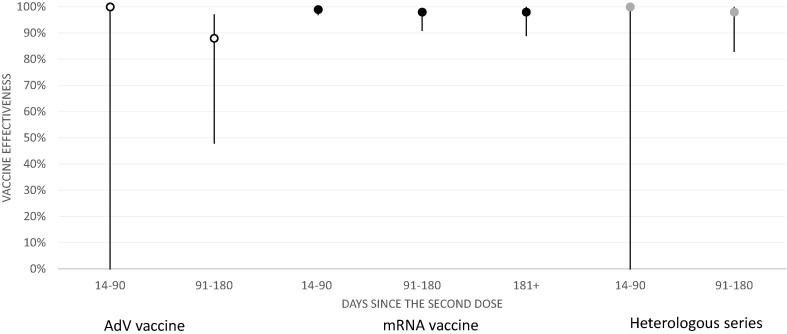
There were 220 and 35 Covid-19-related hospitalizations in the unvaccinated and vaccinated. Effectiveness against hospitalization was 88% or above for all series during the first ten months of the campaign (Fig. 3 , Supplementary Table 3).
Fig. 3.
Effectiveness of AdV (white), mRNA (black) and heterologous (grey) vaccine series against Covid-19-related hospitalization among healthcare workers (N = 427 905) in Finland, 27 Dec 2020 – 26 Oct 2021.
During the Delta period VE was similar to that in the Pre-Delta period (Supplementary Tables 2–3). Brand-specific mRNA VE estimates were also comparable (Supplementary Table 4).
4. Discussion
This study’s aim was to analyze the effectiveness of Covid-19 vaccines in HCWs during the first 10 months of the vaccination campaign in Finland. We observed high early VE against SARS-CoV-2 infection after the second dose with significant waning after 3 months. However, Covid-19 vaccines maintained excellent effectiveness against Covid-19 hospitalization. We found no meaningful differences between mRNA and AdV vaccine series, although current knowledge is that mRNA vaccine provides better protection [2], [11]. As in Denmark and Sweden, we observed similar protection levels after mRNA and heterologous vaccine series [12], [13].
We did not detect changes in VE following the emergence of Delta, indicating that the decrease in VE against infection is due to waning of vaccine-induced immunity, which has also been seen elsewhere [2], [4], [5], [11], [14]. The waning against infection was slightly faster than that observed in a clinical trial of Comirnaty [5] and among HCWs in the USA [14], but comparable to the waning detected among 18–64-year-olds in the UK [2]. Because boosters enhance protection against infection [6], HCWs are likely to benefit from receiving boosters. Additionally, Covid-19 vaccines might also reduce transmission of SARS-CoV-2 [15] and therefore giving boosters to HCWs could indirectly protect patients.
Although in agreement with the literature, our findings may be prone to bias. The HCWs with the longest post-vaccination follow-up are those at high risk of work-related infection, such as intensive care unit nurses. Therefore, our study may underestimate the average VE in HCWs 6 months after the second dose. Furthermore, the HCWs who were vaccinated first received their second dose 3–4 weeks after the first one, while those vaccinated later received their second dose 12 weeks after the first one. Thus, the length of follow-up after receipt of the second dose differs between these groups. As it has been shown that, compared to the standard interval, an extended dosing interval induces greater antibody response and possibly greater VE [16], [17], [18], this could have introduced further underestimation of the average VE 6 months after the second dose.
Strengths of this analysis are the size and representativeness of the cohort and the register-based study design, which has already been evaluated and applied in previous studies [9, [9], [19]. Furthermore, as the threshold for SARS-CoV-2 testing, offered free of charge, was very low in Finland through 26 August 2021, it can be assumed that most of the symptomatic infections that occurred by then were registered, both in the unvaccinated and the vaccinated. Unfortunately, the study data did not include information on the HCWs’ current status and field of employment so it was impossible to assess their risk of work-related infection.
5. Conclusions
We observed waning of Covid-19 VE against SARS-CoV-2 infection three and six months after the second dose, while high VE against hospitalization sustained beyond six months. Boosters may be beneficial for HCWs to enhance protection against infection and to decrease transmission of SARS-CoV-2 to patients. However, level and duration of protection after a booster dose are presently unknown.
6. Authors’ contributions
EP, UB, TL, HN and AAP conceptualized the study. HS and TOL identified the medical conditions predisposing to severe Covid-19. UB conducted the statistical analysis. EP reviewed the literature and drafted the manuscript. All authors gave comments and revised the manuscript.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Arto Palmu reports a relationship with Sanofi Pasteur Inc that includes: funding grants. Arto Palmu reports a relationship with GlaxoSmithKline USA that includes: funding grants. Arto Palmu reports a relationship with Pfizer that includes: funding grants.
Acknowledgements
The authors thank Esa Ruokokoski, Jonas Sundman, Oskari Luomala, Teemu Möttönen and Tuomo Nieminen from the Finnish Institute for Health and Welfare for their assistance in managing the study data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.12.032.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta K-D, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med [Internet]. 2021 Oct 14 [cited 2021 Nov 1]; Available from: https://www.nature.com/articles/s41591-021-01548-7 [DOI] [PMC free article] [PubMed]
- 3.Keehner J., Horton L.E., Binkin N.J., Laurent L.C., Pride D., Longhurst C.A., et al. Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. N Engl J Med. 2021;385(14):1330–1332. doi: 10.1056/NEJMc2112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Alroy-Preis S., et al. Protection Across Age Groups of BNT162b2 Vaccine Booster against Covid-19 [Preprint] Epidemiology. 2021 doi: 10.1056/NEJMoa2114255. http://medrxiv.org/lookup/doi/10.1101/2021.10.07.21264626 Oct [cited 2021 Oct 14]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolmas koronarokoteannos - Infektiotaudit ja rokotukset - THL [Internet]. Terveyden ja hyvinvoinnin laitos. [cited 2021 Nov 1]. Available from: https://thl.fi/fi/web/infektiotaudit-ja-rokotukset/rokotteet-a-o/koronavirusrokotteet-eli-covid-19-rokotteet-ohjeita-ammattilaisille/kolmas-koronarokoteannos. Finnish.
- 8.Vaccination order and at-risk groups for COVID-19 - Infectious diseases and vaccinations - THL [Internet]. Finnish Institute for Health and Welfare (THL), Finland. [cited 2021 Apr 6]. Available from: https://thl.fi/en/web/infectious-diseases-and-vaccinations/what-s-new/coronavirus-covid-19-latest-updates/transmission-and-protection-coronavirus/vaccines-and-coronavirus/vaccination-order-and-at-risk-groups-for-covid-19.
- 9.Baum U., Poukka E., Palmu A.A., Salo H., Lehtonen T.O., Leino T. Effectiveness of vaccination against SARS-CoV-2 infection and Covid-19 hospitalization among Finnish elderly and chronically ill – An interim analysis of a nationwide cohort study [Preprint] Infectious Diseases (except HIV/AIDS) 2021 doi: 10.1371/journal.pone.0258704. http://medrxiv.org/lookup/doi/10.1101/2021.06.21.21258686 Jun [cited 2021 Nov 1]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministry of Social Affairs and Health, Finland. Communicable Diseases Act (1227/2016) [Internet]. Ministry of Social Affairs and Health, Finland; 2016 [cited 2021 Nov 1]. Available from: https://www.finlex.fi/en/laki/kaannokset/2016/en20161227.pdf.
- 11.Andrews N., Tessier E., Stowe J., Gower C., Kirsebom F., Simmons R., et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK [Preprint] Epidemiology. 2021 http://medrxiv.org/lookup/doi/10.1101/2021.09.15.21263583 Sep [cited 2021 Nov 1]. Available from: [Google Scholar]
- 12.Gram MA, Nielsen J, Schelde AB, Nielsen KF, Moustsen-Helms IR, Bjørkholt Sørensen AK, et al. Vaccine effectiveness when combining the ChAdOx1 vaccine as the first dose with an mRNA COVID-19 vaccine as the second dose [Preprint]. Epidemiology; 2021 Jul [cited 2021 Nov 1]. Available from: http://medrxiv.org/lookup/doi/10.1101/2021.07.26.21261130.
- 13.Nordström P., Ballin M., Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: A nationwide cohort study. The Lancet Regional Health - Europe. 2021;11:100249. doi: 10.1016/j.lanepe.2021.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilishvili T., Gierke R., Fleming-Dutra K.E., Farrar J.L., Mohr N.M., Talan D.A., et al. Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel. N Engl J Med. 2021;385(25):e90. doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Gier B, Andeweg S, Backer JA, RIVM COVID-19 surveillance and epidemiology team, Hahné SJM, van den Hof S, et al. Vaccine effectiveness against SARS-CoV-2 transmission to household contacts during dominance of Delta variant (B.1.617.2), August-September 2021, the Netherlands [Preprint]. Epidemiology; 2021 Oct [cited 2021 Nov 1]. Available from: http://medrxiv.org/lookup/doi/10.1101/2021.10.14.21264959. [DOI] [PMC free article] [PubMed]
- 16.Skowronski DM, Setayeshgar S, Febriani Y, Ouakki M, Zou M, Talbot D, et al. Two-dose SARS-CoV-2 vaccine effectiveness with mixed schedules and extended dosing intervals: test-negative design studies from British Columbia and Quebec, Canada [Preprint]. Infectious Diseases (except HIV/AIDS); 2021 Oct [cited 2021 Nov 1]. Available from: http://medrxiv.org/lookup/doi/10.1101/2021.10.26.21265397.
- 17.Payne RP, Longet S, Austin JA, Skelly D, Dejnirattisai W, Adele S, et al. Sustained T Cell Immunity, Protection and Boosting Using Extended Dosing Intervals of BNT162b2 mRNA Vaccine. SSRN Journal [Preprint]. 2021 [cited 2021 Nov 1]; Available from: https://www.ssrn.com/abstract=3891065.
- 18.Parry H, Bruton R, Stephens C, Brown K, Amirthalingam G, Hallis B, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people [Preprint]. Infectious Diseases (except HIV/AIDS); 2021 May [cited 2021 Nov 1]. Available from: http://medrxiv.org/lookup/doi/10.1101/2021.05.15.21257017.
- 19.Baum U., Auranen K., Kulathinal S., Syrjänen R., Nohynek H., Jokinen J. Cohort study design for estimating the effectiveness of seasonal influenza vaccines in real time based on register data: The Finnish example. Scand J Public Health. toukokuuta. 2020;48(3):316–322. doi: 10.1177/1403494818808635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.