Abstract
Background and Aims
Vitamin D downregulates the in vitro expression of the gut-tropic integrin α4β7 on immune cells. The clinical relevance of this finding in patients with inflammatory bowel disease [IBD] is unclear. We tested the hypothesis that vitamin D is associated with α4β7 immunophenotypes and risk of vedolizumab [anti-α4β7] failure in IBD.
Methods
We performed single-cell immunophenotyping of peripheral and intestinal immune cells using mass cytometry [CyTOF] in vedolizumab-naïve patients with IBD [N = 48]. We analysed whole-genome mucosal gene expression [GSE73661] from GEMINI I and GEMINI long-term safety [LTS] to determine the association between vitamin D receptor [VDR] and integrin alpha-4 [ITGA4] and beta-7 [ITGB7] genes. We estimated the odds of vedolizumab failure with low pre-treatment vitamin D in a combined retrospective and prospective IBD cohort [N = 252] with logistic regression.
Results
Immunophenotyping revealed that higher 25[OH]D was associated with decreased α4β7+ peripheral blood mononuclear cells [R = -0.400, p <0.01] and α4β7+ intestinal leukocytes [R = -0.538, p = 0.03]. Serum 25[OH]D was inversely associated with α4β7+ peripheral B cells and natural killer [NK] cells and α4β7+ intestinal B cells, NK cells, monocytes, and macrophages. Mucosal expression of VDR was inversely associated with ITGA4 and ITGB7 expression. In multivariate analysis, 25[OH]D <25 ng/mL was associated with increased vedolizumab primary non-response during induction (odds ratio [OR] 26.10, 95% confidence interval [CI] 14.30–48.90, p <0.001) and failure at 1-year follow-up [OR 6.10, 95% CI 3.06–12.17, p <0.001].
Conclusions
Low serum 25[OH]D is associated with α4β7+ immunophenotypes and predicts future vedolizumab failure in patients with IBD.
Podcast
This article has an associated podcast which can be accessed at https://academic.oup.com/ecco-jcc/pages/podcast
Keywords: Vitamin D, integrins, vedolizumab, inflammatory bowel disease, clinical outcomes
1. Introduction
The integrin α4β7 expressed on gut-tropic immune cells binds to mucosal addressin cell adhesion molecule 1 [MAdCAM-1] and facilitates leukocyte trafficking to the gut leading to intestinal inflammation.1 Vedolizumab, a humanised monoclonal antibody to α4β7, is approved for the treatment of inflammatory bowel disease [IBD]2,3 and is thought to selectively inhibit gut leukocyte trafficking. Only about 40% of IBD patients achieve clinical remission with vedolizumab, and a significant proportion of patients lose response over time.4,5 The mechanisms underlying vedolizumab failure are unclear. Studies identifying modifiable risk factors predicting response to vedolizumab therapy are needed to elucidate mechanisms of treatment failure, enhance the precision of patient selection for therapy, and ultimately optimise the therapeutic efficacy of vedolizumab in patients with IBD.
Vitamin D status is a well-recognised risk factor implicated in inflammatory bowel disease.6 Genetic polymorphisms in the vitamin D receptor [VDR] have been linked to disease susceptibility to ulcerative colitis and Crohn’s disease.7,8 Low vitamin D levels in patients with inflammatory bowel disease have been associated with active inflammation, disease severity, poor quality of life, and adverse clinical outcomes.9,10 Previous mechanistic studies have suggested that the protective associations of higher vitamin D levels in patients with IBD are in part mediated through anti-inflammatory cytokine profiles11 and antimicrobial peptides.12 Previous observations have also suggested that pre-treatment vitamin D levels may affect response to therapy. For example, a higher pre-treatment vitamin D level in patients with IBD was associated with increased odds of primary response to anti-tumour necrosis factor [TNF] therapy.13 Whether pre-treatment vitamin D levels are associated with response to vedolizumab therapy in patients with IBD has not been previously explored.
The imprinting of the gut-tropic integrin α4β7 on leukocytes is regulated by gut lamina propria-derived CD103+ retinoic acid-metabolising dendritic cells in mesenteric lymph nodes.14 Interestingly, vitamin D has been identified as a regulator of integrin α4β7 expression on immune cells in vitro. In one study,15 in the presence of retinoic acid, the active form of vitamin D (1,25[OH]2D3) downregulated α4β7 expression on naïve T cells in a dose-dependent manner. In another study,16 addition of 1,25[OH]2D3 to retinoic acid and interleukin [IL]-2 inhibited the in vitro production of proinflammatory cytokines and integrin α4β7 expression on innate lymphoid cells [ILCs]. It is unclear whether the effects of vitamin D on integrin α4β7 expression on immune cells can be recapitulated in vivo and is clinically relevant in IBD. Specifically, it is unknown whether vitamin D levels correlate with α4β7 expression on peripheral and intestinal immune cells in patients with IBD.
Given that low vitamin D is associated with increased inflammation and poor clinical outcomes in patients with IBD, that vitamin D can downregulate the in vitro expression of α4β7 on immune cells, and that α4β7 immune cell expression in IBD patients may predict vedolizumab response,17 we hypothesised that low 25[OH]D is associated with increased gut-tropic α4β7+ immunophenotypes and predicts future vedolizumab therapy failure in patients with IBD. To test this hypothesis, we performed single-cell immunophenotyping of peripheral and intestinal immune cells in vedolizumab-naïve IBD patients, analysed the association of mucosal gene expression of VDR and vitamin D metabolism enzymes with integrin gene expression, and performed retrospective and prospective cohort analyses in IBD patients treated with vedolizumab, to estimate the odds of future vedolizumab failure with low pre-treatment serum 25[OH]D.
2. Methods
2.1. Single-cell immunophenotyping of peripheral and intestinal immune cells in IBD patients using mass cytometry [CyTOF]
Blood and intestinal biopsies were obtained from IBD patients [blood from 48 patients of whom 12 patients had biopsies] enrolled in the Stanford IBD Registry and biobank [IRB 28427]. Peripheral blood mononuclear cells [PBMCs] were isolated from whole blood using the Ficoll-Paque method as previously described.18 Intestinal leukocytes were isolated from intestinal biopsies using enzymatic treatment.19 PBMCs and intestinal leukocytes were cryopreserved at -80°C until analysis. Immunophenotyping of PBMC and intestinal leukocytes was performed using mass cytometry [CyTOF] at the Stanford Human Immune Monitoring Center according to our previously published protocols.20 All antibody conjugates were validated for accurate detection of their respective antigens and to ensure minimal isotope spillover, by us, the Stanford Human Immune Monitoring Center, and/or in the literature, using flow cytometry with antibody clones and mass cytometry with antibody–metal conjugates. Beads [Fluidigm, cat. #201078] were spiked into each sample for subsequent normalisation using the Helios instrument software, and no cell stimulation or barcoding was used. Bead-normalised sample files were obtained from the Helios instrument using on-board software.
FlowJo was used for cleaning up files, concatenating files, and calculating manual gates and statistics. Doublets were carefully gated out in all samples. Cytobank was used to perform viSNE, CITRUS, and Spade analyses. viSNE analyses were run on live human single cells concatenated from individual samples by group. α4β7hi cells were identified, and subsequently canonical cell populations were gated from this subset as previously described. viSNE21 was run on live human single cells concatenated from individual samples by clinical group. In total, 15,000 events were randomly subsampled from each file of samples concatenated by clinical group, and clustering based on 16 canonical lineage antigens [CD3, CD4, CD8, CD11b, CD11c, CD14, CD16, CD19, CD20, CD25, CD27, CD45RO, CD56, CD123, CD127, and HLA-DR] was run on all concatenated files in parallel for blood or tissue samples using a random seed, 1000 iterations, perplexity of 30, and theta of 0.5 in each of the two runs. We generated tSNE plots to display α4β7 cell surface protein expression gradients among immune cell subsets (CD4 T cells, CD8 T cells, T regulatory Cells, B cells, natural killer [NK] cells, monocytes, macrophages, and innate lymphoid cells) as outlined in a previous study by our group.20 We subsequently plotted serum 25[OH]D with percentage of α4β7+ cells from PBMCs, intestinal leukocytes, and individual immune subsets, and calculated Pearson’s correlation coefficients for normally distributed datasets and Spearman’s correlation coefficients for non-normally distributed datasets. In our CyTOF correlation analyses, we adjusted for potential confounders such as age, sex, ethnicity, active endoscopic inflammation defined as a Mayo score ≥2 in ulcerative colitis [UC] and a Simple Endoscopic Score [CD-SES] >6 in Crohn’s disease [CD] patients, and medications, using multivariate regression. Finally, we analysed expression of other leukocyte trafficking markers [CCR9, CCR1, and GPR15] on immune cell subsets and correlated these immunophenotypes with serum 25[OH]D.
2.2. Mucosal gene expression analysis of vitamin D receptor, vitamin D metabolism enzymes, and integrin gene expression
We used the NCBI Gene Expression Omnibus [GEO] database to download GSE73661 for correlation analyses between the vitamin D pathway and integrin alpha-4 [ITGA4] and beta-7 [ITGB7] genes. GSE73661 was derived from baseline colon biopsies in 41 ulcerative colitis patients with active disease who were enrolled in two phase III vedolizumab clinical trials (GEMINI I and GEMINI long-term safety [[LTS]).22 Pearson correlations were computed after gene expression values were log2-normalised and scaled using rcorr from the Hmisc package in R. p-values were adjusted for multiple comparisons using the Benjamini‐Hochberg method. All correlation analyses were performed and visualised using ggplot2 and R 3.6.3.
2.3. Retrospective and prospective cohort analyses of IBD patients treated with vedolizumab
To test the hypothesis that low serum 25[OH]D is associated with increased risk of vedolizumab failure in patients with IBD, we performed a combined cohort analysis using two independent IBD cohorts [retrospective and prospective cohorts]. Our study was approved by the Stanford University Institutional Review Board [IRB] under protocol 28427. For both cohorts, we included patients who: were 18 years or older with inflammatory bowel disease [ulcerative colitis or Crohn’s disease]; were previously vedolizumab-naïve and were started on vedolizumab therapy as part of standard of care determined by an IBD specialist based on symptoms (Ulcerative Colitis Disease Activity Index [UCDAI] of total score >2 with all individual categories >1, Harvey‐Bradshaw Index [HBI] >7); had faecal calprotectin >250 μg/g or active endoscopic inflammation [if repeat endoscopy performed] defined as Mayo score ≥2, Crohn’s Disease Simple Endoscopic Score [CD-SES] >6; had available pre-treatment serum 25[OH]D measured before vedolizumab initiation [no more than 2 weeks before vedolizumab]; and had response rates measured at induction [WWeek 14] and maintenance therapy at 1-year follow-up. Patients without IBD, less than 18 years of age, without vedolizumab treatment, or who did not have available serum 25[OH]D before vedolizumab initiation were excluded. Our exposure was low pre-treatment serum 25[OH]D level [measured before of starting vedolizumab] defined as <25 ng/mL [lower limit of normal at Stanford laboratory]. Our primary outcome was vedolizumab failure at 1-year follow-up, which included patients who were both primary non-responders and patients who responded during induction but later failed during maintenance therapy at 1-year follow-up. Primary non-response was defined by any of the following at or before Week 14 of induction: ongoing symptoms [UCDAI total score >2 with all individual categories >1, HBI >7]; faecal calprotectin >250 μg/g; or active endoscopic inflammation [if repeat endoscopy performed] defined as Mayo score ≥2, Crohn’s disease simple endoscopic score [CD-SES] >6. Vedolizumab failure at 1 year was defined by: UCDAI >2 with all individual categories >1, HBI [HBI] >7; faecal calprotectin >250 μg/g; or active endoscopic inflammation defined as Mayo score ≥2, Crohn’s Disease Simple Endoscopic Score [CD-SES] >6 at any time during induction therapy [up to Week 14] or maintenance therapy up to 1-year follow-up. Our retrospective cohort was derived from the Stanford Research Repository [STARR] database.
Patients were screened by ICD code [Crohn’s disease ICD code K50.xx and ulcerative colitis ICD code K51.xx], and vedolizumab use and serum 25[OH]D was confirmed by chart review. For our prospective cohort, vedolizumab-naïve patients who were started on vedolizumab as part of standard of care and had pre-treatment serum 25[OH]D levels checked, were enrolled in our IBD registry and longitudinally followed and assessed for primary non-response at Week 14 and vedolizumab failure during 1-year follow-up after vedolizumab initiation. For both cohorts, we collected baseline clinical data including patient demographics [age, sex, ethnicity], IBD subtype and characteristics per the Montreal classification, body mass index [BMI], smoking and alcohol use status, laboratory values [pre-treatment serum 25[OH]D, CRP, albumin, creatinine, faecal calprotectin], baseline endoscopic inflammation (Mayo endoscopic score for ulcerative colitis, and simple endoscopic score [SES-CD] for Crohn’s disease), and medications (current steroids, current mesalamine use, current 6-mercaptopurine use/azathioprine, current methotrexate, previous anti-TNF failure, previous ustekinumab, previous tofacitinib, current opioid use, current non-steroidal anti-inflammatory drug [NSAID] use, and current vitamin D supplementation [not including multivitamin]). We defined baseline active endoscopic inflammation as a Mayo endoscopic score ≥2 for ulcerative colitis patients and a simple endoscopic score [SES-CD] >6 for Crohn’s disease. We recorded the rates of total vedolizumab failure at 1-year follow-up, primary non-response at Week 14, and time to vedolizumab failure. Since this was an observational study, patients with low serum 25[OH]D received vitamin D supplementation as part of standard of care. Based on two previously developed and validated scoring tools for predicting response to vedolizumab therapy in patients with ulcerative colitis23 and Crohn’s disease,24 we included IBD disease duration ≥2 years, previous anti-TNF failure, baseline active endoscopic inflammation, baseline albumin level, previous bowel surgery, previous fistulising disease, and baseline C-reactive protein [CRP] as covariates in our logistic regression models for vedolizumab response.
2.4. Statistical and sensitivity analyses
The rate of primary and secondary outcomes, predictive value of clinical variables on primary and secondary outcomes, odds ratio [OR] with its 95% confidence interval [CI], and p-values were calculated using Statistics/Data Analysis [Stata/IC 15.1 for Windows, College Station, TX]. Dichotomous variables were analysed for outcomes using the chi square test or the Fisher’s exact test where appropriate, and continuous variables were analysed using Student’s t tests if normally distributed, or the Wilcoxon signed-rank test for non-normal data. Correction for multiple testing was performed using the Bonferroni correction.
For our multivariate analyses, model building was based on forward stepwise logistic regression, with a p-value of 0.05 required for entry, and known predictors were also included. We tested the significance of a mediation effect using the Sobel, Aroian, and Goodman tests, which assess for interactions or statistically significant mediator variables for our primary outcome of vedolizumab failure. We performed time-to-event analyses to compare rates [hazard ratios] of primary non-response at Week 14 and vedolizumab failure at 1-year follow-up, between IBD patients with a pre-treatment serum 25[OH]D <25 ng/mL versus 25[OH]D ≥25 ng/mL. Time-to-event analyses were performed using GraphPad Prism [version 8.3; GraphPad Software Inc., La Jolla, CA]. To test the robustness of our findings, we performed several sensitivity analyses. Our first sensitivity analysis involved using different serum 25[OH]D thresholds [from <10 to <60 ng/mL with increments of 5 ng/mL] to define a low vitamin status in our multivariate model for the outcome of vedolizumab failure. In our second sensitivity analysis, we performed our multivariate model separately in patients from retrospective versus prospective cohorts. Third, we performed our multivariate model separately in patients with ulcerative colitis versus Crohn’s disease by combining patients from both retrospective and prospective datasets. Fourth, we analysed our multivariate model according to endoscopic inflammation severity [moderate/severe versus quiescent/mild] using patients from both retrospective and prospective cohorts. Finally, we compared our results using vitamin D to predict vedolizumab failure with previously published vedolizumab prediction and clinical decision support tool [CDST] scores as previously described for ulcerative colitis23 and Crohn’s disease.24 In these previously published CDST, low scores corresponded to decreased risk of vedolizumab response, whereas higher scores corresponded to higher probability of vedolizumab response.
3. Results
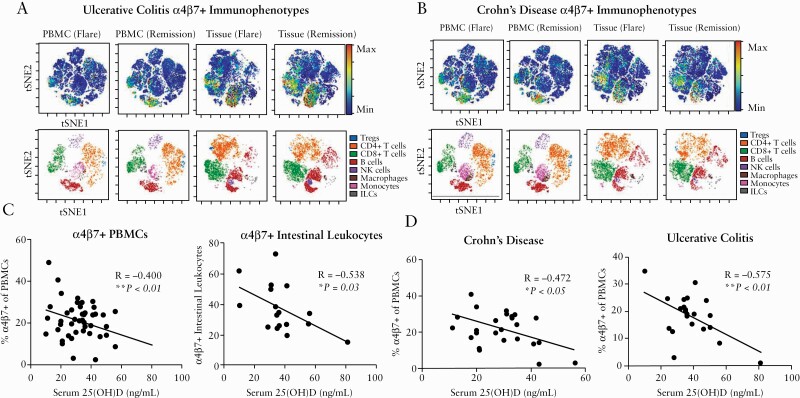
3.1. Single-cell immunophenotyping of peripheral and intestinal immune cells in IBD patients using mass cytometry [CyTOF] reveals an inverse correlation between 25[OH]D and α4β7+ immunophenotypes
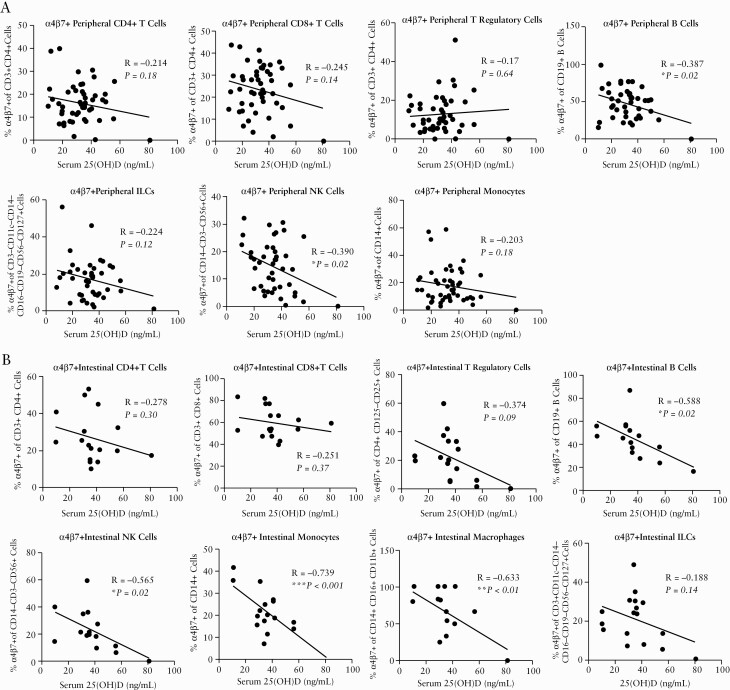
The baseline clinical characteristics of the 48 vedolizumab-naïve IBD patients are summarised in Supplementary Table 1, available as Supplementary data at ECCO-JCC online. The single-cell distribution of α4β7 cell surface protein expression on various immune subsets in patients with IBD is summarised in Figure 1. Our multivariate correlation analysis is summarised in Supplementary Table 2, available as Supplementary data at ECCO-JCC online. After adjusting for potential confounders such as age, sex, ethnicity, active endoscopic inflammation, and medications, serum 25[OH]D was inversely associated with α4β7+ peripheral blood mononuclear cells [PBMC] [R = -0.400, p <0.01] and α4β7+ intestinal leukocytes [R = -0.538, p = 0.03]. Mayo endoscopic scores [R =-0.324, p = 0.82] and simple endoscopic scores [R = 0.038, p = 0.90] did not statistically correlate with percentage of α4β7+ PBMCs in patients with IBD [Supplementary Figure 1, available as Supplementary data at ECCO-JCC online]. In cross-sectional analysis [Supplementary Figure 2, available as Supplementary data at ECCO-JCC online], serum 25 [OH]D was not associated with total peripheral CD4 T cells, CD8 T cells, T regulatory cells, innate lymphoid cells, NK cells, B cells, or monocytes. Serum 25[OH]D was inversely associated with total intestinal CD8 T cells [R = -0.573, p = 0.02] and intestinal macrophages [R = -0.555, p = 0.03], but not associated with total intestinal CD4 T cells, T regulatory cells, innate lymphoid cells, NK cells, B cells, or monocytes. In subgroup analysis, serum 25[OH]D was inversely associated with α4β7+ PBMCs in both patients with ulcerative colitis [R = -0.575, p <0.01] and Crohn’s disease [R = -0.472, p <0.05].
Figure 1.
Single-cell immunophenotyping by mass cytometry [CyTOF] reveals [A] α4β7 immunophenotypes in patients with Crohn’s disease, [B] α4β7 immunophenotypes in patients with ulcerative colitis, [C] α4β7 expression on peripheral and intestinal immune cells in patients with IBD, and [D] α4β7 expression on peripheral blood mononuclear cells [PBMC] in patients with Crohn’s disease and ulcerative colitis. IBD, inflammatory bowel disease.
In analyses focused on specific immune subsets [Figure 2], higher serum 25[OH]D was associated with decreased peripheral α4β7+ B cells [R = -0.387, p = 0.02] and peripheral α4β7+ NK cells [R = -0.390, p = 0.02] and decreased intestinal α4β7+ B cells [R = -0.588, p = 0.02], intestinal α4β7+NK cells [R = -0.565, p = 0.02], intestinal α4β7+ monocytes [R = -0.739, p <0.001], and intestinal α4β7+ macrophages [R = -0.633, p <0.01]. Immunophenotyping [Supplementary Figure 3, available as Supplementary data at ECCO-JCC online] based on other leukocyte trafficking markers [CCR9, CCR1, and GPR15] did not reveal associations between serum 25[OH]D and PBMCs or intestinal leukocytes.
Figure 2.
Single-cell immunophenotyping by mass cytometry [CyTOF] of [A] peripheral immune cells [CD4 T cells, CD8 T cells, T regulatory cells, B cells, and ILCs], NK cells, and monocytes] and [B] intestinal immune cells [CD4 T cells, CD8 T cells, T regulatory cells, B cells, NK cells, monocytes, macrophages, and ILCs]. ILCs, innate lymphoid cells.
3.2. Vitamin D receptor and vitamin D inactivating enzyme CYP24A1 gene expression are associated with ITGA4 and ITGB7 mucosal expression in patients with IBD
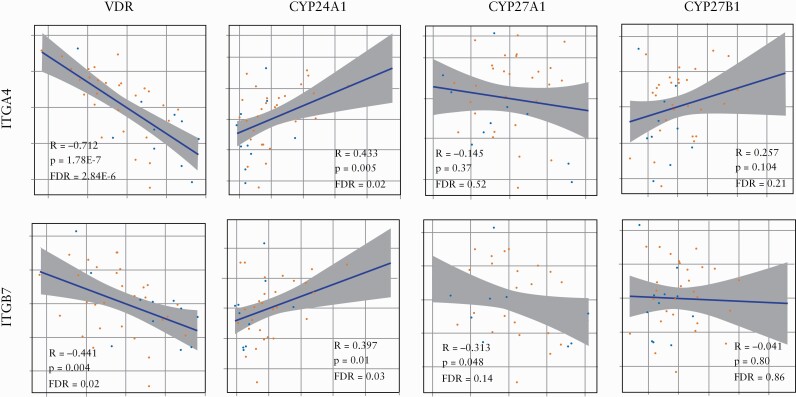
Whole genome mucosal gene expression analysis [GSE73661] from baseline colon biopsies in 41 ulcerative colitis patients with active disease, who were enrolled in two phase III vedolizumab clinical trials [GEMINI I and GEMINI LTS],22 is summarised in Supplementary Table 3, available as Supplementary data at ECCO-JCC online. In gene expression correlation analyses [Figure 3], vitamin D receptor [VDR] expression was inversely associated with expression of integrin subunits α4 [ITGA4, R =- 0.712, FDR = 5.33x10-6] and β7 [ITGB7, R = -0.441, FDR = 0.03]. Conversely, gene expression of the vitamin D inactivating enzyme vitamin D3 24-hydroxylase [CYP24A1] was positively associated with gene expression of α4 [ITGA4, R = 0.434, FDR = 0.03] and β7 [ITGB7, R = 0.397, FDR = 0.04]. Gene expression of vitamin D activating enzymes [CYP27A1 and CYP27B1] was not associated with ITGA4 or ITGB7 gene expression. VDR expression was inversely associated with CCR1 expression [R = -0.562, FDR <0.01], whereas CYP24A1 was positively associated with CCR1 expression [R = 0.418, FDR = 0.03]. Expression of VDR and vitamin D metabolism enzymes [CYP27A1, CYP27B1, and CYP24A1] was not associated with leukocyte trafficking markers CCR9 or GPR15.
Figure 3.
Association of gene expression of gut-tropic integrins ITGA4 [A] and ITGB7 [B] with vitamin D receptor [VDR] and vitamin D metabolism enzymes [CYP24A1, CYP27A1 and CYP27B1]. Pearson correlations were done using log2-normalised scaled values for each gene and adjusted for multiple testing using the Benjamini‐Hochberg method.
3.3. Low pre-treatment serum 25[OH]D is associated with increased odds of vedolizumab failure at 1-year follow-up in patients with IBD
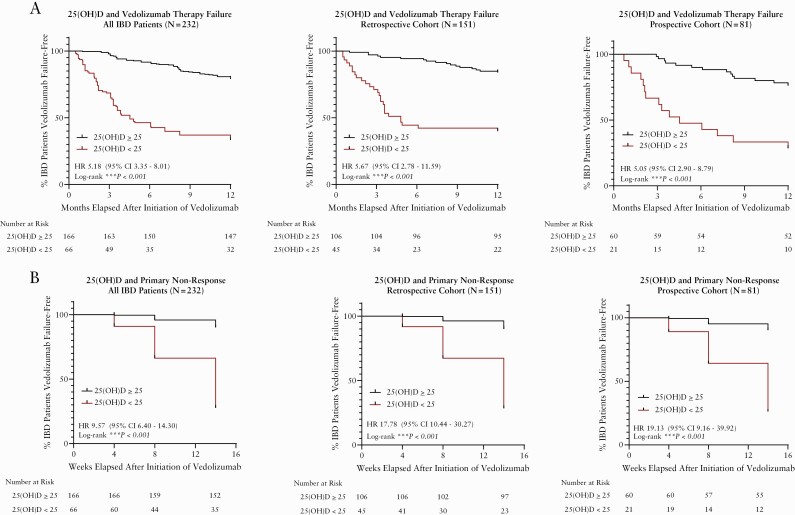
The baseline clinical characteristics of all IBD patients included [combined retrospective and prospective cohorts] are summarised in Table 1. Supplementary Table 4, available as Supplementary data at ECCO-JCC online, summarises rates of vedolizumab primary non-response during induction, vedolizumab failure at 1-year follow-up, rates of IBD surgery, and medications after vedolizumab failure. A total of 73 patients [30%] failed vedolizumab at 1-year follow-up, of whom 45 patients failed during induction therapy [primary non-responders]. Among patients who failed vedolizumab at 1-year follow-up, 22 patients [30.1%] underwent surgery, 42 patients [57.6%] were on steroids [38 were continued on steroids, four were started on new steroids], 26 [36.5%] were switched to ustekinumab, 15 [20.5%] were switched to an anti-TNF agent, and six [8.2%] were switched to tofacitinib. Table 2 summarises our univariate and multivariate logistic regression analysis for clinical predictors of vedolizumab failure at 1-year follow-up for all IBD patients. In univariate logistic regression, 25[OH]D <25 ng/mL [OR 6.77, 95% CI 3.50‐13.10, p <0.001], active endoscopic inflammation [OR 3.43, 95% CI 1.83‐6.43, p <0.001], and previous anti-TNF therapy [OR 2.31, 95% CI 1.15‐4.63, p = 0.019] were associated with increased odds of vedolizumab failure at 1-year follow-up. In multivariate analysis, 25[OH]D <25 ng/mL [OR 6.10, 95% CI 3.06‐12.17, p <0.001] and active endoscopic inflammation [OR 2.98, 95% CI 1.50‐5.93, p = 0.002] were independently associated with vedolizumab failure. Figure 4A summarises the time-to-event analyses comparing rates of vedolizumab failure at 1-year follow-up among IBD patients with a pre-treatment serum 25[OH]D <25 ng/mL versus 25[OH]D ≥25 ng/mL. A pre-treatment serum 25[OH]D <25 ng/mL was associated with greater rates [shorter time to event] of vedolizumab failure [HR 5.18, 95% CI 3.35‐8.01, p <0.001].
Table 1.
Baseline clinical characteristics of inflammatory bowel disease [IBD] patients initiating vedolizumab therapy.
| Clinical variables | All IBD | Vedolizumab | Vedolizumab | p-Value |
|---|---|---|---|---|
| patients | responders | non-responders | ||
| N = 232 | N = 159 | N = 73 | ||
| Age, years [mean ± SD] | 41.2 [±16.8] | 40.9 [±16.6] | 41.5 [±17.4] | 0.800 |
| Sex | ||||
| Male, no. [%] | 116 [50.0] | 70 [44.0] | 29 [39.7] | 0.130 |
| Female, no. [%] | 116 [50.0] | 89 [56.0] | 44 [60.3] | |
| Race | ||||
| White, no. [%] | 172 [74.1] | 120 [75.5] | 52 [71.2] | 0.802 |
| Hispanic, no. [%] | 21 [9.1] | 13 [8.1] | 8 [11.0] | 0.984 |
| Black, no. [%] | 5 [2.2] | 3 [1.9] | 1 [1.4] | 0.963 |
| Asian, no. [%] | 28 [12.1] | 20 [12.6] | 8 [11.0] | 0.912 |
| Pacific Islander, no. [%] | 1 [0.01] | 1 [0.01] | 0 [0] | 0.320 |
| Unknown, no. [%] | 5 [2.2] | 5 [2.2] | 0 [0] | 0.442 |
| Ulcerative colitis | ||||
| Total, no. [%] | 122 [52.6] | 84 [52.8] | 39 [53.4] | 0.636 |
| E1, no. [%] | 4 [1.7] | 3 [1.9] | 1 [1.4] | 0.963 |
| E2, no. [%] | 42 [18.1] | 28 [17.6] | 14[ 19.2] | 0.953 |
| E3, no. [%] | 76 [32.8] | 54 [34.0] | 22 [30.1] | 0.500 |
| Crohn's disease | ||||
| Total, no. [%] | 111 [47.8] | 75 [47.2] | 36 [49.3] | 0.894 |
| L1, no. [%] | 13 [5.6] | 11 [6.9] | 2 [2.8] | 0.200 |
| L2, no. [%] | 15 [6.5] | 9 [5.7] | 6 [8.2] | 0.648 |
| L3, no. [%] | 83 [35.8] | 51 [32.1] | 32 [43.8] | 0.281 |
| L4, no. [%] | 0 [0] | 0 [0] | 0 [0] | --- |
| Perianal disease | 50 [21.6] | 32[ 20.1] | 18 [24.7] | 0.471 |
| B1, no. [%] | 20 [8.6] | 15 [9.4] | 5 [6.8] | 0.656 |
| B2, no. [%] | 35 [15.1] | 23 [14.5] | 12 [16.4] | 0.968 |
| B3, no. [%] | 74 [31.9] | 40 [25.2] | 34 [46.6] | 0.181 |
| BMI, kg/m2 [mean ± SD] | 25.0 [±6.2] | 24.7 [±5.7] | 25.7 [±7.1] | 0.844 |
| Current smoker, no. [%] | 4 [1.7] | 1 [0.6] | 3 [4.1] | 0.090 |
| Current alcohol use, no. [%] | 95 [40.9] | 66 [41.5] | 29 [39.7] | 0.492 |
| Pre-treatment 25[OH]D, ng/mL [mean ± SD] | 33.7 [±13.7] | 36.5 [±11.7] | 28.4 [±15.6] | <0.001 |
| 25[OH]D <25 ng/mL, no. [%] | 66 [28.4] | 25[15.7] | 41 [56.2] | |
| 25[OH]D ≥ 25 ng/mL, no. [%] | 166 [65.9] | 134 [84.3] | 32 [43.8] | |
| Creatinine, mg/dL [mean ± SD] | 0.81 [±0.3] | 0.84 [±0.4] | 0.75 [±0.2] | 0.470 |
| Albumin, g/L [mean ± SD] | 3.5 [±0.5] | 3.6 [±0.5] | 3.4 [±0.6] | 0.960 |
| C-reactive protein, mg/dl [mean ± SD] | 3.66 [±6.9] | 3.30 [±5.7] | 4.3 [±8.7] | 0.783 |
| Faecal calprotectin, µg/g [mean ± SD] | 665.5 [±653.9] | 551.9 [±549.0] | 892.9 [±785.7] | 0.985 |
| Baseline endoscopic inflammationa | ||||
| Quiescent/mild, no. [%] | 112 [48.3] | 94 [59.1] | 18 [24.7] | <0.001 |
| Moderate/severe, no. [%] | 120 [51.7] | 65[ 40.9] | 55 [75.3] | <0.001 |
| IBD medication history | ||||
| Current steroids, no. [%] | 103 [44.4] | 65 [40.9] | 38 [52.1] | 0.387 |
| Current 5-ASA, no. [%] | 63 [27.2] | 42 [26.4] | 21 [28.8] | 0.993 |
| Current 6MP/azathioprine, no. [%] | 30 [12.9] | 19 [11.9] | 11 [15.1] | 0.973 |
| Current methotrexate, no. [%] | 21 [9.1] | 15 [9.4] | 6 [8.2] | 0.906 |
| Previous anti-TNF failure, no. [%] | 167 [72.0] | 107 [67.3] | 60 [82.3] | 0.017 |
| Previous ustekinumab, no. [%] | 0 [0] | 0 [0] | 0 [0] | --- |
| Previous tofacitinib, no. [%] | 0 [0] | 0 [0] | 0 [0] | --- |
| Current opioids, no. [%] | 19 [8.2] | 13 [8.2] | 6 [8.2] | 0.890 |
| Current NSAIDs, no. [%] | 34 [14.7] | 20 [12.6] | 14 [19.2] | 0.562 |
| Current vitamin D supplementation, no. [%] | 127 [54.7] | 87 [54.7] | 40 [54.8] | 0.277 |
SD, standard deviation; BMI, body mass index; ASA; aminosalicylic acid; NSAID, non-steroidal anti-inflammatory drug; CD-SES, Crohn’s Disease Simple Endoscopic Score.
aEndoscopic inflammation: quiesecent [Mayo = 0, CD-SES = 0‐2], mild [Mayo = 1, CD-SES 3‐6], moderate [Mayo = 2, CD-SES = 7-15]; severe [Mayo = 3, CD-SES >15].
Table 2.
Univariate and multivariate predictors of vedolizumab failure in patients with inflammatory bowel disease [IBD] at 1-year follow-up.
| Clinical variables | All IBD patients, N = 252 | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Odds ratio | 95% CI | p-Value | Odds ratio | 95% CI | p-Value | |
| IBD disease duration ≥2 years | 0.79 | 0.41–1.53 | 0.483 | |||
| Fistulising disease | 1.82 | 0.69–4.79 | 0.184 | |||
| Low serum 25[OH]D | 6.77 | 3.50–13.10 | <0.001 | 6.10 | 3.06–12.17 | <0.001 |
| Albumin [g/L] | 0.65 | 0.33–1.04 | 0.068 | |||
| C-reactive protein [mg/dL] | 1.02 | 0.97–1.07 | 0.394 | |||
| Active endoscopic inflammation | 3.43 | 1.83–6.43 | <0.001 | 2.98 | 1.50–5.93 | 0.002 |
| Previous bowel surgery | 2.58 | 0.97–7.61 | 0.175 | |||
| Previous anti-TNF failure | 2.31 | 1.15–4.63 | 0.019 | 1.94 | 0.90–4.20 | 0.092 |
| Current vitamin D supplementation | 0.73 | 0.41–1.29 | 0.278 | 0.65 | 0.34–1.20 | 0.205 |
Figure 4.
Time-to-event analyses for [A] vedolizumab therapy failure at 1-year follow-up and [B] vedolizumab primary non-response during induction therapy.
3.4. Low pre-treatment serum 25[OH]D is associated with increased odds of vedolizumab primary non-response during induction therapy in patients with IBD
Table 3 summarises our univariate and multivariate logistic regression analysis for clinical predictors of vedolizumab primary non-response during induction therapy [14 weeks after vedolizumab initiation] in IBD patients. In univariate logistic regression, 25[OH]D <25 ng/mL [OR 26.87, 95% CI 10.60‐58.35, p <0.001], active endoscopic inflammation [OR 3.34, 95% CI 1.55‐7.17, p = 0.002], previous bowel surgery [OR 7.88, 95% CI 3.13‐19.80, p <0.001], and previous anti-TNF therapy [OR 2.48, 95% CI 1.04‐5.91, p = 0.04] were associated with increased odds of vedolizumab primary non-response at Week 14. In multivariate analysis, 25[OH]D <25 ng/mL [OR 26.10, 95% CI 14.30‐48.90, p <0.001] and previous bowel surgery [OR 9.45, 95% CI 4.62‐28,87, p <0.001] were independently associated with vedolizumab primary non-response, whereas vitamin D supplementation use before vedolizumab initiation was independently associated with decreased risk [OR 0.36, 95% CI 0.14‐0.95, p = 0.039] of vedolizumab primary non-response. Figure 4B summarises the time-to-event analyses comparing rates of vedolizumab primary non-response at Week 14 among IBD patients with a pre-treatment serum 25[OH]D <25 ng/mL versus 25[OH]D ≥25 ng/mL. A pre-treatment serum 25[OH]D <25 ng/mL was associated with greater rates [shorter time to event] of primary non-response [HR 9.57, 95% CI 6.40‐14.30, p <0.001].
Table 3.
Univariate and multivariate predictors of vedolizumab primary non-responders during induction [Week 14].
| Clinical variables | All IBD patients, N = 252 | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Odds ratio | 95% CI | p-Value | Odds ratio | 95% CI | p-Value | |
| IBD disease duration ≥ 2years | 0.77 | 0.22–1.12 | 0.080 | |||
| Fistulising disease | 1.97 | 0.85–4.56 | 0.112 | |||
| Low serum 25[OH]D | 24.87 | 10.60–58.35 | <0.001 | 26.10 | 14.30–48.90 | <0.001 |
| Albumin [g/L] | 0.56 | 0.30–1.05 | 0.071 | |||
| C-reactive protein [mg/dL] | 0.98 | 0.92–1.05 | 0.604 | |||
| Active endoscopic inflammation | 3.34 | 1.55–7.17 | 0.002 | 2.51 | 0.87–7.29 | 0.090 |
| Previous bowel surgery | 7.88 | 3.13–19.80 | <0.001 | 9.45 | 4.62–28.87 | <0.001 |
| Previous anti-TNF failure | 2.48 | 1.04 –5.91 | 0.041 | 1.48 | 0.50–4.41 | 0.482 |
| Current vitamin D supplementation | 0.64 | 0.33–1.25 | 0.192 | 0.36 | 0.14–0.95 | 0.039 |
IBD, inflammatory bowel disease; TNF, tumour necrosis factor.
3.5. Sensitivity analyses
In our sensitivity analyses evaluating different serum 25[OH]D thresholds to define a low vitamin D status [Supplementary Table 5, available as Supplementary data at ECCO-JCC online], serum 25[OH]D was significantly associated with vedolizumab failure starting at 25[OH]D <15 ng/mL and extending to <40 ng/mL in our combined cohorts. Lower 25[OH]D thresholds were associated with larger effect sizes [odds ratios]. In our sensitivity analyses [Supplementary Table 6, available as Supplementary data at ECCO-JCC online] comparing our results based on type of cohort [retrospective versus prospective], low vitamin D remained an independent predictor of vedolizumab failure at 1-year follow-up in both retrospective [OR 4.45, 95% CI 1.82–10.84, p = 0.001] and prospective cohorts [OR 15.58, 95% CI 3.93–61.75, p <0.001]. When we compared our results based on IBD subtype [Supplementary Table 7, available as Supplementary data at ECCO-JCC online], the effect size of this association of low vitamin D with vedolizumab failure was greater in ulcerative colitis [OR 15.15, 95% CI 4.85–47.35, p <0.001] compared with Crohn’s disease [OR 3.25, 95% CI 1.31–8.09, p = 0.011]. Given that baseline endoscopic inflammation was also associated with increased risk of future vedolizumab failure, we performed sensitivity analyses [Supplementary Table 8, available as Supplementary data at ECCO-JCC online] separating patients with moderate/severe versus quiescent/mild endoscopic inflammation. Low pre-treatment serum 25[OH]D was associated with increased risk of vedolizumab failure in IBD patients with moderate/severe [OR 4.41, 95% CI 1.94–9.99, p <0.001] and quiescent/mild endoscopic inflammation [OR 11.56, 95% CI 3.27–40.91, p <0.001]. We performed formal mediation analyses using the Sobel, Aroian, and Goodman tests to assess for interactions [mediator variables] with 25[OH]D and our primary outcome of vedolizumab failure. Our formal mediation analyses are summarised in Supplementary Table 9, available as Supplementary data at ECCO-JCC online. None of the covariates was found to have statistically significant [p <0.05] mediator effects on serum 25[OH]D and risk of vedolizumab failure by Sobel, Aroian, and Goodman tests.
Supplementary Table 10, available as Supplementary data at ECCO-JCC online, summarises our analysis comparing our vitamin D vedolizumab clinical outcomes with previously published23,24 vedolizumab prediction and clinical decision support tool [CDST] models. In these CDST models, a lower score corresponded with lower probability of vedolizumab response whereas a higher score was associated with higher probability of vedolizumab response. In our cohorts, patients with ulcerative colitis in the low CDST score group [<26] had a greater proportion of patients with low vitamin D [27.4% vs 14.3%, p <0.05], vedolizumab primary non-response [20.5% vs 7.1%, p <0.01], and vedolizumab failure at 1 year [34.2% vs 7.1%, p <0.01] compared with the high CDST group [>32]. Likewise, patients with Crohn’s disease in the low CDST score group [<13] included a greater proportion of patients with low vitamin D [47.8% vs 14.8%, p <0.001], vedolizumab primary non-response [39.1% vs 11.1%, p <0.01], and vedolizumab failure at 1 year [69.6% vs 14.8%, p <0.001] compared with high CDST [>19]. Taken together, using vitamin D levels to risk-stratify patients to predict probability of vedolizumab failure correlated well with these previous CDST models.
4. Discussion
In this multicohort study, we demonstrate novel associations between serum 25[OH]D and α4β7 cell surface expression on PBMCs and intestinal leukocytes and an association between VDR and vitamin D-inactivating enzyme CYP24A1 with ITGA4 and ITGB7 mucosal gene expression. Our findings also identify pre-treatment serum 25[OH]D as a novel biomarker and a potential modifiable risk factor in predicting response to vedolizumab therapy.
Since low vitamin D predicted vedolizumab failure in patients with IBD, we were interested in exploring which α4β7+ immune cell subsets were inversely associated with vitamin D. Our data demonstrated that innate immune cells [monocytes, macrophages, NK cells] and B cells were inversely associated with vitamin D. Although our study did not evaluate whether and how these vitamin D-associated α4β7+ innate immune cells and B cells were mediating the risk of future vedolizumab failure, more recent studies have suggested that innate immune cells25–27 and B cells27 may predict the therapeutic efficacy of vedolizumab. In a prospective study of IBD patients treated with vedolizumab by Zeissig et al.,25 a decrease in M1/M2 macrophage ratio and macrophage-associated gene signatures were associated with response to vedolizumab. Furthermore, in a study by Verstockt et al.,27 M1 macrophages were enriched in vedolizumab non-responders, whereas naïve B cells were enriched in responders. Extending beyond the known α4β7 role on gut T cell trafficking, and understanding the function of α4β7+ innate immune cells and B cells in the pathogenesis of IBD, may reveal insights into the mechanisms of anti-integrin therapy failure.
Our finding that low pre-treatment 25[OH]D is associated with future vedolizumab failure in patients with IBD may have several explanations. First, vitamin D may negatively regulate α4β7 expression in patients with IBD. By reducing the frequency of α4β7+ immune cells able to migrate to the gut, higher serum 25[OH]D levels may work synergistically with vedolizumab to inhibit immune cell trafficking to the gastrointestinal tract and hence reduce gastrointestinal inflammation. This association with vitamin D may be specific to α4β7, as there was no association with the other leukocyte trafficking markers CCR9, CCR1, and GPR15. Although the exact mechanisms of how vitamin D regulates α4β7 immunophenotypes are unclear and beyond the scope of this study, the biological plausibility of this relationship is supported by earlier studies. Vitamin D has been shown to decrease the maturation and activation of dendritic cells, the master regulators of α4β7 imprinting on immune cells.28 Decreased dendritic cell maturation may in turn result in attenuated α4β7 imprinting. Another potential mechanism is that vitamin D may reduce levels of IL-7, which has been shown to induce expression and activation of α4β7, promoting immune cell homing to the intestinal mucosa.29 Vitamin D supplementation has been shown previously to reduce serum IL-7 levels.30 Second, vitamin D may mediate the risk of vedolizumab failure through α4β7-independent mechanisms. An earlier study by Ananthakrishnan et al.31 demonstrated that gut microbiome function predicts response to anti-integrin therapy in IBD. Both the vitamin D receptor and vitamin D have been shown be major factors in shaping the gut microbiome. The vitamin D receptor plays a role in regulation of gut microbiota composition32 and in promoting healthy microbial metabolites.33 Likewise, vitamin D has been shown to modulate the gut microbiota in IBD.34,35 Vitamin D may mediate the risk of vedolizumab failure through regulation of the gut microbiome. Third, an alternative explanation is that IBD patients with low pre-treatment serum 25[OH]D had more severe baseline disease and thus were more likely to fail vedolizumab therapy. Our analysis did confirm that baseline active endoscopic inflammation was an independent predictor of vedolizumab failure, but we adjusted for this covariate in our multivariate models and sensitivity analyses and serum 25[OH]D remained an independent predictor of vedolizumab failure.
Our study has several strengths. First, our findings are novel. To our knowledge, this is the first study to demonstrate an association between 25[OH]D and α4β7+ immunophenotypes in patients with IBD and to highlight a role of pre-treatment 25[OH]D in predicting vedolizumab response. Whereas other groups have shown that vitamin D regulates in vitro α4β7 expression, our study directly translates these findings to patients with IBD. Second, we applied innovative techniques such as deep immunophenotyping with mass cytometry and whole-genome mucosal gene expression analyses to evaluate the association of vitamin D and VDR with α4β7 expression in patients with IBD. Third, our findings are both scientifically and clinically impactful. Our study highlighted the need to better understand vitamin D regulation of gut trafficking molecules on innate immune cells and B cells and the functional consequences of these immune cell subsets in IBD, which represent conceptual advances beyond the field’s focus on T cell biology. Furthermore, our study suggests that pre-treatment 25[OH]D could be used as a novel biomarker to better risk-stratify patients with IBD who would most benefit from vedolizumab therapy. Given that serum 25[OH]D is an easily modifiable risk factor through diet and vitamin D supplementation, our study also raises the possibility of therapeutic interventions related to vitamin D. Well-powered, randomised, controlled trials evaluating the clinical benefit of concomitant vitamin D supplementation with vedolizumab induction therapy in patients with IBD are warranted.
Our study also has several limitations that warrant attention. First, our study was observational and may not establish causation or account for residual unmeasured confounders. However, our study met several Bradford Hill criteria36 to support a plausible causal relationship.37 First, the effect sizes for risk of vedolizumab failure were strong, and even stronger for primary non-response. Second, the relationship between low serum 25[OH]D and future vedolizumab failure was reproducible in an independent prospective verification cohort. Third, the association with 25[OH]D was specific to α4β7 and not other leukocyte-trafficking markers. Fourth, there was temporality in our association, as the exposure of low pre-treatment 25[OH]D preceded the outcome of future vedolizumab failure. Fifth, we demonstrated a dose-response relationship. Lower 25[OH]D thresholds were associated with much larger effect sizes for the outcome of vedolizumab failure. We previously discussed several potential mechanisms to support the biological plausibility of vitamin D in regulating α4β7 immunophenotypes and affecting the risk of vedolizumab failure. Sixth, our clinical study was conducted at a single centre, which limits the generalizability of our findings. However, as a tertiary referral centre for a large northern California population, our patients are ethnically diverse and representative of the US population. Finally, due to study design, we were unable to determine the mechanisms of how low vitamin D leads to vedolizumab failure and how vitamin D alterations of α4β7 immunophenotypes mediate this risk. Future mechanistic studies and prospective validation of our CyTOF findings are warranted.
In conclusion, we demonstrate that serum 25[OH]D is inversely associated with α4β7 expression on PBMCs and intestinal leukocytes, that VDR is inversely associated with mucosal gene expression of ITGA4 and ITGB7, and that low serum 25[OH]D is associated with increased risk of future vedolizumab failure among patients with IBD. Future studies are warranted to elucidate the exact mechanisms of these associations and to determine whether therapeutic interventions with vitamin D could be used to mitigate the increased risk of vedolizumab failure associated with low pre-treatment vitamin D levels.
Supplementary Material
Funding
This project was in part supported by a Chan Zuckerberg Biohub Physician Scientist Scholar Award [JG], NIH NIDDK LRP Award Grant number [L30 DK126220] [JG], NIH R01 DK101119 [AH], and the Ann and Bill Swindells Charitable Trust as well as Leslie and Douglas Ballinger [AH].
Conflict of Interest
The authors have no conflicts of interests or financial disclosures relevant to this manuscript.
Author Contributions
JG and AH planned and designed the study; SJSR and YH performed mass cytometry; LB performed the whole-genome gene expression analyses; JG and SJSR performed the CyTOF and vitamin D analyses; JG, SL, TB, AP, and AS performed the retrospective chart review for the discovery cohort, JG, TB, AP, and AS processed the prospective data from the Stanford IBD Registry; JG performed clinical study statistical and sensitivity analyses; JG drafted the manuscript; AH and SRS provided critical review of the manuscript; all authors interpreted the results and contributed to critical review of the manuscript; JG had full access to the study data and takes responsibility for the integrity of the data and accuracy of the analysis.
References
- 1. Habtezion A, Nguyen LP, Hadeiba H, Butcher EC. Leukocyte trafficking to the small intestine and colon. Gastroenterology 2016;150:340–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 3. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 4. Barré A, Colombel JF, Ungaro R. Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther 2018;47:896–905. [DOI] [PubMed] [Google Scholar]
- 5. Biemans VB, van der Woude CJ, Dijkstra G, et al. Vedolizumab for inflammatory bowel disease: two-year results of the Initiative on Crohn and Colitis [ICC] Registry, a nationwide prospective observational cohort study: ICC Registry–Vedolizumab. Clin Pharmacol Ther 2020;107:1189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gubatan J, Moss AC. Vitamin D in inflammatory bowel disease: more than just a supplement. Curr Opin Gastroenterol 2018;34:217–25. [DOI] [PubMed] [Google Scholar]
- 7. Xia SL, Yu LQ, Chen H, et al. Association of vitamin D receptor gene polymorphisms with the susceptibility to ulcerative colitis in patients from Southeast China. J Recept Signal Transduct Res 2015;35:530–5. [DOI] [PubMed] [Google Scholar]
- 8. Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut 2000;47:211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gubatan J, Mitsuhashi S, Zenlea T, Rosenberg L, Robson S, Moss AC. Low serum vitamin D during remission increases risk of clinical relapse in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2017;15:240–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gubatan J, Chou ND, Nielsen OH, Moss AC. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther 2019;50:1146–58. [DOI] [PubMed] [Google Scholar]
- 11. Gubatan J, Mitsuhashi S, Longhi MS, et al. Higher serum vitamin D levels are associated with protective serum cytokine profiles in patients with ulcerative colitis. Cytokine 2018;103:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gubatan J, Mehigan GA, Villegas F, et al. Cathelicidin mediates a protective role of vitamin D in ulcerative colitis and human colonic epithelial cells. Inflamm Bowel Dis 2020;26:885–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winter RW, Collins E, Cao B, Carrellas M, Crowell AM, Korzenik JR. Higher 25-hydroxyvitamin D levels are associated with greater odds of remission with anti-tumour necrosis factor-α medications among patients with inflammatory bowel diseases. Aliment Pharmacol Ther 2017;45:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agace WW, Persson EK. How vitamin A metabolizing dendritic cells are generated in the gut mucosa. Trends Immunol 2012;33:42–8. [DOI] [PubMed] [Google Scholar]
- 15. Sigmundsdottir H, Pan J, Debes GF, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol 2007;8:285–93. [DOI] [PubMed] [Google Scholar]
- 16. Ruiter B, Patil SU, Shreffler WG. Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells. Clin Exp Allergy 2015;45:1214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuchs F, Schillinger D, Atreya R, et al. Clinical response to vedolizumab in ulcerative colitis patients is associated with changes in integrin expression profiles. Front Immunol 2017;8:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fuss IJ, Kanof ME, Smith PD, Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol 2009.. doi: 10.1002/0471142735.im0701s85. [DOI] [PubMed] [Google Scholar]
- 19. Selby WS, Janossy G, Bofill M, Jewell DP. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut 1984;25:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rubin SJS, Bai L, Haileselassie Y, et al. Mass cytometry reveals systemic and local immune signatures that distinguish inflammatory bowel diseases. Nat Commun 2019;10:2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amir el-AD, Davis KL, Tadmor MD, et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol 2013;31:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arijs I, De Hertogh G, Lemmens B, et al. Effect of vedolizumab [anti-α4β7-integrin] therapy on histological healing and mucosal gene expression in patients with UC. Gut 2018;67:43–52. [DOI] [PubMed] [Google Scholar]
- 23. Dulai PS, Singh S, Casteele NV, et al. Development and validation of clinical scoring tool to predict outcomes of treatment with vedolizumab in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020;18:2952–61.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dulai PS, Boland BS, Singh S, et al. Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with Crohn’s disease. Gastroenterology 2018;155:687–95.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeissig S, Rosati E, Dowds CM, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut 2019;68:25–39. [DOI] [PubMed] [Google Scholar]
- 26. Osterman MT, Gordon IO, Davis EM, et al. Mucosal biomarker of innate immune activation predicts response to vedolizumab in Crohn’s disease. Inflamm Bowel Dis 2020;26:1554‐61. [DOI] [PubMed] [Google Scholar]
- 27. Verstockt B, Verstockt S, Veny M, et al. Expression levels of 4 genes in colon tissue might be used to predict which patients will enter endoscopic remission after vedolizumab therapy for inflammatory bowel diseases. Clin Gastroenterol Hepatol 2020;18:1142–51.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pedersen AW, Holmstrøm K, Jensen SS, et al. Phenotypic and functional markers for 1α, 25-dihydroxyvitamin D3-modified regulatory dendritic cells. Clin Exp Immunol 2009;157:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cimbro R, Vassena L, Arthos J, et al. IL-7 induces expression and activation of integrin α4β7 promoting naive T-cell homing to the intestinal mucosa. Blood 2012;120:2610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alkhedaide AQH, Alshehri ZS, Soliman MM, Althumali KW, Abu-Elzahab HS, Baiomy AAA.. Vitamin D3 supplementation improves immune and inflammatory response in vitamin D deficient adults in Taif, Saudi Arabia. Biomed Res 2016;27. ISSN: 0970-938X. [Google Scholar]
- 31. Ananthakrishnan AN, Luo C, Yajnik V, et al. Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe 2017;21:603–10.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bakke D, Chatterjee I, Agrawal A, Dai Y, Sun J. Regulation of microbiota by vitamin D receptor: a nuclear weapon in metabolic diseases. Nuclear Recept Res 2018;5:101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chatterjee I, Lu R, Zhang Y, et al. Vitamin D receptor promotes healthy microbial metabolites and microbiome. Sci Rep 2020;10:7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Battistini C, Ballan R, Herkenhoff ME, Saad SMI, Sun J. Vitamin D modulates intestinal microbiota in inflammatory bowel diseases. Int J Mol Sci 2021;22:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garg M, Hendy P, Ding JN, Shaw S, Hold G, Hart A. The effect of vitamin D on intestinal inflammation and faecal microbiota in patients with ulcerative colitis. J Crohns Colitis 2018;12:963–72. [DOI] [PubMed] [Google Scholar]
- 36. Hill AB. The environment and disease: association or causation? 1965. J R Soc Med 2015;108:32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. VanderWeele TJ. Can sophisticated study designs with regression analyses of observational data provide causal inferences? JAMA Psychiatry 2020. doi: 10.1001/jamapsychiatry.2020.2588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.