ABSTRACT
Background
The “carnivore diet,” based on animal foods and excluding most or all plant foods, has attracted recent popular attention. However, little is known about the health effects and tolerability of this diet, and concerns for nutrient deficiencies and cardiovascular disease risk have been raised.
Objectives
We obtained descriptive data on the nutritional practices and health status of a large group of carnivore diet consumers.
Methods
A social media survey was conducted 30 March–24 June, 2020 among adults self-identifying as consuming a carnivore diet for ≥6 mo. Survey questions interrogated motivation, dietary intake patterns, symptoms suggestive of nutritional deficiencies or other adverse effects, satisfaction, prior and current health conditions, anthropometrics, and laboratory data.
Results
A total of 2029 respondents (median age: 44 y, 67% male) reported consuming a carnivore diet for 14 mo (IQR: 9–20 mo), motivated primarily by health reasons (93%). Red meat consumption was reported as daily or more often by 85%. Under 10% reported consuming vegetables, fruits, or grains more often than monthly, and 37% denied vitamin supplement use. Prevalence of adverse symptoms was low (<1% to 5.5%). Symptoms included gastrointestinal (3.1%–5.5%), muscular (0.3%–4.0%), and dermatologic (0.1%–1.9%). Participants reported high levels of satisfaction and improvements in overall health (95%), well-being (66%–91%), various medical conditions (48%–98%), and median [IQR] BMI (in kg/m2) (from 27.2 [23.5–31.9] to 24.3 [22.1–27.0]). Among a subset reporting current lipids, LDL-cholesterol was markedly elevated (172 mg/dL), whereas HDL-cholesterol (68 mg/dL) and triglycerides (68 mg/dL) were optimal. Participants with diabetes reported benefits including reductions in median [IQR] BMI (4.3 [1.4–7.2]), glycated hemoglobin (0.4% [0%–1.7%]), and diabetes medication use (84%–100%).
Conclusions
Contrary to common expectations, adults consuming a carnivore diet experienced few adverse effects and instead reported health benefits and high satisfaction. Cardiovascular disease risk factors were variably affected. The generalizability of these findings and the long-term effects of this dietary pattern require further study.
Keywords: low-carbohydrate diet, ketogenic diet, meat, animal-based foods, micronutrients, obesity, diabetes, cardiovascular disease risk
In a survey of >2000 adults following a “carnivore diet” (i.e., one that aims to avoid plant foods), health benefits and satisfaction were generally reported.
Introduction
Dietary variety is near-universally recommended in National Guidelines to satisfy human nutritional needs (1, 2). Consumption of a variety of food groups from both plant and animal food sources has been linked to favorable health outcomes in epidemiologic studies (3) and clinical trials (4–6) and is expected to satisfy the recommended DRIs of macronutrients (i.e., protein, carbohydrates, and fats), micronutrients (i.e., vitamins and minerals), and food components (e.g., dietary fiber).
Nevertheless, restrictive diets have long been promoted for various health and philosophical reasons. One notable eating pattern, a vegan diet, that eliminates animal foods has been promoted for ethical (7), environmental (8), and health (9) benefits—including reduction in BMI, improvement in serum lipids, and cancer prevention (9). However, these reported benefits may be confounded by dietary and nondietary health behaviors, and negative effects have also been reported (10). Vegan diet consumers may not meet DRIs for vitamin B-12, calcium, and protein, and adverse events, such as an increased incidence of bone fractures (11), have been observed.
Recently, popular interest has grown in an opposite eating pattern, a carnivore diet, which aims to eliminate most or all plant-based foods. Numerous social media groups (e.g., https://www.facebook.com/groups/worldcarnivoretribe/) have been formed, with membership of many thousands in the United States and other nations, to promote this diet for health and other benefits. Whereas diets high in animal foods have been commonly discouraged owing to high saturated fats content and low density of essential nutrients and bioactive compounds (e.g., fiber, phytonutrients) (12), little is known about the health status of people habitually following a carnivore eating pattern. According to a common view, long-term consumption of a strictly animal-based diet would be associated with significant nutritional deficiencies (13) and confer negative health effects compared with a plant-based diet (14, 15), including poor gut and gut-microbiota health (16–19), an adverse cardiovascular disease risk pattern (20, 21), and other chronic health complications (22).
What little evidence exists for the sustainability of carnivore diets derives from historic reports on Arctic or nomadic societies, clinical case studies, and accounts on nutrition therapy for diabetes mellitus in the preinsulin era c.1920. Early reports by Arctic researchers and population surveys provide evidence that animal-based diets with little to no plant matter were consumed by traditional populations at high latitude during most of the year (23–25), and were associated with good health and longevity (26, 27). Inspired by their experiences, 2 Arctic explorers participated in a study, partly conducted under strict inpatient observation, of a diet containing only meat (27). After 1 y, they reported good health and displayed no clinical evidence of any vitamin deficiency, although a negative calcium balance was reported (28). Of note, the animal-based diets consumed in this study incorporated both lean and fatty meats, including a variety of organ meats; these were frequently boiled, and sometimes consumed raw, with potential implications for the availability of low-concentration or labile nutrients. Likewise inspired by observations on an indigenous diet in St. Lucia, Dr. John Rollo in 1797 successfully treated 2 patients with diabetes with a diet consisting only of meat and fat. Rollo recommended near-complete elimination of plant foods (29), a prescription that was widely adopted and empirically optimized to prolong the life of people with diabetes in the 19th century. Recognizing the link between carbohydrate intake and glucosuria, some physicians allowed intake of low-carbohydrate vegetables (30), whereas others promoted a strictly meat- and fat-based approach for diabetes management [e.g., Cantani, Primavera (31)]. With the discovery of insulin, these dietary approaches were abandoned in favor of less restrictive mixed diets (32), and modern research on an animal-based diet is sparse.
Although several contemporary treatments of obesity or type 2 diabetes promote high intake of meat and fat (e.g., the Atkins diet) (33), these diets typically include, or reintroduce after short periods, consumption of low-carbohydrate vegetables and low-sugar fruits. Whereas a recent perspective suggests that all essential nutrients can be obtained from a carnivore diet (34), few empirical data are available. Therefore, the aim of this study was to characterize the motivation, dietary behaviors, self-perceived health status, and satisfaction of a large group of adults habitually consuming a carnivore diet, and thereby to provide insights into this poorly characterized dietary approach.
Methods
Design
Using an online survey, we collected self-reported data from respondents who self-identified as following a carnivore diet for a minimum of 6 mo. Our aims were to 1) characterize the diet consumed by parti-cipants, 2) describe perceived health status and changes in health since starting the diet, 3) assess perceived symptoms of nutritional deficiencies or other adverse effects, and 4) evaluate the satisfaction and acceptance of consuming a carnivore diet. The study was approved by the Boston Children's Hospital Institutional Review Board. Electronic consent was obtained from all respondents, with no restrictions on data use. The only identifiable data collected were participants’ email addresses; these were removed for de-identification upon completion of data collection and elimination of duplicate responses.
Participants and enrollment
Respondents were recruited from open social media communities (World Carnivore Tribe, Facebook, 23%; Instagram, 18%; r/Zerocarnb, Reddit, 7%; Zeroing in on Health, Facebook, 5%; Twitter, 5%; other, 42%). Inclusion criteria were age ≥ 18 y and consumption of a carnivore diet for ≥6 mo by self-report. After 2 eligibility questions, a link to the study survey was displayed and sent by e-mail, with ≤3 reminders provided over a 1-mo period. Survey data acquisition was conducted between 30 March and 24 June, 2020. As such, data collection occurred during the period of the COVID-19 pandemic when most people were in lockdown.
Of an initial 3883 respondents, 1418 were excluded owing to age < 18 y (n = 4), diet duration < 6 mo (n = 156), incomplete screening information (n = 113), declining participation (n = 87), or not starting the main survey (n = 1058). Duplicate survey responses (n = 28) were identified on the basis of e-mail addresses and deleted. Participants who did not provide at least a diet start date and food intake frequency information (n = 126) in the main survey, or whose diet start date was within 6 mo of survey participation (n = 282), were excluded from the analysis. A total of 2029 (52%) respondents were eligible and willing to participate and provided sufficient information to be included in the study.
Data collection and treatment
Survey instruments
Data were collected and managed with research electronic data capture (REDCap, version 10.0.23; Vanderbilt University) tools hosted at Boston Children's Hospital (35). Survey questions were developed in consultation with members of the carnivore diet community and addressed several domains: 1) current intake frequency of main food groups and relevant food items, as well as food preparation and related considerations; 2) chronic medical conditions and medication use, anthropometric and laboratory data, perceived health and well-being, and perceived symptoms of nutritional deficiencies or other adverse effects—including in the present and before starting the diet; 3) diet satisfaction and social support; and 4) sociodemographic data. Multiple-choice questions were used to solicit specific habits, reasons for choosing the diet, medical conditions, and medication intake. Modified Likert scales were used to estimate the frequency of intake of prespecified food categories; satisfaction; and changes in health, well-being, chronic conditions, and symptoms. Highest educational attainment was categorized as primary (primary school or less), secondary (secondary school, including high school), postsecondary [intermediate between secondary and university (e.g., some college and technical training)], or tertiary (completed college, university, graduate school, or equivalent). Participants were asked to specify their income category as lower, middle, or upper. Race and ethnicity were self-reported with multiple-choice and free-text options. Self-reported anthropometric and laboratory data were collected for the following timeframes: Prior: within 1 and 5 y before starting the diet, respectively; Present: within a year of taking the survey and ≥3 mo after starting the carnivore diet. Participants were asked to specify source of anthropometrics as clinician or self-measured, and data were prioritized in that order.
Units of measure
To account for the international background of people within the social media communities, respondents were able to select among unit systems (metric, imperial, international system of units, conventional); data were collected accordingly and converted to metric and conventional units with the following conversion factors: height—inches to centimeters, 2.54; weight—pounds to kilograms, 0.45; glucose—mmol/L to mg/dL, 18; total cholesterol (TC), LDL-cholesterol, and HDL-cholesterol—mmol/L to mg/dL, 38.67; triglycerides (TG)—mmol/L to mg/dL, 88.57; β-hydroxybutyrate—mg/dL to mmol/L, 0.096; C-reactive protein (CRP)—nmol/L to mg/dL, 0.105; creatinine (Cr)—μmol/L to mg/dL, 0.113; alanine aminotransferase, aspartate aminotransferase, and γ-glutamyltransferase (GGT)—μkat/L to U/L, 60. Glycated hemoglobin (HbA1c) was converted from mmol/mol to percentages by using the formula HbA1c (%) = 0.09148 × HbA1c (mmol/mol) + 2.152, and from estimated average glucose (eAG; mmol) with the formula HbA1c (%) = (eAG*18.02 + 47.6)/28.7, as proposed by the International Federation of Clinical Chemistry and Laboratory Medicine for the standardization of HbA1c (http://www.ngsp.org/ifccngsp.asp).
Calculated variables
Time on the diet and timing of anthropometric and laboratory data were calculated with reported dates and the survey date. Height was averaged if >1 value was reported. BMI (in kg/m2) was calculated by dividing weight (kg) by the square of height (m). Symptoms consistent with nutrient deficiency or other adverse effects were assessed categorically; Supplemental Table 1lists all symptoms queried. Changes in symptoms were grouped into 2 categories: stable/improved and new/deteriorated. Respondents were designated as having diabetes if they reported using the carnivore diet as a way to manage or reverse diabetes, if they had ever been prescribed any oral or injectable diabetes medications, or if they reported an HbA1c ≥6.5%. Respondents were designated as not using any vitamins if they answered “never” to both using a multivitamin and using any other vitamin supplement.
Statistical analysis
Analyses were performed using SPSS version 27 (SPSS, IBM Corporation). Continuous data were visually assessed for normality. Owing to skewed data distribution, we used nonparametric testing and report medians and IQRs. Statistical significance was defined as a 2-tailed P value < 0.01. Present and prior (prediet) data were compared by Wilcoxon rank-link test for respondents with data at both time points. Comparisons were made between respondents reporting never taking any vitamin supplements and the rest of the group, and between respondents with and without a diabetes diagnosis, by chi-square test for nominal variables and Mann–Whitney U test for numeric variables. Because all information was not available for all participants, the number of answers is reported for each variable, and percentages based on number of respondents are given.
Results
Participants
Table 1 lists descriptive characteristics. Most respondents were from the United States, Canada, Europe, or Australia; 67% were male; 83% were white and non-Hispanic; and 64% completed college or the equivalent. Income spanned all classes with 14% reporting low, 66% middle, and 20% high income. Seven women were pregnant and 10 were breastfeeding at median 12 mo [IQR: 9–18 mo] postpartum. The median [IQR] time following a carnivore diet was 14 mo [9–20 mo]; 93% of participants stated health reasons as their motivation for beginning the diet.
TABLE 1.
Participant characteristics
| Characteristics | Responses, n | Finding, n (%) or median [IQR] | Range |
|---|---|---|---|
| Time on carnivore diet, mo | 2029 | 14 [9–20] | 6–337 |
| Anthropometrics | |||
| Sex | 2002 | ||
| Male | 1347 (67) | ||
| Female | 651 (33) | ||
| Other | 4 (0.2) | ||
| Age, y | 1991 | 44 [34–54] | 18–85 |
| Height, cm | 1818 | 175 [168–183] | 147–203 |
| Weight, kg | 1699 | 76 [66–86] | 38–176 |
| BMI, kg/m2 | 1682 | 24.3 [22.1–27.0] | 13.7–56.6 |
| Sociodemographics | |||
| Country of residence | 1891 | ||
| United States/Canada | 1205 (64) | ||
| Europe/United Kingdom | 217 (11) | ||
| Australia | 146 (8) | ||
| Other | 323 (17) | ||
| Race | 1889 | ||
| White, non-Hispanic | 1573 (83) | ||
| Black | 16 (0.9) | ||
| Hispanic or Latino | 74 (4) | ||
| Asian | 59 (3) | ||
| Other | 167 (9) | ||
| Education | 1890 | ||
| Primary or less | 15 (0.8) | ||
| Secondary | 179 (9) | ||
| Postsecondary | 484 (26) | ||
| Tertiary | 1212 (64) | ||
| Income | 1888 | ||
| Low | 261 (14) | ||
| Middle | 1244 (66) | ||
| High | 383 (20) | ||
| Reproductive status (female or other) | 653 | ||
| Pregnant | 7 (1) | ||
| Breastfeeding | 10 (2) | ||
| Motivation | |||
| Aiming for ketosis | 2025 | 832 (41) | |
| Reason for carnivore | 2029 | ||
| Health/body weight | 1879 (93) | ||
| Food preference | 671 (33) | ||
| Curiosity | 303 (15) | ||
| Ethics | 185 (9) | ||
| Other | 256 (13) | ||
| Health reasons | 1879 | ||
| Body weight/composition | 1572 (84) | ||
| Athletic performance | 869 (46) | ||
| Focus/energy | 1398 (74) | ||
| Allergies/skin/autoimmunity | 1131 (60) | ||
| Digestive health | 969 (52) | ||
| Mental health | 848 (45) | ||
| Diabetes | 232 (12) | ||
| Other | 297 (16) | ||
Food intake and eating habits
Table 2 and Supplemental Table 2 show the intake of food groups and individual foods. Red meat other than pork (e.g., beef, lamb, venison, buffalo, goat) was the most commonly consumed food, followed by eggs and nonmilk dairy, whereas pork, poultry, and seafood were less frequently eaten. Weekly or more frequent consumption was reported for organ meat by 42%, and for nonmilk dairy by 72%. Less than 10% of respondents consumed starchy vegetables, nonstarchy vegetables, fruits, or grains more often than once monthly, and 37% reported no use of any vitamin supplements. Use of other over-the-counter supplements (e.g., dietary, herbal, digestive enzymes, antioxidants) was denied by 75%. Alcohol was rarely consumed, with 63% reporting frequency of less than once a month or never. More than 50% of participants drank coffee at least daily. In contrast to the typical Western/Standard American diets, few individuals following the carnivore diet reported consuming fast foods.
TABLE 2.
Frequency of food intake1
| Intake frequency, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Food class | n | Each meal | ≥Daily | >Weekly | Weekly | >Monthly | Monthly | <Monthly | Never |
| Meat | |||||||||
| Red meat, nonpork | 2027 | 39 | 46 | 14 | 0.3 | 0.3 | 0.1 | 0.2 | 0.1 |
| Pork | 2005 | 2 | 11 | 38 | 15 | 11 | 9 | 8 | 7 |
| Poultry | 2007 | 0.5 | 2 | 20 | 18 | 18 | 14 | 19 | 8 |
| Seafood, fish | 2015 | 0.5 | 3 | 17 | 19 | 19 | 15 | 19 | 7 |
| Seafood, nonfish | 2014 | 0.1 | 0.7 | 8 | 11 | 16 | 17 | 27 | 20 |
| Organ meat | 2017 | 1 | 5 | 18 | 18 | 14 | 10 | 14 | 20 |
| Processed meats | 2020 | 0.1 | 3 | 16 | 11 | 16 | 12 | 20 | 22 |
| Dairy | |||||||||
| Eggs | 2023 | 7 | 37 | 33 | 4 | 5 | 4 | 4 | 5 |
| Dairy, nonmilk | 2023 | 7 | 36 | 24 | 5 | 7 | 4 | 5 | 11 |
| Dairy, milk | 2009 | 0.9 | 8 | 6 | 2 | 4 | 4 | 10 | 65 |
| Nuts | 2020 | 0.1 | 1 | 5 | 3 | 5 | 8 | 14 | 64 |
| Fruits/vegetables | |||||||||
| Vegetables, nonstarchy | 2021 | 0.0 | 0.5 | 2 | 3 | 3 | 5 | 17 | 69 |
| Vegetables, starchy | 2024 | 0.0 | 0.1 | 1 | 2 | 2 | 4 | 17 | 74 |
| Legumes | 2018 | 0.0 | 0.0 | 0.0 | 0.3 | 0.3 | 1 | 9 | 89 |
| Fruit | 2018 | 0.1 | 0.7 | 2 | 2 | 3 | 6 | 20 | 66 |
| Grains | 2016 | 0.0 | 0.1 | 0.6 | 1 | 2 | 4 | 14 | 79 |
| Sugars | |||||||||
| Sugar | 2017 | 0.0 | 0.7 | 1.0 | 1 | 2 | 4 | 13 | 78 |
| Honey | 2013 | 0.1 | 1 | 2 | 2 | 3 | 4 | 14 | 74 |
| Noncalorie sweeteners | 2017 | 0.7 | 9 | 7 | 3 | 4 | 4 | 8 | 65 |
| Miscellaneous | |||||||||
| Exceptions2 | 2027 | 0.3 | 1 | 5 | 9 | 10 | 11 | 31 | 33 |
| Multivitamin usage | 1988 | 0.7 | 8 | 3 | 1 | 1 | 0.9 | 4 | 80 |
| Other vitamin usage | 1706 | 2 | 29 | 11 | 2 | 3 | 1 | 5 | 47 |
Participants were asked to report intake frequency of the listed food groups and items on an 8-point scale. For visualization, the response frequencies are color-coded dark gray if ≥70%, with increasing brightness if 40%–69%, 20%–39%, 10%–19%, 5%–9%, 1%–4%, and <1%.
Frequency of making exceptions from the carnivore diet.
Most participants reported eating 1–3 times daily (differentiation between meals and snacks was not queried), including 16% three times per day, 64% twice per day, and 17% once per day. Generally, more participants reported choosing cuts of meat with high (61%) or moderate (37%) fat content as opposed to lean cuts (2%). Seventy-six percent reported a preference for consuming meat at raw, rare, or medium-rare doneness. Intention to achieve nutritional ketosis was reported by 41%, among whom 41% monitored ketone concentrations; 56% intended to achieve a medium or high amount of salt intake, as commonly recommended in the setting of low-carbohydrate diets that are associated with increased natriuresis (36). Additional dietary data are available in Supplemental Table 3.
Perceived health-related outcomes
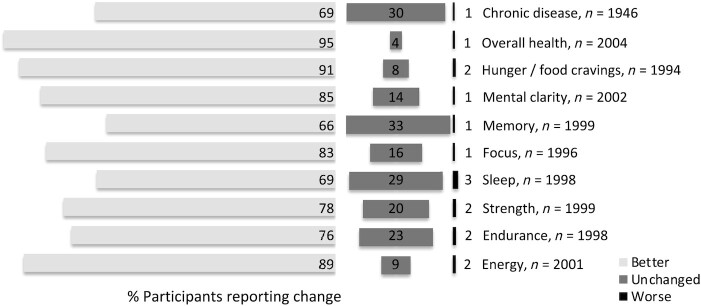
Participants reported improvements in chronic medical conditions, general health, and aspects of well-being such as energy, sleep, strength, endurance, mental clarity, memory, and focus (Figure 1). Table 3 lists the prevalence of specific medical conditions and changes in their severity. Although most queried medical conditions improved with the diet, lipid abnormalities were variably affected: 56% of participants reported resolution or improvement, 18% stability, and 27% new occurrence or worsening. Ophthalmologic conditions were improved or unchanged with equal frequency.
FIGURE 1.
Reported changes in health status. Participants were asked to rate their current overall health and well-being on a 3-point scale as better (light gray bars), unchanged (dark gray), or worse (black) compared with the time before starting the carnivore diet. Percentage of respondents is given.
TABLE 3.
Self-reported prevalence of and changes in chronic conditions and medication usage1
| Prevalence, n (%) | Changes when following diet, % | |||||
|---|---|---|---|---|---|---|
| Chronic condition | Resolved | Improved | Unchanged | Worsened | New | |
| Obesity/overweight | 928 (46) | 52 | 41 | 5 | 1 | 0.2 |
| Underweight | 100 (5) | 52 | 28 | 14 | 5 | 1 |
| Lipid abnormalities | 429 (21) | 27 | 29 | 18 | 19 | 8 |
| Hypertension | 374 (18) | 61 | 32 | 7 | 0.3 | 0.0 |
| Cardiovascular | 126 (6) | 41 | 43 | 15 | 0.8 | 0.8 |
| Diabetes/insulin resistance | 402 (20) | 74 | 24 | 1 | 0.0 | 0.0 |
| Gastrointestinal | 531 (26) | 59 | 38 | 1 | 1 | 0.2 |
| Endocrinologic | 191 (9) | 40 | 48 | 12 | 0.5 | 0.0 |
| Autoimmune | 369 (18) | 36 | 53 | 11 | 0.0 | 0.0 |
| Musculoskeletal | 502 (25) | 42 | 54 | 4 | 0.0 | 0.2 |
| Neurological | 89 (4) | 42 | 42 | 16 | 1 | 0.0 |
| Cognitive | 100 (5) | 42 | 54 | 4 | 0.0 | 0.0 |
| Psychiatric | 479 (24) | 48 | 48 | 4 | 0.0 | 0.0 |
| Respiratory | 354 (17) | 51 | 34 | 14 | 0.0 | 0.0 |
| Urologic | 181 (9) | 76 | 16 | 8 | 0.0 | 0.6 |
| Dermatologic | 690 (34) | 44 | 48 | 7 | 0.6 | 0.1 |
| Ophthalmologic | 327 (16) | 12 | 36 | 51 | 0.6 | 0.6 |
| Hematologic | 127 (6) | 66 | 18 | 14 | 0.0 | 2 |
| Oncologic | 75 (4) | 41 | 12 | 47 | 0.0 | 0.0 |
| Other | 208 (10) | 42 | 45 | 13 | 0.0 | 1 |
| Diabetes medications | Discontinued | Decreased | Unchanged | Increased | New | |
|---|---|---|---|---|---|---|
| Insulin | 29 (1) | 522 | 38 | 3 | 0.0 | 7 |
| Insulin (T2DM only) | 13 (0.6) | 92 | 0.0 | 0.0 | 0.0 | 8 |
| Diabetes injectables, other | 16 (0.8) | 100 | 0.0 | 0.0 | 0.0 | 0.0 |
| Oral diabetes medications | 82 (4) | 84 | 14 | 2 | 0.0 | 0.0 |
Participants were asked if they had ever suffered from or taken any of the listed conditions or medications. n (%) of positive responses is given in the first column (prevalence) and is the denominator for percentages in the subsequent columns. Positive respondents were then asked to rate the severity of each condition relative to the time before starting the carnivore diet on a 5-point scale. For visualization, response frequencies are color-coded dark gray if ≥70%, and in increasing brightness if 40%–69%, 20%–39%, 10%–19%, 5%–9%, 1%–4%, and <1%. T2DM, type 2 diabetes mellitus.
Includes people with type 1 diabetes mellitus and T2DM.
Table 4 and Supplemental Figure 1 summarize changes in anthropometrics and laboratory studies. Median [IQR] BMI decreased from 27.2 [23.5–31.9] to 24.3 [22.1–27.0]. As observed with other diets low in carbohydrate, TC and LDL-cholesterol were markedly elevated, whereas HDL-cholesterol and TG were in an optimal range. The Present ratio of TG to HDL-cholesterol was 1.0 [IQR: 0.7–1.5]. CRP and GGT decreased, and other liver enzymes, Cr, and HbA1c were unchanged from pre-diet to current. Coronary artery calcium score was low at Prior (2; IQR: 0–132) and Present (0; IQR: 0–27) among the few respondents reporting this value.
TABLE 4.
Self-reported current and prediet anthropometrics and laboratory studies1
| Data source,2n | Current3 | Prediet3 | |||||
|---|---|---|---|---|---|---|---|
| Measure | Current/prediet/pairs | Median | Q1 | Q3 | Median | Q1 | Q3 |
| Weight, kg | 1699/1333/1235 | 76* | 66 | 86 | 85 | 71 | 101 |
| BMI, kg/m2 | 1682/1315/1229 | 24.3* | 22.1 | 27.0 | 27.2 | 23.5 | 31.9 |
| TC, mg/dL | 467/334/259 | 256* | 214 | 323 | 209 | 175 | 243 |
| LDL-C, mg/dL | 462/326/247 | 172* | 131 | 237 | 126 | 98 | 164 |
| HDL-C, mg/dL | 466/333/256 | 68* | 57 | 84 | 58 | 45 | 73 |
| TG, mg/dL | 465/334/260 | 68* | 50 | 94 | 83 | 58 | 122 |
| HbA1c, % | 340/204/158 | 5.3* | 5.0 | 5.5 | 5.3 | 5.1 | 5.7 |
| CRP, mg/dL | 210/75/73 | 0.7 (0.8)* | 0.3 | 1.5 (2.0) | 1.0 | 0.3 (0.4) | 3.3 |
| Cr, mg/dL | 435/307/244 | 0.9 | 0.8 | 1.1 | 0.9 | 0.8 | 1.1 |
| ALT, U/L | 336/247/190 | 26 | 19 | 35 | 25 | 19 (20) | 35 |
| AST, U/L | 305/229/177 | 23 | 18 (19) | 28 | 22 | 18 (19) | 30 |
| GGT, U/L | 159/99/74 | 15* | 11 (12) | 20 (21) | 18 (19) | 13 (14) | 24 |
| CAC | 118/55/15 | 0 (81) | 0 (12) | 27 (401) | 2 (55) | 0 | 132 (182) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAC, coronary artery calcium score; Cr, creatinine; CRP, C-reactive protein; GGT, γ-glutamyltransferase; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; Q, quartile; TC, total cholesterol; TG, triglyceride.
Number of participants who reported data for the following time points: current/prediet/both (pairs).
Medians and quartiles are given for the entire sample, and in parentheses for participants with available pre- and postdiet measures (pairs) when >5% discrepant.
*P < 0.01, paired comparison.
Among the 262 respondents with type 1 diabetes mellitus or type 2 diabetes mellitus (T2DM), prior BMI, HbA1c, and TG were higher than among those without diabetes. Respondents with diabetes experienced relatively large median [IQR] reductions in BMI (4.3 [1.4–7.2]) and HbA1c (−0.4% [0% to 1.7%]) (Supplemental Table 4). Diabetes medication use was significantly reduced. All respondents with diabetes discontinued noninsulin injection agents, 84% discontinued oral medications, and 92% of participants with T2DM discontinued insulin (Table 3).
Reported prevalence of adverse effects or symptoms consistent with nutritional deficiency was generally low, as shown in Supplemental Table 1, and commonly preceded the diet. New or worsened diarrhea occurred in 5.5%, constipation in 3.1%, weight gain in 2.3%, muscle cramps in 4.0%, hair loss or thinning in 1.9%, insomnia in 1.7%, dry skin in 1.4%, itchiness in 1.1%, heart rate changes in 1.1%, brittle fingernails in 1.0%, and menstrual irregularity in 1.0%. Worsening or incidence of any of the other assessed symptoms occurred in <1% of participants. Prevalence and incidence of symptoms were not increased compared with the overall group in participants who denied intake of vitamin supplements or denied consuming organ meat or dairy.
Satisfaction and support
Participants reported high levels of overall satisfaction with the diet (Supplemental Table 5). The majority perceived no impact on their social life, and neutral or positive supportiveness from social contacts. Medical providers were perceived as neutral, supportive, and unsupportive by generally similar proportions.
Discussion
In this social media–based survey, a self-selected group of adults consuming a carnivore diet for ≥6 mo reported perceived good health status, perceived absence of symptoms of nutritional deficiencies, and high satisfaction with this eating pattern. To our knowledge, this is the first modern report on a large group of people habitually consuming few plant foods, a dietary pattern broadly considered incompatible with good health.
Weight loss and other health benefits were most frequently indicated as the motivation for adoption of a carnivore diet. In accordance with this possibility, respondents reported substantial BMI reduction and improvements in physical and mental well-being, overall health, and numerous chronic medical conditions. Respondents with diabetes reported special benefit, including greater weight loss than the overall group, and marked reductions in diabetes medication usage and HbA1c—notable findings in view of the generally low success of lifestyle interventions for obesity and diabetes (37, 38). Although we did not formally assess macronutrient intake, carbohydrate content in meat and other animal-based foods is minimal, and inherent limits to protein intake exist. Both ancestral data (39) and self-reported preference of fatty cuts of meat in our survey suggest high fat intake with the carnivore diet. As such, the macronutrient composition of a carnivore diet would likely correspond to other very-low-carbohydrate diets (e.g., ketogenic, Atkins). For this reason, studies of these diets may provide relevant comparisons. In meta-analyses of trials for T2DM, low- compared with high-carbohydrate diets produced greater weight loss (40–42), lower HbA1c (40–46), and reduction in usage of glucose-lowering medications (41, 43, 45, 46), consistent with our observations. Although general dietary adherence and glycemic effects diminish over time (47), the findings of 1 recent nonrandomized trial suggest that a very-low-carbohydrate diet may be sustainable and efficacious when combined with high-intensity individual support (48).
Consistent with other low-carbohydrate diet studies (40–45), respondents reported a mixed blood lipid pattern: LDL-cholesterol, a major conventional cardiovascular disease risk factor, was markedly elevated whereas HDL-cholesterol and TG were favorable. However, LDL-cholesterol elevation, when associated with low TG, may reflect large, buoyant lipoprotein particles, possibly comprising a relatively low-risk subtype (49). Indeed, the low ratio of TG to HDL-cholesterol is suggestive of high insulin sensitivity and good cardiometabolic health (50). However, it is unclear whether this apparent benefit of the diet, together with the reported weight reduction and improved glycemic control (in the subset with diabetes), would counterbalance or outweigh any increased risk from LDL-cholesterol elevation. For individuals with a more extreme LDL-cholesterol response, drug treatment could be considered—an option that is generally more effective and better tolerated than drug treatment of insulin-resistance dyslipidemia.
Beyond macronutrient composition, elimination of allergenic, inflammatory, or other food components may provide potential health benefits to individuals following a carnivore diet. Food allergies and sensitivities are common, and predominantly related to plant foods (51). Some plant chemicals may produce adverse effects through other mechanisms, such as lecithin in beans, cyanogenic glycosides in certain seeds, and glycoalkaloids in potatoes. Indeed, >50% of survey participants started the carnivore diet to improve allergic, skin, or autoimmune conditions, or digestive health, and many reported improvements in inflammatory conditions and related symptoms. Conversely, dietary intake may be low for vitamins that are typically derived from plant foods (e.g., fruits, vegetables, nuts, seeds, and grains) or from nutritional fortification of staple foods (e.g., milk, juices, cereals, pastas, and other grain products) (52, 53). In addition, often unquantified phytochemicals (e.g., polyphenols, alkylresorcinols, phytosterols) are largely absent from the diet. Although these phytochemicals do not have DRIs, they have been linked to cardiometabolic benefits (54, 55). In people who eat meat only with exclusion of dairy (∼30% in this survey), calcium intake might also be low, as illustrated by the low intake and negative calcium balance in 2 Arctic explorers (28). Although essential nutrients can presumably be derived in sufficient amounts from animal foods (34), they are present in less commonly consumed parts of the animal, such as fat and organ meats (vitamins A and D), or bone (calcium), or may be reduced during food preparation (vitamin C) (34). Vitamin C is of particular interest, because meats are not formally considered a good source of vitamin C (i.e., they contain <10% of the DRI per serving) (56). Typical symptoms of deficiencies in these vitamins would include dermatological, cognitive, or neurological symptoms, as listed in Supplemental Table 1. A worsening or new presentation of these symptoms was reported in <2% of survey participants, whereas the majority of participants reported improvements, resolution, or no change—regardless of intake of vitamins, organ meat, or dairy. Given the self-reported nature of these findings, it remains unclear whether clinical or subclinical symptoms of nutrient deficiency are present. Research is needed to clarify the absence of perceived symptoms of nutrient deficiencies and the underlying biochemical processes that govern nutrient needs with the long-term consumption of a carnivore diet. It is possible that requirements for some micronutrients may be lower than those established in DRIs for the general population (57), related to remodeling of the gut microbiome, whole-body metabolism, and nutrient utilization in the setting of a low-carbohydrate carnivore diet, analogous to observations with a vegan diet (58).
Respondents reported high levels of satisfaction, and little social impact, from following a carnivore diet. Notably, medical providers were perceived as supportive, neutral, or unsupportive at generally similar proportions despite the discrepancy of the carnivore diets from dietary guidelines. Whereas meat is more expensive than grains and starchy foods, it may be less expensive on a caloric basis, depending on location and specific comparisons, than fresh fruits and nonstarchy vegetables (59), and cost may be in addition offset by decreased expenditure for diabetes and other medications. Our respondents spanned low to high income classes, suggesting against major financial barriers to the diet.
Our study does not address several important concerns related to consumption of an animal-based diet. Intensive animal production, typically with use of commodity grains and soy for feed, causes significant environmental harms and raises ethical issues about animal treatment. These concerns may be mitigated, to some degree, with integrated, pasture-based agricultural systems (60) and other interventions, such as the use of algal feed additives to reduce greenhouse gas production (61, 62). For context, industrial-scale, commodity grain monoculture may also cause substantial environmental impacts and loss of wildlife.
These findings must be interpreted cautiously in view of several major design limitations. Our survey assessed the perception of individuals following a carnivore diet and did not objectively assess diet, nutrient status, health-related outcomes, or confounding health-associated behaviors; and no physiological or biochemical measurements were obtained. These self-reported data are prone to recall and reporting bias, especially for the prediet information. Specifically, participants may have started the diet during a time of poor health and perceived subsequent regression to the mean as a benefit of the diet and being online community members may have resulted in over-reporting of adherence and perceived beneficial effects. Because no validated instruments are available to assess food intake frequency in a carnivore population, we used modified Likert scales. We did not assess portion size or other quantitative intake characteristics, nor did we use other dietary instruments for a more detailed characterization of the diet; these comprise topics of future investigations. Finally, the generalizability of the findings is unknown owing to the existence of selection bias, because individuals who experienced adverse effects or lack of health benefits are likely to have abandoned the diet and would therefore not have been captured in this survey. Adults adhering to a carnivore diet and responding to this online survey represent a special subpopulation with high levels of motivation and other health-related behaviors (e.g., physical activity, consumption of relatively whole, unprocessed foods). Therefore, respondents likely differ from the general population in ways that could influence the effectiveness, practicality, and safety of a carnivore diet. Related to this point, we did not obtain detailed information on diet and lifestyle habits before beginning a carnivore diet.
Our study reports on a large group of participants following a carnivore diet, with perceived health benefits and absence of symptoms consistent with nutritional deficiencies, providing insights into a poorly characterized dietary approach. However, the data are limited by several major design limitations inherent to the survey design. A clearer understanding of the long-term safety and benefits of a carnivore diet, exact dietary habits of people following this diet, and the generalizability of our findings, must await additional research.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Shawn Baker and Travis Statham for input in developing the survey instruments, online distribution of the survey, and critical review of the manuscript, and Shui Yu for statistical verification. The authors’ responsibilities were as follows—BSL and OHH: conducted the research and analyzed the data; BSL, JTM, and OHH: wrote the paper; DSL: revised the paper; BSL: had primary responsibility for the final content; and all authors: designed the research and read and approved the final manuscript.
Notes
BSL and OHH were supported by National Institute of Diabetes and Digestive and Kidney Diseases grants K23 DK119546 and R03 DK123541 to study a low-carbohydrate diet. JTM was supported by National Center for Complementary and Integrative Health training grant T32AT004094. The funders had no involvement in the design, implementation, analysis, and interpretation of the data.
Author disclosures: DSL reports royalties for books that recommend a carbohydrate-modified diet; his spouse owns a nutrition education and consulting business. All other authors report no conflicts of interest.
Supplemental Figure 1 and Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: Cr, creatinine; CRP, C-reactive protein; eAG, estimated average glucose; GGT, γ-glutamyltransferase; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, Low-density lipoprotein; TC, total cholesterol; TG, triglyceride; T2DM, type 2 diabetes mellitus.
Contributor Information
Belinda S Lennerz, Email: belinda.lennerz@childrens.harvard.edu, New Balance Foundation Obesity Prevention Center, Boston Children's Hospital, Boston, MA, USA; Division of Endocrinology, Boston Children's Hospital, Boston, MA, USA; Department of Pediatrics, Harvard Medical School, Boston, MA, USA.
Jacob T Mey, Integrated Physiology and Molecular Medicine, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Owen H Henn, New Balance Foundation Obesity Prevention Center, Boston Children's Hospital, Boston, MA, USA; Division of Endocrinology, Boston Children's Hospital, Boston, MA, USA.
David S Ludwig, New Balance Foundation Obesity Prevention Center, Boston Children's Hospital, Boston, MA, USA; Division of Endocrinology, Boston Children's Hospital, Boston, MA, USA; Department of Pediatrics, Harvard Medical School, Boston, MA, USA.
Data Availability
Survey instruments and data described in the article, code book, and analytic code will be made available upon request.
References
- 1. US Department of Agriculture and US Department of Health and Human Services (DHHS) . Dietary Guidelines for Americans, 2020–2025. [Internet]. 9th ed. Washington (DC): USDA and US DHHS; 2020. Available from: https://dietaryguidelines.gov(accessed October 5, 2021). [Google Scholar]
- 2. Davis C, Etta S. Dietary recommendations and how they have changed over time. In: Frazão E, editor. America's eating habits: changes and consequences. Washington (DC): USDA Economic Research Service; 1999. p. 33–50. [Google Scholar]
- 3. O'Connor LE, Hu EA, Steffen LM, Selvin E, Rebholz CM. Adherence to a Mediterranean-style eating pattern and risk of diabetes in a U.S. prospective cohort study. Nutr Diabetes. 2020;10(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MMet al. . A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117–24. [DOI] [PubMed] [Google Scholar]
- 6. Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra Jet al. . Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. [DOI] [PubMed] [Google Scholar]
- 7. Janssen M, Busch C, Rodiger M, Hamm U. Motives of consumers following a vegan diet and their attitudes towards animal agriculture. Appetite. 2016;105:643–51. [DOI] [PubMed] [Google Scholar]
- 8. Errickson F, Kuruc K, McFadden J. Animal-based foods have high social and climate costs. Nature Food. 2021;2(4):274–81. [DOI] [PubMed] [Google Scholar]
- 9. Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640–9. [DOI] [PubMed] [Google Scholar]
- 10. Tong TYN, Appleby PN, Bradbury KE, Perez-Cornago A, Travis RC, Clarke R, Key TJ. Risks of ischaemic heart disease and stroke in meat eaters, fish eaters, and vegetarians over 18 years of follow-up: results from the prospective EPIC-Oxford study. BMJ. 2019;366:l4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tong TYN, Appleby PN, Armstrong MEG, Fensom GK, Knuppel A, Papier K, Perez-Cornago A, Travis RC, Key TJ. Vegetarian and vegan diets and risks of total and site-specific fractures: results from the prospective EPIC-Oxford study. BMC Med. 2020;18(1):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barnard ND, Leroy F. Children and adults should avoid consuming animal products to reduce risk for chronic disease: YES. Am J Clin Nutr. 2020;112(4):926–30. [DOI] [PubMed] [Google Scholar]
- 13. Calton JB. Prevalence of micronutrient deficiency in popular diet plans. J Int Soc Sports Nutr. 2010;7(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melina V, Craig W, Levin S. Position of the Academy of Nutrition and Dietetics: vegetarian diets. J Acad Nutr Diet. 2016;116(12):1970–80. [DOI] [PubMed] [Google Scholar]
- 15. Crosby L, Davis B, Joshi S, Jardine M, Paul J, Neola M, Barnard ND. Ketogenic diets and chronic disease: weighing the benefits against the risks. Front Nutr. 2021;8:702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koponen KK, Salosensaari A, Ruuskanen MO, Havulinna AS, Männistö S, Jousilahti P, Palmu J, Salido R, Sanders K, Brennan Cet al. . Associations of healthy food choices with gut microbiota profiles. Am J Clin Nutr. 2021;114(2):605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daïen CI, Pinget GV, Tan JK, Macia L. Detrimental impact of microbiota-accessible carbohydrate-deprived diet on gut and immune homeostasis: an overview. Front Immunol. 2017;8:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang H, Greenwood DC, Risch HA, Bunce D, Hardie LJ, Cade JE. Meat consumption and risk of incident dementia: cohort study of 493,888 UK Biobank participants. Am J Clin Nutr. 2021;114(1):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang Y, Cao D, Chen Z, Chen B, Li J, Guo J, Dong Q, Liu L, Wei Q. Red and processed meat consumption and cancer outcomes: umbrella review. Food Chem. 2021;356:129697. [DOI] [PubMed] [Google Scholar]
- 20. Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr. 2014;112(5):762–75. [DOI] [PubMed] [Google Scholar]
- 21. Crimarco A, Springfield S, Petlura C, Streaty T, Cunanan K, Lee J, Fielding-Singh P, Carter MM, Topf MA, Wastyk HCet al. . A randomized crossover trial on the effect of plant-based compared with animal-based meat on trimethylamine-N-oxide and cardiovascular disease risk factors in generally healthy adults: Study With Appetizing Plantfood—Meat Eating Alternative Trial (SWAP-MEAT). Am J Clin Nutr. 2020;112(5):1188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Norat T. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6(6):e20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heinbecker P. Studies on the metabolism of Eskimos. J Biol Chem. 1928;80(2):461–75. [Google Scholar]
- 24. Krogh A, Krogh MJ. A study of the diet and metabolism of Eskimos undertaken in 1908 on an expedition to Greenland. Medd Grønland. 1914;51(1):1–52. [Google Scholar]
- 25. Håglin L. Nutrient intake among Saami people today compared with an old, traditional Saami diet. Arctic Med Res. 1991;(Suppl):741–6. [PubMed] [Google Scholar]
- 26. Sköld P, Axelsson P. The northern population development; colonization and mortality in Swedish Sapmi, 1776–1895. Int J Circumpolar Health. 2008;67(1):29–44. [PubMed] [Google Scholar]
- 27. McClellan WS, Du Bois EF. Clinical calorimetry: XLV. Prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87(3):651–68. [Google Scholar]
- 28. McClellan WS, Rupp VR, Toscani V. Clinical calorimetry. XLVI. Prolonged meat diets with a study of the metabolism of nitrogen, calcium and phosphorus. J Biol Chem. 1930;87(3):669–80. [Google Scholar]
- 29. Rollo J. Account of two cases of diabetes mellitus, with remarks. Ann Med (Edinb). 1797;2:85–105. [PMC free article] [PubMed] [Google Scholar]
- 30. Brunton TL. Lectures on the pathology and treatment of diabetes mellitus. BMJ. 1874;1(686):221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ebstein W. Über die Lebensweise der Zuckerkranken. Wiesbaden, Germany: J.F. Bergmann; 1898. p. 15–20. [Google Scholar]
- 32. Lennerz BS, Koutnik AP, Azova S, Wolfsdorf JI, Ludwig DS. Carbohydrate restriction for diabetes: rediscovering centuries-old wisdom. J Clin Invest. 2021;131(1):e142246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Atkins RC. Atkins’ diet revolution: the high calorie way to stay thin forever. New York: David McKay Inc. Publishers; 1972. [Google Scholar]
- 34. O'Hearn A. Can a carnivore diet provide all essential nutrients?. Curr Opin Endocrinol Diabetes Obes. 2020;27(5):312–16. [DOI] [PubMed] [Google Scholar]
- 35. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeFronzo RA. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia. 1981;21(3):165–71. [DOI] [PubMed] [Google Scholar]
- 37. Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, Bowman JD, Pronk NP. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–67. [DOI] [PubMed] [Google Scholar]
- 38. Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115(9):1447–63. [DOI] [PubMed] [Google Scholar]
- 39. Institute of Medicine of the National Academies . Protein and amino acids. In: Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): National Academies Press; 2005. p. 692–3. [Google Scholar]
- 40. Choi YJ, Jeon S-M, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12(7):2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, Gibson AA. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;139:239–52. [DOI] [PubMed] [Google Scholar]
- 42. Kirk JK, Graves DE, Craven TE, Lipkin EW, Austin M, Margolis KL. Restricted-carbohydrate diets in patients with type 2 diabetes: a meta-analysis. J Am Diet Assoc. 2008;108(1):91–100. [DOI] [PubMed] [Google Scholar]
- 43. Huntriss R, Campbell M, Bedwell C. The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr. 2018;72(3):311–25. [DOI] [PubMed] [Google Scholar]
- 44. van Zuuren EJ, Fedorowicz Z, Kuijpers T, Pijl H. Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: a systematic review including GRADE assessments. Am J Clin Nutr. 2018;108(2):300–31. [DOI] [PubMed] [Google Scholar]
- 45. Meng Y, Bai H, Wang S, Li Z, Wang Q, Chen L. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017;131:124–31. [DOI] [PubMed] [Google Scholar]
- 46. Snorgaard O, Poulsen GM, Andersen HK, Astrup A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2017;5(1):e000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Wyk HJ, Davis RE, Davies JS. A critical review of low-carbohydrate diets in people with type 2 diabetes. Diabet Med. 2016;33(2):148–57. [DOI] [PubMed] [Google Scholar]
- 48. Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, Volek JS, Phinney SD, McCarter JP. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. 2019;10:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ahmadi S-A, Boroumand M-A, Gohari-Moghaddam K, Tajik P, Dibaj S-M. The impact of low serum triglyceride on LDL-cholesterol estimation. Arch Iran Med. 2008;11(3):318–21. [PubMed] [Google Scholar]
- 50. Quispe R, Manalac RJ, Faridi KF, Blaha MJ, Toth PP, Kulkarni KR, Nasir K, Virani SS, Banach M, Blumenthal RSet al. . Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: the Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis. 2015;242(1):243–50. [DOI] [PubMed] [Google Scholar]
- 51. Shahali Y, Dadar M. Plant food allergy: influence of chemicals on plant allergens. Food Chem Toxicol. 2018;115:365–74. [DOI] [PubMed] [Google Scholar]
- 52. Rafferty K, Watson P, Lappe JM. The selection and prevalence of natural and fortified calcium food sources in the diets of adolescent girls. J Nutr Educ Behav. 2011;43(2):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fulgoni VL 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: where do Americans get their nutrients?. J Nutr. 2011;141(10):1847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Belobrajdic DP, Bird AR. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr J. 2013;12(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang Y-J, Gan R-Y, Li S, Zhou Y, Li A-N, Xu D-P, Li H-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20(12):21138–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Descalzo AM, Rossetti L, Grigioni G, Irurueta M, Sancho AM, Carrete J, Pensel NA. Antioxidant status and odour profile in fresh beef from pasture or grain-fed cattle. Meat Sci. 2007;75(2):299–307. [DOI] [PubMed] [Google Scholar]
- 57. Institute of Medicine (US) Committee on Use of Dietary Reference Intakes in Nutrition Labeling . A brief review of the history and concepts of the Dietary Reference Intakes. [Internet]. In: Dietary Reference Intakes: guiding principles for nutrition labeling and fortification. Washington (DC): National Academies Press (US); 2003. Available from: https://www.ncbi.nlm.nih.gov/books/NBK208878/(accessed October 5, 2021). [PubMed] [Google Scholar]
- 58. Hovinen T, Korkalo L, Freese R, Skaffari E, Isohanni P, Niemi M, Nevalainen J, Gylling H, Zamboni N, Erkkola Met al. . Vegan diet in young children remodels metabolism and challenges the statuses of essential nutrients. EMBO Mol Med. 2021;13(2):e13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dimbleby H. National Food Strategy; Escaping the Junk Food Cycle. In: National Food Strategy: independent review. [Internet]. 2021. p. 43–55.. Available from: https://www.nationalfoodstrategy.org/wp-content/uploads/2021/07/National-Food-Strategy-The-Plan.pdf. [Google Scholar]
- 60. Rowntree JE, Paig LS, Maciel ICF, Thorbecke M, Rosenzweig ST, Hancock DW, Guzman A, Raven MR. Ecosystem impacts and productive capacity of a multi-species pastured livestock system. Front Sustain Food Syst. 2020;4:544984. [Google Scholar]
- 61. Thompson JR, Rowntree JE. Methane sources, quantification, and mitigation in grazing beef systems. Appl Anim Sci. 2020;36(4):556–73. [Google Scholar]
- 62. Roque BM, Venegas M, Kinley RD, de Nys R, Duarte TL, Yang X, Kebreab E. Red seaweed (Asparagopsistaxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS One. 2021;16(3):e0247820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Survey instruments and data described in the article, code book, and analytic code will be made available upon request.