Abstract
Background
Veno-arterial extracorporeal membrane oxygenation (VA ECMO) is increasingly utilized in patients with cardiogenic shock due to improved technology and outcomes. Peripheral VA ECMO offers several advantages over central ECMO and is becoming increasingly popular. However, when configured via the femoral vessels, retrograde flow to the descending aorta and arch of aorta competes with antegrade ventricular output and can be associated with a watershed phenomenon and increased risk of neurologic and visceral injury.
Case summary
In this case series, we report three patients who were supported with peripheral VA ECMO for cardiogenic shock. All three were successfully weaned from peripheral VA ECMO; however, they had developed bilateral lower limb paralysis. Magnetic resonance imaging revealed spinal cord infarction in all three patients. All patients subsequently succumbed to multiorgan failure and did not survive to hospital discharge.
Discussion
The use of mechanical circulatory support, in particular, peripheral ECMO, has escalated with advances in technology, better understanding of cardiac physiology and improving outcomes. Spinal cord infarction is a rare but serious complication of peripheral VA ECMO support with only a few case reports published. Further studies are needed to identify the exact cause and prevention of this rare but often terminal complication. Through this series of three patients supported on peripheral VA ECMO complicated by spinal cord infarction, we review previously published reports, analyse possible mechanisms, and propose alternate management strategies to be considered in patients at risk.
Keywords: Spinal cord infarction, Veno-arterial extracorporeal membrane oxygenation, Case report
Learning points
Spinal cord infarction due to relative hypoperfusion caused by watershed phenomenon of peripheral veno-arterial extracorporeal membrane oxygenation is a rare but catastrophic complication.
Prevention strategies may include alteration of cannulation, generation of artificial pulse, or use of catheter-based axial flow circulatory support.
Introduction
Peripheral veno-arterial extracorporeal membrane oxygenation (VA ECMO) for the support of cardiogenic shock, offers several advantages over central ECMO in that it can be deployed more rapidly and avoids sternotomy. In the setting of post-cardiotomy support including primary graft failure after heart transplantation, peripheral VA ECMO keeps the chest free of cannulae, allowing the sternum to be closed and reducing the risks of bleeding and infection. However, retrograde flow to the descending aorta and arch of aorta can be associated with neurological complications, such as embolic phenomena to the viscera and brain.1 A less well-studied complication is that of a watershed phenomenon when the retrograde flow competes with antegrade ventricular output.2 In this case series, we outline our experience of three cases of this rare complication, with further discussion of the aetiology and possible prevention strategies.
Timeline
| Day 1 | ECMO-wean | Clinical course | Mortality | |
|---|---|---|---|---|
| Patient 1 | Patient undergoes orthotopic heart transplantation (OHTx). Surgery complicated by primary graft failure requiring peripheral veno-arterial extracorporeal membrane oxygenation (VA ECMO). | Day 10: Weaned off VA ECMO and decannulated. Developed right ventricular dysfunction over the next 12 h requiring temporary right ventricular assist device (RVAD). | Days 10–15: On temporary RVAD. Sedation weaned. Noted to have flaccid paralysis of lower limbs. Magnetic resonance imaging (MRI) was performed and confirms spinal cord infarction. | 6 weeks later: Multiorgan failure. Treatment withdrawn. |
| Patient 2 | Patient undergoes coronary artery bypass grafting + aortic valve replacement + maze procedure. Requires intra-aortic balloon pump (IABP) to be weaned off cardiopulmonary bypass. Develops low cardiac output over the next few hours requiring peripheral VA ECMO with removal of IABP. | Day 10: Weaned from peripheral VA ECMO and decannulated uneventfully. Noticed to have bilateral lower limb paralysis. MRI reveals spinal cord infarction. | — | Day 13: Biliary sepsis and renal failure requiring haemofiltration. Treatment withdrawn. |
| Patient 3 | Patient undergoes OHTx on a background of post-partum cardiomyopathy, supported for 7 months on left ventricular assist device. Develops primary graft failure within 12 h requiring peripheral VA ECMO support. | Day 13: Weaned from peripheral VA ECMO and decannulated successfully | Day 20: Noticed to have bilateral lower limb paralysis. MRI revealed spinal cord infarction. | At 3 months: Develops sepsis and multiorgan failure and succumbs to it. |
Case presentation
Case 1
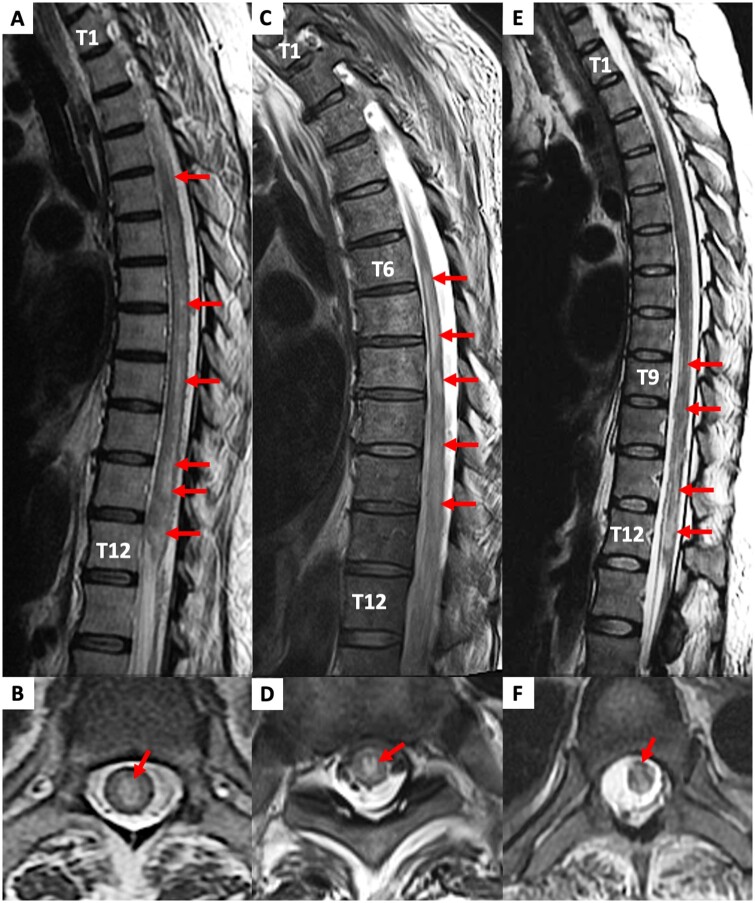
A 49-year-old Caucasian female with hypertrophic cardiomyopathy underwent orthotopic heart transplantation (OHTx) complicated by primary graft dysfunction. Veno-arterial extracorporeal membrane oxygenation was instituted intraoperatively due to failure to wean from cardiopulmonary bypass. She remained stable in the intensive care unit requiring a median dose of 4 µg/min epinephrine and 2 µg/min norepinephrine over the next 10 days. At that time, she was able to be weaned from ECMO and decannulated. However, over the next 12 h, she developed worsening right ventricular dysfunction necessitating the insertion of a temporary right ventricular assist device (Jostra-Rotaflow). She remained stable over the next 5 days and her sedation was weaned. At this time, it was noted that she had flaccid paralysis and areflexia of her lower limbs. Upper limb function was preserved. After a further 5 days, the patient was uneventfully weaned from mechanical support. Magnetic resonance imaging (MRI) revealed extensive central spinal cord oedema from T1-2 level to the conus suggestive of infarction (Figure 1A and B). Over the next 6 weeks, the patient developed multi-organ failure and sepsis and treatment was withdrawn.
Figure 1.
Magnetic resonance images of spinal cord infarcts in Patient 1 (A, B), Patient 2 (C, D), and Patient 3 (E, F).
Case 2
A 72-year-old Caucasian male underwent coronary artery bypass grafting, aortic valve replacement, and Maze procedure. He required an intra-aortic balloon pump (IABP) to be weaned off cardiopulmonary bypass. Over the next few hours, he had persistently low cardiac output (on 4 µg/min of epinephrine and 14 µg/min of norepinephrine), acidosis (pH 7.2), and rising lactate (8 mmol/L) was placed on peripheral VA ECMO and the IABP was removed. Due to ongoing severe left ventricular dysfunction (requiring up to 4 µg/min of epinephrine and 24 µg/min of norepinephrine) and an inability to wean off ECMO, 6 days after the index operation, a repeat coronary angiogram was performed. This demonstrated patent grafts to the circumflex and first diagonal territories. However, an ostial left main stenosis, which had not been previously demonstrated, was noted. He underwent repeat surgery the following day with a bypass graft to the left anterior descending artery. He was subsequently weaned from VA ECMO and decannulated uneventfully 72 h later (a total of 10 days on VA ECMO). At this time, he was noticed to have bilateral lower limb paralysis. Spinal MRI revealed extensive central thoracic cord T2-weighted signal hyperintensity, swelling and diffusion restriction from T6 down to the conus, consistent with spinal cord infarction (Figure 2C and D). Over the next 3 days, he developed biliary sepsis with rising lactate, rising vasopressor requirements, and renal failure requiring haemofiltration. Treatment was withdrawn in view of these complications.
Figure 2.
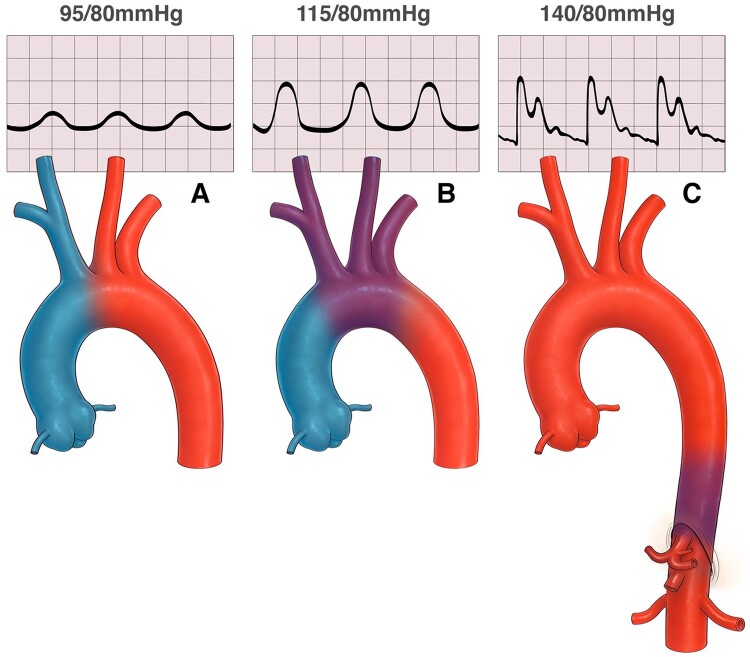
Illustration of alterations in watershed zone as ventricle recovers. First panel (A) depicts poor ventricular function where most of the vascular run off is supplied by oxygenated blood from the retrograde flow of the peripheral extracorporeal membrane oxygenation. Second panel (B) shows a slightly improved ventricular function whereby the watershed zone has moved up into the aortic arch. Third panel (C) shows much improved ventricular and pulmonary function with the watershed zone low in the thoracic aorta.
Case 3
A 38-year-old Caucasian female with post-partum cardiomyopathy, supported for 7 months on continuous flow left ventricular assist device (HeartWare, Medtronic, MA, USA), underwent OHTx. She developed primary graft failure within 12 h requiring the institution of peripheral VA ECMO. She was successfully weaned and decannulated after 13 days on VA ECMO. Sedation was subsequently weaned over the next week, but she was noticed to have paralysis of bilateral lower limbs. Spinal MRI demonstrated hyperintense T1-weighted non-enhancing lesions predominantly affecting the central cord grey matter from T9 to T12, suggestive of cord infarction (Figure 1E and F). Her intensive care unit stay was protracted, and she eventually succumbed to sepsis and multi-organ failure 3 months after her initial heart transplantation.
All three patients were on mechanical ventilation when VA ECMO was instituted and remained sedated and on mechanical ventilation for the duration of the VA ECMO run. They had all had a normal neurological status prior to their operation, having presented from home for surgery. Veno-arterial extracorporeal membrane oxygenation was instituted within 12 h of surgery for all three patients. None of the patients required left ventricular venting. All patients received a downflow cannula into the superficial femoral artery and none were complicated by lower limb ischaemia. All patients had pulmonary artery catheters placed as part of their surgery, although these are generally removed after 7 days to reduce infection risk. Veno-arterial extracorporeal membrane oxygenation was instituted using HLS Bioline coated cannulae (Getinge AB, Gothenburg, Sweden), a Bio-Medicus Pump driver and head (550 Bio-Console, Medtronic Inc., Dublin, Ireland), and Quadrox D oxygenator (Getinge AB, Gothenburg, Sweden). Parameters were adjusted to achieve adequate end-organ perfusion maintaining mean blood pressure above 65 mmHg, mixed venous oxygen saturation above 70% and aiming for a cardiac index above 2.2 L/min/body surface area (m2), as per our hospital protocol. This generally requires flows of 3.5–5 L/min to achieve these parameters and the flow is controlled by manually adjusting the revolutions per minute on the centrifugal pump console, and modified by utilising vasodilator infusions to reduce afterload as required. When possible we aim to maintain some pulsatility and utilise intravenous inotropic infusions to achieve this, as well as regular echocardiography to identify (and maintain) aortic valve opening. Heparin was commenced at 12 h post-institution of VA ECMO if there was no surgical bleeding, aiming for an activated partial thromboplastin time of 60–80 s. None of the patients had any other embolic phenomena to suggest that the spinal cord injury was the result of an embolic shower, although this cannot be excluded.
There was no suspicion of spinal cord injury in any of the three patients until they were weaned from sedation. This occurred at about 15 days post-operatively in the two female heart transplant patients and at 10 days in the male post-cardiotomy patient. The actual timing of the spinal cord injury is unknown. In identifying that the patients had profound lower limb weakness, the following differential diagnoses were considered: intensive care myopathy, critical illness polyneuropathy, ischaemic stroke, and disc herniation. However, the clinical features were highly suspicious of a spinal cord pathology and so spinal cord MRI was performed confirming the diagnosis in each patient.
Discussion
In this case series, we report a rare complication of spinal cord infarction in three patients who were supported with peripheral VA ECMO support for post-cardiotomy cardiogenic shock. Although all three patients had cardiac recovery and were successfully weaned from VA ECMO, as their sedation was lightened it became evident that they could not move their legs. Magnetic resonance imaging confirmed spinal cord infarction in all three patients.
The use of mechanical circulatory support, in particular ECMO, has escalated with advances in technology, better understanding of the physiology and improving outcomes. Peripheral ECMO is increasingly popular because it is relatively easy to insert, can be done percutaneously, is a relatively low cost circulatory support option, and has lower bleeding and infection risks that central VA ECMO. However, the retrograde flow through the descending aorta has been associated with neurological complications such as embolic strokes,1 and as reported here, spinal cord infarction.
Spinal cord infarction is a rare complication of peripheral ECMO with only a few case reports (Table 1).2–6 In some, IABP support was used concurrently with VA ECMO, potentially impacting the aetiology of the injury.2,3 The overall incidence has been reported as 0.3% in the largest series published to date from the La Pitie Salpetriere Hospital in Paris, France with an impressive survival rate of 83% (5/6 patients).2 We report an incidence of 0.6%—three cases of spinal cord injury in the last 10 years at our institution during which time we have had 504 patients on VA ECMO. In contrast, all three of our patients succumbed to multiorgan failure and sepsis. It is possible that the incidence of spinal cord injury is much higher with patients on ECMO dying of other complications before diagnosis of a spinal cord infarction.
Table 1.
Literature review
| Author, year | Age, gender | Indication for ECMO | Cardiac arrest | ECMO config-uration | Conco- mitant IABP | Duration of VA ECMO (days) | Days to first evidence of neurological deficit/days to radiological diagnosis of SCI | Level of SCI on MRI | Hospital survival | Neurological function/recovery |
|---|---|---|---|---|---|---|---|---|---|---|
| Oda et al.,4 2010 | 6 years, male | H1N1 influenza myocarditis | No | Peripheral femoral | No | 4.5 | NR/7.5 | T4-5 | Yes | Paraplegia/no |
| Samadi et al.,3 2016 | 37 years, female | Viral cardiomyopathy | No | Peripheral femoral | Yes | 10 | 10/13 | T1 to conus | Yes | Paraplegia/no |
| Samadi et al.,3 2016 | 43 years, female | Cardiogenic shock after AMI and cardiotomy | Yes | Peripheral femoral | Yes | 9 | 7/NR | Conus | Yes | LL weakness/yes |
| Samadi et al.,3 2016 | 19 years, female | Bridge-to-destination after peri-partum cardiomyopathy | Yes | NR | Yes | 3 | 10/NR | C5 to conus | NR | Paraplegia/no |
| Shin et al.,6 2016 | 81 years, female | Cardiogenic shock after AMI and cardiac arrest | No | Peripheral femoral | No | NR | 1/NR | T5 to conus | Yes | Paraplegia/no |
| Magnusson et al.,5 2018 | 28 years, female | Fulminant perimyocarditis | Yes | Peripheral (NR) | No | 21 | NR/NR | T6 to conus | Yes | Paraplegia/no |
| Le Guennec et al.,2 2020 | 35 years, male | Cocaine-induced cardiomyopathy | Yes | Peripheral femoral | No | 4 | 23/59 | T1 to conus | Yes | Paraplegia/UL only |
| Le Guennec et al.,2 2020 | 48 years, male | Pneumococcal pneumonia septic cardiomyopathy | Yes | Peripheral femoral | No | 29 | 11/59 | T9 to conus | Yes | Paraplegia/no |
| Le Guennec et al.,2 2020 | 56 years, male | Cardiogenic shock after AMI and cardiac arrest | Yes | Peripheral femoral | No | 2 | 3/3 | T10 to conus | Yes | Paraplegia/no |
| Le Guennec et al.,2 2020 | 62 years, male | PGD after OHTx | No | Peripheral femoral | No | 47 | 22/55 | T6 to conus | No | Paraplegia/no |
| Le Guennec et al.,2 2020 | 43 years, male | Cardiogenic shock after AMI | No | Peripheral femoral | Yes | 4 | 6/6 | Conus | Yes | LL weakness/yes |
| Le Guennec et al.,2 2020 | 62 years, male | Ischaemic dilated cardiomyopathy and PGD after OHTx | No | Peripheral femoral | Yes | 13 | 7/50 | T12 to conus | Yes | LL pain and weakness/yes |
| Current study | 49 years, female | PGD after OHTx | No | Peripheral femoral | No | 10 | 15/24 | T2 to conus | No | Paraplegia/no |
| Current study | 73 years, male | Post-cardiotomy cardiogenic shock | No | Peripheral femoral | No | 10 | 10/12 | T6 to conus | No | Paraplegia/no |
| Current study | 38 years, female | PGD after OHTx | No | Peripheral femoral | No | 13 | 19/70 | T9 to conus | No | Paraplegia/no |
AMI, acute myocardial infarction; ECMO, extra-corporeal membrane oxygenation; IABP, intra-aortic balloon pump; LL, lower limb; MRI, magnetic resonance imaging; NR, not reported; OHTx, orthotopic heart transplantation; PGD, primary graft dysfunction, SCI, spinal cord infarction, UL, upper limb, VA, veno-arterial.
Potential aetiologies for spinal cord infarction in these patients include: (i) relative hypoperfusion of the spinal cord caused by the watershed phenomenon of peripheral VA ECMO; (ii) hypoperfusion due to vasopressors or haemodynamic instability; and (iii) embolic phenomena particularly related to ECMO cannulation, IABP insertion or use. Although we have not been able to confirm watershed infarction with autopsy, the absence of any other embolic phenomena and the distribution of the spinal cord injury on MRI makes us highly suspicious that that was the mechanism in these three patients. However, this does highlight the often multifactorial aetiology of this complication and the difficulty in making an early diagnosis. Furthermore, VA ECMO circuitry is a contra-indication to MRI. Thus, even if clinical suspicion arose, the diagnosis of spinal cord infarction would not be able to be made until successful wean from VA ECMO. In that situation, possible management strategies to mitigate spinal cord damage could include increasing perfusion pressure, and cerebrospinal fluid drainage. As no specific treatment exists for this condition, close monitoring and avoiding potential aetiological factors is vital.
Watershed phenomenon in peripheral ECMO is well described. Well oxygenated blood from the ECMO outflow cannula competes with native cardiac output (Figure 2). Depending on the functional status of the left ventricle, the competing zone may be anywhere from the ascending aorta to the renal arteries. If the native lung function is poor, then the heart ejects relatively deoxygenated blood. As ventricular function improves, the competing zone moves further distally beyond the aortic arch. If the native cardiac output is poorly oxygenated due to compromised respiratory function, differential cyanosis can result, also known as ‘Harlequin’ syndrome. This watershed zone is a potential area of low flow giving rise to relative ischaemia and infarction and has been imaged with aortography, computed tomography, ultrasound, and computational fluid dynamics.7–9 Furthermore, as native cardiac function improves, and ECMO flows are reduced, the watershed zone enlarges particularly during diastole leading to a potentially larger vascular bed at risk (Figure 2A–C).
Vasopressor support in the setting of a watershed zone is likely to cause vasoconstriction and exacerbate the relative ischaemia in that zone. The arterial blood supply of the spinal cord is potentially tenuous with only three main longitudinal vessels arising from the intracranial portion of the vertebral artery—the anterior spinal artery which supplies the ventral two-thirds of the cord and two posterior spinal arteries which supply the posterior third. They are supplemented by segmental arteries of which the largest, the artery of Adamkiewicz arises variably from T9 to L5 which is likely to be at risk as the watershed zone extends down the thoracic aorta.
Further studies are needed to identify the exact cause and prevention of this rare but often terminal complication. In particular, the management of the watershed zone needs to be addressed. The dynamic nature of this phenomenon warrants continuous monitoring and imaging which has been successfully performed with contrast-enhanced ultrasound at the bedside.7
Management options for watershed zone hypoperfusion is to alter the cannulation strategy in these patients to veno-arterial-venous cannulation, particularly early on in their ECMO run when ventricular and pulmonary function is at its worst.8 In such a cannulation strategy, oxygenated blood from the ECMO circuit is returned to the iliac artery as retrograde flow and to the superior vena cava via an internal jugular return line, ensuring that oxygenated blood is passing through the native left ventricle, thereby eliminating deoxygenated blood ejecting from the heart. This would eliminate a deoxygenated watershed zone, although it would not address potential areas of low flow or turbulence in the watershed zone. Another potential management option that has been suggested is to artificially pulse the ECMO flow as the left ventricle improves, reducing the size of the mixing zone and stabilising its position further upstream.9 A further option would be to avoid VA ECMO altogether and provide circulatory support with an Impella (Abiomed, MA, USA) device. This catheter-based axial flow circulatory support device has been used in post-cardiotomy cardiogenic shock with success and can deliver up to 5.0 L/min flow.10 The catheter tip enters the left ventricular cavity via the aortic valve and flow is directed to the ascending aorta. By design, this would not result in a watershed phenomenon in the descending aorta. However, we are not aware of this form of left ventricular support having been used for primary graft failure after heart transplantation.
Conclusion
In this case series, we outline a rare complication of VA ECMO, spinal cord infarction. This catastrophic complication highlights the need to further improve the way we administer VA ECMO, and the way we monitor these patients. Consideration should also be given to alternative circulatory support measures in these cardiogenic shock patients.
Lead author biography

Shivanand Gangahanumaiah is the transplant and cardiothoracic fellow at The Alfred Hospital in Melbourne Australia. He graduated from Christian Medical College, Vellore, India and obtained his MS (Gen Surg) from Bangalore Medical College. Following that he trained at Christian Medical College Vellore to obtain his MCh (Cardiothoracic Surgery) and continued there as an Assistant Professor of Cardiothoracic Surgery. Before coming to Australia, he was working at Sagar Hospital, India as a Consultant Cardiac Surgeon.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The patients reported in this case are deceased. Despite the best efforts of the authors, they have been unable to contact the patients' next-of-kin to obtain consent for publication. Agreement to wave consent for publicatino has been agreed by a local ethics committee. Every effort has been made to anonymise the case. This situation has been discussed with the editors.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1. Wilcox C, Etchill E, Giuliano K, Mayasi Y, Gusdon AM, Cho CW. et al. Acute brain injury in postcardiotomy shock treated with venoarterial extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 2021;35:1989–1996. [DOI] [PubMed] [Google Scholar]
- 2. Le Guennec L, Shor N, Levy B, Lebreton G, Leprince P, Combes A. et al. Spinal cord infarction during venoarterial-extracorporeal membrane oxygenation support. J Artif Org 2020;30:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Samadi B, Nguyen D, Rudham S, Barnett Y.. Spinal cord infarct during concomitant circulatory support with intra-aortic balloon pump and veno-arterial extracorporeal membrane oxygenation. Crit Care Med 2016;44:e101–e105. [DOI] [PubMed] [Google Scholar]
- 4. Oda T, Yasunaga H, Tsutsumi Y, Shojima T, Zaima Y, Nishino H. et al. A child with influenza A (H1N1)-associated myocarditis rescued by extracorporeal membrane oxygenation. J Artif Organs 2010;13:232–234. [DOI] [PubMed] [Google Scholar]
- 5. Magnusson P, Levin C, Mattsson G, Vest AR.. A case of fulminant perimyocarditis leading to extensive ECMO treatment and spinal injury resulting in paraplegia. Clin Case Rep 2018;6:2471–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shin B, Cho YH, Choi J-H, Yang JH.. Spinal cord infarction in a patient undergoing veno-arterial extracorporeal membrane oxygenation. Acute Crit Care 2018;33:187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchtele N, Staudinger T, Schwameis M, Schörgenhofer C, Herkner H, Hermann A. et al. ; UltraECMO investigators. Feasibility and safety of watershed detection by contrast-enhanced ultrasound in patients receiving peripheral venoarterial extracorporeal membrane oxygenation: a prospective observational study. Crit Care 2020;24:126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Napp LC, Kuhn C, Hoeper MM, Vogel-Claussen J, Haverich A, Schäfer A. et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol 2016;105:283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevens MC, Callaghan FM, Forrest P, Bannon PG, Grieve SM.. Flow mixing during peripheral veno-arterial extra corporeal membrane oxygenation—a simulation study. J Biomech 2017;55:64–70. [DOI] [PubMed] [Google Scholar]
- 10. David C-H, Quessard A, Mastroianni C, Hekimian G, Amour J, Leprince P. et al. Mechanical circulatory support with the Impella 5.0 and the Impella left direct pumps for postcardiotomy cardiogenic shock at La Pitié-Salpêtrière Hospital. Eur J Cardiothorac Surg 2020;57:183–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.