Abstract
Activation of Akt by the phosphatidylinositol 3′-OH kinase (PI3K) results in the inhibition of proapoptotic signals and the promotion of survival signals (L. P. Kane et al., Curr. Biol. 9:601–604, 1999; G. J. Kops et al., Nature 398:630–634, 1999). Evidence supporting the importance of the PI3K/Akt signaling pathway in tumorigenesis stems from experiments with transgenic mice bearing polyomavirus middle T antigen under the control of the mouse mammary tumor virus long terminal repeat promoter. Mammary epithelium-specific expression of polyomavirus middle T antigen results in the rapid development of multifocal metastatic mammary tumors, whereas transgenic mice expressing a mutant middle T antigen decoupled from the phosphatidylinositol 3′-OH kinase (MTY315/322F) develop extensive mammary gland hyperplasias that are highly apoptotic. To directly assess the role of Akt in mammary epithelial development and tumorigenesis, we generated transgenic mice expressing constitutively active Akt (HAPKB308D473D or Akt-DD). Although expression of Akt-DD interferes with normal mammary gland involution, tumors were not observed in these strains. However, coexpression of Akt-DD with MTY315/322F resulted in a dramatic acceleration of mammary tumorigenesis correlated with reduced apoptotic cell death. Furthermore, coexpression of Akt-DD with MTY315/322F resulted in phosphorylation of the FKHR forkhead transcription factor and translational upregulation of cyclin D1 levels. Importantly, we did not observe an associated restoration of wild-type metastasis levels in the bitransgenic strain. Taken together these observations indicate that activation of Akt can contribute to tumor progression by providing an important cell survival signal but does not promote metastatic progression.
The growth and development of the mammary gland is regulated by a complex set of factors including hormones, cell-substratum interactions, and growth factors and their associated receptors. Activation of growth factor receptors leads to the recruitment of a number of cytoplasmic signaling molecules, including the phosphatidylinositol 3′-OH kinase (PI3K). Recruitment of the PI3K to the cell membrane by these activated growth factors or docking molecules then results in the activation of a number of molecules. PI3K-dependent generation of phosphatidylinositol 3′ phosphate provides docking sites for several Pleckstrin homology (PH) domain-harboring molecules including Akt (also known as protein kinase B [PKB]) as well as its upstream kinases, PDK1 and the proposed PDK2 (2, 16). These latter enzymes phosphorylate Akt at threonine 308 and serine 473, respectively, causing full Akt activation (1, 2). Activation of Akt subsequently results in the inhibition of proapoptotic signals from such proteins as BAD (9), caspase 9 (4), and the forkhead transcription factor family (3, 22, 34) and the promotion of survival signals from such proteins as NF-κB (20). Although evidence suggests roles for PI3K and Akt in normal mammary development (15) and tumorigenesis (5, 30, 31, 35), the role of these signaling molecules in these processes remains to be elucidated.
Evidence supporting the importance of the PI3K/Akt signaling pathway in tumorigenesis stems from experiments with transgenic mice bearing polyomavirus (PyV) middle T antigen (mT) under the control of the mouse mammary tumor virus long terminal repeat promoter (MMTV-LTR). The MMTV-LTR is transcriptionally active throughout mammary development, and its transcriptional activity increases during pregnancy (26). Mammary epithelium-specific expression of PyV mT results in the rapid development of multifocal metastatic mammary tumors (18) due to its ability to associate with and activate the Src family kinases, PI3K, and the Shc adapter protein (6, 7, 14). In contrast to the rapid tumor progression observed in transgenic mice carrying the PyV mT oncogene (MT634), transgenic mice expressing a mutant mT decoupled from the PI3K pathway (MMTV/MTY315/322F) develop extensive mammary gland hyperplasias that are highly apoptotic (35). Focal mammary tumors do eventually arise in these strains and are further correlated with upregulation of the ErbB-2 and ErbB-3 growth factor receptors (35). In addition, these tumors show defects in metastatic progression (35).
The defects in tumor progression in the mutant mT strain suggested that Akt may play important roles in tumorigenesis by inhibiting apoptosis and/or promoting metastasis. In this report we show that activation of Akt alone can interfere with the apoptotic process of mammary gland involution and promote tumor progression by providing an important cell survival signal but does not promote metastasis. The dramatic acceleration of tumor progression in these strains was further correlated with the phosphorylation of FKHR, a member of the forkhead class of transcription factors, and induction of cyclin D1. Together these observations suggest that activation of Akt provides complementary cell survival signals that are required for mammary tumorigenesis.
MATERIALS AND METHODS
Generation and identification of transgenics.
The cDNA encoding HAPKB308D473D was subcloned into pMMTV-SV40Pa (p206) (18). This construct was prepared and injected as previously described (35). Transgenic progeny were identified by Southern analysis using the EcoRI-BamHI (3900 to 4775) fragment of p206 (18) as a probe. Akt-MTY315/322F bitransgenics were generated by crossing MMTV/MTY315/322F males to MMTV/Akt7 females and were subsequently identified by identical Southern analysis.
Histology and apoptosis assays.
Lower left mammary fat pad tissues were fixed in 4% paraformaldehyde, blocked in paraffin, sectioned at 5 μm, stained with hematoxylin and eosin, and examined. Whole-mount preparations were prepared from the lower right mammary fat pad as previously described (35). In situ apoptosis assays were performed with the Apoptag Peroxidase In Situ Apoptosis Detection Kit (Intergen) as described previously (35).
RNA isolation and analysis.
RNA was isolated from mammary glands and analyzed by RNase protection using simian virus 40 (SV40) polyadenylation-specific (SPA) and PGK-1 ribonucleotide protection probes as previously described (35).
Protein extraction and analysis.
Tissue from various organs was flash frozen in liquid nitrogen and stored at −80°C or immediately lysed. Protein lysates were prepared as previously described (35). All immunoblots and immunoprecipitations were carried out as previously described (35) with the following exceptions. Antihemagglutinin (anti-HA) immunoblot analyses of mammary tissue from the FVB/n, Akt7, MTY315/322F, and Akt7 × MTY315/322F strains were performed on 250 μg of total protein lysate. Lysates were precleared in protein G-Sepharose and subjected to anti-HA immunoblot analysis with HA-11 monoclonal antibody (Babco) (1:1,000). PyV mT was immunoprecipitated from 2 mg of total protein lysate with 2 μg of mouse monoclonal Pab762 (courtesy of S. Dilworth) and subjected to anti-mT immunoblot analysis with rat monoclonal Pab701 (1:1,000). Anticytokeratin immunoblot analysis was carried out on 250 μg of total protein lysate using Troma-1 rat monoclonal antibody from ascites (1:50). Cyclin D1 analysis was carried out on 50 μg of total protein using the anti-cyclin D1 72-13G monoclonal antibody from Santa Cruz. FKHR analysis was carried out on 50 μg of total protein using the anti-FKHR N-18 polyclonal antibody from Santa Cruz and the anti-phospho-FKHR (Ser256) antibody from New England Biolabs.
Akt immunoblotting was carried out on total lysate using the anti-Akt antibody from New England Biolabs. Akt kinase activity assays were carried out on immunoprecipitates from total lysate using the anti-PH domain PKB antibody from UBI and the cross-tide peptide as substrate. Glycogen synthase kinase 3 (GSK-3) analysis was carried out on total lysate using anti-GSK-3 antibodies from New England Biolabs.
RESULTS AND DISCUSSION
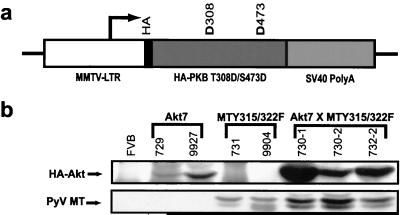
To further assess the importance of the PI3K/Akt signaling pathway in PyV mT-induced tumorigenesis and metastasis, we derived transgenic mice that express a constitutively active version of Akt (HAPKB308D473D or Akt-DD), which mimics the active phosphorylated state of the protein (1), in the mammary gland (Fig. 1a). To distinguish between the transgene-derived and endogenous Akt protein, an HA epitope tag was placed in frame at the amino terminus of the activated Akt protein (Fig. 1a). Initially, 11 activated MMTV/Akt founder lines were derived. Nine of these lines passed the transgene to their offspring, and a screen for expression of the activated Akt transgene revealed expression in the mammary gland in three of these lines (Table 1). The tissue specificity of transgene protein product expression of two of these lines (MMTV/Akt7 and MMTV/Akt10) was determined, and the higher expresser (MMTV/Akt7) was chosen for further study (Table 2). To confirm that activated Akt protein product was expressed in the mammary epithelium of transgenic mice, multiple mammary tissue extracts from the Akt7 line were subjected to anti-HA immunoblot analysis. The results revealed that virgin mammary glands from these strains were expressing significant levels of the transgene-derived Akt protein (Fig. 1b).
FIG. 1.
Activated Akt transgene expression. (a) Structure of the MMTV/Akt transgene. The Bluescript vector backbone is represented by a thin line on either side of the expression cassette, with the white region corresponding to the MMTV-LTR derived from plasmid pAp, the black portion corresponding to the hemagglutinin tag, the dark grey region corresponding to the Akt (HAPKBT308D/S473D) cDNA with aspartate substitutions at amino acid positions 308 and 473, and the mid-grey region corresponding to the transcriptional processing sequences derived from the SV40 early transcription unit. The transcription start site is indicated by an arrow. (b) Immunoblot analysis of expression of HAPKB and PyV mT in bitransgenic Akt7 × MTY315/322F strains. Note that Akt7 × MTY315/322F tumor samples coexpress both Akt and PyV mT proteins. The numbers above each lane indicate individual mouse identification numbers.
TABLE 1.
MMTV/Akt transgene expression in mammary glanda
| Line | Expressionb |
|---|---|
| Akt1 | − |
| Akt2 | − |
| Akt3 | − |
| Akt4 | − |
| Akt5c | ND |
| Akt6 | − |
| Akt7 | ++ |
| Akt8 | − |
| Akt9 | + |
| Akt10 | + |
| Akt11c | ND |
Expression of the Akt transgene in the mammary gland was determined via RNase protection analysis on 20 μg of total RNA with a probe directed against the SV40 poly(A) region of the transgene.
Relative levels of transgene expression: ND, no data; −, not detected; +, low; ++, high.
Strain did not pass transgene.
TABLE 2.
MMTV/Akt transgene expression in MMTV/Akt7 tissuesa
| Sex | Tissue | Expressionb in strain:
|
|
|---|---|---|---|
| Akt7 | Akt10 | ||
| Female | Mammary gland | ++ | + |
| Brain | − | − | |
| Heart | − | − | |
| Kidney | − | − | |
| Liver | − | − | |
| Lung | − | − | |
| Ovary | − | − | |
| Salivary | − | − | |
| Spleen | − | − | |
| Thymus | − | − | |
| Male | Epididymis | ++++ | +++ |
| Seminal vesicles | ++ | ++++ | |
| Testes | − | − | |
Expression of the Akt transgene was determined by Western blot analysis using the HA-11 monoclonal antibody (Babco) on 250 μg of total protein lysate precleared in protein G-Sepharose.
Relative levels of transgene expression: −, not detected; +, low; ++, intermediate; +++, high; ++++, very high.
To ascertain whether elevated expression of activated Akt could interfere with normal mammary gland development, whole-mount analyses of both virgin and involuting mammary glands were conducted. Virgin female glands from MMTV/Akt strains were histologically and morphologically identical to FVB/n female controls (Fig. 2a and b). Consistent with these observations, female virgin Akt transgenic mice have yet to develop mammary tumors after a year of observation. This observation is further supported by the observation that multiparous Akt transgenic females, which would have undergone multiple periods of high transgene expression, have also failed to exhibit tumors. Given the importance of apoptotic cell death in mammary gland involution, we next examined whether mammary gland involution was adversely affected in the activated Akt strain. To explore this possibility, mammary glands from the wild-type and activated Akt strains were examined at 1, 3, and 7 days postparturition. In contrast to wild-type control animals, which exhibited extensive involution at 1 and 3 days postparturition (Fig. 3a, c, e, and g), the Akt7 animals displayed a dramatic defect in mammary gland involution (Fig. 3b, d, f, and h). However, the Akt7 mammary glands eventually underwent full involution at 7 days postparturition (data not shown), likely due to a drop in the hormonally responsive MMTV-driven transgene expression in the activated Akt strain.
FIG. 2.
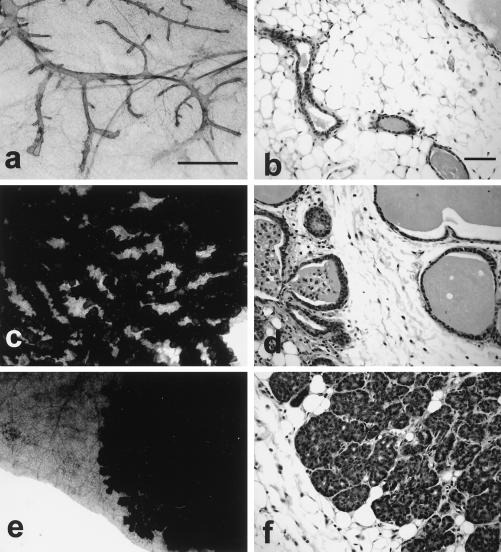
Coexpression of Akt and mutant PyV mT oncogene results in the induction of multifocal mammary tumors. These digital images illustrate the histological patterns observed in the Akt7 (a and b), MTY315/322F (c and d), and Akt7 × MTY315/322F (e and f) bigenic mice. Note that the whole-mount preparations (a, c, and e) demonstrate that the Akt strains have a relatively normal mammary tree (a) compared to the cystic hyperplasias seen in the MTY315/322F strains at the same age (c) (8 weeks) (scale bar = 1 mm). In contrast, the bigenic mammary gland does not fill the fat pad (e) and is a solid mass at this age (f). The histological patterns seen at high magnification (scale bar = 0.01 mm) demonstrate that the Akt7 strain has a normal epithelium (b), while the MTY315/322F strain has a cystic hyperplasia of the ducts and glands without significant atypia (d). In contrast, the Akt7 × MTY315/322F cross has acinar or lobular hyperplasia with low-grade atypia at 8 weeks (f). Normal mammary gland morphologies for the FVB strain can be viewed at the following website: http://ccm.ucdavis.edu/tgmouse/wmtable.htm.
FIG. 3.
Mammary epithelial expression of Akt results in defect in mammary gland involution. Digital images of the involution patterns in wild-type (a, c, e, and g) and Akt7 (b, d, f, and h) mammary glands. The images compare whole-mount preparations (a, e, b, and f) of the mammary gland (scale bar = 1 mm) with the histological pattern (c, d, g, and h) (scale bar = 0.1 mm) on days 1 (a to d) and 3 (e to h) of involution. Note the delayed involution in the Akt7 mouse mammary gland (b to h).
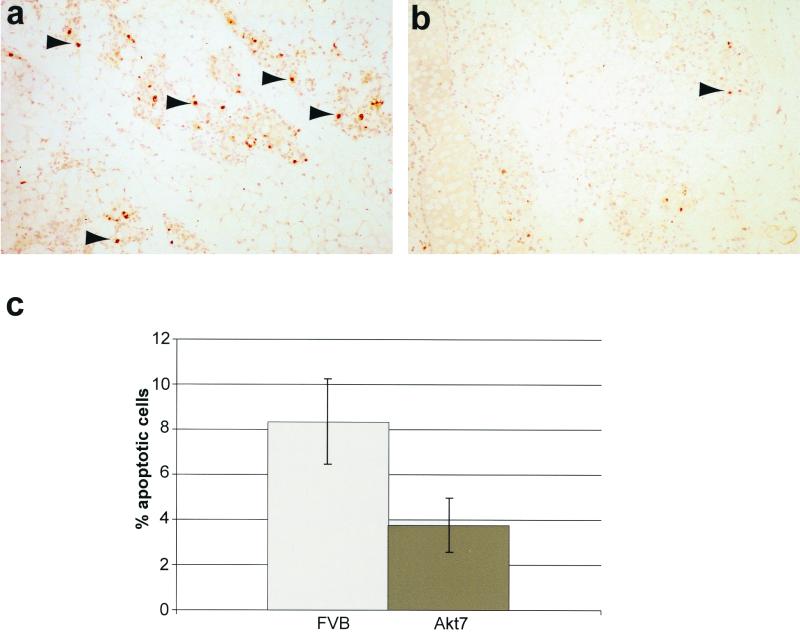
To assess whether the observed delay in mammary gland involution was due to a defect in the induction of apoptotic cell death, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) analyses were conducted on involuting mammary epithelium derived from FVB/n and Akt7 strains (Fig. 4). The results revealed that mammary glands derived from the involuting FVB/n glands exhibited elevated levels of apoptotic cell death relative to mammary epithelium of the Akt7 strain (compare Fig. 4a and b). Taken together, these observations argue that activation of Akt can interfere with normal mammary gland involution by attenuating apoptotic death in the involuting mammary gland.
FIG. 4.
Mammary epithelial expression of Akt results in decreased mammary gland apoptosis during involution. (a and b) TUNEL analysis of involuting mammary glands from FVB/n (a) and Akt7 (b) at 3 days postparturition. Arrows indicate representative apoptotic cells. (c) Mammary apoptotic indices of FVB/n and Akt7 at 3 days postparturition. Values shown represent the percentage of total cells stained positive for apoptosis by TUNEL assay in age-matched singly parous female mice at 15 weeks of age.
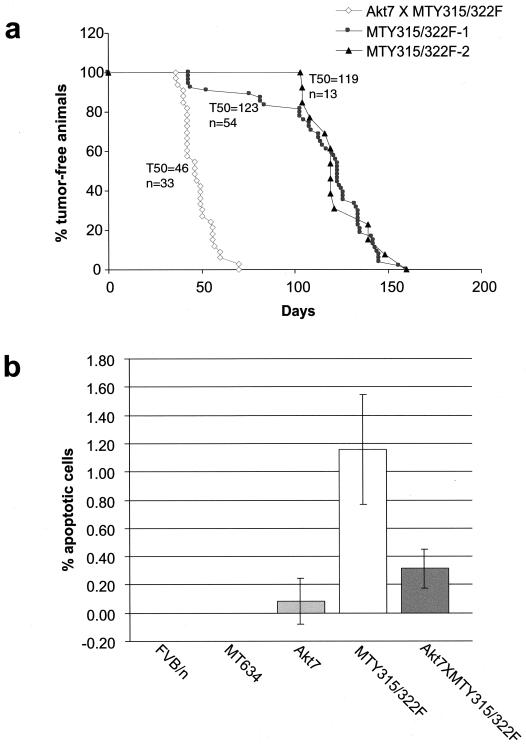
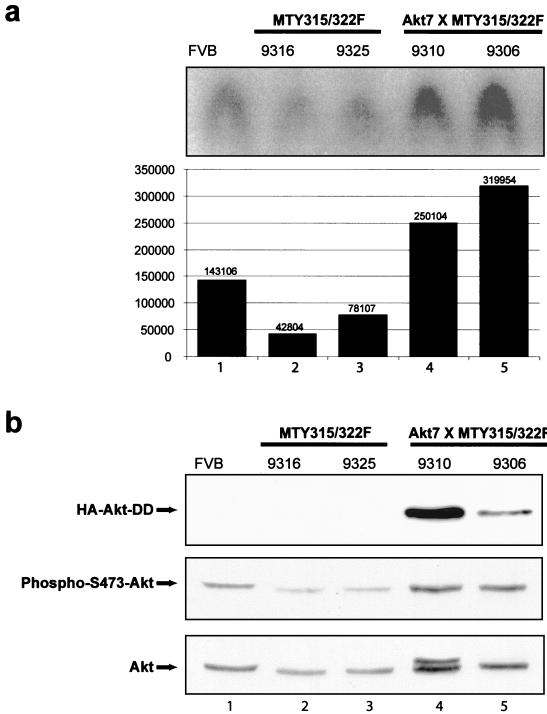
Although these data suggest that the Akt-DD mutant can interfere with apoptotic cell death during mammary gland involution, its role in mammary tumorigenesis is unclear. To explore whether active Akt expression could complement the defect in tumorigenesis exhibited by transgenic mice expressing the mutant PyV mT decoupled from the PI3K/Akt signaling pathway, bitransgenics expressing both the Akt transgene and the mutant mT transgene (MTY315/322F) were derived (Fig. 1b) and monitored for tumor formation by physical palpation. The results of these analyses revealed that bitransgenic mice developed multifocal mammary tumors with 100% penetrance with an average latency of 46 days (Fig. 5a). In contrast, physical palpation of two independent cohorts of female mice carrying the mutant PyV mT transgene alone revealed a significant delay in the onset of tumor formation with average latencies of 123 and 119 days, respectively (Fig. 5a). In addition, these tumors were focal in nature, arising next to hyperplastic mammary epithelium. Consistent with these kinetic analyses, whole-mount analyses of virgin mammary glands of bitransgenic mice revealed a dramatic difference in the extent of tumor growth (compare Fig. 2e and f to c and d). In contrast to the diffuse cystic hyperplasias exhibited by the mutant PyV mT strains, female transgenic mice coexpressing the mutant PyV mT and activated Akt transgenes exhibited polyclonal differentiated carcinomas. In agreement with these analyses, these lesions could be subcutaneously transplanted into syngeneic recipients. To confirm that bitransgenics expressing MTY315/322F and activated Akt exhibited elevated Akt kinase activity, we examined the total Akt kinase activity against a peptide substrate in virgin FVB/n, MTY315/322F, and bitransgenic mammary glands. These studies revealed an approximately fivefold increase in the total Akt kinase activity in the bitransgenic mammary glands as compared to those of MTY315/322F transgenics (Fig. 6a). The minimal increases in endogenous Akt phosphorylation (Fig. 6b) would suggest that the majority of the Akt kinase activity is derived from the activated mutant. However, these results do not completely preclude a mechanism whereby endogenous Akt is in some way activated via the combination of Akt-DD and MTY315/322F and contributes to tumor formation.
FIG. 5.
Mammary tumor kinetics and apoptotic indices in transgenic strains. (a) Mammary tumor kinetics of MTY315/322F and Akt7 × MTY315/322F strains. Two different kinetics curves are shown for the MTY315/322F strain, from the original published data (MTY315/322F-1) and confirmatory data us (MTY315/322F-2), to account for possible differences in palpation technique between researchers. The age indicated is that at which a mammary tumor is first palpable in each transgenic strain. The number of animals analyzed for each strain (n) and the median age at which tumors were palpable are also shown. (b) Mammary apoptotic indices of FVB/n, MT634 (wild-type mT), Akt7, MTY315/322F, and Akt7 × MTY315/322F strains. Values shown represent the percentage of total cells stained positive for apoptosis by TUNEL assay in virgin female mice at 10 to 12 weeks of age.
FIG. 6.
Akt kinase activity in transgenic strains. (a) Total Akt kinase activity analysis in 8- to 10-week-old virgin females from FVB/n (lane 1), MTY135/322F (lanes 2 and 3), and bitransgenic Akt7 × MTY315/322F (lanes 4 and 5) strains. Assays were conducted using the cross-tide peptide as an Akt kinase substrate. Kinase activities were quantified by phosphorimager analysis and are represented here both graphically and numerically. (b) Immunoblot analysis of expression of HA-Akt-DD, phospho-S473-Akt, and Akt in 8- to 10-week-old virgin females from FVB/n (lane 1), MTY135/322F (lanes 2 and 3), and bitransgenic Akt7 × MTY315/322F (lanes 4 and 5) strains. All tissues were derived from 8- to 10-week-old virgin mammary glands. The arrows indicate the migration of transgenic HA-Akt-DD (upper panel), phospho-S473-Akt (middle panel), and total Akt (bottom panel). The numbers above each lane indicate individual mouse identification numbers.
As the mammary epithelial hyperplasias associated with the mutant PyV mT strains exhibit elevated levels of apoptotic cell death, we measured the degree of apoptotic cell death in mammary glands derived from the mutant PyV mT or bitransgenic mice. The results revealed that mammary epithelial expression of activated Akt resulted in a dramatic repression of the high rates of apoptotic cell death in PyV mT mutant tissue decoupled from the PI3K (Fig. 5b). Taken together, these observations argue that the dramatic acceleration of mammary tumorigenesis exhibited by these strains is due to the ability of activated Akt to suppress the elevated apoptotic cell death displayed by mutant PyV mT mammary epithelium.
Although the active, transgenic Akt is able to complement the mutant PyV mT strains for the induction of mammary tumors, only 20% of the tumor-bearing mice have developed lung metastases more than 8 weeks after the initial palpation of the mammary tumor (n = 10) at tumor loads comparable to those observed in mice expressing wild-type mT at similar time points. The penetrance of the metastatic phenotype is comparable to the 30% metastasis levels exhibited by the parental mutant PyV strains. In contrast, 100% of mice expressing wild-type mT show multiple lung metastases at comparable time points and tumor loads (18). These observations argue that while expression of active Akt can complement the defect in mammary tumor progression, it is unable to rescue the defect in metastatic progression.
To further explore the molecular basis for the observed cooperative interaction between activated Akt and the mutant PyV mT oncogene, we assessed the status of some of the known targets of Akt, including BAD (9), I-κ-B (20), and the FKHR forkhead transcription factor (34).
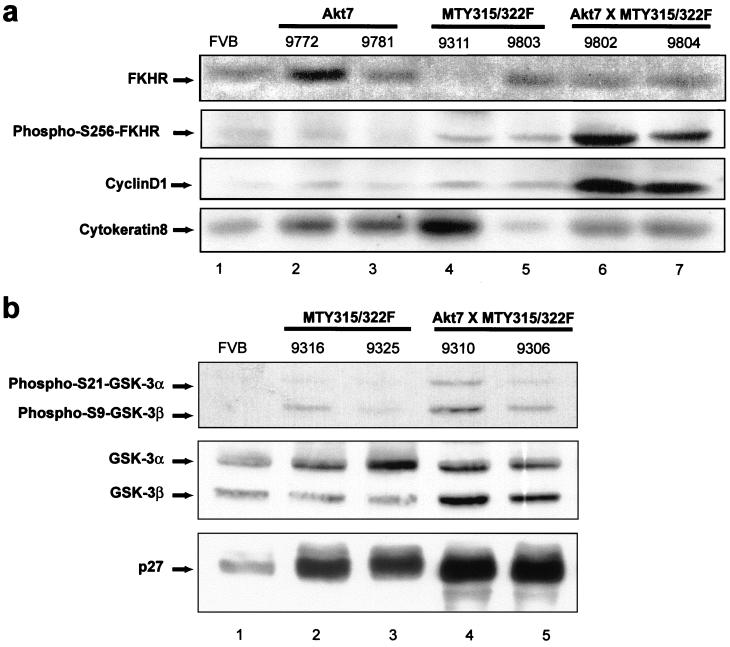
No significant differences in either BAD-Ser136 phosphorylation or I-κ-B levels were observed between the various transgenic strains (data not shown). Caspase 9, another Akt substrate (4), was not examined, as the Akt phosphorylation site found in human caspase 9 is absent in mouse caspase 9 (17). However, analysis of protein lysates from mammary tissues of 8-week-old virgin FVB/n, Akt7, mutant PyV mT, and bigenic mice subjected to immunoblot analyses with phospho-specific antisera to serine 256 of FKHR (Fig. 7a) revealed that the mammary tissue samples derived from the bitransgenic animals expressed elevated levels of phosphorylated FKHR protein relative to the other tissue samples (second panel). The differences in the phosphorylation status of FKHR proteins were not due to levels of FKHR protein, since most of the tissues expressed comparable levels of FKHR protein (upper panel). In addition, the differences in the phosphorylation status could not be due to variation in epithelial content, since these samples expressed comparable levels of cytokeratin 8 (lower panel). Consistent with these observations, we have demonstrated an identical pattern of FKHR phosphorylation in a second independent cohort of samples (data not shown). To further explore this observation we examined the status of p27 (Kip1), as forkhead transcription factors have been shown to target expression of the cell cycle regulator p27 (13, 23, 24). In particular, adenoviral expression of a constitutively active version of FKHR in the human renal cancer cell line 786-O cells induces expression of p27 (24). However examination of p27 levels by Western blotting revealed no apparent decreases in p27 levels in the bitransgenic animals as compared to MTY315/322F and FVB/n controls (Fig. 7b, lower panel). This apparent discrepancy may be due to the different nature of the tissues and signals involved in these experiments.
FIG. 7.
Coexpression of activated Akt and MTY315/322F results in FKHR phosphorylation at serine 256 and increased cyclin D1 levels but does not affect GSK-3 phosphorylation or p27 levels. (a) Immunoblot analysis of expression of FKHR, phospho-FKHR (Ser256), cyclin D1, and cytokeratin in 8- to 10-week-old virgin females from FVB/n (lane 1), Akt7 (lanes 2 and 3), MTY135/322F (lanes 4 and 5), and bitransgenic Akt7 × MTY315/322F (lanes 6 and 7) strains. All tissues were derived from 8- to 10-week virgin mammary glands. The arrows indicate the migration of FKHR (upper panel), phospho-FKHR (Ser256) (second panel), cyclin D1 (third panel), and cytokeratin proteins (lower panel). The numbers above each lane indicate individual mouse identification numbers. (b) Immunoblot analysis of expression of phospho-S21-GSK-3α, phospho-S9-GSK-3β, GSK-3α/β, and p27 in 8- to 10-week-old virgin females from FVB/n (lane 1), MTY135/322F (lanes 2 and 3), and bitransgenic Akt7 × MTY315/322F (lanes 4 and 5) strains. All tissues were derived from 8- to 10-week virgin mammary glands. The arrows indicate the migration of phospho-S21-GSK-3α and phospho-S9-GSK-3β (upper panel), GSK-3α/β (middle panel), and p27 (lower panel). The numbers above each lane indicate individual mouse identification numbers.
Nevertheless, another potential target for the PI3K/Akt kinase axis is the cell cycle machinery. Indeed, it has previously been demonstrated that suppression of the PI3K signaling pathway by expression of the PTEN tumor suppressor results in downregulation of cyclin D1 expression and cell cycle arrest (32, 36). To determine whether the levels of cyclin D1 could be influenced by Akt activation, the identical set of mammary tissues were subjected to immunoblot analyses with cyclin D1-specific antibodies. The results of these analyses revealed that the bitransgenic tissues coexpressing both Akt-DD and the mutant PyV mT oncogene exhibited dramatically elevated levels of cyclin D1 (Fig. 7a, third panel). The differences in cyclin D1 protein were not due to increased levels of cyclin D1 transcripts, since these samples expressed comparable levels of cyclin D1 transcript (data not shown). Taken together these observations suggest that activation of FKHR and cyclin D1 proteins are involved in promoting tumor progression in these strains.
The studies outlined above provide compelling evidence that expression of activated Akt is involved in promoting tumor progression by providing a critical cell survival pathway. Consistent with this contention, mammary epithelial expression of Akt can result in profound delays in mammary gland involution, a process involving extensive apoptotic cell death. Moreover, coexpression of activated Akt can suppress the elevated rates of apoptotic cell death that are observed in mammary epithelial hyperplasias induced by the mutant PyV mT decoupled from the PI3K signaling pathway. However, because mammary epithelial expression of activated Akt does not result in the induction of mammary tumors itself, tumorigenesis requires the constitutive activation of other signaling pathways that are recruited by the mutant PyV mT oncogene, including the Src family kinases and Shc/Grb2/Ras pathway. Consistent with this view, we have observed that efficient phosphorylation of the FKHR protein requires the concerted activation of both Akt and the mutant PyV mT oncogene (Fig. 7). A similar requirement for coactivation of Akt and mutant PyV mT was also noted for the induction of cyclin D1. In this regard, it has recently been reported that the cooperation of Ras and Akt are required for the efficient transformation of primary glial cells (19). A potential mechanism for the increased levels of cyclin D1 was suggested by the ability of Akt and mitogen-activated protein kinases to phosphorylate and inhibit GSK-3 (8, 33), which has been shown to target cyclin D1 for proteasomal degradation (12). However, analysis of GSK-3 phosphorylation showed no significant increases in the bitransgenic strain as compared to FVB/n and MTY315/322F controls, once differences in GSK-3 levels were accounted for (Fig. 7b, upper and middle panels). Even so, these results suggest that the concerted activation of both cell survival and proliferative signaling pathways may be a common requirement for oncogenic transformation of primary cells.
Although our studies suggest that activated Akt can cooperate with these signaling pathways to efficiently induce mammary tumorigenesis, the observed low rates of metastasis suggest the involvement of other Akt-independent signals downstream of middle T in the potent metastatic phenotype exhibited by wild-type PyV mT. While these signals are in all likelihood PI3K dependent, we cannot exclude the possibility that signaling molecules other than PI3K may bind to and be activated via the 315 and 322 phosphorylated tyrosine residues. However, PI3K activation does modulate the activity of members of the Rho family of GTP-binding proteins (21, 25, 27, 29) and the integrin-linked kinase (11). This modulation is highly relevant, as the roles of these sets of signaling molecules in cell migration and adhesion implicates them in metastatic progression (10, 28). Further exploration of these PI3K-dependent pathways will provide important insight into the molecular basis of the metastatic phenotype.
ACKNOWLEDGMENTS
We thank Dinsdale Gooden for oligonucleotide synthesis and Brian Allore for automated DNA sequence analysis (MOBIX Central Facility, McMaster University). We are grateful to S. Dilworth for generously providing the PAb701 and PAb762 anti-PyV mT antibodies. We are also grateful to Monica Graham and Judy Walls for technical support.
This work was supported by grants awarded to W.J.M. by the United States Army Medical Research's Breast Cancer Research Program and the Canadian Breast Cancer Research Initiative and by NCIC and MRC grants awarded to J.R.W. W.J.M. is a recipient of a Medical Research Council of Canada Scientist award, J.R.W. is a recipient of an MRC Senior Scientist award, and J.N.H. is supported by a scholarship from the United States Army Medical Research's Breast Cancer Research Program.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 4.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 5.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 6.Courtneidge S A, Heber A. An 81 kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell. 1987;50:1031–1037. doi: 10.1016/0092-8674(87)90169-3. [DOI] [PubMed] [Google Scholar]
- 7.Courtneidge S A, Smith A E. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984;3:585–591. doi: 10.1002/j.1460-2075.1984.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 9.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 10.Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol. 1999;9:319–323. doi: 10.1016/s0962-8924(99)01612-8. [DOI] [PubMed] [Google Scholar]
- 11.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diehl J A, Cheng M, Roussel M F, Sherr C J. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dijkers P F, Medema R H, Pals C, Banerji L, Thomas N S, Lam E W, Burgering B M, Raaijmakers J A, Lammers J W, Koenderman L, Coffer P J. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27 (KIP1) Mol Cell Biol. 2000;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dilworth S M, Brewster C E, Jones M D, Lanfrancone L, Pelicci G, Pelicci P G. Transformation by polyoma virus middle T-antigen involves the binding and tyrosine phosphorylation of Shc. Nature. 1994;367:87–90. doi: 10.1038/367087a0. [DOI] [PubMed] [Google Scholar]
- 15.Farrelly N, Lee Y J, Oliver J, Dive C, Streuli C H. Extracellular matrix regulates apoptosis in mammary epithelium through a control on insulin signaling. J Cell Biol. 1999;144:1337–1348. doi: 10.1083/jcb.144.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 17.Fujita E, Jinbo A, Matuzaki H, Konishi H, Kikkawa U, Momoi T. Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem Biophys Res Commun. 1999;264:550–555. doi: 10.1006/bbrc.1999.1387. [DOI] [PubMed] [Google Scholar]
- 18.Guy C T, Cardiff R D, Muller W J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland E C, Celestino J, Dai C, Schaefer L, Sawaya R E, Fuller G N. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 20.Kane L P, Shapiro V S, Stokoe D, Weiss A. Induction of NF-κB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 21.Karnam P, Standaert M L, Galloway L, Farese R V. Activation and translocation of Rho (and ADP ribosylation factor) by insulin in rat adipocytes. Apparent involvement of phosphatidylinositol 3-kinase. J Biol Chem. 1997;272:6136–6140. doi: 10.1074/jbc.272.10.6136. [DOI] [PubMed] [Google Scholar]
- 22.Kops G J, de Ruiter N D, De Vries-Smits A M, Powell D R, Bos J L, Burgering B M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 23.Medema R H, Kops G J, Bos J L, Burgering B M. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers W R. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nobes C D, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108(Pt.1):225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- 26.Pattengale P K, Stewart T A, Leder A, Sinn E, Muller W, Tepler I, Schmidt E, Leder P. Animal models of human disease. Pathology and molecular biology of spontaneous neoplasms occurring in transgenic mice carrying and expressing activated cellular oncogenes. Am J Pathol. 1989;135:39–61. [PMC free article] [PubMed] [Google Scholar]
- 27.Reif K, Nobes C D, Thomas G, Hall A, Cantrell D A. Phosphatidylinositol 3-kinase signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- 28.Sander E E, Collard J G. Rho-like GTPases: their role in epithelial cell-cell adhesion and invasion. Eur J Cancer. 1999;35:1302–1308. doi: 10.1016/s0959-8049(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 29.Sarner S, Kozma R, Ahmed S, Lim L. Phosphatidylinositol 3-kinase, Cdc42, and Rac1 act downstream of Ras in integrin-dependent neurite outgrowth in N1E-115 neuroblastoma cells. Mol Cell Biol. 2000;20:158–172. doi: 10.1128/mcb.20.1.158-172.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shayesteh L, Lu Y, Kuo W L, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills G B, Gray J W. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 31.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 32.Sun H, Lesche R, Li D M, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland C, Cohen P. The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 1994;338:37–42. doi: 10.1016/0014-5793(94)80112-6. [DOI] [PubMed] [Google Scholar]
- 34.Tang E D, Nunez G, Barr F G, Guan K L. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 35.Webster M A, Hutchinson J N, Rauh M J, Muthuswamy S K, Anton M, Tortorice C G, Cardiff R D, Graham F L, Hassell J A, Muller W J. Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weng L P, Smith W M, Dahia P L, Ziebold U, Gil E, Lees J A, Eng C. PTEN suppresses breast cancer cell growth by phosphatase activity-dependent G1 arrest followed by cell death. Cancer Res. 1999;59:5808–5814. [PubMed] [Google Scholar]