Abstract
Conventional calpains are ubiquitous calcium-regulated cysteine proteases that have been implicated in cytoskeletal organization, cell proliferation, apoptosis, cell motility, and hemostasis. There are two forms of conventional calpains: the μ-calpain, or calpain I, which requires micromolar calcium for half-maximal activation, and the m-calpain, or calpain II, which functions at millimolar calcium concentrations. We evaluated the functional role of the 80-kDa catalytic subunit of μ-calpain by genetic inactivation using homologous recombination in embryonic stem cells. The μ-calpain-deficient mice are viable and fertile. The complete deficiency of μ-calpain causes significant reduction in platelet aggregation and clot retraction but surprisingly the mutant mice display normal bleeding times. No detectable differences were observed in the cleavage pattern and kinetics of calpain substrates such as the β3 subunit of αIIbβ3 integrin, talin, and ABP-280 (filamin). However, μ-calpain null platelets exhibit impaired tyrosine phosphorylation of several proteins including the β3 subunit of αIIbβ3 integrin, correlating with the agonist-induced reduction in platelet aggregation. These results provide the first direct evidence that μ-calpain is essential for normal platelet function, not by affecting the cleavage of cytoskeletal proteins but by potentially regulating the state of tyrosine phosphorylation of the platelet proteins.
The calpains are a family of calcium-dependent neutral cysteine proteases present in essentially all tissues of higher animals (8, 34, 37). Calpain homologues distantly related to the catalytic subunits of conventional calpains are also found in lower organisms such as parasites, insects, nematodes, fungi, and yeast (34). They are believed to play functionally important roles in diverse biological processes such as reorganization of cortical cytoskeleton, cell motility, cell proliferation, apoptosis, and hemostasis (9, 27, 31, 39). Calpains are divided into two broad classes, ubiquitous and tissue specific. Calpain I (also referred to as μ-calpain) and calpain II (also referred to as m-calpain) are expressed in all tissues in varying amounts and share ∼61% sequence identity (20). Both the μ- and m-calpains contain an 80-kDa catalytic subunit that forms a heterodimer with the regulatory 30-kDa subunit (34). The 80-kDa catalytic subunits of the μ- and m-calpains are products of separate but closely related genes (referred to as Capn1 and Capn2, respectively), while the 30-kDa subunit (encoded by the Capn4 gene) is common to both (34). The μ-calpain is fully active in micromolar concentrations of calcium, while the m-calpain requires millimolar calcium concentrations for full activation. Larger tissue-specific calpains have been cloned from stomach and smooth muscle tissues (35, 37). Mutations of the muscle-specific Capn3 (calpain 3 gene) have been shown to cause one form of limb-girdle muscular dystrophy type 2A (30). More recently, several groups have identified CAPN10 (calpain 10) as the target gene for mutations in the type 2 or non-insulin-dependent diabetes mellitus, thus underscoring the importance of calpains in the regulation of fundamental signaling pathways (2, 16, 25). Although much biochemical information has been accumulated on the combined effects of μ- and m-calpains, their individual physiological functions have not been identified. In order to obtain an in vivo model of μ-calpain deficiency, we disrupted the μ-calpain catalytic subunit gene (Capn1) in the mouse. Here, we describe the in vivo consequences of disruption of the μ-calpain gene in mice. Our data provide the first unambiguous proof of the essential role of μ-calpain in platelet aggregation, clot retraction, and tyrosine phosphorylation of platelet proteins. Since the μ-calpain gene is expressed in many cell types, our findings may be of significance not only in blood coagulation and platelet physiology but also in the areas of cell motility, cancer (metastasis), inflammation, and other integrin-dependent processes.
MATERIALS AND METHODS
Generation of targeted embryonic stem cells and mutant mice.
A 2.3-kb mouse μ-calpain cDNA was amplified by reverse transcriptase PCR (RT-PCR) using a total spleen RNA as a template. Total RNA was first reverse transcribed using a random hexamer primer (Promega) and Moloney murine leukemia virus reverse transcriptase (Gibco BRL). Overlapping cDNA fragments were then amplified using the Expand Long Template PCR system (Boehringer Mannheim). The following primers were designed based on the rat and mouse μ-calpain cDNA sequences: Cal 1 (263 to 282, 5′-CTACGGAACTGCTGTCAAAC) and Cal 2 (970 to 989, 5′-TCCATCTTGACCCTCAGCTG); Cal 6 (1872 to 1892, 5′-GTTCACCATGCTGCGGCACGA) and Cal 7 (941 to 958, 5′-GGAACAAAGTGGACCCCT). A genomic clone of 28 kb was isolated by screening a 129-Sv genomic library (Stratagene) and was characterized. An XbaI fragment (5.5 kb) containing exons 1 to 3 (upstream arm) was cloned at the XbaI site in the pPNT targeting vector. A SalI fragment (2.4 kb) containing exon 5 and a segment of exon 4 was cloned at the XhoI site as the downstream arm. The vector was linearized using SspI and electroporated into the R1 ES cell line. Six correctly targeted clones were identified by PCR and confirmed by Southern blotting. PCR analysis was performed using primers specific for Neo on the 5′ end and exon 6 on the 3′ end. Two separate ES cell clones heterozygous for disrupted alleles were microinjected into the blastocysts of C57BL/6J mice. Male chimeras were then bred with female C57BL/6J mice to confirm germ line transmission, and the resulting heterozygotes were mated to generate homozygotes for the mutation in the μ-calpain gene. Southern, Northern, and Western blotting and casein zymography were performed using standard protocols. To ensure that the design of the targeting construct did not result in the expression of low abundance, truncated μ-calpain transcripts, RT-PCR of μ-calpain was performed using total RNA pooled from different tissues (Fig. 1e). The following primers were used: Cal 1 (exon 1) and Cal 2 (exon 7); Cal 10 (exon 2; 5′-ACAGACATCTGCCAGGGAGC) and Cal 19 (exon 8; 5′-CCAGTTTGGTGAATTCACGG); Cal 18 (exon 6; 5′-GGGTGAAGTGGAGTGGAAAGGA) and Cal 6 (exon 16; 5′-GTTCACCATGCTGCGGCACGA). The RT-PCR of m-calpain (primers: mCal 1 [5′-GAGGTGGTGGTGGACGACAG] and maCal 2 [5′-TTTCTGCAGGCTTCCTGAAC]), G3PDH (primers: Clonetech 5′-RACE kit), and the 30-kDa regulatory subunit of calpain (primers: 30K-1 [5′-CTCCGCCTCCACGTAGTCAT] and 30K-2 [5′-GCTATCAGGGACTAGCCAGT]) was performed using gene-specific primers.
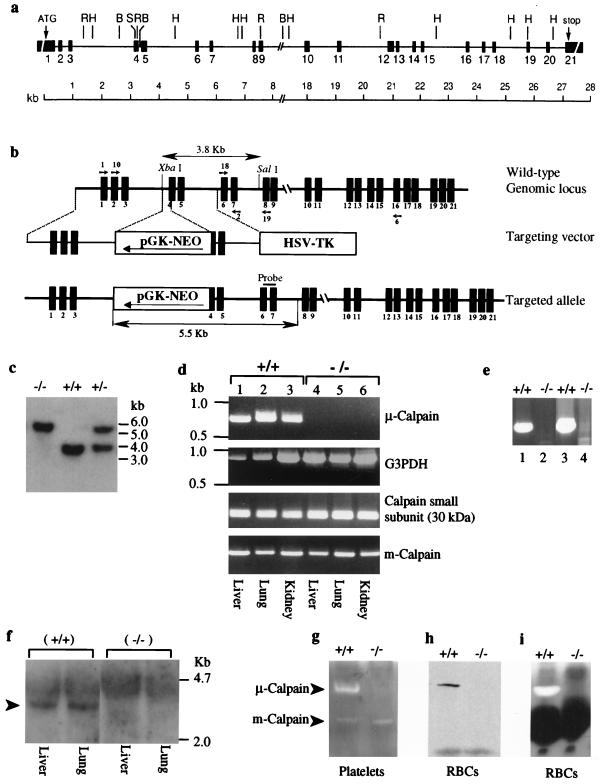
FIG. 1.
Targeted disruption of the Capn1 locus and generation of mutant mice. (a) Schematic representation of the genomic locus of mouse μ-calpain drawn to scale. Sequence information for the intron-exon boundaries is shown in Table 1. The restriction sites shown on the map are as follows: BamHI (B), HindIII (H), and SalI (S). (b) The Capn1 locus (wild type; top line), the targeting vector (middle line), and the disrupted Capn1 locus (bottom line). Shown is the expected size of an XbaI and SalI fragment that hybridized with the probe for the wild-type locus (top) and the mutated allele (bottom). Small arrows indicate the positions of calpain primers that were used to conduct the RT-PCR analysis. (c) Southern blot analysis of genomic DNA isolated from the F2 generation of Capn1+/− mice was digested with XbaI and SalI and was blotted and hybridized with the probe shown in panel b. (d) RT-PCR analysis of mouse μ-calpain using primers Cal 1 and Cal 2. Glyceraldehyde 3-phosphate dehydrogenase (G3PDH) was used as a control to normalize the samples. The calpain regulatory 30-kDa subunit and the m-calpain catalytic subunit were amplified from the total RNA of liver, lung, and kidney of wild-type and Capn1−/− mice using gene-specific primers as described in Materials and Methods. (e) To rule out the possibility that alternate splicing may have occurred and produced low levels of truncated transcripts, RT-PCR analysis was conducted using total RNA pooled from liver, lung, and kidney. Lanes 1 and 2 show the results obtained using primer pair Cal 10 and Cal 19 (upstream transcript), and lanes 3 and 4 show the results obtained with primer pair Cal 18 and Cal 6 (downstream transcript). (f) Northern blot analysis of total RNA isolated from liver and lung of Capn1+/+ and Capn1−/− mice. The blot was hybridized with a cDNA probe (1.5 kb; exons 2 to 16) of murine μ-calpain. (g) Casein zymogram showing the distribution of μ-calpain and m-calpain in the platelet extract of Capn1+/+ and Capn1−/− mice. Note that only the μ-calpain activity was lost in the Capn1−/− mice. (h) Western blot analysis of μ-calpain in the whole red blood cell lysate of Capn1+/+ (left lane) and Capn1−/− (right lane) mice. (i) Casein zymogram of calpain activity in the red blood cell lysate of Capn1+/+ (left lane) and Capn1−/− (right lane) mice. The dark band represents the position of hemoglobin in the red cell lysate.
Preparation of washed platelets and PRP.
Blood was collected from the inferior vena cava of mice anesthetized with the pentobarbitol sodium (Nembutal)-ketamine mixture. Blood (∼700 μl) was withdrawn into a syringe containing 25 μl of heparin (1,000 U/ml) and 5 U of apyrase/ml. Blood was pooled from several mice in a 15-ml tube, and an equal volume of phosphate-buffered saline was added. The sample was centrifuged at 1,000 × g for 10 min and the resulting platelet-rich plasma (PRP) was centrifuged at 3,000 × g for 7 min. Sedimented platelets were resuspended in Tyrode's buffer (10 mM HEPES [pH 7.4], 5.56 mM glucose, 137 mM NaCl, 12 mM NaHCO3, 2.7 mM KCl, 0.36 mM NaH2PO4, 1 mM MgCl2), counted, and diluted to 1.5 × 108/ml. All steps were performed at 25 to 30°C.
Platelet aggregation and tail-bleeding measurements.
Aggregation and ATP secretion of washed and recalcified (2.0 mM) platelets were measured by light scattering in a Chrono-log lumiaggregometer (Model 560VS/490-2D). Platelets were induced to aggregate by the addition of thrombin (Hematologic Technologies, Inc.), ADP (Sigma), collagen (Sigma), and A23187 ionophore (Sigma) with stirring at 37°C. For tail-bleeding experiments, 8- to 12-week-old Capn1+/+ (36 mice) and Capn1−/− (52 mice) mice were anesthetized and 6 min later their tails were transected 0.2 cm from the tip with a scalpel blade. The tail was placed in a solution of phosphate-buffered saline at 37°C, and the time taken for the blood flow to cease was recorded. Where necessary, experiments were terminated by cauterization at 600 s to prevent death.
Clot retraction assay.
Clot retraction was measured essentially as described before (15) by mixing the following: 200 μl of PRP from Capn1 null and wild-type mice, 750 μl of 53 μM Na2HPO4-12 μM KH2PO4, 5 μl of erythrocytes (to enhance clot contrast for photography), and 50 μl of thrombin (1 or 10 nM). A glass rod was placed in each glass test tube and incubated at ambient temperature for 1 to 12 h. Clot formation and subsequent clot retraction were recorded visually at various time intervals prior to photography.
Cleavage of talin, filamin, and β3 integrin and tyrosine phosphorylation.
Purified platelets (200 μl at 5 × 108 platelets/ml) were calcified (1.0 mM CaCl2) and stimulated using calcium ionophore A23187 (1.0 μM) and thrombin (10 nM) at 37°C with constant stirring. Samples were withdrawn at the indicated time intervals, immediately solubilized in the 5× gel-loading buffer (250 mM Tris-HCl [pH 6.8], 5 mM sodium vanadate, 10 mM EDTA, 5 mM phenylmethylsulfonyl fluoride, 10 mM benzimidine, 25% glycerol, 15% sodium dodecyl sulfate [SDS], 2.5% β-mercaptoethanol), and boiled at 95°C for 5 min. Proteins were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) (6% gel) and visualized by Coomassie staining of the gel. Cleavage of talin was analyzed by Western blotting using a monoclonal anti-talin 8d4 antibody (Sigma), and β3 integrin was detected using a polyclonal anti-β3C antibody (raised against the C-terminal 20 amino acids of the β3 integrin cytoplasmic domain) (10). Another polyclonal antibody, anti-Nβ3 (N-20), raised against the N-terminal domain of β3 integrin (Santa Cruz) was used to normalize the amount of β3 integrin in the Western blots. For antiphosphotyrosine blots, total platelet protein extract was analyzed by SDS-PAGE (7% gel), transferred to the nitrocellulose, and immunoblotted with an antiphosphotyrosine monoclonal antibody 4G10 (Upstate Biotechnology Inc.).
Immunoprecipitation assays.
Equal numbers of platelets from wild-type and Capn1−/− mice were activated with either 10 nM thrombin or 1 μM calcium ionophore A23187 for 30 s, 1 min, and 3 min. Platelets were solubilized with an equal volume of double-strength modified RIPA buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 4 mM EDTA, 4 mM EGTA, 20 μg of aprotinine/ml, 2 mM phenylmethylsulfonyl fluoride, 200 μM leupeptin, 4 mM sodium orthovanadate, 4 mM benzamidine, 2 mM sodium fluoride, 2 mM sodium pyrophosphate, and 0.2% sodium deoxycholate). Immunoprecipitations were performed using biotin-conjugated anti-integrin β3 antibody (Becton Dickinson) for 12 h at 4°C, followed by incubation with streptavidin-conjugated agarose beads (Pierce) for 2 h at 4°C. Immunoprecipitates were washed with two volumes of RIPA buffer (as described above) and analyzed by SDS-PAGE (8% gel). Immunoprecipitates were transferred to nitrocellulose, and immunoblotting was performed using an antiphosphotyrosine antibody, 4G10 (Upstate Biotechnology Inc.). The same blot was stripped and reprobed with an anti-β3 integrin antibody, Nβ3 (Santa Cruz), to ensure an equal amount of β3 integrin in each lane.
RESULTS AND DISCUSSION
Although numerous attempts have been made to delineate the biological function of μ-calpain and m-calpain using cysteine protease inhibitors, the results have often been confounded due to the lack of specificity of these inhibitors. We set out to determine the specific function of μ-calpain by genetic inactivation of its expression in mice. Overlapping mouse μ-calpain large subunit cDNA clones were isolated by RT-PCR of BALB/c mouse spleen RNA. The overlapping cDNA sequence (GenBank accession number AF084459) extends 2,311 nucleotides and encodes a protein of 713 amino acids consistent with the reported cDNA sequence of the mouse μ-calpain catalytic subunit (26). A mouse 129 strain λ genomic library (Stratagene) was screened, and the complete genomic structure of the μ-calpain large subunit was assembled using established methods. The mouse μ-calpain large subunit gene spans ∼28 kb and consists of 21 exons (Fig. 1a and Table 1). A targeting vector was constructed that will delete the 5′ segment of exon 4 (amino acids 153 to 160: LWQFGEWV) as well as disrupt the gene by inserting the pGK-Neo cassette (Fig. 1b). Exon 4 encodes a critical part of the catalytic domain of μ-calpain (17, 36). Two embryonic stem cell clones bearing the targeted allele were used to generate chimeric mice, which were then mated to generate heterozygous animals. Wild-type, heterozygous, and homozygous mice were borne at the expected Mendelian ratio from the heterozygous mating. A Neo-specific probe was used to confirm unique integration in the genome. Correct targeting of Capn1 was confirmed by Southern blotting (Fig. 1c) using a probe derived from exons 6 and 7 (Fig. 1b). Northern blot analysis of liver and lung tissues indicated a complete absence of μ-calpain transcript (∼2.8 kb) when probed with a cDNA fragment of 1.5 kb from exons 2 to 16 of Capn1 (Fig. 1f). The absence of μ-calpain transcript in the homozygous mutant mice was further confirmed by RT-PCR analysis of liver, lung, and kidney tissues (Fig. 1d). In order to check any differentially spliced transcript, RT-PCR was carried out from the regions upstream and downstream of pGK-Neo insertion (Fig. 1e). The transcripts encoding m-calpain and the regulatory 30-kDa subunit were unaltered in the μ-calpain null tissues (Fig. 1d).
TABLE 1.
Mouse μ-calpain large subunit gene exon donor and acceptor sequences
| 5′ donor site (exon/intron) | Intron no. (∼kb) | 3′ Acceptor site (intron/exon) | Exon no. (bp) |
|---|---|---|---|
| 1 (NDa) | |||
| CCTACGgtaaga | 1 (0.14) | ccgtgggtcttgtagGAACTG | 2 (70) |
| CACTGGgtaggc | 2 (0.25) | tctttcctattccagGGGACT | 3 (119) |
| TTCCAGgtgaag | 3 (2.2) | ttgtccctcccacagCTGTGG | 4 (134) |
| TGCTAAgtgagt | 4 (0.1) | tggagctgcccacagAGTGAA | 5 (169) |
| ATTAATgtgagt | 5 (1.7) | cgtttctttccgtagATCTCC | 6 (84) |
| AAGCAGgtattt | 6 (0.4) | cacttttgggaacagGTAACT | 7 (86) |
| TGACAGgtaagt | 7 (1.4) | gcctgcttcccacagCTCCTA | 8 (75) |
| GTTCTGgtaagc | 8 (0.10) | cccttgggccctcagGATGTC | 9 (161) |
| ACCCAGgtgagt | 9 (10.2) | cctctcctcctgcagCTACCT | 10 (176) |
| TACCAGgttagc | 10 (1.1) | ctgtctctcttgcagGTCCCT | 11 (12) |
| CGGGAGgtaggt | 11 (1.9) | tcttttctcctccagCTGGCG | 12 (210) |
| ACCCAGtgagta | 12 (0.1) | tctctgctctgcaggGAACTA | 13 (38) |
| GATGAGgtacgt | 13 (0.5) | ccgtttcgtgtacagAAAGTT | 14 (68) |
| GGGGATgtaagt | 14 (0.2) | cttctcttcccctagGACATG | 15 (58) |
| GCAAACgtaagg | 15 (1.5) | tgttgtttgtttcagACAAAG | 16 (69) |
| ATGGATgtatcc | 16 (0.5) | ttaacatcagccaagCGAGAT | 17 (69) |
| TACCTGgtaggt | 17 (0.3) | tatccttccacacagACCATC | 18 (79) |
| CTGCAGgtgagg | 18 (1.1) | cccctctctgctcagGCTTCA | 19 (117) |
| TGTTCCgtgagt | 19 (0.3) | ttttcttgaacacagGGTTTT | 20 (58) |
| ATTTAAggtgag | 20 (0.6) | ctgtcttgccctgcaGTGGCT | 21 (ND) |
ND, not determined.
The enzyme activity of μ-calpain was measured in the platelets of mutant mice by casein zymography. Casein zymograms of wild-type platelets showed an asymmetric distribution of calpain activity: ∼80% μ-calpain and ∼20% m-calpain (Fig. 1g). In the μ-calpain null platelets, the band corresponding to μ-calpain activity was absent whereas the m-calpain activity remained essentially unaltered (Fig. 1g). Because the available monoclonal antibody reacts with all isoforms of calpains, we could not perform Western blotting on μ-calpain null platelets since both μ- and m-calpains migrate at the same position during electrophoresis under denaturing conditions. Alternatively, we examined the mature erythrocytes from μ-calpain null mice by Western blotting using the same monoclonal antibody that reacts with both calpains. As shown in Fig. 1h, neither μ-calpain nor m-calpain was present in the mature erythrocytes. Indeed, the casein zymography confirmed that the mature erythrocytes from wild-type mice contain the μ-calpain exclusively, whereas the μ-calpain null erythrocytes are completely devoid of any calpain activity (Fig. 1i). Taken together, these results demonstrate the development of a murine model system that selectively lacks the μ-calpain activity systemically.
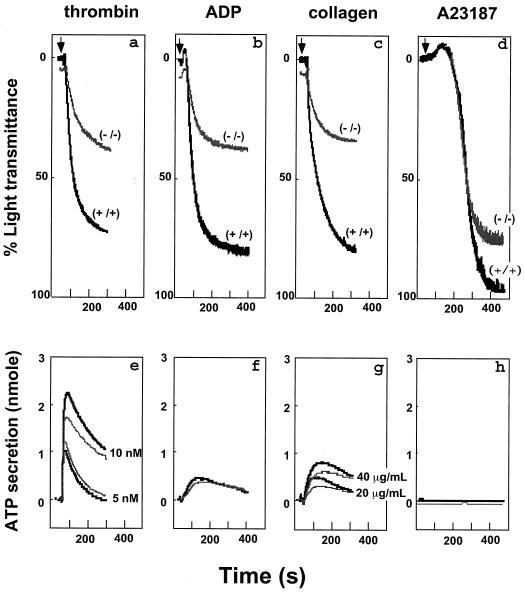
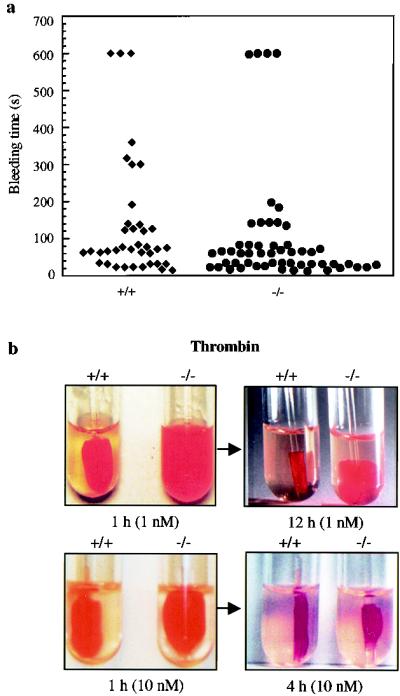
Because ∼80% of the total calpain activity in mouse platelets is contributed by the μ-calpain (Fig. 1g) and the calpains are known to play a major role in platelet physiology, we evaluated the effects of μ-calpain deficiency on platelet function. Activation of platelets involves a sequence of cytoskeletal reorganization events that include release of granule contents, engagement of integrins to form platelet-platelet aggregrates, and eventual formation of a stable hemostatic plug (33). Calcium-dependent calpains proteolyze a wide range of cytoskeletal proteins, including the β3 subunit of αIIbβ3 integrin, that have been proposed to play an essential role in platelet granule secretion, aggregation, and retraction of fibrin-bound blood clots (9, 10, 31, 32, 41). Therefore, we examined the effects of μ-calpain deficiency on platelet function. Platelet aggregation was reduced by 50 to 60% in response to thrombin (10 nM), ADP (20 μM), collagen (20 μg/ml), and calcium ionophore A23187 (1.0 μM) (Fig. 2, top panels). In separate experiments (data not shown), a comparable diminution in the platelet aggregation (∼55%) was observed at a lower concentration of the calcium ionophore A23187 (0.1 μM). In contrast, relatively small differences were observed in the ATP secretion from dense granules upon thrombin and collagen activation whereas no detectable differences were apparent in the ADP- and calcium ionophore A23187-treated platelets (Fig. 2, bottom panels). We then measured the bleeding time in Capn1−/− mice using the transected tail method. The bleeding times were essentially normal (Fig. 3a). The platelet numbers in the peripheral blood of 8- to 12-week-old mice were comparable between wild-type (1.13 × 106/μl) and homozygous (1.07 × 106/μl) mice. Significantly, however, the Capn1−/− platelets were defective in retracting clots, especially when the clot formation was induced with 1.0 nM thrombin (Fig. 3b). Since the clot retraction is an important part of thrombus consolidation and is dependent upon αIIbβ3 integrin function (18, 21, 40), our results suggest that μ-calpain plays a role in αIIbβ3 integrin-mediated signaling in murine platelets.
FIG. 2.
Effects of μ-calpain deficiency on platelet aggregation and granule secretion. Aggregation (top panel) and granule secretion (bottom panel) responses of washed platelets (1.5 × 108/ml) from Capn1+/+ (black) and Capn1−/− (gray) mice are shown. (a) Thrombin, 10 nM. (b) ADP, 20 μM. (c) Collagen, 20 μg/ml. (d) Calcium ionophore A23187, 1 μM. In separate experiments, calcium ionophore at 0.1 μM was also used and produced an aggregation response consistent with other agonists. All measurements of platelet aggregation and granule secretion (19) were performed on the apyrase-treated (5 U/ml) platelets. Data are representative of four experiments.
FIG. 3.
Effects of μ-calpain deficiency on hemostasis and clot retraction. (a) Bleeding time was measured as described in Materials and Methods. Each symbol represents bleeding time measurement on a single Capn1+/+ mouse (left) and Capn1−/− mouse (right). (b) Photographs show the extent of in vitro clot retraction using PRP from wild-type and calpain null mice. Samples were treated with either 1.0 or 10 nM thrombin. As mentioned in Materials and Methods, 5 μl of red blood cells was added to enhance the color contrast for photography. The defective clot retraction in the μ-calpain null platelets was not influenced by the addition of red blood cells. Each photograph is representative of three independent experiments.
It is noteworthy here that the ∼50% impaired platelet-platelet aggregate formation occurs under saturating agonist concentrations for thrombin, ADP, and collagen. In other words, the addition of more agonist does not overcome this deficient aggregation, demonstrating that impairment cannot be overcome by eventual activation of m-calpain. Thus, the most obvious biological function of μ-calpain is its role in controlling the very late events of platelet aggregation. Remarkably, early events of platelet activation such as shape change, granule release, and the initial rate of platelet aggregation are unaffected (the initial slopes start out identically); however, the final extent of platelet aggregation and clot retraction are both severely deficient. Therefore, it is quite clear that the high-affinity μ-calpain isoform is essential for the proper functioning of the later calcium-dependent cytoskeletal, integrin, and contractility apparatus in murine platelets.
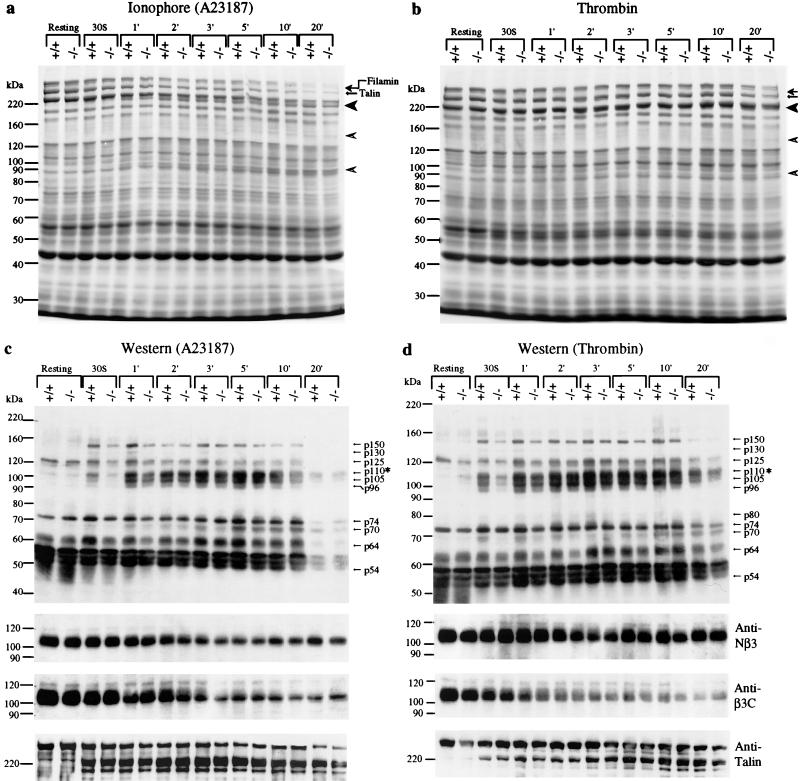
To further investigate the molecular basis of μ-calpain-mediated signaling in platelets, we examined the proteolysis of known calpain substrates such as β3 integrin, filamin, and talin in μ-calpain null platelets. Talin (270 kDa) and filamin (280 kDa) link the cytoplasmic domain of β3 integrin to the actin cytoskeleton and are cleaved upon platelet activation (3, 9, 12). Similarly, the cytoplasmic domain of β3 integrin itself undergoes calpain-dependent proteolysis upon platelet activation (10). Surprisingly, the time courses of proteolysis of talin (Fig. 4a to d), filamin (Fig. 4a and b), and the β3 integrin cytoplasmic domain (Fig. 4c and d) were indistinguishable in Capn1+/+ and Capn1−/− platelets. This result suggests that the dysfunctions of Capn1−/− platelets are not due to the cleavage of the β3 cytoplasmic domain of αIIbβ3 integrin. These results also suggest either that μ-calpain is not essential for this thrombin- and ionophore (A23187)-induced cleavage or that a possible compensation of μ-calpain activity by m-calpain occurs in the Capn1−/− platelets. Alternatively, these proteins may not be authentic substrates of μ-calpain in murine platelets and an independent proteolytic mechanism might exist that mediates cleavage of these proteins upon agonist-dependent activation of mouse platelets.
FIG. 4.
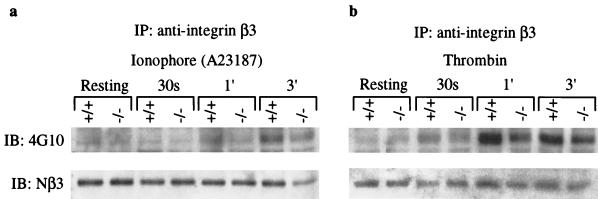
Proteolysis and tyrosine phosphorylation of platelet proteins. Washed platelets were activated by either calcium ionophore A23187 (1 μM) or thrombin (10 nM) at 37°C with stirring, and samples were taken out at indicated times for gel electrophoresis. (a) SDS-PAGE and Coomassie blue-stained 6% gel of total platelet lysate (40 μl). Calcium ionophore A23187 was used as an agonist. Arrows indicate the positions of intact filamin and talin. The 190-kDa fragment of talin (solid arrowhead) and the 130- and 93-kDa cleavage products of filamin (open arrowheads) are shown. (b) SDS-PAGE and Coomassie blue-stained 6% gel of total platelet lysate (40 μl). Thrombin was used as an agonist. Other symbols are the same as shown in the previous panel. (c and d) Western blot analysis of total platelet protein samples shown in panels a and b. (c) Calcium ionophore A23187 treatment. (d) Thrombin-treated platelets. An equal amount of platelet lysate, normalized by protein concentration, was analyzed by SDS-PAGE (7% gel), transferred to nitrocellulose, and probed with an antiphosphotyrosine monoclonal antibody (4G10). Note that the asterisk at p110 indicates the position of the β3 subunit of αIIbβ3 integrin. The same blots were stripped and reprobed to examine the proteolysis of β3 integrin and talin using defined antibodies. The bottom three panels show the results of Western blots using antibodies against β3 integrin and talin. The anti-Nβ3 antibody is specific for the N-terminal region of β3 integrin and was used to determine the total amount of β3 integrin in each lane. The anti-β3C antibody is cleavage sensitive and detects only the intact C terminus of β3 integrin (10). The anti-talin 8d4 antibody (Sigma) recognized the intact talin and cleaved the 190-kDa fragment but not the 50-kDa fragment. The same results were obtained in four independent experiments.
Studies with pharmacological inhibitors of tyrosine kinases have shown that the platelet agonists trigger inside-out signaling via αIIbβ3 integrin by tyrosine phosphorylation of proteins in the molecular mass range of 54, 60, 64, 75, and 130 to 140 kDa (6, 7, 13, 14). Moreover, the cytoplasmic domain of β3 integrin is itself tyrosine phosphorylated as a consequence of outside-in signaling and plays an important role in the regulation of integrin-myosin interaction during clot retraction (18, 22). Most agonists, except collagen, activate platelets by binding to G protein coupled receptors, and the agonist-induced platelet aggregation and secretion parallel this activity with a concomitant increase in the tyrosine phosphorylation of multiple proteins (24, 33). These phosphorylation events occur in distinct temporal waves. The early tyrosine phosphorylation of proteins such as p21ras GAP (4), cortactin (11), p60src (5), and RAFTK/Pyk2 (29) follows engagement of αIIbβ3 integrin with fibrinogen resulting in platelet aggregation-dependent tyrosine phosphorylation of several proteins, such as focal adhesion kinase, p72syk, vinculin, paxillin, and the cytoplasmic domain of β3 integrin itself (7, 22, 23, 28, 38). Calpain-dependent proteolysis of phosphotyrosine phosphatase 1B has also been implicated in the regulation of tyrosine phosphorylation following platelet aggregation (41).
We explored the mechanism of μ-calpain-mediated signaling by Western blotting using antiphosphotyrosine antibodies. Time course analysis revealed that the level of tyrosine phosphorylation of several proteins was significantly reduced (∼70% reduction) in μ-calpain null platelets upon their activation with either calcium ionophore or thrombin (Fig. 4c and d). Striking differences in tyrosine phosphorylation were seen at 30 s after the addition of ionophore or thrombin, the time period that encompasses the initiation of platelet aggregation. The most notable phosphorylation difference was observed for the band corresponding to the β3 subunit of αIIbβ3 integrin (∼70% reduction), although several other as yet unidentified polypeptides ranging in molecular mass from 54 to 150 kDa were also underphosphorylated during the initial phase of platelet activation (Fig. 4c and d). To confirm that the β3 integrin cytoplasmic domain is underphosphorylated, we immunoprecipitated the β3 integrin from wild-type and μ-calpain null platelets and immunoblotted with antiphosphotyrosine antibodies. Indeed, the β3 subunit of αIIbβ3 integrin is significantly underphosphorylated at tyrosine residues as a consequence of μ-calpain deficiency (Fig. 5a and b). We suggest that the underphosphorylation of β3 integrin at one or both tyrosine residues in its cytoplasmic domain may lead to defective platelet aggregation and clot retraction (18, 21). Together, our results provide the first direct evidence for the functional requirement of μ-calpain in the agonist-induced activation of tyrosine phosphorylation during platelet activation. How the μ-calpain activity modulates tyrosine phosphorylation of platelet proteins, including β3 integrin, remains an issue of fundamental importance and requires an extensive biochemical analysis of the μ-calpain-deficient platelets in terms of defined effects of μ-calpain on specific modulatory molecules and signaling pathways.
FIG. 5.
Immunoprecipitation and tyrosine phosphorylation of the β3 subunit of αIIbβ3 integrin. The β3 integrin was immunoprecipitated using biotin-conjugated anti-mouse antibody against the integrin β3 chain. Immunoprecipitates (IP) were analyzed by SDS-PAGE (8% gel), transferred to a nitrocellulose membrane, and blotted (IB) an antiphosphotyrosine antibody, 4G10 (upper panel). The same blot was stripped and blotted with an anti-Nβ3 antibody to normalize the amount of β3 integrin in each lane (lower panel).
The biological function of calpains has been extensively investigated using synthetic inhibitors of calpain activity. Because the calpain inhibitors cannot distinguish between μ- and m-calpains, the specific role of each calpain remains to be established in platelet secretion, aggregation, and spreading (9). Our findings begin to resolve some of these questions, and the Capn1−/− mice would be useful in evaluating the function of μ-calpain in apoptosis, cell shape regulation, the pathogenesis of Alzheimer's disease, and numerous other cellular processes. Recently, the genetic disruption of the small regulatory subunit of murine calpain (Capn4) has been reported (1). The small subunit is required for the activity of μ-calpains as well as m- calpains, and the Capn4−/− embryos die at midgestation with defects in vasculogenesis and erythropoiesis (1). Since our μ-calpain null mice have no apparent embryological defects, this would suggest that m-calpain compensates for μ-calpain in Capn1−/− mice. The future generation of m-calpain-deficient mice may also allow assessment of the individual and specific contributions of both μ-and m-calpains in a diverse array of biologically important processes. Combined with the availability of mice specifically lacking μ-calpain, as reported here, we may finally begin to comprehend the biological function of conventional calpains in mammalian physiology.
ACKNOWLEDGMENTS
This work was partially supported by grants from the National Institutes of Health to A.H.C. and A.K.
We thank Toshihiko Hanada, Hani Hassoun, and David Liu for many helpful suggestions during the course of these studies. We are also grateful to D. Marie-Mironchuk for help with the artwork, Xiaoping Du of the University of Illinois, Chicago, for the gift of the anti-β3C antibody, and Gary Sclar for help with the microinjection experiments.
M.A. and S.S.A. contributed equally to this work.
REFERENCES
- 1.Arthur J S C, Elce J S, Hegadorn C, Williams K, Greer P A. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–4481. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baier L J, Permana P A, Yang X, Pratley R E, Hanson R L, Shen G Q, Mott D, Knowler W C, Cox N J, Horikawa Y, Oda N, Bell G I, Bogardus C. A calpain-10 gene polymorphism is associated with reduced muscle mRNA levels and insulin resistance. J Clin Investig. 2000;106:R69–R73. doi: 10.1172/JCI10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderwood D A, Zent R, Grant R, Rees D J, Hynes R O, Ginsberg M H. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 4.Cichowski K, McCormick F, Brugge J S. p21rasGAP association with Fyn, Lyn, and Yes in thrombin-activated platelets. J Biol Chem. 1992;267:5025–5028. [PubMed] [Google Scholar]
- 5.Clark E A, Brugge J S. Redistribution of activated pp60c-src to integrin-dependent cytoskeletal complexes in thrombin-stimulated platelets. Mol Cell Biol. 1993;13:1863–1871. doi: 10.1128/mcb.13.3.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark E A, Shattil S J, Brugge J S. Regulation of protein tyrosine kinases in platelets. Trends Biochem Sci. 1994;19:464–469. doi: 10.1016/0968-0004(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 7.Clark E A, Shattil S J, Ginsberg M H, Bolen J, Brugge J S. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin alpha IIb beta 3. J Biol Chem. 1994;269:28859–28864. [PubMed] [Google Scholar]
- 8.Croall D E, DeMartino G N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991;71:813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- 9.Croce K, Flaumenhaft R, Rivers M, Furie B, Furie B C, Herman I M, Potter D A. Inhibition of calpain blocks platelet secretion, aggregation, and spreading. J Biol Chem. 1999;274:36321–36327. doi: 10.1074/jbc.274.51.36321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du X, Saido T C, Tsubuki S, Indig F E, Williams M J, Ginsberg M H. Calpain cleavage of the cytoplasmic domain of the integrin beta 3 subunit. J Biol Chem. 1995;270:26146–26151. doi: 10.1074/jbc.270.44.26146. [DOI] [PubMed] [Google Scholar]
- 11.Fox J E, Lipfert L, Clark E A, Reynolds C C, Austin C D, Brugge J S. On the role of the platelet membrane skeleton in mediating signal transduction. Association of GP IIb-IIIa, pp60c-src, pp62c-yes, and the p21ras GTPase-activating protein with the membrane skeleton. J Biol Chem. 1993;268:25973–25984. [PubMed] [Google Scholar]
- 12.Hayashi M, Suzuki H, Kawashima S, Saido T C, Inomata M. The behavior of calpain-generated N- and C-terminal fragments of talin in integrin-mediated signaling pathways. Arch Biochem Biophys. 1999;371:133–141. doi: 10.1006/abbi.1999.1427. [DOI] [PubMed] [Google Scholar]
- 13.Hers I, Donath J, Litjens P E M H, van Willigen G, Akkerman J W. Inhibition of platelet integrin αIIbβ3 by peptides that interfere with protein kinases and the β3 tail. Arterioscler Thromb Vasc Biol. 2000;20:1651–1660. doi: 10.1161/01.atv.20.6.1651. [DOI] [PubMed] [Google Scholar]
- 14.Hers I, Donath J, van Willigen G, Akkerman J W. Differential involvement of tyrosine and serine/threonine kinases in platelet integrin alphaIIbbeta3 exposure. Arterioscler Thromb Vasc Biol. 1998;18:404–414. doi: 10.1161/01.atv.18.3.404. [DOI] [PubMed] [Google Scholar]
- 15.Hodivala-Dilke K M, McHugh K P, Tsakiris D A, Rayburn H, Crowley D, Ullman-Cullere M, Ross F P, Coller B S, Teitelbaum S, Hynes R O. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Investig. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horikawa Y, Oda N, Cox N J, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner T H, Mashima H, Schwarz P E, del Bosque-Plata L, Horikawa Y, Oda Y, Yoshiuchi I, Colilla S, Polonsky K S, Wei S, Concannon P, Iwasaki N, Schulze J, Baier L J, Bogardus C, Groop L, Boerwinkle E, Hanis C L, Bell G I. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163–175. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 17.Hosfield C M, Elce J S, Davies P L, Jia Z. Crystal structure of calpain reveals the structural basis for Ca(2+)-dependent protease activity and a novel mode of enzyme activation. EMBO J. 1999;18:6880–6889. doi: 10.1093/emboj/18.24.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins A L, Nannizzi-Alaimo L, Silver D, Sellers J R, Ginsberg M H, Law D A, Phillips D R. Tyrosine phosphorylation of the beta3 cytoplasmic domain mediates integrin-cytoskeletal interactions. J Biol Chem. 1998;273:13878–13885. doi: 10.1074/jbc.273.22.13878. [DOI] [PubMed] [Google Scholar]
- 19.Kahn M L, Diacovo T G, Bainton D F, Lanza F, Trejo J, Coughlin S R. Glycoprotein V-deficient platelets have undiminished thrombin responsiveness and do not exhibit a Bernard-Soulier phenotype. Blood. 1999;94:4112–4121. [PubMed] [Google Scholar]
- 20.Kawasaki H, Kawashima S. Regulation of the calpain-calpastatin system by membranes (review) Mol Membr Biol. 1996;13:217–224. doi: 10.3109/09687689609160599. [DOI] [PubMed] [Google Scholar]
- 21.Law D A, DeGuzman F R, Heiser P, Ministri-Madrid K, Killeen N, Phillips D R. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 22.Law D A, Nannizzi-Alaimo L, Phillips D R. Outside-in integrin signal transduction. Alpha IIb beta 3-(GP IIb IIIa) tyrosine phosphorylation induced by platelet aggregation. J Biol Chem. 1996;271:10811–10815. doi: 10.1074/jbc.271.18.10811. [DOI] [PubMed] [Google Scholar]
- 23.Lipfert L, Haimovich B, Schaller M D, Cobb B S, Parsons J T, Brugge J S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parise L V. Integrin alpha(IIb)beta(3) signaling in platelet adhesion and aggregation. Curr Opin Cell Biol. 1999;11:597–601. doi: 10.1016/s0955-0674(99)00018-6. [DOI] [PubMed] [Google Scholar]
- 25.Permutt M A, Bernal-Mizrachi E, Inoue H. Calpain 10: the first positional cloning of a gene for type 2 diabetes? J Clin Investig. 2000;106:819–821. doi: 10.1172/JCI11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirier C, Poussard S, Faust D M, Imaizumi-Scherrer T, Weiss M C, Ducastaing A, Montarras D, Pinset C, Guenet J L. Mapping, cloning, cDNA sequence, and expression of the gene encoding the mouse micromolar calpain large subunit. Mamm Genome. 1998;9:388–389. doi: 10.1007/s003359900776. [DOI] [PubMed] [Google Scholar]
- 27.Potter D A, Tirnauer J S, Janssen R, Croall D E, Hughes C N, Fiacco K A, Mier J W, Maki M, Herman I M. Calpain regulates actin remodeling during cell spreading. J Cell Biol. 1998;141:647–662. doi: 10.1083/jcb.141.3.647. . (Erratum, 141:1287.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabinowich H, Lin W C, Manciulea M, Herberman R B, Whiteside T L. Induction of protein tyrosine phosphorylation in human natural killer cells by triggering via alpha 4 beta 1 or alpha 5 beta 1 integrins. Blood. 1995;85:1858–1864. [PubMed] [Google Scholar]
- 29.Raja S, Avraham S, Avraham H. Tyrosine phosphorylation of the novel protein-tyrosine kinase RAFTK during an early phase of platelet activation by an integrin glycoprotein IIb-IIIa-independent mechanism. J Biol Chem. 1997;272:10941–10947. doi: 10.1074/jbc.272.16.10941. [DOI] [PubMed] [Google Scholar]
- 30.Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, Brenguier L, Devaud C, Pasturaud P, Roudaut C, et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27–40. doi: 10.1016/0092-8674(95)90368-2. [DOI] [PubMed] [Google Scholar]
- 31.Schoenwaelder S M, Yuan Y, Cooray P, Salem H H, Jackson S P. Calpain cleavage of focal adhesion proteins regulates the cytoskeletal attachment of integrin alphaIIbbeta3 (platelet glycoprotein IIb/IIIa) and the cellular retraction of fibrin clots. J Biol Chem. 1997;272:1694–1702. doi: 10.1074/jbc.272.3.1694. [DOI] [PubMed] [Google Scholar]
- 32.Schoenwaelder S M, Yuan Y, Jackson S P. Calpain regulation of integrin alpha IIb beta 3 signaling in human platelets. Platelets. 2000;11:189–198. doi: 10.1080/09537100050057620. [DOI] [PubMed] [Google Scholar]
- 33.Shattil S J, Kashiwagi H, Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–2657. [PubMed] [Google Scholar]
- 34.Sorimachi H, Ishiura S, Suzuki K. Structure and physiological function of calpains. Biochem J. 1997;328:721–732. doi: 10.1042/bj3280721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorimachi H, Saido T C, Suzuki K. New era of calpain research. Discovery of tissue-specific calpains. FEBS Lett. 1994;343:1–5. doi: 10.1016/0014-5793(94)80595-4. [DOI] [PubMed] [Google Scholar]
- 36.Strobl S, Fernandez-Catalan C, Braun M, Huber R, Masumoto H, Nakagawa K, Irie A, Sorimachi H, Bourenkow G, Bartunik H, Suzuki K, Bode W. The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium. Proc Natl Acad Sci USA. 2000;97:588–592. doi: 10.1073/pnas.97.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki K, Sorimachi H, Yoshizawa T, Kinbara K, Ishiura S. Calpain: novel family members, activation, and physiologic function. Biol Chem Hoppe-Seyler. 1995;376:523–529. doi: 10.1515/bchm3.1995.376.9.523. [DOI] [PubMed] [Google Scholar]
- 38.Vostal J G, Shulman N R. Vinculin is a major platelet protein that undergoes Ca(2+)-dependent tyrosine phosphorylation. Biochem J. 1993;294:675–680. doi: 10.1042/bj2940675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang K K. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- 40.Ylanne J, Chen Y, O'Toole T E, Loftus J C, Takada Y, Ginsberg M H. Distinct functions of integrin alpha and beta subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol. 1993;122:223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan Y, Dopheide S M, Ivanidis C, Salem H H, Jackson S P. Calpain regulation of cytoskeletal signaling complexes in von Willebrand factor-stimulated platelets. Distinct roles for glycoprotein Ib-V-IX and glycoprotein IIb-IIIa (integrin alphaIIbbeta3) in von Willebrand factor-induced signal transduction. J Biol Chem. 1997;272:21847–21854. doi: 10.1074/jbc.272.35.21847. [DOI] [PubMed] [Google Scholar]