Abstract
Dyspnoea self-management is often suboptimal for patients with COPD. Many patients with COPD experience chronic dyspnoea as distressing and disabling, especially during physical activities. Breathing therapy is a behavioural intervention that targets reducing the distress and impact of dyspnoea on exertion in daily living.
Using a qualitative design, we conducted interviews with 14 patients after they participated in a novel mind–body breathing therapy intervention adjunct, capnography-assisted respiratory therapy (CART), combined with outpatient pulmonary rehabilitation. Comprehensive CART consisted of patient-centred biofeedback, tailored breathing exercises, a home exercise programme and motivational interviewing counselling. We assessed participants’ perceptions and reported experiences to gauge the acceptability of CART and refine CART based on feedback. Constant comparative analysis was used to identify commonalities and themes.
We identified three main themes relating to the acceptability and reported benefits of CART: (1) self-regulating breathing; (2) impact on health; and (3) patient satisfaction. Our findings were used to refine and optimise CART (i.e. its intensity, timing and format) for COPD.
By addressing dysfunctional breathing behaviours and dysregulated interoception, CART offers a promising new paradigm for relieving dyspnoea and related anxiety in patients with COPD.
Short abstract
Capnography-assisted respiratory therapy (CART) is a new adjunctive mind–body therapy. Patients with COPD find CART to be acceptable and to complement pulmonary rehabilitation. https://bit.ly/3iP4glN
Introduction
COPD is the third leading cause of death in the world [1, 2]. COPD is characterised by airflow limitation and dysfunctional breathing, which leads to abnormal levels of carbon dioxide (CO2) and oxygen (O2). In particular, an abnormally rapid, upper thoracic-dominant breathing pattern, characteristic of COPD, allows insufficient time to empty the lungs [3–5], contributing to abnormal CO2 levels and dyspnoea [6]. Dyspnoea, which is laboured, uncomfortable breathing, is the primary symptom of COPD; it is a powerfully aversive symptom that is experienced as suffocation and/or air hunger and unsatisfied inspiration, provoking intense anxiety and distress [7, 8, 9]. Airway hypocapnia (low CO2) associated with hyperventilation worsens airway secretions and bronchoconstriction increasing the work of breathing and dyspnoea sensation [5, 6, 10]. Dysfunctional breathing patterns (e.g. tachypnea, shallow breathing pattern [7]) are associated with a vicious cycle of dyspnoea, anxiety, and physical activity and exercise limitations [5]. Tachypnea due to exercise intolerance and emotional stress can therefore lead to neuromechanical uncoupling (failure of the ventilatory pump and CO2 retention) [3, 11] with associated fear, and in some cases ultimately emergency medical care [3]. Management of dyspnoea in COPD populations continues to be suboptimal, limited by low uptake of pulmonary rehabilitation and few treatment options [7].
Breathing therapy is an important component of pulmonary rehabilitation and self-management interventions to help patients alleviate cycles of heightened dyspnoea and related anxiety [12–15]. Expert consensus promotes more psychological and educational breathlessness services for dyspnoea remediation [16]. Because respiratory muscles are under both brainstem and skeletal muscle control, patients can learn to control their respiratory rate, flow and depth to help manage their symptoms [6, 17–19]. However, the effects of breathing therapy on dyspnoea and related disability in COPD have been equivocal, possibly hampered by the wide variation in breathing exercises and protocols studied [20, 21]. Therefore, more evidence about the effectiveness of breathing therapy as an adjunct to exercise training is needed to guide clinical practice and care delivery [22–24].
Capnography-assisted respiratory training (CART) is a novel approach to addressing underlying dysfunctional breathing patterns and abnormal CO2 levels in patients with COPD. CART is a comprehensive, multi-component, patient-centred intervention that targets optimal CO2 levels (eucapnic or balanced breathing) and learning more functional breathing habits through real-time breathing biofeedback, breathing exercises and counselling to treat dyspnoea and associated anxiety symptoms. Patient-centred care is a quality indicator defined as “providing the care that the patient needs in the manner the patient desires at the time the patient desires… including the ability to be active partners in their care, and the opportunity to share in treatment decisions” [25]. The aims of this study were to assess the acceptability of CART, when combined with outpatient pulmonary rehabilitation, in adults with COPD, and refine CART based on feedback from participants. Acceptability is “the perception among implementation stakeholders that a given treatment, service, practice, or innovation is agreeable, palatable, or satisfactory” [26].
Methods
Participants
Participants were a subset of patients from a randomised controlled trial (RCT) of multi-component CART [27]. Using a purposive sampling approach, all participants who were assigned to the active CART intervention and received ≥1 CART session were invited to participate in post-treatment interviews. The RCT inclusion criteria were: 1) over 40 years of age; 2) COPD as defined by forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) of <0.70 or as shown on chest computed tomography; 3) medically cleared to participate in pulmonary rehabilitation; and 4) English speaking. Exclusion criteria included: 1) 24-hour supplemental oxygen use; 2) cognitive impairment as measured by ≤23/30 on the Mini Mental State Examination (MMSE) [28]; 3) actively being treated for lung cancer; 4) morbidly obese (body mass index >40); 5) currently smoking; and 6) history of myocardial infarction in the past 3 months. The study was approved by NYU Langone Health's Institutional Review Board.
Assessment
A qualitative design was used to gather descriptive data on patients’ experiences with CART. Open-ended questions provided an opportunity for greater understanding of acceptability, satisfaction and addressable contextual challenges of CART intervention to support its further development and implementation [29].
Trained interviewers conducted 15–30-min semi-structured, in-depth interviews post-intervention, either in person or by phone. Participants were asked a series of open-ended questions developed from our previous qualitative research: [30–34]:
Please tell me about your experience with breathing therapy (CART).
How did you feel about the time and effort involved to participate in the treatment?
What can we do to improve the breathing therapy for future participants?
How satisfied were you with the treatment? Was it what you were expecting?
Would you recommend this treatment to others? Why or why not?
Please tell me how the format of the breathing therapy sessions made you feel.
Were there any challenges that made it difficult to participate in the breathing therapy intervention?
What was your experience with the breathing exercises at home?
What things helped you and hindered you to do the breathing exercises at home?
Do you think that the breathing therapy helped you? If so, how?
The intervention evaluation questions were developed based on the Social Cognitive Theory with a focus on questions related to how the intervention addressed self-management strategies, including self-efficacy and behaviour change [35, 36]. The interview guide was amended as new concepts were identified in order to further explore and understand emerging theoretical constructs. All interviews were audio-recorded and transcribed [37].
CART intervention
CART is a patient-centred intervention, with sessions delivered to individuals (rather than a group format). Sessions were ∼1 hour in duration, offered weekly for 6 weeks, for a total of 6 h. A portable capnograph (CapnoTrainer; Better Physiology, Cheyenne, WY, USA) was used in-session only to provide real-time visual biofeedback of end-tidal CO2 (ETCO2), respiratory rate, rhythm, depth of breathing and ratio of inspirations to expirations in order to guide breathing therapy and measure progress. CART consisted of 10 tailored core breathing exercises focused on lengthening the exhale and reinforcing and nudging more functional breathing behaviours (table 1). Motivational Interviewing (MI), a client-centred counselling style, served as the foundation of CART to establish a collaborative, therapeutic relationship and motivate behaviour change [38]. Counselling and debriefing after each breathing exercise facilitated participants’ learning to: 1) identify dysfunctional breathing patterns; 2) explore and link ETCO2 and respiratory rate changes to dyspnoea symptoms; and 3) recognise false or catastrophic beliefs about their breathing. CART was implemented by a pulmonary rehabilitation clinician (a registered occupational therapist) formally trained in breathing behaviour analysis and educational capnography. A tailored home exercise programme (HEP) included daily voice-recorded, guided and mindful breathing exercises via audio-files or an app, and tailored goals facilitated by pictorial instructions of ribcage breathing exercises and an exercise log; no capnography biofeedback was given. A minimum of 10 min of daily breathing exercises was encouraged for homework (≥60 min per week) using a pulse oximeter to monitor and record heart rate and oxygen saturation. Home exercises were tailored and collaboratively chosen from the 10 core breathing exercises after they were introduced and successfully practised in-session with the therapist.
TABLE 1.
CART core breathing exercises
| 1. | Slow respiration facilitated by supine and forward leaning resting postures, and tactile and verbal cueing [39]. |
| 2. | Nasal breathing promoted by correct positioning of the tongue [40]. |
| 3. | Ribcage stretches coordinated with the exhalation phase [41]. |
| 4. | Control pause, part of a 3-phase breathing cycle of inspiration, expiration, then a pause, held for a few seconds to lengthen the exhalation [42]. |
| 5. | Volume-regulated breathing to promote awareness of optimal breath size. |
| 6. | Breathing interoceptive awareness for improved body listening and trust [43, 44]. Interoception is the “process by which the nervous system senses, interprets, and integrates signals originating from within the body” [45]. |
| 7. | Breath counting, e.g. “in, out, 1; in, out, 2” and so on for up to 5 or 10 counts. |
| 8. | Humming, a type of resistance breathing, practised with participants’ choice of music [46, 47]. |
| 9. | Pursed-lips breathing with tailored (≤5 min) physical challenges (e.g. bending, walking or stair-climbing) [22]. |
| 10. | Brief mindful breathing (≤5 min) guided with adapted scripts [48, 49]. Mindfulness is “the awareness that arises from paying attention on purpose, in the present moment, nonjudgmentally to experiences that unfold” [50]. |
CART: capnography-assisted respiratory training.
Data analysis
To assess themes related to acceptability and addressable challenges, we used a constant comparative analytical method in which data were continually compared to explain behaviour and further refine categories and theoretical concepts [37, 51–53]. Qualitative analyses of transcribed interviews were conducted using an inductive approach whereby, through an iterative process of analysis, acceptability themes emerged from the content of the participants’ comments [54]. Two raters independently developed an initial set of core codes, informed by our prior qualitative research [33, 34, 55]. For each core code, raters developed secondary codes that represented either more specific or restricted aspects of the phenomenon, to contextualise it further. Data analyses were guided by a codebook that included definitions of codes; definitions were refined with emerging insights throughout the coding process. Discrepancies in coding were resolved through open discussion with the coding team. Coded transcripts were analysed with Dedoose™ software [56]. To examine demographic and clinical characteristics, we used mean±sd for continuous variables and frequencies (%) for categorical variables.
Results
14 adults with COPD completed semi-structured interviews from August 2018 through September 2019. Of the 31 participants from the RCT, 22 were randomised 2:1 to CART+pulmonary rehabilitation and nine were randomised to receive pulmonary rehabilitation alone. 20 participants began CART+pulmonary rehabilitation; two participants were withdrawn after randomisation because they were ineligible to receive pulmonary rehabilitation. 70% of participants who began CART were interviewed. Six eligible participants were not interviewed because they became ill (n=3); had social issues (n=1); were lost to follow-up (n=1); or lacked interest (n=1). Of those interviewed, 13 participants completed all 6 CART sessions; one participant completed only three sessions because of illness. Participants’ age range was 48–84 years; post-bronchodilator FEV1/FVC was 0.53 (0.13). Participants’ other characteristics are presented in table 2.
TABLE 2.
Sample demographics and clinical characteristics
| Total | |
| Subjects | 14 |
| Sex | |
| Female | 9 (64.3) |
| Age years | 73.57±10.23 |
| Home oxygen use | |
| Yes | 2 (14.3) |
| Post-bronchodilator FEV1 % predicted | 53.36±23.95 |
| COPD severity | |
| Mild (GOLD 1) | 2 (14.3) |
| Moderate (GOLD 2) | 6 (42.9) |
| Severe (GOLD 3) | 5 (35.7) |
| Very severe (GOLD 4) | 1 (7.1) |
| mMRC dyspnoea | 3.21±1.05 |
| Smoking pack-years | 46.08±29.81 |
| BMI kg·m−2 | 25.49±4.14 |
| Congestive heart failure comorbidity | |
| Yes | 3 (21.4) |
| Marital status | |
| Married | 5 (35.7) |
| Ethnicity | |
| Caucasian | 13 (92.9) |
| African American | 1 (7.1) |
| Hispanic | 2 (14.3) |
| Education | |
| Greater than high school | 10 (71.4) |
| Taking medication for anxiety and/or depression | |
| Yes | 4 (36.4) |
Data are presented as mean±sd for continuous variables and n (%) for categorical variables unless stated otherwise. FEV1: forced expiratory volume in 1 s; GOLD: Global Initiative for Chronic Obstructive Lung Disease; mMRC: Modified Medical Research Council dyspnoea scale; BMI: body mass index.
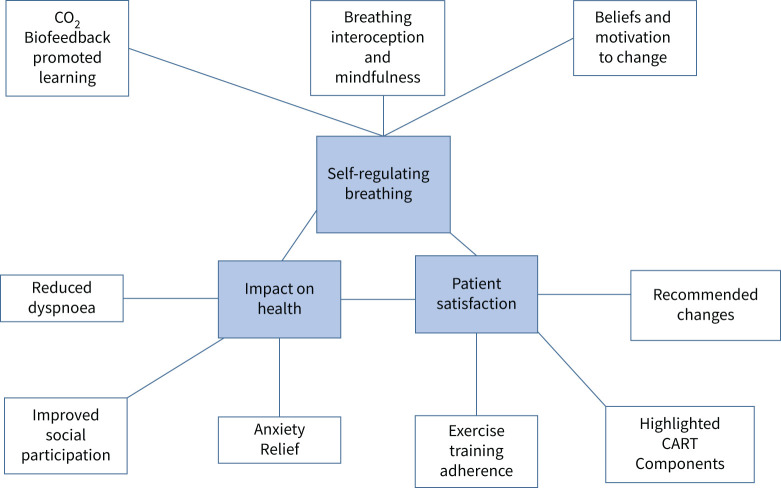
We identified three main themes and nine sub-themes in the data (figure 1): Self-regulating Breathing, Impact on Health, and Patient Satisfaction.
FIGURE 1.
Concept map of themes and sub-themes relating to learning self-regulated breathing patterns in COPD. CART: capnography-assisted respiratory training.
Theme: self-regulating breathing
Sub-theme: CO2 biofeedback promoted learning
Capnography biofeedback was reported to facilitate perceptual learning and reinforce more efficient, eucapnic breathing. Participants said that capnographs simplified breathing physiology to promote increased awareness and ability to exhale (see table 3 for exemplar quotes). Participants described learning to identify and unlearn their dysfunctional breathing habits. “Through immediate feedback”, CART was described as a process of discovery and “learning through experience”; “a total new area of learning”. Participants explored new states of consciousness and relaxation. Some participants learned to identify and allow the breathing reflex, “to be conscious of how the diaphragm was moving and kicking in”, and began to link their breathing patterns with symptoms. Participants described gaining better awareness and understanding of the challenge of exhaling air in COPD.
TABLE 3.
Exemplar quotes
|
Theme: Self-regulating breathing The experience of learning more self-regulated breathing patterns | |
| Sub-theme: CO2 biofeedback | I was amazed by the graphs on the computer – an immediate feedback. And there was a connection seeing that with the slow breathing. I have also seen the screen on her computer and how I can reach different levels of carbon dioxide being exhaled and the width and height of the breath; the inhale and the exhalation; and the impact on my body as far as the relaxation and the rhythmic quality…. Not only do I see it, I feel it. It gives you an idea of what you're doing right and wrong. It was very helpful to me to see the pattern of my breathing on the readout. It was a concrete example of my inhales and exhales and my timing. There was no cheating the device. From week to week, I could see my progress. The one thing I really enjoyed was the capnometer and seeing the breaths. |
| Sub-theme: breathing interoception and mindfulness | [There were] so many different aspects of breathing and mindfulness… I was able to understand the connection between the way I was breathing and my body; becoming more aware of the impact of my thinking, my feelings, my bodily position, and their impact on my breathing, and also the exhale, because I've had a big problem with exhaling. I can't get out air…. I've learned to look at what's happening in the here and the now [and] what the impact is on me…. I think what I have learned is how I can regulate it [my breathing] rather than having it just be stuck. I think that has been extraordinarily helpful. Because I could kind of almost trust my lungs a little bit better. Well I became much more aware of my gasping for air even though I had plenty of air in me. It was sort of an automatic response when I try to exhale fully. |
|
Sub-theme:
beliefs and motivation to change breathing |
That to me was a big kind of leap forward in terms of how I am going to live with it – come to face with it…. I've got to be breathing through my nose. Even though I get the oxygen when I'm working out, I'll still be breathing mostly through my mouth. So I have to be conscious that I'm doing that. There was a great deal of resistance when I started. And then I felt there was a buildup and…. growing effect and profit; [I realised] that you have to keep at it. So then I found there was a time of the day that became easier to do it [home exercises]…. I think the success of the programme is tied to desire. What do you want? In Spanish, we say what's your gana?… What moves you? I'm enjoying them [home-based breathing exercises]. I'm still doing them. I'm committed to continuing to do those as long as I can continue to breathe. If I don't continue to do them, I'm afraid I'm going to lapse back into the short breathing. Being with [the therapist] was very positive…. It motivated me to go home and take what I've learned with me. I felt like everything about that was positive and we were on the same page and it was very beneficial. |
|
Theme: impact on health The perceived clinical benefit of adjunctive CART | |
| Sub-theme: reduced dyspnoea | I've learned where I was getting shortness of breath on a daily basis. For example, I didn't realise that it was because I was holding my breath. I wasn't breathing properly…. I think I know now when I hit a stride and I'm doing it [breathing] properly. I'm not forcing the breath. I can feel it…. It seems to be paying off. The thing I got out of it was that I didn't realise that I was breathing the way I was. Like I would hold my breath sometimes, like I said on exertion…. So, now with walking and moving, I'm conscious of my breathing and I'm more in control…. Especially when I feel like my breathing is getting a little out of control, I've learned how to relax and slow down. I think if I'm walking up an incline or rushing, being able to regulate my breathing you know will help me feel more confident that I'm not going to run out of breath, or I may not have to slow down. |
| Sub-theme: anxiety relief | One of the keys to that was that when I stood up straight, I could feel the benefit to the body. I didn't need worries. I knew it. Even when I'm in public, I hold my body more openly…. It's more open and things are easier. As far as feeling stressors and pressures, knowing that I can breathe into my ribcage and dissipate some of the tension is very beneficial. Because I think having trouble breathing is definitely a very scary thing. It helped me concentrate more on my breathing …. If I can't take a deep breath, I get frightened. So, it probably kind of snowballs. But now I'm learning…. There's no doubt, sometimes I start to hyperventilate a little bit, but that doesn't usually last long…. It's like all stops because you are concentrating on [breathing], and it's peaceful there. So, I really liked that part. It stays with you. Because you know, if you can be calm and you can be peaceful, you can be breathing all right. And you won't be feeling those frightening feelings. It helped me to control my breathing more, and to breathe slower, and what to do if I felt really short of breath and not panic. I have such a cough. It helped not only with the breathing itself, but even with the calming of the nerves. It made me aware of how fast I was breathing when I got nervous or anxious. |
| Sub-theme: improved social participation | The interactions with my family have improved because I'm not upsetting everybody because I have a respiratory problem. I control my problem by controlling the way I laugh and the way I talk. The way I control my breathing affects the whole family.… It's very important that I've learned to control my breathing because so often I'm at a family gathering, all eyes would be on me because I'd be coughing and wheezing and having to use my nebuliser and that is all done with now, because I'm aware. I stop myself and let someone else have the floor…. So it taught me how to slow down when I'm talking, and I'm now having fewer distressing episodes. |
|
Theme: Patient satisfaction Perceived satisfaction of CART | |
| Sub-theme: highlighted CART components | Learning how to breathe [was the best part about the process of participating]. What I liked best was probably learning how to relax more with my breathing, instead of getting a little bit nervous when I would get out of breath and start coughing.… I've become aware of my breathing and I think I was breathing much more shallow before and I would always hold my breath on exertion. When somebody would say take a deep breath, I would take it straight from my [upper] chest. I would never even think of feeling my ribs and my diaphragm expanding. And that was the best part of it. The techniques and recovery positions are very helpful. I purposefully learned to breathe with walking outdoors. She [my therapist] made me feel very comfortable and confident that I would get better. I wanted to mention my therapist who I found to be an excellent and patient teacher and instructor in breaking down the process of breathing and developing exercises that I could use to make my breathing more efficient. |
| Sub-theme: recommended changes | I thought it was awesome. It showed me techniques that I could use throughout I guess the rest of my life…. I [recommend to] get the information out there because I'm sure there are a lot of people who can benefit from it. I think it was excellent. I think if possible it should be given to everyone with breathing problems … it is so helpful and it causes people to become more aware…. It has been very, very enlightening and very helpful. Six [sessions] is just too short. |
| Sub-theme: exercise training adherence | It helped me to get along with and to keep up and not give up. I probably would have enjoyed and realised the benefit of rehab – the exercising – without the CART program. I probably would have enjoyed CART without the rehab. But the two of them together! |
CO2: carbon dioxide; CART: capnography-assisted respiratory training.
The CO2 biofeedback reportedly helped participants to link CO2 changes to somatic symptoms (e.g. dyspnoea, visceral sensations of breathing and muscle spasms). Participants gained new awareness as they could see breath-by-breath, in real time, the pattern and regularity of their breathing. The visual feedback of capnographs reinforced optimal breathing patterns and provided a way to gauge progress between sessions. Participants appreciated being able to see improvements in their CO2 and respiratory rate values. Through biofeedback, some participants articulated new connections between their posture, the way they were breathing, and the relative intensity of dyspnoea and emotions. Others learned to make connections about balanced CO2 levels and their symptoms.
Participants gained awareness of dysfunctional breathing habits. Importantly, 43% of participants said they learned to slow their breathing down. Prolonged exhalations, in particular, could help them to normalise ETCO2 levels. Participants learned to attend to body cues to know when to slow down their breathing and speech. CO2 biofeedback helped to reinforce more optimal breathing patterns; for example, allowing rather than forcing the breath. They learned to read body cues to recognise eucapnic, unforced breathing.
Sub-theme: breathing interoception and mindfulness
Most participants (86%) expressed a profound and positive change in their interoceptive awareness of breathing (i.e. an enhanced ability to sense their internal body and how they were breathing) [57]. Breathing exercises helped some participants to better appreciate how the mind and body interact. Participants’ new attention to breathing sensations, without worrying, seemed to help them to gain insight into the nature and sources of their breathing difficulty. They described increased attention to bodily cues as an antidote to more automatic habits of distressed breathing and functioning. This aware state (metacognition) allowed them to reconnect with and observe their body breathing itself; to attune and distinguish different breathing sensations.
Breathing awareness offered participants focused, visceromotor cues to follow the breath as the body breathes more freely and rhythmically without conscious management; as opposed to purposefully manipulating, forcing or controlling the breath when anxious. For some participants, this new way of regulating attention to their breathing helped them to regain trust in their bodies and to develop greater self-efficacy to recover from dyspnoea and prevent a breathing crisis.
Several participants described managing dyspnoea at night challenging. CART exercises helped some participants fall asleep more easily at night. For example, two participants described how recorded therapist-guided breathing exercises helped them to internalise a different way to relieve dyspnoea at night. Another participant learned to turn attention towards dyspnoea and body tension, rather than distract herself, to release the holding pattern, relieve her dyspnoea and prepare for sleep.
Participants also developed more open awareness of how they were breathing with physical activities – breathing irregularities, breath sounds, ease, rate; and whether their breathing was predominately nasal or open-mouth. Many participants expressed a growing understanding of the value of breath awareness as a vehicle to better notice their condition, breathing difficulty and triggers for shortness of breath (e.g. strong feelings or breath-holding); this led to increased problem-solving and confidence in their physical abilities. This new insight and interoceptive awareness of breathing helped them to adjust their behaviour to optimise their breathing mechanics and airflow, especially with exertion and under stress.
Sub-theme: beliefs and motivation to change breathing
Motivation to change breathing behaviour was closely tied to awareness, goals and positive reinforcement (e.g. feeling better and seeing improvement on capnograph targets of CO2 levels, such as more rhythmical, slower breaths). Participants expressed motivation to use the breathing exercises to manage dyspnoea once they felt the health benefits. One participant described CART as life transforming in helping him to learn to live better with COPD. Homework breathing exercises were communicated as an important component of CART participation. Some participants continued to use voice-recorded, guided breathing exercises after CART. The home exercises were well accepted with high reported motivation and adherence. Many participants expressed a new awareness of a need and value in continuing with breathing exercises at home as a daily practice for well-being to manage, and prevent relapse of, their COPD. Furthermore, 64% specified that they continued to perform the breathing exercises post-CART. Some indicated that performing these home exercises had become a habit, and for at least one it was an enjoyable habit. The desire to keep up with breathing and physical exercises post-rehabilitation was expressed by several participants.
As part of the learning process, some participants described how they overcame initial resistance to changing and adopting new habits. They expressed persevering with their home breathing exercises with new set routines because they could see they helped; this outweighed the costs of the time involved. Motivation was also tied to their therapeutic partnerships with the CART therapist. The MI emphasis on listening for accurate understanding of the client's perspective emerged in participants’ comments. Their statements suggested that participants felt the therapist valued their ideas and input and made an effort to understand their point of view.
Theme: impact on health
Participants said that CART helped to relieve their dyspnoea and anxiety, and improve their quality of life (physical function, sleep and social participation).
Sub-theme: reduced dyspnoea
Participants (64%) explicitly described less dyspnoea with exertion. The breathing exercises were perceived as tools to improve quality of living with shortness of breath. As a result, they reported less disability with engaging in physical activities. Participants described re-interpreting their dyspnoea as a breathing pattern problem that can be ameliorated, which was an important step in the process of their dyspnoea management. For example, one participant described unlearning breath-holding and learning to use purse-lip breathing instead with daily physical activities, such as when emptying the dishwasher, to better self-regulate his airflow and prolong exhalation. Improved breathing and emotional self-regulation led to improved exercise capacity for many, such as improved ability to climb stairs, take their dog for a walk, walk longer distances, or walk up an incline with more speed, less dyspnoea and a reduced need to stop during physical activities.
Sub-theme: anxiety relief
Several participants (71%) reported less dyspnoea anxiety and related emotional distress after CART. They learned to manage dyspnoea triggers (e.g. laughing and nervousness with lying down flat at night) and worry less. Improved breathing self-regulation helped to break the anxiety cycle. Their improved ability to read their body signals and self-regulate their breathing pattern helped to prevent dyspnoea from escalating and promoted quicker breathing recovery.
Participants said breathing exercises helped them to slow their breathing, relieve stress responses, and manage feelings of panic and nervousness. They learned to listen to their bodies to identify symptoms of hypocapnia and dysfunctional breathing patterns (e.g. chest tightness, dyspnoea, anxiety and muscle spasms). Participants’ new awareness of shallow breathing and hyperventilation was an important new skill to cue more eucapnic (efficient) breathing and promote breathing recovery. Feelings of peacefulness and calm were positively reinforcing to them, promoting new breathing pattern habit formation. They expressed gaining improved breathing confidence (self-efficacy) with physical exertion, such as walking up a hill, or rushing.
Sensing visceral sensations of the body breathing (e.g. the lower ribs expanding and contracting) was reported to be comforting, and to relieve muscle tension, helping to break the cycle of dyspnoea and anxiety. For example, one participant described the calming of the mind and relief of anxious and scared feelings by bringing her focus on the body breathing on its own.
43% explicitly described how anxiety was closely linked with their dysfunctional breathing patterns. They described a chain of dyspnoea and chest tightness triggering emotions (e.g. fright or fear) and escalating symptoms, which they were learning to overcome.
Sub-theme: improved social participation
Participants reported improved social functioning with newly learned breathing habits. Improved mobility and stamina facilitated participants’ social functioning and ability to visit and engage with family members. More energy (expressed by 21% of participants) may have helped them to socialise more with others. For some, more functional breathing habits translated into improved social function and participation.
Participants described more positive social interactions and feelings of well-being. They reported being able to regulate their breathing to talk more easily. They learned to tune into body cues to know when to take a break from talking or slow their breathing to avoid a breathing crisis. Participants learned to coordinate their breathing with talking to reduce coughing, dyspnoea and other symptoms of dysfunctional breathing. They learned to manage dyspnoea triggers, such as laughing and strong emotions, for improved engagement (and less avoidance) in social situations through improved breathing pattern regulation and interoceptive awareness. One participant said that her family members were more at ease in her company and worried less about contributing to a dyspnoea crisis.
Theme: patient satisfaction
Sub-theme: highlighted CART components
Most participants (93%) expressed high satisfaction with the CART intervention. Many explicitly recommended that CART be made available to all patients with breathing difficulties. They identified learning how to breathe more easily and comfortably as the best part of the intervention. Some described valuing their gains in breathing awareness and being less anxious about their breathing. One participant expressed how important it was to him to have understood more about breathing physiology and mechanics.
Several said they found slow breathing exercises the most helpful. Breathing exercises highlighted by participants included: interoceptive awareness, recovery positions, humming, ribcage stretches and homework. Participants highlighted worrying less about their breathing, having more breath control, and being able to relieve tension and break the chain of dyspnoea–anxiety–coughing as key benefits. Participants especially found nasal and pursed-lips breathing helpful to slow their breathing down. They expressed satisfaction with unlearning dysfunctional breathing patterns, such as breath-holding and thoracic-dominant breathing patterns, that triggered dyspnoea and coughing. Many expressed satisfaction to have gained new breathing habits for a lifetime. Several participants recognised the therapeutic relationship with their therapist as being important in their learning and change process.
Sub-theme: recommended changes to CART
A majority of participants (57%) recommended more breathing sessions (8–12 sessions instead of six sessions). They recommended more sessions because they felt they could have made more progress with learning functional breathing habits. Three participants (21%) recommended CART be scheduled before exercise training. Two participants suggested that CART sessions be scheduled before exercise training, on the same day, when they had more energy and to afford them more practice opportunities of newly learned breathing techniques. A third person suggested the entire CART programme be offered before an exercise training programme phase begins. Two participants recommended more breathing coaching in the context of physical activities (e.g. lifting weights and bending) to promote learning and reinforcement for generalisation to daily living. Two participants also requested more education about the physiology of breathing and the role of CO2.
Sub-theme: exercise training adherence
CART was perceived as complementing pulmonary rehabilitation exercise training. Participants reported CART helped to improve their exercise perseverance. Participants expressed gaining more insight into their breathing challenges with exercise and how to relieve dyspnoea.
Discussion
The aims of our qualitative study were to gain a deeper understanding of participants’ experience and acceptability of CART, a new adjunctive mind–body therapy to pulmonary rehabilitation, and refine CART based on feedback. Primary quantitative data from the parent study demonstrated good CART session adherence and improved pulmonary rehabilitation attendance associated with CART [27]. Qualitative data added a more in-depth understanding of patients’ perceptions and how they perceived change occurring. All but one participant reported high satisfaction with CART. Health outcomes reported were reduced dyspnoea, improved mood (less anxiety and negative emotions), improved social participation, increased physical activity tolerance and improved ability to fall asleep. Components of the CART programme especially highlighted by participants were the helpful in-session breathing computer biofeedback and tools to facilitate home breathing exercises for dyspnoea relief. Overall participants reported that the home breathing exercises were easy to implement independently. Participants also reported improved ability to sense and regulate their breathing patterns, especially slow their breathing rate. They reported mindful breathing generated feelings of calm and peacefulness, and a new sense of trust and connectedness with their bodies for greater well-being. Some participants also reported improved ability to persevere with and tolerate exercise as a promising benefit of CART. Specific feedback will be applied to: 1) lengthen the CART programme to eight sessions to allow more breathing practice with physical activities; 2) schedule CART before pulmonary rehabilitation; and 3) provide breathing (respiratory rate) biofeedback and automated adherence checks with a device for home-based exercises.
Dysfunctional, inefficient breathing patterns are especially prevalent in adults with COPD [58–60]. They include such patterns as hyperventilation, thoracic-dominant breathing, tachypnea, open-mouth breathing, thoraco-abdominal asynchrony and deep sighing [58]. By addressing dysfunctional behavioural breathing habits, CART may reduce physiological impairment (hypocapnia, hypercapnia, lung hyperinflation, impaired respiratory muscle function and inefficient recovery from a breathing challenge) associated with both dyspnoea and comorbid anxiety.
The high acceptability of CART, based on participants’ high reported satisfaction and positive feedback, is consistent with previous research in adults with asthma and panic. For example, a previous study of 120 adults with asthma found good acceptability of CART, as measured by high treatment completion (92.7%) and home exercise adherence (70.1%). Mean treatment expectancy and credibility ratings (of 8/9) of the first session also indicated high acceptability [17]. Similarly, in another CART study of 20 adults with panic disorder, they found 100% therapy session attendance and 91% homework exercise completion rates [61]. Our findings of high acceptability of CART in COPD offers the first evidence to support making this adjunctive breathing therapy more available to patients.
Several mechanisms may have contributed to patient-reported health benefits of our modified CART intervention for COPD. CART emphasised eucapnic breathing and efficient breathing mechanics to reduce dyspnoea and anxiety symptoms. Some participants learned to link ETCO2 changes to dyspnoea symptoms. Similarly, in patients with panic disorder, Meuret et al. (2010) [62] found that ETCO2 mediated changes in anxiety control in a CART treatment group (unlike a cognitive therapy control group). Improved expectation of control of breathing pattern and re-evaluation of dyspnoea threat in COPD may have reduced learned helplessness and blunted stress reactions [63–65]. CART may work in part by giving patients back a perceived sense of control when anticipating and experiencing aversive dyspnoea sensations enabling them to build resilience to episodes of worsened breathing. Breathing exercises may also have raised a low arterial CO2 tension (PaCO2) set point via desensitisation (i.e. addressing a sensitised suffocation alarm system) [61]. Exercises may have also improved dyspnoea by reducing tension in respiratory muscles [66].
By providing interoceptive exposure to breathing sensations and higher ETCO2 levels, CART may have also reduced anxiety sensitivity (“tendency of certain individuals to view interoceptive sensations as dangerous or threatening”) [67]. Meuret et al. (2009) [68] found ETCO2 significantly mediated their CART intervention effects on anxiety sensitivity (specifically fear of bodily symptoms) in panic disorder; higher ETCO2 reduced fear of bodily symptoms. Similarly, Giardino et al. (2010) [69], in a study of COPD (with and without panic disorder) and healthy matched controls, used respiratory loads to study anxiety sensitivity and dyspnoea (n=28). Groups did not differ on a respiratory load detection task (p=0.20). However, they found patients with COPD and panic had significantly higher anxiety sensitivity scores (p<0.001) at baseline and reported greater dyspnoea to inspiratory respiratory loads (compared to the other two groups). The investigators attributed higher anxiety sensitivity to the differences in dyspnoea ratings in the COPD and panic group compared to the COPD group without panic disorder and the healthy control group.
CART may have also improved regulation of breathing interoception to promote resilience (better adaption to the stress of dyspnoea). A recent study by Haase et al. [65] found that individuals with low resilience (as measured by the Connor–Davidson Resilience Scale) had significantly less body awareness and were less responsive to interoceptive breathing signals potentially contributing to greater body prediction errors and anxiety. In particular, they found low-resilience individuals had greater activation of the middle insula and thalamus in anticipating aversive dyspnoea compared to normal and high-resilience individuals [65]. They argued that in less resilient individuals, exaggerated and inefficient neural processing in the limbic system resulted from a mismatch of actual versus anticipated body states; and that difficulty monitoring body stimuli contributed to a less adaptive response to stressful breathing sensations. By being more in tune with their bodies after CART, participants in our study may have better predicted breathing challenges and more effectively employed relief strategies in anticipation of dyspnoea with exercise and other physical activities.
Participants reported decreased dyspnoea and related anxiety as a benefit of CART. In contrast, other mind–body interventions in COPD, such as mindfulness and mindfulness-based stress reduction (MBSR), have not found any evidence for improvements in dyspnoea intensity [70–72]. Further, unlike other mind–body study interventions in COPD, CART exercises focused primarily on awareness of breathing sensations and patterns for improved emotional and breathing self-regulation. We attribute reported improvements in dyspnoea on CART's body awareness focus on breathing, which we believe is important in addressing dysfunctional breathing and promoting desensitisation (reducing negative or exaggerated emotional responses) to dyspnoeic and somatic symptoms [69, 71].
Brief (≤5 min) mindful breathing exercises were incorporated into CART sessions and homework as one component (but not a main focus). In contrast, other COPD mindfulness studies had a primary focus on mindfulness exercises and shifted traditional body awareness focus away from breathing to other bodily sensations such as heartbeat, blood flow and contact of the feet with the ground [70, 71]. One exception is the COPD yoga study by Donesky–Cuenco et al. [73], which found an Iyengar yoga intervention (consisting of poses and timed breathing) improved dyspnoea distress as well as exercise and functional performance compared to a usual care group (n=29). However, participants reported difficulty carrying out the yoga exercises independently at home. CART's delivery to individuals allowed tailoring of breathing exercises and home programme to facilitate independence and confidence. In contrast to our study, all other mind–body study interventions in COPD were delivered in groups [70–73].
To the best of our knowledge, this is the first qualitative study to evaluate the acceptability of CART in adults with COPD. A limitation of our study was that four participants who received (at least one) CART session were not interviewed; their perceptions of CART may have been different. Repeated interviews with longer follow-up were not possible but could have provided a more comprehensive qualitative evaluation of CART acceptability. Future research is needed to evaluate the efficacy of CART in symptom management and improving pulmonary rehabilitation outcomes and utilisation in COPD. It will also be important to study the individual effects of CART separate from pulmonary rehabilitation in a future study.
Conclusion
Our primary finding was that patients with COPD found tailored CART, delivered to individuals, to be acceptable. Specific feedback will be applied to optimise and refine CART dose (lengthen the programme and offer it before pulmonary rehabilitation) and provide more quality and adherence monitoring of home-based exercises. This study addressed the need for new, patient-centred comprehensive breathing therapy and mind–body approaches for relieving distressing dyspnoea and anxiety symptoms in chronic lung diseases [44]. CART holds promise for improving pulmonary rehabilitation implementation and symptom management, thereby enhancing quality of life and reducing disability in patients with COPD. CART, therefore, warrants further investigation.
Acknowledgements
The authors wish to thank our participants for sharing their insights of living with COPD. We would also like to thank Danielle Veltri and Alexandra Gordon (Rehabilitation Medicine, NYU Grossman School of Medicine) for assisting with conducting the interviews. We wish to thank Peter Litchfield (Graduate School of Behavioral Health Sciences, Cheyenne, Wyoming) and Roger Price (Breathing Well Pty Ltd, Australia) for their expert training in capnography biofeedback and dysfunctional breathing behaviour analysis.
Footnotes
Provenance: Submitted article, peer reviewed.
This study is registered at www.clinicaltrials.gov with identifier number NCT03457103.
Conflict of interest: A.M. Norweg has nothing to disclose.
Conflict of interest: A. Skamai has nothing to disclose.
Conflict of interest: S. Kwon declares grants from the National Institute on Disability, Independent Living, and Rehabilitation Research.
Conflict of interest: J. Whiteson has nothing to disclose.
Conflict of interest: K. MacDonald has nothing to disclose.
Conflict of interest: F. Haas has nothing to disclose.
Conflict of interest: E.G. Collins has nothing to disclose.
Conflict of interest: R.M. Goldring has nothing to disclose.
Conflict of interest: J. Reibman has nothing to disclose.
Conflict of interest: Y. Wu has nothing to disclose.
Conflict of interest: G. Sweeney has nothing to disclose.
Conflict of interest: A. Pierre has nothing to disclose.
Conflict of interest: A.B. Troxel has nothing to disclose.
Conflict of interest: L. Ehrlich-Jones has nothing to disclose.
Conflict of interest: N.M. Simon has nothing to disclose.
Support statement: This study was funded under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) (grant number 90SFGE0003). NIDILRR is a centre within the Administration for Community Living (ACL), Dept of Health and Human Services (HHS). The contents of this paper do not necessarily represent the policy of NIDILRR, ACL or HHS. This work was also supported in part by grant 1R34AT010673-01A1 from National Institutes of Health, National Center for Complementary & Integrative Health. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for chronic obstructive pulmonary disease: US preventive services task force recommendation statement. JAMA 2016; 315: 1372–1377. doi: 10.1001/jama.2016.2638 [DOI] [PubMed] [Google Scholar]
- 2.Terzikhan N, Verhamme KM, Hofman A, et al. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam study. Eur J Epidemiol 2016; 31: 785–792. doi: 10.1007/s10654-016-0132-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kummer F. Panic attacks in COPD and the somato-psycho-somatic feedback. Eur Respir J 2010; 36: 457.; author reply 457–458. doi: 10.1183/09031936.00045310 [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell DE, Banzett RB, Carrieri-Kohlman V, et al. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc 2007; 4: 145–168. doi: 10.1513/pats.200611-159CC [DOI] [PubMed] [Google Scholar]
- 5.Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med 2006; 119: 10 Suppl. 1, 21–31. doi: 10.1016/j.amjmed.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 6.Macklem PT. Therapeutic implications of the pathophysiology of COPD. Eur Respir J 2010; 35: 676–680. doi: 10.1183/09031936.00120609 [DOI] [PubMed] [Google Scholar]
- 7.O'Donnell DE, James MD, Milne KM, et al. The pathophysiology of dyspnea and exercise intolerance in chronic obstructive pulmonary disease. Clin Chest Med 2019; 40: 343–366. doi: 10.1016/j.ccm.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 8.Laviolette L, Laveneziana P. Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J 2014; 43: 1750–1762. doi: 10.1183/09031936.00092613 [DOI] [PubMed] [Google Scholar]
- 9.Reardon JZ, Lareau SC, ZuWallack R. Functional status and quality of life in chronic obstructive pulmonary disease. Am J Med 2006; 119: 10 Suppl. 1, 32–37. [DOI] [PubMed] [Google Scholar]
- 10.Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med 2002; 347: 43–53. doi: 10.1056/NEJMra012457 [DOI] [PubMed] [Google Scholar]
- 11.O'Donnell DE, Ora J, Webb KA, et al. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir Physiol Neurobiol 2009; 167: 116–132. doi: 10.1016/j.resp.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 12.Mularski RA, Reinke LF, Carrieri-Kohlman V, et al. An official American Thoracic Society workshop report: assessment and palliative management of dyspnea crisis. Ann Am Thorac Soc 2013; 10: S98–106. doi: 10.1513/AnnalsATS.201306-169ST [DOI] [PubMed] [Google Scholar]
- 13.Effing TW, Bourbeau J, Vercoulen J, et al. Self-management programmes for COPD: moving forward. Chron Respir Dis 2012; 9: 27–35. doi: 10.1177/1479972311433574 [DOI] [PubMed] [Google Scholar]
- 14.Hillegass EA. Breathing retraining for individuals with chronic obstructive pulmonary disease: a role for clinicians. Chron Respir Dis 2009; 6: 43–44. doi: 10.1177/1479972308098670 [DOI] [PubMed] [Google Scholar]
- 15.Jerath R, Crawford MW, Barnes VA, et al. Self-regulation of breathing as a primary treatment for anxiety. Appl Psychophysiol Biofeedback 2015; 40: 107–115. doi: 10.1007/s10484-015-9279-8 [DOI] [PubMed] [Google Scholar]
- 16.Man WD, Chowdhury F, Taylor RS, et al. Building consensus for provision of breathlessness rehabilitation for patients with chronic obstructive pulmonary disease and chronic heart failure. Chron Respir Dis 2016; 13: 229–239. doi: 10.1177/1479972316642363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritz T, Rosenfield D, Steele AM, et al. Controlling asthma by training of Capnometry-Assisted Hypoventilation (CATCH) vs slow breathing: a randomised controlled trial. Chest 2014; 146: 1237–1247. doi: 10.1378/chest.14-0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins EG, Langbein WE, Fehr L, et al. Can ventilation-feedback training augment exercise tolerance in patients with chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2008; 177: 844–852. doi: 10.1164/rccm.200703-477OC [DOI] [PubMed] [Google Scholar]
- 19.Raupach T, Bahr F, Herrmann P, et al. Slow breathing reduces sympathoexcitation in COPD. Eur Respir J 2008; 32: 387–392. doi: 10.1183/09031936.00109607 [DOI] [PubMed] [Google Scholar]
- 20.Cahalin LP, Braga M, Matsuo Y, et al. Efficacy of diaphragmatic breathing in persons with chronic obstructive pulmonary disease: a review of the literature. J Cardiopulm Rehabil 2002; 22: 7–21. doi: 10.1097/00008483-200201000-00002 [DOI] [PubMed] [Google Scholar]
- 21.Norweg A, Collins EG. Evidence for cognitive-behavioral strategies improving dyspnea and related distress in COPD. Int J Chron Obstruct Pulmon Dis 2013; 8: 439–451. doi: 10.2147/COPD.S30145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 23.Marciniuk DD, Goodridge D, Hernandez P, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J 2011; 18: 69–78. doi: 10.1155/2011/745047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012; 185: 435–452. doi: 10.1164/rccm.201111-2042ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis K, Schoenbaum SC, Audet AM. A 2020 vision of patient-centered primary care. J Gen Intern Med 2005; 20: 953–957. doi: 10.1111/j.1525-1497.2005.0178.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011; 38: 65–76. doi: 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norweg A, Whiteson J, Wu Y, et al. Feasibility of capnography-assisted respiratory therapy in chronic obstructive pulmonary disease. J Respir Crit Care Med 2020; 201: A6108. [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 29.Dillman DA, Smyth, JD, Christian, LM. Internet, Phone, Mail, and Mixed-Mode Surveys: The tailored Design Method. 4th Edn. Hoboken, NJ, John Wiley & Sons, 2014. [Google Scholar]
- 30.Islam N, Patel S, Brooks-Griffin Q, et al. Understanding barriers and facilitators to breast and cervical cancer screening among Muslim women in New York City: perspectives from key informants. SM J Community Med 2017; 3: 1022. [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon SC, Kranick JA, Bougrab N, et al. Development and assessment of a Helicobacter pylori medication adherence and stomach cancer prevention curriculum for a Chinese American immigrant population. J Cancer Educ 2019; 34: 519–525. doi: 10.1007/s13187-018-1333-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi SS, Wyatt LC, Patel S, et al. A faith-based intervention to reduce blood pressure in underserved metropolitan New York immigrant communities. Prev Chronic Dis 2019; 16: E106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norweg A, Bose P, Snow G, et al. A pilot study of a pulmonary rehabilitation programme evaluated by four adults with chronic obstructive pulmonary disease. Occup Ther Int 2008; 15: 114–132. doi: 10.1002/oti.251 [DOI] [PubMed] [Google Scholar]
- 34.Norweg A, Hass F, Whiteson JH, et al. Respiratory patient perspectives on exercising with music: a qualitative study. Am J Respir Crit Care Med 2019; 199: A3738. [Google Scholar]
- 35.Bandura A. Self-Efficacy: The Exercise of Control. New York, W.H. Freeman Co, 1997. [Google Scholar]
- 36.Bandura A. Health promotion from the perspective of social cognitive theory. Psychol Health 1998; 13: 623–649. doi: 10.1080/08870449808407422 [DOI] [Google Scholar]
- 37.Harris T. Grounded theory. Nurs Stand 2015; 29: 32–39. doi: 10.7748/ns.29.35.32.e9568 [DOI] [PubMed] [Google Scholar]
- 38.Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. New York, The Guilford Press, 2013. [Google Scholar]
- 39.Migliore A. Management of dyspnea guidelines for practice for adults with chronic obstructive pulmonary disease. Occup Ther Health Care 2004; 18: 1–20. doi: 10.1080/J003v18n03_01 [DOI] [PubMed] [Google Scholar]
- 40.Kim EJ, Choi JH, Kim KW, et al. The impacts of open-mouth breathing on upper airway space in obstructive sleep apnea: 3-D MDCT analysis. Eur Arch Oto-Rhino-l 2011; 268: 533–539. doi: 10.1007/s00405-010-1397-6 [DOI] [PubMed] [Google Scholar]
- 41.de Sa RB, Pessoa MF, Cavalcanti AGL, et al. Immediate effects of respiratory muscle stretching on chest wall kinematics and electromyography in COPD patients. Respir Physiol Neurobiol 2017; 242: 1–7. doi: 10.1016/j.resp.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 42.Courtney R, Cohen M. Investigating the claims of Konstantin Buteyko, M.D., Ph.D.: the relationship of breath holding time to end tidal CO2 and other proposed measures of dysfunctional breathing. J Altern Complement Med 2008; 14: 115–123. doi: 10.1089/acm.2007.7204 [DOI] [PubMed] [Google Scholar]
- 43.Paulus MP. The breathing conundrum-interoceptive sensitivity and anxiety. Depress Anxiety 2013; 30: 315–320. doi: 10.1002/da.22076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farb N, Daubenmier J, Price CJ, et al. Interoception, contemplative practice, and health. Front Psychol 2015; 6: 763. doi: 10.3389/fpsyg.2015.00763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalsa SS, Adolphs R, Cameron OG, et al. Interoception and mental health: a roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3: 501–513. doi: 10.1016/j.bpsc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weitzberg E, Lundberg JO. Humming greatly increases nasal nitric oxide. Am J Respir Crit Care Med 2002; 166: 144–145. doi: 10.1164/rccm.200202-138BC [DOI] [PubMed] [Google Scholar]
- 47.Maniscalco M, Pelaia G, Sofia M. Exhaled nasal nitric oxide during humming: potential clinical tool in sinonasal disease? Biomarkers Med 2013; 7: 261–266. doi: 10.2217/bmm.13.11 [DOI] [PubMed] [Google Scholar]
- 48.Williams M, Penman D. Mindfulness: An Eight-Week Plan for Finding Peace in a Frantic World. New York, Rodale Inc, 2011. [Google Scholar]
- 49.McCown D, Reibel D, Micozzi M. Teaching Mindfulness: A Practical Guide for Clinicians and Educators. New York, NY, Springer, 2011. [Google Scholar]
- 50.Paulson S, Davidson R, Jha A, et al. Becoming conscious: the science of mindfulness. Ann NY Acad Sci 2013; 1303: 87–104. doi: 10.1111/nyas.12203 [DOI] [PubMed] [Google Scholar]
- 51.Creswell JW. Qualitative Inquiry & Research Design. Thousand Oaks, SAGE Publications, 2007. [Google Scholar]
- 52.Glaser B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. Hawthorne, Aldine, 1967. [Google Scholar]
- 53.Blumer H. Symbolic Interactions: Perspective and Method. Englewood Cliffs, Prentice Hall, 1969. [Google Scholar]
- 54.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006; 3: 77–101. doi: 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 55.Islam NS, Patel S, Wyatt LC, et al. Sources of health information among select Asian American immigrant groups in New York City. Health Commun 2016; 31: 207–216. doi: 10.1080/10410236.2014.944332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dedoose. Version 7.0 ed. Los Angeles, CA: SocioCultural Research Consultants, LLC, 2016.
- 57.Seth AK, Tsakiris M. Being a beast machine: the somatic basis of selfhood. Trends Cogn Sci 2018; 22: 969–981. doi: 10.1016/j.tics.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 58.Boulding R, Stacey R, Niven R, et al. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev 2016; 25: 287–294. doi: 10.1183/16000617.0088-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vidotto LS, Carvalho CRF, Harvey A, et al. Dysfunctional breathing: what do we know? J Bras Pneumol 2019; 45: e20170347. doi: 10.1590/1806-3713/e20170347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Law N, Ruane LE, Low K, et al. Dysfunctional breathing is more frequent in chronic obstructive pulmonary disease than in asthma and in health. Respiratory Physiol Neurobiol 2018; 247: 20–23. doi: 10.1016/j.resp.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 61.Meuret AE, Wilhelm FH, Ritz T, et al. Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. J Psychiatr Res 2008; 42: 560–568. doi: 10.1016/j.jpsychires.2007.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meuret AE, Rosenfield D, Seidel A, et al. Respiratory and cognitive mediators of treatment change in panic disorder: evidence for intervention specificity. J Consult Clin Psychol 2010; 78: 691–704. doi: 10.1037/a0019552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baratta MV, Maier SF. New tools for understanding coping and resilience. Neurosci Lett 2019; 693: 54–57. doi: 10.1016/j.neulet.2017.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maier SF, Seligman ME. Learned helplessness at fifty: insights from neuroscience. Psychol Rev 2016; 123: 349–367. doi: 10.1037/rev0000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haase L, Stewart JL, Youssef B, et al. When the brain does not adequately feel the body: links between low resilience and interoception. Biol Psychol 2016; 113: 37–45. doi: 10.1016/j.biopsycho.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ritz T, Meuret AE, Bhaskara L, et al. Respiratory muscle tension as symptom generator in individuals with high anxiety sensitivity. Psychosom Med 2013; 75: 187–195. doi: 10.1097/PSY.0b013e31827d1072 [DOI] [PubMed] [Google Scholar]
- 67.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry 2006; 60: 383–387. doi: 10.1016/j.biopsych.2006.03.042 [DOI] [PubMed] [Google Scholar]
- 68.Meuret AE, Rosenfield D, Hofmann SG, et al. Changes in respiration mediate changes in fear of bodily sensations in panic disorder. J Psychiatr Res 2009; 43: 634–641. doi: 10.1016/j.jpsychires.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giardino ND, Curtis JL, Abelson JL, et al. The impact of panic disorder on interoception and dyspnea reports in chronic obstructive pulmonary disease. Biol Psychol 2010; 84: 142–146. doi: 10.1016/j.biopsycho.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 70.Farver-Vestergaard I, O'Toole MS, O'Connor M, et al. Mindfulness-based cognitive therapy in COPD: a cluster randomised controlled trial. Eur Respir J 2018; 51: 1702082. doi: 10.1183/13993003.02082-2017 [DOI] [PubMed] [Google Scholar]
- 71.Chan RR, Giardino N, Larson JL. A pilot study: mindfulness meditation intervention in COPD. Int J Chron Obstruct Pulmon Dis 2015; 10: 445–454. doi: 10.2147/COPD.S73864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mularski RA, Munjas BA, Lorenz KA, et al. Randomised controlled trial of mindfulness-based therapy for dyspnea in chronic obstructive lung disease. J Altern Complement Med 2009; 15: 1083–1090. doi: 10.1089/acm.2009.0037 [DOI] [PubMed] [Google Scholar]
- 73.Donesky-Cuenco D, Nguyen HQ, Paul S, et al. Yoga therapy decreases dyspnea-related distress and improves functional performance in people with chronic obstructive pulmonary disease: a pilot study. J Altern Complement Med 2009; 15: 225–234. doi: 10.1089/acm.2008.0389 [DOI] [PMC free article] [PubMed] [Google Scholar]