Abstract
Background
The very unprecedented virus causing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has continued causing catastrophes in economy and loss of human lives. Despite countries’ urgent and resilient public health actions against the COVID-19 pandemic, the disease is causing a large number of deaths. However, predictors of mortality among hospitalized COVID-19 patients have not been well investigated in Ethiopia. Therefore, this study aimed to identify the predictors of mortality among hospitalized COVID-19 patients at a tertiary care hospital in Ethiopia.
Methods
A hospital-based retrospective cohort design study was implemented among hospitalized COVID-19 patients at a tertiary care hospital in Harar, Ethiopia from March 20 to August 20, 2021. Data of 531 admitted patients were entered using Epi-data 3.1 and exported to STATA 14.2 for analysis. Binary logistic regression was used to identify significant predictors of outcome variables with an adjusted odds ratio (AOR) with a 95% confidence interval.
Results
Of the total 531 study participants, 101 deaths occurred. The mortality rate was 16.2 per 1000 person-days of observation with median survival time of 44 days with IQR [28, 74]. Smoking history [AOR=2.55, 95% CI (1.15, 5.65)], alcohol history [AOR=2.3, 95% CI (1.06, 4.97)], comorbidities [AOR=2.95, 95% CI (1.26, 6.91)], and increasing oxygen saturation [AOR=0.92, 95% CI (0.89, 0.95)], and lymphocyte count [AOR=0.90, 95% CI (0.88, 0.97)] were independent significant predictors of death from Covid-19.
Conclusion
The incidence of mortality among hospitalized COVID-19 patients was found to be high. Devising individual, tailored management for patients with “risk” behaviors, comorbid conditions, and poor prognostic markers such as lymphopenia and low oxygen saturation, may reduce the incidence of death among hospitalized COVID-19 patients.
Keywords: mortality rate, predictors, COVID-19, SARS-COV-2
Introduction
The very unprecedented virus causing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which causes coronavirus disease 2019 (COVID-19) was detected in Wuhan, China in December 2019.1 The virus spread throughout the world and was categorized as a pandemic by the World Health Organization (WHO) in March 2020.2 Coronavirus causes respiratory diseases and most commonly spreads from individual-to-individual through infected air precipitation, people making contact with fingers or faces that contain the virus and then touching their eyes, nose, or mouth with unclean hands.2
As of November 08, 2021, COVID-19 had infected more than 250,742,353 people globally, of whom about 5,067,409 had died, and 226,971,946 had recovered from COVID-19. In Ethiopia, 367,210 people have been infected by the virus of whom 6542 died, giving overall mortality rate of 18 deaths per 1000 infected persons.3
Adding to the current COVID-19 pandemic, based on the recent epidemiological update by the WHO, as of June 22, 2021, four SARS-CoV-2 variants of concern (VOCs) have been identified since the beginning of the pandemic. Alpha: first variant of concern described in the United Kingdom (UK) in late December 2020, Beta: first reported in South Africa in December 2020, Gamma: first reported in Brazil in early January 2021, Delta: first reported in India in December 2020.4,5 This VOC has the potential to spread faster with higher mortality than first seen in the pandemic to date.6
Studies revealed that the outcome of COVID-19 varies and is mainly determined by individual characteristics. The outcome of COVID-19 infection could include spontaneous recovery, respiratory complications that require mechanical ventilation, sepsis and septic shock, and multi-organ failure, including acute kidney injury and cardiac injury and death.7–10 Clinical features like fever, shortness of breath, deranged laboratory findings have been identified as indicators of poor prognosis.11,12
Older age and underlying comorbidities such as hypertension, cardiovascular disease and diabetes, have been reported as risk factors for severe disease and death.10,13 To mitigate some of the risks in patients with chronic disease, technological innovation such as telemedicine along with public health strategies were considered in some countries.14 Telemedicine offsets the decline in outpatient visits during COVID-19 while providing critical patient continuity and limiting exposure to health systems and healthcare workers.15
Non-pharmacological measures such as lockdown and travel restrictions have shown a positive result in terms of containing the transmission of the virus. Studies revealed countries that implemented an immediate application of lockdown reduced deaths compared to countries that delayed the application of this strong containment measure.16,17 Vaccines have been found to be highly efficacious at preventing symptomatic disease, as shown by clinical trials18,19 and real-world evidence.20–22 As of November 05, 2021 in Ethiopia, 5,237,750 doses of COVID-19 vaccines had been administered.23
Literature has indicated that COVID-19-related morbidities and mortalities in sub-Saharan African countries are less as compared to higher income countries.24,25 Different assumptions for this have been forwarded, including the higher proportion of younger population in SSA, under-reporting of mortality, low coverage of COVID-19 testing, different immune co-activation status and exposure to non-pathogenic corona viruses.26–29
Having knowledge of the incidence and predictors of death from COVID-19 infection in our setup is the key to make an informed decision to obtain the most favorable outcome of treatment for each patient. Previously conducted studies in Ethiopia are not adequate in addressing important clinical predictors of severity and mortalities from COVID-19 infection.30,31 Therefore, the aim of the study was to investigate the factors associated with mortality in hospitalized COVID-19 patients at a tertiary care hospital in Ethiopia.
Methods and Materials
Study Area, Period, and Design
A hospital-based retrospective cohort study was conducted in Harari Regional State, Ethiopia from March, 2020 to August, 2021. The region is one of the ten regional administrations in Ethiopia and located 522 kilometers from Addis Ababa to the east of the country. Hiwot Fana Specialized University Hospital is one of the largest referral and teaching hospitals in the region serving the large majority of Eastern Ethiopia estimated to be more than six million population in the catchment areas. The hospital is one of the ten regional centers designated by the Ethiopia Ministry of Health to treat COVID-19 cases in Eastern Ethiopia.
Population and Sample
The source population of the study was all COVID-19 patients admitted to the hospital. The study population was all COVID-19 hospitalized patients, between March, 2020 and 20 August, 2021. All COVID-19 patients admitted during the study period were included in the study using a retrospective approache. Those patients who had incomplete data for variable of interest were excluded from the study.
The sample size was determined using double population proportion under the assumptions of type-I error=0.05, power (1-β=0.8), proportion of survival group= 0.57, and proportion of deceased patients= 0.43.32 The sample size was determined to be 400. Finally, by considering 10% of loss of follow-up, the sample was determined to be 440. However, to increase the precision of the result we included all 531 COVID-19 patients whose treatment outcome was identified before 20 August 2021.
Study Variables and Measurements
The outcome variable was survival status of COVID-19 cases (discharged vs died). The independent variables included socio-demographic characteristics such as gender, age, marital status, occupation, residence, COVID-19 clinical characteristics, comorbidities, and clinical outcomes, use of ventilators, COVID-19 clinical management, laboratory findings, and imaging findings. Diagnosis of COVID-19 was defined as the patient having a positive result on the oropharyngeal swab for SARS-CoV-2 by reverse transcriptase polymerase chain reaction (RT-PCR). Common radiological assessment included chest radiograph and computed tomography of the chest based on clinical decision-making. COVID-19 clinical stages are defined as follows.33
Asymptomatic infection: individuals who test positive for SARS-CoV-2 using a virology test (ie, a nucleic acid amplification test [NAAT] or an antigen test) but who have no symptoms that are consistent with COVID-19.
Mild illness: individuals who have any of the various signs and symptoms of COVID-19 (eg, fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell) but who do not have shortness of breath, dyspnea, or abnormal chest imaging.
Moderate illness: individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have oxygen saturation (SpO2) ≥94% on room air at sea level.
Severe illness: individuals with clinical signs of pneumonia (fever, cough, dyspnea, fast breathing) plus SpO2 <94% on room air, respiratory rate >30 breaths/min, or lung infiltrates >50%
Critical illness: individuals who have respiratory failure, septic shock, and/or multiple organ dysfunctions.
Equipment Used and Preparation Made on the SARS-COV-2 Assay Using RT-PCR
Viral transport medium (VTM), swab sticks, ice-box, ice-pads, tongue depressor, marker, requisition form, and personal protective equipment (PPE) were prepared and used beforehand. The VTM that contains three mL fluids composed of gelatin and antimicrobial agents in a buffered salt solution was used. This VTM was used to prevent the sample collected from drying, maintains the viability of the virus, and avoids the growth of contaminants. The used swabs are made up of rayon with a plastic shaft. Ice-box and ice-pads are used for maintaining a cold chain during sample transportation from the sample collection area to the laboratory center.34 The ice-pad was filled with water and stored in the freezer (−20°C) before and after use.
Sample contamination of the nasal vestibule was avoided by sterile openening of the outer case of the swab, inserting the swab into the mouth by slightly elevating the tip of the nose, and keeping the tip of the swab in the oropharynx for a few seconds, then rotating to achieve the highest absorption of oropharyngeal secretions.35,36
Data Collection Procedure and Quality Control
A checklist was prepared and used to collect relevant data by reviewing patient charts. Health professionals were assigned as data collectors and data were extracted by reviewing follow-up charts and cards of patients. Two-day training was given to the data collectors on the objective of the study and how to review the documents as per the data extraction checklist. The principal investigator supervised the data collectors closely. Adequacy of the checklist was evaluated and ambiguous questions were modified before the actual data collection. Besides, daily monitoring of data for completeness and consistency was done.
Statistical Analysis
Data were entered using Epi-data 3.1 and exported to STATA 14.2 for analysis. Descriptive statistics were conducted using frequency and percentages for categorical measurements and mean and median were used to summarize continuous variables.
The important assumptions of Chi-square were checked before statistical modeling where chi-square assumptions were; sample was randomly drawn from the population and all expected values were are at least 5. In addition, important assumptions such as multicollinearity, independence of errors, and linearity in the Logit for continuous variables of logistic regression were checked on top of modeling. Moreover, a receiver operating characteristic curve (ROC-curve) was used to assess the trade-off between sensitivity and specificity. For each predictor, bi-variable binary logistic model was fitted. In bi-variable analysis, predictors with a p-value less than 0.2 were included in multivariable model. Finally, multivariable logistics regression model was fitted for clinical outcome of COVID-19 patients (discharged and death) with covariates fulfilling the prerequisite. The association between outcome and predictors was presented by adjusted odds ratios (AORs) with 95% confidence interval. The statistically significant association between outcome variable and predictors was detected by using 95% confidence interval. The confidence interval which does not include the null hypothesis value means there is statistically significant association. Moreover, after fitting the model, goodness of the final model was checked by using the Hosmer-Lemeshow test. The Hosmer-Lemeshow statistic indicates a good fit at p-value of 0.05 or greater.37 If the confidence interval does not contain the null hypothesis value, the results are statistically significant.
Ethical Statement
Haramaya University College of Health and Medical Sciences Institutional Health Research Ethical Review Committee (IHRERC) ethically cleared the paper. A letter of permission was sent from the College of Health to Hiwot Fana specialized University Hospital. Due to difficulty in reaching patients to get informed consent, data were obtained from patients’ medical records anonymously guaranteeing information confidentiality, and all data extraction processes were conducted per the declaration of Helsinki.
Results
Socio-Demographic Characteristics
Data of 531 patients who were at COVID-19 treatment center between May 2020 and August 2021 were collected. The final analysis was done on the data of 531 patients. The median age of study subjects was 40 years with an interquartile range (IQR) of 28–60 years (Table 1).
Table 1.
Baseline Socio-Demographic Characteristics of Covid-19 Patients in Hiwot Fana Specialized University Hospital Treatment Center, Harar, Ethiopia
| Variables | Frequency (%) |
|---|---|
| Sex | |
| Male | 342 (64.41) |
| Female | 189 (35.59) |
| Age group | |
| 1–25 | 83 (19.67) |
| 26 −50 | 218 (51.66) |
| 51–65 | 64 (15.17) |
| >65 | 57 (13.51) |
| Ethnicity | |
| Harari | 294 (55.37) |
| Oromo | 224 (42.8) |
| Other | 13 (2.44) |
| Marital status | |
| Single | 79 (14.88) |
| Married | 393 (74.01) |
| Divorced | 4 (0.75) |
| Widowed | 11 (2.07) |
| Separated | 44 (8.29) |
| Occupational status | |
| Employed | 82 (17.40) |
| Unemployed | 154 (32.70) |
Note: Other: Somali, Amhara.
Clinical and Baseline Behavioral Characteristics
The median respiratory rate of the patients was 8 BR with IQR: (24, 40). The median heart rate of the study participants was 100bit/m with [IQR: 219 to 464]. In the baseline of all, 116 (21.85%) of study subjects were alcohol consumers (Table 2). One hundred and one (19.06%) study participants were in the critical COVID-19 clinical stage and 84 (15.85%) were in severe COVID-19 clinical stage (Table 3).
Table 2.
The Median with Interquartile Range of Clinical Biomarker Characteristics of Covid-19 Patients in Hiwot Fana Specialized University Hospital Treatment Center, Harar, Ethiopia
| Variables | Median | IQR |
|---|---|---|
| Partial oxygen saturation | 89 | [76, 95] |
| Alanine transferase | 39 | [76, 95] |
| Aspartate | 45 | [32, 65] |
| Lactate dehydrogenase | 45 | [36, 150] |
| Bilirubin | 0.6 | [0.60, 0.90] |
| Creatinine | 0.9 | [0.70, 1.30] |
| Bun level | 45 | [28, 62] |
| Neutrophil | 78.70 | [62.70, 88.82] |
| Lymphocyte | 15.30 | [8.10, 27.00] |
| Leucocyte | 8.46 | [5.67, 13.07] |
| Hemoglobin | 12.9 | [10.9, 14.76] |
| Platelets level | 276.5 | [201.5, 352, 25] |
Table 3.
Frequencies and Percentages of Clinical and Baseline Behavioral Characteristics of Covid-19 Patients in Hiwot Fana Specialized University Hospital Treatment Center, Harar, Ethiopia
| Variables | Frequency (%) |
|---|---|
| Baseline smoking | |
| Yes | 191 (35.97) |
| No | 340 (64.03) |
| COVID-19 clinical stage | |
| Mild | 154 (29.06) |
| Moderate | 191 (35.96) |
| Severe | 85 (16.00) |
| Critical | 101 (19.02) |
| Medication history | |
| Yes | 217 (40.87) |
| No | 314 (59.13) |
| Cough | |
| Yes | 417 (21.47) |
| No | 114 (78.53) |
| Fatigue | |
| Yes | 160 (30.13) |
| No | 371 (89.87) |
| Fever | |
| Yes | 173 (32.58) |
| No | 358 (67.42) |
| Diarrhea | |
| Yes | 33 (6.21) |
| No | 498 (93.79) |
| Asymptomatic | |
| Yes | 70 (13.18) |
| No | 461 (86.82) |
| X-ray finding | |
| Bilateral consolidation | 110 (20.72) |
| Bronchopneumonia | 22 (4014) |
| Opacity | 22 (4014) |
| Effusion | 8 (1.51) |
| Normal | 354 (66.67) |
| Other | 6 (1.13) |
| Comorbidity | |
| Yes | 280 (41.27) |
| No | 251 (52.73) |
| Dexamethasone | |
| Yes | 96 (18.08) |
| No | 435 (81.92) |
| Mechanical ventilation | |
| Yes | 339 (63.84) |
| No | 192 (36.14) |
| Immediate cause of death | |
| Multi-organ failure | 52.47 |
| Acute respiratory distress syndrome | 47.53 |
| Shortness of breath | |
| Yes | 186 (35.03) |
| No | 345 (64.97) |
Incidence of Mortality
The patients were followed for a minimum of 1 and a maximum of 74 days with a median follow-up time of 8 days [IQR, (5, 14)]. During the follow-up time of 531 study participants, 101 patients died. The study demonstrated that the incidence of mortality was 16.2 per 1000 person-days of observation with median survival time of 44 days with IQR [28, 74].
Model Assumption and ROC Curve
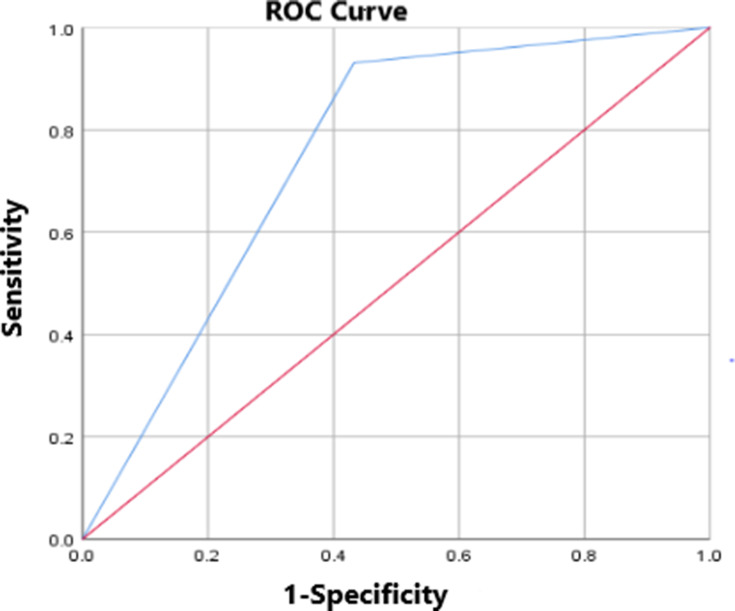
Multicollinearity assumption was checked by using variance inflation factor (VIF) where variance inflation factor (VIF) is a measure of the amount of multicollinearity in a set of multiple regression variables. For this study the VIF of predictors was less than 3 so we can conclude that there was no multicollinearity (Table 4). The Tietjen-Moore test was used to detect strongly influential outliers in the data set. Moreover, linearity in the Logit assumption was checked using the Box-Tidwell variables transformation. Finally, ROC curve was used to see the trade-off between sensitivity and specificity. The result from ROC curve showed that the curve far from the curve 45-degree diagonal of the ROC space in the right side. So we are confident that the predicted probabilities from the model can accurately take on all possible values between 0 and 1 or it is (Figure 1).
Table 4.
Variance Inflation Factor (VIF) of Predictors Associated with Covid-19 for Patients at Treatment Center in Hiwot Fana Specialized University Hospital Treatment Center, Harar, Ethiopia
| Variables | Variance Inflation Factor (VIF) |
|---|---|
| Occupational status | 1.23 |
| Alcohol utilization | 1.45 |
| Medication history | 2.10 |
| Cough | 1.55 |
| Fever | 1.06 |
| Comorbidity | 1.15 |
| Age category | 2.03 |
| Respiratory rate | 1.82 |
| Heart rate | 1.04 |
| Partial Oxygen saturation | 1.26 |
| Creatinine | 2.01 |
| Lymphocyte | 1.76 |
| Dexamethasone | 1.45 |
Figure 1.
Receiver operating characteristic curve for death among Covid-19 patients at a tertiary care hospital in Harar, Ethiopia.
Predictors of Mortality
From the final multi-variable binary logistic regression model: smoking status, alcohol consumption, having comorbidity, oxygen saturation, and lymphocyte count had independent significant association with death from COVID-19 (P<0.05).
The risk of death among COVID-19 patients who had smoking history was 2.55 [AOR=2.55 CI (1.15, 5.65)] times higher when compared with non-smokers. In addition, of “risk” behavior factors, risk of dying was 2.3 [AOR=2.3, CI (1.06, 4.97)] times more likely among COVID-19 individuals who had history of alcohol consumption when compared to their counterparts. The risk of death among COVID-19 patients who had comorbidities was 0.95[AOR=2.95, CI (1.26, 6.91)] times more likely when compared with patients who had no comorbidity. The current study indicated that increment in oxygen saturation level to optimum stage decreases risk of death from COVID-19 by 8% [AOR=0.92 CI (0.89, 0. 95)]. In addition, the current study revealed that one unit increment in lymphocytes among COVID-19 hospitalized patients decreases risk of death by 10%(AOR=0.90, 0.88–0. 97)] (Table 5).
Table 5.
Predictors Associated with Covid-19 for Patients at Treatment Center in Hiwot Fana Specialized University Hospital Treatment Center, Harar, Ethiopia
| Variables | Covid-19 Outcome | COR With 95% CI | AOR With 95% CI | P-value | |
|---|---|---|---|---|---|
| Death | Discharged | ||||
| Occupational status | |||||
| Employed | 75 | 206 | 1 | 1 | |
| Unemployed | 26 | 224 | 0.31[0.19, 0.51] | 1.22 [0.48,3.04] | 0.21 |
| Smoking Status | |||||
| Yes | 63 | 127 | 4.12[2.62, 6.50] | 2.55 [1.15, 5.65]* | 0.02 |
| No | 37 | 303 | 1 | 1 | |
| Alcohol utilization | |||||
| Yes | 42 | 74 | 3.42[2.14, 5.46] | 2.30 [1.06,4.97]* | 0.03 |
| No | 59 | 356 | 1 | 1 | |
| Medication history | |||||
| Yes | 77 | 140 | 6.64[4.02, 10.96] | 1.25[0.52, 3.01] | 0.26 |
| No | 24 | 290 | 1 | 1 | |
| Cough | |||||
| Yes | 88 | 329 | 2.07[1.11, 3.87] | 1.08[0.36, 3.22] | 0.34 |
| No | 33 | 101 | 1 | 1 | |
| Fever | |||||
| Yes | 45 | 117 | 3.33[2.13, 5.20] | 1.40[0.66, 2.95] | 0.22 |
| No | 56 | 313 | 1 | 1 | |
| Comorbidity | |||||
| Yes | 94 | 186 | 17[7.98, 38.86] | 2.96[1.26, 6.92]* | 0.01 |
| No | 26 | 206 | 1 | 1 | |
| Dexamethasone | |||||
| Yes | 34 | 62 | 3.02[1.80, 4.92] | 1.63 [0.74, 3.57] | 0.44 |
| No | 67 | 368 | 1 | 1 | |
| Age category (in years) | |||||
| 1–25 | 96 | 9 | 1 | 1 | |
| 25–55 | 229 | 44 | 1.56[0.70, 3.55] | 0.44[0.16, 2.58] | 0.36 |
| 56–65 | 56 | 24 | 3.98[1.85, 859] | 0.57[0.18, 3.34] | 0.53 |
| >65 | 49 | 24 | 5.22[2.25, 12.09] | 0.93[0.14, 5.96] | 0.37 |
| Respiratory rate | – | – | 1.06[1.04, 1.08] | 1.03[0.99, 1.07] | 0.13 |
| Heart rate | – | – | 1.03[1.02,1.04] | 1.02[0.98, 1.03] | 0.47 |
| Partial Oxygen saturation | – | – | 0.90[0.88, 0.92] | 0.93[0.88, 0.96]* | 0.00 |
| Creatinine | – | – | 1.56[1.22, 2.00] | 1.09[0.88, 1.34] | 0.41 |
| Lymphocyte | – | – | 0.90[0.88, 0.94] | 0.92[0.88, 0.97]* | 0.00 |
Note: *Statistically significant variables (p< 0.05).
Discussion
The study demonstrated that the incidence of mortality was 16.2 per 1000 person-days of observation with median survival time of 44 days with IQR [28, 74]. Smoking status, alcohol history, comorbidity, oxygen saturation, and lymphocytes had significant association with mortality.
In this study, the proportion of mortality was 19.2%. This is comparable with the study finding from Italy (16.7%).38 However, the present finding is inconsistent with studies from Peru,39 France,40 and reports from multiple other countries.41 Pradhan and Olsson confirmed that deaths from COVID-19 are varied across the world.42 This death burden variation could be due to socio-demographic differences such as patients’ age, gender, living environment, personal behaviors, pre-COVID-19 medical conditions, and setting in which the patients received the care.41,43–45
COVID-19 patients who had smoking history had higher risk of death (2.55 times) than non-smoking COVID-19 patients. This finding is in line with several studies conducted in different parts of the world.46–52 This could be due to the fact that cigarette smoking increases ACE2 expression at the apical surface of the airway epithelium that results in the exacerbation of viral entry that imposes poor clinical progression of people with SARS-CoV-2.53,54 In addition, cigarette smoking is associated with poor lung function that could worsen in COVID 19 infections and finally result in death. In addition, both smoking cigarettes55–57 and SARS-COV-258–60 affect lung functions. Thus, the overlapping effect of the two conditions could exacerbate lung impairment that in turn results in poor prognosis of COVID-19 smoker patients.
Even if gender was not significant in the final model, the majority of deaths occurred among males compared to females. The studies suggest that more males are dying from COVID-19.42,61,62 This could be due to variation in immune system responses that may contribute to viral load, clinical stages and outcomes, and hormonal effects.42,63,64 Moreover, males had higher probability of requiring intensive care treatment,65 due to developing severe conditions,66 than females.
COVID-19 patients who had alcohol history had 2.3 times higher risk of dying compared to non-alcohol consuming COVID-19 patients. This finding is aligned with studies conducted in India,67 United States,52 and United Kingdom.68,69 This could be explained by the fact that alcohol consumption intensifies organ sicknesses, especially liver and heart, and leads to increased risk of severity of disease70–72 and could also independently affect the clinical outcome of the patients.
COVID-19 patients who had comorbidities had 2.95 times higher risk of death than patients who had no comorbidity on presentation. This finding is in line with findings from73 Denmark,74 Romania,75 Italy,76,77 Mexico,78 and Michigan state, USA.79 This could be due to the fact that co-existence of SARS-COV-2 infection with other disease could have immunosuppressive effect and low treatment response that might negatively affect the treatment outcome of COVID-19 patients.80,81 Fatality could be exacerbated due to cytokine storm, an acute hyperinflamatory response formation accounted for by comorbidities among COVID-19 patients.82
Oxygen saturation increment by one unit decreases risk of death by 8% among COVID-19 patients. This finding is supported by study findings from Italy,76,83,84 Peru,39,85 Mexico,86 Iran and Afghanistan.87 It is the fact that low oxygen saturation, hypoxia leads to progression of COVID-19 critical stages,88 thus, low level of oxygen saturation could result in early death of COVID-19 patients from the alveoli damage that impairs oxygen exchange with carbon dioxide.89
Furthermore, lymphocyte increment by one unit decreases the risk of death by 10% among COVID-19 hospitalized patients. This finding is supported by studies conducted in Italy,76 Mexico,90 Spain,91 France,40 and Wuhan.92 Studies showed that as COVID-19 clinical stages advance, the lymphocyte count decreases,93–95 thus, as the clinical stages of COVID-19 patients advance to severe and critical, the clinical outcome could be bad. This study may serve as a basis for similar setups because there has been no prior information such as this from similar setups. Even if this study has paramount strengths, it would not be free from limitations. These limitations could be missing some important factors associated with COVID-19-related deaths such as ambient temperature, atmospheric pollution, social distancing, and obesity due to nature of secondary data. Another limitation could be that this study was conducted in only a single center.
Conclusion
This study demonstrated that the incidence of mortality was 16.2 per 1000 person-days of observation with median survival time of 44 days. The predictors of mortality among COVID-19 patients were current smoking, alcohol drinking, having comorbidity conditions; low oxygen saturation, and low lymphocyte count. Therefore, patients with risk behaviors, comorbidities, lymphopenia, and hypoxia need close monitoring and management during health care service in clinical setups.
Data Sharing Statement
The supporting data of this study are available on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pamuk S, Özkan A, Polat B. Epidemiology, pathogenesis, diagnosis and management of COVID-19. Turk J Ear Nose Throat. 2020;30(Supp 1):1–9. doi: 10.5606/Tr-ENT.2020.25338 [DOI] [Google Scholar]
- 3.Worldometer. Covid-19 Coronavirus Pandemic. Available from: https://www.worldometers.info/coronavirus/. Accessed November 8, 2021.
- 4.Aleem A, Akbar Samad AB, Slenker AK. Emerging Variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). In: StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC; 2021. [PubMed] [Google Scholar]
- 5.Prevention CfDCa. SARS-CoV-2 Variant Classifications and Definitions; 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html. Accessed November 26, 2021.
- 6.Grint DJ, Wing K, Williamson E, et al. Case fatality risk of the SARS-CoV-2 variant of concern B.1.1.7 in England, 16 November to 5 February. Euro Surveill. 2021;26(11). doi: 10.2807/1560-7917.es.2021.26.11.2100256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157–1165. doi: 10.1681/ASN.2020030276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72(6):1864–1872. doi: 10.1016/j.jvs.2020.04.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maru EH, Leulseged TW, Hassen IS, et al. Predictors of death in severe COVID-19 patients at millennium COVID-19 care center in Ethiopia: a case-control study. medRxiv. 2020. doi: 10.1101/2020.10.07.20205575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhaskar S, Rastogi A, Chattu VK, et al. Key strategies for clinical management and improvement of healthcare services for cardiovascular disease and diabetes patients in the Coronavirus (COVID-19) settings: recommendations from the REPROGRAM consortium. Front Cardiovasc Med. 2020;7:112. doi: 10.3389/fcvm.2020.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhaskar S, Nurtazina A, Mittoo S, Banach M, Weissert R. Editorial: telemedicine During and Beyond COVID-19. Front Public Health. 2021;9:233. doi: 10.3389/fpubh.2021.662617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu Y, Chen X, Shi W. Impacts of social and economic factors on the transmission of coronavirus disease 2019 (COVID-19) in China. J Popul Econ. 2020;33(4):1127–1172. doi: 10.1007/s00148-020-00778-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coccia M. The relation between length of lockdown, numbers of infected people and deaths of Covid-19, and economic growth of countries: lessons learned to cope with future pandemics similar to Covid-19 and to constrain the deterioration of economic system. Sci Total Environ. 2021;775:145801. doi: 10.1016/j.scitotenv.2021.145801 [DOI] [Google Scholar]
- 18.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernal JL, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–1735. doi: 10.1016/S0140-6736(21)00790-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GAVI. COVAX vaccine roll-out Ethiopia; 2021.
- 24.Fonte L, Acosta A, Sarmiento ME, Ginori M, García G, Norazmi MN. COVID-19 lethality in Sub-Saharan Africa and helminth immune modulation. Front Immunol. 2020;11:2459. doi: 10.3389/fimmu.2020.574910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diop BZ, Ngom M, Biyong CP, Biyong JNP. The relatively young and rural population may limit the spread and severity of COVID-19 in Africa: a modelling study. BMJ Glob Health. 2020;5(5):e002699. doi: 10.1136/bmjgh-2020-002699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutman JR, Lucchi NW, Cantey PT, et al. Malaria and parasitic neglected tropical diseases: potential syndemics with COVID-19? Am J Trop Med Hyg. 2020;103(2):572. doi: 10.4269/ajtmh.20-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hays R, Pierce D, Giacomin P, Loukas A, Bourke P, McDermott R. Helminth coinfection and COVID-19: an alternate hypothesis. PLoS Negl Trop Dis. 2020;14(8):e0008628. doi: 10.1371/journal.pntd.0008628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolin E, Burgers WA, Sturrock ED, et al. Prospects for SARS-CoV-2 diagnostics, therapeutics and vaccines in Africa. Nat Rev Microbiol. 2020;18(12):690–704. doi: 10.1038/s41579-020-00441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mbow M, Lell B, Jochems SP, et al. COVID-19 in Africa: dampening the storm? Science. 2020;369(6504):624–626. doi: 10.1126/science.abd3902 [DOI] [PubMed] [Google Scholar]
- 30.Alemu Y. Predictors associated with COVID-19 deaths in Ethiopia. Risk Manag Healthc Policy. 2021;13:2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiluf Ebuy Abrahaa ZG, Gebrecherkosa T, Kebedea Y, et al. Clinical features and risk factors associated with morbidity and mortality among patients with COVID-19 in northern Ethiopia. Int J Infect Dis. 2021;105:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chilimuri S, Sun H, Alemam A, et al. Predictors of mortality in adults admitted with COVID-19: retrospective cohort study from New York City. West J Emerg Med. 2020;21(4):779. doi: 10.5811/westjem.2020.6.47919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Cilinical managment of COVID-19; May 27, 2020.
- 34.Shrestha LB, Pokharel K. Standard operating procedure for specimen collection, packaging and transport for diagnosis of SARS-COV-2. JNMA J Nepal Med Assoc. 2020;58(228):627. doi: 10.31729/jnma.5260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torretta S, Zuccotti G, Cristofaro V, et al. Diagnosis of SARS-CoV-2 by RT-PCR using different sample sources: review of the literature. Ear Nose Throat J. 2021;100(2_suppl):131S–138S. doi: 10.1177/0145561320953231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torretta S, Zuccotti G, Cristofaro V, et al. Nonserologic test for COVID‐19: How to manage? Head Neck. 2020;42(7):1552–1554. doi: 10.1002/hed.26270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordon-Cardo C, Pujadas E, Wajnberg A, et al. COVID-19: staging of a new disease. Cancer Cell. 2021;38(5):594–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grippo F, Navarra S, Orsi C, et al. The role of COVID-19 in the death of SARS-CoV-2–positive patients: a study based on death certificates. J Clin Med. 2020;9(11):3459. doi: 10.3390/jcm9113459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mejía F, Medina C, Cornejo E, et al. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS One. 2020;15(12):e0244171. doi: 10.1371/journal.pone.0244171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assaad S, Zrounba P, Cropet C, Blay J-Y. Consortium O-s. Mortality of patients with solid and haematological cancers presenting with symptoms of COVID-19 with vs without detectable SARS-COV-2: a French nationwide prospective cohort study. Br J Cancer. 2021;125(5):658–671. doi: 10.1038/s41416-021-01452-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382(25):e102. doi: 10.1056/NEJMoa2007621 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Pradhan A, Olsson P-E. Sex differences in severity and mortality from COVID-19: are males more vulnerable? Biol Sex Differ. 2020;11(1):53. doi: 10.1186/s13293-020-00330-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du R-H, Liang L-R, Yang C-Q, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. doi: 10.1183/13993003.00524-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Narayan RK, Kulandhasamy M, et al. COVID-19 pandemic: insights into molecular mechanisms leading to sex-based differences in patient outcomes. Expert Rev Mol Med. 2021;23:e7. doi: 10.1017/erm.2021.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian T, Zhang J, Hu L, et al. Risk factors associated with mortality of COVID-19 in 3125 counties of the United States. Infect Dis Pover. 2021;10(1):3. doi: 10.1186/s40249-020-00786-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grundy EJ, Suddek T, Filippidis FT, Majeed A, Coronini-Cronberg S. Smoking, SARS-CoV-2 and COVID-19: a review of reviews considering implications for public health policy and practice. Tob Induc Dis. 2020;18:58. doi: 10.18332/tid/124788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umnuaypornlert A, Kanchanasurakit S, Lucero-Prisno DEI, Saokaew S. Smoking and risk of negative outcomes among COVID-19 patients: a systematic review and meta-analysis. Tob Induc Dis. 2021;19:09. doi: 10.18332/tid/132411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simons D, Shahab L, Brown J, Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7). Addiction. 2021;116(6):1319–1368. doi: 10.1111/add.15276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patanavanich R, Glantz SA. Smoking is associated with worse outcomes of COVID-19 particularly among younger adults: a systematic review and meta-analysis. BMC Public Health. 2021;21(1):1554. doi: 10.1186/s12889-021-11579-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eakin MN, Neptune E. Smoking and COVID-19: the real deal. Ann Am Thorac Soc. 2021;18(10):1610–1613. doi: 10.1513/AnnalsATS.202012-1537PS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dai M, Tao L, Chen Z, et al. Influence of cigarettes and alcohol on the severity and death of COVID-19: a multicenter retrospective study in Wuhan, China. Front Physiol. 2020;11:588553. doi: 10.3389/fphys.2020.588553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki A, Efird JT, Redding TS, et al. COVID-19-associated mortality in US Veterans with and without SARS-CoV-2 infection. Int J Environ Res Public Health. 2021;18(16):8486. doi: 10.3390/ijerph18168486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heijink IH, Hackett T-L, Pouwels SD. Effects of cigarette smoking on SARS-CoV-2 receptor ACE2 expression in the respiratory epithelium. J Pathol. 2021;253(4):351–354. doi: 10.1002/path.5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5). doi: 10.1183/13993003.00688-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tantisuwat A, Thaveeratitham P. Effects of smoking on chest expansion, lung function, and respiratory muscle strength of youths. J Phys Ther Sci. 2014;26(2):167–170. doi: 10.1589/jpts.26.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De AK, Tripathi MM. Smoking and lung functions in sportsmen. Br J Sports Med. 1988;22(2):61. doi: 10.1136/bjsm.22.2.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meo SA, AlShehri KA, AlHarbi BB, et al. Effect of Shisha (Waterpipe) smoking on lung functions and fractional exhaled nitric oxide (FeNO) among Saudi young adult Shisha smokers. Int J Environ Res Public Health. 2014;11(9):9638–9648. doi: 10.3390/ijerph110909638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frija-Masson J, Debray M-P, Gilbert M, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56(2):2001754. doi: 10.1183/13993003.01754-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35(3):266–271. doi: 10.1007/s12250-020-00207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pasero D, Sanna S, Liperi C, et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2021;49(5):1055–1060. doi: 10.1007/s15010-020-01561-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fortunato F, Martinelli D, Lo Caputo S, et al. Sex and gender differences in COVID-19: an Italian local register-based study. BMJ Open. 2021;11(10):e051506. doi: 10.1136/bmjopen-2021-051506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raimondi F, Novelli L, Ghirardi A, et al. Covid-19 and gender: lower rate but same mortality of severe disease in women—an observational study. BMC Pulm Med. 2021;21(1):96. doi: 10.1186/s12890-021-01455-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442–447. doi: 10.1038/s41577-020-0348-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brady E, Nielsen MW, Andersen JP, Oertelt-Prigione S. Lack of consideration of sex and gender in COVID-19 clinical studies. Nat Commun. 2021;12(1):1–6. doi: 10.1038/s41467-021-24265-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ueyama H, Kuno T, Takagi H, et al. Gender difference is associated with severity of coronavirus disease 2019 infection: an insight from a meta-analysis. Crit Care Explor. 2020;2(6):e0148–e0148. doi: 10.1097/CCE.0000000000000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saurabh S, Verma MK, Gautam V, et al. Tobacco, alcohol use and other risk factors for developing symptomatic COVID-19 vs asymptomatic SARS-CoV-2 infection: a case-control study from western Rajasthan, India. Trans R Soc Trop Med Hyg. 2021;115(7):820–831. doi: 10.1093/trstmh/traa172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marjot T, Moon AM, Cook JA, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74(3):567–577. doi: 10.1016/j.jhep.2020.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan X, Liu Z, Poulsen KL, et al. Alcohol consumption is associated with poor prognosis in obese patients with COVID-19: a Mendelian randomization study using UK Biobank. Nutrients. 2021;13(5):1592. doi: 10.3390/nu13051592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dawson DA, Li T-K, Grant BF. A prospective study of risk drinking: At risk for what? Drug Alcohol Depend. 2008;95(1):62–72. doi: 10.1016/j.drugalcdep.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Engen PA, Green SJ, Voigt RM, Forsyth CB, Keshavarzian A. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res. 2015;37(2):223–236. [PMC free article] [PubMed] [Google Scholar]
- 72.Rehm J. The risks associated with alcohol use and alcoholism. Alcohol Res Health. 2011;34(2):135–143. [PMC free article] [PubMed] [Google Scholar]
- 73.Moon AM, Webb GJ, Aloman C, et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73(3):705–708. doi: 10.1016/j.jhep.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reilev M, Kristensen KB, Pottegård A, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49(5):1468–1481. doi: 10.1093/ije/dyaa140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pantea Stoian A, Pricop-Jeckstadt M, Pana A, et al. Death by SARS-CoV 2: a Romanian COVID-19 multi-centre comorbidity study. Sci Rep. 2020;10(1):21613. doi: 10.1038/s41598-020-78575-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benelli G, Buscarini E, Canetta C, et al. SARS-COV-2 comorbidity network and outcome in hospitalized patients in Crema, Italy. Medrxiv. 2020. doi: 10.1101/2020.04.14.20053090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barbu MG, Thompson RJ, Thompson DC, Cretoiu D, Suciu N. The impact of SARS-CoV-2 on the most common comorbidities–A retrospective study on 814 COVID-19 deaths in Romania. Original Research. Front Med. 2020;7:581. doi: 10.3389/fmed.2020.567199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105(8):2752–2761. doi: 10.1210/clinem/dgaa346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krishnan S, Patel K, Desai R, et al. Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia. J Clin Anesth. 2020;67:110005. doi: 10.1016/j.jclinane.2020.110005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jordan RE, Adab P, Cheng K. Covid-19: Risk Factors for Severe Disease and Death. British Medical Journal Publishing Group; 2020. [DOI] [PubMed] [Google Scholar]
- 81.Thakur B, Dubey P, Benitez J, et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep. 2021;11(1):8562. doi: 10.1038/s41598-021-88130-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhaskar S, Sinha A, Banach M, et al. Cytokine STORM in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Capra R, De Rossi N, Mattioli F, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Covino M, De Matteis G, Santoro M, et al. Clinical characteristics and prognostic factors in COVID-19 patients aged ≥80 years. Geriatr Gerontol Int. 2020;20(7):704–708. doi: 10.1111/ggi.13960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Díaz-Vélez C, Urrunaga-Pastor D, Romero-Cerdán A, et al. Risk factors for mortality in hospitalized patients with COVID-19 from three hospitals in Peru: a retrospective cohort study. F1000Research. 2021;10:224. doi: 10.12688/f1000research.51474.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friedman J, Calderón-Villarreal A, Bojorquez I, Vera Hernández C, Schriger DL, Tovar Hirashima E. Excess out-of-hospital mortality and declining oxygen saturation: the sentinel role of emergency Medical Services data in the COVID-19 crisis in Tijuana, Mexico. Ann Emerg Med. 2020;76(4):413–426. doi: 10.1016/j.annemergmed.2020.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eskandarian R, Sani ZA, Behjati M, et al. Identification of clinical features associated with mortality in COVID-19 patients. medRxiv. 2021. doi: 10.1101/2021.04.19.21255715 [DOI] [Google Scholar]
- 88.Grieb P, Swiatkiewicz M, Prus K, Rejdak K. Hypoxia may be a determinative factor in COVID-19 progression. Curr Res Pharmacol Drug Discover. 2021;2:100030. doi: 10.1016/j.crphar.2021.100030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mason RJ. Thoughts on the alveolar phase of COVID-19. m J Physiol Lung Cell Mol Physiol. 2020;319(1):L115–L120. doi: 10.1152/ajplung.00126.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rizo-Téllez SA, Méndez-García LA, Flores-Rebollo C, et al. The neutrophil-to-monocyte ratio and lymphocyte-to-neutrophil ratio at admission predict in-hospital mortality in Mexican patients with severe SARS-CoV-2 infection (Covid-19). Microorganisms. 2020;8(10):1560. doi: 10.3390/microorganisms8101560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cantenys‐Molina S, Fernández‐Cruz E, Francos P, et al. Lymphocyte subsets early predict mortality in a large series of hospitalized COVID‐19 patients in Spain. Clin Exp Immunol. 2021;203(3):424–432. doi: 10.1111/cei.13547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang K, Qiu Z, Liu J, et al. Analysis of the clinical characteristics of 77 COVID-19 deaths. Sci Rep. 2020;10(1):16384. doi: 10.1038/s41598-020-73136-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):1–3. doi: 10.1038/s41392-019-0089-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y, Yang T, Wang S, et al. The value of lymphocyte count in determining the severity of COVID-19 and estimating the time for nucleic acid test results to turn negative. Bosnian J Basic Med Sci. 2021;21(2):235–241. doi: 10.17305/bjbms.2020.4868 [DOI] [PMC free article] [PubMed] [Google Scholar]