Abstract
Background
Patients with advanced biliary tract cancer who progress on first-line therapy have limited treatment options. The TreeTopp study assessed varlitinib, a reversible small molecule pan-human epidermal growth factor receptor inhibitor, plus capecitabine in previously treated advanced biliary tract cancer.
Patients and methods
This global, double-blind, randomized, placebo-controlled phase II study enrolled patients with confirmed unresectable or metastatic biliary tract cancer and disease progression after one prior line of gemcitabine-containing chemotherapy. Patients received oral varlitinib 300 mg or placebo twice daily (b.i.d.) for 21 days, plus oral capecitabine 1000 mg/m2 b.i.d. on days 1-14, in 21-day treatment cycles. Co-primary endpoints were objective response rate and progression-free survival (PFS) according to RECIST v1.1 by Independent Central Review.
Results
In total, 127 patients received varlitinib plus capecitabine (n = 64) or placebo plus capecitabine (n = 63). The objective response rate was 9.4% with varlitinib plus capecitabine versus 4.8% with capecitabine alone (odds ratio 2.28; P = 0.42). Median PFS was 2.83 versus 2.79 months [hazard ratio (HR), 0.90; 95% confidence interval (CI), 0.60-1.37; P = 0.63] and overall survival was 7.8 versus 7.5 months (HR, 1.11; 95% CI, 0.69-1.79; P = 0.66), respectively. In a subgroup analysis, the addition of varlitinib appeared to provide a PFS benefit in female patients (median, 4.1 versus 2.8 months; HR, 0.59; 95% CI, 0.28-1.23) and those with gallbladder cancer (median, 2.9 versus 1.6 months; HR, 0.55; 95% CI, 0.26-1.19). Grade ≥3 treatment-emergent adverse events were reported in 65.6% of patients receiving varlitinib plus capecitabine versus 58.7% of those receiving capecitabine alone.
Conclusions
In patients with advanced biliary tract cancer, second-line treatment with varlitinib plus capecitabine was well tolerated but did not improve efficacy versus capecitabine alone. A PFS benefit was suggested in female patients and those with gallbladder cancer.
Key words: advanced, biliary tract cancer, capecitabine, metastatic, phase II randomized trial, second-line, TreeTopp, varlitinib
Highlights
-
•
In advanced biliary tract cancer, second-line varlitinib plus capecitabine did not improve efficacy versus capecitabine alone.
-
•
Varlitinib was well tolerated in combination with capecitabine.
-
•
Subgroup analyses suggested varlitinib plus capecitabine may benefit female patients and those with gallbladder cancer.
Introduction
Biliary tract cancer encompasses a spectrum of malignancies including cholangiocarcinoma, gallbladder cancer, and cancers of the ampulla of Vater, which typically present at an advanced clinical stage with a highly aggressive disease course and a poor prognosis.1,2 Gemcitabine plus cisplatin is the standard first-line treatment for advanced biliary tract cancer,3,4 and is associated with a median overall survival (OS) of <12 months.5 Evidence for second-line treatments is limited, although a randomized, phase III study recently demonstrated a modest OS benefit with 5-fluorouracil plus oxaliplatin (FOLFOX) in addition to active symptom control versus active symptom control alone.6 Pemigatinib7 and infigratinib,8 both fibroblast growth factor receptor (FGFR) inhibitors, and ivosidenib,9 an isocitrate dehydrogenase (IDH) 1 inhibitor, have also shown therapeutic potential in the second-line setting for the small subgroups of patients with cholangiocarcinoma who have FGFR2 gene fusions/rearrangements or IDH1 mutations, respectively. Nonetheless, there is an urgent need for more effective and well tolerated therapies for patients with previously treated, advanced biliary tract cancer.
The human epidermal growth factor receptor (HER) family is a group of receptor tyrosine kinases consisting of four members: HER1 (epidermal growth factor receptor; EGFR), HER2, HER3, and HER4.10 Aberrant expression and/or activation of the HER family receptor proteins has been implicated in the pathogenesis of many cancer types.11 In biliary tract cancer, a meta-analysis found that overexpression rates of HER2 and HER3 were 26.5% and 27.9%, and amplification rates were 30.1% and 26.5%, respectively.12 Subgroup analyses showed that the rates of HER2 overexpression and amplification were greater in extrahepatic biliary tract cancer subtypes including gallbladder cancer (19.9% and 22.5%, respectively) compared with intrahepatic cholangiocarcinoma (4.8% and 17.6%, respectively).12
Overexpression of EGFR and HER4 has also been detected in biliary tract cancer.13, 14, 15 Investigations of EGFR and EGFR/HER2 inhibitors in unselected populations of patients with biliary tract cancer, however, have shown disappointing results.16, 17, 18, 19, 20 Given the heterogeneity of biliary tract cancers,21 it is likely that careful patient selection based on tumor profiling may be necessary in order to demonstrate clinical activity. Another consideration is that heterodimerization of EGFR with HER2, HER3, or HER422 might limit the antitumor effect of EGFR inhibition, suggesting that pan-HER inhibition could improve the efficacy of EGFR inhibition through limiting receptor cross-talk signaling.
Varlitinib is a reversible small molecule pan-HER inhibitor targeting EGFR, HER2, and HER4.23 By potently antagonizing EGFR, HER2, and HER4, varlitinib also effectively inhibits heterodimers with HER3, which lacks a kinase domain.24 Preclinically, varlitinib inhibited proliferation and enhanced apoptosis in cholangiocarcinoma cell lines, suppressed tumor growth in a cholangiocarcinoma xenograft model, and showed synergism in combination with phosphatidylinositol 3-kinase (PI3K) inhibition.23 Early clinical studies suggested that varlitinib had promising antitumor activity in combination with gemcitabine plus cisplatin or 5-fluorouracil/capecitabine plus a platinum compound as first-line treatment for advanced biliary tract cancer.25,26
Here we report results from the TreeTopp (TREatmEnT OPPortunity with varlitinib in biliary tract cancer) study, which aimed to determine the safety and efficacy of varlitinib in combination with capecitabine for second-line treatment of advanced biliary tract cancer.
Methods
Study design and patients
This was a double-blind, randomized, placebo-controlled, phase II study with a single-arm, open-label safety lead-in, conducted across 56 sites in the United States (n = 11), Japan (n = 7), Australia (n = 5), Hong Kong (n = 2), Singapore (n = 2), South Korea (n = 15), Taiwan (n = 5), Hungary (n = 2), Poland (n = 2), and Spain (n = 5). The study is registered at ClinicalTrials.gov (NCT03093870).
Eligible patients were of or older than the legal age in the respective country at the time of written informed consent and had: histologically or cytologically confirmed unresectable or metastatic biliary tract cancer, including intrahepatic or extrahepatic cholangiocarcinoma, gallbladder cancer, carcinoma of the ampulla of Vater; received only one prior line of systemic therapy (≥6 doses) which must have contained gemcitabine; radiological evidence of disease progression after receiving first-line therapy; radiographically measurable disease (RECIST v1.1); no evidence of biliary duct obstruction unless the obstruction was controlled by local treatment or the biliary tree could be decompressed by endoscopic or percutaneous stenting with subsequent reduction in bilirubin to ≤1.5 × upper limit of normal; Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; and adequate hepatic and hematological function.
Key exclusion criteria were receipt of anticancer therapy, radiation, or local treatment within 3 weeks before receiving the first dose of study treatment; two or more peritoneal metastases or ascites at baseline as assessed by Independent Central Review (ICR; ascites that could be attributed to non-malignant causes or minimal ascites not requiring paracentesis were permitted); major surgical procedure within 14 days before receiving the first dose of study treatment; metastatic brain lesions, including asymptomatic and well controlled lesions.
All patients provided written informed consent before screening. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The study protocol, informed consent form, and patient information sheet were approved by local Institutional Review Boards. The first patient was enrolled on 10 May 2018 and the last patient completed the study on 11 December 2019.
Randomization and masking
Patients in the safety lead-in were not randomized. In the randomized part of the study, eligible patients were assigned in a 1 : 1 ratio using a computer-generated, blocked randomization list stratified by primary tumor location (gallbladder versus non-gallbladder) and geographical region (US versus non-US). Investigators, site staff, and patients were all blinded to randomized study treatment.
Procedures
In the safety lead-in, patients received oral varlitinib 300 mg twice daily (b.i.d.) every day for 21 days, plus oral capecitabine 1000 mg/m2 b.i.d. on days 1-14, in 21-day treatment cycles. A data safety monitoring board meeting was planned after the first 12 patients completed one cycle of treatment to review the safety and tolerability data before commencement of the randomized part of the study. In the randomized part of the study, patients received either oral varlitinib 300 mg or matching placebo b.i.d. every day for 21 days, in addition to oral capecitabine 1000 mg/m2 b.i.d. on days 1-14, in 21-day treatment cycles.
Study treatment was administered until disease progression, unacceptable toxicity, withdrawal of consent, or death. Radiographic tumor assessments were carried out using computed tomography or magnetic resonance imaging at baseline and every 6 weeks (±5 days) thereafter until the end of treatment visit in the safety lead-in and until disease progression in the randomized part of study. Radiological data were assessed at local sites in the safety lead-in and by ICR in the randomized part of the study. After disease progression, patients in the randomized part of the study were followed for survival every 12 weeks until death or the data cut-off. All patients were monitored continuously for safety throughout the study and at 28 days after the last dose of study treatment or within 1 day before the start of a new antitumor treatment, whichever came first.
Outcomes
In the randomized part of the study, the co-primary endpoints were objective response rate (ORR) and progression-free survival (PFS) determined according to RECIST version 1.1 by ICR. ORR was defined as the proportion of patients achieving a complete response (CR) or partial response (PR) at ≥1 visit. PFS was defined as the time from randomization to objective disease progression or death from any cause in the absence of disease progression. Secondary endpoints included OS and adverse events. OS was defined as the time from randomization to death due to any cause. In both parts of the study, adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Statistical analysis
The safety lead-in was not formally powered to assess any statistical hypothesis. Between 12 and 20 patients were planned to be enrolled. Patients in this arm were to be replaced if varlitinib compliance was <85% in the first 14 days.
In the randomized part of the study, a Hochberg procedure was used in order to maintain an overall, one-sided 10% type I error rate, whereby the primary objective was deemed to have been met if either co-primary endpoint was significant at the one-sided 5% level, or if both endpoints were significant at the one-sided 10% significance level. It was estimated that a sample of 120 patients was required to provide approximately 80% power to detect a true 17% difference in response rate based on a one-sided 5% significance level and assuming a 27% response rate with varlitinib and a 10% response rate with placebo. To ensure adequate data were available to evaluate the effects of varlitinib on both co-primary endpoints, the data cut-off for the primary analysis was to be the later of 3 months after enrollment of the last patient or when 70% (n = 84) of patients had experienced a PFS event. Based on a minimum of 84 PFS events, the trial would have at least 80% power to detect a true hazard ratio (HR) of 0.58 for PFS, based on a one-sided 5% significance level.
Although treatment allocation was stratified by both primary tumor location and geographical region, the latter was removed as a factor for the statistical analyses in a subsequent protocol amendment, due to the low number of US patients enrolled. ORR was analyzed using an exact binomial test stratified by primary tumor location. PFS and OS curves were estimated using Kaplan–Meier methodology. HRs and corresponding two-sided 95% confidence intervals (CIs) and P values were determined using a log-rank test stratified by primary tumor location. Although the study was not powered to evaluate subgroup interactions, prespecified subgroup analyses of PFS were carried out according to the following factors: region, primary tumor location, sex, race, baseline ECOG performance status, extent of disease, and age. For each subgroup, HRs and associated CIs were calculated using a log-rank test and presented on a forest plot.
Results
Safety lead-in
Of 27 patients who were screened, 24 were enrolled and received at least one dose of varlitinib plus capecitabine. All patients were discontinued from study treatment, most commonly due to radiographic disease progression (41.7%) and adverse events (29.2%). Baseline characteristics are presented in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100314.
All patients had at least one treatment-emergent adverse event (TEAE) and 18 (75.0%) had at least one grade ≥3 TEAE (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100314). The most frequent TEAEs were nausea (62.5%), fatigue (58.3%), and diarrhea (54.2%). Only one grade ≥3 TEAE was reported in more than two patients (hyponatraemia, 16.7%). Six patients had a TEAE leading to discontinuation that was considered related to treatment (asthenia, toxic leukoencephalopathy, alanine aminotransferase increased, nausea, worsening nausea, and hyperbilirubinaemia in one patient each). There were no treatment-related deaths.
One patient (4.2%) had a PR, five (20.8%) had stable disease, nine (37.5%) had progressive disease, and nine (37.5%) were not assessable.
Randomized study
Patients
A total of 127 patients were randomized to receive varlitinib plus capecitabine (n = 64) or placebo plus capecitabine (n = 63). All randomized patients received at least one dose of study treatment. Patient disposition is shown in Figure 1.
Figure 1.
Patient disposition: randomized population.
Overall, patients were mostly male (60.6%) and Asian (70.1%). Baseline characteristics were generally well balanced between groups (Table 1). There was a lower proportion of females in the varlitinib plus capecitabine arm compared with the placebo plus capecitabine arm (31.3% versus 47.6%). At the data cut-off for the survival analysis (15 July 2020), the median (quartile 1-quartile 3) duration of follow-up was 7.5 (4.8-11.7) months.
Table 1.
Baseline characteristics: randomized population
| Characteristics | Varlitinib + capecitabine (n = 64) | Placebo + capecitabine (n = 63) |
|---|---|---|
| Median age, years (range) | 63 (31-79) | 64 (36-82) |
| Male, n (%) | 44 (68.8) | 33 (52.4) |
| Race, n (%) | ||
| White | 21 (32.8) | 16 (25.4) |
| Black/African American | 1 (1.6) | 0 (0) |
| Asian | 42 (65.6) | 47 (74.6) |
| Median body mass index, kg/m2 (range) | 24.3 (16-44) | 22.5 (15-38) |
| Geographical location, n (%) | ||
| Asia-Pacific | 46 (71.9) | 48 (76.2) |
| Europe | 11 (17.2) | 9 (14.3) |
| United States | 7 (10.9) | 6 (9.5) |
| Primary tumor location, n (%) | ||
| Intra-hepatic duct | 26 (40.6) | 29 (46.0) |
| Extra-hepatic duct | 8 (12.5) | 12 (19.0) |
| Gallbladder | 18 (28.1) | 16 (25.4) |
| Ampulla of Vater and hilar | 12 (18.8) | 6 (9.5) |
| Recurrent disease, n (%) | 17 (26.6) | 22 (34.9) |
| Extent of disease, n (%) | ||
| Locally advanced | 5 (7.8) | 3 (4.8) |
| Metastatic | 59 (92.2) | 60 (95.2) |
| Baseline ECOG PS, n (%) | ||
| 0 | 37 (57.8) | 26 (41.3) |
| 1 | 27 (42.2) | 37 (58.7) |
| First-line therapy, n (%) | ||
| Gemcitabine + cisplatin | 58 (90.6) | 57 (90.5) |
| Gemcitabine + FOLFOX | 0 (0) | 1 (1.6) |
| Gemcitabine + oxaliplatin | 1 (1.6) | 2 (3.2) |
| Gemcitabine monotherapy | 1 (1.6) | 0 (0) |
| Other | 4 (6.3) | 3 (4.8) |
| Estimated PFS on first-line therapy, months, median (range)a | 6.4 (1-36) | 7.8 (1-55) |
ECOG PS, Eastern Cooperative Oncology Group performance status; PFS, progression-free survival.
Estimated from reported start date of first-line treatment and reported date of progression following first-line treatment.
Treatment exposure
The median (range) exposure to study treatment was 2.4 (0.1-13.8) months for varlitinib and 1.7 (0.1-9.1) months for capecitabine in the varlitinib plus capecitabine arm, and 2.8 (0.0-12.8) months with placebo and 1.8 (0.0-6.2) months with capecitabine in the placebo plus capecitabine arm.
Efficacy
The ORR was 9.4% with varlitinib plus capecitabine and 4.8% with placebo plus capecitabine, but the difference did not reach statistical significance (odds ratio 2.28; 95% CI 0.46-14.76; P = 0.42; Table 2). No patients achieved a CR.
Table 2.
Summary of responses: randomized population
| Responses, n (%) | Varlitinib + capecitabine (n = 64) | Placebo + capecitabine (n = 63) |
|---|---|---|
| Objective response | 6 (9.4) | 3 (4.8) |
| Complete response | 0 (0) | 0 (0) |
| Partial response | 6 (9.4) | 3 (4.8) |
| Stable disease | 29 (45.3) | 34 (54.0) |
| Progressive disease | 24 (37.5) | 24 (38.1) |
| Early death | 4 (6.3) | 0 (0) |
| RECIST v1.1 progression | 20 (31.3) | 24 (38.1) |
| Non-evaluable | 5 (7.8) | 2 (3.2) |
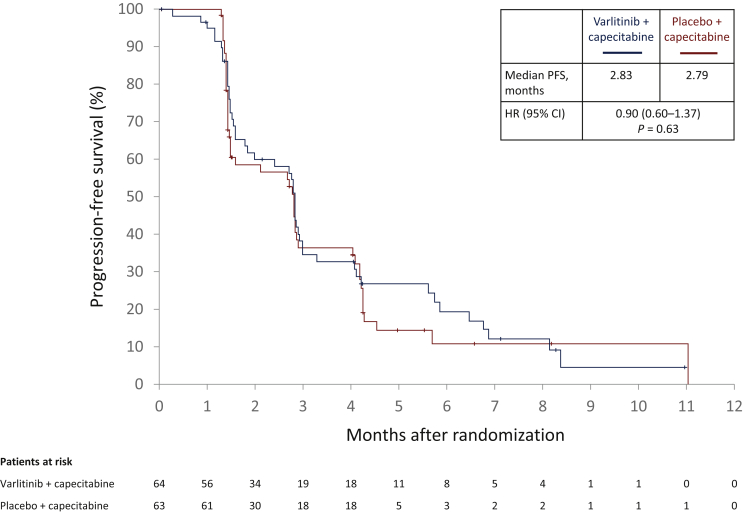
Median PFS was 2.83 months with varlitinib plus capecitabine versus 2.79 months with placebo plus capecitabine (HR, 0.90; 95% CI 0.60-1.37; P = 0.63; Figure 2). The proportion of patients with progression events was 76.6% versus 74.6%, respectively; 23.4% versus 25.4% had censored observations, and 15.6% versus 22.2% were progression-free at the last radiologic assessment, respectively. Median OS was 7.8 months with varlitinib plus capecitabine versus 7.5 months with placebo plus capecitabine (HR, 1.11; 95% CI 0.69-1.79; P = 0.66; Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100314). The proportion of patients who died was 60.9% versus 55.6%, respectively; 39.1% versus 44.4% were censored, of whom 26.6% versus 34.9% were alive at the data cut-off, respectively.
Figure 2.
Progression-free survival: randomized population.
P value was derived from a log-rank test stratified by primary tumor location.
CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.
Prespecified subgroup analyses suggested that the addition of varlitinib may provide a PFS benefit in female patients (median, 4.1 versus 2.8 months; HR, 0.59; 95% CI 0.28-1.23) and those with gallbladder cancer (median, 2.9 versus 1.6 months; HR, 0.55; 95% CI 0.26-1.19; Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100314).
Safety
A summary of TEAEs is provided in Table 3. The most frequent TEAEs with varlitinib plus capecitabine were nausea (51.6%), blood bilirubin increased (43.8%), and diarrhea (40.6%). A slightly greater proportion of patients in the varlitinib plus capecitabine arm had at least one grade ≥3 TEAE compared with those in the placebo plus capecitabine arm (65.6% versus 58.7%). Increased blood bilirubin, anemia, and hyperbilirubinemia were the most frequently reported grade ≥3 TEAEs in the varlitinib plus capecitabine arm. Dose interruptions or modifications were required by 81.3% and 23.4% of patients receiving varlitinib plus capecitabine, and 65.1% and 4.8% of those receiving placebo plus capecitabine, respectively. Three patients had a TEAE leading to discontinuation that was considered as treatment related (vomiting, biliary tract infection, and cholangiolitis in one patient each). Only one death was considered possibly related to treatment (cholangiolitis in the varlitinib plus capecitabine arm).
Table 3.
Adverse events: randomized population
| Event, n (%) | Varlitinib + capecitabine (n = 64) | Placebo + capecitabine (n = 63) |
|---|---|---|
| Any TEAE | 64 (100) | 59 (93.7) |
| Grade ≥3 TEAE | 42 (65.6) | 37 (58.7) |
| Serious TEAE | 25 (39.1) | 27 (42.9) |
| Treatment-related TEAE leading to treatment discontinuation | 3 (4.7) | 0 (0) |
| Treatment-related TEAE leading to death | 1 (1.6) | 0 (0) |
| TEAEs in ≥20 patients | ||
| Nausea | 33 (51.6) | 14 (22.2) |
| Blood bilirubin increased | 28 (43.8) | 14 (22.2) |
| Diarrhea | 26 (40.6) | 16 (25.4) |
| Decreased appetite | 25 (39.1) | 11 (17.5) |
| Vomiting | 22 (34.4) | 11 (17.5) |
| Palmar-plantar erythrodysesthesia syndrome | 20 (31.3) | 19 (30.2) |
| Abdominal pain | 16 (25.0) | 12 (19.0) |
| Fatigue | 16 (25.0) | 12 (19.0) |
| Pyrexia | 15 (23.4) | 11 (17.5) |
| Grade ≥3 TEAEs in ≥5 patients | ||
| Blood bilirubin increased | 9 (14.1) | 7 (11.1) |
| Anemia | 7 (10.9) | 6 (9.5) |
| Hyperbilirubinemia | 6 (9.4) | 1 (1.6) |
| Abdominal pain | 5 (7.8) | 5 (7.9) |
| Vomiting | 5 (7.8) | 2 (3.2) |
| Alanine transaminase increased | 5 (7.8) | 1 (1.6) |
| Cholangiolitis | 4 (6.3) | 4 (6.3) |
| Asthenia | 4 (6.3) | 1 (1.6) |
| Palmar-plantar erythrodysesthesia syndrome | 2 (3.1) | 3 (4.8) |
| Serious TEAEs in >2 patients receiving varlitinib | ||
| Blood bilirubin increased | 5 (7.8) | 5 (7.9) |
| Cholangiolitis | 4 (6.3) | 4 (6.3) |
| Vomiting | 4 (6.3) | 2 (3.2) |
| Hyperbilirubinemia | 4 (6.3) | 0 (0) |
| Pyrexia | 3 (4.7) | 0 (0) |
TEAE, treatment-emergent adverse event.
Discussion
The TreeTopp study showed that the addition of varlitinib to capecitabine did not statistically improve ORR, PFS, or OS compared with capecitabine alone in patients with advanced biliary tract cancer and disease progression following first-line treatment. Following a review of these results, a pre-planned, second part of the study was cancelled. Varlitinib in combination with capecitabine was generally well tolerated.
Currently, the standard treatment for advanced biliary tract cancer in the second-line setting is FOLFOX chemotherapy.3 This is based on evidence from a randomized, phase III study (ABC-06) showing that the addition of FOLFOX to active symptom control modestly prolonged OS compared with active symptom control alone (6.2 versus 5.3 months; adjusted HR, 0.69; 95% CI 0.50-0.97; P = 0.031).6 The median PFS noted in TreeTopp (2.83 months) was lower than with FOLFOX (4.0 months) in the ABC-06 study. Radiological evaluations were obtained every 6 weeks in TreeTopp compared with every 12 weeks in the ABC-06 trial, however, which may have impacted these results. Targeted therapies have shown encouraging efficacy in patients with biliary tract cancers with actionable mutations. For example, FGFR and IDH mutations have been reported in 11% and 20%, respectively, of intrahepatic cholangiocarcinomas, but are rarely found in other biliary tract cancers.27 In phase II studies in patients with previously treated advanced cholangiocarcinoma and FGFR fusions or rearrangements, pemigatinib and infigratinib resulted in ORRs of 35.5% and 23.1%, respectively.7,8 A phase III study in patients with previously treated IDH1-mutant cholangiocarcinoma reported a significant improvement in median PFS with ivosidenib compared with placebo (2.7 months versus 1.4 months; HR 0.37; P = 0.0001), although the ORR in the ivosidenib group was only 2%.9
Although HER provides a rational target in advanced biliary tract cancer, consistent with our findings, previous studies of HER-targeted agents, including cetuximab and panitumumab (EGFR antibodies),16 erlotinib (an EGFR inhibitor),17, 18, 19 lapatinib (an EGRF/HER2 inhibitor),20 and afatinib (a pan-HER inhibitor)28 have also shown negative results. There was, however, no patient selection based on biomarkers in these studies. Similarly, as our study was based on the hypothesis that pan-HER inhibition targeting EGFR, HER2, and HER4 would improve outcomes in patients with previously treated biliary tract cancer, the design did not include patient selection according to HER status. Interestingly, small studies including patients with biliary tract cancer and HER2 mutations or overexpression have suggested promising efficacy with HER2-targeted therapies.29,30 As HER2 overexpression occurs in only around one-quarter of biliary tract cancers,12 selecting patients with HER2 aberrations may increase the likelihood of observing a therapeutic effect compared with an unselected patient population. In this regard, the SUMMIT basket trial investigated monotherapy with neratinib, a pan-HER kinase inhibitor, in pretreated patients with HER2-mutated advanced biliary tract cancer.31 While the observed efficacy, however, was considered similar to current standards of care, with an ORR of 16%, the trial did not meet the criteria for further expansion.
The prespecified subgroup analysis suggested that varlitinib may provide benefit in female patients and those with gallbladder cancer; however, the 95% CIs of the HRs crossed 1. Gallbladder cancer is more common in females compared with males,32 and HER2 overexpression is more frequently observed in gallbladder cancer compared with intrahepatic cholangiocarcinoma.12 Therefore, we speculate that it may be easier to observe the antitumor effect of varlitinib in selected patients with HER2 overexpression. Nevertheless, no strong conclusions can be drawn from the present study regarding the suitability of varlitinib in these patients.
Our study was limited by the lack of patient selection at inclusion and further studies of varlitinib will require adequate patient selection according to HER status. Although varlitinib is not being investigated further for the second-line treatment of biliary tract cancer in combination with capecitabine, preclinical findings support the future investigation of varlitinib in combination with other agents, for example, PI3K inhibitors.23 Future studies with targeted agents such as varlitinib for preselected patients with HER alterations are encouraged.
Acknowledgements
The authors wish to thank all patients, investigators, and coordinators who participated in the study. Medical writing support was provided by Dr Sharon Gladwin, PhD from Rude Health Consulting, and was funded by ASLAN Pharmaceuticals.
Funding
This trial was funded by ASLAN Pharmaceuticals Pte. Ltd.
Disclosures
MMJ has served as a consultant/steering committee member for Taiho, QED, AstraZeneca, Meclun, TransThera, Bristol Myers Squibb (BMS), EMD Serono, Incyte, Nucana, and PCI, and has received research funding from EMD Serono, Merck, QED, Novartis, AstraZeneca, Basilea, Eli Lilly, ASLAN, Incyte, and Agios, and honoraria/speaker bureau fees from QED, Agios, and Incyte. D-YO has served as a consultant/advisory board member for AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho, ASLAN, Halozyme, Zymeworks, BMS/Celgene, BeiGene, Basilea, and Turning Point, and has received research grants from AstraZeneca, Novartis, Array, Eli Lilly, Servier, BeiGene, Merck Sharp & Dohme (MSD), and Handok. MI has served as an advisory board member for Eisai, Eli Lilly Japan, ASLAN, GlaxoSmithKline, Nihon Servier, Chugai, BMS, Novartis, Bayer, and Takeda, has received honoraria from Eisai, MSD, Eli Lilly Japan, Yakult, Teijin Pharma, Astellas, EA Pharma, Sumitomo Dainippon, Otsuka, Yakult, Nihon Servier, Taiho, Chugai, BMS, Novartis, Bayer, and Takeda, and has received research funding from Eisai, Merck Serono, MSD, Eli Lilly Japan, Yakult, Ono, ASLAN, J-Pharma, AstraZeneca, EA Pharma, Pfizer, Merus N.V., Nihon Servier, Delta-Fly Pharma, Chiome Bioscience, Chugai, BMS, Novartis, Bayer, and Takeda. W-PY has served as an advisory board member for ASLAN. NM is a consultant for ASLAN. BL is a former employee (at the time of the study) and current shareholder of ASLAN. CF and KH are employees and shareholders of ASLAN.
Supplementary data
References
- 1.Augustine M.M., Fong Y. Epidemiology and risk factors of biliary tract and primary liver tumors. Surg Oncol Clin N Am. 2014;23:171–188. doi: 10.1016/j.soc.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Groot Koerkamp B., Fong Y. Outcomes in biliary malignancy. J Surg Oncol. 2014;110:585–591. doi: 10.1002/jso.23762. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network Hepatobiliary Cancers (Version 3.2021) https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf Available at.
- 4.Valle J.W., Borbath I., Khan S.A., et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 5.Valle J., Wasan H., Palmer D.H., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 6.Lamarca A., Palmer D.H., Wasan H.S., et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abou-Alfa G.K., Sahai V., Hollebecque A., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Javle M.M., Roychowdhury S., Kelley R.K., et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J Clin Oncol. 2021;39:265. [Google Scholar]
- 9.Abou-Alfa G.K., Macarulla T., Javle M.M., et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H., Berezov A., Wang Q., et al. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117:2051–2058. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar R., George B., Campbell M.R., et al. HER family in cancer progression: From discovery to 2020 and beyond. Adv Cancer Res. 2020;147:109–160. doi: 10.1016/bs.acr.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Galdy S., Lamarca A., McNamara M.G., et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev. 2017;36:141–157. doi: 10.1007/s10555-016-9645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pignochino Y., Sarotto I., Peraldo-Neia C., et al. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer. 2010;10:631. doi: 10.1186/1471-2407-10-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin W. ErBb family proteins in cholangiocarcinoma and clinical implications. J Clin Med. 2020;9:2255. doi: 10.3390/jcm9072255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshikawa D., Ojima H., Iwasaki M., et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418–425. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizzo A., Frega G., Ricci A.D., et al. Anti-EGFR monoclonal antibodies in advanced biliary tract cancer: a systematic review and meta-analysis. In Vivo. 2020;34:479–488. doi: 10.21873/invivo.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J., Park S.H., Chang H.M., et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181–188. doi: 10.1016/S1470-2045(11)70301-1. [DOI] [PubMed] [Google Scholar]
- 18.El-Khoueiry A.B., Rankin C., Siegel A.B., et al. S0941: a phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br J Cancer. 2014;110:882–887. doi: 10.1038/bjc.2013.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiorean E.G., Ramasubbaiah R., Yu M., et al. Phase II trial of erlotinib and docetaxel in advanced and refractory hepatocellular and biliary cancers: Hoosier Oncology Group GI06-101. Oncologist. 2012;17:13. doi: 10.1634/theoncologist.2011-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramanathan R.K., Belani C.P., Singh D.A., et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol. 2009;64:777–783. doi: 10.1007/s00280-009-0927-7. [DOI] [PubMed] [Google Scholar]
- 21.Personeni N., Lleo A., Pressiani T., et al. Biliary tract cancers: molecular heterogeneity and new treatment options. Cancers. 2020;12(11):3370. doi: 10.3390/cancers12113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37:S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 23.Dokduang H., Jamnongkarn W., Promraksa B., et al. In vitro and in vivo anti-tumor effects of pan-HER inhibitor varlitinib on cholangiocarcinoma cell lines. Drug Des Dev Ther. 2020;14:2319–2334. doi: 10.2147/DDDT.S250061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sierke S.L., Cheng K., Kim H.H., Koland J.G. Biochemical characterization of the protein tyrosine kinase homology domain of the ErbB3 (HER3) receptor protein. Biochem J. 1997;322:757–763. doi: 10.1042/bj3220757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh D.Y., Yong W.P., Chen L.T., et al. A multicenter, phase Ib/2 study of varlitinib plus gemcitabine and cisplatin (gem/cis) for treatment of naïve, advanced, or metastatic biliary tract cancer (BTC) J Clin Oncol. 2019;37:319. Abstract 319. [Google Scholar]
- 26.Tan A.C., Oh D.Y., Chao Y., et al. AB062. P-33. Efficacy and safety of varlitinib, a reversible pan-HER tyrosine kinase inhibitor, in combination with platinum-based regimens in biliary tract cancers: a pooled analysis from three phase 1 studies. HepatoBiliary Surg Nutr. 2019;8:AB062. [Google Scholar]
- 27.Javle M., Bekaii-Saab T., Jain A., et al. Biliary cancer: utility of next-generation sequencing for clinical management. Cancer. 2016;122:3838–3847. doi: 10.1002/cncr.30254. [DOI] [PubMed] [Google Scholar]
- 28.Moehler M., Maderer A., Ehrlich A., et al. Safety and efficacy of afatinib as add-on to standard therapy of gemcitabine/cisplatin in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, phase I trial with an extensive biomarker program. BMC Cancer. 2019;19:55. doi: 10.1186/s12885-018-5223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javle M., Churi C., Kang H.C., et al. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol. 2015;8:58. doi: 10.1186/s13045-015-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong H., Jeong J.H., Kim K.P., et al. Feasibility of HER2-targeted therapy in advanced biliary tract cancer: a prospective pilot study of trastuzumab biosimilar in combination with gemcitabine plus cisplatin. Cancers. 2021;13:161. doi: 10.3390/cancers13020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harding JJ, Cleary JM, Quinn DI, et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: results from the phase 2 SUMMIT ‘basket’ trial. Paper presented at the Gastrointestinal Cancers Symposium, January 15-17, 2021 (virtual): Poster 320.
- 32.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.