Abstract
Alzheimer’s disease (AD) is a chronic, progressive, and fatal neurodegenerative disorder affecting cognition, behavior, and function, being one of the most common causes of mental deterioration in elderly people. Once thought as being just developed because of β amyloid depositions or neurofibrillary Tau tangles, during the last decades, numerous AD-related targets have been established, the multifactorial nature of AD became evident. In this context, the one drug-one target paradigm has resulted in being inefficient in facing AD and other disorders with complex etiology, opening the field for the emergence of the multitarget approach. In this review, we highlight the recent advances within this area, emphasizing in hybridization tools of well-known chemical scaffolds endowed with pharmacological properties concerning AD, such as curcumin-, resveratrol-, chromone- and indole-. We focus mainly on well established and incipient AD therapeutic targets, AChE, BuChE, MAOs, β-amyloid deposition, 5-HT4 and Serotonin transporter, with the aim to shed light about new insights in the AD multitarget therapy.
Keywords: Alzheimer disease, multi-target directed ligands, cholinesterase inhibitors, serotonin transporter, 5-HT receptors, β – amyloid aggregation, tau protein, monoamine oxidase
1. INTRODUCTION
According to the World Alzheimer Report (2019), around 50-60% of the overall dementias correspond to Alzheimer’s disease (AD). Although it has been for years a major health concern in developed economies, it is increasing in the developing countries as life expectancy increases. Even though WHO estimates that around 47 million people currently suffer from AD, this number is expected to double every 20 years; thus, the AD population could reach 75 million by 2030 [1]. Despite multiple efforts carried out within the last decades by universities, foundations, research centers and pharmaceutical companies, the detailed pathogenesis of AD is still unclear, and the underlying mechanism leading to this disease is not yet fully understood. Unfortunately, given the continuous failures in clinical trials, pharmaceutical companies are pulling out AD research, and it has been 17 years since the last drug, memantine, reached the market in 2003 [2].
AD is a progressive neurodegenerative disease resulting in the irreversible loss of neurons, particularly in the cortex and hippocampus [3]. Symptoms may include progressive loss of memory, cognition, motor, and functional capacity, often accompanied by behavioral disturbance such as aggression, depression and wandering [4], being the most common cause of dementia among elderly people [5].
Many authors defined AD as a heterogeneous disease caused by a combination of environmental and genetic factors, being the age one of the most important risk factors for the development of the disease [6, 7]. Some of the predisposing factors of this pathology include vascular disease [8], diabetes [9], depression [10], and hypertension [11]. On the other hand, many lifestyle modifications such as physical activity, sleep, feeding, smoking, alcohol, and intellectual stimulation are thought to have an impact cognitive impairment [12] even though more evidence is still needed. So far, AD has been related to several altered brain functions, including extracellular plaques containing abnormal deposits of beta-amyloid peptides [13-16], the hyperphosphorylated form of the microtubular Tau protein involving twisted fibers [17-20], inflammation [21-24], oxidative stress [25-28], cholinergic neuron damage (cholinergic hypothesis) [29-31], serotonin misregulation (serotoninergic hypothesis) [32-34], and many others [35-39].
Despite many years of evidence suggesting a connection between amyloid plaques or neurofibrillary tangles as the earliest lesions in AD, the role of such processes remains controversial [40] even though there is no doubt that those aggregates promote inflammation responses and activate neurotoxic pathways, leading to dysfunction and death of brain cells. In this line, the inflammatory process significantly contributes to AD pathogenesis [41]. In a recent review [42], the importance of understanding the inflammation process was explained, suggesting that the control of interactions between the immune and nervous system could be a key to the prevention or delaying of most late-onset central nervous system (CNS) diseases, including AD. Authors concluded that the brain can no longer be viewed as an immune-privileged organ.
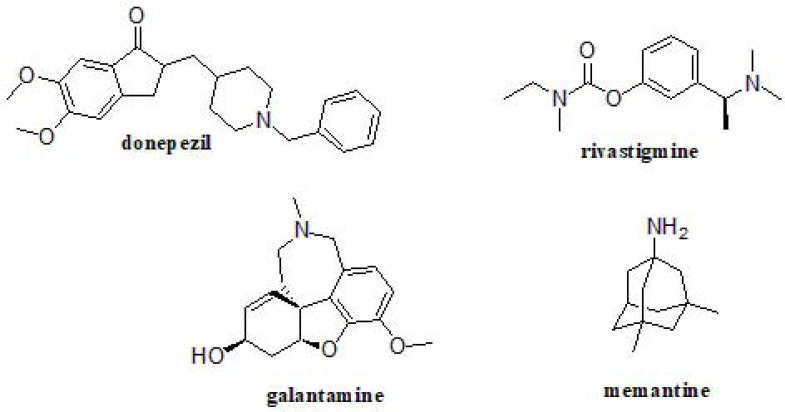
At present, only 4 drugs have been approved for AD treatment, acetylcholinesterase inhibitors donepezil, rivastigmine, galantamine and the N-methyl-d-aspartate (NMDA) receptor antagonist memantine (Fig. 1), and they only address associated symptomatology without halting or reversing the disease progression [43]. To this day, AD has also been related to additional targets/functions, whose misregulation has been proposed to lead to AD onset. These include ApoE [44], dopamine D2 receptor [45], γ-aminobutyric acid receptor [46], acetylcholinesterase and butyrylcholinesterase [4], β- and γ-secretases [47, 48], serotonin 5-HT6 and 5-HT4 receptors [49, 50], serotonin transporter [51], or SRFP1 [52]. After providing this big picture, the only clear issue is that we are facing a multifactorial disorder, which cannot be managed by drugs acting at just a single level.
Fig. (1).
Approved drugs for AD treatment.
The “one drug-one target” paradigm has not succeeded and does not provide a solution in the treatment of complex and multifactorial diseases like AD [7, 53]. In this context, the multitarget approach has recently emerged as a potential solution by using multi-target directed ligands (MTDLs) [54, 55]. Thus, the aims of this proposal are based on the design of new drug candidates simultaneously modulating different biological targets involved in the neurodegenerative AD cascade. Due to the complex etiology and multifactorial nature of this disease, various hypotheses have been proposed in an attempt to address it, although none of them is able to explain the onset and progression of the disease on its own [56].
1.1. Cholinergic Hypothesis
The cholinergic hypothesis is based on the association between low levels of acetylcholine (ACh) and a decline in learning, cognitive function and memory in AD patients [57-60]. It has been demonstrated that the dysfunction and neuronal loss in basal forebrain regions are directly related to the expression and activity of choline acetyltransferase (ChAT) and acetylcholinesterase (AChE), specific enzymes related to CNS functions. Their activities play an essential role in cholinergic transmission, showing variations in the cerebral cortex and the hippocampus in AD-suffering subjects [61]. The presynaptic cholinergic deficit is associated with a marked loss of cholinergic cells from the nucleus basalis of Meynert, decrease of ACh releasing and reuptaking [62]. The cholinergic hypothesis has not had widespread support because the AChE inhibitor-based AD treatment only brings a slight symptomatic improvement, failing in curing or preventing the disease progression [57, 60].
1.2. Amyloid Hypothesis
Another plausible and widely studied cause of AD is based on the amyloid cascade hypothesis. The accumulation of the hydrophobic amyloid-beta (Aβ, Aβ40 and Aβ42) peptides resulting in self-aggregation and insoluble plaques formation is still considered to be the main feature of AD etiology [63-65]. It is originated from the proteolytic cleavages of the transmembrane amyloid precursor protein (APP) by specific secretases (β-, and γ-secretase) [66, 67]. Aβ fibrils accumulation is thus considered an early toxic event that activates neurotoxic pathways. Some studies suggest that these oligomers can destroy the integrity of the cell membrane and disrupt the steady-state of brain cells [68-70], leading to brain cell dysfunction and death [71]. Some authors indicate that Aβ aggregates can also induce oxidative stress [72, 73], initiate an inflammatory response [41, 74, 75], and alter calcium homeostasis [70, 76]. Furthermore, Selkoe [77] emphasizes that the word “cause” of AD pathology cannot necessarily be directly applied to the Aβ accumulation, due to the existence of some genetic mutations or polymorphisms that can produce an increase in other peptide accumulation (presenilin or apolipoprotein E) [78, 79]. Despite what was previously indicated, the self-aggregation of Aβ itself is insufficient to explain the accumulation of the peptide in specific brain regions of AD patients. The “metal hypothesis of AD” is based on the effects of Aβ accumulations (as senile plaques) promoted by Aβ-metal interactions. The metal ion content of the brain are essential trace elements that are stringently regulated with virtual no passive flux of metals from the circulation to the brain, but interestingly, elevated concentration of copper, zinc, and iron have been detected in amyloid plaques, which induces the protein to precipitate into metal-enriched masses [80]. However, the mechanism of how these metals bind to and promote its aggregation is still unknown [80-82]. A plausible approach may be modulating these interactions by metal chelators, and indeed, this is considered another promising strategy for AD treatment.
1.3. Tau Hypothesis
The Tau protein is an important component of the neuronal cytoskeleton, being its principal activities related to stabilizing microtubules [83], cell shape maintaining and axonal transport [84]. In the normal brain, the balance between Tau phosphorylation and dephosphorylation is a dynamic process that causes conformational and structural changes, regulating the stability of the cytoskeleton and axonal morphology [85-88]. The imbalance in the action of different kinases and phosphatases is one of the possible proposals of Tau hyperphosphorylation [89, 90]. During the development of AD, Tau begins to phosphorylate in a massive way, which triggers its collapse and intracellular aggregation to form neurofibrillary tangles (NFTs) [91]. A progressive neuronal degeneration is the start of alteration leading to degradation of the cytoskeletons. In other words, these fibrils create a physical barrier within the neuron that generates a toxic medium with a high concentration of NFTs. Some authors [92-94] exposed that NFTs are inert and do not have influence in microtubules assembly, but they choke the affected neurons and facilitate cell death by acting as a space-occupying lesion. In a review [90], the authors summarize the evidences and therapeutic approaches that linked Tau misregulation to AD pathogenesis. One approach is the use of kinase inhibitors and phosphatase activators [95, 96], however, these enzymes are present in nearly every cell in the body and the problem would be finding molecules that alter the activi-ty specifically of the target enzyme without affecting the others. Identifying key sites of Tau in order to develop small molecule anti-aggregators is still a hopeful field of research [97].
Certainly, another proposed approach involving Tau and Amyloid hypotheses is immunotherapy, which is the use of immunity-enhancing techniques as a medical treatment. Huge advances in immunotherapy AD research have been achieved within the last decade [98, 99], supported by several Clinical Trials and the recent FDA approval of Aducanumab. However, in order to stick to the script, such an interesting approach will not be considered here as it falls far beyond the scope of this review.
Indeed, it is worth mentioning that the Tau hypothesis alone is inadequate to explain all the symptomatic conditions observed in AD, so it is not surprising that drugs targeting Tau protein itself have not achieved any relevant progress.
1.4. Serotonergic Hypothesis
Depression may be one of the initial symptoms of neurodegenerative disorders, and it is regarded as a risk factor for later development of dementias, being depressed mood in elders associated with an increased risk of AD [100]. Nowadays, our concept of the nature of the relationship between cognitive impairment and the serotonin system is evolving, thus the serotonergic hypothesis of AD is slowly emerging [101], as long as more and more researchers worldwide are suggesting AD modulators based on monoamine oxidase (MAO) inhibitors, serotonin reuptake inhibitors (SSRIs) [102-104], and 5-HT4 and 5-HT6 modulators [49, 105]. According to many authors, the accumulation of Aβ-amyloid could be a secondary effect of reduced monoamine neurotransmitters [101].
The MAO enzyme exists as two isoforms, MAO-A and MAO-B, and their principal activities are related to catalyzing the oxidation of monoamines and are thus responsible for the metabolism of neurotransmitters such as serotonin, noradrenaline, and dopamine [106]. They are located in the CNS and in peripheral tissues. Some studies revealed that MAOs are associated with psychiatric and neurological disorders, including AD [107-110]. MAO-A inhibitors are used as antidepressants and anti-anxiety agents, while MAO-B inhibitors have been revealed to be useful in neurodegenerative disorders such as Parkinson´s disease and AD, also inhibiting their associate oxidative damage [111, 112]. In summary, simultaneous inhibition of both MAOs, have been suggested to provide additional benefits in AD therapy. Along with MAO, 5-HT4 receptor (5-HT4R) ligands have also been proposed in AD research since many studies have shown the involvement of 5-HT4R in cognitive processes. Moreover, many authors provided important findings suggesting that 5HT4R agonists may also affect the amyloid β-peptide pathway, supporting the serotonergic approach in AD [113].
The scope of this review is to describe some widely studied bioactive structures: curcumin-, resveratrol-, chromone-, and indole-derivatives as MTDLs for Alzheimer’s Disease, mainly oriented to interact with the aforementioned targets, included or not in the previously described hypotheses. This literature review is focused in identifying small molecular fragments as promising starting points for biological target modulation [7, 114] with the aim of shifting the current paradigm towards a disease-modifying strategy.
In this review, we summarize the latest medicinal chemistry goals in AD-related MTDLs development: small molecule fragment with known biological activities combined through hybridization or fine chemical tuning, in order to develop true MTDLs to face such devastating disease from a multifactorial point of view.
2. CURCUMIN AND CURCUMINOIDS HYBRIDS
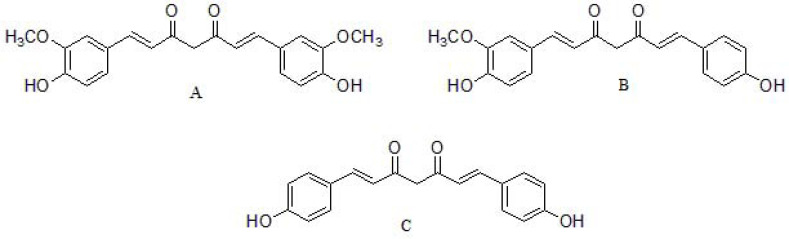
The major curcuminoids present in turmeric (curcuma longa) are curcumin, demethoxycurcumin and bisdemethoxycurcumin [115] (Fig. 2), being curcumin the most bioactive component [116]. Curcuminoids from turmerichave shown anti-inflammatory, antioxidant, anticancer, antimicrobial, and neuroprotective effects, among others [117]. These compounds display different chemical functions: a methoxy phenolic group; α, β-unsaturated β-diketo linker, and keto-enol tautomerism having a predominant keto form in acidic and neutral solutions, and stable enol form in alkaline medium. The aromatic groups confer hydrophobicity, the linker brings flexibility and tautomeric structures also influence the hydrophobicity and polarity [118]. However, curcumin exhibits several limitations, such as chemical instability, poor solubility in water, low bioavailability, and fast metabolism under physiological conditions, thereby resulting in a rapid systematic elimination and limiting its application as a drug candidate [119]. In this sense, it is reasonable to design curcumin analogs able to enhance the aforementioned drawbacks. Several groups tested curcumin derivatives using cells and mouse models of AD and reported that curcumin derivatives have strong anti-amyloid beta aggregation property, are able to cross the blood-brain barrier (BBB), ameliorates cognitive decline, and improve synaptic functions in a mouse model [120-124]. Besides, curcumin itself also exert MAO-B inhibitory capabilities, in addition to the ability to degrade Tau neurofibrillary tangles [125], although the mechanism of action of such processes are not fully understood.
Fig. (2).
Chemical structures of (A) curcumin, (B) demethoxycurcumin and (C) bisdemethoxycurcumin.
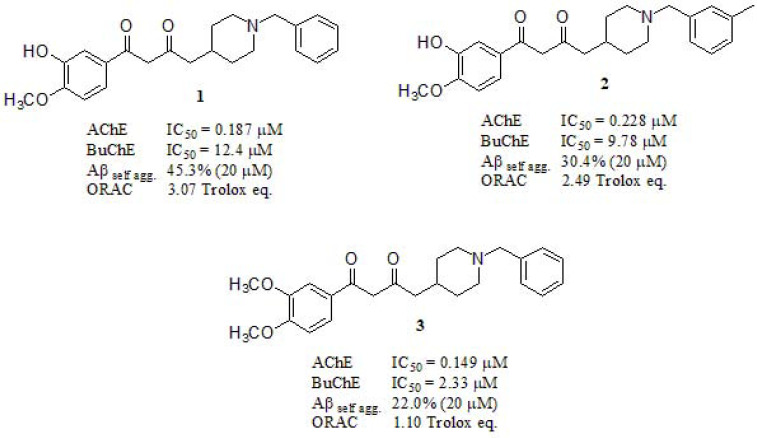
Yan et al. [126] reported the synthesis and biological activities of MTDLs based on chimerical structures consistent in donepezil-curcumin fused scaffolds. The most active studied compounds 1, 2, and 3 were evaluated in AChE and butyrylcholinesterase (BuChE) inhibition, BuChE /AChE selectivity, Aβ1-42 self-aggregation inhibition and antioxidant effects (Fig. 3). Compound 1 was revealed as potent AChEi (IC50 = 187 nM) compared to the rest of the series (donepezil (DPZ) AChE IC50 = 37 nM as reference), and the highest selectivity ratio (BuChE /AChE: 66.3) which was significantly better than Tacrine and Galantamine (selectivity: 0.15 and 25.3, respectively) although still far away from DPZ (selectivity: 85.4). Inhibitory activity against Aβ1-42 self-aggregation was evaluated employing curcumin as reference (54.9% at 20µM). Compounds 1, 2 and 3 displayed 45.3%, 30.4% and 22.0%, respectively, evidencing the importance of the hydroxy group in the Aβ1-42 self-aggregation inhibitory activity. They also conducted an oxygen radical absorbance capacity assay (ORAC), evaluating their antioxidant activity in vitro with Trolox as standard. All compounds exhibited good ORAC values of 1.01 – 3.07 Trolox equivalent (expressed as µM of Trolox eq/ µM tested compounds). It is known that curcumin (2.52 Trolox eq.) displays potent antioxidant activity, but compound 1, featuring a hydroxyl group at the meta position, displayed a stronger one.
Fig. (3).
Multi-target directed ligands based on donepezil and curcumin scaffolds reported by Yan et al.
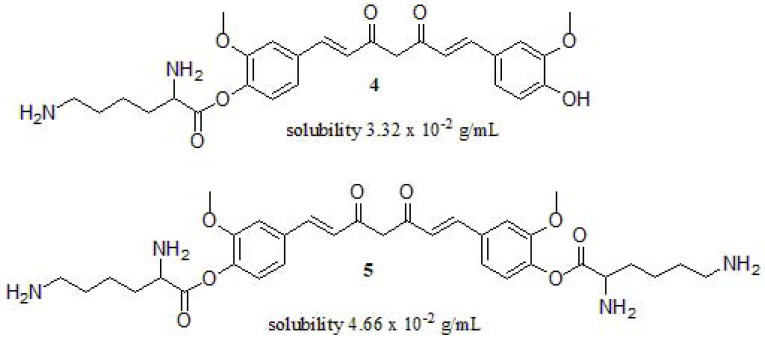
Due to the poor solubility and oral bioavailability of curcumin, scientists have seen the need to modify its structure to improve these deficiencies. In this way, Wang et al. [127] designed and synthesized L-lysine-functionalized curcumin derivatives to improve their water-solubility and inhibition of amyloid fibrillation in vitro, using Hen egg-white lysozyme (HEWL) as a model protein (Fig. 4). They used Nα-Fmoc-Nε-Boc-L-lysine as a novel water-soluble amino acid derivative. Compounds 4 and 5 exhibited enhanced solubility (3.32 x 10-2 g/mL and 4.66 x 10-2 g/mL, respectively) in water compared to curcumin (1-10 µg/mL) [128]. Moreover, these compounds showed amyloid fibrillation inhibition of HEWL when the concentration of 4 and 5 reach to 20.14 mM and 49.62 mM, respectively. In addition, the lag phase duration of 4 (e.g., 7.3 days) is longer than 5 (e.g., 6.2 days), the authors attributed it to the phenolic hydroxyl group and the charged amino acid, concluding that it is an effective way to improve its solubility.
Fig. (4).
Water-soluble functionalized curcumin derivatives reported by Wang et al.
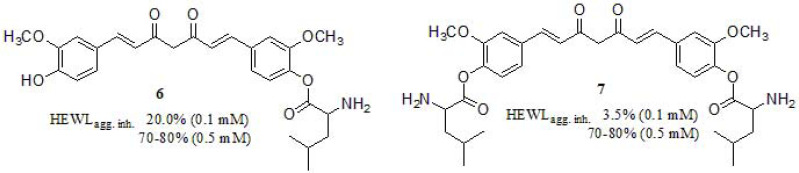
In a recent work, Cui et al. [129] studied and synthesized water-soluble curcumin derivatives based on Boc-L-isoleucine (Fig. 5). The inhibitory potency of the monosubstituted derivative 6 on the formation of HEWL amyloid fibrils was superior to the disubstituted counterpart 7 at low concentration, suggesting the importance of the free hydroxyl group in the aromatic ring (20% and 3.5% at 0.1 mM; both reached to 70-80% at 0.5 mM). Regarding the solubility profile, both derivatives exhibited enhanced solubility 3.05 mg/mL and 2.12 mg/mL in water respect to curcumin [128]. It is worth mentioning that both derivatives displayed low cytotoxicity in HeLa cell line, above 70% viability at 10-50 µM.
Fig. (5).
Functionalized curcumin derivatives described by Cui and coworkers.
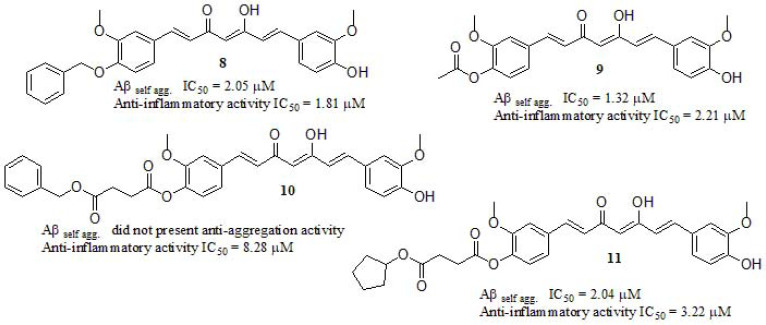
Many authors proposed that the intractable nature of the Aβ plaques and tangles stimulates a chronic inflammatory reaction to clear this debris [22, 130-139]. These plaques depositions in the brain stimulate an inflammatory response generating the overexpression of proinflammatory mediators, such as the neuroinflammatory interleukin [140], playing a key role in inflammatory and anti-inflammatory processes in AD. Interleukin-6 (IL-6) is a soluble mediator with a pleiotropic effect on inflammation, immune response, and hematopoiesis [141-144]. Inhibition of IL-6 secretion is frequently used as a readout of anti-inflammatory activity. In this line, Lakey-Beitia et al. [140] reported new curcumin derivatives synthesized by etherification, and esterification of curcumin and benzyl bromide, acetyl chloride, 4-(benzyloxy)-4-oxobutanoic acid, and 4-(cyclopentyloxy)-4-oxobutanoic acid, displaying anti-aggregation capabilities and anti-inflammatory activity (Fig. 6). In order to evaluate the IL-6 production, lipopolysaccharide-stimulated macrophages were used. Compounds 8, 9, and 11 exhibited more potent anti-inflammatory activity compared to curcumin (IC50 = 8.25 µM), while compound 10 displayed a similar effect. The introduction of a benzyl moiety liked through an ether bond in one of the curcumin rings (8) led to the most potent anti-inflammatory derivative, but the presence of a bulky diester group was conducted to less active derivatives 10 and 11. They concluded that hydroxyl groups on the aromatic rings of the curcumin were the pharmacophore required to diminish the IL-6 production. Regarding the anti-aggregation profile in vitro, compounds 8, 9, and 11 inhibited the Aβ aggregation in a concentration-dependent manner, with IC50 values ranging from 1.32 to 2.05 µM, showing an amyloid anti-aggregation effect in the same magnitude as the standard curcumin (IC50 = 1.4 µM) but, surprisingly compound 10 did not present anti-aggregation activity.
Fig. (6).
Curcumin derivatives reported by Lakey-Beitia et al.
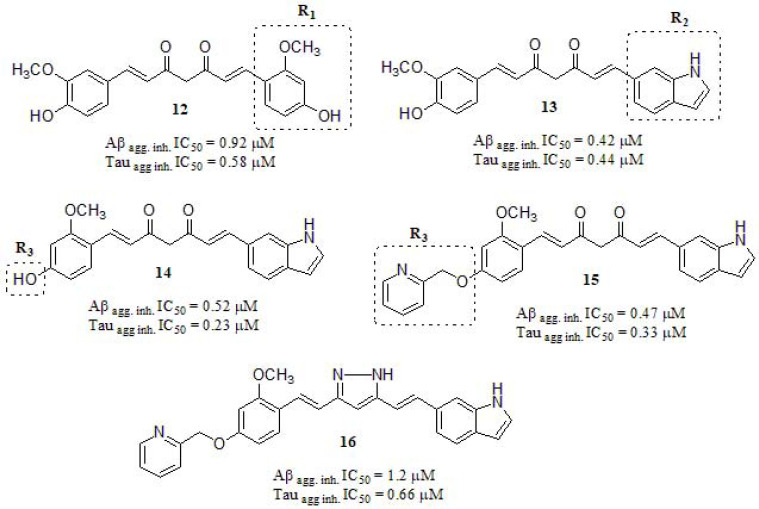
To delve into this concept, Okuda et al. [145] designed a series of asymmetric curcumin derivatives by different strategies, and the most active compounds are summarized in (Fig. 7). Firstly, a compound series were synthesized by changing the hydroxyl and methoxyl substitution pattern on one of the aromatic moieties (12), showing that the inhibitory activity on Aβ aggregation increased when these substituents were located in meta position to each other, displaying higher inhibitory activity compared to curcumin (IC50 = 5.4 µM). Next, by only exchanging one aromatic ring for other cyclic structures, curcumin derivative 13 was achieved with interesting results, suggesting that a bicyclic structure may increase the inhibitory activity, especially in Tau aggregation. Combining the aforementioned results, compound 14 was designed and synthesized. Taking into account that in animal models [146-150], curcumin undergoes rapid metabolic reduction and conjugation, resulting in poor systematic bioavailability after oral administration [151], they introduced various residues in order to protect the residual phenolic hydroxyl group (14) from being metabolized, although just one compound (15) exerted comparable inhibitory activity to 14. Additional performed experiments were related to obtaining the pharmacokinetic profile. Each compound was orally administered to rats at 50 mg/kg. The Cmax of 15 was found to be 20-fold lower than that of curcumin (5.7 ± 3.3 ng/mL at 30 min and 125 ± 65 ng/mL at 15 min), but the concentration in the brain was 13-fold lower compared to curcumin itself. In order to achieve a more convenient pharmacokinetic profile, many structural changes were necessary. They modified the central diketone skeleton in 15 by introducing a pyrazole ring (16). Although the IC50 = 1.2 µM for Aβ aggregation and IC50 = 0.66 µM for Tau aggregation cannot be denoted as a stunning result, the concentration of 16 in the brain was 300-fold higher than that of 15 and 20-fold higher than that of curcumin.
Fig. (7).
Curcumin asymmetric derivatives as amyloid β and tau aggregation inhibitors.
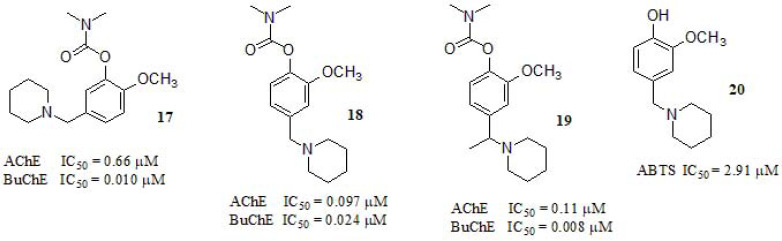
Li et al. [82] synthesized and evaluated MTDLs based on rivastigmine and curcumin hybrids. Rivastigmine demonstrated unique central selective towards AChE and BuChE inhibitory activity free of hepatic metabolism, while curcumin represents a neuro-protective agent, with a variety of functions (Fig. 8). Compound 18 was the most potent AChE inhibitor by 20-fold compared to the reference compound (rivastigmine, IC50 = 2.07 µM). Regarding the AChE inhibitory profile, the position of the aminoalkyl group in the benzene ring resulted crucial for the inhibition potency. While the derivate 17 displayed only moderate micromolar activity, shifting this group to the 4-position conducted to nanomolar IC50 values, as shown in (Fig. 8) (compounds 18 and 19). On the other hand, all compounds exerted good inhibitory activity regarding BuChE with compounds 17 and 19 showing 40-50-fold improvement respect to rivastigmine (BuChE IC50 = 0.37 µM). Aβ aggregation inhibitory profile of compounds 17, 18, and 19 were qualitatively evaluated by Transmission Electron Microscopy (TEM). As depicted in this work, after incubating Aβ1-40 along with the selectedmolecules, the reduction in Aβ1-40 deposition was evident, as only a few fibbers could be observed, which was similar to curcumin and indicated that all compounds were also endowed with potent Aβ anti-aggregation capabilities. Interestingly, the addition of rivastigmine had little effect on its aggregation. In a further assay designed to shed light on initial metabolism, compound 18 was incubated with rat cortex homogenate (AChE) and the extract was analyzed by HPLC-PS after 24 h, in which the prodrug activation process was confirmed by obtaining compound 20. This compound showed potent ABTS [2,20-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] radical cation scavenging capacity (IC50 = 2.91 µM) respect to melatonin control (IC50 = 1.92 µM), and moderate copper ion chelating activity in vitro.
Fig. (8).
Multi-target direct ligands based on rivastigmine and curcumin hybrids investigated by Li et al.
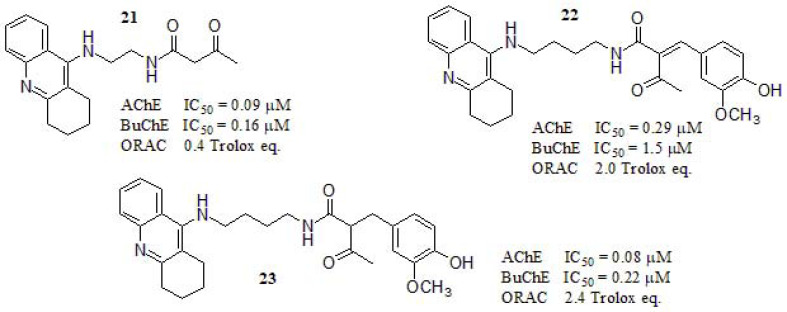
In 2017, Liu et al. [152] reported the synthesis and biological evaluation of tacrine-curcumin derivatives as MTDLs (Fig. 9) along with a deep molecular modeling study in order to rationalize their results. They evaluated in vitro the AChE and BuChE inhibition and the most active compounds were selected for further investigation. The AChE inhibitory activity of 21 and 23 was higher than the tacrine (IC50 = 0.10 µM). Compound 21 showed higher inhibitory activity against both enzymes compared to the other compounds. The authors attributed this result to the absence ofthe aromatic ring at the end of the side chain and the smaller structure, making it suitable for the accommodation into the catalytic gorge of AChE, since this is relatively narrow and with large steric hindrance. Regarding the composition of AChE and BuChE, they mainly differ in their amino acid composition at the mid gorge level. While AChE has several aromatic residues, those in BuChE are replaced by smaller aliphatic counterparts resulting in a larger pocket in the latter. The molecular modeling study of compound 21 showed interactions with Trp84 and Phe330 through a π- π staking due to the relatively small side chain on the tacrine derivative and could smoothly enter the catalytic active site (CAS) pocket. On the other hand, compound 23 has an aromatic ring at the end of the side chain, resulting in a stabilized interaction by hydrogen bonding between the carbonyl group and Tyr121 residue, so that the ligand can be perfectly located in the gorge of AChE with the benzene ring binding to CAS, and the tacrine moiety binding to the peripheral anionic site (PAS). It is, therefore, understandable why 23 presented the most potent activity in the AChE enzymatic assay. The antioxidant capabilities of 21, 22, and 23 were determined by ORAC, using curcumin and tacrine as positive and negative controls, respectively. Curcumin was a potent scavenger of peroxyl radical (3.1 trolox eq.). Compounds 22 and 23 showed potent ability to scavenge reactive oxygen species (2.0 and 2.4, respectively) while compound 21 exhibited a weak ROS scavenger profile (0.4) in the same order to tacrine (0.3).
Fig. (9).
Fusion of tacrine and curcumin as actives hybrids.
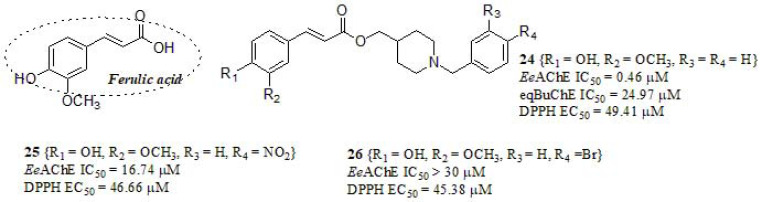
By taking advantage of the aforementioned properties of curcumin derivatives as MTDLs, Dias et al. [153] designed a series of compounds based on the combination of feruloyl subunit present in curcumin, and N-benzylpiperidine (a pharmacophoric subunit from DPZ) with the aim to obtain MTDLs as promising leads prototypes for AD (Fig. 10). Compounds were evaluated as EeAChE inhibitors, resulting in two active compounds (24, 25) in relation to curcumin as reference (EeAChE IC50 = 132.12 µM), even though still far from DPZ (EeAChE IC50 = 0.026 µM). On the other hand, compound 24 was also active in equine serum butyrylcholinesterase (eqBuChE), exhibiting a discrete value compared to standard DPZ (eqBuChE IC50 = 4.69 µM for DPZ) but more active than curcumin (eqBuChE IC50 > 300 µM for curcumin). The authors found in a substrate competition assay that compound 24 followed a non-competitive inhibition mechanism (complemented by molecular docking studies), and interestingly, they conclude that the substitutions on the aromatic ring of the N-benzylpiperidine lead to a decrease in the AChE activity independent of its ability to donating or withdrawing electrons, or its size. The antioxidant activity of compounds was evaluated in vitro by using the radical scavenging DPPH assay; compounds 24, 25, and 26 displayed antioxidant profile and were effective in scavenging free radicals compared to Ferulic acid, iso-ferulic acid, and trolox as standards (DPPH EC50 = 35.54 µM, > 100 µM, and 40.86 µM, respectively). It is worth mentioning that compounds bearing a ferulic acid moiety displayed 100-fold more potency in radical scavenging compared to its iso-ferulic acid counterparts, settling the evidence about the importance of curcuminoid framework as an antioxidant. The neurotoxic effects of compounds 24 and 25 were evaluated in SH-SY5Y cells and they showed the absence of cytotoxic and pro-oxidant effects, and authors suggest that the ferulic acid subunit contributes to counteract the ROS formation. The ability of compounds 24-26 to chelate biometals was studied by UV-Vis spectroscopy: all compounds were able to chelate only Cu+2 and Fe+2, but no Fe+3 and Zn+2. Taking these results into consideration, selected compounds were evaluated in vivo, and they showed that compound 24 displayed significant anti-inflammatory activity in different animal models, highlighting this compound as a potential multifunctional lead for AD treatment.
Fig. (10).
Feruloyl-donepezil hybrids as MTDLs synthesized by Dias et al.
3. RESVERATROL HYBRIDS
Resveratrol is a stilbene which contains two aromatic rings linked by an ethylene bridge. This compound exists in two geometric isomers, cis-(Z) and trans-(E), as shown in (Fig. 11). This compound is found in many vegetables such as peanuts, pistachios, grapes, red and white wine, blueberries, cranberries, and even cocoa and dark chocolate. Some of its studied biological properties include anti-cancer, anti-inflammatory, anti-aging, cardioprotective, antioxidant, chelating, and scavenging capability towards reactive ox-ygen species. Multiple studies detail the ability of resveratrol and its derivatives to inhibit amyloid β aggregation, although their underlying mechanism of action is not well understood [154]. The versatile function of these compounds in plant defense mechanisms as phytoalexins to fight fungal infection, ultraviolet radiation, stress, and injury confers them promising potential as pharmaceutical agents. This framework has attracted lots of interest in order to understand their biosynthetic pathways and their biological properties. One major limitation in the use of resveratrol as a therapeutic agent is associated with their inherent poor aqueous solubility and low bioavailability [155]. The studies of resveratrol and several other stilbenes in AD models suggest that stilbenes may be very effective modulators of AD development and progression, depending on their bioavailability and activity in vivo [156]. To solve the bioavailability and solubility concerns of resveratrol, several drug delivery systems have recently been developed, such as encapsulation in liposomal formulations [157-160], use of cyclodextrin complex as a drug carrier for enhanced binding to the protein [161-164], and solid lipid nanoparticles to enhance matrix-based delivery [165-168], among others [169].
Fig. (11).
Isomers of resveratrol.
Resveratrol is also associated with the activation of silent information regulator-1 (SIRT1), and it plays a critical role in neuronal protection as it regulates reactive oxygen species (ROS), nitric oxide (NO), proinflammatory cytokine production, and Aβ expression in AD patients brains [170]. SIRT1 was found to be essential for synaptic plasticity, cognitive functions [171-173], modulation of learning and memory function [174-176]. In a recent review, the importance of the neuroprotective role of resveratrol towards the activation of SIRT1 was discussed, even though the mechanisms of action are still unclear and the anti-inflammatory and antioxidant action of this molecule may be independent of SIRT1 [170]. The challenge of devising resveratrol derivatives is mainly focused on obtaining compounds with improved efficiency, low toxicity, better bioavailability, and solubility for developing more active drugs for clinical application [177].
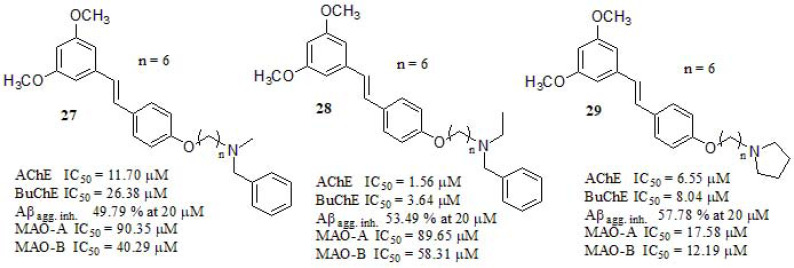
In a recent paper, Pan et al. [178] described the synthesis and evaluation of resveratrol-based compounds as MTDLs. Inhibitory activities against AChE and BuChE were tested along to tacrine and galantamine as reference standards (Fig. 12). Compounds 27, 28, and 29 displayed higher inhibitory activity against cholinesterases than resveratrol (AChE IC50 = 165.24 µM, BuChE IC50 = 752.46 µM), indicating that the introduction of amino group side chains may result in increasing the inhibitory capability of the target compounds. In their original contribution, the authors evaluated different chain lengths and found that a six-carbon linker between the trans-stilbene moiety and the amino group was the optimal length for biological activity. Besides, they explored different terminal amines resulting in compound 28 as the most potent (almost 8-fold more potent than 27), concluding that the methylene group could increase the lipophilicity leading to a rise in AChE inhibitory potency [179, 180]. Compound 28 was selected for kinetic measurements using Lineweaver-Burk plots, the authors found in the graphical representation of the steady-state for the inhibition of AChE that both slopes and intercepts were increased at increasing concentration of the inhibitor, concluding that compound 28 was a mixed-type inhibitor, which could bind to the CAS and the PAS sites of AChE. Besides, the inhibition of Aβ42 self-induced aggregation was compared with resveratrol (68.51% at 20 µM) and curcumin (52.21% at 20 µM) as reference compounds, while 28 and 29 displayed a similar inhibition profile. Compound 29 endowed with the terminal cyclic amine displayed stronger inhibitory activity compared to the open-chain amino derivative 28. Authors also pointed out that MAO-A inhibitory ability of compounds was not relevant and apparently lacked a structure-activity relationship, while their MAO-B inhibitory activity was relatively potent and could be related to the length of the alkyl chain, resulting in a n = 3 carbon spacer compound (data not shown) exerting the best MAO-B inhibition (IC50 = 5.01 µM). While compounds 27 and 28 did not display relevant activity against MAOs compared to iproniazid as reference (MAO-A IC50 = 6.58 µM; MAO-B IC50 = 7.82 µM). Eventually, compound 29 displayed significant inhibition towards both MAO-A and MAO-B at the same time, it showed no toxicity in the SH-SY5Y neuroblastoma cell line at 1-50 µM.
Fig. (12).
Resveratrol derivatives as MTDLs against AD.
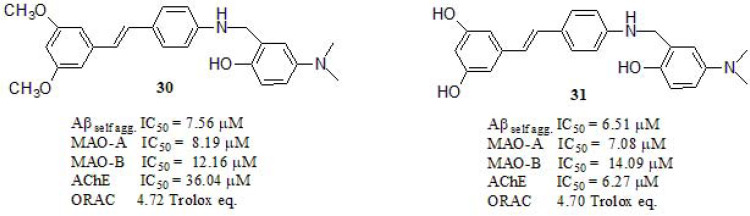
Considering that biometal (Fe, Cu, and Zn) ions may be crucial participants in pathological processes of AD, Lu et al. [181] combined resveratrol and clioquinol, a well-known metal chelator, to obtain a novel series of derivatives expected to behave as biometal chelators, antioxidants, and inhibitors of Aβ aggregation (Fig. 13). Compounds 30 (79.50% at 20 µM) and 31 (78.06% at 20 µM) exhibited stronger Aβ aggregation inhibition than curcumin (52.77% at 20 µM, IC50 = 12.35 µM) and resveratrol (69.73% at 20 µM, IC50 = 15.11 µM). Regarding the antioxidant activity, compounds 30 and 31 exhibited strong but lower antioxidant capacity compared to resveratrol (5.92 trolox eq.) as a reference compound. Metal-Chelating properties of compounds were studied by UV−vis spectroscopy and the ability of 30 and 31 to complex biometals such as Cu (II), Fe (II), Fe (III) and Zn (II) was measured. Their results indicated the formation of 30-Cu (II) and 31-Cu (II) complexes, with 3:1 and 1:1 stoichiometry, respectively. Moreover, the ability of these compounds to inhibit Cu (II)-induced Aβ aggregation was investigated by ThT fluorescence and TEM. In the presence of Cu (II) well-defined Aβ fibrils were observed, while fewer fibrils were present when compounds 30 and 31 were added to the samples, demonstrating its capabilities in disassembling the highly structured fibrils induced by Cu (II). The MAO inhibitory ability of compounds was evaluated using ladostigil as reference (MAO-B inhibitor, IC50 = 37.1 µM) and clorgyline (MAO-A irreversible and selective inhibitor, IC50 = 4.1 nM), and both displayed a strong balance in MAO inhibitory activity. Furthermore, compounds 30 and 31 exhibited moderate AChE inhibitory activity. Intracellular antioxidant activity was evaluated in the SH-SY5Y cell line, resulting in 30 and 31 activity more potent than Trolox, indicating that resveratrol derivatives have the potential to be efficient multifunctional agents. Finally, compound 30 was able to cross the blood-brain barrier in vitro and did not exhibit any acute toxicity in mice at doses of up to 2000 mg/kg.
Fig. (13).
Fusion of resveratrol and clioquinol as MTDLs.
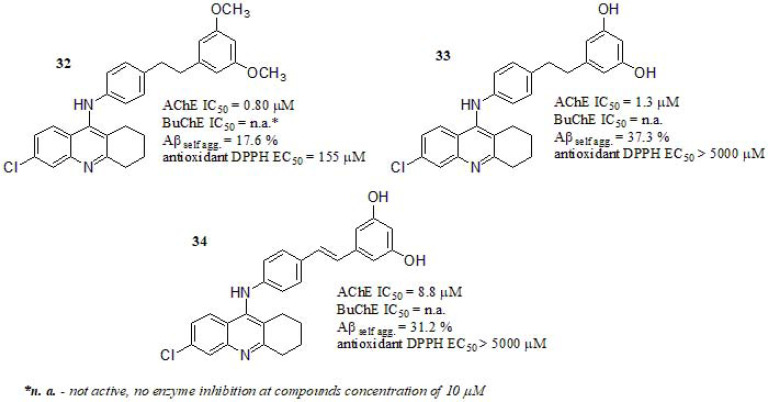
Jeřábek et al. [182] fused the cholinesterase inhibitor drug tacrine with resveratrol, designing a series of new MTDLs. All compounds carried a 6-chlorotacrine fragment connected to a resveratrol derivative moiety. Among other selected compounds (Fig. 14) only 32 and 33 showed significant AChE inhibitory activity compared to tacrine as reference (tacrine AChE IC50 = 0.5 µM; 6-chlorotacrine AChE IC50 = 0.07 µM; data taken from ref [183].). Compound 34, with a double bond, has a higher degree of structural rigidity in contrast with the other derivatives, displaying a weak AChE inhibition. Additionally, docking investigation revealed that chlorine in 6-position allows compounds to establish Van der Waals interactions in with AChE hydrophobic residues of the active site. Since chlorine can decrease the electron density on the aromatic ring in tacrine moiety, it favors π electron interaction with nearby residues [183, 184].All compounds evaluated in BuChE displayed no enzyme inhibition when tested at 10 μM. In this sense, compounds 33 and 34 were the most active inhibitors even though it was not possible to establish a correlation between the rigid fragment and the anti-amyloid properties, however, the presence of resorcinol ring (1,3-dihydroxybenzene) seems to be important for the possibility of establishing hydrogen-bond interactions. This is clearly seen in compound 32, endowed with a 2,4-dimethoxy substituent on the phenyl ring, exhibiting lower inhibitory activity on Aβ self-aggregation. However, compounds 33 and 34 with a resorcinol moiety displayed a similar Aβ42 inhibitory profile than resveratrol as reference (Aβ42 self-aggregation % inhibition = 30.0%). The antioxidant activity of compounds 32, 33, and 34 was assessed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) in an antioxidant assay, expressed as the concentration that causes a 50% decrease in the DPPH activity (EC50 values) with Trolox as a reference compound. Compounds 33 and 34 carrying free hydroxyl groups on the phenyl ring were detrimental to the free radical scavenging efficacy, nevertheless, derivative 32 with two methoxyl groups, showed reasonable antioxidant activity although lower than that of resveratrol (EC50 = 25.6 µM). A clear cytotoxic effect was evident for compound 32 when assessing cerebellar granule neurons of rat at 5 µM concentration, compound 33 showed neurotoxic only at the highest tested concentrations (25 and 50 µM). Compound 34 showed no clear neurotoxicity at all tested concentrations. Finally, the authors found general hepatotoxicity for all derivatives, attributing it to the presence of hepatotoxic tacrine fragment.
Fig. (14).
Resveratrol-Tacrine hybrids reported by Jeřábek et al.
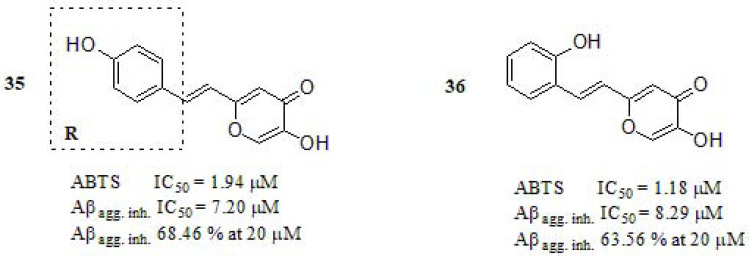
In 2018, a significant advance was conducted by Cheng et al. [185] reporting the synthesis and in vitro evaluation of hybrids merging maltol and resveratrol as MTDLs (Fig. 15) [186-188]. The ABTS radical scavenging method was used to determine the antioxidant capacity. Compounds 35 and 36 exhibited excellent antioxidant activity even higher than trolox (IC50 = 3.89 µM), showing that a modification in the substitution pattern of the benzene ring by fluoro, ethoxy, or methoxy resulted in a decrease of the antioxidant activity (compounds not included in this discussion). The Aβ1-42 self-aggregation inhibition profile of 35 and 36 resulted to be more potent than resveratrol and curcumin, used as positive controls (IC50=11.89 and 18.73 μM, respectively). Biometals (copper, iron, and zinc) were able to facilitate Aβ aggregation thought binding to three histidines (H6, H13, and H14) of the Aβ1-42 peptide [189]. The TEM experiment demonstrated a disaggregation of Aβ fibrils, indicating that compounds 35 and 36 can efficiently chelate Fe+3, Cu+2, and inhibit Fe+3/Cu+2 –induced Aβ aggregation.
Fig. (15).
Novel maltol-resveratrol hybrids as MTDLs reported by Cheng et al.
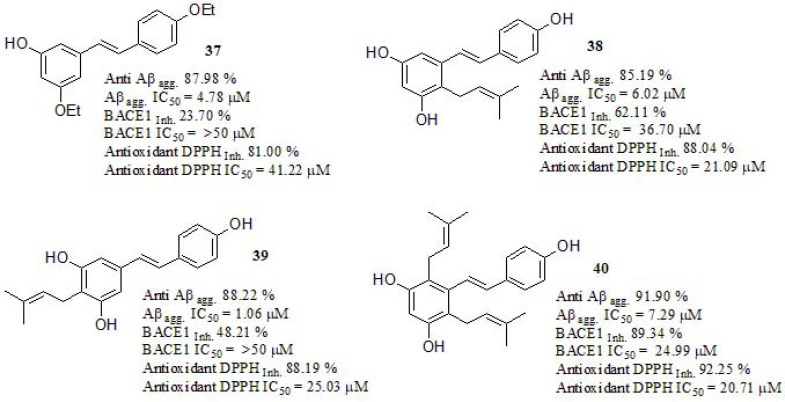
Synthesis and evaluation of prenylated resveratrol derivatives were recently discussed by Puksasook et al. [190] (Fig. 16). Prenylation consists of the addition of a hydrophobic prenyl chain, as a natural active moiety of a β-secretase (BACE1) inhibitor [191, 192]. The Aβ1-42 aggregation inhibition was evaluated using curcumin as a positive control (IC50 = 0.77 µM, Anti Aβagg. 87.98% at 100 µM). The best result was obtained from derivative 39, bearing a geranyl group at the C-4 position on the resorcinol ring, showing a similar effect than curcumin, followed by 37 bearing a prenyl group at the same position. Authors confirmed by molecular docking study that prenyl group at C-4 was less effective than a geranyl group at the same position, this may be due to the shorted alkyl side chain leading to less hydrophobic interactions. The inhibition of BACE1 was carried out using β-secretase inhibitor IV (Calbiochem®) as a reference compound (IC50 = 0.015 µM, β secretase inhibition 96.51% at 50 µM). Compound 40 was the most potent BACE1 inhibitor. The freeradical scavenging activity was evaluated using DPPH according to a modified version of the Brand-Williams method [193]. Compounds 38, 39, and 40 showed stronger activity than 37. Authors attributed this result to the free -OH groups that were essential for the antioxidant activity because these can donate hydrogen atoms and stabilize electrons by conjugation [194]. The IC50 values were compared to vitamin C (IC50 = 21.63 µM, antioxidant DPPH inhibition 95.78% at 100 µM) used as a positive control resulting in compound 40 as the most potent antioxidant. Neuronal viability assay was carried out using the P-19-derived neuron cell line. Compound 37 (>100% neuron viability at 1 nM to 10µM concentrations), promoted high viability of the cultured neurons, while compounds 38, 39, and 40 geranylated resveratrol derivatives showed stronger neurotoxicity at 1 nM (% viability 51.25 ± 13.12, 70.07 ± 36.33 and 34.17 ± 29.98, respectively). The prenyl substituent at the C-4 position in compound 37 might play an important role in neuronal viability. The neuroprotective ability of compound 37 was evaluated in a serum deprivation model using P-19 derived neurons cultured in a concentration of 1 nM and 10 µM. Compound 37 significantly protected the cultured neurons against serum deprivation at 50.59 ± 3.98 and 53.19 ± 12.48% viability, respectively (assumed ROS toxicity from serum-depravation induced oxidative stress), and it was more effective than resveratrol (37.41 ± 4.40% viability), and comparable to that of the quercetin positive control (58.04 ± 9.20% viability). Finally, the neuritogenic activity of compound 37 caused more branching numbers (9.33) than the control (2.12), and longer neurites (109.74 µM) than the positive control quercetin (104.33 µM).
Fig. (16).
Prenylated and geranylated resveratrol derivatives.
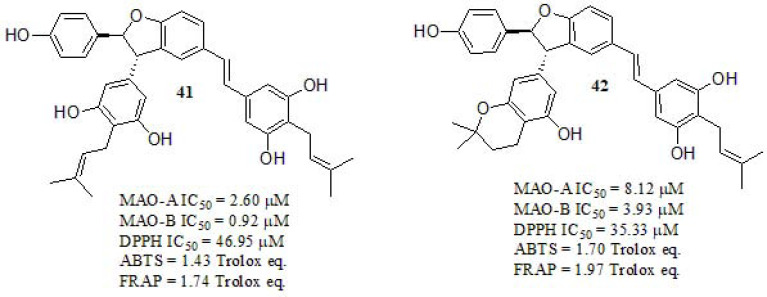
Tang et al. [195] designed and studied isoprenylated resveratrol dimers (Fig. 17). The inhibitory activities against MAOs were evaluated in vitro using p-tyramine as a nonselective substrate of MAO-A and MAO-B. Compounds 41 and 42 displayed enhanced inhibition towards MAO-B respect to the A isoform. In addition, in order to evaluate the antioxidant activities of those, three independent approaches were used: DPPH and ABTS radical scavenging methodsand Ferric ion reducing antioxidant power (FRAP) assay. DPPH radical scavenging revealed that compounds 41 and 42 are endowed with significant antioxidant activity in relation to Trolox (IC50 = 49.77µM). ABTS and FRAP antioxidant analysis showed a similar trend of free radical scavenging activity. Potential toxicity effects were evaluated in PC12 and BV2 cells. Compounds 41 and 42 were tested in their capacities of protecting PC12 cells against oxidative stress associated death by H2O2. The results showed that these compounds could significantly inhibit cell death at concentrations ranging from 6.25 to 25 μM. Both compounds exhibited very low toxicity in PC12 and BV2 cell lines. The neuroprotective effect was evaluated against oxidative injuries in PC12 cells by using oligomycin-A and rotenone as toxic lesions simulation [196-198]. Both compounds exerted relatively poor neuroprotective activity against rotenone-induced cell damage, while they showed moderate to high neuroprotective activity against oligomycin-A. As depicted in (Fig. 17), compound 41 displayed improved biological activities while its BBB crossing capabilities were enhanced respect to 42.
Fig. (17).
Isoprenylation-Resveratrol dimer derivatives described by Tang et al.
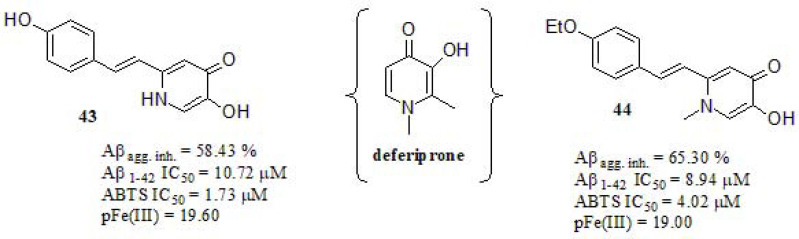
Xu et al. [199] integrated resveratrol and deferiprone (a known iron metal chelator) scaffolds in a novel series, with the aim of developing new MTDLs for AD. (Fig. 18). The Aβ self-induced aggregation inhibition profile was tested by using the ThT based fluorometric assay. Compound 44 displayed stronger Aβ inhibitory activity in relation to resveratrol and curcumin (64.08% and 56.44%, respectively), while compound 43 exhibited similar behavior. The antioxidant activity was determined by the ABTS radical scavenging method employing Trolox as a positive control. Compound 43 showed higher antioxidant activity in relation to the reference (IC50 = 3.89 µM), while compound 44 exerted a similar effect. As expected, compound 43 demonstrated improved antioxidant properties. The pFe(III) values were determined by fluorescence spectroscopy along to deferiprone, which was used as a reference compound [200]. Compounds 43 and 44 displayed closely related Fe(III) scavenging properties in relation to deferiprone (pFe(III) = 20.60).
Fig. (18).
Deferiprone-resveratrol hybrids.
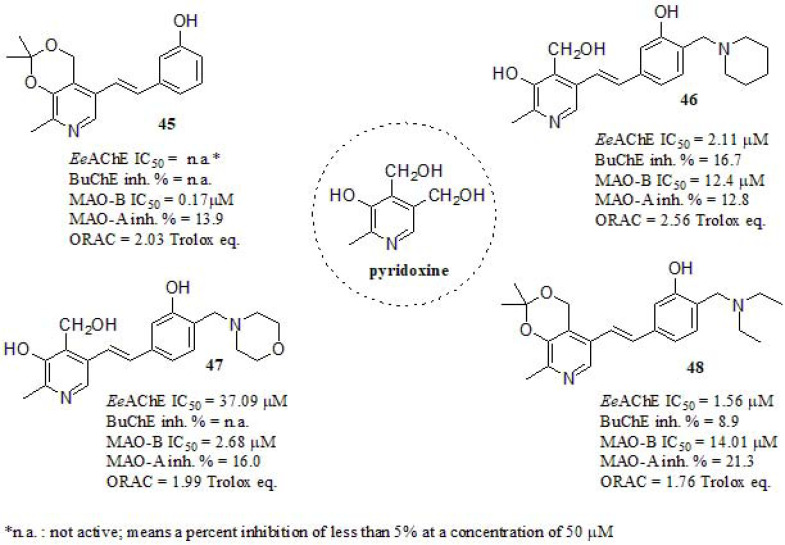
Yang et al. [201] investigated a series of pyridoxine-resveratrol hybrids by introducing Mannich base moieties. According to them, hybrids containing phenolic Mannich base moieties may exhibit good antioxidant properties [202], AChE inhibitory activity [203], and metal chelating properties [204]. Vitamin B6 (pyridoxine) has a critical function in cellular metabolism and stress response. Furthermore, it also behaves as a potent antioxidant that effectively quenches reactive oxygen species [205]. The inhibition of cholinesterases was evaluated in vitro using AChE from electrophorus electricus (EeAChE) and BuChE from rat serum (Fig. 19). Compound 45 was inactive as EeAChEi, while compound 48 displayed the strongest EeAChE inhibitory activity in the series even if lower than DPZ (IC50 = 23.0 nM). On the other hand, compound 46 bearing a piperidine unit showed stronger inhibition in EeAChE than the structurally related compound 47, differing only in oxygen in the morpholine moiety. In order to explore the mechanism of action of these hybrids, a kinetic study was carried out for compound 46, indicating a mixed-type inhibition and supporting a dual-site binding to both CAS and PAS of AChE. All compounds were inactive or weak as BuChE inhibitors. The MAOs inhibition activity were evaluated using clorgyline (MAO-B IC50 = 8.85 µM; MAO-A IC50 = 7.9 nM), rasagiline (MAO-B IC50 = 0.044 µM; MAO-A IC50 = 0.71 µM), and iproniazid (MAO-B IC50 = 4.32 µM; MAO-A IC50 = 1.37 µM) for comparative purposes. All tested compounds showed much stronger inhibitory activities towards MAO-B than MAO-A. The intermediate 45 showed the highest MAO-B inhibition activity, followed by 47, suggesting that the Mannich base moiety was detrimental for MAO-B inhibition (reminding us all the key importance of extending the biological assays to the intermediates). The antioxidant activity of those was evaluated by the ORAC fluorescein method. All compounds exhibited good ORAC values ranging from 1.76 – 2.56 compared to resveratrol (ORAC = 5.60 trolox eq.), also isopropylidene-protected derivative 48 showed slightly weaker antioxidant activity than 46, what could be related to the hydroxyl of lacking the latter.
Fig. (19).
Pyridoxine-Resveratrol hybrids Mannich base derivatives.
4. CHROMONE DERIVATIVES
Chromones are a group of oxygen-containing heterocyclic compounds (Fig. 20), widespread and naturally occu-rring. It represents an unusual group of structurally diverse secondary metabolites, derived from the convergence of multiple biosynthetic pathways that are widely distributed through the plant and animal kingdoms [206]. Chromone scaffold ((4H)-1-benzopyran-4-one) has also been extensively recognized as a key pharmacophore [207-214]. The chromone ring is the core fragment of several flavonoid derivatives, such as flavones and isoflavones [215]. The structural diversity of chromones in nature allows their division into simple and fused chromones. These heterocycles have attracted much attention because they show a variety of pharmacological properties such as anti-inflammatory effect [216, 217], analgesic [218, 219], metal chelating ability [220], antioxidant [221, 222], antimicrobial [223-225], antifungal [226, 227] and neuroprotective effects [228, 229], among others [230-232]. In recent years, many research groups optimized their chemical structure in order to develop new derivatives for the potential AD therapy, being the main hallmark related to its neuroprotective capability, cholinesterases (ChEs) inhibitory capabilities, MAO inhibition, and amyloid β aggregation inhibitory activities [228, 233]. Furthermore, Reis and coworkers [234] showed that chromone is a privileged scaffold for the development of novel MAO-B inhibitors, highlighting the effect of the substituent nature located at C3- and/or C6-positions of the benzopyrone ring. Otherwise, chromone derivatives have also been applied to the preparation of fluorescent probes due to its photochemical properties [214]. The chromone core is found in flavones and isoflavones and they are preferential scaffolds for the development of MAO inhibitors [235].
Fig. (20).
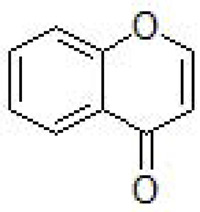
Chromone.
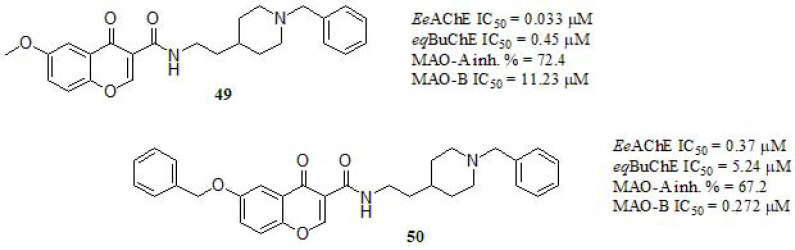
Li et al. [236, 237] reported the synthesis of tacrine-flavonoids hybrids as multifunctional ChEs inhibitors. Their results showed that the chromone framework contributes to the bioactivities of flavonoids hybrids. Based on such findings, in recent work, Wang et al. [238] reported a series of chromone-donepezil hybrids (Fig. 21) and their inhibitory activity against eqBuChE and EeAChE were evaluated. Compound 49, carrying a 6-methoxy substituent at the chromone moiety displayed the stronger inhibition, similar to DPZ in AChE but much stronger in BuChE (eqBuChE: IC50 = 2.47 μM, EeAChE: IC50 = 0.032 μM). Compound 50 exerted significant inhibitory activity to both ChEs even though the effect was less. Indeed, since the only difference between both is the OBn group, the steric drawbacks of the latter turns clear. Both compounds endowed with N-ethylcarboxamide linker between the benzylpiperidine and chromone moieties exhibited higher inhibitory activity than the others lacking this spacer (data not shown). Regarding the MAOs, the position and nature of the substituents resulted in a shift of its inhibitory profile. Compounds 49 and 50 showed weak inhibition of MAO-A. However, in MAO-B the inhibitory strength was directly related to the length of the alkylene chain. Compound 50 displayed higher MAO-B inhibitory potency respect to iproniazid (IC50 = 6.93 µM) and similar to pargyline (IC50 = 0.12 µM) as reference compounds. Compound 50 was selected for kinetic study to the inhibition of ChEs and MAO-B. Interestingly, a mixed-type inhibitory behavior was found in AChE while in BuChE, a competitive mechanism was pointed out. In addition, the kinetic profile of 50 towards MAO-B was compatible with competitive inhibition. Molecular modeling supported the aforementioned outcomes. Moreover, compound 50 could penetrate the BBB to target the enzyme in the CNS and showed low cell toxicity in rat pheochromocytoma (PC12) cells in vitro. These results shed light on these multifunctional agents that may contribute to the field of multitarget directed ligands for potential AD therapy.
Fig. (21).
Chromone and benzylpiperidine moieties of donepezil as multifunctional agents.
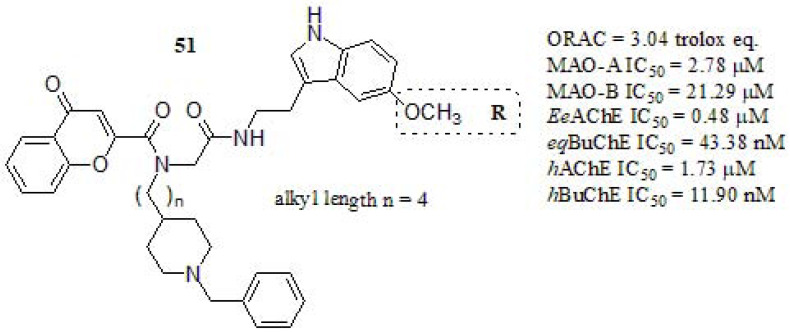
Pachón-Angona et al. [239] combined donepezil + chromone + melatonin as scaffolds, prepared by multicomponent reaction (MCR) synthetic strategy, transforming three or more starting material into new products in a one-pot procedure (Fig. 22) [240, 241]. In a first trial, the antioxidant behavior of such compounds was carried out by the ORAC-FL method. Ferulic acid and Melatonin were used as positive references (ORAC values of 3.74 [242] and 2.45 [242], Trolox eq. respectively). Compound 51 exhibited strong antioxidant power, higher than melatonin and similar to Ferulic acid. However, the other compounds with a linker length of n = 1,2 displayed more potent antioxidant capabilities than Ferulic acid (ORAC = 6.52 Trolox eq.; n = 2 and R = H). The MAO activity was evaluated in vitro, by using clorgyline and pargyline as references. Compound 51 showed moderate MAO-A inhibition, less active than clorgyline (IC50 = 0.05 µM), and lower MAO-B inhibitory activity compared to pargyline (IC50 = 0.08 µM). The ChEs inhibitory activity was evaluated for EeAChE and eqBuChE using DPZ and tacrine as references. Compound 51 showed strong eqBuChE inhibition, stronger than DPZ (IC50 = 840 nM) even though diminished respect to tacrine (IC50 = 5.1 nM). Regarding the structure-activity relationship, considering the same substituent, the most potent inhibitor was those with a n = 2 linker (IC50 = 6.29 nM, and R = OCH(CH3)2) while those with n = 3, and n = 4 displayed lower potency. On the other hand, 51 resulted a moderated AChE inhibitor. The most potent compounds were those bearing propoxy or isopropoxy substituents at the indole ring (IC50 = 0.08 µM and IC50 = 0.09 µM, respectively). Finally, in molecular docking simulation was noticed that in ChEs the chain ending in pyrrole and chromone ring were crucial for the binding to the active site of the enzyme. Furthermore, the MAO analysis revealed that the N-benzylpiperidine chain was a required feature to achieve good inhibitory profiles.
Fig. (22).
Donepezil + chromone + melatonin hybrids as multitarget agents for AD.
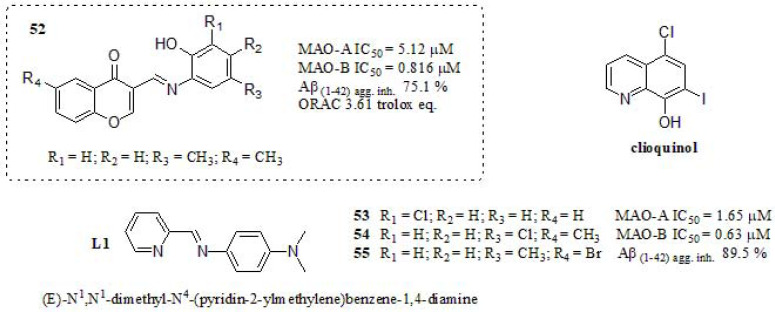
In 2017, Li et al. [229] described the synthesis of chromone derivatives combining the pharmacophore moiety L1, a previously reported to regulate metal-induced Aβ aggregation, ROS production, and neurotoxicity in vitro [243], and clioquinol (Fig. 23). The inhibitory activities against MAOs were measured and compared to those of rasagiline (MAO-A IC50 = 49.7 µM and MAO-B IC50 = 7.47 µM) and iproniazid (MAO-A IC50 = 6.46 µM and MAO-B IC50 = 7.98 µM). Compound 52 displayed strong inhibitory values as MAOs inhibitors. The nature of substituent and their position generated changes regarding the structure-activity relationship. The most potent and selective MAO-A inhibitor was compound 53 (IC50 = 1.65 µM, R1 = Cl, R2 = H, R3 = H, and R4 =H). Moreover, 54 (IC50 = 0.634 µM, R1 = H, R2 = H, R3 = Cl, and R4 = CH3) displayed the most potent inhibitory activity towards MAO-B. Compound 52 exhibited moderate Aβ aggregation inhibition even though stronger than curcumin and resveratrol (46.5% and 57.2%, respectively). The stronger Aβ aggregation inhibitor was compound 55, exhibiting 89.5% inhibition (R1 = H, R2 = H, R3 = CH3, and R4 = Br) even though it does not meet a multi-target feature. ThT binding assay and TEM were used to identify the degree of Aβ aggregation [244]. On the basis of the results, they concluded that compound 52 was capable of inhibiting Cu+2 induced Aβ aggregation, exhibiting significant antioxidant activity, metal chelation capabilities, H2O2-induced intracellular ROS accumulation reduction properties, and was able to cross the BBB (showed Pe values > 4.0). It is worth to mention that it did not show significant toxicity in PC12 cells, suggesting that further investigation and comprehension of this scaffold may achieve advancements in AD multitarget therapy.
Fig. (23).
Chromone derivatives reported by Li et al.
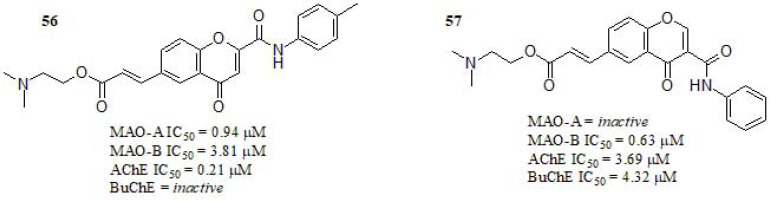
Reis et al. [234] reported a series of chromone 2- and 3-phenylcarboximide derivatives (Fig. 24). Regarding the inhibitory activity towards ChEs, compound 56 displayed submicromolar activity towards AChE and inactivity towards BuChE. Furthermore, compound 57 displayed bifunctional ChEs inhibitory activity in the low micromolar range, while compound 56 bearing a methyl group in the para position of the chromone exocyclic phenyl ring and two methyl group linked to the tertiary amine, also showed submicromolar MAO-A values and micromolar MAO-B values even though still far from clorgyline (IC50 = 0.0045 μM), and rasagiline (IC50 = 0.050 μM).
Fig. (24).
Chromone 2- and 3-phenylcarboximide derivatives.
Compared with previous works of this group regarding similar structures, this result was less remarkable in regard to MAO-B inhibitory activity [245-248]. Compound 57 carrying no substituent at the exocyclic ring, resulted inactive in MAO-A and while acted as a selective MAO-B inhibitor. A kinetic study was performed in both MAO-A and MAO-B. The results showed that 56 and 57 behave as competitive MAO inhibitors. The evaluation of the AChE inhibition mechanism of 56 showed a mixture of competitive and non-competitive mechanisms. Most promising chromones were screened towards human BACE-1, however, none of the compounds displayed relevant potency (IC50 > 10 μM). The cytotoxicity profile was evaluated in differentiated human neuroblastoma (SH-SY5Y) and human hepatocarcinoma (HepG2) cell lines, being both clinically relevant. Compound 57 presented a wider safety profile and promising safety margin.
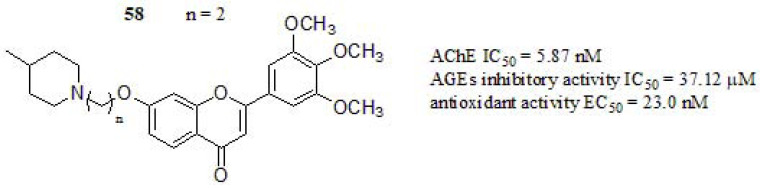
Starowicz et al. [249] studied the ability of various spices and herbs that are characteristic of European cuisineto inhibit the formation of advanced glycation end products (AGEs) and their antioxidant capacity. Glycation is defined as a reaction that leads to the formation of an irreversible structure called AGEs and a high concentration of those could initiate actions leading to various disorders, such as AD, atherosclerosis, diabetes, kidney disease, and chronic heart failure [250, 251]. The research group of Singh and coworkers [59] focused their efforts on the design and synthesis of chromen-4-one derivatives, making modifications at different positions of the “skeleton key” [252]. The inhibition towards AChE was determined and compound 58 (Fig. 25) exhibited the stronger inhibitory profile, higher than DPZ (IC50 = 12.7 nM) as standard. However, a further increase in carbon spacer (n = 6, 8) reduced the activity by 3-folds (IC50 range from 48.1 to 67.2 nM). Thus, n = 2 and n = 3 spacer chain length was optimal considering cyclic aminoalkyl groups for AChE inhibitory profile. Anti-glycation assay was performed according to the method reported by Matsuura et al. [253] with slight modifications. Compound 58 displayed significant inhibitory activity compared to aminoguanidine as reference drug (AGEs IC50 = 40.0 µM). Respect to in vitro antioxidant activity, compound 58 showed lower antioxidant activity compared to ascorbic acid (EC50 = 20.0 nM). Furthermore, the authors concluded that the conjugation system of chromen-4-one moiety appears to be crucial to their radical scavenging behavior. The kinetic study of compound 58 exhibited a mixed-type inhibition, which could bind with both CAS and PAS of the enzyme. Likewise, docking studies revealed the dual binding property as it interacted with both CAS as well as PAS via a hydrogen bond, π-π aromatic, and hydrophobic interactions, complementing the previous information.
Fig. (25).
MTDLs based on chromen-4-one reported by Singh et al.
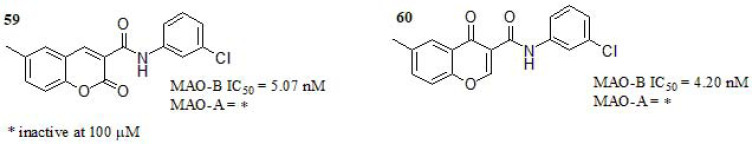
Coumarin and chromone are two structural isomers that exhibit relevant pharmacological activities [230, 254, 263, 255-262]. Fonseca and coworkers [264] performed a comparative study of coumarin- and chromone-3-phenyl carboxamide scaffolds and its structure-activity relationship (SAR) as MAOs inhibitors (Fig. 26). Firstly, the authors carried out a docking study of ligand-target recognition using the principal skeleton of both series of compounds. The binding modes analysis did not reveal significant differences in coumarin- and chromone- scaffolds. Consequently, the design of new derivatives was focused on i) the effects of the different substituents at the benzopyrone ring; ii) substituent position and its capabilities as electron-donating or withdrawing entities; iii) whether the position of the carbonyl group in the isomeric structures display some impact. Compounds 59 and 60 bearing a meta chlorine substituent at the benzamide portion showed stronger MAO-B inhibition compared to standard drugs deprenyl (IC50 = 16.73 nM) and safinamide (IC50 = 23.07 nM). The SAR analysis of remaining compounds (not presented here), bearing para substituents resulted in a decrease of activity, and the presence of a hydroxyl group either in meta or para position also resulted in activity decreasing. The position of the carbonyl group in coumarin or chromone moiety was apparently not relevant. Both compounds were inactive towards MAO-A. Eventually, the kinetic study of both compounds revealed a noncompetitive inhibition mechanism.
Fig. (26).
Coumarin versus chromone scaffold reported by Fonseca et al.
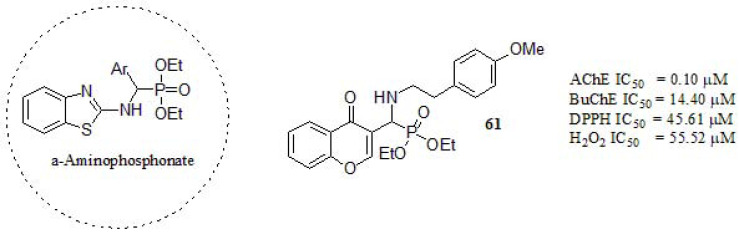
In a recent article, Shaikh et al. [265] designed a series of chromone-derived aminophosphonates in a one-pot reaction, catalyzed by porcine pancreatic lipase under solvent-free conditions. The α-aminophosphonates are a class of compounds with promising biological and pharmacologicalimportance as anti-AD agents [266]. Compound 61 (Fig. 27) was the most potent AChE inhibitor compared to tacrine (IC50 = 0.29 µM), galantamine (IC50 = 3.64 µM) and rivastigmine (IC50 = 5.21 µM), showing higher activity towards AChE than BuChE. As an important observation regarding ChEs inhibitory activity, aliphatic amines displayed a stronger inhibitory profile towards AChE, while aromatic ones showed better performance in BuChE inhibition. The kinetic study of ChEs revealed a mixed-type inhibition, which is in agreement with the molecular docking results [237]. Besides, the antioxidant activity was evaluated against DPPH and hydrogen peroxide scavenging method. Compound 61 exhibited the greatest radical elimination and high scavenging activity comparable to Ascorbic acid (DPPH IC50 = 42.28 µM; H2O2 IC50 = 51.45 µM). Finally, 61 showed significant DNA damage protection activity.
Fig. (27).
α-Aminophosphonate -functionalized chromone as MTDLs.
5. INDOLE DERIVATIVES
Indole is a planar heterocyclic molecule in which a benzene ring is fused to a pyrrole ring through 2 and 3 positions of the latter (Fig. 28). Due to the delocalization of π- electrons, it undergoes electrophilic substitution reactions, being a widely used chemical scaffold in medicinal chemistry. Its relevance in biological systems relies on being built into proteins through the indolic amino acid tryptophan [267]. Thus, indole moiety is considered a biologically accepted pharmacophore in medical compounds [268, 269].
Fig. (28).
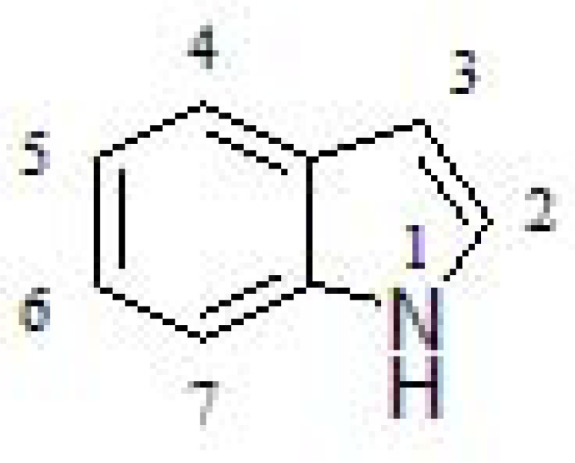
Indole structure.
Indole is a prominent phytoconstituent across various plant species and is produced by a variety of bacteria [270]. The indole-derived phytoconstituents and bacterial metabolites are a result of biosynthesis via the coupling of tryptophan with other amino acids. For this reason, it is a constituent of flower perfumes, pharmacologically active indole alkaloids, and some animal hormones or neurotransmitters such as serotonin [271] and melatonin [272]. Some naturally occurring indole alkaloids have gained FDA approval, including vincristine, vinblastine, vinorelbine, and vindesine for its anti-tumor activity [273, 274], ajmaline for its anti-arrhythmic activity [275-277], and physostigmine for glaucoma [278]. Taking inspiration from these natural compounds, several synthetic drugs were synthesized having reached the patient's bedside, such as indomethacin (NSAID) [279], ondansetron (chemotherapy-induced nausea and vomiting) [280], fluvastatin (hypercholesterolemia) [281], zafirlukast (leukotriene receptor antagonist) [282], etc. The success of the above-mentioned compounds indicates the importance of the ring system in multi-disciplinary fields, including the pharmaceutical and agrochemical industry.
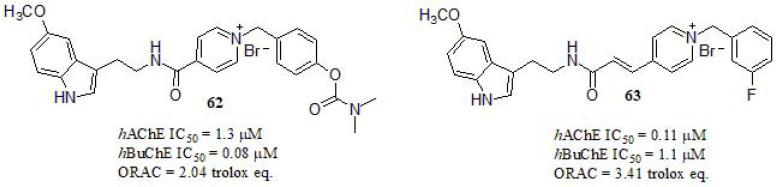
Luo et al. [283] reported the synthesis of multifunctional hybrids based on melatonin-benzylpyridinium bromides (Fig. 29) and their cholinergic activities were evaluated. The most promising derivative was compound 63, showing significant inhibitory activity in AChE even though 10-fold lower than DPZ as reference compound (IC50 = 0.014 µM). Otherwise, hybrid 62 exhibited a stronger inhibitory activity to BuChE, resulting in 70-fold higher than DPZ (IC50 = 5.6 µM). The authors highlighted the relevance of substituentsat the main moieties, as different substitutions with varied electronic properties showed a little fluctuation on the inhibitory activity, except for the introduction of -cyano group at -para position in the benzylpyridinium moiety (AChE: IC50 = 22.9 µM; BuChE: IC50 >100 µM). On the other hand, regarding the indole moiety, 5-methoxy substituent had no influence on the inhibitory activity of ChEs compared to the corresponding unsubstituted hybrid. The evaluation of the antioxidant activity was carried out by using oxygen radical absorbance capacity by fluorescence (ORAC-FL) method [284]. Melatonin, an endogenous neurohormone with strong antioxidant properties [285], was tested as reference (2.34 trolox eq.), and compound 62 exhibited a comparable activity. Compound 63, with an extra double bond within the spacer of both moieties, showed the most potent antioxidant activity. Furthermore, derivatives bearing such 5-methoxy group displayed enhanced activity respect to the unsubstituted one. A kinetic study was performed for compound 63. In AChE, the Lineweaver-Burk plots indicated a mixed-type inhibition, which suggested that compound 63 could be able to interact with CAS and PAS of AChE. A different behavior was obtained for BuChE, showing different Km and Vmax at different concentrations; in this case, compound 63 might act as a competitive inhibitor of the BuChE isozymes. Cell viability and neuroprotection studies were assayed in the human neuroblastoma cell line SH-SY5Y. MTT assay was used to examine the potential cytotoxic effects with no toxicity displayed for 62 and 63 at the range of concentrations studied (1-50 μM). Furthermore, both compounds were tested for their capacity to protect human SH-SY5Y cells against oxidative stress-associated death induced by H2O2. Compounds 62 and 63 showed neuroprotective effects at concentrations ranging from 1 to 10 µM. While compound 62 showed higher protective capability in comparison with the reference melatonin (at 10 µM).
Fig. (29).
Melatonin-benzyl pyridinium bromides derivates synthesized by Luo et al.
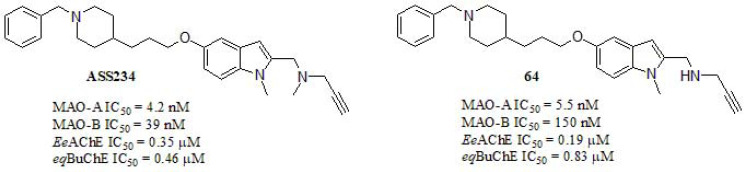
In addition to the aforementioned, another target that could play significant roles in the pathophysiology of the neurological diseases correspond to monoamine oxidase inhibitors (IMAOs). MAO is the enzyme that catalyzes the oxidative deamination of a variety of biogenic and xenobiotic amines [286], due to alterations in other neurotransmitter systems, especially serotoninergic and dopaminergic, which are also thought to be related to many behavioral disturbances observed in AD patients [287]. In this line, Bautista-Aguilera et al. [288] described the synthesis and pharmacological evaluation of novel hybrids designed through a combination of the previously reported [287] N-[(5-benzyloxy-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn- 1-amine and 1-benzylpiperidine, fragment present in DPZ (Fig. 30). Among the synthesized hybrids, the most promising derivative exhibited potent and moderated values as both MAOs enzymes and ChEs inhibitors, respectively. Compound 64 resulted to be the stronger MAO-A and MAO-B inhibitor compared to DPZ (MAO-A IC50 = 850 µM; MAO-B IC50 = 15 µM) and as well as and BuChE inhibitor in relation to the same reference (EeAChE IC50: 0.013 µM; eqBuChE IC50: 0.84 µM). The resulting pharmacological evaluation indicated the 1-benzylpiperidin-4-yl unit plays a key role in the AChE inhibitory activity, suggesting that this moiety mediates the binding to the enzyme. According to the design, the results showed that the linker length did not seem to be a decisive factor for the inhibitory potency against ChEs, whereas it seems to have a relevant effect in MAOs. Otherwise, the replacement of piperidine for bioisostere piperazine had a drastic reduction in the inhibitory activity, resulting in inactive compounds for ChEs (data not shown). A number of dual binding site AChE inhibitors have been found to exhibit a significant inhibitory activity on Aβ self-aggregation, thus compound 64 exhibits a significant inhibitory effect of Aβ-self-induced aggregation and human AChE-dependent aggregation, being more potent (human AChE-dependent) than the parent compound DPZ. The inhibition values of Aβ inhibition for compound 64 were 47.8% self-induced and 32.4% AChE-induced. This behavior may be explained through the kinetic study that exhibited a mixed-type inhibition. Molecular modeling suggests that 64 mimics the binding mode of DPZ in the crystal structure of AChE.
Fig. (30).
MTDLs based on donepezil and indole scaffolds reported by Bautista-Aguilera et al.
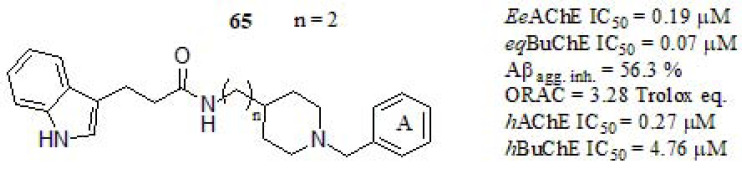
Several studies have documented the key activity of melatonin in scavenging a variety of reactive oxygen species, and moderate inhibition of Aβ aggregation affecting the synthesis and maturation of APP [289], which play an important role in AD. In this line, Wang et al. [290] described the synthesis and biological evaluation of donepezil-melatonin derivatives (Fig. 31), focused on taking advantage of the potential neurogenic profile of melatonin-based hybrids, which are endowed with additional anticholinergic properties. The activity of compound 65 against EeAChE showed a significant inhibitory profile, higher than tacrine (IC50 = 0.23 µM), although lower than DPZ (IC50 = 0.04 µM). Furthermore 65 showed a strong eqBuChE inhibition respect to donepezil (IC50 = 3.36 µM) and similar to tacrine (IC50 = 0.05 µM). According to the mentioned results, a modification in the indole ring with a methoxyl group showed a higher inhibitory potency than the compound without substituent (data not shown). Besides, the effect of the alkyl linker length influences the observed activities (n in (Fig. 30). Kinetic analysis and molecular modeling studies revealed that compound 65 acted as a mixed-type AChE inhibitor, binding simultaneous CAS and PAS of the enzyme. The inhibition of Aβ1-42 self-aggregation of 65 was improved respect to curcumin (45.2% at 20 µM) and resveratrol (43.5% at 20 µM). For the remaining compounds (not considered in this discussion), the effect of an electron-donating group at the benzene ring (A) might not be favorable for Aβ1-42 aggregation inhibition. Likewise, compound 65 exhibited significant antioxidant activity by ORAC assay respect to melatonin (2.3 trolox eq.), it may chelate metal ions, reduce oxygen stress induced PC12 cell death, and penetrate the BBB.
Fig. (31).
Donepezil-melatonin derivatives reported by Wang et al.
Several studies reported that Phosphodiesterase’s (PDE) inhibitors, such as sildenafil [291], tadalafil [292], and icariin [293], also displayed potent anti-AD effects in different mouse models of AD, significantly reversing cognitive impairment and improving learning and memory [294]. To illustrate this, Puzzo et al. [295] reported that sildenafil was beneficial against a mouse model of amyloid deposition, given that it produced amelioration of synaptic function and memory associated with a reduction of Aβ levels. In 2012, García-Osta et al. [296] revised Phosphodiesterase 5 (PDE5) inhibitors properties, and could act via anti-amyloid mechanisms, exhibit good BBB penetration, decrease p-Tau levels, shed light in their pharmacokinetics, safety and efficacy in vivo in animal models, but highlighted the lack of clinical trials in AD patients. Furthermore, Fiorito and coworkers [297] proposed PDE5 inhibitors as promising therapeutic agents for the treatment of AD. They synthesized quinoline derivatives with prominent outcomes in PDE5 inhibition and promising result in an in vivo mouse model of AD. In addition, Prickaerts et al. [298] carried out a study of rats in the object recognition task, suggested that PDE5 inhibitors improve processes of consolidation of object information, while AChE inhibitors improve processes of consolidation of object information. Therefore, AChE/PDE5 dual inhibitors could play a synergistic anti-AD effect and may supply a new perspective and breakthrough for the treatment of AD [294].
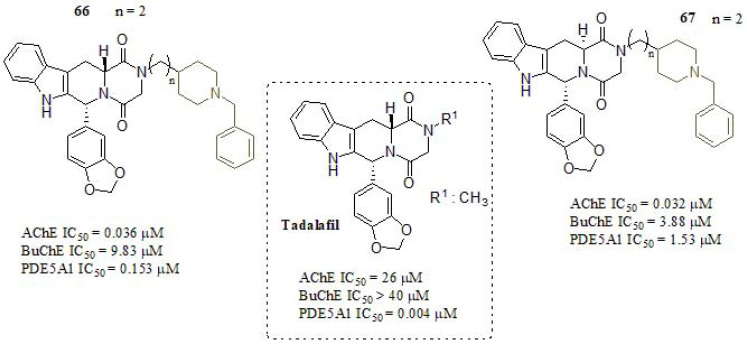
According to the aforementioned information, Mao et al. [294] described a series of novel tadalafil derivatives in order to seek dual-target AChE/PDE5 inhibitors as candidate drugs for potential AD therapy. The design of such derivates was based in PDE5 inhibitory activity presented in the tadalafil scaffold, by only varying the different substituent attached at the N-atom of piperazine-2,5-dione, incorporating different moieties such as morpholine, benzylpyridine, dimethylamine, benzylamine, and benzylpiperidine derivates. These results showed that the substituents in the R1 group (Fig. 32) and absolute configuration (R, R) remarkably affected the AChE inhibitory activities. Compounds 66 and 67 exhibited the strongest AChE inhibitory values, with nanomolar IC50 values. The results showed that the chain length (n= 2) between both moieties, tadalafil, and 1-benzylpiperidine, played a pivotal role in the AChE activities, so the optimal chain length was established as two methylenes (n = 2). Furthermore, the influence of stereochemistry on AChE inhibition was considered a key factor. The diastereoisomers 66 and 67 showed almost the same AChE inhibitory activity, comparable in potency to DPZ and huperzine A (IC50 = 0.013 µM and IC50 = 0.084 µM, respectively). However, both derivatives exhibited weak BuChE inhibitory activity. PDE5 inhibitory activity was determined by an IMAP-FP (immobilized metal ion affinity-based fluorescence polarization) assay [299, 300].The results showed that most of the tested compounds presented values ranging between 0.032 - 23.20 µM. In this context, the chain length presented no obvious influence on PDE5 type PDE5A1 inhibition. Moreover, compounds bearing aryl methyl and pyridyl substituents at piperidine nitrogen exhibited higher inhibitory activity than unsubstituted ones. Finally, 66 and 67, exhibited good to moderate PDE5A1 inhibitory activity respect to the other derivatives studied. Besides, the BBB crossing capabilities, 7.67 and 9.25 Pe (10-6 cm s-1) respectively, indicated that both compounds could be considered as potential dual-target AChE/PDE5 inhibitors.
Fig. (32).
Tadalafil derivates as AChE/PDE5 dual inhibitors.
The serotonergic system has been widely studied and well documented related to AD progression [301]. The modulation of 5-HT4 and 5-HT6 receptors have been recently proved to enhance cognition in AD models [302]. 5-HT4 receptors (5-HT4R) control brain functions, such as learning, memory, feeding, and mood behavior. In the AD context, activation of 5-HT4R can promote the nonamyloidogenic cleavage (APP), leading to the formation of a neurotrophic protein, sAPPa [303-305]. On the other hand, 5-HT6 receptors (5-HT6R) play a role in functions like motor control, cognition, and memory [302].
A new proposal for combining 5HT4 affinity along with nanomolar AChE inhibition was reported by Lecoutey et al. [105] in 2014 with the design and synthesis of donecopride (Fig. 33). RS67333 [306] is a potent 5HT4 antagonist that had been investigated as a potential antidepressant [307], nootropic [308], and as a potential treatment of AD [308]. Interestingly, RS67333 was also established as a low micromolar AChE inhibitor by the aforementioned authors [105]. This finding led them to pharmacomodulate it in order to enhance AChE inhibition profile with no significant effect on 5HT4 antagonism, while micromolar BuChE inhibition was also achieved (AChE IC50 = 16.0 nM; BuChE IC50 = 3.5 µM; 5HT4 Ki = 6.6 nM). Moreover, sAPPα increasing capabilities of donecopride were also demonstrated (EC50 = 11.3 nM) [105]. According to the authors, donecopride is able to exert not only a symptomatic effect but also a disease-modifying effect against AD. Among a wide number of tested molecules [309], donecopride was selected for studies in vivo, showing no effect on the spontaneous locomotor activity at the maximum dose of 10 mg/kg. At 0.3 and 1 mg/kg a precognitive effect with an improvement in memory performances was observed, along with an antiamnesic effect by scopolamine-induced spontaneous alternation deficit. Moreover, they also suggested a slight antidepressant effect by a decreased time of immobility during a forced swimming test. Later on, donecopride was found to display potent anti-amnesic properties in AD animal models, preserving learning capabilities, including working and long-term spatial memories. Clinical trials will soon be undertaken to confirm these findings in a First in Human study [310].
Fig. (33).
Donecopride, RS67333 and donepezil hybrid designed by Lecoutey et al.
Lalut et al. [305] designed a series of derivatives based on donecopride fine-tuning [105, 311] (Fig. 34). By replacing the benzene ring by an indole residue, they obtained MTDLs with enhanced biological activities. Compounds 68, 69, 70, and 71 were evaluated in their capacity to inhibit hAChE and to bind guinea pig (gp)5-HT4R. All compounds displayed a decreased affinity for 5-HT4R respect to donecopride (Ki = 9.5 nM) being 71, which showed the strongest inhibitory profile. The SAR analysis revealed that a cycloalkyl or an alkyl substituent on the piperidine ring improved the affinity for this receptor compared to N-benzyl ring. Besides, substituents (chloro and methoxy) present in the indole moiety, did not significantly influence the activity. For AChE inhibition compounds 69, 70, and 71 displayed low IC50 values, in the same order of donecopride (IC50 = 16 nM) and DPZ (IC50 = 6.0 nM). In this case, N-Bn substituent greatly increased AChE inhibition in relation to a cycloalkyl or alkyl substituents. While substitution pattern or nature at the indole moiety seems to have little influence on activity, N-substituents can dramatically decrease it. Concerning kinetic studies, compounds showed non – competitive inhibitions type, therefore interacting with PAS and anionic subsite of AChE. Finally, compound 69 displayed a protective effect against dizocilpine-induced impairment in the passive avoidance test in mice [312, 313].
Fig. (34).
Donecopride derivates as MTDL reported by Lalut et al.
Previous studies reported that C5-Substituted indole compounds containing a propyl spacer connected to different moieties such as piperazines and arylpiperazines, were endowed with serotoninergic activity [314-316]. Likewise, other studies have also reported AChE inhibitory activity in compounds containing these skeletons [104, 234, 317, 318]. Intending to combine such discoveries, Rodriguez-Lavado et al. [104] recently reported the synthesis, and in vitro evaluation of a new series of indolylpropyl benzamidopiperazines as promising MTDLs with dual activity against hSERT and hAChE (Fig. 35). Compounds 73 and 74 displayed an inhibitory profile in AChE in the same order of magnitude as DPZ (IC50 = 2.17 nM). The substituents R1 and R2 remarkably affected the inhibitory profile in AChE. In this sense: i) the unsubstituted compound (R1 = R2 = H) showed no inhibitory response; ii) almost all compounds with a methoxyl group at the 5-indolic position were inactive; iii) R1 = F or H resulted in a moderate to very active compounds depending on R2 substituent, as in the case of compounds 73 and 74. The authors thus explained how the appropriate substitution pattern can make a difference between inactive and very active compounds. On the other hand, compounds 72 and 75 showed a high affinity towards SERT, similar to citalopram (IC50 = 3.0 nM), both of them carrying R1 = F (a small and electron-withdraw atom) and R2 = 2-Br or 4-Br (bulky atom).
Fig. (35).
Indolylpropyl benzamidopiperazines derivates with AChE and SERT activities reported by Rodríguez-Lavado et al.
As expected, C5-Fluorine indole derivatives displayed nanomolar SERT affinity, being this an extensively reported property of fluorinated indoles [315, 319-321]. Interestingly, such fluorinated derivatives were also among the most active towards hAChE. None of the most active dual compounds resulted to be toxic at the studied concentration range in both HEK-293 and SH-5YSY cells. Molecular docking studies for both targets strongly supported the experimental results. Unfortunately, just one compound of the series resulted in significant β-amyloid self-aggregation inhibition (data not shown).
For clarity purposes, activities for some selected compounds endowed with promising multitarget capabilities are summarized in Table (1).
Table 1.
In Vitro Values for Selected MTDLs.
| Comp. | AChE IC50 (µM) | BuChE IC50 (µM) | MAO-A IC50 (µM) | MAO-B IC50 (µM) | ORAC (Trolox Equivalent) | Other Activity | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | 0.187 | 12.4 | n.d. | n.d. | 3.07 | Aβself agg. 45.3% | [126] |
| 2 | 0.228 | 9.78 | n.d. | n.d. | 2.49 | Aβself agg. 30.4% | [126] |
| 3 | 0.149 | 2.33 | n.d. | n.d. | 1.10 | Aβself agg. 22.0% | [126] |
| 21 | 0.09 | 0.16 | n.d. | n.d. | 0.4 | n.d. | [152] |
| 22 | 0.29 | 1.5 | n.d. | n.d. | 2.0 | n.d. | [152] |
| 23 | 0.08 | 0.22 | n.d. | n.d. | 2.4 | n.d. | [152] |
| 24 | 0.46a | 24.97b | n.d. | n.d. | n.d. | DPPH EC50 = 49.41 µM | [153] |
| 27 | 11.70 | 26.38 | 90.35 | 40.29 | n.d. | Aβself agg 49.8% | [178] |
| 28 | 1.56 | 3.64 | 89.65 | 58.31 | n.d. | Aβself agg 53.5% | [178] |
| 29 | 6.55 | 8.04 | 17.58 | 12.19 | n.d. | Aβself agg 57.8% | [178] |
| 30 | 36.04 | n.d. | 8.19 | 12.16 | 4.72 | Aβ IC50 = 7.56µM | [181] |
| 31 | 6.27 | n.d. | 7.08 | 14.09 | 4.70 | Aβ IC50 = 6.51µM | [181] |
| 46 | 2.11a | 16.7 | 12.8c | 12.4 | 2.56 | n.d. | [201] |
| 47 | 37.09a | n.a. | 16.0c | 2.68 | 1.99 | n.d. | [201] |
| 48 | 1.56a | 8.9 | 21.3c | 14.01 | 1.76 | n.d. | [201] |
| 49 | 0.033a | 0.45b | 72.4c | 11.23 | n.d. | n.d. | [238] |
| 50 | 0.37a | 5.24b | 67.2c | 0.272 | n.d. | n.d. | [238] |
| 51 | 1.73 0.48a | 1.19 x10-2 4.34 x10-2b | 2.78 | 21.29 | 3.04 | n.d. | [239] |
| 62 | 1.30 | 0.08 | n.d. | n.d. | 2.04 | n.d. | [283] |
| 63 | 0.11 | 1.1 | n.d. | n.d. | 3.41 | n.d. | [283] |
| 64 | 0.19a | 0.83b | 5.5 x10-3 | 150 x10-3 | n.d. | n.d. | [288] |
| 65 | 0.19a 0.27 | 0.07b 4.76 | n.d. | n.d. | 3.28 | Aβself agg 56.3% | [290] |
| 66 | 0.036 | 9.83 | n.d. | n.d. | n.d. | PDE5A1 IC50 = 0.15 µM | [294] |
| 67 | 0.032 | 3.88 | n.d. | n.d. | n.d. | PDE5A1 IC50 = 1.53 µM | [294] |
n.d.: non determined, n.a.: not active, a. Electrophorus electricus Ee, b. equine, c. MAO-A inhibition %.
CONCLUSION
AD is still an incurable disorder mainly due to its multifactorial nature and complex etiology. The more efforts are made by research groups and pharmaceutical companies to understand the underlying mechanism and find a disease- modifying treatment, the more AD-related targets are discovered. Therefore, there is no reason we can expect a solution provided by the ‘one drug-one target’ paradigm. Within the last years, the so-called multitarget paradigm has emerged to stay. In order to shed some light on the recent advances within this field, four biologically active scaffolds (curcumin-, resveratrol- chromone- and indole-) have been selected pointing to the simultaneous interaction towards many AD-related targets/functions, emphasizing on cholinesterases (AChE and BuChE), MAOs (MAO-A/B), 5-HT4, SERT, β-amyloid self-aggregation and radical scavenging activity. While many of them are well known AD-related targets, others have not still been so deeply explored. We sincerely hope that this review will help other researchers worldwide to develop future improvements within this exciting field since much more efforts are needed to make this multitarget approach evolve into new drugs that can eventually be used in clinical trials and finally reach the market for the overcoming of such devastating disease.
ACKNOWLEDGEMENTS
We thank Prof. Ricardo Tapia for his valuable contribution to this review. This work was supported by FONDECYT Postdoctoral Grant 3180602.
LIST OF ABBREVIATIONS
- WHO
World Health Organization
- AD
Alzheimer’s Disease
- CNS
Central Nervous System
- NMDA
N-methyl-D-aspartate
- MTDL
Multi-target Directed Ligands
- ACh
Acetylcholine
- ChAT
Choline Acetyltransferase
- AChE
Acetylcholinesterase
- Aβ
Amyloid beta
- APP
Amyloid Precursor Protein
- NFTs
Neurofibrillary Tangles
- MAO
Monoamine Oxidase
- SRRIs
Serotonin Reuptake Inhibitors
- 5-HT4R
5-HT4R 5-HT4 Receptor
- BBB
Blood-brain Barrier
- BuChE
Butyrylcholinesterase
- DPZ
Donepezil
- ORAC
Oxygen Radical Absorbance Capacity Assay
- HEWL
Hen Egg White Lysozyme
- IL-6
Interleukin-6
- TEM
Transmission Electron Microscopy
- ABTS
[2,20-Azinobis-(3-Ethylbenzothiazoline-6-sulfonic Acid)]
- CAS
Catalytic Active Site
- PAS
Peripheral Anionic Site
- SIRT1
Silent Information Regulator 1
- ROS
Reactive Oxygen Species
- NO
Nitric Oxide
- DPPH
2,2-Diphenyl-1-Picrylhydrazyl
- FRAP
Ferric ion Reducing Antioxidant Power
- EeAChE
Electrophorus Electricus AChE
- ChEs
Cholinesterases
- eqBuChE
Equine BuChE
- MCR
Multicomponent Reaction
- AGEs
Advanced Glycation End Products
- SAR
Structure-Activity Relationship
- NSAID
Indomethacin
- PDE
Phosphodiesterase’s
- PDE5
Phosphodiesterase 5
- IMAP-FP
Immobilized Metal ion Affinity-based Fluorescence Polarization
- GP
Guinea Pig.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Alzheimer’s Disease International. World Alzheimer Report 2019: Attitudes to Dementia. London: Alzheimer’s Disease International; 2019. [Google Scholar]
- 2.Reisberg B., Doody R., Stöffler A., Schmitt F., Ferris S., Möbius H.J. Memantine Study Group. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 3.Nussbaum R.L., Ellis C.E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 2003;348(14):1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 4.Francis P.T., Palmer A.M., Snape M., Wilcock G.K. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J. Neurol. Neurosurg. Psychiatry. 1999;66(2):137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dias K.S.T., Viegas C., Jr Multi-target directed drugs: a modern approach for design of new drugs for the treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2014;12(3):239–255. doi: 10.2174/1570159X1203140511153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridge P.G., Mukherjee S., Crane P.K., Kauwe J.S.K., Consortium A.D.G. Alzheimer’s disease genetics consortium. Alzheimer’s disease: analyzing the missing heritability. PLoS One. 2013;8(11):e79771–e79771. doi: 10.1371/journal.pone.0079771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oset-Gasque M.J., Marco-Contelles J. Alzheimer’s Disease, the “One-Molecule, one-target” paradigm, and the multitarget directed ligand approach. ACS Chem. Neurosci. 2018;9(3):401–403. doi: 10.1021/acschemneuro.8b00069. [DOI] [PubMed] [Google Scholar]
- 8.Panpalli A.M., Karaman Y., Guntekin S., Ergun M.A. Analysis of genetics and risk factors of Alzheimer’s Disease. Neuroscience. 2016;325:124–131. doi: 10.1016/j.neuroscience.2016.03.051. [DOI] [PubMed] [Google Scholar]
- 9.Baglietto-Vargas D., Shi J., Yaeger D.M., Ager R., LaFerla F.M. Diabetes and Alzheimer’s disease crosstalk. Neurosci. Biobehav. Rev. 2016;64:272–287. doi: 10.1016/j.neubiorev.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Li P., Hsiao I-T., Liu C-Y., Chen C-H., Huang S-Y., Yen T-C., Wu K-Y., Lin K-J. Beta-amyloid deposition in patients with major depressive disorder with differing levels of treatment resistance: a pilot study. EJNMMI Res. 2017;7(1):24. doi: 10.1186/s13550-017-0273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janelidze S., Stomrud E., Palmqvist S., Zetterberg H., van Westen D., Jeromin A., Song L., Hanlon D., Tan H.C.A., Baker D., Blennow K., Hansson O. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci. Rep. 2016;6:26801. doi: 10.1038/srep26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D., Ballard C., Banerjee S., Burns A., Cohen-Mansfield J., Cooper C., Fox N., Gitlin L.N., Howard R., Kales H.C., Larson E.B., Ritchie K., Rockwood K., Sampson E.L., Samus Q., Schneider L.S., Selbæk G., Teri L., Mukadam N. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 13.Masters C.L., Selkoe D.J. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2(6):a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGeer P.L., McGeer E.G. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126(4):479–497. doi: 10.1007/s00401-013-1177-7. [DOI] [PubMed] [Google Scholar]
- 15.Lee M., McGeer E., McGeer P.L. Activated human microglia stimulate neuroblastoma cells to upregulate production of beta amyloid protein and tau: implications for Alzheimer’s disease pathogenesis. Neurobiol. Aging. 2015;36(1):42–52. doi: 10.1016/j.neurobiolaging.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Blennow K., Mattsson N., Schöll M., Hansson O., Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol. Sci. 2015;36(5):297–309. doi: 10.1016/j.tips.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Wang J-Z., Xia Y-Y., Grundke-Iqbal I., Iqbal K. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimers Dis. 2013;33(Suppl. 1):S123–S139. doi: 10.3233/JAD-2012-129031. [DOI] [PubMed] [Google Scholar]
- 18.Spillantini M.G., Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013;12(6):609–622. doi: 10.1016/S1474-4422(13)70090-5. [DOI] [PubMed] [Google Scholar]
- 19.Pîrşcoveanu D.F.V., Pirici I., Tudorică V., Bălşeanu T.A., Albu V.C., Bondari S., Bumbea A.M., Pîrşcoveanu M. Tau protein in neurodegenerative diseases - a review. Rom. J. Morphol. Embryol. 2017;58(4):1141–1150. [PubMed] [Google Scholar]
- 20.Gao Y., Tan L., Yu J-T., Tan L. Tau in Alzheimer’s disease: mechanisms and therapeutic strategies. Curr. Alzheimer Res. 2018;15(3):283–300. doi: 10.2174/1567205014666170417111859. [DOI] [PubMed] [Google Scholar]
- 21.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 2006;12(9):1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 22.Heneka M.T., O’Banion M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007;184(1-2):69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Heppner F.L., Ransohoff R.M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16(6):358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 24.Su C., Zhao K., Xia H., Xu Y. Peripheral inflammatory biomarkers in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Psychogeriatrics. 2019;19(4):300–309. doi: 10.1111/psyg.12403. [DOI] [PubMed] [Google Scholar]
- 25.Yan M.H., Wang X., Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W-J., Zhang X., Chen W-W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016;4(5):519–522. doi: 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019;20(3):148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butterfield D.A., Mattson M.P. Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer’s disease. Neurobiol. Dis. 2020;138:104795. doi: 10.1016/j.nbd.2020.104795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohno M., Sametsky E.A., Younkin L.H., Oakley H., Younkin S.G., Citron M., Vassar R., Disterhoft J.F. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer’s disease. Neuron. 2004;41(1):27–33. doi: 10.1016/S0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 30.Chen X-Q., Mobley W.C. Exploring the Pathogenesis of Alzheimer Disease in basal forebrain cholinergic neurons: converging insights from alternative hypotheses. Front. Neurosci. 2019;13:446. doi: 10.3389/fnins.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peña-Bautista C., Flor L., López-Nogueroles M., García L., Ferrer I., Baquero M., Vento M., Cháfer-Pericás C. Plasma alterations in cholinergic and serotonergic systems in early Alzheimer Disease: Diagnosis utility. Clin. Chim. Acta. 2020;500:233–240. doi: 10.1016/j.cca.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Lorke D.E., Lu G., Cho E., Yew D.T. Serotonin 5-HT2A and 5-HT6 receptors in the prefrontal cortex of Alzheimer and normal aging patients. BMC Neurosci. 2006;7:36. doi: 10.1186/1471-2202-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoury R., Grysman N., Gold J., Patel K., Grossberg G.T. The role of 5 HT6-receptor antagonists in Alzheimer’s disease: an update. Expert Opin. Investig. Drugs. 2018;27(6):523–533. doi: 10.1080/13543784.2018.1483334. [DOI] [PubMed] [Google Scholar]
- 34.Demir E.A., Tutuk O., Dogan H., Tumer C. Depression in Alzheimer’s Disease: The roles of cholinergic and serotonergic systems. In: Wisniewski T., editor. Brisbane (AU): 2019. [PubMed] [Google Scholar]
- 35.Tarditi A., Caricasole A., Terstappen G. Therapeutic targets for Alzheimer’s disease. Expert Opin. Ther. Targets. 2009;13(5):551–567. doi: 10.1517/14728220902865614. [DOI] [PubMed] [Google Scholar]
- 36.Pulina M.V., Hopkins M., Haroutunian V., Greengard P., Bustos V. C99 selectively accumulates in vulnerable neurons in Alzheimer’s disease. Alzheimers Dement. 2020;16(2):273–282. doi: 10.1016/j.jalz.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Kwak S., Weiss J.H. Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr. Opin. Neurobiol. 2006;16(3):281–287. doi: 10.1016/j.conb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Fukumoto H., Cheung B.S., Hyman B.T., Irizarry M.C. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 2002;59(9):1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 39.Dingwall C. Spotlight on BACE: the secretases as targets for treatment in Alzheimer disease. J. Clin. Invest. 2001;108(9):1243–1246. doi: 10.1172/JCI14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 41.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S.T., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O’Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uliassi E., Prati F., Bongarzone S., Bolognesi M.L. 2017. 10 - Medicinal chemistry of hybrids for neurodegenerative diseases; Decker, M. B. T.-D. of H. M. for D. D., Ed; Elsevier, pp. 259–277. [Google Scholar]
- 44.Long J.M., Maloney B., Rogers J.T., Lahiri D.K. Novel upregulation of amyloid-β precursor protein (APP) by microRNA-346 via targeting of APP mRNA 5′-untranslated region: Implications in Alzheimer’s disease. Mol. Psychiatry. 2019;24(3):345–363. doi: 10.1038/s41380-018-0266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ambrée O., Richter H., Sachser N., Lewejohann L., Dere E., de Souza Silva M.A., Herring A., Keyvani K., Paulus W., Schäbitz W-R. Levodopa ameliorates learning and memory deficits in a murine model of Alzheimer’s disease. Neurobiol. Aging. 2009;30(8):1192–1204. doi: 10.1016/j.neurobiolaging.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Wu Z., Guo Z., Gearing M., Chen G. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s [corrected] disease model. Nat. Commun. 2014;5:4159. doi: 10.1038/ncomms5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haass C. Take five--BACE and the gamma-secretase quartet conduct Alzheimer’s amyloid beta-peptide generation. EMBO J. 2004;23(3):483–488. doi: 10.1038/sj.emboj.7600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishnaswamy S., Verdile G., Groth D., Kanyenda L., Martins R.N. The structure and function of Alzheimer’s gamma secretase enzyme complex. Crit. Rev. Clin. Lab. Sci. 2009;46(5-6):282–301. doi: 10.3109/10408360903335821. [DOI] [PubMed] [Google Scholar]
- 49.Lezoualc’h F. 5-HT4 receptor and Alzheimer’s disease: the amyloid connection. Exp. Neurol. 2007;205(2):325–329. doi: 10.1016/j.expneurol.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Alloza M., Hirst W.D., Chen C.P.L-H., Lasheras B., Francis P.T., Ramírez M.J. Differential involvement of 5-HT(1B/1D) and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer’s disease. Neuropsychopharmacology. 2004;29(2):410–416. doi: 10.1038/sj.npp.1300330. [DOI] [PubMed] [Google Scholar]
- 51.Mössner R., Schmitt A., Syagailo Y., Gerlach M., Riederer P., Lesch K.P. The serotonin transporter in Alzheimer’s and Parkinson’s disease. J. Neural Transm. Suppl. 2000;60(60):345–350. doi: 10.1007/978-3-7091-6301-6_24. [DOI] [PubMed] [Google Scholar]
- 52.Esteve P., Rueda-Carrasco J., Inés Mateo M., Martin-Bermejo M.J., Draffin J., Pereyra G., Sandonís Á., Crespo I., Moreno I., Aso E., Garcia-Esparcia P., Gomez-Tortosa E., Rábano A., Fortea J., Alcolea D., Lleo A., Heneka M.T., Valpuesta J.M., Esteban J.A., Ferrer I., Domínguez M., Bovolenta P. Elevated levels of Secreted-Frizzled-Related-Protein 1 contribute to Alzheimer’s disease pathogenesis. Nat. Neurosci. 2019;22(8):1258–1268. doi: 10.1038/s41593-019-0432-1. [DOI] [PubMed] [Google Scholar]
- 53.Kumar A., Tiwari A., Sharma A. Changing paradigm from one target one ligand towards multi-target directed ligand design for key drug targets of Alzheimer Disease: an important role of in silico methods in multi-target directed ligands design. Curr. Neuropharmacol. 2018;16(6):726–739. doi: 10.2174/1570159X16666180315141643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavalli A., Bolognesi M.L., Minarini A., Rosini M., Tumiatti V., Recanatini M., Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008;51(3):347–372. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- 55.León R., Garcia A.G., Marco-Contelles J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013;33(1):139–189. doi: 10.1002/med.20248. [DOI] [PubMed] [Google Scholar]
- 56.Rajasekhar K., Govindaraju T. Current progress, challenges and future prospects of diagnostic and therapeutic interventions in Alzheimer’s Disease. RSC Advances. 2018;8:23780–23804. doi: 10.1039/C8RA03620A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stanciu G.D., Luca A., Rusu R.N., Bild V., Beschea Chiriac S.I., Solcan C., Bild W., Ababei D.C. Alzheimer’s Disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules. 2019;10(1):10. doi: 10.3390/biom10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terry A. V, Buccafusco J. J. The Cholinergic hypothesis of age and alzheimer´s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 59.Singh M., Kaur M., Singh N., Silakari O. Exploration of multi-target potential of chromen-4-one based compounds in Alzheimer’s disease: Design, synthesis and biological evaluations. Bioorg. Med. Chem. 2017;25(24):6273–6285. doi: 10.1016/j.bmc.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 60.Ferreira-Vieira T.H., Guimaraes I.M., Silva F.R., Ribeiro F.M. Alzheimer’s disease: targeting the cholinergic system. Curr. Neuropharmacol. 2016;14(1):101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartus R.T., Dean R.L., III, Beer B., Lippa A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 62.Martorana A., Esposito Z., Koch G. Beyond the cholinergic hypothesis: do current drugs work in Alzheimer’s disease? CNS Neurosci. Ther. 2010;16(4):235–245. doi: 10.1111/j.1755-5949.2010.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Kant R., Goldstein L.S.B., Ossenkoppele R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020;21(1):21–35. doi: 10.1038/s41583-019-0240-3. [DOI] [PubMed] [Google Scholar]
- 64.Ricciarelli R., Fedele E. The amyloid cascade hypothesis in alzheimer’s disease: it’s time to change our mind. Curr. Neuropharmacol. 2017;15(6):926–935. doi: 10.2174/1570159X15666170116143743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murphy M.P., LeVine H., III Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010;19(1):311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andrew R.J., Kellett K.A.B., Thinakaran G., Hooper N.M. A greek tragedy: The growing complexity of alzheimer amyloid precursor protein proteolysis. J. Biol. Chem. 2016;291(37):19235–19244. doi: 10.1074/jbc.R116.746032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Müller U.C., Deller T., Korte M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017;18(5):281–298. doi: 10.1038/nrn.2017.29. [DOI] [PubMed] [Google Scholar]
- 68.Stefani M. Biochemical and biophysical features of both oligomer/fibril and cell membrane in amyloid cytotoxicity. FEBS J. 2010;277(22):4602–4613. doi: 10.1111/j.1742-4658.2010.07889.x. [DOI] [PubMed] [Google Scholar]
- 69.Mucke L., Selkoe D.J. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2012;2(7):a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verma M., Vats A., Taneja V. Toxic species in amyloid disorders: Oligomers or mature fibrils. Ann. Indian Acad. Neurol. 2015;18(2):138–145. doi: 10.4103/0972-2327.144284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lobello K., Ryan J.M., Liu E., Rippon G., Black R. Targeting Beta amyloid: a clinical review of immunotherapeutic approaches in Alzheimer’s disease. Int. J. Alzheimers Dis. 2012;2012:628070. doi: 10.1155/2012/628070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varadarajan S., Yatin S., Aksenova M., Butterfield D.A. Review: Alzheimer’s amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000;130(2-3):184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- 73.Ferreira S.T., Lourenco M.V., Oliveira M.M., De Felice F.G. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front. Cell. Neurosci. 2015;9:191. doi: 10.3389/fncel.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Golde T.E. Alzheimer disease therapy: can the amyloid cascade be halted? J. Clin. Invest. 2003;111(1):11–18. doi: 10.1172/JCI200317527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garwood C.J., Ratcliffe L.E., Simpson J.E., Heath P.R., Ince P.G., Wharton S.B. Review: Astrocytes in Alzheimer’s disease and other age-associated dementias: a supporting player with a central role. Neuropathol. Appl. Neurobiol. 2017;43(4):281–298. doi: 10.1111/nan.12338. [DOI] [PubMed] [Google Scholar]
- 76.Small D.H., Mok S.S., Bornstein J.C. Alzheimer’s disease and Abeta toxicity: from top to bottom. Nat. Rev. Neurosci. 2001;2(8):595–598. doi: 10.1038/35086072. [DOI] [PubMed] [Google Scholar]
- 77.Selkoe D.J. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J. Alzheimers Dis. 2001;3(1):75–80. doi: 10.3233/JAD-2001-3111. [DOI] [PubMed] [Google Scholar]
- 78.Jagust W.J., Mormino E.C. Lifespan brain activity, β-amyloid, and Alzheimer’s disease. Trends Cogn. Sci. (Regul. Ed.) 2011;15(11):520–526. doi: 10.1016/j.tics.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Musiek E.S., Holtzman D.M. Three dimensions of the amyloid hypothesis: time, space and ‘wingmen’. Nat. Neurosci. 2015;18(6):800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bush A.I., Tanzi R.E. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics. 2008;5(3):421–432. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bush A.I. Metal complexing agents as therapies for Alzheimer’s disease. Neurobiol. Aging. 2002;23(6):1031–1038. doi: 10.1016/S0197-4580(02)00120-3. [DOI] [PubMed] [Google Scholar]
- 82.Li Y., Peng P., Tang L., Hu Y., Hu Y., Sheng R. Design, synthesis and evaluation of rivastigmine and curcumin hybrids as site-activated multitarget-directed ligands for Alzheimer’s disease therapy. Bioorg. Med. Chem. 2014;22(17):4717–4725. doi: 10.1016/j.bmc.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 83.Buée L., Bussière T., Buée-Scherrer V., Delacourte A., Hof P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 2000;33(1):95–130. doi: 10.1016/S0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 84.Iqbal K., Liu F., Gong C-X., Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010;7(8):656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Billingsley M. L., Kincaid R. L. Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem. J. 1997;323(Pt 3):577–591. doi: 10.1042/bj3230577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sánchez C., Díaz-Nido J., Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog. Neurobiol. 2000;61(2):133–168. doi: 10.1016/S0301-0082(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 87.Benítez-King G., Ortiz-López L., Morales-Mulia S., Jiménez-Rubio G., Ramírez-Rodríguez G., Meza I. Phosphorylation-dephosphorylation imbalance of cytoskeletal associated proteins in neurodegenerative diseases. Recent Patents CNS Drug Discov. 2006;1(2):219–230. doi: 10.2174/157488906777452776. [DOI] [PubMed] [Google Scholar]
- 88.Nirschl J.J., Ghiretti A.E., Holzbaur E.L.F. The impact of cytoskeletal organization on the local regulation of neuronal transport. Nat. Rev. Neurosci. 2017;18(10):585–597. doi: 10.1038/nrn.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plattner F., Angelo M., Giese K.P. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J. Biol. Chem. 2006;281(35):25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- 90.Himmelstein D.S., Ward S.M., Lancia J.K., Patterson K.R., Binder L.I. Tau as a therapeutic target in neurodegenerative disease. Pharmacol. Ther. 2012;136(1):8–22. doi: 10.1016/j.pharmthera.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naseri N.N., Wang H., Guo J., Sharma M., Luo W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019;705:183–194. doi: 10.1016/j.neulet.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gong C-X., Grundke-Iqbal I., Iqbal K. Targeting tau protein in Alzheimer’s disease. Drugs Aging. 2010;27(5):351–365. doi: 10.2165/11536110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 93.Iqbal K., Zaidi T., Bancher C., Grundke-Iqbal I. Alzheimer paired helical filaments. Restoration of the biological activity by dephosphorylation. FEBS Lett. 1994;349(1):104–108. doi: 10.1016/0014-5793(94)00650-4. [DOI] [PubMed] [Google Scholar]
- 94.Alonso A. del C., Li B., Grundke-Iqbal I., Iqbal K. Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc. Natl. Acad. Sci. USA. 2006;103(23):8864–8869. doi: 10.1073/pnas.0603214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kickstein E., Krauss S., Thornhill P., Rutschow D., Zeller R., Sharkey J., Williamson R., Fuchs M., Köhler A., Glossmann H., Schneider R., Sutherland C., Schweiger S. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc. Natl. Acad. Sci. USA. 2010;107(50):21830–21835. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hübinger G., Geis S., LeCorre S., Mühlbacher S., Gordon S., Fracasso R.P., Hoffman F., Ferrand S., Klafki H.W., Roder H.M. Inhibition of PHF-like tau hyperphosphorylation in SH-SY5Y cells and rat brain slices by K252a. J. Alzheimers Dis. 2008;13(3):281–294. doi: 10.3233/JAD-2008-13306. [DOI] [PubMed] [Google Scholar]
- 97.Mukrasch M.D., Biernat J., von Bergen M., Griesinger C., Mandelkow E., Zweckstetter M. Sites of tau important for aggregation populate beta-structure and bind to microtubules and polyanions. J. Biol. Chem. 2005;280(26):24978–24986. doi: 10.1074/jbc.M501565200. [DOI] [PubMed] [Google Scholar]
- 98.Rosenmann H., Grigoriadis N., Karussis D., Boimel M., Touloumi O., Ovadia H., Abramsky O. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch. Neurol. 2006;63(10):1459–1467. doi: 10.1001/archneur.63.10.1459. [DOI] [PubMed] [Google Scholar]
- 99.Boimel M., Grigoriadis N., Lourbopoulos A., Haber E., Abramsky O., Rosenmann H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp. Neurol. 2010;224(2):472–485. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 100.Meltzer C.C., Smith G., DeKosky S.T., Pollock B.G., Mathis C.A., Moore R.Y., Kupfer D.J., Reynolds C.F., III Serotonin in aging, late-life depression, and Alzheimer’s disease: the emerging role of functional imaging. Neuropsychopharmacology. 1998;18(6):407–430. doi: 10.1016/S0893-133X(97)00194-2. [DOI] [PubMed] [Google Scholar]
- 101.Vakalopoulos C. Alzheimer’s Disease: The alternative serotonergic hypothesis of cognitive decline. J. Alzheimers Dis. 2017;60(3):859–866. doi: 10.3233/JAD-170364. [DOI] [PubMed] [Google Scholar]
- 102.Toda N., Tago K., Marumoto S., Takami K., Ori M., Yamada N., Koyama K., Naruto S., Abe K., Yamazaki R., Hara T., Aoyagi A., Abe Y., Kaneko T., Kogen H. Design, synthesis and structure-activity relationships of dual inhibitors of acetylcholinesterase and serotonin transporter as potential agents for Alzheimer’s disease. Bioorg. Med. Chem. 2003;11(9):1935–1955. doi: 10.1016/S0968-0896(03)00091-9. [DOI] [PubMed] [Google Scholar]
- 103.Smith G.S., Barrett F.S., Joo J.H., Nassery N., Savonenko A., Sodums D.J., Marano C.M., Munro C.A., Brandt J., Kraut M.A., Zhou Y., Wong D.F., Workman C.I. Molecular imaging of serotonin degeneration in mild cognitive impairment. Neurobiol. Dis. 2017;105:33–41. doi: 10.1016/j.nbd.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodríguez-Lavado J., Gallardo-Garrido C., Mallea M., Bustos V., Osorio R., Hödar-Salazar M., Chung H., Araya-Maturana R., Lorca M., Pessoa-Mahana C.D., Mella-Raipán J., Saitz C., Jaque P., Reyes-Parada M., Iturriaga-Vásquez P., Pessoa-Mahana H. Synthesis, in vitro evaluation and molecular docking of a new class of indolylpropyl benzamidopiperazines as dual AChE and SERT ligands for Alzheimer’s disease. Eur. J. Med. Chem. 2020;198:112368. doi: 10.1016/j.ejmech.2020.112368. [DOI] [PubMed] [Google Scholar]
- 105.Lecoutey C., Hedou D., Freret T., Giannoni P., Gaven F., Since M., Bouet V., Ballandonne C., Corvaisier S., Malzert Fréon A., Mignani S., Cresteil T., Boulouard M., Claeysen S., Rochais C., Dallemagne P. Design of donecopride, a dual serotonin subtype 4 receptor agonist/acetylcholinesterase inhibitor with potential interest for Alzheimer’s disease treatment. Proc. Natl. Acad. Sci. USA. 2014;111(36):E3825–E3830. doi: 10.1073/pnas.1410315111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang L., Lu C., Sun Y., Mao F., Luo Z., Su T., Jiang H., Shan W., Li X. Multitarget-directed benzylideneindanone derivatives: anti-β-amyloid (Aβ) aggregation, antioxidant, metal chelation, and monoamine oxidase B (MAO-B) inhibition properties against Alzheimer’s disease. J. Med. Chem. 2012;55(19):8483–8492. doi: 10.1021/jm300978h. [DOI] [PubMed] [Google Scholar]
- 107.Walker W.H., Kearney E.B., Seng R.L., Singer T.P. The covalently-bound flavin of hepatic monoamine oxidase. 2. Identification and properties of cysteinyl riboflavin. Eur. J. Biochem. 1971;24(2):328–331. doi: 10.1111/j.1432-1033.1971.tb19690.x. [DOI] [PubMed] [Google Scholar]
- 108.Chiba K., Trevor A., Castagnoli N., Jr Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem. Biophys. Res. Commun. 1984;120(2):574–578. doi: 10.1016/0006-291X(84)91293-2. [DOI] [PubMed] [Google Scholar]
- 109.Wang Y., Sun Y., Guo Y., Wang Z., Huang L., Li X. Dual functional cholinesterase and MAO inhibitors for the treatment of Alzheimer’s disease: synthesis, pharmacological analysis and molecular modeling of homoisoflavonoid derivatives. J. Enzyme Inhib. Med. Chem. 2016;31(3):389–397. doi: 10.3109/14756366.2015.1024675. [DOI] [PubMed] [Google Scholar]
- 110.Uddin M.S., Kabir M.T., Rahman M.M., Mathew B., Shah M.A., Ashraf G.M. TV 3326 for Alzheimer’s dementia: a novel multimodal ChE and MAO inhibitors to mitigate Alzheimer’s-like neuropathology. J. Pharm. Pharmacol. 2020;72(8):1001–1012. doi: 10.1111/jphp.13244. [DOI] [PubMed] [Google Scholar]
- 111.Chen J.J., Swope D.M., Dashtipour K. Comprehensive review of rasagiline, a second-generation monoamine oxidase inhibitor, for the treatment of Parkinson’s disease. Clin. Ther. 2007;29(9):1825–1849. doi: 10.1016/j.clinthera.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 112.Haefely W., Burkard W.P., Cesura A.M., Kettler R., Lorez H.P., Martin J.R., Richards J.G., Scherschlicht R., Da Prada M. Biochemistry and pharmacology of moclobemide, a prototype RIMA. Psychopharmacology (Berl.) 1992;106(Suppl.):S6–S14. doi: 10.1007/BF02246225. [DOI] [PubMed] [Google Scholar]
- 113.Maillet M., Robert S.J., Lezoualc’h F. New insights into serotonin 5-HT4 receptors : a novel therapeutic target for Alzheimer’s disease? Curr. Alzheimer Res. 2004;1(2):79–85. doi: 10.2174/1567205043332252. [DOI] [PubMed] [Google Scholar]
- 114.Agatonovic-Kustrin S., Kettle C., Morton D.W. A molecular approach in drug development for Alzheimer’s disease. Biomed. Pharmacother. 2018;106:553–565. doi: 10.1016/j.biopha.2018.06.147. [DOI] [PubMed] [Google Scholar]
- 115.Agarwal S., Mishra R., Gupta A.K., Gupta A. Elsevier; 2018. Chapter 5 - turmeric: isolation and synthesis of important biological molecules; Tewari, A., Tiwari, S. B. T.-S. of M. A. from P., Eds; pp. 105–125. [Google Scholar]
- 116.Kocaadam B., Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017;57(13):2889–2895. doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- 117.Menon V.P., Sudheer A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 118.Amalraj A., Pius A., Gopi S., Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives - A review. J. Tradit. Complement. Med. 2016;7(2):205–233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wiggers H.J., Zaioncz S., Cheleski J., Mainardes R.M., Khalil N.M. Curcumin, a multitarget phytochemical: challenges and perspectives. 2017;53 doi: 10.1016/B978-0-444-63930-1.00007-7. [DOI] [Google Scholar]
- 120.Reddy P.H., Manczak M., Yin X., Grady M.C., Mitchell A., Tonk S., Kuruva C.S., Bhatti J.S., Kandimalla R., Vijayan M., Kumar S., Wang R., Pradeepkiran J.A., Ogunmokun G., Thamarai K., Quesada K., Boles A., Reddy A.P. Protective effects of indian spice curcumin against amyloid-β in Alzheimer’s Disease. J. Alzheimers Dis. 2018;61(3):843–866. doi: 10.3233/JAD-170512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cole G.M., Teter B., Frautschy S.A. In: Springer U.S., editor. Boston, MA: 2007. Neuroprotective effects of curcumin bt - the molecular targets and therapeutic uses of curcumin in health and disease; Aggarwal, B. B., Surh, Y.-J., Shishodia, S; pp. 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xiong Z., Hongmei Z., Lu S., Yu L. Curcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s Disease. Pharmacol. Rep. 2011;63(5):1101–1108. doi: 10.1016/S1734-1140(11)70629-6. [DOI] [PubMed] [Google Scholar]
- 123.Liu Z-J., Li Z-H., Liu L., Tang W-X., Wang Y., Dong M-R., Xiao C. Curcumin attenuates beta-amyloid-induced neuroinflammation via Activation of peroxisome proliferator-activated receptor-gamma function in a rat model of Alzheimer’s Disease. Front. Pharmacol. 2016;7:261. doi: 10.3389/fphar.2016.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomas P., Wang Y-J., Zhong J-H., Kosaraju S., O’Callaghan N.J., Zhou X-F., Fenech M. Grape seed polyphenols and curcumin reduce genomic instability events in a transgenic mouse model for Alzheimer’s disease. Mutat. Res. 2009;661(1-2):25–34. doi: 10.1016/j.mrfmmm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 125.Farooqui A. A. Curcumin in neurological disorders curcumin neurol. Curcumin Neurol. Psychiatr. Disord. Neurochem. Pharmacol. Prop. 2019:45–62. [Google Scholar]
- 126.Yan J., Hu J., Liu A., He L., Li X., Wei H. Design, synthesis, and evaluation of multitarget-directed ligands against Alzheimer’s disease based on the fusion of donepezil and curcumin. Bioorg. Med. Chem. 2017;25(12):2946–2955. doi: 10.1016/j.bmc.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 127.Wang S., Peng X., Cui L., Li T., Yu B., Ma G., Ba X. Synthesis of water-soluble curcumin derivatives and their inhibition on lysozyme amyloid fibrillation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018;190:89–95. doi: 10.1016/j.saa.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 128.Jagannathan R., Abraham P.M., Poddar P. Temperature-dependent spectroscopic evidences of curcumin in aqueous medium: a mechanistic study of its solubility and stability. J. Phys. Chem. B. 2012;116(50):14533–14540. doi: 10.1021/jp3050516. [DOI] [PubMed] [Google Scholar]
- 129.Cui L., Wang S., Zhang J., Wang M., Gao Y., Bai L., Zhang H., Ma G., Ba X. Effect of curcumin derivatives on hen egg white lysozyme amyloid fibrillation and their interaction study by spectroscopic methods Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2019;223 doi: 10.1016/j.saa.2019.117365. [DOI] [PubMed] [Google Scholar]
- 130.McGeer E.G., McGeer P.L. Inflammatory processes in Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(5):741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 131.Walker D.G., Lue L-F. Investigations with cultured human microglia on pathogenic mechanisms of Alzheimer’s disease and other neurodegenerative diseases. J. Neurosci. Res. 2005;81(3):412–425. doi: 10.1002/jnr.20484. [DOI] [PubMed] [Google Scholar]
- 132.Vasto S., Candore G., Duro G., Lio D., Grimaldi M.P., Caruso C. Alzheimer’s disease and genetics of inflammation: a pharmacogenomic vision. Pharmacogenomics. 2007;8(12):1735–1745. doi: 10.2217/14622416.8.12.1735. [DOI] [PubMed] [Google Scholar]
- 133.Vasto S., Candore G., Listì F., Balistreri C.R., Colonna-Romano G., Malavolta M., Lio D., Nuzzo D., Mocchegiani E., Di Bona D., Caruso C. Inflammation, genes and zinc in Alzheimer’s disease. Brain Res. Brain Res. Rev. 2008;58(1):96–105. doi: 10.1016/j.brainresrev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 134.Cameron B., Landreth G.E. Inflammation, microglia, and Alzheimer’s disease. Neurobiol. Dis. 2010;37(3):503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Broussard G.J., Mytar J., Li R.C., Klapstein G.J. The role of inflammatory processes in Alzheimer’s disease. Inflammopharmacology. 2012;20(3):109–126. doi: 10.1007/s10787-012-0130-z. [DOI] [PubMed] [Google Scholar]
- 136.Rubio-Perez J.M., Morillas-Ruiz J.M.A. A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Delaby C., Gabelle A., Blum D., Schraen-Maschke S., Moulinier A., Boulanghien J., Séverac D., Buée L., Rème T., Lehmann S. Central nervous system and peripheral inflammatory processes in Alzheimer’s Disease: biomarker profiling approach. Front. Neurol. 2015;6:181. doi: 10.3389/fneur.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Porcelli S., Crisafulli C., Donato L., Calabrò M., Politis A., Liappas I., Albani D., Atti A.R., Salfi R., Raimondi I., Forloni G., Papadimitriou G.N., De Ronchi D., Serretti A. Role of neurodevelopment involved genes in psychiatric comorbidities and modulation of inflammatory processes in Alzheimer’s disease. J. Neurol. Sci. 2016;370:162–166. doi: 10.1016/j.jns.2016.09.053. [DOI] [PubMed] [Google Scholar]
- 139.Bisht K., Sharma K., Tremblay M-È. Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol. Stress. 2018;9:9–21. doi: 10.1016/j.ynstr.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lakey-Beitia J., González Y., Doens D., Stephens D.E., Santamaría R., Murillo E., Gutiérrez M., Fernández P.L., Rao K.S., Larionov O.V., Durant-Archibold A.A. Assessment of novel curcumin derivatives as potent inhibitors of inflammation and Amyloid-β Aggregation in Alzheimer’s Disease. J. Alzheimers Dis. 2017;60(s1):S59–S68. doi: 10.3233/JAD-170071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tanaka T., Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol. Res. 2014;2(4):288–294. doi: 10.1158/2326-6066.CIR-14-0022. [DOI] [PubMed] [Google Scholar]
- 143.Ding C., Cicuttini F., Li J., Jones G. Targeting IL-6 in the treatment of inflammatory and autoimmune diseases. Expert Opin. Investig. Drugs. 2009;18(10):1457–1466. doi: 10.1517/13543780903203789. [DOI] [PubMed] [Google Scholar]
- 144.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res. Ther. 2006;8(Suppl. 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Okuda M., Hijikuro I., Fujita Y., Teruya T., Kawakami H., Takahashi T., Sugimoto H. Design and synthesis of curcumin derivatives as tau and amyloid β dual aggregation inhibitors. Bioorg. Med. Chem. Lett. 2016;26(20):5024–5028. doi: 10.1016/j.bmcl.2016.08.092. [DOI] [PubMed] [Google Scholar]
- 146.Ravindranath V., Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16(3):259–265. doi: 10.1016/0300-483X(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 147.Baum L., Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J. Alzheimers Dis. 2004;6(4):367–377. doi: 10.3233/JAD-2004-6403. [DOI] [PubMed] [Google Scholar]
- 148.Potter P.E. Curcumin: a natural substance with potential efficacy in Alzheimer’s disease. J. Exp. Pharmacol. 2013;5:23–31. doi: 10.2147/JEP.S26803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y., Yin H., Wang L., Shuboy A., Lou J., Han B., Zhang X., Li J. Curcumin as a potential treatment for Alzheimer’s disease: a study of the effects of curcumin on hippocampal expression of glial fibrillary acidic protein. Am. J. Chin. Med. 2013;41(1):59–70. doi: 10.1142/S0192415X13500055. [DOI] [PubMed] [Google Scholar]
- 150.Giacomeli R., Izoton J.C., Dos Santos R.B., Boeira S.P., Jesse C.R., Haas S.E. Neuroprotective effects of curcumin lipid-core nanocapsules in a model Alzheimer’s disease induced by β-amyloid 1-42 peptide in aged female mice. Brain Res. 2019;1721:146325. doi: 10.1016/j.brainres.2019.146325. [DOI] [PubMed] [Google Scholar]
- 151.Vareed S.K., Kakarala M., Ruffin M.T., Crowell J.A., Normolle D.P., Djuric Z., Brenner D.E. Pharmacokinetics of Curcumin Conjugate Metabolites in Healthy Human Subjects. Cancer Epidemiol. biomarkers Prev. a Publ. Am. Assoc. Cancer Res. cosponsored by. Am. Soc. Prev. Oncol. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Z., Fang L., Zhang H., Gou S., Chen L. Design, synthesis and biological evaluation of multifunctional tacrine-curcumin hybrids as new cholinesterase inhibitors with metal ions-chelating and neuroprotective property. Bioorg. Med. Chem. 2017;25(8):2387–2398. doi: 10.1016/j.bmc.2017.02.049. [DOI] [PubMed] [Google Scholar]
- 153.Dias K.S.T., de Paula C.T., Dos Santos T., Souza I.N.O., Boni M.S., Guimarães M.J.R., da Silva F.M.R., Castro N.G., Neves G.A., Veloso C.C., Coelho M.M., de Melo I.S.F., Giusti F.C.V., Giusti-Paiva A., da Silva M.L., Dardenne L.E., Guedes I.A., Pruccoli L., Morroni F., Tarozzi A., Viegas C., Jr Design, synthesis and evaluation of novel feruloyl-donepezil hybrids as potential multitarget drugs for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017;130:440–457. doi: 10.1016/j.ejmech.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 154.Al-Edresi S., Alsalahat I., Freeman S., Aojula H., Penny J. Resveratrol-mediated cleavage of amyloid β1-42 peptide: potential relevance to Alzheimer’s disease. Neurobiol. Aging. 2020;94:24–33. doi: 10.1016/j.neurobiolaging.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 155.Jeandet P., Sobarzo-Sánchez E., Silva A.S., Clément C., Nabavi S.F., Battino M., Rasekhian M., Belwal T., Habtemariam S., Koffas M., Nabavi S.M. Whole-cell biocatalytic, enzymatic and green chemistry methods for the production of resveratrol and its derivatives. Biotechnol. Adv. 2020;39:107461. doi: 10.1016/j.biotechadv.2019.107461. [DOI] [PubMed] [Google Scholar]
- 156.Richard T., Pawlus A.D., Iglésias M.L., Pedrot E., Waffo-Teguo P., Mérillon J.M., Monti J.P. Neuroprotective properties of resveratrol and derivatives. Ann. N. Y. Acad. Sci. 2011;1215:103–108. doi: 10.1111/j.1749-6632.2010.05865.x. [DOI] [PubMed] [Google Scholar]
- 157.Amri A., Chaumeil J.C., Sfar S., Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release. 2012;158(2):182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 158.Isailović B.D., Kostić I.T., Zvonar A., Đorđević V.B., Gašperlin M., Nedović V.A., Bugarski B.M. Resveratrol loaded liposomes produced by different techniques. Innov. Food Sci. Emerg. Technol. 2013;19:181–189. doi: 10.1016/j.ifset.2013.03.006. [DOI] [Google Scholar]
- 159.Davidov-Pardo G., McClements D.J. Resveratrol encapsulation: designing delivery systems to overcome solubility, stability and bioavailability issues. Trends Food Sci. Technol. 2014;38:88–103. doi: 10.1016/j.tifs.2014.05.003. [DOI] [Google Scholar]
- 160.Huang M., Liang C., Tan C., Huang S., Ying R., Wang Y., Wang Z., Zhang Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019;10(10):6447–6458. doi: 10.1039/C9FO01338E. [DOI] [PubMed] [Google Scholar]
- 161.Lucas-Abellán C., Fortea I., López-Nicolás J.M., Núñez-Delicado E. Cyclodextrins as resveratrol carrier system. Food Chem. 2007;104:39–44. doi: 10.1016/j.foodchem.2006.10.068. [DOI] [Google Scholar]
- 162.Lu Z., Cheng B., Hu Y., Zhang Y., Zou G. Complexation of resveratrol with cyclodextrins: solubility and antioxidant activity. Food Chem. 2009;113:17–20. doi: 10.1016/j.foodchem.2008.04.042. [DOI] [Google Scholar]
- 163.Ansari K.A., Vavia P.R., Trotta F., Cavalli R. Cyclodextrin-based nanosponges for delivery of resveratrol: in vitro characterisation, stability, cytotoxicity and permeation study. AAPS PharmSciTech. 2011;12(1):279–286. doi: 10.1208/s12249-011-9584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Soo E., Thakur S., Qu Z., Jambhrunkar S., Parekh H.S., Popat A. Enhancing delivery and cytotoxicity of resveratrol through a dual nanoencapsulation approach. J. Colloid Interface Sci. 2016;462:368–374. doi: 10.1016/j.jcis.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 165.Teskač K., Kristl J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010;390(1):61–69. doi: 10.1016/j.ijpharm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 166.Gokce E.H., Korkmaz E., Dellera E., Sandri G., Bonferoni M.C., Ozer O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: evaluation of antioxidant potential for dermal applications. Int. J. Nanomedicine. 2012;7:1841–1850. doi: 10.2147/IJN.S29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Neves A.R., Queiroz J.F., Reis S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnology. 2016;14:27. doi: 10.1186/s12951-016-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Loureiro J.A., Andrade S., Duarte A., Neves A.R., Queiroz J.F., Nunes C., Sevin E., Fenart L., Gosselet F., Coelho M.A.N., Pereira M.C. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s Disease. Molecules. 2017;22(2):22. doi: 10.3390/molecules22020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rege S.D., Geetha T., Griffin G.D., Broderick T.L., Babu J.R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front. Aging Neurosci. 2014;6:218. doi: 10.3389/fnagi.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gomes B.A.Q., Silva J.P.B., Romeiro C.F.R., Dos Santos S.M., Rodrigues C.A., Gonçalves P.R., Sakai J.T., Mendes P.F.S., Varela E.L.P., Monteiro M.C. Neuroprotective mechanisms of resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018;2018:8152373. doi: 10.1155/2018/8152373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Michán S., Li Y., Chou M.M-H., Parrella E., Ge H., Long J.M., Allard J.S., Lewis K., Miller M., Xu W., Mervis R.F., Chen J., Guerin K.I., Smith L.E.H., McBurney M.W., Sinclair D.A., Baudry M., de Cabo R., Longo V.D. SIRT1 is essential for normal cognitive function and synaptic plasticity. J. Neurosci. 2010;30(29):9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wu D., Qiu Y., Gao X., Yuan X-B., Zhai Q. Overexpression of SIRT1 in mouse forebrain impairs lipid/glucose metabolism and motor function. PLoS One. 2011;6(6):e21759. doi: 10.1371/journal.pone.0021759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cao W., Dou Y., Li A. Resveratrol boosts cognitive function by targeting SIRT1. Neurochem. Res. 2018;43(9):1705–1713. doi: 10.1007/s11064-018-2586-8. [DOI] [PubMed] [Google Scholar]
- 174.Gao J., Wang W-Y., Mao Y-W., Gräff J., Guan J-S., Pan L., Mak G., Kim D., Su S.C., Tsai L-H. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466(7310):1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang R., Zhang Y., Li J., Zhang C. Resveratrol ameliorates spatial learning memory impairment induced by Aβ1-42 in rats. Neuroscience. 2017;344:39–47. doi: 10.1016/j.neuroscience.2016.08.051. [DOI] [PubMed] [Google Scholar]
- 176.Cao Y., Yan Z., Zhou T., Wang G. SIRT1 regulates cognitive performance and ability of learning and memory in diabetic and nondiabetic models. J. Diabetes Res. 2017;2017:7121827. doi: 10.1155/2017/7121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu Y., Liu Y., Chen H., Yao X., Xiao Y., Zeng X., Zheng Q., Wei Y., Song C., Zhang Y., Zhu P., Wang J., Zheng X. Synthetic resveratrol derivatives and their biological activities: a review. Open J. Med. Chem. 2015;05:97–105. doi: 10.4236/ojmc.2015.54006. [DOI] [Google Scholar]
- 178.Pan L.F., Wang X.B., Xie S.S., Li S.Y., Kong L.Y. Multitarget-Directed Resveratrol Derivatives: Anti-Cholinesterases, Anti-β-Amyloid aggregation and monoamine oxidase inhibition properties against Alzheimer’s Disease. MedChemComm. 2014;5:609–616. doi: 10.1039/C3MD00376K. [DOI] [Google Scholar]
- 179.Tumiatti V., Rosini M., Bartolini M., Cavalli A., Marucci G., Andrisano V., Angeli P., Banzi R., Minarini A., Recanatini M., Melchiorre C. Structure-activity relationships of acetylcholinesterase noncovalent inhibitors based on a polyamine backbone. 2. Role of the substituents on the phenyl ring and nitrogen atoms of caproctamine. J. Med. Chem. 2003;46(6):954–966. doi: 10.1021/jm021055+. [DOI] [PubMed] [Google Scholar]
- 180.Piazzi L., Belluti F., Bisi A., Gobbi S., Rizzo S., Bartolini M., Andrisano V., Recanatini M., Rampa A. Cholinesterase inhibitors: SAR and enzyme inhibitory activity of 3-[omega-(benzylmethylamino)alkoxy]xanthen-9-ones. Bioorg. Med. Chem. 2007;15(1):575–585. doi: 10.1016/j.bmc.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 181.Lu C., Guo Y., Yan J., Luo Z., Luo H.B., Yan M., Huang L., Li X. Design, synthesis, and evaluation of multitarget-directed resveratrol derivatives for the treatment of Alzheimer’s disease. J. Med. Chem. 2013;56(14):5843–5859. doi: 10.1021/jm400567s. [DOI] [PubMed] [Google Scholar]
- 182.Jeřábek J., Uliassi E., Guidotti L., Korábečný J., Soukup O., Sepsova V., Hrabinova M., Kuča K., Bartolini M., Peña-Altamira L.E., Petralla S., Monti B., Roberti M., Bolognesi M.L. Tacrine-resveratrol fused hybrids as multi-target-directed ligands against Alzheimer’s disease. Eur. J. Med. Chem. 2017;127:250–262. doi: 10.1016/j.ejmech.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 183.Nepovimova E., Uliassi E., Korabecny J., Peña-Altamira L.E., Samez S., Pesaresi A., Garcia G.E., Bartolini M., Andrisano V., Bergamini C., Fato R., Lamba D., Roberti M., Kuca K., Monti B., Bolognesi M.L. Multitarget drug design strategy: quinone-tacrine hybrids designed to block amyloid-β aggregation and to exert anticholinesterase and antioxidant effects. J. Med. Chem. 2014;57(20):8576–8589. doi: 10.1021/jm5010804. [DOI] [PubMed] [Google Scholar]
- 184.Galdeano C., Viayna E., Sola I., Formosa X., Camps P., Badia A., Clos M.V., Relat J., Ratia M., Bartolini M., Mancini F., Andrisano V., Salmona M., Minguillón C., González-Muñoz G.C., Rodríguez-Franco M.I., Bidon-Chanal A., Luque F.J., Muñoz-Torrero D. Huprine-tacrine heterodimers as anti-amyloidogenic compounds of potential interest against Alzheimer’s and prion diseases. J. Med. Chem. 2012;55(2):661–669. doi: 10.1021/jm200840c. [DOI] [PubMed] [Google Scholar]
- 185.Cheng G., Xu P., Zhang M., Chen J., Sheng R., Ma Y. Resveratrol-maltol hybrids as multi-target-directed agents for Alzheimer’s disease. Bioorg. Med. Chem. 2018;26(22):5759–5765. doi: 10.1016/j.bmc.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 186.Murakami K., Ishida K., Watakabe K., Tsubouchi R., Haneda M., Yoshino M. Prooxidant action of maltol: role of transition metals in the generation of reactive oxygen species and enhanced formation of 8-hydroxy-2′-deoxyguanosine formation in DNA. Biometals. 2006;19(3):253–257. doi: 10.1007/s10534-005-6998-y. [DOI] [PubMed] [Google Scholar]
- 187.Kang K.S., Yamabe N., Kim H.Y., Yokozawa T. Role of maltol in advanced glycation end products and free radicals: in vitro and in vivo studies. J. Pharm. Pharmacol. 2008;60(4):445–452. doi: 10.1211/jpp.60.4.0006. [DOI] [PubMed] [Google Scholar]
- 188.Kontoghiorghes G.J. Advances on chelation and chelator metal complexes in medicine. Int. J. Mol. Sci. 2020;21(7):E2499. doi: 10.3390/ijms21072499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rauk A. The chemistry of Alzheimer’s disease. Chem. Soc. Rev. 2009;38(9):2698–2715. doi: 10.1039/b807980n. [DOI] [PubMed] [Google Scholar]
- 190.Puksasook T., Kimura S., Tadtong S., Jiaranaikulwanitch J., Pratuangdejkul J., Kitphati W., Suwanborirux K., Saito N., Nukoolkarn V. Semisynthesis and biological evaluation of prenylated resveratrol derivatives as multi-targeted agents for Alzheimer’s disease. J. Nat. Med. 2017;71(4):665–682. doi: 10.1007/s11418-017-1097-2. [DOI] [PubMed] [Google Scholar]
- 191.Wollack J.W., Zeliadt N.A., Mullen D.G., Amundson G., Geier S., Falkum S., Wattenberg E.V., Barany G., Distefano M.D. Multifunctional prenylated peptides for live cell analysis. J. Am. Chem. Soc. 2009;131(21):7293–7303. doi: 10.1021/ja805174z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ribaudo G., Coghi P., Zanforlin E., Law B.Y.K., Wu Y.Y.J., Han Y., Qiu A.C., Qu Y.Q., Zagotto G., Wong V.K.W. Semi-synthetic isoflavones as BACE-1 inhibitors against Alzheimer’s disease. Bioorg. Chem. 2019;87:474–483. doi: 10.1016/j.bioorg.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 193.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 194.Kim S.H., Naveen Kumar Ch., Kim H.J., Kim D.H., Cho J., Jin C., Lee Y.S. Glucose-containing flavones their synthesis and antioxidant and neuroprotective activities. Bioorg. Med. Chem. Lett. 2009;19(21):6009–6013. doi: 10.1016/j.bmcl.2009.09.062. [DOI] [PubMed] [Google Scholar]
- 195.Tang Y-W., Shi C-J., Yang H-L., Cai P., Liu Q-H., Yang X-L., Kong L-Y., Wang X-B. Synthesis and evaluation of isoprenylation-resveratrol dimer derivatives against Alzheimer’s disease. Eur. J. Med. Chem. 2019;163:307–319. doi: 10.1016/j.ejmech.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 196.Wei Z., Bai O., Richardson J.S., Mousseau D.D., Li X-M. Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. J. Neurosci. Res. 2003;73(3):364–368. doi: 10.1002/jnr.10668. [DOI] [PubMed] [Google Scholar]
- 197.Gal S., Zheng H., Fridkin M., Youdim M.B.H. Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases. In vivo selective brain monoamine oxidase inhibition and prevention of MPTP-induced striatal dopamine depletion. J. Neurochem. 2005;95(1):79–88. doi: 10.1111/j.1471-4159.2005.03341.x. [DOI] [PubMed] [Google Scholar]
- 198.Woo J.H., Lee J.H., Kim H., Park S.J., Joe E-H., Jou I. Control of inflammatory responses: a new paradigm for the treatment of chronic neuronal diseases. Exp. Neurobiol. 2015;24(2):95–102. doi: 10.5607/en.2015.24.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xu P., Zhang M., Sheng R., Ma Y. Synthesis and biological evaluation of deferiprone-resveratrol hybrids as antioxidants, Aβ1-42 aggregation inhibitors and metal-chelating agents for Alzheimer’s disease. Eur. J. Med. Chem. 2017;127:174–186. doi: 10.1016/j.ejmech.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 200.Ma Y., Xie Y., Hider R.C. A novel fluorescence method for determination of pFe3+. Analyst (Lond.) 2013;138(1):96–99. doi: 10.1039/C2AN36186H. [DOI] [PubMed] [Google Scholar]
- 201.Yang X., Qiang X., Li Y., Luo L., Xu R., Zheng Y., Cao Z., Tan Z., Deng Y. Pyridoxine-resveratrol hybrids Mannich base derivatives as novel dual inhibitors of AChE and MAO-B with antioxidant and metal-chelating properties for the treatment of Alzheimer’s disease. Bioorg. Chem. 2017;71:305–314. doi: 10.1016/j.bioorg.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 202.Park D.H., Venkatesan J., Kim S-K., Ramkumar V., Parthiban P. Antioxidant properties of Mannich bases. Bioorg. Med. Chem. Lett. 2012;22(20):6362–6367. doi: 10.1016/j.bmcl.2012.08.080. [DOI] [PubMed] [Google Scholar]
- 203.Roman G. Mannich bases in medicinal chemistry and drug design. Eur. J. Med. Chem. 2015;89:743–816. doi: 10.1016/j.ejmech.2014.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.B̈uÿukkidan N., Öozer S. Synthesis and characterization of Ni(II) and Cu(II) complexes derived from novel phenolic mannich bases. Turk. J. Chem. 2013;37:101–110. [Google Scholar]
- 205.Mooney S., Leuendorf J.E., Hendrickson C., Hellmann H. Vitamin B6: a long known compound of surprising complexity. Molecules. 2009;14(1):329–351. doi: 10.3390/molecules14010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Khadem S., Marles R.J. Chromone and flavonoid alkaloids: occurrence and bioactivity. Molecules. 2011;17(1):191–206. doi: 10.3390/molecules17010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mitra I., Saha A., Roy K. Development of multiple QSAR models for consensus predictions and unified mechanistic interpretations of the free-radical scavenging activities of chromone derivatives. J. Mol. Model. 2012;18(5):1819–1840. doi: 10.1007/s00894-011-1198-x. [DOI] [PubMed] [Google Scholar]
- 208.Elgazwy A-S.S.H., Edrees M.M., Ismail N.S.M. Molecular modeling study bioactive natural product of khellin analogues as a novel potential pharmacophore of EGFR inhibitors. J. Enzyme Inhib. Med. Chem. 2013;28(6):1171–1181. doi: 10.3109/14756366.2012.719504. [DOI] [PubMed] [Google Scholar]
- 209.Helguera A.M., Pérez-Garrido A., Gaspar A., Reis J., Cagide F., Vina D., Cordeiro M.N.D.S., Borges F. Combining QSAR classification models for predictive modeling of human monoamine oxidase inhibitors. Eur. J. Med. Chem. 2013;59:75–90. doi: 10.1016/j.ejmech.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 210.Gobbi S., Hu Q., Zimmer C., Engel M., Belluti F., Rampa A., Hartmann R.W., Bisi A. Exploiting the chromone scaffold for the development of inhibitors of corticosteroid biosynthesis. J. Med. Chem. 2016;59(6):2468–2477. doi: 10.1021/acs.jmedchem.5b01609. [DOI] [PubMed] [Google Scholar]
- 211.Pires A.D.R.A., Lecerf-Schmidt F., Guragossian N., Pazinato J., Gozzi G.J., Winter E., Valdameri G., Veale A., Boumendjel A., Di Pietro A., Pérès B. New, highly potent and non-toxic, chromone inhibitors of the human breast cancer resistance protein ABCG2. Eur. J. Med. Chem. 2016;122:291–301. doi: 10.1016/j.ejmech.2016.05.053. [DOI] [PubMed] [Google Scholar]
- 212.Mohadeszadeh M., Iranshahi M. Recent Advances in the catalytic one-pot synthesis of flavonoids and chromones. Mini Rev. Med. Chem. 2017;17(14):1377–1397. doi: 10.2174/1389557517666170315124951. [DOI] [PubMed] [Google Scholar]
- 213.Mathew B., Dev S., Joy M., Mathew G.E., Marathakam A., Krishnan G.K. Refining the structural features of chromones as selective MAO-B inhibitors: exploration of combined pharmacophore-based 3D-QSAR and quantum chemical studies. ChemistrySelect. 2017;2:11645–11652. doi: 10.1002/slct.201701213. [DOI] [Google Scholar]
- 214.Silva C.F.M., Pinto D.C.G.A., Silva A.M.S. Chromones: privileged scaffolds for the production of multi-target-directed-ligand agents for the treatment of Alzheimer’s disease. Expert Opin. Drug Discov. 2018;13(12):1141–1151. doi: 10.1080/17460441.2018.1543267. [DOI] [PubMed] [Google Scholar]
- 215.Liu Q., Qiang X., Li Y., Sang Z., Li Y., Tan Z., Deng Y. Design, synthesis and evaluation of chromone-2-carboxamido-alkylbenzylamines as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2015;23(5):911–923. doi: 10.1016/j.bmc.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 216.Silva C.F.M., Pinto D.C.G.A., Silva A.M.S. Chromones: A Promising Ring System for New Anti-inflammatory Drugs. ChemMedChem. 2016;11(20):2252–2260. doi: 10.1002/cmdc.201600359. [DOI] [PubMed] [Google Scholar]
- 217.Opretzka L.C.F., Espírito-Santo R.F.D., Nascimento O.A., Abreu L.S., Alves I.M., Döring E., Soares M.B.P., Velozo E.D.S., Laufer S.A., Villarreal C.F. Natural chromones as potential anti-inflammatory agents: Pharmacological properties and related mechanisms. Int. Immunopharmacol. 2019;72:31–39. doi: 10.1016/j.intimp.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 218.Shaveta, Singh A., Kaur M., Sharma S., Bhatti R., Singh P. Rational design, synthesis and evaluation of chromone-indole and chromone-pyrazole based conjugates: identification of a lead for anti-inflammatory drug. Eur. J. Med. Chem. 2014;77:185–192. doi: 10.1016/j.ejmech.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 219.Thombre N.A., Gaikwad S.M., Chaudhari K.S. A review on analgesic herbals. Pharma Tutor. 2019:7. [Google Scholar]
- 220.Grazul M., Budzisz E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord. Chem. Rev. 2009;253:2588–2598. doi: 10.1016/j.ccr.2009.06.015. [DOI] [Google Scholar]
- 221.Gomes A., Neuwirth O., Freitas M., Couto D., Ribeiro D., Figueiredo A.G.P.R., Silva A.M.S., Seixas R.S.G.R., Pinto D.C.G.A., Tomé A.C., Cavaleiro J.A.S., Fernandes E., Lima J.L.F.C. Synthesis and antioxidant properties of new chromone derivatives. Bioorg. Med. Chem. 2009;17(20):7218–7226. doi: 10.1016/j.bmc.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 222.Demetgül C., Beyazit N. Synthesis, characterization and antioxidant activity of chitosan-chromone derivatives. Carbohydr. Polym. 2018;181:812–817. doi: 10.1016/j.carbpol.2017.11.074. [DOI] [PubMed] [Google Scholar]
- 223.Ali T.E-S., Ibrahim M.A. Synthesis and antimicrobial activity of chromone-linked 2-pyridone fused with 1,2,4-Triazoles, 1,2,4-Triazines and 1,2,4-Triazepines ring systems. J. Braz. Chem. Soc. 2010;21:1007–1016. doi: 10.1590/S0103-50532010000600010. [DOI] [Google Scholar]
- 224.Kavitha P., Saritha M., Laxma Reddy K. Synthesis, structural characterization, fluorescence, antimicrobial, antioxidant and DNA cleavage studies of Cu(II) complexes of formyl chromone Schiff bases. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013;102:159–168. doi: 10.1016/j.saa.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 225.Dofe V.S., Sarkate A.P., Lokwani D.K., Kathwate S.H., Gill C.H. Synthesis, antimicrobial evaluation, and molecular docking studies of novel chromone based 1,2,3-triazoles. Res. Chem. Intermed. 2017;43:15–28. doi: 10.1007/s11164-016-2602-z. [DOI] [Google Scholar]
- 226.Prakash O., Kumar R., Parkash V. Synthesis and antifungal activity of some new 3-hydroxy-2-(1-phenyl-3-aryl-4-pyrazolyl) chromones. Eur. J. Med. Chem. 2008;43(2):435–440. doi: 10.1016/j.ejmech.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 227.Abdel G.S.B., Mugisha P.J., Wilcox J.C., Gado E.A.M., Medu E.O., Lamb A.J., Brown R.C.D. Convenient one-pot synthesis of chromone derivatives and their antifungal and antibacterial evaluation. Synth. Commun. 2013;43:1549–1556. doi: 10.1080/00397911.2011.647222. [DOI] [Google Scholar]
- 228.Yoon J.S., Lee M.K., Sung S.H., Kim Y.C. Neuroprotective 2-(2-phenylethyl)chromones of Imperata cylindrica. J. Nat. Prod. 2006;69(2):290–291. doi: 10.1021/np0503808. [DOI] [PubMed] [Google Scholar]
- 229.Li F., Wu J.J., Wang J., Yang X.L., Cai P., Liu Q.H., Kong L.Y., Wang X.B. Synthesis and pharmacological evaluation of novel chromone derivatives as balanced multifunctional agents against Alzheimer’s disease. Bioorg. Med. Chem. 2017;25(14):3815–3826. doi: 10.1016/j.bmc.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 230.Keri R.S., Budagumpi S., Pai R.K., Balakrishna R.G. Chromones as a privileged scaffold in drug discovery: a review. Eur. J. Med. Chem. 2014;78:340–374. doi: 10.1016/j.ejmech.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 231.Gaspar A., Matos M.J., Garrido J., Uriarte E., Borges F. Chromone: a valid scaffold in medicinal chemistry. Chem. Rev. 2014;114(9):4960–4992. doi: 10.1021/cr400265z. [DOI] [PubMed] [Google Scholar]
- 232.Makhaeva G.F., Boltneva N.P., Lushchekina S.V., Rudakova E.V., Serebryakova O.G., Kulikova L.N., Beloglazkin A.A., Borisov R.S., Richardson R.J. Synthesis, molecular docking, and biological activity of 2-vinyl chromones: Toward selective butyrylcholinesterase inhibitors for potential Alzheimer’s disease therapeutics. Bioorg. Med. Chem. 2018;26(16):4716–4725. doi: 10.1016/j.bmc.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 233.Baptista F.I., Henriques A.G., Silva A.M.S., Wiltfang J., da Cruz e Silva O.A. Flavonoids as therapeutic compounds targeting key proteins involved in Alzheimer’s disease. ACS Chem. Neurosci. 2014;5(2):83–92. doi: 10.1021/cn400213r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Reis J., Cagide F., Valencia M.E., Teixeira J., Bagetta D., Pérez C., Uriarte E., Oliveira P.J., Ortuso F., Alcaro S., Rodríguez-Franco M.I., Borges F. Multi-target-directed ligands for Alzheimer’s disease: Discovery of chromone-based monoamine oxidase/cholinesterase inhibitors. Eur. J. Med. Chem. 2018;158:781–800. doi: 10.1016/j.ejmech.2018.07.056. [DOI] [PubMed] [Google Scholar]
- 235.Guglielmi P., Carradori S., Ammazzalorso A., Secci D. Novel approaches to the discovery of selective human monoamine oxidase-B inhibitors: is there room for improvement? Expert Opin. Drug Discov. 2019;14(10):995–1035. doi: 10.1080/17460441.2019.1637415. [DOI] [PubMed] [Google Scholar]
- 236.Li S-Y., Wang X-B., Xie S-S., Jiang N., Wang K.D.G., Yao H-Q., Sun H-B., Kong L-Y. Multifunctional tacrine-flavonoid hybrids with cholinergic, β-amyloid-reducing, and metal chelating properties for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013;69:632–646. doi: 10.1016/j.ejmech.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 237.Li R-S., Wang X-B., Hu X-J., Kong L-Y. Design, synthesis and evaluation of flavonoid derivatives as potential multifunctional acetylcholinesterase inhibitors against Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2013;23(9):2636–2641. doi: 10.1016/j.bmcl.2013.02.095. [DOI] [PubMed] [Google Scholar]
- 238.Wang X.B., Yin F.C., Huang M., Jiang N., Lan J.S., Kong L.Y. Chromone and donepezil hybrids as new multipotent cholinesterase and monoamine oxidase inhibitors for the potential treatment of Alzheimer’s Disease. RSC Med. Chem. 2020;11:225–233. doi: 10.1039/C9MD00441F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pachón-Angona I., Refouvelet B., Andrýs R., Martin H., Luzet V., Iriepa I., Moraleda I., Diez-Iriepa D., Oset-Gasque M.J., Marco-Contelles J., Musilek K., Ismaili L. Donepezil + chromone + melatonin hybrids as promising agents for Alzheimer’s disease therapy. J. Enzyme Inhib. Med. Chem. 2019;34(1):479–489. doi: 10.1080/14756366.2018.1545766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dömling A., Wang W., Wang K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012;112(6):3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Akritopoulou-Zanze I. Isocyanide-based multicomponent reactions in drug discovery. Curr. Opin. Chem. Biol. 2008;12(3):324–331. doi: 10.1016/j.cbpa.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 242.Benchekroun M., Ismaili L., Pudlo M., Luzet V., Gharbi T., Refouvelet B., Marco-Contelles J. Donepezil-ferulic acid hybrids as anti-Alzheimer drugs. Future Med. Chem. 2015;7(1):15–21. doi: 10.4155/fmc.14.148. [DOI] [PubMed] [Google Scholar]
- 243.Hindo S.S., Mancino A.M., Braymer J.J., Liu Y., Vivekanandan S., Ramamoorthy A., Lim M.H. Small molecule modulators of copper-induced Abeta aggregation. J. Am. Chem. Soc. 2009;131(46):16663–16665. doi: 10.1021/ja907045h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jakob-Roetne R., Jacobsen H. Alzheimer’s disease: from pathology to therapeutic approaches. Angew. Chem. Int. Ed. Engl. 2009;48(17):3030–3059. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- 245.Alcaro S., Gaspar A., Ortuso F., Milhazes N., Orallo F., Uriarte E., Yáñez M., Borges F. Chromone-2- and -3-carboxylic acids inhibit differently monoamine oxidases A and B. Bioorg. Med. Chem. Lett. 2010;20(9):2709–2712. doi: 10.1016/j.bmcl.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 246.Gaspar A., Reis J., Fonseca A., Milhazes N., Viña D., Uriarte E., Borges F. Chromone 3-phenylcarboxamides as potent and selective MAO-B inhibitors. Bioorg. Med. Chem. Lett. 2011;21(2):707–709. doi: 10.1016/j.bmcl.2010.11.128. [DOI] [PubMed] [Google Scholar]
- 247.Gaspar A., Silva T., Yáñez M., Vina D., Orallo F., Ortuso F., Uriarte E., Alcaro S., Borges F. Chromone, a privileged scaffold for the development of monoamine oxidase inhibitors. J. Med. Chem. 2011;54(14):5165–5173. doi: 10.1021/jm2004267. [DOI] [PubMed] [Google Scholar]
- 248.Reis J., Cagide F., Chavarria D., Silva T., Fernandes C., Gaspar A., Uriarte E., Remião F., Alcaro S., Ortuso F., Borges F. Discovery of new chemical entities for old targets: insights on the lead optimization of chromone-based monoamine oxidase B (MAO-B) inhibitors. J. Med. Chem. 2016;59(12):5879–5893. doi: 10.1021/acs.jmedchem.6b00527. [DOI] [PubMed] [Google Scholar]
- 249.Starowicz M., Zieliński H. Inhibition of advanced glycation end-product formation by high antioxidant-leveled spices commonly used in european cuisine. Antioxidants. 2019;8(4):8. doi: 10.3390/antiox8040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Uribarri J., del Castillo M.D., de la Maza M.P., Filip R., Gugliucci A., Luevano-Contreras C., Macías-Cervantes M.H., Markowicz Bastos D.H., Medrano A., Menini T., Portero-Otin M., Rojas A., Sampaio G.R., Wrobel K., Wrobel K., Garay-Sevilla M.E. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015;6(4):461–473. doi: 10.3945/an.115.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sadowska-Bartosz I., Bartosz G. Effect of glycation inhibitors on aging and age-related diseases. Mech. Ageing Dev. 2016;160:1–18. doi: 10.1016/j.mad.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 252.Singh M., Silakari O. Design, synthesis and biological evaluation of novel 2-phenyl-1-benzopyran-4-one derivatives as potential poly-functional Anti-Alzheimer’s agents. . RSC Advances. 2016;6:108411–108422. doi: 10.1039/C6RA17678J. [DOI] [Google Scholar]
- 253.Matsuura N., Aradate T., Sasaki C., Kojima H., Ohara M., Hasegawa J., Ubukata M. Screening system for the maillard reaction inhibitor from natural product extracts. J. Health Sci. 2002;48:520–526. doi: 10.1248/jhs.48.520. [DOI] [Google Scholar]
- 254.Budzisz E. Synthesis, reactions and biological activity of phosphorus-containing derivatives of chromone and coumarin. Phosphorus Sulfur Silicon Relat. Elem. 2004;179:2131–2147. doi: 10.1080/10426500490475139. [DOI] [Google Scholar]
- 255.Zwergel C., Valente S., Salvato A., Xu Z., Talhi O., Mai A., Silva A., Altucci L., Kirsch G. Novel benzofuran–chromone and –coumarin derivatives: synthesis and biological activity in k562 human leukemia cells. MedChemComm. 2013;4:1571–1579. doi: 10.1039/c3md00241a. [DOI] [Google Scholar]
- 256.Bubols G.B., Vianna D. da R., Medina-Remon A., von Poser G., Lamuela-Raventos R.M., Eifler-Lima V.L., Garcia S.C. The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem. 2013;13(3):318–334. doi: 10.2174/138955713804999775. [DOI] [PubMed] [Google Scholar]
- 257.Medina F.G., Marrero J.G., Macías-Alonso M., González M.C., Córdova-Guerrero I., Teissier García A.G., Osegueda-Robles S. Coumarin heterocyclic derivatives: chemical synthesis and biological activity. Nat. Prod. Rep. 2015;32(10):1472–1507. doi: 10.1039/C4NP00162A. [DOI] [PubMed] [Google Scholar]
- 258.Singh A., Bimal D., Kumar R., Maikhuri V.K., Thirumal M., Senapati N.N., Prasad A.K. Synthesis and antitubercular activity evaluation of 4-furano-coumarins and 3-furano-Chromones. Synth. Commun. 2018;48:2339–2346. doi: 10.1080/00397911.2018.1480041. [DOI] [Google Scholar]
- 259.Baruah P., Rohman M.A., Yesylevskyy S.O., Mitra S. Therapeutic potency of substituted chromones as Alzheimer’s drug: Elucidation of acetylcholinesterase inhibitory activity through spectroscopic and molecular modelling investigation. Bioimpacts. 2019;9(2):79–88. doi: 10.15171/bi.2019.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Menezes J.C.J.M.D.S., Diederich M.F. Natural dimers of coumarin, chalcones, and resveratrol and the link between structure and pharmacology. Eur. J. Med. Chem. 2019;182:111637. doi: 10.1016/j.ejmech.2019.111637. [DOI] [PubMed] [Google Scholar]
- 261.Hussain M.I., Syed Q.A., Khattak M.N.K., Hafez B., Reigosa M.J., El-Keblawy A. Natural product coumarins: biological and pharmacological perspectives. Biologia (Bratisl.) 2019;74:863–888. doi: 10.2478/s11756-019-00242-x. [DOI] [Google Scholar]
- 262.Borges F., Roleira F., Milhazes N., Santana L., Uriarte E. Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity. Curr. Med. Chem. 2005;12(8):887–916. doi: 10.2174/0929867053507315. [DOI] [PubMed] [Google Scholar]
- 263.Srivastava P., Vyas V.K., Variya B., Patel P., Qureshi G., Ghate M. Synthesis, anti-inflammatory, analgesic, 5-lipoxygenase (5-LOX) inhibition activities, and molecular docking study of 7-substituted coumarin derivatives. Bioorg. Chem. 2016;67:130–138. doi: 10.1016/j.bioorg.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 264.Fonseca A., Reis J., Silva T., Matos M.J., Bagetta D., Ortuso F., Alcaro S., Uriarte E., Borges F. Coumarin versus chromone monoamine oxidase b inhibitors: quo vadis? J. Med. Chem. 2017;60(16):7206–7212. doi: 10.1021/acs.jmedchem.7b00918. [DOI] [PubMed] [Google Scholar]
- 265.Shaikh S., Dhavan P., Ramana M. M. V., Jadhav B. L. Design, synthesis and evaluation of new chromone-derived aminophosphonates as potential acetylcholinesterase inhibitor. Mol. Divers. 2020 doi: 10.1007/s11030-020-10060-y. [DOI] [PubMed] [Google Scholar]
- 266.Valasani K.R., Hu G., Chaney M.O., Yan S.S. Structure-based design and synthesis of benzothiazole phosphonate analogues with inhibitors of human ABAD-Aβ for treatment of Alzheimer’s disease. Chem. Biol. Drug Des. 2013;81(2):238–249. doi: 10.1111/cbdd.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hopkins F.G., Cole S.W. A contribution to the chemistry of proteids: Part I. A preliminary study of a hitherto undescribed product of tryptic digestion. J. Physiol. 1901;27(4-5):418–428. doi: 10.1113/jphysiol.1901.sp000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sravanthi T.V., Manju S.L. Indoles - A promising scaffold for drug development. Eur. J. Pharm. Sci. 2016;91:1–10. doi: 10.1016/j.ejps.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 269.Kochanowska-Karamyan A.J., Hamann M.T. Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010;110(8):4489–4497. doi: 10.1021/cr900211p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chadha N., Silakari O. Elsevier; 2018. Chapter 8 - Indoles: as multitarget directed ligands in medicinal chemistry; Silakari, O. B. T.-K. H. C. for D. M. M., Ed. pp. 285–321. [Google Scholar]
- 271.de Sá Alves F.R., Barreiro E.J., Fraga C.A.M. From nature to drug discovery: the indole scaffold as a ‘privileged structure’. Mini Rev. Med. Chem. 2009;9(7):782–793. doi: 10.2174/138955709788452649. [DOI] [PubMed] [Google Scholar]
- 272.Wu Y-J. In: Gribble G. W., Heidelberg S.B., editors. Berlin, Heidelberg: 2010. New Indole-Containing Medicinal Compounds BT - Heterocyclic Scaffolds II: reactions and applications of indoles. pp. 1–29. [Google Scholar]
- 273.Andreani A., Burnelli S., Granaiola M., Leoni A., Locatelli A., Morigi R., Rambaldi M., Varoli L., Landi L., Prata C., Berridge M.V., Grasso C., Fiebig H-H., Kelter G., Burger A.M., Kunkel M.W. Antitumor activity of bis-indole derivatives. J. Med. Chem. 2008;51(15):4563–4570. doi: 10.1021/jm800194k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yan J., Chen J., Zhang S., Hu J., Huang L., Li X. Synthesis, evaluation, and mechanism study of novel indole-chalcone derivatives exerting effective antitumor activity through microtubule destabilization in vitro and in vivo. J. Med. Chem. 2016;59(11):5264–5283. doi: 10.1021/acs.jmedchem.6b00021. [DOI] [PubMed] [Google Scholar]
- 275.Lenox H., Dick H., Mccawley E.L. Clinical pharmacologic observations of the effects of ajmaline in chronic atrial fibrillation. Clin. Pharmacol. Ther. 1963;4:315–320. doi: 10.1002/cpt196343315. [DOI] [PubMed] [Google Scholar]
- 276.Mohan J.C., Kaul U., Bhatia M.L. Acute effects of anti-arrhythmic drugs on cardiac pacing threshold. Acta Cardiol. 1984;39(3):191–201. [PubMed] [Google Scholar]
- 277.Yang Z-H., Wang S-B., Du G-H. In: Ajmaline BT - Natural small molecule drugs from plants; Du, G.-H. Singapore S., editor. Singapore: 2018. pp. 5–11. [DOI] [Google Scholar]
- 278.Batiha G.E-S., Alkazmi L.M., Nadwa E.H., Rashwan E.K., Beshbishy A.M., Shaheen H., Wasef L. Physostigmine: a plant alkaloid isolated from physostigma venenosum: a review on pharmacokinetics, pharmacological and toxicological activities. J. Drug Deliv. Ther. 2020:10. doi: 10.22270/jddt.v10i1-s.3866. [DOI] [Google Scholar]
- 279.Harman R.E., Meisinger M.A., Davis G.E., Kuehl F. A. J. The metabolites of indomethacin, a new anti-inflammatory drug. J. Pharmacol. Exp. Ther. 1964;143:215–220. [PubMed] [Google Scholar]
- 280.Meiri E., Jhangiani H., Vredenburgh J.J., Barbato L.M., Carter F.J., Yang H-M., Baranowski V. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr. Med. Res. Opin. 2007;23(3):533–543. doi: 10.1185/030079907X167525. [DOI] [PubMed] [Google Scholar]
- 281.Jacobson T.A., Chin M.M., Fromell G.J., Jokubaitis L.A., Amorosa L.F. Fluvastatin with and without niacin for hypercholesterolemia. Am. J. Cardiol. 1994;74(2):149–154. doi: 10.1016/0002-9149(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 282.Dicpinigaitis P.V., Dobkin J.B., Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough-variant asthma. J. Asthma. 2002;39(4):291–297. doi: 10.1081/JAS-120002285. [DOI] [PubMed] [Google Scholar]
- 283.Luo X-T., Wang C-M., Liu Y., Huang Z-G. New multifunctional melatonin-derived benzylpyridinium bromides with potent cholinergic, antioxidant, and neuroprotective properties as innovative drugs for Alzheimer’s disease. Eur. J. Med. Chem. 2015;103:302–311. doi: 10.1016/j.ejmech.2015.08.052. [DOI] [PubMed] [Google Scholar]
- 284.Zheng H., Youdim M.B.H., Fridkin M. Site-activated multifunctional chelator with acetylcholinesterase and neuroprotective-neurorestorative moieties for Alzheimer’s therapy. J. Med. Chem. 2009;52(14):4095–4098. doi: 10.1021/jm900504c. [DOI] [PubMed] [Google Scholar]
- 285.Chojnacki J.E., Liu K., Yan X., Toldo S., Selden T., Estrada M., Rodríguez-Franco M.I., Halquist M.S., Ye D., Zhang S. Discovery of 5-(4-hydroxyphenyl)-3-oxo-pentanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide as a neuroprotectant for Alzheimer’s disease by hybridization of curcumin and melatonin. ACS Chem. Neurosci. 2014;5(8):690–699. doi: 10.1021/cn500081s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Dringenberg H.C. Alzheimer’s disease: more than a ‘cholinergic disorder’ - evidence that cholinergic-monoaminergic interactions contribute to EEG slowing and dementia. Behav. Brain Res. 2000;115(2):235–249. doi: 10.1016/S0166-4328(00)00261-8. [DOI] [PubMed] [Google Scholar]
- 287.Bolea I., Juárez-Jiménez J., de Los Ríos C., Chioua M., Pouplana R., Luque F.J., Unzeta M., Marco-Contelles J., Samadi A. Synthesis, biological evaluation, and molecular modeling of donepezil and N-[(5-(benzyloxy)-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine hybrids as new multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of Alzheimer’s disease. J. Med. Chem. 2011;54(24):8251–8270. doi: 10.1021/jm200853t. [DOI] [PubMed] [Google Scholar]
- 288.Bautista-Aguilera O.M., Esteban G., Bolea I., Nikolic K., Agbaba D., Moraleda I., Iriepa I., Samadi A., Soriano E., Unzeta M., Marco-Contelles J. Design, synthesis, pharmacological evaluation, QSAR analysis, molecular modeling and ADMET of novel donepezil-indolyl hybrids as multipotent cholinesterase/monoamine oxidase inhibitors for the potential treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014;75:82–95. doi: 10.1016/j.ejmech.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 289.Stevens C.B., Hanna J.M.J., Jr, Lammi R.K. Synthesis of tetrahydroxybiphenyls and tetrahydroxyterphenyls and their evaluation as amyloid-β aggregation inhibitors. Bioorg. Med. Chem. Lett. 2013;23(6):1703–1706. doi: 10.1016/j.bmcl.2013.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wang J., Wang Z-M., Li X-M., Li F., Wu J-J., Kong L-Y., Wang X-B. Synthesis and evaluation of multi-target-directed ligands for the treatment of Alzheimer’s disease based on the fusion of donepezil and melatonin. Bioorg. Med. Chem. 2016;24(18):4324–4338. doi: 10.1016/j.bmc.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 291.Cuadrado-Tejedor M., Hervias I., Ricobaraza A., Puerta E., Pérez-Roldán J.M., García-Barroso C., Franco R., Aguirre N., García-Osta A. Sildenafil restores cognitive function without affecting β-amyloid burden in a mouse model of Alzheimer’s disease. Br. J. Pharmacol. 2011;164(8):2029–2041. doi: 10.1111/j.1476-5381.2011.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.García-Barroso C., Ricobaraza A., Pascual-Lucas M., Unceta N., Rico A.J., Goicolea M.A., Sallés J., Lanciego J.L., Oyarzabal J., Franco R., Cuadrado-Tejedor M., García-Osta A. Tadalafil crosses the blood-brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology. 2013;64:114–123. doi: 10.1016/j.neuropharm.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 293.Jin F., Gong Q-H., Xu Y-S., Wang L-N., Jin H., Li F., Li L-S., Ma Y-M., Shi J-S. Icariin, a phosphodiesterase-5 inhibitor, improves learning and memory in APP/PS1 transgenic mice by stimulation of NO/cGMP signalling. Int. J. Neuropsychopharmacol. 2014;17(6):871–881. doi: 10.1017/S1461145713001533. [DOI] [PubMed] [Google Scholar]
- 294.Mao F., Wang H., Ni W., Zheng X., Wang M., Bao K., Ling D., Li X., Xu Y., Zhang H., Li J. Design, Synthesis, and biological evaluation of orally available first-generation dual-target selective inhibitors of acetylcholinesterase (AChE) and Phosphodiesterase 5 (PDE5) for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2018;9(2):328–345. doi: 10.1021/acschemneuro.7b00345. [DOI] [PubMed] [Google Scholar]
- 295.Puzzo D., Staniszewski A., Deng S.X., Privitera L., Leznik E., Liu S., Zhang H., Feng Y., Palmeri A., Landry D.W., Arancio O. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer’s disease mouse model. J. Neurosci. 2009;29(25):8075–8086. doi: 10.1523/JNEUROSCI.0864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.García-Osta A., Cuadrado-Tejedor M., García-Barroso C., Oyarzábal J., Franco R. Phosphodiesterases as therapeutic targets for Alzheimer’s disease. ACS Chem. Neurosci. 2012;3(11):832–844. doi: 10.1021/cn3000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Fiorito J., Saeed F., Zhang H., Staniszewski A., Feng Y., Francis Y.I., Rao S., Thakkar D.M., Deng S-X., Landry D.W., Arancio O. Synthesis of quinoline derivatives: discovery of a potent and selective phosphodiesterase 5 inhibitor for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013;60:285–294. doi: 10.1016/j.ejmech.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Prickaerts J., Sik A., van der Staay F.J., de Vente J., Blokland A. Dissociable effects of acetylcholinesterase inhibitors and phosphodiesterase type 5 inhibitors on object recognition memory: acquisition versus consolidation. Psychopharmacology (Berl.) 2005;177(4):381–390. doi: 10.1007/s00213-004-1967-7. [DOI] [PubMed] [Google Scholar]
- 299.Sportsman J.R., Gaudet E.A., Boge A. Immobilized metal ion affinity-based fluorescence polarization (IMAP): advances in kinase screening. Assay Drug Dev. Technol. 2004;2(2):205–214. doi: 10.1089/154065804323056549. [DOI] [PubMed] [Google Scholar]
- 300.Nunes I.K. da C., de Souza E.T., Cardozo S.V.S., Carvalho V.F., Romeiro N.C., Silva P.M.R.E., Martins M.A., Barreiro E.J., Lima L.M. Synthesis, pharmacological profile and docking studies of new sulfonamides designed as phosphodiesterase-4 inhibitors. PLoS One. 2016;11(10):e0162895. doi: 10.1371/journal.pone.0162895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Claeysen S., Bockaert J., Giannoni P. Serotonin: A New Hope in Alzheimer’s Disease? ACS Chem. Neurosci. 2015;6(7):940–943. doi: 10.1021/acschemneuro.5b00135. [DOI] [PubMed] [Google Scholar]
- 302.Lalut J., Karila D., Dallemagne P., Rochais C. Modulating 5-HT4 and 5-HT6 receptors in Alzheimer’s disease treatment. Future Med. Chem. 2017;9(8):781–795. doi: 10.4155/fmc-2017-0031. [DOI] [PubMed] [Google Scholar]
- 303.Cachard-Chastel M., Lezoualc’h F., Dewachter I., Deloménie C., Croes S., Devijver H., Langlois M., Van Leuven F., Sicsic S., Gardier A.M. 5-HT4 receptor agonists increase sAPPalpha levels in the cortex and hippocampus of male C57BL/6j mice. Br. J. Pharmacol. 2007;150(7):883–892. doi: 10.1038/sj.bjp.0707178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Cochet M., Donneger R., Cassier E., Gaven F., Lichtenthaler S.F., Marin P., Bockaert J., Dumuis A., Claeysen S. 5-HT4 receptors constitutively promote the non-amyloidogenic pathway of APP cleavage and interact with ADAM10. ACS Chem. Neurosci. 2013;4(1):130–140. doi: 10.1021/cn300095t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lalut J., Santoni G., Karila D., Lecoutey C., Davis A., Nachon F., Silman I., Sussman J., Weik M., Maurice T., Dallemagne P., Rochais C. Novel multitarget-directed ligands targeting acetylcholinesterase and σ1 receptors as lead compounds for treatment of Alzheimer’s disease: Synthesis, evaluation, and structural characterization of their complexes with acetylcholinesterase. Eur. J. Med. Chem. 2019;162:234–248. doi: 10.1016/j.ejmech.2018.10.064. [DOI] [PubMed] [Google Scholar]
- 306.Eglen R.M., Bonhaus D.W., Johnson L.G., Leung E., Clark R.D. Pharmacological characterization of two novel and potent 5-HT4 receptor agonists, RS 67333 and RS 67506, in vitro and in vivo. Br. J. Pharmacol. 1995;115(8):1387–1392. doi: 10.1111/j.1476-5381.1995.tb16628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lucas G., Rymar V.V., Du J., Mnie-Filali O., Bisgaard C., Manta S., Lambas-Senas L., Wiborg O., Haddjeri N., Piñeyro G., Sadikot A.F., Debonnel G. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55(5):712–725. doi: 10.1016/j.neuron.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 308.Lamirault L., Simon H. Enhancement of place and object recognition memory in young adult and old rats by RS 67333, a partial agonist of 5-HT4 receptors. Neuropharmacology. 2001;41(7):844–853. doi: 10.1016/S0028-3908(01)00123-X. [DOI] [PubMed] [Google Scholar]
- 309.Rochais C., Lecoutey C., Gaven F., Giannoni P., Hamidouche K., Hedou D., Dubost E., Genest D., Yahiaoui S., Freret T., Bouet V., Dauphin F., Sopkova de Oliveira Santos J., Ballandonne C., Corvaisier S., Malzert-Fréon A., Legay R., Boulouard M., Claeysen S., Dallemagne P. Novel multitarget-directed ligands (MTDLs) with acetylcholinesterase (AChE) inhibitory and serotonergic subtype 4 receptor (5-HT4R) agonist activities as potential agents against Alzheimer’s disease: the design of donecopride. J. Med. Chem. 2015;58(7):3172–3187. doi: 10.1021/acs.jmedchem.5b00115. [DOI] [PubMed] [Google Scholar]
- 310.Rochais C., Lecoutey C., Hamidouche K., Giannoni P., Gaven F., Cem E., Mignani S., Baranger K., Freret T., Bockaert J., Rivera S., Boulouard M., Dallemagne P., Claeysen S. Donecopride, a Swiss army knife with potential against Alzheimer’s disease. Br. J. Pharmacol. 2020;177(9):1988–2005. doi: 10.1111/bph.14964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Freret T., Bouet V., Quiedeville A., Nee G., Dallemagne P., Rochais C., Boulouard M. Synergistic effect of acetylcholinesterase inhibition (donepezil) and 5-HT(4) receptor activation (RS67333) on object recognition in mice. Behav. Brain Res. 2012;230(1):304–308. doi: 10.1016/j.bbr.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 312.Maurice T., Hiramatsu M., Itoh J., Kameyama T., Hasegawa T., Nabeshima T. Behavioral evidence for a modulating role of sigma ligands in memory processes. I. Attenuation of dizocilpine (MK-801)-induced amnesia. Brain Res. 1994;647(1):44–56. doi: 10.1016/0006-8993(94)91397-8. [DOI] [PubMed] [Google Scholar]
- 313.Maurice T., Junien J.L., Privat A. Dehydroepiandrosterone sulfate attenuates dizocilpine-induced learning impairment in mice via sigma 1-receptors. Behav. Brain Res. 1997;83(1-2):159–164. doi: 10.1016/S0166-4328(97)86061-5. [DOI] [PubMed] [Google Scholar]
- 314.Modh R.P., Kumar S.P., Jasrai Y.T., Chikhalia K.H. Design, synthesis, biological evaluation, and molecular modeling of coumarin-piperazine derivatives as acetylcholinesterase inhibitors. Arch. Pharm. (Weinheim) 2013;346(11):793–804. doi: 10.1002/ardp.201300242. [DOI] [PubMed] [Google Scholar]
- 315.Heinrich T., Böttcher H., Gericke R., Bartoszyk G.D., Anzali S., Seyfried C.A., Greiner H.E., Van Amsterdam C. Synthesis and structure--activity relationship in a class of indolebutylpiperazines as dual 5-HT(1A) receptor agonists and serotonin reuptake inhibitors. J. Med. Chem. 2004;47(19):4684–4692. doi: 10.1021/jm040793q. [DOI] [PubMed] [Google Scholar]
- 316.Modica M.N., Intagliata S., Pittalà V., Salerno L., Siracusa M.A., Cagnotto A., Salmona M., Romeo G. Synthesis and binding properties of new long-chain 4-substituted piperazine derivatives as 5-HT₁A and 5-HT₇ receptor ligands. Bioorg. Med. Chem. Lett. 2015;25(7):1427–1430. doi: 10.1016/j.bmcl.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 317.Oliveira C., Bagetta D., Cagide F., Teixeira J., Amorim R., Silva T., Garrido J., Remião F., Uriarte E., Oliveira P.J., Alcaro S., Ortuso F., Borges F. Benzoic acid-derived nitrones: A new class of potential acetylcholinesterase inhibitors and neuroprotective agents. Eur. J. Med. Chem. 2019;174:116–129. doi: 10.1016/j.ejmech.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 318.Ghanei-Nasab S., Khoobi M., Hadizadeh F., Marjani A., Moradi A., Nadri H., Emami S., Foroumadi A., Shafiee A. Synthesis and anticholinesterase activity of coumarin-3-carboxamides bearing tryptamine moiety. Eur. J. Med. Chem. 2016;121:40–46. doi: 10.1016/j.ejmech.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 319.Evrard D.A., Zhou P., Yi S.Y., Zhou D., Smith D.L., Sullivan K.M., Hornby G.A., Schechter L.E., Andree T.H., Mewshaw R.E. Studies towards the next generation of antidepressants. Part 4: derivatives of 4-(5-fluoro-1H-indol-3-yl)cyclohexylamine with affinity for the serotonin transporter and the 5-HT1A receptor. Bioorg. Med. Chem. Lett. 2005;15(4):911–914. doi: 10.1016/j.bmcl.2004.12.064. [DOI] [PubMed] [Google Scholar]
- 320.Ojeda-Gómez C., Pessoa-Mahana H., Iturriaga-Vásquez P., Pessoa-Mahana C.D., Recabarren-Gajardo G., Méndez-Rojas C. Synthesis and biological screening of novel indolalkyl arenes targeting the serotonine transporter. Arch. Pharm. (Weinheim) 2014;347(3):174–184. doi: 10.1002/ardp.201300321. [DOI] [PubMed] [Google Scholar]
- 321.Pessoa-Mahana H., Silva-Matus P., Pessoa-Mahana C. D., Chung H., Iturriaga-Vásquez P., Quiroz G., Möller-Acuña P., Zapata-Torres G., Saitz-Barría C., Araya-Maturana R., Reyes-Parada M. Synthesis and docking of novel 3-indolylpropyl derivatives as new polypharmacological agents displaying affinity for 5-HT(1A) R/SERT. Arch. Pharm. (Weinheim). 2017:350. doi: 10.1002/ardp.201600271. [DOI] [PubMed] [Google Scholar]