Abstract
Migraine is a common chronic neurovascular disease characterized by headaches. Calcitonin gene-related peptide (CGRP) signaling in the trigeminovascular system plays a critical role in the development of migraine. The monoclonal antibodies against CGRP and its receptor have been used clinically for the prevention of migraine; however, they may not be a cost-effective option for patients with low-frequency episodic migraine. Thus, it is quite valuable to search for an alternative strategy to downregulate CGRP signaling. Uncariae Ramulus Cum Uncis (UR) has a long-term history for the treatment of cardiovascular and central nervous systems disorders in China and Eastern Asia. Several clinical studies showed that famous herbal formulas comprising UR were able to improve headaches in migraineurs. In addition, increasing in vivo studies further indicated that migraine-related changes, such as CGRP increase, inflammation, nitric oxide increase, and spontaneous behavior problems could be reduced by UR extraction and its active constituents. In this review, we summarize the pathophysiological factors affecting abnormal CGRP release in the trigeminovascular system during a migraine, and for the first time, analyze the effects of UR on these factors and evaluate the potentials of UR for the treatment of migraine.
Keywords: Migraine, CGRP, trigeminovascular system, uncariae ramulus cum uncis, inflammation, nitric oxide
1. INTRODUCTION
Migraine is a common, chronic, intermittently disabling neurovascular disease, which is characterized by mild or severe headaches. The attacks of migraine headaches usually occur on one side of the head and last from hours to days, seriously resulting in a decrease in an individual’s quality of life. The associated symptoms, including scintillations, numbness, weakness, photophobia, nausea, and vomiting, are common [1-3]. Before headache, up to one-third of patients have migraine aura, such as visual, tactile, or behavioral symptoms. The estimated global prevalence of migraine is 15.1% and is more prevalent than diabetes, epilepsy, and asthma [4, 5]. Previously, migraine was considered to result primarily from vascular dysregulation because it was thought to arise from a spasm of a cerebral artery by local hypoxia, followed by the headache from rebound meningeal vasodilation [6]. However, headache onset preceded meningeal vasodilation was observed in migraineurs, which overthrow the previous hypothesis. Up till now, the pathophysiology of migraine is not fully understood. Current studies demonstrate that the onset of migraine is linked to the abnormal secretion of calcitonin gene-related peptides (CGRP) from trigeminal nerves in the trigeminovascular system [7-10], suggesting that CGRP within the trigeminovascular system plays a central role in migraine.
Clinically, previous non-steroidal anti-inflammatory drugs, such as aspirin, indomethacin, diclofenac, and ibuprofen, are generally used for relieving the pain in migraine but cannot cure or prevent the disease [11] Table (1). Besides, they have low efficacy and are prone to drug resistance with adverse effects. Nowadays, CGRP targeting therapies are designed specifically to act on the trigeminovascular system and prevent migraine attacks, offering dramatic improvements over existing medicine and little or no side effects. Monoclonal antibodies targeting the trigeminal sensory neuropeptide CGRP (Eptinezumab, Galcanezumab, and Fremanezumab) or the CGRP receptor (Erenumab) are effective to prevent the migraine headache before they occur, whereas CGRP receptor antagonists (Gepants) work effectively for acute relief of migraine attacks [12]. Although anti-CGRP therapy is the first to be used clinically for the prevention of episodic migraine (<15 days/month) and chronic migraine (15 or >15 days/month), these kinds of antibodies need to be administered monthly by subcutaneous injection and may not be a cost-effective option for patients with low-frequency episodic migraine [12]. Therefore, searching for herbal medicines targeting CGRP signaling may be the promising alternative strategy for the treatment of migraine.
Table 1.
Drugs for the treatment or prevention of migraine.
| Drug | Action Mechanism | Indication |
|---|---|---|
| Aspirin | Nonsteroidal anti-inflammatory drug | Acute treatment |
| Indomethacin | Nonsteroidal anti-inflammatory drug | Acute treatment |
| Diclofenac | Nonsteroidal anti-inflammatory drug | Acute treatment |
| Ibuprofen | Nonsteroidal anti-inflammatory drug | Acute treatment |
| Flunarizine | Calcium channel blocker | Prevention |
| Triptans | 5-HT1B/D receptor agonist | Acute treatment |
| Lasmiditan | 5-HT1F receptor agonist | Acute treatment |
| Gepants | CGRP receptor antagonist | Acute treatment |
| Fremanezumab | Monoclonal antibody targeting CGRP | Prevention |
| Galcanezumab | Monoclonal antibody targeting CGRP | Prevention |
| Eptinezumab | Monoclonal antibody targeting CGRP | Prevention |
| Erenumab | Monoclonal antibody targeting CGRP receptor | Prevention |
Uncariae Ramulus Cum Uncis (UR, Gouteng in Chinese) is one of the medicinal herbs, which has a long-term history for the treatment of central nervous and cardiovascular systems disorders in China and Eastern Asia [13, 14]. UR is one of the major components of classical, traditional Chinese medicine (TCM) formula Tianma Gouteng decoction/granule that is widely used for reducing headaches in migraineurs [15, 16]. In Japan, UR is a key component of the famous Kampo medicine formula, known as Yokukansan, that is reported to markedly improve the frequency and severity of chronic migraine [17]. Previous studies indicated that UR extraction and its active components were able to reduce NO release [18], inflammation [19, 20], CGRP increase and spontaneous behavior problems in migraine rat model [21]. Thus, it is of great significance to preform systematic analysis for the effects of UR on CGRP signaling. In this review, we will summarize the pathophysiological factors affecting abnormal CGRP release in the trigeminovascular system during migraine, and, for the first time, analyze the effects of UR on these factors and further evaluate the therapeutic potentials of UR for the treatment of migraine.
2. TRIGEMINOVASCULAR SYSTEM
The trigeminovascular system consists of neurons in the trigeminal nerve that densely innervate the meningeal vasculature. The trigeminal nerve is the fifth cranial nerve (CN V) that contains both motor and sensory nerves. The sensory part is responsible for sensation in the face and head. The cell body of the trigeminal nerve sits in the trigeminal ganglion (TG). The three major branches of the trigeminal nerve include the ophthalmic division (V1), the maxillary division (V2), and the mandibular division (V3) that converges on TG. Sensory nerve fibers related to migraine pain are from the ophthalmic division (V1) of the trigeminal nerve. These trigeminal nerves include thinly non-myelinated C-fibers and primary afferent nociceptive myelinated Aδ- fibers, which are pseudo-unipolar nerves, with cell bodies in TG and bifurcated axons extending towards central and peripheral areas [22]. This specific morphology allows the release of neurotransmitters to the peripheral and central parts for bidirectional communication [23]. In the periphery sites, almost all vasculatures included meningeal and cerebral arteries are innervated by these sensory nerve fibers. The projections from TG converge at trigeminal nucleus caudalis (TNC) of the brainstem. Second order TNC neurons convey trigeminal nociceptive transmission from the dura mater into ventroposterior medial nucleus of the thalamus. Thalamus has bidirectional connections with multiple functionally distinct sensory cortical sites, such as the insula, somatosensory cortex, amygdala, thus combining nociceptive inputs with various sensory responses [24].
3. CGRP AND ITS RECEPTOR
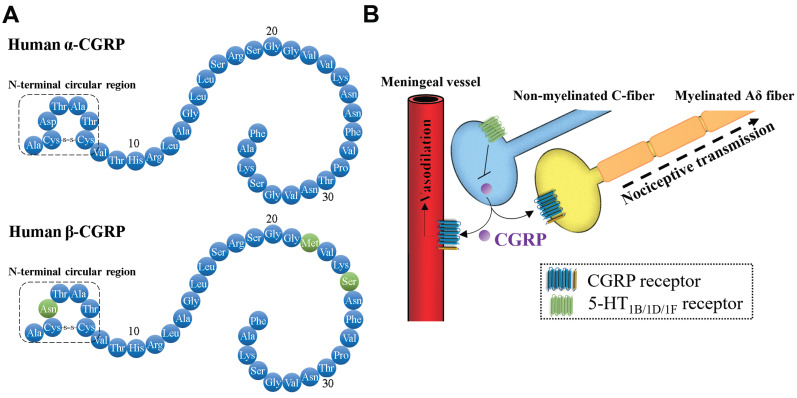
CGRP is a member of the calcitonin family of peptides, which was identified in 1982 [25]. It is a 37-amino acid neuropeptide formed via tissue-specific alternative splicing of mRNA. There are two CGRP isoforms, α-CGRP and β-CGRP (Fig. 1). α-CGRP differs from β-CGRP by three amino acids in humans, but there are no significant differences in the roles of α- and β-CGRP. Although the function of CGRP is mainly associated with sensory Aδ- and C-fibers, it is also found to be linked to smooth muscles in the vasculature and heart [26], suggesting the potential roles of CGRP in pain sensory and cardiovascular regulation. In the trigeminovascular system, CGRP can be released from the cell body, the peripheral end, and the central end of non-myelinated C-fibers. CGRP pro-peptide synthesis mainly occurs at the cell body of non-myelinated C-fibers, subsequently, the pro-peptide is cleaved into the mature form, which is stored in large vesicles at the endings [27]. The activation of calcium-dependent pathways following nerve depolarization mediates exocytosis to release CGRP [28]. In addition, several endogenous substances, such as nerve growth factors, NO, glucocorticoids, and steroid hormones, are reported to promote the release and synthesis of CGRP in the sensory nerves via the activation of the mitogen-activated protein kinase (MAPK) signaling pathway [29].
Fig. (1).
The roles of CGRP in trigeminovascular system. (A) Mature peptide of human α-CGRP and β-CGRP. (B) In the trigeminovascular system, the released CGRP from non-myelinated C-fiber can bind to the CGRP receptor in vascular endothelial cells and vascular smooth muscle cells, subsequently promote the production of NO to trigger vasodilation. In addition, CGRP bind to the CGRP receptor in the terminal of nociceptive transmission from trigeminal nerves to the sensory cortex. Activation of 5-HT1B/1D/1F receptors signaling can block the release of CGRP from C-fiber. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
CGRP triggers cellular responses through binding to the CGRP receptor, which is composed of a calcitonin receptor- like receptor (CRLR) and a receptor activity-modifying protein (RAMP1) [30]. CRLR is one of G protein-coupled receptors, which contains seven transmembrane domains, a long extracellular N-terminus, and a short intracellular C-terminus. RAMP1 is an integral membrane protein with single transmembrane domain with a short intracellular C-terminus region and an extracellular N-terminal. CGRP receptor complex linked to receptor component protein (RCP) can be coupled with G proteins to initiate the cellular signal cascades. CGRP receptors are found in not only the trigeminovascular system but also other body parts, suggesting that CGRP modulates a variety of physiological functions [31].
Since CGRP receptors are present in middle cerebral, middle meningeal, pial, and superficial temporal vessels [32], CGRP can be released from perivascular sensory nerve terminals to plasma, which contributes to its role in vasodilation [27]. The plasma levels of CGRP in healthy people are quite low due to the rapid metabolic clearance of plasma CGRP [33]. Previous studies demonstrated that the intravenous injection of CGRP induced a decline in blood pressure in normotensive and hypertensive animals and humans, suggesting that CGRP is a strong vasodilator [34]. NO-independent or -dependent pathways have been demonstrated to be involved in CGRP/CGRP receptor-induced vasodilation. In the majority of tissues, NO-independent vasodilation is observed in the vascular smooth muscle cells, where binding of CGRP to its receptor increases intracellular cAMP level through G-protein coupled adenylate cyclase [35, 36]. The increased cAMP level activates protein kinase A (PKA) and further, in turn, phosphorylates and opens potassium-sensitive ATP channels, thus relaxes vascular smooth muscle cells. NO-dependent vasodilation occurs in the vascular endothelial cells. The binding of CGRP with CGRP receptor results in the activation of cAMP/PKA/endothelial NO synthases (eNOS) signaling and further promotes the generation and release of NO, acting to relax the underlying vascular smooth muscles. In addition to the vasodilator, CGRP from the terminals of C-fibers also act as a pain-signaling neurotransmitter to sensitize the adjacent Aδ fiber terminals. In the peripheral of trigeminal nerve, myelinated Aδ-fibers also express the components of the CGRP receptor [37, 38]. The released CGRP from the terminals of C-fibers can bind to the CGRP receptor on the terminals of Aδ-fibers for nociceptive transmission [39] (Fig. 1).
4. ABNORMAL CGRP RELEASE IN THE TRIGEMINOVASCULAR SYSTEM DURING MIGRAINE
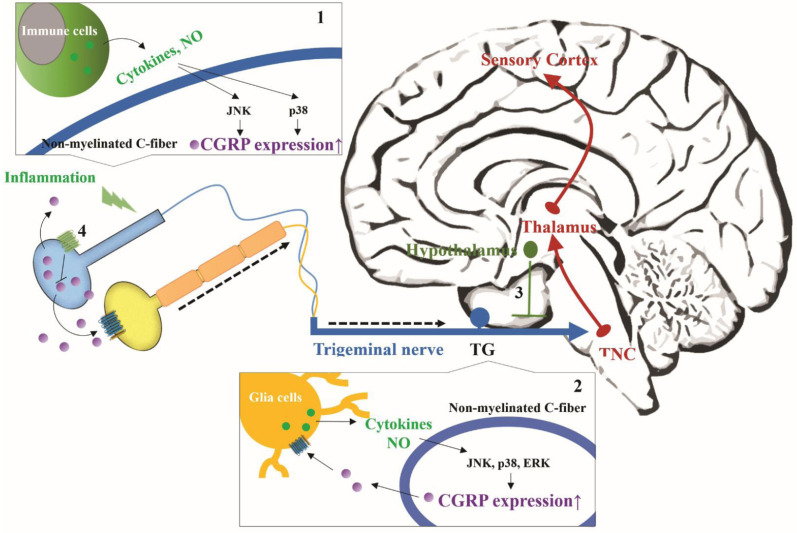
During a migraine attack, the levels of CGRP in blood from the jugular vein of patients are increased. Further studies found that activated sensory neurons in the trigeminovascular system release more CGRP from their peripheral trigeminal nerve terminals during the migraine [40]. Anti-CGRP monoclonal antibodies can prevent repeated CGRP-induced trigeminal nociceptive transmission, but they are large molecules that do not cross the blood-brain barrier, further suggesting that abnormal increase of CGRP occurs in the periphery. However, blocking CGRP by monoclonal antibodies selectively reduces the migraine headache, but not other pains [41], revealing that the effects of CGRP on the nociceptive transmission exhibit the difference between meningeal and non-meningeal peripheral nerves. Thus, it is generally well accepted that CGRP-mediated nociceptive transmission is a critical factor in the attacks of migraine. Notably, the study of Oleson et al. indicated that direct intravenous injection of CGRP is able to induce the migraine- like headache in individuals susceptible to migraine, but not in healthy people [42], revealing that CGRP does not directly cause migraine pain. Edvinsson et al. proposed that migraine attack may initiate in the regions of the central nervous system (dorsal pons, hypothalamus, and thalamus); these regions activate trigeminal nerves and trigger trigeminal nociceptive transmission [43]. The pain transmission, in turn stimulates the initiation regions and re-activates trigeminal nerves. They thought that this cycle confers the persistent activation of CGRP circuits within the trigeminal nerves to amplify migraine pain. However, the abnormal release of CGRP from trigeminal nerves is also considered to result from peripheral sensitization [44]. The peripheral sensitization can reduce the firing threshold of the peripheral sensory nerves to enhance the release of CGRP, and can increase the sensitivity of peripheral afferent nerves to nociceptive stimulus, thus enlarging pain signals [23]. The peripheral sensitization is caused by the increased activity of peripheral nerves in response to inflammation response. In addition, it should not be ignored that other factors such as serotonin, NO, and melatonin can affect the abnormal release of CGRP from trigeminal nerves during migraine. Some other relevant drugs have also proven to reduce migraine pain (Fig. 2).
Fig. (2).
The pathophysiological factors involved in abnormal CGRP release in the trigeminovascular system during migraine. Pro-inflammatory cytokines and NO can enhance CGRP expression during migraine. Their secretion may result from (1) the local inflammation at the end of C-fiber and (2) satellite glial cells within TG. (3) 5-HT signaling and (4) melatonin signaling can down-regulate CGRP expression. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4.1. Serotonin
Serotonin (5-hydroxytryptamine, 5-HT) has long been linked to migraine pathophysiology. 5-HT is a monoamine neurotransmitter, which is synthesized from L-tryptophan in serotonergic neurons. Serotonergic neurons have been found in TG and raphe nuclei located in the brainstem [45]. In addition, 5-HT is stored in platelets and is released into vessels during vasoconstriction and migraine [46]. The injection of 5-HT relieves migraine headache in patients but triggers some side effects such as nausea, faintness, paraesthesia, and dyspnea [47]. 5-HT triggers diverse cellular effects via binding to 5-HT receptors, which are a group of G protein- coupled receptor. 5-HT1B, 5-HT1D, and 5-HT1F receptors are expressed in various locations of the trigeminovascular system, including centrally in TNC and peripherally in the end of trigeminal nerves [48, 49]. 5-HT receptor signaling can inhibit adenylate cyclase. Triptans are a family of 5-HT1B/1D receptor agonists used to treat acute migraine [50]. They are considered to act directly on the peripheral site of the trigeminovascular system because their hydrophobic structure allows poor penetration into the blood-brain barrier [51]. This type of agonist is substantiated by the efficacy of CGRP antagonists and antibodies due to their serious cardiovascular side effects. Lasmiditan is a new approved 5-HT1F receptor agonist for the treatment of acute migraine [49]. It can bypass the blood-brain barrier and work in the central and peripheral parts of the trigeminovascular system without the adverse effects of Triptans. The activation of 5-HT1B/1D/1F receptors signaling has been proven to be able to regulate the release of CGRP negatively.
4.2. NO
NO is a free radical gas, which is generated from L-arginine via different types of NOS. It plays distinct roles in the endocrine, nervous, and immune systems [52]. NTG is an NO donor and has been commonly used as an inducer for migraine model in the animal studies. The administration of NTG into animals or human migraineurs can induce migraine-like symptoms, increase the generation of oxidative stress as well as the expression of CGRP [53, 54]. NOS inhibitor NG-Methyl-L-arginine (L-NMMA) is reported to effectively reduce the attacks of migraine without aura [53]. In the trigeminovascular system, NO and CGRP can amplify each other’s levels [55]. Through acting on endothelial CGRP receptors, released CGRP can trigger the activation of downstream signaling cascades, resulting in the increase of eNOS-mediated NO generation. Released NO not only leads to vasodilation but also diffuses to the sensory non-myelinated C-fibers endings, where it can promote the release of CGRP, revealing that the physiological NO plays a certain role in trigeminovascular system. In addition, satellite glial cells are found to distribute within TG and express the CGRP receptor [56]. CGRP from the trigeminal nerve can activate these TG glia cells to release NO. However, the implication of this communication is still poorly understood. Inflammation is the major pathological source to generate a high level of NO (micromolar), which is considered to contribute to the development of migraine and various chronic diseases [57]. The pathological NO level is considered as one of the major mediators in neuronal damages, including hypoxia ischemic injury, oxidative stress, glutamate neurotoxicity, and neurodegeneration [58]. Increasing evidence suggest that levels of CGRP positively correlate with inflammation [59]. The pathological NO can stimulate the activation of ERK, JNK, and p38 signaling to increase the expression of CGRP [60].
4.3. Pro-inflammatory Cytokines
The levels of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-alpha (TNFα), are increased during migraine attacks [5]. Satellite glial cells, around the cell body of trigeminal nerves, are considered to secrete these pro-inflammatory cytokines in response to CGRP [5]. Complete Freund’s Adjuvant contains inactivated Mycobacterium, commonly used for a non-specific stimulator of the local immune response. The injection of Complete Freund’s Adjuvant to the dura mater can trigger increased CGRP expression in rat TG [61]. Satellite glial cells within TG are found to increase IL1β expression after the administration of Complete Freund’s Adjuvant, revealing that local inflammation causes the peripheral sensitization of trigeminal sensor nerve and further indirectly activates these TG glial cells. The injection of a glial inhibitor minocycline into rat TG site is reported to reduce CGRP-induced thermal nociception, glial activity, and pro-inflammatory cytokines increase, suggesting that satellite glial cells, within TG, contribute to the CGRP-induced pain [62]. In addition, IL-1β and TNFα can stimulate the expression and release of CGRP from sensory neurons [63, 64]. Further study indicates that anti-inflammatory agent, thymoquinone, is able to reduce CGRP increase in plasma, TG, and brain stem in NTG-induced migraine rat model [65]. On the other hand, one of the CGRP functions may act as the mediator in the inflammation. Previous study shows that continued administration of low concentration of CGRP into the paws of rats leads to peripheral sensitization, thereby significantly reducing the response threshold to a mechanical stimulus [66]. CGRP can induce the migration of eosinophil and stimulate T cells adhesion at the inflammation site [67]. Thus, the trigeminovascular system may communicate with the immune system via CGRP. Notably, CGRP may trigger the continued peripheral sensitization of trigeminal nerves, probably through activating satellite glial cells or immune cells to release pro-inflammatory cytokines.
4.4. Melatonin
Melatonin is a biogenic hormone, which is synthesized from 5-HT in the pineal gland. Melatonin secretion is mainly controlled by an endogenous circadian oscillator within the suprachiasmatic nucleus in the hypothalamus [68]. Melatonin receptors, MT1 and MT2 are G protein-coupled receptors and distribute in the brain and peripheral organs [69]. Low urinary levels of the metabolite of melatonin have been found in migraine patients [70]. Some clinical studies suggest that melatonin can help prevent or treat migraine attacks [71, 72]. Melatonin is able to decrease iNOS activity, NO, and IL-1β release in the cultured peripheral blood mononuclear cells from migraine patients [72]. In addition to anti-inflammation activity, melatonin treatment can inhibit CGRP-induced vasodilation in rat middle cerebral arteries [73] and significantly reduce capsaicin-induced neuronal activation in rat TNC [74]. Thus, melatonin signaling, having an anti-excitatory effect on brain activity, may confer inhibitory signal to the active trigeminal nerve for preventing the peripheral sensitization [75].
5. THE THERAPEUTIC EFFECTS OF TIANMA GOUTENG DECOCTION/GRANULE AND YOKUKANSAN ON MIGRAINE
According to the ancient record of TCM, Tianma Gouteng decoction/granule is a classical formula that comprises of UR and other herbs, wildly used for the treatment of headaches [13, 17]. Through the analysis of Tianma Gouteng decoction/granule by ultra-high performance liquid chromatography coupled with quadrupole-tandem time-of-flight mass spectrometry, three UR components, including rhynchophylline, isorhynchophylline, and isocorynoxeine, were identified from 17 major peaks [76]. In clinical studies, Tianma Gouteng decoction exhibited the high efficiency to reduce headaches in migraine patients (93.10% in 58 cases and 92.65% in 68 cases) [15, 16]. In NTG-induced migraine rats, the isolated fraction from Tianma Gouteng decoction/granule could significantly decrease the frequency of head scratching and mediate the abnormal levels of vasoactive neurotransmitters, such as NO, and 5-HT, in serum and brain [77]. Similarly, in patients with hypertension, Tianma Gouteng decoction/granule is able to decrease plasma NO [78]. NO level positively affects CGRP generation in trigeminal nerves, whereas reducing NO level has been proven to reduce CGRP levels and migraine attacks [53]. These studies support the therapeutic effects of Tianma Gouteng decoction/granule on migraine-induced headaches. In addition, a popular Japanese Kampo formula Yokukansan also contains UR, originally used to treat insomnia, irritability, neurosis, and night crying in infants. Recently, several clinical reports indicated that Yokukansan was effective to reduce the frequency and severity of migraine [17, 79, 80]. Notably, UR is the only same ingredient in both formulas Table (2), revealing UR as a critical herb in both formulas for the treatment of migraine.
Table 2.
The effects and composition of Tianma Gouteng decoction, Diaoteng San, and Yokukansan.
|
Traditional Herbal Formula |
Components | Symptom Treatment |
|---|---|---|
| Tianma Gouteng decoction/ granules | UR, Gastrodiae rhizoma, Scutellariae radix, Eucommiae cortex, Cyathulae radix, Loranthus parasiticus, Haliotidis concha, Gardeniae fructus, Leonuri herba, Caulis polygoni multiflori, and Poria cocos. | Headache, dizziness, insomnia, lassitude in legs, red tongue with scanty coating, and wiry-rapid pulse. |
| Yokukansan | UR, Atractylodis lanceae rhizome, Poria, Cnidii rhizome, Angelicae radix, Bupleuri radix, and Glycyrrhizae radix. | Insomnia, neurosis, night crying, and irritability in children. |
6. CHEMICAL CONSTITUENTS OF UR
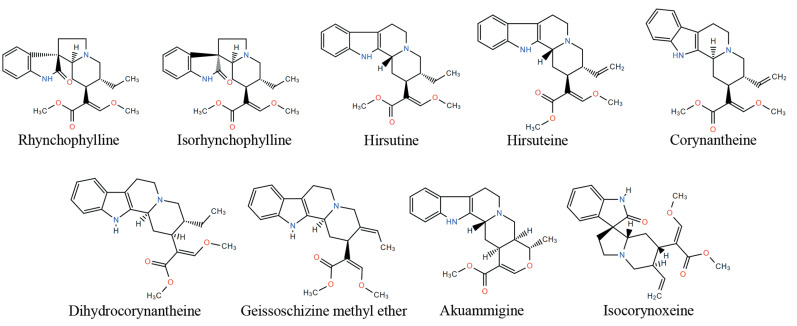
UR belongs to the plant of Rubinaceae genus, which mainly distributes in the torrid zone and the south area of China [81]. The extraction from dry hooked stem of three types of UR, including Uncaria rhynchophylla Miq Jacks, Uncaria sinensis (Oliv) Havil, and Uncaria macrophylla Wall, has been wildly used for the treatment of brain and cardiovascular diseases, such as headache, convulsions, numbness, lightheadedness, and hypertension [82]. According to the current investigation, indole alkaloids are considered as the major bioactive components of UR, which contribute to the therapeutic effects of UR [83-85]. The total alkaloid content in UR is about 0.2%, in which rhynchophylline and isorhynchophylline are the major alkaloids. However, due to quick degradation of isorhynchophylline in water, UR is usually added at last for preparing TCM decoction when the herbs of the prescriptions are decocted. UR also contains a variety of alkaloid structures, such as hirsutine, hirsuteine corynantheine, dihydrocorynantheine, akuammigine, isocorynoxeine, and geissoschizine methyl ether (Fig. 3). In addition, UR contains melatonin and trace components, such as ptcropodic acid and mitraphyllic acid [86].
Fig. (3).
Representative chemical structures of alkaloids of UR.
7. THE EFFECTS OF UR COMPONENTS ON INFLAMMATION
The extracts of UR have been reported to reduce NO and pro-inflammatory cytokines from various inflammation. In the study of Kim et al., the aqueous extracts of UR displayed inhibitory activities on lipopolysaccharide (LPS)-induced NO production in murine RAW 264.7 macrophages via blocking NF-кB pathway [87]. Similarly, Kang et al. found that hexane extracts of UR reduced the generation of NO and TNFα in LPS-stimulated murine BV2 microglial cells via blocking the NF-кB pathway [88]. They further thought that the anti-neuroinflammatory effects of UR extractions contribute to preventing focal cerebral ischemic injuries in mice. Indole alkaloids are major bioactive components from UR extracts, which play a vital role in reducing the inflammatory response. Rhynchophylline is one of the major alkaloids in UR. Yuan et al. reported that rhynchophylline could suppress LPS-induced inflammatory response of mouse N9 microglia by blocking NF-кB-mediated inducible NO synthase (iNOS) protein expression [89]. In the study of Song et al., rhynchophylline also reduced LPS-induced inflammatory response in primary microglia by blocking the NF-кB signaling pathway [90]. Isorhynchophylline is an isomeric structure of rhynchophylline that exhibited more potent inhibition of LPS-stimulated microglial activation than rhynchophylline [89]. Jung et al. found that hirsutine could inhibit inflammation-mediated neurotoxicity and microglial activation [20]. NF-кB is a transcriptional factor that mediates the expression of pro-inflammatory cytokines, iNOS, and cyclooxygenase-2 (COX-2). iNOS and COX-2 are the major enzymes for NO generation [91]. These reports suggest that the UR extracts can reduce NO and pro-inflammatory cytokines from different inflammation via the similar mechanism even though peripheral inflammation is mainly involved in the initiation of migraine attack. These anti-inflammatory activities of UR may confer to the partial effects of the traditional herbal formula of UR on the treatment of migraine.
8. THE EFFECTS OF UR COMPONENTS ON 5-HT AND MELATONIN SIGNALING
UR has been reported to act on the 5-HT receptors. Through a 5-HT receptor binding assay, Kanatani et al. found that UR indole alkaloids, geissoschizine methyl ether, corynantheine and dihydrocorynantheine, could be partial agonists for the 5-HT receptor [92], revealing that corynantheine-type structure has the binding affinity with 5-HT receptor. Through hamster ovary cells expressing 5-HT1A receptors and competitive binding assays, Nish et al. also found that UR extracts could exhibit a partial agonistic effect on 5-HT1A receptors [93]. Jung et al. reported that the aqueous extract of UR exhibited the anxiolytic-like effect in rats, whereas this effect was reduced by WAY100635, a 5-HT1A receptor antagonist [94]. Furthermore, through testing the 5-HT1A receptor binding ability of alkaloids, they found that geissoschizine methyl ether could potently bind to 5-HT1A receptors and act as a potent 5-HT1A receptor agonist [95]. In addition, Uncarialins A-I are newly identified monoterpenoid indole alkaloids from UR, and were also found to be natural agonists of the 5-HT1A receptor [96]. However, 5-HT1A receptor does not distribute in the trigeminal nerves, its agonist was reported to have no therapeutic effects on migraine attacks. Notably, geissoschizine methyl ether can also bind to 5-HT1B receptor [95] but lack further studies to support its agonist effects on 5-HT1B or other migraine-related 5-HT receptors [95]. Interestingly, Xian et al. reported that the intragastric administration of isorhynchophylline significantly enhanced the levels of 5-HT in the mouse hippocampus [97]. However, brain 5-HT cannot bypass the blood-brain barrier to affect the peripheral nerve. 5-HT is the precursor of melatonin, revealing that isorhynchophylline may enhance the generation of melatonin in hippocampus.
In addition to 5-HT receptors, recent studies show that UR constituents also have agonistic activity on melatonin receptor. Through bioassay, Geng et al. found that the extracts of UR stem and hook exhibited agonistic effects on MT1 and MT2 melatonin receptors [98]. They further identified that two UR flavanols, catechin and epicatechin had agonistic activity on melatonin receptors. In addition, Zhang et al. reported that the extracts of different UR species also displayed agonistic effects on melatonin receptors [99]. Several new alkaloids isolated from UR show agonistic activities on both MT1 and MT2 melatonin receptors [100, 101]. The increase in melatonin signaling by these UR components may confer inhibitory signal to the active trigeminal nerve and further suppress the abnormal release of CGRP.
9. THE EFFECTS OF UR COMPONENTS ON CGRP RELEASE IN THE TRIGEMINOVASCULAR SYSTEM
The major UR alkaloid, rhynchophylline, was reported to reduce CGRP expression via inhibiting ERK, p38, and JNK MAPK signaling in the trigeminovascular system. MAPK pathways are involved in the upregulation of CGRP in the trigeminal nerve [102]. Lai et al. reported that NTG could induce migraine-related phenotypes, such as abnormal electroencephalogram and spontaneous behavior problems in rats, whereas the administration of a high dose of rhynchophylline attenuated them [21]. They found that rhynchophylline could attenuate the concentration of CGRP in peripheral blood in this migraine model, revealing that rhynchophylline may inhibit abnormal CGRP release from non-myelinated C-fibers in trigeminovascular system. Through western blotting, they found that NTG significantly increased the activity of ERK, p38, and JNK MAPKs in TNC tissue where the CGRP-expressing trigeminal nerves also divide, whereas rhynchophylline reduced their activation in a dose-dependent manner. Furthermore, they observed that the nuclear translocation of NF-кB increased in model group but decreased in rhynchophylline treatment group. In sensory neurons, NF-кB can positively mediate the expression of CGRP [64]; blocking NF-кB activation has been provided to effectively attenuate NTG-induced migraine [90, 103]. Thus, these studies provide the solid evidence to support the fact that rhynchophylline can suppress CGRP expression in the trigeminovascular system via inhibiting the MAPKs/NF-кB pathway.
CONCLUSION
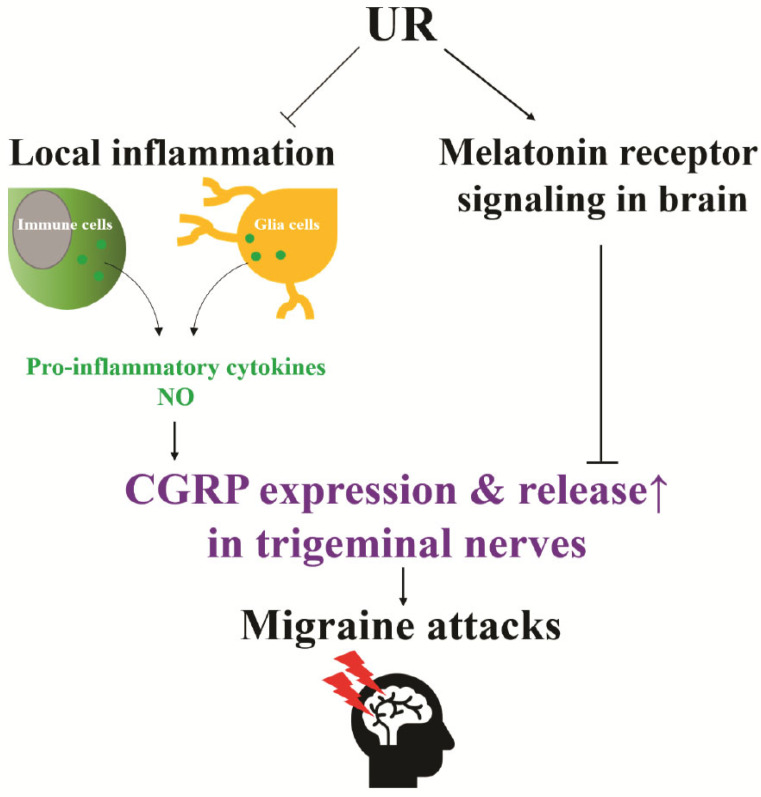
The strong evidence shows that CGRP plays a critical role in the sensitization of trigeminal nociceptive neurons, contributing to the pain experienced in migraine. However, there are various pathophysiological factors affecting abnormal CGRP release in the trigeminovascular system during migraine. Thus, it will be a promising strategy to target these factors to reduce migraine pain and frequency. Tianma Gouteng decoction and Yokukansan composed of UR show clinical efficacy to reduce the symptoms of migraine. Bioactive components of UR have multiple beneficial effects that include reducing inflammation-derived NO and pro-inflammatory cytokines, as well as enhancing melatonin signaling (Fig. 4). These events may contribute to the down-regulation of CGRP levels in the trigeminovascular system, suggesting the potentials of UR for reducing peripheral sensitization and the treatment of migraine attacks. Besides, UR also can increase brain 5-HT level and act as the agonist of melatonin receptors; it may be helpful to prevent migraines via enhancing the inhibitory signal from the hypothalamus. Further studies on UR and its major components are warranted to fully illustrate the underlying molecular mechanisms, pharmacokinetics, and toxicological profiles of these naturally occurring compounds and their potentials for the treatment and prophylaxis of migraine.
Fig. (4).
The effects of UR on down-regulating CGRP expression in the trigeminal nerve via reducing inflammation-derived NO and pro-inflammatory cytokines, as well as enhancing melatonin signaling. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by the grants from The Science and Technology Development Fund, Macau SAR (File no. 0020/2018/A, 0039/2018/A1, 071/2017/A2, and SKL-QRCM(UM)-2020-2022) and the Research Fund of University of Macau (File no. MYRG2019-00143-ICMS).
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
References
- 1.Schürks M., Diener H.C., Goadsby P. Update on the prophylaxis of migraine. Curr. Treat. Options Neurol. 2008;10(1):20–29. doi: 10.1007/s11940-008-0003-3. [DOI] [PubMed] [Google Scholar]
- 2.Yu S.Y., Cao X.T., Zhao G., Yang X.S., Qiao X.Y., Fang Y.N., Feng J.C., Liu R.Z., Steiner T.J. The burden of headache in China: validation of diagnostic questionnaire for a population-based survey. J. Headache Pain. 2011;12(2):141–146. doi: 10.1007/s10194-011-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipton R.B., Bigal M.E., Diamond M., Freitag F., Reed M.L., Stewart W.F., Group A.A. AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 4.Steiner T.J., Stovner L.J., Birbeck G.L. Migraine: the seventh disabler. Headache. 2013;53(2):227–229. doi: 10.1111/head.12034. [DOI] [PubMed] [Google Scholar]
- 5.Edvinsson L., Haanes K.A., Warfvinge K. Does inflammation have a role in migraine? Nat. Rev. Neurol. 2019;15(8):483–490. doi: 10.1038/s41582-019-0216-y. [DOI] [PubMed] [Google Scholar]
- 6.The International Classification of Headache Disorders. 3rd edition. Cephalalgia: 2018. Headache Classification Committee of the International Headache Society (IHS). . [DOI] [PubMed] [Google Scholar]
- 7.Fusayasu E., Kowa H., Takeshima T., Nakaso K., Nakashima K. Increased plasma substance P and CGRP levels, and high ACE activity in migraineurs during headache-free periods. Pain. 2007;128(3):209–214. doi: 10.1016/j.pain.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Jang M.U., Park J.W., Kho H.S., Chung S.C., Chung J.W. Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral Dis. 2011;17(2):187–193. doi: 10.1111/j.1601-0825.2010.01717.x. [DOI] [PubMed] [Google Scholar]
- 9.Goadsby P.J., Edvinsson L., Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28(2):183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 10.Uzar E., Evliyaoglu O., Toprak G., Acar A., Yucel Y., Calisir T., Cevik M.U., Tasdemir N. Increased asymmetric dimethylarginine and nitric oxide levels in patients with migraine. J. Headache Pain. 2011;12(2):239–243. doi: 10.1007/s10194-011-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carville S., Padhi S., Reason T., Underwood M. Guideline Development Group. Diagnosis and management of headaches in young people and adults: summary of NICE guidance. BMJ. 2012;345:e5765. doi: 10.1136/bmj.e5765. [DOI] [PubMed] [Google Scholar]
- 12.The Lancet Neurology. Complicated decisions on new migraine-prevention therapies. Lancet Neurol. 2019;18(3):221. doi: 10.1016/S1474-4422(19)30041-9. [DOI] [PubMed] [Google Scholar]
- 13.Chen B., Wang Y., He Z., Wang D., Yan X., Xie P. Tianma Gouteng decoction for essential hypertension: Protocol for a systematic review and meta-analysis. Medicine (Baltimore) 2018;97(8):e9972. doi: 10.1097/MD.0000000000009972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X., Zhang S. Pharmacology of traditional Chinese medical formulae. Higher Education Press; 1994. [Google Scholar]
- 15.Guangxi C. Pharmacology of traditional Chinese medical formulae. Higher Education Press; 2012. [Google Scholar]
- 16.Nai-qin L. The Analysis of 58 Examples on Healing Migraine by using Tianma Gouteng Drink Addition or Subtraction. Journal of Practical Traditional Chinese Internal Medicine. 2011;(5):51. [Google Scholar]
- 17.Akiyama H., Hasegawa Y. Effectiveness of the traditional Japanese Kampo medicine Yokukansan for chronic migraine: A case report. Medicine (Baltimore) 2019;98(36):e17000. doi: 10.1097/MD.0000000000017000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y.B., Yang W.Z., Yao C.L., Feng R.H., Yang M., Guo D.A., Wu W.Y. New triterpenic acids from Uncaria rhynchophylla: chemistry, NO-inhibitory activity, and tandem mass spectrometric analysis. Fitoterapia. 2014;96:39–47. doi: 10.1016/j.fitote.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Yuan D., Ma B., Wu C., Yang J., Zhang L., Liu S., Wu L., Kano Y. Alkaloids from the leaves of Uncaria rhynchophylla and their inhibitory activity on NO production in lipopolysaccharide-activated microglia. J. Nat. Prod. 2008;71(7):1271–1274. doi: 10.1021/np8000305. [DOI] [PubMed] [Google Scholar]
- 20.Jung H.Y., Nam K.N., Woo B.C., Kim K.P., Kim S.O., Lee E.H. Hirsutine, an indole alkaloid of Uncaria rhynchophylla, inhibits inflammation-mediated neurotoxicity and microglial activation. Mol. Med. Rep. 2013;7(1):154–158. doi: 10.3892/mmr.2012.1135. [DOI] [PubMed] [Google Scholar]
- 21.Lai T., Chen L., Chen X., He J., Lv P., Ge H. Rhynchophylline attenuates migraine in trigeminal nucleus caudalis in nitroglycerin-induced rat model by inhibiting MAPK/NF-кB signaling. Mol. Cell. Biochem. 2019;461(1-2):205–212. doi: 10.1007/s11010-019-03603-x. [DOI] [PubMed] [Google Scholar]
- 22.Iyengar S., Johnson K.W., Ossipov M.H., Aurora S.K. CGRP and the Trigeminal System in Migraine. Headache. 2019;59(5):659–681. doi: 10.1111/head.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basbaum A.I., Bautista D.M., Scherrer G., Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noseda R., Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154(Suppl. 1):S44–S53. doi: 10.1016/j.pain.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Amara S.G., Jonas V., Rosenfeld M.G., Ong E.S., Evans R.M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 26.Csillik B., Tajti L., Kovács T., Kukla E., Rakic P., Knyihár-Csillik E. Distribution of calcitonin gene-related peptide in vertebrate neuromuscular junctions: relationship to the acetylcholine receptor. J. Histochem. Cytochem. 1993;41(10):1547–1555. doi: 10.1177/41.10.8245413. [DOI] [PubMed] [Google Scholar]
- 27.Brain S.D., Grant A.D. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004;84(3):903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 28.Russell F.A., King R., Smillie S.J., Kodji X., Brain S.D. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 2014;94(4):1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durham P.L., Russo A.F. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J. Neurosci. 2003;23(3):807–815. doi: 10.1523/JNEUROSCI.23-03-00807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poyner D.R., Sexton P.M., Marshall I., Smith D.M., Quirion R., Born W., Muff R., Fischer J.A., Foord S.M. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002;54(2):233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 31.Arulmani U., Maassenvandenbrink A., Villalón C.M., Saxena P.R. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur. J. Pharmacol. 2004;500(1-3):315–330. doi: 10.1016/j.ejphar.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 32.Oliver K.R., Wainwright A., Edvinsson L., Pickard J.D., Hill R.G. Immunohistochemical localization of calcitonin receptor-like receptor and receptor activity-modifying proteins in the human cerebral vasculature. J. Cereb. Blood Flow Metab. 2002;22(5):620–629. doi: 10.1097/00004647-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Kraenzlin M.E., Ch’ng J.L., Mulderry P.K., Ghatei M.A., Bloom S.R. Infusion of a novel peptide, calcitonin gene-related peptide (CGRP) in man. Pharmacokinetics and effects on gastric acid secretion and on gastrointestinal hormones. Regul. Pept. 1985;10(2-3):189–197. doi: 10.1016/0167-0115(85)90013-8. [DOI] [PubMed] [Google Scholar]
- 34.Struthers A.D., Brown M.J., Macdonald D.W., Beacham J.L., Stevenson J.C., Morris H.R., MacIntyre I. Human calcitonin gene related peptide: a potent endogenous vasodilator in man. Clin. Sci. (Lond.) 1986;70(4):389–393. doi: 10.1042/cs0700389. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimoto R., Mitsui-Saito M., Ozaki H., Karaki H. Effects of adrenomedullin and calcitonin gene-related peptide on contractions of the rat aorta and porcine coronary artery. Br. J. Pharmacol. 1998;123(8):1645–1654. doi: 10.1038/sj.bjp.0701805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weston C., Winfield I., Harris M., Hodgson R., Shah A., Dowell S.J., Mobarec J.C., Woodlock D.A., Reynolds C.A., Poyner D.R., Watkins H.A., Ladds G. Receptor activity-modifying protein-directed G protein signaling specificity for the calcitonin gene-related peptide family of receptors. J. Biol. Chem. 2016;291(42):21925–21944. doi: 10.1074/jbc.M116.751362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eftekhari S., Warfvinge K., Blixt F.W., Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J. Pain. 2013;14(11):1289–1303. doi: 10.1016/j.jpain.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Goadsby P.J., Holland P.R., Martins-Oliveira M., Hoffmann J., Schankin C., Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol. Rev. 2017;97(2):553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messlinger K. The big CGRP flood - sources, sinks and signalling sites in the trigeminovascular system. J. Headache Pain. 2018;19(1):22. doi: 10.1186/s10194-018-0848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hay D.L., Garelja M.L., Poyner D.R., Walker C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 2018;175(1):3–17. doi: 10.1111/bph.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melo-Carrillo A., Noseda R., Nir R.R., Schain A.J., Stratton J., Strassman A.M., Burstein R. Selective inhibition of trigeminovascular neurons by fremanezumab: A Humanized monoclonal anti-CGRP antibody. J. Neurosci. 2017;37(30):7149–7163. doi: 10.1523/JNEUROSCI.0576-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lassen L.H., Haderslev P.A., Jacobsen V.B., Iversen H.K., Sperling B., Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 43.Edvinsson L., Haanes K.A., Warfvinge K., Krause D.N. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat. Rev. Neurol. 2018;14(6):338–350. doi: 10.1038/s41582-018-0003-1. [DOI] [PubMed] [Google Scholar]
- 44.Iyengar S., Ossipov M.H., Johnson K.W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158(4):543–559. doi: 10.1097/j.pain.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berman N.E., Puri V., Chandrala S., Puri S., Macgregor R., Liverman C.S., Klein R.M. Serotonin in trigeminal ganglia of female rodents: relevance to menstrual migraine. Headache. 2006;46(8):1230–1245. doi: 10.1111/j.1526-4610.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 46.Mück-Seler D., Deanović Z., Dupelj M. Platelet serotonin (5-HT) and 5-HT releasing factor in plasma of migrainous patients. Headache. 1979;19(1):14–17. doi: 10.1111/j.1526-4610.1979.hed1901014.x. [DOI] [PubMed] [Google Scholar]
- 47.Lance J.W. 5-Hydroxytryptamine and its role in migraine. Eur. Neurol. 1991;31(5):279–281. doi: 10.1159/000116754. [DOI] [PubMed] [Google Scholar]
- 48.Lanfumey L., Hamon M. 5-HT1 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004;3(1):1–10. doi: 10.2174/1568007043482570. [DOI] [PubMed] [Google Scholar]
- 49.Vila-Pueyo M. Targeted 5-HT1F therapies for migraine. Neurotherapeutics. 2018;15(2):291–303. doi: 10.1007/s13311-018-0615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juhasz G., Zsombok T., Jakab B., Nemeth J., Szolcsanyi J., Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25(3):179–183. doi: 10.1111/j.1468-2982.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 51.Ahn A.H., Basbaum A.I. Where do triptans act in the treatment of migraine? Pain. 2005;115(1-2):1–4. doi: 10.1016/j.pain.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dawson T.M., Dawson V.L. Nitric oxide synthase: role as a transmitter/mediator in the brain and endocrine system. Annu. Rev. Med. 1996;47:219–227. doi: 10.1146/annurev.med.47.1.219. [DOI] [PubMed] [Google Scholar]
- 53.Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol. Ther. 2008;120(2):157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Iversen H.K., Olesen J., Tfelt-Hansen P. Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain. 1989;38(1):17–24. doi: 10.1016/0304-3959(89)90067-5. [DOI] [PubMed] [Google Scholar]
- 55.Pradhan A.A., Bertels Z., Akerman S. Targeted nitric oxide synthase inhibitors for migraine. Neurotherapeutics. 2018;15(2):391–401. doi: 10.1007/s13311-018-0614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J., Vause C.V., Durham P.L. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 2008;1196:22–32. doi: 10.1016/j.brainres.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flynn M.G., Markofski M.M., Carrillo A.E. Elevated inflammatory status and increased risk of chronic disease in chronological aging: Inflamm-aging or Inflamm-inactivity? Aging Dis. 2019;10(1):147–156. doi: 10.14336/AD.2018.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chong C.M., Ai N., Ke M., Tan Y., Huang Z., Li Y., Lu J.H., Ge W., Su H. Roles of nitric oxide synthase isoforms in neurogenesis. Mol. Neurobiol. 2018;55(3):2645–2652. doi: 10.1007/s12035-017-0513-7. [DOI] [PubMed] [Google Scholar]
- 59.Williamson D.J., Hargreaves R.J. Neurogenic inflammation in the context of migraine. Microsc. Res. Tech. 2001;53(3):167–178. doi: 10.1002/jemt.1081. [DOI] [PubMed] [Google Scholar]
- 60.Bellamy J., Bowen E.J., Russo A.F., Durham P.L. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur. J. Neurosci. 2006;23(8):2057–2066. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lukács M., Haanes K.A., Majláth Z., Tajti J., Vécsei L., Warfvinge K., Edvinsson L. Dural administration of inflammatory soup or Complete Freund’s Adjuvant induces activation and inflammatory response in the rat trigeminal ganglion. J. Headache Pain. 2015;16:564. doi: 10.1186/s10194-015-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Afroz S., Arakaki R., Iwasa T., Oshima M., Hosoki M., Inoue M., Baba O., Okayama Y., Matsuka Y. CGRP induces differential regulation of cytokines from satellite glial cells in trigeminal ganglia and orofacial nociception. Int. J. Mol. Sci. 2019;20(3):E711. doi: 10.3390/ijms20030711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bowen E.J., Schmidt T.W., Firm C.S., Russo A.F., Durham P.L. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J. Neurochem. 2006;96(1):65–77. doi: 10.1111/j.1471-4159.2005.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hou L., Li W., Wang X. Mechanism of interleukin-1 beta-induced calcitonin gene-related peptide production from dorsal root ganglion neurons of neonatal rats. J. Neurosci. Res. 2003;73(2):188–197. doi: 10.1002/jnr.10651. [DOI] [PubMed] [Google Scholar]
- 65.Kilinc E., Tore F., Dagistan Y., Bugdayci G. Thymoquinone inhibits neurogenic inflammation underlying migraine through modulation of calcitonin gene-related peptide release and stabilization of meningeal mast cells in glyceryltrinitrate-induced migraine model in rats. Inflammation. 2020;43(1):264–273. doi: 10.1007/s10753-019-01115-w. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura-Craig M., Gill B.K. Effect of neurokinin A, substance P and calcitonin gene related peptide in peripheral hyperalgesia in the rat paw. Neurosci. Lett. 1991;124(1):49–51. doi: 10.1016/0304-3940(91)90819-F. [DOI] [PubMed] [Google Scholar]
- 67.Springer J., Geppetti P., Fischer A., Groneberg D.A. Calcitonin gene-related peptide as inflammatory mediator. Pulm. Pharmacol. Ther. 2003;16(3):121–130. doi: 10.1016/S1094-5539(03)00049-X. [DOI] [PubMed] [Google Scholar]
- 68.Pévet P. Melatonin. Dialogues Clin. Neurosci. 2002;4(1):57–72. doi: 10.31887/DCNS.2002.4.1/ppevet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin J.J., Lin Y., Zhao T.Z., Zhang C.K., Zhang T., Chen X.L., Ding J.Q., Chang T., Zhang Z., Sun C., Zhao D.D., Zhu J.L., Li Z.Y., Li J.L. Melatonin suppresses neuropathic pain via MT2-dependent and -independent pathways in dorsal root ganglia neurons of mice. Theranostics. 2017;7(7):2015–2032. doi: 10.7150/thno.19500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masruha M.R., de Souza Vieira D.S., Minett T.S., Cipolla-Neto J., Zukerman E., Vilanova L.C., Peres M.F. Low urinary 6-sulphatoxymelatonin concentrations in acute migraine. J. Headache Pain. 2008;9(4):221–224. doi: 10.1007/s10194-008-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peres M.F., Zukerman E., da Cunha T.F., Moreira F.R., Cipolla-Neto J. Melatonin, 3 mg, is effective for migraine prevention. Neurology. 2004;63(4):757. doi: 10.1212/01.WNL.0000134653.35587.24. [DOI] [PubMed] [Google Scholar]
- 72.Ansari M., Karkhaneh A., Kheirollahi A., Emamgholipour S., Rafiee M.H. The effect of melatonin on gene expression of calcitonin gene-related peptide and some proinflammatory mediators in patients with pure menstrual migraine. Acta Neurol. Belg. 2017;117(3):677–685. doi: 10.1007/s13760-017-0803-x. [DOI] [PubMed] [Google Scholar]
- 73.Viswanathan M. Melatonin inhibits calcitonin gene-related peptide-induced vasodilation and increase in cAMP in rat middle cerebral arteries. Eur. J. Pharmacol. 2001;415(2-3):247–250. doi: 10.1016/S0014-2999(01)00826-3. [DOI] [PubMed] [Google Scholar]
- 74.Tanuri F.C., de Lima E., Peres M.F., Cabral F.R., da Graça Naffah-Mazzacoratti M., Cavalheiro E.A., Cipolla-Neto J., Zukerman E., Amado D. Melatonin treatment decreases c-fos expression in a headache model induced by capsaicin. J. Headache Pain. 2009;10(2):105–110. doi: 10.1007/s10194-009-0097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emet M., Ozcan H., Ozel L., Yayla M., Halici Z., Hacimuftuoglu A. A review of melatonin, its receptors and drugs. Eurasian J. Med. 2016;48(2):135–141. doi: 10.5152/eurasianjmed.2015.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Y.Y., Liu L.F., Yue R.Q., Xu J., Ho A., Li M., Han Q.B. Full component analysis of Tianma-Gouteng-Yin. Chin. Med. 2016;11:44. doi: 10.1186/s13020-016-0115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lou Z., Xia B., Zhao J., Huang Y., Hu M., Zhang G. The selection and content determination of an anti-migraine effective fraction separation from tianma gouteng decocition by macroporous resin. J. Exp. Biol. 2014;2:4. [Google Scholar]
- 78.Wang J., Feng B., Yang X., Liu W., Liu Y., Zhang Y., Yu G., Li S., Zhang Y., Xiong X. Tianma gouteng yin as adjunctive treatment for essential hypertension: a systematic review of randomized controlled trials. Evid. Based Complement. Alternat. Med. 2013;2013:706125. doi: 10.1155/2013/706125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitsufuji T., Yamamoto T., Hayashi Y.J.J.J.A.O.P.M. Use of yokukansan foe the treatment of medication overuse headache. 2013;28:47–49. [Google Scholar]
- 80.Kimura Y., Shimizu S., Tanaka A., Suzuki M., Kinebuchi A., Inaki K., Sato H.J.K.M. Efficacy of Yokukansan-based prescriptions for the treatment of patients with headache. 2008;59(2):265–271. [Google Scholar]
- 81.Shi J.S., Yu J.X., Chen X.P., Xu R.X. Pharmacological actions of Uncaria alkaloids, rhynchophylline and isorhynchophylline. Acta Pharmacol. Sin. 2003;24(2):97–101. [PubMed] [Google Scholar]
- 82.Ndagijimana A., Wang X., Pan G., Zhang F., Feng H., Olaleye O. A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia. 2013;86:35–47. doi: 10.1016/j.fitote.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 83.Ho T.Y., Tang N.Y., Hsiang C.Y., Hsieh C.L. Uncaria rhynchophylla and rhynchophylline improved kainic acid-induced epileptic seizures via IL-1β and brain-derived neurotrophic factor. Phytomedicine. 2014;21(6):893–900. doi: 10.1016/j.phymed.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 84.Wu L.Z., Xiao X.M. Evaluation of the effects of Uncaria rhynchophylla alkaloid extract on LPS-induced preeclampsia symptoms and inflammation in a pregnant rat model. Braz. J. Med. Biol. Res. 2019;52(6):e8273. doi: 10.1590/1414-431x20198273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li H.Q., Ip S.P., Zheng G.Q., Xian Y.F., Lin Z.X. Isorhynchophylline alleviates learning and memory impairments induced by aluminum chloride in mice. Chin. Med. 2018;13:29. doi: 10.1186/s13020-018-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen G., Huo Y., Tan D.X., Liang Z., Zhang W., Zhang Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003;73(1):19–26. doi: 10.1016/S0024-3205(03)00252-2. [DOI] [PubMed] [Google Scholar]
- 87.Kim J.H., Bae C.H., Park S.Y., Lee S.J., Kim Y. Uncaria rhynchophylla inhibits the production of nitric oxide and interleukin-1β through blocking nuclear factor κB, Akt, and mitogen-activated protein kinase activation in macrophages. J. Med. Food. 2010;13(5):1133–1140. doi: 10.1089/jmf.2010.1128. [DOI] [PubMed] [Google Scholar]
- 88.Kang B.K., Kim M.K., Kim S.Y., Lee S.J., Choi Y.W., Choi B.T., Shin H.K. Anti-Neuroinflammatory effects of Uncaria sinensis in LPS-stimulated BV2 microglia cells and focal cerebral ischemic mice. Am. J. Chin. Med. 2015;43(6):1099–1115. doi: 10.1142/S0192415X15500639. [DOI] [PubMed] [Google Scholar]
- 89.Yuan D., Ma B., Yang J.Y., Xie Y.Y., Wang L., Zhang L.J., Kano Y., Wu C.F. Anti-inflammatory effects of rhynchophylline and isorhynchophylline in mouse N9 microglial cells and the molecular mechanism. Int. Immunopharmacol. 2009;9(13-14):1549–1554. doi: 10.1016/j.intimp.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 90.Song Y., Qu R., Zhu S., Zhang R., Ma S. Rhynchophylline attenuates LPS-induced pro-inflammatory responses through down-regulation of MAPK/NF-κB signaling pathways in primary microglia. Phytother. Res. 2012;26(10):1528–1533. doi: 10.1002/ptr.4614. [DOI] [PubMed] [Google Scholar]
- 91.Neeb L., Reuter U. Nitric oxide in migraine. CNS Neurol. Disord. Drug Targets. 2007;6(4):258–264. doi: 10.2174/187152707781387233. [DOI] [PubMed] [Google Scholar]
- 92.Kanatani H., Kohda H., Yamasaki K., Hotta I., Nakata Y., Segawa T., Yamanaka E., Aimi N., Sakai S. The active principles of the branchlet and hook of Uncaria sinensis Oliv. examined with a 5-hydroxytryptamine receptor binding assay. J. Pharm. Pharmacol. 1985;37(6):401–404. doi: 10.1111/j.2042-7158.1985.tb03023.x. [DOI] [PubMed] [Google Scholar]
- 93.Terawaki K., Ikarashi Y., Sekiguchi K., Nakai Y., Kase Y. Partial agonistic effect of yokukansan on human recombinant serotonin 1A receptors expressed in the membranes of Chinese hamster ovary cells. J. Ethnopharmacol. 2010;127(2):306–312. doi: 10.1016/j.jep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Jung J.W., Ahn N.Y., Oh H.R., Lee B.K., Lee K.J., Kim S.Y., Cheong J.H., Ryu J.H. Anxiolytic effects of the aqueous extract of Uncaria rhynchophylla. J. Ethnopharmacol. 2006;108(2):193–197. doi: 10.1016/j.jep.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 95.Nishi A., Yamaguchi T., Sekiguchi K., Imamura S., Tabuchi M., Kanno H., Nakai Y., Hashimoto K., Ikarashi Y., Kase Y. Geissoschizine methyl ether, an alkaloid in Uncaria hook, is a potent serotonin ₁A receptor agonist and candidate for amelioration of aggressiveness and sociality by yokukansan. Neuroscience. 2012;207:124–136. doi: 10.1016/j.neuroscience.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 96.Liang J.H., Luan Z.L., Tian X.G., Zhao W.Y., Wang Y.L., Sun C.P., Huo X.K., Deng S., Zhang B.J., Zhang Z.J., Ma X.C. Uncarialins A-I, monoterpenoid indole alkaloids from Uncaria rhynchophylla as natural agonists of the 5-HT1A receptor. J. Nat. Prod. 2019;82(12):3302–3310. doi: 10.1021/acs.jnatprod.9b00532. [DOI] [PubMed] [Google Scholar]
- 97.Xian Y.F., Fan D., Ip S.P., Mao Q.Q., Lin Z.X. Antidepressant-like effect of isorhynchophylline in mice. Neurochem. Res. 2017;42(2):678–685. doi: 10.1007/s11064-016-2124-5. [DOI] [PubMed] [Google Scholar]
- 98.Geng C.A., Yang T.H., Huang X.Y., Ma Y.B., Zhang X.M., Chen J.J. Antidepressant potential of Uncaria rhynchophylla and its active flavanol, catechin, targeting melatonin receptors. J. Ethnopharmacol. 2019;232:39–46. doi: 10.1016/j.jep.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 99.Zhang J.G., Huang X.Y., Ma Y.B., Chen J.J., Geng C.A. UFLC-PDA-MS/MS profiling of Seven uncaria species integrated with melatonin/5-Hydroxytryptamine receptors agonistic assay. Nat. Prod. Bioprospect. 2020;10(1):23–36. doi: 10.1007/s13659-020-00230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geng C.A., Huang X.Y., Ma Y.B., Hou B., Li T.Z., Zhang X.M., Chen J.J. (±)-Uncarilins A and B, Dimeric isoechinulin-type alkaloids from uncaria rhynchophylla. J. Nat. Prod. 2017;80(4):959–964. doi: 10.1021/acs.jnatprod.6b00938. [DOI] [PubMed] [Google Scholar]
- 101.Zhang J.G., Huang X.Y., Ma Y.B., Zhang X.M., Chen J.J., Geng C.A. Dereplication-guided isolation of a new indole alkaloid triglycoside from the hooks of Uncaria rhynchophylla by LC with ion trap time-of-flight MS. J. Sep. Sci. 2018;41(7):1532–1538. doi: 10.1002/jssc.201701175. [DOI] [PubMed] [Google Scholar]
- 102.Lei L., Yuan X., Wang S., Zhang F., Han Y., Ning Q., Luo G., Lu S. Mitogen-activated protein kinase pathways are involved in the upregulation of calcitonin gene-related peptide of rat trigeminal ganglion after organ culture. J. Mol. Neurosci. 2012;48(1):53–65. doi: 10.1007/s12031-012-9772-y. [DOI] [PubMed] [Google Scholar]
- 103.Yin Z., Fang Y., Ren L., Wang X., Zhang A., Lin J., Li X. Atorvastatin attenuates NF-kappaB activation in trigeminal nucleus caudalis in a rat model of migraine. Neurosci. Lett. 2009;465(1):61–65. doi: 10.1016/j.neulet.2009.08.081. [DOI] [PubMed] [Google Scholar]