Abstract
Two Ras effector pathways leading to the activation of Raf-1 and phosphatidylinositol 3-kinase (PI3K) have been implicated in the survival signaling by the interleukin 3 (IL-3) receptor. Analysis of apoptosis suppression by Raf-1 demonstrated the requirement for mitochondrial translocation of the kinase in this process. This could be achieved either by overexpression of the antiapoptotic protein Bcl-2 or by targeting Raf-1 to the mitochondria via fusion to the mitochondrial protein Mas p70. Mitochondrially active Raf-1 is unable to activate extracellular signal-related kinase 1 (ERK1) and ERK2 but suppresses cell death by inactivating the proapoptotic Bcl-2 family member BAD. However, genetic and biochemical data also have suggested a role for the Raf-1 effector module MEK-ERK in apoptosis suppression. We thus tested for MEK requirement in cell survival signaling using the interleukin 3 (IL-3)-dependent cell line 32D. MEK is essential for survival and growth in the presence of IL-3. Upon growth factor withdrawal the expression of constitutively active MEK1 mutants significantly delays the onset of apoptosis, whereas the presence of a dominant negative mutant accelerates cell death. Survival signaling by MEK most likely results from the activation of ERKs since expression of a constitutively active form of ERK2 was as effective in protecting NIH 3T3 fibroblasts against doxorubicin-induced cell death as oncogenic MEK. The survival effect of activated MEK in 32D cells is achieved by both MEK- and PI3K-dependent mechanisms and results in the activation of PI3K and in the phosphorylation of AKT. MEK and PI3K dependence is also observed in 32D cells protected from apoptosis by oncogenic Raf-1. Additionally, we also could extend these findings to the IL-3-dependent pro-B-cell line BaF3, suggesting that recruitment of MEK is a common mechanism for survival signaling by activated Raf. Requirement for the PI3K effector AKT in this process is further demonstrated by the inhibitory effect of a dominant negative AKT mutant on Raf-1-induced cell survival. Moreover, a constitutively active form of AKT synergizes with Raf-1 in apoptosis suppression. In summary these data strongly suggest a Raf effector pathway for cell survival that is mediated by MEK and AKT.
Signal transduction downstream of the interleukin 3 (IL-3) receptor occurs through the activation of the two Ras effectors Raf-1 (9) and phosphatidylinositol 3-kinase (PI3K) (23) as well as independently of Ras through stimulation of JAK kinases and results in proliferation, differentiation, and cell survival (17). Published work has implicated both AKT and Raf-1 in cell survival signaling by IL-3. We have shown previously that expression of an oncogenic form of Raf-1 significantly delayed the onset of apoptotic cell death in the IL-3-dependent cell line 32D upon growth factor removal (12). Further work demonstrated that Raf-1 could synergize in this process with Bcl-2 (53), and dissection of this interaction indicated a role for Bcl-2 in the mitochondrial translocation of Raf-1, where it mediated inactivation of the proapoptotic protein BAD (54). These data thus identified a novel Raf-1 effector pathway(s) at the mitochondrial level that did not involve MEK1 and -2 (16). This extracellular signal-related kinase (ERK) activator is essential, however, for Raf-1-induced proliferation, differentiation, and transformation (13, 27, 31, 32, 49). More recently, the mitogenic cascade (Raf-MEK-ERK) has been implicated in the apoptosis suppression by B-Raf (19). Moreover, in Drosophila melanogaster apoptosis regulation is achieved mainly via the Raf-MEK-ERK cascade by modulating two target genes, hid and grim, whose mammalian counterparts are still elusive (4, 5, 34).
In the present study we have used the IL-3-dependent promyeloid cell line 32D (24) to evaluate the role of the MEK-ERK module in apoptosis suppression. Upon growth factor removal these cells arrest in the G1 phase of the cell cycle and within 24 h >80% of the cells die through apoptosis (12). Using the MEK-specific inhibitor U0126 (1, 21) we show that MEK-dependent signals are required for growth and survival in the presence of IL-3. The survival activity of MEK can also be demonstrated following the overexpression of constitutively active mutants of the kinase. Consistent results were also obtained in the pro-B cell line BaF3 as well as in NIH 3T3 fibroblasts. Furthermore, the reversion of the protective effect by the PI3K inhibitor LY29004 suggested the requirement for PI3K-dependent signals in survival signaling by IL-3 as well as by oncogenic versions of MEK1 or Raf-1. The involvement of one potential PI3K effector, the serine-threonine kinase AKT, is shown by experiments demonstrating that a constitutively active mutant of AKT synergizes with oncogenic Raf-1 in apoptosis suppression whereas a dominant negative mutant blocks survival signaling by MEK. Consistent with a requirement for AKT downstream of MEK we are able to show that PI3K is constitutively active in v-raf- or MEK-expressing cells and both U0126 and LY290004 abolished phosphorylation of serine 473 on AKT.
MATERIALS AND METHODS
Plasmids.
The retroviral expression construct for dominant interfering (pBabe-puro MEK LIDA) and constitutively active (pBabe-puro MEK-LIDEMANE) MEK1 have been described before (13). Constitutive activation of rabbit mitogen-activated protein kinase kinase 1 (MEK1) results from an exchange of the two Raf-1 phosphorylation sites on serine 218 and 222 to glutamic acid. pBabe-puro ΔStuI-MEK-LIDEMANE was generated by releasing the insert through digestion with the restriction endonucleases BamHI and EcoRI (partial). The isolated fragment was recloned into the corresponding sites of pBluescript (Stratagene), and the resulting construct was cut again with StuI to release a 60-bp fragment coding for an alpha helix (36). After religation the mutated insert was released and shuttled back into the pBabe-puro vector (40). Expression constructs for hemagglutinin-tagged versions of wild-type, kinase inactive, and constitutively active forms of AKT (protein kinase B α) in pCMV5 have been described before (3, 38). For our experiments the inserts were released with a BgIII/XbaI digest, blunt ended, and recloned into the SnaBI site of pBabe-puro. The retroviral construct EHneo (49), which expresses gag-v-Raf, was used for the generation of v-raf-expressing viruses. The constitutively active form of ERK2 generated by a fusion of ERK2 to its upstream activator MEK1 has been published (42). All DNAs have been purified using Qiagen maxiprep columns according to the manufacturer's instructions.
Cell culture.
All experiments described in this paper have been performed with 32D cells obtained from J. S. Greenberger. 32D and BaF3 cells were grown in RPMI 1640 (Gibco) supplemented with 10% heat-inactivated (45 min at 56°C) fetal calf serum, 100 U of penicillin and streptomycin per ml, 2 mM l-glutamine, and 15% WEHI-conditioned medium at 37°C, with 5% CO2 and 90% humidity. Every three days cells were split at a ratio of 1:10 and refed with fresh medium. NIH 3T3 fibroblasts and the amphotropic packaging cell line Phoenix ampho was routinely maintained in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% heat-inactivated (45 min at 56°C) fetal calf serum, 100 U of penicillin and streptomycin per ml, and 2 mM l-glutamine.
Establishment of MEK-expressing cells.
Phoenix cells (70% confluent) were transfected in a single well of a flat-bottom six-well tissue culture plate with 2.5 μg of purified DNA following a modified CaPO4 protocol (10). Twenty-four hours after transfection cells were refed with 2 ml of fresh medium overnight. The following day tissue culture supernatants were cleared from cells by centrifugation (800 × g, 5 min at 20°C) and sterile filtered through a 0.45-μm-pore-size filter attached to a syringe. 32D and BaF3 cells to be infected were seeded at 0.5 × 106 cells per ml, to which 1 ml of the virus supernatant, 250 μl of WEHI-conditioned medium, and 4 μg of Polybrene/ml were added. Twenty-four hours later the infection was resupplied with 2 ml of fresh 32D cell culture medium. Selection of cells carrying the pBabe-puro expression constructs was started by adding 2 μg of puromycin/ml. Selection medium was changed every other day.
Cell survival assay.
For the analysis of cell survival and intracellular signaling pathways cells were washed three times in tissue culture medium without WEHI cell supernatant. A quantity of 0.5 × 106 cells in a total volume of 1 ml were dispensed into a single well of a flat-bottom 24-well tissue culture plate. Cell viability was routinely assessed by staining the cells in trypan blue (Sigma).
Protein analysis.
Cells were washed twice in phosphate-buffered saline (PBS) without calcium and magnesium, and equal numbers of cells were lysed by direct addition of protein sample buffer (60 mM Tris-HCl [pH 6.8], 10% [wt/vol] glycerin, 3% sodium dodecyl sulfate [wt/vol], 5% 2-mercaptoethanol [wt/vol], and 0.05% [wt/vol] bromphenol blue) and boiling at 100°C for 5 min. Cell extracts were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a nitrocellulose membrane in a buffer containing 25 mM Tris (pH 8), 190 mM glycine, and 20% methanol using a tank blot procedure. Blotting was performed at 800 mA for 45 min. All further incubations were carried out at room temperature on a platform shaker. The membrane was blocked for 1 h in PBS supplemented with 0.05% Tween 20 (vol/vol) and 5% (wt/vol) nonfat dry milk (Carnation). Incubation with the primary antibody appropriately diluted in PBS supplemented with 0.05% Tween 20 (vol/vol) and 1.6% (wt/vol) dry milk powder was carried out for 1 h followed by three washes (10 min each) with PBS containing 0.05% Tween 20 (wt/vol). Next the blot was incubated with anti-mouse or anti-rabbit horseradish peroxidase-linked F(ab′)2 fragments (Amersham) diluted 1:3,000 in PBS supplemented with 0.05% Tween 20 (vol/vol) and 1.6% (wt/vol) dry milk powder for 45 min. Following three washes the bands were visualized using the enhanced chemiluminescence detection system. For specific antibodies the following modifications were performed according to the manufacturer's instructions: nonfat dry milk was replaced by 5% bovine serum albumin (Sigma) and incubation with the primary antibody was performed overnight at 4°C.
For the determination of MEK activity cells were lysed at 4°C in NP-40 buffer (10 mM HEPES [pH 7.4], 145 mM KCl, 5 mM MgCl2, 1 mM EGTA, 0.2% Igepal, aprotinin [10 mg/ml], Pefabloc [100 μg/ml], leupeptin [1 mg/ml], 1 mM Na-O-vanadate, 1 mM NaF). Following centrifugation for 10 min at 12,000 × g 1 μg of MEK-specific antibody and 20 μl of protein A-agarose were added to the precleared lysates. Immunoprecipitation was carried out for 2 h at 4°C. Immunoprecipitates were washed twice in lysis buffer and twice in kinase buffer (25 mM HEPES [pH 7.4], 150 mM NaCl, 25 mM glycero-phosphate, 1 mM dithiothreitol, 5 mM MgCl2). For the kinase reaction 30 μl of a mix containing kinase buffer, 10 μM ATP, and 1 μg of purified bacterially expressed kinase dead ERK2 (25) was added to the immunoprecipitates and the reaction was started by the addition of 10 μCi of [γ-32P]ATP. The reaction was carried out for 20 min at room temperature under constant agitation and terminated by the addition of 30 μl of protein sample buffer.
For the determination of PI3K activity p85 was immunoprecipitated using a rabbit polyclonal antiserum (UBI 06–195), which results in the coprecipitation of p110. Further analysis was performed as described (55).
The following primary antibodies were used in our experiments: MEK1 (C-18; rabbit polyclonal antiserum; Santa Cruz), AKT1 and -2 (sc-8312; rabbit polyclonal antiserum; Santa Cruz), ERK1 (sc-94; rabbit polyclonal antiserum; Santa Cruz), pERK (sc-7383; mouse monoclonal antibody; Santa Cruz), pAKT Ser473 (9271s; rabbit polyclonal antiserum; New England Biolabs), p85 (06–195; rabbit polyclonal antiserum; UBI), anti-myc tag (9E10; mouse monoclonal antibody; Institut für Medizinische Strahlenkunde und Zellforschung).
RESULTS
IL-3-induced growth and survival of 32D cells depend on MEK and PI3K signaling.
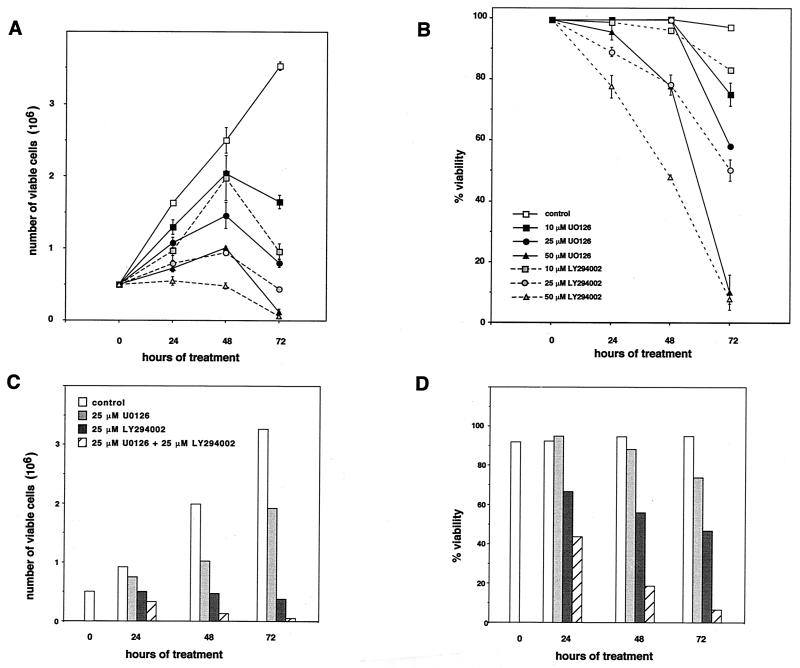
Previous work identified the Raf effector kinase module MEK-ERK as essential for Raf-1-mediated transformation, proliferation, and differentiation (13, 26, 31, 32, 49). Contrary to this, the analysis of Raf-1-mediated apoptosis suppression had suggested the existence of a MEK-ERK-independent pathway for survival signaling by Raf-1 requiring mitochondrial localization of the kinase (54). However, Drosophila genetics (4, 5, 34) and analysis of apoptosis suppression by B-Raf (19) had strongly suggested a role for MEK-ERK-dependent signals in this process. We thus tested the MEK requirement for growth and survival using the IL3-dependent immortalized myeloblast-like cell line 32D (54). Cells growing in the presence of 15% WEHI-conditioned medium as the source of IL-3 cells were treated with a single dose of the MEK inhibitor U0126 (21), which blocks Raf-dependent activation as well as downstream signaling of the dual-specificity kinase MEK (1) or the inhibitor of PI3K, LY29004 (52). Cell viability as measured by trypan blue exclusion and cell counts was determined every 24 h. As shown in Fig. 1A and B interference with either signaling pathway affected not only cell growth in a dose-dependent fashion but also cell survival. The presence of a single agent at higher concentrations was sufficient to completely block survival or proliferation, suggesting that both pathways are jointly required to sustain viability and growth. To exclude the possibility that inhibitory effects seen with high concentrations of U0126 resulted from unspecific toxicity we also tested the combination of both inhibitors at lower concentrations. As already demonstrated above (Fig. 1B), incubation of cells with U0126 at a concentration of 25 μM had little effect on cell survival at early time points (Fig. 1D). However, when combined with LY294002, which at this concentration already significantly affected cell proliferation, the presence of U0126 resulted in a further significant decrease in cell viability (Fig. 1D) as well as cell growth (Fig. 1C).
FIG. 1.
Effect of MEK and PI3K inhibitors on IL-3-induced survival and growth. 32D cells (0.5 × 106) were seeded in triplicate in the presence of 15% WEHI-conditioned medium as the source of IL-3 into the wells of a 24-well plate. Cell numbers (A and C) and cell viabilities (B and D) were determined at the time points indicated. Inhibitors for MEK (U0126) and PI3K (LY294002) were dissolved in DMSO and added once at time point 0 h. Control, DMSO control corresponding to the highest inhibitor concentration. Mean values and standard deviations (error bars) are shown.
Expression of activated MEK results in apoptosis suppression.
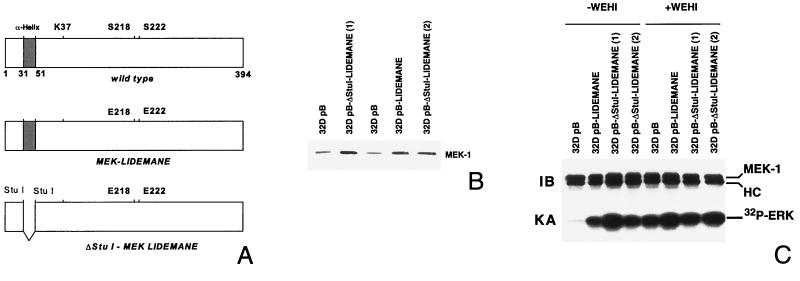
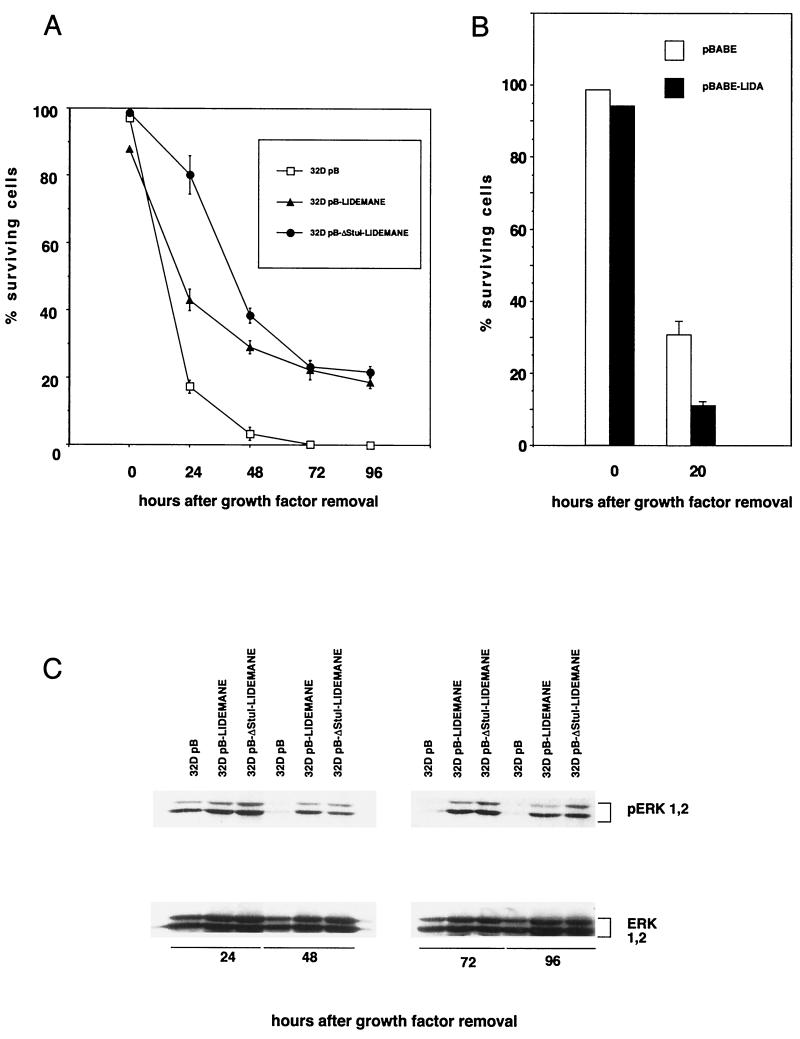
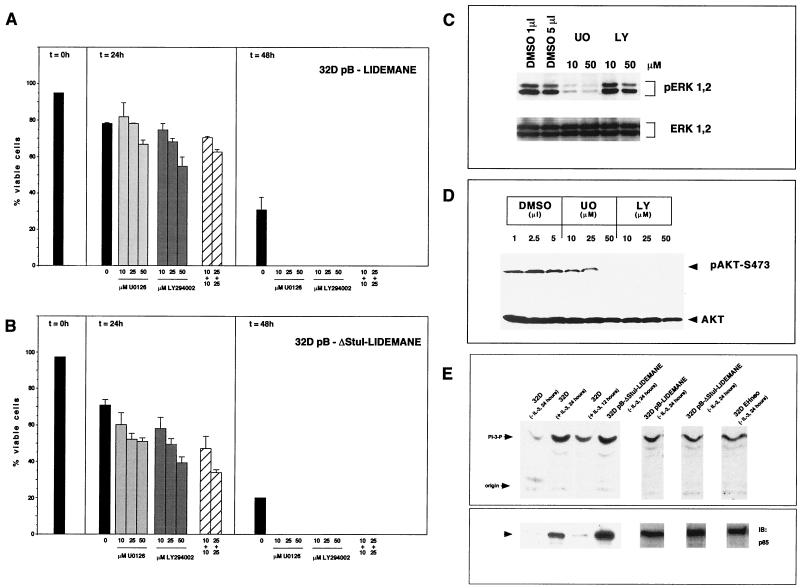
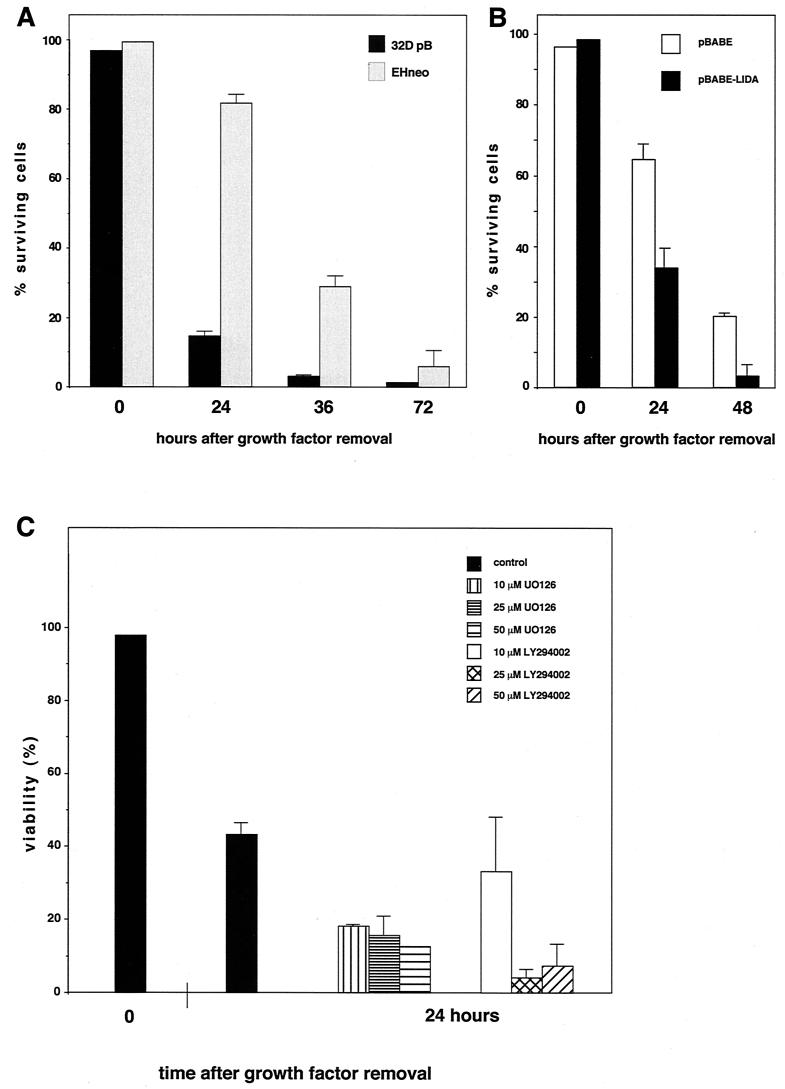
To further analyze the role of MEK in apoptosis suppression we generated 32D cells expressing activated versions of the rabbit mitogen-activated protein kinase kinase MEK1 (Fig. 2A). Two mutants were used in our studies which both contain an exchange of the Raf-1 phosphorylation site serines 218 and 222 in the sequence LIDSMANS to glutamic acid, resulting in the constitutive activation of the kinase (13) (Fig. 2A). Further mutational analysis of the protein had revealed an important function of an alpha helix in the negative regulation of the kinase, leading to increased kinase activity upon removal of the coding region for this stretch of 20 amino acids flanked by restriction sites for the endonuclease StuI (36) (Fig. 2A). Both mutants were cloned into the retroviral expression vector pBabe-puro (40), and virus supernatants generated following transient transfection of the packaging cell line Phoenix were then used to infect parental 32D cells. Following puromycin, selection expression of the MEK transgene in the resulting cell pools was verified by Western blotting (Fig. 2B). To analyze MEK activity in these cell pools immune complex kinase assays were performed using purified bacterially expressed ERK2 as the substrate. Our data show that mutant MEK1 was active in the absence of cytokine stimulation (Fig. 2C). We next addressed the question of whether presence of the constitutively active kinase conferred a survival advantage to 32D cells upon removal of IL-3. We have shown previously that growth factor withdrawal results in G1 arrest and within 24 h in the death of 32D cells as indicated by trypan blue exclusion (12). The dying cells showed all morphological and biochemical changes characteristic for apoptosis (12). 32D pB cells carrying only the vector, or 32D LIDEMANE and 32D ΔStuI-LIDEMANE cells expressing the two MEK1 mutants, were extensively washed and reseeded in medium without IL-3. As shown in Fig. 3A, 24 h later growth factor removal had resulted in a dramatic loss of cell viability in the control cells, which was much less pronounced in the 32D LIDEMANE cells and almost not existent in 32D cells expressing the ΔStuI-LIDEMANE mutant. After prolonged absence of the growth factor both mutants showed a similar protective effect. To further substantiate a role for MEK in survival signaling by IL-3 we also analyzed 32D cells expressing a dominant negative mutant of MEK (13). As expected from the results obtained with the MEK inhibitor, the presence of this mutant accelerated cell death (Fig. 3B).
FIG. 2.
Generation and analysis of MEK-expressing 32D cells. Activated MEK1 was generated by replacement of the two Raf-1 phosphorylation site serines 218 and 222 in the catalytic domain with glutamic acid (MEK-LIDEMANE) and additionally by deleting amino acids 32 to 51 in the N terminus of MEK1, which results in a further enhancement of the kinase activity (ΔStuI-MEK-LIDEMANE). 32D cells stably expressing the vector control or the MEK mutants were generated by retroviral infection, and MEK1 overexpression was confirmed by Western blot analysis of total cell lysates (B). To measure MEK1 kinase activity cells were washed three times in tissue culture medium without IL-3 and then incubated for 24 h in the presence (+WEHI) or absence (−WEHI) of WEHI-conditioned medium. Following cell lysis MEK1 was immunoprecipitated and assayed in an immune complex kinase assay using bacterially expressed ERK2 as the substrate (C).
FIG. 3.
Expression of activated MEK delays apoptotic cell death of 32D cells following growth factor removal and results in prolonged activation of ERK1 and -2. Cells (0.5 × 106) expressing empty vector (pB), activated mutants of MEK (LIDEMANE and ΔStuI-LIDEMANE) or a dominant negative form of MEK (LIDA) were seeded in triplicate into the wells of a 24-well plate. At the time points indicated cell viabilities were determined (A and B) or cell lysates were prepared by washing the cells in PBS and directly lysing them in protein sample buffer (C). The activation status of ERK1 and -2 was analyzed using phosphorylation-specific antibodies. Identical results were obtained in three independent experiments. (A and B) Error bars, standard deviations.
Apoptosis suppression by MEK1 coincides with the prolonged activation of ERK1 and -2 and requires MEK1 kinase activity.
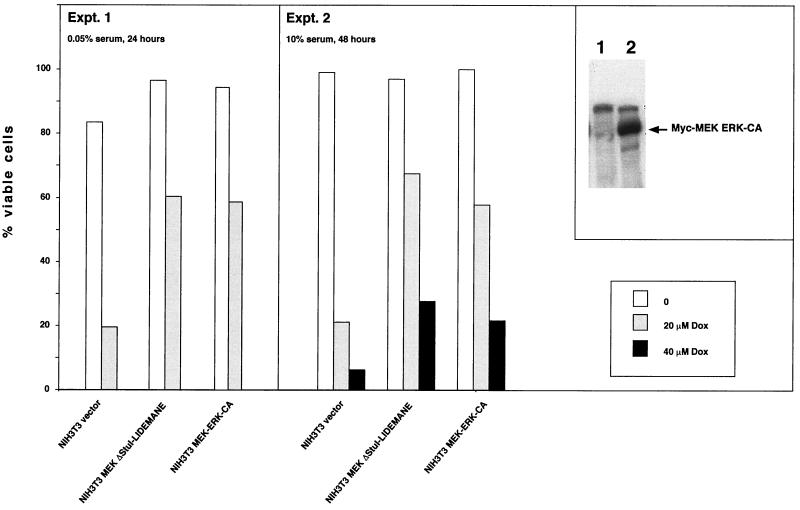
To test whether the expression of the MEK1 mutants resulted in the activation of ERK1 and -2 in vivo antibodies were employed which specifically detect MEK1-mediated phosphorylations on ERK1 and -2, which are essential for activation (2, 41). As shown in Fig. 3C expression of activated MEK1 lead to the phosphorylation of ERK1 and -2 which was independent of the presence of growth factors. This protein modification was still maintained 96 h after growth factor removal, suggesting that ERK activation might be essential for apoptosis suppression by MEK1. To directly test the survival activity of activated ERK we used NIH 3T3 cells expressing a recently described constitutively active form of ERK2 (42). Expression of this mutant protected NIH 3T3 cells against doxorubicin-induced cell death under conditions of low and high serum concentrations as efficiently as activated MEK (Fig. 4). These data thus suggest that apoptosis suppression by MEK may be mediated through activation of ERKs.
FIG. 4.
Expression of constitutively active ERK inhibits doxorubicin-induced apoptosis of NIH 3T3 cells. Confluent monolayers of NIH 3T3 cells expressing either a constitutively active version of MEK (ΔStuI-LIDEMANE) or myc-tagged ERK2 (MEK-ERK-CA) were treated with the apoptosis inducer doxorubicin for 24 h (cells additionally serum starved) or 48 h (cells growing in full medium). At the end of the treatment all cells were collected and analyzed for viability by trypan blue staining. (Inset) Western blot analysis of the myc-tagged mutant ERK2 protein. Expression of activated ΔStuI-LIDEMANE was evident from the transformed morphology of the cells.
To confirm the requirement of MEK1 kinase for the survival of 32D cells expressing oncogenic MEK1, the synthetic inhibitor U0126 was used. The substance has been shown previously not only to prevent phosphorylation-dependent activation of MEK by Raf-1 but also to interfere with signaling from activated MEK1 (1, 22). To test for MEK1 requirement 32D LIDEMANE cells were depleted of growth factors and incubated in the presence of increasing amounts of the inhibitor. As shown in Fig. 5A this treatment with U0126 significantly decreased the percentage of viable cells, especially at later time points, confirming that the protective effect observed in these cells depended upon signaling by active MEK1. Identical results were obtained with 32D ΔStuI-LIDEMANE cells (Fig. 5B).
FIG. 5.
(A and B) Apoptosis suppression by activated MEK is blocked by inhibitors of MEK and PI3K signaling. Cells (0.5 × 106) were seeded in triplicate into the wells of a 24-well plate. Inhibitors for MEK (U0126) and PI3K (LY294002) were dissolved in DMSO and added once at time point 0 h. Control, DMSO control corresponding to the highest inhibitor concentration. Mean values and standard deviations (error bars) are shown. (C) Presence of the PI3K inhibitor LY29004 does not affect ERK1 and -2 phosphorylation. (D) Expression of activated MEK results in the phosphorylation of AKT. Following a 24-h treatment with the MEK inhibitor U0126 or the PI3K inhibitor LY29004 cells were washed with PBS and cell lysates were prepared by boiling in protein sample buffer. Immunoblots were probed with antibodies specific for the phosphorylated forms of ERK1 and -2 (C) or AKT (D). Control immunoblots were carried out with antibodies recognizing total ERK or AKT protein. (E) PI3K is constitutively active in MEK- and Raf-transformed 32D cells. PI3K was immunoprecipitated from the indicated cell lines and assayed for activity.
Inhibitors of PI3K activation negatively affect survival activity of MEK1.
Recent work has linked the PI3K-mediated activation of AKT to the suppression of apoptosis in various cell systems under different death stimuli (14) (Fig. 1). The protective effect in this case may result from the phosphorylation of the proapoptotic protein BAD (15, 18, 47) as well as of transcriptional targets involved in cell survival control (8, 14, 43). AKT requires the successive action of lipid and protein kinases for activation (29, 39). The PI3K-AKT cascade represents a Ras effector pathway working parallel to the Raf-MEK-ERK module and was therefore not necessarily expected to play a role in MEK-induced cell survival. The inhibitor of PI3K activation, LY294002 (52), however, enhanced apoptotic cell death in these cells in a dose-dependent fashion (Fig. 5A and B), suggesting a requirement for PI3K. Similar results were also obtained with the chemically unrelated inhibitor wortmannin (data not shown). These effects were not caused by an unspecific blockage of MEK1-induced ERK phosphorylation, as demonstrated in Fig. 5C. To confirm that PI3K was really activated in these cells, lysates were probed with phosphorylation-specific antibodies against serine residue 473 on AKT. AKT activation requires binding of the PI3K-modified lipid phosphatidylinositol 3,4,5-triphosphate to the pleckstrin homology region in AKT, resulting in the membrane translocation, where further activation requires phosphorylation of serines 308 and 473 by PDK1 and PDK2, respectively (29). We thus checked the phosphorylation status of serine 473. For this purpose growth factor-deprived 32D MEK LIDEMANE cells were either kept in the presence of dimethyl sulfoxide (DMSO) used as a solvent in our experiments or exposed to increasing concentrations of the inhibitors U0126 or LY29004 for 24 h. Immunoblot analysis of lysates from these cells showed that serine 473 phosphorylation persisted in control cells but was efficiently blocked by interference with either MEK or PI3K signaling (Fig. 5D). Consistent with our observation that lower concentrations of U0126 have little inhibitory effect on MEK-induced survival at this time point we also failed to detect a significant effect on AKT phosporylation.
To directly analyze the activation status of PI3K, PI3K was immunoprecipitated using a polyclonal antibody against p85 and assayed for activity. High constitutive PI3K activity was detected in 32D cells growing in the presence of IL-3 as well as in IL-3-deprived 32D cells expressing activated MEK or an oncogenic form of Raf-1 (12) (Fig. 5E). No PI3K activity was detectable in IL-3-starved parental 32D cells. Immunoblotting failed to detect expression of p85. p85 has been shown previously to be required for the activation as well as for the stabilization of the catalytic subunit (22, 51, 56). Taken together these data further support the role of Raf-MEK-dependent signals for the activation of PI3K.
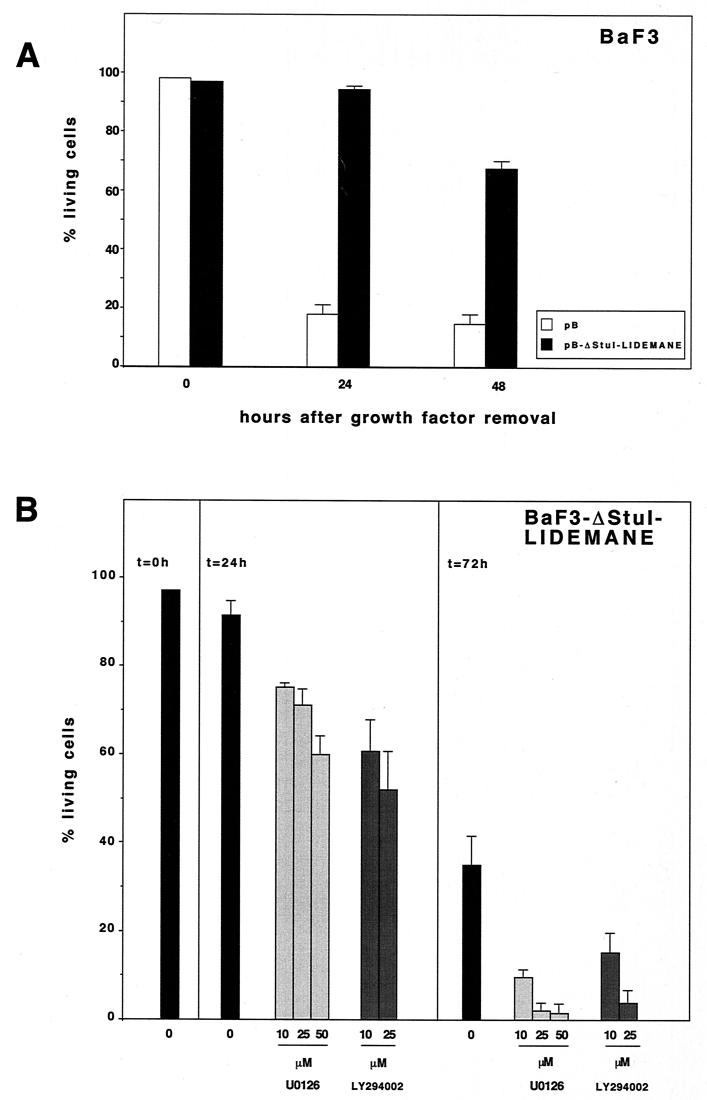
Having already demonstrated survival activity for activated MEK in 32D and NIH 3T3 cells, we also included in our analyses the IL-3-dependent bone marrow-derived pro-B-cell line BaF3. Through retroviral infection we generated BaF3 cells expressing ΔStuI-LIDEMANE and assayed the effect of the transgene on the induction of apoptotic cell death following IL-3 withdrawal. The presence of activated MEK delayed the onset of apoptotic cell death (Fig. 6A) as shown before for NIH 3T3 and 32D cells. Consistent with our findings obtained in 32D cells we also observed that the survival activity of MEK in BaF3 cells depended on MEK and PI3K activity (Fig. 6B). These findings are in support of a more general cell survival pathway employing MEK and perhaps the subsequent activation of PI3K-AKT.
FIG. 6.
Expression of constitutively active MEK protects IL-3-dependent BaF3 cells from growth factor withdrawal-induced apoptosis (A) by MEK- and PI3K-dependent mechanisms (B). BaF3 cells expressing an activated version of MEK1 (ΔStuI-LIDEMANE) were generated by retroviral infection. A total of 0.5 × 106 MEK-ΔStuI-LIDEMANE-expressing BaF3 cells or BaF3 cells expressing the retroviral control vector pBabe-puro were washed free of growth factor and seeded in triplicate into the wells of a 24-well plate. Cell viabilities were determined at the time points indicated. (B) Inhibitors for MEK (U0126) or PI3K (LY294002) were dissolved in DMSO and added once at the start of the experiment. Control, DMSO control corresponding to the highest inhibitor concentration. Mean values and standard deviations (error bars) are shown.
Requirement for MEK and AKT in survival signaling by oncogenic Raf-1.
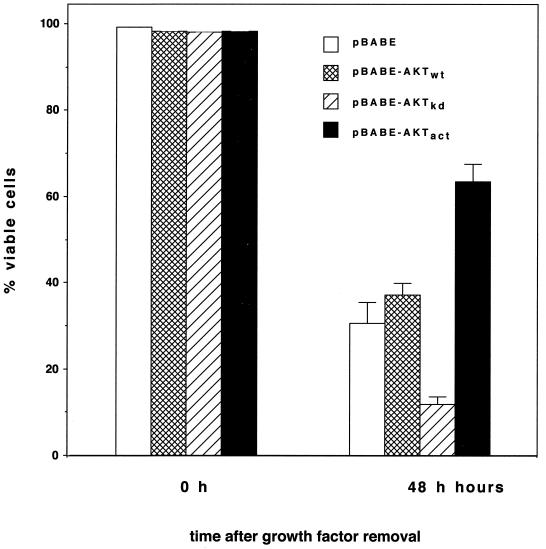
We next addressed the question of whether the survival activity of oncogenic Raf-1 was similarly dependent upon MEK and AKT. For this purpose we generated a pool of 32D cells expressing the retroviral expression construct EHneo coding for gag-v-raf. As shown in Fig. 7A, the presence of activated Raf-1 significantly delayed the onset of apoptosis following growth factor removal. The requirement for MEK in survival signaling by Raf-1 could be demonstrated by the use of a dominant negative mutant of MEK which reversed the antiapoptotic effect of Raf-1 (Fig. 7B). Using the same approach as above we were also able to show that blocking MEK as well as PI3K by chemical inhibitors signaling reversed the antiapoptotic effect of v-raf (Fig. 7C). To further test the requirement of AKT in survival signaling by transforming Raf, we generated 32D cells expressing an activated or a kinase-inactive form of AKT together with activated Raf-1. As demonstrated in Fig. 8 coexpression of the activated mutant of AKT further delayed the onset of apoptotic cell death, whereas the presence of the inhibitory mutant of AKT resulted in a significant reduction in the survival activity of Raf-1. These data further confirm the involvement of PI3K-AKT-dependent pathways in survival signaling by Raf-1 and MEK.
FIG. 7.
Expression of oncogenic v-raf (EHneo) results in apoptosis suppression (A), which is reversed by the expression of a dominant negative mutant of MEK (B) or inhibitors of MEK or PI3K (C). 32D cells expressing an activated version of Raf-1 were generated by retroviral infection with the gag-v-raf-expressing virus EHneo. pBABE-puro control vector or pBABE-puro expressing a dominant negative mutant of MEK was introduced by retrovirus transfer. For survival assays cells were washed free of growth factor and 0.5 × 106 cells were seeded in triplicate into the wells of a 24-well plate. Cell viabilities were determined at the time points indicated. (C) Inhibitors for MEK (U0126) or PI3K (LY294002) were dissolved in DMSO and added once at the start of the experiment. Control, DMSO control corresponding to the highest inhibitor concentration. Mean values and standard deviations (error bars) are shown.
FIG. 8.
AKT is required for apoptosis suppression by activated Raf-1. 32D EHneo cells expressing oncogenic gag-v-raf were infected with a retroviral construct for wild-type (wt), kinase-dead (kd), or activated (act) AKT (protein kinase B α). A total of 0.5 × 106 cells were washed free of growth factor and seeded in triplicate into the wells of a 24-well plate. Cell viabilities were determined at the time points indicated. Mean values and standard deviations (error bars) are shown.
DISCUSSION
Analysis of cytokine receptor-initiated cell survival signaling revealed the existence of two major signaling pathways downstream of the IL-3 receptor which are essential for apoptosis suppression, resulting in the activation of the protein serine-threonine kinases Raf-1 and AKT (11, 18). Raf-1 has been identified initially as an essential membrane-proximal integration point of receptor signaling that funnels signals into the MEK-ERK effector module (16). Activation of the Raf-MEK-ERK cascade was shown to be essential for the ability of Raf-1 to mediate proliferation, differentiation, and transformation (13, 26, 31, 32, 49). However, dissection of cell survival signaling by activated Raf-1 had suggested that Bcl-2-mediated mitochondrial translocation provided access to novel effector pathways since Raf-1 in this cellular location was unable to trigger the activation of the MEK effector kinases ERK1 and -2 (54). Using the chemical inhibitor U0126 and a dominant negative mutant of MEK we demonstrate in our experiments an essential role for MEK1 in apoptosis suppression by activated Raf-1. Furthermore, we also show that both MEK1 and Raf-1 activate PI3K and require PI3K-AKT for apoptosis suppression. Thus, while mitochondrial Raf-1 may be a direct BAD kinase, our data point to a novel indirect mechanism by which a Raf-1-MEK1-dependent effector pathway would lead to the recruitment of PI3K-AKT.
Over the last 2 years increasing evidence has accumulated suggesting various levels of cross-talk between the Raf-1-MEK-ERK and the PI3K-AKT pathways, which also may play a role in apoptosis regulation. It has been suggested that these signaling cascades may reconverge immediately downstream of the receptor (44, 58). The proapoptotic Bcl-2 family member BAD might provide another convergence point. Phosphorylation of BAD after growth factor stimulation occurs on serines 112 and 136 (57). Whereas residue 136 is an established AKT phosphorylation site, the search for the 112 kinase also provided evidence for the involvement of ERKs or ERK-regulated kinases in this process (7, 20, 27, 45, 46). Previous work also identified Raf-1 as a potential BAD kinase (54). However, no data on the Raf-1 phosphorylation sites have been published so far. Our own attempts to phosphorylate purified bacterially expressed glutathione S-transferase–BAD proteins in vitro only detected marginal phosphorylation of the substrate compared to activated AKT (A. von Gise, P. Lorenz, U. R. Rapp, and J. Troppmair, unpublished data). This observation initially prompted us to look for means by which Raf-1 might recruit PI3K-AKT-dependent signals to achieve BAD phosphorylation. It has been suggested that both kinases may physically interact (58). However, at least in the cell system where this interaction was analyzed coexpression of activated AKT with Raf-1 resulted in the phosphorylation of Raf-1, which negatively affects signaling through Raf-1 (44, 58). While we also observed complex formation between Raf-1 and AKT in 32D cells (data not shown) our results point to an indirect mechanism involving PI3K that would connect these two kinases. The current understanding of the nature of these presumably parallel signaling pathways makes it unlikely that PI3K activation is directly triggered by MEK or ERK, and thus additional intermediates have to be considered. One possible route involves the autocrine or paracrine secretion of a growth factor, resulting in the activation of PI3K (28, 37, 50). Our attempt to interfere with autocrine signals using suramin (6) led to an approximately 50% reduction in the viability of 32D ΔStuI-LIDEMANE cells at concentrations ranging from 75 to 100 μM following a 48-h treatment (A. von Gise, U. R. Rapp, and J. Troppmair, unpublished data). This inhibitor interfered with both cell survival and proliferation. A similar dependence on autocrine signals has been suggested in the analysis of the effect of a conditional MEK mutant on cell cycle progression (48). Existence of autocrine growth factor loops has been demonstrated before in fibroblast cells transformed by v-raf (33) or an estrogen receptor-regulated form of active Raf-1 (ER-Raf) (37). v-raf induced transforming growth factor α in rat fibroblast cells (28), whereas ER-Raf induced the synthesis of heparin binding epidermal growth factor (EGF). Heparin-binding EGF upon binding to the EGF receptor triggers the activation of Jun N-terminal protein kinase kinases (37). Both IL-3 and granulocyte-macrophage colony-stimulating factor (CSF) have been shown to support the growth of 32D cells (25). We therefore used an RNase protection approach (Riboquant; PharMingen) to analyze MEK-expressing 32D cells for production of these cytokines. However, we failed to detect mRNAs for IL-3 or granulocyte-macrophage CSF as well as for IL-11, IL-7, macrophage or granulocyte CSF, leukocyte inhibitory factor, IL-6, or stem cell factor (F. Berberich-Siebelt and J. Troppmair, unpublished data). It therefore remains to be established which signaling molecules link Raf-1-MEK1 with PI3K-AKT in these hematopoietic cells.
The cooperation of Raf-1 and MEK with PI3K-AKT in apoptosis suppression might be even more complex since evidence has also been put forward in support of a requirement for mitochondrial Raf-1 downstream of AKT in 32D cells (35). In these experiments expression of a dominant negative mutant of mitochondrially located Raf-1 rendered AKT-expressing cells susceptible to apoptosis induction following growth factor removal. Similar results have been obtained previously in the case of Bcl-2 (54). This argues for the existence of distinct Raf-1-specific effector pathways at the mitochondrial level which cannot be targeted by either AKT or Bcl-2. We expect that the further analysis of the localization and activation status of the Raf-1–AKT complex will provide insight into the mutual interdependence of these two kinases.
Despite our demonstration of a requirement for PI3K-AKT in the survival signaling downstream of MEK in two different hematopoietic cell lines, a previous analysis of MEK-dependent pathways in cell survival signaling in Rat-1 cells failed to detect any PI3K involvement (19). In these experiments the block of apoptosis inhibition was mapped downstream of cytochrome c release from mitochondria which, however, can be efficiently inhibited by AKT (30). Preliminary analysis of the cytochrome c release from mitochondria in ΔStuI-LIDEMANE-expressing 32D cells indicates no loss of mitochondrial cytochrome c up to 24 h after growth factor removal, suggesting that MEK functions in this process similar to AKT (Y. Le Mellay and J. Troppmair, unpublished data). Thus, while our data confirm the survival activity of MEK, it remains to be established whether the requirement of PI3K-AKT is restricted to cells of the hematopoietic lineage.
In summary our data provide evidence for a novel mechanism by which activation of a single pathway results in the coactivation of a parallel signaling cascade that is jointly required to achieve a certain biological effect. While this kind of cross talk has been observed among the evolutionarily highly conserved MAPK cascades, our findings indicate that this mechanism may also extend to additional receptor-initiated signaling pathways.
ACKNOWLEDGMENTS
We thank the following colleagues for providing essential reagents: Sally Cowley and Chris Marshall for the pBabe-puro MEK-LIDEM ANE and pBabe-puro MEK-LIDA constructs, H. Land for the pBabe-puro vector, Melanie Cobb for the constitutively active form of ERK2, and Gary P. Nolan for the amphotropic packaging cell line Phoenix ampho. We are also grateful to Tamara Potapenko and Véronique Le Mellay for technical help and to all members of the Troppmair lab for continuous support. This also extends to R. Wetzker, Jena, Germany, for helpful discussions.
This work was supported in part by a grant from the Interdisziplinäres Zentrum für Klinische Forschung “Pathogenese von Vaskulopathien und fehlgesteuerten Immunreaktionen” to J.T. and by grants from the Deutsche Krebshilfe (10-1446-Ra4) and the Deutsche Forschungsgemeinschaft (Ra 642/6-1).
REFERENCES
- 1.Ahn, N. G., T. S. Nahreini, N. S. Tolwinski, and K. A. Resing. Pharmacologic inhibitors of MKK1 and MKK2. Methods Enzymol., in press. [DOI] [PubMed]
- 2.Alessi D R, Saito Y, Campbell D G, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall C J, Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andjelkovic M, Alessi D R, Meier R, Fernandez A, Lamb N J, Frech M, Cron P, Cohen P, Lucocq J M, Hemmings B A. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann A, Agapite J, McCall K, Steller H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95:331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann A, Agapite J, Steller H. Mechanisms and control of programmed cell death in invertebrates. Oncogene. 1998;17:3215–3223. doi: 10.1038/sj.onc.1202586. [DOI] [PubMed] [Google Scholar]
- 6.Betsholtz C, Johnsson A, Heldin C H, Westermark B. Efficient reversion of simian sarcoma virus-transformation and inhibition of growth factor-induced mitogenesis by suramin. Proc Natl Acad Sci USA. 1986;83:6440–6444. doi: 10.1073/pnas.83.17.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonni A, Brunet A, West A E, Datta S R, Takasu M A, Greenberg M E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 8.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 9.Carroll M P, Clark-Lewis I, Rapp U R, May W S. Interleukin-3 and granulocyte-macrophage colony-stimulating factor mediate rapid phosphorylation and activation of cytosolic c-raf. J Biol Chem. 1990;265:19812–19817. [PubMed] [Google Scholar]
- 10.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleveland J L, Dean M, Rosenberg N, Wang J Y, Rapp U R. Tyrosine kinase oncogenes abrogate interleukin-3 dependence of murine myeloid cells through signaling pathways involving c-myc: conditional regulation of c-myc transcription by temperature-sensitive v-abl. Mol Cell Biol. 1989;9:5685–5695. doi: 10.1128/mcb.9.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleveland J L, Troppmair J, Packham G, Askew D S, Lloyd P, Gonzalez-Garcia M, Nunez G, Ihle J N, Rapp U R. v-raf suppresses apoptosis and promotes growth of interleukin-3-dependent myeloid cells. Oncogene. 1994;9:2217–2226. [PubMed] [Google Scholar]
- 13.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 14.Datta S R, Brunet A, Greenberg M E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 15.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 16.Daum G, Eisenmann-Tappe I, Fries H W, Troppmair J, Rapp U R. The ins and outs of Raf kinases. Trends Biochem Sci. 1994;19:474–480. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 17.de Groot R P, Coffer P J, Koenderman L. Regulation of proliferation, differentiation and survival by the IL-3/IL-5/GM-CSF receptor family. Cell Signal. 1998;10:619–628. doi: 10.1016/s0898-6568(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 18.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 19.Erhardt P, Schremser E J, Cooper G M. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol. 1999;19:5308–5315. doi: 10.1128/mcb.19.8.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang X, Yu S, Eder A, Mao M, Bast R C, Jr, Boyd D, Mills G B. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene. 1999;18:6635–6640. doi: 10.1038/sj.onc.1203076. [DOI] [PubMed] [Google Scholar]
- 21.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, Copeland R A, Magolda R L, Scherle P A, Trzaskos J M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 22.Fruman D A, Mauvais-Jarvis F, Pollard D A, Yballe C M, Brazil D, Bronson R T, Kahn C R, Cantley L C. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85alpha. Nat Genet. 2000;26:379–382. doi: 10.1038/81715. [DOI] [PubMed] [Google Scholar]
- 23.Gold M R, Duronio V, Saxena S P, Schrader J W, Aebersold R. Multiple cytokines activate phosphatidylinositol 3-kinase in hemopoietic cells. Association of the enzyme with various tyrosine- phosphorylated proteins. J Biol Chem. 1994;269:5403–5412. [PubMed] [Google Scholar]
- 24.Greenberger J S, Sakakeeny M A, Humphries R K, Eaves C J, Eckner R J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci USA. 1983;80:2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagemann D, Troppmair J, Rapp U R. Cot protooncoprotein activates the dual specificity kinases MEK-1 and SEK-1 and induces differentiation of PC12 cells. Oncogene. 1999;18:1391–1400. doi: 10.1038/sj.onc.1202431. [DOI] [PubMed] [Google Scholar]
- 26.Hartkamp J, Troppmair J, Rapp U R. The JNK/SAPK activator mixed lineage kinase 3 (MLK3) transforms NIH 3T3 cells in a MEK-dependent fashion. Cancer Res. 1999;59:2195–2202. [PubMed] [Google Scholar]
- 27.Hayakawa J, Ohmichi M, Kurachi H, Kanda Y, Hisamoto K, Nishio Y, Adachi K, Tasaka K, Kanzaki T, Murata Y. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000;60:5988–5994. [PubMed] [Google Scholar]
- 28.Heidecker G, Cleveland J L, Beck T, Huleihel M, Kolch W, Storm S M, Rapp U R. Role of raf and myc oncogenes in signal transduction. In: Colburn N, editor. Genes and signal transduction in multistage carcinogenesis. New York, N.Y: Marcel Dekker, Inc; 1989. pp. 339–374. [Google Scholar]
- 29.Hemmings B A. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy S G, Kandel E S, Cross T K, Hay N. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerkhoff E, Rapp U R. Cell cycle targets of Ras/Raf signalling. Oncogene. 1998;17:1457–1462. doi: 10.1038/sj.onc.1202185. [DOI] [PubMed] [Google Scholar]
- 32.Kerkhoff E, Rapp U R. Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol Cell Biol. 1997;17:2576–2586. doi: 10.1128/mcb.17.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keski-Oja J, de Larco J E, Rapp U R, Todaro G J. Murine sarcoma growth factors affect the growth and morphology of cultured mouse epithelial cells J. Cell Physiol. 1980;104:41–46. doi: 10.1002/jcp.1041040107. [DOI] [PubMed] [Google Scholar]
- 34.Kurada P, White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95:319–329. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- 35.Majewski M, Nieborowska-Skorska M, Salomoni P, Slupianek A, Reiss K, Trotta R, Calabretta B, Skorski T. Activation of mitochondrial Raf-1 is involved in the antiapoptotic effects of Akt. Cancer Res. 1999;59:2815–2819. [PubMed] [Google Scholar]
- 36.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy S A, Samuels M L, Pritchard C A, Abraham J A, McMahon M. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 1995;9:1953–1964. doi: 10.1101/gad.9.16.1953. [DOI] [PubMed] [Google Scholar]
- 38.Meier R, Alessi D R, Cron P, Andjelkovic M, Hemmings B A. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J Biol Chem. 1997;272:30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 39.Meier R, Hemmings B A. Regulation of protein kinase B. J Recept Signal Transduct Res. 1999;19:121–128. doi: 10.3109/10799899909036639. [DOI] [PubMed] [Google Scholar]
- 40.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pages G, Brunet A, L'Allemain G, Pouyssegur J. Constitutive mutant and putative regulatory serine phosphorylation site of mammalian MAP kinase kinase (MEK1) EMBO J. 1994;13:3003–3010. doi: 10.1002/j.1460-2075.1994.tb06599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson M J, Stippec S A, Goldsmith E, White M A, Cobb M H. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr Biol. 1998;8:1141–1150. doi: 10.1016/s0960-9822(07)00485-x. [DOI] [PubMed] [Google Scholar]
- 43.Romashkova J A, Makarov S S. NF-kappaB is a target of AKT in antiapoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 44.Rommel C, Clarke B A, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos G D, Glass D J. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 45.Scheid M P, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: involvement of MEK upstream of Bad phosphorylation Proc. Natl Acad Sci USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheid M P, Schubert K M, Duronio V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J Biol Chem. 1999;274:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- 47.Songyang Z, Baltimore D, Cantley L C, Kaplan D R, Franke T F. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Treinies I, Paterson H F, Hooper S, Wilson R, Marshall C J. Activated MEK stimulates expression of AP-1 components independently of phosphatidylinositol 3-kinase (PI3-kinase) but requires a PI3-kinase signal to stimulate DNA synthesis. Mol Cell Biol. 1999;19:321–329. doi: 10.1128/mcb.19.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Troppmair J, Bruder J T, Munoz H, Lloyd P A, Kyriakis J, Banerjee P, Avruch J, Rapp U R. Mitogen-activated protein kinase/extracellular signal-regulated protein kinase activation by oncogenes, serum, and 12-O-tetradecanoylphorbol-13-acetate requires Raf and is necessary for transformation. J Biol Chem. 1994;269:7030–7035. [PubMed] [Google Scholar]
- 50.Troppmair J, Hartkamp J, Rapp U R. Activation of NF-kappa B by oncogenic Raf in HEK 293 cells occurs through autocrine recruitment of the stress kinase cascade. Oncogene. 1998;17:685–690. doi: 10.1038/sj.onc.1201981. [DOI] [PubMed] [Google Scholar]
- 51.Vanhaesebroeck B, Waterfield M D. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 52.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 53.Wang H G, Miyashita T, Takayama S, Sato T, Torigoe T, Krajewski S, Tanaka S, Hovey L, 3rd, Troppmair J, Rapp U R, et al. Apoptosis regulation by interaction of Bcl-2 protein and Raf-1 kinase. Oncogene. 1994;9:2751–2756. [PubMed] [Google Scholar]
- 54.Wang H G, Rapp U R, Reed J C. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 55.Wellbrock C, Schartl M. Activation of phosphatidylinositol 3-kinase by a complex of p59fyn and the receptor tyrosine kinase Xmrk is involved in malignant transformation of pigment cells. Eur J Biochem. 2000;267:3513–3522. doi: 10.1046/j.1432-1327.2000.01378.x. [DOI] [PubMed] [Google Scholar]
- 56.Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr G A, Backer J M. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110α catalytic subunit by the p85 regulatory subunit. Mol Cell Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 58.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]