Abstract
Rotavirus is a major cause of severe pediatric diarrhea worldwide. In 2006, 2 live, oral rotavirus vaccines, Rotarix and RotaTeq, were licensed for use in infants and were rapidly adopted in many high- and middle-income settings where efficacy had been demonstrated in clinical trials. Following completion of successful trials in low-income settings, the World Health Organization (WHO) recommended rotavirus vaccination for all infants globally in 2009. In 2018, 2 new rotavirus vaccines, Rotasiil and Rotavac, were prequalified by WHO, expanding global availability. As of March 2021, rotavirus vaccines have been introduced nationally in 106 countries. Since, Rotavirus vaccines have demonstrated effectiveness against severe disease and mortality, even among age groups in eligible for vaccination. Cross-genotypic protection has been demonstrated, and the favorable benefit-risk profile of these vaccines continues to be confirmed. Ongoing research seeks to better understand reasons for the geographic disparities in effectiveness observed, in order to optimize vaccine strategies worldwide.
Keywords: rotavirus, rotavirus vaccines, vaccine effectiveness, pediatric gastroenteritis, vaccine-preventable disease, acute gastroenteritis
Rotavirus is a leading cause of severe pediatric diarrhea worldwide, estimated to have caused 258 million diarrheal episodes and >128 000 associated deaths among children <5 years of age in 2016 [1]. The mortality burden of rotavirus falls most heavily on developing countries where access to healthcare is suboptimal [1, 2]. Rotavirus has been estimated to be the leading cause of pediatric diarrheal deaths in countries with low- to high-middle sociodemographic index (SDI), and as the third leading cause of pediatric diarrheal deaths in high SDI countries [3]. The proportion of rotavirus illness among infants and young children hospitalized for severe diarrhea prior to widespread introduction of rotavirus vaccine has been found to be similar across geographies and in some studies was highest in the highest industrialization strata [4, 5], suggesting that traditional measures to improve hygiene and sanitation and access to safe water are unlikely to fully control the disease. Initial rotavirus infections occur early in life, and in the prevaccine era, nearly all children suffered at least 1 rotavirus infection by the age of 5 years [4]. Rotavirus infection confers partial immunity, with the level of protection against disease increasing with each subsequent infection [6]. Vaccination, through mimicking the effects of natural rotavirus infection, is considered the best means of control of rotavirus disease [7, 8].
EVIDENCE AND IMPACT OF THE FIRST 2 GLOBALLY LICENSED ROTAVIRUS VACCINES: ROTARIX AND ROTATEQ
In 2006, 2 live, oral rotavirus vaccines, Rotarix (GlaxoSmithKline) and RotaTeq (Merck), were licensed for use in infants based on data from trials conducted in the United States, Europe, and Latin America [9, 10]. Due to the experience with RotaShield, a tetravalent reassortant rhesus rotavirus vaccine that was withdrawn from the US market in 1999 because it carried a risk of 1 additional case of intussusception (a form of bowel obstruction) per 10 000 vaccinated infants, large clinical trials for both Rotarix and RotaTeq (60 000–70 000 infants each) were conducted to examine safety [11]. Neither vaccine was found to cause an increased risk of intussusception in clinical trials, and both vaccines were highly efficacious against severe rotavirus gastroenteritis [9, 10]. On the strength of these data, in 2006 the World Health Organization (WHO) recommended rotavirus vaccines for use in high- and middle-income settings [12]. However, because of concerns about the efficacy of oral rotavirus vaccines in low-income settings, WHO recommended additional trials to examine vaccine efficacy in these settings [12].
Rotavirus vaccine uptake in the Americas was rapid, and evidence quickly accumulated on the effectiveness of these vaccines in reducing hospitalizations and deaths from pediatric gastroenteritis [13–19]. In the United States, an analysis of hospital discharge data showed significant reductions in rotavirus hospitalizations in 2008 as compared to prevaccine years, even among older children and young adults 3–24 years of age, who would not have been vaccinated [14]; similar results were seen when the analysis was extended to 2010 [15]. These findings, which suggested that rotavirus vaccination may also offer indirect protection to older children and adults by reducing overall levels of community transmission of rotavirus, provided important evidence of the far-reaching benefits of rotavirus vaccine introduction. The potential life-saving impact of rotavirus vaccination was first demonstrated in Mexico, where an analysis of pediatric diarrheal mortality found significant declines after the introduction of rotavirus vaccine [16, 19].
One question that would urgently impact the viability of rotavirus vaccines as a means of disease control was the extent to which these vaccines could provide cross-genotype protection, particularly against strains not included in the vaccine. Rotavirus genotypes are defined by 2 outer capsid proteins: VP4 (which defines the G type) and VP7 (which defines the P type) [20, 21]. Although a limited number of strains tend to account for the bulk of infections (G1P[8], G2P[4], G3P[8], G4P[8], G9P[8], and G12P[8]), there are numerous other strains that circulate at a lower frequency, and overall circulating patterns vary geographically and over time [22, 23]. Rotarix is based on an attenuated G1P[8] human rotavirus, whereas RotaTeq is a pentavalent vaccine containing bovine-human reassortant virus with the G1, G2, G3, G4, and P[8] antigens [9, 10]. Encouragingly, data from a US-based active surveillance platform demonstrated high vaccine effectiveness of both vaccines against a variety of strains, including nonvaccine-type strains [17, 24]. Similarly, data from an efficacy trial in Africa found Rotarix efficacy to be comparable across vaccine- and nonvaccine-type strains [25]. Results from postlicensure evaluations in Latin America further confirmed the cross-protection conferred by vaccination [26–29].
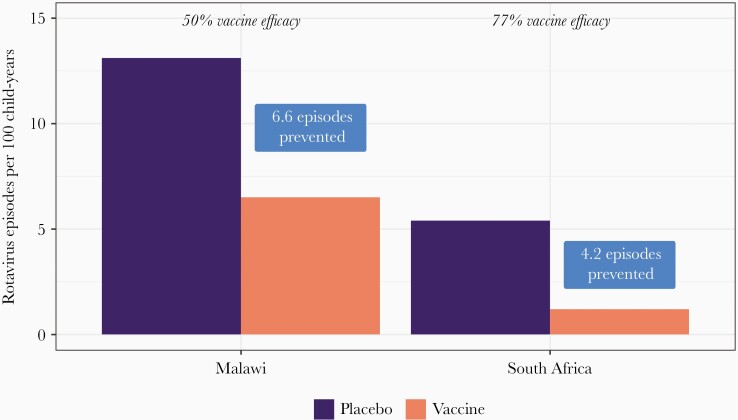
In 2009, after the completion of additional clinical trials showing vaccine efficacy in low-income countries of Africa and Asia, WHO recommended rotavirus vaccines for inclusion in national immunization programs worldwide [7]. This expansion opened up rotavirus vaccine recommendations to areas most in need of intervention, as it has been estimated that 65% of rotavirus deaths occur in just 10 countries—all in Africa and Asia [2]. Vaccine efficacy in clinical trials of Rotarix and RotaTeq in Asia and Africa ranged from 51% to 64%, moderate in comparison to results from the initial trials in high-income countries, in which efficacy was >85% [9, 10, 25, 30, 31]. However, given the higher burden of rotavirus disease in these settings, even a vaccine with modest efficacy can have a substantial public health impact. As an example, when comparing results from Malawi, a lower-resource country, with those from South Africa, a higher-resource country, it is clear that despite lower efficacy in Malawi, the health impact in terms of episodes of severe rotavirus disease averted by vaccination of 100 infants was greater in Malawi as compared to South Africa, which had a higher estimated efficacy [25] (Figure 1). The effectiveness and public health impact of these vaccines continues to be borne out in postintroduction evaluations, with evidence suggesting that although effectiveness tends to be lower in countries with high all-cause child mortality burden, impact of rotavirus vaccine in terms of reducing rotavirus-associated hospitalizations and deaths tends to be greater in these high child-mortality countries as compared to other settings [32, 33].
Figure 1.
Vaccine impact (episodes prevented) and vaccine efficacy by country: Malawi and South Africa. Adapted from Madhi et al 2010 [25] analysis of clinical trial data collected 2005–2007.
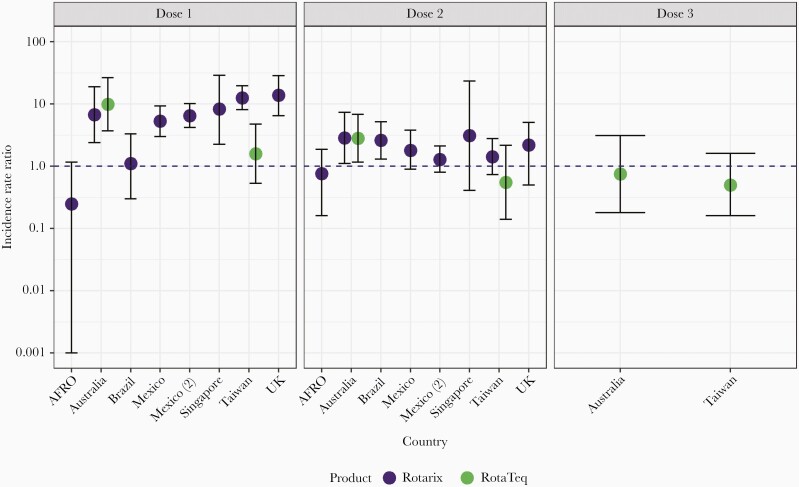
To further support expansion of Rotarix and RotaTeq into additional national vaccination programs, more evidence was needed on the safety of these 2 vaccines during routine use. Although no evidence of an association with intussusception was noted during clinical trials, these studies were not powered to detect an increased risk smaller than 1 per 10 000 children vaccinated. Postintroduction evaluations in several high- and middle-income countries found an even smaller but significant risk of intussusception associated with both vaccines, approximately 1–6 additional cases per 100 000 infants vaccinated [34–42]. However, as the benefits of vaccination still exceeded the possible intussusception risk [43], no changes were made to overall recommendations. Furthermore, later analysis of data from 7 African countries using Rotarix found no evidence of a significantly increased risk of intussusception after vaccination [44], nor did another analysis of data from South Africa [45] (Figure 2). These analyses reaffirm the favorable safety profile of rotavirus vaccines and suggest that the benefits of rotavirus vaccination far outweigh the associated risks.
Figure 2.
Estimates of incidence rate ratio for intussusception in the 1–7 days following rotavirus vaccine administration, using the self-controlled case series method, by country and dose [34, 36, 39, 40–42]. Study data from 2006 through 2016. Dots indicate point estimates for incidence rate ratios, and lines and whiskers indicate 95% confidence intervals. Abbreviations: AFRO = African Rotavirus Surveillance Network Countries (Ethiopia, Ghana, Kenya, Malawi, Tanzania, Zambia, and Zimbabwe); UK = United Kingdom.
EXPANDING GLOBAL ROTAVIRUS VACCINE CHOICE: IMPLEMENTATION OF ROTAVAC AND ROTASIIL
In 2018, interruptions to the global rotavirus vaccine supply underscored the importance of having multiple affordable vaccine options available to countries. That same year, 2 Indian-manufactured rotavirus vaccines were prequalified by WHO: Rotasiil (Serum Institute) and Rotavac (Bharat Biotech), both live, oral vaccines given in a 3-dose infant schedule. Rotasiil is based on a bovine-human reassortant strain and contains G1, G2, G3, G4, and G9 antigens, while Rotavac is based on a naturally occurring neonatal strain of G9P[11] [46, 47]. These vaccines have several features that make them attractive to many countries: Rotasiil is heat-stable for extended periods of time at high ambient temperatures, while Rotavac requires only a 5-drop dose, and both vaccines are available at a relatively low cost compared to Rotarix and RotaTeq [48, 49]. Clinical trials for these vaccines were conducted in India and Niger, and both demonstrated similar efficacy as was seen for Rotarix and RotaTeq in Asia and Africa [47, 50–52]. Both vaccines are in routine use in India, and each has also been adopted in a handful of other countries in Africa and Asia [53]. One country where a substantial impact might be expected is the Democratic Republic of Congo, which has both a large birth cohort and a high burden of rotavirus disease [2, 54, 55]. Given that Rotavac and Rotasiil were prequalified only in 2018, the evidence base for safety and effectiveness in routine usage is still being built. However, several postlicensure evaluations of Rotavac usage in India have been published, all showing no increased risk of intussusception associated with vaccination [56–58]. Additional evaluations of Rotavac and Rotasiil are underway to further assess the safety and effectiveness of both vaccines under real-world conditions of use.
NATIONAL VACCINES: ROTAVIN AND LANZHOU LAMB ROTAVIRUS VACCINE
In addition to Rotasiil and Rotavac, 2 other indigenously produced rotavirus vaccines are licensed and in use in their countries of origin: Rotavin (POLYVAC) is licensed for use in Vietnam, and Lanzhou Lamb Rotavirus Vaccine (LLRV; Lanzhou Institute of Biological Products) is licensed for use in China [59–63]. Although neither vaccine is currently part of any national immunization program, both are available on the private market in their respective countries of origin. Rotavin has demonstrated immunogenicity in a phase 2 clinical trial and has been introduced into the immunization schedule in selected areas of 2 provinces, where its impact and effectiveness are being evaluated [59]. A phase 3 trial of a liquid presentation of the vaccine has been completed, and results are pending publication [64]. No efficacy data are available for LLRV, but vaccine effectiveness estimates have varied from 35% to 77% [63, 65–68]. Nationally produced vaccines are an important element in ensuring broad global access to affordable vaccines, and more data on these 2 nationally licensed products will inform the global research agenda.
RESEARCH GAPS AND FUTURE DIRECTIONS
Recently, there has been increased scientific interest in possible off-target effects of rotavirus vaccination. The most well-documented such effect is a decrease in seizure hospitalizations following rotavirus immunization, which has been observed in several countries through both cohort studies and ecological analyses [69–73]. It is hypothesized that this effect is mediated through reduction in rotavirus disease, which has been shown to cause seizures in addition to (or sometimes in the absence of) gastrointestinal illness [74, 75]. Two autoimmune diseases, celiac disease (CD) and type 1 diabetes (T1D) have also been linked to rotavirus infection, although evidence suggests that the etiology of both autoimmune conditions is multifactorial [74, 76]. Two studies have found that rotavirus vaccination may have some protective effect against CD, in conjunction with other factors [77, 78]. Although 2 analyses have found rotavirus vaccination to be associated with reduced T1D diagnosis [79, 80], several other studies have shown no significant effect [77, 81–83]. Further research will be necessary to better elucidate possible relationships of rotavirus vaccination to CD and T1D, as well as to identify any other possible unanticipated benefits of rotavirus vaccination [74–76].
Another area of ongoing research involves the differential effectiveness of rotavirus vaccine by setting, whereby higher effectiveness is demonstrated in higher-income settings as compared to lower-income settings. Although this phenomenon has been well documented, the exact reasons for this disparity, and the best interventions, are still not well defined. Multiple possible factors have been identified, such as those that may act directly on vaccine virus in the gut (eg, maternal antibodies, breast milk, stomach acid, and oral polio vaccine [OPV]), as well as factors that act to impair general immune response (eg, malnutrition, microbiome, and coinfections such as human immunodeficiency virus [HIV]) [84–92]. Available evidence suggests that delaying rotavirus vaccination schedules may contribute to enhanced immune response due to waning interference from maternal antibodies [93], but it is not known if this would translate into increased effectiveness, and any changes to vaccine schedules must also be balanced with practical and logistical concerns, as well as the desire to protect infants early in life. Similarly, while OPV has been well documented to interfere with rotavirus vaccine immunogenicity when coadministered (the converse does not occur) [85], this finding does not suggest a clear public health intervention. Several interventions specific to the time of vaccination have been tested, for instance withholding breastfeeding and adding nutritional supplementation, but these were found to have little or no effect on rotavirus vaccine immune response [88, 94]. Research also suggests that susceptibility to rotavirus (and thus live rotavirus vaccines) varies by histo-blood group antigens in a rotavirus P-genotype–dependent way; the expression of these antigens is governed by polymorphisms in 2 genes, the prevalence of which varies by population [95–107]. As a specific example, the genotype conferring increased susceptibility to P[6] rotaviruses is more common in Africa, a setting where both increased circulation of P[6] rotaviruses and moderate efficacy of current rotavirus vaccines have been observed. Taken together, the evidence suggests a need for holistic interventions, such as those that could improve overall infant nutritional status, and a potential role for parenterally administered rotavirus vaccines, which would not be subject to some of the same limitations as the current oral vaccines.
Indeed, there are several rotavirus vaccines under development that are designed for parenteral administration; another candidate that has completed phase 2 trials is being developed for neonatal administration [108]. The most advanced parenteral vaccine candidates are 2 subunit vaccines: one contains the P[8] antigen, and the other contains P[4], P[6], and P[8] antigens, both being developed by PATH and both demonstrating immunogenicity [109, 110]. RV3-BB, the rotavirus vaccine candidate being developed for neonatal administration, is based on a naturally attenuated neonatal strain of G3P[6] rotavirus that was initially isolated in Australia [111, 112]. A phase 2b trial demonstrated both safety and efficacy of this vaccine when given on a schedule that includes a birth dose (along with doses at 8 and 14 weeks of age) as well as a more standard infant schedule at 8, 14, and 18 weeks of age (although the trial was not powered to detect extremely rare side effects such as intussusception) [111].
CONCLUSIONS
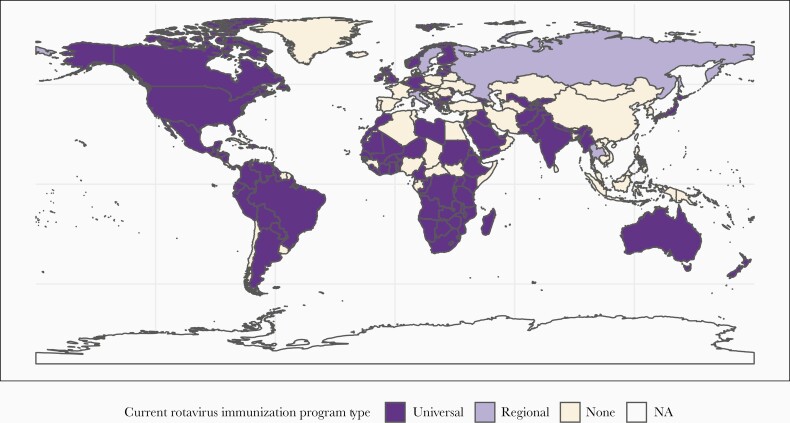
As of March 2021, rotavirus vaccines have been introduced nationally in 106 countries, and regionally within 4 countries (Figure 3) [53]. It is not yet clear what impact the coronavirus disease 2019 (COVID-19) pandemic may have had on rotavirus vaccine coverage or burden, but encouragingly, despite the pandemic, several countries introduced rotavirus vaccines into their national schedules during 2020. Although great progress has been made over the past 15 years, there is a notable gap evident: in much of Asia, an area with a substantial rotavirus burden, no national immunization program is in place. A 2018 analysis estimated that early introduction of rotavirus vaccines throughout Asia could have averted >700 000 hospitalizations and approximately 35 000 deaths among children <5 years of age, with impact concentrated in the highest-burden countries [113]. As we look forward towards the next 15 years of rotavirus vaccines, our goal must be to close these regional gaps to protect more children from severe gastroenteritis. With multiple planned introductions in 2021, including in Bangladesh, a country with an extraordinarily high rotavirus burden [114], we can look forward to continued progress in improving child health.
Figure 3.
Map of rotavirus vaccine introductions by country, with program status (universal vs regional vs none).
Notes
Disclaimer. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 15th International Conference on Diarrheal Disease and Nutrition, 28–30 January 2020, Dhaka, Bangladesh.
References
- 1. GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18:1211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance N. global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 2016; 62(Suppl 2):S96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD Diarrhoeal Disease Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 2017; 17:909–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kapikian AZ, Shope RE. Rotaviruses, reoviruses, coltiviruses, and orbiviruses. In: Baron S, ed. Medical microbiology. Galveston, TX: University of Texas Medical Branch at Galveston, 1996. [PubMed] [Google Scholar]
- 5. Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE; Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 2013; 8:e72788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Velázquez FR, Matson DO, Calva JJ, et al. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med 1996; 335:1022–8. [DOI] [PubMed] [Google Scholar]
- 7. Meeting of the Strategic Advisory Group of Experts on immunization, October 2009 - conclusions and recommendations. Wkly Epidemiol Rec 2009; 84:517–32. [PubMed] [Google Scholar]
- 8. Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec 2013; 88:49–64. [PubMed] [Google Scholar]
- 9. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. ; Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11–22. [DOI] [PubMed] [Google Scholar]
- 10. Vesikari T, Matson DO, Dennehy P, et al. ; Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23–33. [DOI] [PubMed] [Google Scholar]
- 11. Murphy TV, Gargiullo PM, Massoudi MS, et al. ; Rotavirus Intussusception Investigation Team. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 2001; 344:564–72. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization. Rotavirus vaccines. Wkly Epidemiol Rec 2007; 82:285–95. [PubMed] [Google Scholar]
- 13. Tate JE, Cortese MM, Payne DC, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J 2011; 30:S56–60. [DOI] [PubMed] [Google Scholar]
- 14. Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis 2011; 204:980–6. [DOI] [PubMed] [Google Scholar]
- 15. Gastañaduy PA, Curns AT, Parashar UD, Lopman BA. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA 2013; 310:851–3. [DOI] [PubMed] [Google Scholar]
- 16. Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med 2010; 362:299–305. [DOI] [PubMed] [Google Scholar]
- 17. Payne DC, Boom JA, Staat MA, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009–2011. Clin Infect Dis 2013; 57:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Payne DC, Staat MA, Edwards KM, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties, 2006–2009. Clin Infect Dis 2011; 53:245–53. [DOI] [PubMed] [Google Scholar]
- 19. Richardson V, Parashar U, Patel M. Childhood diarrhea deaths after rotavirus vaccination in Mexico. N Engl J Med 2011; 365:772–3. [DOI] [PubMed] [Google Scholar]
- 20. Estes MK, Palmer EL, Obijeski JF. Rotaviruses: a review. In: Cooper M, Hofschneider PH, Koprowski H, et al. , eds. Current topics in microbiology and immunology: Vol 105. Berlin, Heidelberg: Springer, 1983:123–84. [DOI] [PubMed] [Google Scholar]
- 21. Hoshino Y, Kapikian AZ. Classification of rotavirus VP4 and VP7 serotypes. Arch Virol Suppl 1996:12:99–111. [DOI] [PubMed]
- 22. Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol 2005; 15:29–56. [DOI] [PubMed] [Google Scholar]
- 23. Esona MD, Gautam R. Rotavirus. Clin Lab Med 2015; 35:363–91. [DOI] [PubMed] [Google Scholar]
- 24. Payne DC, Selvarangan R, Azimi PH, et al. Long-term consistency in rotavirus vaccine protection: RV5 and RV1 vaccine effectiveness in US children, 2012–2013. Clin Infect Dis 2015; 61:1792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. [DOI] [PubMed] [Google Scholar]
- 26. Pringle KD, Patzi M, Tate JE, et al. Sustained effectiveness of rotavirus vaccine against very severe rotavirus disease through the second year of life, Bolivia 2013–2014. Clin Infect Dis 2016; 62(Suppl 2) :S115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel MM, Patzi M, Pastor D, et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ 2013; 346:f3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA 2009; 301:2243–51. [DOI] [PubMed] [Google Scholar]
- 29. Patel M, Pedreira C, De Oliveira LH, et al. Effectiveness of pentavalent rotavirus vaccine against a diverse range of circulating strains in Nicaragua. Clin Infect Dis 2016; 62(Suppl 2):S127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. [DOI] [PubMed] [Google Scholar]
- 31. Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615–23. [DOI] [PubMed] [Google Scholar]
- 32. Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis 2017; 215:1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burnett E, Parashar UD, Tate JE. Real-world effectiveness of rotavirus vaccines, 2006-19: a literature review and meta-analysis. Lancet Glob Health 2020; 8:e1195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel MM, López-Collada VR, Bulhões MM, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med 2011; 364:2283–92. [DOI] [PubMed] [Google Scholar]
- 35. Shui IM, Baggs J, Patel M, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. JAMA 2012; 307:598–604. [DOI] [PubMed] [Google Scholar]
- 36. Carlin JB, Macartney KK, Lee KJ, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s national immunization program. Clin Infect Dis 2013; 57:1427–34. [DOI] [PubMed] [Google Scholar]
- 37. Weintraub ES, Baggs J, Duffy J, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med 2014; 370:513–9. [DOI] [PubMed] [Google Scholar]
- 38. Yih WK, Lieu TA, Kulldorff M, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med 2014; 370:503–12. [DOI] [PubMed] [Google Scholar]
- 39. Yung CF, Chan SP, Soh S, Tan A, Thoon KC. Intussusception and monovalent rotavirus vaccination in Singapore: self-controlled case series and risk-benefit study. J Pediatr 2015; 167:163–8.e1. [DOI] [PubMed] [Google Scholar]
- 40. Stowe J, Andrews N, Ladhani S, Miller E. The risk of intussusception following monovalent rotavirus vaccination in England: a self-controlled case-series evaluation Ref. No: JVAC-D-16-01124. Vaccine 2016; 34:6115. [DOI] [PubMed] [Google Scholar]
- 41. Huang WT, Juan YC, Liu CH, Yang YY, Chan KA. Intussusception and Kawasaki disease after rotavirus vaccination in Taiwanese infants. Vaccine 2020; 38:6299–303. [DOI] [PubMed] [Google Scholar]
- 42. Velazquez FR, Colindres RE, Grajales C, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J 2012; 31:736–44. [DOI] [PubMed] [Google Scholar]
- 43. Patel MM, Haber P, Baggs J, Zuber P, Bines JE, Parashar UD. Intussusception and rotavirus vaccination: a review of the available evidence. Expert Rev Vaccines 2009; 8:1555–64. [DOI] [PubMed] [Google Scholar]
- 44. Tate JE, Mwenda JM, Parashar UD. Intussusception after rotavirus vaccination in Africa. N Engl J Med 2018; 379:1288–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Groome MJ, Tate JE, Arnold M, et al. Evaluation of intussusception after oral monovalent rotavirus vaccination in South Africa. Clin Infect Dis 2020; 70:1606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coldiron ME, Guindo O, Makarimi R, et al. Safety of a heat-stable rotavirus vaccine among children in Niger: data from a phase 3, randomized, double-blind, placebo-controlled trial. Vaccine 2018; 36:3674–80. [DOI] [PubMed] [Google Scholar]
- 47. Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. ; India Rotavirus Vaccine Group. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet 2014; 383:2136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. World Health Organization. WHO Prequalified Vaccines. https://extranet.who.int/gavi/PQ_Web/Default.aspx?nav=1. Accessed 14 March 2019.
- 49. UNICEF Supply Division. Rotavirus vaccine: supply and demand update, February 2020. https://www.unicef.org/supply/sites/unicef.org.supply/files/2020-03/rotavirus-vaccine-supply-and-demand-update_0.pdf. Accessed March 2021. [Google Scholar]
- 50. Isanaka S, Guindo O, Langendorf C, et al. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. N Engl J Med 2017; 376:1121–30. [DOI] [PubMed] [Google Scholar]
- 51. Kulkarni PS, Desai S, Tewari T, et al. ; SII BRV-PV author group. A randomized phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine 2017; 35:6228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. ; India Rotavirus Vaccine Group. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine 2014; 32(Suppl 1):A110–6. [DOI] [PubMed] [Google Scholar]
- 53. Johns Hopkins Bloomberg School of Public Health. International Vaccine Access Center (IVAC). www.view-hub.org. Accessed March 2021.
- 54. Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 2018; 172:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pukuta ES, Esona MD, Nkongolo A, et al. Molecular surveillance of rotavirus infection in the Democratic Republic of the Congo August 2009 to June 2012. Pediatr Infect Dis J 2014; 33:355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reddy SN, Nair NP, Tate JE, et al. Intussusception after rotavirus vaccine introduction in India. N Engl J Med 2020; 383:1932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Early Rollout of ROTAVAC® Indian Network. Assessment of risk of intussusception after pilot rollout of rotavirus vaccine in the Indian public health system. Vaccine 2020; 38:5241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. INCLEN Intussusception Surveillance Network Study Group. Risk of intussusception after monovalent rotavirus vaccine (Rotavac) in Indian infants: a self-controlled case series analysis. Vaccine 2021; 39:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dang DA, Nguyen VT, Vu DT, et al. ; Rotavin-M1 Vaccine Trial Group. A dose-escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin-M1) in Vietnamese children. Vaccine 2012; 30(Suppl 1):A114–21. [DOI] [PubMed] [Google Scholar]
- 60. Luan le T, Trang NV, Phuong NM, et al. Development and characterization of candidate rotavirus vaccine strains derived from children with diarrhoea in Vietnam. Vaccine 2009; 27(Suppl 5):F130–8. [DOI] [PubMed] [Google Scholar]
- 61. Li D, Xu Z, Xie G, et al. Genotype of rotavirus vaccine strain LLR in China is G10P[15] [in Chinese]. Bing Du Xue Bao 2015; 31:170–3. [PubMed] [Google Scholar]
- 62. Li JS, Cao B, Gao HC, et al. Faecal shedding of rotavirus vaccine in Chinese children after vaccination with Lanzhou lamb rotavirus vaccine. Sci Rep 2018; 8:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fu C, Wang M, Liang J, He T, Wang D, Xu J. Effectiveness of Lanzhou lamb rotavirus vaccine against rotavirus gastroenteritis requiring hospitalization: a matched case-control study. Vaccine 2007; 25:8756–61. [DOI] [PubMed] [Google Scholar]
- 64. Center for Research and Production of Vaccines and Biologicals, Vietnam. Phase III study of liquid formulation of ROTAVIN. https://clinicaltrials.gov/ct2/show/NCT03703336. Accessed 9 May 2019.
- 65. Fu C, Dong Z, Shen J, et al. Rotavirus gastroenteritis infection among children vaccinated and unvaccinated with rotavirus vaccine in Southern China: a population-based assessment. JAMA Netw Open 2018; 1:e181382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fu C, He Q, Xu J, et al. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine 2012; 31:154–8. [DOI] [PubMed] [Google Scholar]
- 67. Fu C, Tate JE, Jiang B. Effectiveness of Lanzhou lamb rotavirus vaccine against hospitalized gastroenteritis: further analysis and update. Hum Vaccin 2010; 6:953. [DOI] [PubMed] [Google Scholar]
- 68. Zhen SS, Li Y, Wang SM, et al. Effectiveness of the live attenuated rotavirus vaccine produced by a domestic manufacturer in China studied using a population-based case-control design. Emerg Microbes Infect 2015; 4:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Burke RM, Tate JE, Dahl RM, Aliabadi N, Parashar UD. Rotavirus vaccination is associated with reduced seizure hospitalization risk among commercially insured U.S. children. Clin Infect Dis 2018; 67:1614–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pardo-Seco J, Cebey-López M, Martinón-Torres N, et al. Impact of rotavirus vaccination on childhood hospitalization for seizures. Pediatr Infect Dis J 2015; 34:769–73. [DOI] [PubMed] [Google Scholar]
- 71. Payne DC, Baggs J, Zerr DM, et al. Protective association between rotavirus vaccination and childhood seizures in the year following vaccination in US children. Clin Infect Dis 2014; 58:173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pringle KD, Burke RM, Steiner CA, Parashar UD, Tate JE. Trends in rate of seizure-associated hospitalizations among children <5 years old before and after rotavirus vaccine introduction in the United Sates, 2000–2013. J Infect Dis 2018; 217:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sheridan SL, Ware RS, Grimwood K, Lambert SB. Febrile seizures in the era of rotavirus vaccine. J Pediatric Infect Dis Soc 2016; 5:206–9. [DOI] [PubMed] [Google Scholar]
- 74. Gómez-Rial J, Sánchez-Batán S, Rivero-Calle I, et al. Rotavirus infection beyond the gut. Infect Drug Resist 2019; 12:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rivero-Calle I, Gómez-Rial J, Martinón-Torres F. Systemic features of rotavirus infection. J Infect 2016; 72(Suppl):S98–S105. [DOI] [PubMed] [Google Scholar]
- 76. Gómez-Rial J, Rivero-Calle I, Salas A, Martinón-Torres F. Rotavirus and autoimmunity. J Infect 2020; 81:183–9. [DOI] [PubMed] [Google Scholar]
- 77. Hemming-Harlo M, Lähdeaho ML, Mäki M, Vesikari T. Rotavirus vaccination does not increase type 1 diabetes and may decrease celiac disease in children and adolescents. Pediatr Infect Dis J 2019; 38:539–41. [DOI] [PubMed] [Google Scholar]
- 78. Kemppainen KM, Lynch KF, Liu E, et al. Factors that increase risk of celiac disease autoimmunity after a gastrointestinal infection in early life. Clin Gastroenterol Hepatol 2017; 15:694–702.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Perrett KP, Jachno K, Nolan TM, Harrison LC. Association of rotavirus vaccination with the incidence of type 1 diabetes in children. JAMA Pediatrics 2019; 173, :280–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rogers MAM, Basu T, Kim C. Lower incidence rate of type 1 diabetes after receipt of the rotavirus vaccine in the United States, 2001–2017. Sci Rep 2019; 9:7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Burke RM, Tate JE, Dahl RM, et al. Rotavirus vaccination and type 1 diabetes risk among us children with commercial insurance. JAMA Pediatr 2020; 174:383–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Glanz JM, Clarke CL, Xu S, et al. Association between rotavirus vaccination and type 1 diabetes in children. JAMA Pediatr 2020; 174:455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vaarala O, Jokinen J, Lahdenkari M, Leino T. Rotavirus vaccination and the risk of celiac disease or type 1 diabetes in Finnish children at early life. Pediatr Infect Dis J 2017; 36:674–5. [DOI] [PubMed] [Google Scholar]
- 84. Velasquez DE, Parashar U, Jiang B. Decreased performance of live attenuated, oral rotavirus vaccines in low-income settings: causes and contributing factors. Expert Rev Vaccines 2018; 17:145–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Patel M, Steele AD, Parashar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine 2012; 30(Suppl 1):A30–5. [DOI] [PubMed] [Google Scholar]
- 86. Naylor C, Lu M, Haque R, et al. ; PROVIDE Study Teams. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine 2015; 2:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Perez-Schael I, Salinas B, Tomat M, et al. Efficacy of the human rotavirus vaccine RIX4414 in malnourished children. J Infect Dis 2007; 196:537–40. [DOI] [PubMed] [Google Scholar]
- 88. Groome MJ, Moon SS, Velasquez D, et al. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull World Health Organ 2014; 92:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gruber JF, Hille DA, Liu GF, et al. Heterogeneity of rotavirus vaccine efficacy among infants in developing countries. Pediatr Infect Dis J 2017; 36:72–8. [DOI] [PubMed] [Google Scholar]
- 90. Moon SS, Groome MJ, Velasquez DE, et al. Prevaccination rotavirus serum IgG and IgA are associated with lower immunogenicity of live, oral human rotavirus vaccine in South African infants. Clin Infect Dis 2016; 62:157–65. [DOI] [PubMed] [Google Scholar]
- 91. Gastañaduy PA, Steenhoff AP, Mokomane M, et al. Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: a multisite prospective case-control study. Clin Infect Dis 2016; 62(Suppl 2):S161–7. [DOI] [PubMed] [Google Scholar]
- 92. Ali SA, Kazi AM, Cortese MM, et al. Impact of different dosing schedules on the immunogenicity of the human rotavirus vaccine in infants in Pakistan: a randomized trial. J Infect Dis 2014; 210:1772–9. [DOI] [PubMed] [Google Scholar]
- 93. Armah G, Lewis KD, Cortese MM, et al. A randomized, controlled trial of the impact of alternative dosing schedules on the immune response to human rotavirus vaccine in rural Ghanaian infants. J Infect Dis 2016; 213:1678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lazarus RP, John J, Shanmugasundaram E, et al. The effect of probiotics and zinc supplementation on the immune response to oral rotavirus vaccine: a randomized, factorial design, placebo-controlled study among Indian infants. Vaccine 2018; 36:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nordgren J, Sharma S, Bucardo F, et al. Both Lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus genotype-dependent manner. Clin Infect Dis 2014; 59:1567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Payne DC, Currier RL, Staat MA, et al. Epidemiologic association between FUT2 secretor status and severe rotavirus gastroenteritis in children in the United States. JAMA Pediatr 2015; 169:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yang TA, Hou JY, Huang YC, Chen CJ. Genetic susceptibility to rotavirus gastroenteritis and vaccine effectiveness in Taiwanese children. Sci Rep 2017; 7:6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kazi AM, Cortese MM, Yu Y, et al. Secretor and salivary ABO blood group antigen status predict rotavirus vaccine take in infants. J Infect Dis 2017; 215:786–9. [DOI] [PubMed] [Google Scholar]
- 99. Lee B, Dickson DM, deCamp AC, et al. Histo-blood group antigen phenotype determines susceptibility to genotype-specific rotavirus infections and impacts measures of rotavirus vaccine efficacy. J Infect Dis 2018; 217:1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ramani S, Giri S. Influence of histo blood group antigen expression on susceptibility to enteric viruses and vaccines. Curr Opin Infect Dis 2019; 32:445–52. [DOI] [PubMed] [Google Scholar]
- 101. Armah GE, Cortese MM, Dennis FE, et al. Rotavirus vaccine take in infants is associated with secretor status. J Infect Dis 2019; 219:746–9. [DOI] [PubMed] [Google Scholar]
- 102. Magwira CA, Kgosana LP, Esona MD, Seheri ML. Low fecal rotavirus vaccine virus shedding is significantly associated with non-secretor histo-blood group antigen phenotype among infants in northern Pretoria, South Africa. Vaccine 2020; 38:8260–3. [DOI] [PubMed] [Google Scholar]
- 103. Wang JX, Chen LN, Zhang CJ, et al. Genetic susceptibility to rotavirus infection in Chinese children: a population-based case-control study. Hum Vaccin Immunother 2021; 17:1803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. MacDonald J, Groome MJ, Mans J, Page N. FUT2 secretor status influences susceptibility to VP4 strain-specific rotavirus infections in South African children. Pathogens 2020; 9:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cantelli CP, Velloso AJ, Assis RMS, et al. Rotavirus A shedding and HBGA host genetic susceptibility in a birth community-cohort, Rio de Janeiro, Brazil, 2014–2018. Sci Rep 2020; 10:6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Williams FB, Kader A, Colgate ER, et al. Maternal secretor status affects oral rotavirus vaccine response in breastfed infants in Bangladesh [published online ahead of print 11 March 2020]. J Infect Dis 2021; 224:1147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Boniface K, Byars SG, Cowley D, Kirkwood CD, Bines JE. Human neonatal rotavirus vaccine (RV3-BB) produces vaccine take irrespective of histo-blood group antigen status. J Infect Dis 2020; 221:1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Burke RM, Tate JE, Kirkwood CD, Steele AD, Parashar UD. Current and new rotavirus vaccines. Curr Opin Infect Dis 2019; 32:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Groome MJ, Fairlie L, Morrison J, et al. Safety and immunogenicity of a parenteral trivalent P2-VP8 subunit rotavirus vaccine: a multisite, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2020; 20: 851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Groome MJ, Koen A, Fix A, et al. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2017; 17:843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bines JE, At Thobari J, Satria CD, et al. Human neonatal rotavirus vaccine (RV3-BB) to target rotavirus from birth. N Engl J Med 2018; 378:719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bines JE, Danchin M, Jackson P, et al. ; RV3 Rotavirus Vaccine Program. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2015; 15:1389–97. [DOI] [PubMed] [Google Scholar]
- 113. Burnett E, Tate JE, Kirkwood CD, et al. Estimated impact of rotavirus vaccine on hospitalizations and deaths from rotavirus diarrhea among children <5 in Asia. Expert Rev Vaccines 2018; 17:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Satter SM, Aliabadi N, Gastanaduy PA, et al. An update from hospital-based surveillance for rotavirus gastroenteritis among young children in Bangladesh, July 2012 to June 2017. Vaccine 2018; 36:7811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]