Abstract
In this study, we investigate the role of the receptor-like protein tyrosine phosphatase CD148 in T-cell activation. Overexpression of CD148 in the Jurkat T-cell line inhibited activation of the transcription factor nuclear factor of activated T cells following T-cell receptor (TCR) stimulation but not following stimulation through a heterologously expressed G protein-coupled receptor, the human muscarinic receptor subtype 1. Using a tetracycline-inducible expression system, we show that the TCR-mediated activation of both the Ras and calcium pathways was inhibited by expression of CD148 at levels that approximate those found in activated primary T cells. These effects were dependent on the phosphatase activity of CD148. Analysis of TCR-induced protein tyrosine phosphorylation demonstrated that most phosphoproteins were unaffected by CD148 expression. However, phospholipase Cγ1 (PLCγ1) and LAT were strikingly hypophosphorylated in CD148-expressing cells following TCR stimulation, whereas the phosphorylation levels of Slp-76 and Itk were modestly reduced. Based on these results, we propose that CD148 negatively regulates TCR signaling by interfering with the phosphorylation and function of PLCγ1 and LAT.
Engagement of the T-cell receptor (TCR) initiates a cascade of biochemical events that culminates in transcription of cytokine genes, cell proliferation, and acquisition of T-cell effector functions (reviewed in references 36 and 44). Protein tyrosine phosphorylation is a driving force in signal transduction from the cell surface to the nucleus. This is achieved primarily by regulating the activity of enzymes such as kinases and phospholipases or by creating binding sites for proteins containing Src homology 2 (SH2) domains or phosphotyrosine-binding domains, thereby altering subcellular localization or recruitment into multiprotein complexes.
The earliest events in TCR signaling are dependent on tyrosine kinases of the Src and Syk families and eventually lead to activation of the Ras pathway and mobilization of intracellular calcium, two events crucial for transcription of the interleukin-2 gene. Ligation of the TCR stimulates the autophosphorylation of the Src family kinase member Lck in its activation loop, increasing its kinase activity (42). Activated Lck phosphorylates tyrosine residues contained within immunoreceptor tyrosine-based activation motifs of the CD3 and TCRζ chains, which subsequently recruit ZAP-70, a member of the Syk family of tyrosine kinases, via its SH2 domains. ZAP-70 is subsequently phosphorylated and activated by Lck. These two kinases phosphorylate numerous downstream substrates, including the adapter proteins LAT and Slp-76, which nucleate a variety of signaling complexes crucial for T-cell activation. Lck, ZAP-70, LAT, and Slp-76 are required for the phosphorylation and activation of phospholipase Cγ1 (PLCγ1) (4, 10, 44, 50). Activated PLCγ1 cleaves the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), leading to the release of calcium from intracellular stores and the activation of protein kinase C, respectively. DAG can induce the activation of Ras through the recently identified RasGRP protein, which plays a critical role in T-cell development (8a).
Since protein tyrosine phosphorylation is a fundamental mechanism driving T-cell activation, it is crucial that it is tightly regulated to ensure adequate T-cell responses without generating autoimmunity. Indeed, T-cell activation is controlled by a delicate balance of positive and negative regulators. Protein tyrosine phosphatases (PTPs) are obvious candidates for controlling the magnitude and specificity of tyrosine phosphorylation and thus are likely to play important roles in regulating T-cell responses. PTPs can be classified either as receptor-like or intracellular, based on their localization. Intracellular PTPs are found in the cytoplasm or associated with intracellular membranes, contain a single phosphatase domain, and very often contain domains implicated in protein-protein interactions. Receptor-like PTPs (RPTPs) possess extracellular domains that vary substantially in their structure and can contain motifs that resemble fibronectin type III-like domains or immunoglobulin-like domains. Most RPTPs contain two tandem phosphatase domains in their intracellular portion, with only the membrane-proximal domain possessing significant enzymatic activity. While the role of the second catalytically inactive domain is unclear, it has been postulated to influence the substrate specificity of the phosphatase.
PTPs can both positively and negatively regulate lymphocyte activation. CD45 is an RPTP constitutively expressed exclusively in cells of hematopoietic origin and is required for the initiation of TCR signaling by dephosphorylating a negative regulatory tyrosine in the C-terminal tail of Lck (42). CD45 may also negatively regulate Lck by dephosphorylating the tyrosine in the activation loop (2, 8, 42), thereby attenuating Lck activity. CD148 is another RPTP which is widely but not exclusively expressed in cells of the immune system. CD148 expression is low in resting T cells but is upregulated following T-cell activation (40). The extracellular domain of CD148 consists of a series of fibronectin type III-like repeats, while the cytoplasmic domain is unusual in that it contains only a single phosphatase domain. CD148 was originally isolated from fibroblasts, where it was reported to be upregulated in dense, as opposed to sparse, cultures and was hypothesized to play a role in contact-mediated growth arrest (33). Consistent with this result, inducible expression of CD148 in several breast cancer cell lines dramatically inhibited cell growth (23). In T cells, transient overexpression of CD148 in the Jurkat T-cell line inhibited upregulation of the lymphocyte activation marker CD69 and nearly completely abolished inducible tyrosine phosphorylation following TCR stimulation (41). However, this occurred only with the highest levels of CD148 expression, which are likely to be superphysiologic and may not accurately reflect the true function of CD148.
In order to better characterize the function of CD148 in T-cell activation, we established an inducible CD148 expression system in the Jurkat line, which, like many transformed cell lines, has little CD148 expression. The level of CD148 in these cells approximates that found on activated primary T cells. We find that, in this context, CD148 inhibits IP3 production, calcium mobilization, and activation of the Ras pathway as measured by CD69 upregulation and phosphorylation of the extracellular signal-regulated kinase (ERK). This inhibition of TCR signaling is dependent on the phosphatase activity of CD148, since there is no effect with expression of a catalytically inactive mutant. However, we find that the tyrosine phosphorylation of only a few proteins is affected by CD148 expression, the most prominent being LAT and PLCγ1. Thus, we conclude that CD148 is a negative regulator of T-cell activation, most likely at the level of LAT and PLCγ1 rather than at the most proximal events in TCR signaling.
MATERIALS AND METHODS
Antibodies.
The anti-Jurkat TCRβ monoclonal antibody (MAb) C305 (47) was used for stimulation of Jurkat cells. Phycoerythrin (PE)-conjugated MAb to human CD148 (clone A3) (40) was previously reported. Fluorescein isothiocyanate (FITC)-conjugated MAb to CD69 was from Becton Dickinson. Anti-phospho-ERK1/2 was from New England Biolabs, and the anti-ERK antibody was from Zymed. Antiphosphotyrosine MAbs 4G10 and RC20 were from Upstate Biotechnology and Transduction Laboratories, respectively. Antibodies to Src Tyr416 were from BioSource. Antibodies to LAT, Myc, and PLCγ1 were from Upstate Biotechnology. Sheep polyclonal antisera to Slp-76 (30) and to SLAP-130 (31) were gifts from G. Koretzky (University of Pennsylvania). Rabbit antisera to ZAP-70 (no. 1600) (10) and to Pyk2 (nos. 1 and 600) (35) and the anti-TCRζ MAb 6B10.2 (45) were previously described. Anti-Lck MAb (clone 1F6) was obtained from J. Bolen (DNAX Research Institute, Palo Alto, Calif.). Cbl antibodies were purchased from Santa Cruz Biotechnology. The MAb to Vav (clone 24C1) was raised against amino acids 565 to 592 of Vav. A polyclonal antiserum to Itk (43) was a gift from M. Tomlinson (DNAX Research Institute).
Plasmids.
The pEF-BOS/CD148 expression construct (41) was previously reported. pEF-BOS/CD148CS was generated by PCR mutagenesis of the codon encoding cysteine 1239 (TGC), changing it to a serine (TCC). The nuclear factor of activated T cells (NFAT)-luciferase reporter construct has been previously described (37). The plasmids pUHD172–1neo (encoding the reverse tetracycline-controlled transactivator [rtTA] [14]) and pUHD10–3 (the tetracycline response plasmid [13]) were a gift from H. Bujard (Zentrum fur Molekulare Biologie, Heidelberg, Germany). pUHD10–3/CD148 and pUHD10–3/CD148CS were constructed by cloning the XbaI/ClaI fragment from pEF-BOS/CD148 or pEF-BOS/CD148CS into XbaI/ClaI sites introduced into the multiple cloning site of pUHD10–3. pTK-Hyg was obtained from Clontech. To generate the plasmid encoding the glutathione S-transferase (GST)–CD148 fusion protein, the CD148 cytoplasmic domain was amplified by PCR using the following primers: AGAAAGAAGAGGAAAGATGCAAA (5′) and CGACGGTCTGGTTCACTCC (3′). The PCR product was then cloned into the vector pGEX-2TK. GST-CD148(DA) was made through PCR mutagenesis of the codon encoding aspartic acid 1205 (GAC), changing it to an alanine (GCC). The GST fusion proteins were induced and purified as previously described (38).
Cell culture.
Jurkat, Myc-tagged LAT-reconstituted JCaM2, and J-HM1–2.2 (the Jurkat cell line stably transfected with the human muscarinic receptor [12]) were maintained in RPMI 1640 containing 10% fetal calf serum. The tetracycline-inducible CD148 stable cell lines were maintained in RPMI 1640 containing 10% tetracycline-free fetal calf serum (Clontech), 2 mg of G418/ml, and 300 μg of hygromycin/ml. Activated peripheral T cells were obtained by culturing freshly isolated human peripheral blood mononuclear cells in RPMI 1640 containing 10% fetal calf serum, 50 μM 2-mercaptoethanol, and 1 μg of phytohemagglutinin/ml. Each culture medium was supplemented with 2 mM glutamine, penicillin, and streptomycin. Inducible expression of CD148 was obtained by adding 1 μg of doxycycline/ml to the culture media for 2 days.
Cell transfection.
All transfections were performed by electroporating 2 × 107 Jurkat cells resuspended in 400 μl of serum-free RPMI 1640 with the indicated amount of DNA in a 0.4-cm-diameter cuvette using the Gene Pulser (Bio-Rad Laboratories) at a setting of 250 V and 960 μF. The tetracycline-inducible stable lines were generated by transfection of Jurkat cells first with the pUHD172–1neo plasmid, followed by selection in 2 mg of G418/ml. One functional clone (JrtTA7.6) was selected for subsequent cotransfection with pHUD10–3CD148 or pHUD10–3CD148CS (20 μg) and pTK-Hyg (2 μg), followed by selection in 300 μg of hygromycin/ml. Myc-tagged LAT-reconstituted JCaM2 cells were generated as previously described (10).
NFAT-luciferase assay.
J-HM1–2.2 was transiently transfected with 20 μg of the NFAT-luciferase reporter plasmid and 20 μg of empty vector (pEFBOS), pEFBOS/CD148, or pEFBOS/CD148CS. Sixteen to twenty hours following transfection, cells were harvested and stimulated in triplicate with medium, anti-TCR MAb C305 (1:1,000), carbachol (100 μM), or a combination of phorbol myristate acetate (PMA) (25 ng/ml) and ionomycin (1 μM). After 6 h, the cells were lysed and assayed for luciferase activity as previously described (37).
CD69 upregulation.
Cells were either left untreated or were stimulated with anti-TCR MAb C305 (1:1,000) or PMA (25 ng/ml). After 14 h, the cells were analyzed for CD69 expression by staining with an FITC-conjugated antibody to CD69, followed by flow cytometry analysis on a FACScan.
Measurement of inositol phosphate production and intracellular calcium mobilization.
To measure inositol phosphate production, cells were metabolically loaded with [3H]myo-inositol and cultured overnight. Triplicate samples of the cells were either left untreated or stimulated with the anti-TCR MAb C305 (1:1,000) for 10 min, followed by lysis of the cells and isolation of soluble total inositol phosphates by anion-exchange chromatography as previously described (19). To analyze intracellular calcium mobilization, cells were loaded with the fluorescent calcium indicator dye Indo-1 (Molecular Probes) and were treated either with the anti-TCR MAb C305 (1:1,000) or ionomycin (1 μM). The fluorescence at 400- and 500-nm wavelengths was measured using a Hitachi F-4500 fluorescence spectrophotometer, and the intracellular free calcium concentration was calculated based on the ratio of the fluorescence at 400 and 500 nm (16).
Cell stimulation, lysate preparation, immunoprecipitation, and Western blotting.
Cells were washed with warmed phosphate-buffered saline (PBS) and either were left untreated or were stimulated for the indicated period with the anti-TCR MAb C305 (1:500) in PBS at 37°C. Cells were lysed in buffer containing 1% NP-40, 10 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, and a cocktail of protease and phosphatase inhibitors, followed by incubation on ice for 20 min. Nuclei and particulate were removed by centrifugation. To immunoprecipitate specific proteins, the lysates were incubated with the indicated antibodies and protein A- or protein G-Sepharose beads for 2 h at 4°C. The immunoprecipitates were washed three times with lysis buffer, followed by separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transfer to Immobilon-P membrane (Millipore). The membranes were then probed with the indicated primary antibody, followed by a horseradish peroxidase-conjugated secondary antibody. The proteins were then visualized by enhanced chemiluminescence (Amersham).
In vitro kinase assay of ZAP-70.
ZAP-70 immunoprecipitates were washed twice with NP-40 lysis buffer; twice with 10 mM Tris (pH 7.5) and 0.5 M LiCl; and once with kinase buffer containing 10 mM Tris (pH 7.5), 10 mM MgCl2, and 10 mM MnCl2. The kinase assay was performed at room temperature for 5 min in 50 μl of kinase buffer containing 2 μg of GST-band 3 fusion protein (54), 20 μM ATP, and 10 μCi of [γ-P32]ATP (3,000 Ci/mmol). The reactions were stopped by adding 2× SDS sample buffer and boiling. The reactions were separated by SDS-PAGE and were transferred to Immobilon-P membrane (Millipore). The phosphorylated GST-band 3 was visualized by autoradiography.
In vitro phosphatase assay.
Cells were stimulated with pervanadate (100 μM Na3VO4 and 10 μM H2O2 in PBS) for 10 min, and postnuclear lysates were prepared. Immunoprecipitations were performed with the indicated antibodies and were then washed twice with lysis buffer containing vanadate, twice with lysis buffer lacking vanadate, and once with phosphatase buffer (150 mM NaCl, 50 mM Tris [pH 6.8], 1 mM EDTA, 10 mM dithiothreitol). The immunoprecipitates were divided into three, and an in vitro phosphatase assay was performed at 37°C for 10 min by the addition of 5 μg of the indicated GST-CD148 fusion protein in 30 μl of phosphatase buffer. The proteins were then resolved by SDS-PAGE and analyzed by Western blotting.
RESULTS
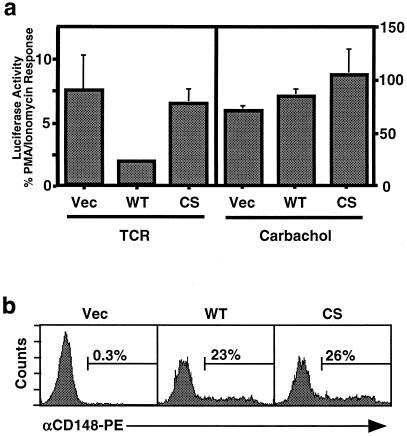
A well-characterized functional readout of T-cell activation is increased activity of the transcription factor NFAT (9), which is dependent both on the activation of Ras and on a sustained calcium flux (48, 49). Induction of NFAT following TCR stimulation requires early tyrosine phosphorylation events (36). However, NFAT can also be activated by G protein-coupled receptors in a tyrosine kinase-independent manner, as demonstrated in the Jurkat line J-HM1–2.2, which is stably transfected with the G protein-coupled human muscarinic receptor (7, 12, 15). To assess whether CD148 affects NFAT activation in T cells, J-HM1–2.2 was transiently transfected with expression constructs containing either wild-type CD148, a catalytically inactive version of CD148 in which the essential catalytic cysteine was mutated to serine, or the empty vector, along with an NFAT-luciferase reporter plasmid. Luciferase activity was measured following stimulation with the anti-TCR antibody, carbachol (which activates the human muscarinic receptor), or PMA and ionomycin, which induce Ras activation and calcium mobilization, respectively, while bypassing the requirement for proximal tyrosine phosphorylation events. Equivalent expression of the transfected proteins was confirmed by flow cytometry (Fig. 1b). The results demonstrated that expression of CD148 inhibited TCR-mediated NFAT activation but had no effect on carbachol-mediated NFAT activation (Fig. 1a) nor on PMA- and ionomycin-induced NFAT activity (data not shown). These results suggested that CD148 interferes with proximal tyrosine phosphorylation events at the cell membrane.
FIG. 1.
Effect of CD148 on NFAT activation in J-HM1–2.2 cells. (a) J-HM1–2.2 cells were cotransfected with 20 μg of NFAT-luciferase reporter construct together with 20 μg of empty pEF-BOS expression vector (Vec) or the expression plasmid pEF-BOS/CD148 or pEF-BOS/CD148CS containing cDNA encoding wild-type CD148 (WT) or a catalytically inactive mutant (CS), respectively. The following day, equivalent numbers of cells were stimulated in triplicate for 6 h with anti-TCR MAb, carbachol, or PMA plus ionomycin. The results are reported as the TCR response or the carbachol response as a percentage of the PMA-plus-ionomycin response. Data are representative of at least three independent experiments. (b) CD148 expression levels in the transfectants were examined by staining the transfectants with a PE-conjugated antibody specific for CD148, followed by flow cytometry. The numbers above the bars refer to the percentage of live cells expressing CD148.
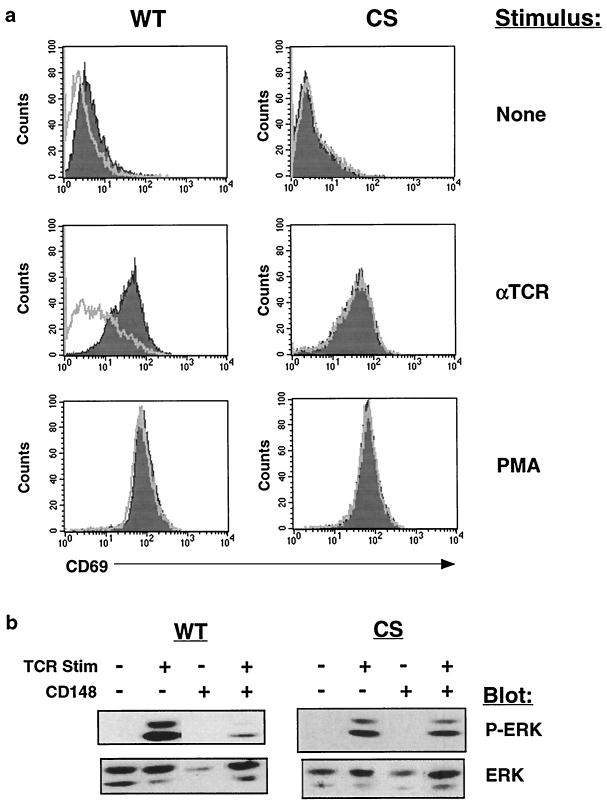
Jurkat cells express nearly undetectable levels of CD148, and we have been unable to generate Jurkat cell lines stably expressing CD148, possibly as a result of the ability of CD148 to repress cell growth. In order to study the effect of CD148 on tyrosine phosphorylation of specific proteins involved in TCR signaling, we established a tetracycline-inducible CD148 expression system in Jurkat cells. A Jurkat cell line stably expressing the rtTA (14) was transfected with expression constructs containing either wild-type CD148 or catalytically inactive CD148 under the control of a promoter that is activated by rtTA in the presence of the tetracycline analog doxycycline, and stable lines were generated. We obtained several wild-type and catalytically inactive lines that consistently upregulated CD148 following treatment with doxycycline, as determined by flow cytometry (Fig. 2a). TCR levels were not affected by treatment with doxycycline (data not shown). Importantly, the level of CD148 expressed in these cell lines approximated the level of CD148 in activated human peripheral T cells and thus was not grossly overexpressed (Fig. 2b). CD148 phosphatase activity was detected in CD148 immunoprecipitates isolated only from the wild-type lines and only following doxycycline treatment (data not shown). The results in the following experiments were obtained with the L19 wild-type line; however, both wild-type lines produced similar results. Prior to each individual experiment, the levels of inducible expression were assessed by flow cytometry and were found to be similar (data not shown). Following stimulation through the TCR, cells upregulate expression of the surface marker CD69 (17), for which activation of the Ras pathway is both necessary and sufficient (5). To determine the effect of CD148 on CD69 upregulation, the cell lines were induced with doxycycline followed by treatment with media, anti-TCR antibody, or PMA, and CD69 expression was analyzed by flow cytometry. Expression of wild-type CD148 inhibited CD69 upregulation following TCR stimulation but not following treatment with PMA, which activates Ras independent of the early tyrosine phosphorylation events at the membrane (Fig. 3a). The catalytically inactive CD148 mutant had no effect on CD69 upregulation, indicating that CD148 phosphatase activity was required to suppress the TCR activation effects. Cross-linking CD148 with immobilized antibodies partially reversed the inhibition of CD69 upregulation in response to TCR stimulation (data not shown), consistent with previous studies of CD148 (41). This could result from dimerization of CD148, leading to inhibition of its catalytic activity, as has been reported with other RPTPs (22, 29). To further address the effect of CD148 on the Ras pathway, we examined the activation of the mitogen-activated protein kinase ERK following TCR stimulation. ERK activation occurs via phosphorylation on both tyrosine and threonine and is dependent on Ras activation (20). Expression of CD148 but not of the catalytically inactive mutant resulted in the reduced activation of ERK, as assessed by blotting with an antiserum specific for dually phosphorylated ERK (Fig. 3b). A time course of TCR stimulation revealed that phospho-ERK was reduced at all time points examined (data not shown). Therefore, we conclude that CD148 can inhibit activation of the Ras pathway in T cells.
FIG. 2.
Analysis of inducible CD148 expression in stably transfected Jurkat cell lines. (a) Stably transfected cell lines containing the rtTA and either wild-type CD148 (WT) or catalytically inactive CD148 (CS) driven by a tetracycline-responsive promoter were left untreated or were treated with 1 μg of doxycycline/ml. After 48 h, the cells were stained with a PE-conjugated antibody specific for CD148 and were analyzed by flow cytometry. The shaded histogram represents the untreated cells, and the empty histogram represents the doxycycline-treated cells. L19 and L12 represent two individual WT lines. (b) Human peripheral blood leukocytes stimulated for 2 days with phytohemagglutinin were stained with an FITC-conjugated anti-CD3 antibody and either a PE-conjugated antibody to CD148 (empty histogram) or a PE-conjugated isotype matched control antibody (shaded histogram) and were subsequently analyzed by flow cytometry. The histograms represent CD3-positive cells.
FIG. 3.
Expression of CD148 inhibits TCR-mediated CD69 upregulation and ERK phosphorylation. (a) The wild-type CD148 (WT) and catalytically inactive CD148 (CS) stable lines were either untreated or were induced for 48 h with doxycycline. Subsequently, the cells were stimulated with anti-TCR MAb (1:1,000), 25 ng of PMA/ml or were left unstimulated. Fourteen hours later, the cells were stained with an FITC-conjugated antibody to CD69 followed by flow cytometry. The shaded histogram represents the uninduced cells, while the empty histogram represents the doxycycline-induced cells. The stimulus is noted to the right of the histograms. (b) The stable lines were induced with doxycycline as described for panel a. Subsequently, the cells were left unstimulated or were stimulated for 2 min with anti-TCR MAb, and postnuclear lysate was analyzed by Western blotting with an antiserum against phospho-ERK (P-ERK). The blot was then stripped and reprobed with an antiserum against total cellular ERK. The blotting antisera are noted to the right of the blots. Stim, stimulation. + and − represent presence and absence of TCR stimulation or of CD148.
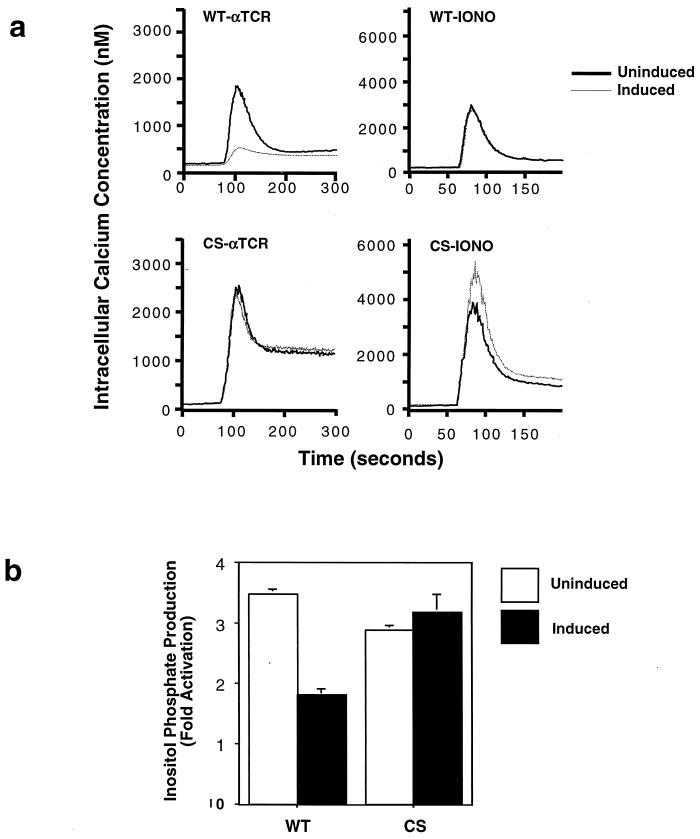
In addition to stimulating the Ras pathway, TCR engagement leads to an increase in intracellular calcium, which facilitates NFAT nuclear translocation via the calcium-dependent serine/threonine phosphatase calcineurin (36). Calcium mobilization is triggered by IP3, one of the products of activated PLCγ1. In order to assess whether CD148 influenced the calcium response, we analyzed both the production of inositol phosphates and the increase in intracellular calcium following TCR stimulation. Inducible expression of wild-type CD148 but not of the mutant significantly reduced the rise in intracellular calcium following treatment with anti-TCR antibody (Fig. 4a). This reduction was not due to an inherent defect in calcium stores, as CD148 expression did not influence calcium release following treatment of the cells with the calcium ionophore ionomycin. Consistent with the attenuated calcium flux, the generation of inositol phosphates was also reduced in the presence of wild-type CD148 (Fig. 4b). Thus, the CD148-mediated reduction in NFAT activity is likely to be due to the inhibition of both the Ras and calcium pathways.
FIG. 4.
CD148 inhibits calcium mobilization and inositol phosphate production following TCR stimulation. The stable lines were induced with doxycyline as described for Fig. 3a. (a) The cells were loaded with the calcium indicator dye Indo-1, stimulated at the 60-s time point with either anti-TCR MAb (αTCR) or 1 μM ionomycin (IONO), and the concentration of intracellular calcium was calculated based on the fluorescence at 400- and 500-nm wavelengths. The dotted line represents the doxycycline-induced cells, while the solid line represents the uninduced cells. WT, wild type; CS, catalytically inactive mutant. (b) Equivalent numbers of cells were loaded with [3H]myo-inositol and were left unstimulated or were stimulated with anti-TCR MAb for 10 min. Soluble inositol phosphates were extracted, and their levels were measured by scintillation counting. The graphs indicate the fold increase in the total amount of inositol phosphates following stimulation. The empty bars represent the uninduced cells, while the solid bars represent the induced cells. The experiment was performed in triplicate twice with the WT cells and once with the CS cells.
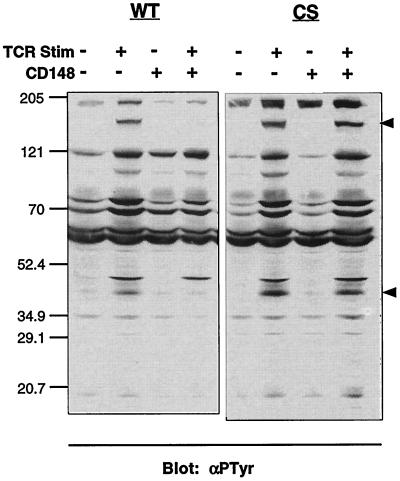
The above results suggest that the biochemical basis for the inhibitory effect of CD148 on T-cell activation lies in the proximal protein tyrosine phosphorylation events that occur following TCR ligation. Therefore, we analyzed the phosphorylation status of various proteins known to be upstream of the Ras and calcium pathways. Probing postnuclear lysates from the wild-type- and mutant-inducible stables following TCR stimulation with an antiphosphotyrosine antibody revealed that there was still significant inducible tyrosine phosphorylation of cellular proteins in the presence of CD148 (Fig. 5). However, several bands displayed a considerable reduction in intensity. In particular, a band at ∼150 kDa, corresponding to the molecular weight of PLCγ1, exhibited almost no phosphorylation, and a band at ∼36 to 38 kDa, corresponding to the molecular weight of LAT, was also hypophosphorylated. Some bands showed small reductions in antiphosphotyrosine reactivity in the presence of wild-type CD148, while others were completely unaffected. This result suggests that CD148 does not globally inhibit inducible protein tyrosine phosphorylation but rather targets specific proteins in the TCR signaling pathway.
FIG. 5.
Effect of CD148 on inducible tyrosine phosphorylation. The stable cell lines were induced with doxycycline as described for Fig. 3a, and equivalent numbers of cells were left unstimulated or were stimulated with anti-TCR MAb for 3 min. Postnuclear lysates were separated by SDS-PAGE and analyzed by Western blotting with the antiphosphotyrosine antibody 4G10 (αPTyr). The molecular weight markers (in thousands) are noted to the left of the blot, and the upper and lower arrows to the right of the blot correspond to the molecular weights of PLCγ1 and LAT, respectively. WT; wild type; CS; catalytically inactive mutant; Stim, stimulation; −, absence of TCR or CD148; +, presence of TCR or CD148.
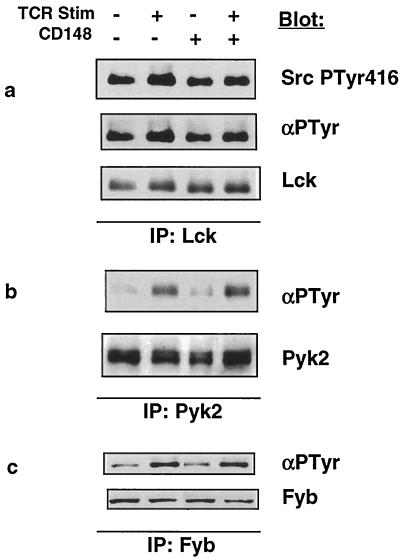
A more extensive analysis of the phosphorylation state of individual proteins was undertaken by immunoprecipitating each protein in the presence or absence of induced CD148 following TCR stimulation and blotting for phosphotyrosine. The Src family tyrosine kinase Lck is rapidly activated following TCR stimulation via autophosphorylation of tyrosine 394 in the activation loop of the kinase domain. An antiserum raised against the corresponding phosphotyrosine in the Src tyrosine kinase (Tyr416) cross-reacts with the highly conserved phosphotyrosine 394 in Lck (18; data not shown). Blotting Lck isolated from stimulated cells with or without CD148 with this antiserum demonstrated that CD148 did not affect the phosphorylation of tyrosine 394 in Lck (Fig. 6a, top panel). Similarly, blotting Lck with an antiphosphotyrosine antibody revealed no difference in Lck tyrosine phosphorylation (Fig. 6a, middle panel). Reprobing the blot with an antibody against Lck demonstrated equal protein levels (Fig. 6a, bottom panel). Pyk2 is a member of the focal adhesion kinase family of tyrosine kinases and is tyrosine phosphorylated in Jurkat cells following TCR ligation. Pyk2 phosphorylation is mediated by the Src family kinase member Fyn but not by Lck (35). Another protein that is selectively phosphorylated by Fyn is the adapter protein SLAP-130/Fyb (6). Figure 6b and c revealed that there was no difference in the inducible phosphorylation of Pyk2 or SLAP-130/Fyb when CD148 was expressed, implying that Fyn activity was not affected by CD148. Thus, unlike the RPTP CD45, CD148 does not appear to influence the activation of Src family kinase members Lck and Fyn.
FIG. 6.
Analysis of Lck, Pyk2, and SLAP-130/Fyb tyrosine phosphorylation. The wild-type (WT) CD148 stable cell line was induced with doxycycline as described for Fig. 3a. Equivalent numbers of cells were left unstimulated or were stimulated for 3 min with anti-TCR MAb, and postnuclear lysates were prepared. Immunoprecipitations (IPs) were performed with antibodies against Lck (a), Pyk2 (b), or SLAP-130/Fyb (c); the immunoprecipitates were separated by SDS-PAGE and were analyzed by Western blotting using antiphosphotyrosine antibodies 4G10 (Lck and SLAP-130/Fyb) and RC20 (Pyk2). The blots were subsequently stripped and reprobed with the antibodies used in the immunoprecipitation to control for protein level. The blotting antibodies are noted to the right of the blots. Stim, stimulation; −, absence of TCR or CD148; +, presence of TCR or CD148.
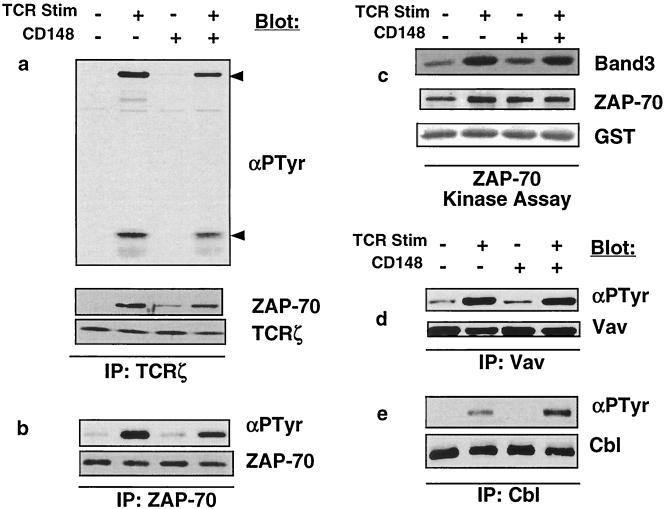
Activated Lck phosphorylates tyrosines contained in the immunoreceptor tyrosine-based activation motif sequences within TCRζ, creating a docking site for the ZAP-70 tyrosine kinase, resulting in its recruitment to TCRζ and its phosphorylation and activation by Lck. Activated ZAP-70 then proceeds to phosphorylate a multitude of enzymes and adapter proteins crucial for T-cell activation. TCRζ isolated from stimulated cells displayed a minimal reduction in tyrosine phosphorylation when CD148 was expressed and recruited slightly less ZAP-70 (Fig. 7a). Examination of total cellular ZAP-70 from stimulated cells also revealed a small decrease in ZAP-70 tyrosine phosphorylation (Fig. 7b); however, there was very little reduction of inducible ZAP-70 kinase activity when CD148 was expressed (Fig. 7c). Moreover, analysis of the ZAP-70 substrate Vav demonstrated no difference in its tyrosine phosphorylation in the presence of CD148 (Fig. 7d). Furthermore, another ZAP-70 substrate, Cbl, displayed a modest yet reproducible hyperphosphorylation in the presence of CD148 (Fig. 7e). Based on these results, we conclude that CD148 does not have a substantial functional effect on TCRζ and ZAP-70.
FIG. 7.
Analysis of TCRζ, ZAP-70, Vav, and Cbl phosphorylation and of ZAP-70 kinase activity. The wild-type (WT) CD148 stable cell line was induced with doxycycline as described for Fig. 3a. Equivalent numbers of cells were left unstimulated or were stimulated for 3 min with anti-TCR MAb, and postnuclear lysates were prepared. Immunoprecipitations (IPs) were performed with antibodies against TCRζ (a), ZAP-70 (b), Vav (d), or Cbl (e); the immunoprecipitates were separated by SDS-PAGE and were analyzed by Western blotting using the antiphosphotyrosine antibody 4G10 (αPTyr). The blots were subsequently stripped and were reprobed with the antibodies used in the immunoprecipitation to control for protein level. The TCRζ immunoprecipitates were also blotted with ZAP-70 to demonstrate that nearly equivalent amounts of ZAP-70 coimmunoprecipitate with TCRζ. The upper and lower arrows to the right of the blot in panel a correspond to ZAP-70 and TCRζ, respectively. The blotting antibodies are noted to the right of the blots. (c) To assess ZAP-70 kinase activity, immunoprecipitations were performed with antibodies against ZAP-70, followed by an in vitro kinase assay with GST-band 3 as a substrate. The kinase assay was separated by SDS-PAGE and transferred to an Immobilon-P membrane. The in vitro phosphorylated band 3 was detected by autoradiography (top panel). The membrane was then probed with antibodies to ZAP-70 (middle panel) and to GST (bottom panel) to control for protein levels of the kinase and the substrate. Similar results were obtained using GST-LAT as a substrate (data not shown). Stim, stimulation; −, absence of TCR or CD148; +, presence of TCR or CD148.
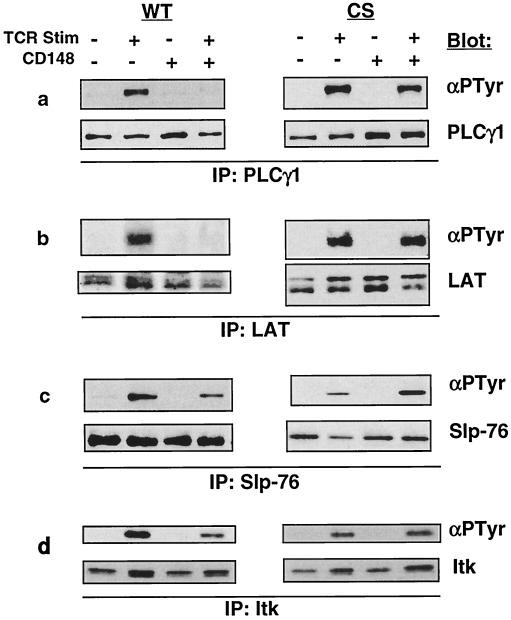
PLCγ1 is inducibly tyrosine phosphorylated following TCR stimulation, and this phosphorylation is required to activate its enzymatic activity. Consistent with the results of the antiphosphotyrosine blot of postnuclear lysate, PLCγ1 tyrosine phosphorylation was nearly completely abolished in the presence of CD148 (Fig. 8a). The pathway in T cells leading to optimal PLCγ1 phosphorylation and activation is known to be dependent on the adapter proteins LAT and Slp-76 and the Tec family tyrosine kinase member Itk, all of which are inducibly tyrosine phosphorylated following TCR stimulation. The prevailing model postulates that phosphorylated LAT and Slp-76 provide docking sites for PLCγ1 via its several SH2 domains and also recruit Itk, which directly phosphorylates PLCγ1 (44). Analysis of the phosphorylation state of LAT revealed that it also was hypophosphorylated in the presence of CD148 (Fig. 8b), while the phosphorylation levels of Slp-76 and Itk were modestly reduced (Fig. 8c and d). The diminished phosphorylation of these proteins is consistent with the reduction in both the Ras and calcium pathways and suggests that LAT and/or PLCγ1 may be direct CD148 substrates. Indeed, both LAT and PLCγ1 can serve as substrates for a GST-CD148 fusion protein in vitro (Fig. 9). Based on the above results, we conclude that CD148 does not globally inhibit tyrosine phosphorylation in Jurkat cells following stimulation through the TCR but rather specifically inhibits phosphorylation of PLCγ1, LAT, Slp-76, and Itk.
FIG. 8.
Analysis of PLCγ1, LAT, Slp-76, and Itk phosphorylation. The stable cell lines were induced with doxycycline as described for Fig. 3a. Equivalent numbers of cells were left unstimulated or were stimulated for 3 min with anti-TCR MAb, and postnuclear lysates were made. Immunoprecipitations (IPs) were performed with antibodies against PLCγ1 (a), LAT (b), Slp-76 (c), or Itk (d); the immunoprecipitates were separated by SDS-PAGE and were analyzed by Western blotting using the antiphosphotyrosine antibody 4G10 (αPTyr). The blots were subsequently stripped and reprobed with the antibodies used in the immunoprecipitation to control for protein level. The blotting antibodies are noted to the right of the blots. WT, wild type CD148; CS, catalytically inactive mutant; Stim, stimulation; −, absence of TCR or CD148; +, presence of TCR or CD148.
FIG. 9.
CD148 can dephosphorylate LAT and PLCγ1 in vitro. Myc-tagged LAT-reconstituted JCaM2 cells (a) and Jurkat cells (b) were stimulated with pervanadate for 10 min, and postnuclear lysates were prepared. Immunoprecipitations (IPs) were performed with antibodies against Myc (a) or PLCγ1 (b). The immunoprecipitates were divided into three, and an in vitro phosphatase assay was performed by adding either wild-type GST-CD148 (WT), a catalytically inactive GST-CD148 (DA), or phosphatase buffer (−). The proteins were then resolved by SDS-PAGE and analyzed by Western blotting with the antiphosphotyrosine antibody 4G10 (αPTyr) or antibodies against LAT and PLCγ1 to control for protein levels. The blotting antibodies are noted to the right of the blots.
DISCUSSION
We have established an inducible expression system in Jurkat cells to further define the regulation of TCR signaling events by the RPTP CD148. We found that expression of CD148 at levels that approximate those found in activated primary T cells inhibited a variety of events downstream of TCR stimulation, including CD69 upregulation, ERK phosphorylation, inositol phosphate production, and calcium mobilization, further substantiating the role of CD148 as a negative regulator of TCR signaling. In addition, we show that CD148 influenced the tyrosine phosphorylation of a restricted set of proteins rather than globally inhibiting phosphorylation. While the phosphorylation states of most proteins remained largely unchanged when CD148 was expressed, tyrosine phosphorylation of LAT and PLCγ1 was substantially reduced. Thus, it is likely that CD148 exhibits some degree of substrate specificity and inhibits T-cell activation at the level of LAT and PLCγ1 rather than inhibiting the earliest events of T-cell activation.
The transmembrane adapter protein LAT and the cytoplasmic adapter protein Slp-76 are both absolutely required for T-cell development and activation and, in particular, the phosphorylation and activation of PLCγ1 (4, 10, 34, 50, 51). PLCγ1 undergoes inducible tyrosine phosphorylation following TCR stimulation (46), and this phosphorylation is required to stimulate its catalytic activity (24, 32) through a mechanism that is poorly understood. The products of phospholipase activity, DAG and IP3, result in the activation of protein kinase C and the release of calcium from intracellular stores, respectively. Itk is a member of the Tec family of tyrosine kinases, and studies of Itk-deficient mice reveal that Itk is important for PLCγ1 phosphorylation, IP3 production, and calcium mobilization following TCR stimulation (27). The prevailing model that accounts for the requirement of LAT, Slp-76, and Itk for PLCγ1 phosphorylation and activation is as follows: after TCR stimulation, PLCγ1 binds to phosphorylated tyrosine 132 in LAT via its N-terminal SH2 domain, resulting in its recruitment to the membrane in close proximity to its substrate, PIP2 (53). Slp-76 is also recruited to LAT through Gads, an adapter protein that binds to phosphorylated LAT via its SH2 domain and to Slp-76 via its SH3 domains (1, 26, 28). Itk is then recruited to the complex by interacting with phosphorylated tyrosines within Slp-76 via its SH2 domain (39) and is likely to phosphorylate and activate PLCγ1. Thus, the reduced phosphorylation of LAT, Slp-76, and Itk in CD148-expressing cells correlates with the reduction in PLCγ1 phosphorylation and the attenuation of downstream pathways.
It is not possible to conclusively determine the direct substrates for CD148 based on the above results; however, several scenarios can be envisioned. One is that LAT could be the primary substrate of CD148, with the possibility that specific phosphotyrosine residues within LAT may be better substrates than others. Along these lines, it has recently been demonstrated that CD148 can dephosphorylate specific phosphotyrosine residues within the platelet-derived growth factor receptor (PDGFR), with the tyrosine that binds to PLCγ1 within the PDGFR being a preferred target (25). Although this may suggest that CD148 targets phosphotyrosines contained within consensus PLCγ1 SH2 domain binding motifs, it should be noted that the PLCγ1-binding tyrosine targeted by CD148 in the PDGFR binds to the C-terminal PLCγ1 SH2 domain, while residues surrounding tyrosine 132 in LAT, which binds to PLCγ1, resemble a PLCγ1 N-terminal SH2 domain binding motif. Reduced phosphorylation of LAT could result in the reduced recruitment and phosphorylation of Slp-76 and Itk, culminating in a dramatic reduction in the phosphorylation and activation of PLCγ1. Interestingly, the phenotype of CD148-expressing cells resembles that of the LAT-deficient cell line JCaM2 in that TCRζ and ZAP-70 phosphorylation is unaffected, Slp-76 and PLCγ1 phosphorylation is impaired, and Cbl phosphorylation is augmented. A second possibility is that PLCγ1 is a major CD148 substrate. CD148 and PLCγ1 are each expressed in a variety of tissues. CD148 is upregulated in dense, as opposed to sparse, cultures of several fibroblast lines, and was hypothesized to play a role in the inhibition of cell growth during contact inhibition (33). Furthermore, inducible expression of CD148 has been shown to inhibit growth of several breast cancer cell lines (23). PLCγ1 is coupled to many growth factor receptors, and substantial evidence suggests that it plays a role in the promotion of cell growth (3). Since the substrate of activated PLCγ1 is found in the membrane, PLCγ1 could be a candidate substrate for receptor tyrosine phosphatases. The above correlative evidence, coupled with our observation that CD148 can dephosphorylate LAT and PLCγ1 in vitro, suggests that either protein could be a primary CD148 substrate. However, we have been unable to isolate LAT or PLCγ1 using a CD148 substrate-trapping mutant in which an aspartic acid necessary for catalytic activity was mutated to an alanine (11). Nevertheless, the substrate specificity of CD148 is likely to be rather selective and different from that of the RPTP CD45, which is expressed in T cells at much higher levels and directly regulates only Src family kinases.
Why CD148 affects only a subset of proteins, and not other membrane-associated phosphoproteins, is an intriguing matter. One attractive possibility is that CD148 is localized to the glycolipid- and cholesterol-enriched membrane microdomains (GEMs) to which many signaling proteins are recruited following TCR stimulation and where the bulk of functional LAT constitutively resides (52). However, we did not detect CD148 in GEM fractions in either stimulated or unstimulated cells following sucrose density centrifugation (data not shown), and CD148 does not possess a juxtramembrane cysteine that could be palmitoylated and thus targeted to GEMs. Further microscopic analysis will be required to ensure that CD148 does not colocalize with GEMs in T cells. Another possibility is that tyrosine phosphorylation of CD148 could target it to SH2 domain-containing proteins, such as Itk, Slp-76, or PLCγ1. Such a mechanism has been shown to target RPTPα to its substrate, Src (55). CD148 has previously been reported to be tyrosine phosphorylated (21), and we have observed inducible tyrosine phosphorylation of a catalytically inactive EGFR-CD148 chimera following TCR stimulation (data not shown).
While both we and Tangye et al. (41) have reached the conclusion that CD148 negatively regulates TCR signaling, our results differ from theirs in that they found that CD148 nearly completely inhibited inducible phosphorylation of most phosphoproteins following TCR engagement. The most likely explanation for this discrepancy is that the previous studies used a transient overexpression system in which CD148 levels considerably exceeded the physiologic levels found in activated primary T cells and that these high levels resulted in substantial substrate promiscuity. In our inducible cell lines, the CD148 levels are close to the endogenous levels found in activated human T cells, and the biochemical characterization of our cells is thus more likely to be an accurate reflection of the function of CD148.
In conclusion, we have demonstrated that CD148 negatively regulates TCR signaling and selectively interferes with the phosphorylation of a subset of proteins involved in T-cell activation, the most prominent being PLCγ1 and LAT. The in vivo role of CD148 in shaping the immune response is likely to be an interesting area of future study.
ACKNOWLEDGMENTS
We thank G. Koretzky, J. Bolen, M. Tomlinson, and H. Bujard for reagents; L. Kane and M. Kuhne for critical reading of the manuscript; and members of the Weiss lab for helpful suggestions.
J. E. Baker is a research associate and A. Weiss is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Asada H, Ishii N, Sasaki Y, Endo K, Kasai H, Tanaka N, Takeshita T, Tsuchiya S, Konno T, Sugamura K. Grf40, a novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT. J Exp Med. 1999;189:1383–1390. doi: 10.1084/jem.189.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashwell J D, D'Oro U. CD45 and Src-family kinases: and now for something completely different. Immunol Today. 1999;20:412–416. doi: 10.1016/s0167-5699(99)01505-4. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter G, Ji Q. Phospholipase C-gamma as a signal-transducing element. Exp Cell Res. 1999;253:15–24. doi: 10.1006/excr.1999.4671. [DOI] [PubMed] [Google Scholar]
- 4.Clements J L, Yang B, Ross-Barta S E, Eliason S L, Hrstka R F, Williamson R A, Koretzky G A. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 5.D'Ambrosio D, Cantrell D A, Frati L, Santoni A, Testi R. Involvement of p21ras activation in T cell CD69 expression. Eur J Immunol. 1994;24:616–620. doi: 10.1002/eji.1830240319. [DOI] [PubMed] [Google Scholar]
- 6.da Silva A J, Li Z, de Vera C, Canto E, Findell P, Rudd C E. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc Natl Acad Sci USA. 1997;94:7493–7498. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai D M, Newton M E, Kadlecek T, Weiss A. Stimulation of the phosphatidylinositol pathway can induce T-cell activation. Nature. 1990;348:66–69. doi: 10.1038/348066a0. [DOI] [PubMed] [Google Scholar]
- 8.D'Oro U, Ashwell J D. The CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J Immunol. 1999;162:1879–1883. [PubMed] [Google Scholar]
- 8a.Dower N A, Stang S L, Bottorff D A, Ebinu J O, Oikie P, Ostergaard H L, Stone J C. Ras GRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 9.Durand D B, Shaw J P, Bush M R, Replogle R E, Belagaje R, Crabtree G R. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988;8:1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finco T S, Kadlecek T, Zhang W, Samelson L E, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 11.Flint A J, Tiganis T, Barford D, Tonks N K. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci USA. 1997;94:1680–1685. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldsmith A M, Desai D M, Schultz T, Weiss A. Function of a heterologous muscarinic receptor in T cell antigen receptor signal transduction mutants. J Biol Chem. 1989;264:17190–17197. [PubMed] [Google Scholar]
- 13.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 15.Graber M, June C H, Samelson L E, Weiss A. The protein tyrosine kinase inhibitor herbimycin A, but not genistein, specifically inhibits signal transduction by the T cell antigen receptor. Int Immunol. 1992;4:1201–1210. doi: 10.1093/intimm/4.11.1201. [DOI] [PubMed] [Google Scholar]
- 16.Grynkiewicz G, Poenie M, Tsien R Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 17.Hara T, Jung L K, Bjorndahl J M, Fu S M. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J Exp Med. 1986;164:1988–2005. doi: 10.1084/jem.164.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardwick J S, Sefton B M. The activated form of the Lck tyrosine protein kinase in cells exposed to hydrogen peroxide is phosphorylated at both Tyr-394 and Tyr-505. J Biol Chem. 1997;272:25429–25432. doi: 10.1074/jbc.272.41.25429. [DOI] [PubMed] [Google Scholar]
- 19.Imboden J B, Stobo J D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985;161:446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izquierdo M, Leevers S J, Marshall C J, Cantrell D. p21ras couples the T cell antigen receptor to extracellular signal-regulated kinase 2 in T lymphocytes. J Exp Med. 1993;178:1199–1208. doi: 10.1084/jem.178.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jallal B, Mossie K, Vasiloudis G, Knyazev P, Zachwieja J, Clairvoyant F, Schilling J, Ullrich A. The receptor-like protein-tyrosine phosphatase DEP-1 is constitutively associated with a 64-kDa protein serine/threonine kinase. J Biol Chem. 1997;272:12158–12163. doi: 10.1074/jbc.272.18.12158. [DOI] [PubMed] [Google Scholar]
- 22.Jiang G, den Hertog J, Su J, Noel J, Sap J, Hunter T. Dimerization inhibits the activity of receptor-like protein-tyrosine phosphatase-alpha. Nature. 1999;401:606–610. doi: 10.1038/44170. [DOI] [PubMed] [Google Scholar]
- 23.Keane M M, Lowrey G A, Ettenberg S A, Dayton M A, Lipkowitz S. The protein tyrosine phosphatase DEP-1 is induced during differentiation and inhibits growth of breast cancer cells. Cancer Res. 1996;56:4236–4243. [PubMed] [Google Scholar]
- 24.Kim H K, Kim J W, Zilberstein A, Margolis B, Kim J G, Schlessinger J, Rhee S G. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65:435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- 25.Kovalenko M, Denner K, Sandström J, Persson C, Gross S, Jandt E, Vilella R, Böhmer F, Ostman A. Site-selective dephosphorylation of the platelet-derived growth factor beta-receptor by the receptor-like protein-tyrosine phosphatase DEP-1. J Biol Chem. 2000;275:16219–16226. doi: 10.1074/jbc.275.21.16219. [DOI] [PubMed] [Google Scholar]
- 26.Law C L, Ewings M K, Chaudhary P M, Solow S A, Yun T J, Marshall A J, Hood L, Clark E A. GrpL, a Grb2-related adaptor protein, interacts with SLP-76 to regulate nuclear factor of activated T cell activation. J Exp Med. 1999;189:1243–1253. doi: 10.1084/jem.189.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K Q, Bunnell S C, Gurniak C B, Berg L J. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S K, Fang N, Koretzky G A, McGlade C J. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 29.Majeti R, Bilwes A M, Noel J P, Hunter T, Weiss A. Dimerization-induced inhibition of receptor protein tyrosine phosphatase function through an inhibitory wedge. Science. 1998;279:88–91. doi: 10.1126/science.279.5347.88. [DOI] [PubMed] [Google Scholar]
- 30.Motto D G, Ross S E, Wu J, Hendricks-Taylor L R, Koretzky G A. Implication of the GRB2-associated phosphoprotein SLP-76 in T cell receptor-mediated interleukin 2 production. J Exp Med. 1996;183:1937–1943. doi: 10.1084/jem.183.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musci M A, Hendricks-Taylor L R, Motto D G, Paskind M, Kamens J, Turck C W, Koretzky G A. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J Biol Chem. 1997;272:11674–11677. doi: 10.1074/jbc.272.18.11674. [DOI] [PubMed] [Google Scholar]
- 32.Nishibe S, Wahl M I, Hernández-Sotomayor S M, Tonks N K, Rhee S G, Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990;250:1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- 33.Ostman A, Yang Q, Tonks N K. Expression of DEP-1, a receptor-like protein-tyrosine-phosphatase, is enhanced with increasing cell density. Proc Natl Acad Sci USA. 1994;91:9680–9684. doi: 10.1073/pnas.91.21.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt F W, Geha R S. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 35.Qian D, Lev S, van Oers N S, Dikic I, Schlessinger J, Weiss A. Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. J Exp Med. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro V S, Mollenauer M N, Greene W C, Weiss A. c-rel regulation of IL-2 gene expression may be mediated through activation of AP-1. J Exp Med. 1996;184:1663–1669. doi: 10.1084/jem.184.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 39.Su Y W, Zhang Y, Schweikert J, Koretzky G A, Reth M, Wienands J. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur J Immunol. 1999;29:3702–3711. doi: 10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 40.Tangye S G, Phillips J H, Lanier L L, de Vries J E, Aversa G. CD148: a receptor-type protein tyrosine phosphatase involved in the regulation of human T cell activation. J Immunol. 1998;161:3249–3255. [PubMed] [Google Scholar]
- 41.Tangye S G, Wu J, Aversa G, de Vries J E, Lanier L L, Phillips J H. Negative regulation of human T cell activation by the receptor-type protein tyrosine phosphatase CD148. J Immunol. 1998;161:3803–3807. [PubMed] [Google Scholar]
- 42.Thomas M L, Brown E J. Positive and negative regulation of Src-family membrane kinases by CD45. Immunol Today. 1999;20:406–411. doi: 10.1016/s0167-5699(99)01506-6. [DOI] [PubMed] [Google Scholar]
- 43.Tomlinson G M, Kurosaki T, Berson A E, Fujii G H, Johnston J A, Bolen J B. Reconstitution of Btk signaling by the atypical tec family tyrosine kinases Bmx and Txk. J Biol Chem. 1999;274:13577–13585. doi: 10.1074/jbc.274.19.13577. [DOI] [PubMed] [Google Scholar]
- 44.Tomlinson G M, Lin J, Weiss A. Lymphocytes with a complex: adapter proteins in antigen receptor signaling. Immunol Today. 2000;21:584–591. doi: 10.1016/s0167-5699(00)01716-3. [DOI] [PubMed] [Google Scholar]
- 45.van Oers N S, von Boehmer H, Weiss A. The pre-T cell receptor (TCR) complex is functionally coupled to the TCR-zeta subunit. J Exp Med. 1995;182:1585–1590. doi: 10.1084/jem.182.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss A, Koretzky G, Schatzman R C, Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc Natl Acad Sci USA. 1991;88:5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss A, Stobo J D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodrow M, Clipstone N A, Cantrell D. p21ras and calcineurin synergize to regulate the nuclear factor of activated T cells. J Exp Med. 1993;178:1517–1522. doi: 10.1084/jem.178.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodrow A M, Rayter S, Downward J, Cantrell D A. p21ras function is important for T cell antigen receptor and protein kinase C regulation of nuclear factor of activated T cells. J Immunol. 1993;150:3853–3861. [PubMed] [Google Scholar]
- 50.Yablonski D, Kuhne M R, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Sommers C L, Burshtyn D N, Stebbins C C, DeJarnette J B, Trible R P, Grinberg A, Tsay H C, Jacobs H M, Kessler C M, Long E O, Love P E, Samelson L E. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W, Trible R P, Samelson L E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Trible R P, Zhu M, Liu S K, McGlade C J, Samelson L E. Association of Grb2, Gads, and phospholipase C-γ1 with phosphorylated LAT tyrosine residues. J Biol Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 54.Zhao Q, Weiss A. Enhancement of lymphocyte responsiveness by a gain-of-function mutation of ZAP-70. Mol Cell Biol. 1996;16:6765–6774. doi: 10.1128/mcb.16.12.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng X M, Resnick R J, Shalloway D. A phosphotyrosine displacement mechanism for activation of Src by PTPalpha. EMBO J. 2000;19:964–978. doi: 10.1093/emboj/19.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]