Abstract
Background
Inadvertent perioperative hypothermia is a phenomenon that can occur as a result of the suppression of the central mechanisms of temperature regulation due to anaesthesia, and of prolonged exposure of large surfaces of skin to cold temperatures in operating rooms. Inadvertent perioperative hypothermia has been associated with clinical complications such as surgical site infection and wound‐healing delay, increased bleeding or cardiovascular events. One of the most frequently used techniques to prevent inadvertent perioperative hypothermia is active body surface warming systems (ABSW), which generate heat mechanically (heating of air, water or gels) that is transferred to the patient via skin contact.
Objectives
To assess the effectiveness of pre‐ or intraoperative active body surface warming systems (ABSW), or both, to prevent perioperative complications from unintended hypothermia during surgery in adults.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 9, 2015); MEDLINE (PubMed) (1964 to October 2015), EMBASE (Ovid) (1980 to October 2015), and CINAHL (Ovid) (1982 to October 2015).
Selection criteria
We included randomized controlled trials (RCTs) that compared an ABSW system aimed at maintaining normothermia perioperatively against a control or against any other ABSW system. Eligible studies also had to include relevant clinical outcomes other than measuring temperature alone.
Data collection and analysis
Several authors, by pairs, screened references and determined eligibility, extracted data, and assessed risks of bias. We resolved disagreements by discussion and consensus, with the collaboration of a third author.
Main results
We included 67 trials with 5438 participants that comprised 79 comparisons. Forty‐five RCTs compared ABSW versus control, whereas 18 compared two different types of ABSW, and 10 compared two different techniques to administer the same type of ABSW. Forced‐air warming (FAW) was by far the most studied intervention.
Trials varied widely regarding whether the interventions were applied alone or in combination with other active (based on a different mechanism of heat transfer) and/or passive methods of maintaining normothermia. The type of participants and surgical interventions, as well as anaesthesia management, co‐interventions and the timing of outcome measurement, also varied widely. The risk of bias of included studies was largely unclear due to limitations in the reports. Most studies were open‐label, due to the nature of the intervention and the fact that temperature was usually the principal outcome. Nevertheless, given that outcome measurement could have been conducted in a blinded manner, we rated the risk of detection and performance bias as high.
The comparison of ABSW versus control showed a reduction in the rate of surgical site infection (risk ratio (RR) 0.36, 95% confidence interval (CI) 0.20 to 0.66; 3 RCTs, 589 participants, low‐quality evidence). Only one study at low risk of bias observed a beneficial effect with forced‐air warming on major cardiovascular complications (RR 0.22, 95% CI 0.05 to 1.00; 1 RCT with 12 events, 300 participants, low‐quality evidence) in people at high cardiovascular risk. We found no beneficial effect for mortality. ABSW also reduced blood loss during surgery but the magnitude of this effect seems to be irrelevant (MD ‐46.17 mL, 95% CI ‐82.74 to ‐9.59; I² = 78%; 20 studies, 1372 participants). The same conclusion applies to total fluids infused during surgery (MD ‐144.49 mL, 95% CI ‐221.57 to ‐67.40; I² = 73%; 24 studies, 1491 participants). These effects did not translate into a significant reduction in the number of participants being transfused or the average amount of blood transfused. ABSW was associated with a reduction in shivering (RR 0.39, 95% CI 0.28 to 0.54; 29 studies, 1922 participants) and in thermal comfort (standardized mean difference (SMD) 0.76, 95% CI 0.29 to 1.24; I² = 77%, 4 trials, 364 participants).
For the comparison between different types of ABSW system or modes of administration of a particular type of ABSW, we found no evidence for the superiority of any system in terms of clinical outcomes, except for extending systemic warming to the preoperative period in participants undergoing major abdominal surgery (one study at low risk of bias).
There were limited data on adverse effects (the most relevant being thermal burns). While some trials included a narrative report mentioning that no adverse effects were observed, the majority made no reference to it. Nothing so far suggests that ABSW involves a significant risk to patients.
Authors' conclusions
Forced‐air warming seems to have a beneficial effect in terms of a lower rate of surgical site infection and complications, at least in those undergoing abdominal surgery, compared to not applying any active warming system. It also has a beneficial effect on major cardiovascular complications in people with substantial cardiovascular disease, although the evidence is limited to one study. It also improves patient's comfort, although we found high heterogeneity among trials. While the effect on blood loss is statistically significant, this difference does not translate to a significant reduction in transfusions. Again, we noted high heterogeneity among trials for this outcome. The clinical relevance of blood loss reduction is therefore questionable. The evidence for other types of ABSW is scant, although there is some evidence of a beneficial effect in the same direction on chills/shivering with electric or resistive‐based heating systems. Some evidence suggests that extending systemic warming to the preoperative period could be more beneficial than limiting it only to during surgery. Nothing suggests that ABSW systems pose a significant risk to patients.
The difficulty in observing a clinically‐relevant beneficial effect with ABSW in outcomes other than temperature may be explained by the fact that many studies applied concomitant procedures that are routinely in place as co‐interventions to prevent hypothermia, whether passive or active warming systems based in other physiological mechanisms (e.g. irrigation fluid or gas warming), as well as a stricter control of temperature in the context of the study compared with usual practice. These may have had a beneficial effect on the participants in the control group, leading to an underestimation of the net benefit of ABSW.
Plain language summary
Body warming of people undergoing surgery to avoid complications and increase comfort after surgery
Review question
We reviewed the effects of warming the body by transferring heat through the skin surface to prevent complications caused by unintended low body temperature (hypothermia) in adults undergoing surgery.
Background
Sedatives and anaesthesia interfere with temperature regulatory responses and so can cause unplanned hypothermia during surgery and immediately after surgery. Long periods of exposure of large surfaces of skin to cold temperatures in operating rooms can also contribute to this effect. Hypothermia can make the recovery process more uncomfortable for the patients, as they often wake with chills and shivering, an involuntary response to cold to increase the production of body heat. Hypothermia may also be related to undesirable events such as infections and complications of the wound, complications of the heart and circulation, increased bleeding and a greater need for blood transfusions.
To avoid this unintended hypothermia, several different types of active warming systems are used to transfer heat to the body of the patient through the skin, either immediately before or during surgery, or both.
Study characteristics
The review includes 67 randomized controlled trials (5438 people). The trials included patients of all ages and both genders undergoing all types of surgery. The evidence was from studies available to October 2015. Forty‐five trials compared a warming system to a control intervention, 18 compared different types of warming systems, and 10 compared different modalities of the same warming system. Forced‐air warming was the most studied system.
Key results
Active warming had some beneficial clinical effects on the patient. It reduced the risk of a major complication of heart and circulation in one trial in people with substantial disease of that system, but the evidence remains inconclusive. Active warming reduced the rate of infection and complications of surgical wounds.. This effect was shown in two quite large trials in people undergoing abdominal surgery; forced‐air warming was applied exclusively before the operation in one study, while in the other it was applied during the operation. Patients receiving active warming systems had about one‐third the risk of postsurgical chills or shivering compared to those receiving control treatment (29 trials, 1922 people). Thermal comfort was increased for the patient compared with the control intervention (10 trials involving 700 people). On the other hand, warming made little or no difference to the risk of death, blood loss or the need for a blood transfusion. We found no differences in the number of non‐fatal heart attacks, in anxiety or in pain, compared with people in the control groups.
The trials in the review did not allow us to identify which warming system was better. However, there was an indication from one trial at low risk of bias that results were better when systemic warming was extended to the period before the operation in people undergoing major abdominal surgery. We could only get limited information from the study reports regarding adverse effects. In some cases the trials reported that there had been no adverse effects.
Quality of the evidence
The quality of the evidence was low for surgical site infections and complications of the heart and circulation. This is because very few trials with few events reported on these outcomes, although they were at low risk of bias. Patients differed in the types of surgery, with different complexities and duration, the type of anaesthesia, patient age, the severity of the condition and other illnesses. The trials did not last long, which made it difficult to detect clinical effects. These outcomes are also strongly influenced by other management components during the operation that we did not evaluate in this review. While some studies applied a single intervention, others used two or more interventions in combination, and/or included other methods of passive warming. The control group did not always consist of a 'pure control' without active heating, and sometimes patients also received another intervention as part of usual care. All these reasons may explain the diversity that we observed for some outcomes among the studies. The temperature of the control group may also have been more strictly controlled, as there is now widespread awareness of the risk of hypothermia.
Summary of findings
Summary of findings for the main comparison. Active body surface warming systems compared to control for preventing inadvertent perioperative hypothermia in adults.
| Active body surface warming systems compared to control for preventing inadvertent perioperative hypothermia in adults | ||||||
| Patient or population: adults undergoing surgery Settings: Inpatients Intervention: Active body surface warming systems (ABSW) Comparison: Control (no active warming) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Active warming systems | |||||
| Infection and complications of the surgical wound | 157 per 1000 | 57 per 1000 (31 to 104) | RR 0.36 (0.20 to 0.66) | 589 (3 studies) | ⊕⊕⊝⊝ low1 | |
| Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, and non‐fatal cardiac arrest) | 63 per 1000 | 14 per 1000 (3 to 63) | RR 0.22 (0.05 to 1) | 300 (1 study) | ⊕⊕⊝⊝ low1 | |
| All‐cause mortality | 16 per 1000 | 16 per 1000 (4 to 63) | RR 1.01 (0.26 to 4) | 500 (2 studies) | ⊕⊕⊝⊝ low1 | |
| Participants transfused | 291 per 1000 | 259 per 1000 (163 to 413) | RR 0.79 (0.50 to 1.23) | 621 (8 studies) | ⊕⊕⊝⊝ moderate2 | |
| Chills/shivering | 212 per 1000 | 83 per 1000 (59 to 115) |
RR 0,39 (0,28 to 0,54) |
1922 (29 studies) | ⊕⊕⊝⊝ high3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The number of events is low. 2Although some of the included studies had a high risk of bias (Johansson 1999 had a high risk of detection bias due to inadequate blinding of the participants and physicians, and Kabbara 2002 had a high risk of selection bias), we estimate that it is unlikely that further studies show a beneficial effect on this outcome although the precise effect estimate may change to some extent depending on clinical circumstances. 3Although four out of 29 of the included studies (Camus 1993b; Fallis 2006; Ng 2003; Wongprasartsuk 1998) had a high risk of bias, our overall confidence in the effect estimate remains high. A sensitivity analysis excluding those trials (data not shown) did not change the estimate and the sample size remains above 250 events.
Background
Description of the condition
Regulation of temperature
In healthy individuals, the mean body temperature varies between 36.1ºC and 37.4ºC. Maintaining body temperature means maintaining a balance between production and loss of heat. Heat is generated continuously as a product of the body’s metabolism. Regulation of temperature is done through a feedback mechanism in the central nervous system. The hypothalamus acts as a 'biological thermostat', noting temperature changes and initiating thermal regulation aimed at increasing or decreasing overall body temperature.
During rest (anaesthesia would be an extreme case of rest), the greatest amount of heat comes from the metabolic activity of the brain and the other major organs. All heat generated by metabolism is dissipated to the environment (mainly through the skin) in order to maintain a stable thermal condition.
The effects of anaesthesia on thermal regulation
In clinical doses, both sedatives and anaesthesia inhibit thermal regulatory responses (primarily vasoconstriction). The physiological thermal regulating mechanisms are not shut off but the thermal thresholds through which the usual responses start are altered. In this way, general anaesthesia produces vasodilatation by depressing vasoconstrictor responses. Since the thermal regulation mechanisms are inhibited, the central compartment goes through a progressive loss of heat, which is transmitted to the peripheral compartment. The speed of this transfer as well as the amount of heat lost depends on the difference in temperature between the two compartments. Vasodilatation in the peripheral compartment brings about the loss of heat to the environment, which as a consequence helps to cool down the central compartment. This process of caloric transfer is known as redistribution.
The combination of reduced heat production and surgical, anaesthetic and environmental factors that increase heat loss can cause hypothermia in the patient. Intraoperative hypothermia is defined as a central body temperature below 36ºC.
At the beginning of general anaesthesia, the overall body temperature does not change, since the temperature loss in the central compartment is picked up by the peripheral compartment. By the second hour of anaesthesia the heat loss in the central compartment is slower, and in this phase the loss of body heat to the environment is more important. Overall temperature decreases when more heat is lost than is generated. The people who are most susceptible to heat loss are the elderly, patients at higher anaesthetic risk (American Society of Anesthesiologists (ASA) grade 3 to 4), cachectics, burn victims, people with hypothyroidism, and those affected by corticoadrenal insufficiency.
Perioperative hypothermia complications
Hypothermia may increase morbidity as a result of altering various systems and functions within the organism.
Cardiac complications are the principal cause of morbidity during the postoperative phase. Prolonged ischaemia is usually associated with cellular damage, and for this reason it is important to prevent factors that can lead to this complication, such as decreased body temperature. Hypothermia stimulates and amplifies adrenergic responses with the release of noradrenaline, which results in peripheral vasoconstriction and hypertension (Sessler 1991; Sessler 2001) and increases the chances of myocardial ischaemia.
Some studies have shown that intraoperative hypothermia, accompanied by vasoconstriction, constitutes an independent factor that slows wound healing and increases the incidence of surgical site infection (Kurz 1996; Melling 2001).
Even moderate hypothermia (35ºC) can alter physiologic coagulation mechanisms by affecting platelet function and modifying enzymatic reactions. Decreased platelet activity produces an increase in bleeding and greater need for transfusion (Rajagopalan 2008). Moderate hypothermia can also reduce the metabolic rate, manifesting as a prolonged effect of certain drugs used during anaesthesia and some uncertainty about their effects. This is particularly significant in elderly people (Heier 1991; Heier 2006; Leslie 1995).
Patients often comment on shivering upon awakening from anaesthesia, identifying this as one of the most uncomfortable immediate postoperative experiences. Shivering is a response to cold and is the result of involuntary muscular activity, the purpose of which is to increase metabolic heat (Sessler 2001).
Due to the above reasons, inadvertent non‐therapeutic hypothermia is considered an adverse effect of general and regional anaesthesia (Bush 1995; Putzu 2007; Sessler 1991). The monitoring of body temperature is essential for maintaining normothermia during surgery and for timely detection of the appearance of unintended hypothermia. As a result, the monitoring of body temperature is included as one of the items in the surgical safety checklist of the World Health Organization guidelines (WHO 2015). This checklist is intended to reduce the rate of major surgical complications.
Description of the intervention
The goal of preserving a patient's body temperature during anaesthesia and surgery is to minimize heat loss by reducing radiation and convection from the skin, evaporation from exposed surgical areas, and cooling caused by the introduction of cold intravenous fluids. Interventions used to maintain body temperature can be classified as follows:
i) Interventions that decrease loss of heat through redistribution (i.e. preoperative pharmacologic vasodilatation and prewarming the skin prior to anaesthesia).
ii) Passive warming systems aimed at reducing heat loss and thus preventing hypothermia, including interventions at above environmental temperatures; passive isolation by covering the exposed body surface; and a closed or semi‐closed anaesthesia circuit with low flows.
iii) Active warming systems aimed at transferring heat to the patient. The effectiveness of these systems depends on various factors such as the design of the device, the type of heat transfer, placement of the system over the patient and, most importantly, the total body area covered in the heat exchange. The following systems are used for active warming: infrared lights, electric blankets, mattresses or blankets with warm‐water circulation, forced‐air warming or convective air‐warming transfer, warming of intravenous and irrigation fluids, warming and humidifying of anaesthetic air, and carbon dioxide (CO₂) warming in laparoscopic surgery.
How the intervention might work
For the purposes of this review, we have focused only on those active warming systems that transfer heat through the skin (active body surface warming systems (ABSW)) using a mechanical system. We expect that keeping body temperature from falling under certain levels should prevent perioperative vasoconstriction, leading to less catecholamine release, and hypertension.
Maintaining temperature through a mechanical heat transference (air‐based or water‐based) system should prevent perioperative complications more efficiently that just passively preventing a person's loss of heat, as happens with thermal isolation. Adequately‐warmed people should also maintain their platelet activity, preventing them from excessive bleeding and the need for transfusions.
Why it is important to do this review
The clinical effectiveness of the different types of warming devices that can be used has been assessed in a very extensive guideline commissioned by the National Institute for Health and Clinical Excellence in the UK (NICE 2008). The report concludes that there is sufficient evidence of clinical effectiveness and cost effectiveness for recommendations to be made on the use of forced‐air warming (the most widely investigated ABSW) to prevent and treat perioperative hypothermia. Nevertheless, most of the data come from intermediate outcomes such as temperature. The report's search is only current until the year 2007, when much research, especially with new systems, has been published since that date. Given this, our review evaluates the efficacy and safety of these ABSW systems focusing exclusively on relevant clinical outcomes other than temperature.
Other Cochrane reviews have provided evidence for the efficacy and safety of passive methods such as thermal insulation (Alderson 2014) and non‐cutaneous active systems, such as warmed gases or intravenous fluids (Birch 2011; Campbell 2015). Other reviews have also addressed pharmacological interventions to prevent specific complications derived from hypothermia, such as shivering (Lewis 2015).
Objectives
To assess the effectiveness of pre‐ or intraoperative active body surface warming systems (ABSW), or both, to prevent perioperative complications from unintended hypothermia during surgery in adults.
Methods
Criteria for considering studies for this review
Types of studies
We include only randomized controlled trials (RCTs) assessing the efficacy and safety of pre‐ and/or intraoperative active body surface warming systems to prevent complications due to heat loss and hypothermia during surgery.
We have excluded RCTs where the aim was to treat rather than prevent unintended hypothermia. This topic is covered in a separate review (Warttig 2014). We have also excluded trials of rewarming in induced hypothermia.
We have included RCTs where the intervention was applied preoperatively, intraoperatively, or preoperatively and intraoperatively. We exclude RCTs where the intervention was applied exclusively in the postoperative phase, as this usually corresponds to surgery with intentional hypothermia.
We define the:
Preoperative phase, as the hour before induction of anaesthesia (when the patient is prepared for surgery on the ward or in the emergency department)
Intraoperative phase, as total anaesthesia time
We have excluded RCTs comparing an ABSW system to another active warming system not covered in this review (i.e. warming of intravenous or irrigation fluids, etc.) except when the latter intervention was applied as a co‐intervention simultaneously in both study groups; in this case, we classified the trial as a comparison between ABSW versus control.
Types of participants
We only included adults undergoing a scheduled surgery (including ambulatory surgery), except surgery using intended hypothermia (such as off‐pump surgery and certain neurosurgical interventions).
Types of interventions
The protocol for the review (Urrútia 2011) originally intended to cover all active warming systems to prevent unintended hypothermia. Subsequently, we limited the focus of the review to ABSW systems, and have amended the review accordingly (See Differences between protocol and review).
For the purposes of this review, we include the following ABSW systems: electric blankets, electric heated mattresses and pads, warm‐water circulation systems (mattresses, blankets or garments), other conductive warming systems (such as resistive conductive polymer blankets and mattresses) and forced‐air warming systems. All these systems have in common that the transfer of heat to the recipient is achieved by skin contact.
We have not considered other active warming systems based on distinct mechanisms (such as fluid warming, infrared lights, anaesthetic air warming and warm CO₂ in laparoscopic surgery) in this review, as they are covered in separate reviews (Birch 2011; Campbell 2015).
The comparisons of interest in this review are:
ABSW versus control (generally involving a passive warming system, warmed cotton blankets or thermal insulation) (ABSW versus CTRL)
ABSW versus any other ABSW (alone or in combination with other active warming systems) (ABSW1 versus ABSW2)
Different modalities of an intervention with a particular ABSW (ABSWa versus ABSWb)
To define the comparisons of interest, we have not taken into account the co‐interventions (which could consist of passive or other types of active warming systems) that were applied to all participants (both study groups). Rather, we have considered only those interventions that were randomly assigned to each study group.
Types of outcome measures
Eligible studies had to include relevant clinical outcomes other than measuring temperature or other physiologic parameters alone.
Primary outcomes
Surgical site infection and complications (wound healing and dehiscence)
Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke and non‐fatal cardiac arrest)
All‐cause mortality
Secondary outcomes
Transfusions (number of participants transfused; blood product usage)
Blood loss
Intraoperative intravenous (IV) fluids infused
Other cardiovascular complications (bradycardia, hypotension, arrhythmias)
Participant‐reported outcomes (anxiety, thermal comfort, thermal sensation, pain)
Shivering (number of participants)
Pressure sores and ulcers
Adverse effects (including thermal burns)
Search methods for identification of studies
Electronic searches
We searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, Issue 9, 2015); MEDLINE (PubMed) (1964 to October 2015), EMBASE (Ovid) (1980 to October 2015), CINAHL (Ovid) (1982 to October 2015). All the searches were designed and executed by the Trials Search Co‐ordinator of the Cochrane Anaesthesia Review Group (CARG) in three consecutive phases (up to August 2009, up to April 2011 and up to October 2013) and thereafter by the information specialist at the Iberoamerican Cochrane Center (up to October 2015). Our search strategies can be found in the Appendices (CENTRAL Appendix 1; MEDLINE Appendix 2; EMBASE Appendix 3; CINAHL Appendix 4).
Our search strategy used free text and controlled language (MeSH terms) for those terms and descriptors concerning interventions (warming systems) and indications (surgery, hypothermia), and methodologic filters for an exhaustive identification of the selected studies (clinical trials). We did not apply restrictions regarding publication status or by sample size.
To screen the results of this search, we did not use a specific software to manage the references, but did this manually (using Word files), except for the last update where we used Endnote.
Searching other resources
In addition:
We performed a search for other reviews and health technology assessment reports about this topic, and a manual review of all the bibliographic references in all these reviews and reports;
We screened all the reference lists of the RCTs identified during the review process.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Several authors (GU, HP, EM) and collaborators (SC, BN and EP) in pairs, independently reviewed the results of the bibliographic searches to select the articles to be included in the review. We only included those studies that fulfilled all the eligibility criteria of the review.
The pairs of authors initially reviewed titles and abstracts and obtained the full text if more detailed information was required to determine if the trial met the inclusion criteria. Each of these authors documented the reasons for trial exclusion when appropriate (see Appendix 5 for a copy of the Study Selection Form). We resolved disagreements by discussion and consensus between authors, with the collaboration of a third author among the specialists (JC, PP and LM).
Where there was insufficient published information in order to make a decision about inclusion, GU contacted the first author of the relevant trial.
Data extraction and management
Several authors, in pairs (MR, GU, EM, HP, PA) extracted data independently from the selected trials using a standardized data extraction form. A copy of this form is in Appendix 6. We resolved disagreements by discussion and consensus between authors, with the collaboration of a third author from among the specialists (JC, PP and LM).
These authors entered the retrieved data from manuscripts into Review Manager 5 (Revman 2014). Where necessary, we contacted the authors of the original publications to obtain additional data about the design of the study and results.
We extracted the following data from each study:
General information, such as title, first author, contact address, publication source, publication year, country.
Methodological characteristics and study design.
Clinical and demographic characteristics of study participants.
Description of the intervention and the control. We collected information about the type of surgery, duration, surgical team experience, and prophylactic antibiotic administration, when available.
Outcomes measures as noted above.
Results for each study group.
Assessment of risk of bias in included studies
Two pairs of authors (MR, GU, EM, HP) independently assessed risks of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by involving a third assessor among the specialists (JC, PP, and LM).
We considered a trial as having a low risk of bias if we assessed all of the following criteria as adequate, and as having a high risk of bias if we assessed one or more of the following criteria as inadequate:
Sequence generation (checking for possible selection bias). We have described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We have assessed the methods as: adequate (any truly random process, e.g. random‐number table, computer random‐number generator); inadequate (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or unclear.
Allocation concealment (checking for possible selection bias). We have described for each included study the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We have assessed the methods as: adequate (e.g. telephone or central randomization, consecutively‐numbered sealed opaque envelopes); inadequate (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); unclear.
Blinding of participants and personnel (checking for possible performance bias). We have described for each included study all the methods used, if any, to blind participants and personnel from knowledge of which intervention a participant received. We have also provided information on whether the intended blinding was effective. Where blinding was not possible, we have appraised whether the lack of blinding was likely to have introduced bias.
Blinding of outcome assessment (checking for possible detection bias). We have described for each included study all the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We have also provided information on whether the intended blinding was effective. Where blinding was not possible, we have assessed whether the lack of blinding was likely to have introduced bias.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). We have described for each included study and for each outcome the completeness of data, including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or could be supplied by the trial authors, we have re‐included missing data in the analyses. We have considered intention‐to‐treat (ITT) analysis as adequate if all dropouts or withdrawals were accounted for, and as inadequate if the number of dropouts or withdrawals was not stated, or if the reason for any dropouts or withdrawals was not stated.
Selective reporting. We have reported for each included study which outcomes of interest declared in the Methods section were later unreported in the Results section. When we did not have access to the protocol or the register of the trial, selective reporting was labelled as "unclear". .
Other sources of bias. We have described for each included study any important concerns we have about other possible sources of bias. We have assessed whether each study was free of other problems that could put it at risk of bias as: low risk, high risk or unclear.
With reference to (1) to (7) above, we have assessed the likely magnitude and direction of the bias and whether we consider it likely to have impacted on the findings.
We have also assessed the quality of the evidence, per outcome and overall, using the GRADE system (Guyatt 2008). We include a 'Summary of findings' table (SoF), with some of the most relevant outcomes. This table does not include adverse effects (safety), due to the absence of data in the studies.
Measures of treatment effect
We have analysed the results of the trials using Review Manager 5, following the recommendations given by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We have measured treatment effect by risk ratios for dichotomous variables, and by mean differences or standardised mean differences for continuous variables. We used 95% confidence intervals (CIs) to indicate the precision of the estimates.
Unit of analysis issues
The unit of analysis was always the participant, as all RCTs included in the review had a parallel design.
Dealing with missing data
Given the low proportion of missing data and their even distribution across groups, we conducted all analysis with the available data in the trials, without imputations for missing values.
Assessment of heterogeneity
Before obtaining pooled estimates of global and specific effects for each type of intervention, we have carried out a statistical heterogeneity analysis assessing the value of the I² statistic, thereby estimating the percentage of total variance across studies that is due to heterogeneity rather than to chance (Higgins 2002). We have considered a value greater than 30% as indicating statistically significant heterogeneity. When heterogeneity was present, we attempted to explore it by considering the clinical features and design of the trials.
Assessment of reporting biases
Due to the sparse number of studies assessing the same outcomes, we did not use statistical techniques to assess publication bias. For selective reporting bias, we recorded the number of studies that reported results of each outcome specified by the authors in the Methods section of the study report.
Data synthesis
We have estimated the effect of the ABSW systems through meta‐analyses, using the random‐effects model applied to the intervention effect indicators (risk ratio and mean difference), using Review Manager 5 software. We conducted all main analyses on an 'available case' basis, analysing data as presented in the individual reports.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses for the comparison ABSW versus control for all outcomes. We applied a test for subgroup differences based on the I² value. We have analysed the following subgroups:
Type of anaesthesia (general or combined anaesthesia versus exclusively regional anaesthesia).
Timing of application of the intervention (preoperatively, intraoperatively, or both preoperatively and intraoperatively).
We did not run the other planned subgroup analyses, based on type of surgery and use of premedications.
Sensitivity analysis
We had planned several sensitivity analyses to investigate potential sources of heterogeneity:
According to the risk of bias (including only trials at low risk of bias).
According to the statistical model, using a fixed‐effect model.
Including only studies where duration of surgery was longer than 120 minutes, as a surrogate for higher surgery risk.
According to imputation method to derive an intention‐to‐treat analysis, using a 'best case/worst case' imputation of missing data. Given the low proportion of missing data and their distribution, we changed the main analyses to a per protocol analysis, which rendered the sensitivity analyses by method of imputation irrelevant.
Results
Description of studies
Results of the search
The searches retrieved 2170 unique references (records) that were carefully screened. We used the most comprehensive guideline available on this topic (NICE 2008) as an aid not only in the identification of studies but to decide on their eligibility. We required the full text for all potentially relevant studies published after the date of search of the guideline, and for others where some doubts persisted. We assessed 111 full‐text articles for eligibility, and selected 66 articles covering 67 RCTs (one paper reported two different RTCs conducted simultaneously by the same team) for inclusion (see Figure 1).
1.
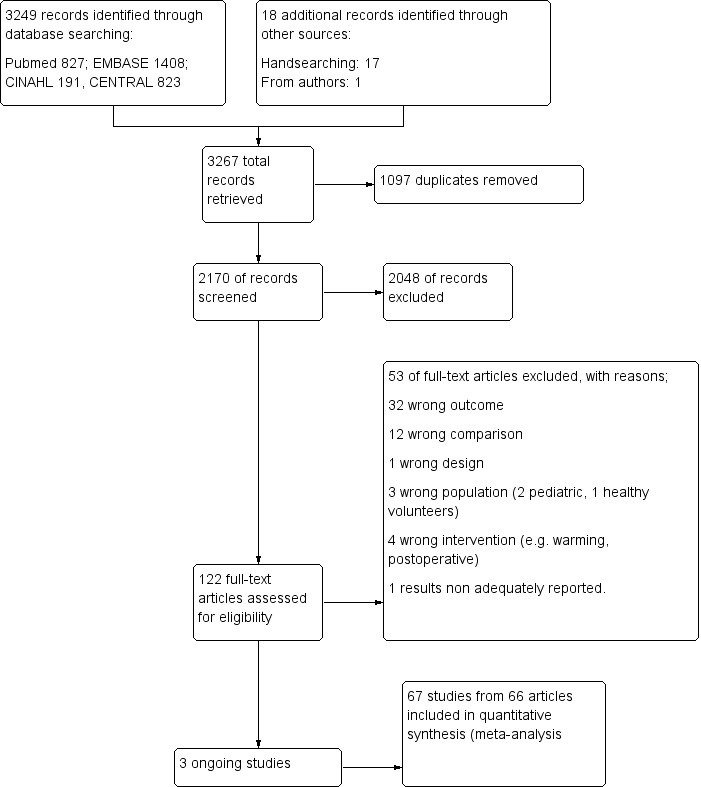
Study flow diagram.
Included studies
We included 67 RCTs (one article includes two RCTs: Camus 1993a; Camus 1993b) with 5438 participants, comprising 79 comparisons of interest:
Forty‐five RCTs (47 comparisons after merging 11 interventions arms into five, and four control arms into two) correspond to comparison type 1 (ABSW versus control) (Bennett 1994; Benson 2012; Bock 1998; Butwick 2007; Campos‐Suárez 1997; Camus 1993a; Camus 1993b; Camus 1995; Camus 1997; Casati 1999b; Chakladar 2014; Chung 2012; D'Angelo Vanni 2007; Fallis 2006; Fossum 2001; Frank 1997; Horn 2002; Horn 2012; Johansson 1999; Just 1993; Kabbara 2002; Kiessling 2006; Krenzinschek 1995; Kurz 1995; Kurz 1996; Leeth 2010; Lindwall 1998; Mason 1998; Melling 2001; Mogera 1997; Ng 2003; O'Brien 2010; Paris 2014; Peña García 1996; Persson 2001; Pu 2014; Rasmussen 1998; Rathinam 2009; Schmied 1996; Scott 2001; Steinbrook 1997; Wongprasartsuk 1998; Yamakage 1995; Yildirim 2012; Zhao 2005). Two studies comprised 3 branches, providing 2 comparisons type 1 (ABSW vs control) in a single trial (Melling 2001; Rasmussen 1998). The most studied warming system was by large forced‐air warming (FAW) in 38 RCTs, followed by electric blankets in three. The rest (one study each) were: resistive warming mattress, water garment, warmed foam pad, warming pad, heated circulating water system, and radiant heating. For the intervention used as a control, there was wide variability among studies, with some RCTs where participants did not receive any kind of active warming ('pure controls'), and many others where they could receive either warmed cotton blankets, thermal insulation, and/or warming of intravenous and irrigation fluids and blood (which are active warming systems not covered by this review). Finally, in one study (Bock 1998), participants in the control group received an ABSW during the intraoperative period as a co‐intervention (an intervention that was used systematically in all study participants). A few studies used a sham ABSW procedure (Butwick 2007; Chung 2012; Kurz 1996), while all the rest were open‐label.
Eighteen RCTs (22 comparisons) corresponded to comparison type 2 (ABSW versus another type of ABSW) (Calcaterra 2009; Elmore 1998; Hasegawa 2012 (three comparisons); Hofer 2005 (three comparisons); Janicki 2001; Kim 2014; Lee 2004; Leung 2007; Matsukawa 1994; Melling 2001; Moysés 2014; Ng 2006; Pagnocca 2009; Suraseranivongse 2009; Tanaka 2013; Torrie 2005; Vassiliades 2003; Zangrillo 2006). As for the specific comparisons made in these studies, nine compared FAW versus a circulating water‐based system (mattress, wraps, garments and pads), four compared FAW versus radiant heating, three compared FAW versus resistive heating, two compared FAW versus electric heating pads, one compared FAW with thermally‐conductive foam pads. Two trials compared a circulating‐water‐based system versus carbon‐fibre resistive heating and one compared thermal blankets versus thermal mattress.
Ten RCTs (12 comparisons) corresponded to comparison type 3 (same ABSW: two different ways of administration) (Andrzejowski 2008; Camus 1993b; Casati 1999a; D'Angelo Vanni 2007; Horn 2012; Peña García 1996; Perl 2014; Winkler 2000; Wong 2007; Yamakage 1995).
Most of the included studies reported only one comparison (two‐arm RCTs), but 14 trials reported two or more comparisons in the same article (Bennett 1994; Camus 1993b; Chung 2012; D'Angelo Vanni 2007; Hasegawa 2012; Hofer 2005; Horn 2012; Melling 2001; Ng 2003; Paris 2014; Peña García 1996; Perl 2014; Rasmussen 1998; Yamakage 1995). Of these, we excluded five comparisons and merged others for practical reasons. One study (Negishi 2003), was included as a secondary reference of another trial (Hasegawa 2012), since it was a preliminary report of the completed trial. Therefore, its data were not included in the analysis, since it included the same participants.
The sample size in each study ranged from 14 (Yamakage 1995) to 416 (Melling 2001). Thirty‐three (49%) studies included 50 participants or fewer, 21 (31%) between 51 and 100, and 13 (20%) had more than 100. The average sample size was 81 participants per study. The mean age of the participants ranged from 30 (Fallis 2006) to 73 years (Torrie 2005).
By operating time
Seven studies exclusively considered the preoperative period (prewarming) when the intervention was applied and assessed (Camus 1995; Chung 2012; Fossum 2001; Horn 2012; Just 1993; Leeth 2010; Melling 2001), and nine reported having used warming systems during both pre‐ and intraoperative periods of surgery (Andrzejowski 2008; Benson 2012; Bock 1998; D'Angelo Vanni 2007; Horn 2002; Perl 2014; Rathinam 2009; Wong 2007; Wongprasartsuk 1998). The remaining 51 studies reported having used warming systems during the intraoperative period of surgery, starting after the induction of anaesthesia.
By anaesthesia type
In 41 trials the participants were operated on under general anaesthesia, under spinal/epidural anaesthesia in 16 trials, under regional anaesthesia in one, and in eight trials the participants received different types of anaesthesia (Frank 1997; Hasegawa 2012; Krenzinschek 1995; Lee 2004; Lindwall 1998; Scott 2001; Steinbrook 1997; Tanaka 2013). In one trial it was unclear what type of anaesthesia was administered (Melling 2001).
By duration of the surgical intervention
In 38 trials the surgery had a mean duration of 120 minutes or longer (Andrzejowski 2008; Bennett 1994; Bock 1998; Campos‐Suárez 1997; Camus 1993a; Camus 1993b; Camus 1995; Camus 1997; Elmore 1998; Frank 1997; Hasegawa 2012; Hofer 2005; Janicki 2001; Just 1993; Kabbara 2002; Kiessling 2006; Kim 2014; Kurz 1995; Kurz 1996; Lee 2004; Leung 2007; Lindwall 1998; Mason 1998; Matsukawa 1994; Mogera 1997; Moysés 2014; Pagnocca 2009; Peña García 1996; Pu 2014; Rasmussen 1998; Rathinam 2009; Suraseranivongse 2009; Tanaka 2013; Vassiliades 2003; Wong 2007; Wongprasartsuk 1998; Zangrillo 2006; Zhao 2005). In 23 RCTs, it was less than 120 minutes on average (Benson 2012;Casati 1999a; Casati 1999b; Chakladar 2014; Chung 2012;D'Angelo Vanni 2007; Fallis 2006; Fossum 2001; Horn 2002;Horn 2012; Johansson 1999; Melling 2001;Ng 2003; Ng 2006; O'Brien 2010; Paris 2014;Perl 2014; Persson 2001; Schmied 1996; Scott 2001; Torrie 2005;Winkler 2000; Yildirim 2012). In six studies, duration of surgery was not reported (Calcaterra 2009; Krenzinschek 1995; Leeth 2010; Steinbrook 1997; Yamakage 1995; Butwick 2007).
There was a wide range of types of surgeries across the studies, including open abdominal surgery in 23 studies, laparoscopic abdominal surgery in two, caesarean section in six, total hip arthroplasty in seven, other types of orthopaedic surgery in six, off‐pump coronary artery by‐pass in five, thoracic surgery in two, transurethral resection of the prostate in one, and neurosurgery in one. Thirteen studies reported a mixture of gynaecological, orthopaedic, laparoscopic, breast, head‐neck, plastic, vascular and/or general surgical procedures.
Regarding the American Society of Anaesthesiologists (ASA) physical status classification, 19 studies included participants with ASA I to II status, while 24 studies reported having included participants with ASA I to III status. Two studies included only ASA III status participants (Campos‐Suárez 1997; Hofer 2005) and four studies included participants with ASA I to IV status. Eighteen studies did not state the ASA status of the participants.
Concerning the geographical setting of the included studies, 30 were conducted in Europe, 18 in North America (USA and Canada), 13 in Asia, two in Australia, and three in South America (Brazil). The vast majority were single‐centre trials, and six were multicentre (only two were international).
Fourty‐for trials included all participants in the analyses, either because they had no missing data or because they conducted an ITT analysis. Of the 23 trials with missing data, the proportion of lost participants was higher than 10% in five (Elmore 1998; Frank 1997; Kiessling 2006; Leeth 2010; Scott 2001).
For further details see Characteristics of included studies.
Excluded studies
We excluded 53 studies, mainly because of the lack of data on the outcomes of interest for the review (most of them reported exclusively on temperature, which is the most studied outcome in this context), or because they addressed a comparison not covered by this review in 12 studies. We excluded other studies for a variety of reasons (pediatric population, healthy volunteers, wrong interventions such as rewarming, or wrong design). For further details see Characteristics of excluded studies.
Studies awaiting classification
Three studies are awaiting classification (Kaudasch 1996; Leben 1997; Xu 2004). For further details see Characteristics of studies awaiting classification.
Risk of bias in included studies
The main limitation of the majority of the studies is the small sample size, which limits the likelihood of detecting any difference in the clinical outcomes considered in this review, as almost all of the trials were designed with temperature as the principal outcome. Some of the outcomes considered in this review (blood loss, transfusion or intravenous fluid requirements) were recorded not as an outcome but as descriptive information in the baseline characteristics of the study population.
We summarize the risk of bias in the 'Risk of bias' graph (Figure 2) and the 'Risk of bias' summary (Figure 3). The reasons for classification of the risk of bias are provided in the tables Characteristics of included studies.
2.
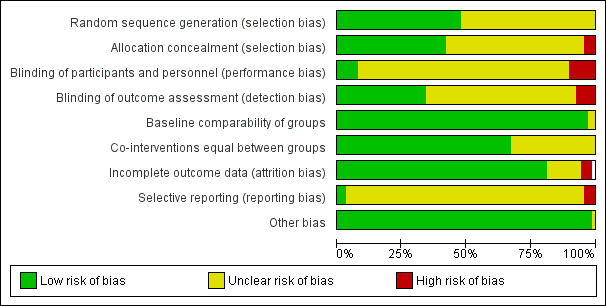
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
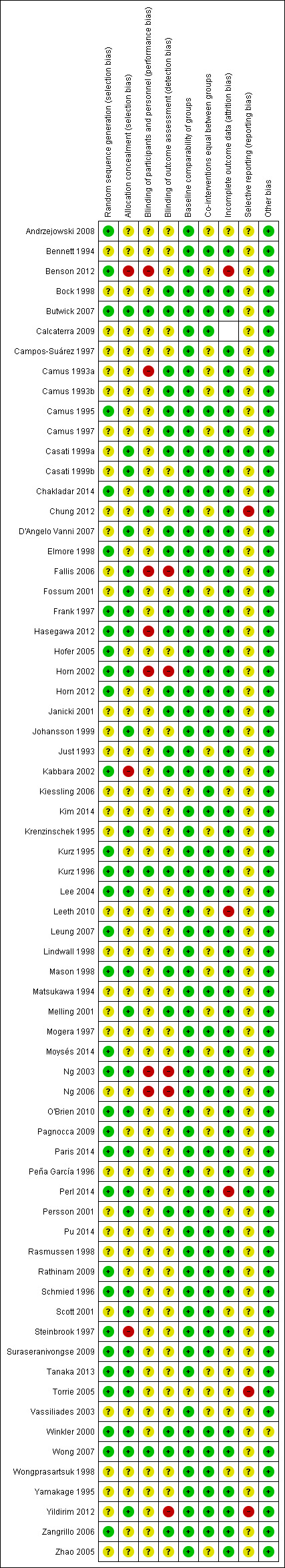
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The included studies had a low risk of bias for baseline comparability (we found no obvious differences among study groups based on the information provided in the table of participants' characteristics), attrition bias (as expected, due to the study setting and the short duration of follow‐up, the number of losses and dropouts was irrelevant) and co‐interventions common to both groups (although this is very difficult to assess in detail, given the concurrence of numerous interventions, not necessarily reported, which could potentially affect the results). We rated the risk of bias as low to unclear for random sequence generation and for allocation concealment due to lack of details, selective reporting, and blinding of participants and personnel. We judged it as unclear to high for blinding of outcome assessment, as most of the studies had an open design (no measures were implemented to guarantee an objective assessment of outcomes) or it was not mentioned at all in the publications. Finally, for most of the comparisons there were not enough studies to assess publication bias. For the comparison and the outcome with most studies (chills/shivering) we did not detect publication bias (Figure 4).
4.
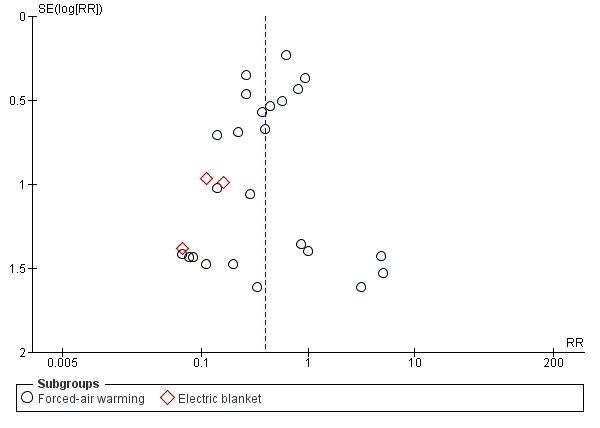
Funnel plot of comparison: 1 Active warming systems vs control (no active warming), outcome: 1.12 Chills/shivering.
Allocation
Twenty‐three RCTs reported the mechanism for generating the sequence of random assignment to the interventions (computer‐based in the majority of cases, but also 'drawing lots' in two studies, 'flipping a coin' in one, and rolling a modified dice in one), while the other 44 did not provide further details (there was only a mention about randomization in the title or the text of the article).
Twenty‐nine RCTs provided details on allocation concealment, the majority having used the sealed opaque envelopes system to administer the assignments (although not all of the publications specified that it was opaque sequentially‐numbered and sealed envelopes). In four trials there was no concealment at all, while the remaining 34 RCTs did not provide details on this procedure.
Blinding
Blinding could not be implemented due to the nature of the interventions, except for the four trials that used a sham intervention in the control group (Butwick 2007; Chung 2012; Kurz 1996; Wong 2007). Consequently, most of the trials had an open‐label design, although in the majority there was no mention of this domain. We assume these trials had an open‐label design, as temperature was the principal outcome and it was measured in a variety of ways that probably were not subject to bias. However, in 24 studies an evaluation was conducted by a blinded assessor of at least one outcome of interest. Again, it should be noted that the level of details provided to ensure the effectiveness of blinding is very low in most of the studies.
With regard to blinding, our 'Risk of bias' assessment depends on the nature of the outcome (although most of them were subjective outcomes) and the type of comparison we were appraising. For instance, for comparison type 1, where an ABSW system was compared with a control, we rated the lack of evidence about a blinded outcome assessment as being at high risk of bias. On the other hand, for comparison types 2 and 3 where different active interventions were being compared between them, we have rated it as being at unclear risk.
Incomplete outcome data
Given the nature of the studies conducted in surgical participants and with a very short follow‐up period (hours or at most a few days), the reported losses and dropouts were irrelevant. Although in some cases neither this information nor the basis for the analysis of the results (intention‐to‐treat versus valid cases, or the assumption for the missing values) were clearly reported, this is unlikely to be a major problem for this review.
Selective reporting
We found no evidence of selective reporting in most of the trials. It should be noted that temperature was the primary outcome in most studies, while clinical outcomes were all evaluated as secondary, and in many trials were not prespecified as outcomes.
Effects of interventions
See: Table 1
Comparison type 1: Active warming versus control
Primary outcome 1: Surgical site infection and complications (wound healing and dehiscence)
Three trials (Kurz 1996; Melling 2001; Pu 2014) including 589 participants showed a significant benefit of forced‐air warming (FAW) over control in the incidence of surgical site infection and complications (risk ratio (RR) 0.36, 95% confidence interval (CI) 0.20 to 0.66; P = 0.0008; I² = 0%) (Analysis 1.1).
1.1. Analysis.
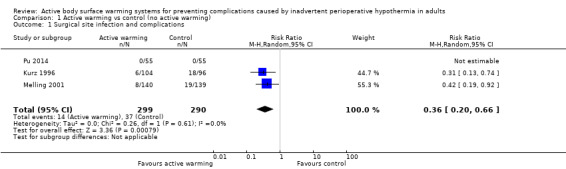
Comparison 1 Active warming vs control (no active warming), Outcome 1 Surgical site infection and complications.
Primary outcome 2: Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke and non‐fatal cardiac arrest)
A single trial (Frank 1997) assessed major cardiovascular complications (labelled in the trial as 'morbid cardiac events') and compared FAW to control in participants with documented coronary artery disease or at high risk for coronary disease. The trial reported a statistically significant reduction in perioperative morbid cardiac events (defined as cardiac arrest, myocardial infarction, or unstable angina/ischaemia occurring in the first 24 hours postoperatively) with ABSW (reported P value < 0.02). Nevertheless, our own analysis showed a marginally non‐significant reduction in risk of major cardiovascular complications in the active warming group (12 events; RR 0.22, 95% CI 0.05 to 1.00; 1 study, 300 participants) (Table 2). There was no evidence of an effect of active warming on non‐fatal myocardial infarction (one event, RR 0.37, 95% CI 0.02 to 9.03; P = 0.54). The trial showed a marginally non‐significant reduction in risk of non‐fatal cardiac arrest in the active warming group (nine events; RR 0.22, 95% CI 0.05 to 1.00; P = 0.05).
1. Data and analyses for outcomes with only one RCT: comparison 1.
| Outcome or Subgroup | Study | Intervention | Control | Effect estimate (95% CI) | ||
| n | N | n | N | |||
| Forced‐air warming (FAW) versus control (no ABSW) | ||||||
| Morbid cardiac events (includes cardiac arrest, myocardial infarction, or unstable angina/ischaemia occurring in the first 24 hours postoperatively) | Frank 1997 | 2 | 142 | 10 | 158 | RR = 0.22 (0.05 to 1.00) |
| Electrocardiographic cardiac events (includes myocardial ischaemia or ventricular tachycardia occurring either intraoperatively or 24 hours postoperatively) | Frank 1997 | 22 | 142 | 38 | 158 | RR = 0.64 (0.40 to 1.03) |
| Postoperative morbid cardiac and electrocardiographic events | Frank 1997 | 11 | 142 | 33 | 158 | RR = 0.37 (0.19 to 0.71) |
| Pressure sores and ulcers | Scott 2001 | 9 | 161 | 17 | 163 | RR = 0.54 (0.25 to 1.17) |
RR: risk ratio; CI: confidence interval;
This trial also found that in the intra‐ and 24‐hours postoperative period, the control group had a greater incidence of electrocardiograph (ECG) events (myocardial ischaemia or ventricular tachycardia) (60 events, 300 participants; RR 0.64, 95% CI 0.40 to 1.03) (Table 2). According to the trial, the difference was statistically significant only for postoperatively ECG events, with a lower rate with FAW (32 events; reported P value < 0.02).
ABSW also reduced the combination of postoperative ECG or morbid cardiac events (44 events, 300 participants; RR 0.37, 95% CI 0.19 to 0.71; P = 0.003) (Table 2).
Primary outcome 3: All‐cause mortality
Two trials (Frank 1997; Kurz 1996) (500 participants) assessed this outcome and found no significant differences between FAW and control (eight events; RR 1.01, 95% CI 0.26 to 4.00; P = 0.99). In Frank 1997 the four events observed (two in each group) occurred on the fifth hospital day or beyond, and only one was reported to be related to an ischaemic cardiac event. Results were homogeneous between the trials (I² = 0%) (Analysis 1.2).
1.2. Analysis.
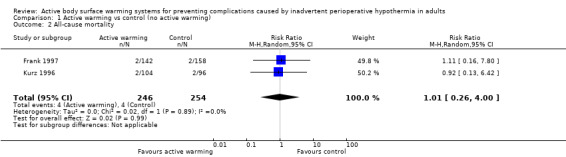
Comparison 1 Active warming vs control (no active warming), Outcome 2 All‐cause mortality.
Secondary outcome 1: Transfusions (number of participants transfused; blood product usage)
Eight trials (Bennett 1994; Frank 1997; Johansson 1999; Kurz 1996; Mogera 1997; Peña García 1996; Schmied 1996; Zhao 2005) with 779 participants assessed the amount of blood products (mainly red blood cells) transfused during surgery, showing a consistent reduction in the amount of blood transfused between FAW and control groups (mean difference (MD) ‐54.58, 95% CI ‐92.57 to ‐16.58; P = 0.005; I² = 0%) (Analysis 1.3).
1.3. Analysis.

Comparison 1 Active warming vs control (no active warming), Outcome 3 Blood transfusions during surgery and up to 48 hours post‐surgery (ml).
Eight trials (Bennett 1994; Bock 1998; Campos‐Suárez 1997; Chakladar 2014; Johansson 1999, Kabbara 2002; Kurz 1996; Schmied 1996) (621 participants) assessed the number of participants that received intraoperative transfusions, showing no differences between FAW and control (RR 0.79, 95% CI 0.50 to 1.23; I² = 39%) (Analysis 1.4).
1.4. Analysis.
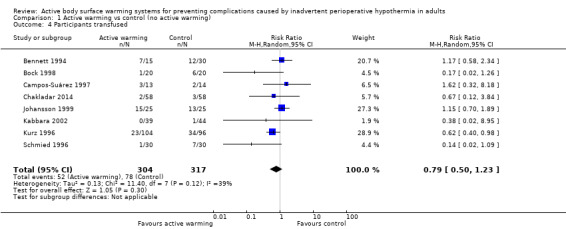
Comparison 1 Active warming vs control (no active warming), Outcome 4 Participants transfused.
Secondary outcome 2: Blood loss (ml)
The amount of blood loss during surgery was assessed in 22 trials. Eighteen of these trials (Bock 1998; Butwick 2007; Campos‐Suárez 1997; Casati 1999b; Chung 2012; Frank 1997; Johansson 1999; Kabbara 2002; Lindwall 1998; Mason 1998; Mogera 1997; Persson 2001; Peña García 1996; Pu 2014; Rathinam 2009; Schmied 1996; Steinbrook 1997; Zhao 2005) (1103 participants) assessed the effect of FAW, showing a reduction in the blood lost in the FAW group compared to the control group, of little clinical relevance (MD ‐50.77, 95% CI ‐88.43 to ‐13.10; P = 0.008). There was high heterogeneity among studies (I² = 78%) (Analysis 1.5).
1.5. Analysis.
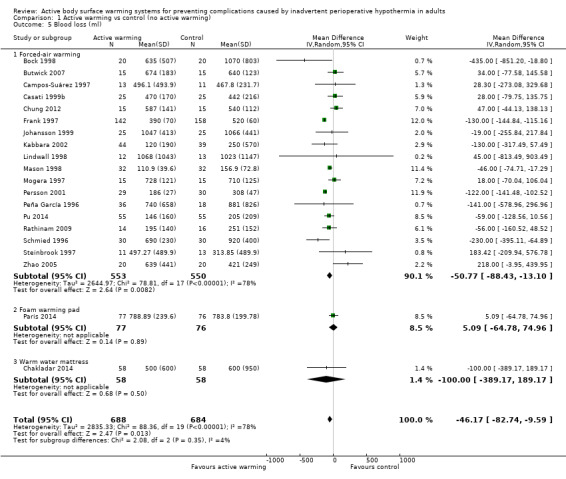
Comparison 1 Active warming vs control (no active warming), Outcome 5 Blood loss (ml).
An additional study with FAW (Horn 2012) concluded that intraoperative blood loss was comparable between groups (no raw data provided).
Three other studies compared different ABSWs with a control: one study with 100 participants used circulating warmed‐water pads (plus warmed IV fluids) (Kiessling 2006) (results only in graph with no raw data available), one study with 153 participants used foam warming pads (Paris 2014), and one study with 116 participants used a warm‐water mattress (Chakladar 2014). None of these studies found a statistically significant difference between groups.
The pooled analysis of all 20 RCTs (1372 participants) for which raw data were available showed a reduction in blood loss in the ABSW group compared to the control group, of little clinical relevance (MD ‐46.17, 95% CI ‐82.74 to ‐9.59; P = 0.000) (Analysis 1.5).
Secondary outcome 3: Intraoperative fluids infused (ml)
The amount of fluids (crystalloids or colloids or both) infused during surgery was assessed in 24 trials (Bock 1998; Butwick 2007; Campos‐Suárez 1997; Camus 1993a; Camus 1993b; Camus 1995; Casati 1999b; Chakladar 2014; Chung 2012; D'Angelo Vanni 2007; Frank 1997; Johansson 1999; Just 1993; Kabbara 2002; Kurz 1995; Kurz 1996; Mason 1998; Mogera 1997; Pu 2014; Rasmussen 1998; Rathinam 2009; Schmied 1996; Steinbrook 1997; Zhao 2005) (1491 participants), that showed a significant reduction in fluid transfusion for the intervention group compared to the control group (MD ‐144.49, 95% CI ‐221.57 to ‐67.40; P = 0.00001; I² = 73%) (Analysis 1.6).
1.6. Analysis.
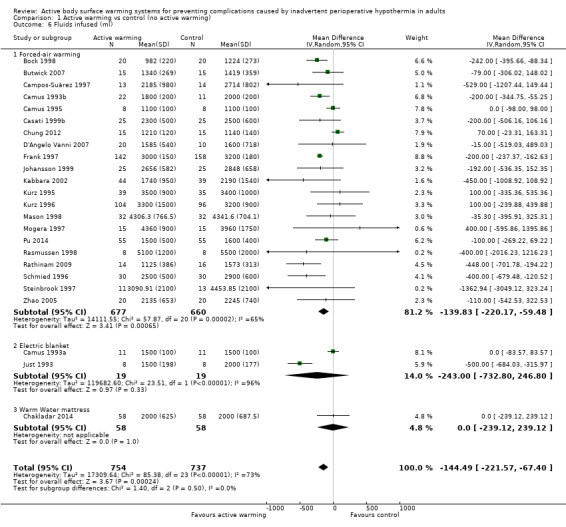
Comparison 1 Active warming vs control (no active warming), Outcome 6 Fluids infused (ml).
Twenty‐one of these trials assessed the effect of FAW versus control (1337 participants), showing a similar reduction in the fluids infused in the FAW group (MD ‐139.83, 95% CI ‐220.17 to ‐59.48; P value = 0.0001; I² = 65%). Two additional studies with FAW (Camus 1997; Horn 2012) concluded that intraoperative fluids administered were comparable between groups (no raw data provided).
The two studies that compared an electric blanket to a control (Camus 1993a; Just 1993) with 19 participants in each arm, showed a no reduction in the fluids infused with active warming (MD ‐243.00, 95% CI ‐772.80 to 246.80).
Secondary outcome 4: Other cardiovascular complications (bradycardia, hypotension, arrhythmias)
No studies reported this outcome.
Secondary outcome 5: Participant‐reported outcomes (anxiety, thermal comfort, pain)
The degree of participant anxiety was assessed in one trial (O'Brien 2010) (130 participants) through a visual analogue scale (VAS), that did not show differences between FAW and control (MD 0.40, 95% CI ‐0.57 to 1.37; P = 0.42).
Thermal comfort was assessed in 10 trials (700 participants), all of them comparing FAW with a control. Four RCTs (364 participants) used a Likert scale where higher values meant a higher degree of satisfaction (Benson 2012; Fossum 2001; Leeth 2010; O'Brien 2010). The pooled analysis found a higher satisfaction with FAW compared with control (standardized mean difference (SMD) 0.76; 95% IC 0.29 to 1.24; P = 0.005), although there was high heterogeneity (I² = 77%) (Analysis 1.7).
1.7. Analysis.
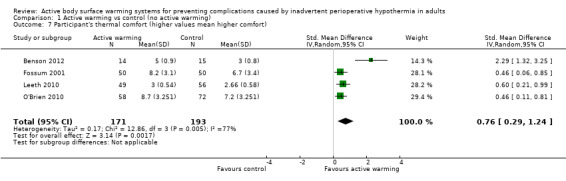
Comparison 1 Active warming vs control (no active warming), Outcome 7 Participant's thermal comfort (higher values mean higher comfort).
Six other studies (336 participants) used a verbal numerical scale where 0 mm was 'worst imaginable cold', 50 mm was 'thermoneutral', and 100 mm was 'insufferably hot' (Butwick 2007; Chung 2012; Krenzinschek 1995; Kurz 1996; Wongprasartsuk 1998; Yamakage 1995). The pooled analysis did not find differences in satisfaction between and control (MD 1,13 , 95% CI ‐0,61 to 2,87; P = 0.20), although there was extremely high heterogeneity (I² = 97%) (Analysis 1.8). Exclusion of the outlier trial (Kurz 1996) led to similar results, with moderate heterogeneity (MD 0,36, 95% CI ‐0,27 to 0,98; P= 0.26; I² = 56%).
1.8. Analysis.
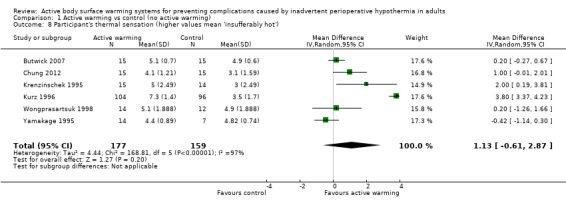
Comparison 1 Active warming vs control (no active warming), Outcome 8 Participant's thermal sensation (higher values mean 'insufferably hot').
Four additional studies (Fallis 2006; Horn 2002; Horn 2012; Kabbara 2002) reported that postoperative thermal comfort scores were no different between groups (no raw data were provided). Another study (Kurz 1995) reported that participants assigned to FAW reported significantly higher thermal comfort scores than those allocated to the control group (no raw data provided).
Pain was assessed in seven trials (Benson 2012; Fossum 2001; Frank 1997; Horn 2002; Krenzinschek 1995; Pu 2014; Wongprasartsuk 1998) (624 participants), all of them comparing FAW to control. There were no statistically significant differences between the two interventions (MD ‐0.24, 95% CI ‐1.12 to 0.64). The results were quite heterogeneous (I² = 84%) (Analysis 1.9). An additional trial (Fallis 2006) that assessed pain (no raw data provided) observed that there was a statistically significant up‐trend over time for pain scores in both groups, but that there was no significant difference over time between the two groups (P = 0.302). Two other studies (Kurz 1995; Kurz 1996) stated that "pain scores and the amount of opioid administered were virtually identical in the two groups at every postoperative measurement" (no raw data provided).
1.9. Analysis.
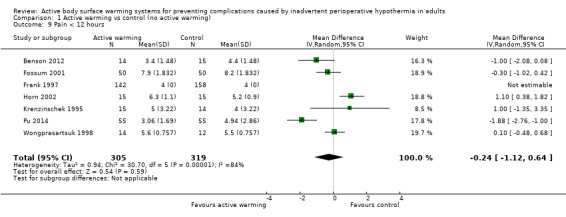
Comparison 1 Active warming vs control (no active warming), Outcome 9 Pain < 12 hours.
Secondary outcome 6: Shivering (number of participants)
There were 29 trials (Bock 1998; Butwick 2007; Camus 1993a; Camus 1993b; Camus 1995; Camus 1997; Casati 1999b; Chakladar 2014; Chung 2012; D'Angelo Vanni 2007; Fallis 2006; Fossum 2001; Frank 1997; Horn 2002; Horn 2012; Just 1993; Krenzinschek 1995; Mason 1998; Mogera 1997; Ng 2003; Persson 2001; Pu 2014; Rasmussen 1998; Rathinam 2009; Steinbrook 1997; Wongprasartsuk 1998; Yamakage 1995; Yildirim 2012; Zhao 2005) (1922 participants) assessing chills and shivering, that showed that participants receiving active warming systems had about one‐third the risk of chills/shivering compared to those receiving control treatment (RR 0.39, 95% CI 0.28 to 0.54; P < 0.00001; I² = 28%) (Analysis 1.10).
1.10. Analysis.
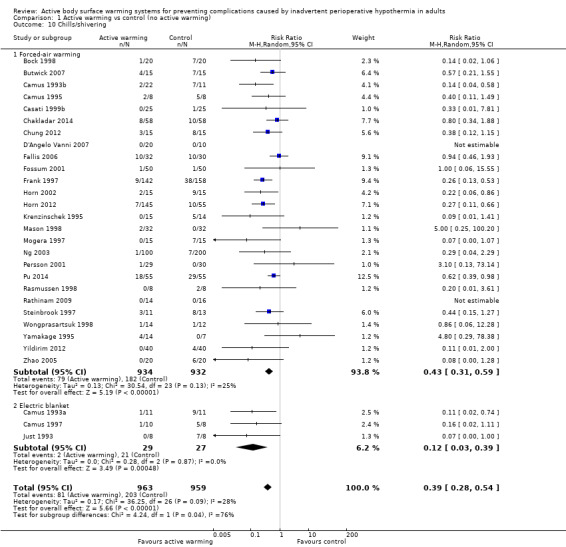
Comparison 1 Active warming vs control (no active warming), Outcome 10 Chills/shivering.
Most of the trials (26 trials, 1866 participants) used FAW as active warming, with similar results (RR 0.43, 95% CI 0.31 to 0.59; P < 0.00001; I² = 25%). An additional study (Kurz 1995) also found a favourable result with FAW (no raw data were provided).
Three trials (Camus 1993a; Camus 1997; Just 1993) (56 participants) used electric blankets, with even larger differences between groups (RR 0.12, 95% CI 0.03 to 0.39; P = 0.0005; I² = 0%).
Secondary outcome 7: Pressure sores and ulcers
Pressure ulcers were assessed in a single trial (Scott 2001) (324 participants) where the risk of pressure ulcers in the FAW group was half of that in the control group, albeit with no statistical significance (RR 0.54, 95% CI 0.25 to 1.17; P = 0.12) (Table 2).
Secondary outcome 8: Adverse effects (including thermal burns)
Three studies (Bennett 1994; Kabbara 2002; Mason 1998) stated narratively (no data presented) that there were no complications attributable to ABSW.
Other outcomes not included in the review
A variety of additional outcomes have been assessed in the studies included in the review (see table Characteristics of included studies). These include the percentage of participants who became hypothermic during the study, coagulation markers, haemoglobin level, heart rate and blood pressure, dose of analgesics, and postoperative recovery, among many others.
Comparison type 2: Active warming (1) versus active warming (2)
2.1 Forced‐air warming (FAW) versus electric heating (EHS) or resistive heating systems (RHS)
Primary outcome 1: Surgical site infection and complications (wound healing and dehiscence)
One trial with 59 participants comparing FAW versus carbon‐fibre resistive heating blankets (Hofer 2005) assessed the rate of surgical site infection (major sternal infection), and found no significant difference between FAW and EHS (two events; RR 1.03, 95% CI 0.07 to 15.77; P = 0.98) (Table 3).
2. Data and analyses for outcomes with only one RCT: comparison 2.
| Outcome or Subgroup | Study | Intervention | Control | Effect estimate (95% CI) | ||
| n or mean (SD) | N | n or mean (SD) | N | |||
| FAW/Convective air warming versus electric or resistive heating systems | ||||||
| Infection of surgical wound (major sternal infection) | Hofer 2005 | 1 | 29 | 1 | 30 | RR = 1.03 (0.07 to 15.77) |
| Blood products transfused (packed red blood cells) | Leung 2007 | 100 (276.7) | 30 | 50 (159.2) | 30 | MD = 50.00 (‐64.23 to 164.23) |
| Perioperative RBC transfusion (mL) | Hofer 2005 | 1,097 (874) | 29 | 986 (744) | 30 | MD = 111.00 (‐303.81 to 525.81) |
| Participants transfused (allogenic transfusion) | Hofer 2005 | 14 | 29 | 12 | 30 | RR = 1.29 (0.74 to 2.27) |
| Forced‐air warming (FAW) versus warm water circulation systems | ||||||
| Postoperative cardiac complications (defined as angina, myocardial infarction, cardiac arrest, unstable ventricular tachycardia, or congestive heart failure) | Elmore 1998 | 2 | 50 | 0 | 50 | RR = 5.00 (0.25 to 101.58) |
| All‐cause mortality | Elmore 1998 | 2 | 50 | 0 | 50 | RR = 5.00 (0.25 to 101.58) |
| Cardiac complications or death | Elmore 1998 | 4 | 50 | 0 | 50 | RR = 9.00 (0.50 to 162.89) |
| Thermal comfort (VAS scale) | Kim 2014 | 5 (0.5) | 23 | 4 (0.79) | 23 | MD = 1.00 (0.62 to 1.38) |
| Forced‐air warming (FAW) versus radiant heat | ||||||
| Participant's comfort (thermal sensation) | Lee 2004 | 49 (5) | 29 | 48 (14) | 30 | MD = 1.00 (‐4.33 to 6.33) |
| Resistive heating systems versus warm water circulation systems | ||||||
| Participants transfused (allogenic transfusion) | Hofer 2005 | 12 | 30 | 5 | 29 | RR = 2.32 (0.93 to 5.76) |
| Perioperative blood loss (mL) | Hofer 2005 | 2300.0 (788.0) | 30 | 1497.0 (497.0) | 29 | MD = 803.00 (467.99 to 1138.01) |
| Blood transfusions during surgery (mL) | Hofer 2005 | 1097.0 (874.0) | 30 | 431.0 (387.0) | 29 | MD = 666.00 (323 to 1001) |
| Thermal mattress vs thermal blanket (not specified) | ||||||
| Fluids infused | Moysés 2014 | 3023.7 (1160.5) | 19 | 2878.9 (1376.7) | 19 | MD = 144.80 (‐664.82 to 954.42) |
| Blood transfusions (mL) | Moysés 2014 | 589.9 (398.3) | 9 | 412.3 (157.0) | 8 | MD = 177.60 (‐104.44 to 459.64) |
RR: risk ratio; CI: confidence interval; MD: mean difference; RBC: red blood cell
Primary outcome 2: Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke and non‐fatal cardiac arrest)
The included studies did not address these outcomes.
Primary outcome 3: All‐cause mortality
The included studies did not provide data for this outcome.
Secondary outcome 1: Transfusions (number of participants transfused; blood product usage)
One trial (60 participants) comparing FAW versus electric heating pad (Leung 2007) assessed the amount of blood products transfused intraoperatively; it showed no statistically significant difference in units of blood transfusions during surgery between participants who received FAW and those who received EHS (MD 50.00, 95% CI ‐64.23 to 164.23; P = 0.39). Similarly, one trial with 59 participants (Hofer 2005) found no statistically significant difference in the volume (ml) of blood/plasma/platelet transfusions during surgery between participants who received FAW and those who received EHS (MD 111.00, 95% CI ‐303.81 to 525.81; P = 0.60) (Table 3).
For the number of participants receiving transfusions, the same study (Hofer 2005) found no statistically significant differences between those who received FAW and those who received EHS (RR 1.29, 95% CI 0.74 to 2.27; P = 0.37) (Table 3).
Secondary outcome 2: Blood loss (ml)
Five trials with 270 participants (Hasegawa 2012; Hofer 2005; Leung 2007; Ng 2006; Tanaka 2013) assessed the amount of blood lost during surgery, and found no statistically significant differences between FAW and electric or resistive heating systems (MD ‐3.68, 95% CI ‐55.43 to 48.07; P value = 0.28; I² = 21%) (Analysis 2.1).
2.1. Analysis.
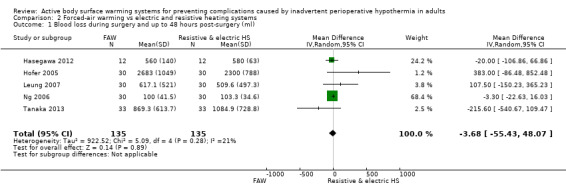
Comparison 2 Forced‐air warming vs electric and resistive heating systems, Outcome 1 Blood loss during surgery and up to 48 hours post‐surgery (ml).
Secondary outcome 3: Intraoperative fluids infused (ml)
Three trials (184 participants) (Leung 2007; Ng 2006; Tanaka 2013) examined the amount of fluids transfused during surgery and found no statistically significant differences for participants who received FAW and those who received electric or resistive heating systems (MD 101.59, 95% CI ‐14.67 to 217.85; I² = 0%) (Analysis 2.2). An additional study (Hasegawa 2012) that reported intraoperative fluids infused as ml/kg/hr stated that this was similar between groups (no raw data).
2.2. Analysis.
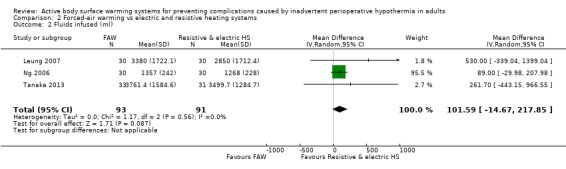
Comparison 2 Forced‐air warming vs electric and resistive heating systems, Outcome 2 Fluids infused (ml).
Secondary outcome 4: Other cardiovascular complications (bradycardia, hypotension, arrhythmias)
No studies reported on this outcome.
Secondary outcome 5: Participant‐reported outcomes (anxiety, thermal comfort, pain)
Thermal comfort was assessed in two studies (120 participants) (Leung 2007;Ng 2006) that showed no statistically significant difference in thermal comfort between FAW and electric or resistive heating systems (MD 0.07, 95% CI ‐0.21 to 0.35; P = 0.61; I² = 0%) (Analysis 2.3).
2.3. Analysis.
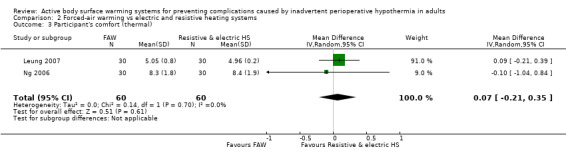
Comparison 2 Forced‐air warming vs electric and resistive heating systems, Outcome 3 Participant's comfort (thermal).
No studies reported on anxiety or pain.
Secondary outcome 6: Shivering (number of participants)
Two studies (120 participants) (Leung 2007; Ng 2006) examined the occurrence of chills/shivering. There was no statistically significant difference in this outcome measure for participants who received FAW and those who received electric or resistive heating systems (RR 1.31, 95% CI 0.30 to 5.74; I² = 0%) (Analysis 2.4).
2.4. Analysis.
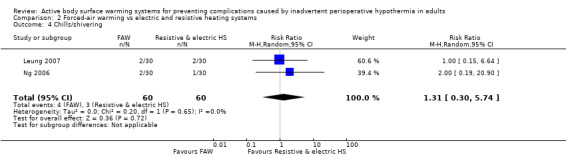
Comparison 2 Forced‐air warming vs electric and resistive heating systems, Outcome 4 Chills/shivering.
Secondary outcome 7: Pressure sores and ulcers
The included studies did not provide data for this outcome.
Secondary outcome 8: Adverse effects (including thermal burns)
Only one study (Hasegawa 2012) stated that they detected no complications related to any of the warming methods.
2.2 Forced‐air warming (FAW) versus warm‐water circulation systems (WWCS)
Primary outcome 1: Surgical site infection and complications (wound healing and dehiscence)
Three trials (208 participants) (Calcaterra 2009; Elmore 1998; Hofer 2005) assessed the rate of surgical site infection, and found no statistically significant difference between FAW and WWCS (RR 3.00, 95% CI 0.62 to 14.53; P = 0.17; I² = 0%) (Analysis 3.1).
3.1. Analysis.
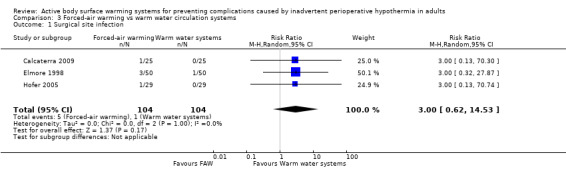
Comparison 3 Forced‐air warming vs warm water circulation systems, Outcome 1 Surgical site infection.
Primary outcome 2: Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke and non‐fatal cardiac arrest)
A single study with 100 participants (Elmore 1998) assessed the occurrence of major cardiovascular complications (defined as postoperative angina, myocardial infarction, cardiac arrest, unstable ventricular tachycardia, or congestive heart failure). The event was observed in two participants who received FAW and none in those who received WWCS (RR 5.00, 95% CI 0.25 to 101.58; P = 0.29) (Table 3).
Primary outcome 3: All‐cause mortality
There was no statistically significant difference in all‐cause mortality between participants who received FAW and those who received WWCS in the one trial (100 participants) (Elmore 1998) reporting this outcome (two events; RR 5.00, 95% CI 0.25 to 101.58; P = 0.29) (Table 3).
The combination of both cardiovascular complications and death in Elmore 1998 also yielded to a statistically non‐significant result (RR 9.00, 95% CI 0.50 to 162.89; P = 0.14) (Table 3).
Secondary outcome 1: Transfusions (number of participants transfused; blood product usage)
Transfusions during surgery (N of participants) was assessed in two studies (108 participants) (Calcaterra 2009; Hofer 2005), and found no statistically significant differences between FAW and WWCS (RR 1.59, 95% CI 0.48 to 5.24; P = 0.45; I² = 81%), although one of the trials detected a difference in favour of WWCS (Hofer 2005).
Three trials (Hofer 2005; Kim 2014; Matsukawa 1994) assessed blood transfusions during surgery (ml) in 144 participants, and found that similar volumes were transfused in the FAW and WWCS groups (MD 136.21, 95% CI ‐119.10 to 391.51; P = 0.30; I² = 86%) (Analysis 3.3).
3.3. Analysis.
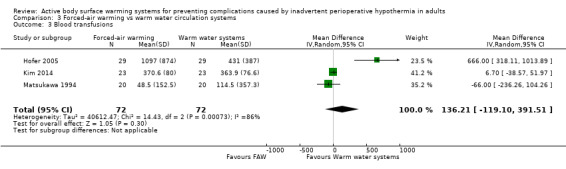
Comparison 3 Forced‐air warming vs warm water circulation systems, Outcome 3 Blood transfusions.
Secondary outcome 2: Blood loss (ml)
Six studies (299 participants) (Calcaterra 2009; Hofer 2005; Janicki 2001; Kim 2014; Matsukawa 1994; Vassiliades 2003) assessed blood loss during surgery, and found no statistically significant differences between FAW and WWCS (MD 38.08, 95% CI ‐298.39 to 374.54; P = 0.82). Heterogeneity was highly significant (I² = 90%) (Analysis 3.4).
3.4. Analysis.
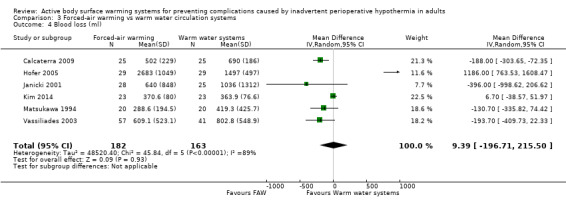
Comparison 3 Forced‐air warming vs warm water circulation systems, Outcome 4 Blood loss (ml).
One study comparing intraoperative forced‐air warming mattress versus circulating‐water mattress (Suraseranivongse 2009) found no statistically significant differences between both groups (P value for the comparison between median values = 0.962).
Secondary outcome 3: Intraoperative fluids infused (ml)
Four studies (230 participants) (Elmore 1998; Janicki 2001; Kim 2014; Zangrillo 2006) found that similar but higher volumes were transfused in the WWCS arm than in the FAW groups (MD ‐215.11, 95% CI ‐519.20 to 88.99; I² = 39%) (Analysis 3.5).
3.5. Analysis.
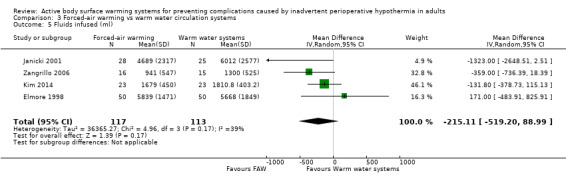
Comparison 3 Forced‐air warming vs warm water circulation systems, Outcome 5 Fluids infused (ml).
Secondary outcome 4: Other cardiovascular complications (bradycardia, hypotension, arrhythmias)
No studies reported on this outcome.
Secondary outcome 5: Participant‐reported outcomes (anxiety, thermal comfort, pain)
Only one study with 46 participants reported on thermal comfort (Kim 2014), and found a statistically significant improvement with FAW compared to WWCS (MD 1.00, 95% CI 0.62 to 1.38) (Table 3).
Secondary outcome 6: Shivering (number of participants)
Data were available only for the incidence of chills/shivering. Three studies (123 participants) (Janicki 2001; Kim 2014; Matsukawa 1994) found no statistically significant differences between FAW and WWCS (RR 1.84, 95% CI 0.17 to 19.64; I² = 81%) (Analysis 3.6).
3.6. Analysis.
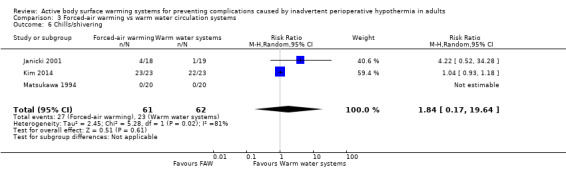
Comparison 3 Forced‐air warming vs warm water circulation systems, Outcome 6 Chills/shivering.
Secondary outcome 7: Pressure sores and ulcers
No studies reported on this outcome.
Secondary outcome 8: Adverse effects (including thermal burns)
One study (Suraseranivongse 2009) reported that no pressure‐heat burn was found in the FAW mattress group while burns were found in five participants in the WWCS group.
2.3 Forced‐air warming (FAW) versus radiant heating
Primary outcome 1: Surgical site infection and complications (wound healing and dehiscence)
The included studies did not provide data for this outcome.
Primary outcome 2: Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke and non‐fatal cardiac arrest)
The included studies did not provide data for this outcome.
Primary outcome 3: All‐cause mortality
The included studies did not provide data for this outcome.
Secondary outcome 1: Transfusions (number of participants transfused; blood product usage)
The included studies did not provide data for this outcome.
Secondary outcome 2: Blood loss (ml)
The included studies did not provide data for this outcome.
Secondary outcome 3: Intraoperative fluids infused (ml)
The included studies did not provide data for this outcome.
Secondary outcome 4: Other cardiovascular complications (bradycardia, hypotension, arrhythmias)
The included studies did not provide data for this outcome.
Secondary outcome 5: Participant‐reported outcomes (anxiety, thermal comfort, pain)
Thermal comfort was assessed in one study (59 participants) (Lee 2004) that showed no significant difference in thermal comfort between FAW and radiant heat (MD 1.00, 95% CI ‐4.33 to 6.33; P = 0.71) (Table 3).
No studies reported on anxiety or pain.
Secondary outcome 6: Shivering (number of participants)
Two studies (115 participants) (Lee 2004; Torrie 2005) examined the occurrence of chills/shivering. There was no significant difference in this outcome measure between participants who received FAW and those who received radiant heat (RR 1.22, 95% CI 0.25 to 6.08; P = 0.81; I² = 0%) (Analysis 4.1).
4.1. Analysis.
Comparison 4 Forced‐air warming vs radiant heat, Outcome 1 Chills/shivering.
Secondary outcome 7: Pressure sores and ulcers
The included studies did not provide data for this outcome.
Secondary outcome 8: Adverse effects (including thermal burns)
The included studies did not provide data for this outcome.
2.4 Resistive heating systems (RHS) versus warm‐water circulation systems (WWCS)
Primary outcome 1: Surgical site infection and complications (wound healing and dehiscence)
The included studies did not address these outcomes.
Primary outcome 2: Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke and non‐fatal cardiac arrest)
The included studies did not address these outcomes.
Primary outcome 3: All‐cause mortality
The included studies did not provide data for this outcome.
Secondary outcome 1: Transfusions (number of participants transfused; blood product usage)
Disposable circulating‐water warming garment (WWCS) worked better than RHS (resistive heating electric carbon‐fibre blankets) in the different modalities of transfusion considered. Consequently, participants in the RHS group received significantly more blood transfusions during surgery, compared to the group who received WWCS (MD 666.00, 95% CI 323 to 1001; P = 0.0003; 1 study, 59 participants) (Hofer 2005) (Table 3).
The number of participants being transfused (allogenic transfusion) in Hofer 2005 was higher with RHS compared to those with WWCS (RR 2.32, 95% CI 0.93 to 5.76), but the difference was not statistically significant.
Secondary outcome 2: Blood loss (ml)
Participants receiving RHS had significantly greater perioperative blood loss, compared to those receiving WWCS (MD 803.00, 95% CI 467.99 to 1138.01; P value < 0.00001; 1 study, 59 participants) (Hofer 2005) (Table 3).
Secondary outcome 3: Intraoperative fluids infused (mL)
No data were available to assess the amount of fluids transfused during surgery.
Secondary outcome 4: Other cardiovascular complications (bradycardia, hypotension, arrhythmias)
The included studies did not provide data for this outcome.
Secondary outcome 5: Participant‐reported outcomes (anxiety, thermal comfort, pain)
The included studies did not consider these outcomes.
Secondary outcome 6: Shivering (number of participants)
The included studies did not provide data for this outcome.
Secondary outcome 7: Pressure sores and ulcers
The included studies did not provide data for this outcome.
Secondary outcome 8: Adverse effects (including thermal burns)
The included studies did not provide data for this outcome.
Comparison type 3: Different modalities of the same type of active warming system
Eleven RCTs (701 participants) compared different modalities of the same ABSW system (Andrzejowski 2008; Camus 1993b; Casati 1999a; D'Angelo Vanni 2007; Horn 2012; Pagnocca 2009; Peña García 1996; Matsukawa 1994; Winkler 2000; Yamakage 1995; Wong 2007). Five were two‐arm RCTs comparing two different modes of administration of the same active warming system (Andrzejowski 2008; Casati 1999a; Pagnocca 2009; Winkler 2000; Wong 2007), while the rest included other arms using either a control group (included in comparison type 1) or a different ABSW system (included in comparison type 2).
Nine studies used FAW and compared: FAW (pre‐ and intraoperative) versus FAW intraoperative (Andrzejowski 2008; D'Angelo Vanni 2007); FAW versus insulated FAW (Camus 1993b); FAW of either the two upper limbs versus FAW of the unoperated limb (Casati 1999a); FAW plus circulating warmed‐water blanket or mattress versus circulating warmed‐water blanket or mattress (Matsukawa 1994; Pagnocca 2009); FAW 'aggressive' versus FAW 'conventional' (Winkler 2000); FAW upper body versus FAW lower body (Yamakage 1995); and FAW intraoperative plus a conductive carbon polymer mattress (pre‐ and postoperative) versus FAW intraoperative (Wong 2007). One additional study compared convective heating (not defined) plus warmed IV fluids versus convective heating (Peña García 1996).
This third comparison is of less interest to this review in relation to the previous two that evaluate the effectiveness of active warming and the relative efficacy between different types of active warming. Moreover, available data on clinical outcomes in this comparison are sparse, since all studies were focused on evaluating the effect on temperature. It is therefore unsurprising that, due to the lack of statistical power, we found no statistically significant results in favour of one particular mode of intervention, apart from one study (Wong 2007). This study, at low risk of bias, showed that extending systemic warming to the perioperative period had additional beneficial effects in terms of lower blood loss (median: 200 mL (range 5 – 1000) versus 400 mL (range 50 – 2300); P = 0.011) and global complication rates (32% versus 54%; P = 0.027) (surgical site infection was included in what the author assesses as global complication rates) (see Table 4). Participants in the intervention group showed no differences in blood loss nor need of a blood transfusion.
3. Data and analyses for comparison 3.
| Outcome or Subgroup | Study | FAW | FAW + CCPM | Effect estimate (95%CI) | ||
| FAW (intraOp) ±conductive carbon polymer mattress (preOp) | ||||||
| N of participants with a complication | Wong 2007 | 30 | 56 | 15 | 47 | RR = 0.60 (0.37 to 0.97) |
| Infection and complications of the surgical wound | Wong 2007 | 15 | 56 | 6 | 47 | RR = 0.40 (0.17 to 0.94) |
| Deaths | Wong 2007 | 1 | 47 | 2 | 56 | RR = 060 (0.06 to 6.37) |
| Participants transfused | Wong 2007 | 19 | 56 | 11 | 47 | RR = 0.69 (0.37 to 1.30) |
| Pressure ulcers | Wong 2007 | 0 | 56 | 1 | 47 | RR = 3.56 (0.15 to 85.45) |
| Cardiac complications | Wong 2007 | 2 | 56 | 0 | 47 | RR = 0.04 (0.01 to 0.28) |
RR: risk ratio; CI: confidence interval
Subgroup analyses by anaesthesia type (active warming versus control)
The test for subgroup differences was not significant except for the outcomes: blood loss during surgery, fluids transfused during surgery, and pain. See Analysis 5.1 to Analysis 5.13
5.1. Analysis.

Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 1 Surgical site infection and complications.
5.13. Analysis.
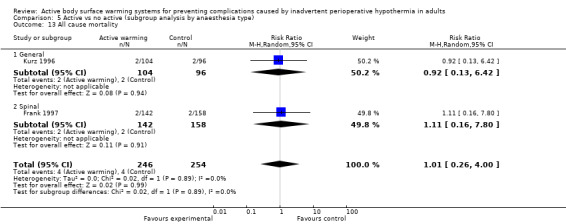
Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 13 All cause mortality.
The reduction in blood loss during surgery observed in the main analyses was maintained in the subgroups of general anaesthesia, combined anaesthesia and unknown anaesthesia. In the subgroup of spinal anaesthesia, there were no differences in blood loss between the active warming system and the control (MD 34.34; 95% CI ‐22.95 to 91.63; I² = 0%).
The reduction in fluids transfused during surgery observed in the main analyses was observed in all the subgroups, although the subgroups of combined and unknown anaesthesia presented higher magnitudes of reduction in fluids than the subgroups of general and spinal anaesthesia.
The results for pain are difficult to interpret because of the low number of trials that could be meta‐analysed. One trial in the subgroup of spinal anaesthesia reported lower levels of pain in the control arm with respect to the active arm (MD 1.10, 95% CI 0.38 to 1.82), in contrast with the statistically non‐significant differences observed in the subgroups of general and combined anaesthesia.
Subgroup analyses by timing of intervention (active warming versus control)
Most trials implemented the interventions intraoperatively, and only six trials had exclusively a preoperative intervention (Camus 1995; Fossum 2001; Horn 2012; Just 1993; Leeth 2010; Melling 2001), while eight trials implemented the intervention both pre‐ and intraoperatively (Benson 2012; Bock 1998; Chung 2012; D'Angelo Vanni 2007; Horn 2002;; Wong 2007; Wongprasartsuk 1998). For this reason, only some outcomes had data for a test of subgroup differences. The test for subgroup differences was not significant for any outcome. See Analysis 6.1 to Analysis 6.13.
6.1. Analysis.
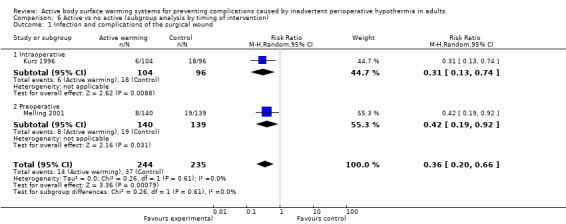
Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 1 Infection and complications of the surgical wound.
6.13. Analysis.

Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 13 All‐cause mortality.
Sensitivity analyses (active warming versus control)
Including only trials at low risk of bias: this sensitivity analyses could not be performed because there were not enough trials at low risk of bias within each category, according to the comparison type.
Analyses using a fixed‐effect model: we found no relevant differences with respect to the main analyses, except for data on blood loss and blood transfusions in the comparison FAW versus warm‐water circulation systems.
Restricted to trials with duration of more than 120 minutes: this sensitivity analysis, restricted to 13 trials, found results similar to the main analyses for all outcomes. See Analysis 7.1 to Analysis 7.12.
7.1. Analysis.
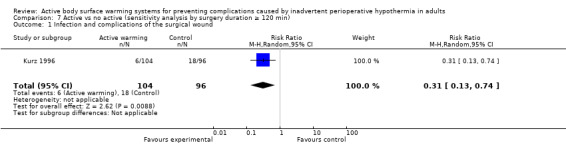
Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 1 Infection and complications of the surgical wound.
7.12. Analysis.
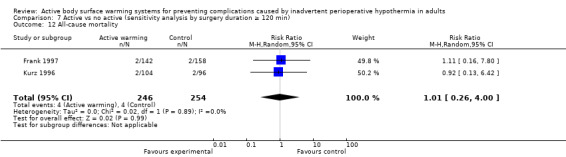
Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 12 All‐cause mortality.
Discussion
One of the main challenges, and potential limitations, of this review is the great clinical variability or heterogeneity found among the numerous studies identified, hindering both the synthesis and interpretation of results. This variability affects both the specific modalities of interventions being evaluated in the intervention (ABSW) and control groups, as well as the study populations, settings and methods.
In relation to the interventions, while some studies applied a single intervention, others used two or more interventions in combination (e.g. a combination of an active body surface warming system and passive warming or thermal insulation, or a combination of ABSW with another type of active warming not covered by this review, such as warming of intravenous or irrigation fluids). Moreover, for comparison type 1 (ABSW versus control), the most important comparison of this review, the control group did not always consist of a 'pure control' without any active heating but sometimes participants received (like the intervention group) a co‐intervention (for example, warming of irrigation and intravenous fluids) as part of usual care. This contamination of the control group is greater in more recent studies, to the extent that there is widespread awareness of the risk of hypothermia and measures have increasingly been established to prevent it as part of usual care. It is therefore unlikely that future studies with pure control will be carried out for ethical reasons.
In addition to the potential interaction with other components of usual care in surgery, the short duration of the studies due to the specific condition being addressed (perioperative complications) and the principal outcome assessed in the majority of the studies (temperature) make it difficult to detect effects of a clinically relevant magnitude. Finally, it should also be noted that some of the outcomes assessed in this review (e.g. surgical site infection, cardiovascular events, mortality, pain, etc.) are strongly influenced not only by the interventions aimed at preventing unintended hypothermia but also by other perioperative management components that are not evaluated in this review. Particularly, the strict intraoperative temperature control performed in the included studies, which seems not to be the case in routine practice (Torossian 2007), may have limited the potential to detect relevant clinical effects of ABSW.
In relation to the study population, there is a wide variety of type and duration of surgery (complexity), type of anaesthesia, severity of participant illness and co‐morbidities, etc. The large clinical variability between studies obliges us to create broad categories in which to classify and analyse the studies. The above‐mentioned condition particularly affects the comparison between ABSW and control.
There are also few available data on clinical outcomes that have been commonly assessed in many of the identified studies, limiting the power to detect potential differences. This is particularly relevant for the primary outcomes. Moreover, the specific way of defining outcomes is often not detailed or is diverse across studies, which raises some doubts and limits the interpretation of pooled analysis. All the RCTs measured and evaluated temperature in different ways, mostly as the main outcome. The goal of this review was to assess the effects of an intervention aimed at preventing hypothermia on clinical outcomes, beyond temperature. However, although the review identified a large number of RCTs that fulfilled this eligibility criterion, the data on outcomes other than temperature are sparse, often focusing on minor outcomes, such as total blood loss or total infused IV fluids, or on outcomes that are difficult to quantify and compare, such as shivering.
Summary of main results
Most information in this review comes from comparison type 1 between active body surface warming systems and a control group without application of any ABSW system. This is the most important type of comparison because it answers the question about the consequences of implementing ABSW interventions before and/or during surgery in terms of clinical benefits rather than temperature.
Since forced‐air warming (FAW) was by far the most widely evaluated intervention, the results of this review basically apply to this type of intervention. FAW was shown to be more effective than usual care in reducing the rates of surgical site infection and complications. This effect was demonstrated in two quite large RCTs (Kurz 1996; Melling 2001) in participants undergoing abdominal surgery (under general anaesthesia), and FAW was applied exclusively in the preoperative phase in one study (Melling 2001), while in the other it was applied intraoperatively (Kurz 1996) .
Only one study at low risk of bias assessed and observed a beneficial effect with FAW on major cardiovascular complications (Frank 1997), in participants with documented high cardiovascular risk.
ABSW also reduced blood loss during surgery but the magnitude of these effects (which was only apparent after the pooled analysis) seems to be clinically irrelevant (a difference of ‐57 mL between groups) and was highly heterogeneous among studies. We came to similar conclusion about total fluids transfused during surgery (a difference of ‐132 mL in favour of ABSW). These effects did not translate into a significant reduction in the number of participants being transfused or the average amount of blood transfused.
The most favourable effect with ABSW (especially with FAW but also with electric blankets) was observed for shivering (a statistically significant reduction in the number of participants experiencing shivering) and on thermal comfort.
We found no differences in the incidence of pressure ulcers, non‐fatal myocardial infarction, anxiety, pain or mortality.
In comparison type 2 between different types of ABSW, we found no evidence of superiority for any system in terms of clinical outcomes, due to the sparse data that are available.
For comparison type 3 on specific modes of administration of a particular ABSW, limited evidence from a single study at low risk of bias shows better results in reducing blood loss and complication rates (mainly surgical site infection) in favour of extending systemic warming to the perioperative period in participants undergoing major abdominal surgery.
Finally, with regard to safety, although nothing suggests that ABSW involves a significant risk to patients, the potential risks with FAW have been documented. Wood 2014 reports that FAW contaminates ultra‐clean air ventilation which, in theory, would be associated with an increased risk of surgical site infection. Future studies should therefore explore this risk, at least in situations where contamination of the operative field may be critical.
Overall completeness and applicability of evidence
The available data on important clinical outcomes (other than temperature, which has been extensively reported in previous reviews) that are of major interest for clinicians and patients are still very sparse, limiting the possibility of reaching firm conclusions. We had expected that, after the publication of the extensive NICE guideline (NICE 2008), new studies in this area would provide evidence on these outcomes, but this has not been the case. The exception is the interest in measuring thermal comfort and shivering, two patient‐centred outcomes that, while important, are of secondary clinical importance in relation to other outcomes that are directly related to the effects of hypothermia on the wound, the risk of cardiovascular events or exposure to allogenic transfusion. Nevertheless, it seems there is a widespread belief that keeping temperature within the normal range (preventing unintended hypothermia) during surgery is also beneficial to patients. This is a very reasonable pathophysiological hypothesis that has not been clearly demonstrated in a consistent manner by well‐designed clinical trials with blinded assessment of outcomes and sufficiently powered to detect clinically important differences. Furthermore, these studies need to be fairly large in order to detect the beneficial effect of the specific ABSW component in terms of the aforementioned clinical outcomes. It is possible that these studies will not be conducted for practical reasons, especially the comparison between ABSW and a control group without an active warming system. In addition, since the clinical consequences of hypothermia may be due to factors that can be controlled by a variety of diverse interventions (e.g. pharmacological, etc.), which are not covered by this review but are used as part of routine practice, it becomes very difficult to demonstrate the net effect provided the ABSW system.
The patient populations were fairly representative of people undergoing a wide range of elective surgery, with a range of anaesthetic techniques and co‐interventions aimed at heat conservation. The evidence therefore seems directly applicable to current practice.
Quality of the evidence
The quality of the evidence in the included studies was low to moderate. This was due to a moderate risk of bias and to the small number of participants and events for most patient‐important outcomes across comparisons. There were important limitations regarding the consistency of results for some of the outcomes. We found high heterogeneity for some outcomes, suggesting that the clinical and methodological variability observed in the included studies influenced the effects of ABSW. Indirectness was not an issue. We did not detect publication bias.
Potential biases in the review process
We followed the guidance from theCochrane Handbook for Systematic Reviews of Interventions for this review. We attempted to minimize bias in a number of ways; two authors assessed eligibility for inclusion, carried out data extraction and assessed risks of bias. Each pair of authors worked independently. There were no language, publication status, or sample size restrictions. However, there may be many unpublished trials and we are unable to assess publication bias properly because of the inclusion of a small number of trials for most comparisons and outcomes in this review.
Agreements and disagreements with other studies or reviews
The results of this review do not contradict the findings or the recommendations established by the existing guidance on the subject (NICE 2008). The NICE guideline concluded in favour of the use of forced‐air warming (FAW) and the results of our review, although not adding much conclusive evidence in terms of clinical outcomes, does not change the import of the current guidelines.
Authors' conclusions
Implications for practice.
Forced‐air warming (FAW), applied in the surgical pre‐ or intraoperative phases or both, seems to have a beneficial effect in terms of a lower rate of surgical site infection and complications, at least in people undergoing abdominal surgery with risk of infection, compared to not applying any active warming system. Intraoperative FAW also seems to have a beneficial effect in terms of lower rates of major cardiovascular complications when applied to people with documented substantial cardiovascular risk. It also improves patient comfort, as it maintains the core temperature within the normal range (data not shown but supported by many other reviews and reports focused on the effect on temperature, such as NICE 2008). The effects on blood loss and transfusion are not clear, as the observed reduction in blood loss does not result in a reduction in transfusion rates. The evidence for other types of active body surface warming systems (ABSW) is scant.. In this review, we have not considered the costs associated with each ABSW system, which could be a determining factor when deciding the method that should be used to maintain normothermia.
Implications for research.
There is still a need for larger studies of high quality and focused on clinically relevant outcomes. Although it is unlikely that these studies will be conducted to compare active body surface warming systems (ABSW) versus control, mainly for practical and ethical reasons, it is possible that future studies comparing different types of active warming systems (whether cutaneous or not) will emerge. These studies should undertake a blinded assessment of the outcomes, ideally incorporating a cost‐effectiveness analysis and rigorously assessing the potential risks of the medical devices. In addition, the reporting should be improved by adhering to CONSORT standards (Schulz 2010), as well as to the TIDieR guideline for better reporting of non‐pharmacological interventions (Hoffmann 2014).
Acknowledgements
We would like to acknowledge Andrew Smith (content editor), Nathan Pace (statistical editor), David Leaper, Kate Leslie (peer reviewers), Yusra Badr, Sara Yaron, and Janet Wale (representatives of the Cochrane Consumer Network) for their help and editorial advice during the preparation of the protocol for the review.
We also want to thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia Review Group (CARG)), Karen Hovhannisyan (Trials Search Co‐ordinator, CARG) for their assistance, and Kate Cahill, who copyedited our work.
In addition, we acknowledge Sonia Cibrián (SC), Fritz E Gempeler, Betina Nishishinya (BN), Ekaterina Popova (EP), Robin WM Vernooij, and Carlos Álvarez for their help at various stages in the process of the review (screening references, extracting data, or commenting on the drafts).
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Body Temperature, this term only #2 MeSH descriptor Heating, this term only #3 MeSH descriptor Rewarming explode all trees #4 (Active warming system*) or ((Mattress* or blanket*) near (warm water or Electric)) or Forced‐air warming or (((Intravenous or irrigation) near fluid*) and warming) or (CO2 near warming) or (an?esthetic near warming) or ((thermal or temperature) near manag*) or (warming or blanket*):ti,ab #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Surgical Procedures, Operative, this term only #7 MeSH descriptor Operating Rooms explode all trees #8 MeSH descriptor Recovery Room explode all trees #9 ((operat* or recovery) near room*):ti,ab #10 MeSH descriptor General Surgery, this term only #11 MeSH descriptor Intraoperative Complications explode all trees #12 MeSH descriptor Postoperative Complications, this term only #13 MeSH descriptor Preoperative Care explode all trees #14 MeSH descriptor Postoperative Care explode all trees #15 MeSH descriptor Intraoperative Care explode all trees #16 ((operat* or surg*) near complic*):ti,ab or (surg* or operat*):ti,ab or (post?operativ* or pre?operativ* or peri?operativ*):ab #17 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18 (#5 AND #17) #19 BairHugger OR Bair Hugger OR ThermaCare OR Gaymar OR Optisan OR WarmAir OR FilteredFlow OR WarmTouch OR CareDrape OR Life‐Air OR Snuggle Warm OR Warm‐Gard #20 (#18 OR #19)
Appendix 2. MEDLINE (Ovid SP) search strategy
1. Heating/ or Body Temperature/ or Rewarming/ 2. (Active warming system* or ((Mattress* or blanket*) adj3 (warm water or Electric)) or Forced‐air warming or (((Intravenous or irrigation) adj3 fluid*) and warming) or (CO2 adj6 warming) or (an?esthetic adj6 warming) or ((thermal or temperature) adj3 manag*)).mp. or (warming or blanket*).ti,ab. 3. 1 or 2 4. Surgical Procedures, Operative/ or General Surgery/ or exp Postoperative Complications/ or exp Intraoperative Complications/ or exp Perioperative Care/ or exp Postoperative Care/ or exp Preoperative Care/ 5. ((operat* or recovery) adj3 room*).ti. or ((operat* or surg*) adj3 complic*).ab. or (surg* or operat*).ab. or (post?operativ* or pre?operativ* or peri?operativ*).ab. 6. 4 or 5 7. 6 and 3 8. (BairHugger or Bair Hugger or ThermaCare or Gaymar or Optisan or WarmAir or FilteredFlow or WarmTouch or CareDrape or Life‐Air or Snuggle Warm or Warm‐Gard).mp. 9. 8 or 7 10. ((randomiserandomised controlled trial or controlled clinical trial).pt. or randomiserandomised.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 11. 10 and 9
Appendix 3. EMBASE (Ovid SP) search strategy
1. (Active warming system* or ((Mattress* or blanket*) adj3 (warm water or Electric)) or Forced‐air warming or (((Intravenous or irrigation) adj3 fluid*) and warming) or (CO2 adj6 warming) or (an?esthetic adj6 warming) or ((thermal or temperature) adj3 manag*)).mp. or (warming or blanket*).ti,ab. 2. exp warming/ or heating/ or body temperature/ 3. 1 or 2 4. ((operat* or recovery) adj3 room*).ti,ab. 5. ((operat* or surg*) adj3 complic*).mp. or (surg* or operat*).ti,ab. or ((post?operativ* or pre?operativ* or peri?operativ*) adj3 complicat*).mp. 6. surgery/ or operating room/ or recovery room/ or exp perioperative complication/ or exp postoperative complication/ or exp preoperative care/ or exp postoperative care/ 7. 6 or 4 or 5 8. 3 and 7 9. (BairHugger or Bair Hugger or ThermaCare or Gaymar or Optisan or WarmAir or FilteredFlow or WarmTouch or CareDrape or Life‐Air or Snuggle Warm or Warm‐Gard).mp. 10. 8 or 9 11. ((((singl* or doubl* or tripl*) adj3 blind) or crossover).ti,ab. or multicenter.ab. or placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animal not (human and animal)).sh. 12. 11 and 10
Appendix 4. CINAHL (EBSCOhost) search strategy
S1 (MH "Body Temperature") or (MH "Core Body Temperature") S2 (MH "Heating") S3 (MH "Warming Techniques") or (MM "Hypothermia Treatment (Iowa NIC)") S4 TX Active warming system* or TX ( ((Mattress* or blanket*) and (warm water or Electric)) ) or TX Forced‐air warming or TX ( (((Intravenous or irrigation) and fluid*) and warming) ) or TX CO2 N3 warming or TX an?esthetic N3 warming or TX ( ((thermal or temperature) and manag*) ) or TI (warming or blanket* ) or AB ( warming or blanket* ) S5 S1 or S2 or S3 or S4 S6 (MH "Surgery, Operative") or (MH "Operating Rooms") S7 (MH "Post Anesthesia Care Units") or (MH "Patients' Rooms") S8 TI ( (operat* or recovery) and room* ) or AB ( (operat* or recovery) and room* ) S9 (MH "Postoperative Complications+") S10 AB ( post?operativ* or pre?operativ* or peri?operativ* ) or AB ( surg* or operat* ) or TI ( surg* or operat*) or TX ( (operat* or surg*) and complic* ) S11 S6 or S7 or S8 or S9 or S10 S12 S5 and S11 S13 TX BairHugger OR Bair Hugger OR ThermaCare OR Gaymar OR Optisan OR WarmAir OR FilteredFlow OR WarmTouch OR CareDrape OR Life‐Air OR Snuggle Warm OR Warm‐Gard S14 S12 or S13 S15 (MM "Random Assignment") S16 MH "Clinical Trials+" S17 (MM "Placebos") or (MM "Multicenter Studies") or (MM "Crossover Design") S18 (MM "Double‐Blind Studies") or (MM "Single‐Blind Studies") or (MM "Triple‐Blind Studies") S19 AB random* or TI trail* or AB ( placebo* or mulicenter or crossover ) S20 AB ( (double or single or triple) and (blind* or mask*) ) or TI ( (double or single or triple) and (blind* or mask*)) S21 S15 or S16 or S17 or S18 or S19 or S20 S22 S14 and S21
Appendix 5. Study Selection Form
Study identification
First author:
Title:
Journal:
Year:
Volume:
Number:
Pages:
Study eligibility
1.‐ Randomised controlled trial (RCT)? YES /NO
2.‐ Assessing an (pre)intra‐operative warming system (IWS) aimed at reducing heat loss and prevent hypothermia? YES/NO
3.‐ Compasion of interest? YES/NO
Comparison type num. 1: AWBS versus CTRL (no intervention / sham)
Tick the AWBS used in the intervention group:
Infrared light
Electric blankets
Warm water circulation systems
Forced‐air warming systems
Other (specify)
Comparison type num. 2 & 3: ABSW (1) versus ABSW (2)
Tick both ABSW being compared:
Infrared light
Electric blankets
Warm water circulation systems
Forced‐air warming systems
Other (specify)
4.‐ Patients undergoing a scheduled major or conventional surgery? YES/ NO
5.‐ Assessing a relevant outcome? YES/NO
Primary:
Infection and complications of the surgical wound (wound healing and dehiscence)
Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke and non‐fatal cardiac arrest)
All cause mortality
Secondary:
Transfusions (number of patients transfused; blood product usage
Blood loss (mL)
Intraoperative fluids infused (mL)
Other cardiovascular complications (bradycardia, hypotension, arrhythmias)
Patient reported outcomes (anxiety, thermal comfort, pain)
Shivering (number of patients)
Pressure sores and ulcers
Adverse effects (including thermal burns)
Final decision on eligibility:
INCLUDED
To be included the answer to all items should be YES
EXCLUDED
AWAITING ASSESSMENT
Appendix 6. Data Extraction Form
Study identification
First author:
Title:
Journal:
Year:
Volume:
Number:
Pages:
Specify if there are other papers providing relevant information on methods and/or results related to this same study:
Interventions:
| Intervention | Duration | |
| Intervention group |
|
|
| Control group |
Elegibility criteria
Inclusion criteria:
Exclusion criteria:
Outcomes
Tick all relevant outcomes measured by the RCT:
-
Primary:
Infection and complications of the surgical wound (wound healing and dehiscence)
Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke and non‐fatal cardiac arrest)
All cause mortality
-
Secondary:
Transfusions (number of patients transfused; blood product usage)
Blood loss (mL)
Intraoperative fluids infused (mL)
Other cardiovascular complications (bradycardia, hypotension, arrhythmias)
Patient reported outcomes (anxiety, thermal comfort, pain)
Shivering (number of patients)
Pressure sores and ulcers
Adverse effects (including thermal burns)
Other (specify): ________________________________________________
Study quality assessment(risk of bias)
1.‐ How was the treatment allocation schedule generated?
Computer generated sequence, random number tables, lot drawing, coin tossing, shuffling cards, throwing dice(A)
Case number, date of birth, date of Admission, alternation (B)
Unclear, other(C)
Notes:
2.‐ Was the allocation sequence properly concealed?
Central randomization, coded drug boxes, sealed opaque envelopes(A)
Open allocation sequence, procedures based on inadequate generation(B)
Unclear, other(C)
Notes:
3.‐ To what extent the study groups were comparable in terms of prognostic factors at base‐line?
Notes:
4.‐ Description of losses and drop‐outs and its causes:
Are the losses and drop‐out properly described?
Number of patients randomized
Number of patients analysed
Verify the number of losses and drop‐outs for each outcome
Have the causes of losses and drop‐out been described?
Specify:
Notes:
5.‐ Are co‐interventions comparable in both groups? YES/NO
Notes:
6.‐ Was there any attempt to assess the outcomes in a blinded manner? YES/NO
Notes:
Summary of risk of bias assessment:
(A)LOW risk of bias
(B) MODERATE risk of bias
(C) HIGH risk of bias
Risk of bias Table
| Item | Judgment | Description |
|
Adequate sequence generation? |
||
|
Allocation concealment? |
||
|
Blinding? |
||
|
Incomplete outcome data addressed? |
||
|
Free of selective reporting? |
Outcome Data: All Patients Group
| |
Outcome measure (Binary) |
Control group | Experimental group | |||||
| Events | Total patients | Events | Total patients | |||||
| Primary outcomes | 1 | Wound infection | ||||||
| 2 | Other complications of surgical wound | |||||||
| 3 | All cause mortality | |||||||
| 4 | Major cardiac events (specify) | |||||||
| Secindary outcomes | 5 | Transfussions (Num. patients) | ||||||
| 6 | Other cardiovascular complications | |||||||
| 7 | Burns | |||||||
| 8 | Pressure ulcers | |||||||
| 9 | Shivering (Num. patients) | |||||||
| OTHER: _________________________________ | ||||||||
|
Ouctome measures (Continous) |
Mean | SD | Total patients | Mean | SD | Total patients | ||
| 10 | Blood transfused intra & postoperative (<48h) (mL) | |||||||
| 11 | Blood transfused (Units) | |||||||
| 12 | Fluids (IV) infused (mL) | |||||||
| 13 | Shivering (score) | |||||||
| 14 | Thermal comfort (score) | |||||||
| 15 | Pain (score) | |||||||
| OTHER: _________________________________ | ||||||||
| Central temperature at the beginning of surgery | ||||||||
| at 30 min | ||||||||
| at 60 min | ||||||||
| at 90 min | ||||||||
| at 120 min | ||||||||
| at 150 min | ||||||||
| at 180 min | ||||||||
| at the end of surgery | ||||||||
| Peripheral temperature at the beginning of surgery | ||||||||
| at 30 min | ||||||||
| at 60 min | ||||||||
| at 90 min | ||||||||
| at 120 min | ||||||||
| at 150 min | ||||||||
| at 180 min | ||||||||
| at the end of surgery | ||||||||
Characteristics of the study
| Study identification | |
| Methods | Design: Follow‐up: Withdrawals: Setting: N Total: Funding: |
| Intervention (ABSW) (n =) (describe) |
Timing: Preop / Intraop / Postop |
| Control (n =) (describe) |
Timing: Preop / Intraop / Postop |
| Co‐interventions Room temperature (describe) |
|
| Patient’s temperature measurement (techniques) |
|
| Participants | Age: Gender: Type of surgery: Duration of surgery: Type of anaesthesia: ASA grade (1‐4): |
| Grades of surgery: |
Minor / Intermediate / Major / Major plus |
| Outcomes | |
| Notes | Power calculation |
Data and analyses
Comparison 1. Active warming vs control (no active warming).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection and complications | 3 | 589 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.66] |
| 2 All‐cause mortality | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.26, 4.00] |
| 3 Blood transfusions during surgery and up to 48 hours post‐surgery (ml) | 8 | 779 | Mean Difference (IV, Random, 95% CI) | ‐54.58 [‐92.57, ‐16.58] |
| 4 Participants transfused | 8 | 621 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.50, 1.23] |
| 5 Blood loss (ml) | 20 | 1372 | Mean Difference (IV, Random, 95% CI) | ‐46.17 [‐82.74, ‐9.59] |
| 5.1 Forced‐air warming | 18 | 1103 | Mean Difference (IV, Random, 95% CI) | ‐50.77 [‐88.43, ‐13.10] |
| 5.2 Foam warming pad | 1 | 153 | Mean Difference (IV, Random, 95% CI) | 5.09 [‐64.78, 74.96] |
| 5.3 Warm water mattress | 1 | 116 | Mean Difference (IV, Random, 95% CI) | ‐100.0 [‐389.17, 189.17] |
| 6 Fluids infused (ml) | 24 | 1491 | Mean Difference (IV, Random, 95% CI) | ‐144.49 [‐221.57, ‐67.40] |
| 6.1 Forced‐air warming | 21 | 1337 | Mean Difference (IV, Random, 95% CI) | ‐139.83 [‐220.17, ‐59.48] |
| 6.2 Electric blanket | 2 | 38 | Mean Difference (IV, Random, 95% CI) | ‐243.00 [‐732.80, 246.80] |
| 6.3 Warm Water mattress | 1 | 116 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐239.12, 239.12] |
| 7 Participant's thermal comfort (higher values mean higher comfort) | 4 | 364 | Std. Mean Difference (IV, Random, 95% CI) | 0.76 [0.29, 1.24] |
| 8 Participant's thermal sensation (higher values mean 'insufferably hot') | 6 | 336 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐0.61, 2.87] |
| 9 Pain < 12 hours | 7 | 624 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐1.12, 0.64] |
| 10 Chills/shivering | 29 | 1922 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.28, 0.54] |
| 10.1 Forced‐air warming | 26 | 1866 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.31, 0.59] |
| 10.2 Electric blanket | 3 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.03, 0.39] |
Comparison 2. Forced‐air warming vs electric and resistive heating systems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss during surgery and up to 48 hours post‐surgery (ml) | 5 | 270 | Mean Difference (IV, Random, 95% CI) | ‐3.68 [‐55.43, 48.07] |
| 2 Fluids infused (ml) | 3 | 184 | Mean Difference (IV, Random, 95% CI) | 101.59 [‐14.67, 217.85] |
| 3 Participant's comfort (thermal) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.21, 0.35] |
| 4 Chills/shivering | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.30, 5.74] |
Comparison 3. Forced‐air warming vs warm water circulation systems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 3 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.62, 14.53] |
| 2 Participants transfused | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.48, 5.24] |
| 3 Blood transfusions | 3 | 144 | Mean Difference (IV, Random, 95% CI) | 136.21 [‐119.10, 391.51] |
| 4 Blood loss (ml) | 6 | 345 | Mean Difference (IV, Random, 95% CI) | 9.39 [‐196.71, 215.50] |
| 5 Fluids infused (ml) | 4 | 230 | Mean Difference (IV, Random, 95% CI) | ‐215.11 [‐519.20, 88.99] |
| 6 Chills/shivering | 3 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.17, 19.64] |
3.2. Analysis.
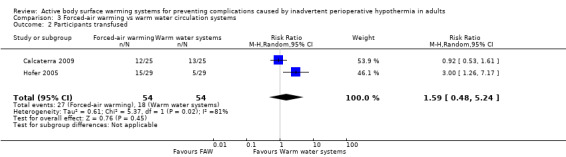
Comparison 3 Forced‐air warming vs warm water circulation systems, Outcome 2 Participants transfused.
Comparison 4. Forced‐air warming vs radiant heat.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chills/shivering | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.25, 6.08] |
Comparison 5. Active vs no active (subgroup analysis by anaesthesia type).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection and complications | 2 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.66] |
| 1.1 General | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.74] |
| 1.2 Not stated | 1 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.19, 0.92] |
| 2 Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, and non‐fatal cardiac arrest) | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 1.00] |
| 3 Non‐fatal myocardial infarction | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.03] |
| 4 Non‐fatal cardiac arrest | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 1.00] |
| 5 Blood transfusions during surgery and up to 48 hours post‐surgery (mL) | 5 | 404 | Mean Difference (IV, Random, 95% CI) | ‐35.82 [‐82.87, 11.23] |
| 5.1 General | 4 | 354 | Mean Difference (IV, Random, 95% CI) | ‐37.35 [‐88.35, 13.66] |
| 5.2 Spinal | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐25.0 [‐240.82, 190.82] |
| 6 Participants transfused | 6 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 6.1 General | 5 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.44, 1.46] |
| 6.2 Spinal | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.70, 1.89] |
| 7 Blood loss during surgery ‐ mL | 16 | 963 | Mean Difference (IV, Random, 95% CI) | ‐57.02 [‐97.69, ‐16.35] |
| 7.1 General | 9 | 448 | Mean Difference (IV, Random, 95% CI) | ‐80.71 [‐147.89, ‐13.53] |
| 7.2 Spinal | 4 | 160 | Mean Difference (IV, Random, 95% CI) | 34.34 [‐22.95, 91.63] |
| 7.3 Combined | 2 | 325 | Mean Difference (IV, Random, 95% CI) | ‐129.95 [‐144.78, ‐115.11] |
| 7.4 Unknown | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐56.0 [‐160.52, 48.52] |
| 8 Fluids transfused during surgery ‐ mL | 17 | 1079 | Mean Difference (IV, Random, 95% CI) | ‐178.22 [‐275.00, ‐79.44] |
| 8.1 General | 11 | 589 | Mean Difference (IV, Random, 95% CI) | ‐201.50 [‐364.99, ‐38.01] |
| 8.2 Spinal | 4 | 160 | Mean Difference (IV, Random, 95% CI) | ‐43.94 [‐187.94, 100.07] |
| 8.3 Combined | 1 | 300 | Mean Difference (IV, Random, 95% CI) | ‐198.00 [‐237.37, ‐162.63] |
| 8.4 Unknown | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐448.0 [‐701.78, ‐194.22] |
| 9 Participant's anxiety and state | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.57, 1.37] |
| 10 Participant's comfort (thermal) (higher values mean higher comfort) | 10 | 700 | Std. Mean Difference (IV, Random, 95% CI) | 0.77 [0.19, 1.36] |
| 10.1 General | 4 | 431 | Std. Mean Difference (IV, Random, 95% CI) | 0.92 [‐0.18, 2.01] |
| 10.2 Spinal | 4 | 110 | Std. Mean Difference (IV, Random, 95% CI) | 0.68 [‐0.32, 1.69] |
| 10.3 Combined | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | 0.78 [0.02, 1.54] |
| 10.4 Unknown | 1 | 130 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.11, 0.81] |
| 11 Pain | 5 | 214 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.71, 0.82] |
| 11.1 General | 2 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.51, 0.39] |
| 11.2 Spinal | 2 | 59 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐2.01, 2.11] |
| 11.3 Combined | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.35, 3.35] |
| 12 Chills/shivering | 25 | 1466 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.25, 0.53] |
| 12.1 General | 15 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.17, 0.48] |
| 12.2 Spinal | 7 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.33, 0.96] |
| 12.3 Combined | 2 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.13, 0.48] |
| 12.4 Unknown | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 All cause mortality | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.26, 4.00] |
| 13.1 General | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.13, 6.42] |
| 13.2 Spinal | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.16, 7.80] |
5.2. Analysis.
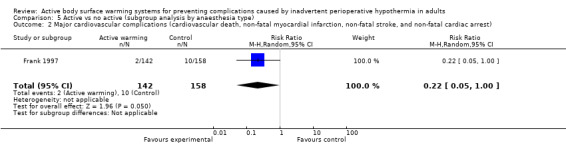
Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 2 Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, and non‐fatal cardiac arrest).
5.3. Analysis.
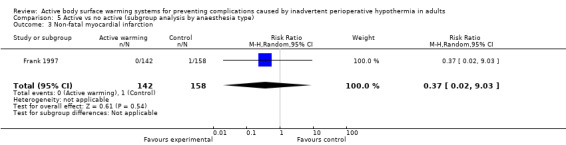
Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 3 Non‐fatal myocardial infarction.
5.4. Analysis.
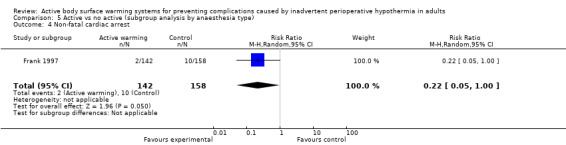
Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 4 Non‐fatal cardiac arrest.
5.5. Analysis.
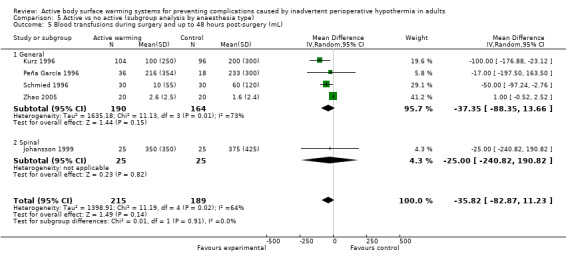
Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 5 Blood transfusions during surgery and up to 48 hours post‐surgery (mL).
5.6. Analysis.
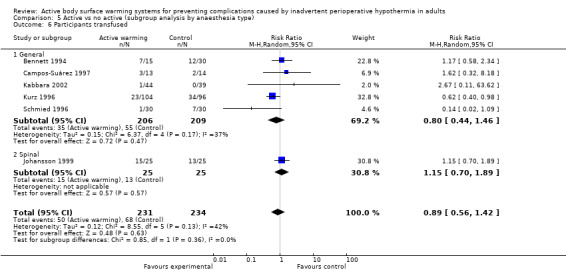
Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 6 Participants transfused.
5.7. Analysis.
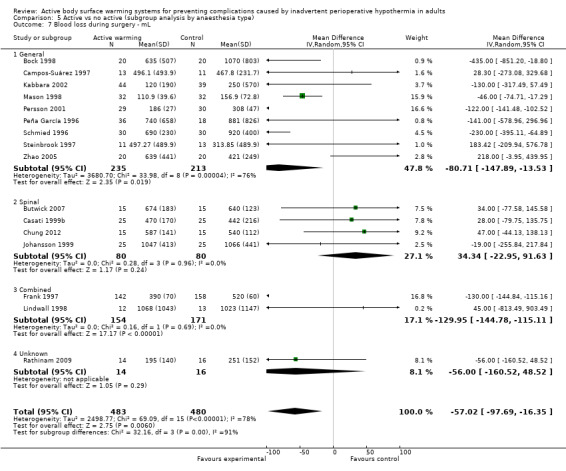
Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 7 Blood loss during surgery ‐ mL.
5.8. Analysis.
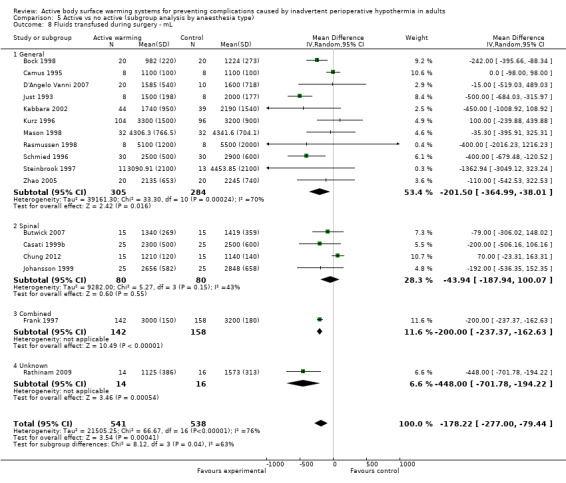
Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 8 Fluids transfused during surgery ‐ mL.
5.9. Analysis.
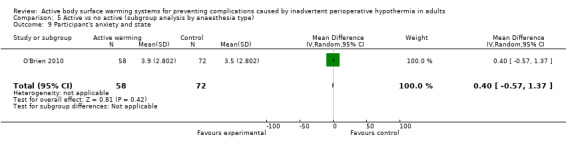
Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 9 Participant's anxiety and state.
5.10. Analysis.
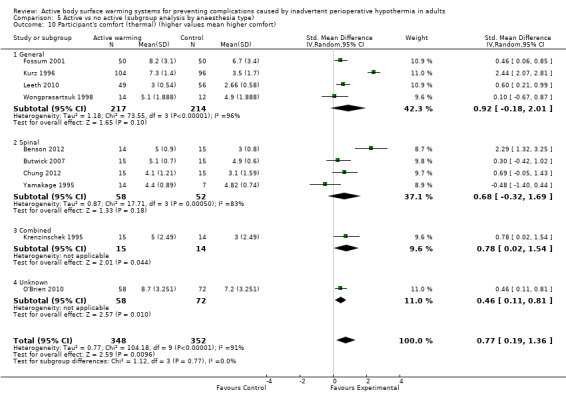
Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 10 Participant's comfort (thermal) (higher values mean higher comfort).
5.11. Analysis.

Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 11 Pain.
5.12. Analysis.
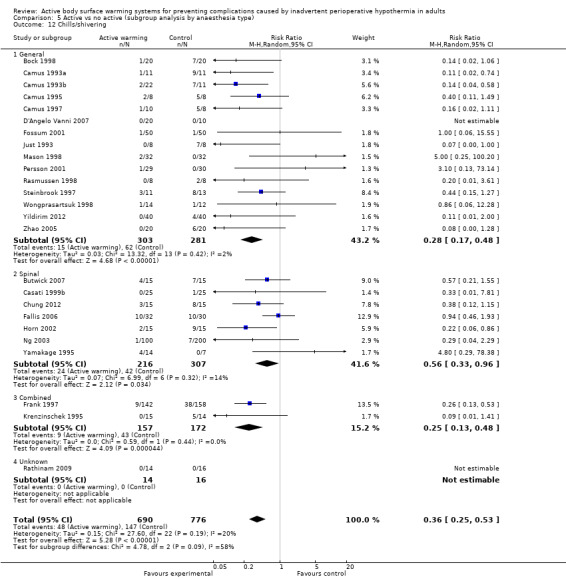
Comparison 5 Active vs no active (subgroup analysis by anaesthesia type), Outcome 12 Chills/shivering.
Comparison 6. Active vs no active (subgroup analysis by timing of intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection and complications of the surgical wound | 2 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.66] |
| 1.1 Intraoperative | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.74] |
| 1.2 Preoperative | 1 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.19, 0.92] |
| 2 Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, and non‐fatal cardiac arrest) | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 1.00] |
| 3 Non‐fatal myocardial infarction | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.03] |
| 4 Non‐fatal cardiac arrest | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 1.00] |
| 5 Blood transfusions during surgery and up to 48 hours post‐surgery (mL) | 5 | 404 | Mean Difference (IV, Random, 95% CI) | ‐35.82 [‐82.87, 11.23] |
| 6 Participants transfused | 6 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 7 Blood loss during surgery ‐ mL | 16 | 963 | Mean Difference (IV, Random, 95% CI) | ‐57.02 [‐97.69, ‐16.35] |
| 7.1 Intraoperative | 14 | 893 | Mean Difference (IV, Random, 95% CI) | ‐66.70 [‐106.62, ‐26.79] |
| 7.2 Pre + Intraoperative | 2 | 70 | Mean Difference (IV, Random, 95% CI) | ‐149.47 [‐613.68, 314.75] |
| 8 Fluids transfused during surgery ‐ mL | 17 | 1079 | Mean Difference (IV, Random, 95% CI) | ‐178.22 [‐275.00, ‐79.44] |
| 8.1 Intraoperative | 12 | 947 | Mean Difference (IV, Random, 95% CI) | ‐205.29 [‐287.91, ‐122.67] |
| 8.2 Preoperative | 2 | 32 | Mean Difference (IV, Random, 95% CI) | ‐243.68 [‐733.52, 246.15] |
| 8.3 Pre + Intraoperative | 3 | 100 | Mean Difference (IV, Random, 95% CI) | ‐68.07 [‐323.57, 187.43] |
| 9 Participant's anxiety and state | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.57, 1.37] |
| 10 Participant's comfort (thermal) (higher values mean higher comfort) | 10 | 700 | Std. Mean Difference (IV, Random, 95% CI) | 0.77 [0.19, 1.36] |
| 10.1 Intraoperative | 5 | 410 | Std. Mean Difference (IV, Random, 95% CI) | 0.73 [‐0.35, 1.82] |
| 10.2 Preoperative | 2 | 205 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [0.25, 0.81] |
| 10.3 Pre + Intraoperative | 3 | 85 | Std. Mean Difference (IV, Random, 95% CI) | 0.99 [‐0.18, 2.17] |
| 11 Pain | 5 | 214 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.71, 0.82] |
| 11.1 Intraoperative | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.35, 3.35] |
| 11.2 Preoperative | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.02, 0.42] |
| 11.3 Pre + Intraoperative | 3 | 85 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐1.04, 1.18] |
| 12 Chills/shivering | 25 | 1466 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.25, 0.53] |
| 12.1 Intraoperative | 17 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.22, 0.63] |
| 12.2 Preoperative | 3 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.10, 1.17] |
| 12.3 Pre + Intraoperative | 5 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.14, 0.63] |
| 13 All‐cause mortality | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.26, 4.00] |
6.2. Analysis.
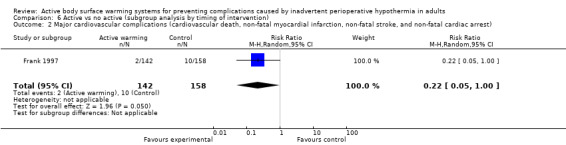
Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 2 Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, and non‐fatal cardiac arrest).
6.3. Analysis.
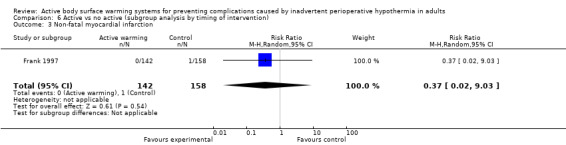
Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 3 Non‐fatal myocardial infarction.
6.4. Analysis.
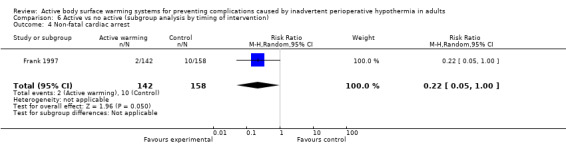
Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 4 Non‐fatal cardiac arrest.
6.5. Analysis.
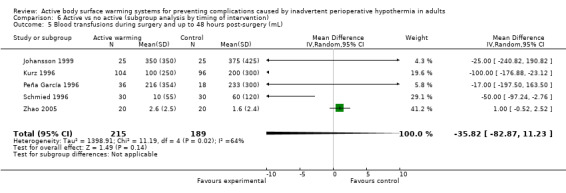
Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 5 Blood transfusions during surgery and up to 48 hours post‐surgery (mL).
6.6. Analysis.
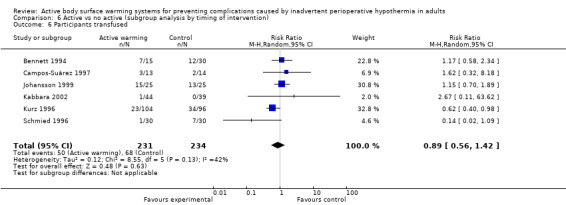
Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 6 Participants transfused.
6.7. Analysis.
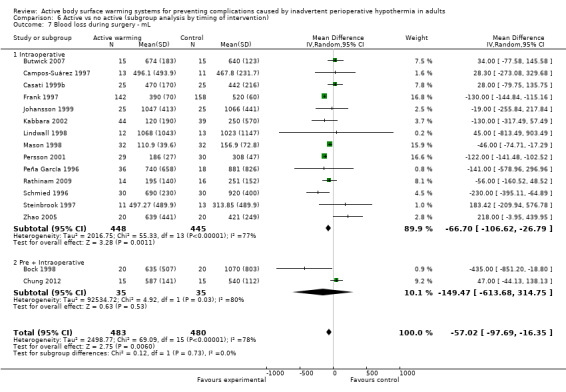
Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 7 Blood loss during surgery ‐ mL.
6.8. Analysis.
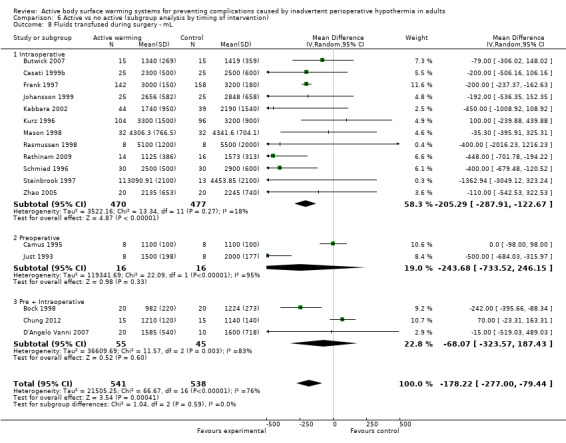
Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 8 Fluids transfused during surgery ‐ mL.
6.9. Analysis.
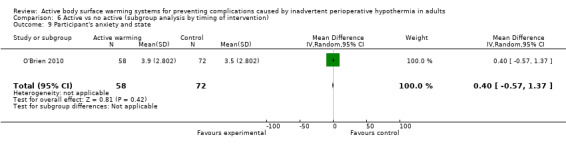
Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 9 Participant's anxiety and state.
6.10. Analysis.
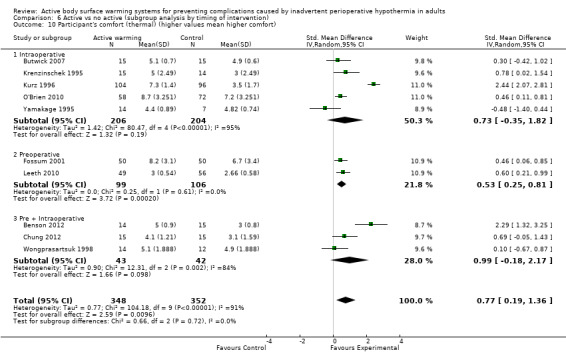
Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 10 Participant's comfort (thermal) (higher values mean higher comfort).
6.11. Analysis.

Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 11 Pain.
6.12. Analysis.

Comparison 6 Active vs no active (subgroup analysis by timing of intervention), Outcome 12 Chills/shivering.
Comparison 7. Active vs no active (sensitivity analysis by surgery duration ≥ 120 min).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection and complications of the surgical wound | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.74] |
| 2 Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, and non‐fatal cardiac arrest) | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 1.00] |
| 3 Non‐fatal myocardial infarction | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.03] |
| 4 Non‐fatal cardiac arrest | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 1.00] |
| 5 Blood transfusions during surgery and up to 48 hours post‐surgery (mL) | 3 | 294 | Mean Difference (IV, Random, 95% CI) | ‐36.25 [‐114.08, 41.58] |
| 6 Participants transfused | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.50, 1.44] |
| 7 Blood loss during surgery ‐ mL | 8 | 577 | Mean Difference (IV, Random, 95% CI) | ‐63.25 [‐133.05, 6.55] |
| 8 Fluids transfused during surgery ‐ mL | 8 | 720 | Mean Difference (IV, Random, 95% CI) | ‐234.63 [‐354.78, ‐114.49] |
| 9 Participant's comfort (thermal) (higher values mean higher comfort) | 2 | 226 | Std. Mean Difference (IV, Random, 95% CI) | 1.30 [‐0.99, 3.59] |
| 10 Pain | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.48, 0.68] |
| 11 Chills/shivering | 9 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.12, 0.51] |
| 12 All‐cause mortality | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.26, 4.00] |
7.2. Analysis.
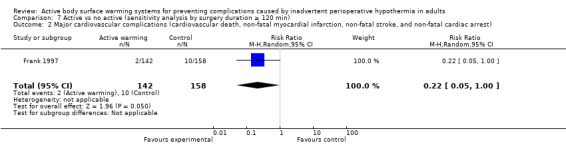
Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 2 Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, and non‐fatal cardiac arrest).
7.3. Analysis.
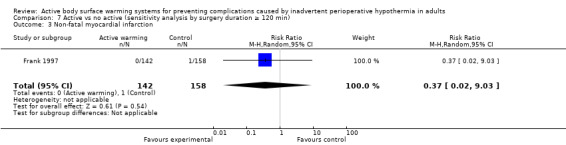
Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 3 Non‐fatal myocardial infarction.
7.4. Analysis.
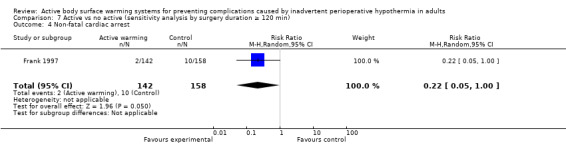
Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 4 Non‐fatal cardiac arrest.
7.5. Analysis.
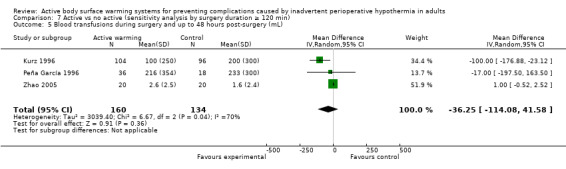
Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 5 Blood transfusions during surgery and up to 48 hours post‐surgery (mL).
7.6. Analysis.
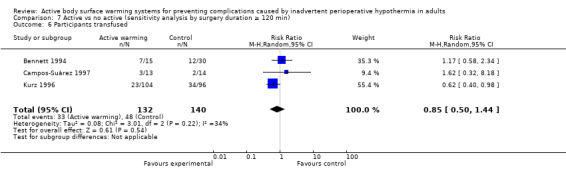
Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 6 Participants transfused.
7.7. Analysis.

Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 7 Blood loss during surgery ‐ mL.
7.8. Analysis.
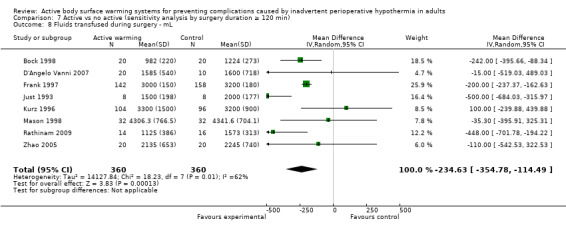
Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 8 Fluids transfused during surgery ‐ mL.
7.9. Analysis.
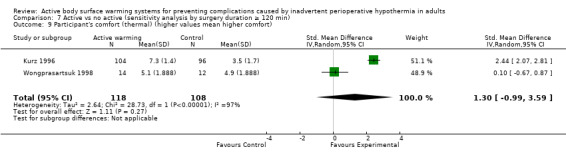
Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 9 Participant's comfort (thermal) (higher values mean higher comfort).
7.10. Analysis.
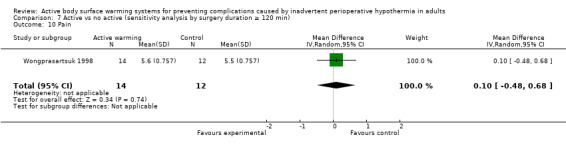
Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 10 Pain.
7.11. Analysis.
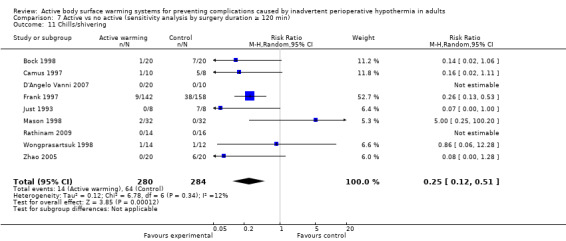
Comparison 7 Active vs no active (sensitivity analysis by surgery duration ≥ 120 min), Outcome 11 Chills/shivering.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrzejowski 2008.
| Methods | Design: RCT Operative phase: pre‐ and intraoperative Withdrawals: none Setting: single centre (UK) Sample size: 68 Funding: Arizant Healthcare UK provided the Bair Paws System and disposables for use in the trial |
|
| Participants | Age (mean): 54/57 years Gender (M/F): 45/23 ASA grade: I ‐ II Surgery type: elective (spinal surgery) Surgery duration (mean): > 2 hrs (131/138 min) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSWa): n = 31 Forced‐air warming (Bair Paws®): prewarming (60 min) and intraoperative (temperature set at 38ºC) Intervention (ABSWb): n = 37 Forced‐air warming (Bair Paws®): intraoperative (temperature set at 38ºC) Body area covered: full body blanket was used for participants having cervical spine surgery and a surgical access warming blanket for those undergoing lumbar spine surgery Co‐interventions: not stated Room temperature: 20.7ºC /20.9ºC |
|
| Outcomes | Shivering Fluids infused |
|
| Notes | Comparison 3 (FAW pre+intraoperative vs FAW intraoperative) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8 participants lost due to surgery cancellation |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | |
Bennett 1994.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (UK) Sample size: 45 Funding: Augustine Medical donated the Bair Hugger blankets and Mallinkrodt the Mono‐therm temperature probes |
|
| Participants | Age (mean): 71 years (range 59 – 88) Gender (M/F): 30/15 ASA grade: not stated Surgery type: elective (hip arthroplasty) Surgery duration (mean): > 2 hrs (2 ‐ 2½ hrs) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 15 Convective warm air blanket (Bair Hugger®, Augustine Medical, USA) Duration: after induction until end of surgery Temperature (max.) at 43°C Body area covered: spread over the trunk, upper limbs and head Control 1: n = 15 Metallized plastic garment (Thermolire®, Techstyles, USA) Duration: after induction until end of surgery Body area covered: head, upper limbs, exposed part of trunk and the non‐operated lower limb Control 2: n = 15 "No form of intraoperative warming" Co‐interventions: all participants received an IV infusion of Hartmann's solution (at ambient temperature) at a rate of 6ml/ Kg/1h. Blood was warmed to 37ºC before infusion Room temperature: 19° to 21ºC |
|
| Outcomes | Blood transfusions (ml) N of participants transfused (The paper states that "no complications with FAW were observed") |
|
| Notes |
Comparison 1 The two control groups have been merged in the analysis leaving 1 single comparison |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | |
Benson 2012.
| Methods | Design: RCT Operative phase: pre‐ and intraoperative Withdrawals: 1 (not informed) Setting: 1 centre (Canada) Sample size: 30 Funding: grant from the Fort Garry Branch Royal Canadian Legion Poppy |
|
| Participants | Age (mean): 68.0/68.5 years Gender (M/F): 12/18 ASA grade: I ‐ III Surgery type: elective (total knee arthroplasty) Surgery duration (mean): < 2 hrs (60.1/61.9 mins anaesthesia duration) Anaesthesia type: spinal |
|
| Interventions |
Intervention (ABSW): n = 15 Forced‐air warming gown connected to a portable warming unit capable of generating up to 1000 BTU per hr (Bair Paws® patient adjustable Warming System, Arizant HealthCare, Eden Prairie, MN. Gown model 81001, unit model 875) Temperature (max.) at 43ºC Duration: perioperative Body area covered: not stated Control: n = 15 Hospital gown and prewarmed standard cotton blanket Duration: perioperative Control body area covered: spread over the trunk, upper limbs and head * Note: Each group retained the same warming method throughout the perioperative period (defined here as from the time of preoperative preparation in the day surgery through to discharge from the PACU) Co‐interventions: not stated Room temperature: 20.5ºC |
|
| Outcomes | Postoperative pain (at 12 and 24 hours) (VNRS 0 ‐ 10) Thermal comfort (Likert 1 ‐ 5) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Lot drawing with coloured papers in a bag |
| Allocation concealment (selection bias) | High risk | There was no allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment was done at PACU, but there is no mention of blinding |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There are several outcomes with incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | |
Bock 1998.
| Methods | Design: RCT Ooperative phase: pre‐ and intraoperative Withdrawals: 1 participant (control group) Setting: 1 centre (Germany) Sample size: 40 Funding: not reported |
|
| Participants | Age (mean): 43/49 years (range 19 ‐ 78) Gender (M/F): 21/19 ASA grade: I – III Surgery type: major abdominal surgery for cancer or inflammatory bowel disease (elective?) Surgery duration: > 2 hrs (4 ‐ 4.3 hrs) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW + co‐intervention): n = 20 Forced‐air warming (WarmTouch® system) + circulating‐water mattress (as a co‐intervention) Duration: 30 minutes before induction and during anaesthesia Temperature: 40° ‐ 42ºC Area covered: arms and chest using forced air; abdomen and legs using blankets Proportion covered: ≥ 50% Control (co‐intervention): n = 20 "Passive protection against heat loss" + circulating‐water mattress (as a co‐intervention) Duration: during anaesthesia Temperature: 39°C Area covered: abdomen and legs/2 blankets; arms and chest covered with blankets Proportion covered ≥ 50% Co‐interventions: 2 layers of blankets and fluid‐warming devices Room temperature: 22ºC |
|
| Outcomes | Blood loss (ml) N of participants transfused Fluids infused (ml) Shivering (present/absent) Other outcomes reported not included in the review:
|
|
| Notes |
Comparison 1 This is the only trial in this comparison where the control group receives an ABSW as concomitant treatment (ABSW1 + ABSW2 co‐intervention vs ABSW2 co‐intervention) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blood loss and shivering were assessed by an independent anaesthetist who was not involved in the study and took care of the participant during surgery or during the participant`s stay in the PACU (simple blind) |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Co‐interventions were comparable between groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | . |
Butwick 2007.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (USA) Sample size: 30 Funding: Department of Anesthesia, Stanford University Medical Center. Dr Carvalho's work is supported by a Building Interdisciplinary Careers in Women's Health Research grant from the Office of Research on Women's Health and National Institute of Child Health and Human Development of the National Institutes of Health (5K12 HD043452) |
|
| Participants | Age: 36/32 years Gender (M/F): 0/30 ASA grade: I ‐ II Surgery type: elective caesarean section for delivery Surgery duration: < 2 hrs (41/52 mins) Anaesthesia type: spinal |
|
| Interventions |
Intervention (ABSW): n = 15 Forced‐air warming unit with lower‐body warming cover (Bair Hugger®; Augustine Medical, Eden Prairie, MN) using a model 501 Duration: not stated Temperature: 43ºC Area covered: lower body Control: n = 15 Sham forced‐air warming (identical cover applied with forced‐air warming unit switched off) Duration: not stated Temperature: not stated Area covered: lower body Co‐interventions: a warmed cotton blanket was placed over the forced‐air warming cover of participants in both study groups, and a second warmed cotton blanket was placed over the upper body with arms positioned on arm rests. Room temperature: 23ºC |
|
| Outcomes | Shivering (4‐point scale, where 0 = 'no shivering' and 4 = 'gross muscular activity involving the whole body') Pain (VNRS 0 ‐ 100) (* no data provided) Thermal comfort (VNRS, where 0 as 'worst imaginable cold', 50 as 'thermoneutral' and 100 mm as 'insufferably hot') Fluids infused (ml) Blood loss (ml) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham intervention in the control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded investigator assessed oral temperature, shivering, and thermal comfort scores at 15‐min intervals until discharge from the PACU. However, this proved difficult due to the noise the forced‐air warming unit produced when active |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants finished the study |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | |
Calcaterra 2009.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (USA) Sample size: 50 Funding: study supported by a grant from Kimberly‐Clark Inc. |
|
| Participants | Age (mean): 62.7/61.7 Gender (M/F): 30/20 ASA grade: not stated Surgery type: off‐pump CABG Surgery duration: not stated Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1): n = 25 Kimberly‐Clark® patient warming system (conductive) Duration: not stated Temperature: not stated Area covered: not stated Intervention (ABSW2): n = 25 Forced‐air warming (Bair Hugger®) Duration: not stated Temperature: not stated Area covered: not stated Co‐interventions: before admission to the operation room participants were kept warm using Bair‐Hugger®. All participants received warmed intravenous fluids during surgery Room temperature: 36ºC |
|
| Outcomes | Wound infections Blood loss ('cell saver volume') (cc) Blood products transfusions (ml) N of participants transfused Other outcomes reported not included in the review:
|
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | |
Campos‐Suárez 1997.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 3/30 (10%) Setting: 1 centre (Spain) Sample size: 30 Funding: not stated |
|
| Participants | Age (mean): 64.3/66.6 years Gender (M/F): 15/12 ASA grade: III Surgery type: elective (abdominal surgery involving laparotomy) Surgery duration (mean): > 2 hrs (235 min) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW: n = 13 Forced‐air warming system (Bair Hugger®, Augustine Medical Inc.) Duration: 235.76 mins (SD 62.9) Temperature: not stated Area covered: not stated Control: n = 14 Routine care Duration: 235.71 mins (SD 67.1) Temperature: not stated Area covered: not stated Co‐interventions: not stated Room temperature: not stated |
|
| Outcomes | Blood loss during surgery (ml) Transfusions during surgery (N of participants) Fluids infused (crystalloids; colloids) (ml) |
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were excluded because the disease could not be surgically remedied |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | |
Camus 1993a.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (France) Sample size: 22 Funding: not stated |
|
| Participants | Age (mean): 46/51 years Gender (M/F): 10/12 ASA grade: I ‐ II Surgery type: elective abdominal surgery Surgery duration (mean): > 2 hrs (185/195 mins duration of anaesthesia) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 11 Electric warming blanket (CM‐AN 220®, Chromex, Le Mans, France) Temperature set at 42°/43ºC Duration: not stated Body area covered: legs up to the pubis Control: n = 11 "No hypothermia prevention" Co‐interventions: Intravenous fluids were infused at ambient temperature but irrigation infusions were warmed to 37ºC Room temperature (mean): 20.3ºC |
|
| Outcomes | Shivering (absent/present) Fluids infused (total) (L) |
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Shivering was evaluated by an independent observer blinded to the treatment |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | ‐ |
Camus 1993b.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (France) Sample size: 33 Funding: not stated |
|
| Participants | Age (mean): 47/53/54 years Gender (M/F): 21/12 ASA grade: I ‐ II Surgery type: elective abdominal surgery Surgery duration (mean): > 2 hrs (187 to 237 mins duration of anaesthesia) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSWa): n = 11 Forced‐air warming device (Bair Hugger® Model 200, Augustine Medical, Eden Prairie, MN) covered by 2 cotton sheets ("Insulated BH") Temperature set at 43ºC Duration: not stated Body area covered: legs Intervention (ABSWb): n=11 Lower body forced‐air blower cover attached to a Bair Hugger® Model 200 (Augustine Medical, Eden Prairie, MN) which was set on 'high' (43ºC) Temperature set at 43ºC Duration: not stated Body area covered: legs Control: n = 11 "No hypothermia prevention" Co‐interventions: Irrigation solutions were warmed to 37ºC Room temperature: 21.5ºC |
|
| Outcomes | Shivering (present/absent) Fluids infused (total) (L) |
|
| Notes |
Comparison 1 The 2 intervention groups have been merged in the analysis, giving 1 single comparison Comparison 3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Shivering was evaluated by an independent observer blinded to the treatment |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | |
Camus 1995.
| Methods | Design: RCT Operative phase: preoperative Withdrawals: none Setting: 1 centre (France) Sample size: 16 Funding: Mallinckrodt products donated thermocouples |
|
| Participants | Age (mean): 44 yrs Gender (M/F): 5/11 ASA grade: I – II Surgery type: elective laparoscopy cholecystectomy Surgery duration (mean): > 2 hrs (122/132 mins duration of anaesthesia) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 8 Forced‐air warming (Bair Hugger® model 500, Augustine Medical, Inc., Eden Prairie, MN) + cotton sheet over the cover Duration: 60 mins before induction of anaesthesia Temperature setting at 41°C Body area covered: up to the shoulders Proportion covered ≥ 50% Control: n = 8 Wool blanket (usual treatment) during the same pre‐induction period Duration: 60 mins before induction of anaesthesia Body area covered: not stated Proportion covered: not stated Co‐interventions: Participants in both groups were subsequently covered only with a single layer of surgical draping. No special precautions were taken intraoperatively to avoid hypothermia IV fluids were infused at ambient temperature. In the PACU, all participants were actively rewarmed |
|
| Outcomes | Shivering (present/absent) Fluids infused (total) (L) |
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Shivering assessed by a blinded investigator |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized patients were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | |
Camus 1997.
| Methods | Design: RCT Operative phase:intraoperative Withdrawals: none Setting: 1 centre (France) Sample size: 18 Funding: not stated |
|
| Participants | Age (range): 50 (24 – 65) years Gender (M/F): not stated ASA grade: not stated Surgery type: elective (non‐haemorrhagic abdominal surgery in the supine position lasting > 2 hrs) Surgery duration: > 2 hrs (eligibility: "at least two hours") Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 10 2 electric blankets (Electro Concept®, model cb2 and cb3) + single cotton sheet between skin and blankets Duration: during surgery Body area covered: legs to the pubis + head, trunk and arms Temperature setting at 40°C Control: n = 8 "No special precautions were taken to prevent hypothermia" Co‐interventions: anaesthetic gases were not actively warmed and IV fluids were infused at room temperature |
|
| Outcomes | Shivering (present/absent) Thermal skin lesions Fluids infused (no data reported) |
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent observer blinded |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | ‐ |
Casati 1999a.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (Italy) Sample size: 48 Funding: not stated |
|
| Participants | Age (average): 66/68 Gender (M/F): not stated ASA grade: I ‐ III Surgery type: elective (total hip arthroplasty) Surgery duration: < 2 hrs (66/100 mins) Anaesthesia type: spinal‐epidural |
|
| Interventions |
Intervention (ABSWa): n = 24 Forced‐air warming of either of the 2 upper limbs (Bair Hugger®, Augustine Medical, Eden Prairie, MN.) Duration: after loss of sensation until end of surgery Body area covered: 2 upper limbs Proportion covered: not stated Intervention (ABSWb): n = 24 Forced‐air warming of the lower limb not involved in the surgical procedure (Bair Hugger®, Augustine Medical, Eden Prairie, MN.) Duration: after loss of sensation until end of surgery Body area covered: lower limb not involved in surgical procedure Proportion covered: not stated Co‐interventions: IV infusion of lactate Ringer’s solution warmed at 37°C was given throughout surgery. Autologous blood was warmed to 37°C before infusion Room temperature: 21° ‐ 23°C |
|
| Outcomes | Blood loss Fluids infused intraoperatively Shivering |
|
| Notes | Comparison 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelope assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The occurrence of shivering, nausea, vomiting, and other undesired side effects were recorded by an observer who was blinded to the intraoperative warming treatment. |
| Baseline comparability of groups | Low risk | To a high degree according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Low risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting. |
| Other bias | Low risk | |
Casati 1999b.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (Italy) Sample size: 50 Funding: not stated |
|
| Participants | Age (mean): 67 yrs ASA: I ‐ III Sex (M/F): not stated Type of surgery: elective (total hip replacement) Surgery duration (mean): < 2 hrs (100/105 mins) Type of anaesthesia: spinal‐epidural |
|
| Interventions |
Intervention (ABSW): n = 25 Forced‐air warming of the 2 upper limbs (Bair Hugger®, Augustine Medical, Eden Prairie, MN.) Control: n = 25 Reflective blanket covering the trunk, the 2 upper limbs and the non‐operated lower limb Cointerventions: none described Room temperature: 21°‐ 23°C |
|
| Outcomes | Shivering (present/absent) Blood loss (ml) Fluids infused (crystalloids) (L) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelope assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1. |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Chakladar 2014.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 1 (surgery postponed) Setting: 1 centre (UK) Sample size: 119 Funding: none reported |
|
| Participants | Age (mean): 34 yrs Gender: all women ASA grade: I ‐ III Surgery type: scheduled cesarean section Surgery duration (mean): 43 mins Anaesthesia type: epidural |
|
| Interventions |
Intervention (ABSW): n = 58 Under‐body resistive warming mattress Temperature set at : 40°C Duration: intraoperative Body area covered: under‐body Control: n = 58 “Not warmed with mattress” Duration: intraoperative Body area covered: NA Co‐interventions: most women received fluid warming Room temperature (mean): 22.9°‐ 23ºC |
|
| Outcomes | Fluids infused (L) Blood loss (L) N of participants transfused (RBC) Transfusions (units) Shivering (4‐point scale) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind assessment of outcomes |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Warm fluids available to women who may have required it |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for participants not analysed. ITT performed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Chung 2012.
| Methods | Design: RCT Operative phase: preoperative Withdrawals: not stated Setting: 1 centre (Korea) Sample size: 45 Funding: not stated |
|
| Participants | Age (mean): 31.8/32.5/31.9 years Gender (M/F): 0/45 ASA grade: I ‐ II Surgery type: elective (caesarean section) Surgery duration (mean): < 2 hrs (41.5/45.7 mins) Anaesthesia type: spinal |
|
| Interventions |
Intervention (ABSW): n = 15 Upper body forced‐air warming unit (Bair HuggerⓇ; Augustine Medical, Eden Prairie, MN) + IV fluids at room temperature Temperature set at 43ºC Duration: not stated Body area covered: not stated Control: n = 15 Forced‐air warming unit switched off (sham FAW) + warmed IV fluids (40ºC) during the 15 mins before spinal anaesthesia * Note: This group is excluded from this review as it includes a specific active warming system in the control group Control: n = 15 Forced‐air warming unit switched off (sham FAW) + IV fluids at room temperature Co‐interventions: not stated Room temperature: not stated |
|
| Outcomes | Shivering (4‐point scale) Thermal comfort (VAS 0 ‐ 100) Pain (ephedrine dose) Fluids infused (total) (ml) Blood loss (ml) |
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Yes, the control group received same intervention (forced‐air system), but they kept it switched off |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were reported |
| Selective reporting (reporting bias) | High risk | Nausea and vomiting are reported as secondary outcomes, but no data are provided |
| Other bias | Low risk | |
D'Angelo Vanni 2007.
| Methods | Design: RCT Operative phase: pre‐ and intraoperative Withdrawals: not stated Setting: 1 centre (Brasil) Sample size: 30 Funding: not stated |
|
| Participants | Age (mean): 39/34/44 years Gender (M/F): 0/30 ASA grade: I ‐ II Surgery type: elective (lower abdominal surgery lasting at least 1 hr) Surgery duration (mean): < 2 hrs (91/98 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSWa): n = 10 Preoperative and intraoperative active skin‐surface warming with a forced‐air warming blanket (WarmTouch® model 5200, Mallinckrodt Medical). A cotton sheet was interposed between the skin and the WarmTouch blanket, which was covered by 1 cotton sheet to reduce heat loss from the cover to the environment Temperature set at 42°‐ 46ºC Duration: 60 mins before induction of anaesthesia and after 5 mins of the induction of anaesthesia Body area covered: up to the shoulders Intervention (ABSWb): n = 10 Intraoperative active skin‐surface warming with a forced‐air warming blanket (WarmTouch® model 5200, Mallinckrodt Medical). Temperature set at 42°‐ 46ºC Intraoperative duration: after 5 mins of the induction of anaesthesia Body area covered: up to the shoulders Control: n = 10 2 cotton sheets. No special precautions were taken to avoid hypothermia Duration: during the surgery Body area covered: thorax, shoulders, arms, and hands Co‐interventions: IV fluids were kept at operating room temperature before infusion Room temperature: 20º ‐ 23ºC |
|
| Outcomes | Shivering (present/absent) Fluids infused (total) (ml) Other outcomes reported not included in the review:
|
|
| Notes |
Comparison 1 The 2 intervention groups have been merged in the analysis, giving 1 single comparison Comparison 3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In the PACU, shivering was evaluated by an independent observer who was blinded to the study treatment. |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Elmore 1998.
| Methods | Design: RCT Operative phase: intra‐ and postoperative Withdrawals: 17/100 (17%) Setting: 1 centre (USA) Sample size: 100 Funding: research grant from the Penn State Geisinger Health System |
|
| Participants | Age (average): 68 years Gender (M/F): 85/15 ASA grade: not stated Surgery type: elective (infrarenal aortic surgery) Surgery duration: > 2 hrs (4.0/4.2 hrs) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1): n = 50 Forced‐air warming blanket (Bair Hugger®, Augustine Medical, Eden Prarie, MN) Duration: not stated Temperature: set at high until the participant's temperature reached 37.5°C Body area covered: upper body Intervention (ABSW2): n = 50 Circulating‐water mattress (Aquamatic K Thermia American Hospital Supply Corp, Cincinnati, Ohio) Duration: not stated Temperature: heating unit temperature set at the maximum setting of 41°C Co‐interventions: preoperatively participants were covered with warm cotton blankets. All participants received inhaled gases warmed with the humidifier set at 38°C and received warmed fluids IV Room temperature: 20°C |
|
| Outcomes | Cardiac events (angina, myocardial infarction, cardiac arrest, unstable ventricular tachycardia or congestive heart failure) Deaths Wound infection Fluids infused (cell saver; crystalloids) (ml) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The cardiologist who interpreted the Holter recordings was blinded to the warming method of each participant |
| Baseline comparability of groups | Low risk | To a high extent according to table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100 randomized participants, 83 analysed. Causes for exclusion described, but it is clear from which arm the losses come. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Fallis 2006.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: not stated Setting: 2 acute‐care hospitals (Canada) Sample size: 62 Funding: supported by the Health Sciences Center Foundation, Winnipeg, Manitoba. Equipment was donated by Associated Health Systems Inc. (Bair Hugger devices) and Alaris Medical (IV AC thermometers) |
|
| Participants | Age (mean): 30 years Gender (M/F): 0/62 ASA grade: not stated Surgery type: elective cesarean delivery in low‐risk pregnant women Surgery duration < 2 hrs (70.6/79.5 mins) (total time in OR) Anaesthesia type: neuraxial spinal |
|
| Interventions |
Intervention (ABSW): n = 32 Forced‐air warming blanket (Bair Hugger® Model 500, Arizant Healthcare, Eden Prairie, MN) Duration: following the insertion of the spinal needle and until the mother left the OR Temperature: warming unit turned on “high” (˜43°C) Body area covered: upper torso and arms Proportion covered: not stated Control: n = 30 Warmed cotton blankets Duration: following the insertion of the spinal needle and until the mother left the OR Co‐interventions: participants in both groups received IV fluids from the IV warming cupboard Room temperature: 21.6°C |
|
| Outcomes | Thermal comfort (VNRS 0 ‐ 10 scale) (reported only in narratively) Shivering (4‐point scale) (no data reported) Pain (VAS 0 ‐ 10) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocked randomization |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Fossum 2001.
| Methods | Design: RCT Operative phase: preoperative Withdrawals: none Setting: 1 centre (USA) Sample size: 100 Funding: Augustine medical‐equipment & financial support |
|
| Participants | Age (mean): 45.2 years Gender (M/F): 57/43 ASA grade: I ‐ III Surgery type: not stated (gynaecologic, orthopaedic, urological surgery) Surgery duration: Study reports anaesthesia time minimum = 1 hr and maximum = 3 hrs Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 50 Forced‐air warming (Bair Hugger® model # 505) with a single‐layer cotton blanket placed over Duration: 45 mins (in the preoperative holding area) FAW was set at medium operating temperature of 38 ± 3°C Body area covered: not stated Control: n = 50 Warmed single cotton blanket Duration: 45 mins (in the preoperative holding area) Warmed at 66° Body area covered: not stated Co‐interventions: not stated |
|
| Outcomes | Thermal comfort (VNRS 0 ‐ 10) Shivering (present/absent) Pain (Likert 0 ‐ 10) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Letters containing the assignment were shuffled before the participant's consent form was signed and randomly chosen by the investigator consenting the participant |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Frank 1997.
| Methods | Design: RCT Operative phase: intraoperative Follow‐up: Withdrawals: 30/300 (10%) Setting: 1 centre (USA) Sample size: 300 participants > 60 yrs at high risk of cardiovascular events Funding: National Institutes of Health grant GM38177 and Mallinckrodt Medical Inc. Dr Fleisher was supported by the Richard Ross Clinician Scientist Award of The Johns Hopkins University School of Medicine. The ambulatory ECG recorders were donated by Spacelabs Medical Inc |
|
| Participants | Age (mean): 71 years Gender (M/F): 167/133 ASA grade: I ‐ IV Surgery type: elective (peripheral vascular, abdominal or thoracic procedures) Surgery duration: > 2 hrs (3.4/3.6 hrs ) Anaesthesia type: general and epidural |
|
| Interventions |
Intervention (ABSW): n = 142 Forced‐air warming cover (Mallinckrodt Medical®) Duration: not stated Temperature: Set to maintain core temp at 37°C Body area covered: legs and trunk Proportion covered: not stated Control: n = 158 1 layer of paper of surgical field Duration: not stated Control body area covered: not stated Proportion covered: not stated Co‐interventions: IV fluids and blood were warmed, and a heat‐moisture exchanger (Thermovent) was used in the respiratory circuit |
|
| Outcomes | All‐cause mortality Cardiac events (electrocardiographic and morbid intraoperative and postoperative events: unstable angina/ischaemia, cardiac arrest, or myocardial infarction) Pain (VAS 0 ‐ 10) 30 and 90 mins postoperatively and the day after surgery Shivering (present/absent) Blood loss (ml) Fluids infused (crystalloids) (ml) Transfusions (RBC) (Units) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence. |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not informed of their treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Morbid cardiac events were determined by a blinded investigator. All ECGs and enzyme CK data were masked, as well as the Holter tapes |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30 participants withdrawn because Holter monitoring data were missing (n = 15 hypothermic group, n = 15 in normothermic group) |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Hasegawa 2012.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (Japan) Sample size: 36 Funding: not stated |
|
| Participants | Age (mean range): 59 ‐ 64 Gender (M/F): 21/15 ASA grade: I ‐ II Surgery type: elective (major abdominal surgery) Surgery duration (mean): > 2 hrs (241/250/241 mins) Anaesthesia type: epidural and general |
|
| Interventions |
Intervention (ABSW1): n = 12 Lower body forced‐air warming device (Bair Hugger®, Arizant Healthcare, Inc., MN) Temperature set at 43ºC Duration: started at induction of general anaesthesia and maintained throughout surgery Body area covered: 15%/20% Intervention (ABSW2): n = 12 A pair of circulating‐water leg wraps (RaprRound Body Wraps, Gaymar Industries, NY, USA) + a full‐length circulating‐water mattress (Gaymar) set to 42ºC (circulating‐water group) Temperature set at 42ºC Duration: started at induction of general anaesthesia and maintained throughout surgery Body area covered: 30% of skin surface Intervention (ABSW3): n=12 Carbon‐fibre resistive‐heating blankets (SmartCare, Geratherm Medical AG, Germany) set to 42ºC Temperature set at 42ºC Duration: started at induction of general anaesthesia and maintained throughout surgery Body area covered: left arm, chest, and both legs Co‐interventions: not stated Room temperature: 20.7ºC ‐ 20.9ºC |
|
| Outcomes | Blood loss (ml/kg) Complications Fluids infused (ml/kg/hr) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 2 (x 3 comparisons) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated codes |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding methods were described, but all outcomes were objective (temperature and blood loss) |
| Baseline comparability of groups | Low risk | Participant demographic and morphometric characteristics and type of surgery were similar across each group |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomized were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Hofer 2005.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 2/90 (2%) Setting: 1 centre (Switzerland) Sample Size: 90 Funding: "this study was performed without any financial support from manufacturers or the pharmaceutical industry. Material support was provided by Soma Pharm AG, Switzerland, for the Thermamed SmartCare OP system (Medeqco, Bad Oeynhausen, Germany) and by Homedica AG, Switzerland/MTRE Advanced Technologies Ltd, Israel, for the Allon 2001 system. None of the authors is related to or has financial interests in the manufacturers of the products studied. Also, no specific institutional funding was necessary because all authors are regularly employed at their institutions" |
|
| Participants | Age: 64 ‐ 66 years Gender (M/F): 72/18 ASA grade: III Surgery type: off‐pump coronary artery bypass grafting Surgery duration: > 2 hrs (232 /249 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1): n = 29 Convective air‐warming system (Warm‐Touch® system; Mallinckrodt Inc, St Louis, Mo) by using a total body garment before OPCABG and a sterile lower body blanket until the end of the operation after the preparation of venous grafts Temperature: the system was set to 42ºC Intervention (ABSW2): n=30 Resistive‐heating electric carbon‐fibre blankets (Thermamed SmartCare OP® system; Medeqco, Bad Oeynhausen, Germany), the upper extremities can be completely covered and the neck, body trunk, and lower extremities can be partially covered for warming during OPCABG Temperature: the system was set to 42ºC Intervention (ABSW3): n=29 Disposable circulating‐water warming garment (Allon 2001® system; MTRE Advanced Technologies Ltd, Or‐ Akiva Industrial Park, Israel) can be wrapped around the participant’s body, covering the back and upper parts of the extremities Temperature: the system was set to 36.7ºC Co‐interventions: Upon arrival in the OR, all participants were covered with warmed sheets Room temperature: 22.2ºC |
|
| Outcomes | Blood loss (perioperative) (ml) Perioperative transfusions (N of participants; RBC ml) Complications: burns and decubitus ulcers Fluids infused (plasma) (ml) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 2 (x 3 comparisons) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants (2.2%) were excluded from the study protocol after randomization as a result of conversion to cardiopulmonary bypass during the operation |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Horn 2002.
| Methods | Design: RCT Operative phase: pre‐ and intraoperative Withdrawals: none Setting: 1 centre (Germany) Sample size: 30 Funding: supported by Augustine Medical, Inc. (Eden Prairie, MN), NIH Grant GM 58273 (Bethesda, MD), the Joseph Drown Foundation (Los Angeles, CA), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY). Mallinckrodt Anesthesiology Products, Inc. (St. Louis, MO) donated the thermocouples used |
|
| Participants | Age (mean): 31 ‐ 33 years Gender (M/F): 0/30 ASA grade: I ‐ II Surgery type: elective (cesarean delivery) Surgery duration (mean): < 2 hrs ( 37/38 mins) Anaesthesia type: epidural |
|
| Interventions |
Intervention (ABSW): n = 15 Forced‐air cover. Model 501 (Bair Hugger®, Augustine Medical, Eden Prairie, MN) Temperature set at 43ºC Duration: 15 mins (before insertion of the epidural catheter) of forced‐air prewarming combined with intraoperative warming (during cesarean delivery) Body area covered: over the upper body Control: n = 15 Single cotton blanket Duration: not stated Body area covered: not stated Co‐interventions: all IV fluids administered were warmed to 37ºC Room temperature: 24ºC |
|
| Outcomes | Shivering (4‐point scale) Thermal comfort (sensation) (VAS 0 ‐ 100) (no data provided) Pain (VAS 0 ‐ 100) (no data provided) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Sequentially‐number opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1. |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Horn 2012.
| Methods | Design: RCT Operative phase: preoperative Withdrawals: none Setting: 1 centre (Germany) Sample size: 200 Funding: no funding or competing interests declared |
|
| Participants | Age (mean): 49/55 years Gender (M/F): 61/139 ASA grade: I ‐ II Surgery type: elective (laparoscopic cholecystectomy; inguinal hernia repair; breast surgery; minor orthopaedic surgery; and ENT surgery) Surgery duration: < 2 hrs (60/65 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSWa): n = 52 Forced‐air warming (Level 1 Snuggle Warm Upper Body Blanket; Smiths Medical, Rockland, MA, USA) for 10 mins preoperative, set at 44ºC during the warming period Intervention (ABSWb): n = 43 Same (forced‐air warming) for 20 mins preoperatively Intervention (ABSWc): n = 50 Same (forced‐air warming) for 30 mins preoperatively Body area covered: whole body Control: n = 55 Passive insulation with a single cotton blanket Co‐interventions: all IV fluids administered were warmed to 39ºC. In all groups, participants were covered with cotton blankets intra‐ and postoperatively Room temperature: 23ºC |
|
| Outcomes | Shivering (4‐point scale) Thermal comfort (sensation) (VAS 0 ‐ 100) Blood loss (no data provided) Fluids infused (no data provided) Other outcomes reported not included in the review:
|
|
| Notes |
Comparison 1 The 3 intervention groups have been merged in the analysis, giving 1 single comparison Comparison 3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by rolling a modified dice with 4 faces, each representing 1 of the 4 treatment groups |
| Allocation concealment (selection bias) | Unclear risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not possible to blind participants or personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After the pre‐warming procedure, participants were transferred to theatre. General anaesthesia was induced by an anaesthetist blinded to the pre‐warming randomization. Shivering was assessed by an independent blinded observer |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Janicki 2001.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 7/60 (12%) Setting: 1 centre (USA) Sample Size: 60 Funding: MTRE Advanced Technologies Ltd., Or‐Akiva, Israel |
|
| Participants | Age (range): 54.5 years Gender (M/F): 29/24 ASA grade: II – IV Surgery type: elective (open abdominal surgery) Surgery duration (mean): > 2 hrs (299/361 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1): n = 25 Whole‐body water‐garment warmer (Allon, MTRE, Advanced Technologies, Or‐Akiva, Israel) Duration: participants were placed in the garment before induction of anaesthesia, and the warming was continued intraoperatively until the transfer from the OR table to the stretchers at the end of surgery when the garment was removed Temperature: the garment was prefilled with water at 36.8°C Body area covered: lower and upper extremities, upper anterior, lateral portions of the chest Proportion covered: 70% ‐ 80% Intervention (ABSW2): n = 30 Upper‐body forced‐air warming using a convective air‐warming system consisting of the Bair‐Hugger® Warming (Augustine Medical, Eden Prairie, MN) Duration: the warming blanket was positioned on the participant, and warming was started after induction of anaesthesia and monitor placement and was continued until the end of surgery Temperature: set at 43°C and reduced to 36°C if participant core temperature > 37°C Body area covered: upper body Proportion covered: 20% ‐ 40% Co‐interventions: warming of all IV fluids Room temperature: 20ºC |
|
| Outcomes | Blood loss (ml) Fluids infused (crystalloids) (ml) Shivering (present/absent) Thermal comfort (VAS 0 ‐ 10) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were periodically assessed (every 15 mins for 1 hr and every 30 mins thereafter) by nursing staff (blinded as to the type of warming used perioperatively) for shivering, requirement for use of additional warming devices |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From 60 participants initially enrolled in the study, 5 were removed because of the shorter‐than‐expected duration of their surgical procedure (> 120 mins), and 2 because of unplanned extension of surgery to the rectal area that interfered with the rectal temperature sensor |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Johansson 1999.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 7/50 Setting: 1 centre (Sweden) Sample Size: 50 Funding: County Council of Ostergötland |
|
| Participants | Age (range): 67 ‐ 69 years Gender (M/F): 21/29 ASA grade: not stated Surgery type: elective (total unilateral primary hip arthroplasty) Surgery duration (mean): < 2 hrs (100/102 mins) Anaesthesia type: spinal |
|
| Interventions |
Intervention (ABSW): n = 25 Upper‐body forced‐air warming (Bair Hugger®, Augustine Medical, Eden Prairie, MN) Duration: at arrival at the operation theatre until 2 hours after Temperature: Setting at 36.8°C Body area covered: from the shoulders to the waist, including the arms Proportion covered: not stated Control: n = 25 Cotton blanket Duration: not stated. Temperature: Setting at 43°C; reduced to 'medium': 36°C if participant core temperature > 37°C Body area covered: not stated Proportion covered: not stated Co‐interventions: All participants rested on pre‐warmed gel‐filled mattresses and all infused fluids and blood were warmed Room temperature: 20.9 ºC |
|
| Outcomes | Blood loss (during surgery; postoperative; total) (ml) N of participants transfused Transfusions (units) Fluids infused (crystalloids; colloids) (ml) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sequentally‐numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 randomized participants were excluded, 1 suffering excessive intraoperative bleeding from iatrogenic damage to a major artery, and 6 due to missing laboratory data |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Just 1993.
| Methods | Design: RCT Operative phase: preoperative Withdrawals: none Setting: 1 centre (USA) Sample size: 16 Funding: not stated |
|
| Participants | Age (range): 64 (60/68 years) Gender (M/F): 8/8 ASA grade: I – II Surgery type: elective (total hip arthroplasty) Surgery duration: > 2 hrs ( 174/180 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 8 Electric blanket (CM‐AN 220, Chromex, Le Mans, France) + sheet (warmed) Duration: 90 mins preoperative Temperature: set at 42°– 43°C Body area covered: during surgery shoulders and thorax covered Proportion covered: not stated Control: n = 8 Paper shirt covered with cotton sheet Duration: until during surgery Control body area covered: during surgery ‐ covered shoulders and thorax Proportion covered: not stated Co‐interventions: Not stated |
|
| Outcomes | Shivering (present/absent) Thermal comfort of prewarming Fluids infused (total) (ml) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Shivering assessed by blinded investigator |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | None reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Kabbara 2002.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 4/87 (4.6%) Setting: 1 centre (USA) Sample size: 83 Funding: MetroHealth Foundation, Chester Summer Scholar Program, MetroHealth Medical Center, Cleveland, Ohio, and the Department of Anesthesia, Metro‐Health Medical Center, Cleveland, Ohio |
|
| Participants | Age (range): 43.5 (41/46 years) Gender (M/F): 26/57 ASA grade: I – III Surgery type: elective (gynaecologic, orthopaedic, otolaryngologic, plastic, or general surgery) Surgery duration (mean range): > 2 hrs (131/149 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 45 Forced‐air warming using either a commercial upper‐ or lower‐body blanket (Bair Hugger®, Augustine Medical, Inc., Eden Prairie, MN) Duration: not stated Temperature: set at 43°C Body area and proportion covered: the amount of surface area warmed (%) was estimated for each participant as follows: arm, 9%; leg, 18%; trunk, 36%; head, 9%; and genitals, 1% Control: n = 42 Standard hospital blankets Duration: not stated Co‐interventions: fluids were infused at room temperature Room temperature: 21ºC |
|
| Outcomes | Blood loss (ml) Transfusions (RBC) (units) Thermal comfort in post‐surgical waking‐up ('warm/cold/comfortable') Fluids infused (crystalloids; colloids) (ml) (The paper states that there were no thermal injuries) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of computer‐generated random numbers was used for group assignment |
| Allocation concealment (selection bias) | High risk | Once consent was obtained, the anaesthesia team was notified of group assignment before surgery based on the table of random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Postoperatively, sublingual temperature (IVAC thermistor) was recorded by the PACU nurses who were unaware of group assignments |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Kiessling 2006.
| Methods | Design: RCT Operative phase: intraoperative and postoperative Withdrawals: 11 converted to ECC (6 from intervention and 5 from control group) (11%) Setting: 1 centre (Germany) Sample size: 100 Funding: Departamental Source |
|
| Participants | Age (average): 66.1/68.3 Gender (M/F): 63/27 ASA grade: I – III Surgery type: elective (OFCABG) Surgery duration: > 2 hrs (231/238 mins length of OR stay) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 50 Allon Thermowrap® system (pads with temperature‐controlled water circulation; the pads are gummed to the participants and follow the form of the body surface) Body area covered: back, legs, and arms Proportion: approx. 65% of the body surface Temperature: "the control unit works with ceramic heat exchangers which produce the required water temperature effectively and rapidly. Working range for the instrument is from 30° to 40°C" Control: n = 50 Insulation pads + warm IV fluids (at 37°C) Co‐interventions: not stated Room temperature: 25ºC |
|
| Outcomes | Blood loss (ml) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Unclear risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not included in the results were participants who had to be converted to ECC. 6 participants were rejected from the Thermowrap group and 5 from the control group |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Kim 2014.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (Korea) Sample size: 46 Funding: Konkuk University |
|
| Participants | Age (average): 74 yrs Gender (M/F): 15/31 ASA grade: I ‐ III Surgery type: elective total knee arthroplasty Anaesthesia duration (mean): > 2hrs (150 mins) Anaesthesia type: spinal |
|
| Interventions |
Intervention (ABSW1): n = 23 Forced‐air warming system (Bair Hugger® Model 505, Arizant Healthcare, Eden Prairie, MN) placed over anterior chest, upper limbs, neck Applied after induction of anaesthesia Blower set at high temperature (43ºC) Intervention (ABSW2): n = 23 Circulating‐water mattress (Blanketrol® II, Cincinnati Sub‐Zero, Cincinnati, USA) set at 41ºC, placed over operating table. Warming started 10 mins after the participant was transferred to operating table Co‐interventions: all IV fluids were warmed to 37ºC with an infusion warmer Room temperature (OR and recovery): 21° ‐ 23ºC and 24° ‐ 26ºC |
|
| Outcomes | Other cardiovascular complications: bradycardia, hypotension Blood loss (ml) Fluids infused (crystalloids) (ml) Thermal comfort (VAS 0 ‐ 10) Shivering (4‐point scale) |
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Krenzinschek 1995.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: not stated Setting: 1 centre (USA) Sample size: 29 Funding: Supported in part by National Institutes of Health Grant No.GM 38177 and Mallinckrodt Medical Inc |
|
| Participants | Age (average): 69 years Gender (M/F): 16/13 ASA grade: not stated Surgery type: elective (vascular, thoracic or abdominal surgery) Surgery duration: not reported Anaesthesia type: general and regional |
|
| Interventions |
Intervention (ABSW): n = 15 Intraoperative upper‐ or lower‐body forced‐air warming (Warm Touch®, Mallinckrodt, Inc.) + 2h postoperative (full body) Duration: not stated Temperature: the temperature and air flow were set to high or adjusted to medium to maintain core temperature at or near 37ºC. If core temperature exceeded 37ºC, the blower was turned off and the blanket left in place Body area covered: upper‐ or lower‐body warming blanket over the participant Proportion covered: not stated Control: n = 14 Routine care with 1 layer of paper surgical drapes (intraoperative). During the postoperative period, either 1 or 2 warmed cotton blankets were placed over the participants at PACU nurse's discretion Co‐interventions: not stated. After 2 hrs postoperative, care was similar in both groups Room temperature: not stated |
|
| Outcomes | Shivering (present/absent) Pain score (VNRS 0 ‐ 10) Thermal comfort in post‐surgical waking‐up (VAS 0 ‐ 10) |
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Kurz 1995.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (Austria) Sample size: 74 Funding: Supported by a grant from the Max Kade Foundation and the Joseph Drown Foundation and the National Institutes of Health (GM49670) |
|
| Participants | Age (mean range): 57 ‐ 59 years Gender (M/F): 39/35 ASA grade: I ‐ III Surgery type: elective colorectal surgery Surgery duration (mean): > 2 hrs (3.3/3.5 hrs) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 39 Upper‐body forced‐air cover (Bair Hugger®, Augustine Medical, Inc) Temperature set at 40ºC Duration: not stated Body area covered: upper body Control: n = 35 Routine thermal management Participants in this group were allowed to become hypothermic (34ºC), then FAW was instituted to prevent further hypothermia Co‐interventions: fluid was heated to 37ºC in the FAW group but not in the control group Room temperature: 21° ‐ 22ºC intraoperatively and 23° ‐ 25ºC postoperatively |
|
| Outcomes | Fluids infused (total) (ml) Shivering (grade 1 ‐ 3) Pain (VAS 0 ‐ 100) (reported narratively) Thermal comfort (VAS 0 ‐ 100) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported (most probably not concealed) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | Yes |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Kurz 1996.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: multicentre (3 centres) (Austria) Sample size: 200 Funding: Supported in part by grants (GM49670 and GM27345) from the National Institutes of Health, by the Joseph Drown and Max Kade Foundations, and by Augustine Medical, Inc. |
|
| Participants | Age (mean range): 59 ‐ 61 years Gender (M/F): 108/92 ASA grade: not stated Surgery type: elective colorectal resection for cancer or inflammatory bowel disease Surgery duration (mean): > 2 hrs (3.1 hrs) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 104 Forced‐air cover (Bair Hugger®, Augustine Medical, Inc) + IV fluid warmer Participants' core temperature was maintained near 36.5ºC Temperature set at 40ºC Duration: not stated Body area covered: upper body Control: n = 96 Sham: "a forced‐air cover was positioned over the upper body set to deliver air at the ambient temperature, and IV fluids were administered through a fluid warmer that was not activated". Participants' core temperature was allowed to decrease to approximately 34.5ºC Co‐interventions: as the study had a double‐blind design, different co‐interventions were supposedly avoided Room temperature: 21.9° ‐ 22.1ºC |
|
| Outcomes | Wound infection Thermal comfort (VAS 0 ‐ 100) Pain (VAS 0 ‐ 100) Shivering (4‐point scale) Fluids infused (crystalloids; colloids) (ml) N of participants transfused Transfusion (RBC) (units) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated codes |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham intervention in the control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The number of wound infections was evaluated by an observer unaware of the participants' temperatures and group assignments |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Lee 2004.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 1 Setting: multicentre (2 centres) (Australia) Sample size: 60 Funding: supported by a grant from Fisher and Paykel (Suntouch® manufacturer) |
|
| Participants | Age (mean range): 53 ‐ 56 years Gender (M/F): 28/32 ASA grade: not stated Surgery type: elective or emergency non‐cardiac surgery Surgery duration (mean): > 2 hrs (130/133 mins) Anaesthesia type: general (31%), spinal (37%) and other (not specified) (32%) |
|
| Interventions |
Intervention (ABSW1): n = 30 Radiant warming (Suntouch®) directed at the palm of the hand Intervention (ABSW2): n=30 Forced‐air warming (Bair Hugger®) (upper or lower body) Co‐interventions: IV fluid warming was standard for all participants |
|
| Outcomes | Thermal comfort (VAS 0 ‐ 100) Shivering (present/absent) (The paper states that there were no complications attributable to active warming in either group) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number tables |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors mention that participants were blind to group assignment, but there are no data on personnel blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was excluded for not meeting all the inclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Leeth 2010.
| Methods | Design: RCT Operative phase: preoperative Withdrawals: 37/147 (25%) not included in data analysis. Setting: 1 centre (USA) Sample size: 147 Funding: the forced‐air warming gowns were provided for the study at a discounted purchase price, the cost of which were absorbed by the study unit |
|
| Participants | Age (mean): 43/44 years Gender (M/F): 44/60 ASA grade: I ‐ III Surgery type: elective (head‐neck, upper extremity, core, and lower extremity surgery) Surgery duration: not stated Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 49 Forced‐air warming gowns: Bair Paws forced‐air warming gown (Arizant, Inc, Eden Prairie, MN) Duration: not stated Temperature: not stated Body area covered: not stated Control: n = 56 Warmed cotton blankets Duration: not stated Co‐interventions: not stated Room temperature: not stated |
|
| Outcomes | Thermal comfort (Likert 1 ‐ 5) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 142 participants randomized, 105 analysed. No description of causes or groups they belonged to |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Leung 2007.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: no Setting: 1 centre (Hong Kong) Sample size: 60 Funding: not stated |
|
| Participants | Age (range): 65 years (64.1/66.1) Gender (M/F): 39/21 Exclusion: pregnancy, core temp ≥ 37.5°C ASA grade: I ‐ III Surgery type: elective laparotomy: Pancreatic (n = 8); Gastric (n = 16); Hepatobiliary (n = 19); Colectomy (n = 13); Abdominal aortic aneurism (n = 3); Cystectomy (n = 1) Surgery duration: > 2 hrs (258/271 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1): n = 30 Upper‐body forced‐air warming (Bair Hugger®, Augustine Medical model 500/OR, Prairie, MN) A cotton blanket was folded once to make 2 layers in thickness, with the forced‐air warming blanket sandwiched between the 2 layers. Duration (mean): 271 minutes. After induction until end of surgery Temperature setting at 43°C Body area covered: Intervention (ABSW2): n = 30 Electric heating pad (Operatherm® 202+ prewarmed gel pad) For the heating‐pad group, the 104 X 45 cm pad was placed on the operating table and a pre‐warmed gel pad was placed on top of it, as suggested by the manufacturer, covered in turn with a sheet. The participant then lay on the hospital bed sheet and a double‐folded cotton blanket was applied to cover the anterior chest and both arms, as for the forced‐air group Duration (mean): 271 mins Temperature setting at 39°C Body area covered: Co‐interventions: All IV fluids were warmed to 37ºC with an infusion warmer Ambient temperature: 21.1° ‐ 22.1 ºC |
|
| Outcomes | Thermal comfort (VAS 0 ‐ 10) Shivering (present/absent) Blood loss (ml) Fluids infused (crystalloids; colloids) (ml) Transfusion (RBC) (mL) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated by drawing lots |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Lindwall 1998.
| Methods | Design: RCT Operative phase:intraoperative Withdrawals: 3/28 (11%) Setting: 1 centre (Sweden) Sample size: 25 Funding: not stated |
|
| Participants | Age (mean range): 65 ‐ 66 years Gender (M/F): not stated ASA grade: I ‐ IV Surgery type: elective (extensive thoracoabdominal surgery: oesophageal, rectal or bladder carcinoma) Surgery duration (mean range): > 2 hrs (280/287 mins) Anaesthesia type: general and regional |
|
| Interventions |
Intervention (ABSW): n = 12 Upper or lower forced‐air warming (Bair Hugger®, Model 500, Augustine Medical, USA) Duration: started before induction of anaesthesia and stopped at end of operation Temperature setting at 43°C (SD 2.3) Body area covered: upper or lower body Proportion covered: 30% ‐ 40% Control: n = 13 Standard passive management consisting in insulation with double layers of terry cloth plus operation drapes covering the whole body Duration: not stated Temperature setting at 39°C Co‐interventions: Active fluid warming in both groups (38° – 39°C) Room temperature: 22ºC |
|
| Outcomes | Blood loss (ml) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants withdrawn from the study because they did not fulfill the inclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Mason 1998.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre? (USA) Sample size: 64 Funding: not stated |
|
| Participants | Age (mean range): 38.5 ‐ 40.7 years Gender (M/F): 9/55 Exclusion: not stated ASA grade: not stated Surgery type: elective (Roux‐en‐y gastric bypass surgery for morbid obesity) Surgery duration: > 2 hrs (156 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 32 Forced‐air warming system (Bair Hugger®, Augustine Medical, Inc., Eden Prairie, MN) Duration: started before induction of anaesthesia and stopped at end of operation Temperature setting at 43°C (SD 2.3) Body area covered: Control: n = 32 Warmed cotton blankets Duration: not stated Temperature: not stated Body area covered: Co‐interventions: Not reported Room temperature: 20.9ºC |
|
| Outcomes | Blood loss (ml) Fluids infused (total) (cc) Shivering (4‐point scale) (The paper states that no complications with either study group were observed") Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated codes |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The same observer for all study participants was a registered nurse who was unaware of the group assignment |
| Baseline comparability of groups | Low risk | Yes |
| Co‐interventions equal between groups | Unclear risk | No reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Matsukawa 1994.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (Japan) Sample size: 40 Funding: not stated |
|
| Participants | Age (mean): 61.8/61.3 years Gender (M/F): 27/13 ASA grade: I ‐ II Surgery type: elective (open abdominal surgery) Surgery duration (mean): > 2 hrs (166/171 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1+ABSW2): n = 20 Forced‐air warming device set at 38ºC (Bair Hugger®, Augustine Medical, Eden Prairie, MN) plus circulating‐water blanket warming set at 37ºC (KRthermia RK600, Baxter Health Care). Duration: not stated Body area covered: thoracic region and upper limbs Intervention (ABSW2): n = 20 Circulating‐water blanket warming set at 37 ºC (KRthermia RK600, Baxter Health Care) , with no forced‐air device. Co‐interventions: Room temperature: 25.7 ‐ 25.5ºC |
|
| Outcomes | Blood loss (ml) Transfusion (ml) Shivering (present/absent) |
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Melling 2001.
| Methods | Design: RCT Operative phase: preoperative Withdrawals: 4 Setting: 1 centre (UK) Sample size: 420 Funding: Smith & Nephew foundation; Augustine Medical inc |
|
| Participants | Age (range): not stated Gender (M/F): 174/242 ASA grade: not stated Surgery type: elective (mixed of clean surgery: breast, varicose veins, or hernia) Surgery duration: < 2 hrs (48/49.5 mins) Anaesthesia type: not stated/unclear |
|
| Interventions |
Intervention (ABSW1): n = 139 "Systemic warming": forced‐air warming blanket Duration: 30 mins preoperative (the blanket was left on until just before surgery) (average: 38.73 min) Body area covered: whole body Proportion covered: not stated Intervention (ABSW2): n = 140 "Local warming": non‐contact radiant heat dressing, to just the planned wound area Duration 30 mins preoperative (average 44.94 mins Control: n = 141 Unwarmed cotton blankets (usual care) Duration: 30 mins (average: 38.73 mins) Control body area covered: not stated Proportion covered: wound treated only Co‐interventions: not stated Same standard preoperative care Room temperature: not stated |
|
| Outcomes | Wound infection Other outcomes reported not included in the review:
|
|
| Notes |
Comparison 1 (x 2 comparisons) Comparison 2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A single trained observer unaware of treatment allocation reviewed participants at 2 and 6 weeks postoperatively |
| Baseline comparability of groups | Low risk | To a high degree according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants in the control group and 1 in the local warming group were lost to follow‐up. 1 participant's surgery was cancelled |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Mogera 1997.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (India) Sample size: 30 Funding: not reported |
|
| Participants | Age: 46/48 yrs Gender (M/F): 18/12 ASA grade: not reported Surgery type: elective intracranial surgery (neurosurgery) lasting at least 4 hours Surgery duration (mean): > 2 hrs (240/251 mins duration of anaesthesia) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 15 Forced‐air warming intraoperatively (Bair Hugger®, Augustine Medical, USA Model 500) set at medium (36.5° ‐ 38ºC), covering the trunk, upper and lower limbs Control: n = 15 "No active warming" (a cotton sheet was placed over the trunk, upper and lower limbs) Co‐interventions: Inspiratory gases were not actively or passively warmed. All participants received an IV infusion and blood (whenever necessary) at ambient temperature Room temperature: 19° ‐ 21ºC |
|
| Outcomes | Infused fluids (ml) Blood loss (ml) Blood transfusion (Units) Shivering (absent/mild/severe) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Baseline comparability of groups | Low risk | Yes |
| Co‐interventions equal between groups | Low risk | No |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Moysés 2014.
| Methods | Design: RCT Operative phase: intra‐ and postoperative Withdrawals: none Setting: 1 centre (Brazil) Sample size: 38 Funding: none reported |
|
| Participants | Age (mean): 57.5 yrs Gender (M/F): 21/17 ASA grade: not reported Surgery type: open gastrointestinal surgery Surgery duration (mean): > 2 hrs (214.6/291.6 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1): n = 19 Thermal blanket (no further details are provided) Temperature set at: 38ºC Duration: intraoperative + during recovery Body area covered: lower limbs Intervention (ABSW2): n = 19 Thermal mattress (no further details are provided) Temperature set at : 37ºC beginning and 38ºC end Duration: intraoperative + during recovery Body area covered: underneath Co‐interventions: none reported Room temperature (mean): 20.7° ‐ 23.3°C |
|
| Outcomes | Fluids infused (crystalloids; starch) Transusion (RBC; plasma) |
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of envelopes |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study is described as double‐blind but no details are provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study is described as double‐blind but no details are provided |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | No details are reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Ng 2003.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (Singapore) Sample size: 300 Funding: not stated |
|
| Participants | Age (mean range): 65 ‐ 66 years Gender (M/F): not stated ASA grade: I ‐ II Surgery type: elective (unilateral total knee replacement) Surgery duration (mean): < 2 hrs (1.28/1.41 hrs) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 100 Forced‐air warming blanket (Bair Hugger®, Augustine Medical model 540 Torso, Augustine Medical Inc, Eden Prairie, MN) with 1 cotton blanket (4 layers in thickness) Duration (mean): not stated Temperature setting at 38°C Body area covered: from the level of the iliac crests extending to both shoulders and the neck Proportion covered: not stated Control 1 (reflective insulation): n = 100 Reflective‐blanket (Thermadrape, 4 x 4 ft Blanket T 1300, Concepts Inc, Roanoke, TX) with 1 cotton blanket (4 layers in thickness) Duration (mean): not stated Body area covered: from the level of the iliac crests extending to both shoulders and the neck Proportion covered: not stated Control 2 (passive insulation): n = 100 2 cotton blankets (8 layers in thickness) Body area covered: from the level of the iliac crests extending to both shoulders and the neck Co‐interventions: In all 3 groups, the participants laid on the operating table, which was lined with a warm‐water circulating blanket (Gaymar‐model MTA 4702, Gaymar Inc, Buffalo, NY) set at 37°C Room temperature (OR and recovery): 19ºC and 22ºC |
|
| Outcomes | Shivering Other outcomes reported not included in the review:
|
|
| Notes |
Comparison 1 The 2 intervention groups have been merged in the analysis, giving 1 single comparison |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Equally randomized into 3 groups using the sealed‐envelope method |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No masking procedures are described |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Ng 2006.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: None Setting: multicentre (Hong Kong) Sample size: 60 Funding: not stated |
|
| Participants | Age (mean): 67.4 years Gender (M/F): 17/43 ASA grade: I ‐ III Surgery type: elective (total knee replacement) Surgery duration: < 2 hrs (90 mins) Anaesthesia type: spinal‐epidural |
|
| Interventions |
Intervention (ABSW1): n = 30 Upper‐body forced‐air warming (Bair Hugger®, Augustine Medical, Inc., Eden Prairie, MN) + a cotton blanket was folded once to make 2 layers in thickness, with the forced‐air warming blanket sandwiched between the 2 layers Duration: after induction until end of surgery Temperature setting at 43°C Body area covered: not stated Intervention (ABSW2): n = 30 Electric heating pads (Operatherm 20+ prewarmed gel) Duration: not stated Temperature setting at 39°C Body area covered: not stated Co‐interventions: all IV fluids were warmed to 37ºC with an infusion warmer (BW 485 l, Biegler GmbH, Austria) Room temperature: 20° ± 1ºC |
|
| Outcomes | Thermal comfort in post‐surgical waking‐up (VAS 0 ‐ 10) Shivering (present/absent) Blood loss (ml) Fluids infused (crystalloids) (ml) |
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blinded |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
O'Brien 2010.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: not stated Setting: 1 centre (USA) Sample size: 130 Funding: funded, in part, by an educational grant from Arizant Healthcare Inc. |
|
| Participants | Age (mean): 36 years Gender (M/F): 86/44 ASA grade: not stated Surgery type: elective (outpatient orthopaedic surgery: knee or shoulder) Surgery duration (mean): < 2 hrs (103 mins) Anaesthesia type: regional |
|
| Interventions |
Intervention (ABSW): n = 58 Bair PAWS (Patient Adjustable Warming System) (Arizant Healthcare, Inc, Eden Prairie, MN) Duration (mean): not stated Temperature: 40 ± 3ºC on average Body area covered: not stated Control: n = 72 1 warmed blanket and 1 ambient‐temperature cotton blanket Duration (mean): not stated Co‐interventions: not stated Room temperature: not stated |
|
| Outcomes | Thermal comfort (VAS 0 ‐ 100) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Pagnocca 2009.
| Methods | Design: RCT Operative phase: intra‐ and postoperative Withdrawals: not stated Setting: 1 centre (Brazil) Sample size: 43 Funding: not stated |
|
| Participants | Age (mean range): 49 ‐ 54 years Gender (M/F): 16/27 ASA grade: I ‐ III Surgery type: elective (exploratory xyphopubic laparotomy) Surgery duration: > 2 hrs (229/268 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1+ABSW2): n = 19 "Conductive+convective group": circulating‐water mattress + forced‐air warming blanket Duration (mean): not stated Temperature: 42ºC Body area covered (forced‐air warming blanket): over the thorax and upper limbs Proportion covered: not stated Intervention (ABSW1): n = 24 "Conductive group": circulating‐water mattress covered with a cotton sheet Duration (mean): not stated Temperature: 37ºC Body area covered: not applicable In the conductive group, the circulating‐water mattress was covered with a cotton sheet, participants were covered up to the cervical region with a simple surgical field until exposure of the abdomen for the xyphopubic incision Co‐interventions: not stated Room temperature: 22ºC |
|
| Outcomes | Complaints of feeling cold Shivering ("tremors") (present/absent) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Flipping of a coin |
| Allocation concealment (selection bias) | Unclear risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Paris 2014.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (USA) Sample size: 226 (73 in an arm not part of this review) Funding: Medline Industries donated the warming pads and temperature sensing catheters |
|
| Participants | Age (mean): 31 yrs Gender (M/F): all women ASA grade: not reported Surgery type: elective cesarean delivery Surgery duration (mean): < 2 hrs (40 mins) Anaesthesia type: epidural |
|
| Interventions |
Intervention (ABSW): n = 77 Warm foam pad Temperature set at : 40.3ºC Duration: intraoperative Body area covered: under body Control (usual care): n = 76 "Usual care" for all participants in the OR as follows: 1 warm blanket applied to lower extremities and 1 warm blanket applied across maternal upper chest and arms Duration: intraoperative Co‐interventions: usual care for all participants in the OR as follows: 1 warm blanket applied to lower extremities and 1 warm blanket applied across maternal upper chest and arms. In the PACU, the nurse implemented the use of rescue blankets only when participants complained of being cold or shivered, or both. Room temperature (mean): 21.1ºC |
|
| Outcomes | Fluids infused (total) (ml) Blood loss (ml) Pain scores (reported as "maximum pain category: minimal, moderate, severe") Thermal comfort (VAS 0 ‐ 10, then dichotomized: 'complaint of feeling cold', and 'complaint of feeling hot') Other outcomes reported not included in the review:
|
|
| Notes |
Compasion 1 A 3rd control arm (Warmed IV fluids) was excluded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Perl 2014.
| Methods | Design: RCT Operative phase: pre‐ and intraoperative Withdrawals: 21 were excluded Setting: multi‐centre (3 sites) (Germany, Belgium, and Spain) Sample size: 63 Funding: none declared |
|
| Participants | Age (mean range): 43 ‐ 52 years Gender (M/F): 53/15 ASA grade: I ‐ III Surgery type: elective surgery under general anaesthesia that was scheduled to last between 30 mins and 120 mins (50% ‐ 57% abdominal) Surgery duration (mean): < 2 hrs (60 ‐ 69 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW preoperative): n = 31 Participants were prewarmed in the holding area with a prewarming suit (Mistral‐Air™ Premium Warming Suit, The 37Company, Amersfoort, The Netherlands) and actively warmed with forced air (Mistral Air™ warming unit, The 37Company), for 30 ‐ 60 mins prior to induction of anaesthesia Control 1 (preoperative) : n = 32 Participants in the control group were covered up preoperatively according to the local standard with a hospital duvet on the ward *Control 2 (passive warming preoperative) : n=27 There was a passive prewarming group were participants were covered up preoperatively in the holding area with a Mistral‐Air™ Premium Warming Suit (The 37Company, Amersfoort, The Netherlands) * Note: This group is excluded from the review Co‐intervention: All participants were actively warmed during surgery immediately after induction of anaesthesia using forced air with an upper‐body blanket or lower‐body blanket (Thermoflect®, Amersfoort, The Netherlands) All intraoperative administered IV fluids were warmed to 37°C by an infusion warmer. The temperature in the holding area was not standardised |
|
| Outcomes | Thermal comfort (10‐point scale) Modified Aldrete Score Shivering Adverse effects (skin lesions or burns) |
|
| Notes | Comparison 3 (Convective pre‐ + FAW intraop. vs FAW intraop.) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Centralised via web page |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21 participants out of 90 (23%) were excluded: reasons given in Fig. 1 |
| Selective reporting (reporting bias) | Low risk | Accessible information in clinicaltrials.gov |
| Other bias | Low risk | |
Persson 2001.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 3/59 (5%) Setting: 1 centre (Sweden) Sample size: 59 Funding: not stated |
|
| Participants | Age (mean): 48.0 ‐ 48.3 years Gender (M/F): 0/59 ASA grade: I ‐ II Surgery type: elective (subtotal hysterectomy) Surgery duration (mean): < 2 hrs (79/91 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 29 Upper‐body forced‐air warming blanket (Warm‐Touch®, Mallinckrodt, USA) connected to a forced‐air blower (Warm‐TouchTM, Mallinckrodt, USA) Duration (mean): started approximately 15 mins after induction of general anaesthesia Temperature: set at 43° ‐ 46°C Body area covered: over the chest adjacent to the skin covering both arms Proportion covered: not stated Control: n = 30 Cotton blankets Body area covered: upper body Co‐interventions: Both participant groups had cotton blankets covering the body after surgery. No active warming was provided in the PACU Room temperature: 23.4° ‐ 23.5ºC |
|
| Outcomes | Blood loss (ml) Pain (VAS 0 ‐ 10) Shivering (the paper states that "shivering was reported in one patient in the warm group, but none in the control group") Other outcomes reported not included in the review:
|
|
| Notes |
Comparison 1 Results presented in graphs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses recording data of interest were blinded to the study |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant in the warm group was excluded because of intubation difficulties. 2 participants in the warm group were excluded as a result of an altered surgical procedure and re‐operation due to bleeding within 12 hrs respectively. In the postoperative period, 11 participants in the warm group and 7 in the control group did not fully complete the 48‐hr study protocol because of postoperative nausea and vomiting |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Peña García 1996.
| Methods | Design: RCT Operative phase: intra‐ and postoperative Withdrawals: not stated Setting: 1 centre (Spain) Sample size: 72 Funding: not stated |
|
| Participants | Age (mean): 60/61 years Gender (M/F): 63/9 ASA grade: I ‐ III Surgery type: elective (thoracic surgery, lateral thoracotomy) Surgery duration (mean): > 2 hrs (152/171 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1): n = 18 Convective warming blanket Duration (mean): started approximately 15 mins after induction of general anaesthesia Temperature: set at 37.8°C Body area covered: lower body Proportion covered: not stated Intervention (ABSW1 + Control 1): n = 18 Convective warming blanket + blood infusion warmer Temperature: set at 37.8°C. Body area covered: lower body Proportion covered: *Control 1: n = 18 IV fluids warmed with an infusion warmer * Note: we have excluded this group from the review, leaving Control 2 as "true control" Control 2: n = 18 Usual care (non‐active warming) Co‐interventions: not stated Room temperature: 22ºC |
|
| Outcomes | Blood loss (ml) Transfusions (ml) |
|
| Notes |
Comparison 1 The 2 intervention groups have been merged in the analysis, and a control arm (control 1) has been excluded, giving 1 single comparison Comparison 3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Pu 2014.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (China) Sample size: 110 Funding: In part by the Science and Technology Commission of Shanghai Jiao Tong University (Project Jyh0913) |
|
| Participants | Age (mean): 68 yrs Gender (M/F): 60/50 ASA grade: I ‐ II Surgery type: open and laparoscopic surgery for gastrointestinal tumours Surgery duration (mean): > 2 hrs (146/149 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 55 Forced‐air warming disposable underbody warming blanket (Model 545, 585, 3MTM Bair Hugger®, Saint Paul, MN, USA) with reusable forced‐air warming system (Model 750, 3MTM Bair Hugger®) for either horizontal position or lithotomy position Temperature set at: up to 41ºC Body area covered: legs up to the pubis Control: n = 55 Warm quilt Co‐interventions: The CO₂ used for maintaining pneumoperitoneum was not prewarmed (room temperature) and the fluids intake during the operation were room‐temperature crystalloid solutions Room temperature: 22° ‐ 24 ºC |
|
| Outcomes | Fluids infused (total) (ml) Blood loss (ml) Wound infection Shivering (VAS 0 ‐ 10) Pain (VAS 0 ‐ 10) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Rasmussen 1998.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (Denmark) Sample size: 24 Funding: not stated |
|
| Participants | Age (mean): 71/66/63 years Gender (M/F): 10/14 ASA grade: I ‐ II Surgery type: elective major abdominal surgery (colonic resections or rectal amputations) Surgery duration (mean): > 2 hrs ("surgery lasting at least two hours") Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1): n = 8 Forced‐air warming (Bair Hugger® model 500, Augustine Medical, Eden Prairie, MN, USA) Temperature set at 43ºC Duration: not stated Body area covered: upper extremities and upper thorax *Intervention (ABSW2): n = 8 Oesophageal heat exchanger CF1 (Granulab International BV, Amersfoort Netherlands), connected to an oesophageal double‐lumen, coaxial tube, circulated with warmed water in a closed system Temperature set at 41ºC Duration: not stated * Note: we have excluded this group from the review Control: n = 8 "No active warming" Co‐interventions: blood transfusions were given at 37 ºC through a heating‐device. The remaining IV fluids were given at room temperature. In the OR all participants were placed on a gel mattress prewarmed at 40 ºC Room temperature: 22.3/21.5/22.1ºC |
|
| Outcomes | Shivering (present/absent) Fluids infused (total) (ml) |
|
| Notes | Comparison 1 (x2 comparisons) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1. |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomized were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Rathinam 2009.
| Methods | Design: RCT Operative phase: pre‐ and intraoperative Withdrawals: 7 Setting: 1 centre (UK) Sample size: 30 Funding: not stated |
|
| Participants | Age (mean range): 66 ‐ 69 years Gender (M/F): 22/9 (* error in table 1) ASA grade: not stated Surgery type: elective (major thoracic surgical procedures) Surgery duration: > 2 hrs (140/45 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 14 Forced‐air warming (Warm Touch®, Mallinckrodt Medical) Duration (mean): started approximately 15 mins after induction of general anaesthesia Temperature: set at 38°C Body area covered: lower half of the body from iliac crest with extensions to cover the chest and abdomen outside the area of sterile preparation. FAW blankets were replaced by cotton blankets at the end of the procedure Proportion covered: not stated Control: n = 16 Padded blankets (Mediwrap®) Duration (mean): Mediwrap blankets were applied 30 mins prior to transfer to the OR. This was continued during positioning of participants for epidural (allowing exposure of thoracic spine), induction of anaesthesia, and positioning for surgery. A flap of the blanket was cut open to allow access for surgery. At the end of the procedure this flap was placed back and fastened with tapes to be continued into the postoperative period Temperature: set at 37.8°C Co‐interventions: Measures to prevent hypothermia like fluid warmers (set at 38ºC), low flow anaesthesia, heat and moisture exchange filters in the breathing circuits were used in both groups Room temperature: 22ºC |
|
| Outcomes | Shivering (present/absent) Fluids infused (total) (ml) Blood loss (ml) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants were treatment failures and 1 died |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Schmied 1996.
| Methods | Design: RCT Operative phase: intra + post‐operative Withdrawals: none Setting: multicentre (2) (Austria and USA) Sample size: 60 Funding: Augustine Medical Inc, the Joseph Drown and Max Kade Foundations, and National Institutes of Health grant RO 1GM49670. Mallinckrodt Anesthesia Products Inc, donated thermocouples and thermometers. The authors do not consult for, accept honoraria from, or own shares or share options in any anaesthesia‐ or surgery‐related company |
|
| Participants | Age (mean): 63 years Gender (M/F): 23/37 ASA grade: I ‐ III Surgery type: elective (primary unilateral total hip arthroplasty) Surgery duration (mean range): < 2 hrs (85/87 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 30 Upper‐body forced‐air warming cover (Bair‐Hugger®, Augustine Medical, Eden Prairie, MN) and a warmer set to "high" + intravenous fluids warmed to 37ºC Duration (mean): not stated Temperature: 37ºC Body area covered: upper body Proportion covered: not stated Control: n = 30 "Hypothermia group" (active skin and fluid warming was avoided) Co‐interventions: Low‐molecular weight heparin (5000 IU every 8 hrs) starting 2 hrs before surgery Room temperature: 21ºC |
|
| Outcomes | Fluids infused (crystalloids; colloids) (intraoperatively and postoperatively) Blood loss (cumulative) (ml) N of participants transfused Transfusion (RBC) (ml/participant) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was based on computer‐generated codes sealed in sequentially‐numbered, opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Randomization was based on computer‐generated codes sealed in sequentially‐numbered, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Scott 2001.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 14/338 (4%) Setting: 1 centre (UK) Sample size: 338 Funding: not stated |
|
| Participants | Age (mean): 68.4/68.2 years Gender (M/F): 149/175 ASA grade: I ‐ IV Surgery type: elective (orthopaedic, colorectal, gastrointestinal, urology, vascular) Surgery duration (mean): < 2 hrs (111/115 mins) Anaesthesia type (n): general or regional |
|
| Interventions |
Intervention (ABSW): n = 161 Forced‐air warming device + IV fluids warmed Temperature set at: not stated Duration: not stated Body area covered: not stated Control: n = 163 "Standard care that included automatic regulation of ambient temperature, minimal patient exposure during preparation time, and storage of the blankets in warming units for immediate postoperative use". IV infusions and blood products were warmed at clinical discretion Co‐interventions: not stated Room temperature: not stated |
|
| Outcomes | Pressure ulcers | |
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocked randomization |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding could not be implemented due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14 participants withdrew because of change in surgical procedure (n = 5), cancellation of surgery (n = 6), or a communication breakdown (n = 3) |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Steinbrook 1997.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 3/27 (11%) Setting: 1 centre (USA) Sample size: 27 Funding: not stated |
|
| Participants | Age (mean): 38 ‐ 54 years Gender (M/F): not stated ASA grade: I ‐ III Surgery type: elective (major intra‐abdominal surgery) Surgery duration: not stated Anaesthesia type: general and epidural |
|
| Interventions |
Intervention (ABSW): n = 11 Forced‐air warmer (Model 500, Bair Hugger®, Augustine Medical, Inc., Eden Prairie, MN) was employed to maintain oesophageal temperature as close to 37°C as possible + IV fluids were warmed to 37ºC Duration (mean): not stated Body area covered: not stated Control: n = 13 Routine thermal care Co‐interventions: Inspired gases were not heated Room temperature (OR and PACU): 20° ‐ 22ºC |
|
| Outcomes | Blood loss (ml) Fluids infused (ml) Shivering (present/absent) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Toss of a coin |
| Allocation concealment (selection bias) | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were withdrawn from the study because of changes in the protocol |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Suraseranivongse 2009.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (Thailand) Sample size: 44 Funding: Siriraj Research Development Fund (University in Thailand) |
|
| Participants | Age (mean): 68.7/69.3 Gender (M/F): 29/15 ASA grade: I ‐ III Surgery type: elective (aortic surgery and revascularization of the lower extremities) Surgery duration (mean: > 2 hrs (5.38/5.43 hrs) Anaesthesia type: epidural |
|
| Interventions |
Intervention (ABSW1): n = 22 Warming with a full‐length custom‐made, reusable forced‐air warming mattress (Warm Touch 5900, Tyko‐Mallinkrodt Anesthesiology product, US) set to 43ºC A surgical sheet is placed on top of the mattress with the edges tucked underneath to prevent air leak Duration (mean): not stated Body area covered: not stated Intervention (ABSW2): n = 22 Warming with a full‐length circulating‐water mattress with 2 surgical sheets on top to prevent heat burn Temperature set to 38°C Duration (mean): not stated Body area covered: not stated Co‐interventions: all fluids were warmed to 37°C Room temperature: 22ºC |
|
| Outcomes | Blood loss (median) Fluids infused (ml/kg/hr) Pressure‐heat burns Other outcomes reported not included in the review:
|
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomization based on random‐number table |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Participants were premedicated with 5 mg of midazolam or 0.5 mg of lorazepam before surgery All fluids were warmed to 37°C |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Tanaka 2013.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 6 participants were excluded Setting: 1 centre (Japan) Sample size: 70 Funding: not reported |
|
| Participants | Age (mean): 55 ‐ 60 yrs Gender (M/F): 17/47 ASA grade: I ‐ III Surgery type: elective major abdominal surgery Surgery duration (mean): > 2 hrs ( 260/290 mins) Anaesthesia type: epidural and general |
|
| Interventions |
Intervention (ABSW1): n = 33 Convective warming (Bair Hugger® system: Arizant Heatlhcare, Inc, USA) Temperature set at: 43ºC Duration: intraoperative Body area covered: upper body Intervention (ABSW2): n = 31 Resistive‐heating blanket (SmartCare®, Geratherm Medical AG, Germany) Temperature set at : 42ºC Duration: intraoperative Body area covered: upper body Co‐interventions: cotton blanket to sandwich the intervention. Room temperature (mean): 22° ‐ 24ºC |
|
| Outcomes | Blood loss (ml) Fluids infused (ml) |
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization code |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed and sequentially‐numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | No details are provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 participants were excluded (2 and 4), and reasons given in Fig. 1 |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Torrie 2005.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (USA) Sample size: 60 Funding: not stated |
|
| Participants | Age (mean): 73/72 years Gender (M/F): 60/0 ASA grade: I ‐ III Surgery type: elective (transurethral resection of the prostate) Surgery duration (mean): < 2 hrs (50/56 mins anaesthesia duration) Anaesthesia type: spinal |
|
| Interventions |
Intervention (ABSW1): n = 32 Upper‐body forced‐air warming (Bair‐ Hugger®; Augustine Medical, Eden Prairie, MN) Temperature set at 43 ºC Duration: not stated Body area covered: not stated Intervention (ABSW2): n = 28 Radiant warming, directed at the palm of the hand (Suntouch) Temperature set at 41ºC Duration: not stated Body area covered: not stated Co‐interventions: IV fluids were warmed to 41°C (hotline directly connected to intravenous catheter) + irrigation fluids were warmed to 42 ºC (warming cabinet) Room temperature: 23ºC |
|
| Outcomes | Thermal comfort (VAS 0 ‐ 10) Shivering (present/absent) Fluids infused (L) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Unclear risk | Not reported |
| Co‐interventions equal between groups | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided regarding the principles of the analyses performed, nor about the missing values of the variables or the imputing of missing values |
| Selective reporting (reporting bias) | High risk | Methods report participant´s comfort as an outcome, but no data are provided in Results Section about it. |
| Other bias | Low risk | |
Vassiliades 2003.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 4/98 (4%) Setting: 1 centre (USA) Sample size: 98 Funding: Medivance (Louisville, CO) provided financial support, in the form of donated equipment, and technical assistance |
|
| Participants | Age (mean): 64.3 ‐ 65.8 years Gender (M/F): 68/30 ASA grade: not stated Surgery type: elective (OPCABG) Surgery duration: > 2 hrs (171.6/181.8 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1): n = 41 Arctic Sun system (Medivance, Louisville, CO) (2 pads, Arctic Sun Energy Transfer Pads® with temperature‐controlled water flowing through the pads) No additional methods of warming, including a whole‐body heating blanket, were used Duration (mean): not stated Body area covered: participant’s back Intervention (ABSW2): n = 57 Forced‐air warming blanket + infusing warm IV fluids Duration (mean): not stated Body area covered: not stated Co‐interventions: IV fluid warmers, water blankets, or forced‐air warmers were not used in the intervention group, but were used in the control group Room temperature: 22.5° ‐ 24.3ºC |
|
| Outcomes | Blood loss (reported in a narrative manner) Fluids infused (reported in a narrative manner) |
|
| Notes |
Comparison 2 Results in graphs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | None reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants (2 conventional and 2 Arctic Sun) were converted to cardiopulmonary bypass intra‐operatively and withdrawn from the study |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Winkler 2000.
| Methods | Design: RCT Operative phase:intraoperative Withdrawals: 1 Setting: 1 centre (Austria) Sample size: 150 Funding: not stated |
|
| Participants | Age (mean): 65/64 years Gender (M/F): 65/84 ASA grade: I ‐ III Surgery type: elective (total hip arthroplasty) Surgery duration (mean): < 2 hrs (97/102 mins) Anaesthesia type: spinal |
|
| Interventions |
Intervention (ABSWa): n = 75 Aggressive upper‐ and lower‐body forced‐air covers connected to individual forced‐air heaters (Bair‐ Hugger®; Augustine Medical, Eden Prairie, MN). Temperature of the warmers was adjusted as necessary to maintain 36.5ºC Duration: not stated Body area covered: not stated Intervention (ABSWb): n = 75 Conventional upper‐ and lower‐body forced‐air covers connected to individual forced‐air heaters (Bair‐ Hugger®; Augustine Medical, Eden Prairie, MN) Temperature of the warmers was adjusted, as necessary, to maintain 36ºC Duration: not stated Body area covered: not stated Co‐interventions: IV fluids were warmed to 37°C Room temperature: 23ºC |
|
| Outcomes | Core temperature Blood loss |
|
| Notes | Comparison 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The surgeons were blinded to group assignment and perioperative core temperature, as were the observers who weighed gauze‐sponges and calculated blood recovered by a red‐blood‐cell scavenging system. Results were analysed after completion of data collection and an audit confirming integrity of the randomization process |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants assigned to conventional warming had mean intraoperative core temperatures 36.5°C; similarly, 4 participants assigned to aggressive warming had mean intraoperative core temperatures 36.0°C Data from these participants were included in the analysis on the basis of their intended treatments. 1 conventionally‐warmed participant returned emergently to the operating room after several hours of recovery because of a surgical complication. His data from the initial surgery were included in the analysis; however, his postoperative data were not. All other participants were treated per randomization and completed the study |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Unclear risk | We were not able to assess other bias with the information that was provided in the article. |
Wong 2007.
| Methods | Design: RCT Operative phase: pre‐, intra‐ and postoperative Withdrawals: 2/103 Setting: 1 centre (UK) Sample size: 103 Funding: not stated |
|
| Participants | Age (mean range): 60.5 ‐ 63.0 years Gender (M/F): 53/50 ASA grade: I ‐ III Surgery type: elective (major open abdominal surgery) Surgery duration (mean): > 2 hrs (3.0/3.5 hrs theatre time) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW periop + co‐FAW intraop): n = 47 Conductive carbon polymer mattress (Inditherm® warming mattress, Rotherham, UK) 2 hrs before transfer from the ward to the operating theatre, during surgery and up to 2 hrs after surgery Temperature: set at 40°C Theatre time (mean): 3.0 hrs Body area covered: not stated Control (co‐FAW intraop): n = 56 In the control group, the mattresses were switched off Theatre time (mean): 3.5 hrs Body area covered: not stated Co‐interventions: all the participants in this study were warmed during surgery ("It was standard practice to deliver systemic warming during all major surgery using a forced‐air warming device (Bair Hugger; Arizant Healthcare, Eden Prairie, Minnesota, USA) set at 40°C and with a fluid warmer") Room temperature: not stated |
|
| Outcomes | Blood loss Need for blood transfusion Postoperative complications (surgical site infection, chest infections, ileus, urinary tract infections, pelvic collection, cardiac complications, Clostridium difficile diarrhoea, and pressure ulcers) Length of hospital stay |
|
| Notes | Comparison 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | In the control group, the mattresses were switched off |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent observer unaware of the participants’ group assignment, evaluated the participants’ surgical wounds, postoperative variables and complications daily during hospitalization and again at 6 – 8 weeks after surgery. The senior surgeons, who were unaware of the participants’ group assignment and core temperatures, decided when to begin feeding after surgery, remove sutures and discharge from hospital |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant In the control group was warmed preoperatively. 1 participant In the intervention group was not warmed preoperatively. These participants, however, were included in the ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Wongprasartsuk 1998.
| Methods | Design: RCT Operative phase: pre‐ and intraoperative Withdrawals: 4/30 (13%) Setting: 1 centre (Australia) Sample size: 26 Funding: not stated |
|
| Participants | Age (mean): 50 years Gender (M/F): 14/12 ASA grade: I ‐ III Surgery type: elective (lower limb orthopaedic surgery) Surgery duration: > 2 hrs (148/163 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 14 Forced‐air warming (Bair Hugger® Augustine Medical, Inc., Eden Prairie, MN) Duration: 30 mins preoperative + during surgery Temperature setting at 18‐19 °C Body area covered: upper body and limbs Proportion covered: < 50% Control: n = 12 2 cotton blankets Co‐interventions: all IV fluids were warmed via a warming coil (heated water‐bath type) Room temperature: 18° ‐ 19ºC |
|
| Outcomes | Thermal comfort (VAS 0 ‐ 10) Shivering (present/absent) Pain (VAS 0 ‐ 10) |
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Yamakage 1995.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: none Setting: 1 centre (Japan) Sample size: 21 Funding: not stated |
|
| Participants | Age (mean): 56.2 years Gender (M/F): 13/8 ASA grade: I ‐ II Surgery type: elective (lower abdomen or lower extremity surgery) Surgery duration (mean): not stated Anaesthesia type: spinal |
|
| Interventions |
Intervention (ABSWa): n = 7 Lower‐body warmed by forced‐air warmer (Bair Hugger®). The Bair Hugger supplied air to a disposable blanket laid over the participant, creating a shell of warm air around the body via flow through linear channels and small openings on the blanket's underside Temperature set at 37ºC Duration: not stated Body area covered: below the T10 dermatome Intervention (ABSWb): n = 7 Upper‐body warmed by forced‐air warmer (Bair Hugger®). The Bair Hugger supplied air to a disposable blanket laid over the participant, creating a shell of warm air around the body via flow through linear channels and small openings on the blanket's underside Temperature set at 37ºC Duration: not stated Body area covered: above the T7 dermatome Control: n = 7 Light blanket upper body Duration: not stated Body area covered: above the T7 dermatome Co‐interventions: not stated Room temperature: 23ºC |
|
| Outcomes | Thermal comfort (VAS 0 ‐ 100) Shivering (VAS 0 ‐ 100) |
|
| Notes |
Comparison 1 The 2 intervention groups have been merged in the analysis, giving 1 single comparison Comparison 3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1. |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Yildirim 2012.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 7 Setting: 1 centre (Turkey) Follow‐up: 60 mins Sample size: 87 Funding: not stated |
|
| Participants | Age (mean): 44.05 ‐ 45.48 years Gender (M/F): 12/68 Surgery type: elective (open cholecystectomy) ASA grade: I (69) ‐ II (11) Surgery duration (mean): < 2 hrs (57 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 40 Warming pad (KanMed® heater device) placed under the participant. The participants in the heated group were applied peripheral warming process at 37°C by using the pad of the heating device (STOCKERT Heater‐Cooler System 3T, Germany) covering the entire operation table during their operations Temperature set at 37ºC Duration: not stated Body area covered: placed under the participant Control: n = 40 No warming process was applied for the control group Co‐interventions: after the operation was completed the participants were covered with the same standard blankets Room temperature: 21.6°/21.4ºC |
|
| Outcomes | Shivering (present/absent) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Closed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded to the allocation of participants |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomized were analysed |
| Selective reporting (reporting bias) | High risk | Cardiovascular complications were addressed as outcome in Methods, but not reported in Results |
| Other bias | Low risk | |
Zangrillo 2006.
| Methods | Design: RCT Operative phase: intraoperative Withdrawals: 9/40 (22.5%) Setting: 1 centre (Italy) Sample size: 40 Funding: not stated |
|
| Participants | Age (mean): 63.9 ‐ 67.3 years Gender (M/F): 26/5 ASA grade: not stated Surgery type: elective (OPCABG) Surgery duration (mean): > 2 hrs (185 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW1): n = 15 Circulating‐water garment system (Allon Thermo‐Wrapping Thermoregulation System; MTRE Advanced Technologies Ltd, Or Akiva, Israel) (microprocessor‐controlled heating) Temperature: set at 37 °C Duration (mean): not stated Body area covered: large area of the body Proportion covered: not stated Intervention (ABSW2): n = 16 Forced‐air warming system. Heat convective transfer with a forced‐air system set at 38°C (Bair Hugger, Sterile Cardiac Access blanket Model 645, Augustine SA, Berne, Switzerland) and conductive transfer with a thermostatic water mattress (Thermostat T1000 JMW Medical System Ltd, Midlothian, UK) Temperature: set at 38°C Duration (mean): not stated Body area covered: torso and legs Proportion covered: not stated Co‐interventions: IV fluids heated by a warmer set at 41°C (Hotline; Sims Medical System, Rockland, MA) Room temperature: not stated |
|
| Outcomes | Fluids infused (crystalloids; plasma expanders; total fluids) (ml) Blood loss (ml) The paper states that none of the participants died in the hospital The paper states that there were no adverse events reported Other outcomes reported not included in the review:
|
|
| Notes | Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label ('risk of bias' judgement depending on the nature of the outcome) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding methods were described, but all outcomes were objective (fluids and blood loss) |
| Baseline comparability of groups | Low risk | Groups were similar for baseline factors |
| Co‐interventions equal between groups | Low risk | All co‐interventions and procedures were equal between groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Equal losses in both groups, due to conversion to CBP surgery |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
Zhao 2005.
| Methods | Design: RCT Operative phase: intra‐ and postoperative Withdrawals: not stated Setting: 1 centre (China) Sample size: 40 Funding: not stated |
|
| Participants | Age (mean range): 44 ‐ 52 years Gender (M/F): 23/17 ASA grade: I ‐ II Surgery type: elective (abdominal surgery) Surgery duration (average): > 2 hrs (204/230 mins) Anaesthesia type: general |
|
| Interventions |
Intervention (ABSW): n = 20 Forced‐air warming blanket (Warmtouch®, Mallinckrodt Medical Inc, St Louis, Mo) + IV fluid warming (Warmflo®, Mallinckrodt Medical Inc, St Louis, Mo) Temperature: set at 42° ‐ 43 °C Duration (mean): not stated Body area covered: from the legs up to the pubis Proportion covered: not stated Control: n = 20 Single layer of cotton sheet Duration (mean): not stated Co‐interventions: not stated Room temperature: 22.4° ‐ 22.5ºC |
|
| Outcomes | Transfusion (Units) Shivering ('absent/mild/medium/severe') Blood loss (ml) Fluids infused (crystalloids; colloids) (ml) Other outcomes reported not included in the review:
|
|
| Notes | Comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Baseline comparability of groups | Low risk | To a high extent according to Table 1 |
| Co‐interventions equal between groups | Unclear risk | None reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol, therefore we cannot exclude risk of selective reporting with the information provided |
| Other bias | Low risk | |
ASEPSIS: A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials Points are given for the need for Additional treatment, the presence of Serous discharge, Erythema, Purulent exudate, and Separation of the deep tissues, the Isolation of bacteria, and the duration of inpatient Stay (ASEPSIS). CABG: coronary artery bypass graft CBP: coronary bypass CK: creatine kinase ECC: Extracorporeal Circulation ECG: electrocardiograph FAW: forced‐air warming hr: hour ICU: intensive care unit ITT: intention‐to‐treat IV: intravenous min(s): minute(s) OPCABG: off‐pump coronary artery bypass graft OR: operating room PACU: perioperative anaesthetic care unit PP: post partum CVP: Central Venous Pressure RBC: red blood cell VAS: visual analogue scale VNRS: verbal numerical rating score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 2009 | Comparison not considered by this review |
| Baxendale 2000 | The trial only included temperature as an outcome |
| Berti 1997 | The trial only included temperature as an outcome |
| Borms 1994 | The trial only included temperature as an outcome |
| Bourke 1984 | Comparison not considered by this review |
| Buggy 1994 | The trial only included temperature as an outcome |
| Cobbe 2012 | The trial was performed in healthy voluntaries |
| De Bernardis 2009 | The trial only included temperature as an outcome |
| Dyer 1986 | Comparison not considered by this review |
| Erickson 1991 | Comparison not considered by this review |
| Fanelli 2009 | Comparison not considered by this review |
| Farley 2004 | Comparison not considered by this review |
| Fleisher 1998 | The trial did not report on outcomes of interest for this review |
| Frank 1995 | The trial only included temperature, neuroendocrine response and blood pressure as outcomes |
| Grocott 2004 | Comparison not considered by this review |
| Harper 2007 | The trial only included temperature as an outcome |
| Hindsholm 1992 | Comparison not considered by this review |
| Hoyt 1993 | Comparison not considered by this review |
| Hynson 1992 | The trial only included temperature as an outcome |
| Insler 2008 | The trial only included temperature as an outcome |
| Janicki 2002 | The trial only included temperature as an outcome |
| Joachimsson 1987 | The trial only included temperature as an outcome |
| Kamitani 1999 | The trial only included temperature as an outcome |
| Karayan 1996 | The trial only included temperature as an outcome |
| Kim 2006 | The trial only included temperature as an outcome |
| Kongsayreepong 2002 | The study was performed in neonates |
| Kurz 1993 | The trial only included temperature as an outcome |
| Lenhardt 1997 | The trial only included temperature as an outcome |
| Matsuzaki 2003 | The trial did not report on outcomes of interest for this review |
| Motamed 2000 | Comparison not considered by this review |
| Murat 1994 | The study population were children + the trial only included temperature as an outcome |
| Müller 1993 | The trial only included temperature as an outcome |
| Müller 1995 | The trial only included temperature as an outcome |
| Nesher 2003 | The trial only included temperature and lab parameters as an outcome |
| Onik 1993 | The trial only included temperature as an outcome |
| Ouellette 1993 | The trial only included temperature as an outcome |
| Patel 1997 | Comparison not considered by this review |
| Radel 1986 | The trial only included temperature as an outcome |
| Radford 1979 | The trial only included temperature as an outcome |
| Rein 2007 | The trial only included temperature as an outcome |
| Russell 1995 | The trial only included temperature as an outcome |
| Salazar 2011 | The trial did not include any relevant outcome included in this review |
| Saldanha 2001 | Participants are rewarmed if hypothermic |
| Sessler 1988 | Comparison not considered by this review |
| Severens 2007 | The intervention was applied postoperatively (rewarming) |
| Sheng 2003 | The trial only included temperature as an outcome |
| Smith 1994 | It reports shivering only by graphics (no raw data available) |
| Smith 2006 | Participants in the control group (routine care) received intraoperative convective or IV fluid warming or both at the discretion of the anaesthesiologist |
| Summers 1990 | The intervention was applied postoperatively |
| Tollofsrud 1984 | The trial only included temperature as an outcome |
| Wagner 2006 | Quasi‐experimental design |
| Whitney 1990 | The trial only included temperature as an outcome |
| Wong 2004 | The trial only included temperature as an outcome |
Characteristics of studies awaiting assessment [ordered by study ID]
Kaudasch 1996.
| Methods | RCT |
| Participants | 24 ASA II and III patients scheduled for elective colon surgery |
| Interventions | Participants were randomly assigned to 1 of 2 groups: control group (n = 12, no warming therapy, upper body covered with a cotton hospital blanket) or a convective warming group (n = 12) |
| Outcomes | According to the abstract, the specific aims of the study were: "drawing up heat balances; and analysing postoperative thermoregulation, oxygen consumption and cardiovascular reactions of mechanically ventilated patients." |
| Notes | Full text not yet available (in German) |
Leben 1997.
| Methods | RCT |
| Participants | 40 patients scheduled for elective lumbar or lower thoracic spine surgery |
| Interventions | Convective heating and warm infusion |
| Outcomes | |
| Notes | No abstract available on Pubmed |
Xu 2004.
| Methods | RCT |
| Participants | 40 ASA I ‐ II patients, aged 21 ‐ 69 years, scheduled for elective abdominal surgery under general anaesthesia |
| Interventions | Participants were randomly divided into 2 groups: control group (n = 20) and warming group (n = 20). In both groups, the participants were covered with surgery blanket. In the warming group, participants were additionally warmed with fluid‐warming device and forced‐air warming system during the operation |
| Outcomes | Core temperature, blood loss, blood transfusion, extubation time, and postoperative shivering |
| Notes | Full text not yet available (in Chinese) |
Differences between protocol and review
We have made the following changes from the published protocol (Urrútia 2011):
We have amended the title of the review by adding 'body surface' (active body surface warming systems), to better define the interventions that are of interest in this review. Initially, we had intended to cover all types of active warming systems, but we was subsequently restricted the scope to those systems that are based on heat transfer through skin contact. We have also made it clear that the aim of the intervention is to prevent 'complications from' unintended hypothermia, rather than preventing hypothermia itself, as this review does not assess the impact on temperature.
Accordingly, we have revised the criteria for considering studies for this review (types of interventions) with a detailed specification of which interventions are covered by this review and which others are excluded (the latter being now covered by a separate reviews that have been cited in the text).
Due to the high variability in the interventions used in the studies (often combined interventions), which also affects the control group, where an active warming system not covered by this review was often used as a co‐intervention, we have clarified this limitation in the text. In particular, this has led to the modification of the Types of interventions section, making clearer the criteria for including or excluding studies depending on whether the co‐interventions were applied equally in both groups as part of routine care.
We have introduced some changes in the Types of outcome measures section. As this review is based on clinical outcomes (rather than temperature), which are sparse and widely scattered across studies, we attempted to maximize the information available on those outcomes that are of some interest to our review. This may have caused some post hoc changes. Thus, in order to minimize the number of outcomes included in the review, we have merged burns into adverse effects, while shivering, blood loss and intraoperative fluids have been added separately as new secondary outcomes. All‐cause mortality is now a secondary outcome.
There has been a change of authors based on their contribution to the review.
We conducted the main analyses on an 'available data' basis, including data the same way they were reported in the trials, instead of by intention‐to‐treat, as planned in the review protocol. We took this decision after discussion, taking into account the burden and likelihood of bias that missing data could represent in the review.
In order to shorten the review and make it more readable, we have withdrawn results on temperature, as this was not among our selected outcomes.
Contributions of authors
Eva Madrid, Gerard Urrútia, Marta Roqué i Figuls, Hector Pardo Hernandez, Pablo Alonso‐Coello, Juan Manuel Campos, Pilar Paniagua, Luz Maestre
Conceiving the review: GU, MR, JMC, PA Co‐ordinating the review: GU Screening search results: GU, MR, EM Organizing retrieval of papers: GU, EM Screening retrieved papers against inclusion criteria: GU, HP, EM and collaborators (SC, BN, EP) Appraising quality of papers: GU, MR, EM , PA Solving disagreements (eligibility and risk of bias): JMC, PP, LM Abstracting data from papers: GU, MR, EM, HP, PA Writing to authors of papers for additional information: GU Data management for the review: HP, EM, MR Entering data into Review Manager (RevMan 5.3): MR, HP, EM RevMan statistical data: MR Other statistical analysis not using RevMan: MR Interpretation of data: MR, GU, PA, EM, JCM, PP, LM Statistical inferences: MR Writing the review: all authors Securing funding for the review: GU, MR Performing previous work that was the foundation of the present study: GU, MR Guarantor for the review (one author): GU Person responsible for reading and checking review before submission: all authors
Sources of support
Internal sources
No sources of support supplied
External sources
Agencia de Calidad del Sistema Nacional de Salud, Ministerio de Sanidad y Política Social, Spain.
Instituto de Salud Carlos III ‐ Fondo de Investigación Sanitaria (grant PI08/90403), Spain.
Biomedical Research Centre Universidad de Valparaiso, Chile, Grant 06/2006 partially supported EM visits to Cochrane Iberoamerican Centre during authoring phase. ., Other.
New Source of support, Other.
Declarations of interest
Eva Madrid has no conflicts of interest to declare.
Gerard Urrútia has received consultant fees from Novartis and payments for a methodological workshops from RIMA, Novartis and GSK (addressed to lab representatives or doctors). His institution has received a grant from Instituto de Salud Carlos III (Grant PI08/90403) (Public funding to produce a technology assessment report on forced‐air warming).
Marta Roqué i Figuls has no conflicts of interest to declare.
Hector Pardo Hernandez has no conflicts of interest to declare.
Pablo Alonso‐Coello has no conflicts of interest to declare.
Joan M Campos has no conflicts of interest to declare.
Pilar Paniagua has received payment from CSL Behringer for development of educational presentations about coagulation management in massive bleeding patients.
Luz Maestre has no conflicts of interest to declare.
New
References
References to studies included in this review
Andrzejowski 2008 {published data only}
- Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post‐induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. British Journal of Anaesthesia 2008;101(5):627‐31. [DOI] [PubMed] [Google Scholar]
Bennett 1994 {published data only}
- Bennett J, Ramachandra V, Webster J, Carli F. Prevention of hypothermia during hip surgery: effect of passive compared with active skin surface warming. British Journal of Anaesthesia 1994;73(2):180‐3. [DOI] [PubMed] [Google Scholar]
Benson 2012 {published data only}
- Benson EE, McMillan DE, Ong B. The effects of active warming on patient temperature and pain after total knee arthroplasty. The American Journal of Nursing 2012;112(5):26‐33. [DOI] [PubMed] [Google Scholar]
Bock 1998 {published data only}
- Bock M, Müller J, Bach A, Böhrer H, Martin E, Motsch J. Effects of preinduction and intraoperative warming during major laparotomy. British Journal of Anaesthesia 1998;80(2):159‐63. [DOI] [PubMed] [Google Scholar]
Butwick 2007 {published data only}
- Butwick AJ, Lipman SS, Carvalho B. Intraoperative forced air‐warming during cesarean delivery under spinal anesthesia does not prevent maternal hypothermia. Anesthesia and Analgesia 2007;105(5):1413‐9. [DOI] [PubMed] [Google Scholar]
Calcaterra 2009 {published data only}
- Calcaterra D, Ricci M, Lombardi P, Katariya K, Panos A, Salerno TA. Reduction of postoperative hypothermia with a new warming device: a prospective randomized study in off‐pump coronary artery surgery. The Journal of Cardiovascular Surgery 2009;50(6):813‐7. [PubMed] [Google Scholar]
Campos‐Suárez 1997 {published data only}
- Campos‐Suárez JM, Casas‐Vila JI, Litvan‐Suquieni H, Villar‐Landeira JM. Air‐convection heater for abdominal surgery. Study of the relation between surgical time and the efficacy of body temperature maintenance [Calentador por convección de aire en cirugía abdominal. Estudio de la relación tiempo quirúrgico‐eficacia del mantenimiento de la temperatura corporal]. Revista Española de Anestesiología y Reanimación 1997;44(2):47‐51. [PubMed] [Google Scholar]
Camus 1993a {published data only}
- Camus Y, Delva E, Just B, Lienhart A. Leg warming minimizes core hypothermiaduring abdominal surgery. Anesthesia and Analgesia 1993;77(5):995‐9. [DOI] [PubMed] [Google Scholar]
Camus 1993b {published data only}
- Camus Y, Delva E, Just B, Lienhart AL. Leg warming minimizes core hypothermiaduring abdominal surgery. Anesthesia and Analgesia 1993;77(5):995‐99. [DOI] [PubMed] [Google Scholar]
Camus 1995 {published data only}
- Camus Y, Delva E, Sessler DI, Lienhart A. Pre‐induction skin‐surface warming minimizes intraoperative core hypothermia. Journal of Clinical Anesthesia 1995;7(5):384‐8. [DOI] [PubMed] [Google Scholar]
Camus 1997 {published data only}
- Camus Y, Delva E, Bossard AE, Chandon M, Lienhart A. Prevention of hypothermia by cutaneous warming with new electric blankets during abdominal surgery. British Journal of Anaesthesia 1997;79(6):796‐7. [DOI] [PubMed] [Google Scholar]
Casati 1999a {published data only}
- Casati A, Baroncini S, Pattono R, Fanelli G, Bonarelli S, Musto P, et al. Effects of sympathetic blockade on the efficiency of forced‐air warming during combined spinal‐epidural anesthesia for total hip arthroplasty. Journal of Clinical Anesthesia 1999;11(5):360‐3. [DOI] [PubMed] [Google Scholar]
Casati 1999b {published data only}
- Casati A, Fanelli G, Ricci A, Musto P, Cedrati V, Altimari G, et al. Shortening the discharging time after total hip replacement under combined spinal/epidural anesthesia by actively warming the patient during surgery. Minerva Anestesiologica 1999;65(7‐8):507‐14. [PubMed] [Google Scholar]
Chakladar 2014 {published data only}
- Chakladar A, Dixon MJ, Crook D, Harper CM. The effects of a resistive warming mattress during caesarean section: a randomised, controlled trial. International Journal of Obstetric Anesthesia 2014;23(4):309‐16. [DOI] [PubMed] [Google Scholar]
Chung 2012 {published data only}
- Chung SH, Lee BS, Yang HJ, Kweon KS, Kim HH, Song J, et al. Effect of preoperative warming during cesarean section under spinal anesthesia. Korean Journal of Anesthesiology 2012;62(5):454‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Angelo Vanni 2007 {published data only}
- D'Angelo Vanni SM, Castiglia YM, Ganem EM, Rodrigues Júnior GR, Amorim RB, Ferrari F, et al. Preoperative warming combined with intraoperative skin‐surface warming does not avoid hypothermia caused by spinal anesthesia in patients with midazolam premedication. São Paulo Medical Journal 2007;125(3):144‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elmore 1998 {published data only}
- Elmore JR, Franklin DP, Youkey JR, Oren JW, Frey CM. Normothermia is protective during infrarenal aortic surgery. Journal of Vascular Surgery 1998;28(6):984‐92. [DOI] [PubMed] [Google Scholar]
Fallis 2006 {published data only}
- Fallis WM, Hamelin K, Symonds J, Wang X. Maternal and newborn outcomes related to maternal warming during cesarean delivery. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2006;35(3):324‐31. [DOI] [PubMed] [Google Scholar]
Fossum 2001 {published data only}
- Fossum S, Hays J, Henson MM. A comparison study on the effects of prewarming patients in the outpatient surgery setting. Journal of Perianesthesia Nursing 2001;16(3):187‐94. [DOI] [PubMed] [Google Scholar]
Frank 1997 {published data only}
- Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997;277(14):1127‐34. [PubMed] [Google Scholar]
Hasegawa 2012 {published data only}
- Hasegawa K, Negishi C, Nakagawa F, Ozaki M. Core temperatures during major abdominal surgery in patients warmed with new circulating‐water garment, forced‐air warming, or carbon‐fiber resistive‐heating system. Journal of Anesthesia 2012;26(2):168‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi C, Hasegawa K, Mukai S, Nakagawa F, Ozaki M, Sessler DI. Resistive‐heating and forced‐air warming are comparably effective. Anesthesia and Analgesia 2003;96(6):1683‐7. [DOI] [PubMed] [Google Scholar]
Hofer 2005 {published data only}
- Hofer CK, Worn M, Tavakoli R, Sander L, Maloigne M, Klaghofer R, et al. Influence of body core temperature on blood loss and transfusion requirements during off‐pump coronary artery bypass grafting: a comparison of 3 warming systems. The Journal of Thoracic and Cardiovascular Surgery 2005;129(4):838‐43. [DOI] [PubMed] [Google Scholar]
Horn 2002 {published data only}
- Horn EP, Schroeder F, Gottschalk A, Sessler DI, Hiltmeyer N, Standl T, et al. Active warming during cesarean delivery. Anesthesia and Analgesia 2002;94(2):409‐14. [DOI] [PubMed] [Google Scholar]
Horn 2012 {published data only}
- Horn EP, Bein B, Bohm R, Steinfath M, Sahili N, Hocker J. The effect of short time periods of pre‐operative warming in the prevention of peri‐operative hypothermia. Anaesthesia 2012;67(6):612‐7. [DOI] [PubMed] [Google Scholar]
Janicki 2001 {published data only}
- Janicki PK, Higgins MS, Janssen J, Johnson RF, Beattie C. Comparison of two different temperature maintenance strategies during open abdominal surgery: upper body forced‐air warming versus whole body water garment. Anesthesiology 2001;95(4):868‐74. [DOI] [PubMed] [Google Scholar]
Johansson 1999 {published data only}
- Johansson T, Lisander B, Ivarsson I. Mild hypothermia does not increase blood loss during total hip arthroplasty. Acta Anaesthesiologica Scandinavica 1999;43(10):1005‐10. [DOI] [PubMed] [Google Scholar]
Just 1993 {published data only}
- Just B, Trévien V, Delva E, Lienhart A. Prevention of intraoperative hypothermia by preoperative skin‐surface warming. Anesthesiology 1993;79(2):214‐8. [DOI] [PubMed] [Google Scholar]
Kabbara 2002 {published data only}
- Kabbara A, Goldlust SA, Smith CE, Hagen JF, Pinchak AC. Randomized prospective comparison of forced air warming using hospital blankets versus commercial blankets in surgical patients. Anesthesiology 2002;97(2):338‐44. [DOI] [PubMed] [Google Scholar]
Kiessling 2006 {published data only}
- Kiessling AH, Isgro F, Lehmann A, Piper S, Blome M, Saggau W. Evaluating a new method for maintaining body temperature during OPCAB and robotic procedures. Medical Science Monitor 2006;12(7):MT39‐42. [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim HY, Lee KC, Lee MJ, Kim MN, Kim JS, Lee WS, et al. Comparison of the efficacy of a forced‐air warming system and circulating‐water mattress on core temperature and post‐anesthesia shivering in elderly patients undergoing total knee arthroplasty under spinal anesthesia. Korean Journal of Anesthesiology 2014;66(5):352‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krenzinschek 1995 {published data only}
- Krenzischek DA, Frank SM, Kelly S. Forced‐air warming versus routine thermal care and core temperature measurement sites. Journal of Post Anesthesia Nursing 1995;10(2):69‐78. [PubMed] [Google Scholar]
Kurz 1995 {published data only}
- Kurz A, Sessler DI, Narzt E, Bekar A, Lenhardt R, Huemer G, et al. Postoperative hemodynamic and thermoregulatory consequences of intraoperative core hypothermia. Journal of Clinical Anesthesia 1995;7(5):359‐66. [DOI] [PubMed] [Google Scholar]
Kurz 1996 {published data only}
- Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical‐wound infection and shorten hospitalization. Study of Wound Infectionand Temperature Group. New England Journal of Medicine 1996;334(19):1209‐15. [DOI] [PubMed] [Google Scholar]
Lee 2004 {published data only}
- Lee L, Leslie K, Kayak E, Myles PS. Intraoperative patient warming using radiant warming or forced‐air warming during long operations. Anaesthesia and Intensive Care 2004;32(3):358‐61. [DOI] [PubMed] [Google Scholar]
Leeth 2010 {published data only}
- Leeth D, Mamaril M, Oman KS, Krumbach B. Normothermia and patient comfort: a comparative study in an outpatient surgery setting. Journal of Perianesthesia Nursing 2010;25(3):146‐51. [DOI] [PubMed] [Google Scholar]
Leung 2007 {published data only}
- Leung KK, Lai A, Wu A. A randomised controlled trial of the electric heating pad vs forced‐air warming for preventing hypothermia during laparotomy. Anaesthesia 2007;62(6):605‐8. [DOI] [PubMed] [Google Scholar]
Lindwall 1998 {published data only}
- Lindwall R, Svensson H, Söderström S, Blomqvist H. Forced air warming and intraoperative hypothermia. The European Journal of Surgery 1998;164(1):13‐6. [DOI] [PubMed] [Google Scholar]
Mason 1998 {published data only}
- Mason DS, Sapala JA, Wood MH, Sapala MA. Influence of a forced air warming system on morbidly obese patients undergoing Roux‐en‐Y gastric bypass. Obesity Surgery 1998;8(4):453‐60. [DOI] [PubMed] [Google Scholar]
Matsukawa 1994 {published data only}
- Matsukawa T, Kashimoto S, Nakamura T, Kume M, Kanda F, Kumazawa T. Effects of a forced‐air system (Bair Hugger, OR‐type) on intraoperative temperature in patients with open abdominal surgery. Journal of Anesthesia 1994;8(1):25‐7. [DOI] [PubMed] [Google Scholar]
Melling 2001 {published data only}
- Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet 2001;358(9285):876‐80. [DOI] [PubMed] [Google Scholar]
Mogera 1997 {published data only}
- Mogera H, Dash H, Chaturvedi A, Tewari R, Bhutara S. Control of body temperature with forced‐air warming system during neurosurgery. Journal of Anaesthesiology Clinical Pharmacology 1997;13(3):207‐12. [Google Scholar]
Moysés 2014 {published data only}
- Moysés AM, Dos Santos Trettene A, Navarro LH, Ayres JA. Hypothermia prevention during surgery: comparison between thermal mattress and thermal blanket. Revista de Escola de Enfermagem USP 2014;48(2):228‐35. [DOI] [PubMed] [Google Scholar]
Ng 2003 {published data only}
- Ng SF, Oo CS, Loh KH, Lim PY, Chan YH, Ong BC. A comparative study of three warming interventions to determine the most effective inmaintaining perioperative normothermia. Anesthesia and Analgesia 2003;96(1):171‐6. [DOI] [PubMed] [Google Scholar]
Ng 2006 {published data only}
- Ng V, Lai A, Ho V. Comparison of forced‐air warming and electric heating pad for maintenance of body temperature during total knee replacement. Anaesthesia 2006;61(11):1100‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2010 {published data only}
- O'Brien D, Greenfield ML, Anderson JE, Smith BA, Morris M. Comfort, satisfaction, and anxiolysis in surgical patients using a patient‐adjustable comfort warming system: a prospective randomized clinical trial. Journal of Perianesthesia Nursing 2010;25(2):88‐93. [DOI] [PubMed] [Google Scholar]
Pagnocca 2009 {published data only}
- Pagnocca ML, Tai EJ, Dwan JL. Temperature control in conventional abdominal surgery: comparison between conductive and the association of conductive and convective warming. Revista Brasileira de Anestesiologia 2009;59(1):56‐66. [DOI] [PubMed] [Google Scholar]
Paris 2014 {published data only}
- Paris LG, Seitz M, McElroy KG, Regan M. A randomized controlled trial to improve outcomes utilizing various warming techniques during cesarean birth. Journal of Obstetric, Gynecologic & Neonatal Nursing 2014;43:719‐28. [DOI] [PubMed] [Google Scholar]
Peña García 1996 {published data only}
- Peña García I, García Miguel M. Preventive measures. The risk of hypothermia during prolonged anesthesia and surgery. Study of methods of warming [Riesgo de hipotermia en anestesia y cirugía prolongadas. Estudio de dos métodos de calentamiento]. Revista de Enfermería 1996;19(211):24‐31. [PubMed] [Google Scholar]
Perl 2014 {published data only}
- Perl T, Peichl LH, Reyntjens K, Deblaere I, Zaballos JM, Bräuer A. Efficacy of a novel prewarming system in the prevention of perioperativehypothermia. A prospective, randomized, multicenter study. Minerva Anestesiol 2014;80(4):436‐43. [PubMed] [Google Scholar]
Persson 2001 {published data only}
- Persson K, Lundberg J. Perioperative hypothermia and postoperative opioid requirements. European Journal of Anaesthesiology 2001;18(10):679‐86. [DOI] [PubMed] [Google Scholar]
Pu 2014 {published data only}
- Pu Y, Cen G, Sun J, Gong J, Zhang Y, Zhang M, et al. Warming with an underbody warming system reduces intraoperative hypothermia in patients undergoing laparoscopic gastrointestinal surgery: A randomized controlled study. International Journal of Nursing Studies 2014;51(2):181‐9. [DOI] [PubMed] [Google Scholar]
Rasmussen 1998 {published data only}
- Rasmussen YH, Leikersfeldt G, Drenck NE. Forced‐air surface warming versusoesophageal heat exchanger in the prevention of peroperative hypothermia. Acta Anaesthesiologica Scandinavica 1998;42(3):348‐52. [DOI] [PubMed] [Google Scholar]
Rathinam 2009 {published data only}
- Rathinam S, Annam V, Steyn R, Raghuraman G. A randomised controlled trial comparing Mediwrap heat retention and forced air warming for maintaining normothermia in thoracic surgery. Interactive Cardiovascular and Thoracic Surgery 2009;9(1):15‐9. [DOI] [PubMed] [Google Scholar]
Schmied 1996 {published data only}
- Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet 1996;347(8997):289‐92. [DOI] [PubMed] [Google Scholar]
Scott 2001 {published data only}
- Scott EM, Leaper DJ, Clark M, Kelly PJ. Effects of warming therapy on pressure ulcers‐a randomized trial. AORN Journal 2001;73(5):921‐7, 929‐33, 936‐8. [DOI] [PubMed] [Google Scholar]
Steinbrook 1997 {published data only}
- Steinbrook RA, Seigne PW. Total‐body oxygen consumption after isoflurane anesthesia: effects of mild hypothermia and combined epidural‐general anesthesia. Journal of Clinical Anesthesia 1997;9(7):559‐63. [DOI] [PubMed] [Google Scholar]
Suraseranivongse 2009 {published data only}
- Suraseranivongse S, Pongraweewan O, Kongmuang B, Tivirach W, Pornboonseram S. A custom‐made forced‐air warming mattress for heat loss prevention during vascular surgery: clinical evaluation. Asian Biomedicine 2009;3(3):299‐307. [Google Scholar]
Tanaka 2013 {published data only}
- Tanaka N, Ohno Y, Hori M, Utada M, Ito K, Suzuki T. A randomised controlled trial of the resistive heating blanket versus the convective warming system for preventing hypothermia during major abdominal surgery. Journal of Perioperative Practice 2013;23(4):82‐6. [DOI] [PubMed] [Google Scholar]
Torrie 2005 {published data only}
- Torrie JJ, Yip P, Robinson E. Comparison of forced‐air warming and radiant heating during transurethral prostatic resection under spinal anaesthesia. Anaesthesia and Intensive Care 2005;33(6):773‐8. [DOI] [PubMed] [Google Scholar]
Vassiliades 2003 {published data only}
- Vassiliades TA Jr, Nielsen JL, Lonquist JL. Evaluation of a new temperature management system during off‐pump coronary artery bypass. Interactive Cardiovascular and Thoracic Surgery 2003;2(4):454‐7. [DOI] [PubMed] [Google Scholar]
Winkler 2000 {published data only}
- Winkler M, Akca O, Birkenberg B, Hetz H, Scheck T, Arkilic CF, et al. Aggressive warming reduces blood loss during hip arthroplasty. Anesthesia and Analgesia 2000;91(4):978‐84. [DOI] [PubMed] [Google Scholar]
Wong 2007 {published data only}
- Wong PF, Kumar S, Bohra A, Whetter D, Leaper DJ. Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. The British Journal of Surgery 2007;94(4):421‐6. [DOI] [PubMed] [Google Scholar]
Wongprasartsuk 1998 {published data only}
- Wongprasartsuk P, Konstantatos A, McRae R. The effect of forced air warming on postoperative oxygen consumption and temperature in elective orthopaedic surgery. Anaesthesia and Intensive Care 1998;26(3):267‐71. [DOI] [PubMed] [Google Scholar]
Yamakage 1995 {published data only}
- Yamakage M, Kawana S, Yamauchi M, Kohro S, Namiki A. Evaluation of a forced air warning system during spinal anesthesia. Journal of Anesthesia 1995;9(1):93‐5. [DOI] [PubMed] [Google Scholar]
Yildirim 2012 {published data only}
- Yildirim S, Unal CB, Dongel I, Duger C, Sahin AF, Ersan I. Effects of intraoperative skin surface warming on postanesthetic recovery and shivering: A prospective, randomized, clinical trial. Health MED 2012;6(10):3340‐5. [Google Scholar]
Zangrillo 2006 {published data only}
- Zangrillo A, Pappalardo F, Talò G, Corno C, Landoni G, Scandroglio A, et al. Temperature management during off‐pump coronary artery bypass graft surgery: a randomizedclinical trial on the efficacy of a circulating water system versus a forced‐air system. Journal of Cardiothoracic and Vascular Anesthesia 2006;20(6):788‐92. [DOI] [PubMed] [Google Scholar]
Zhao 2005 {published data only}
- Zhao J, Luo AL, Xu L, Huang YG. Forced‐air warming and fluid warming minimize core hypothermia during abdominal surgery. Chinese Medical Sciences Journal 2005;20(4):261‐4. [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 2009 {published data only}
- Allen GS. Intraoperative temperature control using the Thermogard system during off‐pump coronary artery bypass grafting. The Annals of Thoracic Surgery 2009;87(1):284‐8. [DOI] [PubMed] [Google Scholar]
Baxendale 2000 {published data only}
- Baxendale B, Giovanelli M, Bennett M. Comparison of the Inditherm Mattress and forced‐air patient warming device during major abdominal and orthopaedic surgery. Available at: http://www.inditherm.co.uk/medical/patient‐warming‐perioperative‐and‐icu‐alpha/comparison‐forced‐air/ 2000 (Accessed 15th April 2016)).
Berti 1997 {published data only}
- Berti M, Casati A, Torri G, Aldegheri G, Lugani D, Fanelli G. Active warming, not passive heat retention, maintains normothermia during combined epidural‐general anesthesia for hip and knee arthroplasty. Journal of Clinical Aanesthesia 1997;9(6):482‐6. [DOI] [PubMed] [Google Scholar]
Borms 1994 {published data only}
- Borms SF, Engelen SL, Himpe DG, Suy MR, Theunissen WJ. Bair hugger forced‐air warming maintains normothermia more effectively than thermo‐lite insulation. Journal of Clinical Anesthesia 1994;6(4):303‐7. [DOI] [PubMed] [Google Scholar]
Bourke 1984 {published data only}
- Bourke DL, Wurm H, Rosenberg M, Russell J. Intraoperative heat conservation using a reflective blanket. Anesthesiology 1984;60(2):151‐4. [DOI] [PubMed] [Google Scholar]
Buggy 1994 {published data only}
- Buggy D, Hughes N. Pre‐emptive use of the space blanket reduces shivering after general anaesthesia. British Journal of Anaesthesia 1994;72(4):393‐6. [DOI] [PubMed] [Google Scholar]
Cobbe 2012 {published data only}
- Cobbe KA, Staso R, Duff J, Walker K, Draper N. Preventing inadvertent hypothermia: comparing two protocols for preoperative forced‐air warming. Journal of Perianesthesia Nursing 2012;27(1):18‐24. [DOI] [PubMed] [Google Scholar]
De Bernardis 2009 {published data only}
- Bernardis RC, Prado Da Silva M, Lauzi Y, Gozzani JL, Lacava Pagnocca M. (Use of forced‐air to prevent intraoperative hypothermia) [Uso da manta termica na prevençao da hipotermia intraoperatoria]. Revista da Associação Brasileira de Medicina Psicossomática 2009;55(4):421‐6. [DOI] [PubMed] [Google Scholar]
Dyer 1986 {published data only}
- Dyer PM, Heathcote PS. Reduction of heat loss during transurethral resection of the prostate. Anaesthesia and Intensive Care 1986;14(1):12‐6. [DOI] [PubMed] [Google Scholar]
Erickson 1991 {published data only}
- Erickson RS, Yount ST. Effect of aluminized covers on body temperature in patients having abdominal surgery. Heart Lung 1991;20(3):255‐64. [PubMed] [Google Scholar]
Fanelli 2009 {published data only}
- Fanelli A, Danelli G, Ghisi D, Ortu A, Moschini E, Fanelli G. The efficacy of a resistive heating under‐patient blanket versus a forced‐airwarming system: a randomized controlled trial. Anesthesia and Analgesia 2009;108(1):199‐201. [DOI] [PubMed] [Google Scholar]
Farley 2004 {published data only}
- Farley DR, Greenlee SM, Larson DR, Harrington JR. Double‐blind, prospective, randomized study of warmed, humidified carbon dioxide insufflation vs standard carbon dioxide for patients undergoing laparoscopic cholecystectomy. Archives of Surgery 2004;139(7):739‐44. [DOI] [PubMed] [Google Scholar]
Fleisher 1998 {published data only}
- Fleisher LA, Metzger SE, Lam J, Harris A. Perioperative cost‐finding analysis of the routine use of intraoperative forced‐air warming during general anesthesia. Anesthesiology 1998;88(5):1357‐64. [DOI] [PubMed] [Google Scholar]
Frank 1995 {published data only}
- Frank SM, Higgins MSM, Breslow MJM, Fleisher LAM, Gorman RBM, Sitzmann JVM, et al. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia: a randomized clinical trial. Anesthesiology 1995;82(1):83‐93. [DOI] [PubMed] [Google Scholar]
Grocott 2004 {published data only}
- Grocott HP, Mathew JP, Carver EH, Phillips‐Bute B, Landolfo KP, Newman MF. A randomized controlled trial of the Arctic Sun Temperature Management System versus conventional methods for preventing hypothermia during off‐pump cardiac surgery. Anesthesia and Analgesia 2004;98(2):298‐302. [DOI] [PubMed] [Google Scholar]
Harper 2007 {published data only}
- Harper C. Is a warming mattress as effective as forced‐air warming in preventing peri‐operative hypothermia?. Anesthesiology 2007;107(A):92. [Google Scholar]
Hindsholm 1992 {published data only}
- Hindsholm KB, Bredahl C, Herlevsen P, Kruhoffer PK. Reflective blankets used for reduction of heat loss during regional anaesthesia. British Journal of Anaesthesia 1992;68(5):531‐3. [DOI] [PubMed] [Google Scholar]
Hoyt 1993 {published data only}
- Hoyt K, Clochesy JM, Shamsali S, Bracken W. Comparison of the effect of insulated and noninsulated head covers on heat loss during abdominal surgery. Nurse Anesthesia 1993;4(1):4‐8. [PubMed] [Google Scholar]
Hynson 1992 {published data only}
- Hynson JM, Sessler DI. Intraoperative warming therapies: a comparison of three devices. Journal of Clinical Anesthesia 1992;4(3):194‐9. [DOI] [PubMed] [Google Scholar]
Insler 2008 {published data only}
- Insler SR, Bakri MH, Nageeb F, Mascha E, Mihaljevic T, Sessler DI. An evaluation of a full‐access underbody forced‐air warming system during near‐normothermic, on‐pump cardiac surgery. Anesthesia & Analgesia 2008;106(3):746‐50. [DOI] [PubMed] [Google Scholar]
Janicki 2002 {published data only}
- Janicki PK, Stoica C, Chapman WC, Wright JK, Walker G, Pai R, et al. Water warming garment versus forced air warming system in prevention of intraoperative hypothermia during liver transplantation: a randomized controlled trial [ISRCTN32154832]. BMC Anesthesiology 2002;2(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joachimsson 1987 {published data only}
- Joachimsson PO, Nystrom SO, Tyden H. Heating efficacy of external heat supply during and after open‐heart surgery with hypothermia. Acta Anaesthesiologica Scandinavica 1987;31(1):73‐80. [DOI] [PubMed] [Google Scholar]
Kamitani 1999 {published data only}
- Kamitani K, Higuchi A, Takebayashi T, Miyamoto Y, Yoshida H. Covering the head and face maintains intraoperative core temperature. Canadian Journal of Anaesthesia 1999;46(7):649‐52. [DOI] [PubMed] [Google Scholar]
Karayan 1996 {published data only}
- Karayan J, Thomas D, Lacoste L, Dhoste K, Ricco JB, Fusciardi J. Delayed forced air warming prevents hypothermia during abdominal aortic surgery. British Journal of Anaesthesia 1996;76(3):459‐60. [DOI] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim JY, Shinn H, Oh YJ, Hong YW, Kwak HJ, Kwak YL. The effect of skin surface warming during anesthesia preparation on preventing redistribution hypothermia in the early operative period of off‐pump coronary artery bypass surgery. European Journal of Cardiothoracic Surgery 2006;29(3):343‐7. [DOI] [PubMed] [Google Scholar]
Kongsayreepong 2002 {published data only}
- Kongsayreepong S, Gunnaleka P, Suraseranivongse S, Pirayavaraporn S, Chowvanayotin S, Montapaneewat, et al. A reusable, custom‐made warming blanket prevents core hypothermia during major neonatal surgery. Canadian Journal of Anaesthesia 2002;49(6):605‐9. [DOI] [PubMed] [Google Scholar]
Kurz 1993 {published data only}
- Kurz A, Kurz M, Poeschl G, Faryniak B, Redl G, Hackl W. Forced‐air warming maintains intraoperative normothermia better than circulating‐water mattresses. Anesthesia and Analgesia 1993;77(1):89‐95. [DOI] [PubMed] [Google Scholar]
Lenhardt 1997 {published data only}
- Lenhardt R, Marker E, Goll V, Tschernich H, Kurz A, Sessler DI, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology 1997;87(6):1318‐23. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2003 {published data only}
- Matsuzaki Y, Matsukawa T, Ohki K, Yamamoto Y, Nakamura M, Oshibuchi T. Warming by resistive heating maintains perioperative normothermia as well as forced air heating. British Journal of Anaesthesia 2003;90(5):689‐91. [DOI] [PubMed] [Google Scholar]
Motamed 2000 {published data only}
- Motamed C, Labaille T, Léon O, Panzani JP, Duvaldestin P, Benhamou D. Core and thenar skin temperature variation during prolonged abdominal surgery: comparison of two sites of active forced air warming. Acta Anaesthesiologica Scandinavica 2000;44(3):249‐54. [DOI] [PubMed] [Google Scholar]
Müller 1993 {published data only}
- Müller CM, Gabriel A, Langenecker S, Hartmann T, Steltzer H, Werba A, et al. Effectiveness of rapid infusion and Bair Hugger systems in maintaining normothermia during orthotopic liver transplantation. Transplantation Proceedings 1993;25(2):1833‐4. [PubMed] [Google Scholar]
Müller 1995 {published data only}
- Müller CM, Langenecker S, Andel H, Nantschev I, Hölzenbein TJ, Zimpfer M. Forced‐air warming maintains normothermia during orthotopic livertransplantation. Anaesthesia 1995;50(3):229‐32. [DOI] [PubMed] [Google Scholar]
Murat 1994 {published data only}
- Murat I, Bernière J, Constant I. Evaluation of the efficacy of a forced‐air warmer (Bair Hugger) during spinal surgery in children. Journal of Clinical Anesthesia 1994;6(5):425‐9. [DOI] [PubMed] [Google Scholar]
Nesher 2003 {published data only}
- Nesher N, Zisman E, Wolf T, Sharony R, Bolotin G, David M, et al. Strict thermoregulation attenuates myocardial injury during coronary artery bypass graft surgery as reflected by reduced levels of cardiac‐specific troponin I. Anesthesia and Analgesia 2003;96(2):328‐35. [DOI] [PubMed] [Google Scholar]
Onik 1993 {published data only}
- Onik GM, Chambers N, Chernus SA, Zemel R, Atkinson D, Weaver ML. Hepatic cryosurgery with and without the Bair Hugger. Journal of Surgical Oncology 1993;52(3):185‐7. [DOI] [PubMed] [Google Scholar]
Ouellette 1993 {published data only}
- Ouellette RG. Comparison of four intraoperative warming devices. AANA Journal 1993;61(4):394‐6. [PubMed] [Google Scholar]
Patel 1997 {published data only}
- Patel N, Smith CE, Knapke D, Pinchak AC, Hagen JF. Heat conservation vs convective warming in adults undergoing elective surgery. Canadian Journal of Anaesthesia 1997;44(6):669‐73. [DOI] [PubMed] [Google Scholar]
Radel 1986 {published data only}
- Radel TJ, Fallacaro MD, Sievenpiper T. The effects of a warming vest and cap during lower extremity orthopedic surgical procedures under general anesthesia. AANA Journal 1986;54(6):486‐9. [PubMed] [Google Scholar]
Radford 1979 {published data only}
- Radford P, Thurlow AC. Metallized plastic sheeting in the prevention of hypothermia during neurosurgery. British Journal of Anaesthesia 1979;51(3):237‐40. [DOI] [PubMed] [Google Scholar]
Rein 2007 {published data only}
- Rein EB, Filtvedt M, Walløe L, Raeder JC. Hypothermia during laparotomy can be prevented by locallyapplied warm water and pulsating negative pressure. British Journal of Anaesthesia 2007;98(3):331‐6. [DOI] [PubMed] [Google Scholar]
Russell 1995 {published data only}
- Russell SH, Freeman JW. Prevention of hypothermia during orthotopic liver transplantation: comparison of three different intraoperative warming methods. British Journal of Anaesthesia 1995;74(4):415‐8. [DOI] [PubMed] [Google Scholar]
Salazar 2011 {published data only}
- Salazar F, Donate M, Boget T, Bogdanovich A, Basora M, Torres F, et al. Intraoperative warming and post‐operative cognitive dysfunction after total knee replacement. Acta Anaesthesiologica Scandinavica 2011;55(2):216‐22. [DOI] [PubMed] [Google Scholar]
Saldanha 2001 {published data only}
- Saldanha C, Argano V, Nunn S, Zaidi A, Hadjinikolau L, Rees G, et al. Convective warming post bypass reduces postoperative bleeding. The Journal of Cardiovascular Surgery 2001;42(3):435‐6. [PubMed] [Google Scholar]
Sessler 1988 {published data only}
- Sessler DI, Olofsson CI, Rubinstein EH. The thermoregulatory threshold in humans during nitrous oxide‐fentanylanesthesia. Anesthesiology 1988;69(3):357‐64. [DOI] [PubMed] [Google Scholar]
Severens 2007 {published data only}
- Severens NM, Marken Lichtenbelt WD, Leeuwen GM, Frijns AJ, Steenhoven AA, Mol BA, et al. Effect of forced‐air heaters on perfusion and temperature distribution during andafter open‐heart surgery. European Journal of Cardiothorac Surgery 2007;32(6):888‐95. [DOI] [PubMed] [Google Scholar]
Sheng 2003 {published data only}
- Sheng Y, Zavisca F, Schonlau E, Desmarattes R, Herron E, Cork R. The effect of preoperative reflective hats and jackets, and intraoperative reflective blankets on perioperative temperature. The Internet Journal of Anesthesiology 2003 (print.ispub.com/api/0/ispub‐article/11444); Vol. 6, issue 2:(Accessed 7th April 2016).
Smith 1994 {published data only}
- Smith I, Newson CD, White PF. Use of forced‐air warming during and after outpatient arthroscopic surgery. Anesthesia and Analgesia 1994;78(5):836‐41. [DOI] [PubMed] [Google Scholar]
Smith 2006 {published data only}
- Smith CE, Sidhu RS, Lucas L, Mehta D, Pinchak AC. Should patients undergoingambulatory surgery with general anesthesia be actively warmed?. Internet Journal of Anesthesiology 2006;12(1):18. [Google Scholar]
Summers 1990 {published data only}
- Summers S, Dudgeon N, Byram K, Zingsheim K. The effects of two warming methods on core and surface temperatures, hemoglobinoxygen saturation, blood pressure, and perceived comfort of hypothermic postanesthesia patients. Journal of Post Anesthesia Nursing 1990;5(5):354‐64. [PubMed] [Google Scholar]
Tollofsrud 1984 {published data only}
- Tollofsrud SG, Gundersen Y, Andersen R. Perioperative hypothermia. Acta Anaesthesiologica Scandinavica 1984;28(5):511‐5. [DOI] [PubMed] [Google Scholar]
Wagner 2006 {published data only}
- Wagner D, Byrne M, Kolcaba K. Effects of comfort warming on preoperative patients. Association of Operating Room Nurses Journal 2006;84(3):427‐48. [DOI] [PubMed] [Google Scholar]
Whitney 1990 {published data only}
- Whitney AM. The efficiency of a reflective heating blanket in preventing hypothermia in patients undergoing intra‐abdominal procedures. AANA Journal 1990;58(3):212‐5. [PubMed] [Google Scholar]
Wong 2004 {published data only}
- Wong A, Walker S, Bradley M. Comparison of a radiant patient warming device with forced air warming during laparoscopic cholecystectomy. Anaesthesia and Intensive Care 2004;32(1):93‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kaudasch 1996 {published data only}
- Kaudasch G, Schempp P, Skierski P, Turner E. The effect of convection warming during abdominal surgery on the early postoperative heat balance. Der Anaesthesist 1996;45(11):1075‐81. [DOI] [PubMed] [Google Scholar]
Leben 1997 {published data only}
- Leben J, Tryba M. Prevention of hypothermia during surgery. Contribution of convective heating system and warm infusion. Annals of the New York Academy of Sciences 1997;813:807‐11. [DOI] [PubMed] [Google Scholar]
Xu 2004 {published data only}
- Xu L, Zhao J, Huang YG, Luo AL. The effect of intraoperative warming on patient core temperature. Chung‐Hua Wai Ko Tsa Chih 2004;42(16):1010‐3. [PubMed] [Google Scholar]
Additional references
Alderson 2014
- Alderson P, Campbell G, Smith AF, Warttig S, Nicholson A, Lewis SR. Thermal insulation for preventing inadvertent perioperative hypothermia. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD009908.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Birch 2011
- Birch DW, Manouchehri N, Shi X, Hadi G, Karmali S. Heated CO2 with or without humidification for minimally invasive abdominal surgery. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD007821.pub2] [DOI] [PubMed] [Google Scholar]
Bush 1995
- Bush HL Jr, Hydo LJ, Fischer E, Fantini GA, Silane MF, Barie PS. Hypothermia during elective abdominal aortic aneurysm repair: the high price of avoidable morbidity. Journal of Vascular Surgery 1995;21(3):392‐400. [PUBMED: 7877221] [DOI] [PubMed] [Google Scholar]
Campbell 2015
- Campbell G, Alderson P, Smith AF, Warttig S. Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD009891.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heier 1991
- Heier T, Caldwell JE, Sessler DI, Miller RD. Mild intraoperative hypothermia increases duration of action and spontaneous recovery of vecuronium blockade during nitrous oxide‐isoflurane anesthesia in humans. Anesthesiology 1991;74:815‐9. [PUBMED: 1673591] [DOI] [PubMed] [Google Scholar]
Heier 2006
- Heier T, Caldwell JE. Impact of hypothermia on the response to neuromuscular blocking drugs. Anesthesiology 2006;104(5):1070‐80. [PUBMED: 16645461] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Leslie 1995
- Leslie K, Sessler DI, Bjorksten AR, Moayeri A. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesthesia and Analgesia 1995;80(5):1007‐14. [PUBMED: 7726398] [DOI] [PubMed] [Google Scholar]
Lewis 2015
- Lewis SR, Nicholson A, Smith AF, Alderson P. Alpha‐2 adrenergic agonists for the prevention of shivering following general anaesthesia. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011107.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Negishi 2003
- Negishi C, Hasegawa K, Mukai S, Nakagawa F, Ozaki M, Sessler DI. Resistive‐heating and forced‐air warming are comparably effective. Anesthesia and Analgesia 2003;96(6):1683‐7. [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. Hypothermia: prevention and management in adults having surgery. www.nice.org.uk/guidance/cg65 (Accessed 7th April 2016). [PubMed]
Putzu 2007
- Putzu M, Casati A, Berti M, Pagliarini G, Fanelli G. Clinical complications, monitoring and management of perioperative mild hypothermia: anesthesiological features. Acta Biomedica 2007;78(3):163‐9. [PUBMED: 18330074] [PubMed] [Google Scholar]
Rajagopalan 2008
- Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008;108(1):71‐7. [PUBMED: 18156884] [DOI] [PubMed] [Google Scholar]
Revman 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sessler 1991
- Sessler DI, Rubinstein EH, Moayeri A. Physiologic responses to mild perianesthetic hypothermia in humans. Anesthesiology 1991;75:594‐610. [PUBMED: 1928769] [DOI] [PubMed] [Google Scholar]
Sessler 2001
- Sessler D. Complications and treatment of mild hypothermia. Anesthesiology 2001;95:531‐43. [PUBMED: 9148354] [DOI] [PubMed] [Google Scholar]
Torossian 2007
- Torossian A, TEMMP (Thermoregulation in Europe Monitoring and Managing PatientTemperature) Study Group. Survey on intraoperative temperature management in Europe. European Journal of Anaesthesiology 2007;24(8):668‐75. [DOI] [PubMed] [Google Scholar]
Warttig 2014
- Warttig S, Alderson P, Campbell G, Smith AF. Interventions for treating inadvertent postoperative hypothermia. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD009892] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2015
- World Health Organization. WHO guidelines for safe surgery. apps.who.int/iris/bitstream/10665/44185/1/9789241598552_eng.pdf 2008.
Wood 2014
- Wood AM, Moss C, Keenan A, Reed MR, Leaper DJ. Infection control hazards associated with the use of forced‐air warming in operating theatres. The Journal of Hospital Infection 2014;88(3):132‐40. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Urrútia 2011
- Urrútia G, Roqué i Figuls M, Campos JM, Paniagua P, Cibrian Sánchez S, Maestre L, et al. Active warming systems for preventing inadvertent perioperative hypothermia in adults. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD009016] [DOI] [PMC free article] [PubMed] [Google Scholar]


