According to WHO, SARS-CoV-2 is estimated to have caused 265 million infections and more than 5 million deaths over the past 2 years. Current vaccines are based on the original SARS-CoV-2 strain and are designed primarily to raise an antibody response against the spike protein (S), although elicited T-cell responses can also contribute to protection from severe disease.
The SARS-CoV-2 RNA polymerase is intrinsically error prone, which results in mutation to the viral genome. In the past year, several variants containing multiple mutations in S have been reported: alpha (B.1.1.7), beta (B.1.351), gamma (P.1), and delta (B.1.617.2). These variants contain mutations in the receptor binding motif, a small 25 amino acid patch at the tip of S that mediates interaction with the ACE2 receptor (one mutation in alpha, three in beta and gamma, and two in delta). These changes can lead to increased transmissibility by increasing affinity to ACE2 (by seven times for alpha, 19 times for both beta and gamma, and double for delta)1 or lead to immune escape. First alpha and then delta variants spread globally causing successive waves of infection, while large localised outbreaks were caused in southern Africa by the beta variant and in South America by the gamma variant.
At present, delta is estimated to have caused more than 99% of infections worldwide; however, a new variant of concern, omicron (B.1.1.529), was reported first in South Africa on Nov 24, 2021,2 but has since been reported in multiple countries. Early reports from South Africa suggest that omicron is highly transmissible, in a population where 60–80% already show serological evidence of previous infection or vaccination, suggesting that omicron is able to break through natural and vaccine-induced immunity; although early reports do not indicate more severe disease.
Omicron contains a large number of mutations in S compared with previous variants of concern, mostly concentrated around the receptor binding motif: 30 amino acid substitutions, deletion of six residues, and insertion of three residues.1 Mutations are also present at other sites (receptor binding domain and N-terminal domain) which might affect neutralising antibodies. There is concern that omicron will lead to increased propensity to infect individuals who have received vaccines, whose antigens are based on the original S sequence.
Here, we report the results of neutralisation assays using an isolate of omicron obtained from an infected case in the UK. Neutralisation assays were done on sera from individuals from the immunology cohort of the Com-COV2 study,3 who were seronegative at enrolment (defined by anti-nucleocapsid IgG). Participants were vaccinated with two doses of Oxford–AstraZeneca's ChAdOx1 nCoV-19 (ChAd; n=22), or two doses of Pfizer–BioNTech's BNT162b2 (BNT; n=21) with a priming interval of 8–11 (median 9) weeks. Samples were obtained 28 days (range 25–32) following the second immunisation (appendix p 1).3
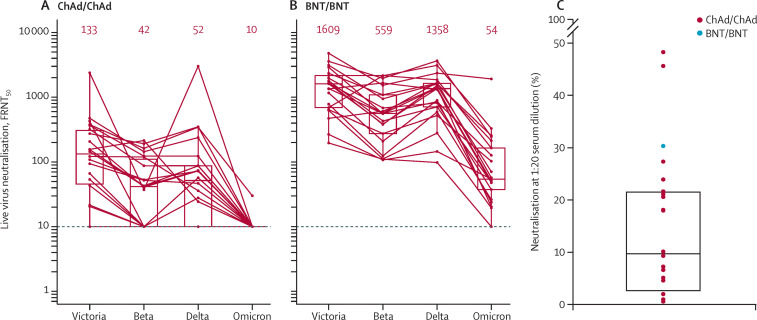
Live virus neutralisation titres against omicron are compared with titres against Victoria, an early pandemic SARS-CoV-2 strain, together with titres against beta and delta variants.
Neutralising titres on sera from participants who had received homologous ChAd dropped to below the detectable threshold in all but one participant (figure A, B ). Median neutralising titres on sera from participants who had received homologous BNT reduced by 29·8 fold from 1609 (Victoria strain) to 54 (omicron variant), with one participant dropping below the detection threshold. In most cases, samples that did not neutralise with 50% focus reduction neutralisation titres at a dilution of less than 1/20 showed some residual neutralising activity (figure 1C).
Figure.
Neutralisation assays of SARS-CoV-2 omicron
Neutralisation of victoria, beta, delta, and omicron using ChAd serum (A) and BNT serum (B). Median values are indicated above each column. The data underpinning the victoria, beta, and delta neutralisation have been previously reported.4 The horizontal dotted line indicates half the value of the lower limit of detection. The red horizontal lines in (A) and (B) represent the assay limit of detection and the red numbers represent the median values of the FRNT50. (C) Percent neutralisation at serum dilution of 1/20 for those sera which did not achieve FRNT50 at 1/20. ChAd=ChAdOx1 nCoV-19. BNT=BNT162b2. FRNT50=50% focus reduction neutralisation titres.
In summary, there was a substantial decrease in neutralisation titre in recipients of both homologous ChAd and BNT primary courses, with evidence of some recipients not neutralising at all. This reduction in neutralisation titre will probably be more pronounced at later timepoints. These data, although derived from a relatively small sample size, are consistent with published data from datasets of similar size.4, 5, 6 Together, the findings suggest that omicron is more antigenically distant from the original SARS-CoV-2 vaccine strain than the previously most distant strains, beta and delta. Preliminary data from the UK Health Security Agency7 have shown reduced effectiveness against symptomatic infection after two doses of ChAd or BNT, suggesting a result of increased breakthrough infections in previously infected or double vaccinated individuals, which could drive a further wave of infection. The effect on disease severity is unknown, although there is currently no evidence of increased potential to cause severe disease, hospitalisation, or death. It could be that other aspects of the immune response such as non-neutralising antibodies and cellular immunity, which are not expected to be as severely affected by this variant, could confer a degree of protection against severe disease. However, it should be noted that higher transmission will inevitably lead to increased numbers of cases and a greater burden on health systems, even without proportional changes in severity.
Possessing a high starting neutralisation titre against early pandemic strains gives a higher level of neutralisation of omicron, which could be obtained by deploying third booster doses of vaccine. There is some reassurance that a third dose of a COVID-19 vaccine does indeed increase vaccine effectiveness against the omicron variant,7 and testing of samples from Cov-BOOST8 will provide further information on the immunology underlying this. Together, these findings will provide further understanding of the potential for a boosting strategy as a control measure for omicron infection and transmission.
Should omicron, as expected, become the dominant strain worldwide, given its antigenic distance from ancestral strains, it could be necessary to produce vaccines tailored to omicron; however, these might be unlikely to give protection against previous strains. This development might stimulate consideration of a switch from the current monovalent vaccine strategy towards multivalent formulations currently used in seasonal influenza vaccines. In the meantime, reaching people who are unvaccinated with current vaccines is a priority, in order to reduce transmission levels and the potential for severe disease in people who are immunologically naive.
Acknowledgments
GRS sits on the GSK Vaccines Scientific Advisory Board and is a founder member of RQ Biotechnology. MDS acts on behalf of the University of Oxford as an investigator on studies funded or sponsored by vaccine manufacturers, including AstraZeneca, GlaxoSmithKline, Pfizer, Novavax, Janssen, Medimmune, and MCM Vaccines. He receives no personal financial payment for this work. AJP reports grants from UK Research and Innovation, Coalition for Epidemic Preparedness Innovations, and National Institute for Health Research. AJP is chair of Department of Health and Social Care (DHSC)'s England Joint Committee on Vaccination and Immunisation (JCVI), but does not participate in discussions on COVID-19 vaccines, and is a member of the WHO's Scientific Advisory Group for Emergencies. The views expressed in this Correspondence are those of the authors and do not necessarily represent the views of the DHSC, JCVI, NIHR, or WHO. The University of Oxford has entered into a partnership with AstraZeneca for the development of a coronavirus vaccine. JSN-V-T is seconded to the DHSC. WD, RHS, and PS contributed equally and are joint first authors. All other authors declare no competing interests. MDS and GRS are joint senior authors.
Supplementary Material
References
- 1.Zahradník J, Tuekprakhon A, Ginn HM, et al. Receptor binding and escape from beta antibody responses drive omicron-B.1.1.529 evolution. bioRxiv. 2021 doi: 10.1101/2021.12.03.471045. published online Dec 7. (preprint). [DOI] [Google Scholar]
- 2.WHO Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
- 3.Stuart ASVS, Shaw RH, Liu X, et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2021 doi: 10.1016/S0140-6736(21)02718-5. published online Dec 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cele S, Jackson L, Khan K, et al. SARS-CoV-2 omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv. 2021 doi: 10.1101/2021.12.08.21267417. published online Dec 9. (preprint). [DOI] [Google Scholar]
- 5.Roessler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 B.1.1.529 variant (omicron) evades neutralization by sera from vaccinated and convalescent individuals. medRxiv. 2021 doi: 10.1101/2021.12.08.21267491. published online Dec 11. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilhelm A, Widera M, Grikscheit K, et al. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. medRxiv. 2021 doi: 10.1101/2021.12.07.21267432. published online Dec 8. (preprint). [DOI] [Google Scholar]
- 7.UK Health Security Agency SARS-CoV-2 variants of concern and variants under investigation in England. 2021. Technical briefing 31. Dec 10, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1040076/Technical_Briefing_31.pdf
- 8.Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021 doi: 10.1016/S0140-6736(21)02717-3. published online Dec 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.