Abstract
The human circadian system consists of the master clock in the suprachiasmatic nuclei of the hypothalamus as well as peripheral molecular clocks located in organs throughout the body. Together, these systems establish normal sleep-wake and eating cycles synced to the day-night cycle. Several biologic and physiologic processes such as body temperature, blood pressure, hormone secretion, gene expression, and immune functions manifest diurnal patterns. Many facets of modern life, such as work schedules, travel, and social engagements, can lead to sleep-wake and eating schedules that are not completely in sync with the biological clock. In its extreme form, this can disrupt and impair physiological and psychological parameters that may ultimately put people at higher risk for chronic diseases like cancer, cardiovascular disease, and metabolic disorders. Understanding the mechanisms that regulate sleep and behavioral effects on sleep and circadian rhythms may ultimately lead to insights on behavioral changes that can lower the risk of these diseases. On February 25, 2021, experts in sleep, circadian rhythms, and chronobiology met virtually for the Keystone eSymposium “Sleep & Circadian Rhythms: Pillars of Health” to discuss the latest research on understanding the bi-directional relationships between sleep, circadian rhythms, and health and disease.
Keywords: appetite control, biomarkers, circadian misalignment, circadian rhythm, food timing, shift work, sleep, sleep duration, sleep homeostasis, social jet lag
Introduction
Circadian rhythms are driven by the circadian clock in our brain: sleep/wake cycles, body temperature, blood pressure, drug metabolism, hormone secretion, kidney function, gene expression, drinking rhythms, and immune system functions all display a diurnal rhythm. The most important cue (zeitgeber) affecting the circadian clock is light.1 In particular, short-wavelength blue light triggers the melanopsin retinal receptors in the eye and entrains the central clock in the suprachiasmatic nuclei (SCN) in the hypothalamus of the brain. This generates circadian activity in SCN neurons that send signals throughout the body. Some evidence suggests timing of other non-photonic stimuli—such as sleep, physical activity, and meals––may also impact the central clock.2 A key marker of the circadian system is melatonin, the production of which is suppressed by light and therefore occurs almost exclusively at night.3 Changes in peripheral melatonin levels provide clues into the function of the circadian system in animal and human studies.
The central clock in the brain acts as a pacemaker for other molecular clock in peripheral organs. These clocks consist of transcriptional activators and repressors, such as CLOCK-BMAL1 and PER-CRY, that coordinate metabolic functions with sleep–wake and feeding–fasting cycles.4 De-synchronization of the central and molecular clocks can impact hormones and cell functions that can affect disease risk.
For many, work schedules, travel, and social engagements mean that sleep and meal times often do not align with biological signals. This can impair circadian rhythms in physiological parameters, such as blood pressure, glucose levels, hormones, and vigilance, thereby contributing to increased chronic disease burden, and increasing the risk of cancer, cardiovascular disease (CVD), and cognitive impairment/accelerated aging.
On February 25, 2021, experts in circadian biology and sleep met virtually for the Keystone Symposium “Sleep & Circadian Rhythms: Pillars of Health” (Box 1). A major goal of the meeting was to bring together sleep and circadian biology researchers to discuss the bi-directional relation between lifestyle behaviors, health outcomes, and sleep and circadian rhythms in humans. The meeting covered the bi-directional relationship between lifestyle and circadian rhythms, exploring both the biologic mechanisms of circadian rhythms as well as how behaviors can alter sleep and circadian rhythms, and ultimately how these impact health.
The first half of the meeting focused on the relationship between circadian rhythm disruptions and chronic disease risk. Presenters discussed recent data on the timing of sleep and food intake on chronic disease risk and elucidated some of the genetic, biologic, and molecular mechanisms underlining the links between lifestyle behaviors and health. During the second half of the meeting, presenters focused on sleep disruption and circadian rhythms and the resulting impacts on health. Presenters also delved into preliminary evidence on restoring circadian rhythms by improving sleep behavior to ameliorate health outcomes.
Circadian rhythms and chronic disease risk
The first half of the conference focused on the association between circadian disruption and chronic disease risk. Speakers presented epidemiologic evidence on the negative effect of circadian disruption on cancer and CVD risk in rotating night shift workers. They also presented data on the genetic and molecular mechanisms that regulate appetite and meal timing and provided evidence for the impact of meal timing on CVD and metabolic disorders.
Impact of circadian disruption on chronic disease risk in night shift workers
Circadian misalignment and ultimately circadian disruption is associated with an increased risk of chronic diseases, including cancer, CVD, and metabolic diseases. One contribution to circadian misalignment in the modern era is increased light at night.5 To investigate the impact of light at night and disrupted circadian patterns on disease, many studies have examined the risk of chronic diseases among night shift workers. Night shift work encompasses a number of circadian disruptions that are tightly linked to the changed light exposure during night work, including disruptions in sleeping, eating, and activity. Night shift workers provide a particularly useful model to understand the impact of wakefulness and light at night for several reasons: (1) they are often chronically exposed to light at night, often several days a month for years; (2) arethey are subjectedare subjected tohigh levels of ambient light particularly at night; and (3) they usually cannot change their exposure, as opposed to people who choose to stay up late in their free time.
Two speakers presented data on the risk of shift work on chronic disease from the Nurses’ Health Studies (NHS).The NHS consists of 3 ongoing cohort studies, dating from 1976. Over 300,000 female nurses have been enrolled, approximately 60% of whom have worked night shifts. Participants provide medical, lifestyle, and behavioral information, as well as blood and urine samples, and are regularly followed up for longitudinal data.
Eva Schernhammer from Harvard Medical School and the University of Vienna discussed her work using the NHS to understand the impact of shift work on cancer risk. A mechanistic link between light at night and breast cancer was proposed over 30 years ago. The melatonin hypothesis purports that increased light at night decreases melatonin levels, which increases estrogen levels and leads to a higher risk of breast cancer.6 The melatonin hypothesis is supported by population-based studies. In the NHS, shift work increased the risk of breast cancer by up to 80%, with risks increasing as the length of time on night shifts increases.7,8 Higher melatonin secretion was associated with a lower breast cancer risk.9,10 An updated analysis of the NHS, with over 24 years of follow-up and 6,000 breast cancer cases confirmed the association between night work and breast cancer risk. The study also demonstrated that breast cancer risk resembles that of non-night working women when shift work ceases.11 Another study correlated high light at night near participants’ homes with a significantly elevated risk for breast cancer. The increased risk was restricted to pre-menopausal women and mostly associated with estrogen receptor positive tumors.12
While the most robust data are for breast cancer, Schernhammer has also shown that shift work increases the risk of colorectal cancer (CRC), endometrial cancer, and lung cancer in the NHS.13–16 In addition, over 20 studies have examined the association between night work and various cancers in men, including prostate, gastrointestinal, and lung cancer, with most demonstrating a dose-response relationship between duration of night work and cancer risk.15–19
In 2007, the International Agency for Research on Cancer (IARC) classified shift work as a Class 2A carcinogen based on the epidemiologic data. This has prompted some countries to compensate night shift workers who develop breast cancer.17 In 2019, the IARC re-evaluated the carcinogenicity of night work due to the large number of new epidemiologic studies that had been published since their initial report.18 The new report reaffirms the classification of night work as a Class 2A carcinogen on the basis of limited evidence that night shift work causes breast, prostate, and CRC, with insufficient evidence for other cancer sites. Current epidemiological data are supported by sufficient evidence in animal models and strong mechanistic evidence in experimental systems. The agency called for more research in humans on the mechanisms underlying this increased risk, such as the impact of night work on oxidative stress, immunosuppression, inflammation, genotoxicity, and epigenetics. Schernhammer stressed that human studies on the effect of shift work on cell proliferation, cell death, and nutrient supply are lacking. She called upon researchers to fill these gaps. Schernhammer ended her presentation with a quote from leading chronobiologist Erhard Haus, who stressed that cancer is just one aspect of the shift work problem.
As Céline Vetter from the University of Colorado, Boulder showed, there is strong evidence for an adverse but modest effect of shift work on heart health as well.19 There is a large amount of heterogeneity between studies, however. To generate more robust data, Vetter conducted a large analysis of over 150,000 participants with 24 years of follow-up data in the NHS2. People who had performed five years of rotating shift work had a 12% increase risk of myocardial infarction. Similar to the findings of cancer studies, longer duration of shift work was associated with a higher risk, with reduction in risk if an individual stopped doing shift work.20 Vetter is also examining other large datasets, such as the UK Biobank, to see if these results can be replicated in other populations. Shift work imposes disruption on several aspects of the circadian rhythm. Typically, the disruption of shift work is modeled by shifting the sleep cycle with respect to melatonin rhythm.
Vetter described other ways to model the disruption, such as repeated phase shifts in the melatonin rhythm as the body continually attempts to re-synchronize with the light-dark cycle and a loss of rhythmicity and/or dampening of the amplitudes of the melatonin cycle.5
Vetter also described some of the mechanistic evidence for circadian misalignment and increased risk of heart disease. In laboratory settings, sleep deprivation has been associated with an increase in biomarkers of CVD risk and blood pressure.21–24 Results in shift-work settings are mixed, likely due to differences in shift-work schedules and study designs.25–29 However, in field settings circadian misalignment and sleep deprivation co-occur and show significant and clinically meaningful inter-individual differences by chronotype.30–34 Vetter proposed that increased heterogeneity in the association between shift work and CVD risk in real-world settings is partially due to higher heterogeneity in chronotype that, together with mixed schedules, creates more variable exposure.31,34,35 While it can be difficult to assess circadian phase in shift workers, there are several behavioral proxies of circadian misalignment36–39 that Vetter hopes can be leveraged in large cohort studies.
Vetter ended by describing efforts to improve heart health by prioritizing alignment of circadian rhythms. Studies have shown that improving sleep hygiene can reduce inflammation, oxidative stress, and insulin resistance, and ameliorate metabolism.40,41 It stands to reason that these effects could cumulatively reduce the risk for heart disease.
Vetter’s work has shown that schedule adjustments among shift workers to remove the most burdensome shifts based on an individual’s chronotype improved sleep quality.42 Since irregular sleep has been associated with higher CVD risk and longer sleep times have been shown to reduced CVD risk, Vetter hopes that interventions like these will ultimately translate to better heart health in this vulnerable population.43
Impacts of meal timing on circadian misalignment and cardiovascular risk
In addition to light, food is a key stimulus for circadian rhythms. Eating patterns in the U.S. have become more erratic over the past several decades44, with a tendency toward later eating times. U.S. adults tend to eat around the clock, with significant day-to-day and weekday-to-weekend variability.36 Eating at unconventional circadian times, such as consuming more calories in the evening or irregular, variable eating patterns can misalign the organ and brain clocks, resulting in cardiometabolic dysfunction and increasing risk of cardiometabolic disease.
Evidence on the role of meal timing in cardiometabolic health is limited. Observational studies suggest an association between later eating and increased cardiometabolic risk.45–49 In addition, those with more regular eating schedules tend to have lower rates of metabolic syndrome50–52 while eating jetlag, i.e., large variability in weekday and weekend eating times, was associated with increased body mass index (BMI).53 Most of these data are from European populations; there are few studies on irregular eating patterns and cardiometabolic risk in the United States. A 2017 statement by the American Heart Association (AHA) called for more population studies to clarify the influence of meal timing, particularly related to the evening meal, on cardiometabolic outcomes.54
Nour Makarem from Columbia University Irving Medical Center has been working to address this gap. She presented data from two studies assessing the associations between eating pattern timing and regularity with cardiometabolic risk factors. Makarem presented unpublished data on one of the first population-based studies on meal time and cardiometabolic health in the United States. The study includes over 12,000 participants in the Hispanic Community Health Study/Study of Latinos to investigate whether nighttime eating is associated with diverse metabolic outcomes such as fasting glucose levels, insulin resistance, and blood pressure.
In another study, Makarem investigated whether variability in daily eating patterns and eating jetlag are associated with cardiometabolic risk in a community cohort of 116 women in the AHA Go Red for Women Strategically Focused Research Network. Over the 1-year follow-up, greater day-to-day variability, eating jetlag in nighttime eating, and span of eating period were associated with higher blood pressure. Greater day-to-day variability and eating jetlag in nighttime eating and timing of the first and last eating session was associated with poor glycemic control. Makarem concluded that an eating pattern characterized by variability in eating timing, duration, and nighttime eating may contribute to cardiometabolic disease (Fig. 1).55
Figure 1.
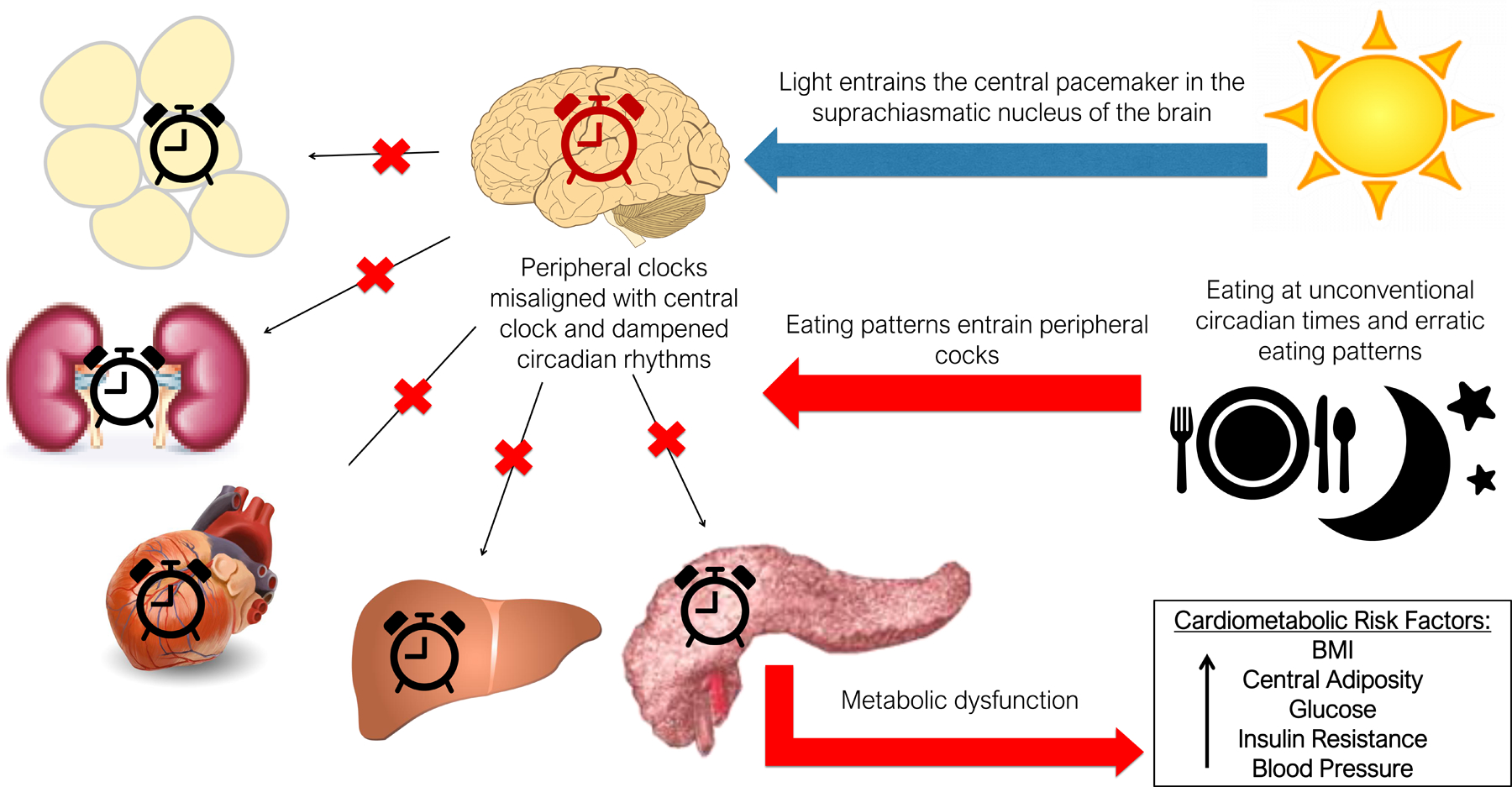
Later eating timing and variable eating patterns may increase cardiometabolic risk via circadian disruption.
Makarem’s results suggest that eating schedules characterized by earlier and regular eating timing and duration and less nighttime eating may lower CVD risk, particularly in women. She stressed that longer-term studies in diverse cohorts are needed to confirm these results.
Regulating eating and appetite: molecular, neurologic, and genetic mechanisms
Several speakers presented data on the mechanisms underlying appetite, eating, and connections to metabolic health.
Jonathan Cedernaes from Northwestern University and Uppsala University discussed his work on understanding the neurological mechanisms that regulate appetite.
In mice, knocking out Clock, a key component of the molecular circadian clock in pacemaker neurons of the SCN, results in an obese phenotype characterized by metabolic syndrome and mistimed feeding.56 Cedernaes is interested in understanding how the central clock in the hypothalamus contributes to disrupted appetite and eating. The central clock has energy-sensing neurons that can regulate functions like insulin sensitivity, insulin output, and glucose production in the liver.57
To study the role of the central clock in appetite, Cedernaes characterized the effects of hypothalamic knockout of the circadian clock gene Bmal1 in mice.58 Similar to Clock knockout mice56, Bmal1 knockout mice had an eating schedule skewed toward the day, the resting period for those animals. They also had an obese phenotype, despite consuming a similar amount of calories as the wild type mice. Cedernaes showed that mistimed feeding can lead to dysregulated metabolism. Mutant mice show increased glucose production by the liver, which was not suppressed by glucose infusion, as well as signs of reduced insulin sensitivity. Time restricted feeding, i.e., feeding mice during the night normalized glucose output as did severing the vagal nerve connecting the brain and the liver.58
Cedernaes next looked at the circadian transcriptional activity in agouti-related protein (AgRP) neurons, which are known to regulate appetite. Knocking out Bmal1 in AgRP neurons increased glucose output, altered the feeding rhythm, and increased body weight. Knockout of Bmal1 changed the diurnal transcriptional activity in AgRP neurons, resulting in upregulation of inflammatory pathways and downregulation of pathways involved in Clock function. He showed that leptin signaling, which is normally increased in the morning during times of fasting, had a more pronounced effect on transcription in Bmal1 knockout AgRP neurons than in wild-type neurons. Cedernaes concluded that the clock gene Bmal1 is essential for the morning/evening response to leptin in AgRP neurons. Ablation of the endogenous clock in AgRP neurons shifts the timing of the transcriptional response to leptin and ultimately leads to mistimed eating and metabolic dysregulation.
Erin Hanlon from the University of Chicago described work on understanding how the endocannabinoid system contributes to food intake. The endocannabinoid (eCB) system is likely best known asasasasasasas the target of Δ−9-tetrahydrocannabinol (THC), an active component of marijuana. The eCB system consists of two g-protein coupled receptors found in both the brain and in peripheral organs involved in energy metabolism. Cannabinoid-1 receptor (CB1) is known to increase appetite and hedonic feeding and has been a drug target for modulating food intake to reduce weight. CB1 inhibitors have been shown to induce weight loss, reduce fat mass, and improve metabolic disorders; however, they were also associated with mood disturbances. The role of cannabinoid-2 receptor (CB2) in appetite is less well understood. CB1 and CB2 are activated by the endogenous ligands 2-arachidonoylglycerol (2-AG) and anandamide (AEA).
Hanlon focused on the role of the endocannabinoid system in feeding and reward, ie, hedonic feeding, during periods of sleep deficiency. Sleep deficiency is known to increase hunger and appetite, particularly for snacks and foods high in hedonic value. She showed that serum levels of the endocannabinoid 2-AG show a robust circadian rhythm in healthy, lean volunteers, with a minimum during the mid-sleep period and a peak in the afternoon The rhythm is distinct from that of other cyclical hormones involved in metabolism such as cortisol and leptin, which peak in early morning and middle of night, respectively. In subjects with restricted sleep (i.e., 4.5 hours vs. 8.5 hours), the circadian rhythm of 2-AG showed a similar shape, but the peak was shifted approximately two hours later and had a higher amplitude.59 The delay in 2-AG peak may affect appetite in the afternoon and evening while the higher peak may contribute to the risk of overeating. Sleep-restricted individuals reported higher rates of hunger and desire to eat and tended to eat more snacks in the afternoon, at about the time that 2-AG levels were highest, compared to individuals with normal sleep.59
Obesity also affected the circadian rhythm of 2-AG. Inindividuals with obesity, the 2-AG rhythm was delayed and the amplitude flattened compared to those without. There was also an association between higher BMI and later 2-AG minimum. This study did not assess whether individuals with obesity ate later than those with normal weight. However, they are consistent with findings that later eating is associated with obesity. It remains to be seen whether the misaligned 2-AG rhythm is a cause or an effect of later eating.60
Hanlon showed that the other endocannabinoid, AEA, also displays a circadian rhythm distinct from that of 2-AG in young, lean volunteers. Unlike 2-AG, the AEA rhythm was not affected by sleep restriction.61 These data suggest that AEA and 2-AG have different roles in physiology, specifically in regard to regulating food intake. Hanlon proposed that AEA may be involved in initiating calorie intake, while 2-AG maintains calorie intake.
While both Cedernaes’ and Hanlon’s work show a connection between eating timing and metabolic dysfunction, questions remain regarding the factors that affect eating times.
Hassan Dashti, from Massachusetts General Hospital, showed that genetic factors also play a role in when people do and can eat, in addition to many behavioral and societal factors. Delineating the genetic factors behind complex human behaviors like food timing can help to identify biological pathways related to food timing, pinpoint molecular targets for drug development, develop tools to predict vulnerable populations, develope personalized treatment approaches, and elucidate correlations and causal effects with other traits and diseases.
Several lines of evidence suggest that food timing may be partially heritable. Food timing-related eating disorders, such as night eating syndrome and sleep-related eating disorder, show strong family-based heritability.62 In the general population, findings from a classical twin study show that food timing heritability ranges from 30–60%, with breakfast and lunch timing showing greater heritability than dinner timing.63
To identify genetic factors associated with food timing, Dashti conducted a genome-wide association study (GWAS) of 190,000 adults in the UK Biobank.64 Food timing information is typically not collected in large cohorts, so Dashti used a proxy phenotype approach, using information on breakfast cereal consumption as a proxy for breakfast skipping. Proxy phenotypes are not uncommon in GWAS studies; for example, education level is often used as a proxy of intelligence.65 Six independent genetic variants were associated with breakfast skipping. These variants were in regions also known to be associated with schizophrenia, caffeine metabolism, carbohydrate metabolism, and a preference for sweet foods. Three genes were known to be involved in regulating the pace of the circadian clock. Breakfast-skipping genes were enriched in the cerebellum, which is implicated in sleep duration, and are shared with other cardiometabolic traits and diseases. Genetics of breakfast skipping were correlated to that of obesity, body fat, lung cancer, rheumatoid arthritis, depressive symptoms, insomnia, and smoking (Fig. 2). The results suggest that there exists a common set of genes that underlie both food timing and obesity.66
Figure 2.
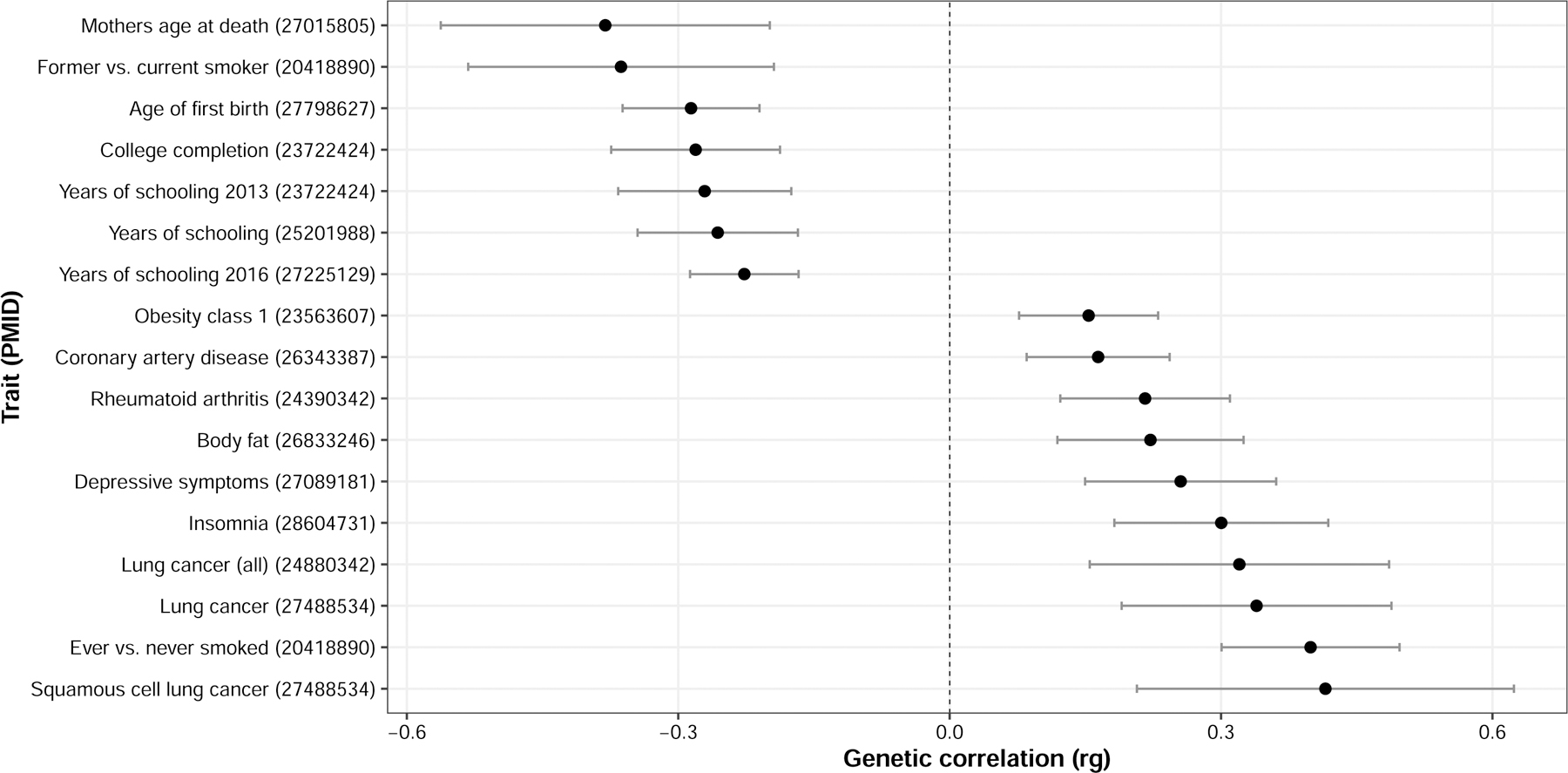
Cross-trait genetic correlations between breakfast skipping and publicly available traits and diseases using LD-score regression. Only significant correlations with P < 0.00022 accounting for 227 tested traits are shown. Point estimates are rg correlations. Error bars indicate 95% CI of rg estimates. PMID = pubmed ID; Rg = correlation estimate.
A separate study further examined at the connection between obesity genetics and food timing in the ChooseWell 365 cohort at Massachusetts General Hospital.67 The cohort consisted of 397 hospital employees, with data on cafeteria purchases, self-reported eating habits, and genome-wide genetic data. Unlike the GWAS, the ChooseWell 365 analysis included objective data on food purchases to estimate food timing. Using three months of food transactions, Dashti showed that employees with higher polygenic obesity risk scores tended to purchase their breakfast meals later. Higher polygenic obesity scores were also associated with more food purchases and lower quality purchases.68
Dashti’s work shows that food timing is at least partially heritable. Genes related to food timing are also involved in circadian rhythms and obesity, supporting the connection between meal timing and cardiometabolic health.
Sleep and circadian disturbance
The second half of the symposium focused on how disturbances to sleep and circadian rhythms can affect health.
Addressing night at light to improve sleep hygiene
Many people are exposed to short-wavelength (blue) light during the day and in the hours before sleep. There is increasing use of light-emitting diodes (LED), which are enriched in short-wavelength light, both inside and outside the home. Outside, LED light sources are replacing older street lamps.69 Inside, people are exposed to short-wavelength light via computers, smartphones, tablets, televisions, and, increasingly, domestic light bulbs. The Sleep in America poll in 2011 found that over 90% of American adults reported using some type of light-emitting electronic device within the hour before bedtime.70 While LED lights are more energy efficient and cost-effective than older light sources, they are also likely impacting human sleep and health. This is because the human circadian photoreceptor system, via intrinsically photosensitive retinal ganglion cells, shows peak sensitivity to ~450–480 nm light within the short-wavelength portion of the visible spectrum.71
Ari Shechter from Columbia University presented work on understanding whether reducing exposure to short-wavelength light during the evening preceding bedtime can improve sleep. In laboratory settings, exposure to short-wavelength/blue light in the evening from LED-backlit computers and self-luminous personal devices such as tablets was associated with factors that can contribute to impaired sleep initiation and worsened sleep quality, including decreased and delayed melatonin secretion, and increased neurocognitive arousal, core body temperature, and heart rate.72–78
As the deleterious effects of short-wavelength/blue light on sleep have become more widely known, the use of blue-light filters on electronic devices in the evening has been embraced by some people. Devices have also begun automatically changing their wavelength distribution after a set time. However, a recent study showed that even when blue-light filters are used, the blue light emitted can still be above the threshold expected to adversely impact the circadian system.79
Shechter is interested in determining whether wearable lenses that block or reduce transmission of short-wavelength/blue light to the retina, and ultimately the circadian and sleep-regulating regions of the hypothalamus, can mitigate some of the adverse impacts of exposure to this light on sleep. Studies have shown that blue-blocking lenses protect against light-induced melatonin suppression and improve self-reported and objective sleep measures. For example, blue-blocking lenses have been shown to maintain melatonin secretion in people exposed to nighttime LED light80 and to improve sleep in individuals with sleep difficulties.81 Shechter’s work has shown that using blue-blocking lenses in the evening can improve insomnia symptoms and increase objective sleep duration in individuals with insomnia.82
To understand the current state of the evidence for blue-blocking lenses on sleep, Shechter and colleagues conducted a systematic review and meta-analysis on studies using the blue-blocking lenses approach during the evening hours to determine their effect on nocturnal sleep. Among the studies that included objective estimates of sleep, there were small-to-medium combined effect sizes for improvements in sleep efficiency and total sleep time (Fig. 3). Comparatively larger effects were seen for subjective measures of sleep, with medium-to-large combined effects sizes observed for Pittsburgh Sleep Quality Index ratings and self-reported total sleep time.83 Overall, there is some modest evidence that this approach can be beneficial for nocturnal sleep, particularly in individuals with sleep disturbances (e.g., insomnia symptoms, delayed sleep phase syndrome) or psychiatric conditions (e.g., bipolar disorder, attention-deficit hyperactive disorder).
Figure 3.
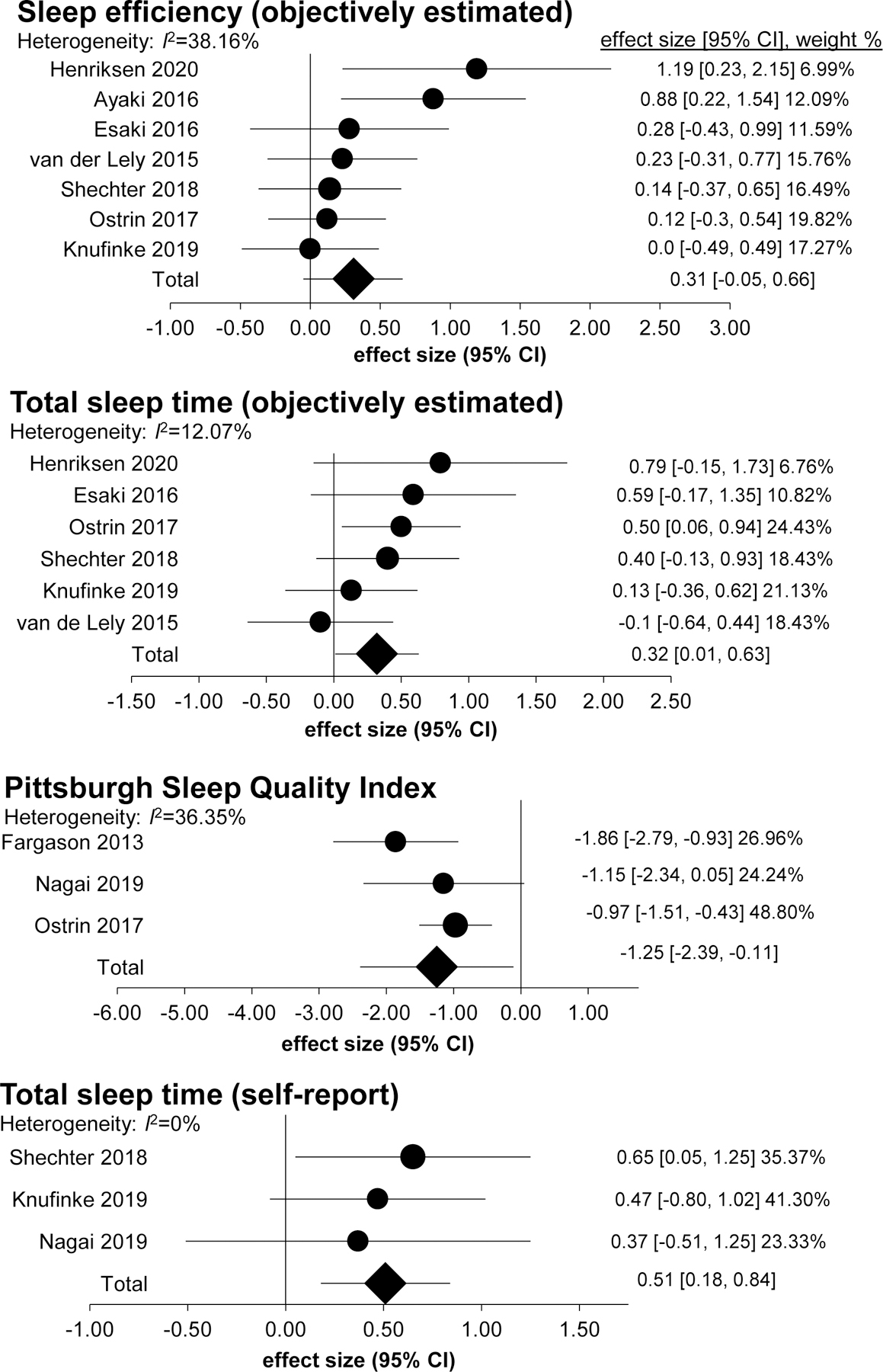
Forest plots of combined effects sizes from the meta-analyses of “blue blocker” interventions at night and their effects on sleep. Shown are the findings from objectively-estimated measures of sleep efficiency and total sleep time, and self-report measures of sleep quality (Pittsburgh Sleep Quality Index rating) and total sleep time. From Ref. 71.
Shechter noted that there was significant heterogeneity in the literature with regard to study designs, populations tested, duration of intervention, and the spectral and light intensity transmission of the lenses. Future studies should include standardized sleep-related outcomes, should include assessments of melatonin and other circadian markers to determine the intervention’s potential biological mechanisms of action, and should include assessments of ambient light levels and spectral composition.
Metabolic biomarkers of sleep and circadian disruption
Christopher Depner from the University of Utah presented work on developing metabolic biomarkers of sleep and circadian disruption. Depner hopes that a biomarker can provide a robust measure of circadian disruption for real-world studies. This can help bridge the rigorous controlled laboratory studies that link insufficient sleep to markers of metabolic dysfunction and the large epidemiologic studies that show associations between insufficient sleep and chronic metabolic diseases.84–89
Depner described his work using untargeted plasma metabolomics to identify small molecular biomarkers of circadian timing and insufficient sleep. In a randomized cross-over study of healthy volunteers, participants completed 5-day conditions with 9-hour or 5-hour time in bed sleep opportunities. Melatonin levels were measured hourly in dim-light conditions, with melatonin onset and offset used as a correlate for circadian timing. During the 9-hour sleep condition, melatonin onset and offset were similar to that of the baseline, control period while melatonin onset shifted approximately two hours later during the 5-hour sleep condition.90 Statistically significant metabolomics-based biomarker models of melatonin onset and offset were developed. However, Depner stressed that the models need to be more robust for clinical settings. He believes that free access to food during the study may have confounded the ability to tease out the influence of sleep timing from food intake on these metabolomics-based biomarkers.91 A separate study looking at transcription-based biomarkers has identified a more robust biomarker of circadian timing than Depner’s metabolomics-based approach.92
Depner also used plasma metabolomics to identify biomarkers of insufficient sleep. A biomarker model of 65 metabolites showed 74% accuracy in identifying insufficient sleep among all samples and 80% accuracy among fasted samples, suggesting food intake acutely impacted these metabolite leveles. 91 In fasted samples, biomarkers of insufficient sleep correlated with reduced insulin sensitivity. This is consistent with previous findings that show insulin sensitivity is reduced during insufficient sleep.93 This correlation was lost, however, when controlling for body weight and energy balance, suggesting that the biomarker is impacted by positive energy balance and weight-gain that occur during insufficient sleep. Depner is currently working to disentangle the impact of insufficient sleep from that of positive energy balance on the metabolome. He noted that it is important to think about the impact of food on sleep-related markers in real-world studies and stressed the need for trials that control food intake and monitor longer-term sleep trajectories.
Nonneuronal cells in sleep homeostasis: the role of astrocytes
Ashley Ingiosi from Washington State University presented work on understanding the role of astrocytes in regulating sleep. Even though glial cells are estimated to make up more than half of the cells in the brain, much less is known about their role in sleep compared to neurons. Ingiosi is specifically interested in glial cells called astrocytes. Several lines of evidence suggest that astrocytes can detect and integrate neural signals and alter neuronal activity; they surround synapses, interact with the neurovasculature, and express receptors for neurotransmitters.94 Evidence also shows that astrocytes can influence sleep and sleep regulation95–97 and that sleep deprivation can alter the astroglial proteome.98
Ingiosi investigated the role of astrocytes in sleep by monitoring astroglial Ca2+ activity in the frontal cortex of mice (Fig. 4). Unlike neurons, astrocytes are not electrically excitable cells. However, astrocytes use Ca2+ to mediate their functions, and previous studies showed that monitoring Ca2+ dynamics can be used to study astroglial activity.99 In addition, astroglial Ca2+ has been shown to play a role in the release of sleep-promoting substances.100 In vivo Ca2+ imaging of astrocytes in the frontal cortex of unanesthetized, freely behaving mice revealed that astroglial Ca2+ activity changed dynamically across naturally cycling sleep/wake states. Astroglial Ca2+ activity was aligned with neuronal EEG and electromyography (EMG) determinations of arousal state. Ingiosi showed that astroglial Ca2+ activity was greatest during wakefulness and was lowest during rapid eye movement (REM) sleep. High-resolution two-photon microscopy showed that Ca2+ dynamics were more robust in the astroglial processes than in the cell body. In addition, the synchrony of astroglial Ca2+ activity was greatest during wakefulness, which differs from that of neuronal electrical activity, suggesting that astrocytes may play a more direct role in sleep/wakeregulation previously thought.
Figure 4.
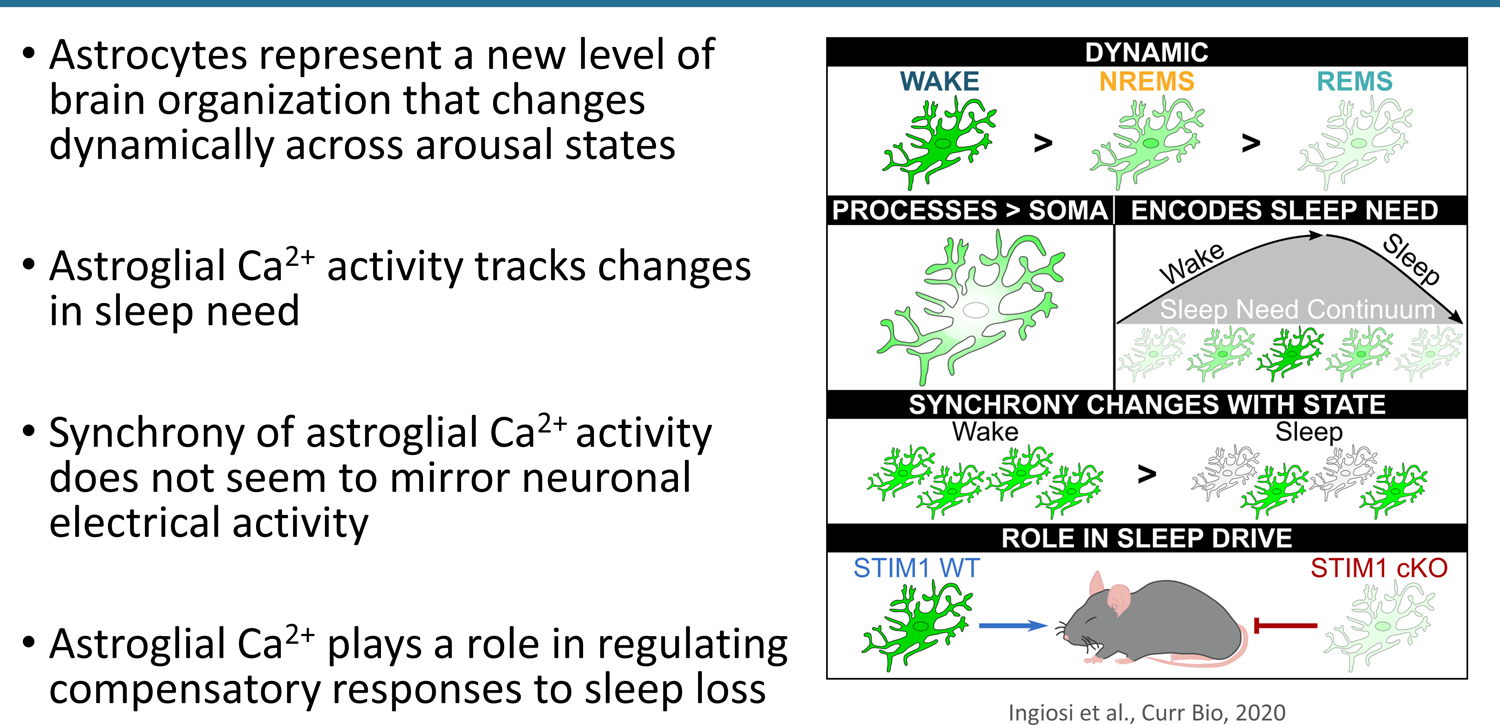
Astroglial Ca2++ activity contributes to sleep regulation.
Ingiosi also showed that astroglial Ca2+ activity changed with sleep need and sleep deprivation. Astroglial Ca2+ activity was higher in sleepy mice compared to rested mice; this effect was more pronounced in sleep-deprived mice.101
Finally, Ingiosi showed that astroglial Ca2+ activity can impact sleep homeostasis. Inhibiting replenishment of astroglial Ca2+ stores through conditional knockout of stromal interaction molecule 1 (Stim1) in astrocytes impaired sleep deprivation-induced Ca2+ increases in astrocytes. After sleep deprivation, mice with impaired astroglial Ca2+ activity did not sleep as long as wild type mice. They also displayed lower levels of sleepiness after sleep deprivation, as assessed by non-REM delta power. There was no impact of impaired astroglial Ca2+ activity on baseline sleep/wake behavior, activity patterns, or body temperature, which suggests that astroglial Ca2+ activity—as mediated by STIM1—plays a selective role in compensatory responses to sleep loss. Overall, Ingiosi proposed that astrocytes represent a new level of brain organization that changes dynamically across arousal states and plays a role in sleep homeostasis.101
High nighttime blood pressure and dementia risk
Blood pressure has a diurnal rhythm. Many people experience a 10% to 20% dip in blood pressure during the night and an increase in the morning after waking. Abnormalities in this diurnal pattern have been associated with CVD and cerebrovascular dysfunction. People who do not experience a nocturnal dip in blood pressure, known as non-dippers, and people who experience a nightly rise in blood pressure, known as reverse dippers, are more likely to have hypertension and a higher risk of heart failure and stroke.102 Reverse dipping is also associated with markers of small vessel cerebrovascular disease and impaired cognitive function in hypertensive individuals.103
Xiao Tan from Uppsala University presented work on the association between nighttime blood pressure and risk of dementia. Tan’s work has investigated whether non-dipping and reverse dipping are a risk factor for dementia. The study looked at approximately 1000 participants in the Uppsala Longitudinal Study of Adult Men, a cohort of 50-year-old men in Sweden that began in 1970. Average nighttime and daytime blood pressure values were measured in participants at ages 70 and 77 years. Tan showed that nocturnal blood pressure dipping decreased with age: 25% of 70-year-olds and 46% of 77-year-olds showed reduced dipping; reverse dipping was also associated with dementia risk. More specific, reverse dippers, i.e., those who had higher blood pressure in the night compared to the day had a 64% greater risk of dementia, in particular Alzheimer’s disease. This effect was independent of hypertension or other common risk factors.104 Tan noted that it may be worth investigating whether taking blood pressure medication in the evening, rather than earlier in the day, to lower nighttime blood pressure could lower risk of dementia.
There are a few possible mechanisms that can explain the link between high nocturnal blood pressure and Alzheimer’s disease. First, given the link between reverse dipping and white matter hyperintensities93, high nighttime blood pressure may cause cerebrovascular injuries that may lead to white matter damage. Second, sleep disorders may play a role. A separate study has shown that 75% of reverse dippers also suffer from obstructive sleep apnea (OSA).105 This could contribute to hypoxia in the brain, which may lead to accumulation of amyloid106, as well as increased arousal, which has been shown to increase tau protein.107 Tan stressed that the diurnal pattern of blood pressure is often overlooked in clinical settings, where blood pressure is typically measured during the day. This could result in health problems going undetected.
The bi-directional relationship between non-apnea sleep disorders and stroke
Animal models have shown that sleep disruption can mediate both stroke risk and post-stroke recovery.108,109 Human studies on the relationship between sleep disruption and stroke have largely focused on sleep apnea; data on non-sleep apnea disorders are sparse.
Elie Gottlieb from the Florey Institute of Neuroscience and the University of Melbourne presented work aimed at understanding the bi-directional relationship between non-apnea sleep disorders and ischemic stroke. A systemic review by Gottleib110 showed that examined the bi-directional relationship between non-apnea sleep and circadian rhythms in stroke patients. Sixty-seven studies with sleep or circadian rhythm metrics before or after stroke were included. Most prospective cohort studies reported a J-shaped relationship between sleep duration and stroke risk, though few studies adjusted for comorbidities like depression or OSA, which might be driving this relationship. In studies that examined the impact of non-apnea sleep disorders on ischemic stroke, sleep-related movement disorders, insomnia, REM behavior disorder, and hypersomnia were associated with increased risk of ischemic stroke. Studies that looked at sleep after stroke found that sleep efficiency decreased in the months following stroke, and patients experienced de novo sleep disorders. No studies assessed sleep for more than 1 year post-stroke, so it is unclear whether these effects on sleep are transient and potentially related to hospitalization or if they are chronic. No studies assessed objectively measured circadian rhythm dysfunction on stroke risk; however, seven studies measured endogenous circadian rhythm post-stroke. All reported significant reductions to melatonin relative to controls.
To try to understand the mechanism of sleep-related disorders after stroke, Gottlieb characterized regional brain volumes and fiber-specific white matter correlates of objectively measured sleep in 112 ischemic stroke patients and 40 matched controls. At three months after stroke,patients with long sleep duration showed cortico–ponto–cerebellar tract degeneration, which may be indicative of sleep–wake pathology. Stroke patients with normal sleep duration had extensive, diffuse white matter degeneration, which may be typical post stroke and not related to sleep. Stroke patients with poor sleep efficiency also showed a decrease in ipsilesional thalamus volume and increase in caudate volume (ipsilesional and contralateral) compared to matched controls.111
Gottlieb is currently conducting a long-term study with 4 years of follow up to understand whether the sleep-wake impairments endured after stroke resolve over time.
Impact of the COVID-19 lockdowns on sleep
The COVID-19 lockdowns in the spring of 2020 brought about a sudden change in social behavior patterns and work schedules. Christine Blume from the University of Basel presented her work on understanding the effect of the lockdowns on sleep quality, sleep behavior, social jetlag, and wellbeing via a one-time survey in three European countries (Austria, Germany, Switzerland, 435 respondents) in March and April 2020. Overall, there was an improvement of biological and social rhythms. This was accompanied by a decrease in social jetlag, the difference in timing of sleep on work vs free days, and in social sleep restriction, primarily driven by later and longer sleep time on work days. There was a median increase in sleep duration of approximately 13 minutes.112 Other studies that have looked at the impact of the lockdowns on sleep with data available before and after the lockdown have likewise found increases in sleep time and decreases in social jetlag.113,114
Participants in Blume’s study also reported a slight decrease in sleep quality. This was somewhat unexpected since aligning external and internal rhythms would be expected to increase sleep quality. However, the decrease in sleep quality was tied to the effects of the pandemic as sleep quality was closely associated with self-perceived burden related to finances, childcare, and the pandemic situation. The decrease in sleep quality was partially mitigated in people who spent more time outside and exercised.112 A separate study also reported a decrease in sleep quality due to the pandemic. In this study, people with poor sleep quality before the pandemic reported an improvement in sleep quality during the pandemic while those with good pre-pandemic sleep quality reported a decrease in sleep quality.115
Blume concluded that overall, lockdowns had a positive effect on sleep timing and duration. Although sleep quality decreased, this was dependent on pre-pandemic sleep quality, and coping mechanisms like spending time outside and staying active may mitigate these effects. While the effects of the pandemic represent a specific event that may prove to be short-lived, Blume hopes that studies like these can prompt broader discussions on how society can support good sleep, for example, by increasing flexibility in work schedules or adapting schedules to an individual’s chronotype.
Conclusions
In modern societies, there is often a mismatch between the biological and social drivers that regulate sleep timing. This can lead to social jetlag (i.e., different sleep timing on work days and free days) and/or social sleep restriction (i.e., unequal sleep duration on work days and free days, often to compensate for sleep deficit during work days) and further influence timing of other behavioral patterns, such as eating. Sleep restriction, delayed eating, and social jetlag havehavehavehavehavejetlaghave been linked to negative health effects, such as depressive symptoms, poor performance at school/work, and chronic metabolic disease. In this symposium, experts in sleep and circadian rhythms have highlighted associations and demonstrated causal pathways linking sleep and circadian disruptions to disease risk. They have additionally recommended avenues for future research and potential applications for risk reduction and treatment. This meeting served as a preview to a future Keystone symposium (Sleep & Circadian Rhythms: Maintaining Tempo for Optimal Health) on the latest research in sleep and circadian rhythms to promote interactions and collaborative work in these overlapping fields.
Acknowledgments
C.V.’s work is funded by the NIH, the University of Colorado Boulder, and the Colorado Clinical and Translational Sciences Initiative. N.M.’s work is funded by the NIH (R00 HL148511) and the American Heart Association.
Footnotes
Competing interests
C.V. serves on the scientific advisory boards of Circadian Lighting Inc., a Diabetes UK funded study, and Chronsulting GmbH. C.V. has also served as a paid consultant to the DoE, and NIOSH.
References
- 1.Vetter C, Pattison PM, Houser K, et al. 2021. A Review of Human Physiological Responses to Light: Implications for the Development of Integrative Lighting Solutions. LEUKOS 0: 1–28. [Google Scholar]
- 2.F B., Js S, An C, et al. 2015. Clocking in: chronobiology in rheumatoid arthritis. Nat. Rev. Rheumatol 11:. [DOI] [PubMed] [Google Scholar]
- 3.Hsu C-N & Tain Y-L. 2020. Light and Circadian Signaling Pathway in Pregnancy: Programming of Adult Health and Disease. Int. J. Mol. Sci 21:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bass J 2012. Circadian topology of metabolism. Nature 491: 348–356. [DOI] [PubMed] [Google Scholar]
- 5.Vetter C 2020. Circadian disruption: What do we actually mean? Eur. J. Neurosci 51: 531–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cos S, González A, Martínez-Campa C, et al. 2006. Estrogen-signaling pathway: a link between breast cancer and melatonin oncostatic actions. Cancer Detect. Prev 30: 118–128. [DOI] [PubMed] [Google Scholar]
- 7.Schernhammer ES, Laden F, Speizer FE, et al. 2001. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J. Natl. Cancer Inst 93: 1563–1568. [DOI] [PubMed] [Google Scholar]
- 8.Schernhammer ES, Kroenke CH, Laden F, et al. 2006. Night Work and Risk of Breast Cancer. Epidemiology 17: 108–111. [DOI] [PubMed] [Google Scholar]
- 9.Schernhammer ES & Hankinson SE. 2005. Urinary melatonin levels and breast cancer risk. J. Natl. Cancer Inst 97: 1084–1087. [DOI] [PubMed] [Google Scholar]
- 10.Schernhammer ES & Hankinson SE. 2009. Urinary melatonin levels and postmenopausal breast cancer risk in the Nurses’ Health Study cohort. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol 18: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wegrzyn LR, Tamimi RM, Rosner BA, et al. 2017. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies. Am. J. Epidemiol 186: 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James P, Bertrand KA, Hart JE, et al. 2017. Outdoor Light at Night and Breast Cancer Incidence in the Nurses’ Health Study II. Environ. Health Perspect 125: 087010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schernhammer ES, Laden F, Speizer FE, et al. 2003. Night-shift work and risk of colorectal cancer in the nurses’ health study. J. Natl. Cancer Inst 95: 825–828. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan AN, Hankinson SE & Schernhammer ES. 2007. Night shift work and the risk of endometrial cancer. Cancer Res 67: 10618–10622. [DOI] [PubMed] [Google Scholar]
- 15.Schernhammer ES, Feskanich D, Liang G, et al. 2013. Rotating night-shift work and lung cancer risk among female nurses in the United States. Am. J. Epidemiol 178: 1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papantoniou K, Devore EE, Massa J, et al. 2018. Rotating night shift work and colorectal cancer risk in the nurses’ health studies. Int. J. Cancer 143: 2709–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise J 2009. Danish night shift workers with breast cancer awarded compensation. BMJ 338: b1152. [DOI] [PubMed] [Google Scholar]
- 18.IARC Monographs Vol 124 group. 2019. Carcinogenicity of night shift work. Lancet Oncol 20: 1058–1059. [DOI] [PubMed] [Google Scholar]
- 19.Torquati L, Mielke GI, Brown WJ, et al. 2018. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand. J. Work. Environ. Health 44: 229–238. [DOI] [PubMed] [Google Scholar]
- 20.Vetter C, Devore EE, Wegrzyn LR, et al. 2016. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA 315: 1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leproult R, Holmbäck U & Van Cauter E. 2014. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63: 1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright KP, Drake AL, Frey DJ, et al. 2015. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain. Behav. Immun 47: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris CJ, Purvis TE, Hu K, et al. 2016. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. U. S. A 113: E1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris CJ, Purvis TE, Mistretta J, et al. 2017. Circadian Misalignment Increases C-Reactive Protein and Blood Pressure in Chronic Shift Workers. J. Biol. Rhythms 32: 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puttonen S, Viitasalo K & Härmä M. 2011. Effect of shiftwork on systemic markers of inflammation. Chronobiol. Int 28: 528–535. [DOI] [PubMed] [Google Scholar]
- 26.Hulsegge G, Picavet HSJ, van der Beek AJ, et al. 2019. Shift work, chronotype and the risk of cardiometabolic risk factors. Eur. J. Public Health 29: 128–134. [DOI] [PubMed] [Google Scholar]
- 27.Johnson CY, Tanz LJ, Lawson CC, et al. 2020. Night shift work and cardiovascular disease biomarkers in female nurses. Am. J. Ind. Med 63: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjorvatn B, Axelsson J, Pallesen S, et al. 2020. The Association Between Shift Work and Immunological Biomarkers in Nurses. Front. Public Health 8: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen JO, Nilsen KB, Hopstock LA, et al. 2021. Shift work, low-grade inflammation, and chronic pain: a 7-year prospective study. Int. Arch. Occup. Environ. Health [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamble KL, Motsinger-Reif AA, Hida A, et al. 2011. Shift work in nurses: contribution of phenotypes and genotypes to adaptation. PloS One 6: e18395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juda M, Vetter C & Roenneberg T. 2013. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J. Biol. Rhythms 28: 141–151. [DOI] [PubMed] [Google Scholar]
- 32.Vetter C & Schernhammer ES. 2015. Early, but not late chronotypes, are up during their biological night when working the night shift. Occup. Environ. Med 72: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korsiak J, Tranmer J, Leung M, et al. 2018. Actigraph measures of sleep among female hospital employees working day or alternating day and night shifts. J. Sleep Res 27: e12579. [DOI] [PubMed] [Google Scholar]
- 34.Kervezee L, Gonzales-Aste F, Boudreau P, et al. 2021. The relationship between chronotype and sleep behavior during rotating shift work: a field study. Sleep [DOI] [PubMed] [Google Scholar]
- 35.Fischer D, Vetter C, Oberlinner C, et al. 2016. A unique, fast-forwards rotating schedule with 12-h long shifts prevents chronic sleep debt. Chronobiol. Int 33: 98–107. [DOI] [PubMed] [Google Scholar]
- 36.Gill S & Panda S. 2015. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roenneberg T, Allebrandt KV, Merrow M, et al. 2012. Social jetlag and obesity. Curr. Biol. CB 22: 939–943. [DOI] [PubMed] [Google Scholar]
- 38.Brown LA, Fisk AS, Pothecary CA, et al. 2019. Telling the Time with a Broken Clock: Quantifying Circadian Disruption in Animal Models. Biology 8:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer D, Vetter C & Roenneberg T. 2016. A novel method to visualise and quantify circadian misalignment. Sci. Rep 6: 38601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowther ME, Ferguson SA, Vincent GE, et al. 2021. Non-Pharmacological Interventions to Improve Chronic Disease Risk Factors and Sleep in Shift Workers: A Systematic Review and Meta-Analysis. Clocks Sleep 3: 132–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neil-Sztramko SE, Pahwa M, Demers PA, et al. 2014. Health-related interventions among night shift workers: a critical review of the literature. Scand. J. Work. Environ. Health 40: 543–556. [DOI] [PubMed] [Google Scholar]
- 42.Vetter C, Fischer D, Matera JL, et al. 2015. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr. Biol. CB 25: 907–911. [DOI] [PubMed] [Google Scholar]
- 43.Daghlas I, Dashti HS, Lane J, et al. 2019. Sleep Duration and Myocardial Infarction. J. Am. Coll. Cardiol 74: 1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kant AK & Graubard BI. 2015. 40-Year Trends in Meal and Snack Eating Behaviors of American Adults. J. Acad. Nutr. Diet 115: 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berg C, Lappas G, Wolk A, et al. 2009. Eating patterns and portion size associated with obesity in a Swedish population. Appetite 52: 21–26. [DOI] [PubMed] [Google Scholar]
- 46.Kutsuma A, Nakajima K & Suwa K. 2014. Potential Association between Breakfast Skipping and Concomitant Late-Night-Dinner Eating with Metabolic Syndrome and Proteinuria in the Japanese Population. Scientifica 2014: 253581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leech RM, Timperio A, Worsley A, et al. 2019. Eating patterns of Australian adults: associations with blood pressure and hypertension prevalence. Eur. J. Nutr 58: 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marinac CR, Sears DD, Natarajan L, et al. 2015. Frequency and Circadian Timing of Eating May Influence Biomarkers of Inflammation and Insulin Resistance Associated with Breast Cancer Risk. PloS One 10: e0136240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahleova H, Lloren JI, Mashchak A, et al. 2017. Meal Frequency and Timing Are Associated with Changes in Body Mass Index in Adventist Health Study 2. J. Nutr 147: 1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sierra-Johnson J, Undén A-L, Linestrand M, et al. 2008. Eating meals irregularly: a novel environmental risk factor for the metabolic syndrome. Obes. Silver Spring Md 16: 1302–1307. [DOI] [PubMed] [Google Scholar]
- 51.Pot GK, Hardy R & Stephen AM. 2014. Irregular consumption of energy intake in meals is associated with a higher cardiometabolic risk in adults of a British birth cohort. Int. J. Obes. 2005 38: 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pot GK, Hardy R & Stephen AM. 2016. Irregularity of energy intake at meals: prospective associations with the metabolic syndrome in adults of the 1946 British birth cohort. Br. J. Nutr 115: 315–323. [DOI] [PubMed] [Google Scholar]
- 53.Zerón-Rugerio MF, Longo-Silva G, Hernáez Á, et al. 2020. The Elapsed Time between Dinner and the Midpoint of Sleep is Associated with Adiposity in Young Women. Nutrients 12:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.St-Onge M-P, Ard J, Baskin ML, et al. 2017. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 135: e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makarem N, Sears DD, St-Onge M-P, et al. 2020. Habitual Nightly Fasting Duration, Eating Timing, and Eating Frequency are Associated with Cardiometabolic Risk in Women. Nutrients 12:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turek FW, Joshu C, Kohsaka A, et al. 2005. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cedernaes J, Waldeck N & Bass J. 2019. Neurogenetic basis for circadian regulation of metabolism by the hypothalamus. Genes Dev 33: 1136–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cedernaes J, Huang W, Ramsey KM, et al. 2019. Transcriptional Basis for Rhythmic Control of Hunger and Metabolism within the AgRP Neuron. Cell Metab 29: 1078–1091.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanlon EC, Tasali E, Leproult R, et al. 2016. Sleep Restriction Enhances the Daily Rhythm of Circulating Levels of Endocannabinoid 2-Arachidonoylglycerol. Sleep 39: 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanlon EC, Leproult R, Stuhr KL, et al. 2020. Circadian Misalignment of the 24-hour Profile of Endocannabinoid 2-Arachidonoylglycerol (2-AG) in Obese Adults. J. Clin. Endocrinol. Metab 105:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanlon EC 2020. Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide). Psychoneuroendocrinology 111: 104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Reardon JP, Peshek A & Allison KC. 2005. Night eating syndrome : diagnosis, epidemiology and management. CNS Drugs 19: 997–1008. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Minguez J, Dashti HS, Madrid-Valero JJ, et al. 2019. Heritability of the timing of food intake. Clin. Nutr. Edinb. Scotl 38: 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bycroft C, Freeman C, Petkova D, et al. 2018. The UK Biobank resource with deep phenotyping and genomic data. Nature 562: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rietveld CA, Esko T, Davies G, et al. 2014. Common genetic variants associated with cognitive performance identified using the proxy-phenotype method. Proc. Natl. Acad. Sci 111: 13790–13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dashti HS, Merino J, Lane JM, et al. 2019. Genome-wide association study of breakfast skipping links clock regulation with food timing. Am. J. Clin. Nutr 110: 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levy DE, Gelsomin ED, Rimm EB, et al. 2018. Design of ChooseWell 365: Randomized controlled trial of an automated, personalized worksite intervention to promote healthy food choices and prevent weight gain. Contemp. Clin. Trials 75: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dashti HS, Hivert M-F, Levy DE, et al. 2020. Polygenic risk score for obesity and the quality, quantity, and timing of workplace food purchases: A secondary analysis from the ChooseWell 365 randomized trial. PLoS Med 17: e1003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Falchi F, Cinzano P, Elvidge CD, et al. 2011. Limiting the impact of light pollution on human health, environment and stellar visibility. J. Environ. Manage 92: 2714–2722. [DOI] [PubMed] [Google Scholar]
- 70.Gradisar M, Wolfson AR, Harvey AG, et al. 2013. The Sleep and Technology Use of Americans: Findings from the National Sleep Foundation’s 2011 Sleep in America Poll. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 9: 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thapan K, Arendt J & Skene DJ. 2001. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol 535: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cajochen C, Münch M, Kobialka S, et al. 2005. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab 90: 1311–1316. [DOI] [PubMed] [Google Scholar]
- 73.Cajochen C, Frey S, Anders D, et al. 2011. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J. Appl. Physiol. Bethesda Md 1985 110: 1432–1438. [DOI] [PubMed] [Google Scholar]
- 74.Chang A-M, Aeschbach D, Duffy JF, et al. 2015. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl. Acad. Sci. U. S. A 112: 1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wood B, Rea MS, Plitnick B, et al. 2013. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl. Ergon 44: 237–240. [DOI] [PubMed] [Google Scholar]
- 76.Figueiro M & Overington D. 2016. Self-luminous devices and melatonin suppression in adolescents. Light. Res. Technol 48: 966–975. [Google Scholar]
- 77.Grønli J, Byrkjedal IK, Bjorvatn B, et al. 2016. Reading from an iPad or from a book in bed: the impact on human sleep. A randomized controlled crossover trial. Sleep Med 21: 86–92. [DOI] [PubMed] [Google Scholar]
- 78.Chinoy ED, Duffy JF & Czeisler CA. 2018. Unrestricted evening use of light-emitting tablet computers delays self-selected bedtime and disrupts circadian timing and alertness. Physiol. Rep 6: e13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rea MS, Nagare R & Figueiro MG. 2020. Predictions of melatonin suppression during the early biological night and their implications for residential light exposures prior to sleeping. Sci. Rep 10: 14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Lely S, Frey S, Garbazza C, et al. 2015. Blue blocker glasses as a countermeasure for alerting effects of evening light-emitting diode screen exposure in male teenagers. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med 56: 113–119. [DOI] [PubMed] [Google Scholar]
- 81.Burkhart K & Phelps JR. 2009. Amber lenses to block blue light and improve sleep: a randomized trial. Chronobiol. Int 26: 1602–1612. [DOI] [PubMed] [Google Scholar]
- 82.Shechter A, Kim EW, St-Onge M-P, et al. 2018. Blocking nocturnal blue light for insomnia: A randomized controlled trial. J. Psychiatr. Res 96: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shechter A, Quispe KA, Mizhquiri Barbecho JS, et al. 2020. Interventions to reduce short-wavelength (“blue”) light exposure at night and their effects on sleep: A systematic review and meta-analysis. SLEEP Adv 1:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cappuccio FP, D’Elia L, Strazzullo P, et al. 2010. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 33: 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baden MY, Hu FB, Vetter C, et al. 2020. Sleep Duration Patterns in Early to Middle Adulthood and Subsequent Risk of Type 2 Diabetes in Women. Diabetes Care 43: 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spiegel K, Knutson K, Leproult R, et al. 2005. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J. Appl. Physiol. Bethesda Md 1985 99: 2008–2019. [DOI] [PubMed] [Google Scholar]
- 87.Leproult R & Van Cauter E. 2010. Role of sleep and sleep loss in hormonal release and metabolism. Endocr. Dev 17: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reutrakul S & Van Cauter E. 2018. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism 84: 56–66. [DOI] [PubMed] [Google Scholar]
- 89.Depner CM, Stothard ER & Wright KP. 2014. Metabolic consequences of sleep and circadian disorders. Curr. Diab. Rep 14: 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Markwald RR, Melanson EL, Smith MR, et al. 2013. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. U. S. A 110: 5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Depner CM, Cogswell DT, Bisesi PJ, et al. 2020. Developing preliminary blood metabolomics-based biomarkers of insufficient sleep in humans. Sleep 43:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wittenbrink N, Ananthasubramaniam B, Münch M, et al. 2018. High-accuracy determination of internal circadian time from a single blood sample. J. Clin. Invest 128: 3826–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eckel RH, Depner CM, Perreault L, et al. 2015. Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Curr. Biol. CB 25: 3004–3010. [DOI] [PubMed] [Google Scholar]
- 94.Allen NJ & Barres BA. 2009. Neuroscience: Glia - more than just brain glue. Nature 457: 675–677. [DOI] [PubMed] [Google Scholar]
- 95.Halassa MM, Florian C, Fellin T, et al. 2009. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ingiosi AM, Raymond RM, Pavlova MN, et al. 2015. Selective contributions of neuronal and astroglial interleukin-1 receptor 1 to the regulation of sleep. Brain. Behav. Immun 48: 244–257. [DOI] [PubMed] [Google Scholar]
- 97.Pelluru D, Konadhode RR, Bhat NR, et al. 2016. Optogenetic stimulation of astrocytes in the posterior hypothalamus increases sleep at night in C57BL/6J mice. Eur. J. Neurosci 43: 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim J-H, Kim J-H, Cho Y-E, et al. 2014. Chronic sleep deprivation-induced proteome changes in astrocytes of the rat hypothalamus. J. Proteome Res 13: 4047–4061. [DOI] [PubMed] [Google Scholar]
- 99.Guerra-Gomes S, Sousa N, Pinto L, et al. 2017. Functional Roles of Astrocyte Calcium Elevations: From Synapses to Behavior. Front. Cell. Neurosci 11: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawamata H, Ng SK, Diaz N, et al. 2014. Abnormal intracellular calcium signaling and SNARE-dependent exocytosis contributes to SOD1G93A astrocyte-mediated toxicity in amyotrophic lateral sclerosis. J. Neurosci. Off. J. Soc. Neurosci 34: 2331–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ingiosi AM, Hayworth CR, Harvey DO, et al. 2020. A Role for Astroglial Calcium in Mammalian Sleep and Sleep Regulation. Curr. Biol. CB 30: 4373–4383.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ingelsson E, Björklund-Bodegård K, Lind L, et al. 2006. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA 295: 2859–2866. [DOI] [PubMed] [Google Scholar]
- 103.Chesebro AG, Melgarejo JD, Leendertz R, et al. 2020. White matter hyperintensities mediate the association of nocturnal blood pressure with cognition. Neurology 94: e1803–e1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tan X, Sundström J, Lind L, et al. 2021. Reverse Dipping of Systolic Blood Pressure Is Associated With Increased Dementia Risk in Older Men: A Longitudinal Study Over 24 Years. Hypertens. Dallas Tex 1979 77: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 105.Genta-Pereira DC, Furlan SF, Omote DQ, et al. 2018. Nondipping Blood Pressure Patterns Predict Obstructive Sleep Apnea in Patients Undergoing Ambulatory Blood Pressure Monitoring. Hypertens. Dallas Tex 1979 72: 979–985. [DOI] [PubMed] [Google Scholar]
- 106.Cermakova P, Eriksdotter M, Lund LH, et al. 2015. Heart failure and Alzheimer’s disease. J. Intern. Med 277: 406–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benedict C, Blennow K, Zetterberg H, et al. 2020. Effects of acute sleep loss on diurnal plasma dynamics of CNS health biomarkers in young men. Neurology 94: e1181–e1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zunzunegui C, Gao B, Cam E, et al. 2011. Sleep disturbance impairs stroke recovery in the rat. Sleep 34: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao B, Kilic E, Baumann CR, et al. 2008. Gamma-hydroxybutyrate accelerates functional recovery after focal cerebral ischemia. Cerebrovasc. Dis. Basel Switz 26: 413–419. [DOI] [PubMed] [Google Scholar]
- 110.Gottlieb E, Landau E, Baxter H, et al. 2019. The bidirectional impact of sleep and circadian rhythm dysfunction in human ischaemic stroke: A systematic review. Sleep Med. Rev 45: 54–69. [DOI] [PubMed] [Google Scholar]
- 111.Gottlieb E, Egorova N, Khlif MS, et al. 2020. Regional neurodegeneration correlates with sleep-wake dysfunction after stroke. Sleep 43:. [DOI] [PubMed] [Google Scholar]
- 112.Blume C, Schmidt MH & Cajochen C. 2020. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr. Biol. CB 30: R795–R797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wright KP, Linton SK, Withrow D, et al. 2020. Sleep in university students prior to and during COVID-19 Stay-at-Home orders. Curr. Biol. CB 30: R797–R798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leone MJ, Sigman M & Golombek DA. 2020. Effects of lockdown on human sleep and chronotype during the COVID-19 pandemic. Curr. Biol. CB 30: R930–R931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kocevska D, Blanken TF, Van Someren EJW, et al. 2020. Sleep quality during the COVID-19 pandemic: not one size fits all. Sleep Med 76: 86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


