Abstract
Purpose:
To test effects of positron emission tomography (PET)-based bone marrow-sparing (BMS) image-guided intensity modulated radiation therapy (IMRT) on efficacy and toxicity for patients with locoregionally advanced cervical cancer.
Methods and Materials:
In an international phase II/III trial, patients with stage IB-IVA cervical carcinoma were treated with either PET-based BMS-IG-IMRT (PET-BMS-IMRT group) or standard image-guided IMRT (IMRT group), with concurrent cisplatin (40 mg/m2 weekly), followed by brachytherapy. The phase II component non-randomly assigned patients to PET-BMS-IMRT or standard IMRT. The phase III trial randomized patients to PET-BMS-IMRT vs. IMRT, with a primary endpoint of progression-free survival (PFS) but was closed early for futility. Phase III patients were analyzed separately and in combination with phase II patients, comparing acute hematologic toxicity, cisplatin delivery, PFS, overall survival (OS), and patterns of failure. In a post-hoc exploratory analysis, we investigated the association between pretreatment absolute lymphocyte count (ALC) and OS.
Results:
In total, 101 patients were enrolled on the phase II/III trial, including 29 enrolled in phase III (PET-BMS-IMRT group: 16; IMRT group: 13) before early closure. Median follow-up was 33 months for phase III patients and 39 months for all patients. PFS and OS at 5 years for all patients were 73.6% (95% CI 64.9%, 84.3%) and 84.0% (95% CI 76.0%, 92.9%), respectively. There were no differences in number of cisplatin cycles, OS, PFS, or patterns of failure between groups for the combined cohort. The incidence of acute grade ≥ 3 neutropenia was significantly lower in the PET-BMS-IMRT group compared to IMRT for randomized patients (19% versus 54%, χ2 p=0.048) and in the combined cohort (13% vs. 35%, χ2 p=0.01). Patients with pretreatment ALC ≤ 1.5 k/μL had non-significantly worse OS on multivariable analysis (HR 2.85, 95% CI 0.94, 8.62, adjusted p-value p=0.216), compared to patients with ALC > 1.5 k/μL. There was no difference in post-treatment ALC by treatment group.
Conclusions:
PET-BMS-IMRT significantly reduced acute grade ≥ 3 neutropenia, but not treatment-related lymphopenia, compared to standard IMRT. We found no evidence that PET-BMS-IMRT impacted chemotherapy delivery or long-term outcomes, and weak evidence of an association between pre-treatment ALC and OS.
Keywords: IMRT, cervical cancer, bone marrow sparing, image-guided radiation therapy, hematologic toxicity
INTRODUCTION
Cervical cancer remains a major source of mortality for women worldwide, particularly in environments of limited resources1,2. For patients with locoregionally advanced disease, cisplatin-based chemoradiation therapy (CRT) has been the standard of care3,4 since 1999. However, the risk of disease recurrence and cancer death remains high, and treatment-related toxicity can both limit optimal treatment delivery and substantially impair quality of life5.
Reducing the toxicity of radiation through advanced technologies is an important strategy to improve tolerance to intensive therapeutic regimens.6–8 Image-guided intensity modulated radiation therapy (IG-IMRT) allows for the delivery of highly conformal radiation dose to the target structures while decreasing dose to nearby normal tissues, including bowel, rectum, and bone marrow.9 Multiple studies have found that reducing radiation dose to normal tissues yields lower rates of toxicity compared to conventional treatment in patients with gynecologic cancers10–14. In the postoperative setting, the TIME-C and PARCER trials both found significantly less gastrointestinal (GI) toxicity using IMRT compared to conventional (3D) techniques.15, 16 However, evidence from randomized trials to support routine use of this approach in patients treated with definitive intent has been sparse.17,18
By reducing dose to bone marrow, it has been hypothesized that positron emission tomography (PET)-based bone marrow sparing (BMS) IG-IMRT could permit higher tolerance to cytotoxic chemotherapy, particularly with concurrent and/or adjuvant doublets, due to sparing of myeloid precursors concentrated in haematopoietically active subregions19. This effect was shown, for example, in a previous report of the phase II Interna-tional Evaluation of Radiotherapy Technology Effectiveness in Cervical Cancer (INTERTECC) results20, and recently replicated in a randomized trial.21 However, little is known about the effect of pelmic BMS with respect to lymphocytes, a question of potential importance in the immunotherapy era. For example, several reports have associated lower bone marrow radiation doses with reduced incidence of lymphopenia in patients with various cancers, including in the pelvis.22–24 In cervical cancer, lymphopenia has been associated with poorer outcomes in patients undergoing CRT.25–27 Moreover, the presence of tumor-infiltrating lymphocytes (TILS) appears to be associated with improved survival.28 Thus, a potential benefit of IG-BMS-IMRT could be realized by sparing bone marrow lymphoblasts, by potentiating, or mitigating suppression of, the anti-tumor immune response.
In 2010, a large retrospective study found that IMRT was associated with improved overall and cause-specific survival compared to a non-IMRT cohort treated definitively.29 Phase 3 of the (INTERTECC) trial was originally designed to randomize PET-BMS-IMRT vs. 3DRT, with a primary endpoint of progression-free survival (PFS), based on the hypothesis that PET-BMS-IMRT would improve both target localization and chemotherapy tolerance.30 After interim review, the trial was closed prematurely due to futility and initiation of the contemporaneous NRG-GY006 trial.8 Here we report long-term outcomes from the (INTERTECC) trial, with emphasis on the effect of PET-BMS-IMRT on acute lymphocyte counts and the prognostic role of pretreatment lymphopenia.
METHODS
Study Design and Participants
The (INTERTECC) trial was an international, multicenter randomized phase II/III trial designed to test the hypothesis that PET-BMS-IMRT would improve PFS compared to standard therapy for cervical cancer patients, by improving target coverage and tolerance to concurrent chemotherapy. Patients were recruited at participating sites in the United States, Czech Republic, United Kingdom, India, and China at the time of initial consultation. Initial results for the phase II portion of the study (INTERTECC-2) were reported previously.20
Eligibility criteria included biopsy-proven carcinoma of the cervix, FIGO 2009 stage IB-IVA, ability to undergo PET/CT imaging, and medical fitness for pelvic CRT. Patients with prior RT or chemotherapy were excluded, as were patients with para-aortic, inguinal, or distant metastases. Patients with clear cell or small cell neuroendocrine carcinoma were also ineligible. In contrast to the phase 2 component of the study (INTERTECC-2), INTER-TECC-3 excluded patients treated post-hysterectomy. Patients treated post-hysterectomy in phase 2 were excluded from the combined analysis reported here. The pretreatment assessment included a full medical history, physical examination, demographic and health information questionnaire, quality of life assessment, and screening laboratory values. Staging with computed tomography (CT) or magnetic resonance imaging (MRI) in addition to 18F-fluorodeoxyglucose (FDG) PET/CT was required.
The study was supported by the National Cancer Institute (INTERTECC and INTERTECC) and approved by each participating center’s Institutional Review Board. The trial was registered on clinical trials.gov (NCT01554397). All patients signed written informed consent.
Procedures
In phase III, patients were randomly assigned in a 3:2 fashion31 to either experimental treatment with PET-BMS-IMRT with sparing of active bone marrow (defined as the subregion with a standardized uptake value (SUV) on PET/CT greater than the mean value over the total bone marrow volume) or standard therapy consisting of IMRT without BMS. Both arms received standard concurrent systemic therapy with cisplatin (40 mg/m2 for planned 6 cycles). All patients were prescribed a dose of 45.0 to 50.4 Gy in 25–28 daily fractions to the planning target volume (PTV). Patients with gross lymphadenopathy were treated with a simultaneous integrated boost (SIB) regimen of 47.6 Gy in 1.7 Gy fractions to the gross tumor and elective nodal regions and 54.0 to 59.4 Gy in 1.93 to 2.12 Gy fractions to grossly abnormal lymph nodes. Chemotherapy was held if patients developed grade 4 neutropenia or thrombocytopenia, febrile neutropenia, renal failure, grade 2 neurotoxicity, or grade ≥ 3 nausea lasting > 24 hours. CT simulation and target volume delineation were performed as previously published20. Pelvic marrow and active marrow mean doses were constrained to <27 Gy and <28.5 Gy, respectively, along with V10 <90% and V20 <75%. Bowel dose was also constrained to bowel volume receiving ≥45 Gy (V45) <200 cm3. Brachytherapy boost was recommended to start after the delivery of at least 39.6 Gy of external beam RT. Interstitial brachytherapy was allowed to treat disease that would be inadequately covered with intracavitary treatment at the discretion of the treating physician. The protocol specified that the total duration of radiation should be ≤60 days, with treatment >66 days representing unacceptable variation. All institutions underwent central credentialing through the Advanced Technology Consortium (ATC; Houston, TX and St. Louis, MO). All IMRT plans were centrally reviewed. In phase II, patients were non-randomly allocated to IMRT or PET-BMS-IMRT, with PET-BSM-IMRT assigned based on availability of PET/CT and patient’s optional participation in an imaging sub-study.20
Statistical Analyses
The phase III trial planned to enroll 415 patients with an expected 163 events to determine if IG-BMS-IMRT improves median PFS from 3.2 to 5.0 years with 80% power and alpha = 0.05, corresponding to a hazard ratio of 0.64, and an interim analysis after 82 events. The primary endpoint was PFS, defined as time to first evidence of cancer progression or death from any cause. Secondary outcomes included overall survival (OS), cumulative incidence of locoregional (pelvic) failure, cumulative incidence of distant metastases. Additional aims were to compare hematologic toxicity and total chemotherapy dose between study arms.
Event times were calculated as the time from trial registration to the date of first evidence of disease progression or death from any cause. Event times were censored for surviving patients if there was no evidence of disease progression at the last recorded follow-up or if the patient was lost to follow-up. PFS and OS were estimated with Kaplan-Meier methods. Cumulative incidence functions were estimated for locoregional failure (LRF) and distant failure. Differences in baseline characteristics between groups were assessed using chi-square (categorical) or t-tests (continuous). Differences in acute toxicity and chemotherapy delivery between groups were assessed using chi-square tests. Differences in pre- versus post-treatment mean blood counts were assessed with paired t-tests.
Phase III initiation coincided with rapid community adoption of IMRT and diminishing equipoise among participating sites regarding conventional RT. Therefore, the protocol was amended in July 2015 to permit non-BMS IMRT in the control arm (“usual care”) of the trial, in effect isolating the question of BMS. After quality assurance review was conducted on the first 29 patients, the protocol review and monitoring committee recommended study closure due to futility. Due to low patient numbers, we did not compare outcomes in the PET-BMS-IMRT vs. IMRT arms for phase III. Patients from the phase II and III components of the trial were pooled for the final analyses, and differences in time to events were analyzed using the log rank test or multivariable Cox proportional hazards models. Multivariable Cox models were used to test for effects on cause-specific hazards for LRF and distant failure. In an exploratory analysis, we tested for effects of pre-treatment absolute lymphocyte count (ALC) on OS and PFS. Multivariable models included pre-treatment lymphopenia and pre-specified clinically relevant covariables (stage and age). The initial plan was to utilize a lymphopenia threshold of 1.0 k/μL, consistent with prior studies27, but additional thresholds were subsequently considered based on a low number of patients with pre-treatment ALC < 1.0 k/μL. The next value chosen was the first quartile (1.5 k/μl) for ALC in the combined cohort, followed by a search between 1.3–1.7 k/μl. The final multivariable p-value was adjusted to account for the search for the optimal cutoff value.32
RESULTS
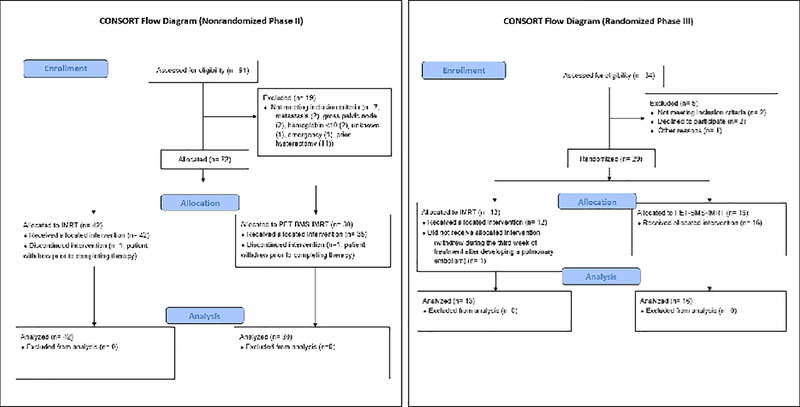
In phase III, 29 patients were enrolled between January 2016 and June 2018 and randomized to standard therapy arm (n=13) or to PET-BMS-IMRT (n=16) (Figure 1). In phase II, 84 patients were enrolled between October 2011 and April 2015, and 83 patients were non-randomly allocated to IMRT or PET-BMS-IMRT, 11 of whom were treated post-hysterectomy and were excluded for the present analysis. In total there were 101 analyzable patients (55 treated with IMRT, 46 treated with PET-BMS-IMRT). Median follow-up time for the 29 patients in phase III was 33 months and for all patients was 39 months. Baseline demographic and tumor variables were balanced for both phase III patients and all trial patients, with the exception of bone marrow dose for both phase III only and combined cohorts and tumor stage for the combined analysis, with a higher proportion of patients (Table 1). Patients in the PET/CT-BMS-IMRT group had significantly lower bone marrow dose as assessed by V10, V20, V30, V40, and overall mean dose compared to IMRT (Table 1). Following EBRT, 97/101 (96%) patients received a high dose rate brachytherapy boost. Of the 4 patients who did not receive brachytherapy, 3 had withdrawn from the study prior to completion of EBRT. Patients received a median dose of 30 Gy (interquartile range (IQR) 28.0, 30.0) in a median 5 fractions (IQR 4, 6). Out of the 97 patients who received brachytherapy, details of the technique were available for 39 patients. Of those with available details, 33 received intracavitary only brachytherapy, 1 received interstitial brachytherapy, and 5 received hybrid intracavitary/interstitial therapy (3 in the IMRT group and 2 in the PET-BMS-IMRT group). Overall duration of radiotherapy was a mean 48.6 days in the PET-BMS-IMRT group, compared to 50.1 days in the IMRT group (p= 0.49).
Figure 1:
CONSORT flow diagram for the INTERTECC phase II/III trial. Left panel: nonrandomized phase II component; right panel: randomized phase III component.
Table 1:
Cohort sample characteristics.
| PHASE III | PHASE II/III | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| IMRT | PET-BMS-IMRT | All | P | IMRT | PET-BMS-IMRT | All | P | |
| N | 13 | 16 | 29 | 55 | 46 | 101 | ||
| AGE, (MEAN, (SD)) | 53.6 (11.4) | 45.6 (11.4) | 49.2 (11.9) | 0.07 | 54.1 (12.9) | 51.2 (12.1) | 52.8 (12.6) | 0.26 |
| RACE | 0.54 | |||||||
| ASIAN | 0 (0) | 0 (0) | 0 (0) | 11 (20.0) | 7 (15.2) | 18 (17.8) | 0.14 | |
| HISPANIC | 2 (15.4) | 5 (31.2) | 7 (24.1) | 3 (5.5) | 9 (19.6) | 12 (11.9) | ||
| OTHER/MIXED | 2 (15.4) | 3 (18.8) | 5 (17.2) | 3 (5.5) | 4 (8.7) | 7 (6.9) | ||
| WHITE | 9 (69.2) | 8 (50.0) | 17 (58.6) | 38 (69.1) | 26 (56.5) | 64 (63.4) | ||
| BODY MASS INDEX (MEAN (SD)) | 31.6 (9.7) | 32.0 (8.3) | 31.8 (8.8) | 0.91 | 27.8 (7.0) | 29.2 (7.48) | 28.4 (7.2) | 0.34 |
| KARNOFSKY PERFORMANCE STATUS | 0.33 | 0.45 | ||||||
| 70 | 0 (0.0) | 1 (6.2) | 1 (3.4) | 0 (0.0) | 1 (2.2) | 1 (1.0) | ||
| 80 | 2 (15.4) | 0 (0.0) | 2 (6.9) | 4 (7.3) | 1 (2.2) | 5 (5.0) | ||
| 90 | 3 (23.1) | 5 (31.2) | 8 (27.6) | 22 (40.0) | 17 (37.0) | 39 (38.6) | ||
| 100 | 8 (61.5) | 10 (62.5) | 18 (62.1) | 29 (52.7) | 27 (58.7) | 56 (55.4) | ||
| FIGO STAGE | 0.39 | 0.046* | ||||||
| IB1 | 1 (7.7) | 3 (18.8) | 4 (13.8) | 1 (1.8) | 3 (6.5) | 4 (4.0) | ||
| IB2 | 2 (15.4) | 3 (18.8) | 5 (17.2) | 5 (9.1) | 7 (15.2) | 12 (11.9) | ||
| IIA | 2 (15.4) | 0 (0.0) | 2 (6.9) | 3 (5.5) | 1 (2.2) | 4 (4.0) | ||
| IIB | 7 (53.8) | 9 (56.2) | 16 (55.2) | 41 (74.5) | 25 (54.3) | 66 (65.3) | ||
| IIIB | 0 (0.0) | 1 (6.2) | 1 (3.4) | 3 (5.5) | 10 (21.7) | 13 (12.9) | ||
| IVA | 1 (7.7) | 0 (0.0) | 1 (3.4) | 2 (3.6) | 0 (0.0) | 2 (2.0) | ||
| GRADE | 1.00 | 0.52 | ||||||
| 1 | 0 (0) | 0 (0) | 0 (0) | 3 (5.5) | 1 (2.2) | 4 (4.4) | ||
| 2 | 5 (28.5) | 6 (37.5) | 11 (37.9) | 28 (50.1) | 16 (34.8) | 44 (43.6) | ||
| 3 | 6 (54.5) | 7 (53.8) | 13 (44.8) | 18 (32.7) | 16 (34.8) | 34 (33.7) | ||
| UNKNOWN | 2 (15.4) | 3 (18.8) | 5 (17.2) | 6 (10.9) | 13 (28.3) | 19 (18.8) | ||
| TUMOR HISTOLOGY | 0.99 | 0.50 | ||||||
| SQUAMOUS CELL CARCINOMA | 8 (61.5) | 11 (68.8) | 19 (65.5) | 47 (85.5) | 36 (78.3) | 83 (82.2) | ||
| ADENOCARCINOMA | 5 (38.5) | 5 (31.3) | 10 (34.5) | 8 (14.5) | 10 (21.7) | 18 (17.8) | ||
| CHEMOTHERAPY CYCLES RECEIVED | 0.51 | 0.38 | ||||||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.8) | 0 (0.0) | 1 (1.0) | ||
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 2 | 1 (7.7) | 0 (0.0) | 1 (3.4) | 3 (5.5) | 0 (0.0) | 3 (3.0) | ||
| 3 | 1 (7.7) | 1 (6.2) | 2 (6.9) | 5 (9.1) | 2 (4.3) | 7 (6.9) | ||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (5.5) | 4 (8.7) | 7 (6.9) | ||
| 5 | 3 (23.1) | 7 (43.8) | 10 (34.5) | 35 (63.6) | 30 (65.2) | 65 (64.4) | ||
| 6 | 8 (61.5) | 8 (50.0) | 16 (55.2) | 8 (14.5) | 10 (21.7) | 18 (17.8) | ||
| SIB PRESCRIBED | 5 (38.5) | 2 (12.5) | 7 (24.1) | 9 (16.4) | 3 (6.5) | 12 (11.9) | 0.23 | |
| PELVIC BONE MARROW DOSE (%) | ||||||||
| MEAN V10 (SD) | 86.5 (4.4) | 81.5 (6.3) | 83.7 (6.0) | 0.02* | 86.7 (3.3) | 78.9 (6.7) | 78.9 (6.7) | <0.001* |
| MEAN V20 (SD) | 67.3 (7.2) | 60.6 (8.6) | 63.6 (8.6) | 0.03* | 70.0 (4.8) | 56.9 (8.8) | 56.9 (8.8) | <0.001* |
| MEAN V30 (SD) | 39.2 (4.8) | 36.66 (6.6) | 37.8 (5.9) | 0.25 | 43.3 (6.4) | 36.9 (6.2) | 36.9 (6.2) | <0.001* |
| MEAN V40 (SD) | 16.2 (3.8) | 16.2 (7.0) | 16.2 (5.7) | 1.00 | 20.32 (7.0) | 16.7 (5.8) | 16.7 (5.8) | 0.007* |
| MEAN DOSE, GY (SD) | 26.0 (1.7) | 24.6 (2.6) | 25.2 (2.3) | 0.12 | 27.1 (1.6) | 24.0 (2.0) | 24.0 (2.0) | <0.001* |
| MEAN RT DURATION, DAYS (SD) | 52.2 (16.9) | 48.0 (10.4) | 49.9 (13.6) | 0.42 | 48.6 (10.9) | 50.1 (10.3) | 49.3 (10.6) | 0.49 |
Data presented as N(%) unless otherwise specified. SD: standard deviation. IQR: interquartile range. FIGO: International Federation of Gynecology and Obstetrics 2009. IMRT: intensity modulated radiation therapy. PET-BMS-IMRT = positron emission tomography-guided bone marrow sparing IMRT. SIB = simultaneous integrated boost. D95, D97, D99: radiation dose delivered to 95%, 97%, and 99% of the PTV, respectively. V10, V20, V30, V40 = volume receiving 10, 20, 30, 40 Gy, respectively.
Effects of PET-BMS-IMRT on Acute Toxicity and Chemotherapy Delivery
For patients enrolled on the phase III trial, the incidence of grade ≥ 3 neutropenia was significantly lower in the PET-BMS-IMRT group compared to the IMRT group (19% versus 54%, χ2 p=0.048; Fisher’s exact p=0.064; relative risk (RR) 0.35, 95% confidence interval (CI) 0.11, 1.09); Table 2). In contrast, grade ≥ 3 lymphopenia, thrombocytopenia, and anemia were not significantly different between groups. The majority of patients in both groups (93.8% for PET-BMS-IMRT; 84.6% for IMRT) received at least 5 cycles of concurrent weekly cisplatin (Table 1).
Table 2:
Acute grade ≥ 2 and grade ≥ 3 toxicity. Data presented as n (% of the total N for the relevant treatment group).
| PHASE III | PHASE II/III | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| IMRT (n=13) | PET-BMS-IMRT (n=16) | P | IMRT (n=55) | PET-BMS-IMRT (n=46) | P | |
| TOXICITY | ||||||
| Neutropenia | ||||||
| Grade ≥ 2 | 9 (69%) | 8 (50%) | 0.30 | 31 (56%) | 22 (48%) | 0.39 |
| Grade ≥ 3 | 7 (54%) | 3 (19%) | 0.048* | 19 (35%) | 6 (13%) | 0.01* |
| Lymphopenia | ||||||
| Grade ≥ 2 | 4 (31%) | 5 (31%) | 0.98 | 17 (31%) | 16 (35%) | 0.68 |
| Grade ≥ 3 | 1 (8%) | 1 (6%) | 0.88 | 6 (11%) | 4 (9%) | 0.75 |
| Thrombocytopenia | ||||||
| Grade ≥ 2 | 1 (8%) | 2 (13%) | 0.36 | 6 (11%) | 7 (15%) | 0.56 |
| Grade ≥ 3 | 0 (0%) | 1 (6%) | 0.67 | 4 (7%) | 1 (2%) | 0.37 |
| Anemia | ||||||
| Grade ≥ 2 | 6 (46%) | 10 (63%) | 0.38 | 23 (42%) | 21 (46%) | 0.70 |
| Grade ≥ 3 | 1 (8%) | 3 (19%) | 0.39 | 1 (2%) | 3 (7%) | 0.33 |
| Leukopenia | ||||||
| Grade ≥ 2 | 8 (62%) | 12 (75%) | 0.44 | 41 (75%) | 31 (67%) | 0.43 |
| Grade ≥ 3 | 5 (38%) | 7 (44%) | 0.77 | 21 (38%) | 14 (30%) | 0.42 |
| Any Hematologic Toxicity | ||||||
| Grade ≥ 2 | 10 (77%) | 14 (88%) | 0.45 | 47 (85%) | 38 (83%) | 0.70 |
| Grade ≥ 3 | 7 (54%) | 9 (56%) | 0.90 | 25 (45%) | 17 (37%) | 0.39 |
| Gastrointestinal Toxicity | ||||||
| Grade ≥ 2 | 3 (23%) | 2 (13%) | 0.44 | 17 (31%) | 17 (37%) | 0.51 |
| Grade ≥ 3 | 0 (0%) | 0 (0%) | - | 1 (2%) | 2 (4%) | 0.59 |
Variable was unbalanced (P<.05) between groups.
In the comparison (non-randomized) of PET-BMS-IMRT vs. IMRT for all patients, we also found significantly reduced grade ≥ 3 neutropenia (13% vs. 35%, χ2 p=0.01; RR 0.38, 95% CI 0.16, 0.87). Fewer patients 6/46 (13%) in the PET-BMS-IMRT group required granulocyte colony stimulating factor support compared to 19/55 (35%) in the IMRT group (Fisher exact p-value = 0.02). However, we found no significant differences in other types of hematologic toxicity, including lymphopenia (Table 2). There was also no association between treatment and either ALC nadir (p=0.93) or ALC at 1 month following treatment (p= 0.10). No significant differences were found in the proportion receiving at least 5 cycles of concurrent weekly cisplatin (78.2% for PET-BMS-IMRT; 87.0% for IMRT; χ2 p=0.25) (Table 1). Grade ≥ 2 GI toxicity also did not differ between groups (38% vs. 31%, respectively; χ2 p=0.51) (Table 2).
Long-Term Outcomes by Intervention
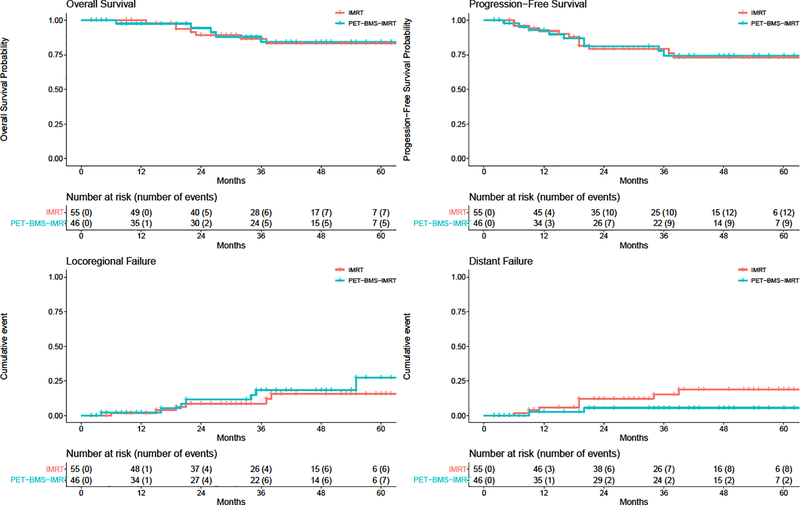
In all patients (phase II/III combined), PFS estimates at 3 and 5 years were 77.0% (95% CI 68.3%, 86.8 %) and 73.6% (95% CI 64.3%, 84.3%) and OS estimates at 3 and 5 years were 85.7% (95% CI 78.1%, 94.0%) and 84.0% (95% CI 76.0%, 92.9%) (Figure 2). In the univariable comparison (non-randomized) of PET-BMS-IMRT vs. IMRT for all patients, we found no difference according to treatment in PFS (HR 1.01; 95% CI 0.43, 2.36; p = 0.98) or OS (HR 0.79; 95% CI 0.26, 2.42; p=0.68); similar results were found on multivariable analysis (Table 3). Similarly, we observed no differences in LRF (HR 1.49, 95% CI 0.50, 4.42, p=0.48) or distant failure (HR 0.31, 95% CI 0.07, 1.46, p=0.14). Outcomes for only phase III, with both arms combined, are presented in Supplemental Figure 1. Nine patients out of the total 112 (8%) in the combined phase II/III group experienced a late grade 3 toxicity (4 in the IMRT group and 5 in the PET-BMS-IMRT group, χ2 p=0.53). All of the late grade 3 toxicities were in the gastrointestinal and/or genitourinary systems. In the phase III group alone, 1 patient in the IMRT group and 1 patient in the PET-BMS-IMRT group experienced a grade 3 toxicity (χ2 p=0.88). There were no grade 4 or 5 events.
Figure 2:
Survival and cancer recurrence outcomes by treatment group (red= IMRT, blue= IG-IMRT). Top row: Overall survival (OS, left) and progression free survival (PFS, right). Bottom row: cumulative incidence of locoregional failure (left) and distant failure (right).
Table 3:
Multivariable Cox modeling of OS and PFS for lymphopenia. Age is analyzed continuously per decade.
| COVARIABLE | OVERALL SURVIVAL N=101 | PROGRESSION-FREE SURVIVAL N=101 | ||
|---|---|---|---|---|
|
|
||||
| Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | |
| PRE-TREATMENT LYMPHOCYTE COUNT | ||||
| > 1.5 K/μL | Reference | Reference | - | |
| ≤ 1.5 K/μL | 2.85 (0.94, 8.62) | 0.06 | 1.72 (0.69, 4.31) | 0.25 |
| IG-IMRT | ||||
| NO | Reference | Reference | - | |
| YES | 0.76 (0.23, 2.49) | 0.66 | 0.98 (0.41, 2.35) | 0.97 |
| AGE (PER 10 YEARS) | 1.06 (0.69, 1.63) | 0.79 | 0.91 (0.64, 1.29) | 0.60 |
| FIGO STAGE | ||||
| STAGE I-IIA | Reference | Reference | - | |
| STAGE IIB | 0.54 (0.16, 1.89) | 0.34 | 0.75 (0.28, 2.04) | 0.58 |
| STAGE III-IVA | 1.07 (0.19, 6.14) | 0.94 | 0.76 (0.18, 3.20) | 0.71 |
Effect of Lymphopenia on Long-Term Outcomes
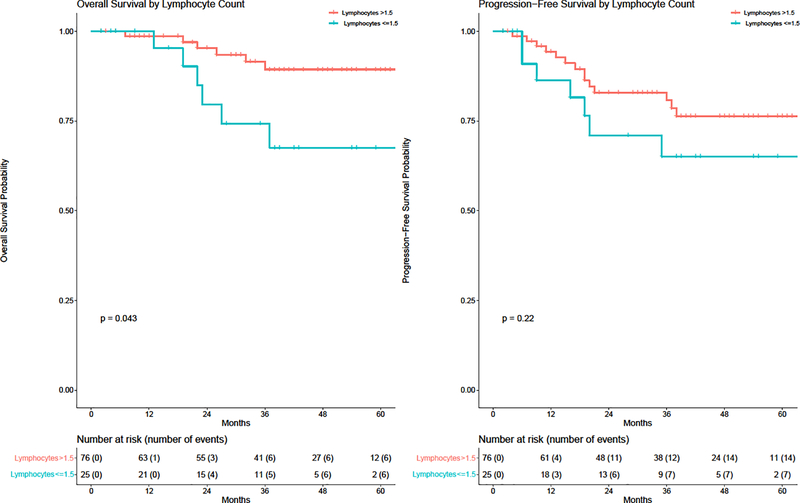
Prior to treatment, the mean ALC for all patients (phase II/III combined) was 2.1 (standard deviation (SD) 0.74). At 1-month post-treatment, the mean ALC was significantly lower compared to baseline (mean difference 1.16 k/μL, p<0.001). On initial analysis, only 4 patients met our initially specified ALC lymphopenia threshold < 1.0 k/μL, so we investigated alternative thresholds starting from the 1st quartile value (1.5 k/μL) and local to this threshold in the range from 1.3–1.7, with the caveat that the findings are exploratory. The optimal cutoff was 1.5 k/μL; at this threshold, pretreatment lymphopenia was associated with significantly worse OS on log-rank test (p=0.04, Figure 3) but nonsignificantly worse OS on both univariable (HR 2.92; unadjusted 95% CI 0.98, 8.71) and multivariable (HR 2.85, unadjusted 95% CI 0.94, 8.62) Cox analyses controlling for age, stage, and treatment (Table 3). To account for the consideration of multiple potential cutoffs, the adjusted p-value based on the multivariable model using the optimal cutoff of 1.5 k/μl did not reach statistical significance (p=0.216). Moreover, lymphopenia defined this way was not significantly associated with PFS on univariable (HR 1.75, 95% CI 0.71, 4.34 p=0.27) or multivariable analysis (HR 1.72, 95% CI 0.69, 4.31, p=0.25). Baseline demographic and tumor variables were otherwise comparable between groups according to this definition (Supplementary Table 1). The neutrophil to lymphocyte ratio was also not associated with OS (HR 1.09, 95% CI 0.82, 1.43, p= 0.58) or PFS (HR 0.92, 95% CI 0.69, 1.23, p=0.57).
Figure 3:
Overall survival (OS, left) and progression free survival (PFS, right) by baseline absolute lymphocyte count ≤ 1.5 vs. > 1.5 k/μL. P-values are from log-rank test.
DISCUSSION
The phase III (INTERTECC-3) trial encountered a number of obstacles leading to early termination, including loss of equipoise an competition with other trials which hindered accrual. In particular, the change in the control arm of the trial to allow non-BMS IMRT placed a large burden on bone marrow dose reduction with PET-BMS-IMRT alone to drive differences in PFS in the setting of weekly cisplatin, which was ultimately considered futile. While this hypothesis still has merit, the effects of PET-BMS-IMRT are likely to be more pronounced in direct comparison to 3D-CRT and/or in the setting of highly myelotoxic chemotherapy.
Our experience offers some insight into the conduct of technology trials in a rapidly changing therapeutic landscape. When we initially activated the trial, large multi-center trials testing IMRT for intact cervix cancer were lacking. Evidence at the time supported the hypothesis that PET-BMS-IMRT in particular could decrease acute toxicity,19 enabling improved delivery of concurrent chemotherapy. Moreover, large retrospective analyses had indicated that IMRT could improve long-term outcomes.29 However, coinciding with the rapid adoption of IMRT, other trials emerged that were better suited to address the questions originally posed in this study8, and ultimately superseded it in priority.
Still, there are several important takeaways from the trial, both positive and negative. First, we confirmed that the use of IMRT, with or without BMS, provides excellent long-term outcomes, with high treatment tolerance and low rates of long-term toxicity, in a multi-institutional international setting. Second, even in a small randomized sample, we were able to confirm a specific effect of PET-BMS-IMRT on reducing acute grade ≥ 3 neutropenia, which was first identified in NTCP models33 and later substantiated in the initial report of INTERTECC-2.20 Of note, a recent larger randomized trial of BMS- IG-IMRT found reductions in neutropenia and other acute hematologic toxicities, but not cisplatin delivery; long-term outcomes and effects on lymphopenia were not reported.21 Thus there is robust evidence that reducing pelvic bone marrow dose at least reduces high grade acute neutropenia. Moreover, there is evidence in settings involving multi-agent chemotherapy7 including anal cancer,34 that PET-BMS-IMRT can support delivery of highly myelotoxic regimens.
Our findings are also notable for the lack of any observed effect of PET-BMS-IMRT on acute lymphopenia, despite confirming significant acute declines in lymphocyte counts that persisted at one month following completion of CRT. This topic has taken on greater relevance in the era of immunotherapy and in the wake of repeated studies indicating associations between lymphopenia and poorer long-term outcomes.25–27, 35–43. In an exploratory analysis, however, we found only weak evidence to support the hypothesis that acute lymphopenia is associated with poorer OS. In any event, it would not appear from our data that PET-BMS-IMRT can produce better long-term outcomes by reducing the incidence of treatment-induced lymphopenia. This may be secondary to the effects of chemoradiation on regions outside the bone marrow, including lymph nodes, circulating peripheral lymphocytes, or GI lymphatics.35,44,45 While other technologies could allow for “lymphocyte-sparing RT,”39,46,47 not all are appropriate for definitive management of cervical cancer.
Despite the increasing use of IMRT in gynecologic cancers, the clinical impact of BMS remains unclear. On the one hand, multiple studies support the hypothesis that BMS can reduce acute hematologic toxicity and facilitate delivery of highly myelotoxic regimens.7,21,34 However, in this study, with standard weekly cisplatin, BMS had no apparent impact on the number of cycles delivered, although the small sample size would have limited our ability to discern modest effects. Moreover, not all studies have found high rates of acute neutropenia with 3D-CRT and weekly cisplatin alone; for example, in the study by Torres et al., grade ≥ 3 neutropenia was 13%.48 It is conceivable then that non-bone marrow sparing IMRT could increase hematologic toxicity compared to conventional RT, although other evidence speaks against that hypothesis13. The complexity of using PET/CT (which is unavailable at many centers) for the purpose of bone marrow delineation also weighs against the approach, although alternatives such as atlas-based bone marrow sparing8,49 or CT-based plans sparing subcortical bone regions21, can be performed without excessive effort or cost. Lastly, sparing bone marrow may have other indirect benefits, including reduced risk of pelvic fractures50; however, we were unable to assess that endpoint in this study.
Our study has several important limitations, including early termination leading to a small sample of randomized patients, precluding any definite conclusions about the effect of PET-BMS-IMRT on our primary endpoint, PFS, relative to usual care. The inclusion of nonrandomized phase II patients also has the potential to introduce bias, although it is not clear in which direction this would be. There were no obvious measured imbalances between treatment groups, although unmeasured confounding cannot be excluded. The analysis of lymphopenia was not defined pre hoc and is best regarded as exploratory, particularly as we were unable to test pre-defined thresholds. However, as other studies have reported this association25–27, our findings can be taken in context with other literature, and further efforts seem warranted to confirm the finding and explore related interventions.
CONCLUSIONS
Patients with locoregionally advanced cervical cancer treated with IMRT and concurrent cisplatin in this multicenter, international, prospective trial had excellent outcomes with low rates of long-term toxicity. Over 80% of patients received at least 5 cycles of cisplatin. PET-based BMS- IMRT was associated with a significant reduction in grade ≥3 neutropenia. We found weak evidence supporting an association between pretreatment lymphocyte count and OS; further validation should be undertaken. However, PET-BMS-IMRT did not reduce acute treatment-induced lymphopenia. Ongoing trials will hopefully further elucidate the value of IMRT and BMS IG-IMRT approaches.
Supplementary Material
Acknowledgments
Funding Statement: The study was supported by the National Cancer Institute (1R01CA197059-01 and 1R21CA162718-01)
Footnotes
Conflict of Interest Notification: There are no actual or potential conflicts of interest
Data Sharing Statement: Anonymized data can be shared upon reasonable request.
Clinical Trial Information: The trial was registered on clinical trials.gov (NCT01554397)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Heal. 2020. doi: 10.1016/S2214-109X(19)30482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buskwofie A, David-West G, Clare CA. A Review of Cervical Cancer: Incidence and Disparities. J Natl Med Assoc. 2020. doi: 10.1016/j.jnma.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 3.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of Radiation Therapy Oncology Group Trial (RTOG) 90–01. J Clin Oncol. 2004. doi: 10.1200/JCO.2004.07.197 [DOI] [PubMed] [Google Scholar]
- 4.Rose PG, Bundy BN, Watkins EB, et al. Concurrent Cisplatin-Based Radiotherapy and Chemotherapy for Locally Advanced Cervical Cancer. N Engl J Med. 1999. doi: 10.1056/nejm199904153401502 [DOI] [PubMed] [Google Scholar]
- 5.Green JA, Kirwan JJ, Tierney J, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005. doi: 10.1002/14651858.CD002225.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dueñas-González A, Zarbá JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011. doi: 10.1200/JCO.2009.25.9663 [DOI] [PubMed] [Google Scholar]
- 7.Mell LK, Xu R, Yashar CM, et al. Phase 1 Trial of Concurrent Gemcitabine and Cisplatin with Image Guided Intensity Modulated Radiation Therapy for Locoregionally Advanced Cervical Carcinoma. Int J Radiat Oncol Biol Phys. 2020;107(5):964–973. doi: 10.1016/j.ijrobp.2020.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusufaly T, Miller A, Medina-Palomo A, et al. A Multi-atlas Approach for Active Bone Marrow Sparing Radiation Therapy: Implementation in the NRG-GY006 Trial. Int J Radiat Oncol Biol Phys. 2020;108(5):1240–1247. doi: 10.1016/j.ijrobp.2020.06.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002. doi: 10.1016/S0360-3016(01)02785-7 [DOI] [PubMed] [Google Scholar]
- 10.Chen MF, Tseng CJ, Tseng CC, Yu CY, Wu C Te, Chen WC. Adjuvant concurrent chemoradiotherapy with intensity-modulated pelvic radiotherapy after surgery for high-risk, early stage cervical cancer patients. Cancer J. 2008. doi: 10.1097/PPO.0b013e318173a04b [DOI] [PubMed] [Google Scholar]
- 11.Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003. doi: 10.1016/S0360-3016(03)00325-0 [DOI] [PubMed] [Google Scholar]
- 12.Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002. doi: 10.1016/S0360-3016(01)02785-7 [DOI] [PubMed] [Google Scholar]
- 13.Brixey CJ, Roeske JC, Lujan AE, Yamada SD, Rotmensch J, Mundt AJ. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002. doi: 10.1016/S0360-3016(02)03801-4 [DOI] [PubMed] [Google Scholar]
- 14.Gandhi AK, Sharma DN, Rath GK, et al. Long Term Clinical Outcome and Late Toxicity of Intensity Modulated Versus Conventional Pelvic Radiation Therapy for Locally Advanced Cervix Carcinoma. J Clin DIAGNOSTIC Res. 2019. doi: 10.7860/jcdr/2019/40260.12741 [DOI] [PubMed] [Google Scholar]
- 15.Klopp AH, Yeung AR, Deshmukh S, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG oncology-RTOG 1203. J Clin Oncol. 2018. doi: 10.1200/JCO.2017.77.4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chopra S, Dora T, Gupta S, et al. Phase III Randomized Trial of Postoperative Adjuvant Conventional Radiation (3DCRT) versus Image Guided Intensity Modulated Radiotherapy (IG-IMRT) in Cervical Cancer (PARCER): Final Analysis. Int J Radiat Oncol. 2020. doi: 10.1016/j.ijrobp.2020.07.2069 [DOI] [Google Scholar]
- 17.Lin Y, Chen K, Lu Z, et al. Intensity-modulated radiation therapy for definitive treatment of cervical cancer: A meta-analysis. Radiat Oncol. 2018. doi: 10.1186/s13014-018-1126-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A Trial Comparing Intensity Modulated Radiation Therapy (IMRT) With Conventional Radiation Therapy in Stage IIB Carcinoma Cervix - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00193804. Accessed March 26, 2021.
- 19.Rose BS, Liang Y, Lau SK, et al. Correlation between radiation dose to 18F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012. doi: 10.1016/j.ijrobp.2011.09.048 [DOI] [PubMed] [Google Scholar]
- 20.A Trial Comparing Intensity Modulated Radiation Therapy (IMRT) With Conventional Radiation Therapy in Stage IIB Carcinoma Cervix ; a. Full Text View - ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT00193804. Accessed March 26, 2021.
- 21.Huang J, Gu F, Ji T, Zhao J, Li G. Pelvic bone marrow sparing intensity modulated radiotherapy reduces the incidence of the hematologic toxicity of patients with cervical cancer receiving concurrent chemoradiotherapy: a single-center prospective randomized controlled trial. Radiat Oncol. 2020. doi: 10.1186/s13014-020-01606-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman NB, Anderson JL, Sherry AD, Osmundson EC. Dosimetric analysis of lymphopenia during chemoradiotherapy for esophageal cancer. J Thorac Dis. 2020. doi: 10.21037/jtd.2020.03.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuncman Ł, Stawiski K, Masłowski M, Kucharz J, Fijuth J. Dose–volume parameters of MRI-based active bone marrow predict hematologic toxicity of chemoradiotherapy for rectal cancer. Strahlentherapie und Onkol. 2020. doi: 10.1007/s00066-020-01659-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sini C, Fiorino C, Perna L, et al. Dose-volume effects for pelvic bone marrow in predicting hematological toxicity in prostate cancer radiotherapy with pelvic node irradiation. Radiother Oncol. 2016. doi: 10.1016/j.radonc.2015.11.020 [DOI] [PubMed] [Google Scholar]
- 25.Choi CH, Kang H, Kim WY, et al. Prognostic Value of Baseline Lymphocyte Count in Cervical Carcinoma Treated With Concurrent Chemoradiation. Int J Radiat Oncol Biol Phys. 2008. doi: 10.1016/j.ijrobp.2007.09.024 [DOI] [PubMed] [Google Scholar]
- 26.Hoskin PJ, Rojas AM, Peiris SN, Mullassery V, Chong IY. Pre-treatment Haemoglobin and Peripheral Blood Lymphocyte Count as Independent Predictors of Outcome in Carcinoma of Cervix. Clin Oncol. 2014. doi: 10.1016/j.clon.2013.11.023 [DOI] [PubMed] [Google Scholar]
- 27.Wu ES, Oduyebo T, Cobb LP, et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol. 2016. doi: 10.1016/j.ygyno.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gooden MJM, De Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br J Cancer. 2011. doi: 10.1038/bjc.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidd EA, Siegel BA, Dehdashti F, et al. Clinical Outcomes of Definitive Intensity-Modulated Radiation Therapy With Fluorodeoxyglucose-Positron Emission Tomography Simulation in Patients With Locally Advanced Cervical Cancer. Int J Radiat Oncol Biol Phys. 2010. doi: 10.1016/j.ijrobp.2009.06.041 [DOI] [PubMed] [Google Scholar]
- 30.Liang Y, Bydder M, Yashar CM, et al. Prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic malignancies. Int J Radiat Oncol Biol Phys. 2013. doi: 10.1016/j.ijrobp.2012.04.044 [DOI] [PubMed] [Google Scholar]
- 31.Dumville JC, Hahn S, Miles JNV, Torgerson DJ. The use of unequal randomisation ratios in clinical trials: A review. Contemp Clin Trials. 2006. doi: 10.1016/j.cct.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 32.Davies RB. Hypothesis testing when a nuisance parameter is present only under the alternative. Biometrika. 1987. March 1;74(1):33–43. [Google Scholar]
- 33.Rose BS, Aydogan B, Liang Y, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011. doi: 10.1016/j.ijrobp.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arcadipane F, Silvetti P, Olivero F, et al. Bone Marrow-Sparing IMRT in Anal Cancer Patients Undergoing Concurrent Chemo-Radiation: Results of the First Phase of a Prospective Phase II Trial. Cancers (Basel). 2020. November 9;12(11):3306. doi: 10.3390/cancers12113306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.So TH, Chan SK, Chan WL, et al. Lymphopenia and Radiation Dose to Circulating Lymphocytes With Neoadjuvant Chemoradiation in Esophageal Squamous Cell Carcinoma. Adv Radiat Oncol. 2020. doi: 10.1016/j.adro.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abravan A, Faivre-Finn C, Kennedy J, McWilliam A, van Herk M. Radiotherapy-Related Lymphopenia Affects Overall Survival in Patients With Lung Cancer. J Thorac Oncol. 2020. doi: 10.1016/j.jtho.2020.06.008 [DOI] [PubMed] [Google Scholar]
- 37.Cho O, Chun M, Chang SJ, Oh YT, Noh K. Prognostic value of severe lymphopenia during pelvic concurrent chemoradiotherapy in cervical cancer. Anticancer Res. 2016. [PubMed] [Google Scholar]
- 38.Lee BM, Byun HK, Seong J. Significance of lymphocyte recovery from treatment-related lymphopenia in locally advanced pancreatic cancer. Radiother Oncol. 2020. doi: 10.1016/j.radonc.2020.07.026 [DOI] [PubMed] [Google Scholar]
- 39.Wild AT, Herman JM, Dholakia AS, et al. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients with Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2016. doi: 10.1016/j.ijrobp.2015.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin JY, Hu C, Xiao Y, et al. Higher Radiation Dose to Immune System is Correlated With Poorer Survival in Patients With Stage III Non–small Cell Lung Cancer: A Secondary Study of a Phase 3 Cooperative Group Trial (NRG Oncology RTOG 0617). Int J Radiat Oncol. 2017. doi: 10.1016/j.ijrobp.2017.06.351 [DOI] [Google Scholar]
- 41.Ladbury CJ, Rusthoven CG, Camidge DR, Kavanagh BD, Nath SK. Impact of Radiation Dose to the Host Immune System on Tumor Control and Survival for Stage III Non-Small Cell Lung Cancer Treated with Definitive Radiation Therapy. Int J Radiat Oncol Biol Phys. 2019. doi: 10.1016/j.ijrobp.2019.05.064 [DOI] [PubMed] [Google Scholar]
- 42.Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014. doi: 10.1016/j.ijrobp.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 43.Campian JL, Sarai G, Ye X, Marur S, Grossman SA. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014. doi: 10.1002/hed.23535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013. doi: 10.3109/07357907.2012.762780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018. doi: 10.1016/j.critrevonc.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 46.Lambin P, Lieverse RIY, Eckert F, et al. Lymphocyte-Sparing Radiotherapy: The Rationale for Protecting Lymphocyte-rich Organs When Combining Radiotherapy With Immunotherapy. Semin Radiat Oncol. 2020. doi: 10.1016/j.semradonc.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellsworth SG. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv Radiat Oncol. 2018. doi: 10.1016/j.adro.2018.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torres MA, Jhingran A, Thames HD, et al. Comparison of Treatment Tolerance and Outcomes in Patients With Cervical Cancer Treated With Concurrent Chemoradiotherapy in a Prospective Randomized Trial or With Standard Treatment. Int J Radiat Oncol Biol Phys. 2008. doi: 10.1016/j.ijrobp.2007.05.028 [DOI] [PubMed] [Google Scholar]
- 49.Li N, Noticewala SS, Williamson CW, et al. Feasibility of atlas-based active bone marrow sparing intensity modulated radiation therapy for cervical cancer. Radiother Oncol. 2017. doi: 10.1016/j.radonc.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 50.Vitzthum LK, Park H, Zakeri K, et al. Risk of Pelvic Fracture With Radiation Therapy in Older Patients. Int J Radiat Oncol. 2020;106(3):485–492. doi: 10.1016/j.ijrobp.2019.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.