Abstract
Eicosanoid signaling controls a wide range of biological processes from blood pressure homeostasis to inflammation and resolution thereof to the perception of pain and to cell survival itself. Disruption of normal eicosanoid signaling is implicated in numerous disease states. Eicosanoid signaling is facilitated by G-protein-coupled, eicosanoid-specific receptors and the array of associated G-proteins. This review focuses on the expression, characterization, regulation, and mechanism of action of non-prostanoid, eicosanoid receptors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-021-00630-6.
Keywords: Receptor, Eicosanoid, Resolvin, Protectin, G-protein, Signaling
Introduction
Eicosanoids are signaling molecules derived from the oxidation of polyunsaturated fatty acids (PUFA). The term eicosanoid derives from the ancient Greek word eikosi, referring to the number 20 and was originally used to refer to lipids similar in structure to the twenty-carbon arachidonic acid. The term has been since expanded to include shorter and longer chain fatty acids that are also involved in signaling. They play a number of biological roles including initiation and resolution of inflammation, blood flow and blood pressure homeostasis, as well as pain perception. They also play a role in the progression of many disease states. These short-lived biomolecules typically act as autocrine or paracrine signaling agents for which their metabolic products may also serve as signaling molecules in their own right. This review is an extension of a companion article (Biringer 2020) with a focus on signaling by the non-prostanoid eicosanoids. The focus of this manuscript is primarily on human receptors for these eicosanoids but includes data for other organisms with significant sequence similarity when the data for humans is lacking.
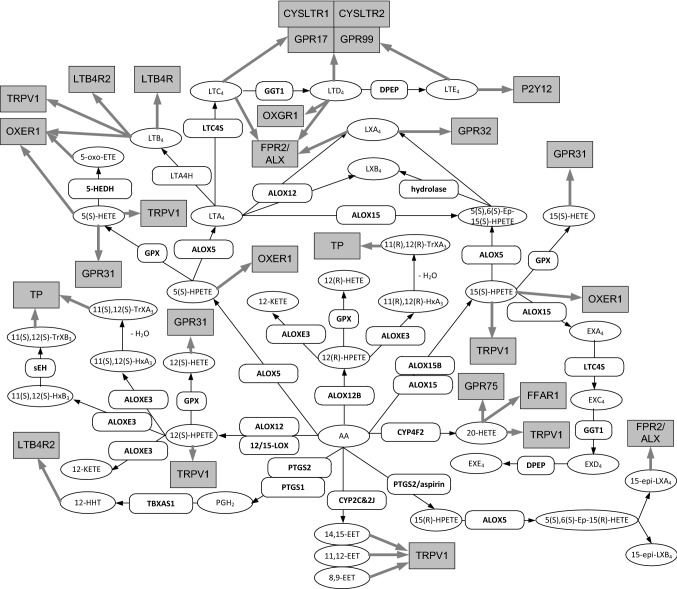
Eicosanoid signaling occurs through specific G-protein coupled receptors (see Figs. 1 and 2). Eicosanoid receptors are typically multi-pass, heptahelical membrane proteins, belonging to the G-protein coupled receptor 1 family (GPCR) of proteins. These are by far amongst the most abundant membrane proteins known (Binda et al. 2014). The biological result of eicosanoid action on cells is not only cell type and tissue dependent, but also dependent on which eicosanoid is acting on a particular receptor. Eicosanoid action diversity is also defined by the diversity of G-proteins that most receptors are able to couple to, leading to actuation of different signaling pathways by the same receptor (Table 1). Some receptors have the capability to form heterodimers with other receptors and in doing so modify their own or the hetero-partner’s activity. In contrast to this diversity of action, many receptors show commonalities in their regulation. Most eicosanoid receptors show agonist-induced desensitization that is usually accompanied by receptor phosphorylation by various protein kinases. Often times phosphorylation leads to uptake into punctate vesicles through a variety of different mechanisms for temporary storage or degradation.
Fig. 1.
Metabolic pathways for non-prostanoid eicosanoids derived from arachidonic acid and their receptors. Gene designations are given for the participating enzymes (rounded boxes), accepted acronyms are given for metabolites (ovals) and accepted receptor acronyms in grey squares. Black arrows indicate enzymatic reactions and grey arrows indicate receptor binding. Enzymes are as follows: 12/15-LOX, 12/15-lipoxygenase; 5-HEDH, 5-hydroxyeicosanoid dehydrogenase; ALOX12, Arachidonate 12(S)-lipoxygenase; ALOX12B, arachidonate 12(R)-lipoxygenase; LTC4S, leukotriene C4 synthase; ALOX15, arachidonate 15-lipoxygenase-1; ALOX15B, arachidonate 15-lipoxygenase-2; ALOX5, Arachidonate 5-lipoxygenase; ALOXE3, arachidonate lipoxygenase 3; CYP2C, cytochrome P450 C2; CYP4F2, cytochrome P450 4F; CYP2J, cytochrome P450 J2; DPEP, dipeptidase; GPX, glutathione peroxidase; LTA4H, Leukotriene A-4 hydrolase; LTC4S, leukotriene C4 synthase; PTGS1, Prostaglandin G/H Synthase 1; PTGS2, Prostaglandin G/H Synthase 2; PTGS2/aspirin, PTGS2 acetylated by aspirin; sEH, soluble epoxide hydrolase; TBXAS1, Thromboxane A Synthase 1. Abbreviations for metabolites: 8,9-EET, 8,9-EET, 8,9-epoxy-5Z,11Z,14Z-eicosatrienoic acid; 11,12-EET, 11,12-epoxy-5Z,8Z,14Z-eicosatrienoic acid; 14,15-EET, 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid; 11(R),12(R)-HXA3, 11(R),12(R)-Hepoxilin A3; 11(S),12(S)-HXA3, 11(S),12(S)-Hepoxilin A3; 11(S),12(S)-HXB3, 11(S),12(S)-Hepoxilin B3; 11(R),12(R)-TrXA3, 11(R),12(R)-trioxilin A3;11(S),12(S)-TrXA3, 11(S),12(S)-trioxilin A3; 11(S),11(S)-TrXB3, 11(S),12(S)-trioxilin B3; 5-oxo-ETE, 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid; 12(S)-HETE, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid; 12(S)-HPETE, 12S-hydroperoxy-5Z,8Z,10E,14Z-eicosatetraenoic acid; 5(S)-HETE, 5S-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid; 5(S)-HPETE, 5S-hydroperoxy-6E,8Z,11Z,14Z-eicosatetraenoic acid; 15(S)-HPETE, 15S-hydroperoxy-5Z,8Z,11Z,13E- eicosatetraenoic acid; 15(S)-HPETE, 15S-hydroperoxy-5Z,8Z,11Z,13E- eicosatetraenoic acid; 15(R)-HETE, 15R-hydroxy-5Z,8Z,11Z,13E- eicosatetraenoic acid;12(R)-HPETE, 12R-hydroperoxy-5Z,8Z,10E,12R,14Z- eicosatetraenoic acid; 12(R)-HETE, 12R-hydroxy-5Z,8Z,10E,12R,14Z- eicosatetraenoic acid; 12-KETE, 12-oxo-5Z,8Z,10E,14Z-eicosatetraenoic acid;12-HHT, 12-Hydroxy-5,8,10-heptadecatrienoic acid; AA, arachidonic acid; 20-HETE, 20-Hydroxyeicosatetraenoic acid; 5(S),6(S)-Ep-15(R)-HETE, 5S,6S-epoxy-15(R)-hydroxy-7E,9E,11Z,13E-eicosatetraenoic acid; 5(S),6(S)-Ep-15(S)-HETE, 5S,6S-epoxy-15(S)-hydroxy-7E,9E,11Z,13E-eicosatetraenoic acid; 15-epi-LXA4, 15-epi-lipoxin A4; 15-epi-LXB4, 15-epi-lipoxin B4; LTA4, leukotriene A4; LTB4, leukotriene B4; LTC4, leukotriene C4; LTD4, leukotriene D4; LTE4, leukotriene E4; LXA4, lipoxin A4; LXB4, lipoxin B4; EXA4, eoxin A4; EXC4, eoxin C4; EXD4, eoxin D4; EXE4, eoxin E4; PGH2, prostaglandin H2. Receptors are as follows: CYSLTR1, cysteinyl leukotriene receptor 1; CYSLTR2, cysteinyl leukotriene receptor 2; FFAR1, free fatty acid receptor 1; FPR2/ALX, N-formyl peptide receptor 2; GPR17, uracil nucleotide/cysteinyl leukotriene receptor; GPR31, 12S-hydroxy-5,8,10,14-eicosatetraenoic acid receptor; GPR32, Probable G-protein coupled receptor 32; GPR75, Probable G-protein coupled receptor 75; LTB4R, Leukotriene B4 receptor 1; LTB4R2, leukotriene B4 receptor 2; OXER1, oxoeicosanoid receptor 1; OXGR1 (GPR99), 2-oxoglutarate receptor 1; P2Y12, P2Y purinoceptor 12; TP, Thromboxane A2 receptor (TBXAR2); TRPV1, Transient receptor potential cation channel subfamily V member 1
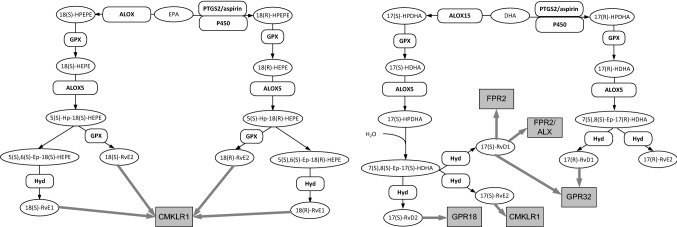
Fig. 2.
Metabolic pathways for non-prostanoid eicosanoids derived from eicosapentaenoic acid (EPA) and dicosahexaenoic acid (DHA). Gene designations are given for the participating enzymes (rounded boxes), accepted acronyms are given for metabolites (ovals) and accepted receptor acronyms in grey squares. Black arrows indicate enzymatic reactions and grey arrows indicate receptor binding. Enzymes are as follows: ALOX, unspecified lipoxygenase; ALOX15, arachidonate 15-lipoxygenase-1; ALOX5, Arachidonate 5-lipoxygenase; GPX, glutathione peroxidase; Hyd, unspecified hydrolase; P450, unspecified P450 enzyme; PTGS2/aspirin, PTGS2 acetylated by aspirin. Abbreviations for metabolites: 11(R),12(R)-HXA3, 11(R),12(R)-Hepoxilin A3; 11(R),12(R)-TrXA3, 11(R),12(R)-trioxilin A3; 11(R),12(S)-TrXB3, 11R,12(S)-trioxilin B3; 11(S),12(S)-HXA3, 11(S),12(S)-Hepoxilin A3; 11(S),12(S)-HXB3, 11(S),12(S)-Hepoxilin B3; 11(S),12(S)-TrXA3, 11(S),12(S)-trioxilin A3; 12(S)-HETE, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid; 12(S)-HPETE, 12S-hydroperoxy-5Z,8Z,10E,14Z-eicosatetraenoic acid; 15(R)-HETE, 15R-hydroxy-5Z,8Z,11Z,13E- eicosatetraenoic acid; 15(S)-HPETE, 15S-hydroperoxy-5Z,8Z,11Z,13E- eicosatetraenoic acid; 15(S)-HPETE, 15S-hydro peroxy-5Z,8Z,11Z,13E- eicosatetraenoic acid; 17(R)-HDHA, 17R-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid; 17(R)-HPDHA, 17R-hydroperoxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid; 17(R)-RvD1, 17(R)-resolvin D1; 17(R)-RvE2, 17(R)-resolvin E2; 17(S)-HDHA, 17S-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid; 17(S)-HPDHA, 17S-hydroperoxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid; 17(S)-HPDHA, 17S-hydroperoxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid; 17(S)-RvD1, 17(S)-resolvin D1; 17(S)-RvD2, 17(S)-resolvin D2; 17(S)-RvE2, 17(S)-resolvin E2; 18(R)-HEPE, 18R-hydroxy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid; 18(R)-HPEPE, 18R-hydroperoxy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid; 18(R)-RvE2, 18(R)-resolvin E2; 18(R)-RvE2, 18(R)-resolvin E2; 18(S)-HEPE, 18S-hydroxy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid; 18(S)-HPEPE, 18S-hydroperoxy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid; 18(S)-RvE1, 18(S)-resolvin E1;18(S)-RvE2, 18(S)-resolvin E2; 5(S),6(S)-Ep-18(R)-HEPE, 5S,6S-epoxy,18R-hydroxy-7E,9E,11Z,14Z,16E-eicosapentaenoic acid; 5(S),6(S)-Ep-18(S)-HEPE, 5S,6S-epoxy,18S-hydroxy-7E,9E,11Z,14Z,16E-eicosapentaenoic acid; 5(S)-Hp-18(R)-HEPE, 5S-hydroperoxy-18R-hydroxy-(6E,8Z,11Z,14Z,16E)-icosapentaenoate; 5(S)-Hp-18(S)-HEPE, 5S-hydroperoxy-18S-hydroxy-(6E,8Z,11Z,14Z,16E)-icosapentaenoate; 5-oxo-ETE, 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid; 5S-HETE, 5S-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid; 5(S)-HPETE, 5S-hydroperoxy-6E,8Z,11Z,14Z-eicosatetraenoic acid; 7(S),8(S)-Ep-17(R)-HDHA, 7S,8S-epoxy-17R-hydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic; 7(S),8(S)-Ep-17(S)-HDHA, 7S,8S-epoxy-17S-hydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic; DHA, docosahexaenoic acid; EPA, Eicosapentaenoic acid. Receptors are as follows: CMKLR1, Chemokine-Like Receptor 1; FPR2/ALX, N-formyl peptide receptor 2; GPR18, N-Arachidondyl Glycine Receptor; GPR32, G-Protein Receptor 32
Table 1.
Signaling pathways and G-protein association for Eicosanoid receptors
| Receptor | signaling pathway | G-protein mediation | Reference |
|---|---|---|---|
| GPR31 | IP3↑ Ca2+↑ | Gαq | Guo et al. (2011) |
| cAMP↓ | Gαi | Mashiko et al. (2019) | |
| rho | Gα11/13 | Mashiko et al. (2019) | |
| LTB4R2 | IP3↑ Ca2+↑ | Gαi | Okuno et al. (2008); Arcemisbéhère et al. (2010) |
| IP3↑ Ca2+↑ | Gαq | Okuno et al. (2008); Arcemisbéhère et al. (2010) | |
| OXER1 | IP3↑ Ca2+↑ | Gβγ | Hosoi et al. (2002, 2005); Jones et al. (2003 |
| IP3↑ Ca2+↑ | Gαi | Hosoi et al. (2002, 2005); Jones et al. (2003) | |
| GPR75 | IP3↑ Ca2+↑ | Gαq11 | Garcia et al. (2017) |
| FFAR1/GPR40 | IP3↑ Ca2+↑ | Gαq/Gβγ | Itoh et al. (2003); Briscoe et al. (2003) |
| IP3↑ Ca2+↑ | Gαi | Itoh et al. (2003); Briscoe et al. (2003) | |
| TRPV1 | Ca2+↑, Na+↑ | none | Marsh et al. (1987) |
| LTB4R | cAMP↓ | Gαi | Saeki and Yokomizo (2017); Malfacini et al. (2019); Kuniyeda et al. (2007) |
| IP3↑ Ca2+↑ | Gαq | Saeki and Yokomizo (2017); Malfacini et al. (2019); Kuniyeda et al. (2007) | |
| IP3↑ Ca2+↑ | Gα16 | Saeki and Yokomizo (2017); Malfacini et al. (2019); Kuniyeda et al. (2007) | |
| CYSLTR1 | Ca2+↑ | Gαq11, | Capra et al. (2003) |
| Ca2+↑ | Gαio | Capra et al. (2003) | |
| Ca2+↑ | Gβγ | Capra et al. (2003) | |
| cAMP↓ Ca2+↑ | Gαq | Parmentier et al. (2012) | |
| cAMP↓ Ca2+↑ | Gαi | Parmentier et al. (2012) | |
| Ca2+↑ | Gαi3 | Adolfsson et al. (1996) | |
| CYSLT2R | IP3↑ Ca2+↑ | Gαq11 | Sarau et al. (1999); Moore et al. (2016) |
| cAMP↓ | Gαio | Mellor et al. (2003) | |
| OXGR1 | cAMP↑ | Gαq | Inbe et al. (2004) |
| cAMP↑ | Gαs | Inbe et al. (2004) | |
| Ca2+↑ | Gαq | Steinke et al. (2014); He et al. (2004) | |
| GPR17 | cAMP↓ | Gαi | Hennen et al. (2013); Simon et al. (2015) |
| cAMP↑ | Gαs | Hennen et al. (2013); Simon et al. (2015) | |
| IP3↑ Ca2+↑ | Gαq | Hennen et al. (2013); Simon et al. (2015) | |
| P2Y12 | Ca2+↑ | Gαi | Soulet et al. (2004) |
| IP3↑ | Gαi | Bodor et al. (2003) | |
| cAMP↓ | Gαi | Kauffenstein et al. (2004) | |
| FPR2/ALX | Ca2+↑ | Gαi | Le et al. (2002) |
| cAMP↓ Ca2+↑ | Gαi | Ge et al. 2020 | |
| GPR18 | Ca2+↑ | Gαi | Kohno et al. 2006 |
| GPR32 | Ca2+↑ | Gαi/o | Hodges et al. (2013) |
| CMKLR1a | Ca2+↑ | Gαi | Arita et al. (2005), Wittamer et al. (2004) |
aExperimental evidence for chemerin signaling is available, but only indirect support for RvE1 signaling is found (see text)
ETE, HETE and oxidized HETE receptors
Introduction
The unstable hydroperoxyeicosatetraenoic acids (HPETE) and their hydroxyeicosatetraenoic acid (HETE) reduction products are notable not only for their function in signaling, but also as precursors for lipoxins, eoxins, leukotrienes, and various other oxidized PUFA products (Brash, 1999). As an example, 15(S)-HPETE and 12(S)-HETE are involved in cell survival mechanisms (Tang et al. 1996) and the binding of monocytes to vascular tissue (Natarajan and Nadler, 2004; Sultana et al. 1996). Both 5-HPETE and 12(S)-HPETE are also involved in modulating neurotransmission (Piomelli et al. 1987). Further, the oxidized derivative of the former, 5-oxo-hydroxyeicosatetraenoic acid (5-oxo-ETE), is a notable activator of neutrophils (Powell et al. 1993). These oxidized derivatives of arachidonic acid are formed enzymatically or through non-enzymatic lipid peroxidation mechanisms (review, Powell and Rokach 2015).
GPR31
Introduction
Human 12-(S)-hydroxy-5,8,10,14-eicosatetraenoic acid (12(S)-HETE) receptor (hGPR31) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. It binds various hydroxyeicosatetraenoic acids with a particular affinity for 12(S)-HETE which stimulates the mitogen-activated protein kinase (MAPK) pathway leading to activation of signal-regulated kinase 1 and 2 (ERG1/2), MAPK/ERK kinase (MEK), and the NF-κB transcription factor (Guo et al. 2011). GPR31 is intimately involved in pro-inflammatory processes as well as cell mobility and long-term cell survival (Guo et al. 2011; Fretland et al. 1995). This receptor is also activated through proton binding, thus serving as an extracellular proton sensor (Goldsmith and Dhanasekaran, 2007; Mashiko et al. 2019).
hGPR31 (UniProtKB-O00270) is translated as a 319 amino acid polypeptide with a calculated molecular weight of 35.1 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 16 residues and a cytosolic C-terminal with 34 residues. There are no additional isoforms and one known coding single nucleotide polymorphic (SNP) variant (H91R) for which no functional abnormalities have been reported (Zingoni et al. 1997; https://genecards.org, Stelzer et al. 2016; Landrum et al. 2016https://www.ncbi.nlm.nih.gov/clinvar/). There are no reported X-ray structures, however, there is one model available on the SWISS-Model site using P2Y purinoceptor (27.8% sequence homology; template PDB entry 4XNV) to serve as a working model (https://swissmodel.expasy.org/, Waterhouse et al. 2018).
There are several N- and O-glycosylations predicted by sequence, but none are confirmed experimentally. Here, and for all receptors discussed in this document, N-glycosylation was predicted using NetNGlyc analysis (http://www.cbs.dtu.dk/services/NetNGlyc/, Blom et al. 2004, Steentoft et al. 2013) and O-glycosylation was predicted using NetOGlyc analysis (http://www.cbs.dtu.dk/services/NetOGlyc/, Steentoft et al. 2013). There are numerous potential phosphorylation sites on the hGPR31 receptor based on motifs, but none are specifically confirmed experimentally. Phosphorylation was predicted for this and the other receptors discussed here using the NetPhos 3.1 server (http://www.cbs.dtu.dk/services/NetPhos/, Blom et al. 1999), a conservative minimum score of 0.9, and the availability of sites based on topology predictions (UniProtKB) for all but GRK kinases. GRK phosphorylation sites are predicted using the GPS server (http://gps.biocuckoo.cn/, Xue et al. 2011). Predicted posttranslational modifications for this receptor and all others discussed here are given in the supplement, Table S1.
Expression and characterization
The hGPR31 receptor is expressed primarily in the immune system, but is also found in the gastrointestinal tract, and male- and female-specific tissues (http://proteinatlas.org; Uhlén et al. 2015; Town et al. 1983; Sharif et al. 2000).
Ligand binding properties for GPR31 have been characterized with human recombinant protein and recombinant GPR31-bovine Gαi fusion protein expressed in Chinese hamster ovary (CHO) cells (Fig. 1, Table 2). 12(S)-HETE binds tightly (Kd = 4.8 nM) in a regio- and stereo-specific manner with much higher signaling efficiency than either 15(S)-HETE and 5(S)-HETE (Guo et al. 2011). In addition to 12(S)-HETE activation, protons also stimulate GPR31. Proton-induced signaling exhibits an EC50 equivalent to pH 5.6 for a GPR31-Gαi fusion protein and around pH 5.8 for the recombinant GPR31 receptor alone (Mashiko et al. 2019).
Table 2.
ETE, HETE and oxidized HETE receptors
| Receptor/Expression | Parameter measured | 12(S)-HETE | 12(S)-HPETE | 12(R)-HETE | 15(S)-HETE | 5(S)-HETE | 5(S)-HPETE | 5-OXO-ETE | 5,15-di-HETE | LTB4 | 12-HHT | RvE1 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki (nM) | |||||||||||||
| hGPR31/CHOa | Disp | 4 | – | – | – | – | – | – | – | – | – | – | Guo et al. (2011) |
| hLTB4R2/CHOb | Disp | 4000 | 4000 | 6000 | 9000 | – | – | – | – | 180 | – | – | Yokomizo et al. (2001) |
| hLTB4R2/CHOc | Disp | – | – | – | – | – | – | – | – | 21 | 2.3 | – | Okuno et al. (2008) |
| hLTB4R2/monomerd | Disp | – | – | – | – | – | – | – | – | 25 | 3.7 | – | Arcemisbéhère et al. (2010) |
| hLTB4R2/dimerd | Disp | – | – | – | – | – | – | – | – | 21 | 4.4 | – | Arcemisbéhère et al. (2010) |
| hLTB4R/COS-7 | Disp | 6000a | – | 30 | – | – | – | – | – | 0.38 | – | – | Yokomizo et al. (1997) |
| hLTB4R/HEKe | Disp | – | – | – | – | – | – | – | – | 0.08 | – | 34.3 | Serhan et al. (2011) |
| hLTB4R/HEKe | Disp | – | – | – | – | – | – | – | – | 3 | – | 70 | Arita et al. (2007) |
| hOXER1/PMNf | Disp | – | – | – | 970 | – | – | 4 | 61 | – | – | – | O'Flaherty et al. (1998) |
| EC50/IC50 (nM) | |||||||||||||
| hGPR31/CHOg | GTPγS | 0.28 | – | – | 42 | 386 | – | – | – | – | – | – | Guo et al. (2011) |
| hGPR31-Sf9/memh | GTPγS | 100 | – | – | – | – | – | – | – | – | – | – | Mashiko et al. (2019) |
| hLTB4R2/CHOi | Ca2+↑ | 5000 | – | – | > 10,000 | – | – | – | – | 700 | – | – | Yokomizo et al. (2001) |
| hLTB4R2/CHOj | Chemo | 5000 | – | – | 20,000 | – | – | – | – | 60 | – | – | Yokomizo et al. (2001) |
| hLTB4R2/CHOi | Ca2+↑ | – | – | – | – | – | – | – | – | 142 | 19 | – | Okuno et al. (2008) |
| hLTB4R2/CHOi | Ca2+↑ | – | – | – | – | – | – | – | – | 200 | 5 | – | Okuno et al. (2015) |
| hLTB4R2/mBMMCk | GTPγS | 5000 | – | – | – | – | – | – | – | – | – | – | Lundeen et al. (2006) |
| mLTB4R2/300.19 l | Ca2+↑ | – | – | – | – | – | – | – | – | 20 | 6.3 | – | Mathis et al. (2010) |
| hLTB4R2/monomerm | GTPγS | – | – | – | – | – | – | – | – | 70 | – | – | Arcemisbéhère et al. (2010) |
| hLTB4R2/dimerm | GTPγS | – | – | – | – | – | – | – | – | 70 | – | – | Arcemisbéhère et al. (2010) |
| hLTB4R/COS-7n | Ca2+↑+ | – | – | – | – | – | – | – | – | 10 | – | – | Yokomizo et al. (1997) |
| hLTB4R/COS-7 m | GTPγS | – | – | – | – | – | – | – | – | 5 | – | – | Kuniyeda et al. (2007) |
| hLTB4R/CHOn | Ca2+↑ | – | – | – | – | – | – | – | – | 3 | – | – | Okuno et al. (2015) |
| hLTB4R/CHOo | cAMP↓ | – | – | – | – | – | – | – | – | 11.5 | – | 3.2 | Serhan et al. (2011) |
| hLTB4R/HEKo | cAMP↓ | – | – | – | – | – | – | – | – | 0.015 | – | 3.2 | Arita et al. (2007) |
| hOXER1-Gαi1/CHOp | GTPγS | – | – | – | – | 240 | 69 | 5.7 | – | – | – | – | Hosoi et al. (2002) |
| hOXER1/CHOq | cAMP↓ | – | – | – | – | – | – | 33 | – | – | – | – | Hosoi et al. (2002) |
| hOXER1/CHOi | Ca2+↑ | – | – | – | – | 510 | 98 | 5.1 | – | – | – | – | Hosoi et al. (2005) |
| hOXER1/HEKq | cAMP↓ | – | – | – | – | – | – | 0.33 | – | – | – | – | Jones et al. (2003) |
| hOXER1/PMNr | Ca2+↑ | – | – | – | 6300 | – | – | 13 | 100 | – | – | – | O'Flaherty et al. (1998) |
| hOXER1/Neui | Ca2+↑ | – | – | – | – | 150 | – | 2 | 900 | 0.2 | – | – | Powell et al. (1993) |
| hOXER1/Eoss | Migration | – | – | – | – | – | – | 20 | – | – | – | – | Powell et al. (1995) |
Agonist parameters given for Human (h) and murine (m) receptors in the indicated cell line or oligomeric state. Parameter measured: Ca2+↑, increase in [Ca2+]i; cAMP↓, decrease in forskolin induced cAMP accumulation; chemo, chemotactic response; disp, displacement assay; GTPγS, binding of [35S] GTPγS; migration, eosinophil migration; Cell type abbreviations: 300.19, Abelson-transformed murine pre-B lymphoma; BMMC, bone marrow mononuclear cells; CHO, Chinese hamster ovary cells; COS-7, African green monkey kidney cell line; Eos, eosinophils; HEK, Human embryonic kidney 293 cells; Neu, neutrophils; PMN, human polymorphonuclear leukocytes; Sf9, clonal isolate of Spodoptera frugiperda Sf21 cells. Agonist abbreviations: 12(S)-HETE, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid; 12(S)-HPETE, 12S-hydroperoxy-5Z,8Z,10E,14Z-eicosatetraenoic acid; 12(R)-HETE, 12R-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid; 15(S)-HETE, 15(S)-Hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid; 5(S)-HETE, 5S-Hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid; 5(S)-HPETE, 5S-Hydroperoxy-6E,8Z,11Z,14Z-eicosatetraenoic acid; 5-OXO-ETE, 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid; 5,15-di-HETE, 5,15-dihydroxy-6E,8Z,11Z,13E-eicosatetraenoic acid; LTB4, leukotriene B4; 12-HHT, 12-hydroxy-5E,8E,10E-heptadecatrienoic acid; RvE1, resolvin E1. a) Estimated from graph for displacement of 12(S)-[3H]HETE and Ki calculated using the Cheng-Prusoff equation. b) Calculated from the IC50 estimated from a graph for displacement of [3H]LTB4 using Cheng-Prusoff equation for human BLT2 expressed in CHO cells. c) Calculated for displacement of [3H]LTB4 using Cheng-Prusoff equation for human BLT2 and a Kd of 23 (Yokomizo et al. 2001) expressed in CHO. d) Calculated from IC50 for displacement of fluorescent labeled LTB4 using Cheng-Prusoff equation. e) Calculated for displacement of [3H]RvE1. f) Calculated from IC50 estimated from graph for displacement of [3H]-5-oxo-ETE using Cheng-Prusoff equation; OXER1 is the most likely receptor in polymorphonuclear neutrophils (PMN). g) Measured with [35S]-GTPγS binding assay. h) Determined with a [35S]GTPγS binding assay of membranes from Sf9 cells expressing human GP31-bovine G1a fusion protein. i) Measured intracellular Ca2+ increase. j) Chemotactic response. k) Chemotaxis for mouse bone marrow derived mast cells. Note: BLT1 and BLT2 receptors are present, but only BLT2 receptors bind 12-HETE. l) Ca2+ release in murine 300.19 cells. m) Measured with [35S]-GTPγS binding assay in the presence of Gαi. n) Estimated from graph for increasing cytosolic Ca2+. o) Calculated from the inhibition of forskolin-activated cAMP. p) Determined with a [35S]-GTPγS binding assay from a fusion protein expressed in CHO cells and EC50. 5(S)-HPETE binding estimated from the data curve. q) Determined from a reduction in forskolin-induced cAMP production. r) Estimated from graph for increasing cytosolic Ca2+; OXER1 is the most likely receptor in polymorphonuclear neutrophils (PMNs) determined from eosinophil migration
Mechanism of cell activation
The binding of 12(S)-HETE to GPR31 activates protein kinase C-a (PKC-a) and does so through an inositol 1,4,5-trisphosphate (IP3) mediated pathway (Guo et al. 2011) which is consistent with signaling through the G-protein Gαq. This result and additional studies show that 12(S)-HETE ultimately leads to activation of NF-κB, MEK and ERK1/2 by PKC-dependent and -independent mechanisms, the latter involving the activation of Src kinases that lead to phosphorylation of adapter proteins (i.e. shc and Grb2) which in turn promotes activation ERK1/2 via the GTPase Ras (Guo et al. 2011; Liu et al. 1995; Szekeres et al. 2000). The involvement of Src and the additional observation that pertussin toxin inhibits activation of ERK1/2 suggests that signaling occurs through Gαi as well. On the other hand, the naturally occurring the linoleic oxidation product OXLAM, 13-hydroxyoctadecadienoic acid (13-HODE) is a GPR31 antagonist with an IC50 = 4 nM (Liu et al. 1995; Honn 2008). Protons can also activate this receptor in the absence of 12(S)-HETE with maximal activation below pH 5 via both Gαi and Gα11/13, suggesting a concomitant reduction of cAMP or a Rho-mediated signaling respectively (Mashiko et al. 2019). Interestingly, 12(S)-HETE stimulation of GPR31 in lymph endothelial cells leads to a 12-HETER/Rho/ROCK/MYPT signaling cascade that induces myosin light chain 2 (MLC2) function (Nguyen et al. 2016).
Regulation
There are no reports of regulation of GPR31 through phosphorylation or β-arrestin binding. This is likely due to the fact this orphan receptor was only recently shown to be the 12(S)-HETE receptor 12-HETER1 (Guo et al. 2011). A more recent report shows a profound up-regulation of 12-HETER1 in prostate cancer tumors and that it plays a critical role in cancer progression (Honn et al. 2016). Related results are observed in a murine hepatocellular carcinoma model where an increase in 12(S)-HETE serves to increase expression of GPR31 and that GPR31 promotes reoccurrence of hepatocellular carcinoma in nonalcoholic fatty liver disease (Yang et al. 2019).
LTB4R2
Introduction
Human leukotriene B4 receptor 2 (hLTB4R2) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. It binds to and is activated by various HETEs at micromolar levels, 12-Hydroxyheptadecatrienoic acid (12-HHT) at nanomolar levels, as well as various leukotrienes at somewhat higher levels (discussed in a later section). Stimulation of this receptor leads to signaling through MAPK and an increase in intracellular Ca2+ ([Ca2+]i). LTB4R2 has a protective role in allergic airway inflammation and is involved in skin barrier function and wound healing.
hLTB4R2 (BL2, JULF2, UniProtKB-Q9NPC1) is translated as a 358 amino acid polypeptide with a calculated molecular weight of 37.9 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 24 residues and a cytosolic C-terminal with 62 residues. There are no additional isoforms and one reported coding SNP variant (P130S) with no reported effect on biological function (Landrum et al. 2016). There are no reported X-ray structures or models available. There is one potential structural model template, Leukotriene B4 receptor BLT1 in complex with BIIL260 (42.3% sequence homology; PDB entry 5X33, template 5 × 33.1.A), selected by the SWISS-MODEL website (Waterhouse et al. 2018) that can be used as a working model. Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally.
Expression and characterization
LTB4R2 is primarily expressed in the skin but is also found in other tissues in lower amounts, particularly in the immune and digestive systems (Uhlén et al. 2015). Although originally characterized as the low affinity LTB4 receptor (Yokomizo et al. 2000, 2001) it has since been shown to be the primary receptor for 12-HHT (Fig. 1) which shows the tightest binding and lowest EC50 of all agonists examined to date (Okuno et al. 2008, 2015) (Table 2). Interestingly, 12-HHT does not bind to the high affinity LTB4 receptor (LTB4R), further supporting the notion that 12-HHT is the primary agonist for the LTB4R2 receptor (Okuno and Yokomizo 2018).
Mechanism of cell activation
12-HHT activation of LTB4R2 proceeds through both Gαi and Gαq mediated pathways, the former leading to a pertussis toxin-sensitive cAMP reduction and the latter to an increase in IP3 and [Ca2+]i (Okuno et al. 2008; Arcemisbéhère et al. 2010). It is also reported that 12(S)-HETE stimulation of LTB4R2 induces dose-dependent ERK signaling and changes in Protein kinase B (Akt, PKB) phosphorylation in murine mast cells (Lundeen et al. 2006). Park et al. (2019) reveal that 12-HHT stimulation of LTB4R2 in cultures of a KRAS mutant colorectal cancer cell line initiates the same pathways which results in the upregulation of cyclin D1 that in turn enhances the proliferation of these cells. This suggests that LTB4R2 may be a potential therapeutic target for KRAS mutant colorectal cancer. 12-HHT stimulation of overexpressed LTB4R2 transfected into human bronchial epithelial cells enhances migration and proliferation of these cells as well as enhancing the airway epithelial barrier integrity, suggesting that LTB4R2 may be a potential target for asthma treatment (Liu et al. 2018).
In the skin LTB4R2 is expressed on the surface of keratinocytes and is activated by 12-HHT produced by activated platelets. Activation by 12-HHT accelerates keratinocyte migration by inducing the production of tumor necrosis factor α (TNFα), interleukin (IL)-1β, and matrix metalloproteinases (MMPs), thus accelerating wound healing (Liu et al. 2014). Further, the 12-HHT/LTB4R2 axis enhances cell–cell junctions in both intestinal and skin epithelial cells by upregulating CLDN4, an integral membrane channel of tight junctions, and does so via a p38/MAPK signaling pathway (Saeki and Yokomizo 2017; Ishii et al. 2016). In addition, both 12(S)-HETE and 12-HHT recruit mast cells (Lundeen et al. 2006), another required function for wound healing (da Silva et al. 2014) and blood–brain barrier function (Kempuraj et al. 2019).
Regulation
Pretreatment of murine bone marrow mast cells with stem cell factor (SCF) downregulates expression of LTB4R2 thus reducing the cells’ chemotactic response to LTB4R2 agonists (Lundeen et al. 2006). LTB4R2 is upregulated in many cancers, particularly in highly aggressive forms, by mechanisms that are currently not understood (Kim et al. 2012; Seo et al. 2011, 2012). Regulation through phosphorylation, typical for G-protein receptors, has yet to be reported.
OXER1
Introduction
The human oxoeicosanoid receptor 1 (hOXER1) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. It binds peroxy-, dihydroxy-, and oxo-eicosanoid derivatives of arachidonic acid. Stimulation of this receptor leads to activation of various phospholipase Cs (PLC), phosphoinositide 3-kinase (PI3K), ERK 1/2, and p38/MAPK and a reduction in cAMP.
hOXER1 (GPR170, GPR R527, GPR TG1019, GPCR48, UniProtKB-Q8TDS5) is translated as a 423 amino acid polypeptide with a calculated molecular weight of 46.0 kDa. The basic structure consists of seven transmembrane helices with a large N-terminal extracellular domain of 97 residues and a cytosolic C-terminal with 66 residues. There are no additional isoforms and four coding SNP variants reported: M316L (Stelzer et al. 2016), L407V (Jones et al. 2003), A368V, and T334P (Landrum et al. 2016). The biological ramifications of these SNPs are not reported. There are no reported X-ray structures. There is one model available on the SWISS-Model site using P2Y purinoceptor (23.4% sequence homology; PDB entry 4XNV) as a template (Waterhouse et al. 2018) that provides a working model. Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally.
Expression and characterization
hOXER1 is expressed in most tissues with particularly prominent expression in the immune and digestive systems, as well as in liver, and male and female-specific tissues (Uhlén et al. 2015). OXER1 is the primary receptor for 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-oxo-ETE), produced enzymatically in several steps from AA (Fig. 1), with Ki and EC50 values in the low nM range (Hosoi et al. 2002; Jones et al. 2003; O'Flaherty et al. 1998) (Fig. 1, Table 2). It also serves as a receptor for other oxidized AA derivatives, 5(S)-HPETE, 5,15-di-HETE, and 5(S)-HETE, with 10–100 times poorer efficiency than 5-oxo-HETE (Hosoi et al. 2002; Jones et al. 2003; O'Flaherty et al. 1998). 5-oxo-ETE serves as a chemoattractant for basophils, monocytes, neutrophils (Powell et al. 1993) and eosinophils, the effect on the latter being significantly greater than the others (Powell et al. 1995; Powell and Rokach 2013).
Mechanism of cell activation
5-oxo-ETE activation of OXER1 proceeds through both Gαi- and Gβγ-mediated pathways (Hosoi et al. 2002, 2005; Jones et al. 2003) that lead to PLC/Ca2+ mobilization, and MEK/ERK and PI3K/Akt phosphorylation/activation when expressed in CHO cells (Hosoi et al. 2005). Stimulation of OXER1 in prostate cancer cells also promotes cell survival through PKCε that is activated by the diacylglycerol produced by OXER-1-activated PLC-β (Zingoni et al. 1997). Although not specifically stated, activation of PKCε by Ca2+ stimulated by the simultaneous production of IP3 by PLC-β may also be involved.
5-oxo-ETE elicits various responses in human eosinophils, including a very rapid increase in [Ca2+]i and actin polymerization (Czech et al. 1997; Powell et al. 1999), the latter is likely involved in cell migration (Mogilner and Oster 1996). Further, 5-oxo-ETE stimulates the release of the β-integrin CD11b and L-selectin in eosinophils, known mediators for lymphocyte adhesion and infiltration (Mogilner and Oster 1996). Lastly, 5-oxo-ETE also induces expression of urokinase plasminogen activator (uPAR) and secretion of metalloproteinase-9 (MMP-9), the former involved in tissue reorganization and the latter involved in the breakdown of extracellular matrix (Langlois et al. 2006). Taken together, these functions collectively promote the infiltration of eosinophils to the source of 5-oxo-ETE release (see also Powell and Rokach 2020).
Regulation
Typical G-protein regulation through phosphorylation or arrestin binding has not been reported to date. Regulation of OXER1 gene expression also has not been reported. However, androgens, testosterone in particular, antagonize the actions of 5-oxo-ETE on the OXER1 receptor expressed in DU-145 prostate cancer cells (Kalyvianaki et al. 2017, 2019). On the other hand, pretreatment of polymorphonuclear neutrophils (PMN) or eosinophils with granulocyte–macrophage-stimulating factor (GM-CSF) or granulocyte-stimulating factor (G-GSF) increases the potency of 5-oxo-ETE activation of OXER1 (O'Flaherty et al. 1996a, 1996b). The increased potency is manifested through an increase the 5-oxo-ETE-induced phosphorylation and hence activity of MAPKs (O'Flaherty et al. 1996b). The mechanism for this process is unknown.
GPR75
Introduction
The human GPR75 receptor (hGPR75) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. It binds to and is activated by the chemokine CCL5/RANTES and is a highly specific target for 20-HETE. The latter is one of the principal eicosanoids formed from AA by the action of cytochrome P450 enzymes (Fig. 1), in particular, the CYP4A and CYP4F families (Fan and Roman 2017). 20-HETE/hGPR75 axis plays a major role in renal, pulmonary, and cardiac function as well as vascular tone and inflammation (Fan et al. 2016; Roman et al. 2002) and is well established as a potent vasoconstrictor (Yu et al. 2003), particularly in cerebral and renal microvessels (Harder et al. 1995).
hGPR75 (GPR170, GPR R527, GPR TG1019, UniProtKB-O95800) is translated as a 540 amino acid polypeptide with a calculated molecular weight of 59.4 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 46 residues and a large cytosolic C-terminal with 169 residues. There are no additional isoforms and seven coding SNP variants (N78K, P99L, S108T, A116T, T135P, C160G, and L433V) (Sauer et al. 2001; Stelzer et al. 2016) with no reported effect on biological function. There are no reported X-ray structures. Only low quality models are calculated by the SWISS-MODEL website (Waterhouse et al. 2018) thus no working model is available. Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally.
Expression and characterization
hGPR75 is expressed in most tissues with particularly prominent expression in the brain, more in neuron-like cells than in astrocytes (Dedoni et al. 2018), and the endocrine system (Uhlén et al. 2015). In 2006 this receptor was identified as a RANTES/CCL5 chemokine receptor (Ignatov et al. 2006) and only recently as a receptor for 20-HETE. Both agonists show very tight binding with a measured Ki for 20-HETE of 0.1 nM (Garcia et al. 2017) and EC50 values of 0.12 or 0.3 nM, depending on the cell type in which it is expressed (Fig. 1, Table 3) (Ignatov et al. 2006).
Table 3.
EET and 20-HETE receptors
| GPR75 | Parameter measured | Ki (nM) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Receptor/Expression | 20-HETE | |||||||||
| hGPR75/HEMa | disp | 0.1 | Garcia et al. (2017) | |||||||
| EC50/IC50 (nM) | ||||||||||
| hGPR75/CHO-K1b | Ca2+↑ | 0.12 | Ignatov et al. (2006) | |||||||
| hGPR75/CHO-K1c | IP3↑ | 0.3 | Ignatov et al. (2006) | |||||||
| FFAR1 | Parameter measured | Ki (nM) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Receptor/Expression | 20-HETE | 14,15-EET | 11,12-EET | 8,9-EET | AA | Linoleic | α-linolenic | Palmitic | ||
| hFFAR1/HEK293d | Disp | – | 6400 | 2700 | – | – | – | – | – | Park et al. (2018) |
| FFAR1/COSb | Disp | 4.5 | – | – | – | – | – | – | 40 | Tunaru et al. (2018) |
| EC50/IC50 (nM) | ||||||||||
| hFFAR1/CHOb | Ca2+↑ | – | – | 1,400 | 6100 | 2400 | 1800 | 2000 | 6800 | Itoh et al. (2003) |
| hFFAR1/HEK293b | Ca2+↑ | – | 580 | 910 | – | 3900 | – | – | – | Park et al. (2018) |
| FFAR1/COSb | Ca2+↑ | 6300 | – | – | – | > 320,000 | 32,000 | 13,000 | 32,000 | Tunaru et al. (2018) |
| TRPV1 | Parameter measured | Ki (µM) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Receptor/Expression | 20-HETE | 12(S)-HPETE | 15(S)-HPETE | 5(S)-HETE | LTB4 | Capsacin | ||||
| rTRPV1/HEK293e | Disp | – | 0.35 | – | – | – | 2.5 | Shin et al. (2002) | ||
| EC50/IC50 (nM) | ||||||||||
| hTRPV1/HEK293e | Current | 10 | – | – | – | – | 0.3 | Wen et al. (2012) | ||
| hTRPV1/HEK293f | Current | – | 8 | 8.7 | 9.2 | 11.7 | – | Hwang et al. (2000) | ||
| hTRPV1/CHOe | Current | – | – | – | – | – | 0.5 | McIntyre et al. (2001) | ||
| hTRPV1/XOg | Current | – | – | – | – | – | 2.2 | Cortright et al. (2001) | ||
Agonist parameters given for Human (h) and rat (r) receptors in the indicated cell line. Parameter measured: Ca2+↑, increase in [Ca2+]i; Current, current response from whole-cell patch clamp; disp, displacement assay; IP3↑, increase in intracellular IP3. Cell type abbreviations: CHO, Chinese hamster ovary cells; CHO-K1, subclone from the parental CHO cell line; COS, African green monkey kidney cell line; HEK293, Human embryonic kidney 293 cells; HEM, Human Epidermal Melanocytes; XO, xenopus oocytes. Agonist abbreviations: 11,12-EET, 11,12-epoxy-5Z,8Z,14Z-eicosatrienoic acid; 12(S)-HPETE, 12S-hydroperoxy-5Z,8Z,10E,14Z-eicosatetraenoic acid; 14,15-EET, 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid; 15(S)-HPETE, 15S-Hydroperoxy-5Z,8Z,11Z,13E-eicosatetraenoic acid; 20-HETE, 20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid; 5(S)-HETE, 5S-Hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid; 8,9-EET, 8,9-epoxy-5Z,11Z,14Z-eicosatrienoic acid; AA, arachidonic acid LBT4, leukotriene B4. a) Estimated from graph for displacement of [3H]20-HETE and Ki calculated using the Cheng-Prusoff equation. b) Determined from Ca2+ mobilization. c) Determined from IP3 production. d) Determined by displacement of agonist [3H]TAK-875. e) Peak current response from patch clamp of whole cells. f) Estimated from graph of voltage response measured with whole cell voltage clamping. g) Current response for hTRPV1 expressed in xenopus oocytes (XO) measured with inside-out patch activated single channel currents
Mechanism of cell activation
In human microvascular endothelial cells, 20-HETE signaling proceeds through a Gαq11 mediated pathway, where the agonist-induced dissociation of Gαq11 leads to an increase in intracellular IP3 (likely PLC activated) which results in an increase in [Ca2+]i, as expected for Gαq signaling. The Gαq11 dissociation also enhances GPCR-kinase interacting protein-1 (GIT1) which in turn leads to activation of the tyrosine-protein kinase c-Src which activates epidermal growth factor receptor (EGFR). Activation of EGFR precipitates an EGFR/MAPK/IKK/NFκB signaling pathway that induces angiotensin-converting enzyme (ACE) transcription, ultimately leading to the conversion of angiotensin I to the vasoconstrictor angiotensin II. This pathway also induces phosphorylation of a subunit (Maxi-Kβ) of the large-conductance Ca2+ channel BKCa (Garcia et al. 2017). This serves to deactivate the BKCa channel (Vetri et al. 2014), ultimately leading to membrane depolarization, increased [Ca2+]i, and stimulation of the contractile apparatus of vascular smooth muscle. In contrast, 20-HETE acts in an anti-hypertensive role in the kidney by initiating sodium reabsorption (Zhang et al. 2018).
As would be expected, RANTES/CCL5 exhibits similar effects upon binding to GPR75. RANTES/CCL5 activation of GPR75 transfected into HEK293 cells stimulates a release of IP3 in a PLC dependent manner that leads to an increase in [Ca2+]i, consistent with a Gαq signaling pathway (Ignatov et al. 2006). Enhanced phosphorylation (activation) of MAPK and Akt is also observed. This event also occurs in neuroblastoma cells that express GPR75 and no other RANTES/CCL5 receptor (Dedoni et al. 2018). In addition, GPR75 serves as a RANTES/CCL5 receptor in human β-cells where stimulation produces an increase in [Ca2+]i that leads to short-term elevation of insulin at both sub-stimulatory and maximal stimulatory glucose concentrations (Tunaru, 2016). More recent studies confirm that CCL5 stimulation of GPR75 is effective at increasing insulin secretion by β-cells (Gençoğlu et al. 2019). Comparable studies for the effect of 20-HETE on β-cells has not been reported.
In contrast to these studies, one attempt to reproduce the elevation of [Ca2+]i by either RANTES or 20-HETE failed, and the authors suggest that the aforementioned studies were actually monitoring the activity of an unknown receptor (Iyinikkel 2018). However, the radioligand displacement studies and radiolabeled binding to wild type and the absence of binding to a GPR75 knockdown clearly show that 20-HETE is a ligand for GPR75 (Garcia et al. 2017).
Regulation
Regulation by phosphorylation has not been reported to date. Several groups have reported that agonist induced β-arrestin binding does not occur with GPR75 and thus inhibition and downregulation do not follow this pathway (Garcia et al. 2017; Iyinikkel 2018; Southern et al. 2013). However, GPR75 does internalize into punctate vesicles and internalization occurs through a dynamin-dependent pathway (Iyinikkel 2018). Interestingly, internalization occurs in the absence of added agonist, suggesting constitutive activity or activation by a yet-to-be discovered agonist in the media.
FFAR1/GPR40
Introduction
The human free fatty acid receptor 1 (hFFAR1, previously designated as hGPR40) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. It binds to and is activated by the potent agonist 20-HETE, EETs (eicosatetraenoic acids), and free fatty acids derived from dietary triglycerides (see Itoh et al. 2003). FFAR1 plays a major role in glucose metabolism, whereby stimulation leads to the secretion of insulin and glucagon (Tunaru et al. 2018; Trauelsen et al. 2018).
Human FFAR1 (GPR40, FFAR1, UniProtKB-O14842) is translated as a 300 amino acid polypeptide with a calculated molecular weight of 31.5 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 8 residues and a cytosolic C-terminal with 21 residues. There are no additional isoforms and one coding SNP variant (R211H) reported with unknown biological effect (Gerhard et al. 2004). There are several reported X-ray structures, (e.g., PDB reference 4PHU, 5KW2). Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally.
Expression and characterization
hFFAR1 is expressed in only a few tissues with highest expression in bone, brain, pancreas, ovary, and digestive system (Uhlén et al. 2015). It is well established that FFAR1 is a receptor for free long chain fatty acids (FFA) with at least 12 carbons as well as various EETs, all with EC50 values in the low micromolar range (Fig. 1, Table 3) (Mohapatra and Nau 2005; Park et al. 2018; Kotarsky et al. 2003) and 20-HETE (Tunaru et al. 2018) with a similar concentration dependence. A recent report shows that FFAR1 is also activated by lysophosphatidyl choline (LPC) (Drzazga et al. 2018).
Mechanism of cell activation
Although the overall function for FFAR1 is tissue dependent, it clearly signals primarily through a Gαq pathway and partially through Gαi (Itoh et al. 2003; Briscoe et al. 2003), both resulting in an increase in [Ca2+]i. The former proceeds through a lysolecithin-inositol trisphosphate (PLV/IP3) pathway. The latter involves the activation of PLC by the Gβγ subunit released upon Gαi activation, producing IP3 that results in an increase in [Ca2+]i while at the same time directly inhibiting adenylate cyclase, thus reducing intracellular cAMP (Nolan et al. 2006).
The primary function of FFAR1 in the pancreas and the digestive system is the enhancement of glucose-stimulated insulin secretion (GSIS) through stimulation of the respective enteroendocrine cells, albeit through vastly different mechanisms. It is well established that under conditions of elevated plasma glucose, pancreatic β-cells import glucose which serves to drive the synthesis of ATP. This in turn leads to depolarization of the plasma membrane and Ca2+ influx. Increased [Ca2+]i stimulates fusion of insulin containing vesicles with the plasma membrane thus releasing insulin to the circulatory system. In addition to glucose transport proteins, pancreatic β-cells also express high amounts of FFAR1. Activation of this receptor with long chain fatty acids or their oxidized derivatives leads to a Gαq-mediated activation of PLC which, through a PLC/IP3 pathway, increases the release of Ca2+ from the ER, leading to an increase [Ca2+]i that serves to enhance GSIS (Tunaru et al. 2018; Houthuijzen 2016). These activators are unlikely to come directly from dietary fatty acids, as the concentrations necessary to activate FFAR1 (Table 3) are well beyond what would be expected for free fatty acids in plasma. However, localized high concentrations of FFA could be facilitated by intracellular lipases activated by glucose that are in turn transported into the localized extracellular environment. Oxidized derivatives of FFAs can be readily produced by intracellular cytochromes and transported to the localized extracellular environment (Tunaru et al. 2018). The lower EC50 for oxidized derivatives compared to FFAs make the oxidized derivatives the more likely physiological agonists (Tunaru et al. 2018; Itoh et al. 2003; Trauelsen et al. 2018; Park et al. 2018). In addition to β-cell expression, FFAR1 is also expressed in the endocrine cells of the gastrointestinal tract. Here, in response to activation by dietary FFAs or autocrine release of FFAs or oxidized derivatives, FFAR1 stimulates the release of incretins (GLP-1 and GIP) which serve to augment GSIS (Edfalk et al. 2008; Luo et al. 2012).
FFAR1 receptor stimulation is also known to enhance glucagon excretion from α-cells. At face value this seems counterintuitive to the known effects of 20-HETE on insulin secretion from β-cells. However, as Taruelson et al. (2018) explain, any 20-HETE stimulation of FFAR1 is likely to be circumvented by paracrine inhibition of α-cells by β-cell secretory products. However, at low glucose concentrations where insulin is low, circulating long chain fatty acids released from fat stores serve to stimulate FFAR1 to release glucagon.
FFAR1 also serves important functions in other tissues. For example, in bone this receptor exerts a protective effect by inhibiting osteoclast differentiation (Wauquier et al. 2013). In the brain it is involved in antinociceptive activity involving the descending pain control system (Aizawa et al. 2016; Mancini et al. 2015). This receptor has also been shown to be intimately involved in the mediation of ovarian cancer growth (Munkarah et al. 2016).
Regulation
To date, there are no reports describing desensitization of hFFAR1 by phosphorylation, including GRK phosphorylation, that would lead to possible arrestin binding and subsequent internalization. However, the FFAR1 agonist dicosahexaenoic acid (DHA) does cause desensitization in monkey bone marrow derived stromal cells (BMSC) through internalization of FFAR1 (Kaplamadzhiev et al. 2010). Murine FFAR1 expressed in HEK-293 cells does interact with both β-arrestin 1 and 2 (Mancini et al. 2015) when stimulated with the strong synthetic agonist TAK-875, supporting a possible β-arrestin-mediated internalization event. However, hFFAR1 expressed in HeLa cells does not exhibit β-arresting binding (Williams-Bey et al. 2014) when exposed to DHA binding, suggesting some other mechanism for internalization in that system.
Regulation of FFAR1 at the genetic level has also been examined. Following treatment of clonally-expanded monkey bone marrow-derived stromal cells (BMSC) with basic fibroblast growth factor (βFGF), the expression of FFAR1 mRNA and protein increases significantly (Kaplamadzhiev et al. 2010).
TRPV1
Introduction
The human transient receptor potential cation channel subfamily V member 1 receptor (hTRPV1) is a member of the transient receptor potential Ca(2 +) channel (trp-cc) family. It is activated by various oxidized derivatives of both AA and linoleic acid, and various vanilloids and endocannabinoids. TRPV1 functions as a general sensor for noxious stimuli including heat, acid, proinflammatory stimulants, and painful chemical stimulants (review, Szallasi and Blumberg 1999). TRPV1 is a non-selective cation channel with high permeability for divalent cations (Caterina et al. 1997).
hTRPV1 receptor (VR1, Capsaicin receptor, OTRPC1, UniProtKB-Q8NER1) is translated as a 540 amino acid polypeptide with a calculated molecular weight of 95.0 kDa. There is a second isoform (isoform 2, UnitrotKB-Q8NER1-3) that differs in sequence for the first 150 amino acids and four potential isoforms that are computationally mapped (UniProt E7EQ78, I3L1R6, E7ESJ2, and A0A3B3ISI9). Lu et al. (2005) reported a third isoform (UniProtKB—Q52PU4) identical to the canonical isoform with the exception of a missing 60 amino acid corresponding to the loss of exon 7 and dubbed it hTRPV1b in reference to the similarly cleaved rat isoform. Sequence analysis reveals that hTRPV1b and the computationally mapped E7ESJ2 are one in the same. There are 11 known coding SNP variants for hTRPV1: P91S, M315I, T469I, T505A (Cortright et al. 2001), I585V (Hayes et al. 2000), T219A, V288G, V458M, F589L, T612M, and D625N for the canonical isoform with unknown biological impact (Landrum et al. 2016). Coding SNP variants for hTRPV1b or isoform 2 have not been reported to date beyond notation in the Ensembl database. There are no complete X-ray structures reported for any isoform but there is one partial structure for the N-terminus of hTRPV1 (residue 101–365, PDB entry 6L93). There are a number of nearly complete structures reported for rat TRPV1 (rTRPV1) determined using cryo-electron microscopy (e.g., PDB entry 5IRX, residues 110–764, 2.95 Å resolution). The fact that the sequence homology between rTRPV1 and hTRPV1, hTRPV1b and isoform 2 are 86%, 62% and 90% respectively make the rTRVP1 structure a good working model for each of these isoforms. The overall structure is unique among those discussed here in that there are only six transmembrane helices and both of the substantial N- (433 residues) and C- termini (152 residues) are cytosolic (Schumacher and Eilers, 2010).
Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally. However, glycosylation of N604 on rTRPV1 has been confirmed (Rosenbaum et al. 2002; Jahnel et al. 2001; Veldhuis et al. 2012) but predicted probabilities for glycosylation at this site are low for both the human (hTRPV1-1; N604) and rat proteins. Although phosphorylation of hTRPV1 has yet to be reported, the 85.8% homologous rat TRPV1 has been shown to be phosphorylated at S502 (Numazaki et al. 2002; Bhave et al. 2003; Jung et al. 2004), S800 (Numazaki et al. 2002; Bhave et al. 2003), T704 (Jung et al. 2004), S6, T144, T370 and S116 (Bhave et al. 2002), corresponding to S502, S801, T705, S6, T146, T370 and S117 on hTRPV1.
Expression and characterization
hTRPV1 is widely expressed in most tissues with higher expression in female specific tissues, skin, and the digestive tract (Uhlén et al. 2015; Montell 2005). Although widely expressed, nociceptors within these tissues exhibit the highest expression of hTRPV1, transducing a localized response to noxious stimuli (Mandadi and Roufogalis 2008; Ständer et al. 2004). As noted above, TRPV1 is a non-selective cation channel with a preference for divalent cations. The relative permeability of rTRPV1 for cations is Ca2+ > Mg2+ > Na+ ≈ K+ ≈ Cs+ where permeability ratios were determined to be: PCa/PNa = 9.6 and PMg/PNa = 4.99 (Caterina et al. 1997). The expression and function of TRPV1 in nervous tissue is particularly interesting. In human brain tissue hTRPV1 is expressed in all regions with slightly higher amounts in the cerebellum, midbrain, and hypothalamus (Uhlén et al. 2015). Specifically, TRPV1 is primarily expressed in nociceptors (Caterina and Julius 2001) and serves as a sensor for heat, acid, proinflammatory stimulants, as well as noxious xenobiotics such as capsaicin, the spicy hot compound found in chili peppers. hTRPV1b is also expressed in many tissues with particularly high expression in the cerebellum, fetal brain and dorsal root ganglia (Vos et al. 2006). Tissue expression of isoform 2 has not been reported, but it is likely that it too is expressed in neurons. The deletion of exon 7 in TRPV1b is reported to alter the sensitivity to stimulants but this effect is controversial. Lu et al. (2005) indicate that this isoform only responds to heat and not to capsacin or protons as observed for TRPV1 whereas Vos et al. (2006) report that this receptor does not respond to any the stimulants found for TRPV1. Specific agonists for isoform 2 have not been reported.
In vivo, the rTPRV1 receptor is found in oligomers with tetramers as the most prevalent (Kedei et al. 2001). Oligomerization is promoted by the presence of agonist but blocked in the presence of the antagonist capsazepine. Another report reveals that very strongly adhered dimers can survive SDS-PAGE analysis, but only if residue N604 is glycosylated and that the N604S mutant rat TRPV1 only forms monomers, confirming the importance of N604 glycosylation (Rosenbaum et al. 2002). hTRPV1 and hTRPV1b also form multimeric complexes of dimers, trimers and tetramers depending on conditions with tetramers preferred by TRPV1b (Vos et al. 2006). Oligomerization of isoform 2 has not been reported.
The TRPV1 receptor binds to and is activated by several oxidized derivatives of AA (5(S)-HETE, 12(S)-HETE, 15(S)-HETE, 20(S)-HETE, and LTB4) all with similar EC50 values around 10 nM (Fig. 1, Table 3). TRPV1 also binds a number of different oxidized derivatives of linoleic acid (e.g., 9(S)-HODE, ( ±) 13-HODE, and 15(S)-HAEA) with EC50 values in the micromolar range. Further, it binds to and is activated by a variety of cannabinoids with sub-micromolar affinity (De Petrocellis et al. 2000) as well as the non-selective opioid antagonist naloxone (Melkes et al. 2020). It is also a high affinity receptor for the plant-derived vanilloid capsaicin (EC50 = 0.3–2.2 nM), the original namesake for this receptor. These and additional PUFA, N-acyl derivative, and other agonists are discussed in detail in a recent review by Benítez-Angeles et al. (2020). Activation of TRPV1b or isoform 2 by any oxidized PUFA has not been reported.
TRPV1 is also gated by both heat and pH. hTRPV1 gates at approximately 44 °C when expressed in either xenopus oocytes (Cortright et al. 2001) or HEK293 cells (Hayes et al. 2000) and ion currents increase significantly with a moderate increase in temperature. hTRPV1 gates with decreasing pH that begins around pH 6.5 with a pK of 5.4–5.5 (Hayes et al. 2000; McIntyre et al. 2001). Lower pH also sensitizes hTRPV1 to the agonist capsaicin (Cortright et al. 2001). The effect of the potent antagonist capsaizepine on pH gating is controversial. One report indicates that capsaizepine inhibits 90% of the pH-induced ion current at pH 5 (Cortright et al. 2001), whereas another report indicates that no such effect is observed (McIntyre et al. 2001). Protons also reduce the temperature threshold for gating, even at moderately acidic pH (≤ 5.9) (Tominaga et al. 1998). Although there appears to be some synergy in signaling, whether the signaling mechanism for protons, heat or agonist share commonalities remains to be determined.
Mechanism of cell activation
Stimulation of neural cells expressing TRPV1 with an agonist such as capsaicin leads to a rapid influx of cations, particularly Ca2+ and Na+ resulting in depolarization of the membrane (Marsh et al. 1987) and the subsequent release of neurotransmitters and neuromodulators from the nociceptor (Caterina and Julius 2001). The depolarization appears to be bimodal with time constants of 6.7 and 52 ms for rTRPV1 when expressed in HEK293 cells and monomodal with a time constant of 385 ms in native dorsal root ganglion (DRG) neurons. The large difference in depolarization rates may be due to the presence of factors found only in native systems (Gunthorpe et al. 2000). The presence of oligomers in native systems could very well be one of these mitigating factors.
Regulation
Regulation by phosphorylation is a common theme for many receptors. Treatment of rat DRG or HEK293 cells expressing hTRPV1 with phorbol 12-myristate-13-acetate (PMA), an activator of PKC, leads to an average of two-fold enhancement of ion current through TRPV1 when in the presence of capsaicin at less than maximal activation concentrations, but not at maximal concentrations (Vellani et al. 2001). Enhancement of both pH- and heat-induced ion current by PKC activation is also observed. However, PMA does not produce significant ion current in most DRGs or HEK293 cells stably transfected with hTRPV1 in the absence of agonist. These and later results (Bhave et al. 2003) suggest that PKC does not actuate the channel but enhances the action of other stimuli. Similar results are also obtained for rTRPV1 expressed in Xenopus oocytes upon activation of PKC (Premkumar and Ahern 2000). In contrast, however, the ion current in the absence of capsaicin or anandamide is more pronounced. The effect of PMA on rTPRV1 and Ser and Thr to Ala mutants expressed in HEK293 cells reveals that S502 and S800 are the sites of PKC phosphorylation and that each contributes to the total augmentation of capsaicin-induced ion current and reduction of the threshold temperature for heat-induced ion-current (Numazaki et al. 2002).
Phosphorylation of TRPV1 by cAMP-dependent PKA also enhances agonist-, heat- or pH-induced ion current. Exposure of rat nociceptive neurons or HEK293 cells transfected with rTRPV1 to the cAMP activator forskolin potentiates heat-induced ion current and exposure to the PKA inhibitor PKI14-22 prevents potentiation (Rathee et al. 2002). The experiment repeated with mutants T144D, T370D and S502D shows that phosphorylation at each of these sites potentiates heat-induced ion current with phosphorylation at S502 rendering the greatest sensitization. Later work reveals that PKA-dependent phosphorylation of S116 also potentiates capsaicin-induced ion-current (Bhave et al. 2002). Further, sensitization of the rTRPV1 response to both capsaicin and heat by PKC, especially PKCε, involves phosphorylation at S502 or S800 (Numazaki et al. 2002) and this sensitization is not modified in the calcineurin-mediated desensitization (Mohapatra and Nau 2005).
The response of rTRPV1 to capsaicin-induced ion current in both cultured rat neurons and HEK293 cells transfected with rTRPV1 is enhanced through phosphorylation by Ca2+-calmodulin dependent kinase II (CaMKII) (Jung et al. 2004). Data obtained from site specific mutants indicates that phosphorylation at T704 allows for activation but is refractory to sensitization by other kinases (e.g., PKA and PKC) unless S502 is also phosphorylated. Further work reveals that dephosphorylation by the calmodulin-dependent phosphatase calcineurin reverses activation by reducing the ability of rTRPV1 to bind agonist. Hence, the regulation of rTRPV1 is controlled by a balance between calcium-mediated phosphorylation by CaMKII and calcium-mediated dephosphorylation by calcineurin with augmentation of activity by PKA and PKC. The apparent calcium-linked paradox has yet to be explained and may be quite complex; several mechanisms have been proposed (Jung et al. 2004). Interestingly, lack of phosphorylation at either S502 or T704 does not affect the response of rTRPV1 to acid, indicating that agonist-induced and acid-induced sensitization proceed through different pathways (Jung et al. 2004).
Both desensitization and ion selectivity but not expression are functions of the glycosylation state of N604 (Veldhuis et al. 2012). Capsaicin-induced ion fluxes are sustained for wild type rTRPV1 expressed in HEK293 cells whereas mutant (N604T), non-glycosylated rTRPV1 is rapidly desensitized. Both forms of rTRPV1 are equally expressed on the plasma membrane surface and thus the glycosylation state does not control surface expression. Capsaicin activation of the wild type channel exhibits a concentration dependent influx of the di-cationic dye YO-PRO-1, whereas the mutant shows no such influx, thus glycosylation controls the selectivity of the receptor. These results leave open the possibility that maintenance of the glycosylation state could be used for the regulation of this channel in vivo (Veldhuis et al. 2012).
When expressed together, TRPV1b associates with TRPV1 in heteromeric complexes. The net result is a negatively regulatory effect on TRPV1 (Vos et al. 2006; Lu et al. 2005). Thus, TRPV1b may be a regulator of TRPV1 activity in vivo.
Leukotriene receptors
Introduction
Leukotrienes represent a specific class of oxidized derivatives of AA containing three conjugated and one unconjugated double bond of which several are derivatized with glutathione that may be proteolyzed to form different leukotriene products. The synthesis begins with the peroxidation of AA by polyunsaturated fatty acid 5-lipoxygenase (ALOX5) to the highly unstable leukotriene A4 (LTA4) (Fig. 1). LTA4 is readily converted to leukotriene B4 through a step-wise hydrolysis by leukotriene A-4 hydrolase (LTA4H) or conjugated with glutathione by leukotriene C4 synthase (LTC4S) to form leukotriene C4 (LTC4). Stepwise proteolysis by glutathione hydrolase 1 proenzyme (GGT1) and then dipeptidase 1 (DPEP) of the glutathione attached to LTC4 produces leukotriene D4 (LTD4) and leukotriene E4 (LTE4) respectively. LTB4 is a potent chemotaxis stimulating molecule serving to recruit and activate eosinophils, monocytes, and neutrophils (Haeggström 2000; Samuelsson 1983; Crooks and Stockley 1998). The cysteinyl-leukotrienes, LTC4, LTD4 and LTE4, as they are known are potent bronchoconstrictors, serving to increase vascular permeability (Samuelsson 1983).
LTB4R
Introduction
Human leukotriene B4 receptor 1 (hLTB4R) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. It binds to and is strongly activated by leukotriene B4 (LTB4), a potent inflammatory chemoattractant. Stimulation of this receptor leads to signaling through MAPK and PI3K pathways (Tong et al. 2005; Lindsay et al. 1998). LTB4R also has a role in enhancing allergic airway inflammation, inflammatory arthritis and atherosclerosis (Yokomizo et al. 2018).
hLTB4R (BLT1, LTB4-R1, P2Y7, UniProtKB-Q15722) is translated as a 352 amino acid polypeptide with a calculated molecular weight of 37.6 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 19 residues and a long cytosolic C-terminal with 63 residues. There are no additional isoforms and two coding SNP variants reported: L346F (Stelzer et al. 2016) and A79S (Landrum et al. 2016). The biological ramifications of these SNPs are unknown. There are no reported X-ray structures. There is one model available in the SWISS-Model repository using Leukotriene B4 receptor BLT1 in complex with BIIL260 (72.8% sequence homology; PDB entry 5X33) that may serve as a working model (Waterhouse et al. 2018). Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, only some of these have been confirmed experimentally. Mutation of Ser and Thr residues to Ala abolishes agonist-induced internalization, suggesting that the predicted phosphorylation at T308, S310, S313, S314 and T315 are potential sites for phosphorylation (Aratake et al. 2012). Phos-tag gel electrophoresis studies confirm these phosphorylation sites and two more, S320 and T324, where phosphorylation of S310 and T308 are LTB4 induced and the others represent basal phosphorylation (Nakanishi et al. 2018).
Both Resolvin E1 and Resolvin E2 (see below), oxidized derivatives of docosahexaenoic acid, serve as equally potent partial agonists for the LTB4R receptor and thus compete with LTB4 but activate the receptor to a lesser extent than LTB4 (Oh et al. 2012). The primary receptor for these resolvins is CMKLR1 (see below).
Expression and characterization
hLTB4R is primarily expressed in the bone, lymphoid tissue and blood, but is also found in other tissues in lower amounts, particularly in the esophagus and digestive system (Uhlén et al. 2015). LTB4R is the high affinity receptor for leukotriene B4 (LTB4) with a Ki in the sub-nanomolar range, but also exhibits strong binding to 12(R)-HETE (Ki = 30 nM) (Fig. 1, Table 2) (Yokomizo et al. 1997). EC50 values for LTB4 are in the 3–10 mM range (Yokomizo et al. 1997). EC50 values for 12(R)-HETE have yet to be reported.
Mechanism of cell activation
LTB4R is yet another receptor with G-protein promiscuity, depending on the G-protein availability or the cell type in which it is expressed. LTB4R couples mainly with Gαi-like proteins, but also with Gαq and Gα16, leading to a reduction in cAMP facilitated by the former and a PLC/IP3-mediated increase in [Ca2+]i by the latter two (Saeki and Yokomizo 2017; Malfacini et al. 2019; Kuniyeda et al. 2007).
The primary function for LTB4R is its involvement in the immune response which is characterized largely by its stimulation of leukocyte chemotaxis. Activation by LTB4 induces neutrophil swarming (Lämmermann et al. 2013) and vascular smooth muscle cell migration (Bäck et al. 2005) to sites of tissue damage. The former is particularly important in allergic skin inflammation where the scratching response to irritants causes microinjuries that lead to neutrophil infiltration (Sadik et al. 2013). LTB4R activation is also involved in the recruitment of CD4+ and CD8+ T cells to sites of inflammation (Goodarzi et al. 2003; Tager et al. 2003).
Regulation
Ligand activated phosphorylation of LTB4R by either PKC, GRK2, GRK5 or GRK6 results in desensitization. T308 is the major but not the only site for GRK6 phosphorylation. However, only phosphorylation at T308 is associated with desensitization (Gaudreau et al. 2002). Nakanishi et al. (2018) expand these observations and delineate the cellular responses. They show that basal phosphorylation of LTB4R occurs in variable patterns in the absence of LTB4. At low concentrations of LTB4 the receptor adopts a high affinity configuration that stimulates the associated Gαi to exchange out GDP for GTP, initiating chemotaxis. Following deactivation of Gαi by GTP hydrolysis, S310 is phosphorylated which initiates further basal phosphorylation, producing a low affinity form. As migration up the LTB4 concentration gradient continues, the higher LTB4 concentrations stimulate phosphorylation at T308, a signal for further phosphorylation leading to additional responses including degranulation.
Desensitization through internalization is a common feature for GPCRs. Desensitization of LTB4R by ligand-induced, phosphorylation-triggered internalization has been observed. The cytoplasmic C-terminus is likely phosphorylated at residues T308, S310, S313, S314, and T315 and phosphorylation is required for ligand-induced internalization. Specific amounts of required phosphorylation and the importance of phosphorylation of specific residues is not known. The dileucine motif in the C-terminal helix (helix 8) is important in internalization and mutation of these leucines (L304 and L305) to alanines result in rapid ligand-induced internalization, suggesting that this motif inhibits receptor phosphorylation (Aratake et al. 2012). Interestingly, phosphorylation of LTB4R is not required for β-arrestin binding and subsequent β-arrestin-mediated internalization (Jala et al. 2005). Taken together, these results show that there are multiple pathways for LTB4R internalization.
Regulation of LTB4R at the genetic level has also been reported. Upregulation of LTB4R is accomplished through a Iκ kinase β/NF-κB-dependent pathway and upregulation can be induced by treatment with either IL-1β or immune-stimulating lipopolysaccharides (LPS) (Bäck et al. 2005).
Cysteinyl Leukotriene receptor 1 (CYSLTR1)
Introduction
Human cysteinyl leukotriene receptor 1 (hCYSLTR1) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family and a structural homolog of purigenic receptors (P2Y). It binds to and is activated by leukotrienes in the following order of preference leukotriene D4 (LTD4) > > leukotriene E4 (LTE4) ≈ leukotriene C4 (LTC4) > > leukotriene B4 (LTB4) (Lynch et al. 1999). Stimulation of this receptor leads to signaling through a PLC/PI3 pathway that leads to the elevation in [Ca2+]i (Crooke et al. 1989; Watanabe et al. 1990; Ohshima et al. 2002; Yan et al. 2011; Sarau et al. 1999) and phosphorylation/activation of ERK/MAPK and various tyrosine kinases (Jiang et al. 2007; Boehmler et al. 2009). CYSLTR1 plays a major role in inflammation and is implicated in a number of diseases including atropic dermatitis, allergic rhinitis, asthma, and cardiovascular disease (review Yokomizo et al. 2018).
hCYSLTR1 (LTD4 receptor, HG55, HMTMF81, UniProtKB- Q9Y271) is translated as a 337 amino acid polypeptide with a calculated molecular weight of 38.5 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 28 residues and a cytosolic C-terminal with 40 residues. There are no additional isoforms and one coding SNP variant reported (G300S) with unknown biological import (Thompson et al. 2016). There is also one N23 frameshift mutant found in malignant prostate tumors with otherwise unknown significance (Landrum et al. 2016). There are two X-ray structures available (PDB entry 6RZ4 and 6RZY). Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, some of which have been confirmed experimentally. Tyr phosphorylations are predicted for Y30 and Y319 but only Tyr phosphorylation without mention of a specific site has been reported (Naik et al. 2005). Ser phosphorylation by PKC on the C-terminal cytoplasmic tail (S313, S315, or S316) is confirmed (Naik et al. 2005).
Expression and characterization
hCYSLTR1 is primarily expressed in the bone, lymphoid tissue and blood, but is also found in other tissues in lower amounts, particularly in the brain and digestive system (Uhlén et al. 2015). CYSLTR1 is the high affinity receptor for leukotriene D4 (LTD4) with a binding IC50 in the sub-nanomolar range and weaker binding (IC50 in the 300–400 nM range) to LTC4 and LTE4 (Table 4) (Lynch et al. 1999). EC50 values for LTD4 are in the low nM range while others are significantly higher (Fig. 1, Table 4).
Table 4.
Leukotriene and Lipoxin receptors
| CYSLTR1&2 | Parameter measured | Ki (nM) | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor/Expression | LTB4 | LTC4 | LTD4 | LTE4 | |||||||
| hCYSLTR1/HLPM higha | Disp | – | 7.9 | 7.7 | 89 | Capra et al. (1998) | |||||
| hCYSLTR1/HLPM lowa | Disp | – | 96 | 46 | 15 | Capra et al. (1998) | |||||
| hCYSLTR1/COS-7b | Disp | – | – | 25 | – | Dupré et al. (2004) | |||||
| EC50/IC50 (nM) | |||||||||||
| hCYSLTR1/Th2c | Ca2+↑ | – | 8 | 0.8 | 6 | Parmentier et al. (2012) | |||||
| hCYSLTR1/HEK293c | Ca2+↑ | – | 1,483 | 11.6 | 391 | Yan et al. (2011) | |||||
| hCYSLTR1/HEK293c | Ca2+↑ | – | 24 | 3 | 240 | Sarau et al. (1999) | |||||
| hCYSLTR1/COS-7c | Ca2+↑ | – | – | 3.4 | – | Carnini et al. (2011) | |||||
| hCYSLTR2/HEK293Tc | Ca2+↑ | – | 11.2 | 0.9 | 83.2 | Foster et al. (2016) | |||||
| hCYSLTR2/HEK293c | Ca2+↑ | – | 95 | 145 | 1208 | Yan et al. (2011) | |||||
| hCYSLTR2/HEK293Tc | Ca2+↑ | > 3000 | 67 | 104 | 2300 | Heise et al. (2000) | |||||
| hCYSLTR2/HEK293Tc | Ca2+↑ | – | 9 | 4.4 | 293 | Nothacker et al. (2000) | |||||
| hCYSLTR2/COS-7c | Ca2+↑ | – | – | 9 | – | Carnini et al. (2011) | |||||
| hCYSLTR2/HUVECd | Ca2+↑ | – | – | – | – | Carnini et al. (2011) | |||||
| GPR17 | Parameter measured | EC50/IC50 (nM) | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor/Expression | LTB4 | LTC4 | LTD4 | LTE4 | UDP | UDP-Glc | UDP-Gal | MDL29,951 | |||
| hGPR17-1/1321N1e | cAMP↓ | – | 0.33 | 7.2 | – | 1,140 | 12,000 | 1100 | – | Ciana et al. (2006) | |
| hGPR17-1/1321N1e | cAMP↓ | – | 65 | 5.9 | – | 4,600 | 530 | – | – | Ciana et al. (2006) | |
| hGPR17-1/COS-7e | cAMP↓ | – | 14.8 | 4.4 | – | – | – | – | – | Ciana et al. (2006) | |
| hGPR17-1/HEK293e | cAMP↓ | – | – | – | – | 1,060 | 9500 | 729 | – | Ciana et al. (2006) | |
| hGPR17-2/1321N1meme | cAMP↓ | – | 0.68 | 2.1 | – | 264 | 2390 | 295 | – | Pugliese et al. (2009) | |
| hGPR17/1321N1memf | disp | – | – | 10 | – | – | 1600 | – | – | Daniele et al. (2011) | |
| mGPR17/1321N1meme | cAMP↓ | – | 0.74 | 0.63 | 0.31 | – | 55–88 | 68 | – | Lecca et al. (2008) | |
| mGPR17/Oli-neu cellsg | Ca2+↑ | – | – | – | – | – | – | – | 10,000 | Simon et al. (2016) | |
| mGPR17-1/Oli-neu cellsh | DMR↑ | – | – | – | – | – | – | – | 320 | Simon et al. (2016) | |
| mGPR17/Oli-neu cellsi | ERK-P↑ | – | – | – | – | – | – | – | 100 | Simon et al. (2016) | |
| mGPR17/Oli-neu cellsj | cAMP↓ | – | – | – | – | – | – | – | 200 | Simon et al. (2016) | |
| hGPR17-1/HEK293h | DMR↑ | – | – | – | – | – | – | – | 5000 | Hennen et al. (2013) | |
| hGPR17-2/HEK293h | DMR↑ | – | – | – | – | – | – | – | 320 | Hennen et al. (2013) | |
| hGPR17-2/HEK293k | cAMP↓ | – | – | – | – | – | – | – | 6/1000 | Hennen et al. (2013) | |
| hGPR17-2/HEK293l | GTPγS | – | – | – | – | – | – | – | 500 | Hennen et al. (2013) | |
| hGPR17-2/HEK293m | IP↑ | – | – | – | – | – | – | – | 320 | Hennen et al. (2013) | |
| hGPR17-2/HEK293n | Ca2+↑ | – | – | – | – | – | – | – | 60 | Hennen et al. (2013) | |
| P2Y12 | Parameter measured | EC50/IC50 (nM) | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor/Expression | LTE4 | ADP | 2MeSADP | PRPP | |||||||
| hP2Y12-G16alpha/CHOc | Ca2+↑ | 1.3 | – | 0.059 | 7.8 | Nonaka et al. (2005) | |||||
| hP2Y12/purifiedn | GTPhyd↑ | – | 30,000 | 16 | – | Bodor et al. (2003) | |||||
| hP2Y12/COS-7o | IP↑ | – | 2400 | 0.6 | – | Bodor et al. (2003) | |||||
| hP2Y12/1321N1e | cAMP↓ | – | 6.8 | 0.07 | – | Kauffenstein et al. (2004) | |||||
| hP2Y12/CHOp | Akt-P↑ | – | – | 0.7 | – | Soulet et al. (2004) | |||||
| hP2Y12/CHOq | MAPK-P↑ | – | – | 0.6 | – | Soulet et al. (2004) | |||||
| FPR2/ALX | Parameter measured | Ki (nM) | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor/Expression | RvD1 | LTC4 | LTD4 | LXA4 | LXD4 | ATLa1 | ATLa2 | 15-deoxy-LXA4 | 15-epi-LXA4 | ||
| hFPR2/HEK293f | Disp | – | – | – | 0.02 | – | 0.03 | 0.03 | 100 | – | Chiang et al. (2000) |
| hFPR2/PMNf | Disp | – | 39 | 35 | 0.94 | – | – | – | – | – | Chiang et al. (2000) |
| hFPR2/CHOf | Disp | – | – | – | 5.6 | 79.9 | – | – | – | – | Fiore et al. (1994) |
| hFPR2/hum leukocyter | RvD1 | 0.17 | – | – | – | – | – | – | – | – | Krishnamoorthy et al. (2010) |
| mFPR2/CHOk | Disp | – | – | – | 2 | – | – | – | – | – | Takano et al. (1997) |
| EC50/IC50 (nM) | |||||||||||
| hFPR2/THP-1 s | laminin | – | – | – | 0.8 | – | 0.08 | 0.08 | – | – | Maddox et al. (1997) |
| hFPR2/HEK293t | β-arrestin | – | – | – | – | – | 0.0029 | – | – | 0.0029 | Sun et al. (2009) |
| hFPR2/HEK293t | β-arrestin | 0.0012 | – | – | 0.0011 | – | – | – | – | – | Krishnamoorthy et al. (2010) |
| hFPR2/HEK293t | β-arrestin | 0.045 | – | – | – | – | – | – | – | – | Krishnamoorthy et al. (2012) |
| hFPR2/HEK293e | cAMP↓ | – | – | – | – | – | – | – | – | 0.5 | Ge et al. (2020) |
| hFPR2/HEK293c | Ca2+ | – | – | – | – | – | – | – | – | < 10 μM | Ge et al. (2020) |
| OXGR1 | Parameter measured | Ki (nM) | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor/Expression | LTD4 | α-KG | AMP | Adenosine | |||||||
| mOXGR1/HEK293u | disp | 6 | – | 3 | – | Kanaoka et al. (2013) | |||||
| mOXGR1/CHOu | disp | 2 | – | – | – | Kanaoka et al. (2013) | |||||
| EC50/IC50 (nM) | |||||||||||
| hOXGR1/HEK293v | Ca2+↑ | – | 69,000/32,000 | – | – | He et al. (2004) | |||||
| hOXGR1/eosinophilsw | cAMP↑ | ≤ 10 | – | – | – | Steinke et al. (2014) | |||||
| hOXGR1/HEK293c | Ca2+↑ | – | – | 920 | 670 | Inbe et al. (2004) | |||||
Agonist parameters given for Human (h) and murine (m) receptors in the indicated cell line or membrane system. Parameter measured: akt-P↑; increae in akt phosphorylation; β-arrestin, β-arrestin binding; Ca2+↑, increase in calcium; cAMP↓, decrease in cAMP; cAMP↑ increase in cAMP accumulation; disp, displacement assay; DMR↑; increase in dynamic mass redistribution; ERK-P↑, increase in ERK phosphorylation; GTPhyd↑; increase in GTP hydrolysis rate; IP↑; increase in inositol phosphate production; laminin, laminin binding; MAPK-P↑; increase in MAP kinase phosphorylation; RvD1, resolvin D1 binding. Cell type abbreviations: CHO, Chinese hamster ovary cells; COS-7, African green monkey kidney cell line; HEK293, Human embryonic kidney 293 cells; HLPM, Human lung parenchyma membranes; hP2Y12-G16alpha, PPY2-G16alpha fusion protein; HUVEC, Human umbilical vein endothelial cells; Oli-neu, murine oligodendroglial precursor cells; PMN, human polymorphonuclear leukocytes; Th2, T helper 2 cells; THP-1, Human monocyte derived from patient with acute monocytic leukemia. Agonist abbreviations: 2MeSADP, 2-methylthio-adenosine-5'-diphosphate; ALTa1, 15(R/S)-methyl-LXA4, a stable LXA4 (15S-LXA4) analog; ALTLa2, 15-epi-16-(para-fluoro)-phenoxy-LXA4 a stable 15R-LXA4 analog; AMP, adenosine monophosphate; α-KG, α-ketoglutarate; LTB4, leukotriene B4; LTC4, leukotriene C4; LTD4, leukotriene D4; LTE4, leukotriene E4; LXA4, lipoxin A4; LXD4, lipoxin D4; MDL29,951, 2-carboxy-4,6-dichloro-1H-indole-3-propionic acid; PRPP, 5-phosphoribosyl 1-pyrophosphate; RvD1, resolvin D1. a) Determined by displacement of agonist [3H]LTC4 for LTD4 determination and [3H]LTD4 for LTC4 and LTE4 determination for high/low binding sites in human lung parenchyma membranes. b) Determined by displacement of agonist [3H]LTD4. c) Determined from changes in intracellular Ca2+ d) Determined from changes in intracellular Ca2+ in human umbilical vein endothelial cells. e) Inhibition of forskolin-stimulated cAMP production. f) Estimated from graph for displacement of [11,12-3H]-LXA4 and Ki calculated using the Cheng-Prussoff equation. g) Estimated from plot of %Ca2+ release versus agonist concentration. h) Estimated from plot of dynamic mass redistribution (DMR) assay versus agonist concentration. i) Estimated from a plot of ERK 1/2 phosphorylation versus agonist concentration. e) Estimated from a plot of cAMP versus agonist concentration. j) Estimated from a plot of cAMP versus agonist concentration as diminishment/enhancement. k) Estimated from a plot of [35S]GTPγS binding versus agonist concentration. l) Estimated from a plot of total intracellular inositol phosphate accumulation versus agonist concentration. m) Estimated from a plot of total [Ca2+]i accumulation versus agonist concentration. n) Purified hP2Y12 inserted into synthetic vesicles as measured by GTP hydrolysis. o) Inositol phosphate production. p) Akt phosphorylation. q) MAP-kinase phosphorylation. r) [3H]RvD1 binding. s) Adherence of human monocytic cell line THP-1 cell adherence to laminin. t) Binding of β-arrestins. u) Estimated from graph for displacement of [3H]LTE4 and Ki calculated using the Cheng-Prussoff equation. v) [Ca2+]i increase by aqueorin assay/FLIPER assay w) Increase in cAMP production
Mechanism of cell activation
CYSLTR1 signals through several different G-proteins. For example, in the differentiated promonocytic leukemia cell line U937, CYSLTR1 signals simultaneously through multiple pathways including Gαq11, Gαio, Gβγ subunits, as well as isoprenylated proteins such as members of the Ras family (Capra et al. 2003), resulting in a complex response leading to increased [Ca2+]i (Pollock and Creba 1990). Similarly, human TH2 cell CYSLTR1 signals through both Gαq and Gαi (Parmentier et al. 2012), leading to an increase in [Ca2+]i and a decrease in intracellular cAMP. In human epithelial cells signaling is known to occur through Gαi3, stimulating an increase in [Ca2+]i (Adolfsson et al. 1996).
CYSLTR1 plays a significant role in stimulating leukocyte chemotaxis. Activation by LTD4 or other potent agonists are known to enhance recruitment of human eosinophils (Ohshima et al. 2002), monocytes (Woszczek et al. 2008), and TH2 cells (Parmentier et al. 2012). In a complementary fashion, CYSLTR1 also enhances vascular permeability, which in turn promotes leukocyte infiltration (Maekawa et al. 2002; Bochnowicz and Underwood 1995). This receptor is also known to control LTD4 and LTC4 induced vasoconstriction of human saphenous veins (Mechiche et al. 2004). Interestingly, in a mouse model for chronic pulmonary inflammation and fibrosis, CYSLTR1 exhibits an anti-inflammatory role by reducing the magnitude of septal thickening and deposition of reticular fibers (Beller et al. 2004). These results suggest a role for this receptor in the resolution of inflammation in lung tissue.
Regulation
Agonist-induced receptor internalization is a common theme for eicosanoid receptors and quite applicable to CYSLTR1. Stimulation of non-transformed human intestinal endothelial cells (Int 407 cells) with LTD4 but not LTC4 induces tyrosine phosphorylation of this receptor, ultimately leading to a rapid (5 min) internalization and localization to the nucleus. It recycles back to the plasma membrane when LTD4 is removed. Internalization proceeds by clatherin-coated pits and trafficking of the early endosomes containing the receptor is facilitated by both the Ras-related protein Rab-5 and arrestin-3. Further, CYSLTR1 heterodimerizes with CYSLTR2 on the plasma membrane under basal conditions and LTD4 stimulates internalization of CYSLTR1, leaving CYSLTR2 on the plasma membrane. Stimulation of the heterodimers with LTC4 does not result in phosphorylation but does initiate internalization of both receptors without localization to the nucleus. Removal of LTC4 reverses the process (Parhamifar et al. 2010). In contrast, CYSLTR1 can internalize by an arrestin-free internalization process where phosphorylation of S313, S315, or S316, or possible a combination by PKC inhibits internalization (Naik et al. 2005).
Heterodimers in human mast cells that naturally express both receptors modify the receptor response. When mast cells are treated with the shRNA-mediated knockdown of CYSLTR1, the response to LTD4 is abrogated, indicating that CYSLTR1 is the primary receptor for LTD4. Knockdown of CYSLTR2, on the other hand, doubles the response to LTD4 by doubling the surface expression of CYSLTR1, presumably as dimers. Clearly, the heterodimer serves to regulate the cellular response to Cys-leukotrienes by regulating the amount of CYSLTR1 homodimer on the plasma membrane (Jiang et al. 2007). CYSLTR2 is not the only moderating receptor for CYSLTR1. The G-protein coupled receptor GPR17 heterodimerizes with CYSLTR1 and abolishes LTC4- and LTD4-mediated response (e.g. [Ca2+]i). GPR17 expression not only mediates the surface expression of CYSLTR1 but also suppresses LTD4 binding (Maekawa et al. 2009). CYSLTR1 and the generic purinergic G-protein coupled receptors (P2Y), of which GPR17 is a member, also interact. Activation of P2Y receptors with ATP or UDP induces desensitization of CYSLTR1 to LTD4 but does not stimulate internalization. Conversely, stimulation of CYSLTR1 with LTD4 has no effect on P2Y receptor responses and does initiate CYSLTR1 internalization. Apparently, crosstalk between G-proteins is intimately involved in regulation of responses at sites of inflammation (Capra et al. 2005).
Regulation of hCYSLTR1 at the transcriptional, translational, and posttranslational modification levels have been reported. Treatment of human bronchial smooth muscle cells (BSMC) with IL-13, transforming growth factor β (TGFβ) or interferon γ (IFNγ) upregulate the expression of CYCLTR1 but only IL-13 and IFNγ increase the amounts of CYSLTR1 mRNA. Evidently, the upregulation by TGF-β is either a translational or posttranslational event. Treatment of BSMCs with TGF-β or IL-13 increases their proliferation in response to LTD4. These results indicate a synergy between cysteinyl leukotrienes (CysLTs) and specific cytokines in the expression of CYSLTR1 and BSMC proliferation (Espinosa et al. 2003). Others have reported that the IL-13 upregulation of hCYSLTR1 in human monocytes is biphasic in nature and IL-4 also increases CYSLTR1 mRNA levels 150-fold (Shirasaki et al. 2007).
Cysteinyl Leukotriene receptor 2 (CYSLTR2)
Introduction
Human cysteinyl leukotriene receptor 2 (hCYSLTR2) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. It binds to and is activated by leukotrienes in the following order of preference LTC4 ≈ LTD4 > > LTE4. Stimulation of this receptor in turn leads to signaling through a PLC/PI3 pathway that leads to the elevation of [Ca2+]i (Sarau et al. 1999; Moore et al. 2016; Carnini et al. 2011) and phosphorylation/activation of ERK/MAPK (Mehdawi et al. 2017; Mellor et al. 2003). CYSLTR2 plays a major role in inflammation (Mellor et al. 2003) and is implicated in several diseases including asthma (Thompson et al. 2016) and cancer (Moore et al. 2016; Brochu-Bourque et al. 2011).
hCYSLTR2 (CYSLT2, CYSLT2R, UniProtKB- Q9NS75) is translated as a 346 amino acid polypeptide with a calculated molecular weight of 39.6 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 42 residues and a cytosolic C-terminal with 39 residues. There are no additional isoforms and 8 reported coding SNP variants with unknown biological import: M201V (Thompson et al. 2016; Brochu-Bourque et al. 2011), E39Q, F50V, N103S, S236L, H242Q, L278I, R315K (Landrum et al. 2016). There are no X-ray structures available. There is one structural model available on the SWISS-MODEL website (PDB entry 6RZ6) (Waterhouse et al. 2018) to serve as a working model. Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally.
Expression and characterization
hCYSLTR2 is primarily expressed in the brain, blood, and both male and female tissues, but is also found in other tissues in lower amounts, particularly in the muscle, bone, and the endocrine tissue Uhlén et al. 2015). CYSLTR2 is the high affinity receptor for both LTD4 and LTC4 with EC50 values in the nM range (Fig. 1, Table 4).
Mechanism of cell activation
In human umbilical endothelial cells (HUVECs) and transfected HEK293 cells, hCYSLTR2 signals through Gαq11, stimulating a PLC/IP3 cascade leading to an increase in [Ca2+]i and the formation of diacyl glycerol (DAG) (Sarau et al. 1999; Moore et al. 2016). One of the results of the increased [Ca2+]i and DAG is the activation of PKC that in turn activates the MAPK pathway leading to enhanced phosphorylation of p65 (NF-κB family), ERK, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase (JNK) (Brochu-Bourque et al. 2010). Stimulation of hCYSLTR2 with agonist also results in significant transactivation and secretion of IL-8 (Mellor et al. 2003; Brochu-Bourque et al. 2011), a chemokine that induces chemotaxis of neutrophils and other granulocytes and stimulates phagocytosis. Interestingly, in human mast cells, IL-8 production is known to be mediated by the Gαio family of G-proteins (Mellor et al. 2003). Secretion of IL-5, a known mediator in eosinophil activation and immunoglobulin secretion, is also enhanced, but this may involve CYSLTR1/ CYSLTR2 heterodimers.
Like CYSLTR1, CYSLTR2 also promotes vascular permeability, but contrary to CYSLTR1 it promotes significant vasodilation (Hui et al. 2004), an event in keeping with the regulatory role of CYSLTR2 in the CYSLTR1/CYSLTR2 heterodimer discussed above.
LTC4 signaling through CYSLTR2 also promotes the synthesis of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) which catalyzes the oxidation of prostaglandin E2 (PGE2) to its inactive metabolite 15-keto-PGE2 (Mehdawi et al. 2017). PGE2 is well established as an important mediator in inflammation, driving acute inflammation, and a potent tumor promotor (Nakanishi and Rosenberg 2013). By mediating upregulation of 15-PGDH, CYSLTR1 is active in both anti-inflammatory and anti-tumor activity.
Regulation
Regulation of CYSLTR2 is reported to occur at both the surface expression and transcriptional levels. Priming of human mast cells (hMC) with IL-4 for 5 days results in a redistribution of CYSLTR2 to the plasma membrane, without changing the total cellular content of this receptor (Shirasaki et al. 2007; Mellor et al. 2003). Additionally, both LTD4 stimulation and IL-13 upregulate CYSLTR2 mRNA levels (Shirasaki et al. 2007). Further, human endothelial cells produce CYSLTR2 and not CYSLTR1 and IFN-γ enhances CYSLTR2 mRNA production and consequently the CYSLTR2 response to agonist (Woszczek et al. 2007).
2-oxoglutarate receptor 1 (OXGR1, GPR99)
Introduction
Human 2-oxoglutarate receptor 1 (hOXGR1), originally designated as an alpha-ketoglutarate receptor, is a member of the seven transmembrane receptor G-protein coupled receptor 1 family and P2Y subfamily. It binds to and is activated by LTE4 in the low nM region, AMP and adenosine in the hundreds of nM region, and α-ketoglutarate in the tens of µM range (Fig. 1, Table 4). Stimulation of the OXGR1 receptor leads to an increase in [Ca2+]i and cAMP, indicating that it couples through at least 2 different G-proteins (Inbe et al. 2004). OXGR1 has a role in vascular permeability (Bankova et al. 2016; Maekawa et al. 2008; Kanaoka et al. 2013) bronchoconstriction (Laitinen et al. 1993) and inflammation (Salimi et al. 2017; Steinke et al. 2014).
hOXGR1 (GPR99, CYSLT3, P2Y15, 2-oxoglutarate receptor 1, alpha-ketoglutarate receptor 1, UniProtKB- Q96P68) is translated as a 337 amino acid polypeptide with a calculated molecular weight of 38.3 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 34 residues and a cytosolic C-terminal with 32 residues. There are no additional isoforms and no reported coding SNP variants and one L45 frameshift mutant all with unknown effect on function (Landrum et al. 2016). No x-ray structures are available. There is one model available on the SWISS-Model site using P2Y purinoceptor (35.8% sequence homology; PDB entry 4XNV) as a template (Waterhouse et al. 2018) for a working model. Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally.
Expression and characterization
hOXGR1 is expressed in high amounts in the brain, gastrointestinal system, kidney and bladder, male and female tissues, skin and blood as well as lower expression in other tissues (Uhlén et al. 2015). GPR 99 is a high affinity receptor for LTE4 with an of EC50 ≤ 10 nM with much lower affinity for AMP, adenosine, and α-ketoglutarate (Table 4). Although CYSLTR1 and CYSLTR2 also bind LTE4, they do so with much lower efficiency (Fig. 1, Table 4) suggesting that OXGR1 is the more likely LTE4 receptor. Further support for this comes from studies utilizing CYSLTR1/CYSLTR2 double knockout mice where treatment LTE4 elicits an elevated vascular leak response (Maekawa et al. 2008). Definitive proof is evident from a study using OXGR1/CYSLTR1/CYSLTR2 triple knockout mice where the LTE4 response is curtailed by 90% compared to wild-type mice (Kanaoka et al. 2013).
Mechanism of cell activation
To date, few publications present details of specific mechanisms that follow stimulation of OXGR1 and only one describing LTE4 stimulation. Here, the data indicate that both [Ca2+]i and cAMP increase with LTE4 stimulation, suggesting that signaling occurs through both Gαq and Gαs proteins. The authors note that cAMP activation might be a function of the cell type used for the transfection (Inbe et al. 2004). Other studies show that activation with AMP, adenosine, or α-ketoglutarate also results in increased [Ca2+]i, further supporting a Gαq signaling pathway (Steinke et al. 2014; He et al. 2004).
There are several publications that outline the response of OXGR1 to ligands other than LTE4 (see review Rajkumar and Pluznick 2017). However, such findings are beyond the scope of this review and will not be discussed further here.
Regulation
Receptor interaction is involved in the regulation of OXGR1. In CYSLTR1/CYSLTR2 the double knockouts noted above, the effect of LTE4 is enhanced 64-fold, presumably through the action of OXGR1 (Maekawa et al. 2008). This enhancement strongly suggests that both CYSLTR1 and CYSLTR2 cross-regulate the response of OXGR1 (Kanaoka and Boyce 2014).
OXGR1 expression in human lung mast cells is upregulated in response to allergic reactions (Nishi et al. 2016). The particulars of the process are unknown.
Uracil nucleotide/cysteinyl leukotriene receptor (GPR17)
Introduction
Human uracil nucleotide/cysteinyl leukotriene receptor (hGPR17) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family with dual specificity receptor for both uracil nucleotides and cysteinyl leukotrienes, as might be expected for a receptor so closely phylogenetically related to both CYSLTRs and the P2Y purinergic receptors (Abbracchio et al. 2006). There are apparently different binding sites for these two structurally different effector molecules (Abbracchio et al. 2006). The actions of these effector molecules as agonists remains controversial. In terms of biological function, there is significant support for the involvement of GPR17 in oligodendrocyte differentiation and myelination (Marucci et al. 2016; Lu et al. 2018; Franke et al. 2013; Simon et al. 2016).
hGRP17-1(G-protein coupled receptor 17, P2Y-like receptor, R12, UniProtKB-Q13304) is translated as a 367 amino acid polypeptide with a calculated molecular weight of 37.8 kDa. The basic structure consists of seven transmembrane helices with a large N-terminal extracellular domain of 64 residues and a cytosolic C-terminal with 38 residues. There is one additional isoform (hGPR17-2) that is missing the first 28 residues found in the canonical isoform (hGPR17-1). Agonist binding data has been obtained for both isoforms (Table 4). There are two reported coding SNP variants (T27M and T55M), both with unknown effect on function (Stelzer et al. 2016; Landrum et al. 2016). No X-ray structures are available. There is one model available on the SWISS-Model site using the apelin receptor in complex with agonist peptide (31.5% sequence homology; PDB entry 5VBL) as a template (Waterhouse et al. 2018) that provides a working model. Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally.
Expression and characterization
hGPR17 is primarily expressed primarily in the brain with lower amounts in the GI tract, muscle and female tissue and small amounts in most other tissues (Uhlén et al. 2015). Although GPR17 is expressed throughout the brain the highest expression is found in the cerebral cortex, hippocampal formation, amygdala, thalamus, pons, and medulla. Cellular locations include both the plasma membrane and intracellular vesicles.
hGPR17 activation by both leukotrienes and uridine nucleotides have been reported (Table 4). Leukotrienes LTC4, LTD4 and LTE4 all promote [35S]GTPγS binding to the receptor with EC50 values in the sub-nM to low nM range (Ciana et al. 2006; Pugliese et al. 2009; Daniele et al. 2011). The uridine nucleotides UDP, UDP-glucose (UDP-Glc), and UDP-galactose (UDP-Gal) also promote [35S]GTPγS binding albeit in the tens of nM to tens of µM range depending on the cell system employed (Ciana et al. 2006; Pugliese et al. 2009; Daniele et al. 2011; Lecca et al. 2008). Ciana et al (2006) report that UDP, UDP-Glc, UDP-Gal and LTD4 all inhibit forskolin induced cAMP formation and increase [Ca2+]i in 30% of the transfected cells. Pugliese et al. (2009) also report an increase in [Ca2+]i upon stimulation with UDP-Glc. Daniele et al. (2014) report an inhibition of forskolin induced cAMP formation upon stimulation with UDP-Glc and LTD4.
Not all laboratories have been able to reproduce these data. Qi et al. (2013) report that hGPR17 transfected CHO cells stimulated with either LTC4, UDP, UDP-Glc, or UDP-Gal fail to promote inhibition of forskolin-stimulated cAMP accumulation. Similarly, these effectors did not induce inositol phosphate accumulation in COS-7 or HEK293 cells transfected with hGPR17. Similar results have been reported by Simon et al. (2017), where LTD4 and the uridine nucleotides failed to exhibit [35S]GTPγS binding, Ca2+ release, or changes in the dynamic mass redistribution (DMR) in HEK293, 1321N1, or CHO hGPR17 transfectants. They also provide proof that the aforementioned hGPR17 activity does occur in each of these cell systems by showing that the synthetic agonist MDL29,951 produces them.
The inconsistent data for agonist stimulation of GPR17 has led to the proposal of an alternative biological function for GPR17. Maekawa et al. (2009) report that when CYSLTR1 is co-expressed with GPR17 the two receptors colocalize on the cell surface and that specific [3H]LTD4 binding to microsomal membranes and LTD4 elicited ERK phosphorylation is fully inhibited. This and GPR17 knockdown studies indicate a ligand-independent negative regulatory role for GPR17. Similar data has been reported by others (Qi et al. 2013).
Mechanism of cell activation
Although the specific, naturally occurring agonist is in doubt, it is clear that the synthetic agonist MDL29,951 does produce cellular changes when binding to GPR17 and provides insight into the signaling pathways. Hennen et al. (2013) present an extensive study of signaling by hGPR17 transfected into CHO, HEK293, and 1321N1 cell lines (Table 4). In all cases, signaling produces an increase in DMR, [Ca2+]i, inositol phosphate accumulation, and [35S]GTPγS binding. Changes in cAMP are bimodal, where low concentrations of agonist produce a decrease in forskolin-stimulated cAMP accumulation and higher concentrations reverse this trend. In both CHO and HEK293 cells the reversal results in an overall increase in cAMP accumulation over that produced by forskolin induction. Hennen et al. (2013) also characterized the signaling pathways involved in the changes in cAMP, inositol phosphate and [Ca2+]i. Treatment of either HEK293 or CHO cells with PTX eliminates the reduction in cAMP accumulation, indicating that the low agonist concentrations are stimulating signaling through a Gαi pathway. The increase in cAMP at higher agonist concentrations is indicative of Gαs-mediated signaling which is supported by the results from Bioluminescence Resonance Energy Transfer (BRET2) assays. They also show that the inositol phosphate accumulation and the increase in [Ca2+]i is Gαq-mediated, as these increases are blunted by the Gαq-selective inhibitor FR900359. Simon et al. (2015) found similar results for endogenous GPR17 in murine oligodentrocytes (Oli-neu cells) and confirmed both Gαi and Gαq signaling. They did not observe the production of cAMP at higher concentrations of agonist as shown in the aforementioned study. Further, they show that the Gαi path is responsible for diminished myelin protein accumulation and does so by reducing the activity of a cAMP/PKA/cAMP response element binding protein (CREB) cascade.
Regulation
Receptor regulation by β-arrestin-mediated internalization is a common feature of GPCR regulation. Hennen et al. (2013) show that MDL29,951 stimulates GPR17 and promotes β-arrestin2 recruitment, and that this occurs through two mechanisms, one being G-protein dependent and the other G-protein independent. Further, agonist dependent desensitization by β-arrestin occurs both through the binding of β-arrestin itself and through promotion of internalization following binding. Fratangeli et al. (2013) report that both LTD4 and UDP-Glc stimulate a clatherin-mediated internalization of GPR17 in Oli-neu cells, some of which is targeted for lysosomal degradation. Daniele et al. (2014) report that both GRK2 and GRK 5 are involved in GPR17 internalization and show that both kinases are weakly associated with GPR17 under basal conditions. Upon LTD4 binding, GRK2 strongly associates with the receptor resulting in receptor phosphorylation. UDP-Glc binding promotes strong association with GRK5 which also results in receptor phosphorylation. Further they show a weak basal association of β-arrestin with GPR17 and that agonist binding strengthens this association and that the complexes formed through LTD4 activation are more transient than those stimulated by UDP-Glc binding. They suggest that the difference in binding strength leads to the complexes formed through LTD4-mediated internalization remaining close to the plasma membrane.
P2Y purinoceptor 12 (P2Y12)
Introduction
Human P2Y purinoceptor 12 (hP2Y12) is a member of the G-protein coupled receptor 1 family and the P2Y (purinergic receptor) subfamily of receptors. It I well known for its involvement in platelet activation (Abbracchio et al. 2006; Bye et al. 2016; Parke and Storey, 2021) and inflammation (Neves et al. 2010;
Paruchuri et al. 2009; Sasaki et al. 2019). As a member of the purinergic receptor family, much of the data available for this receptor centers on purine ligands, where the most effective endogenous agonist is ADP followed by ATP (Burnstock, 2018). However, there are increasing numbers of reports that implicate LTE4 as a highly effective agonist (Nonaka et al. 2005; Sasaki and Yokomizo, 2019). However, the claim remains controversial (Foster et al. 2013).
hP2Y12 (ADPG-R, P2Y(AC), SP1999, UniProtKB-Q9H244) is translated as a 342 amino acid polypeptide with a calculated molecular weight of 39.4 kDa. The basic structure consists of seven transmembrane helices with a large N-terminal extracellular domain of 64 residues and a cytosolic C-terminal with 21 residues. There are no additional isoforms. There are 35 reported coding SNP variants of which 9 are involved in abnormal platelet function or bleeding disorders (R265P, R265W, P258T, R256Q, I240fs, I200M, C175Y, K174E, R122H) (Landrum et al. 2016). Six X-ray structures are available (e.g., PDB entry 4NTJ). Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally.
Expression and characterization
hP2Y12 is primarily expressed in the brain with lower amounts in the blood, bone marrow and lymphoid tissues, lung (protein), adipose tissue and small amounts in most other tissues (Uhlén et al. 2015). hP2Y12 is expressed throughout the brain in relatively equal amounts with the exception of the cerebellum where the expression is much lower. It is important to note that neural expression of P2Y12 is exclusive to microglia and it functions to enhance neuropathic pain associated with nerve injury (Gu et al. 2016; Kobayashi et al. 2008). Cellular locations have been confirmed for both the plasma membrane and intracellular vesicles (Mundell et al. 2006).
hP2Y12 activation by both adenine nucleotides and LTE4 have been reported (Table 4). The adenine nucleotide ADP induces an increase in GTP hydrolysis and inositol phosphate accumulation with EC50 values of 30 and 2.4 µM respectively (Bodor et al. 2003). ADP has also been reported to inhibit forskolin-induced cAMP accumulation with an EC50 of 6.8 nM (Kauffenstein et al. 2004). The rather wide range of efficacies requires further investigation. Nonaka et al. (2005) report a computer modeling study which indicates that LTE4 should be an effective agonist for P2Y12 and support this assertion with an experimental measurement of LTE4-induced Ca2+ mobilization for which an EC50 of 1.3 nM is apparent. In direct contrast, Foster et al. (2013) report that although they found that ADP and the synthetic agonist 2MeSADP are agonists for hP2Y12, LTE4 is not an agonist. However, there is considerable support for LTE4 acting as an agonist for P2Y12. Neves et al. (2010) have reported that both of the leukotriene receptors CYSLTR1 and CYSLTR2 and the hP2Y12 receptor are expressed on eosinophil granule membranes and that LTC4, LTD4 and LTE4 all stimulate isolated granules to secrete eosinophil cationic protein (ECP). They also show that pre-incubation of the granules with the specific P2Y12 antagonist MRS2395 inhibits the release of ECP by each of the three leukotrienes, indicating that hP2Y12 is clearly involved in the process. Pre-incubation with montelukast, a known antagonist for CYSLTR1 exhibits the same effect. These results and the fact that LTE4 is a poor agonist for CYSLTR1 suggest that the release of ECP in this system may in fact be mediated by a heteromer of hP2Y12 and CYSLTR1. Further support for LTE4 agonism of P2Y12 is found in the report by Austen et al. (2009). Here they show that application of LTE4 to sensitized mouse (BALBc) airways induces eosinophilia, goblet cell metaplasia, and the expression of IL-13, a mediator of allergic inflammation and that these effects are completely blocked by the P2Y12-specific antagonist clopidrogel.
Mechanism of cell activation
In contrast to the classic P2Y receptors that couple with Gαq, P2Y12 couples through the PTX-sensitive Gαi (Paruchuri et al. 2009; Foster et al. 2001; Bodor et al. 2003). Bodor et al. (2003) examined this coupling in detail and found that the most robust coupling occurs through Gαi2 but coupling through Gαi1 and Gαi3 are also effective. Little if any coupling was observed to occur through Gαo or Gαq. An alternative pathway for P2Y12 signaling has also been proposed. Soulet et al. (2004) show that hP2Y12 transfected into CHO cells activates both a cell proliferation pathway and an actin cytoskeleton reorganization pathway. The former pathway proceeds through a Gαi coupled PI3K/Akt/MAPK pathway whereas the latter is Gαi-independent and requires activation of RhoA and Rho-kinase.
Regulation
As typical for GPCRs, agonist-induced desensitization and internalization is observed, however, not all reports agree on the degree of desensitization. Hardy et al. (2005) report that the ADP-induced P2Y12 reduction of forskolin-induced cAMP accumulation is blocked by pretreatment with ADP. They further show that this desensitization is mediated by GRKs rather than classical protein kinase C desensitization (PKC). They note that since both GRK2 and GRK6 are endogenously expressed in platelets, these G-proteins are the likely candidates for desensitization. Later work by the same group (Mundell et al. 2006) reveals that desensitization is accompanied by rapid internalization. They show further that the novel PKCγ is capable of facilitating internalization of P2Y12, but not other, classical PKCs. The fact that PKCγ is highly expressed in the human brain, but only marginally expressed blood suggests that this mechanism may play a role in CNS microglia, but not in platelets. In contrast, Baurand et al. (2005) show that preincubation with ADP does not desensitize the P2Y12 reduction in cAMP accumulation. They do observe rapid, transient internalization of P2Y12, but the receptors are rapidly returned to the plasma membrane such that the overall cell surface expression of P2Y12 is unchanged by ADP. Interestingly, the degree of internalization in both studies is remarkably similar (25–30%), suggesting that the rate of surface return may define the difference between the studies. More recently, Nisar et al. (2012) show that the motif-binding protein NHERF1 has a major role in the internalization of P2Y12 and that β-arrestin promotes the interaction between P2Y12 and NHERF1. The relationship between these findings and the earlier reports remains to be seen. Reports of LTE4-induced internalization have not been published to date.
Lipoxin receptors
Introduction
Lipoxins represent a family of metabolites that are either directly or indirectly produced by lipoxygenases. Lipoxin A4 (LXA4) and lipoxin B4 (LXB4) are produced from arachidonic acid first through the action of 5-lipoxygenase to produce LTA4 that is then converted to both LXA4 and LXB4 by 12-lipoxygenase (Fig. 1). Both lipoxins induce anti-inflammatory and pro-resolution mechanisms including repression of leukocyte-mediated injury and pro-inflammatory cytokine production, as well as inhibition of cell proliferation and migration (Tang et al. 1996; Maderna and Godson 2003). These lipoxins are potent activators of monocytes and stimulate chemotaxis and adherence (Maddox and Serhan 1996; Maddox et al. 1997). The 15(R)-epimers of LXA4 and LXB4 (15-epi-LXA4 and 15-epi-LXB4) are produced from arachidonic acid by aspirin-acetylated cyclooxygenase 2 (COX-2) forming 15(R)-HPETE which then is converted by 5-lipoxygenase into 5(S),6(S)-epoxy-15(R)-ETE and then in turn is enzymatically converted to 15-epi-LXA4 and 15-epi-LXB4 (Romano 2010, 2006) (Fig. 1). Although 15-epi-LXA4 has been shown to possess anti-inflammatory activity like LXA4 (Romano 2010; Gewirtz et al. 1998; Kain et al. 2017), the anti-inflammatory activity of 15-epi-LXB4 is somewhat different from that observed for LXB4 (Maddox et al. 1998). ALX/FPR2 is the primary receptor of LXA4, however, receptors for LXB4 or its 15-epi derivative have yet to be identified.
N-formyl Peptide receptor 2 (FPR2/ALX)
Introduction
Human N-formyl peptide receptor 2 (hFPR2/ALX), is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. It binds to and is activated by LTA4, the aspirin-triggered 15(R)-epimer 15-epi-LTA4, Resolvin D1 (discussed below), and various signal peptides such as annexin in the low µM region (Figs. 1 and 2). This review will only cover the former oxidized lipids. Readers interested in binding and activation by peptides and proteins are referred to the excellent review by Cattaneo et al. (2013). Stimulation of this receptor with eicosanoids is complex and is dependent on cell type and whether homo- or hetero-dimers are present on the plasma membrane (Filep 2013). The unifying theme, however, is the activation of anti-inflammatory/pro-resolving pathways, including leukocyte trafficking, control of neutrophil apoptosis, and macrophage efferocytosis (Cooray et al. 2013).
hFPR2/ALX (ALX, ALX/FPR2, FPRH1, FPRL1, LXA4R, Lipoxin A4 receptor, UniProtKB- P25090) is translated as a 351 amino acid polypeptide with a calculated molecular weight of 39.0 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 27 residues and a cytosolic C-terminal with 45 residues. There are no additional isoforms and one coding SNP variant with unknown biological impact reported (Landrum et al. 2016). There are two reported X-ray structures (PDB entry 6LW5 and 6OMM). Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally. However, N-glycosylation in general has been demonstrated for this receptor, but the specific site has not been confirmed (Chiang et al. 2000).
Expression and characterization
hFPR2/ALX is expressed in high amounts in the immune system and in granulocytes as well as lower expression in other tissues including lung and brain (Uhlén et al. 2015). It binds to and is activated by LTA4, 15-epi-LTA4 and Resolvin D2 (discussed below) in the sub nM range (Figs. 1 and 2, Table 4). Lipid and peptide ligands bind to distinctively different binding sites (Chiang et al. 2006) for which the lipid binding site has been shown to modulate the signaling initiated by the peptide binding site.
Mechanism of cell activation
The mechanism by which cells respond to hFPR2/ALX stimulation is a function of cell type and is context-dependent in terms of formation of homo- and hetero-dimers on the plasma membrane. For example, when LXA4 binds to hFPR2/ALX in human monocytes the cells are activated, whereas in neutrophils the cells are inhibited (Maddox et al. 1997), albeit leading to the same result, resolution of inflammation. Only G-protein coupling through Gαi has been reported for both plasma membrane associated (Le et al. 2002) and nuclear membrane associated hFPR2/ALX where signaling proceeds through an increase in [Ca2+]i. Although all reported signaling mechanisms involve an increase in [Ca2+]i, the calcium source and magnitude of signaling is cell dependent. In human monocytes and goblet cells, a significant portion of the mobilized calcium comes from external sources (Romano et al. 1996; Hodges et al. 2017), whereas in neutrophils [Ca2+]i appear to be regulated intracellularly (Hodges et al. 2017). Complementary to this is the observation, LXA4 stimulates a robust increase in [Ca2+]i in human monocytes and goblet cells, whereas in PMNs only a small increase in [Ca2+]i is observed (Hodges et al. 2017). Similar results are obtained from rat goblet cells when stimulated with Resolvin D1 (RvD1) where the cellular response is mediated by multiple signaling systems including PLC, phospholipase D, and phospholipase A2 and their signaling components ERK1/2 and calmodulin kinase (Lippestad et al. 2017).
15-epi-LXA4 has been shown to regulate the response initiated by the synthetic peptide WKYMVm that binds to a separate peptide binding site on hFPR2/ALX (Ge et al. 2020). Utilizing hFPR2/ALX expressed in HEK293 cells, both 15-epi-LXA4 and WKYMVm were both individually found to reduce cAMP with effective concentrations in the sub nM range, the effect of the former much weaker than the latter. Both were also individually found to increase [Ca2+]i with WKYMVm effective in the sub nM range and 15-epi-LXA4 only effective in the 10’s of mM range. However, when the cells are challenged with 10 nm WKYMVm, the minimum concentration necessary to elicit the greatest effect on [Ca2+]i and cAMP, increasing concentrations of 15-epi-LXA4 modulate the WKYMVm signal in a biphasic manner. In the concentration range of 1 to 100 pM 15-epi-LXA4 reduces the WKYMVm signal to a maximum of 20%. With increasing concentrations in the 1 nm to 1 μM range the WKYMVm signal increased to a maximum of 95% at 1 μM. Clearly 15-epi-LXA4 serves to modulate the WKYMVm signal in an anti-inflammatory mode where the second phase reflects a weak agonistic activity of the ligand itself.
Agonist binding has been shown to facilitate dimer formation which can lead to different signaling pathways depending on whether the dimer is a homodimer of FPR2/ALX or a heterodimer with some other receptor. Agonist binding to cells transfected with only hFPR2/ALX results in the formation of homodimers which in turn signal through a p38/MAPK/MAPKAPK/Hsp27 signaling pathway, ultimately leading to an increase in the production of IL-10, a known anti-inflammatory cytokine (Cooray et al. 2013; Filep 2013). On the other hand, cells transfected with both hFPR2/ALX and human formyl peptide receptor 1 (FPR1) form heterodimers in the presence of agonist and show a preference for the JNK/Caspase 3 pathway which leads to cell apoptosis (Cooray et al. 2013).
Regulation
N-glycosylation is often found to direct plasma membrane expression and intracellular trafficking of receptors. However, for FPR2/ALX expressed in HEK293 cells, deglycosylation significantly reduces the affinity for peptide substrates, but has no effect on lipid effectors (Chiang et al. 2000). This also supports the observation that peptides and lipids have separate binding sites on this receptor.
Agonist-induced internalization is a common pathway for regulating expression of GPCRs. The two common modes for internalization involve either clatherin-coated pits or the formation of caveolae. LXA4-induced internalization of murine FPR2/ALX transfected into HEK and human PMN cells proceeds via the formation of caveolae and internalization requires PKC, suggesting a potential receptor phosphorylation event (Maderna et al. 2010). In contrast, FPR2/ALX transfected into HEK293 cells are internalized via clatherin-mediated dynamin-dependent modality when stimulated with a peptide agonist (Huet et al. 2007).
Regulation at the gene level has also been presented. Primary human monocytes produce large amounts of hFPR2/ALX mRNA and express hFPR2/ALX on their plasma membranes whereas macrophages do not. This is due to the fact that monocytes lose the ability to produce hFPR2/ALX upon differentiation into macrophages. However, stimulation of macrophages with either the INF-γ cytokine or LPS results in a > 80% increase in hFPR2/ALX mRNA, but no increase in translation. This loss in ability to produce hFPR2/ALX is explained by the fact that monocytes and macrophages utilize different promotors for hFPR2/ALX transcription, and the promotor used by macrophages results in a longer 5’-UTR that results in reduced translation (Waechter et al. 2012).
Resolvin receptors
Introduction
Resolvins are oxidized products of ω3 PUFAs that are actively involved in the resolution of inflammation. The D series resolvins are derived from eicosapentaenoic acid (EPA), the E series from docosahexaenoic acid (DHA), and the T series from DHA in aspirin-treated cells; aspirin-acetylated COX-2 alters the pathway for the series. A complete description of all these specialized pro-resolving mediators (SPMs) is beyond the scope of this review. Instead, the focus will be on a few classes, namely the Resolvins D1, D2, E1 and E2 which have been studied to the greatest extent.
N-Arachidondyl Glycine receptor, GPR18
Introduction
Human N-arachidondyl glycine receptor (hGPR18), is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. It binds with high affinity and is activated by Resolvin D2 (RvD2), N-arachidondyl glycine (NAGly) and other endocannabinoids, and to a lesser extent, but at biologically relevant concentrations of Δ9-tetrahydrocannabinol (Δ9-THC). Activation of GPR18 initiates anti-inflammatory/pro-resolving pathways (Serhan and Petasis 2011). The potential for the GPR18 receptor’s role in the pharmacology of pain has also been suggested (Guerrero-Alba et al. 2019).
hGPR18 (GPR18/DRV2, GPCRW, NAGly, UniProtKB- Q14330) is translated as a 331 amino acid polypeptide with a calculated molecular weight of 38.1 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 26 residues and a cytosolic C-terminal with 42 residues. There are no additional isoforms and no coding SNP variants reported (Landrum et al. 2016). There are no reported X-ray structures. There is one potential structural model template, an apelin receptor in complex with agonist peptide (23.9% sequence homology, PDB entry 5VBL) selected by the SWISS-MODEL website (Waterhouse et al. 2018) that should serve as a working model. Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally.
Expression and characterization
hGPR18 is expressed in most tissues with high amounts expressed in lymphoid and bone tissue, and blood with lesser, but significant amounts in testes and vagina (Uhlén et al. 2015). The primary natural agonist for this receptor is RvD2 with a Kd of 9.6 nm, but also binds to Δ9-THC with EC50s in the µM range (Fig. 2, Table 5) (Chiang et al. 2015; Rempel et al. 2014; McHugh et al. 2012).
Table 5.
Resolvin receptors
| GPR18 | Ki /Kd(nM) | References | |||||
|---|---|---|---|---|---|---|---|
| Receptor/Expression | Parameter measured | RvD2 | CBD | Δ9-THC | |||
| hGPR18/CHOa | Bind | 9.6 | – | – | – | Chiang et al. (2015) | |
| EC50/IC50 (nM) | |||||||
|---|---|---|---|---|---|---|---|
| Receptor | Parameter measured | RvD2 | CBD | Δ9-THC | NAGly | References | |
| hGPR18/CHOb | β-arrestin | 0.0002 | – | – | – | Chiang et al. (2015) | |
| hGPR18/CHOb | β-arrestin | – | – | 4,610 | – | Rempel et al. (2014) | |
| hGPR18/HEK293c | MAPK | – | – | 960 | – | Rempel et al. (2014) | |
| hGPR18/CHOb | β-arrestin | – | – | 14,200 | – | Rempel et al. (2013) | |
| hGPR18/HEK293c | MAPK | – | 51,000 | 960 | 45 | McHugh et al. (2012) | |
| hGPR18/CHOd | cAMP↓ | – | 20 | Kohno et al. (2006) | |||
| GPR32 | Ki /Kd(nM) | References | |||||
|---|---|---|---|---|---|---|---|
| Receptor/Expression | Parameter measured | RvD1 | AT-RvD1 | DHA | LXA4 | ||
| hGPR32/CHOe | Bind | 0.17 | – | – | – | Krishnamoorthy et al. (2010) | |
| EC50/IC50 (nM) | |||||||
| hGPR32/CHOb | β-arrestin | 0.0036 | 0.0088 | 0.22 | – | Krishnamoorthy et al. (2012) | |
| hGPR32/CHOb | β-arrestin | – | – | – | 0.034 | Krishnamoorthy et al. (2010) | |
| CMKLR1 | Ki /Kd(nM) | References | |||||
|---|---|---|---|---|---|---|---|
| Receptor/Expression | Parameter measured | RvE1 | 18(S)-RvE1 | RvE2 | chemerin | LTB4 | |
| hCMKLR1/CHOf | Bind | 11.3 | – | – | – | – | Arita et al. (2005) |
| hCMKLR1/CHOf | Bind | 330 | – | – | 429 | > 10,000 | Arita et al. (2007) |
| hCMKLR1/CHO-K1g | Disp | – | – | – | 0.56 | – | de Poorter et al. (2013) |
| EC50/IC50 (nM) | |||||||
| hCMKLR1/CHOb | β-arrestin | 0.137 | 0.00633 | – | – | – | Oh et al. (2011) |
| hCMKLR1/HEK293h | GTPγS | 3 | – | – | – | – | Arita et al. (2005) |
| hCMKLR1/HEK293i | luciferase | 6 | – | – | 20 | – | Arita et al. (2005) |
| hCMKLR1/CHOj | Akt-P | 0.5 | – | – | 0.9 | – | Ohira et al. (2010) |
| hCMKLR1/CHOb | β-arrestin | 0.013 | – | 0.09 | – | – | Oh et al. (2012) |
| hCMKLR1/CHO-K1k | Ca2+↑ | – | – | – | 0.31 | – | de Poorter et al. (2013) |
Agonist parameters given for Human (h) receptors in the indicated cell line. Parameter measured: Akt-P, phosphorylation of Akt; β-arrestin, β-arrestin binding; bind, binding assay; Ca2+↑, increase in [Ca2+]i; cAMP↓, decrease in forskolin induced cAMP accumulation; disp, displacement assay; GTPγS, binding [35S]GTPγS; luciferase, luciferase assay; MAPK, MAP kinase activation assay. Cell type abbreviations: CHO, Chinese hamster ovary cells; CHO-K1, subclone from the parental CHO cell line; HEK293, Human embryonic kidney 293 cells. Agonist abbreviations: RvE1, resolvin E1; RvE2, resolvin E2; LTB4, leukotriene B4. a) Kd for binding [3H]RvD2. b) β-arrestin recruitment assay. c) MAP kinase assay. d) Reduction of forskolin-induced cAMP. e) Kd for binding [3H]RvD1. f) Kd for binding [3H]RvE1. g) Competitive binding of chemerin for bound [125I]-chemerin.h) Estimated from plot of [35S]-GTPgS binding. i) Estimated from plot of the inhibition of TNF-α induced NB-κB luciferase activity. j) Estimated from plot for increase in Akt phosphorylation. k) Determined from increase in [Ca2+]i
Mechanism of cell activation
The mechanism by which cells respond to GPR18 stimulation is a function of cell type, cell line, and specific agonist, and is context-dependent with respect to available G-proteins and potential heterodimer partner. When hGPR18 is transfected into CHO cells, stimulation with NAGly leads to an increase in [Ca2+]i and a decrease in cAMP production, indicating coupling through Gαi due to its PTX sensitivity (Kohno et al. 2006). Although these results have been reported in several publications, there are others that report the inability to initiate signaling by NAGly (Rempel et al. 2014; Finlay et al. 2016). It has been suggested that the lack of activity might be due to the absence of a required interacting protein in the system utilized (Finlay et al. 2016). Stimulation with RvD2 appears to be coupled through a Gαs-like proteins due to its cholera toxin (CTX) sensitivity (Chiang et al. 2015). In mouse and human macrophages, RvD2 stimulation of GPR18 results in an increase in cAMP, fostering a cAMP-PKA signaling pathway that is also coupled through a Gαs, leading to enhanced macrophage phagocytosis (Chiang et al. 2017). The same RvD2-induced signaling pathway is also observed in rat conjunctival goblet cells (Botten et al. 2019).
As observed for other eicosanoid receptors, GPR18 can form heterodimers with other receptors. For example, hGPR18 forms a heterodimer with the human cannabinoid receptor CB2R when co-transfected. Rather than exhibiting a synergic effect upon stimulation with cannabinoids as observed for CB2R transfected along with CB1R, the GPR18/CB2R heterodimer exhibits a reduced response (Reyes-Resina et al. 2018).
As noted above, RvD2/GPR18 signaling proceeds through a cAMP/PKA pathway. This in turn leads to enhanced phosphorylation of cAMP response element-binding protein (CREB), ERK1/2 and the signal transducer and activator of transcription 3 (STAT3). PKA and STAT3 notably enhance macrophage phagocytosis of bacteria (Chiang et al. 2017) and PMN apoptosis as well as reducing PMN infiltration (Chiang et al. 2015) in keeping with this receptor’s involvement in resolution of inflammation. Further, in a murine cecal ligation and puncture (CLP) model, RvD2 stimulation reduces levels of inflammatory cytokines IL-6, IL-10, IL-1β, IL-23, IL17 and TNF α as well as reducing pro-inflammatory mediators PGE2 and LTB4 (Spite et al. 2009). NAGly stimulation of GPR18 also enhances production of LXA4 (Chiang et al. 2015). A known pro-resolving, anti-inflammatory eicosanoid.
Regulation
Regulation of GPR18 is not clearly understood. β-arrestin binding, a common mode to initiate agonist-induced receptor internalization, is a function of which agonist binds to GPR18. Both RvD2 (Chiang et al. 2015) and Δ9-THC initiate β-arrestin binding whereas NAGly does not (Console-Bram et al. 2014; Yin et al. 2009). To date, neither quantitation of nor mechanism for the event has been reported. However, hGPR18 expressed in HEK cells exhibits a rapid, constitutive trafficking from cytosolic stores to the plasma membrane, however, the surface expression is significantly lower than the total hGPR18 cellular content suggesting some form of control mechanism (Finlay et al. 2016).
Transcriptional regulation of GPR18-like signaling is also cell type dependent. mGPR18 mRNA expression in mouse macrophages is inducible and increases 52-fold after treatment of murine bone marrow-derived macrophages (BMM) with LPS, whereas the expression in murine thioglycolate-elicited peritoneal macrophages (TEPM) is unaffected and thus constitutively expressed (Lattin et al. 2008). Out of 75 orphan GPCRs, hGPR18 is the most abundantly over-expressed GPCR in human melanomas. Further, hGPR18 is constitutively active in melanomas and serves to inhibit apoptosis, indicating that it has an important role in the tumor cell survival (Qin et al. 2011).
G-Protein receptor 32, GPR32
Introduction
Human G-protein Receptor 32 (hGPR32) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. It binds with high affinity and is activated by Resolvin D11 (17S, RvD1) and its aspirin-triggered 17R isomer AT-RvD1, both in the pM range, as well as LXA4 and DHA in the sub nM range (Table 5, Figs. 1 and 2). Activation initiates anti-inflammatory/pro-resolving pathways. (Krishnamoorthy et al. 2010; Krishnamoorthy et al. 2011; Serhan et al. 2011).
hGPR32 (Probable G-protein coupled receptor 32, DRV1, UniProtKB- O75388, UniProtKB- H9NIL6) is translated as a 356 amino acid polypeptide with a calculated molecular weight of 40.1 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 44 residues and a cytosolic C-terminal with 36 residues. There are no additional isoforms and one coding SNP variant F327L with unknow biological effect (Stelzer et al. 2016). No X-ray structures are available. There is a potential structural model template cryo-EM structure of formyl peptide receptor 2/lipoxin A4 receptor in complex with Gαi (40.7% sequence homology, PDB entry 6OMM) selected by the SWISS-MODEL website (Waterhouse et al. 2018) that should serve as a working model. Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally.
Expression and characterization
hGPR32 is expressed high amounts in parathyroid tissue and low amounts in male and female tissue and cells of the immune system (http://proteinatlas.org, Uhlén et al. 2015). The primary natural agonists for this receptor are RvD1 with an EC50 of 3.6 pM, AT-RvD1 with an EC50 of 8.8 pM, LXA4 with an EC50 of 34 pM, but also binds DHA with an EC50 of 220 pM (Figs. 1 and 2, Table 5) (Krishnamoorthy et al. 2010, 2012).
Mechanism of cell activation
Activation of hGPR32 with RvD1 in human PMNs blocks actin polymerization (Krishnamoorthy et al. 2010) thereby reducing mobility as expected for a pro-resolving response. Sensitivity to PTX and insensitivity to CTX indicates that the signaling likely proceeds through a Gαi/o mediated pathway. No change in [Ca2+]i is observed as expected for the proposed path, but no change in cAMP levels is observed as would be expected for this pathway. However, RvD1 stimulation of human macrophages that naturally express hGPR32 results in an increase in [Ca2+]i and a change in phenotype towards a pro-resolving M2 phenotype with a reduction in secreted pro-inflammatory cytokines (e.g., IL-1β and IL-8), enhanced phagocytosis, and blocked chemotaxis towards chemoattractants (Schmid et al. 2016). RvD1 stimulation of hGPR32 transfected into human macrophages also enhances phagocytosis (Krishnamoorthy et al. 2010). Similarly, RvD1 stimulation of human goblet cell GPR32 activates extracellular EGFR and phospholipases D, C and A2 which lead to an increase in [Ca2+]i (Dartt et al. 2019). Stimulation also results in activation of extracellular regulated kinase (ERK) 1/2 leading to mucin secretion into the tear film to protect ocular tissue from the environment. At the same time, both RvD1 and AT-RvD1 stimulate GPR32 in conjunctival goblet cells to prevent histamine-stimulated H1 receptor response thus reducing inflammation (Li et al. 2013). Clearly, signaling through GPR32 is complex and at the very least, dependent on cell type and perhaps yet-to-be discovered partners in heteromers.
As noted above, the expression of GPR32 in parathyroid tissue far exceeds that of any other tissue. However, to date there are no reports describing the mechanism or action of GPR32 signaling in this tissue. The only relationship thus far elucidated is that parathyroid hormone (PTH), the major product of the parathyroid gland induces the production of resolvins, including RvD1 to induce efferocytosis of apoptotic osteoblasts (McCauley et al. 2014). It is possible that PTH localized about the parathyroid glands may induce the same effect to prevent the onset of inflammation-induced hyperthyroidism. See Talat et al. (2011) for a discussion of inflammation of the parathyroid gland.
Regulation
Regulation of GPR32 has yet to be described in detail. It is known that RvD1 stimulates receptor reuptake of both hGPR32 and hFPR2/ALX, albeit the uptake of hGPR32 occurs at lower concentrations of agonist and hFPR2/ALX uptake occurs only after stimulation with TNF-α. Interestingly, the surface expression of hFPR2/ALX, but not hGPR32 is up-regulated in the presence of pro-inflammatory regulators (e.g., TNF-α and IL-8). This suggests that hGPR32 is constitutively expressed to convey normal physiological functions (Norling et al. 2012) and hFPR2/ALX responds to acute conditions. In direct support of this hypothesis, the pro-inflammatory mi-RNA-181b down-regulates the surface expression of hFPR2/ALX on human macrophages but has no effect on hGPR32 surface expression (Pierdomenico et al. 2015).
Chemokine-like receptor 1 (CMKLR1)
Introduction
Human Chemokine-like receptor 1 (hCMKLR1), is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. It binds with high affinity and is activated by Resolvin E1 (18(S)-Resolvin E1, RvE1), its aspirin-triggered 18(R)-isomer AT-RvE1 with EC50s in the sub-nM and pM range respectively as well as the chemerin peptide in the low nM range (Table 5, Fig. 2) (Oh et al. 2011; Arita et al. 2005; Ohira et al. 2010). Noteworthy is the fact that although RvE1 binds to the LTB4 receptor LTB4R with a comparable EC50 and that LTB4 binds poorly to hCMKLR1 (Serhan et al. 2011).
hCMKLR1 (hChemR23, DEZ, UniProtKB- Q99788) is translated as a 373 amino acid polypeptide with a calculated molecular weight of 42.3 kDa. The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 41 residues and a long cytosolic C-terminal with 53 residues. There is one confirmed additional isoform (isoform B) that is missing residues 1–2 and there are two coding SNP variants with unknown biological impact reported: M287L, and M289I (Landrum et al. 2016). There are no reported X-ray structures. There is a potential structural model template cryo-EM structure of formyl peptide receptor 2/lipoxin A4 receptor in complex with Gαi (27.8% sequence homology, PDB entry 6OMM) selected by the SWISS-MODEL website (Waterhouse et al. 2018) that should serve as a working model. Predicted posttranslational modifications (PTMs) are presented in the supplement Table S1, none of which have been confirmed experimentally. Only indirect evidence for phosphorylation at S345 and S349 has been presented (Oh et al. 2012).
Expression and characterization
hCMKLR1 is expressed in most tissues with particularly high expression in bone and lymphoid tissue, brain, blood and female tissues (Uhlén et al. 2015). The primary lipid-derived natural agonists are RvE1, AT-RvE1, and RvE2 with EC50s in the sub-nM to low nM range (Fig. 2, Table 5). The peptide chemerin is also a strong agonist with EC50s in the nM range.
Mechanism of cell activation
The cellular response to CMKLR1 activation is agonist and cell-type dependent. Activation of CMKLR1 by either RvE1 or RvE2 elicits similar pro-resolving effects, however, RvE2 is less efficacious and less potent than RvE1, suggesting that this receptor might not be the primary receptor for RvE2 (Oh et al. 2012; Tjonahen et al. 2006). The peptide chemerin can be competed out by RvE1 for hCMKLR1 expressed in HEK cells, indicating that the two agonists bind to the same site on the receptor, however, the response from the chemerin-induced G-protein coupling is nearly 3 times larger than RvE1 and induces a different cellular response. Further, RvE1 stimulates strong NF-κB inhibition and does not promote receptor-initiated increases in extracellular acidification rates (EARs) whereas chemerin does evoke EARs and is ten times weaker in NK-κB inhibition (Arita et al. 2005). Additionally, hCMKLR1 is naturally expressed in human chondrocytes where it initiates a pro-inflammatory rather than a pro-resolving response, producing enhanced levels of pro-inflammatory cytokines including IL-6, IL-8, TNF-α and IL-1β (Berg et al. 2010). Murine plasmacytoid dendridic cells (pDCs) express mCMKLR1 and are recruited to areas of infection by chemerin released by cells in the affected area where the pDCs release the interferons INF-α and INF-β that serve to enhance the inflammatory response (Bondue et al. 2011).
The signaling pathways for RvE1 are not well defined. Thus far it has been shown that RvE1 stimulation of hCMKLR1 expressed in CHO cells do signal through a PI3K/Akt/mTOR pathway (Ohira et al. 2009). Further, hCMKLR1 signaling is PTX sensitive suggesting a Gαi dependent signaling pathway (Arita et al. 2005). Signaling pathways for chemerin, however, are considerably more well defined. Human chemerin and various C-terminal peptides of chemerin (e.g. chemerin 9) stimulate hCMKLR1 with EC50s in the low nM range which lead to coupling with Gαi/o resulting in an increase in [Ca2+]i (Wittamer et al. 2004). It has since been shown that human chemerin 9 (residues 149–157) produces coupling to three subtypes of Gαi (Gαi1, Gαi2, Gαi3) and two isoforms of Gα0 (Gα0a, Gα0b) (De Henau et al. 2016). In human fibroblast-like synoviocytes (FLS) taken from rheumatoid arthritis patients express hCMKLR1 and when stimulated with chemerin, activation of ERK1/2, p38MAPK and Akt is enhanced which in turn induces IL-6 production (Kaneko et al. 2011).
Regulation
Increased hCMKLR1 transcription and subsequent protein expression increases during the differentiation of monocytes to macrophages and is further enhanced in classically activated human M1 macrophages. Further, transcription levels increase after treatment of human monocytes with LPS or inflammatory cytokines (Herová, 2015). Similar results have been reported by others (Arita et al. 2005).
Agonist-induced internalization of CMKLR1 by RvE1 or RvE2 is currently unknown. However, Chemerin-induced internalization has been examined. Downregulation of the surface expression for hCMKLR1 by chemerin is facilitated through an agonist-dependent and clatherin-independent process. Chemerin and the active chemerin-derived nonapeptide chemerin 9 induce internalization of hCMKLR1 expressed in HEK293 cells in a dose-dependent manner with chemerin 9 causing up to 90% internalization (Zhou et al. 2014). Ser to Ala mutations of six potential phosphorylation sites reveal that phosphorylation of S345 and S349 (S343 and S347 in isoform B) are required for internalization to occur. Internalization proceeds through a caveolae-mediated rather than a clatherin-mediated process (Oh et al. 2012). Chemerin 9 and chemerin stimulation of hCMKLR1 expressed in CHO-K1 promotes binding of β-arrestin 1 and 2 as well as internalization, suggesting an arrestin-mediated, clatherin-dependent internalization (De Henau et al. 2016). However, the kinetics of arrestin binding and receptor down-regulation do not correlate, the latter being faster, suggesting another mode for internalization is at work.
Other resolvin effects
Resolvins also serve as agonists, antagonists or antagonist/partial-agonists for several receptors. RvD1 is a potent agonist for FPR2/ALX (see above) with EC50 values comparable to that of the primary agonist LXA4 (Lippestad et al. 2017; Krishnamoorthy et al. 2012, 2010). RvE1 and RvE2 serve as equally potent antagonist/partial-agonists for the LTB4 receptor LTB4R and thus compete with LTB4 but activate the receptor to a lesser extent than LTB4 (Fig. 1, Table 2) (Oh et al. 2012). This suggests that the reduced potency/efficacy of RvE2 may merely reflect a similar antagonist/partial-agonist activity for the CMKLR1 receptor. Interestingly, LTB4 does not bind to CMKLR1 (Arita et al. 2007). RvD1, RvD2 and RvE1 differentially inhibit capsaicin-induced pain through the TRPV1 and TRPA1 receptors. RvD2 is the most potent antagonist with an EC50 of 0.1 nM for TRPV1 and 2.1 nM for TRPA1 (Park et al. 2011).
Hepoxilin and Trioxillin receptors
Introduction
Hepoxilins are short-lived monohydroxy-epoxides formed from the reactive 12(S)- and 12(R)-HPETE products of ALOX12 and ALPX12B respectively to Hepoxilin B3 (HxB3) and Hepoxilin A3 (HxA3) respectively (review, Biringer 2018). Hepoxilins are readily converted to their tri-hydroxy counterparts, trioxilins, spontaneously or through the action a soluble epoxy-hydrolases (Cronin et al. 2011). Hepoxilins stimulate both mobilization of intracellular calcium in neutrophils and enhancement of plasma leakage (Pace-Asciak 2015). Hepoxilins also exhibit significant chemotaxis abilities. For example, gradients of HxA3 have been shown to attract neutrophils across epithelial barriers (Kubala et al. 2014). Trioxilins on the other hand, namely TrXA3 and TrXC3 and HxA3 act as thromboxane antagonists and thus help regulate vascular homeostasis (Siangjong et al. 2017).
The receptors for Hepoxilin and Trioxilin
Specific receptors for hepoxilin agonism have been reported. Both HXA3 and HXB3 trigger sustained calcium mobilization in both CHO cells overexpressing each TRPV1 (discussed above) and TRPA1 receptors and in rodent sensory neurons. The net result of hepoxilin agonism is a profound, persistent tactile allodynia as well as a small, transient heat hyperalgesia (Gregus et al. 2012).
Both hepoxilins and trioxilins bind to thromboxane receptors (see Biringer 2020) and serve to function as natural antagonists rather than agonists (Fig. 1, Table 6). Both classes of molecules have been shown to be antagonists of the Thromboxane receptor TP (Fig. 1, Table 6). The stable hepoxilin analog PBT-3 ([10(S)-hydroxy-11,12-cyclopropyl-eicosa-5Z,8Z,14Z-treinoic acid methyl ester) has been shown to effectively compete with the TP agonists I-BOP and U46619 on human platelet TP receptors and reduces agonist-induced platelet aggregation (Pace-Asciak et al. 2002). PBT-3 antagonizes I-BOP induced intracellular calcium release in human platelets with an IC50 of 7 nM. Further, PBT-3 selectively antagonizes the TPα isoform. In transfected COS-7 cells it competes with the strong TP antagonist [3H]SQ 29,548 with an IC50 of 200 nM for the TPα isoform and an IC50 of 1200 nM for the TPβ isoform; the [3H]SQ 29,548 has similar Kd values for each receptor (Kd = 11–12 nM) (Qiao et al. 2003). Similarly, trioxilin A3 (TXA3) and trioxilin B3 (TXB3) antagonize the U46619-induced intracellular calcium increase in HEK293 cells overexpressing human TPα (Siangjong et al. 2017). TXA3, TXB3, and trioxilin C3 (TXC3) induce vasodilation in U46619-constricted mouse mesenteric arteries with EC50 values of 1.26, 7.15, and 1.00 µM respectively. Cis-epoxy isomers of HXA3 (11(R),12(S)- and 12(R),11(S)-HXA3) also vasodilate U46619-constricted mouse mesenteric arteries (Siangjong et al. 2017).
Table 6.
Trioxilin receptors: TP
| Receptor/Expression | Parameter measured | IC50/EC50(nM) | References | |||||
|---|---|---|---|---|---|---|---|---|
| PBT-3 | 11(S),12(R)-HxA3 | 11(R),12(S)-HxA3 | TrXA3 | TrXB3 | TrXC3 | |||
| hTP/human plateletsa | disp | 8.1 | – | – | – | – | – | Pace-Asciak et al. (2002) |
| hTP/human plateletsb | aggregation | 63 | – | – | – | – | – | Pace-Asciak et al. (2002) |
| hTP/human plateletsc | Ca2+↓ | 7 | – | – | – | – | – | Qiao et al. (2003) |
| hTPa/COS-7d | disp | 200 | – | – | – | – | – | Qiao et al. (2003) |
| hTPb/COS-7d | disp | 1200 | – | – | – | – | – | Qiao et al. (2003) |
| mTP/MMAe | vaso | – | 6300 | 6300 | 1260 | 7150 | 1000 | Xue et al. (2011) |
Agonist parameters given for Human (h) and murine (m) receptors in the indicated cell line. Parameters measured: aggregation, platelet aggregation assay;disp, displacement assay; Ca2+↓, inhibition of Ca2 + release; vaso, vasorelaxation assay. Cell type abbreviations: COS-7, African green monkey kidney cell line; MMA, mouse mesenteric arteries. Agonist abbreviations: HxA3, hepoxilin A3; PBT-3: stable hepoxilin analog [10(S)-hydroxy-11,12-cyclopropyl-eicosa-5Z,8Z,14Z trienoic acid methyl ester; TrXA3, trioxilin A3; TrXB3, trioxilin B3; TrXC3, trioxilin C3. a) IC50 for displacement of 125I-BOP. b) IC50 for inhibition of I-BOP-induced platelet aggregation. c) IC50 for inhibition of calcium release by I-BOP. d) IC50 for displacement of the TP antagonist [3H]29,548 (Kd = 11–12 nM). e) EC50 for vasorelaxation of U46619 (stable TP agonist) induced vasoconstriction of mouse mesenteric arteries (MMA)
Conclusions
In this review a summary of what is known about the expression, characterization, regulation, and mechanism of action of non-prostanoid, eicosanoid receptors with a focus on human receptors is presented. These receptors control numerous biological functions not only through the diversity in eicosanoids themselves, but through the diversity of receptors, heterodimers, and the heterogeneity of G-protein coupling. Commonality is found in regulation where agonist-induced receptor desensitization is accomplished by phosphorylation by PKA, PBK, PKC or a variety of different GRKs. Although much is known about eicosanoid receptor signaling, there are still many missing pieces, in particular with respect to posttranslational modifications and the associated functions thereof. It is our hope that this review will help emphasize these areas and stimulate research to identify these missing pieces of the puzzle.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of Interest
The author declares that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolfsson JL, Ohd JF, Sjölander A. Leukotriene D4-induced activation and translocation of the G-protein alpha i3-subunit in human epithelial cells. Biochem Biophys Res Commun. 1996;226:413–419. doi: 10.1006/bbrc.1996.1370. [DOI] [PubMed] [Google Scholar]
- Aizawa F, Nishinaka T, Yamashita T, Nakamoto K, Kurihara T, Hirasawa A, Kasuya F, Miyata A, Tokuyama S. GPR40/FFAR1 deficient mice increase noradrenaline levels in the brain and exhibit abnormal behavior. J Pharmacol Sci. 2016;132:249–254. doi: 10.1016/j.jphs.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Andrew W, Martino B, Stefan B, Gabriel S, Gerardo T, Rafal GH, Florian T, de Beer TAP, Christine R, Lorenza B, Rosalba L, Torsten S. SWISS-MODEL homology modelling of protein structures and complexes. Nucleic Acids Research. 2018;46(1):296–303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratake Y, Okuno T, Matsunobu T, Saeki K, Takayanagi R, Furuya S, Yokomizo T. Helix 8 of leukotriene B4 receptor 1 inhibits ligand-induced internalization. FASEB J. 2012;26:4068–4078. doi: 10.1096/fj.12-212050. [DOI] [PubMed] [Google Scholar]
- Arcemisbéhère L, Sen T, Boudier L, Balestre MN, Gaibelet G, Detouillon E, Orcel H, Mendre C, Rahmeh R, Granier S, Vivès C, Fieschi F, Damian M, Durroux T, Banères JL, Mouillac B. Leukotriene BLT2 receptor monomers activate the G(i2) GTP-binding protein more efficiently than dimers. J Biol Chem. 2010;285:6337–6347. doi: 10.1074/jbc.M109.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Austen KF, Maekawa A, Kanaoka Y, Boyce JA. The leukotriene E4 puzzle: finding the missing pieces and revealing the pathobiologic implications. J Allergy Clin Immunol. 2009;124:406–414. doi: 10.1016/j.jaci.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäck M, Bu DX, Bränström R, Sheikine Y, Yan ZQ, Hansson GK. Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc Natl Acad Sci U S A. 2005;102:17501–17506. doi: 10.1073/pnas.0505845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankova LG, Lai J, Yoshimoto E, Boyce JA, Austen KF, Kanaoka Y, Barrett NA. Leukotriene E4 elicits respiratory epithelial cell mucin release through the G-protein-coupled receptor, GPR99. Proc Natl Acad Sci U S A. 2016;113:6242–6247. doi: 10.1073/pnas.1605957113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurand A, Eckly A, Hechler B, Kauffenstein G, Galzi JL, Cazenave JP, Léon C, Gachet C. Differential regulation and relocalization of the platelet P2Y receptors after activation: a way to avoid loss of hemostatic properties? Mol Pharmacol. 2005;67:721–733. doi: 10.1124/mol.104.004846. [DOI] [PubMed] [Google Scholar]
- Beller TC, Friend DS, Maekawa A, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc Natl Acad Sci U S A. 2004;101:3047–3052. doi: 10.1073/pnas.0400235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benítez-Angeles M, Morales-Lázaro SL, Juárez-González E, Rosenbaum T. TRPV1: structure, endogenous agonists, and mechanisms. Int J Mol Sci. 2020;21:3421. doi: 10.3390/ijms21103421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg V, Sveinbjörnsson B, Bendiksen S, Brox J, Meknas K, Figenschau Y. Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin(21–157) Arthritis Res Ther. 2010;12:R228. doi: 10.1186/ar3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci U S A. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda C, Génier S, Cartier A, Larrivée JF, Stankova J, Young JC, Parent JL. A G protein-coupled receptor and the intracellular synthase of its agonist functionally cooperate. J Cell Biol. 2014;204:377–393. doi: 10.1083/jcb.201304015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biringer RG. The enzymes of the human eicosanoid pathway. Res Rep Med Sci. 2018;2:106. doi: 10.6084/m9.figshare.11649351. [DOI] [Google Scholar]
- Biringer RG. A review of prostanoid receptors: expression, characterization, regulation, and mechanism of action. J Cell Commun Signal. 2020 doi: 10.1007/s12079-020-00585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- Bochnowicz S, Underwood DC. Dose-dependent mediation of leukotriene D4-induced airway microvascular leakage and bronchoconstriction in the guinea pig. Prostaglandins Leukot Essent Fatty Acids. 1995;52:403–411. doi: 10.1016/0952-3278(95)90069-1. [DOI] [PubMed] [Google Scholar]
- Bodor ET, Waldo GL, Hooks SB, Corbitt J, Boyer JL, Harden TK. Purification and functional reconstitution of the human P2Y12 receptor. Mol Pharmacol. 2003;64:1210–1216. doi: 10.1124/mol.64.5.1210. [DOI] [PubMed] [Google Scholar]
- Boehmler AM, Drost A, Jaggy L, Seitz G, Wiesner T, Denzlinger C, Kanz L, Möhle R. The CysLT1 ligand leukotriene D4 supports alpha4beta1- and alpha5beta1-mediated adhesion and proliferation of CD34+ hematopoietic progenitor cells. J Immunol. 2009;182:6789–6798. doi: 10.4049/jimmunol.0801525. [DOI] [PubMed] [Google Scholar]
- Bondue B, Vosters O, de Nadai P, Glineur S, De Henau O, Luangsay S, Van Gool F, Communi D, De Vuyst P, Desmecht D, Parmentier M. ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS Pathog. 2011;7:e1002358. doi: 10.1371/journal.ppat.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botten N, Hodges RR, Li D, Bair JA, Shatos MA, Utheim TP, Serhan CN, Dartt DA. Resolvin D2 elevates cAMP to increase intracellular [Ca2+] and stimulate secretion from conjunctival goblet cells. FASEB J. 2019;33:8468–8478. doi: 10.1096/fj.201802467R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR, Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- Brochu-Bourque A, Véronneau S, Rola-Pleszczynski M, Stankova J. Differential signaling defects associated with the M201V polymorphism in the cysteinyl leukotriene type 2 receptor. J Pharmacol Exp Ther. 2011;336:431–439. doi: 10.1124/jpet.110.172411. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purine and purinergic receptors. Brain Neurosci Adv. 2018;2:2398212818817494. doi: 10.1177/2398212818817494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye AP, Unsworth AJ, Gibbins JM. Platelet signaling: a complex interplay between inhibitory and activatory networks. J Thromb Haemost. 2016;14:918–930. doi: 10.1111/jth.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra V, Nicosia S, Ragnini D, Mezzetti M, Keppler D, Rovati GE. Identification and characterization of two cysteinyl-leukotriene high affinity binding sites with receptor characteristics in human lung parenchyma. Mol Pharmacol. 1998;5:750–758. doi: 10.1124/mol.53.4.750. [DOI] [PubMed] [Google Scholar]
- Capra V, Accomazzo MR, Ravasi S, Parenti M, Macchia M, Nicosia S, Rovati GE. Involvement of prenylated proteins in calcium signaling induced by LTD4 in differentiated U937 cells. Prostaglandins Other Lipid Mediat. 2003;71:235–251. doi: 10.1016/s1098-8823(03)00045-5. [DOI] [PubMed] [Google Scholar]
- Capra V, Ravasi S, Accomazzo MR, Citro S, Grimoldi M, Abbracchio MP, Rovati GE. CysLT1 receptor is a target for extracellular nucleotide-induced heterologous desensitization: a possible feedback mechanism in inflammation. J Cell Sci. 2005;118(Pt 23):5625–5636. doi: 10.1242/jcs.02668. [DOI] [PubMed] [Google Scholar]
- Carnini C, Accomazzo MR, Borroni E, Vitellaro-Zuccarello L, Durand T, Folco G, Rovati GE, Capra V, Sala A. Synthesis of cysteinyl leukotrienes in human endothelial cells: subcellular localization and autocrine signaling through the CysLT2 receptor. FASEB J. 2011;25:3519–3528. doi: 10.1096/fj.10-177030. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cattaneo F, Parisi M, Ammendola R. Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists. Int J Mol Sci. 2013;14:7193–7230. doi: 10.3390/ijms14047193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212:1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, de la Rosa X, Libreros S, Serhan CN. Novel resolvin D2 receptor axis in infectious inflammation. J Immunol. 2017;198:842–851. doi: 10.4049/jimmunol.1601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Console-Bram L, Brailoiu E, Brailoiu GC, Sharir H, Abood ME. Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br J Pharmacol. 2014;171:3908–3917. doi: 10.1111/bph.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A. 2013;110:18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortright DN, Crandall M, Sanchez JF, Zou T, Krause JE, White G. The tissue distribution and functional characterization of human VR1. Biochem Biophys Res Commun. 2001;281:1183–1189. doi: 10.1006/bbrc.2001.4482. [DOI] [PubMed] [Google Scholar]
- Cronin A, Decker M, Arand M. Mammalian soluble epoxide hydrolase is identical to liver hepoxilin hydrolase. J Lipid Res. 2011;52:712–719. doi: 10.1194/jlr.M009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke ST, Mattern M, Sarau HM, Winkler JD, Balcarek J, Wong A, Bennett CF. The signal transduction system of the leukotriene D4 receptor. Trends Pharmacol Sci. 1989;10:103–107. doi: 10.1016/0165-6147(89)90206-x. [DOI] [PubMed] [Google Scholar]
- Crooks SW, Stockley RA. Leukotriene B4. Int J Biochem Cell Biol. 1998;30:173–178. doi: 10.1016/S1357-2725(97)00123-4. [DOI] [PubMed] [Google Scholar]
- Czech W, Barbisch M, Tenscher K, Schöpf E, Schröder JM, Norgauer J. Chemotactic 5-oxo-eicosatetraenoic acids induce oxygen radical production, Ca2+-mobilization, and actin reorganization in human eosinophils via a pertussis toxin-sensitive G-protein. J Invest Dermatol. 1997;108:108–112. doi: 10.1111/1523-1747.ep12285653. [DOI] [PubMed] [Google Scholar]
- da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014;62:698–738. doi: 10.1369/0022155414545334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele S, Trincavelli ML, Gabelloni P, Lecca D, Rosa P, Abbracchio MP, Martini C. Agonist-induced desensitization/resensitization of human G protein-coupled receptor 17: a functional cross-talk between purinergic and cysteinyl-leukotriene ligands. J Pharmacol Exp Ther. 2011;338:559–567. doi: 10.1124/jpet.110.178715. [DOI] [PubMed] [Google Scholar]
- Daniele S, Trincavelli ML, Fumagalli M, Zappelli E, Lecca D, Bonfanti E, Campiglia P, Abbracchio MP, Martini C. Does GRK-β arrestin machinery work as a "switch on" for GPR17-mediated activation of intracellular signaling pathways? Cell Signal. 2014;26:1310–1325. doi: 10.1016/j.cellsig.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Dartt DA, Hodges RR, Serhan CN. Immunoresolvent resolvin D1 maintains the health of the ocular surface. Adv Exp Med Biol. 2019;1161:13–25. doi: 10.1007/978-3-030-21735-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Henau O, Degroot GN, Imbault V, Robert V, De Poorter C, Mcheik S, Galés C, Parmentier M, Springael JY. Signaling properties of chemerin receptors CMKLR1, GPR1 and CCRL2. PLoS ONE. 2016;11:e0164179. doi: 10.1371/journal.pone.0164179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- de Poorter C, Baertsoen K, Lannoy V, Parmentier M, Springael JY. Consequences of ChemR23 heteromerization with the chemokine receptors CXCR4 and CCR7. PLoS ONE. 2013;8:e58075. doi: 10.1371/journal.pone.0058075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedoni S, Campbell LA, Harvey BK, Avdoshina V, Mocchetti I. The orphan G-protein-coupled receptor 75 signaling is activated by the chemokine CCL5. J Neurochem. 2018;146:526–539. doi: 10.1111/jnc.14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzazga A, Kristinsson H, Sałaga M, Zatorski H, Koziołkiewicz M, Gendaszewska-Darmach E, Bergsten P. Lysophosphatidylcholine and its phosphorothioate analogues potentiate insulin secretion via GPR40 (FFAR1), GPR55 and GPR119 receptors in a different manner. Mol Cell Endocrinol. 2018;472:117–125. doi: 10.1016/j.mce.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Dupré DJ, Le Gouill C, Gingras D, Rola-Pleszczynski M, Stanková J. Inverse agonist activity of selected ligands of the cysteinyl-leukotriene receptor 1. J Pharmacol Exp Ther. 2004;309:102–108. doi: 10.1124/jpet.103.059824. [DOI] [PubMed] [Google Scholar]
- Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa K, Bossé Y, Stankova J, Rola-Pleszczynski M. CysLT1 receptor upregulation by TGF-beta and IL-13 is associated with bronchial smooth muscle cell proliferation in response to LTD4. J Allergy Clin Immunol. 2003;111:1032–1040. doi: 10.1067/mai.2003.1451. [DOI] [PubMed] [Google Scholar]
- Fan F, Roman RJ. GPR75 identified as the first 20-HETE receptor: a chemokine receptor adopted by a new family. Circ Res. 2017;120:1696–1698. doi: 10.1161/CIRCRESAHA.117.311022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Ge Y, Lv W, Elliott MR, Muroya Y, Hirata T, Booz GW, Roman RJ. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci (landmark Ed) 2016;21:1427–1463. doi: 10.2741/4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filep JG. Biasing the lipoxin A4/formyl peptide receptor 2 pushes inflammatory resolution. Proc Natl Acad Sci USA. 2013;110:18033–18034. doi: 10.1073/pnas.1317798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay DB, Joseph WR, Grimsey NL, Glass M. GPR18 undergoes a high degree of constitutive trafficking but is unresponsive to N-Arachidonoyl Glycine. Peer J. 2016;4:e1835. doi: 10.7717/peerj.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Jr, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster HR, Fuerst E, Lee TH, Cousins DJ, Woszczek G. Characterisation of P2Y(12) receptor responsiveness to cysteinyl leukotrienes. PLoS ONE. 2013;8:e58305. doi: 10.1371/journal.pone.0058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster HR, Fuerst E, Branchett W, Lee TH, Cousins DJ, Woszczek G. Leukotriene E4 is a full functional agonist for human cysteinyl leukotriene type 1 receptor-dependent gene expression. Sci Rep. 2016;6:20461. doi: 10.1038/srep20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Parravicini C, Lecca D, Zanier ER, Heine C, Bremicker K, Fumagalli M, Rosa P, Longhi L, Stocchetti N, De Simoni MG, Weber M. Abbracchio MP (2013) Changes of the GPR17 receptor, a new target for neurorepair, in neurons and glial cells in patients with traumatic brain injury. Purinergic Signal. 2013;9:451–462. doi: 10.1007/s11302-013-9366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratangeli A, Parmigiani E, Fumagalli M, Lecca D, Benfante R, Passafaro M, Buffo A, Abbracchio MP, Rosa P. The regulated expression, intracellular trafficking, and membrane recycling of the P2Y-like receptor GPR17 in Oli-neu oligodendroglial cells. J Biol Chem. 2013;288:5241–5256. doi: 10.1074/jbc.M112.404996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretland DJ, Anglin CP, Bremer M, Isakson P, Widomski DL, Paulson SK, Docter SH, Djuric SW, Penning TD, Yu S, McKearn JP. Antiinflammatory effects of second-generation leukotriene B4 receptor antagonist, SC-53228: impact upon leukotriene B4- and 12(R)-HETE-mediated events. Inflammation. 1995;19:193–205. doi: 10.1007/bf01534461. [DOI] [PubMed] [Google Scholar]
- Garcia V, Gilani A, Shkolnik B, Pandey V, Zhang FF, Dakarapu R, Gandham SK, Reddy NR, Graves JP, Gruzdev A, Zeldin DC, Capdevila JH, Falck JR, Schwartzman ML. 20-HETE signals through g-protein-coupled receptor GPR75 (Gq) to affect vascular function and trigger hypertension. Circ Res. 2017;120:1776–1788. doi: 10.1161/CIRCRESAHA.116.310525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreau R, Le Gouill C, Venne MH, Stankova J, Rola-Pleszczynski M. Threonine 308 within a putative casein kinase 2 site of the cytoplasmic tail of leukotriene B(4) receptor (BLT1) is crucial for ligand-induced, G-protein-coupled receptor-specific kinase 6-mediated desensitization. J Biol Chem. 2002;277:31567–31576. doi: 10.1074/jbc.M202723200. [DOI] [PubMed] [Google Scholar]
- Ge Y, Zhang S, Wang J, Xia F, Wan JB, Lu J, Ye RD. Dual modulation of formyl peptide receptor 2 by aspirin-triggered lipoxin contributes to its anti-inflammatory activity. FASEB J. 2020;34(5):6920–6933. doi: 10.1096/fj.201903206R. [DOI] [PubMed] [Google Scholar]
- Gençoğlu H, Şahin K, Jones PM. Determining the insulin secretion potential for certain specific G-protein coupled receptors in MIN6 pancreatic beta cells. Turk J Med Sci. 2019;49:403–411. doi: 10.3906/sag-1712-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard DS, Wagner L, Feingold EA, Shenmen CM, Grouse LH, Schuler G, et al. The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC) Genome Res. 2004;14:2121–2127. doi: 10.1101/gr.2596504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, Madara JL. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest. 1998;101:1860–1869. doi: 10.1172/JCI1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene. 2007;26:3122–3142. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- Goodarzi K, Goodarzi M, Tager AM, Luster AD, von Andrian UH. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol. 2003;4:965–973. doi: 10.1038/ni972. [DOI] [PubMed] [Google Scholar]
- Gregus AM, Doolen S, Dumlao DS, Buczynski MW, Takasusuki T, Fitzsimmons BL, Hua XY, Taylor BK, Dennis EA, Yaksh TL. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc Natl Acad Sci USA. 2012;109:6721–6726. doi: 10.1073/pnas.1110460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Eyo UB, Murugan M, Peng J, Matta S, Dong H, Wu LJ. Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav Immun. 2016;55:82–92. doi: 10.1016/j.bbi.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Alba R, Barragán-Iglesias P, González-Hernández A, Valdez-Moráles EE, Granados-Soto V, Condés-Lara M, Rodríguez MG, Marichal-Cancino BA. Some prospective alternatives for treating pain: the endocannabinoid system and its putative receptors GPR18 and GPR55. Front Pharmacol. 2019;9:1496. doi: 10.3389/fphar.2018.01496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Harries MH, Prinjha RK, Davis JB, Randall A. Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1) J Physiol. 2000;525:747–759. doi: 10.1111/j.1469-7793.2000.t01-1-00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, Hsu A, Zhou S, Maddipati KR, Liu J, Joshi S, Tucker SC, Lee MJ, Honn KV. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J Biol Chem. 2011;286:33832–33840. doi: 10.1074/jbc.M110.216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeggström JZ. Structure, function, and regulation of Leukotriene A4 Hydrolase. Am J Respir Crit Care Med. 2000;161:S25–S31. doi: 10.1164/ajrccm.161.supplement_1.ltta-6. [DOI] [PubMed] [Google Scholar]
- Harder DR, Campbell WB, Roman RJ. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res. 1995;32:79–92. doi: 10.1159/000159080. [DOI] [PubMed] [Google Scholar]
- Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood. 2005;105:3552–3560. doi: 10.1182/blood-2004-07-2893. [DOI] [PubMed] [Google Scholar]
- Hayes P, Meadows HJ, Gunthorpe MJ, Harries MH, Duckworth DM, Cairns W, Harrison DC, Clarke CE, Ellington K, Prinjha RK, Barton AJ, Medhurst AD, Smith GD, Topp S, Murdock P, Sanger GJ, Terrett J, Jenkins O, Benham CD, Randall AD, Gloger IS, Davis JB. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88:205–215. doi: 10.1016/s0304-3959(00)00353-5. [DOI] [PubMed] [Google Scholar]
- He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- Heise CE, O'Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, Williams DL, Jr, Zeng Z, Liu Q, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O'Neill GP, Metters KM, Lynch KR, Evans JF. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- Hennen S, Wang H, Peters L, Merten N, Simon K, Spinrath A, Blättermann S, Akkari R, Schrage R, Schröder R, Schulz D, Vermeiren C, Zimmermann K, Kehraus S, Drewke C, Pfeifer A, König GM, Mohr K, Gillard M, Müller CE, Lu QR, Gomeza J, Kostenis E. Decoding signaling and function of the orphan G protein-coupled receptor GPR17 with a small-molecule agonist. Sci Signal. 2013 doi: 10.1126/scisignal.2004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herová M, Schmid M, Gemperle C, Hersberger M. ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J Immunol. 2015;194:2330–2337. doi: 10.4049/jimmunol.1402166. [DOI] [PubMed] [Google Scholar]
- Hodges RR, Li D, Shatos MA, Bair JA, Lippestad M, Serhan CN, Dartt DA. Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol. 2017;10:46–57. doi: 10.1038/mi.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honn KV (2008) Characterization of a Novel 12(S)-HETE receptor and its role in prostate cancer progression. annual research report for U.S. army medical research and materiel command Fort Detrick, Maryland. Available from https://apps.dtic.mil/dtic/tr/fulltext/u2/a482663.pdf
- Honn KV, Guo Y, Cai Y, Lee MJ, Dyson G, Zhang W, Tucker SC. 12-HETER1/GPR31, a high-affinity 12(S)-hydroxyeicosatetraenoic acid receptor, is significantly up-regulated in prostate cancer and plays a critical role in prostate cancer progression. FASEB J. 2016;30:2360–2369. doi: 10.1096/fj.201500076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T, Koguchi Y, Sugikawa E, Chikada A, Ogawa K, Tsuda N, Suto N, Tsunoda S, Taniguchi T, Ohnuki T. Identification of a novel human eicosanoid receptor coupled to G(i/o) J Biol Chem. 2002;277:31459–31465. doi: 10.1074/jbc.M203194200. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Sugikawa E, Chikada A, Koguchi Y, Ohnuki T. TG1019/OXE, a Galpha(i/o)-protein-coupled receptor, mediates 5-oxo-eicosatetraenoic acid-induced chemotaxis. Biochem Biophys Res Commun. 2005;334:987–995. doi: 10.1016/j.bbrc.2005.06.191. [DOI] [PubMed] [Google Scholar]
- Houthuijzen JM. For better or worse: FFAR1 and FFAR4 signaling in cancer and diabetes. Mol Pharmacol. 2016;90:738–743. doi: 10.1124/mol.116.105932. [DOI] [PubMed] [Google Scholar]
- Huet E, Boulay F, Barral S, Rabiet MJ. The role of beta-arrestins in the formyl peptide receptor-like 1 internalization and signaling. Cell Signal. 2007;19:1939–1948. doi: 10.1016/j.cellsig.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Hui Y, Cheng Y, Smalera I, Jian W, Goldhahn L, Fitzgerald GA, Funk CD. Directed vascular expression of human cysteinyl leukotriene 2 receptor modulates endothelial permeability and systemic blood pressure. Circulation. 2004;110:3360–3366. doi: 10.1161/01.CIR.0000147775.50954.AA. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatov A, Robert J, Gregory-Evans C, Schaller HC. RANTES stimulates Ca2+ mobilization and inositol trisphosphate (IP3) formation in cells transfected with G protein-coupled receptor 75. Br J Pharmacol. 2006;149:490–497. doi: 10.1038/sj.bjp.0706909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbe H, Watanabe S, Miyawaki M, Tanabe E, Encinas JA. Identification and characterization of a cell-surface receptor, P2Y15, for AMP and adenosine. J Biol Chem. 2004;279:19790–19799. doi: 10.1074/jbc.M400360200. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Saeki K, Liu M, Sasaki F, Koga T, Kitajima K, Meno C, Okuno T, Yokomizo T. Leukotriene B4 receptor type 2 (BLT2) enhances skin barrier function by regulating tight junction proteins. FASEB J. 2016;30:933–947. doi: 10.1096/fj.15-279653. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Iyinikkel JR (2018) Identifying novel G protein-coupled receptor targets in pulmonary arterial hypertension: uncovering the role of GPR75. Dissertation, University of Aberdeen,UK. Available from: https://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.774022
- Jahnel R, Dreger M, Gillen C, Bender O, Kurreck J, Hucho F. Biochemical characterization of the vanilloid receptor 1 expressed in a dorsal root ganglia derived cell line. Eur J Biochem. 2001;268:5489–5496. doi: 10.1046/j.1432-1033.2001.02500.x. [DOI] [PubMed] [Google Scholar]
- Jala VR, Shao WH, Haribabu B. Phosphorylation-independent beta-arrestin translocation and internalization of leukotriene B4 receptors. J Biol Chem. 2005;280:4880–4887. doi: 10.1074/jbc.M409821200. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA. CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood. 2007;110:3263–3270. doi: 10.1182/blood-2007-07-100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Holden S, Tenaillon L, Bhatia U, Seuwen K, Tranter P, Turner J, Kettle R, Bouhelal R, Charlton S, Nirmala NR, Jarai G, Finan P. Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils. Mol Pharmacol. 2003;63:471–477. doi: 10.1124/mol.63.3.471. [DOI] [PubMed] [Google Scholar]
- Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- Kain V, Liu F, Kozlovskaya V, Ingle KA, Bolisetty S, Agarwal A, Khedkar S, Prabhu SD, Kharlampieva E, Halade GV. Resolution agonist 15-epi-Lipoxin A4 programs early activation of resolving phase in post-myocardial infarction healing. Sci Rep. 2017;7:9999. doi: 10.1038/s41598-017-10441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyvianaki K, Gebhart V, Peroulis N, Panagiotopoulou C, Kiagiadaki F, Pediaditakis I, Aivaliotis M, Moustou E, Tzardi M, Notas G, Castanas E, Kampa M. Antagonizing effects of membrane-acting androgens on the eicosanoid receptor OXER1 in prostate cancer. Sci Rep. 2017;7:44418. doi: 10.1038/srep44418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyvianaki K, Panagiotopoulos AA, Malamos P, Moustou E, Tzardi M, Stathopoulos EN, Ioannidis GS, Marias K, Notas G, Theodoropoulos PA, Castanas E, Kampa M. Membrane androgen receptors (OXER1, GPRC6A AND ZIP9) in prostate and breast cancer: a comparative study of their expression. Steroids. 2019;142:100–108. doi: 10.1016/j.steroids.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy Asthma Immunol Res. 2014;6:288–295. doi: 10.4168/aair.2014.6.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaoka Y, Maekawa A, Austen KF. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem. 2013;288:10967–10972. doi: 10.1074/jbc.C113.453704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Miyabe Y, Takayasu A, Fukuda S, Miyabe C, Ebisawa M, Yokoyama W, Watanabe K, Imai T, Muramoto K, Terashima Y, Sugihara T, Matsushima K, Miyasaka N, Nanki T. Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13:R158. doi: 10.1186/ar3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplamadzhiev DB, Hisha H, Adachi Y, Ikehara S, Tonchev AB, Boneva NB, Pyko IV, Kikuchi M, Nakaya M, Wakayama T, Iseki S, Yamashima T. Bone marrow-derived stromal cells can express neuronal markers by DHA/GPR40 signaling. Biosci Trends. 2010;4:119–129. [PubMed] [Google Scholar]
- Kauffenstein G, Hechler B, Cazenave JP, Gachet C. Adenine triphosphate nucleotides are antagonists at the P2Y receptor. J Thromb Haemost. 2004;2:1980–1988. doi: 10.1111/j.1538-7836.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Mentor S, Thangavel R, Ahmed ME, Selvakumar GP, Raikwar SP, Dubova I, Zaheer S, Iyer SS, Zaheer A. Mast cells in stress, pain, blood-brain barrier, neuroinflammation and Alzheimer's Disease. Front Cell Neurosci. 2019;13:54. doi: 10.3389/fncel.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Choi JA, Park GS, Kim JH. BLT2 up-regulates interleukin-8 production and promotes the invasiveness of breast cancer cells. PLoS ONE. 2012;7:e49186. doi: 10.1371/journal.pone.0049186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci. 2008;28:2892–2902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, Yasukawa M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun. 2006;347:827–832. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun. 2003;301:406–410. doi: 10.1016/s0006-291x(02)03064-4. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubala SA, Patil SU, Shreffler WG, Hurley BP. Pathogen induced chemo-attractant hepoxilin A3 drives neutrophils, but not eosinophils across epithelial barriers. Prostaglandins Other Lipid Mediat. 2014;108:1–8. doi: 10.1016/j.prostaglandins.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniyeda K, Okuno T, Terawaki K, Miyano M, Yokomizo T, Shimizu T. Identification of the intracellular region of the leukotriene B4 receptor type 1 that is specifically involved in Gi activation. J Biol Chem. 2007;282:3998–4006. doi: 10.1074/jbc.M610540200. [DOI] [PubMed] [Google Scholar]
- Laitinen LA, Laitinen A, Haahtela T, Vilkka V, Spur BW, Lee TH. Leukotriene E4 and granulocytic infiltration into asthmatic airways. Lancet. 1993;341:989–990. doi: 10.1016/0140-6736(93)91073-u. [DOI] [PubMed] [Google Scholar]
- Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Hoover J, Jang W, Katz K, Ovetsky M, Riley G, Sethi A, Tully R, Villamarin-Salomon R, Rubinstein W, Maglott DR. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkv1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois A, Ferland C, Tremblay GM, Laviolette M. Montelukast regulates eosinophil protease activity through a leukotriene-independent mechanism. J Allergy Clin Immunol. 2006;118:113–119. doi: 10.1016/j.jaci.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Lattin JE, Schroder K, Su AI, Walker JR, Zhang J, Wiltshire T, Saijo K, Glass CK, Hume DA, Kellie S, Sweet MJ. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008;4:5. doi: 10.1186/1745-7580-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- Lecca D, Trincavelli ML, Gelosa P, Sironi L, Ciana P, Fumagalli M, Villa G, Verderio C, Grumelli C, Guerrini U, Tremoli E, Rosa P, Cuboni S, Martini C, Buffo A, Cimino M, Abbracchio MP. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS ONE. 2008;3:e3579. doi: 10.1371/journal.pone.0003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Hodges RR, Jiao J, Carozza RB, Shatos MA, Chiang N, Serhan CN, Dartt DA. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. 2013;6:1119–1130. doi: 10.1038/mi.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay MA, Haddad EB, Rousell J, Teixeira MM, Hellewell PG, Barnes PJ, Giembycz MA. Role of the mitogen-activated protein kinases and tyrosine kinases during leukotriene B4-induced eosinophil activation. J Leukoc Biol. 1998;64:555–562. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- Lippestad M, Hodges RR, Utheim TP, Serhan CN, Dartt DA. Resolvin D1 increases mucin secretion in cultured rat conjunctival goblet cells via multiple signaling pathways. Invest Ophthalmol vis Sci. 2017;58:4530–4544. doi: 10.1167/iovs.17-21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Khan WA, Hannun YA, Timar J, Taylor JD, Lundy S, Butovich I, Honn KV. 12(S)-hydroxyeicosatetraenoic acid and 13(S)-hydroxyoctadecadienoic acid regulation of protein kinase C-alpha in melanoma cells: role of receptor-mediated hydrolysis of inositol phospholipids. Proc Natl Acad Sci U S A. 1995;92:9323–9327. doi: 10.1073/pnas.92.20.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Saeki K, Matsunobu T, Okuno T, Koga T, Sugimoto Y, Yokoyama C, Nakamizo S, Kabashima K, Narumiya S, Shimizu T, Yokomizo T. 12-Hydroxyheptadecatrienoic acid promotes epidermal wound healing by accelerating keratinocyte migration via the BLT2 receptor. J Exp Med. 2014;211(6):1063–1078. doi: 10.1084/jem.20132063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Shen J, Yuan H, Chen F, Song H, Qin H, Li Y, Xu J, Ye Q, Li S, Saeki K, Yokomizo T. Leukotriene B4 receptor 2 regulates the proliferation, migration, and barrier integrity of bronchial epithelial cells. J Cell Physiol. 2018;233:6117–6124. doi: 10.1002/jcp.26455. [DOI] [PubMed] [Google Scholar]
- Lu G, Henderson D, Liu L, Reinhart PH, Simon SA. TRPV1b, a functional human vanilloid receptor splice variant. Mol Pharmacol. 2005;67:1119–1127. doi: 10.1124/mol.104.009852. [DOI] [PubMed] [Google Scholar]
- Lu C, Dong L, Zhou H, Li Q, Huang G, Bai SJ, Liao L. G-Protein-coupled receptor Gpr17 regulates oligodendrocyte differentiation in response to lysolecithin-induced demyelination. Sci Rep. 2018;8:4502. doi: 10.1038/s41598-018-22452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeen KA, Sun B, Karlsson L, Fourie AM. Leukotriene B4 receptors BLT1 and BLT2: expression and function in human and murine mast cells. J Immunol. 2006;177:3439–3447. doi: 10.4049/jimmunol.177.5.3439. [DOI] [PubMed] [Google Scholar]
- Luo J, Swaminath G, Brown SP, Zhang J, Guo Q, Chen M, Nguyen K, Tran T, Miao L, Dransfield PJ, Vimolratana M, Houze JB, Wong S, Toteva M, Shan B, Li F, Zhuang R, Lin DC. A potent class of GPR40 full agonists engages the enteroinsular axis to promote glucose control in rodents. PLoS ONE. 2012;7:e46300. doi: 10.1371/journal.pone.0046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KR, O'Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, Connolly BM, Bai C, Austin CP, Chateauneuf A, Stocco R, Greig GM, Kargman S, Hooks SB, Hosfield E, Williams DL, Jr, Ford-Hutchinson AW, Caskey CT, Evans JF. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Colgan SP, Clish CB, Petasis NA, Fokin VV, Serhan CN. Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: design of stable lipoxin B4 analogs with increased biologic activity. FASEB J. 1998;12:487–494. doi: 10.1096/fasebj.12.6.487. [DOI] [PubMed] [Google Scholar]
- Maderna P, Godson C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta. 2003;1639:141–151. doi: 10.1016/j.bbadis.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Maderna P, Cottell DC, Toivonen T, Dufton N, Dalli J, Perretti M, Godson C. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010;24:4240–4249. doi: 10.1096/fj.10-159913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277:20820–20824. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci U S A. 2008;105:16695–16700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa A, Balestrieri B, Austen KF, Kanaoka Y. GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc Natl Acad Sci USA. 2009;106:11685–11690. doi: 10.1073/pnas.0905364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfacini D, Patt J, Annala S, Harpsøe K, Eryilmaz F, Reher R, Crüsemann M, Hanke W, Zhang H, Tietze D, Gloriam DE, Bräuner-Osborne H, Strømgaard K, König GM, Inoue A, Gomeza J, Kostenis E. Rational design of a heterotrimeric G protein α subunit with artificial inhibitor sensitivity. J Biol Chem. 2019;294:5747–5758. doi: 10.1074/jbc.RA118.007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini AD, Bertrand G, Vivot K, Carpentier É, Tremblay C, Ghislain J, Bouvier M, Poitout V. β-Arrestin recruitment and biased agonism at free fatty acid receptor 1. J Biol Chem. 2015;290:21131–21140. doi: 10.1074/jbc.M115.644450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi S, Roufogalis BD. ThermoTRP channels in nociceptors: taking a lead from capsaicin receptor TRPV1. Curr Neuropharmacol. 2008;6:21–38. doi: 10.2174/157015908783769680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh SJ, Stansfeld CE, Brown DA, Davey R, McCarthy D. The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience. 1987;23:275–289. doi: 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- Marucci G, Dal Ben D, Lambertucci C, Santinelli C, Spinaci A, Thomas A, Volpini R, Buccioni M. The G Protein-Coupled receptor GPR17: overview and update. ChemMedChem. 2016;11:2567–2574. doi: 10.1002/cmdc.201600453. [DOI] [PubMed] [Google Scholar]
- Mashiko M, Kurosawa A, Tani Y, Tsuji T, Takeda S. GPR31 and GPR151 are activated under acidic conditions. J Biochem. 2019;166:317–322. doi: 10.1093/jb/mvz042. [DOI] [PubMed] [Google Scholar]
- Mathis SP, Jala VR, Lee DM, Haribabu B. Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. J Immunol. 2010;185:3049–3056. doi: 10.4049/jimmunol.1001031. [DOI] [PubMed] [Google Scholar]
- McCauley LK, Dalli J, Koh AJ, Chiang N, Serhan CN. Cutting edge: parathyroid hormone facilitates macrophage efferocytosis in bone marrow via proresolving mediators resolvin D1 and resolvin D2. J Immunol. 2014;193:26–29. doi: 10.4049/jimmunol.1301945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Page J, Dunn E, Bradshaw HB. Δ(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br J Pharmacol. 2012;165:2414–2424. doi: 10.1111/j.1476-5381.2011.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre P, McLatchie LM, Chambers A, Phillips E, Clarke M, Savidge J, Toms C, Peacock M, Shah K, Winter J, Weerasakera N, Webb M, Rang HP, Bevan S, James IF. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1) Br J Pharmacol. 2001;132:1084–1094. doi: 10.1038/sj.bjp.0703918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechiche H, Candenas L, Pinto FM, Nazeyrollas P, Clément C, Devillier P. Characterization of cysteinyl leukotriene receptors on human saphenous veins: antagonist activity of montelukast and its metabolites. J Cardiovasc Pharmacol. 2004;43:113–120. doi: 10.1097/00005344-200401000-00017. [DOI] [PubMed] [Google Scholar]
- Mehdawi LM, Satapathy SR, Gustafsson A, Lundholm K, Alvarado-Kristensson M, Sjölander A. A potential anti-tumor effect of leukotriene C4 through the induction of 15-hydroxyprostaglandin dehydrogenase expression in colon cancer cells. Oncotarget. 2017 doi: 10.18632/oncotarget.16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkes B, Markova V, Hejnova L, Marek A, Novotny J. Naloxone is a potential binding ligand and activator of the capsaicin receptor TRPV1. Biol Pharm Bull. 2020;43:908–912. doi: 10.1248/bpb.b19-00806. [DOI] [PubMed] [Google Scholar]
- Mellor EA, Frank N, Soler D, Hodge MR, Lora JM, Austen KF, Boyce JA. Expression of the type 2 receptor for cysteinyl leukotrienes (CysLT2R) by human mast cells: functional distinction from CysLT1R. Proc Natl Acad Sci U S A. 2003;100:11589–11593. doi: 10.1073/pnas.2034927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilner A, Oster G. Cell motility driven by actin polymerization. Biophys J. 1996;71:3030–3045. doi: 10.1016/S0006-3495(96)79496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005 doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Moore AR, Ceraudo E, Sher JJ, Guan Y, Shoushtari AN, Chang MT, Zhang JQ, Walczak EG, Kazmi MA, Taylor BS, Huber T, Chi P, Sakmar TP, Chen Y. Recurrent activating mutations of G-protein-coupled receptor CYSLTR2 in uveal melanoma. Nat Genet. 2016;48:675–680. doi: 10.1038/ng.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell SJ, Jones ML, Hardy AR, Barton JF, Beaucourt SM, Conley PB, Poole AW. Distinct roles for protein kinase C isoforms in regulating platelet purinergic receptor function. Mol Pharmacol. 2006;70:1132–1142. doi: 10.1124/mol.106.023549. [DOI] [PubMed] [Google Scholar]
- Munkarah A, Mert I, Chhina J, Hamid S, Poisson L, Hensley-Alford S, Giri S, Rattan R. Targeting of free fatty acid receptor 1 in EOC: A novel strategy to restrict the adipocyte-EOC dependence. Gynecol Oncol. 2016;141:72–79. doi: 10.1016/j.ygyno.2016.02.02. [DOI] [PubMed] [Google Scholar]
- Naik S, Billington CK, Pascual RM, Deshpande DA, Stefano FP, Kohout TA, Eckman DM, Benovic JL, Penn RB. Regulation of cysteinyl leukotriene type 1 receptor internalization and signaling. J Biol Chem. 2005;280:8722–8732. doi: 10.1074/jbc.M413014200. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol. 2013;35:123–137. doi: 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y, Tan M, Ichiki T, Inoue A, Yoshihara JI, Maekawa N, Takenoshita I, Yanagida K, Yamahira S, Yamaguchi S, Aoki J, Nagamune T, Yokomizo T, Shimizu T, Nakamura M. Stepwise phosphorylation of leukotriene B4 receptor 1 defines cellular responses to leukotriene B4. Sci Signal. 2018 doi: 10.1126/scisignal.aao5390. [DOI] [PubMed] [Google Scholar]
- Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- Neves JS, Radke AL, Weller PF. Cysteinyl leukotrienes acting via granule membrane-expressed receptors elicit secretion from within cell-free human eosinophil granules. J Allergy Clin Immunol. 2010;125:477–482. doi: 10.1016/j.jaci.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CH, Stadler S, Brenner S, Huttary N, Krieger S, Jäger W, Dolznig H, Krupitza G. Cancer cell-derived 12(S)-HETE signals via 12-HETE receptor, RHO, ROCK and MLC2 to induce lymph endothelial barrier breaching. Br J Cancer. 2016;115:364–370. doi: 10.1038/bjc.2016.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisar SP, Cunningham M, Saxena K, Pope RJ, Kelly E, Mundell SJ. Arrestin scaffolds NHERF1 to the P2Y12 receptor to regulate receptor internalization. J Biol Chem. 2012;287:24505–24515. doi: 10.1074/jbc.M112.347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi H, Pelleg A, Schulman ES. IgE receptor-mediated histamine release in human lung mast cells: modulation by purinergic receptor ligands. Ann Clin Lab Sci. 2016;46:463–469. [PubMed] [Google Scholar]
- Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55(Suppl 2):S16–S23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- Nonaka Y, Hiramoto T, Fujita N. Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun. 2005;337:281–288. doi: 10.1016/j.bbrc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothacker HP, Wang Z, Zhu Y, Reinscheid RK, Lin SH, Civelli O. Molecular cloning and characterization of a second human cysteinyl leukotriene receptor: discovery of a subtype selective agonist. Mol Pharmacol. 2000;58:1601–1608. doi: 10.1124/mol.58.6.1601. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- O'Flaherty JT, Kuroki M, Nixon AB, Wijkander J, Yee E, Lee SL, Smitherman PK, Wykle RL, Daniel LW. 5-Oxo-eicosatetraenoate is a broadly active, eosinophil-selective stimulus for human granulocytes. J Immunol. 1996;157:336–342. [PubMed] [Google Scholar]
- O'Flaherty JT, Kuroki M, Nixon AB, Wijkander J, Yee E, Lee SL, Smitherman PK, Wykle RL, Daniel LW. 5-Oxo-eicosanoids and hematopoietic cytokines cooperate in stimulating neutrophil function and the mitogen-activated protein kinase pathway. J Biol Chem. 1996;271:17821–17828. doi: 10.1074/jbc.271.30.17821. [DOI] [PubMed] [Google Scholar]
- O'Flaherty JT, Taylor JS, Thomas MJ. Receptors for the 5-oxo class of eicosanoids in neutrophils. J Biol Chem. 1998;273:32535–32541. doi: 10.1074/jbc.273.49.32535. [DOI] [PubMed] [Google Scholar]
- Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. Resolvin E2 formation and impact in inflammation resolution. J Immunol. 2012;188:4527–4534. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima N, Nagase H, Koshino T, Miyamasu M, Yamaguchi M, Hirai K, Yamamoto K, Fujisawa T, Nakagawa N, Kishikawa K, Morita Y. A functional study on CysLT(1) receptors in human eosinophils. Int Arch Allergy Immunol. 2002;129:67–75. doi: 10.1159/000065175. [DOI] [PubMed] [Google Scholar]
- Okuno T, Yokomizo T. Biological functions of 12(S)-hydroxyheptadecatrienoic acid as a ligand of leukotriene B4 receptor 2. Inflamm Regen. 2018;38:29. doi: 10.1186/s41232-018-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T, Iizuka Y, Okazaki H, Yokomizo T, Taguchi R, Shimizu T. 12(S)-Hydroxyheptadeca-5Z, 8E, 10E-trienoic acid is a natural ligand for leukotriene B4 receptor 2. J Exp Med. 2008;205:759–766. doi: 10.1084/jem.20072329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T, Ishitani T, Yokomizo T. Biochemical characterization of three BLT receptors in zebrafish. PLoS ONE. 2015;10:e0117888. doi: 10.1371/journal.pone.0117888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Asciak CR. Pathophysiology of the hepoxilins. Biochim Biophys Acta. 2015;1851:383–396. doi: 10.1016/j.bbalip.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak CR, Reynaud D, Demin P, Aslam R, Sun A. A new family of thromboxane receptor antagonists with secondary thromboxane synthase inhibition. J Pharmacol Exp Ther. 2002;301:618–624. doi: 10.1124/jpet.301.2.618. [DOI] [PubMed] [Google Scholar]
- Parhamifar L, Sime W, Yudina Y, Vilhardt F, Mörgelin M, Sjölander A. Ligand-induced tyrosine phosphorylation of cysteinyl leukotriene receptor 1 triggers internalization and signaling in intestinal epithelial cells. PLoS ONE. 2010;5:e14439. doi: 10.1371/journal.pone.0014439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CK, Xu ZZ, Liu T, Lü N, Serhan CN, Ji RR. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci. 2011;31:18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Herrnreiter A, Pfister SL, Gauthier KM, Falck BA, Falck JR, Campbell WB. GPR40 is a low-affinity epoxyeicosatrienoic acid receptor in vascular cells. J Biol Chem. 2018;293:10675–10691. doi: 10.1074/jbc.RA117.001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Jang JH, Kim JH. Mediatory role of BLT2 in the proliferation of KRAS mutant colorectal cancer cells. Biochim Biophys Acta Mol Cell Res. 2019;1866:329–336. doi: 10.1016/j.bbamcr.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Parker WAE, Storey RF. Novel approaches to P2Y12 inhibition and aspirin dosing. Platelets. 2021;32:7–14. doi: 10.1080/09537104.2020.1714574. [DOI] [PubMed] [Google Scholar]
- Parmentier CN, Fuerst E, McDonald J, Bowen H, Lee TH, Pease JE, Woszczek G, Cousins DJ. Human T(H)2 cells respond to cysteinyl leukotrienes through selective expression of cysteinyl leukotriene receptor 1. J Allergy Clin Immunol. 2012;129:1136–1142. doi: 10.1016/j.jaci.2012.01.057. [DOI] [PubMed] [Google Scholar]
- Parravicini C, Ranghino G, Abbracchio MP, Fantucci P. GPR17: molecular modeling and dynamics studies of the 3-D structure and purinergic ligand binding features in comparison with P2Y receptors. BMC Bioinformatics. 2008;9:263. doi: 10.1186/1471-2105-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206(11):2543–2555. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierdomenico AM, Recchiuti A, Simiele F, Codagnone M, Mari VC, Davì G, Romano M. MicroRNA-181b regulates ALX/FPR2 receptor expression and proresolution signaling in human macrophages. J Biol Chem. 2015;290:3592–3600. doi: 10.1074/jbc.M114.592352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Volterra A, Dale N, Siegelbaum SA, Kandel ER, Schwartz JH, Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987;328:38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Pollock K, Creba J. Leukotriene D4 induced calcium changes in U937 cells may utilize mechanisms additional to inositol phosphate production that are pertussis toxin insensitive but are blocked by phorbol myristate acetate. Cell Signal. 1990;2:563–568. doi: 10.1016/0898-6568(90)90078-o. [DOI] [PubMed] [Google Scholar]
- Powell WS, Rokach J. The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor. Prog Lipid Res. 2013;52:651–665. doi: 10.1016/j.plipres.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell WS, Rokach J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim Biophys Acta. 2015;1851:340–355. doi: 10.1016/j.bbalip.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell WS, Rokach J. Targeting the OXE receptor as a potential novel therapy for asthma. Biochem Pharmacol. 2020 doi: 10.1016/j.bcp.2020.113930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell WS, Gravel S, MacLeod RJ, Mills E, Hashefi M. Stimulation of human neutrophils by 5-oxo-6,8,11,14-eicosatetraenoic acid by a mechanism independent of the leukotriene B4 receptor. J Biol Chem. 1993;268:9280–9286. doi: 10.1016/S0021-9258(18)98347-X. [DOI] [PubMed] [Google Scholar]
- Powell WS, Chung D, Gravel S. 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of human eosinophil migration. J Immunol. 1995;154:4123–4132. [PubMed] [Google Scholar]
- Powell WS, Gravel S, Halwani F. 5-oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of L-selectin shedding, surface expression of CD11b, actin polymerization, and calcium mobilization in human eosinophils. Am J Respir Cell Mol Biol. 1999;20:163–170. doi: 10.1165/ajrcmb.20.1.3141. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Pugliese AM, Trincavelli ML, Lecca D, Coppi E, Fumagalli M, Ferrario S, Failli P, Daniele S, Martini C, Pedata F, Abbracchio MP. Functional characterization of two isoforms of the P2Y-like receptor GPR17: [35S]GTPgammaS binding and electrophysiological studies in 1321N1 cells. Am J Physiol Cell Physiol. 2009;297:C1028–1040. doi: 10.1152/ajpcell.00658.2008. [DOI] [PubMed] [Google Scholar]
- Qi AD, Harden TK, Nicholas RA. Is GPR17 a P2Y/leukotriene receptor? examination of uracil nucleotides, nucleotide sugars, and cysteinyl leukotrienes as agonists of GPR17. J Pharmacol Exp Ther. 2013;347:38–46. doi: 10.1124/jpet.113.207647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao N, Reynaud D, Demin P, Halushka PV, Pace-Asciak CR. The thromboxane receptor antagonist PBT-3, a hepoxilin stable analog, selectively antagonizes the TPalpha isoform in transfected COS-7 cells. J Pharmacol Exp Ther. 2003;307:1142–1147. doi: 10.1124/jpet.103.056705. [DOI] [PubMed] [Google Scholar]
- Qin Y, Verdegaal EM, Siderius M, Bebelman JP, Smit MJ, Leurs R, Willemze R, Tensen CP, Osanto S. Quantitative expression profiling of G-protein-coupled receptors (GPCRs) in metastatic melanoma: the constitutively active orphan GPCR GPR18 as novel drug target. Pigment Cell Melanoma Res. 2011;24:207–218. doi: 10.1111/j.1755-148X.2010.00781.x. [DOI] [PubMed] [Google Scholar]
- Rajkumar P, Pluznick JL. Unsung renal receptors: orphan G-protein-coupled receptors play essential roles in renal development and homeostasis. Acta Physiol (oxf) 2017;220:189–200. doi: 10.1111/apha.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel V, Volz N, Gläser F, Nieger M, Bräse S, Müller CE. Antagonists for the orphan G-protein-coupled receptor GPR55 based on a coumarin scaffold. J Med Chem. 2013;56:4798–4810. doi: 10.1021/jm4005175. [DOI] [PubMed] [Google Scholar]
- Rempel V, Atzler K, Behrenswerth A, Karcz T, Schoeder C, Hinz S, Kaleta M, Thimm D, Kiec-Kononowicz K, Müller CE. Bicyclic imidazole-4-one derivatives: a new class of antagonists for the orphan G protein-coupled receptors GPR18 and GPR55. Med Chem Commun. 2014;5:632–649. doi: 10.1039/C3MD00394A. [DOI] [Google Scholar]
- Reyes-Resina I, Navarro G, Aguinaga D, Canela EI, Schoeder CT, Załuski M, Kieć-Kononowicz K, Saura CA, Müller CE, Franco R. Molecular and functional interaction between GPR18 and cannabinoid CB2 G-protein-coupled receptors. Relev Neurodegen Dis Biochem Pharmacol. 2018;157:169–179. doi: 10.1016/j.bcp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Romano M. Lipid mediators: lipoxin and aspirin-triggered 15-epi-lipoxins. Inflamm Allergy Drug Targets. 2006;5:81–90. doi: 10.2174/187152806776383152. [DOI] [PubMed] [Google Scholar]
- Romano M. Lipoxin and aspirin-triggered lipoxins. Sci World J. 2010;10:1048–1064. doi: 10.1100/tsw.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M, Maddox JF, Serhan CN. Activation of human monocytes and the acute monocytic leukemia cell line (THP-1) by lipoxins involves unique signaling pathways for lipoxin A4 versus lipoxin B4: evidence for differential Ca2+ mobilization. J Immunol. 1996;157:2149–2154. [PubMed] [Google Scholar]
- Rosenbaum T, Awaya M, Gordon SE. Subunit modification and association in VR1 ion channels. BMC Neurosci. 2002;3:4. doi: 10.1186/1471-2202-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadik CD, Sezin T, Kim ND. Leukotrienes orchestrating allergic skin inflammation. Exp Dermatol. 2013;22:705–709. doi: 10.1111/exd.12239. [DOI] [PubMed] [Google Scholar]
- Saeki K, Yokomizo T. Identification, signaling, and functions of LTB4 receptors. Semin Immunol. 2017;33:30–36. doi: 10.1016/j.smim.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Salimi M, Stöger L, Liu W, Go S, Pavord I, Klenerman P, Ogg G, Xue L. Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines. J Allergy Clin Immunol. 2017;140:1090–1100.e11. doi: 10.1016/j.jaci.2016.12.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Sarau HM, Ames RS, Chambers J, Ellis C, Elshourbagy N, Foley JJ, Schmidt DB, Muccitelli RM, Jenkins O, Murdock PR, Herrity NC, Halsey W, Sathe G, Muir AI, Nuthulaganti P, Dytko GM, Buckley PT, Wilson S, Bergsma DJ, Hay DW. Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol Pharmacol. 1999;56:657–663. doi: 10.1124/mol.56.3.657. [DOI] [PubMed] [Google Scholar]
- Sasaki F, Yokomizo T. The leukotriene receptors as therapeutic targets of inflammatory diseases. Int Immunol. 2019;31:607–615. doi: 10.1093/intimm/dxz044. [DOI] [PubMed] [Google Scholar]
- Sauer CG, White K, Stöhr H, Grimm T, Hutchinson A, Bernstein PS, Lewis RA, Simonelli F, Pauleikhoff D, Allikmets R, Weber BH. Evaluation of the G protein coupled receptor-75 (GPR75) in age related macular degeneration. Br J Ophthalmol. 2001;85:969–975. doi: 10.1136/bjo.85.8.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Gemperle C, Rimann N, Hersberger M. Resolvin D1 Polarizes Primary Human Macrophages toward a Proresolution Phenotype through GPR32. J Immunol. 2016;196:3429–3437. doi: 10.4049/jimmunol.1501701. [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Eilers H. TRPV1 splice variants: structure and function. Front Biosci (landmark Ed) 2010;15:872–882. doi: 10.2741/3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JM, Cho KJ, Kim EY, Choi MH, Chung BC, Kim JH. Up-regulation of BLT2 is critical for the survival of bladder cancer cells. Exp Mol Med. 2011;43:129–137. doi: 10.3858/emm.2011.43.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JM, Park S, Kim JH. Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. J Biol Chem. 2012;287:13840–13849. doi: 10.1074/jbc.M111.317131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory–pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif NA, Williams GW, Davis TL. Pharmacology and autoradiography of human DP prostanoid receptors using [(3)H]-BWA868C, a DP receptor-selective antagonist radioligand. Br J Pharmacol. 2000;131:1025–1038. doi: 10.1038/sj.bjp.0703686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki H, Seki N, Fujita M, Kikuchi M, Kanaizumi E, Watanabe K, Himi T. Agonist- and T(H)2 cytokine-induced up-regulation of cysteinyl leukotriene receptor messenger RNA in human monocytes. Ann Allergy Asthma Immunol. 2007;99:340–347. doi: 10.1016/S1081-1206(10)60550-9. [DOI] [PubMed] [Google Scholar]
- Siangjong L, Goldman DH, Kriska T, Gauthier KM, Smyth EM, Puli N, Kumar G, Falck JR, Campbell WB. Vascular hepoxilin and trioxilins mediate vasorelaxation through TP receptor inhibition in mouse arteries. Acta Physiol (oxf) 2017;219:188–201. doi: 10.1111/apha.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K, Hennen S, Merten N, Blättermann S, Gillard M, Kostenis E, Gomeza J. The orphan G protein-coupled receptor GPR17 negatively regulates oligodendrocyte differentiation via Gαi/o and Its downstream effector molecules. J Biol Chem. 2016;291:705–718. doi: 10.1074/jbc.M115.683953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K, Merten N, Schröder R, Hennen S, Preis P, Schmitt NK, Peters L, Schrage R, Vermeiren C, Gillard M, Mohr K, Gomeza J, Kostenis E. The orphan receptor GPR17 is unresponsive to uracil nucleotides and cysteinyl leukotrienes. Mol Pharmacol. 2017;91:518–532. doi: 10.1124/mol.116.107904. [DOI] [PubMed] [Google Scholar]
- Soulet C, Sauzeau V, Plantavid M, Herbert JM, Pacaud P, Payrastre B, Savi P. Gi-dependent and -independent mechanisms downstream of the P2Y12 ADP-receptor. J Thromb Haemost. 2004;2:135–146. doi: 10.1111/j.1538-7836.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- Southern C, Cook JM, Neetoo-Isseljee Z, Taylor DL, Kettleborough CA, Merritt A, Bassoni DL, Raab WJ, Quinn E, Wehrman TS, Davenport AP, Brown AJ, Green A, Wigglesworth MJ, Rees S. Screening β-arrestin recruitment for the identification of natural ligands for orphan G-protein-coupled receptors. J Biomol Screen. 2013;18:599–609. doi: 10.1177/1087057113475480. [DOI] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ständer S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz BM, Luger TA, Metze D, Steinhoff M. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- Steinke JW, Negri J, Payne SC, Borish L. Biological effects of leukotriene E4 on eosinophils. Prostaglandins Leukot Essent Fatty Acids. 2014;91:105–110. doi: 10.1016/j.plefa.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana C, Shen Y, Rattan V, Kalra VK. Lipoxygenase metabolites induced expression of adhesion molecules and transendothelial migration of monocyte-like HL-60 cells is linked to protein kinase C activation. J Cell Physiol. 1996;167:477–487. doi: 10.1002/(SICI)1097-4652(199606)167:3<477::AID-JCP12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Sun YP, Tjonahen E, Keledjian R, Zhu M, Yang R, Recchiuti A, Pillai PS, Petasis NA, Serhan CN. Anti-inflammatory and pro-resolving properties of benzo-lipoxin A(4) analogs. Prostaglandins Leukot Essent Fatty Acids. 2009;81:357–366. doi: 10.1016/j.plefa.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szekeres CK, Tang K, Trikha M, Honn KV. Eicosanoid activation of extracellular signal-regulated kinase1/2 in human epidermoid carcinoma cells. J Biol Chem. 2000;275:38831–38841. doi: 10.1074/jbc.M002673200. [DOI] [PubMed] [Google Scholar]
- Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talat N, Diaz-Cano S, Schulte KM. Inflammatory diseases of the parathyroid gland. Histopathology. 2011;59:897–908. doi: 10.1111/j.1365-2559.2011.04001.x. [DOI] [PubMed] [Google Scholar]
- Tang DG, Chen YQ, Honn KV. Arachidonate lipoxygenases as essential regulators of cell survival and apoptosis. Proc Natl Acad Sci USA. 1996;93:5241–5246. doi: 10.1073/pnas.93.11.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MD, Capra V, Clunes MT, Rovati GE, Stankova J, Maj MC, Duffy DL. Cysteinyl leukotrienes pathway genes, atopic asthma and drug response: from population isolates to large genome-wide association studies. Front Pharmacol. 2016;7:299. doi: 10.3389/fphar.2016.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tong WG, Ding XZ, Talamonti MS, Bell RH, Adrian TE. LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem Biophys Res Commun. 2005;335:949–956. doi: 10.1016/j.bbrc.2005.07.166. [DOI] [PubMed] [Google Scholar]
- Town MH, Casals-Stenzel J, Schillinger E. Pharmacological and cardiovascular properties of a hydantoin derivative, BW 245 C, with high affinity and selectivity for PGD2 receptors. Prostaglandins. 1983;25:13–28. doi: 10.1016/0090-6980(83)90131-4. [DOI] [PubMed] [Google Scholar]
- Trauelsen M, Lückmann M, Frimurer TM, Schwartz TW. The HETE is on FFAR1 and pancreatic islet cells. Cell Metab. 2018;27:273–275. doi: 10.1016/j.cmet.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Tunaru S, Chennupati R, Nüsing RM, Offermanns S. Arachidonic acid metabolite 19(S)-HETE induces vasorelaxation and platelet inhibition by activating prostacyclin (IP) receptor. PLoS One. 2016 doi: 10.1371/journal.pone.01636333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunaru S, Bonnavion R, Brandenburger I, Preussner J, Thomas D, Scholich K, Offermanns S. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat Commun. 2018;9:177. doi: 10.1038/s41467-017-02539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics Tissue-based map of the human proteome. Science. 2015 doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- Veldhuis NA, Lew MJ, Abogadie FC, Poole DP, Jennings EA, Ivanusic JJ, Eilers H, Bunnett NW, McIntyre P. N-glycosylation determines ionic permeability and desensitization of the TRPV1 capsaicin receptor. J Biol Chem. 2012;287:21765–72172. doi: 10.1074/jbc.M112.342022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetri F, Saha Roy Choudhury M, Pelligrino DA, Sundivakkam P. BKCa channels as physiological regulators: a focused review. J Receptor Ligand Channel Res. 2014 doi: 10.2147/JRLCR.S36065. [DOI] [Google Scholar]
- Vos MH, Neelands TR, McDonald HA, Choi W, Kroeger PE, Puttfarcken PS, Faltynek CR, Moreland RB, Han P. TRPV1b overexpression negatively regulates TRPV1 responsiveness to capsaicin, heat and low pH in HEK293 cells. J Neurochem. 2006;99:1088–1102. doi: 10.1111/j.1471-4159.2006.04145.x. [DOI] [PubMed] [Google Scholar]
- Waechter V, Schmid M, Herova M, Weber A, Günther V, Marti-Jaun J, Wüst S, Rösinger M, Gemperle C, Hersberger M. Characterization of the promoter and the transcriptional regulation of the lipoxin A4 receptor (FPR2/ALX) gene in human monocytes and macrophages. J Immunol. 2012;188:1856–1867. doi: 10.4049/jimmunol.1101788. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Shimizu T, Miki I, Sakanaka C, Honda Z, Seyama Y, Teramoto T, Matsushima T, Ui M, Kurokawa K. Characterization of the guinea pig lung membrane leukotriene D4 receptor solubilized in an active form. association and dissociation with an islet-activating protein-sensitive guanine nucleotide-binding protein. J Biol Chem. 1990;265:21237–21241. doi: 10.1016/S0021-9258(17)45351-8. [DOI] [PubMed] [Google Scholar]
- Wauquier F, Philippe C, Léotoing L, Mercier S, Davicco MJ, Lebecque P, Guicheux J, Pilet P, Miot-Noirault E, Poitout V, Alquier T, Coxam V, Wittrant Y. The free fatty acid receptor G protein-coupled receptor 40 (GPR40) protects from bone loss through inhibition of osteoclast differentiation. J Biol Chem. 2013;288:6542–6551. doi: 10.1074/jbc.M112.429084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Östman J, Bubb KJ, Panayiotou C, Priestley JV, Baker MD, Ahluwalia A. 20-Hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of transient receptor potential vanilloid 1 (TRPV1) channel. J Biol Chem. 2012;287:13868–13876. doi: 10.1074/jbc.M111.334896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Bey Y, Boularan C, Vural A, Huang NN, Hwang IY, Shan-Shi C, Kehrl JH. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS ONE. 2014;9:e97957. doi: 10.1371/journal.pone.0097957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittamer V, Grégoire F, Robberecht P, Vassart G, Communi D, Parmentier M. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem. 2004;279:9956–9962. doi: 10.1074/jbc.M313016200. [DOI] [PubMed] [Google Scholar]
- Woszczek G, Chen LY, Nagineni S, Alsaaty S, Harry A, Logun C, Pawliczak R, Shelhamer JH. IFN-gamma induces cysteinyl leukotriene receptor 2 expression and enhances the responsiveness of human endothelial cells to cysteinyl leukotrienes. J Immunol. 2007;178:5262–5270. doi: 10.4049/jimmunol.178.8.5262. [DOI] [PubMed] [Google Scholar]
- Woszczek G, Chen LY, Nagineni S, Kern S, Barb J, Munson PJ, Logun C, Danner RL, Shelhamer JH. Leukotriene D(4) induces gene expression in human monocytes through cysteinyl leukotriene type I receptor. J Allergy Clin Immunol. 2008;121:215–221.e1. doi: 10.1016/j.jaci.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Liu Z, Cao J, Ma Q, Gao X, Wang Q, Jin C, Zhou Y, Wen L, Ren J. GPS 2.1: enhanced prediction of kinase-specific phosphorylation sites with an algorithm of motif length selection. Protein Eng Des Sel. 2011;24:255–260. doi: 10.1093/protein/gzq094. [DOI] [PubMed] [Google Scholar]
- Yan D, Stocco R, Sawyer N, Nesheim ME, Abramovitz M, Funk CD. Differential signaling of cysteinyl leukotrienes and a novel cysteinyl leukotriene receptor 2 (CysLT2) agonist, N-methyl-leukotriene C4, in calcium reporter and β arrestin assays. Mol Pharmacol. 2011;79:270–278. doi: 10.1124/mol.110.069054. [DOI] [PubMed] [Google Scholar]
- Yang F, Zhang Y, Ren H, Wang J, Shang L, Liu Y, Zhu W, Shi X. Ischemia reperfusion injury promotes recurrence of hepatocellular carcinoma in fatty liver via ALOX12-12HETE-GPR31 signaling axis. J Exp Clin Cancer Res. 2019;38:489. doi: 10.1186/s13046-019-1480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, Nguyen DG, Caldwell JS, Chen YA. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem. 2009;284:12328–12338. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T, Kato K, Hagiya H, Izumi T, Shimizu T. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J Biol Chem. 2001;276:12454–12459. doi: 10.1074/jbc.M011361200. [DOI] [PubMed] [Google Scholar]
- Yokomizo T, Nakamura M, Shimizu T. Leukotriene receptors as potential therapeutic targets. J Clin Invest. 2018;128:2691–2701. doi: 10.1172/JCI97946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Alonso-Galicia M, Sun CW, Roman RJ, Ono N, Hirano H, Ishimoto T, Reddy YK, Katipally KR, Reddy KM, Gopal VR, Yu J, Takhi M, Falck JR. 20-hydroxyeicosatetraenoic acid (20-HETE): structural determinants for renal vasoconstriction. Bioorg Med Chem. 2003;11:2803–2821. doi: 10.1016/s0968-0896(03)00192-5. [DOI] [PubMed] [Google Scholar]
- Zhang C, Booz GW, Yu Q, He X, Wang S, Fan F. Conflicting roles of 20-HETE in hypertension and renal end organ damage. Eur J Pharmacol. 2018;833:190–200. doi: 10.1016/j.ejphar.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JX, Liao D, Zhang S, Cheng N, He HQ, Ye RD. Chemerin C9 peptide induces receptor internalization through a clathrin-independent pathway. Acta Pharmacol Sin. 2014;35:653–663. doi: 10.1038/aps.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingoni A, Rocchi M, Storlazzi CT, Bernardini G, Santoni A, Napolitano M. Isolation and chromosomal localization of GPR31, a human gene encoding a putative G protein-coupled receptor. Genomics. 1997;42:519–523. doi: 10.1006/geno.1997.4754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.