Abstract
Background
Developmental dysplasia of hip (DDH) is a common disorder of childhood and has a good prognosis when treated at an early age. In spite of being a significant concern, many children with DDH are not picked early and present late at walking age. In our country, it is presumed to be due to absence of a national policy for screening of DDH. Screening programmes including the combination of clinical and radiological methods in different ways have been suggested. However, the exact method of screening is controversial.
Purpose
To analyze effectiveness and cost-effectiveness of various screening methods for DDH.
Study Design
Systematic review.
Methods
This review was conducted in accordance with PRISMA guidelines. Medline database was explored for original case series and randomized clinical trials. Inclusion criteria were English language, screening for DDH in neonates, sample size more than 500, and studies with minimum duration of one year.
Results
Thirty-four studies were selected to write the manuscript. This included 23 studies looking for effectiveness of a screening programme and 11 studies comparing various outcomes of different screening strategies. A trend favoring universal ultrasound screening was observed
Conclusion
The literature supports universal ultrasound screening and has proved its cost-effectiveness. However, considering the logistic and financial challenges in our country, immediate implementation of universal ultrasound screening seems impractical. In the absence of any current guidelines for screening for DDH in India, we suggest professional organizations involved in the care of children and public health policy-makers to come together to develop national screening guidelines for DDH.
Keywords: Developmental dysplasia of hip, Newborn, Clinical screening, Ultrasonography, Selective, Universal
Introduction
Developmental dysplasia of hip (DDH) is a common disorder of childhood with global incidence of 1.6–28.5 per 1000 live births [1]. It refers to a spectrum of hip problems ranging from stable hip with mild acetabular dysplasia to more severe forms with frank dislocation of hip joint. When detected early, it can be treated effectively with simpler means which are readily available and acceptable to the family and owns good prognosis [2]. Treatment of late-presenting DDH is complex and needs surgical intervention. This increases morbidity, cost of treatment, and overall burden on healthcare [2, 3]. Previous studies have shown an incidence of up to 9.2 per 1000 live births for DDH in India [4, 5]. Looking at the huge population of our country, a large number of cases can be anticipated. In spite of being a significant concern for paediatricians and orthopaedicians, many children with DDH are not picked early and present late at walking age [6]. In India, this can be attributed to absence of a national policy for screening of DDH as clinicians do not feel it obligatory to screen every newborn. Furthermore, the ideal screening method for DDH is yet to be sought. Screening programmes including combination of clinical and radiological methods in different ways have been suggested and are being used in different countries [7, 8]. However, exact method of screening is controversial. Through this study, an attempt was made to analyze effectiveness of various screening methods for DDH in decreasing the rate of late diagnosed DDH (LDD) and their cost-effectiveness was studied.
Materials and Methods
This systematic review was performed as per PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [9]. To avoid ambiguity as much as possible, inclusion and exclusion criteria for selection of studies were decided before initiating literature search. The Medline database was explored using combination of MeSh terms; DDH, newborn, clinical screening, and ultrasonography using PubMed, EMBASE, and Google Scholar. Only original case series and randomized clinical trials were included. Related reviews were searched for additional references. Inclusion criteria were English language, screening for DDH in neonates (age < 4 weeks), sample size more than 500, and studies with minimum duration of 1 year. This included studies looking for diagnostic effectiveness of a screening method for DDH in newborns and those comparing one screening method with another in terms of outcome. Case reports, reviews, letter to the editor, and unpublished data were excluded from final list of studies. Attempt was made to eliminate bias in selecting studies through factors based on reliability, internal consistency, and empirical evidence if available. The original studies with available full-length articles were collected. Bibliography of all eligible studies was explored further to find additional studies. Any difference in opinion between the authors was discussed and resolved. Selected studies were assessed for quality using modified Jadad Scale [10] for randomized-controlled trial and GRADE [11] (Grading of Recommendations, Assessment, Development and Evaluation) for case series and those with better scientific evidence were selected to write the manuscript. The selected effectiveness studies were reviewed for method of screening and its effectiveness in decreasing incidence of LDD. Comparative studies were explored for various outcomes of different screening methods like sensitivity, specificity, treatment rate, mean age at treatment, mean age at first operation, cost-effectiveness of each method, and challenges encountered, in implementation of screening strategy.
Results
Two thousand nine hundred seventy-seven studies were screened initially after online search and manual search of bibliography of individual articles (Fig. 1). After further screening, 375 studies on screening for DDH in neonatal age were identified. 106 studies met inclusion criteria, and after quality assessment, 34 were selected to write the manuscript (Tables 1, 2, 3 and 4) [6, 7, 12–43]. This included 23 studies looking for effectiveness of a screening programme and 11 studies comparing various outcomes of two or more screening strategies. Current review covers around 67 lakh neonates screened by one or more methods and the largest series was by Broadhurst et al. [6]. Duration of studies ranged between 1 and 40 years (mean 10.32 years) and longest study was by Myers et al. [7]. Different age criteria were used for definition of late presentation. Majority considered cases presenting after 3 months as late presentation [13–16, 20–23, 34]. Duppe et al. [37] used the shortest cut off mark of 1 week, whereas Broadhurst et al. [6], Milligan et al. [24], and Donnelly et al. [25] used the longest period of 12 months.
Fig. 1.

Flowchart of literature search as per PRISMA guidelines
Table 1.
Studies evaluating effectiveness of clinical screening alone
| Sr no | Authors (year) | Sample size (N) | Method of screening | Duration of study (years) | Rate of LDD (per 1000 live births) (definition of LDD) | Conclusion |
|---|---|---|---|---|---|---|
| 1 | Lehmann et al. 1981 [12] | 176,000 | UC at birth by orthopaedic surgeon vs orthopaedic resident | 5 |
Surgeon 0.3 Resident 0.8 (> 4 weeks) |
Incidence of LDD decreased and was further less in experienced hands. Recommended UC screening |
| 2 | Wenger et al. 2019 [13] | 1,013,589 | UC in neonates by paediatrician. At 6–8 weeks, 6 months and 10–12 months by GP | 18 |
0.12 (> 2 weeks) |
Incidence of LDD, age at diagnosis and disease severity decreased. Recommends serial clinical hip examination after initial screening at birth |
| 3 | Studer et al. 2016 [14] | > 100,000 | UC in neonates by an experienced health practitioner. At 6–8 weeks and 6–9 months by GP or paediatrician | 11 |
0.77 (> 3 months) |
Incidence of LDD increased despite the screening programme. Advised for increased awareness, education and to avoid inappropriate swaddling |
| 4 | Myers et al. 2009 [7] | 41,563 | UC within a week of delivery by an orthopaedic surgeon | 4 |
0.1 (> 6 months) |
UC screening by experienced hand significantly reduces the incidence of late-presenting (walking) DDH |
UC universal clinical, LDD late diagnosed DDH, GP general practitioner
Table 2.
Studies evaluating effectiveness of universal clinical and selective ultrasound screening
| Sr no. | Authors (year) | Sample size (N) | Method of screening | Duration of study (years) | Rate of LDD (per 1000 live births) (definition of LDD) | Conclusion |
|---|---|---|---|---|---|---|
| 1 | Broadhurst et al. 2019 [6] | 3,635,163 | UC within 72 h and S-USG at 2/6–8 weeks. Further clinical assessment at 6–8 weeks by GP | 26 |
1.28 (> 12 months) |
Incidence of LDD did not reduce. Recommends further research and debate for use of U-USG screening |
| 2 | Talbot et al. 2017 [15] | 64,670 | UC at birth and 6–8 weeks and S-USG at 6–8 weeks | 15 |
0.28 (> 3 months) |
LDD still occurs. Recommends for update of screening programme, review training of physicians and further assessment after 6 week check |
| 3 | Clarke et al. 2012 [16] | 107,440 | UC within 72 h and S-USG at 2/6 weeks. Further clinical assessment at 6 weeks by GP | 20 |
0.34 (> 3 months) |
Recommends UC and S-USG screening, one stop treatment and further clinical monitoring till 12 months |
| 4 | Geertsema et al. 2019 [17] | 3536 | UC at 2–3 weeks and S-USG at 6 weeks/3 months | 4 |
Persistent DDH at 18 months 0.3% |
Delayed S-USG screening at 3 months in children with clinically stable hip but having risk factors for DDH is safe and this approach could prevent overtreatment |
| 5 | Laborie et al. 2014 [18] | 81,564 | UC and S-USG at 1–3 days and further clinical assessment at 6 weeks, 6 months and 1 year | 16 |
0.32 (> 4 weeks) |
Recommends S-USG screening as it gives acceptable rates of early treatment and low rates of LDD |
| 6 | Paton et al. 2002 [19] | 28,676 | UC within 48 h and S-USG at 2/6 weeks. Further clinical assessment at 6 weeks by GP | 8 |
0.87 (> ?) |
Recommended against national S-USG screening as it did not reduce the overall rate of LDD |
| 7 | Lisle et al. 2011 [20] | 30,000 | UC at birth, 6 weeks, 3, 6 and 12 months. S-USG at 6 weeks | 1 |
0.57 (> 3 months) |
Incidence of LDD increased three times the previously established rate. Suggested further research |
| 8 | Chan et al. 1999 [21] | 118,379 | UC at birth and 6 weeks. S-USG at 6 weeks. Further clinical check till 2 ½ years | 5 |
0.19 (> 3 months) |
Recommends UC and S-USG screening with further clinical assessment in later life |
| 9 | Sahin et al. 2004 [22] | 5798 | UC at birth. S-USG at 3–8 weeks. Further clinical assessment at 2, 4, 6, 9 and 18 months | 7 |
1.37 (> 3 months) |
Recommends S-USG screening and serial clinical hip examinations till walking age |
| 10 | Boeree et al. 1994 [23] | 26,952 | UC at birth and S-USG at 2/4–6 weeks and clinical assessment at 6 weeks | 4 |
0.22 (> 3 months) |
Delayed S-USG screening is more effective than UC screening alone |
| 11 | Milligan et al. 2020 [24] | 170,580 | UC and S-USG within 6 weeks and further clinical assessment at 4 months | 6 |
0.30 (> 12 months) |
Continuous monitoring of a S-USG screening programme improves the outcome |
| 12 | Donnelly et al. 2015 [25] | 75,856 | UC at birth, 6–8 weeks and 4 month combined with S-USG within 10 days or at 6 weeks | 2 |
0.42 (> 12 months) |
Recommends additional clinical assessment at 4 months in addition to UC at birth and S-USG |
| 13 | Choudry et al. 2018 [26] | 28,241 | UC within 72 h + S-USG at 2/6–8 weeks. Further clinical assessment at 6–8 weeks by GP | 4 |
– (> 6 weeks) |
A decrease in positive predictive value (PPV) of screening and increase in rate of surgery was observed |
UC universal clinical, S-USG selective ultrasonography, LDD late diagnosed DDH, GP general practitioner
Table 3.
Studies evaluating effectiveness of universal ultrasound screening
| Sr no. | Authors (year) | Sample size (N) | Method of screening | Duration of study (years) | Rate of LDD (per 1000 live births) (definition of LDD) | Conclusion |
|---|---|---|---|---|---|---|
| 1 | Thallinger et al. 2014 [27] | > 80,000 | UC + U-USG at birth and 6–8 weeks | 16 | – | U-USG screening produces a distinct and progressive decrease in number of hip surgeries |
| 2 | Ulziibat et al. 2020 [28] | 176,388 | U-USG at 1–2 days after birth | 2 | – | U-USG screening provides high screening, quality surveillance and appropriate treatment rate. It can be deployed successfully in developing countries |
| 3 | Biedermann et al. 2018 [29] | 28,092 | U-USG at birth and UC + U-USG at 6–8 weeks | 5 | 00 | U-USG screening reduced rate of secondary DDH surgery later in life. Recommends early U-USG screening |
| 4 | Von kries et al. 2003 [30] | 50,000 | UC and U-USG before 6 weeks | 5 |
0.14 (> 9 weeks) |
Recommends early U-USG screening and suggests for training of doctors in doing USG |
| 5 | Marks et al. 1994 [31] | 14,050 | U-USG | 3 | 00 | Recommends U-USG screening |
| 6 | Olsen et al. 2018 [32] | 4245 | UC + U-USG within 3 days | 8 |
0.5 (> 4 weeks) |
Adding U-USG to UC screening doubled the treatment rate without a significant reduction in incidence of LDD |
UC universal clinical, U-USG universal ultrasonography, LDD late diagnosed DDH
Table 4.
Comparative analysis of different screening strategies
| Sr no | Authors | Sample size (N) |
Methods compared | Duration of study (years) |
Definition of LDD | NS | UC | UC + S-USG | UC + U-USG | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tredwell et al. 1990 [33] | – |
NS UC |
– | – | TC 19,101.75$/1000 live births |
TC 3391.74$/1000 live births |
– | – | UC is more cost-effective and has economic benefit of 15,143.71$/1000 live births |
| 2 | Rosendahl et al. 1994 [34] | 11,925 |
UC UC + S-USG UC + U-USG (1st week) |
2 | > 3 months | – |
TR 18 LDD 1.3 |
TR 20 LDD 0.7 |
TR 34 LDD 0.3 |
Effect of USG screening in reducing incidence of LDD was not statistically significant |
| 3 | Geitung et al. 1996 [35] | – |
UC UC + U-USG |
– | – | – | TC 17,205,620 NOK | – | TC 16,900,000 NOK | UC + U-USG is not cost-effective. However, U-USG is cost-effective when UC is omitted. Recommends selective screening |
| 4 | Clegg et al. 1999 [36] | – |
UC UC + S-USG UC + U-USG |
20 | – | – |
SI 6.5/year AFO 12.4 MSC 5110£/1000 live births |
SI 5.4/year AFO 14.2 MSC 3811£/1000 live births |
SI 2.5/year AFO 6.7 MSC 468£ / 1000 live births |
Overall cost for management of DDH was comparable. Recommends universal USG screening |
| 5 | Duppe et al. 2002 [37] | 55,267 |
UC and UC + S-USG (1st week) |
20 | > 1 week | – |
LDD 0.51 TR 19.9 RR 31.7 |
LDD 0.07 TR 6.6 RR 12.9 |
– | Recommends UC + S-USG |
| 6 | Holen et al. 2002 [38] | 15,529 |
UC + S-USG UC + U-USG (1st week) |
5 | > 4 weeks | – | – |
TR 8.6 LDD 0.65 |
TR 9.6 LDD 0.13 |
Recommends S-USG as difference was not statistically significant |
| 7 | Dezateux et al. 2003 [39] | 400,000 |
NS UC UC + S-USG UC + U-USG |
– | – |
TR 0.12% SR 0% SI 0.12% FTO 75% |
PSR 2.1% DR 35% TP 0.04% FP 2.08% TN 97.79% FN 0.078% TR 0.52% SR 0.42% SI 0.10% FTO 78% |
PSR 8.1% DR 60% TP 0.07% FP 8.05% TN 91.82% FN 0.04% TR 0.75% SR 0.70% SI 0.05% FTO 88% |
PSR 7.7% DR 76% TP 0.09% FP 7.57% TN 90.31% FN 0.02% TR 0.54% SR 0.51% SI 0.03% FTO 92% |
Clinical screening alone is only marginally beneficial when compared to no screening. USG-based screenings are more effective |
| 8 | Roovers et al. 2005 [40] | 7236 |
UC UC + U-USG (1, 2, 3 month) |
14 months | > 3 months | – |
TR 27 RR 192 LDD 8 S 76.4% |
– |
TR 46 RR 76 LDD 6 S 88.5% |
U-USG detects more cases of DDH and at an early age. However, it leads to overtreatment. Recommends delayed U-USG at 2–3 months |
| 9 | Gray et al. 2005 [41] | 629 |
UC UC + U-USG |
3 | – | – |
SR 48% SI 8% TC 1488$ / patient |
– |
SR 37% SI 7% TC 1298$ / patient |
U-USG does not increase cost burden on health services and family and decreases SR & SI. Recommends UC + U-USG |
| 10 | Thaler et al. 2011 [42] | 83,016 |
UC And UC + U-USG (1st week) |
8 | – | – |
CR 25.2/year SI 17.8/year TC 410,000 €/year |
– |
CR 7.0/year SI 2.6/year TC 467,000 €/year |
Number of interventions decreased by 75.9% since introduction of U-USG and it is cost-effective. Recommends UC + U-USG |
| 11 | Tschauner et al. 2011 [43] | – |
UC UC + U-USG (< 6 weeks) |
30 | – | – |
AIT 5.5 CR 88.7% OR 11.3% |
– |
AIT 2 CR 98.9% OR 1.1% |
U-USG-based treatment is safer, shorter, and simpler. Recommends UC + U-USG |
NS no screening, UC universal clinical, S-USG selective ultrasonography, U-USG universal ultrasonography, LDD Late Diagnosed DDH (per 1000 live births), TR treatment rate (per 1000 live births), RR referral rate (per 1000 live births), CR close reduction, OR open reduction, SI surgical intervention, TC total cost, PSR positive screening result, DR detection rate, TP true positive, FP false positive, TN true negative, FN false negative, SR splintage rate, FTO favourable treatment outcome, S sensitivity, AIT age at initial treatment (months), AFO age at first operation (months), MSC mean surgical cost
Screening methods discussed for their effectiveness were either universal clinical alone or combination of universal clinical with selective or universal ultrasonography (USG). Four studies observed outcomes of universal clinical neonatal screening alone and analyzed its effectiveness in reducing the incidence LDD (Table 1) [7, 12–14]. Three of them found it effective when compared to earlier data in their respective area before implementing the screening programme [7, 12, 13]. They observed that incidence was further less when screening was done by an experienced physician and when serial clinical hip examinations were done. It also effectively reduced the mean age at diagnosis of DDH and disease severity. However, Studer et al. [14] reported increased incidence of LDD despite universal clinical screening in neonates and repeated hip examination at 6–8 weeks and 6–9 months. They advised for more education and awareness among healthcare workers and for avoiding inappropriate swaddling of infants to reduce incidence of late onset DDH. Mean incidence of LDD after universal clinical neonatal screening alone was 0.37 / 1000 live births (range 0.1–0.77/1000 live births).
Thirteen studies analyzed effectiveness of universal clinical neonatal screening combined with selective USG screening (Table 2) [6, 15–26]. Clinical examination of hip was performed unanimously in the first week of life by all. However, there was discrepancy in timing of doing the USG evaluation. Laborie et al. [18] and Olsen et al. [32] performed the USG scan early in first week, while Geertsema et al. [17] did the same at 3 months. However, majority scanned hip between 3 and 8 weeks of life [15, 20, 22, 24]. Five studies followed a differential strategy and scanned the unstable hips early within 2 weeks, whereas those with normal clinical screening but with risk factors for DDH were scanned at 6 weeks [6, 16, 23, 25, 26]. Eleven out of 13 studies maintained further check by repeat clinical hip examination till age between 6 weeks and 30 months [6, 15–18, 21–26]. This strategy decreased the incidence of LDD when compared with single hip examination; however, none of these were successful in completely eliminating it. Satisfactory outcome of a selective screening programme was reported by eight studies [16–18, 21–25], whereas five suggested further research and debate regarding introduction of universal USG screening [6, 15, 19, 20, 26]. The mean incidence of LDD after universal clinical and selective USG screening was 0.53/1000 live births (range 0.19–1.37/1000 live births).
Six studies reported outcome of universal USG screening of hip for DDH and five observed satisfactory results (Table 3) [27–32]. Two of them claimed that it eliminated incidence of LDD after following newborns till 5 years of age [29, 31]. The incidence of LDD was not mentioned specifically in two studies, but they recommended this method after observing a distinct and progressive decrease in number of hip surgeries and an acceptable treatment rate [27, 28]. They further suggested for training of doctors in doing USG scans. Contradictory to this, Olsen et al. [32] reported significant increase in treatment rate without any significant reduction in incidence of LDD. Mean incidence of LDD after universal USG screening was 0.13/1000 live births (range 00–0.5/1000 live births).
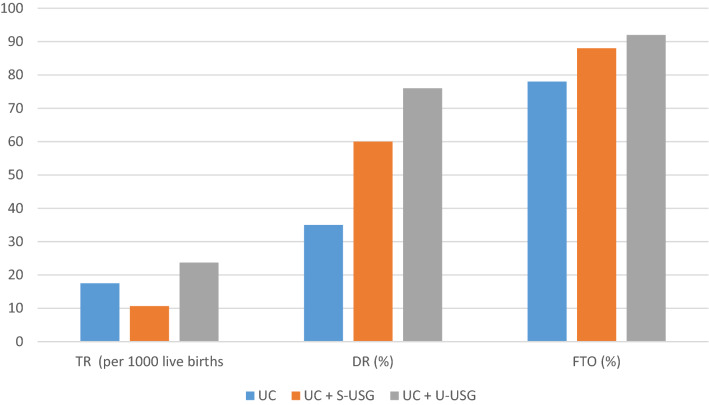
Comparative analysis of different screening strategies was done by 11 studies (Table 4) [33–43]. This included their sensitivity, specificity, overall referral rate, overall treatment rate, overall splintage rate, number of surgical interventions, mean age at initial treatment, favourable treatment outcome, mean age at first operation, incidence of LDD, and cost-effectiveness. Tredwell et al. [33] reported that universal clinical screening alone was more cost-effective when compared to no screening for DDH. Dezateux et al. [39] observed only marginal benefit of isolated clinical screening and found the USG-based screenings more effective. However, a preference between selective and universal USG screening was not made as both methods had their own limitations. For selective USG screening, this included uncertainty about indications of doing scan and ultrasound indications for splint therapy. For universal USG screening, this included high false-positive rate and unnecessary treatment of normal children with consequent increase in complications of abduction splinting like avascular necrosis, femoral nerve palsy, pressure sores, and parental anxiety. Similar results about universal USG screening were observed by Roovers et al. [40] and they suggested for delayed screening at 2–3 months to avoid overtreatment. Overall, universal USG screening showed increase in treatment rate but a better detection rate and favourable treatment outcome (Fig. 2). At the end, six studies reported universal USG to be cost-effective, and supported combination of universal clinical and universal USG screening [33, 36, 40–43]. Two studies suggested against it and recommended for combination of universal clinical and selective USG screening [34, 35, 38].
Fig. 2.
Comparison of outcomes of different screening methods. TR treatment rate, DR detection rate, FTO favourable treatment outcome, UC universal clinical, S-USG selective ultrasonography, U-USG universal ultrasonography
Discussion
Screening of newborns for hip instability is being practiced for several decades with an aim to reduce incidence of late-presenting DDH [7, 8]. However, there is lack of consensus about the ideal method. An ideal screening method should be easy to use, readily available, cost-effective and acceptable to the patient. It should also have adequate sensitivity and specificity to detect the problem. Broadly, screening for DDH involves use of clinical examination of hip, radiological investigations, or combination of both (Table 5). Till now, none of the programmes have been successful in eliminating incidence of late-presenting DDH. Shorter et al. [44] in his Cochrane review, did not find any conclusive evidence to recommend a particular method of screening.
Table 5.
Various screening methods for DDH and their respective mean incidence of late diagnosed DDH
| Method of screening | Description | Method used | Mean incidence of late diagnosed DDH (per 1000 live births) |
|---|---|---|---|
| UC | Clinical hip examination of all newborns for signs of instability and risk factors of DDH |
Barlow test Ortolani test |
0.37 |
| UC + S-USG |
Clinical hip examination of all newborns USG of hip for those with positive clinical screening USG of hip for those with negative hip screening but having one or more risk factors of DDH and those with an inconclusive clinical screening |
Clinical: Barlow test Ortolani sign USG: Graf’s method |
0.53 |
| UC + U-USG |
Clinical hip examination of all newborns USG of hip for all newborns |
Clinical: Barlow test Ortolani sign USG: Graf’s method |
0.13 |
In absence of a national screening programme, risk factor-based screening is resorted to by individual practitioners [45, 46]. Newborns with one or more risk factors for DDH are examined clinically and/or sonographically to confirm hip instability. The knowledge of these risk factors and awareness of possible hip abnormality is a prerequisite to avoid missing DDH. Major known risk factors are family history of DDH and breech presentation. The minor risk factors include first born child, female gender, oligohydramnios, prematurity, twins, excess birth weight, torticollis, and post-natal swaddling [14, 47]. An increased risk of DDH was also observed with idiopathic clubfoot deformity and high altitude [48, 49]. Sixty percent of overall dislocations have been reported to occur in children with one or more risk factors. Furthermore, the risk of DDH increases with increased number of risk factors [50]. However, mere presence of a risk factor does not necessarily indicate presence of DDH. Paton et al. [51] reported much less incidence of dislocation (1 in 75) in infants with risk factor when compared to those with clinical instability (1 in 11). Similarly, DDH is often detected in children without any risk factor. Thus, screening for risk factors alone is not recommended as an ideal method of screening for DDH.
Effectiveness of universal clinical neonatal screening in reducing incidence of late diagnosis of DDH was reported by three out of four studies in current review [7, 12, 13]. Moreover, decrease in age at detection and disease severity was observed [13]. Commonly used clinical tests were Barlow test, Ortolani sign, limited hip abduction, asymmetric thigh folds, and limb length discrepancy. A combination of Ortolani and Barlow test with specificity of 95% is the most commonly used method [12, 13]. They are easy to perform and lack cost issues. However, they are less sensitive (28%) and are unable to pick up irreducible dislocation [13]. Similarly, subluxable hip and dysplasia without dislocation are not detected and may progress and present later [7]. The signs can be very subtle in a newborn posing challenge even to an experienced paediatric orthopaedic surgeon [52]. Other clinical tests may indicate presence of DDH, yet are not suitable as a routine screening test due to poor sensitivity and specificity [53, 54]. Effectiveness of a clinical screening programme alone in reducing the incidence of late presentation of DDH has been refuted by many studies [14]. Even increased incidence in spite of an ongoing clinical neonatal screening programme was observed [14]. However, effectiveness can be improved significantly if clinical screening is done by experienced physicians [12, 15]. The newborn physical examination standards and competencies programme was launched in UK aiming to ensure that all physicians involved in screening programme have adequate training and competency to do that [55]. It is also aimed to assess and audit their performance periodically. Where experienced physicians are not available, paramedics can be involved in the programme after adequate training without compromising the result [56].
DDH includes both morphological abnormality and instability of hip. Unlike an unstable hip, morphological errors are missed by clinical examination. Application of USG for diagnosis of congenital hip dislocation was first reported by Graf in 1980 [57]. Since then, use of USG has decreased the incidence of late presentation and overall, a fall in surgical rates [18, 23, 30]. It is more sensitive and specific when compared to clinical tests and picks up both morphological abnormality as well as instability (Table 6). It is safe, non-invasive and is the preferred radiological tool for screening DDH in children below 6 months of age when femoral head is still cartilaginous. Use of USG can be ‘universal’ or ‘selective’. There is lack of consensus about which method to prefer, definition of an abnormal hip, and importance of visible morphological abnormalities in USG. Controversy also persists regarding the timing of ultrasound screening [12, 15, 17, 20]. Graf et al. suggested USG screening of all newborns in first week of life [58]. Others favour late screening after 6 weeks as most unstable hips detected at birth become stable spontaneously by 4 weeks [23, 31]. In current review, six studies were in favour of early USG screening in first week, whereas seven studies supported screening after 3 weeks [15, 17, 20, 22, 24]. Five studies followed a differential strategy and scanned clinically unstable hips within 2 weeks, whereas delayed screening at 6–8 weeks was done for those with normal clinical screening but with risk factors for DDH [6,16,23,25,26]. USG screening is further criticized for having poor inter-observer and intra-observer reliability. Clarke technique of ultrasound assessment has been claimed to reduce this issue and has lower false-positive rate [59].
Table 6.
Statistical comparison of different methods of neonatal hip screening for DDH
| Presence of risk factor for DDH | Clinical examination (combined Barlow and Ortolani test) | Ultrasound examination (combined static and dynamic) | |
|---|---|---|---|
| Sensitivity (%) | 10 [22] | 62.59 [8], 100 [22], 18.5 [26], (mean 60.36) | 75.78 [8], 100 [22], 47.6 [26], (mean 74.46) |
| Specificity (%) | 98.1 [22] | 99.76 [8], 88.9 [22], 99.6 [26], (mean 96.08) | 99.85 [8], 98.5 [22], 99.6 [26], (mean 99.31) |
| Positive predictive value (%) | 0.9 [22] | 26.18 [8], 1.6 [22], 4.0 [26], (mean 10.59) | 55.08 [8], 50 [22], 16.1 [26], (mean 40.39) |
| Negative predictive value (%) | 99.8 [22] | 99.9 [8], 100 [22] (mean 99.95) | 99.9 [8], 100 [22] (mean 99.95) |
Eight out of thirteen studies in current review favoured use of combined universal clinical neonatal and selective USG screening. It effectively reduced incidence of LDD when compared to universal clinical screening alone [16, 18, 23]. However, Rosendahl et al. [34] found no significant difference when clinical screening was performed by experienced and well-trained physicians. When compared to universal USG screening, rate of LDD in selective USG group was more but was not statistically significant [38]. This method therefore can be more suitable for communities where cost and resource availability is an issue. The Indian experience for selective USG screening in a retrospective cohort of 23,925 term newborns has been encouraging [60]. Selective USG screening gives a low primary treatment rate when compared to universal USG screening (Fig. 2) [16]. Simultaneously, a number of surgical interventions for late presentations are decreased leading to reduced overall cost of diagnosis and management [16, 41]. However, similar rate of late-presenting DDH was reported in England before and after introduction of selective screening programme [6, 15]. To improve the outcome and balance cost-effectiveness, repeat clinical examination beyond neonatal age was advised. American Academy of Pediatrics [61] further advised for a mandatory referral of children with positive clinical findings to an orthopaedic surgeon. Rebello et al. [45] suggested to review dislocatable hips clinically at 2 and 6 weeks and further clinical screening of all children brought to immunization centers. Talbot et al. [15] reported late presentations despite adding clinical evaluation at 6 week and suggested for more screenings in infancy. They associated the initial screening done by less experienced staff with increased incidence of late presentation and recommended that an experienced physician should do the screening. The ongoing selective screening programme in Scotland was upgraded with enhanced detection services [62]. A trained personnel (physiotherapist/physician) was appointed in postnatal ward with responsibility of training and supporting paediatric and maternity staff in doing hip examination, increasing awareness about DDH, and running weekly clinic for secondary screening. This strategy effectively reduced the risk of first surgery for DDH.
Favourable results of universal USG screening were reported by five out of six studies in current review [27–31]. It was more effective in decreasing incidence of LDD when compared to clinical screening alone [27, 29, 30]. Mark et al. [31] and Biedermann et al. [29] found no case of late diagnosis and number of operative procedures decreased by 75.9% in Austria. However, it has not completely eliminated late presentation in other studies [27, 30]. Possible reasons could be technical errors in performing scan or misinterpretation of USG finding. In spite of a right diagnosis, DDH may be inadequately treated or results of treatment may not be satisfactory leading to late presentation. Moreover, onset of DDH itself may be late [63]. Universal USG screening has also been reported to have significant false-positive rate leading to overtreatment [35, 40]. Unnecessary treatment in these otherwise normal children expose them to iatrogenic complications, increases parental anxiety, and cost of treatment. Rosendahl et al. [34] observed a higher negative predictive value for USG but suggested for refining the criteria for initiating therapy to avoid overtreatment. It remains a matter of debate due to economic concerns. It also needs equipment and expert radiologist which may not be readily available and is an important concern in many countries. Furthermore, spontaneous resolution of neonatal hip dysplasia detected on USG has been reported [64]. Olsen et al. [32] reported doubling of treatment rate with universal USG screening without any significant decrease in incidence of late presentation and recommended against it. In current review, 6 out of 11 comparative studies reported universal USG to be cost-effective with better detection rate and supported its use [33, 36, 40–43]. A delayed scan at 2–3 months was suggested to avoid overtreatment [40]. Three comparative studies advised against it [34, 35, 38]. Holen et al. [38] did not find any significant difference in incidence of late presentation when compared with selective USG screening and stressed for a high-quality clinical screening and selective use of USG. Geitung et al. [35] did not find the universal USG screening programme cost-effective when combined with universal clinical screening. However, they observed it to be cost-effective if clinical screening was omitted and ultimately recommended for selective USG screening. At an international interdisciplinary consensus meeting on evaluation of DDH organized in 2018, use of universal USG screening was firmly supported [65]. It was observed that USG screening reduces long-term consequences, is cost-effective, and does not cause overtreatment. Early screening within 6 weeks was recommended and Graf technique was suggested as technique of choice [65].
Cost of running a national screening programme can be a concern particularly in developing nations. It includes expense of training the physician, fee of physician, neonatologist working hour, and cost of ultrasound machine. Expenses involved in DDH care include cost for conservative and operative treatment including splints. Benefit is represented by expected treatment cost at a later date due to missed diagnosis at neonatal age [66]. Cost of surgical intervention is far more when compared to conservative management. An early diagnosis decreases number of late-presenting DDH and cuts down cost of surgical intervention. Economic justification of universal clinical neonatal screening against no screening is well established [33]. However, superiority and cost-effectiveness of universal clinical screening over selective or universal USG screening is debatable. Clegg et al. [36] reported comparable expense of overall management after comparing clinical screening alone with those with addition of selective and universal ultrasound. They recommended for universal USG screening after observing statistically significant reduction in number of surgical interventions per year and mean age at first operation. Holen et al. [38] compared selective and universal USG screening and observed same incidence of late-presenting DDH. They suggested for selective USG screening as it would be less expensive. Bralic et al. [66] did cost–benefit analysis of managing DDH at different ages of presentation, thereby evaluating economic justification for universal USG screening in Croatia. They observed cost difference of US$195,337 in treating DDH at 1 month and 4 months of age. This was 1.6 times the total cost of USG screening of all live birth that year. Furthermore, treatment cost using hip replacement in 165 missed cases alone would have covered total screening cost in entire country. They reported universal USG screening to be highly cost-effective and recommended for its nationwide implementation. A retrospective study from Austria compared the cost-effectiveness of clinical screening alone with universal ultrasound screening and reported a significantly high overall cost of treatment with clinical screening alone [42]. Introduction of ultrasound decreased the rate of splintage in infants and rate of surgical procedures in children over 18 months of age. They also observed that use of ultrasound reduces uncertainty and parental anxiety and may further reduce the cost to health services and family. It was suggested that a recommendation for selective USG screening by earlier studies was due to their failure to acknowledge intermediate findings like number of additional clinical follow-up and number of splints used and due to including patients in learning phase of ultrasound use. In a similar study, Gray et al. [41] reported lower cost of splinting and surgical intervention in universal ultrasound group and concluded against increased cost burden by the use of universal ultrasound method.
For a disorder to undergo nationwide screening, it should have a significant incidence rate and a known natural history, and should be best treated early before usual age of clinical presentation without any screening. Early treatment of DDH is easy to practice, and is known to change its natural history and indications for doing so are well defined. With an incidence up to 9.2 per 1000 live births, DDH in India needs a nationwide screening programme [4, 5, 60]. As discussed earlier, ideal screening method for DDH is still debatable. Literature supports universal USG screening and has proved its cost-effectiveness. However, considering the logistic and financial challenges in our country like shortage of well-trained radiologists and lack of resources, immediate implementation of universal ultrasound screening seems impractical. There are not any current guidelines for DDH screening in India. The time is ripe for professional organizations involved in the care of children and public health policy-makers to come together to develop national screening guidelines for DDH. The authors hope that the current systematic review of literature from countries which have well-established DDH screening programmes may assist them in formulating guidelines which are contextually relevant, practical, and cost-effective for the existing healthcare system in India.
Author Contributions
RAP: data acquisition and data analysis, drafting the manuscript, and proof reading. ANJ: conceptualized the study and made the study design, drafting the manuscript, and proof reading. Both authors read and approved the final draft of manuscript.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Standard Statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed Consent
For this type of study, informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ritesh Arvind Pandey, Email: riteshpandey8262@yahoo.com.
Ashok N. Johari, Email: drashokjohari@hotmail.com
References
- 1.Dezateux C, Rosendahl K. Developmental dysplasia of the hip. Lancet. 2007;369(9572):1541–1552. doi: 10.1016/S0140-6736(07)60710-7. [DOI] [PubMed] [Google Scholar]
- 2.Dunn PM, Evans RE, Thearle MJ, Griffiths HE, Witherow PJ. Congenital dislocation of the hip: early and late diagnosis and management compared. Archives of Disease in Childhood. 1985;60(5):407–414. doi: 10.1136/adc.60.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ning B, Yuan Y, Yao J, Zhang S, Sun J. Analyses of outcomes of one-stage operation for treatment of late-diagnosed developmental dislocation of the hip: 864 hips followed for 3.2–8.9 years. BMC Musculoskeletal Disorders. 2014;15:401. doi: 10.1186/1471-2474-15-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh M, Sharma NK. Spectrum of congenital malformations in the newborn. Indian Journal of Pediatrics. 1980;47:239–244. doi: 10.1007/BF02758201. [DOI] [PubMed] [Google Scholar]
- 5.Gupta AK, Kumari S, Arora PL, Kumar R, Mehtani AK, Sood LK. Hip instability in newborns in an urban community. National Medical Journal of India. 1992;5(6):269–272. [PubMed] [Google Scholar]
- 6.Broadhurst C, Rhodes AML, Harper P, Perry DC, Clarke NMP, Aarvold A. What is the incidence of late detection of developmental dysplasia of the hip in England? A 26-year national study of children diagnosed after the age of one. Bone Joint Journal. 2019;101B(3):281–287. doi: 10.1302/0301-620X.101B3.BJJ-2018-1331.R1. [DOI] [PubMed] [Google Scholar]
- 7.Myers J, Hadlow S, Lynskey T. The effectiveness of a programme for neonatal hip screening over a period of 40 years: A follow-up of the New Plymouth experience. Journal of Bone and Joint Surgery. British Volume. 2009;91(2):245–248. doi: 10.1302/0301-620X.91B2.21300. [DOI] [PubMed] [Google Scholar]
- 8.Mace J, Paton RW. Neonatal clinical screening of the hip in the diagnosis of developmental dysplasia of the hip: a 15-year prospective longitudinal observational study. Bone Joint Journal. 2015;97B:265–269. doi: 10.1302/0301-620X.97B2.34858. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Synthese Library. 2015;4(1):1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 11.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann EC, Street DG. Neonatal screening in Vancouver for congenital dislocation of the hip. Canadian Medical Association Journal. 1981;124(8):1003–1008. [PMC free article] [PubMed] [Google Scholar]
- 13.Wenger D, Duppe H, Nilsson JA, Tiderius CJ. Incidence of late-diagnosed hip dislocation after universal clinical screening in Sweden. JAMA Network Open. 2019;2(11):e1914779. doi: 10.1001/jamanetworkopen.2019.14779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Studer K, Williams N, Antoniou G, et al. Increase in late diagnosed developmental dysplasia of the hip in South Australia: risk factors, proposed solutions. Medical Journal of Australia. 2016;204(6):240. doi: 10.5694/mja15.01082. [DOI] [PubMed] [Google Scholar]
- 15.Talbot C, Adam J, Paton R. Late presentation of developmental dysplasia of the hip. A 15-year observational study. Bone Joint Journal. 2017;99B:1250–1255. doi: 10.1302/0301-620X.99B9.BJJ-2016-1325.R1. [DOI] [PubMed] [Google Scholar]
- 16.Clarke NM, Reading IC, Corbin C, et al. Twenty years’ experience of selective secondary ultrasound screening for congenital dislocation of the hip. Archives of Disease in Childhood. 2012;97:423–429. doi: 10.1136/archdischild-2011-301085. [DOI] [PubMed] [Google Scholar]
- 17.Geertsema D, Meinardi JE, Kempink DRJ, Fiocco M, van de Sande MAJ. Screening program for neonates at risk for developmental dysplasia of the hip: comparing first radiographic evaluation at five months with the standard twelve week ultrasound. A prospective cross-sectional cohort study. International Orthopaedics. 2019;43(8):1933–1938. doi: 10.1007/s00264-018-4089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laborie LB, Markestad TJ, Davidsen H, et al. Selective ultrasound screening for developmental hip dysplasia: effect on management and late detected cases. A prospective survey during 1991–2006. Pediatric Radiology. 2014;44(4):410–424. doi: 10.1007/s00247-013-2838-3. [DOI] [PubMed] [Google Scholar]
- 19.Paton RW, Hossain S, Eccles K. Eight-year prospective targeted ultrasound screening program for instability and at-risk hip joints in developmental dysplasia of the hip. Journal of Pediatric Orthopedics. 2002;22(3):338–341. [PubMed] [Google Scholar]
- 20.Lisle R, Boekelaar M, Stannage K. Delayed diagnosis of developmental dislocation of the hip: the Western Australian experience. ANZ Journal of Surgery. 2012;82:612–615. doi: 10.1111/j.1445-2197.2012.06110.x. [DOI] [PubMed] [Google Scholar]
- 21.Chan A, Cundy PJ, Foster BK, Keane RJ, Byron-Scott R. Late diagnosis of congenital dislocation of the hip and presence of a screening programme: South Australian population-based study. Lancet. 1999;354(9189):1514–1517. doi: 10.1016/S0140-6736(98)12469-8. [DOI] [PubMed] [Google Scholar]
- 22.Sahin F, Aktürk A, Beyazova U, et al. Screening for developmental dysplasia of the hip: results of a 7-year follow-up study. Pediatrics International. 2004;46(2):162–166. doi: 10.1046/j.1442-200x.2004.01855.x. [DOI] [PubMed] [Google Scholar]
- 23.Boeree NR, Clarke NM. Ultrasound imaging and secondary screening for congenital dislocation of the hip. The Journal of Bone and Joint Surgery. 1994;76B:525–533. [PubMed] [Google Scholar]
- 24.Milligan DJ, Cosgrove AP. Monitoring of a hip surveillance programme protects infants from radiation and surgical intervention. Bone Joint Journal. 2020;102B(4):495–500. doi: 10.1302/0301-620X.102B4.BJJ-2019-0809.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnelly KJ, Chan KW, Cosgrove AP. Delayed diagnosis of developmental dysplasia of the hip in Northern Ireland: can we do better? Bone Joint Journal. 2015;97B(11):1572–1576. doi: 10.1302/0301-620X.97B11.35286. [DOI] [PubMed] [Google Scholar]
- 26.Choudry QA, Paton RW. Neonatal screening and selective sonographic imaging in the diagnosis of developmental dysplasia of the hip. Bone Joint Journal. 2018;100B(6):806–810. doi: 10.1302/0301-620X.100B6.BJJ-2017-1389.R1. [DOI] [PubMed] [Google Scholar]
- 27.Thallinger C, Pospischill R, Ganger R, et al. Long-term results of a nationwide general ultrasound screening system for developmental disorders of the hip: the Austrian hip screening program. Journal of Children's Orthopaedics. 2014;8:3–10. doi: 10.1007/s11832-014-0555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulziibat M, Munkhuu B, Schmid R, Baumann T, Essig S. Implementation of a nationwide universal ultrasound screening programme for developmental dysplasia of the neonatal hip in Mongolia. Journal of Children's Orthopaedics. 2020;14(4):273–280. doi: 10.1302/1863-2548.14.200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biedermann R, Riccabona J, Giesinger JM, et al. Results of universal ultrasound screening for developmental dysplasia of the hip: a prospective follow-up of 28 092 consecutive infants. Bone Joint Journal. 2018;100B(10):1399–1404. doi: 10.1302/0301-620X.100B10.BJJ-2017-1539.R2. [DOI] [PubMed] [Google Scholar]
- 30.Von Kries R, Ihme N, Oberle D, et al. Effect of ultrasound screening on the rate of first operative procedures for developmental hip dysplasia in Germany. Lancet 2003;362:1883–1887. [DOI] [PubMed]
- 31.Marks DS, Clegg J, Al-Chalabi AN. Routine ultrasound screening for neonatal hip instability. Can it abolish late-presenting congenital dislocation of the hip? Journal of Bone and Joint Surgery. 1994;76:534–538. [PubMed] [Google Scholar]
- 32.Olsen SF, Blom HC, Rosendahl K. Introducing universal ultrasound screening for developmental dysplasia of the hip doubled the treatment rate. Acta Paediatrica. 2018;107(2):255–261. doi: 10.1111/apa.14057. [DOI] [PubMed] [Google Scholar]
- 33.Tredwell SJ. Economic evaluation of neonatal screening for congenital dislocation of the hip. Journal of Pediatric Orthopedics. 1990;10:327–330. doi: 10.1097/01241398-199005000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Rosendahl K, Markestad T, Lie RT. Ultrasound screening for developmental dysplasia of the hip in the neonate: the effect on treatment rate and prevalence of late cases. Pediatrics. 1994;94:47–52. [PubMed] [Google Scholar]
- 35.Geitung JT, Rosendahl K, Sudmann E. Cost-effectiveness of ultrasonographic screening for congenital hip dysplasia in new-borns. Skeletal Radiology. 1996;25(3):251–254. doi: 10.1007/s002560050074. [DOI] [PubMed] [Google Scholar]
- 36.Clegg J, Bache CE, Raut VV. Financial justification for routine ultrasound screening of the neonatal hip. Journal of Bone and Joint Surgery. British Volume. 1999;81:852–857. doi: 10.1302/0301-620x.81b5.9746. [DOI] [PubMed] [Google Scholar]
- 37.Düppe H, Danielsson LG. Screening of neonatal instability and of developmental dislocation of the hip. A survey of 132,601 living newborn infants between 1956 and 1999. Journal of Bone and Joint Surgery. British Volume. 2002;84(6):878–885. doi: 10.1302/0301-620x.84b6.12326. [DOI] [PubMed] [Google Scholar]
- 38.Holen KH, Tegnander A, Bredland T, et al. Universal or selective screening of the neonatal hip using ultrasound? Journal of Bone and Joint Surgery. British Volume. 2002;84B:886–890. doi: 10.1302/0301-620x.84b6.12093. [DOI] [PubMed] [Google Scholar]
- 39.Dezateux C, Brown J, Arthur R, Karnon J, Parnaby A. Performance, treatment pathways, and effects of alternative policy options for screening for developmental dysplasia of the hip in the United Kingdom. Archives of Disease in Childhood. 2003;88(9):753–759. doi: 10.1136/adc.88.9.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roovers EA, Boere-Boonekamp MM, Castelein RM, Zielhuis GA, Kerkhoff TH. Effectiveness of ultrasound screening for developmental dysplasia of the hip. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2005;90(1):F25–F30. doi: 10.1136/adc.2003.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gray A, Elbourne D, Dezateux C, et al. Economic evaluation of ultrasonography in the diagnosis and management of developmental hip dysplasia in the United Kingdom and Ireland. Journal of Bone and Joint Surgery. American Volume. 2005;87:2472–2479. doi: 10.2106/JBJS.D.01997. [DOI] [PubMed] [Google Scholar]
- 42.Thaler M, Biedermann R, Lair J, Krismer M, Landauer F. Cost-effectiveness of universal ultrasound screening compared with clinical examination alone in the diagnosis and treatment of neonatal hip dysplasia in Austria. Journal of Bone and Joint Surgery. British Volume. 2011;93(8):1126–1130. doi: 10.1302/0301-620X.93B8.25935. [DOI] [PubMed] [Google Scholar]
- 43.Tschauner C, Fürntrath F, Saba Y, Berghold A, Radl R. Developmental dysplasia of the hip: impact of sonographic newborn hip screening on the outcome of early treated decentered hip joints-a single center retrospective comparative cohort study based on Graf's method of hip ultrasonography. Journal of Children's Orthopaedics. 2011;5(6):415–424. doi: 10.1007/s11832-011-0366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shorter D, Hong T, Osborn DA. Screening programmes for developmental dysplasia of the hip in newborn infants. Cochrane Database Systematic Reviews. 2011;9:CD004595. doi: 10.1002/14651858.CD004595.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rebello G, Joseph B. Late presentation of developmental dysplasia of the hip in children from southwest India – Will screening help? Indian Journal of Orthopaedics. 2003;37(4):210–214. [Google Scholar]
- 46.Agarwal A, Gupta N. Risk factors and diagnosis of developmental dysplasia of hip in children. Journal of Clinical Orthopaedics and Trauma. 2012;3(1):10–14. doi: 10.1016/j.jcot.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talbot CL, Paton RW. Screening of selected risk factors in developmental dysplasia of the hip: an observational study. Archives of Disease in Childhood. 2013;98(9):692–696. doi: 10.1136/archdischild-2013-303647. [DOI] [PubMed] [Google Scholar]
- 48.Zhao D, Rao W, Zhao L, et al. Is it worthwhile to screen the hip in infants born with clubfeet? International Orthopaedics. 2013;37(12):2415–2420. doi: 10.1007/s00264-013-2073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao L, Ma Q, Feng X, et al. Screening for developmental dysplasia of the hip in infants in Tibet identifies increased prevalence associated with altitude. Medical Science Monitor. 2019;25:5771–5775. doi: 10.12659/MSM.916456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omeroglu H, Akceylan A, Kose N. Associations between risk factors and developmental dysplasia of the hip and ultrasonographic hip type: a retrospective case control study. Journal of Children's Orthopaedics. 2019;13(2):161–166. doi: 10.1302/1863-2548.13.180174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paton RW, Sriivasan MS, Shah B, et al. Ultrasound screening for hips at risk in developmental dysplasia of the hip: is it worth it? Journal of Bone and Joint Surgery. British Volume. 1999;81:255–258. doi: 10.1302/0301-620x.81b2.8972. [DOI] [PubMed] [Google Scholar]
- 52.Philip H, Joseph BM, Clarke NMP, et al. International Hip Dysplasia Institute (IHDI). Even experts can be fooled: reliability of clinical examination for diagnosing hip dislocations in newborns. Journal of Children's Orthopaedics. 2020;40(8):408–412. doi: 10.1097/BPO.0000000000001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Omeroglu H, Tatlici E, Kose N. Significance of Asymmetry of Groin and Thigh Skin Creases in Developmental Dysplasia of the Hip Revisited: Results of a Comparative Study. Journal of Pediatric Orthopedics. 2020;40(8):e761–e765. doi: 10.1097/BPO.0000000000001531. [DOI] [PubMed] [Google Scholar]
- 54.Senaran H, Ozdemir HM, Ogün TC, Kapicioglu MI. Value of limited hip abduction in developmental dysplasia of the hip. Pediatrics International. 2004;46(4):456–458. doi: 10.1111/j.1442-200x.2004.01931.x. [DOI] [PubMed] [Google Scholar]
- 55.National Screening Committee (2011) NHS Newborn & Infant Physical Examination Screening Programme. http://www.screening.nhs.uk/newborninfantphysical-england. Accessed 26 October 2020.
- 56.Fiddian NJ, Gardiner JC. Screening for congenital dislocation of the hip by physiotherapists. Results of a ten-year study. Journal of Bone and Joint Surgery. British Volume. 1994;76(3):458–459. [PubMed] [Google Scholar]
- 57.Graf R. The diagnosis of congenital hip-joint dislocation by the ultrasonic Combound treatment. Archives of Orthopaedic and Trauma Surgery. 1980;97:117–133. doi: 10.1007/BF00450934. [DOI] [PubMed] [Google Scholar]
- 58.Graf R, Tschauner C, Klapsch W. Progress in prevention of late developmental dislocation of the hip by sonographic newborn hip screening—results of a comparative follow-up-study. Journal of Pediatric Orthopedics. Part B. 1993;2:115–121. [Google Scholar]
- 59.Clarke NM, Harcke HT, McHugh P, et al. Real-time ultrasound in the diagnosis of congenital dislocation and dysplasia of the hip. Journal of Bone and Joint Surgery. British Volume. 1985;67:406–412. doi: 10.1302/0301-620X.67B3.3889008. [DOI] [PubMed] [Google Scholar]
- 60.Kumar RK, Shah P, Ramya AN, Rajan R. Diagnosing developmental dysplasia of hip in newborns using clinical screen and ultrasound of hips—An Indian experience. Journal of Tropical Pediatrics. 2016;62(3):241–245. doi: 10.1093/tropej/fmv107. [DOI] [PubMed] [Google Scholar]
- 61.American Academy of Pediatrics Committee on quality improvement, subcommittee on developmental dysplasia of the hip clinical practice guideline: early detection of developmental dysplasia of the hip. Pediatrics. 2000;105(4):896–905. doi: 10.1542/peds.105.4.896. [DOI] [PubMed] [Google Scholar]
- 62.McAllister DA, Morling JR, Fischbacher CM, Reidy M, Murray A, Wood R. Enhanced detection services for developmental dysplasia of the hip in Scottish children, 1997–2013. Archives of Disease in Childhood. 2018;103(11):1021–1026. doi: 10.1136/archdischild-2017-314354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morris Andrew R, Thomas Joanna MC, Reading Isabel C, Clarke Nicholas MP. Does late hip dysplasia occur after normal ultrasound screening in breech babies? Journal of Pediatric Orthopaedics. 2019;39(4):187–192. doi: 10.1097/BPO.0000000000000903. [DOI] [PubMed] [Google Scholar]
- 64.Tegnander A, Holen KJ, Terjesen T. The natural history of hip abnormalities detected by ultrasound in clinically normal newborns: a 6–8 year radiographic follow-up study of 93 children. Acta Orthopaedica Scandinavica. 1999;70:335–337. doi: 10.3109/17453679908997820. [DOI] [PubMed] [Google Scholar]
- 65.O'Beirne JG, Chlapoutakis K, Alshryda S, et al. International interdisciplinary consensus meeting on the evaluation of developmental dysplasia of the hip. Ultraschall in der Medizin. 2019;40(4):454–464. doi: 10.1055/a-0924-5491. [DOI] [PubMed] [Google Scholar]
- 66.Bralic I, Vrdoljak J, Kovacic L. Ultrasound screening of the neonatal hip: cost-benefit analysis. Croatian Medical Journal. 2001;42(2):171–174. [PubMed] [Google Scholar]