Abstract
Angiostrongylus cantonensis is a zoonotic parasitic helminth that normally resides in the pulmonary arteries and the right ventricle of rats (Rattus sp.), the definitive host, where it causes little disease. Humans, dogs, opossums, and various zoo animals are “accidental” hosts. Here we report verminous meningoencephalomyelitis caused by A. cantonensis in a 9-mo-old male red kangaroo (Macropus rufus). The kangaroo was first presented lethargic, recumbent, and hypothermic, with severe muscle wasting. Within 3 wk, he progressed to non-ambulatory paraparesis and died. Gross examination revealed multifocal areas of dark-brown discoloration, malacia, and cavitation in the brain and the spinal cord. Histologically, there were several sections of nematodes surrounded by extensive areas of rarefaction, hemorrhage, spongiosis, neuronal necrosis, and gliosis. Based on size, morphology, and organ location, the nematodes were identified as subadult males and females. Interestingly, an eosinophilic response was largely absent, and the inflammatory response was minimal. A. cantonensis infection had not been reported previously in a red kangaroo in Louisiana or Mississippi, to our knowledge. Our case reaffirms the widespread presence of the helminth in the southeastern United States and indicates that A. cantonensis should be considered as a differential in macropods with neurologic clinical signs in regions where A. cantonensis is now endemic.
Keywords: Angiostrongylus cantonensis, meningoencephalomyelitis, red kangaroos, zoonotic
Angiostrongylus cantonensis is a zoonotic parasitic nematode (order Strongylida, superfamily Metastrongyloidea, family Angiostrongylidae) that normally resides within the pulmonary arteries and the right ventricle of Norway (Rattus norvegicus) and black (Rattus rattus) rats, the main definitive hosts in North America, in which it causes little clinical disease. Humans, 19 dogs, 15 horses, 25 brushtail possums (Trichosurus vulpecula), 16 fruit bats (Pteropus spp.),1,24 tawny frogmouths (Podargus strigoides),11,18 macropods,17,12,22 and various species of free-ranging and zoo-housed birds and mammals3,10,18 are “accidental” or aberrant hosts. These accidental hosts can acquire infection after ingesting third-stage larvae (L3) present in the intermediate hosts (gastropods), or through transport hosts (frogs, fish, crustaceans). In addition, exposure through direct contact with L3 shed into the environment by infected intermediate hosts has been implicated in human cases linked to handling of gastropods as pets or the ingestion of produce contaminated with larvae. Spontaneous shedding of L3 into the environment by infected gastropods has been reported for various species of metastrongyloids including A. cantonensis, A. costaricensis, and A. vasorum. 6 Tawny frogmouths and Australian marsupials are highly susceptible; however, we found no report of an infection in a red kangaroo.
Here we report verminous meningoencephalomyelitis caused by A. cantonensis in a 9-mo-old (9-mo-out-of-pouch) male red kangaroo (Macropus rufus) from Mississippi. The animal was captive-bred at a private facility in Texas and was raised by the dam for 2–3 mo (out-of-pouch), at which time it was acquired by the owner in rural Mississippi and lived as a companion animal until presentation. Since acquisition, the animal spent most of his time indoors with supervised outside time. The animal was fed Bermuda grass hay, commercial pelleted diet, various fresh produce, table scraps, and had partial access to outdoor grazing. The red kangaroo was first presented to Louisiana State University, Veterinary Teaching Hospital (Baton Rouge, LA, USA) for a 3-d history of lethargy and inappetence. Upon presentation, the kangaroo was recumbent and hypothermic with severe muscle wasting and decreased muscle tone. Withdrawal reflexes were diminished in both hindlimbs with the left hindlimb worse than the right; the left hindlimb had a decreased patellar reflex and decreased conscious proprioception; pupillary light reflexes were also absent. Although the kangaroo was ambulatory, he progressed to non-ambulatory paraparesis within 4 d. Paraparesis continued for up to 2 wk post-hospitalization, with neither improvement nor decline.
Complete blood counts, chemistry panel, urinalysis, fecal examinations, radiographs, and abdominal ultrasound were unremarkable. Serology for toxoplasmosis and leptospirosis was negative. At the end of wk 2, the kangaroo began to decline mentally with decreased appetite and mentation, and paresis progressed further. Prehension, mastication, and deglutition abilities also declined. Respiratory distress, increased bronchovesicular sounds, and wheezes were present on auscultation. Opisthotonos and horizontal nystagmus with fast phase to the left were also observed by this time. The kangaroo became mentally nonresponsive, obtunded, and died by the middle of wk 3.
The kangaroo weighed 5.7 kg at the time of autopsy and was in thin body condition. Gross evaluation did not reveal any major abnormalities; the vessels within the brain and the spinal cord were mildly congested. Tissues were fixed in 10% neutral-buffered formalin. Post-fixation, dark-red to black foci of malacia and cavitation were noted in the brain and spinal cord (Fig. 1A, 1B). Occasionally, slender parasites were associated with these foci. The parasites were extracted by careful dissection. Fixed tissues were processed using routine histologic techniques, paraffin-embedded, sectioned at 4 µm, and stained with hematoxylin and eosin.
Figure 1.
Verminous meningoencephalomyelitis in a red kangaroo. A. Coronal section of the brain at the level of frontal/parietal lobe, with an area of cavitation and necrosis (arrows). B. Section of spinal cord with area of cavitation and necrosis (arrow).
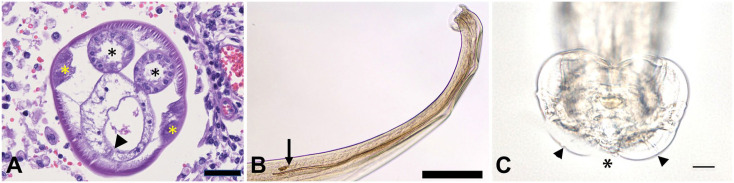
Histologically, multifocal areas of necrosis with sections of metazoan helminth parasites were present within the cerebrum and the cerebellum. These helminths were surrounded by multiple, focally extensive areas of rarefaction, characterized by malacia or necrosis of gray and white matter, abundant hemorrhage, spongiosis, neuronal necrosis, reactive microglial cells, moderate numbers of gitter cells, and rare lymphocytes and plasma cells (Fig. 2A, 2B). Similar to the brain, the cervical, thoracic, lumbar, and sacral spinal cord contained areas of cavitation and necrosis within both the gray and the white matter, especially around the central canal. Several transverse and occasional longitudinal sections of helminth parasites were present within the areas of necrosis (Fig. 2C, 2D). Multifocal areas of the meninges were also expanded by small numbers of cross and tangential sections of helminths mixed with moderate numbers of lymphocytes, plasma cells, macrophages, multinucleate giant cells, rare eosinophils and neutrophils, sometimes accompanied by necrosis, and hemorrhage (Fig. 2E, 2F).
Figure 2.
Verminous meningoencephalomyelitis in a red kangaroo. The areas inside the yellow boxes in panels A, C, and E are presented at higher power in panels B, D, and F, respectively. A, B. Brain section (coronal plane at the level of frontal/parietal lobe) with hemorrhage, necrosis, and transverse sections of a nematode. C, D. Thoracolumbar spinal cord with multifocal cavitation and necrosis with loss of gray and white matter in the tissue adjacent to the central canal. These areas also contain several longitudinal and transverse sections of a nematode. E, F. Meninges of the cerebellum with hemorrhage and transverse sections of nematodes that are surrounded by cellular debris and moderate numbers of macrophages. Bar = 0.5 mm (A, C, E); bar = 50 µm (B, D, F).
The parasites were identified as nematodes based on the presence of a pseudocoelomic body cavity and a gastrointestinal tract. The nematodes were ~125 µm diameter with a 5–8 µm wide eosinophilic, hyalinized, anisotropic, and smooth cuticle lined by coelomyarian–polymyarian musculature (Fig. 3A). An intestinal tract lined by a few multinucleate giant cells with a short and clumped brush border was present within the pseudocoelom (Fig. 3A). Lateral cords were prominent (Fig. 3A). Developing reproductive organs were visible in some sections. Cellular debris, hypertrophied astrocytes, scattered vacuoles containing spheroids, hemorrhage, and moderate-to-large numbers of gitter cells were present in the surrounding tissue. The morphologic characteristics of the nematodes detected on histologic section were typical of metastrongyloids and were consistent with A. cantonensis subadults.
Figure 3.
Verminous meningoencephalomyelitis in a red kangaroo. A. Transverse section of a nematode extracted from the brain or spinal cord; ~125 µm diameter, 5–8 µm wide, eosinophilic, hyalinized, anisotropic, and smooth cuticle lined by coelomyarian–polymyarian musculature. The pseudocoelom contains an intestinal tract lined by a few multinucleate giant cells with brush border. Arrowhead indicates the short, clumped brush border of the intestinal tract. Prominent lateral cords are also present (yellow asterisks). Black asterisks indicate reproductive organs. Bar = 25 µm B. Posterior extremity of the male with long, paired, equal spicules. Black arrow represents incomplete cuticle deposition in the spicule. Bar = 200 µm. C. Copulatory bursa of the male. Arrowheads indicate externodorsal rays. Asterisk indicates indentation of the outline of the bursa where dorsal ray occurs (dorsal ray is not fully visible). Bar = 20 µm.
The liver contained random foci of necrotic hepatocytes that were often surrounded by moderate numbers of viable and degenerate neutrophils. Randomly distributed alveolar spaces contained small numbers of neutrophils, and occasionally, bronchioles and peribronchial spaces were also infiltrated by small numbers of neutrophils. Although microorganisms were not identified on liver culture, mixed bacterial flora, dominated by colonies of Escherichia coli and Micrococcus spp., were recovered on lung culture. These findings were consistent with terminal sepsis, as a sequela of parasitism and debilitation. Based on gross and histologic examination, final morphologic diagnoses of verminous meningoencephalomyelitis, necrotizing hepatitis, and multifocal purulent bronchopneumonia were made.
Subadult male and female worms were dissected from the fixed brain and spinal cord tissue. The worms were 8–12 mm in length. The nematodes lacked a buccal capsule and cuticular synlophe. The male worms had a well-developed but small genital bursa. The dorsal ray was shorter than the external dorsal rays, and long, paired, equal spicules were present (Fig. 3B, 3C). Cuticle deposition in the spicules was not complete. The spicules in 3 of the males were 929–972 μm long; in a fourth male, one spicule was 562 μm and the other was 610 μm long. Females had a short tail with the anus located 40–53 μm from the tail tip; the vaginal opening was located at the posterior-end, 183–203 μm from the tip of the tail.
Differentiation of the various species of Angiostrongylus is based primarily on the morphology of the genital bursa and the spicule length of the adult male worms. 7 In A. cantonensis, the dorsal ray is shorter than the externodorsal rays, and the spicules are large (1.0–1.4 mm long). 7 The length of the spicules in the subadults recovered in our case were slightly (929–972 μm) to significantly (562–610 μm) smaller than those reported for adult A. cantonensis. In the natural rodent definitive host, subadults develop in the arachnoid space and continue to mature as they migrate to the pulmonary arteries. 2 The spicules in subadult males initially lack cuticle and are difficult to discern; they become more visible with cuticle deposition and can be measured in full length at maturity. 2 We ascribed the cause for the shorter than expected length of the spicules in the recovered male subadults to incomplete spicule development. Therefore, identification of A. cantonensis subadults by morphology alone using taxonomic keys based on mature adult worm morphologic characters appears to have the potential to lead to confusion and should be done with some caution. The spicules in all of the Angiostrongylus species occurring in North America measure less than half that of A. cantonensis with the exception of A. blarini occurring in short-tailed shrews (Blarina brevicauda) with spicules of 0.66–0.99 mm. 7 However, the dorsal ray in A. blarini is as long or longer than the externodorsal rays, eliminating it as a possibility in our case. 7 Based on these morphologic characteristics, the worms were identified as A. cantonensis. 7
A. cantonensis is a causative agent of a potentially fatal infectious disease that is globally emerging and now poses a major risk to both wildlife and humans worldwide. 26 Although first discovered in southern China in 1935, 5 it is now widespread in several parts of the world including Southeast Asia, Australia, South America, and Africa. In the continental United States, it was first reported in 1987; nearly 21% of the rats (R. norvegicus) sampled from New Orleans, Louisiana were infected with the nematode. 4 Since then, several human and animal cases have been reported in the United States, with most of the cases being from Hawaii and the southeastern states. 13 Angiostrongylosis is a reportable disease in Hawaii; it is now considered endemic in Louisiana, and the infection has been found in snails and rats. 14 Cases in various aberrant bird and mammalian hosts have also been recorded in Florida, Alabama, Mississippi, Louisiana, Oklahoma, and California. 8 According to a report published in 2017, there have been ~2,800 human cases recorded worldwide. 23 Among infectious diseases, A. cantonensis is the most common cause of eosinophilic meningoencephalitis in humans and other animals worldwide. 22
Red kangaroos are native to central Australia and are one of the largest living marsupials. Although there are reports of A. cantonensis infection in captive red-necked wallaby (Macropus rufogriseus; syn. Bennett’s wallaby), 17 Parma wallaby hybrid (Macropus parma x eugenii), 22 western gray kangaroo (Macropus fuliginosus), 22 and captive rufous bettongs (Aepyprymnus rufescens; syn. rufous rat-kangaroo) 12 from Australia, we found no reports of infection in red kangaroos. Interestingly, in these previous reports, the red-necked wallaby and rufous bettongs were paraparetic and then paralyzed before succumbing,12,17 which is somewhat similar to the disease progression seen in the red kangaroo in our report. Histologically, however, only the brainstem and the cerebellum were found to be infected in the red-necked wallaby case 17 ; cerebrum, cerebellum, spinal cord, and meninges were all affected in the rufous bettongs and the red kangaroo. A. cantonensis usually causes meningitis in the less severe form. 21 However, in the more severe form, it involves the brain and the spinal cord and results in intense eosinophilic meningitis with eosinophilia of the peripheral blood and the cerebrospinal fluid. 20 Interestingly, peripheral eosinophilia was absent, and eosinophilic inflammation was minimal-to-rare in our case.
Although it remains unclear how the kangaroo was infected, it seems plausible that the kangaroo ingested a gastropod intermediate host containing infective L3 or directly ingested L3 on improperly washed produce or grass from grazing outdoors. Vegetables covered in snail slime have also been implicated as a source of infection, as has the slime from hands and feet post-handling or touching infected snails and slugs. 9 As well, rats, the definitive hosts for this nematode, are considered essential for the establishment of A. cantonensis foci in any region. In fact, if A. cantonensis is identified in rats, the nematode is considered endemic. Although A. cantonensis is endemic in Mississippi and Louisiana, the owner did not report a rat infestation on his property. The kangaroo was captive bred in Texas and lived in Mississippi for the majority of its life. It remains unclear as to whether the animal acquired infection in Texas or in Mississippi. Our finding indicates that the susceptible species range of this infection is expanding. Our case also suggests that in places where A. cantonensis is now endemic, A. cantonensis should be considered as a differential for macropods with neurologic clinical signs, even in the absence of clinicopathologic abnormalities.
Acknowledgments
We greatly appreciate the technical service provided by the histopathology laboratory at the Louisiana Animal Disease Diagnostic Laboratory.
Footnotes
Declaration of conflicting interests: The authors declared no conflicting interests with respect to research, authorship, and/or publication of this article.
Funding: Our study was funded by Louisiana State Funds to the Louisiana Animal Disease Diagnostic Laboratory.
ORCID iD: Sonika Patial  https://orcid.org/0000-0002-7373-0835
https://orcid.org/0000-0002-7373-0835
Contributor Information
Sonika Patial, Departments of Comparative Biomedical Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA.
Brooke A. Delcambre, Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA
Peter M. DiGeronimo, Veterinary Clinical Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA Current address: Adventure Aquarium, Camden, NJ, USA.
Gary Conboy, Department of Pathology and Microbiology, Atlantic Veterinary College, Charlottetown, Prince Edward Island, Canada.
Adriano F. Vatta, Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA
Rudy Bauer, Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA.
References
- 1. Barrett JL, et al.Neuro-angiostrongylosis in wild black and grey-headed flying foxes (Pteropus spp). Aust Vet J 2002;80:554–558. [DOI] [PubMed] [Google Scholar]
- 2. Bhaibulaya M. Comparative studies on the life history of Angiostrongylus mackerrasae Bhaibulaya, 1968 and Angiostrongylus cantonensis (Chen, 1935). Int J Parasitol 1975;5:7–20. [DOI] [PubMed] [Google Scholar]
- 3. Burns RE, et al. Cerebral Angiostrongylus cantonensis infection in a captive African pygmy falcon (Polihierax semitorquatus) in southern California. J Vet Diagn Invest 2014;26:695–698. [DOI] [PubMed] [Google Scholar]
- 4. Campbell BG, Little MD. The finding of Angiostrongylus cantonensis in rats in New Orleans. Am J Trop Med Hyg 1988;38:568–573. [DOI] [PubMed] [Google Scholar]
- 5. Chen H-T. Un nouveau nematode pulmonaire Pulmonema cantonensis, n. g., n. sp. des rats de Canton [A new pulmonary nematode of rats, Pulmonema cantonensis n g, n sp from Canton]. Ann Parasitol Hum Comp 1935;13:312–317. French. https://www.parasite-journal.org/articles/parasite/pdf/1935/04/parasite1935134p312.pdf [Google Scholar]
- 6. Conboy G, et al. Spontaneous shedding of metastrongyloid third-stage larvae by experimentally infected Limax maximus. Parasitol Res 2017;116:41–54. [DOI] [PubMed] [Google Scholar]
- 7. Costa JO, et al. Redescription of Angiostrongylus vasorum (Baillet, 1866) and systematic revision of species assigned to the genera Angiostrongylus Kamensky, 1905 and Angiocaulus Schulz, 1951. Revue Med Vet 2003;154:9–16. [Google Scholar]
- 8. Cowie RH. Angiostrongylus cantonensis: agent of a sometimes fatal globally emerging infectious disease (rat lungworm disease). ACS Chem Neurosci 2017;8:2102–2104. [DOI] [PubMed] [Google Scholar]
- 9. Cowie RH. Pathways for transmission of angiostrongyliasis and the risk of disease associated with them. Hawaii J Med Public Health 2013;72(6 Suppl 2):70–74. [PMC free article] [PubMed] [Google Scholar]
- 10. Duffy MS, et al. Parastrongylus cantonensis in a nonhuman primate, Florida. Emerg Infect Dis 2004;10:2207–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gelis S, et al. Neuroangiostrongyliasis and other parasites in tawny frogmouths (Podargus strigoides) in south-eastern Queensland. Aust Vet J 2011;89:47–50. [DOI] [PubMed] [Google Scholar]
- 12. Higgins DP, et al. Neural angiostrongylosis in three captive rufous bettongs (Aepyprymnus rufescens). Aust Vet J 1997;75:564–566. [DOI] [PubMed] [Google Scholar]
- 13. Johnston DI, et al. Review of cases of angiostrongyliasis in Hawaii, 2007–2017. Am J Trop Med Hyg 2019;101:608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim DY, et al. Parastrongylus (=Angiostrongylus) cantonensis now endemic in Louisiana wildlife. J Parasitol 2002;88:1024–1026. [DOI] [PubMed] [Google Scholar]
- 15. Lunn JA, et al. Twenty two cases of canine neural angiostrongylosis in eastern Australia (2002–2005) and a review of the literature. Parasit Vectors 2012;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma G, et al. Tawny frogmouths and brushtail possums as sentinels for Angiostrongylus cantonensis, the rat lungworm. Vet Parasitol 2013;192:158–165. [DOI] [PubMed] [Google Scholar]
- 17. McKenzie RA, et al. Angiostrongylus cantonesis infection of the brain of a captive Bennett’s wallaby (Macropus rufogriseus). Aust Vet J 1978;54:86–88. [DOI] [PubMed] [Google Scholar]
- 18. Monks DJ, et al. Angiostrongylus cantonensis as a cause of cerebrospinal disease in a yellow-tailed black cockatoo (Calyptorhynchus funereus) and two tawny frogmouths (Podargus strigoides). J Avian Med Surg 2005;19:289–293. [Google Scholar]
- 19. Morton NJ, et al. Severe hemorrhagic meningoencephalitis due to Angiostrongylus cantonensis among young children in Sydney, Australia. Clin Infect Dis 2013;57:1158–1161. [DOI] [PubMed] [Google Scholar]
- 20. Pien FD, Pien BC. Angiostrongylus cantonensis eosinophilic meningitis. Int J Infect Dis 1999;3:161–163. [DOI] [PubMed] [Google Scholar]
- 21. Sawanyawisuth KA, Sawanyawisuth KI. Treatment of angiostrongyliasis. Trans R Soc Trop Med Hyg 2008;102:990–996. [DOI] [PubMed] [Google Scholar]
- 22. Spratt DM. Species of Angiostrongylus (Nematoda: Metastrongyloidea) in wildlife: a review. Int J Parasitol Parasites Wildl 2015;4:178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stockdale Walden HD, et al. Geographic distribution of Angiostrongylus cantonensis in wild rats (Rattus rattus) and terrestrial snails in Florida, USA. PLoS One 2017;12:e0177910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warshaw M, et al. Pathology in practice: aberrant cerebral migration of Angiostrongylus cantonensis in an Indian flying fox. J Am Vet Med Assoc 2018;252:545–548. [DOI] [PubMed] [Google Scholar]
- 25. Wright JD, et al. Equine neural angiostrongylosis. Aust Vet J 1991;68:58–60. [DOI] [PubMed] [Google Scholar]
- 26. York EM, et al. Geographic range expansion for rat lungworm in North America. Emerg Infect Dis 2015;21:1234–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]