Abstract
Objectives
Increasing access to oxygen services may improve outcomes among children with pneumonia living in low-resource settings. We conducted a systematic review to estimate the impact and cost-effectiveness of strengthening oxygen services in low-income and middle-income countries with the objective of including oxygen as an intervention in the Lives Saved Tool.
Design
We searched EMBASE and PubMed on 31 March 2021 using keywords and MeSH terms related to ‘oxygen’, ‘pneumonia’ and ‘child’ without restrictions on language or date. The risk of bias was assessed for all included studies using the quality assessment tool for quantitative studies, and we assessed the overall certainty of the evidence using Grading of Recommendations, Assessment, Development and Evaluations. Meta-analysis methods using random effects with inverse-variance weights was used to calculate a pooled OR and 95% CIs. Programme cost data were extracted from full study reports and correspondence with study authors, and we estimated cost-effectiveness in US dollar per disability-adjusted life-year (DALY) averted.
Results
Our search identified 665 studies. Four studies were included in the review involving 75 hospitals and 34 485 study participants. We calculated a pooled OR of 0.52 (95% CI 0.39 to 0.70) in favour of oxygen systems reducing childhood pneumonia mortality. The median cost-effectiveness of oxygen systems strengthening was $US62 per DALY averted (range: US$44–US$225). We graded the risk of bias as moderate and the overall certainty of the evidence as low due to the non-randomised design of the studies.
Conclusion
Our findings suggest that strengthening oxygen systems is likely to reduce hospital-based pneumonia mortality and may be cost-effective in low-resource settings. Additional implementation trials using more rigorous designs are needed to strengthen the certainty in the effect estimate.
Keywords: pneumonia, systematic review, child health, health economics
Key questions.
What is already known?
WHO recommends oxygen therapy for management of hypoxaemia in low-resource settings.
Oxygen can be feasibly introduced and used in low-resource settings for management of hypoxaemia and individual studies have found mortality reductions, though with variable results.
We know little about the cost-effectiveness of investments to increase oxygen utilisation for pneumonia.
What are the new findings?
Interventions to strengthen oxygen systems are likely to reduce pneumonia mortality and these interventions are cost-effective.
There are few published studies examining the effect of oxygen systems on pneumonia mortality in children; all of the studies used an observational, non-randomised design resulting in moderate risk of bias and low certainty in the overall evidence.
What do the new findings imply?
Global health should prioritise oxygen systems strengthening as an intervention to address childhood pneumonia deaths in low-resource settings.
Additional research using more rigorous designs is needed to strengthen the certainty in the estimate of effect.
Background
Pneumonia is the leading infectious cause of mortality among children under-5 in low-income and middle-income countries (LMICs).1 Children with pneumonia are at risk for developing hypoxaemia, or low levels of oxygen in the blood, which greatly increases the likelihood of death.2 Oxygen is an important intervention for patients with hypoxaemia, and therefore, children with pneumonia could greatly benefit from increased access to this life-saving therapy. While oxygen is included as one of the interventions in the Global Action Plan for Pneumonia and Diarrhoea, it has received less attention than other interventions, such as vaccines, breast feeding, indoor air pollution reduction and antibiotics, as evidenced by the lack of global investment and indicators to track oxygen scale-up.3 The lack of prioritisation may be due in part to perceptions that investment in oxygen systems are expensive.
In this paper, we aim to review evidence on the effectiveness of strengthening oxygen systems on mortality for children with pneumonia with the purpose of populating estimates in the Lives Saved Tool (LiST) and estimating the potential public health benefits of increased access to oxygen therapy. The LiST is a model that estimates the impact of scaling up on maternal, newborn and child health, and nutrition interventions in LMICs.4 LiST is often used for strategic planning, programme evaluation, and advocacy by governments, donors and international organisations, and inclusion of oxygen in LiST could support efforts to prioritise it within the context of other child health interventions.5 A previous review completed by Catto et al conservatively estimated that improving oxygen systems could reduce child pneumonia mortality by 20%, saving 68 000–122 000 child lives annually. However, the authors were hampered by lack of effectiveness data from multiple contexts and the resulting evidence was insufficient for inclusion into LiST.6 In this review, we build on this previous work to establish the effectiveness and cost-effectiveness of strengthening oxygen systems on childhood pneumonia mortality in low-ncome and middle-income countries.
Methods
Aims and objectives
The aim of the study was to estimate the impact and cost-effectiveness of improved oxygen systems on pneumonia mortality in children under-5 compared with usual care with the objective of including oxygen as an intervention in the LiST.
Search strategy
We searched two databases (EMBASE and PubMed) for peer-reviewed literature using keywords and MeSH terms related to ‘oxygen’, ‘pneumonia’ and ‘child’ without limitation on language or date. We identified search terms from previous reports and literature reviews, with help from a public health informationist, and tested them to ensure known eligible studies were retrieved. Details of the search strategy and databases searched are presented in online supplemental file 1. AS conducted the search on 31 March 2021. We also reviewed reference lists of included studies and the previous systematic review and contacted corresponding authors and experts in child pneumonia and/or oxygen therapy to identify additional studies not located by the database search.
bmjgh-2021-007468supp001.pdf (59.7KB, pdf)
Results from the searches were exported to Covidence (Veritas Health Innovation, Melbourne, Australia) for managing the review and data extraction. AS and VBC independently screened the abstracts of each study. Studies were included if the study involved children aged 1–59 months with pneumonia, had a comparator or control arm, and included the provision of both oxygen therapy and pulse oximetry as part of the intervention. We excluded studies that were conducted in the intensive care unit, included mechanical ventilation, or studied advanced delivery methods such as continuous positive airway pressure (CPAP) or bubble CPAP. We excluded studies exclusively focused on neonatal populations. If AS and VBC had conflicting decisions on a study, FL reviewed the abstract and provided a final decision. AS and FL conducted a full-text review of studies passing the abstract screening. AS and FL discussed any conflicting reviews and made a joint final decision.
Data extraction
AS and FL extracted study data using a standardised form in Covidence. Key variables extracted include publication details, timing of the study, description of the study population and any subgroups, description of the intervention and context, number of participants and number who died by study arm and mortality impact estimate. Where multiple analyses were reported (eg, on different subpopulations or at different stages of intervention), we first looked for estimates that precisely met our study population (ie, hospitalised children under-5 with pneumonia). If the study included our population of interest, but did not present results specifically for our study population (ie, all paediatric patients instead of under-5), we contacted study authors for clarification or request for reanalysis.
Detailed cost data were also extracted from the full reports, including data on equipment, installation and educational activities, maintenance and ongoing support. Where not published, we contacted study authors to gather data on the costs of programme implementation.
FL assessed risk of bias for all included studies using the quality assessment tool for quantitative studies.7 This tool enables structured evaluation of potential bias in study design, participant selection, confounding, blinding, data collection methods, and withdrawals and drop-outs, has been validated against the Cochrane risk of bias tool and is applicable to all interventional studies.7 8
Data analysis
We present summary details on all studies included in qualitative synthesis, including details on the study design, population, intervention details and context. We included all studies with comparable outcome data in quantitative analysis using generic inverse variance with random effects to calculate a pooled effect estimate with 95% CIs using Review Manager (RevMan V.5.4) (The Cochrane Collaboration, 2020). We expressed the intervention effect as ORs comparing the intervention group to the control group and reported the individual and pooled effect sizes in tables and forest plots. We visually depicted heterogeneity between studies in a forest plot and discussed this heterogeneity with respect to the study context and interventional components in qualitative synthesis but did not attempt quantitative subgroup analysis. To assess outcome reporting bias, FL reviewed study protocols and published reports, comparing the outcomes specified in the protocol (or the Methods section of report if protocol not available) with the outcomes reported in the corresponding report. To assess the certainty of these estimates FL considered each of the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) domains (risk of bias, imprecision, inconsistency, indirectness, publication bias, magnitude of effect and effects of residual confounding) and then gave an overall confidence score of very low, low, moderate or high.9
We calculated cost-effectiveness as disability-adjusted life-years (DALYs) averted per dollar and deaths averted per dollar. Cost data were first categorised into three groups: equipment costs (including freight and customs), implementation (ie, training, installation, maintenance), and solar power where relevant. All study costs were adjusted for inflation and converted to US dollar in the year 2000. The year for study costs were taken as the midpoint of the study.
We estimated number of deaths averted in each study in two steps. We first constructed a counterfactual by dividing the number of pneumonia deaths in the intervention arm by the intervention effect estimate for each study. Then, we took the difference between the observed number of deaths in the intervention arm of the study and the calculated counterfactual estimate to estimate the number of pneumonia deaths averted. To estimate the number of DALYS averted, we multiplied the number of deaths averted by 33, corresponding to the number of DALYs lost due to a death in infancy.10 As all studies did not include solar power equipment as part of the intervention package, we estimated cost-effectiveness of strengthening oxygen systems without solar costs using all studies and cost-effectiveness with solar for only studies that included it.
Cost-effectiveness calculations were conducted in Google Sheets (Alphabet, Mountain View, California, USA).
Results
Search results
Figure 1 presents results from the search results. After removing duplicates, we identified 665 studies for abstract review. Forty-eight studies were included for full text review, and four studies met all criteria for inclusion. No additional studies were identified through expert consultation, and experts reaffirmed that the four studies were the only ones they were aware of.
Figure 1.
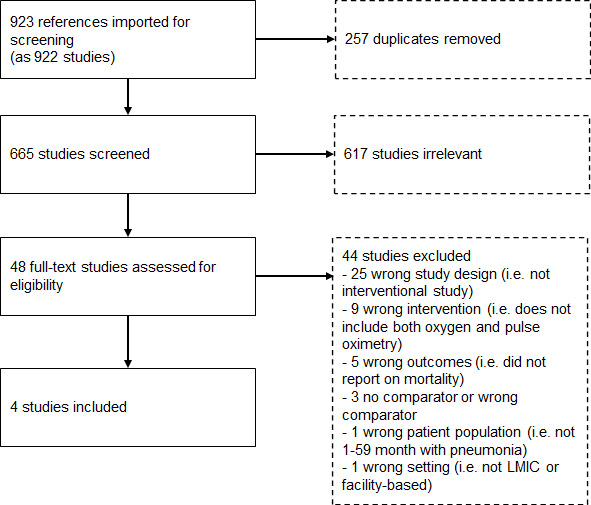
Flow diagram of search results. LMIC, low-and-middle income country.
Study descriptive summaries
Table 1 provides an overview of the four studies included in the review. The studies reviewed included 75 hospitals and 34 485 study participants. Duke et al conducted two non-randomised pre–post prospective oxygen intervention studies in hospitals in Papua New Guinea involving 5 hospitals (2005–2007) and 38 rural health facilities (2015–2017), respectively.11 12 Gray et al conducted a non-randomised controlled prospective evaluation of oxygen systems in 20 (10 intervention, 10 control) hospitals in Laos (2011–2013).13 Graham et al conducted a stepped-wedge cluster-randomised trial in 12 hospitals in Southwest Nigeria (2015–2017) using the stepped wedge design to evaluate pulse oximetry alone compared with full oxygen system and mixed-effects regression to compare against preintervention mortality rates.14
Table 1.
Summary description of included studies
| Study | Country | Study setting | Study design | Study period | No of patients (deaths) | Effect estimate: OR (95% CI) | Quality assessment rating |
| Duke11 | Papua New Guinea | 5 hospitals (3 in highland, 1 coastal and 1 inland) | Prospective before-and-after controlled study | 2005–2007 | 11 291 (489) | 0.64 (0.52 to 0.78) | Moderate |
| Gray13 | Lao PDR | 20 district hospitals | Prospective before-and-after controlled study | 2011–2013 | 1403 (25) | 0.32 (0.13 to 0.80) | Moderate |
| Graham14 | Nigeria | 12 secondary level hospitals in Southwest Nigeria (Oyo, Ondo, Ogun, and Osun states) | Stepped-wedge cluster randomised trial with a prospective before-and-after extended analysis | 2015–2017 | 2858 (195) | 0.46 (0.23 to 0.92) | Strong |
| Duke12 | Papua New Guinea | 38 rural hospitals | Prospective before-and-after controlled study | 2015–2017 | 18 933 (530) | 0.47 (0.39 to 0.57) | Moderate |
Context
The first Papua New Guinea study involved four tertiary (provincial) hospitals and one secondary (district) hospital in highland and lowland areas of Papua New Guinea, each admitting 600–2500 children annually.11 The Lao PDR and Nigerian studies both focused on secondary (district) hospitals that admitted around 50–2500 (median ~350) children annually.13 14 The Lao PDR study involved 20 secondary (district) hospitals (10 intervention, 10 control) distributed across Northern and Southern provinces, representing different climates and disease patterns. The Nigeria study involved 12 secondary level facilities distributed across four states in malaria-endemic south-west Nigeria. The second Papua New Guinea study included 26 primary (health centre) and 12 secondary (district/rural) hospitals, mostly located in remote areas of the highlands and admitting a median 65 (range 0–485) and 375 (range 61–1592) children annually.12
All studies focused activities and evaluation on children, with a particular focus on children under 5 years of age admitted with pneumonia. However, the oxygen systems introduced to facilities served broader newborn, child, and adolescent populations, and those installed in Lao PDR and the smaller facilities in Papua New Guinea also served adults.
Intervention
The improved oxygen systems introduced in all four included studies involved (1) equipment, including oxygen concentrators and handheld pulse oximeters, (2) educational activities for healthcare workers and biomedical engineers/technicians (typically conducted on-site), (3) some degree of ongoing support and supervision; and (4) were implemented using quality improvement approaches (eg, problem solving teams, audit and feedback). However, the specific activities within these core components varied considerably (table 2). The three studies from Papua New Guinea and Lao PDR delivered their oxygen intervention as part of a comprehensive educational programme on hospital care for children, while the Nigeria study focused training more narrowly on oxygen and pneumonia. For example, the Papua New Guinea programme described by Duke included a 5-day comprehensive child health training module delivered by visiting paediatricians at each hospital,12 15 while the Nigeria programme used half-day workshops focused on oximetry and oxygen.16 17 All programmes used quality improvement strategies (eg, problem solving teams, audit and feedback) and included follow-up supervisory and re-educational visits. All programmes used concentrator-based oxygen systems and followed similar design and installation procedures and used the same consultant for senior engineering support. The two most recent programmes included solar power provision to answer implementation questions about how to provide oxygen reliably in small and remote facilities without reliable power.
Table 2.
Description of interventions of included studies
| Study | Intervention components |
| Duke11 |
Equipment and maintenance
Capacity building
Leadership and oversight
Evaluations and assessments
|
| Gray13 |
Equipment and maintenance
Capacity building
Financial
Leadership and oversight
Evaluations and assessments
|
| Graham14 |
Equipment and maintenance
Capacity building
Leadership and oversight
Evaluations and assessments
|
| Duke12 |
Equipment and maintenance
Capacity building
Leadership and oversight
Evaluations and assessments
|
Risk of bias assessment
Based on the design of the studies, we rated the quality of three studies (Duke, Grayand Duke) as moderate and one study as strong (Graham). The three studies were rated as moderate due to having weaker methods in controlling for confounders. All three studies used prospective before-and-after evaluation designs and relied on patient admission and discharge registers to measure mortality rates and with little or no additional data used to control for differences in admission patterns in the preintervention and postintervention periods. Details of the risk of bias assessment is presented in online supplemental file 2.
bmjgh-2021-007468supp002.pdf (96KB, pdf)
Outcome: under-5 pneumonia mortality
Pooled analysis of the four studies found OR 0.52 (95% CI 0.39 to 0.70) for the odds of under-5 pneumonia death comparing improved oxygen systems to standard care (figure 2). Individually, all studies found a reduction in pneumonia mortality when oxygen systems were strengthened with ORs ranging from 0.32 (95% CI 0.13 to 0.83) to 0.64 (95% CI 0.51 to 0.81). Pooled under-5 pneumonia mortality rates reduced from 4.3% to 2.6% following oxygen system strengthening, corresponding to 20 fewer deaths per 1000 cases (from 25 fewer to 14 fewer). Given the general homogeneity in study quality and outcomes, and the low number of studies, we did not conduct subgroup or sensitivity analysis. Using the GRADE, we assessed the overall certainty of the evidence as low due to the observational design of the studies (table 3).
Figure 2.
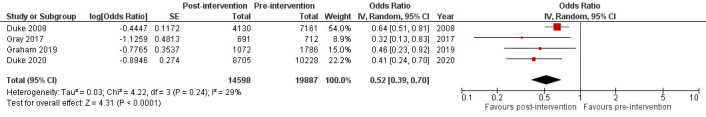
Meta-analysis results and forest plot for under-5 pneumonia mortality.
Table 3.
GRADE assessment of included studies
| Participants (studies) |
Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Magnitude of effect | Effects of residual confounding | Overall certainty of evidence | Study event rates (%) | Relative effect (95% CI) | Anticipated absolute effects | ||
| Risk of death prior to oxygen systems strengthening | Risk of death after oxygen systems strengthening | Risk of death prior to oxygen systems strengthening | Risk difference after oxygen systems strengthening | ||||||||||
| 34 485 (4 observational studies) |
Not serious | Not serious | Not serious | Not serious | No | Not large | No | Low | 860/19887 (4.3%) | 379/14598 (2.6%) | OR 0.52 (0.39 to 0.70) |
43 per 1000 | 20 fewer per 1000 (from 25 fewer to 14 fewer) |
Outcome: under-5 all-cause mortality
Three studies —Duke and Duke in Papua New Guinea and Graham in Nigeria—reported all-cause mortality among paediatric patients admitted to the study facilities. All-cause mortality results from the first study in Papua New Guinea were reported in a separate review.18 The pooled analysis of the studies found OR=0.74 (95% CI 0.59 to 0.94) for the odds of under-5 death comparing improved oxygen systems to standard care (figure 3). Both studies in Papua New Guinea individually found statistically significant differences between the postintervention and preintervention periods of the studies. The odds of mortality in paediatric patients in the period after oxygen systems strengthening relative to the pre-intervention period were 0.72 (95% CI 0.65 to 0.81) in Duke and 0.60 (95% CI 0.45 to 0.80) in Duke. Results from Nigeria did not find a reduction in all-cause paediatric mortality (OR 1.03, 95% CI 0.72 to 1.47).
Figure 3.
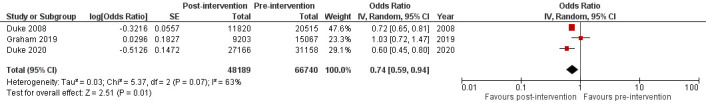
Meta-analysis results and forest plot for paediatric all-cause mortality.
Cost-effectiveness
Table 4 presents programme costs per study facility adjusted to USD in the year 2000. Graham and Duke had the highest per study facility costs –US$57 540 and US$42 432, respectively—due to the costs of solar systems which were not part of the programmes in Duke—US$21 924 per facility—or Gray—US$9448 per facility. Excluding the costs of the solar systems, the programme costs for Graham and Duke were US$19 020 and US$12 912 per facility. The relative costs of oxygen system equipment (including spare parts, ancillary supplies such as nasal prongs, and shipping) accounted for most non-solar programme costs—between 65% and 73%—and implementation costs, such as installation, training and monitoring, were 23%–35%.
Table 4.
Programme costs (in USD in the year 2000)
| Study | No of study facilities | Total programme costs | Per facility costs | ||||||
| Oxygen equipment and supplies | Implementation | Solar | Total | Oxygen equipment and supplies | Implementation | Solar | Total | ||
| Duke11 | 5 | US$71 731 | US$37 890 | N/A | US$109 620 | US$14 346 | US$7578 | N/A | US$21 924 |
| Gray13 | 10 | US$62 977 | US$31 500 | N/A | US$94 477 | US$6298 | US$3150 | N/A | US$9448 |
| Graham14 | 12 | US$167 040 | US$61 200 | US$462 240 | US$690 480 | US$13 920 | US$5100 | US$38 520 | US$57 540 |
| Duke12 | 38 | US$320 720 | US$169 920 | US$1 121 760 | US$1 612 400 | US$8440 | US$4472 | US$29 520 | US$42 432 |
N/A, not available.
Table 5 presents the results of the cost-effectiveness calculations. Across all the studies, we estimate 410 under-5 pneumonia deaths were averted during programme implementation in the studies and approximately 13 526 DALYs averted. We estimate the median cost-effectiveness of strengthening oxygen systems (without solar costs) is US$68 per DALY averted (range: US$44–US$225). For the two studies which included costs of solar power equipment, the cost-effectiveness ranges from US$205 to US$222 per DALY averted. When considering the two Papua New Guinea studies with paediatric all-cause mortality results, we estimate the cost-effectiveness of oxygen systems ranges between US$18 and US$26 per DALY averted. The study in Nigeria did not find a reduction in paediatric all-cause mortality so a cost-effectiveness estimate could not be estimated.
Table 5.
Cost-effectiveness estimates
| Study | OR of postintervention to preintervention | Observed deaths | Estimated counterfactual deaths | Estimated deaths averted | DALYs averted | Cost per DALY averted (without solar) | Cost per DALY averted (with solar) |
| Under-5 pneumonia mortality | |||||||
| Duke11 | 0.64 | 133 | 208 | 75 | 2469 | US$44 | N/A |
| Gray13 | 0.32 | 6 | 19 | 13 | 421 | US$225 | N/A |
| Graham14 | 0.46 | 87 | 189 | 102 | 3370 | US$68 | $205 |
| Duke12 | 0.41 | 153 | 373 | 220 | 7266 | US$68 | $222 |
| Paediatric all-cause mortality | |||||||
| Duke11 | 0.72 | 481 | 668 | 187 | 6173 | US$18 | N/A |
| Duke12 | 0.60 | 867 | 1445 | 578 | 19 074 | US$26 | $85 |
DALYs, disability-adjusted life-years; N/A, not available.
Discussion
Oxygen systems are an essential service for hospital care of children and adults but have not been recognised as a priority until the global COVID-19 pandemic. While oxygen is indicated from a wide variety of acute conditions and essential for safe anaesthesia and surgery, it is particularly critical for the care of children with severe pneumonia where hypoxaemia is common and deadly.19 20 Recent updates to global pneumonia strategies have included oxygen as a priority, but planning and investment cases have been hampered by lack of consensus on the effectiveness and cost-effectiveness of improving oxygen systems.
Our findings suggest that strengthening oxygen systems could reduce hospital-based pneumonia deaths by nearly half and hospital-based paediatric deaths overall by a quarter. One previous review of oxygen for pneumonia in LMICs was conducted by Catto et al.6 At the time of the study’s publication, only one of the studies included in this review was published. Therefore, Catto et al used the Child Health and Nutrition Research Initiative framework to evaluate the effectiveness of oxygen and other dimensions such as feasibility and sustainability. They found the median mortality reduction estimated by experts was 20% (IQR: 10%–35%, min. 0%, max. 50%). Our results fall in the higher end of the estimates found by Catto et al and builds on this work through inclusion of additional studies found through a systematic review and meta-analysis to synthesise the evidence across the studies.
The direction and magnitude of the reported impact of improved oxygen systems on child pneumonia mortality was similar across all four included studies despite variation in intervention design and delivery. A previous mixed-methods review of oxygen systems for paediatric care identified key features that contribute to practice change and sustainability, emphasising the importance of multidisciplinary team-based approaches that address both oxygen supply issues and how oxygen is used.18 While the four included studies in our review varied in strategy, they were all exemplars in this multidisciplinary and systematic approach and we recommend reading the individual study papers to learn more about what works in different contexts.11–14 21–23
Our cost-effectiveness analysis suggests that investments in strengthening oxygen systems are as cost-effective as other prioritised interventions such as vaccines, breastfeeding and indoor air pollution. Figure 4 depicts these results alongside cost-effectiveness results of other child pneumonia interventions found in an analysis conducted by Niessen et al.24 The cost-effectiveness analysis is likely a conservative estimate on the returns on investments to oxygen systems as we included all costs but limited effect calculations to children 1–59 months with pneumonia, for whom the best data on effectiveness exists, over relatively short study periods. However, oxygen systems in all participating facilities served a much broader population, including children with other illnesses, neonates and in some cases adult obstetric and general patients. When we examined cost-effectiveness for all-cause mortality among paediatric admissions—though the evidence was limited to Papua New Guinea—the cost per DALY averted fell by more than half. Our cost-effectiveness calculations were also restricted to the study periods (2–3 years), but we would expect these systems to continue working for at least 5 years with proper maintenance.25 A modelling analysis conducted by Huang et al estimated the cost-effectiveness of solar-powered oxygen systems over a 10-year period and found a cost-effectiveness estimate of US$20 per DALY averted.26 The included studies all used facility-based oxygen system solutions based on oxygen concentrators. While this fitted the clinical quality improvement approach of these small to medium-scale programmes, there are opportunities for increased efficiency by larger scale oxygen systems interventions that include a mix of oxygen supply technologies, policy and market shaping activities, and coordinated supply and distribution mechanisms. For example, while oxygen concentrators have utility in rapid deployment and rural settings, larger scale oxygen production and delivery methods, such as pressure swing adsorption plants and liquid oxygen can provide larger volumes of oxygen at a lower per unit cost and are likely to be more cost-efficient if combined with effective demand forecasting and distribution systems.
Figure 4.
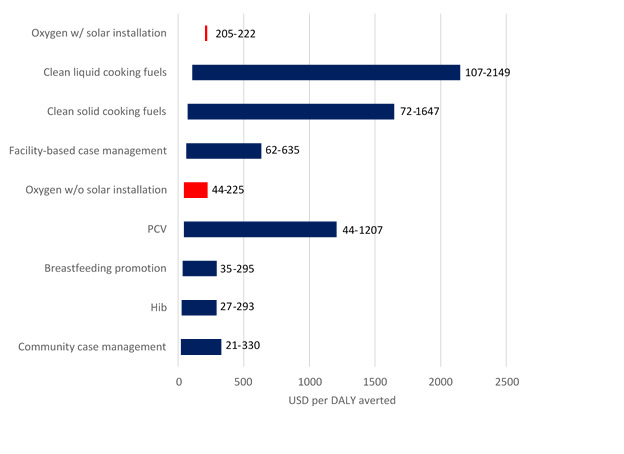
Cost-effectiveness of strengthening oxygen systems (with and without solar) presented alongside other child pneumonia interventions* (in USD in year 2000). *Cost-effectiveness estimates for other child pneumonia interventions were reproduced from Niessen et al.24 PCV, pneumococcal conjugate vaccine; Hib, Haemophilus influenza (H influenzae) type b vaccine.
Our review was limited by the number and quality of the studies. Only four studies examining the effectiveness of strengthening oxygen systems were found during the search with one study conducted in Nigeria, one in Laos and two in Papua New Guinea. Three of the studies used a before-and-after design, and while Graham et al used a stepped-wedge cluster-randomised design, comparison against the preintervention period used a before-and-after approach. While we attempted to isolate the effect on children 1–59 months of age admitted to the facilities with a diagnosis of pneumonia, one of the studies (Duke) did not have age-disaggregated data for paediatric pneumonia admissions. The study author indicated that the vast majority (>90%) of paediatric pneumonia admissions were under-5 (personal correspondence Duke).
Using the GRADE framework, we assess the certainty in the evidence as low—meaning that further research is very likely to have an impact on our confidence in the estimate and change it. The reason for the low rating is primarily due to the observational design of the studies as we had no serious concerns about other characteristics of the study. However, it would be challenging to conduct an individually-randomised trial of oxygen therapy today for ethical reasons. Evidence for the clinical efficacy of oxygen as a medical therapy was established before clinical trials were developed, led by the work of John Scott Haldane and military medics during the first and second World Wars.27–29 As a result, oxygen therapy for treatment of hypoxaemia is standard of care and recommended by leading normative organisations such as WHO.30 Thus, withholding oxygen therapy from hypoxaemic children currently recommended to receive oxygen (ie, a blood oxygen saturation (SpO2) <90%) in order to estimate its clinical efficacy is likely to face ethical challenges. One recent multicentred trial attempted to examine the effect of different oxygen delivery strategies on mortality, including a control arm where children did not receive oxygen unless SpO2 was <80%0.31 The trial was halted by its steering committee before reaching its sample size as the study did not have sufficient funds to continue due to multiple study delays, one of which was a lawsuit over the legality and ethics of the trial.32
Importantly, the studies we reviewed were all assessing the impact of oxygen systems improvement programmes in facilities that lacked oxygen or had very limited access—not the clinical efficacy of oxygen as a medical therapy. Further programme implementation trials using rigorous study designs will continue to be important to generate evidence on successful implementation models, explore the use of oxygen in other settings such as outpatient and emergency referral, shine light on technical, clinical, economic and policy challenges, and contribute to the evidence base on mortality effects.33–37 Despite its ethical challenges, there also remain important areas of research regarding the clinical use of oxygen, including appropriate SpO2 thresholds for prescribing oxygen for different patient groups, health system contexts and geographical altitudes.31 38
Taking into consideration the review findings, we recommend including oxygen therapy as an intervention in LiST and provisionally using the pooled effect estimate and confidence intervals found in this review for the intervention effect and uncertainty parameters in LiST. The process and results of our review followed the intervention review standards for use in LiST described by the Child Health Epidemiology Reference Group (CHERG), and though the certainty of the effect estimate is ‘low’, this does not automatically preclude the intervention from being included in LiST.39 The CHERG guidelines recommend review of interventions graded as ‘low’ be included in the model but the intervention effect size should continue to be studied and as new evidence emerges that changes the effect estimate for oxygen, the parameters in LiST should be updated to reflect the best available evidence. Future research and discussion are also needed to define and measure oxygen therapy coverage to populate LiST coverage estimates.
Conclusions
Strengthening oxygen systems in LMICs appears to reduce hospital-based pneumonia mortality rates in children under-5 and may be cost-effective. Additional implementation studies using more rigorous designs are needed to strengthen the certainty in the effect estimate.
Acknowledgments
We thank Donna Hesson, public health informationist at the Johns Hopkins Bloomberg School of Public Health, who helped develop and conduct our search strategy.
Footnotes
Handling editor: Seye Abimbola
Twitter: @grahamhamish
Contributors: FL conceptualised the study and VBC, AS and FL developed the study protocol. VBC and AS developed the search strategy and screened records. VBC, AS and FL extracted data and FL analysed the data. FL wrote the first draft of the manuscript and all authors provided critical review, important intellectual input and approved the final version of the manuscript for publication. The data were available to all authors on request. FL is the guarantor for the study and accepts full responsibility for the finished work and/or the conduct of the study, had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding: The study was supported by grants from the Bill & Melinda Gates Foundation (INV-001132) and The ELMA Foundation.
Disclaimer: The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: FL is employed by CHAI is who implementing oxygen strengthening programmes across low-resource settings. HRG is a lead investigator for one of the studies included in the review (Graham 2019), coinvestigator for one other study (Duke 2020) and advisor to the Lifebox Foundation, UNICEF and Unitaid on pulse oximetry. AS and VBC declare no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1.McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health 2019;7:e47–57. 10.1016/S2214-109X(18)30408-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazzerini M, Sonego M, Pellegrin MC. Hypoxaemia as a mortality risk factor in acute lower respiratory infections in children in low and middle-income countries: systematic review and meta-analysis. PLoS One 2015;10:e0136166. 10.1371/journal.pone.0136166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization & UNICEF . End preventable deaths: global action plan for prevention and control of pneumonia and diarrhoea 2013.
- 4.Walker N, Tam Y, Friberg IK. Overview of the lives saved tool (LiST). BMC Public Health 2013;13:S1. 10.1186/1471-2458-13-S3-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stegmuller AR, Self A, Litvin K, et al. How is the lives saved tool (list) used in the global health community? Results of a mixed-methods list user study. BMC Public Health 2017;17:773. 10.1186/s12889-017-4750-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catto AG, Zgaga L, Theodoratou E, et al. An evaluation of oxygen systems for treatment of childhood pneumonia. BMC Public Health 2011;11(Suppl 3):S28. 10.1186/1471-2458-11-S3-S28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas BH, Ciliska D, Dobbins M, et al. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004;1:176–84. 10.1111/j.1524-475X.2004.04006.x [DOI] [PubMed] [Google Scholar]
- 8.Armijo-Olivo S, Stiles CR, Hagen NA, et al. Assessment of study quality for systematic reviews: a comparison of the Cochrane collaboration risk of bias tool and the effective public health practice project quality assessment tool: methodological research. J Eval Clin Pract 2012;18:12–18. 10.1111/j.1365-2753.2010.01516.x [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Vist GE, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization, 2009. [Google Scholar]
- 11.Duke T, Wandi F, Jonathan M, et al. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. Lancet 2008;372:1328–33. 10.1016/S0140-6736(08)61164-2 [DOI] [PubMed] [Google Scholar]
- 12.Duke T, Pulsan F, Panauwe D, et al. Solar-powered oxygen, quality improvement and child pneumonia deaths: a large-scale effectiveness study. Arch Dis Child 2021;106:224–30. 10.1136/archdischild-2020-320107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray AZ, Morpeth M, Duke T, et al. Improved oxygen systems in district hospitals in Lao PDR: a prospective field trial of the impact on outcomes for childhood pneumonia and equipment sustainability. BMJ Paediatr Open 2017;1:e000083. 10.1136/bmjpo-2017-000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham HR, Bakare AA, Ayede AI, et al. Oxygen systems to improve clinical care and outcomes for children and neonates: a stepped-wedge cluster-randomised trial in Nigeria. PLoS Med 2019;16:e1002951. 10.1371/journal.pmed.1002951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duke T, Hwaihwanje I, Kaupa M, et al. Solar powered oxygen systems in remote health centers in Papua New Guinea: a large scale implementation effectiveness trial. J Glob Health 2017;7:010411. 10.7189/jogh.07.010411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham HR, Bakare AA, Ayede AI, et al. Oxygen systems to improve clinical care and outcomes for children and neonates: a stepped-wedge cluster-randomised trial in Nigeria. PLoS Med 2019;16:e1002951. 10.1371/journal.pmed.1002951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham HR, Ayede AI, Bakare AA, et al. Improving oxygen therapy for children and neonates in secondary hospitals in Nigeria: study protocol for a stepped-wedge cluster randomised trial. Trials 2017;18:502. 10.1186/s13063-017-2241-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham H, Tosif S, Gray A, et al. Providing oxygen to children in hospitals: a realist review. Bull World Health Organ 2017;95:288–302. 10.2471/BLT.16.186676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subhi R, Adamson M, Campbell H, et al. The prevalence of hypoxaemia among ill children in developing countries: a systematic review. Lancet Infect Dis 2009;9:219–27. 10.1016/S1473-3099(09)70071-4 [DOI] [PubMed] [Google Scholar]
- 20.Graham H, Bakare AA, Ayede AI, et al. Hypoxaemia in hospitalised children and neonates: a prospective cohort study in Nigerian secondary-level hospitals. EClinicalMedicine 2019;16:51–63. 10.1016/j.eclinm.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matai S, Peel D, Wandi F, et al. Implementing an oxygen programme in hospitals in Papua New Guinea. Ann Trop Paediatr 2008;28:71–8. 10.1179/146532808X270716 [DOI] [PubMed] [Google Scholar]
- 22.Duke T, Peel D, Wandi F, et al. Oxygen supplies for hospitals in Papua New Guinea: a comparison of the feasibility and cost-effectiveness of methods for different settings. P N G Med J 2010;53:126–38. [PubMed] [Google Scholar]
- 23.Graham HR, Bakare AA, Gray A, et al. Adoption of paediatric and neonatal pulse oximetry by 12 hospitals in Nigeria: a mixed-methods realist evaluation. BMJ Glob Health 2018;3:e000812. 10.1136/bmjgh-2018-000812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niessen LW, ten Hove A, Hilderink H, et al. Comparative impact assessment of child pneumonia interventions. Bull World Health Organ 2009;87:472–80. 10.2471/BLT.08.050872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley BD, Light JD, Ebonyi AO, et al. Implementation and 8-year follow-up of an uninterrupted oxygen supply system in a hospital in the Gambia. Int J Tuberc Lung Dis 2016;20:1130–4. 10.5588/ijtld.15.0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Mian Q, Conradi N, et al. Estimated cost-effectiveness of Solar-Powered oxygen delivery for pneumonia in young children in low-resource settings. JAMA Netw Open 2021;4:e2114686. 10.1001/jamanetworkopen.2021.14686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grainge C. Breath of life: the evolution of oxygen therapy. J R Soc Med 2004;97:489–93. 10.1177/0141076809701011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Royal Society of Medicine . Reports of societies (oxygen therapy). BMJ 1920;1:150–3. [Google Scholar]
- 29.Haldane JS. The therapeutic administration of oxygen. Br Med J 1917;1:181–3. 10.1136/bmj.1.2928.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization . The clinical use of oxygen in hospitals with limited resources: guidelines for health-care workers, hospital engineers and managers 2012.
- 31.Maitland K, Kiguli S, Olupot-Olupot P, et al. Randomised controlled trial of oxygen therapy and high-flow nasal therapy in African children with pneumonia. Intensive Care Med 2021;47:566–76. 10.1007/s00134-021-06385-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters MJ, Macharia W, Molyneux E. A COASTal view: where prior beliefs and uncertainty collide. Intensive Care Med 2021;47:591–3. 10.1007/s00134-021-06406-1 [DOI] [PubMed] [Google Scholar]
- 33.Fashanu C, Mekonnen T, Amedu J, et al. Improved oxygen systems at hospitals in three Nigerian states: an implementation research study. Pediatr Pulmonol 2020;55. 10.1002/ppul.24694 [DOI] [PubMed] [Google Scholar]
- 34.McCollum ED, Mvalo T, Eckerle M, et al. Bubble continuous positive airway pressure for children with high-risk conditions and severe pneumonia in Malawi: an open label, randomised, controlled trial. Lancet Respir Med 2019;7:964–74. 10.1016/S2213-2600(19)30243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCollum ED, King C, Deula R, et al. Pulse oximetry for children with pneumonia treated as outpatients in rural Malawi. Bull World Health Organ 2016;94:893–902. 10.2471/BLT.16.173401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King C, Boyd N, Walker I, et al. Opportunities and barriers in paediatric pulse oximetry for pneumonia in low-resource clinical settings: a qualitative evaluation from Malawi and Bangladesh. BMJ Open 2018;8:e019177. 10.1136/bmjopen-2017-019177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mian Q, Huang Y, Conroy A, et al. Solar-powered oxygen delivery to treat childhood pneumonia in low-resource settings: a randomised controlled non-inferiority trial and cost-effectiveness study. Lancet Glob Health 2019;7:S10. 10.1016/S2214-109X(19)30095-6 [DOI] [Google Scholar]
- 38.Colbourn T, King C, Beard J, et al. Predictive value of pulse oximetry for mortality in infants and children presenting to primary care with clinical pneumonia in rural Malawi: a data linkage study. PLoS Med 2020;17:e1003300. 10.1371/journal.pmed.1003300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker N, Fischer-Walker C, Bryce J, et al. Standards for CHERG reviews of intervention effects on child survival. Int J Epidemiol 2010;39(Suppl 1):i21–31. 10.1093/ije/dyq036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-007468supp001.pdf (59.7KB, pdf)
bmjgh-2021-007468supp002.pdf (96KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Not applicable.


