Abstract
Cofilin is an essential actin filament severing protein that accelerates the assembly dynamics and turnover of actin networks by increasing the number of filament ends where subunits add and dissociate. It binds filament subunits stoichiometrically and cooperatively, forming clusters of contiguously-bound cofilin at sub-saturating occupancies. Filaments partially occupied with cofilin sever at boundaries between bare and cofilin-decorated segments. Imaging studies concluded that bound clusters must reach a critical size (Cc) of 13–100 cofilins to sever filaments. In contrast, structural and modeling studies suggest that a few or even a single cofilin can sever filaments, possibly with different severing rate constants. How clusters grow through the cooperative incorporation of additional cofilin molecules, specifically if they elongate asymmetrically or uniformly from both ends and if they are modulated by filament shape and external force, also lacks consensus. Here, using hydrodynamic flow to visualize individual actin filaments with TIRF microscopy, we found that neither flow-induced filament bending, tension, nor surface attachment conditions substantially affected the kinetics of cofilin binding to actin filaments. Clusters of bound cofilin preferentially extended toward filament pointed ends and displayed severing competency at small sizes (Cc < 3), with no detectable severing dependence on cluster size. These data support models in which small clusters of cofilin introduce local, but asymmetric, structural changes in actin filaments that promote filament severing with a rate constant that depends weakly on the size of the cluster.
Keywords: tension, curvature, microfluidics, kinetics, fluorescence
Introduction
Actin filaments generate force via polymerization and help drive an array of cellular functions such as endocytosis and cell motility. To help regulate this force generation the actin filament network must be disassembled for subunit recycling. A central player in this process is the conserved, eukaryotic ADF/cofilin family (herein referred to as ‘cofilin’) of actin filament severing proteins.1,2
Extensive biochemical and biophysical analysis of various cofilin isoforms within a given species and across different phyla carried out under a broad range of solution conditions (salt concentration and pH) has revealed unique behaviors of the individual isoforms, but also identified general conserved principles of the cofilin binding and filament severing mechanism. Cofilin binds actin filaments between longitudinal subunits with a 1 cofilin:1 actin subunit stoichiometry.3,4 Bound cofilin changes the helical twist3 and mechanical properties5–10 of filaments. Cofilin binds filaments with positive cooperativity,4,11,12 so clusters of contiguously-bound cofilin form at sub-stoichiometric occupancies.4,11–16 These clusters elongate from their ends through incorporation of additional cofilin molecules. Filament severing occurs preferentially at cluster ends, or “boundaries” (between bare and cofilin-decorated segments)1,16. This boundary severing mechanism renders filaments partially decorated with cofilin more susceptible to severing than bare or fully-decorated ones.1,4–6,16–18
Although a great deal has been learned about the mechanism of cofilin binding, cluster growth and boundary severing, how clusters grow, specifically if they elongate uniformly13,19 or asymmetrically15,20–22 lacks general consensus. Similarly, whether cofilin clusters must reach a critical cluster size to sever filaments,4,13,14,18,20,21,23 and how the severing rate constant scales with the cofilin cluster size also remain to be firmly established. Some studies report symmetrical cluster growth,13,19 while others support asymmetric effects on actin15,20–22 and cluster elongation, with the pointed end-side boundary elongating more rapidly.15,20–22 Similarly, recent fluorescence13,14 and atomic force microscopy20 imaging studies reported that bound cofilin clusters must grow and reach a critical size (Cc) of 13–100 to be severing competent. In contrast, structural studies21 and mathematical modeling of the [cofilin]-dependence of the filament length distribution4,6,22,23 suggest that smaller clusters (Cc = 2–3) sever filaments, with even a single cofilin displaying severing activity,4,12,18,23 albeit less effectively than clusters > 2 cofilins.21,23 These inconsistencies cannot be attributed to specific behaviors of the individual isoforms investigated since conflicting results have been reported for the same isoform. Here, we evaluate the assembly kinetics, elongation, and actin filament severing competency of S. cerevisiae cofilin (herein referred to as cofilin). Binding of fluorescently-labeled cofilin was imaged in real time with TIRF microscopy while applying force with hydrodynamic flow using microfluidics. Cofilin clusters elongated asymmetrically with more rapid growth towards filament pointed ends, and clusters readily sever filaments at small sizes.
Results
Visualization of cofilin binding to actin filaments by fluorescence microscopy and microfluidics
Alexa-488 labeled cofilin binding to actin filaments was measured in real time by TIRF microscopy16 while applying long axis pulling forces (range = 0. 2–20 pN) with hydrodynamic flow (see Methods).19,24,25 Filaments tethered to the surface near their pointed ends fluctuated in and out of the focal plane in the absence of flow. Filaments remained in the plane of focus when under hydrodynamic flow (500 μL min−1, up to 20 pN), which facilitated continuous visualization of the entire filament (Figure 1(A), Sup Movie 1). Fluid flow introduces a tension gradient across the filaments. The filament tensile force scales linearly with their length,19,24–26 peaking at the surface attachment point19,24–26 (see Methods).
Figure 1.
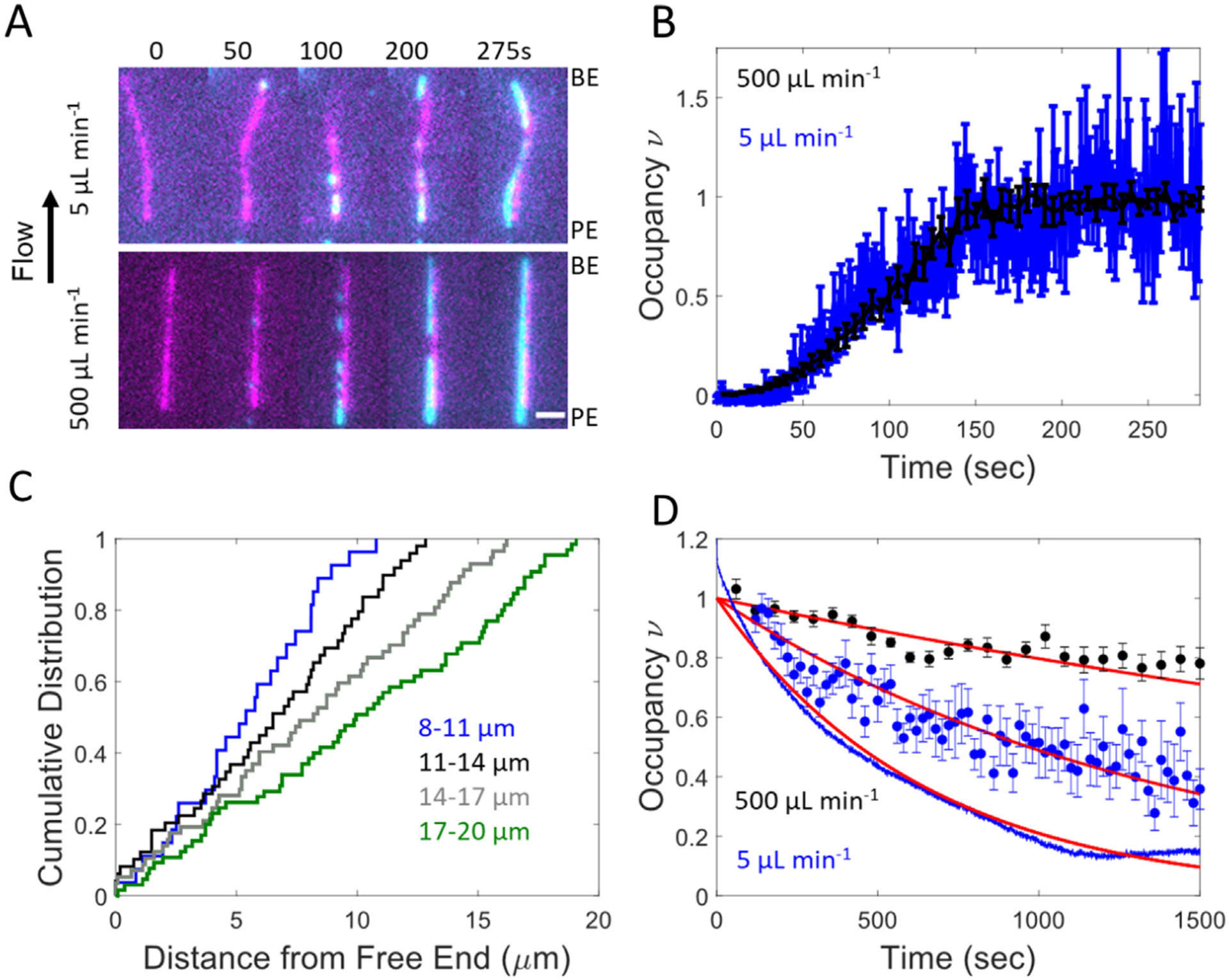
Cofilin association, but not dissociation, is independent of applied flow force and filament bending. (A) Example association experiments where 200 nM cofilin is flown over pointed end tethered actin filaments at 500 (bottom) and 5 (top) μL min−1 flow. Alexa 647-actin filaments are colored magenta and Alexa 488-cofilin is colored cyan. Scale bar is 2 μm. (B) Time courses of normalized actin filament-bound cofilin fluorescence intensity during association in (A) (see Methods). n = 10 and 14 for the 500 (black) and 5 (blue) μL min-1 flow rates, respectively. Only filaments without severing events were analyzed. Uncertainty bars represent the standard error of the mean intensity. Average filament lengths are 9.7 and 7.7 μm for high and low flow, respectively. (C) Cumulative distribution function of the observable cluster formation positions from filament free ends. Positions were determined 15 s after addition of 1 μM cofilin. A total of 19 filaments with 230 clusters were used. Traces correspond to filaments with comparable lengths (blue = 8–11 mm, black = 11–14 mm, gray = 14–17 μm, and green = 17–20 μm). (D) Time courses of competitive cofilin dissociation via stopped flow and fluorescence microscopy. Dissociation of Alexa-488 cofilin was measured under 500 μL min−1 flow (black circles) and 5 μL min−1 flow (blue circles) with 1 μM unlabeled cofilin in the flow buffer. n = 19 and 18 filaments, respectively. Uncertainty bars represent the standard error of the mean. Dissociation of Oregon green-labeled cofilin was measured via fluorescence change with stopped flow (continuous blue line) in solution by competition with unlabeled cofilin (see Methods). All time courses were fit to single exponentials (red lines) yielding cooperative dissociation rate constants of 2.3 × 10−4 s−1 (95% CI 2–2.5 × 10−4) under 500 μL min−1 flow, 7.1 × 10−4 s−1 (95% CI 5.8–8.4 × 10−4) under 5 μL min−1 flow, and 1.6 × 10−3 s−1 (95% CI 1.5–1.6 × 10−3) under stopped flow.
Cofilin cluster formation and elongation depend weakly on filament tension, curvature, and surface tethering
Cofilin binds actin filaments with positive cooperativity,11,12 forming nuclei of contiguously bound cofilin along filaments4,23 that appear initially as puncta when visualized by TIRF microscopy,13,14,16,27 (Figure 1(A), Sup Movie 1). Clusters elongate through the incorporation of additional cofilin molecules at their ends, or boundaries that adjoin bare actin segments (Figure 1(A), discussed below).12,13 Elongating clusters can merge (before filament severing occurs) to form larger clusters, leading to a concomitant reduction in the number of boundaries.
Time courses of cofilin binding to individual actin filaments assayed from the cumulative fluorescence of filament-bound cofilin were comparable at low (5 μL min−1, ≤0.2 pN of force) and high (500 μL min−1, up to 20 pN of force) flow rates and applied forces (Figure 1(B) and Sup Movie 1), suggesting that cluster formation and elongation are independent of filament tension over the experimental range.13,19 Consistent with this interpretation, the cumulative distribution of cofilin cluster formation position along actin filaments scales linearly with the distance from the free end (Figure 1(C)), indicating that clusters form randomly along filaments independent of long-axis tensile force, which scales with filament length. If filament tension over the range evaluated here (0.2–20 pN) inhibited cofilin binding,28 cluster formation would be biased towards the free end (i.e. the origin), where force is lowest.
To assess if filament shape, namely curvature, impacts cofilin association, experiments were carried out with biotinylated filaments adhered to the surface in a random orientation throughout their length (see Methods), such that filaments displayed straight and curved segments (up to 3 μm−1; Sup Figure 1(A)). Cofilin clusters formed with equal probability at curved and straight filament segments (Sup Figure 1 and Sup Movie 2). Cofilin cluster formation and growth along fluctuating or biotin tethered filament segments are also similar (Sup Figure 2). Thus, cofilin cluster formation and elongation depend weakly on the range of filament tensions (up to 20 pN) and curvatures (<3 μm−1, radius of curvature > 300 nm) and the surface attachment conditions evaluated here.
In contrast, cofilin cooperatively dissociated from saturated filaments ~ 2–3-fold more slowly under high fluid flow and applied force (500 μL min−1, up to 20 pN) than under low flow (5 μL min−1, up to 0.2 pN) and in solution (i.e., no force; Figure 1(D)). In solution, cooperative dissociation of Oregon green-labeled cofilin was measured by competition with unlabeled cofilin via stopped flow (see Methods), and was ~2 fold faster compared to low flow (5 μL min−1) measurements (Figure 1(D)).
Together, these observations are consistent in that cooperative dissociation increased with filament bending and decreased with little long-axis filament tension. However, cofilin cooperatively dissociated uniformly from straight filaments under high flow and force (i.e., dissociation was not biased to low- or high-tension regions; Sup Figure 3), suggesting modest or negligible effects of long-axis tension on cofilin dissociation. It is then more likely that filament bending, which occurs readily at low force and in solution, facilitates cofilin cooperative dissociation, whereas tension along a straight filament does not. We note that cooperative cofilin dissociation occurs much more slowly than association, so any effects of filament bending and tension on cooperative dissociation would not significantly contribute to the observed association time courses described above.
Cofilin clusters preferentially elongate towards the filament pointed end
Clusters of bound cofilin elongated from both of their ends (i.e., boundaries; Figure 2(A)) at a constant, [cofilin]-dependent rate (Figure 2(B) and Sup Figure 4). Elongation towards the filament pointed end was 2-fold faster than towards the barbed end (kPE,elong = 6.6 μM s−1; kBE,elong = 3.3 μM s−1). Although clusters grew at a constant rate, there was considerable variance in the elongation rate of any given cluster (Sup Figure 4 and Figure 2(C), (E), (F)). The elongation rate was independent of the distance from the untethered filament barbed end, suggesting that the observed variability did not arise from filament tension (Figure 2(C) and (E)). The variability in cluster growth was less pronounced on unlabeled actin filaments when compared to fluorescently labeled actin filaments (Figure 2(D)–(F) and Sup Figures 4 and 5).
Figure 2.
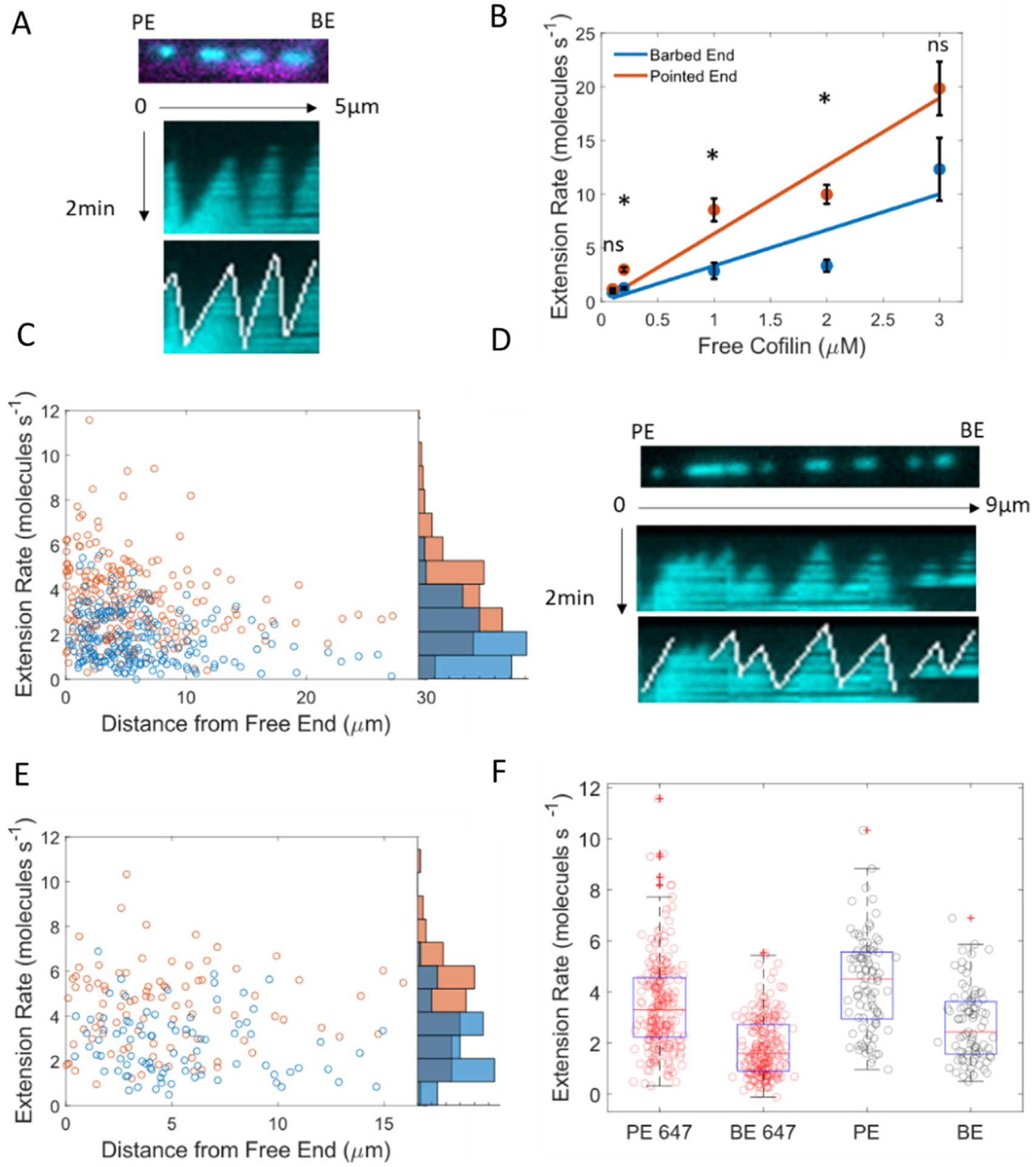
Cofilin forms clusters that preferentially elongate toward the filament pointed end. (A) Cofilin association on 15% Alexa-647-labeled filaments (top). Kymographs of association (middle) and manual tracing of cofilin extension (bottom). (B) Cofilin cluster extension rates towards the barbed (blue) and pointed ends (orange) as measured during association with kymographs. n > 20 clusters for each condition. Slopes of best fit lines yield cluster elongation rates of 3.3 (95% CI 1.8–4.9) and 6.3 (95% CI 4.8–7.8) μM−1 s−1 towards the barded and pointed ends, respectively. Uncertainty bars represent the standard error of the mean for each point. Significance was determined with Dunn’s multiple comparison test. The * indicates p < 0.05. C) Cofilin cluster extension rates towards the barbed (blue) and pointed ends (orange) of Alexa-647-labeled filaments as a function of the distance from the center of the cluster from the barbed end. The scatter along the x-axis is due to the filament length distribution and not tension. D) Cofilin association on unlabeled filaments (top). Kymographs of association (middle) and manual tracking of cofilin extension (bottom). E) Cofilin cluster extension rates towards the barbed (blue) and pointed ends (orange) on unlabeled filaments as a function of the distance of the center of the cluster from the barbed end. F) Box plot of cofilin extension rates during 200 nM association on unlabeled (black points) and Alexa-647 (red points) labeled filaments. The red line represents the median, the blue box indicates the 25th and 75th percentiles, whiskers indicate the data spread excluding outliers, and plus signs indicate outliers. Points were classified as outliers if they were greater than q3 + w × (q3 − q1) or less than q1 − w × (q3 − q1). Here q1, is the first quartile, q2 is the second quartile, and w is the maximum whisker which extends to the points closest to ±2.7σ.
Filaments sever preferentially at the pointed end side of cofilin clusters
Previous studies with human ADF and mouse cofilin suggest that severing occurs preferentially at the pointed end boundary of clusters.13,20 This observation is consistent with larger structural perturbations on the pointed end boundary relative to the barbed end boundary.21,29 To test if this behavior is conserved in non-vertebrate cofilins, we evaluated the severing of filaments partially decorated with S. cerevisiae cofilin under non-equilibrium conditions. Cofilin (1 μM) was rapidly flowed into the microfluidic chamber which contained pointed end tethered actin filaments and incubated for ~ 20 seconds while clusters formed uniformly across actin filaments. Free cofilin was washed out of the chamber (see Methods) and severing events were monitored in real time under high force.
We identified 51 filament severing events: 25 occurred at the pointed end-side of a cofilin cluster and 6 at the barbed end-side (Sup Figure 6). Some severing events (n = 20), could not be classified because clusters and their boundaries were too close to reliably distinguish (Sup Figure 6). Given that 25 out of the 31 (81%) classifiable severing events occurred at the pointed end side of clusters, we favor a mechanism in which filaments sever preferentially at the pointed end side of clusters (for a binomial test p < 0.001), consistent with the reported behavior of human cofilin.13
A few bound cofilins can sever a filament
To determine if clusters of bound cofilin must reach a critical size to promote filament severing as reported,4,13,14,18,20,21,23 we mixed low [cofilin] (50 nM) with filaments that were tethered to the surface near their pointed ends (as described above) or throughout their length and monitored severing events. Under these conditions, nuclei formed, elongated slowly (Sup Figure 3), and severed at their boundaries (Sup Movies 2–4). Low cofilin concentrations (50 nM) were essential to assess if a few cofilin can sever filaments. At higher cofilin concentrations, clusters grew rapidly and merged, lowering the number of boundaries and opportunities for severing as the occupancy increased, thus limiting the [cofilin]-range over which informative measurements could be done.
To determine the sizes of these bound cofilin clusters, the fluorescence intensity was converted to number of cofilin molecules via bleach stepping analysis30 (Figure 3(A) and (B), and Sup Figure 7). Briefly, a separate set of experiments were conducted in which clusters were rapidly photobleached and the loss in fluorescence for each individual cluster was fit to a bleach stepping model (Figure 3(A)).30 The magnitude of the model predicted intensity changes of each step for all the clusters were binned into a histogram and fit to a mixture of two Gaussians (Figure 3(B)). The step size associated with the first Gaussian peak was taken as the brightness of a single cofilin and the second peak was approximately two-fold brighter, consistent with two simultaneous bleach events.30
Figure 3.
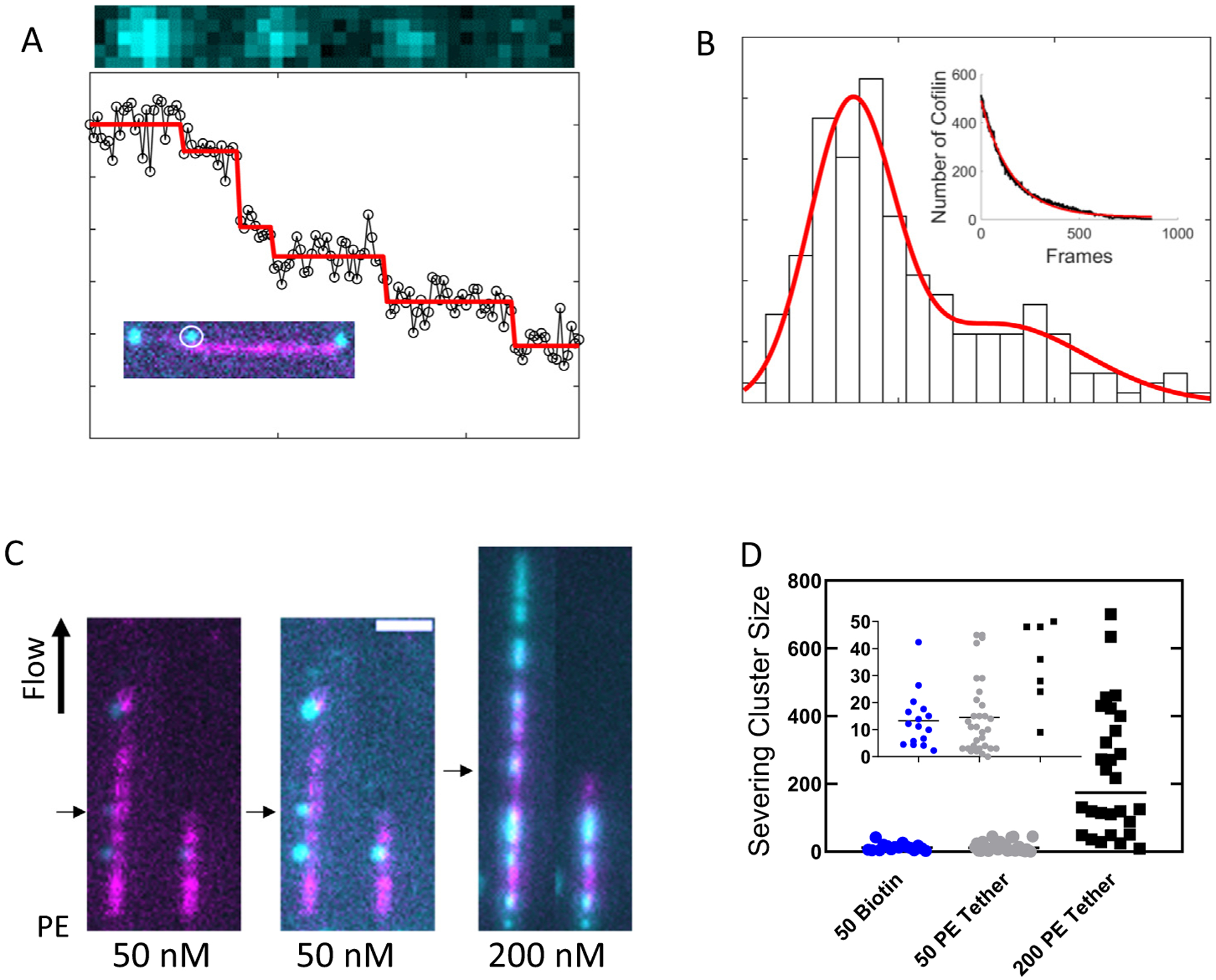
A few cofilins can sever a filament. (A) Photobleaching of a cofilin cluster (black circles) and the predicted bleach steps (red line) (see Methods). (B) Histogram of model fit bleach steps for all the clusters measured during rapid photobleaching on filaments completely adhered to the surface with biotin. The red line represents the best fit of the data to a mixture of Gaussians. The means for the two prominent peaks are 70 and 160. The inset depicts the ensemble bleaching rate for all the clusters. The red line represents the best fit of the data to a single exponential yielding a rate constant of 6.4 × 10−3 frame s−1 which is a combination of bleaching and dissociation. (C) Filament severing during 50 (left) and 200 (right) nM cofilin association. Filaments were attached to the surface near their pointed ends. The middle image is an intensity rescaling of the leftmost image to better visualize the clusters. (D) Size of bound cofilin clusters (measured via fluorescence) prior to observed filament severing events. During 50 nM cofilin severing experiments, filaments were biotinylated throughout their length or tethered at their pointed end. During 200 nM cofilin severing experiments, filaments were only tethered at their pointed ends.
Severed filaments always fragmented adjacent to small clusters (Figure 3(C)), save 1 of 32 observed events at 50 nM cofilin under pointed end tethered conditions (>97% of observed severing events). The mean number of cofilin molecules comprising a cluster immediately (i.e. 1 frame) prior to severing was 13.3 and 14.6 with standard deviations 10.3 and 14 for filaments biotinylated throughout their length and tethered on at their pointed end, respectively (Figure 3(D)). As few as 1 cofilin was observed severing filaments under both attachment conditions, suggesting that a single cofilin can sever an actin filament.4,18,21,23 We note that clusters are growing throughout the experiment, so the mean number of cofilins does not reliably reflect a critical cluster size for severing.
The mean cluster size for a given severing event depended on the cofilin concentration and increased from ~ 13–15 at 50 nM cofilin to 233 with standard deviation 191 at 200 nM cofilin (Sup Movie 3). Similar to 50 nM cofilin experiments, severing events only occurred adjacent to cofilin clusters (28 total severing events up to 20 pN), strongly suggesting that they do not represent random, intrinsic filament severing events (Sup Figure 8).
Cluster size and severing rate are independent
The preceding data indicates that small cofilin clusters can sever filaments, but it does not reveal if or how the severing rate constant depends on the cluster size. If severing is independent of the cofilin cluster size, a single severing rate constant should account for the observed severing events. Conversely, if the severing rate constant scales with the cofilin cluster size, the total number of observed severing events should vary with different cluster size distributions. We measured the number of severing events over time at 2 different cofilin concentrations (50 and 200 nM); the limits over which establish 2 reliably different cluster size distributions (Sup Movie 3). At concentrations lower than 50 nM clusters don’t form with a frequency that permits severing measurements with high enough throughput and at concentrations higher than 200 nM cluster elongation happens fast enough to lower severing frequency without a significant change in cluster size distribution. Thus, we evaluated the limits of our experimental range.
Throughout the duration of the association experiments (Figure 3(C) and (D); discussed above), cofilin clusters grew ~ 4x faster at 200 nM than 50 nM. Once a detectable cluster formed, the time until boundary severing occurred was monitored at filament pointed and barbed ends. Pointed end boundaries exhibited shorter boundary lifetimes, providing further evidence that the pointed end boundaries sever faster than barbed end boundaries (Figure 4(A) and (B)). No detectable change in boundary severing lifetimes was detected across the two free cofilin concentrations (Figure 4(A) and (B)), indicating a weak or no relationship between boundary severing and cluster size.
Figure 4.

Cofilin boundary severing is not strongly dependent on cluster size: (A and B) The lifetime of unsevered boundaries at the pointed end (A) and barbed end (B) of cofilin clusters with 50 nM (black) and 200 nM (blue) free cofilin in solution. 95% confidence bounds are indicated by shaded regions. Kaplan Meir corrections were applied to account for boundaries that were not severed or lost.44
Discussion
Filament bending and long-axis tension do not affect cofilin cluster formation or elongation
Previous studies have evaluated the effects of pN tensions on vertebrate cofilin binding, with conflicting results.13,19,28,31 Long axis pulling forces (30 pN) applied with optical traps slowed mouse cofilin-1 severing 2.5-fold28 (due to a reduction in binding), while forces (30 pN) applied with hydrodynamic flow did not affect mouse cofilin-1 association.13,19 Given this discrepancy, we evaluated the effects of filament tension and bending as well as surface attachment on cofilin binding.
We observed no substantial effects of filament tension (up to 20 pN) on cofilin cluster formation or elongation, consistent with Wioland et al.13,19 who used the same method as we have here. Although filament tension and under twisting are coupled during flow experiments due to uncon-strained rotation of the untethered filament end, elastic mesoscale modeling of actin filaments under tension32–34 indicates that the experimental tensions here are not large enough to significantly under twist actin filaments (Sup Figure 9). Because flow in our experiments introduces minimal filament twisting, potential effects of applied torsional loads on cofilin occupancy32,33 can be dismissed.
Recent modeling studies suggested that filament curvatures could destabilize the actin D-loop of subdomain 2 and enhance cofilin binding.33 We observed no statistical difference between cofilin binding to linear versus curved regions of filaments (<3 μm−1, radius of curvature 300 nm). The curvatures measured here (<3 μm1, radius of curvature > 300 nm) are significantly less curved than the curvature introduced in the modeling study (radius of curvature ~ 50 nm), suggesting that the weak effects of filament curvature reported here are because the filament curvature was too small to affect cofilin binding. At high curvature, the filament twist changes,34 which is predicted to affect cofilin binding32 since filament twist and cofilin occupancy are thermodynamically linked. Large-scale filament and bundle curvature has been observed in cells,35 and may allow contractile forces generated by myosin motors to specifically target cofilin to specific regions and filament populations.
Cofilin clusters elongate preferentially towards the actin filament pointed end
Cofilin binding to actin filaments is considerably slower than the limit for diffusional encounter and is likely limited by filament conformational dynamics.12,18,31 Cofilin clusters elongated ~2 times faster from their pointed ends than their barbed ends (Figure 2). This small but detectable bias in cluster elongation polarity was not observed in other fluorescence studies,13 but atomic force microscopy visualized the helical pitch of cofilactin clusters propagate allosterically 13 subunits into bare, pointed end filament segments.20 Such a large and asymmetric change in filament structure could certainly account for the observed pointed end elongation bias. However, one may anticipate the difference to be more than 2-fold as observed here if 13 subunits adopted the cofilin binding competent conformation.
Filament structures determined by electron cryo-microscopy show that cofilin clusters alter the filament twist 1–2 subunits toward the barbed end side and 2–3 subunits toward the pointed end side.15,21 This small but significant difference, which is considerably smaller than that observed by AFM, can readily account for a 2-fold faster elongation toward the pointed end. Thus, the two-fold difference in barbed versus pointed end-directed cluster elongation is consistent with small local asymmetries at boundaries.
Cofilin clusters sever preferentially at their pointed ends
Clusters of bound cofilin were ~6 times more likely to sever at their pointed end boundaries than at their barbed end side (Sup Figure 6).13 A more rapid severing rate constant at the pointed end side is consistent with structural studies that demonstrated a bound cofilin tilts the unoccupied, pointed end actin subunit, which compromises its stabilizing D-loop contacts.21 In contrast, neither a single bound cofilin or contiguous clusters compromised the D-loop of the neighboring actin subunit at the barbed end.21 We therefore favor a mechanism in which cofilin preferentially binds and severs the pointed end side of clusters because of biased subunit tilting of adjacent, unoccupied actin subunits at the pointed end side of boundaries.
Small cofilin clusters (Cc ≤ 3) can sever filaments.
We observed severing events with only a few bound cofilins (Cc ≤ 3) and as few as only 1 cofilin (Figure 3), suggesting that few are needed to efficiently sever an actin filament. This is at variance with AFM and fluorescence imaging studies that reported cluster sizes of 13,20 23,14 or 10013 cofilins are required for severing. The small critical cluster size observed here for S. cerevisiae cofilin consistent with single molecule, vertebrate cofilin binding studies that observed severing events next to singly bound cofilin31 and equilibrium severing data which is best fit by models in which single human cofilin has severing activity.4,6,18,23
Although our conclusions here differ from previous fluorescence studies with human and S. cerevisiae cofilin,13,14 our experimental observations are consistent with the latter, carried out with the same isoform. Specifically, the study with S. cerevisiae cofilin reported a critical cluster size of 23, but numerous severing events of much smaller clusters contributed to this mean value.14 Small cluster severing events are rare at low cofilin concentrations because there are very few clusters, not because they don’t sever. The fluorescence study reporting a critical cluster size of 100 for human cofilin13 estimated the duration of lag phase in severing during cofilin association experiments. The cofilin severing experiments in our work did not exhibit a lag in boundary severing nor did it produce a detectable difference in boundary severing at two different cluster size extension rates (Figure 4). These differences may be due to the isoforms or fluorescent modification of cofilin. We note, however, other studies favor a small critical cluster size for human cofilin as reported here for S. cerevisiae cofilin.
Given these experimental observations and considering recent electron cryo-microscopy structures of partially decorated cofilactin filaments,21 we favor a general cofilin boundary severing mechanism in which only a few cofilins are sufficient to sever filaments. Filament fragmentation results from structural perturbation of lateral neighboring actin subunits on the pointed end side of clusters.15,21 These perturbations are local (1–3 subunits), occur with single cofilins and small clusters, and do not propagate further into bare filament segments with larger cluster sizes.15,21 If these structural changes do not increase with larger cluster sizes and are the primary cause of severing, severing should not increase with cluster size. Taken together this work demonstrates the relationship between force sensitivity, cofilin binding kinetics, cluster growth, and boundary severing and links these properties to recently established structural observations.
Methods
Protein purification and labeling
Rabbit skeletal muscle actin was purified and labeled with biotin or Alexa 647 NHS ester.6,7 Ca++ actin monomers in G Buffer (5 mM Tris (pH 7.5), 0.2 mM ATP, 0.1 mM CaCl2, 0.5 mM DTT, 1 mM NaN3) were converted to Mg2+ actin by addition of 50 mM MgCl2 and 0.2 mM EGTA for 5 min on ice4 immediately before use. Saccharomyces cerevisiae cofilin with a surface engineered cysteine16 was purified and labeled with Alexa 488 maleimide (70–80 %) or Oregon Green maleimide (33 %).
Microfluidic chambers and slide preparation
Cofilin association experiments were conducted in microfluidic chambers with a single inlet and outlet port sandwiched between a microscope slide and a silanized cover slip.36,37 Microfluidic channels were made by cutting rectangles (1′’ × 1/16′’) into a strip of parafilm with a razor blade,38 sandwiched between a microscope slide, and a silanized cover slip, and melted for 5 seconds with a 100 °C heat block.
Force estimation for filaments under flow
To determine order of magnitude estimates of the forces felt by actin filaments under flow, theory for slender rods parallel to a boundary under Poiselle flow was used.19,24–26 The tension F across an anchored filament under flow reduces linearly from its attachment point, i.e.
| (1) |
Here, d is the distance from the attachment point, v is the fluid velocity (500 μm s−1), η is the viscosity of water (0.0014 kg m−1 s−1), h is the distance of the actin filament from the surface (0.02–0.2 μm), and r is the filament radius (4 nm). The fluid velocity was determined by flowing TetraSpek beads (Thermo Fisher), with a diameter of 100 nm, through the microfluidic chamber.24,37 It follows that a 10 μm filament under to 500 μL min−1 flow experiences a tension of ~9–19 pN at its attachment point. The estimated two-fold range in tension arises from uncertainty in the filament height from the surface.
Cofilin binding increases the actin filament radius ~20–30%.3,8 This change in radius leads to a < 4% difference in tension, based on Eq. (1). Because this difference is small, when calculating filament tension we neglect the contributions from the change in filament radius due to cofilin binding during our experiments.
Surface adsorption of actin filaments
Filaments were tethered to the surface in two different ways- throughout the entire filament length or from seeds which untethered segments were polymerized. When tethering filaments throughout their length, preassembled filaments (containing 15% Alexa 647 labeled and 10% biotinylated) in KMI6.6 buffer (50 mM KCl, 2 mM MgCl2, 2 mM DTT, 0.2 mM ATP, 1 mM NaN3, and 20 mM Imidazole (pH 6.6)) were adhered to a neutravidin-coated coverslip.37 Briefly, 200 μL of 10 μg mL−1 biotin BSA in T50 buffer (50 mM NaCl and 10 mM Tris pH 8) was pipetted into microfluidic chambers and equilibrated at 25 °C for 10 min, followed by a 45 min incubation 25 °C with 200 μL of 0.2% tween in T50 buffer. The channel was washed with 200 μL of T50 buffer and then incubated with 200 μL of 10 μg mL−1 neutravidin in T50 buffer for 10 min. Finally, 200 μL of KMI6.6 buffer was passed through the channel. Then polymerized with addition of 0.1 volume of 10X KMI6.6 polymerizing buffer and equilibrated for 1 h at 25 °C. Filaments were diluted to 10 nM in KMI6.6 buffer supplemented with GODCAT (15 mM glucose, 0.02 mg mL−1 catalase, and 0.1 mg mL−1 glucose catalase), gently pipetted into the microfluidic chamber, and equilibrated for 10 min. KMI6.6-GODCAT buffer with cofilin was flowed at 500 μL min−1 with a syringe pump.
Cofilin binding to pointed end anchored filaments
To prepare pointed end anchored actin filaments, chambers were prepared as described for surface adhered experiments using 1 μg mL−1 biotin. Actin monomers (5 μM, 10% biotinylated) were polymerized with 0.1 volumes of 10X KMI6.6 buffer, equilibrated for 1 hr at 25 °C, then sheared by vigorous pipetting for 1 min. An aliquot of the sheared filaments sample was mixed with 647-Alexa-labeled and unlabeled actin monomers and 0.1 volume of 10X KMI6.6 GODCAT-buffer to yield a final actin concentration of 0.95 μM 15% Alexa-647-labeled monomers and 0.05 μM seeds. This sample was equilibrated for 2–3 min then added to the flow chamber by pipetting and equilibrating for an additional 2 min. KMI6.6-GODCAT buffer with or without 200 nM unlabeled actin monomers was flowed at 5 μL min−1 for 30 min. Alexa-488-labeled cofilin was subsequently added in the KMI6.6-GODCAT buffer with the indicated flow. To ensure that free cofilin concentrations were identical at both low and high flow rate association experiments, cofilin was initially pulsed into the chamber at 500 μL min−1 and then the flow rate was reduced as appropriate (Sup Figure 10). Time courses of cofilin dissociation were measured as follows: Alexa-488-labeled cofilin (1 μM) was flowed for ~ 5 min to achieve saturation. Dissociation was measured by flowing 1 μM unlabeled cofilin at the indicated flow rate.
Image acquisition
Samples were imaged using a sequential two color TIRF microscope (Till iMic) equipped with a 100x NA 1.49 objective (Olympus), and an Andor iXon897 EMCCD camera. 647 and 488 lasers were set to 50.5% and 25% power, respectively. The Electron Multiplier gain was set to 250, with 50 ms exposure times.
Correction for non-uniform illumination
To correct for non-uniform fluorescence illumination (a common problem in TIRF imaging39,40), images of freely diffusing Alexa-488-cofilin were imported into MATLAB and the two dimensional (x, y) fluorescence intensity Ifree(x, y) profile was fitted with a 5th order polynomial using the MATLAB function fit. The best fit resulted in a surface polynomial S(x, y), which was normalized by its maximum intensity and used to correct the images of cofilin flourescence acquired during cofilin binding (Ibind(x, y, t)).
| (2) |
| (3) |
Here ./ represents element by element division, Ibind(x, y, t) is the image stack acquired during cofilin binding experiments, and Ibind_flat(x, y, t) is the corrected image stacks.
Cofilin binding and cluster growth on actin filaments
Image stacks of cofilin association time courses were imported to ImageJ. A box was manually drawn around individual filaments and the mean fluorescence intensity within that box was quantitated for each image. Background fluorescence was determined from a box from an area not containing filaments and subtracted from each respective association or dissociation trace.
To measure bound cofilin cluster growth under high flow, filaments were traced with lines and kymographs were produced in ImageJ. The extensions of the cofilin clusters were found by manually tracing the kymographs in ImageJ.
Cofilin cluster tracking and boundary severing analysis
Experiments conducted with 50 nM cofilin permitted tracking of individual diffraction limited cofilin clusters. Cluster tracking was conducted in ImageJ using the TrackMate plugin.41 For particle detection, a “Laplacian of a Gaussian” filter was chosen with a “blob diameter” of 7 pixels. For tracking, the “Simple LAP tracker” was chosen with a “Linking max distance” of 5 pixels, a “GAP-closing max distance” of 5 pixels, and a “Gap-closing max frame gap” of 2 frames. Tracks were exported as text files and analyzed with MATLAB. Severing events were identified manually. During 200 nM association clusters, grew longer than the diffraction limit and were tracked via manual kymograph tracing.
Bleach step calibration of cofilin brightness
To calibrate the brightness of a single cofilin we applied the bleach step analysis developed by Chen et al.30 Specifically, cofilin clusters were imaged at 10 frames per second to induce photobleaching (Figure 3(A) and Sup Figure 6). Clusters’ positions were monitored with TrackMate and their fluorescence intensity from unprocessed images was monitored with MATLAB. Individual photobleaching events could be resolved as stepwise losses in fluorescence. Steps were determined using the Tdetector algorithm for unequal variances30 (Figure 3(A)). The distribution of these bleach steps was then estimated via a Gaussian mixture model (GMM) using the MATLAB function gmfitdist. The mean of the first Gaussian was determined to be the true step size as the means of successive gaussians were approximately multiples of the first mean. Multiples of the first mean are indicative of events where two or more cofilins were bleached during the same exposure. The photobleaching rate constant was determined by fitting the ensemble bleaching of cluster fluorophores versus the cumulative exposure time to a single exponential.
Actin curvature estimation
To determine the curvature of actin filaments tethered to the surface throughout their length, filament contours were traced with the ImageJ plugin JFilament.42 Convergence was achieved by varying the stretching stiffness “Alpha” between 15 and 100 and the step size parameter “Gamma” between 400 and 1000. All other parameters were set at default values of JFilament. Spacing between discretized points along the contour was kept constant and each point was converted to a curvature using 2D LineCurvature available on the MATLAB file exchange.43 The function evaluates the curvature at a given point by fitting a parameterized 2nd order polynomial to the point and its first nearest neighbors.
| (4) |
| (5) |
The curvature κ is then calculated analytically for each parameterized polynomial.
| (6) |
Dissociation of cofilin via stopped flow
The fluorescence intensity of Oregon-Green-labeled cofilin increases with actin filament binding (Sup Figure 11). This increase in fluorescence permitted the evaluation of cofilin binding and unbinding from actin filaments. Time courses of Oregon-green-labeled cofilin dissociation from actin filaments was measured via competition by mixing an equilibrated mixture of 1.2 μM Oregon-green-labeled cofilin and 0.6 μM actin filaments with 20 μM unlabeled cofilin11,12 at 25 °C in KMI6.6 buffer with an Applied Photophysics SX20 stopped-flow instrument.
Supplementary Material
Acknowledgements
This research was supported by the National Institutes of Health through R35-GM136656 (awarded to E.M.D.L.C.), J.P.B. was partially supported by the Department of Defense Army Research Office through a multidisciplinary university research initiative grant W911NF1410403 (awarded to E.M.D.L.C.), and S. G. was supported by supported by R35 GM136656-01S1. We would like to thank Wenxiang Cao and Nandan Pandit for their help in editing the manuscript.
Footnotes
Declaration of Competing Interest
The authors declare no competing interest.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmb.2021.166833.
References
- 1.De La Cruz EM, (2009). How cofilin severs an actin filament. Biophys. Rev, 1, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanellos G, Frame MC, (2016). Cellular functions of the ADF/cofilin family at a glance. J. Cell Sci, 129, 3211–3218. [DOI] [PubMed] [Google Scholar]
- 3.McGough A, Pope B, Chiu W, Weeds A, (1997). Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J. Cell Biol, 138, 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De La Cruz EM, (2005). Cofilin binding to muscle and non-muscle actin filaments: isoform-dependent cooperative interactions. J. Mol. Biol, 346, 557–564. [DOI] [PubMed] [Google Scholar]
- 5.McCullough BR, Grintsevich EE, Chen CK, Kang HR, Hutchison AL, Henn A, et al. , (2011). Cofilin-linked changes in actin filament flexibility promote severing. Biophys. J, 101, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang HR, Bradley MJ, Cao WX, Zhou KF, Grintsevich EE, Michelot A, et al. , (2014). Site-specific cation release drives actin filament severing by vertebrate cofilin. Proc. Natl. Acad. Sci. USA, 111, 17821–17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prochniewicz E, Janson N, Thomas DD, De La Cruz EM, (2005). Cofilin increases the torsional flexibility and dynamics of actin filaments. J. Mol. Biol, 353, 990–1000. [DOI] [PubMed] [Google Scholar]
- 8.McCullough BR, Blanchoin L, Martiel JL, De la Cruz EM, (2008). Cofilin increases the bending flexibility of actin filaments: implications for severing and cell mechanics. J. Mol. Biol, 381, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan J, Saunders MG, Haddadian EJ, Freed KF, De La Cruz EM, Voth GA, (2013). Molecular origins of cofilin-linked changes in actin filament mechanics. J. Mol. Biol, 425, 1225–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaendtner J, De La Cruz EM, Voth GA, (2010). Actin filament remodeling by actin depolymerization factor/cofilin. Proc. Natl. Acad. Sci. USA, 107, 7299–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De La Cruz EM, Sept D, (2010). The kinetics of cooperative cofilin binding reveals two states of the cofilin-actin filament. Biophys. J, 98, 1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao W, Goodarzi JP, De La Cruz EM, (2006). Energetics and kinetics of cooperative cofilin-actin filament interactions. J. Mol. Biol, 361, 257–267. [DOI] [PubMed] [Google Scholar]
- 13.Wioland H, Guichard B, Senju Y, Myram S, Lappalainen P, Jegou A, et al. , (2017). ADF/Cofilin accelerates actin dynamics by severing filaments and promoting their depolymerization at both ends. Curr. Biol, 27, 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gressin L, Guillotin A, Guerin C, Blanchoin L, Michelot A, (2015). Architecture dependence of actin filament network disassembly. Curr. Biol, 25, 1437–1447. [DOI] [PubMed] [Google Scholar]
- 15.Huehn A, Cao W, Elam WA, Liu X, De La Cruz EM, Sindelar CV, (2018). The actin filament twist changes abruptly at boundaries between bare and cofilin-decorated segments. J. Biol. Chem, 293, 5377–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suarez C, Roland J, Boujemaa-Paterski R, Kang H, McCullough BR, Reymann AC, et al. , (2011). Cofilin tunes the nucleotide state of actin filaments and severs at bare and decorated segment boundaries. Curr. Biol, 21, 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlov D, Muhlrad A, Cooper J, Wear M, Reisler E, (2007). Actin filament severing by cofilin. J. Mol. Biol, 365, 1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrianantoandro E, Pollard TD, (2006). Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell, 24, 13–23. [DOI] [PubMed] [Google Scholar]
- 19.Wioland H, Jegou A, Romet-Lemonne G, (2019). Torsional stress generated by ADF/cofilin on cross-linked actin filaments boosts their severing. Proc. Natl. Acad. Sci. USA, 116, 2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngo KX, Kodera N, Katayama E, Ando T, Uyeda TQ, (2015). Cofilin-induced unidirectional cooperative conformational changes in actin filaments revealed by high-speed atomic force microscopy. Elife, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huehn AR, Bibeau JP, Schramm AC, Cao WX, De La Cruz EM, Sindelar CV, (2020). Structures of cofilin-induced structural changes reveal local and asymmetric perturbations of actin filaments. Proc. Natl. Acad. Sci. USA, 117, 1478–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jermyn AS, Cao W, Elam WA, De La Cruz EM, Lin MM, (2020). Directional allosteric regulation of protein filament length. Phys. Rev. E, 101, 032409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elam WA, Cao W, Kang H, Huehn A, Hocky GM, Prochniewicz E, et al. , (2017). Phosphomimetic S3D cofilin binds but only weakly severs actin filaments. J. Biol. Chem, 292, 19565–19579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Courtemanche N, Lee JY, Pollard TD, Greene EC, (2013). Tension modulates actin filament polymerization mediated by formin and profilin. Proc. Natl. Acad. Sci. USA, 110, 9752–9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jegou A, Carlier MF, Romet-Lemonne G, (2013). Formin mDia1 senses and generates mechanical forces on actin filaments. Nat. Commun, 4, 1883. [DOI] [PubMed] [Google Scholar]
- 26.Brennen C, Winet H, (1977). Fluid-mechanics of propulsion by cilia and flagella. Annu. Rev. Fluid Mech, 9, 339–398. [Google Scholar]
- 27.Chin SM, Jansen S, Goode BL, (2016). TIRF microscopy analysis of human Cof1, Cof2, and ADF effects on actin filament severing and turnover. J. Mol. Biol, 428, 1604–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayakawa K, Tatsumi H, Sokabe M, (2011). Actin filaments function as a tension sensor by tensiondependent binding of cofilin to the filament. J. Cell Biol,, 195, 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hocky GM, Sindelar CV, Cao W, Voth GA, (2021). De La Cruz EM. Structural basis of fast- and slow-severing actin–cofilactin boundaries. J. Biol. Chem, 10.1016/j.jbc.2021.100337. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Deffenbaugh NC, Anderson CT, Hancock WO, (2014). Molecular counting by photobleaching in protein complexes with many subunits: best practices and application to the cellulose synthesis complex. Mol. Biol. Cell, 25, 3630–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayakawa K, Sakakibara S, Sokabe M, Tatsumi H, (2014). Single-molecule imaging and kinetic analysis of cooperative cofilin-actin filament interactions. Proc. Natl. Acad. Sci. USA, 111, 9810–9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schramm AC, Hocky GM, Voth GA, Blanchoin L, Martiel JL, De La Cruz EM, (2017). Actin filament strain promotes severing and cofilin dissociation. Biophys. J, 112, 2624–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schramm AC, Hocky GM, Voth GA, Martiel JL, De La Cruz EM, (2019). Plastic deformation and fragmentation of strained actin filaments. Biophys. J, 117, 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De La Cruz EM, Roland J, McCullough BR, Blanchoin L, Martiel JL, (2010). Origin of twist-bend coupling in actin filaments. Biophys. J, 99, 1852–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medeiros NA, Burnette DT, Forscher P, (2006). Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol, 8, 215–226. [DOI] [PubMed] [Google Scholar]
- 36.Hua B, Han KY, Zhou R, Kim H, Shi X, Abeysirigunawardena SC, et al. , (2014). An improved surface passivation method for single-molecule studies. Nat. Methods, 11, 1233–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandit NG, Cao W, Bibeau J, Johnson-Chavarria EM, Taylor EW, Pollard TD, et al. , (2020). Force and phosphate release from Arp2/3 complex promote dissociation of actin filament branches. Proc. Natl. Acad. Sci. USA, 117, 13519–13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson-Chavarria EM, Agrawal U, Tanyeri M, Kuhlman TE, Schroeder CM, (2014). Automated single cell microbioreactor for monitoring intracellular dynamics and cell growth in free solution. Lab Chip., 14, 2688–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiolka R, Belyaev Y, Ewers H, Stemmer A, (2008). Even illumination in total internal reflection fluorescence microscopy using laser light. Microscopy Res. Tech, 71, 45–50. [DOI] [PubMed] [Google Scholar]
- 40.Zong WJ, Huang XS, Zhang C, Yuan TY, Zhu LL, Fan M, et al. , (2014). Shadowless-illuminated variable-angle TIRF (siva-TIRF) microscopy for the observation of spatial-temporal dynamics in live cells. Biomed. Optics Exp, 5, 1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tinevez JY, Perry N, Schindelin J, Hoopes GM, Reynolds GD, Laplantine E, et al. , (2017). TrackMate: an open and extensible platform for single-particle tracking. Methods, 115, 80–90. [DOI] [PubMed] [Google Scholar]
- 42.Smith MB, Li HS, Shen TA, Huang XL, Yusuf E, Vavylonis D, (2010). Segmentation and tracking of cytoskeletal filaments using open active contours. Cytoskeleton, 67, 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koon D-J. LineCurvature2D. 1.3.0.0 ed. MATLAB CentralFile Exchange, 2011. [Google Scholar]
- 44.Kaplan EL, Meier P, (1958). Nonparametric-estimation from incomplete observations. J. Am. Stat. Assoc, 53, 457–481. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


