Abstract
Background
Understanding immunogenicity and effectiveness of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines is critical to guide rational use.
Methods
We compared the immunogenicity of mRNA-1273, BNT-162b2, and Ad26.COV2.S in healthy ambulatory adults. We performed an inverse-variance meta-analysis of population-level effectiveness from public health reports in > 40 million individuals.
Results
A single dose of either mRNA vaccine yielded comparable antibody and neutralization titers to convalescent individuals. Ad26.COV2.S yielded lower antibody concentrations and frequently undetectable neutralization titers. Bulk and cytotoxic T-cell responses were higher in mRNA1273 and BNT162b2 than Ad26.COV2.S recipients. Regardless of vaccine, <50% of vaccinees demonstrated CD8+ T-cell responses. Antibody concentrations and neutralization titers increased comparably after the first dose of either vaccine, and further in recipients of a second dose. Prior infection was associated with high antibody concentrations and neutralization even after a single dose and regardless of vaccine. Neutralization of Beta, Gamma, and Delta strains were poorer regardless of vaccine. In meta-analysis, relative to mRNA1273 the effectiveness of BNT162b2 was lower against infection and hospitalization, and Ad26COV2.S was lower against infection, hospitalization, and death.
Conclusions
Variation in the immunogenicity correlates with variable effectiveness of the 3 vaccines deployed in the United States.
Keywords: Ad26.COV2.S, BNT162b2, death, effectiveness, hospitalization, immunogenicity, mRNA-1273, neutralization, SARS CoV-2, T cells
SARS-CoV-2 vaccines differ in immunogenicity (by multiple humoral and T-cell measures): mRNA1273 induced the strongest responses following by BNT162b2; Ad26.COV2.S induced weak responses. Differences in immunogenicity predict population-level effectiveness against infection, hospitalization, or death (mRNA1273 > bnt162b2 > Ad26.COV2.S).
Prophylactic vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are being deployed globally to combat the coronavirus disease 2019 (COVID-19) pandemic. A burgeoning body of evidence links the immunogenicity of the different vaccines to the degree of protection from infection or disease, although a precise correlate has not been agreed upon and studies of direct comparisons of vaccines are limited. Vaccination with mRNA-1273 (100 µg, Moderna), BNT162b2 (30 µg, Pfizer), and Ad26.COV2.S (5 × 1010 viral particles, Johnson & Johnson/Janssen) were each shown to be efficacious in reducing the risk of severe disease and infection in randomized clinical trials [1–3], and have received emergency use authorization (EUA) or full approval from the United States Food and Drug Administration (FDA). All 3 vaccines encode a largely similar SARS CoV-2 spike protein homologous to the SARS-CoV-2 strain isolated in Wuhan (China) but differ in dose and mechanism of delivery (mRNA vs adenovirus vectored). Scale-up of vaccination in the general population may plausibly result in outcomes different to the original trials because of broader inclusion in the real world, the evolution of viral variants, and potential variation in vaccine production. Immunogenicity analyses nested within the randomized trials of several vaccines have consistently demonstrated a quantitative association between anti-spike antibodies and/or neutralization titers and infection outcomes [4–7]. In animal models, experimental transfer of antibodies protects from infection [8–10] and in human randomized controlled trials, prophylactic administration of neutralizing antibodies reduces incidence of clinical COVID-19 [11, 12]. However, there is no consensus between vaccine manufacturers on the immune assays to employ, nor in the details of similar assays, for example which viral variant to assess neutralization against. There are few data regarding the comparative immunogenicity of these vaccines, except for indirect inferences from publicly available trial data from manufacturers [13–15] and small recent studies [13]. These factors collectively justify direct comparisons between vaccines in real-world settings using consistent methods. Such studies may provide data to base decisions regarding which vaccine to deploy, and timing of additional booster doses.
We compared the immunogenicity of mRNA1273, BNT162b2, and Ad26.COV2.S during the first months following their deployment in the pandemic in the United States. We found a distinct hierarchy in humoral and cellular immunity, including towards currently circulating viral variants. Moreover, we find that this hierarchy is mirrored by variation in population-scale effectiveness.
METHODS
Cohort Description
Use of human samples was approved by Partners Institutional Review Board (protocol 2020P001081 and 2020P002274). Consenting adults in Chelsea, Massachusetts were enrolled in a study of antibody responses and sampled in August 2020 and/or early 2021 (March or April 2021). Data in this study are also derived from previously reported cohorts of healthy adults who had received vaccination and enrolled in a COVID-19 vaccine biobanking study. Demographic data, information regarding prior SARS CoV-2 testing, symptoms, and exposure were collected as was vaccine-related information. Prepandemic serum samples were obtained from the clinical laboratories at Massachusetts General Hospital as previously described [16]. We grouped patients by time postvaccination using a 7-day window to capture the expected kinetics of antibody production after vaccination. Individuals who had received a single dose of vaccination ≥7 days prior (or had received a second dose < 7 days prior) were analyzed as 1-dose recipients; those who had received 2 doses ≥ 7 days prior were analyzed as 2-dose recipients.
Measurement of Immunogenicity
Anti-spike and anti-nucleocapsid antibodies were measured with the Roche Elecys assay. Additional measurement of receptor-binding domain (RBD) antibodies was performed with a customized enzyme-linked immunosorbent assay (ELISA) assay. Serum neutralization was measured using an extensively validated SARS-CoV-2 pseudovirus neutralization assay [14]. Interferon-γ (IFN-γ) ELISpot assays were performed as previously described [15] according to the manufacturer’s instructions (Mabtech). Detailed methods are provided in the Supplementary Materials.
Meta-analysis of Effectiveness Data
We systematically searched Pubmed, and recent online news (including twitter) for mention of public health reports of breakthrough infection according to vaccine type using the terms “breakthrough,” “effectiveness,” “Moderna,” “mRNA1273,” “Pfizer,” “BNT162b2,” “J&J,” or “Ad26.COV2.S.” We describe these further in Supplementary Methods. Inverse-variance meta-analysis was performed in R using the metagen function.
General Statistical Methods
We performed multivariate linear regression in R version 4.05 using the lm function with log10 transformed spike values or 50% pseudovirus neutralization titer (pNT50) as the dependent variable, and age, sex, days postvaccination, or vaccine group as the independent variables. Graphics were rendered in Prism version 9.0. We modelled kinetics of vaccine response by plotting the individual measures or repeated measures (for donors who had repeated measures performed), and calculating a trend line using the R geom_smooth() function with span 0.1.
RESULTS
We characterized the immunogenicity of mRNA-1273 (Moderna), BNT162b2 (Pfizer-BioNTech), and Ad26.COV2.S (Johnson & Johnson/Janssen) in ambulatory adults enrolled in a community study of healthy individuals in Chelsea, Massachusetts and a biobanking effort among laboratory or health care workers in Boston, Massachusetts. In total we include data from 215 participants who had received 1 (n = 99) or 2 doses (n = 116) of vaccine ≥ 7 days prior, 130 unvaccinated participants with asymptomatic or symptomatic prior infection confirmed by positive anti-nucleocapsid antibody, 112 uninfected (anti-nucleocapsid antibody negative), and 1220 historical controls sampled before the pandemic [16]. The median age of vaccinated participants was 39 years (interquartile range, 31–55 years) and 120/215 (56%) were female. We compared immune responses according to prior infection, and vaccination type and dose, and adjusted for age, sex, and duration (detailed in Supplementary Table 1) after vaccination in all analyses.
Binding Antibody Response to Vaccination
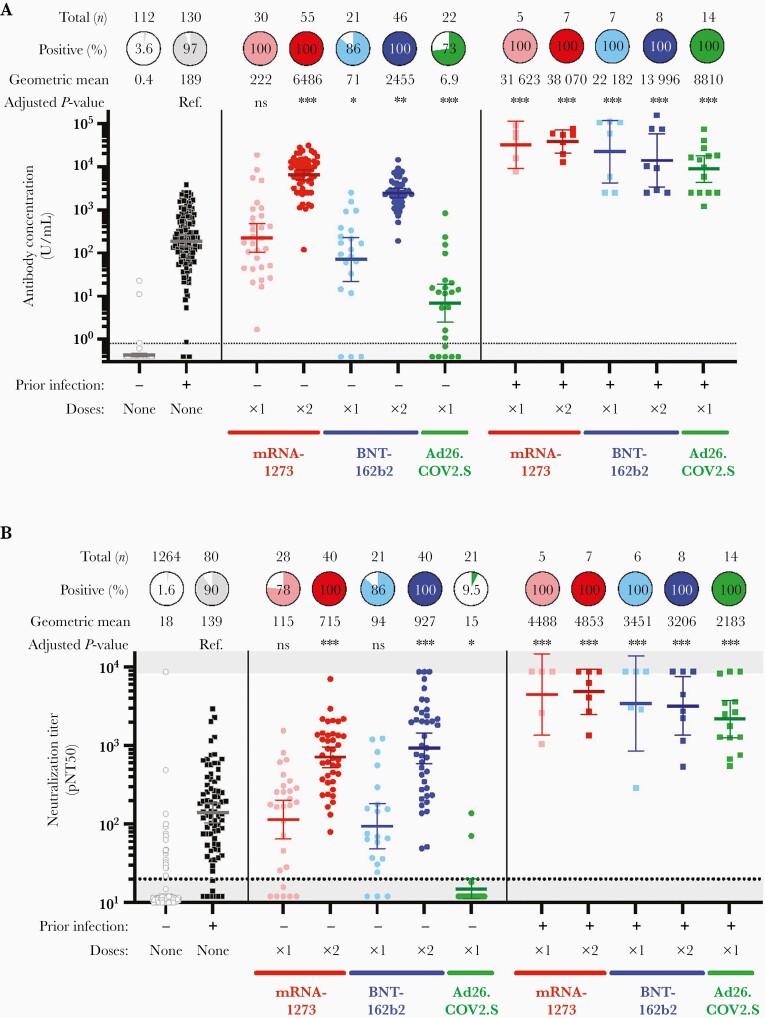
We assessed total immunoglobulin G/M/A (IgG/M/A) binding antibody levels against the SARS-CoV-2 spike protein (Roche Elecsys Anti-SARS-CoV-2 S), and found substantial variation in antibody concentrations depending on prior infection, vaccine type, and vaccine dose (Figure 1A, summarized in a multivariate regression model in Supplementary Table 2). Among participants without prior infection, antibody concentrations after a single dose of mRNA-1273 was comparable to convalescent individuals (geometric mean concentration [GMC], 222 U/mL vs 189 U/mL, adjusted P = .4 for mRNA-1273) and after a single dose of BNT162b2 was lower than convalescent individuals (GMC 71 U/mL vs 189 U/mL, adjusted P = .01 for BNT162b2). Recipients of Ad26.COV2.S without prior infection had an approximately 25-fold lower antibody concentrations than convalescent unvaccinated individuals (GMC 6.9 U/mL vs 189 U/mL, adjusted P < .001). Participants with no prior infection who received a single dose of either of the 3 vaccines were sampled at a comparable duration after vaccination (a median of 22–24 days). A single dose of mRNA-1273 or BNT162b2 induced higher titers than Ad26.COV2.S (adjusted P < .001 for both comparisons; Supplementary Table 3). At a median of 24 days postvaccination, 27.3% (6/22) of Ad26.COV2.S recipients had undetectable antibody levels. Notably, none of 22 Ad26.COV2.S recipients had a major medical comorbidity or received immunosuppressive medications in the prior 6 months. Receipt of both doses of mRNA-1273 or BNT162b2 were associated with substantially higher antibody concentrations than convalescent individuals (GMC 6486 U/mL for mRNA-1273 and GMC 2455 U/mL for BNT162b2 vs 189 U/mL, adjusted P < .001 for both comparisons). Individuals with prior infection who were vaccinated had approximately 2 log10 IU/mL higher antibody concentrations than convalescent individuals regardless of vaccine type, even after a single dose.
Figure 1.
Immunogenicity of mRNA-1273, BNT-162b2, and Ad26.COV2.S in individuals with and without prior SARS-CoV-2 infection. A, Quantitative SARS-CoV-2 spike IgG/A/M antibody concentration (Roche Elecsys Anti-SARS-CoV-2 assay) in U/mL of serum for 112 participants without prior infection or vaccination, 130 with prior infection, and 215 following vaccination. An antibody titer of 0.8 U/mL was considered positive (dotted line). The number of donors, proportion positive, and geometric mean concentration are shown above each group. B, Pseudovirus neutralization titer 50 (pNT50, defined as the titer at which the serum achieves 50% neutralization of SARS-CoV-2 wild-type pseudovirus entry into ACE2-expressing 293T cells) for a subset of the donors above and an additional 1220 prepandemic controls (from Garcia-Beltran et al [16]) used in assay validation and deriving the cut off shown. The threshold for defining positive individuals, denoted by the dotted horizontal line, was a pNT50 of 20 [16]. The number of donors, proportion positive (at a threshold of 1:20), and geometric mean titer are shown above each group. A and B, For each group the horizontal line denotes the geometric mean concentration, and whiskers extend to 95% confidence interval. Asterisks denote P values adjusted for age, sex, and duration after vaccination at time of sampling, relative to unvaccinated individuals with prior infection (Supplementary Tables 2 and 4): ∗ P < .05, ∗∗ P < .01, ∗∗∗ P < .001. Abbreviations: ACE2, angiotensin-converting enzyme 2; IgG/A/M, immunoglobulin G/A/M; ns, not significant; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
We next sought to confirm these results using orthogonal assays. In an independent, validated total IgG/M/A or IgG ELISA, measurement of RBD binding antibodies confirmed the differences above, and IgM and IgA responses were low (Supplementary Figure 1). Further measurement of IgG against RBD multimers also confirmed these findings (Supplementary Figure 2). Control experiments revealed that IgG responses against equivalent RBD multimers of less pathogenic, common coronaviruses OCU43 and HKU1 were comparable between groups, supporting the specificity of these findings and controlling for any sample collection or storage artifacts between recipients of the 3 vaccines (Supplementary Figure 3).
Neutralizing Antibody Responses to Vaccination
We assessed the ability of serum from participants to neutralize SARS-CoV-2 by using a well-characterized assay [14, 16] that utilizes lentivirus pseudotyped with the spike protein of the Wuhan isolate of SARS-CoV-2. Neutralization data were available for 35 Ad26.COV2.S vaccinees and a 80 mRNA-1273 and 75 BNT162b2 vaccinees. Among prepandemic and confirmed uninfected individuals, a neutralization titer (pNT50) cutoff of 20 identified 1.6% of samples as positive. Using this threshold, 90.1% of unvaccinated convalescent individuals demonstrated neutralization (Figure 1B). Following a single dose of mRNA1273 or BNT162b2, neutralization titers were comparable to convalescent individuals (geometric mean titer [GMT] 115 for mRNA1273 and 94 for BNT162b2 vs 139 for convalescent donors, P = ns for both comparisons Supplementary Table 4) and 78% and 86% neutralized virus. Titers were higher after both doses of mRNA vaccine (GMT 715 for mRNA1273 and 927 for BNT162b2, adjusted P < .001 for both), and serum from all 2-dose mRNA vaccinees neutralized wild-type SARS-CoV-2 pseudovirus. In contrast, 9.5% (2/21) Ad26.COV2.S recipients had a neutralization titer > 20. Among individuals with prior infection, receipt of 1 or 2 doses of either of the 3 vaccines generated high neutralization titers that ranged from 46-times higher (for 1 dose Ad26.COV2.S) to 200-times higher (for 2 dose mRNA1273) than unvaccinated individuals with prior infection.
T-Cell Responses to Vaccination
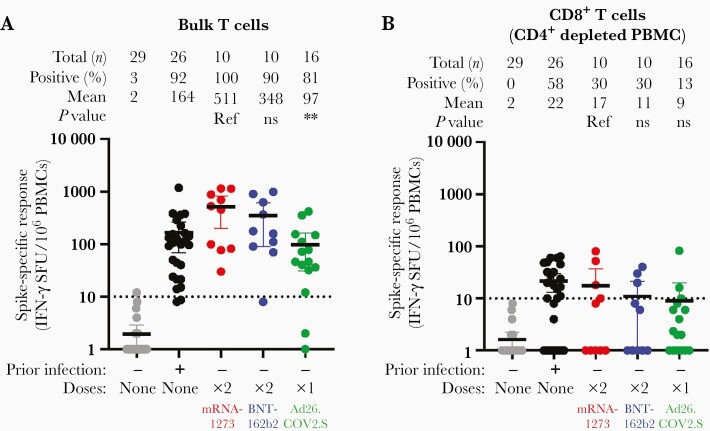
To explore differences in cellular immune response to vaccination, we assessed T-cell responses to SARS-CoV-2 spike peptides (wild-type strain) by IFN-γ ELISpot in 29 unvaccinated individuals without and 26 with prior infection, and 36 vaccinees without prior SARS CoV-2 infection who completed their vaccination schedule (Figure 2). We first examined bulk T-cell responses, which include both CD4+ and CD8+ T cells (Figure 2A). For reference, 92% of unvaccinated participants with prior infection had a measurable response with a mean of 164 spot forming units (SFU) per 106 peripheral blood mononuclear cell (PBMC). Spike-specific bulk T-cell responses varied significantly by vaccine (Kruskall-Wallis P = .007). Bulk T-cell responses were higher and more frequent in recipients of mRNA-1273 or BNT-162b2 than Ad26.COV2.S (mean 511 or 348 vs 97 SFU/106 PBMC, P = .003). Relative to convalescent individuals (adjusting for age and sex) the magnitude of response was higher in mRNA1273 recipients (effect estimate 310 SFU/106 PBMC; 95% confidence interval [CI], 90–530 SFU/106 PBMC; P = .007), tended towards being significantly higher in BNT162b2 recipients (effect estimate 182 SFU/106 PBMC; 95% CI, 28–391 SFU/106 PBMC; P = .08), and was nonsignificantly lower after Ad26.COV2.S (effect estimate −62 SFU/106 PBMC; 95% CI, −239 to 114 SFU/106 PBMC; P = .05).
Figure 2.
T-cell responses to spike peptides after mRNA-1273, BNT-162b2, and Ad26.COV2.S in individuals without prior SARS-CoV-2 infection measured in bulk T cells (A) and CD4-depleted T cells (B). The threshold for defining positive individuals, is denoted by the dotted horizontal line at 10 SFU/106 PBMC. Data are normalized to background dimethyl sulfoxide control responses. ∗∗ denotes P < .01. Abbreviations: IFN-γ, interferon-γ; ns, not significant; PBMC, peripheral blood mononuclear cell; Ref, reference; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFU, spot forming units.
To evaluate anti-spike cytotoxic T-cell responses we measured spike-specific responses after depleting CD4+ cells (Figure 2B). Unvaccinated participants with prior infection had a mean 22 SFU/106 CD4+ depleted PBMC, and 58% had a measurable response. Regardless of vaccine, fewer participants had measurable cytotoxic T-cell responses to spike peptides (30% for mRNA1273, 30% for BNT162b2, and 13% for Ad26.COV2.S) relative to convalescent individuals (58%). The magnitude of responses were higher among recipients of mRNA-1273 (or BNT-162b2) than Ad26.COV2.S (mean 17 or 11 vs 9 SFU/106 PBMC, adjusted P = .04). Neither bulk nor CD4-depleted spike-specific responses correlated with binding antibody titers (data not shown).
Change in Antibody Responses Over Time
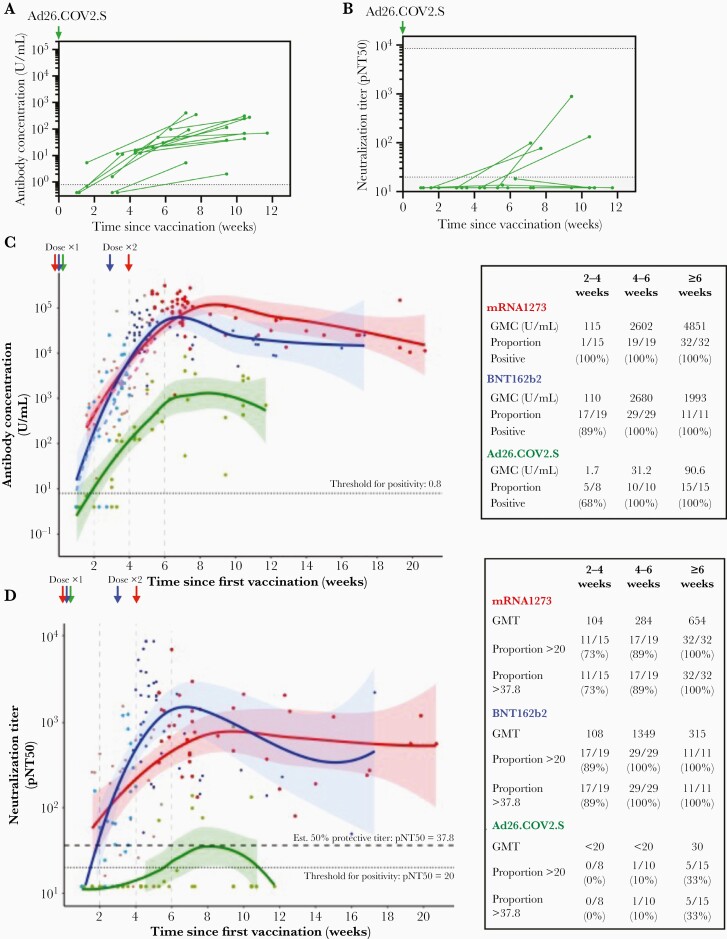
To explore how antibody titers vary over time, we performed 2 additional analyses. First, to assess whether an increase in anti-SARS-CoV-2 antibodies occurs at later time points, which has been described among Ad26.COV2.S recipients [3, 17], we obtained repeat measures in a subset of 15 Ad26.COV2.S recipients without prior infection. For this subset the baseline sampling was at a median 23 days (range, 7–44 days) and the follow-up sampling at a median 66 days (range, 25–82 days) after vaccination. Anti-spike antibody concentrations increased among all Ad26.COV2.S individuals (Figure 3A); however, only 4 individuals had an increase in neutralization, and 73% (11/15) remained with a pNT50 < 20 (Figure 3B). Second, we pooled initial and repeat measures among individuals without prior infection and modelled the kinetics of responses for all 3 vaccines. Over the first approximately 6 weeks following receipt of first dose of vaccination, antibody concentrations increased regardless of vaccine (Figure 3C) but the estimated plateau titers were lower among Ad26.COV2.S recipients. Because Ad26.COV2.S is administered as a single dose, we decided for fair comparison to analyze the kinetics of response for the 3 vaccines in the period just after the first doses and prior to the second mRNA vaccines (ie, the first 4 weeks for BNT162b2 and 5 weeks for mRNA1273). Titers increased for all 3 vaccines, and the rate of increase was not statistically dissimilar between vaccines (interaction P = .85).
Figure 3.
Follow-up measurement of response in Ad26.COV2.S recipients and kinetics of humoral responses to mRNA1273, BNT162b2, and Ad26.COV2.S SARS-CoV-2 vaccines. Longitudinal assessment of SARS-CoV-2 spike IgG/A/M antibody titers (A) and virus neutralization (B) in 15 Ad26CoV2.S vaccinees with baseline and repeat measures. Pooling all data, we modelled the kinetics of antibody concentration and neutralization according to vaccine. C, SARS-CoV-2 spike IgG/A/M antibody levels and (D) virus neutralization for all donors sampled after vaccination, with best-fit lines (Loess fit) over the first 20 weeks following vaccination. C and D, GMC or titer, and proportion positive at threshold indicated are shown for the periods 0–2, 2–4, 4–6, and ≥6 weeks in the table inserts. Each individual point and corresponding fit are colored according to vaccine type (red, mRNA-1273; blue, BNT162b2; and green, Ad26COV2.S); shaded areas denote 95% confidence intervals, and points are additionally shaded according to dose. C, Dashed lines show best-fit lines for the period after receipt of the first dose of mRNA-1273 or BNT162b2. D, Dotted line denotes a pNT50 threshold of 20 derived from study of prepandemic controls, and the upper dashed line denotes a pNT50 titer of 37.8, which represents 20% of the geometric mean neutralization titers of unvaccinated individuals with prior infection and corresponds with 50% estimated protection in Khoury et al [4]. Abbreviations: GMC, geometric mean concentration; IgG/A/M, immunoglobulin G/A/M; pNT50, 50% pseudovirus neutralization titer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Analysis of the kinetics of viral neutralization show similar shapes and time-course as the antibody assay for mRNA-1273 and BNT162b2, with peak values reached 6–10 weeks after the first vaccine (Figure 3D). Such a comparison was not possible for Ad26.COV2.S recipients because most did not neutralize virus at any time point. Prior publications have estimated that 20% of the GMT value of convalescent individuals is a good threshold for predicting titers offering approximately 50% protection from reinfection [4]. In this cohort, 20% of the GMT of convalescent individuals is 27.8, and we used this threshold to show that most recipients of mRNA-1273 (73%, 11/15) or BNT162b2 (89%, 17/19) achieved predicted protective neutralization titers rapidly, before receipt of the second dose of vaccination, and show that these are sustained for several months.
Most reported neutralization data amongst vaccinated individuals are derived from vaccine manufacturer’s study of trial participants. The assays used, in particular the virus strain, varies between studies. Notably, studies of Ad26.COV2.S recipients utilized the Victoria strain of SARS CoV-2 [14], a strain that has been noted to be more easily neutralized [18]. We introduced the Victoria strain-associated S247R mutation into the SARS CoV-2 Wuhan isolate used in this study. Sera from vaccine recipients demonstrated higher neutralization titers against this S247R-containing variant than the original Wuhan strain regardless of vaccine administered (Supplementary Figure 4). Therefore differences in the described neutralization seen in this study versus the Ad26.COV2.S trials may be partially accounted for by use of the S247R viral variant in prior studies.
Neutralization of Viral Variants
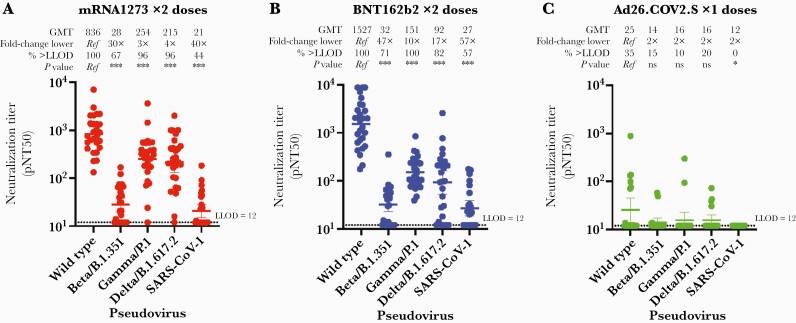
Variants of SARS CoV-2 have arisen over the course of the pandemic. mRNA1273, BNT162b2, and Ad26.COV2.S each encode the wild-type (Wuhan strain) spike protein as the sole viral antigen. We evaluated vaccine-mediated neutralization of the Beta (B.1.351), Gamma (P1), and Delta (B.1.617.2) variants of concern and SARS-COV-1 by serum from volunteers who completed a vaccine series. The overall neutralization of each variant was similar between both mRNA vaccines in that neutralization of the Beta variant was markedly impaired relative to the ancestral Wuhan strain (30-fold reduced GMT for mRNA1273 and 48-fold reduced GMT for BNT162b2), while neutralization of both Gamma and Delta variants was modestly impaired (Figure 4). Serum from Ad26.COV2.S recipients demonstrated low neutralization for all strains, as most individuals had neutralization that was not measurable.
Figure 4.
Neutralization of SARS-CoV-2 variants of concern by sera from healthy donors without prior infection vaccinated with mRNA1273 × 2 doses, n = 27 (A), BNT162b2 × 2 doses, n = 28 (B), or Ad26.COV2.S × 1 dose, n = 20 (C). The pNT50 (defined as the titer at which the serum achieves 50% neutralization of the relevant SARS-CoV-2 wild-type pseudovirus entry into ACE2-expressing 293T cells) is shown for each donor against the SARS-CoV-2 wild-type (Wuhan) strain, the Beta, Gamma, and Delta viral variants, and SARS-CoV-1. The GMT, fold-change relative to neutralization of wild type, proportion above the LLOD (horizontal dotted line at pNT50 = 12), and degree of statistical evidence relative to wild type are shown above each group. Nonparametric (Friedman) test P values relative to wild type are denoted as ns, ∗ P < .05, ∗∗ P < .01, ∗∗∗ P < .001. For each group the horizontal line denotes the geometric mean titer, and whiskers extend to 95% confidence intervals. Abbreviations: GMT, geometric mean titer; LLOD, lower limit of detection; ns, not significant; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Comparison of Population-Level Vaccine Effectiveness Mirrors Differences in Immunogenicity
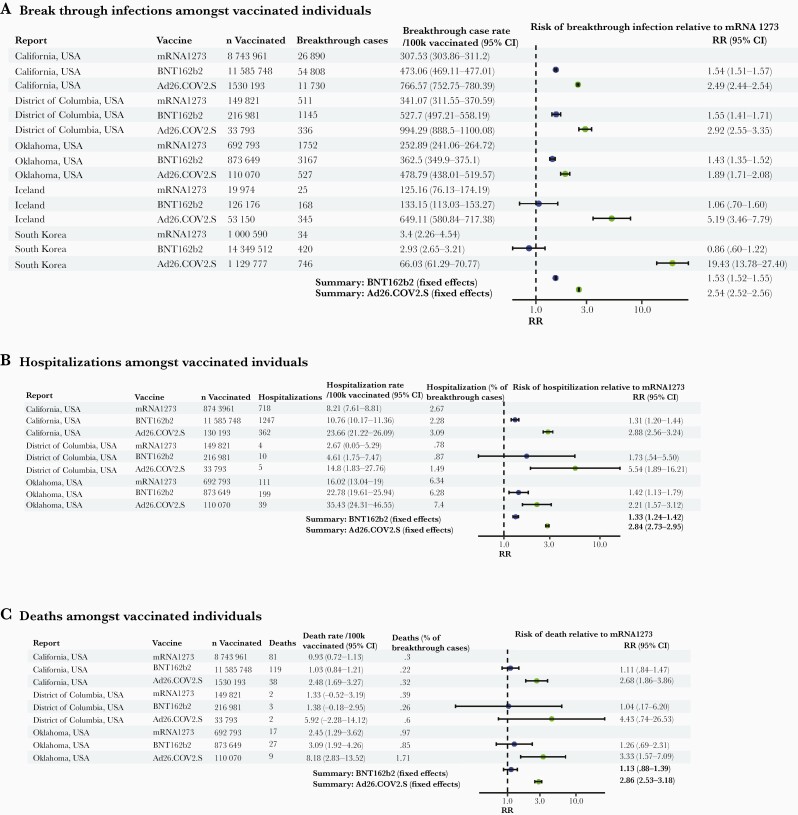
We hypothesized that the observed relative differences in immunogenicity between the 3 studied vaccines may correlate with their relative clinical effectiveness against infection and severe disease (hospitalization and/or death) at the population level. In randomized clinical trials, performed prior to heterologous variants rising to dominate the pandemic, and conducted largely among younger adults without major comorbidities, the primary end-point efficacy was 94.1% for mRNA1273 [1], 94.6% for BNT162b2 [2], and 66.1% for Ad26.COV2.S [3]. However, the differences between these studies with regard to study participant characteristics, study end points (severity of disease and onset relative to vaccination), the prevalence of viral variants, and other factors make direct comparisons between vaccines difficult. We systematically searched for reports of comparative effectiveness and performed a fixed-effects meta-analysis (Figure 5). We identified 5 subnational (US states of California, Oklahoma, and the District of Columbia) or national (Iceland and South Korea) sources of relative effectiveness data reporting: in summary, there were 102 604 breakthrough infections among 40 616 188 individuals who had completed the primary vaccine series (10 607 139 mRNA1273, 27 152 066 BNT162b2, and 2 856 983 Ad26.COV2.S). Three sources reported 2695 hospitalization and 298 deaths among 23 937 009 individuals (9 586 575 mRNA1273, 12 676 378 BNT162b2, and 1 674 056 Ad26.COV2.S). We note that the real-world effectiveness of each vaccine (relative to unvaccinated individuals) has been reported elsewhere and is high and consistent with the trial estimates, justifying global deployment of these vaccines and public health recommendations for universal vaccination and the goal of this analysis was a comparison between the vaccines.
Figure 5.
Forest plot of estimates from national or subnational public health reports of comparative vaccine effectiveness against infection (A), hospitalization (B), or death (C) for mRNA1273, BNT162b2, and Ad26.COV2.S recipients. See Supplementary Material for data sources. Abbreviations: CI, confidence interval; RR, relative risk.
In each of the 5 studies, rates of breakthrough infection were lowest after mRNA1273, higher after BNT162b2, and highest after Ad26.COV2.S, regardless of report, although the absolute rate of breakthrough infection varied widely amongst the 5 reports. In a fixed-effects meta-analysis, the risk of breakthrough infection after BNT162b2 relative to mRNA1272 was 1.53 (95% CI, 1.52–1.55), and after AD26.COV2.S was 2.54 (95% CI, 2.52–2.56). The risk of hospitalization as a proportion of breakthrough cases was not markedly different between the 3 vaccines, but the absolute risk of hospitalization after BNT162b2 relative to mRNA1272 was 1.33 (95% CI, 1.24–1.42) and after AD26.COV2.S was 2.84 (95% CI, 2.73–2.95). Absolute death rates were low amongst vaccinated individuals consistent with very high levels of protection against death, regardless of vaccine. Overall mortality estimates were comparable between BNT162b2 and mRNA1273 recipients (relative risk [RR], 1.13; 95% CI, .88–1.39) but significantly higher after Ad26.COV2.S (RR, 2.86; 95% CI, 2.53–3.18).
DISCUSSION
We studied immunogenicity in a healthy population of individuals receiving 1 of the FDA EUA vaccine during population scale-up. Regardless of immune measure examined, namely anti-spike binding antibodies, anti-RBD binding antibodies, neutralization of wildtype, Beta, Gamma, or Delta strains, bulk T cells, or cytotoxic T cells, we observed a consistent pattern with mRNA1273 being the most immunogenic followed by BNT162b2, and both mRNA vaccines being significantly more immunogenic than Ad26.COV2.S. Although the measures we took are relatively early after vaccination, the differences are likely to be exacerbated by waning immune responses, even over the duration studied here. Similarly, the effectiveness against infection, hospitalization, or death in meta-analysis of population data was highest with mRNA1273, intermediate after BNT162b2, and lowest after Ad26.COV2.S. Differences in the efficacy [1–3] and effectiveness during deployment under EUA of mRNA-1273, BNT162b2, and Ad26.COV2.S vaccines may be, at least in part, due to the variable immunogenicity of the vaccines described here. These data may be important in rationalizing use of the most immunogenic vaccines in high-risk individuals, consideration of prioritizing booster doses for those with the weakest responses to ameliorate hospitalization and death, and tailoring booster plans by vaccine and prior infection.
Among individuals with prior infection, mRNA vaccination conferred higher antibody concentration titers. This supports recent data suggesting a single dose of mRNA vaccine in seropositive convalescent patients elicits comparable antibody titers to seronegative individuals who receive 2 doses of mRNA vaccine [19]. However, we extend these findings by showing that this trend applies to Ad26.COV2.S and is confirmed for all 3 vaccines with neutralization titers regardless of vaccine type and whether a second dose was given. These findings may explain why vaccination in the setting of prior infection appears to be associated with enhanced protection [20]. In settings with limited vaccine supply, consideration to prioritizing individuals without prior infection may be warranted.
We found differences in the immunogenicity and effectiveness of the 3 FDA EUA vaccines in the United States. The higher immunogenicity and significantly enhanced effectiveness of mRNA1273 compared with BNT162b2 may be due to the roughly 3.3-fold higher dose administered in the mRNA1273 regimen. Surprisingly, we observed sustained lower immunogenicity of the Ad26.COV2.S vaccine by multiple measures. These findings contrast with small studies from the manufacturer, which employed a viral variant that was more readily neutralized in vitro than ancestral SARS CoV-2 here and in prior studies but concur with recent studies by Tada and colleagues [13]. These data further highlight the need for immunogenicity assessment using consistent assays to permit comparisons. Consistent with the lower efficacy in trials, breakthrough infection, hospitalization, and death rates after Ad26.COV2.S were significantly higher than after mRNA1273. Additional booster doses have already been recommended for Ad26.COV2.S recipients, regardless of other host factors in several settings, notably in South Korea. Regardless of vaccine, in vitro neutralization of SARS CoV-2 Beta variant was markedly impaired, as has been previously noted. Variants that combine the neutralization evasion (as for the Beta variant) with enhanced transmissibility and pathogenicity (as for the Delta variant) may pose particular challenges to vaccine-induced immunity. Collectively, these data justify careful consideration of how to ensure similarly high levels of protection taking into account the primary vaccine series for individuals, in the setting of evolving variants.
The precise immune correlate of protection has yet to be agreed upon but growing data indicates the utility of anti-spike antibody titers or pseudoneutralization. A role for T cells continues to be explored. The data here are consistent with the central role of CD4+ T-helper cells in antibody induction. However, the marked heterogeneity and low frequency of cytotoxic T cells in this, and other studies [21, 22], makes this specific measure unlikely to be a universal mechanism of protection from severe disease in vaccinated individuals.
Several important limitations of this study are worth highlighting. First, the immunogenicity studies were focused on largely healthy individuals. Further study in the particular risk groups may be warranted. Secondly, we did not measure other features of the immune system such as non-neutralizing antibodies [23] and were limited in being able to examine kinetics of responses without repeat measures or at later time points. Thirdly, the effectiveness studies presented are observational and are not, by virtue of what data are available, reported stratified by age or adjusted for important potential confounders. In the absence of randomized trials comparing these vaccines directly, immunogenicity and observational effectiveness studies will continue to be required. Monitoring of vaccine immunogenicity and effectiveness could be an important strategy to identify individuals at high risk of breakthrough infection.
Taken together, these data demonstrate marked variation in the immunogenicity and effectiveness of the 3 FDA EUA vaccines deployed in the United States and may inform rational and equitable vaccination policy in the United States and elsewhere.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Andrea Nixon and the Massachusetts General Hospital Core Laboratory for assistance in performing assays. We thank Michael Farzan, PhD for providing ACE2-expressing 293T cells, Aaron Schmidt for providing proteins for development of ELISA assays, and Atul Bhan, MD and Mandakolathur Murali, MD for useful and insightful discussion. The RBD ELISA assay was developed by Steve Mullenbrock, Diego Farfan-Arribas, Mark Stump, Nels Pederson, Randy Wetzel, and Roberto Polakiewicz of Cell Signaling Technologies, Danvers, MA. We also thank Kellie Burke, Susan Gonzalez, Dominic Iafrate, Chioma Agugoesi, Katie Tarbox, Kirsten Dickens, Pedro Ojeda, JeanCarlos Cruz, Julian Villalba, Michelle Garlin, Daniel Montes, Karla Gonzalez, Hugh Shirley, Flor Amaya, Mimi Graney, Daniel Cortez, and Minjee Kim for assisting in participant recruitment.
Financial support. This work was supported by the Peter and Ann Lambertus Family Foundation (to A. J. I.); VIC Innovation Fund (to D. J. G., M. C. P., and M. N. P.); Burroughs Wellcome Fund (Career Award in Medical Sciences to G. D. G.); National Institute on Drug Abuse (grant number DP2DA040254 Avenir New Innovator Award to A. B. B.); Massachusetts General Hospital Transformative Scholars Program (to A. B. B.); and the Charles H. Hood Foundation (to A. B. B.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sadoff J, Gray G, Vandebosch A, et al. ; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khoury DS, Cromer D, Reynaldi A, et al. . Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 5. Lustig Y, Sapir E, Regev-Yochay G, et al. . BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med 2021; 9:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feng S, Phillips DJ, White T, et al. ; Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021; 27:2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Earle KA, Ambrosino DM, Fiore-Gartland A, et al. . Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021; 39:4423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMahan K, Yu J, Mercado NB, et al. . Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021; 590:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rogers TF, Zhao F, Huang D, et al. . Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020; 369:956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corbett KS, Nason MC, Flach B, et al. . Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science 2021; 373:eabj0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen MS, Nirula A, Mulligan MJ, et al. ; BLAZE-2 Investigators. Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA 2021; 326:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Brien MP, Forleo-Neto E, Musser BJ, et al. ; Covid-19 Phase 3 Prevention Trial Team. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med 2021; 385:1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tada T, Zhou H, Samanovic MI, et al. . Comparison of neutralizing antibody titers elicited by mRNA and adenoviral vector vaccine against SARS-CoV-2 variants. bioRxiv, doi: 10.1101/2021.07.19.452771, 6 August 2021, preprint: not peer reviewed. [DOI] [Google Scholar]
- 14. Garcia-Beltran WF, Lam EC, St Denis K, John Iafrate A, Naranbhai V, Balazs AB.. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021; 184:2372–83.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nathan A, Rossin EJ, Kaseke C, et al. . Structure-guided T cell vaccine design for SARS-CoV-2 variants and sarbecoviruses. Cell 2021; 184:4401–13.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Beltran WF, Lam EC, Astudillo MG, et al. . COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021; 184:476–88.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stephenson KE, Le Gars M, Sadoff J, et al. . Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 2021; 325:1535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emary KRW, Golubchik T, Aley PK, et al. ; COVID-19 Genomics UK Consortium; AMPHEUS Project; Oxford COVID-19 Vaccine Trial Group. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet 2021; 397:1351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krammer F, Srivastava K, Alshammary H, et al. . Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 2021; 384:1372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gazit S, Shlezinger R, Perez G, et al. . Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. medRxiv, doi: 10.1101/2021.08.24.21262415, 25 August 2021, preprint: not peer reviewed. [DOI] [Google Scholar]
- 21. Sahin U, Muik A, Vogler I, et al. . BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021; 595:572–7. [DOI] [PubMed] [Google Scholar]
- 22. Anderson EJ, Rouphael NG, Widge AT, et al. ; mRNA-1273 Study Group. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020; 383:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alter G, Yu J, Liu J, et al. . Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021; 596:268–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.