Abstract
This comprehensive review establishes the role of vitamin B12 as adjunct therapy for viral infections in the treatment and persistent symptoms of COVID-19, focusing on symptoms related to the muscle–gut–brain axis. Vitamin B12 can help balance immune responses to better fight viral infections. Furthermore, data from randomized clinical trials and meta-analysis indicate that vitamin B12 in the forms of methylcobalamin and cyanocobalamin may increase serum vitamin B12 levels, and resulted in decreased serum methylmalonic acid and homocysteine concentrations, and decreased pain intensity, memory loss, and impaired concentration. Among studies, there is much variation in vitamin B12 doses, chemical forms, supplementation time, and administration routes. Larger randomized clinical trials of vitamin B12 supplementation and analysis of markers such as total vitamin B12, holotranscobalamin, total homocysteine and methylmalonic acid, total folic acid, and, if possible, polymorphisms and methylation of genes need to be conducted with people with and without COVID-19 or who have had COVID-19 to facilitate the proper vitamin B12 form to be administered in individual treatment.
Keywords: cobalamin, COVID-19 symptoms, muscle–gut–brain axis, post COVID-19, viral infections
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), which was recognized by the World Health Organization as a pandemic in early 2020.1 This viral infection often causes respiratory-tract infection symptoms, but it is not limited to impairing lung function, because the infection can systematically affect the organism, undermining gastrointestinal,2 cardiovascular, and renal functions;3 or even the nervous4 and muscular systems.5,6 In addition to patient recovery, a growing concern has been that many patients have presented with post-acute COVID-19, long COVID-19, or persistent post–COVID-19 symptoms, which refer to effects or symptoms of this disease that continue for weeks or months beyond the initial illness.
Long COVID-19 appears to be a multisystem disease with varying symptoms.7,8 The main reported symptoms are shortness of breath, chest pain, headaches, neurocognitive difficulties, depression and other mental health conditions, muscle pains and weakness, gastrointestinal disorders, rashes, metabolic disruption, and thromboembolic conditions.9–11 The symptoms reported either during long COVID-19 seem to directly involve the skeletal muscle–gut–brain axis, which refers to the mutual interaction among these 3 systems.12–14 Even though relationships that consolidate this axis are not yet well established, there is a need to search for strategies that can strengthen it vis-à-vis this infectious disease.
Although population vaccinations are advancing in many countries, studies show that the virus has mutated, still infecting and reinfecting thousands of people, which has led to growing concern among health agencies.15 Nutritional status, chronic diseases, and age have been identified as important variables for the outcome of COVID-19.16 In this sense, the search for nutritional strategies that aim to reduce susceptibility to SARS-CoV-2 infection or the long-term complications of COVID-19 has been constant in several studies.16–18
In this scenario, vitamin B12 (also known as cobalamin) is a water-soluble vitamin that is part of the group of vitamins in the B complex. It has important functions in the blood and cardiovascular system,19 also being involved with the regulation of the immune system and antiviral activity.20,21 Furthermore, this vitamin is an essential nutrient with markedly important functions in the skeletal muscle–gut–brain axis, such as maintenance of skeletal muscle and neurobehavioral parameters22–25 and modulation of gut microbiota.26 Vitamin B12 was ranked among the top 4 substances for potential use in treatment for COVID-19, on the basis of findings from a study carried out with the help of molecular modelling and virtual screening tools, using data on US Food and Drug Administration–approved drugs.27 Thus, vitamin B12 combined with a healthy diet can be an important adjuvant in treating COVID-19 and in patients treated after COVID-19 infection.
The subclinical deficiency rates of vitamin B12 are high in developing countries and vegetarian populations because the main source of this vitamin are animal foods.28–30 In addition, older adults, people who have had bariatric surgery, and those are at increased risk of B12 deficiency, and use of some medications also is a risk factor.31 Vitamin B12 deficiency leads to hematologic, neuropathologic, and cardiovascular disorders, mainly by interfering in the homocysteine (Hcy) metabolism and the methylation reactions of the organism.32,33
Given that vitamin B12 is involved in various functions in the body and is influenced by several clinical conditions known to be at risk, depending on the outcomes of COVID-19, identifying vitamin B12 status is necessary for patients with current COVID-19 infection and those who have had COVID-19. Considering the relationship of vitamin B12 with the muscle–gut–brain axis and its role in viral infections and the immune system, we aimed in this comprehensive review to provide evidence and novel insights into the role of B12 during treatment and persistent symptoms of COVID-19.
SEARCH STRATEGY AND SELECTION CRITERIA
An online literature search was performed in the PubMed, Scopus, Web of Science, and Cochrane Library databases; on Google and ClinicalTrials.gov; and the International Clinical Trials Registry Platform to perform a comprehensive review about the role of vitamin B12 in COVID-19 prognosis. The following Medical Subject Heading and free-text search terms associated with vitamin B12 were input: “vitamin B12” OR “cobalamin” OR “cyanocobalamin” OR “methylcobalamin” OR “adenosylcobalamin” OR “hydroxycobalamin” OR “holotranscobalamin” OR “B12 deficiency” OR “vitamin B12 metabolism,” which were first used singly and then subsequently matched, in turn, with the terms associated with COVID-19 and other respiratory viral infections: “COVID” OR “COVID-19” OR “SARS-CoV-2” OR “SARS-CoV” OR “MERS” OR “respiratory infection” OR “viral infection” OR “viral disease.”34
The third round of searches ensued with terms referring to how the virus gains entry and causes damage in organs. Thus, “ACE-2” OR “gut” OR “brain” OR “muscle” OR “muscle-gut-brain axis” OR “COVID symptoms” OR “long-COVID” OR “post COVID” OR “persistent symptoms COVID” were added, in turn, to the first round of searches.34 The minimum eligible sample size of studies was 20 participants.
Only publications focusing on vitamin B12 relative to COVID-19 prognosis, other viral infections, and diseases with similar symptoms were eligible for inclusion. All searches, including title and abstract screening, were performed by 2 investigators working independently. Any discrepancies were resolved through consensus. Only articles published in English were short-listed; all articles deemed potentially eligible were retrieved for full-text review, and preprint articles were excluded.
VITAMIN B12: FUNCTIONS, SOURCES, AND DEFICIENCY
The term vitamin B12 is generally used to describe cobalamin, which is chemically composed by a heterocyclic corrin ring made up of 4 pyrroles with cobalt at the center of the ring.19 Vitamin B12 comprises many forms, including cyano-, methyl-, deoxyadenosyl-, and hydroxy-cobalamin.35 Cyanocobalamin is the synthetic form of vitamin B12 and can be found in supplements and fortified foods.19
The biggest dietary sources of vitamin B12 are viscera, such as liver (26–58 μg), meat (3–10 μg), dairy foods (0.3–2.4 μg), eggs (1–2.5 μg), poultry (trace amounts to 1 μg) in 100 g wet weight.11,26,36 Bonito fish and clam extracts contain considerable amounts of free vitamin B12, 41 μg and 132 μg/100 g wet weight, respectively.37 Gastrointestinal fermentation supports the growth of these vitamin B12–synthesizing microorganisms, and this vitamin is subsequently absorbed and incorporated into animal tissues, such as those of ruminants.19
Vitamin B12 is not synthesized by plants; therefore, low serum B12 levels may be more prevalent among vegetarians, and especially vegans.32 Vegans and even lacto-ovo-vegetarians with only a small intake of eggs and dairy foods may require supplemental vitamin B12 from fortified foods or supplements.33 Some foods, like cheddar cheese, “veggie burgers,” breakfast cereals, sunflower margarine, yeast extracts, vegetable stock, sausage mixes, and vegetable margarine are fortified with vitamin B12.38,39 The US Institute of Medicine has recommended that adults older than 51 years consume most of their vitamin B12 from fortified foods or supplements, bearing in mind that older adults are at higher risk of B12 deficiency due to the physiological reduction in intrinsic factor secretion necessary for absorbing this vitamin, as well as due to the use of drugs that can reduce the bioavailability of cobalamin.35
Vitamin B12 has also been reported to be present in lower levels in nonanimal foods, including edible algae, some mushrooms, and fermented foods such as tempeh, kimchi, miso, and tea.40 For example, Chlorella,41Spirulina,42 and Porphyra yezoensis, commonly known as purple laver or nori, can produce a cobalamin-like compound, also called pseudo-cobalamin, which has an inactive corrinoid.39
Bacterial vitamin B12 is synthesized by the gut-resident Propionibacterium. Freudenreichii, Lactobacillus reuteri, L.coryniformis, L. plantarum, L. coryniformis, Bifidobacterium animalis, B. infantis, and B. longum, among others, to produce adenosylcobalamin.21 The aforementioned bacteria are recognized for having probiotic activity, which points to the importance of the relationship between vitamin B12 and the gut microbiota, because probiotics are defined as living microorganisms that provide benefits to the host’s health when administered in adequate doses.43
The recommended daily allowance of vitamin B12 is 3–5 μg/day (2.4 μg/day for adults, 1.2 μg/day for children up to 8 years of age, and 2.6 μg/day for pregnant women and breastfeeding mothers).35 The average, Western nonvegetarian diet will contain 5–7 μg/day of vitamin B12, which is sufficient to maintain normal cobalamin homeostasis.44
Dietary cobalamin is released from food proteins by the action of stomach acid, where it is rapidly complexed to haptocorrin or transcobalamin I (a salivary B12-transfer protein). The haptocorrin-B12 complex suffers proteolysis in the duodenum by pancreatic proteases. In the proximal ileum, B12 is released to bind to the gastric intrinsic factor, which is a B12-transfer protein essential for ileal cobalamin absorption. Next, the intrinsic factor-B12 complex can enter mucosal cells in the distal ileum, and it again binds to transcobalamin (a serum B12-transfer protein). Finally, the transcobalamin-B12 complex circulates in the blood and then enters target cells.45 Most of the circulating B12 is bound to haptocorrin, which is unavailable for immediate delivery to cells. Between 10% and 30% of circulating B12 is bound to transcobalamin, forming holotranscobalamin or transcobalamin II.46
Specific blood-transport nonglycosylated protein is synthesized in most tissues that deliver cobalamin to cells by a receptor-mediated endocytosis. This protein binds to cobalamin with a high affinity and is encoded by a gene located on chromosome 22.47
Evidence shows that transcobalamins can suppress systemic inflammation by modulating certain cytokines (ie, interleukin-6), growth factors and other substrates with anti-inflammatory properties under normal physiological conditions. Vitamin B12 can be considered an endogenous negative regulator of nuclear transcription factor-κB (NFκB) through the regulation of nitric oxide, which plays a key role in regulating the immune response to infection.48,49 Vitamin B12 contributes to improving the immune response via an increase in CD8 + T cells and natural killer T cells.20,21 In addition, this vitamin has antioxidant properties through the reduced glutathione-sparing effect: it is capable of increasing the cytosolic bioavailability of reduced glutathione and thus can promote the synthesis of oxidized glutathione.48 In addition, vitamin B12 is recognized to modulate the ecology of the gut microbiota.26
Vitamin B12 is essential for DNA synthesis and regulation. It is involved in many important metabolic pathways, especially in the metabolism of lipids, carbohydrates, and proteins, and plays a central role in hemopoiesis. Methylcobalamin is a cofactor of 2 enzymes present in mammalian cells: methionine synthase and methylmalonyl-CoA mutase enzyme.33 When B12 levels are too low in the body, the result is an increase in methylmalonic acid (MMA) and Hcy concentration due to inhibition of methylmalonyl-CoA mutase and methionine synthase, respectively.33 The increase in Hcy causes folate sequestration and interrupts DNA synthesis. Increased MMA levels cause demyelinating defects in the nervous system and elevate propionic acid, resulting in metabolic acidosis.50
The symptoms of subclinical B12 deficiency are subtle and often not recognized. A B12 deficiency can remain without symptoms for a long time, leading to a chronic deficiency. For many years, B12 concentrations < 148 pmol/L in blood have been identified as being deficient. Nevertheless, due to the limitations of sensitivity and specificity of individual assays, 2 or more biomarkers should be used in combination to accurately diagnose vitamin B12 deficiency, such as direct (total B12 and holotranscobalamin) and functional (Hcy and MMA) biomarkers.51
Vitamin B12 deficiency occurs at all ages (but mainly in the older adult population) and in both sexes, especially in people who have a restricted diet in foods of animal origin either by choice or due to lack of financial resources to purchase these foods.52 Deficiency is much more common in developing countries, starting in early life and persisting across the life span,30 and can be associated with insufficient nutrition or microbial infections. Determining the prevalence of subclinical deficiency of vitamin B12 is challenging, however.45
Studies have shown a high prevalence of vitamin B12 deficiency in populations with different types of vegetarian diets, specifically > 60% in vegans and > 40% in lacto- or ovolactovegetarians.53,54 Vegans have a higher prevalence of B12 deficiency compared with other vegetarians that depends, in part, on dietary rigidity and length of time following this lifestyle.53,55 The lactovegetarian and ovolactovegetarian groups have intermediate vitamin B12 status when compared with vegans and omnivores, because although milk, dairy products, and eggs contain some amount of vitamin B12, the intake of this vitamin through a lacto- or ovolactovegetarian diet is considered limited.53,54 The use of vitamin B12 supplements may be necessary for these groups because the bioavailability of vitamin B12 from supplements is greater than that from foods.53
Low B12 concentrations in the body may be present in different pathophysiological situations, including pregnancy, old age, smoking, and comorbidities such as hypertension, diabetes mellitus, pancreatic insufficiency, autoimmune gastritis, gastrectomy or gastric bypass, diseases or resection of the ileum, bacterial overgrowth, celiac disease, inflammatory bowel disease, or uremia-related malnutrition.22,23,56 Treatments such as antibiotics, proton pump inhibitor medications, antihyperglycemic medicines (eg, metformin), nitrous oxide anesthesia, a nonsteroidal anti-inflammatory drug, some anticonvulsants, and colchicine interfere with B12 absorption and metabolism.33,45 Angiotensin-converting enzyme inhibitors have also been associated with low B12 levels in older adults.57,58
Cobalamin deficiency causes a decrease in hemoglobin levels, characterizing megaloblastic or pernicious anemia with manifestations that include skin pallor, decreased energy and exercise tolerance, fatigue, shortness of breath, and palpitations.59 Scientific evidence indicates that not necessarily the deficiency but the low status of the B12 biomarker has been associated with increased total Hcy level, which is involved in increased generation of reactive oxygen species in lipid peroxidation and tissue damage to the endothelium vascular and thromboembolism.41,51 Hyperhomocysteinemia due to low B12 status leads to an increased risk of several chronic diseases of aging, including cardiovascular disease and osteoporosis; however, these investigations are limited.41,51
The neurologic complications of B12 deficiency occur at a later stage of depletion than the indicators we discuss later in this article and are not specific for this deficiency. Alterations in peripheral nerves, followed by degenerative alterations of the posterior spinal cords and cortical spinal ducts, have been reported, in addition to sensory disturbances in the extremities (tingling and numbness). This deficiency was associated with severe symptoms of depression, suicidal behaviors, reduced cognition, mental fatigue, bad or depressed moods, mania, psychosis, and intense agitation.49
Other symptoms related to cobalamin deficiency are elevated lactic dehydrogenase levels, mechanical hemolysis, thrombocytopenia, intravascular coagulation thrombosis, low reticulocyte count, vasoconstriction, and renal and pulmonary vasculopathy.60,61 Vitamin B12 deficiency may induce macrocytosis, peripheral neuropathy, ataxia, dizziness, cognitive disturbances, depression, delirium, psychosis, paralysis, muscle cramps, fibromyalgia-like symptoms, and fatigue.22–25
ACTION OF VITAMIN B12 ON THE SKELETAL MUSCLE–GUT–BRAIN AXIS
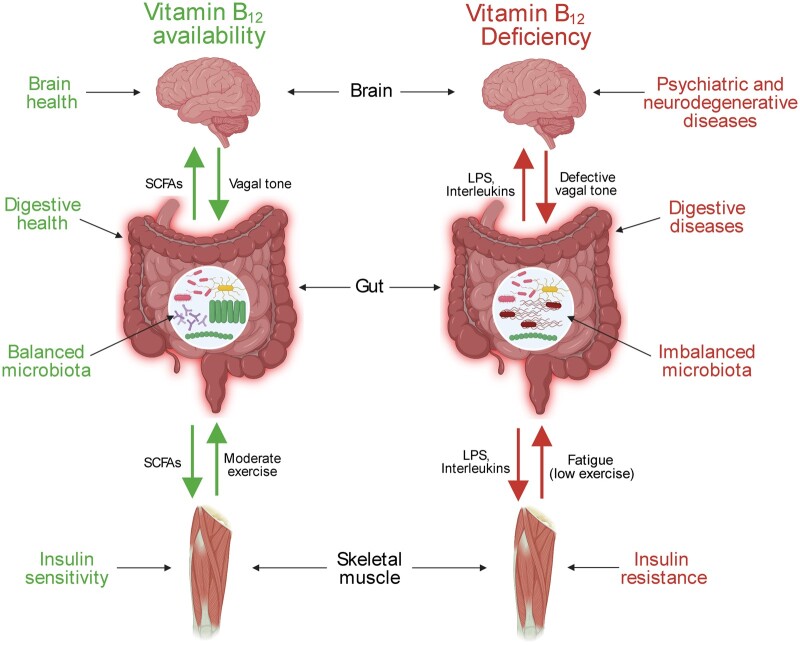
Skeletal muscle, gut, and brain are tissues with a collaborative role in regulating physiological processes, including energy homeostasis. The skeletal muscle–gut–brain axis is a recently introduced concept that is supported by increasing clinical and preclinical evidence showing a close relationship between the muscle–gut and gut–brain axes (Figure 1). 12–14 The skeletal muscle–gut–brain axis is critically regulated by microbiota, a community of gut-resident microorganisms composed of ∼1014 cells classified in > 1000 different bacterial species.13,62 Gut microbiota composition is regulated by several factors, such as diet, drugs, stress, and exercise.12 Homeostatic imbalance in this microbial composition, known as dysbiosis, is related to a sedentary lifestyle and some diseases like Alzheimer’s disease, colitis, and obesity in humans and rodents (Figure 1).14,63–65
Figure 1.
Impact of vitamin B12 on the muscle–gut–brain axis. Abbreviations: LPS, lipopolysaccharide; SCFA, short-chain fatty acid.
Gut microbiota modulate a wide range of functions in the host under normal conditions, including nutritional status, metabolism, and immunity, promoting physical and brain benefits (Figure 1).14,66,67 Microbiota exert their effects through direct cross-talk with tissues other than the intestine. A bidirectional communication linking gut microbiota and muscle, as well as gut microbiota and brain, has been demonstrated.12,13 Moderate physical activity and healthy diets promote a balanced microbiome (eg, a high Bacteroidetes to Firmicutes ratio) along with the production of neurotransmitters (eg, serotonin, dopamine), secondary bile acids, and short-chain fatty acids, including acetate, propionate, and butyrate (Figure 1).13,66 These bacteria-produced compounds positively affect both skeletal muscle and brain, decreasing inflammation and risk of psychiatric and neurodegenerative disorders, as well as increasing insulin sensitivity and glucose control (Figure 1).12,13 Opposite effects are observed in dysbiosis conditions (eg, a low Bacteroidetes to Firmicutes ratio), where high levels of lipopolysaccharides and pro-inflammatory cytokines (eg, interleukin-1β, interleukin-6, tumor necrosis factor α) are produced by gut bacteria (Figure 1).12,13 Skeletal muscle and brain effects on microbiota are exerted by direct physical activity and vagal colonocyte modulation.12,68
The aforementioned evidence shows the close relationship among muscle, gut, and brain, supporting the concept of the muscle–gut–brain axis. Thus, its modulation can be used as a valuable strategy to develop therapies against diseases related to the brain and muscle, such as psychiatric disorders and neurodegenerative and muscular diseases.
Vitamin B12 is exclusively produced by archaea and bacteria69 and is required for DNA and methionine synthesis, as well as catabolism of fatty acids and amino acids.70 This vitamin emerges as a serious candidate to modulate the muscle–gut–brain axis, because of its ability to modulate functions at the muscular, intestinal, and cerebral levels. Treatment with cobalamin improves skeletal muscle dysfunction in patients with hyperhomocysteinemia71 and reduces formalin-induced muscle pain.72 Vitamin B12 deficiency at the brain level is associated with affective disorders, behavior changes, psychosis, cognitive impairment or decline, and dementia (including Alzheimer’s disease and vascular dementia protection).73
Treatment with vitamin B12 in rats with experimental autism improved impaired markers of this neurologic condition.74 Modulation of the muscle–gut–brain axis by vitamin B12 is also possible by acting on gut microbiota. This is based on the fact that resident bacteria in the colon produce cobalamin, which regulates gene expression in gut Bacteroidetes.26 Moreover, vitamin B12 protects from dysbiosis and promotes different microbial responses in a murine model of colitis.63
That vitamin B12 can act at any level of the skeletal muscle–gut–brain axis makes its use an interesting option to treat associated diseases. Thus, cobalamin may be an important agent to prevent or improve neurologic consequences of COVID-19.
VITAMIN B12 ANTIVIRAL ROLE
Adaptive immunity has been associated with low levels of vitamin B12 involving viral infection.75 It seems plausible that severe vitamin B12 deficiency can also be associated with increased risk and severity of infections, because this deficiency affects the functions of phagocytes, production of interferon, replication of viruses, and maturation of T lymphocytes.76–78 In 9 studies, researchers reported the effects of vitamin B12 supplementation on viral diseases (Table 1). 79–87
Table 1.
Main studies showing the role of B12 in parameters of the immune system related to viral infection
| Reference | Design | Target condition | No. of participants | Population (age) | Dose and time | Main results |
|---|---|---|---|---|---|---|
| Iwarson and Lindberg (1977)79 | RCT | Acute viral hepatitis | 40 | 2 groups of patients from the 1972 epidemic with short-incubation hepatitis A (aged 15–45 y) | 1 mg HOCbl, IM, per d for 12 d, followed by 1 mg of oral HOCbl per d for 23 d | A significant return of serum aminotransferase levels to normal was observed in the group treated with coenzyme-B12. |
| Baldewicz et al (2000)80 | RCT | HIV | 159 | Bereaved HIV-1(+) and HIV-1(−) homosexual men (aged 30–40 y) | Observational study | Serum cobalamin level was inversely related to self-reported overall distress level and specifically to depression, anxiety, and confusion subscale scores, as well as to clinically rated depressed and anxious mood. |
| Semeere et al (2012)81 | Cross-sectional study | HIV | 204 | ART-naïve adults (34.4 [SD ± 9.4] y) | 3 doses of 1 mg of parenteral vitamin B12a | HIV-infected, ART-naïve individuals had a lower mean vitamin B12 level (384 pg/mL) than the mean B12 level reported in a population of healthy university students (469 pg/mL). |
| Rocco et al (2013)82 | RCT | Chronic viral hepatitis | 94 | Patients with chronic hepatitis, naïve to antiviral therapy (51–53 y) | 5000 μg of IM vitamin B12a for 4 wk | Vitamin B12 supplementation significantly improved sustained viral response rates in patients with HCV naïve to antiviral therapy. |
| Balfour et al (2014)83 | Multicenter RCT | HIV | 218 | Asymptomatic patients with HIV (38.1 [SD ± 8.9] y). | Vitamin B12b divided into 16 capsules of micronutrient supplements per day for 2 y. | Lower baseline levels of B12 (<133 pmol/L) correlated with lower baseline CD4 count (r = 0.2; P = 0.007) in multiple linear regression adjusted for sex and body mass index, as well as in unadjusted analysis (r = 0.21; P = 0.02). |
| Sugihara et al (2017)84 | Prospective cohort study | Chronic viral hepatitis | 90 | Patients with chronic viral hepatitis and viral-induced cirrhosis (30–88 y) | Observational study | The serum vitamin B12 level was a significant independent predictor for overall survival in patients with chronic viral liver disease. Falsely elevated serum vitamin B12 levels (584.5 pg/mL) were associated with severity and prognosis in viral liver disease. |
| Tenforde et al (2017)85 | Case-cohort study | Patients with HIV with incident TB | 332 | Incident TB after ART initiation in patients infected with HIV (29–41 y), CD4+ T-cell counts <300 cells/mm3 | Observational study | Using established deficiency cutoffs, only 6% of patients with TB and 9% of those without TB were deficient in vitamin B12 (<148 pmol/L; P = 0.39). |
| Shivakoti et al (2019)86 | Random subcohort | HIV | 270 | Adults positive for HIV with CD4 count < 300 cells/mm³ (29–41 y) | Observational study | Deficiency in vitamin B12 (<148 pmol/L) was associated with lower CD4 reconstitution compared with those with sufficient levels. |
| Williams et al (2019)87 | Nonrandomized controlled clinical trial | Norovirus infection | 52 | Healthy adults exposed to norovirus (21–28 y) | Observational study | Norovirus infection elicited a time-limited inflammatory response. However, the concentrations of vitamin B12 did not differ over time within the inflamed group. |
Vitamin B12 form not mentioned.
Dose and vitamin B12 form not mentioned.
Abbreviations: ART, antiretroviral therapy; HOCBL, hydroxycobalamin; HCV, hepatitis C virus; IM, intramuscularly; RCT, randomized clinical trial; TB, tuberculosis.
In this respect, it is useful to note that most studies to date have looked at the association between vitamin B12 levels and the anti-inflammatory and immune-modulating properties of patients infected with HIV. First, a randomized controlled trial (RCT) showed the relationship of a continuous measure of cobalamin level to psychological distress in bereaved HIV-1(+) and HIV-1(−) patients. The mood outcomes in this study were inversely related to levels of serum vitamin B12. Moreover, the lower plasma cobalamin levels also were associated with the presence of symptoms consistent with major depressive disorder.80
The second study was a cross-sectional study81 including antiretroviral therapy (ART)-naïve adults. Logistic regression was used to determine factors associated with suboptimal vitamin B12 levels. Compared with people with normal concentrations of B12, individuals with vitamin B12 deficiency had a longer known duration of HIV infection. In addition, participants eligible for ART (ie, those with CD4 count < 350 cells/µL) with suboptimal B12 had a higher mean rate of CD4 cell population decline than counterparts with normal B12 levels.
In the third study, researchers performed a multicenter clinical trial in asymptomatic patients with HIV with a CD4 T-cell count between 375 and 750 cells/µL at screening evaluation.83 The authors evaluated the effect of high-dose micronutrient supplements (16 capsules/day) on measures of HIV disease progression used to guide ART initiation. The weak but significant correlation of levels of serum vitamin B12 levels with CD4 count observed in this study may suggest either that low B12 levels may predict CD4 decline, or that B12 and CD4 count decline in concert.
More recently, Shivakoti et al86 conducted a secondary analysis of a random subcohort sample from a multinational randomized trial of a combination ART regimen efficacy among 1571 combination-ART–naïve adults. The researchers aimed to investigate the relationship of micronutrients and inflammation with CD4 cell recovery. The results showed that small numbers of participants (17.1%) were deficient in vitamin B12. Therefore, the analysis was not powered to detect significant differences in CD4 count among participants who were B12 deficient and those who were not.
In the same period, Tenford et al85 performed a case-cohort design study to evaluate the association between micronutrient deficiencies and incident tuberculosis in a diverse population with high incidence of HIV, particularly in low- and middle-income countries. The vitamin A and vitamin D levels at ART initiation were independently associated with increased risk of incident tuberculosis in the ensuing 96 weeks. The median values of vitamin B12 did not differ significantly between groups.
In addition, 2 different viral conditions were included in this review: hepatitis and norovirus infection. Three studies investigated the ability of vitamin B12 to repair tissue damage and compensate for diminished hepatic storage during viral hepatitis. In the first RCT, researchers reported the therapeutic effect of coenzyme-B12 in hepatitis A compared with that of hydroxycobalamin after the 1972 epidemic.79 Admitted patients who were born in even-numbered years received hydroxycobalamin. Patients born in odd-numbered years were treated with coenzyme B. All patients had to rest during the period of abnormal serum bilirubin levels. The group treated with coenzyme B showed a tendency to more rapid normalization of aminotransferases than did the patients treated with hydroxycobalamin.
In the second randomized controlled study, researchers compared pegylated interferon-α plus ribavirin (standard of care) with the standard of care plus vitamin B12.82 The results showed that, in patients with chronic hepatitis C virus infection naive to antiviral therapy, vitamin B12 supplementation improved the overall rate of sustained viral response to pegylated interferon-α and ribavirin by 34%. The effect seemed to be particularly pronounced in difficult-to-treat patients, namely, those infected with hepatitis C virus genotype 1 and with a high baseline viral load.
Authors of the third study analyzed the relationship between vitamin B12 levels and liver disease severity and long-term prognosis in patients with chronic viral hepatitis and cirrhosis.84 Consecutive patients with chronic viral hepatitis and viral-induced cirrhosis were prospectively enrolled in this prospective cohort. The authors found that serum vitamin B12 levels were significantly higher in patients with cirrhosis with Child-Pugh C than in patients with chronic hepatitis or Child-Pugh A/B cirrhosis. Therefore, serum vitamin B12 level was a significant independent predictor for overall survival in patients with chronic viral liver disease.
Recently, Williams et al87 evaluated associations between inflammation and micronutrient biomarkers after norovirus exposure. Serum vitamin B12 was analyzed using a competitive protein-binding chemiluminescence immunoassay. Vitamin B12 concentrations did not differ over time within the inflamed group at the end point. The authors suggested that the lower vitamin B12 concentrations at baseline that appeared protective of norovirus infection may have a genetic explanation, such as a single nucleotide polymorphism. Genetic variants of fucosyltransferase 2 have been associated with lower vitamin B12 status, and the homozygous fucosyltransferase 2 nonsecretor genotype is characterized as resistant to norovirus infection.
There is some evidence in the available literature that vitamin B12 is involved with nucleoprotein metabolism, and it is suggested that its administration may accelerate the repair of damaged cells and thus be responsible for the more favorable course of the illness in the group to which it was administered. Moreover, the serum vitamin B12 level seems to be associated with a positive prognosis in some viral conditions. However, the mechanisms behind this association are still uncertain, and more high-quality studies that investigate the underlying mechanisms of this interaction are needed.
EFFECTS OF VITAMIN B12 ON SYMPTOMS DURING AND AFTER COVID-19
COVID-19 affects people of all ages and sexes, but the severity of COVID-19 symptoms predominantly increases in elderly individuals, men, and people with comorbidities such as obesity, malnutrition, hypertension, and diabetes mellitus who generally have inadequate nutritional status and inflammation.88–91
Patients with COVID-19 may present acute polyneuropathy such as Guillain–Barré syndrome and variants, which affect the peripheral nervous system due to an exacerbated immune response to infection or also as a postinfectious immune-mediated response.6,92–94 The most common Guillain–Barré syndrome and variants symptoms are severe back pain and muscle weakness, and there may be long-term complications, including severe disability, pain, and fatigue.92,94
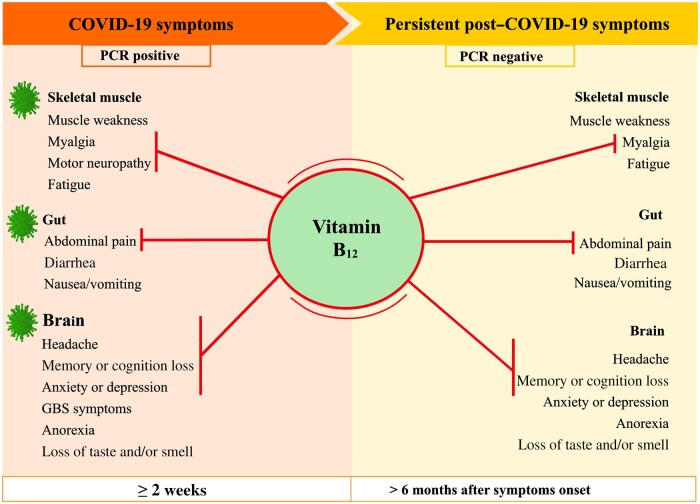
Some COVID-19 symptoms can persist for weeks or months after symptoms onset; this condition is called acute post–COVID-19 (from week 5 to week 12), long COVID-19 (from week 12 to week 24), or persistent post–COVID-19 symptoms (lasting > 24 weeks).8 The symptoms include gastrointestinal symptoms (eg, diarrhea, nausea and vomiting, abdominal pain); neurologic manifestations (eg, concentration impairment, anxiety and depression symptoms, headache, migraine, dementia, stroke, obsessive-compulsive disorder, anorexia, apathy, executive deficits, vertigo, memory or cognition loss, hallucinations, sleep disturbances, post-traumatic stress disorder, loss of taste (ageusia) or of smell (anosmia); neuromuscular disorders (eg, fatigue); and muscular disorders (eg, muscle weakness, myalgia).9,95–98
Various vitamin B12 deficiency symptoms are similar to those found in patients with COVID-19 and post–COVID-19.22–25 Studies tested vitamin B12 supplementation to alleviate some of the symptoms of various diseases that are also present in COVID-19 (Table 2). 23,99–112 Two RCTs99,110 and 5 meta-analyses23,100–102,109 reported benefits of vitamin B12 supplementation in methylcobalamin (0.5–1 mg orally or local injection for 2 weeks to 1 year) and cyanocobalamin (2000 mg orally or 1–1000 mg via intramuscular route for 90 days and 4 months) forms. The benefits were mainly in analgesic action and attenuation of neurologic symptoms.
Table 2.
Effects of vitamin B12 treatment on symptoms related to COVID-19 prognosis
| Reference | Design | Target condition | No. of participants | Population (age) | Dose and time | Main results |
|---|---|---|---|---|---|---|
| Mauro et al (2000)99 | Randomized, double-blind, placebo-controlled trial | Low back pain | 60 | Patients with a proven medical history for back pain, without vitamin B12 deficiency (18–65 y) | 1000 mg daily of cyanocobalamin IM for 2 wk | Alleviating the low back pain and related functional disability, decreasing the consumption of paracetamol |
| Sun et al (2005)100 | Meta-analysis of RCTs | DN | 231 | Patients with diabetic PN, without vitamin B12 deficiency (53–56 y) | 0.5 mg of methylcobalamin injection 3 times per week for 4 wk; or 0.5–500 mg of oral methylcobalamin 3 times a day for 4–16 wk | Improved somatic symptoms, such as pain and paresthesia. In 3 studies, methylcobalamin therapy improved autonomic symptoms (peripheral neurophysiology, oral dryness, and dysuria). |
| Vidal-Alaball et al (2005)101; Butler et al (2006)102; and Wang et al (2018)23 | Meta-analysis of RCTs | Oral vs IM vitamin B12 to treat vitamin B12 deficiency | 153 | Patients with megaloblastic anemia, (16–86 y) | 1000 µg daily of oral vitamin B12a; 2000 µg daily of oral cyanocobalamin, 1000 µg daily IM cyanocobalamin or vitamin B12a for 90 d and 4 mo |
|
| Talaei et al (2009)103 | RCT, single-blind | DN | 100 | Patients with diabetes (duration > 3 years; 18–53 y old) with DN, and without vitamin B12 deficiency | 2 mg IM vitamin B12a twice weekly for 3 mo | Decrease in pain and paresthesia scores, and tingling sensation; no changes in vibration, position, pinprick, and nerve conduction parameters |
| Volkov et al (2009)104 | RCT, double-blind | RAS | 58 | Patients with RAS and without vitamin B12 deficiency (22.54–42.67 y) | 1000 µg daily of sublingual vitamin B12a for 6 mo | Decreases in pain level, number of ulcers, and duration of outbreaks at 5 and 6 mo of treatment, regardless of initial vitamin B12 levels in the blood. |
| Syed et al (2013)105 | RCT | Major depressive disorder | 73 | Patients with depression and low to normal B12 levels (24.28–51.06 y) | 1000 µg IM vitamin B12a every week in addition to the antidepressants (imipramine 100–250 mg/d and fluoxetine 20–40 mg/d) during the 6 wk | Improved depressive symptoms |
| Taneja et al (2013)106 | RCT, double-blind | Diarrhea and acute lower respiratory tract infections | 1000 | North Indian children with or without B12 deficiency (6–30 mo) | 1.8 μg of oral vitamin B12a for 6 mo | The supplementation significantly improved vitamin B12 status, but vitamin B12 administration did not reduce the incidence of diarrhea or lower respiratory infections. |
| Almeida et al (2015)107 | Meta-analysis of RCTs | Major depressive episodes | 1695 | Patients with major depression with or without B12 deficiency (16–85 y) | 0.1–0.5 mg daily of oral vitamin B12a for 52 wk to 7 y, or 1 mg local injection of cyanocobalamin for 4 wk | Vitamin B12 did not decrease the severity of depressive symptoms. |
| Scholten et al (2018)108 | RCT, double-blind | Severe fatigue | 95 | Patients with irritable bowel syndrome or inflammatory bowel disease, and normal vitamin B12 blood levels (18–65 y) | 1000 μg daily of oral vitamin B12a for 8 wk | Increased vitamin B12 blood levels, but not improved fatigue, quality of life, or depressive or anxiety symptoms. |
| Wang et al (2018)109 | Meta-analysis of RCTs | Herpetic neuralgia | 383 | Patients with postherpetic neuralgia and with or without B12 deficiency (47.01–74.70 y) | 1 mg daily of methylcobalamin, local injection, for 2–4 wk | Improved the quality of life, decreased pain intensity and analgesics use |
| Didangelos et al (2021)110 | Randomized, double-blind, placebo-controlled trial | Neurophysiological parameters, pseudomotor function, life quality, and level of pain | 90 | Patients with DN, metformin use, and low vitamin B12 levels (53.4–71.8 y) | 1000 μg daily of oral methylcobalamin for 1 y | Increased plasma B12 levels and improved all neurophysiological parameters, pseudomotor function, pain score, and quality of life, but it did not improve cardiovascular autonomic reflex tests and MNSI. |
| Markun et al (2021)111 | Meta-analysis of RCTs | Cognitive function, depressive symptoms, and fatigue | 6276 | Patients with or without mild cognitive impairment, with or without vitamin B12 deficiency (66–82 y) | 0.1–1 mg daily of oral cyanocobalamin or methylcobalamin; or 1 mg IM cyanocobalamin once or twice weekly up to 2 y | Vitamin B12 supplementation did not improve cognitive function and depressive symptoms, and idiopathic fatigue analysis was not possible. |
| Stein et al (2021)112 | Meta-analysis of RCTs | PN | 2948 | Patients with PN and lowered plasma vitamin B12 level (33–86 y) | 0.75–2 mg daily of oral methylcobalamin for 28–168 d | The presence of PN was associated with lowered B12 levels. B12 treatment showed a nonsignificant association with symptom improvement (eg, numbness, paresthesia, pain, and/or dysesthesia), perhaps due to the low number of studies included in the meta-analysis (n = 4). |
Vitamin B12 form not mentioned.
Abbreviations: DN, diabetic neuropathy; IM, intramuscularly; MNSI, Michigan Neuropathy Screening Instrument Examination; PN, peripheral neuropathy; RAS, recurrent aphthous stomatitis; RCT, randomized controlled trial.
Other meta-analyses107,111,112 and RCTs106,108 did not indicate significant results in relieving pain, fatigue, or neurologic symptoms by supplementation with these vitamin B12 forms. However, the authors of these meta-analyses listed some limitations that open the way for carrying out more RCTs to answer questions: a small number of available trials and high heterogeneity between included studies107; a long time difference between the start of vitamin B12 supplementation and the appearance of measurable benefits; heterogeneity in the included studies; only 1 high-quality RCT evaluated the effects on fatigue, making it impossible to estimate the meta-analysis111; small variations in combined estimates in sensitivity and subgroup analysis; and a low number of studies for subgroup analyses.112
A meta-analysis of observational studies (n = 21 837 people 12–90 years old) revealed a significant inverse association between dietary intake of vitamin B12 and/or vitamin B12 supplementation and the risk of depression in women.10 The most used vitamin B12 forms in the included studies were methylcobalamin and cyanocobalamin. There is still controversy regarding the effectiveness of vitamin B12 used in supplementation; some scientists claim that natural methylcobalamin and adenosylcobalamin forms may have greater vitamin B12 activity than the synthetic cyanocobalamin form.48,113 Cyanocobalamin must be broken down to cobalamin and cyanide must be converted to the active forms of B12 in the human body, whereas genetic single nucleotide polymorphisms can interfere in the metabolism and conversion to intracellular active forms of vitamin B12.113
Nevertheless, Obeid et al114 proposed that cyanocobalamin supplementation (the most stable and inexpensive form) is as effective as the cobalamin coenzyme forms methylcobalamin and adenosylcobalamin. Methylmalonic aciduria and homocystinuria type C protein convert cyanocobalamin into the active methylcobalamin and adenosylcobalamin forms in cells, as long as there is no remethylation disorder cblC in methylmalonic aciduria and homocystinuria type C protein, cblF and cblJ in the protein integral membrane of lysosomal cobalamin transport-escort protein LMBD1 and ATP-binding cassette subfamily D member 4—required for lysosomal release of transport of cobalamin.115,116 The guidelines for diagnosis and management of the cobalamin‐related remethylation disorders recommend treatment with parenteral hydroxylcobalamin in suspected cases of remethylation disorder, the incidence of serious complications, and for significant improvement in patient survival.115
Thus, vitamin B12 can be used in adjunct treatment of mild to severe COVID-19 symptoms, because of its analgesic function and role in neuromuscular disorders (Figure 2). The appropriate choice of the chemical form of vitamin B12, the dose, and treatment time will depend on individual factors such as the type of vitamin B12 deficiency, age, preexisting diseases, type of methylation gene, and medication used.
Figure 2.
Vitamin B12 action on COVID-19 and post–COVID-19 symptoms. Abbreviations: GBS, Guillain–Barré syndrome; PCR, polymerase chain reaction.
Many protocols performed in hospitals in Brazil that receive patients affected by COVID-19 recommend supplementation or intramuscular injection of vitamin B12 in cases where a deficiency or subclinical deficiency of vitamin B12 is identified. This clinical practice may be related to the increase in Hcy in patients with severe COVID-19.117 Pharmacological treatment with B12 usually implements high doses (1000–2000 μg/day) for an average of 1–3 months.22,34,44,118 Vitamin B12 therapy reduces oxidative damage and inflammation levels, both systemically and in the central nervous system, especially associated with folate; it improves microvascular disease associated with hyperhomocysteinemia119; and can alleviate COVID-19 symptoms, thereby improving the prognosis either during or in the post–acute COVID-19 syndrome.
In a cohort study by Tan et al,17 older patients (≥50 years; n = 43) with COVID-19 received a daily oral combination of 500 µg methylcobalamin, 1000 IU of cholecalciferol (vitamin D3), and 150 mg of magnesium oxide before the onset of the primary outcome and during 14 days. These patients had lower required need of oxygen therapy during hospitalization than did the control group (which did not receive the combination).
Jang et al120 evaluated in 6 hospitals in South Korea 80 patients (median age, 63 years) with COVID-19 receiving mechanical ventilatory support, 19 of whom were treated with extracorporeal membrane oxygenation (9.8 days; interquartile range, 7.0–13.7 days). Five patients (31.58%) received vitamin B12 therapy and were successfully weaned off extracorporeal membrane oxygenation (P = 0.013). However, they did not specify the chemical form of vitamin B12 used in hospital treatment. The resolution of lung injury by vitamin B12 may be associated with its reduced glutathione-sparing effect to maintain the antioxidant status, as well as its role as a regulator of NFκB levels affecting the expression of genes encoding pro-inflammatory cytokines.44,48,121
The daily oral supplementation of vitamin B12 (250 µg) in women immunized against influenza A (H1N1) during pregnancy and 3 months postpartum increased the vitamin B12 values in plasma, colostrum, and breast milk; increased H1N1-specific immunoglobulin A responses in the plasma and colostrum of mothers, but not in babies; decreased MMA in mothers and children; and reduced the number of babies with high levels of C-reactive protein.122 The C-reactive protein levels may have an inverse correlation with vitamin B12 concentration.123
Vitamin B12 can block factors that facilitate infection with SARS-CoV-2. Kandeel and Al-Nazawi27 conducted a virtual screening study of the Food and Drug Administration–approved drugs against COVID-19 main protease 3-C-like protease, which plays a key role in viral replication and transcription.124 Vitamin B12 was in the fourth position of docking scores (relative docking score, 1.99) against COVID-19 main protease 3-C-like protease. The authors suggested combining vitamin B12 with nicotinamide (vitamin B3) or drugs against COVID-19, such as ribavirin (an potent antiviral drug, especially vs RNA viruses), and telbivudine (used to treat hepatitis B virus) to treat COVID-19.
Methylcobalamin has a significant affinity to bind to the active site of the nsp12 protein of SARS-CoV-2, with a docking score of −8.193, Glide gscore of −8.263, and Glide energy of −75.794 (Schrödinger, New York, NY). Thus, vitamin B12 may inhibit the RNA‐dependent RNA polymerase activity of nsp12 responsible for the replication of the viral genome.125 In an in silico study by Narayanan and Nair,126 the docking score of −10.008, Glide gscore of −10.008, and Glide energy of −86.131 indicated that vitamin B12 has an affinity for binding with the active site of nsp14 protein from SARS-CoV-2.
The nsp14 protein has 3′ to 5′ exoribonuclease activity responsible for removing mismatches that arise during genome duplication; this action may impair the inhibitory effect of drugs used to treat COVID-19.126 Furthermore, Kaur et al127 proposed that the entry of SARS-CoV-2 facilitated by nsp14 protein in the host cell allows the use of cell S-adenosylmethionine for viral RNA capping, culminating in an increase in the Hcy production and angiotensin-converting enzyme-2 activation, which will facilitate greater viral entry into cells.128,129 It is important to note that the prolonged use of angiotensin-converting enzyme inhibitors to reduce viral infection can reduce vitamin B12 levels (< 200 pmol/L) in older adult patients aged ≥ 65 years.57
In data from 24 262 participants with a mean age of 48.0 (SD, ± 19.0) years, Wolffenbuttel et al130 found that low serum B12 concentrations (< 140 pmol/L) were associated with a moderate increase in all causes of death (eg, chronic lower respiratory diseases, Alzheimer’s disease, influenza and pneumonia, cerebrovascular diseases, diabetes mellitus) and high serum concentrations of MMA and Hcy; the increase in cardiovascular causes of death was associated with both low (< 140 pmol/L) and high (> 700 pmol/L) serum B12 levels.
On the other hand, excess vitamin B12 in the body was also associated with poor outcomes of diseases. Ersöz and Yilmaz131 reported poor prognostic factors (eg, death of patients in the intensive care unit and intubated) in 310 patients from Turkey with COVID-19 (mean age ± SD, 57.02 ± 18.28 years) with high blood vitamin B12 concentration (> 911 pg/mL), and low folate, iron, vitamin D, and hemoglobin levels. Some studies indicated an association between higher B12 levels (1000–1719 pg/mL) and the death of critically ill adult patients in the intensive care unit (mean age ± SD, range, 53.5 ± 12.0 to 66.7 ± 20.0 years).84,132,133 Flores-Guerrero et al134 showed that a plasma vitamin B12 concentration > 455.41 pg/mL was associated with a higher risk of all-cause mortality in 1394 adults (mean age ± SD, 54.6 ± 11.6 years). Excess vitamin B12 is still controversial, and other authors have used distinct values for high plasma B12 levels: >950 pg/mL or 701 pmol/L135; > 771 pg/mL136; > 203 pg/mL or > 601 pmol/L.137
Dalbeni et al138 exposed that 9 of 49 patients with COVID-19–associated pneumonia who did not receive vitamin B12 by any route had excess vitamin B12 in plasma (median, 1315 ng/mL), low arterial oxygenation (median, 202 partial pressure of oxygen/% inhaled oxygen) and were transferred to the intensive care unit or died.138 Some factors in this study limit the establishment of the relationship between the high blood levels of vitamin B12 and intensive therapy or death resulting from COVID-19: 1) 9 patients (small sample size) were older than the 40 recovered patients by an average of 13 years (83.3 vs 70.2 years old, respectively); 2) the increase in vitamin B12 level did not interfere with Hcy values, which were similar between groups (11 μmol/L vs 9 μmol/L) and are within the normal range, 5–15 μmol/L139; and 3) the authors did not verify the values of other vitamin B12 biomarkers, such as MMA. Aging itself predisposes to lower resistance to viral infections.140
The mechanism by which blood excess vitamin B12 occurs in patients in the intensive care unit is not clear, but some factors that may partially explain it include: 1) the elevated plasma levels of the cobalamin-carrier proteins, the transcobalamins I and III; 2) elevated release of vitamin B12 from liver storage and decreased vitamin B12 hepatic clearance; and 3) decreased hepatic production of transcobalamin II with reduction of vitamin B12 peripheral tissues uptake, or reduced affinity of carrier proteins for vitamin B12.44,134,138
Transcobalamin II gene polymorphisms can decrease tissue distribution of cobalamin, even with high serum cobalamin levels.141 The 776GG homozygous variant of 776C>G polymorphism encodes a transcobalamin 2 with a lower binding affinity to vitamin B12, whereas polymorphisms in the FUT 6 gene (ie, rs708686, rs78060698, rs3760775, and rs7788053) elevate vitamin B12 status.142 It is interesting to note that polymorphisms in the ATP-binding cassette subfamily D member 4 protein will affect the transporting vitamin B12 out of lysosomes, and thus intracellular processing of vitamin B12, which may increase serum levels of vitamin B12.142
Several conditions may also result in higher vitamin B12 concentrations in critically ill patients, especially in older adults, such as preexisting diseases (eg, renal failure, hepatic diseases, cancer, Alzheimer’s disease), nutritional status, and inflammatory status (eg, sepsis).44,135 Acute uncontrolled systemic inflammation in severe COVID-19 induces sepsis and multiple organ failure, and can lead to an elevation of vitamin B12 level due to higher levels of transcobalamins I and II, their receptors, and unsaturated B12 binding capacity in the blood.141,143 The inactive form of vitamin B12 bound to transcobalamin I is the main factor that elevates blood vitamin B12 levels.84 However, the mechanisms by which excess vitamin B12 is associated with sepsis remain poorly elucidated and, in these cases, supplementation with vitamin B12 should be individually evaluated considering the aforementioned metabolic and genetic factors.
Patients with severe COVID-19 have elevated levels of high mobility group box 1,144 a potential biomarker of sepsis that is modulated by NFκB.141 The active form of this vitamin in patients with functional transcobalamin II and normal B12 cell metabolism can inhibit production of this biomarker by indirect mechanisms, that is, through downregulation of NFκB levels and increased acetylcholine synthesis, which positively modulates the neuro-immune cholinergic anti-inflammatory pathway.141
Some interventional clinical trials on the effects of vitamin B12 supplementation in combination with other micronutrients and/or medications in cases of COVID-19 are currently being recorded in the International Clinical Trials Registry Platform and ClinicalTrials.gov databases registration numbers NCT04395768 (500 μg methylcobalamin orally, daily, for 14 days), NCT04751669 (9.6 mg cyanocobalamin orally, once a day, for 14 days), and NCT04828538 (1 mg daily oral B12 supplementation for up to 60 days). In addition, 2 registered observational clinical studies will investigate the B12 levels in patients positive for COVID-19 (age range, 21–60 years; Clinical Trials Registry India identification number CTRI/2021/02/030946) and in pregnant women positive for COVID-19 (ClinicalTrials.gov registration number NCT04407572).
However, more RCTs of vitamin B12 supplementation and analysis of various markers (eg, total B12, holotranscobalamin, total Hcy and MMA, total folic acid, and if possible, polymorphism and/or methylation of genes) are needed to precisely identify the status of this micronutrient (before and after) in people with or without COVID-19 and thus facilitate the proper choice of vitamin B12 form to be administered in treatment.
CONCLUSIONS
The evaluation of parameters that determine the deficiency or subclinical levels of vitamin B12 deficiency can be an ally in treating patients affected by COVID-19 or in persistent symptoms of the disease, given the important functions of this vitamin in the skeletal muscle–gut–brain axis.
Vitamin B12 plays an important role in viral infections. The consumption of a healthy diet containing vitamin B12 sources, and especially supplementation with methylcobalamin and cyanocobalamin, are promising alternatives as adjuvants in the treatment of COVID-19, especially in patients with B12 deficiency or deficiency risk. However, establishing doses, intervention times, and mechanisms of action of vitamin B12 against COVID-19 can be a great challenge.
Researchers are encouraged to identify whether the subclinical deficiency or deficiency itself of this vitamin is a risk factor for COVID-19 complications, and it is necessary to carry out intervention studies with vitamin B12 supplementation in both the adjuvant treatment of mild, moderate, and severe COVID-19 and post–COVID-19, with a focus on minimizing symptoms related to the muscle–gut–brain axis.
Acknowledgments
Figure 1 was created with BioRender.com. The authors thank Christopher Quinn from English Consulting Brazil for the English revision.
Author contributions. K.S.B., V.M.C. P.A.F.L., O.G.-Q., and J.S.A. contributed to study conception and data collection. K.S.B., O.G.-Q., R.M.-C., A.E.T., L.P.C., M.E.B.S.Q., S.M.A., and J.S.A. contributed to the investigation, establishing methodology, and writing the original draft of the manuscript. K.S.B., S.M.A., O.G.-Q., and J.S.A. supervised the project and writing, review, and editing of the manuscript.
Funding. This work was supported by the Improvement of Higher Education Personnel Coordination (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil (grant number 0001 to K.S.B.).
Declaration of interest. The authors declare no conflict of interest regarding the publication of this paper.
References
- 1. Cucinotta D, Vanelli M.. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong SH, Lui RN, Sung JJ.. COVID-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744–748. [DOI] [PubMed] [Google Scholar]
- 3. Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berger JR. COVID-19 and the nervous system. J Neurovirol. 2020;26:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ali AM, Kunugi H.. Skeletal muscle damage in COVID-19: a call for action. Medicina (Kaunas). 2021;57:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bahouth S, Chuang K, Olson L, et al. COVID-19 related muscle denervation atrophy. Skeletal Radiol. 2021;50:1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Callard F, Perego E.. How and why patients made long COVID. Soc Sci Med. 2021;268:113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenhalgh T, Knight M, A'Court C, et al. Management of post-acute COVID-19 in primary care. BMJ. 2020;370:m3026. [DOI] [PubMed] [Google Scholar]
- 10. Wu Y, Zhang L, Li S, et al. Associations of dietary vitamin B1, vitamin B2, vitamin B6, and vitamin B12 with the risk of depression: a systematic review and meta-analysis. Nutr Rev. 2021;29:nuab014. doi:10.1093/nutrit/nuab014. [DOI] [PubMed] [Google Scholar]
- 11. Gille D, Schmid A.. Vitamin B12 in meat and dairy products. Nutr Rev. 2015;73:106–115. [DOI] [PubMed] [Google Scholar]
- 12. Cryan JF, O'Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. [DOI] [PubMed] [Google Scholar]
- 13. Przewłócka K, Folwarski M, Kaźmierczak-Siedlecka K, et al. Gut-muscle axis exists and may affect skeletal muscle adaptation to training. Nutrients. 2020;12:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schlegel P, Novotny M, Klimova B, et al. “Muscle-gut-brain axis”: can physical activity help patients with Alzheimer’s disease due to microbiome modulation? J Alzheimers Dis. 2019;71:861–878. [DOI] [PubMed] [Google Scholar]
- 15. Dao TL, Hoang VT, Gautret P.. Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: a narrative review. Eur J Clin Microbiol Infect Dis. 2021;40:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Araújo Morais AH, Aquino JS, Da Silva-Maia JK, et al. Nutritional status, diet and viral respiratory infections: perspectives for severe acute respiratory syndrome coronavirus 2. Br J Nutr. 2021;125:851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan CW, Ho LP, Kalimuddin S, et al. Cohort study to evaluate effect of vitamin D, magnesium, and vitamin B12 in combination on severe outcome progression in older patients with coronavirus (COVID-19). Nutrition. 2020;79-80:111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moscatelli F, Sessa F, Valenzano A, et al. COVID-19: role of nutrition and supplementation. Nutrients. 2021;13:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. Vitamin B12. In: Vitamin and Mineral Requirements in Human Nutrition: Report of a Joint FAO/WHO Expert Consultation. 2nd ed. Bangkok, Thailand: World Health Organization; 1998:279–288. Available at: https://apps.who.int/iris/handle/10665/42716. Accessed August 20, 2021. [Google Scholar]
- 20. Tamura J, Kubota K, Murakami H, et al. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol. 1999;116:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshii K, Hosomi K, Sawane K, et al. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr. 2019;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolffenbuttel BHR, Wouters HJCM, Heiner-Fokkema MR, et al. The many faces of cobalamin (vitamin B12) deficiency. Mayo Clin Proc Innov Qual Outcomes. 2019;3:200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang H, Li L, Quin LL, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst Rev. 2018;2018:CD004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tee LY, Alhamid SM, Tan JL, et al. COVID-19 and undiagnosed pre-diabetes or diabetes mellitus among international migrant workers in Singapore. Front Public Health. 2020;8:584249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kulkarni R, Rajput U, Dawre R, et al. Severe malnutrition and anemia are associated with severe COVID in infants. J Trop Pediatr. 2021;67:fmaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Degnan PH, Taga ME, Goodman AL.. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014;20:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kandeel M, Al-Nazawi M.. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020;251:117627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stabler SP, Allen RH.. Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr. 2004;24:299–326. [DOI] [PubMed] [Google Scholar]
- 29. Shipton MJ, Thachil J.. Vitamin B12 deficiency - a 21st century perspective. Clin Med. 2015;15:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr. 2009;89:693S–696S. [DOI] [PubMed] [Google Scholar]
- 31. Allen LH, Miller JW, De Groot L, et al. Biomarkers of Nutrition for Development (BOND): vitamin B-12 review. J Nutr. 2018;148:1995S–2027S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rizzo G, Laganà AS, Rapisarda AMC, et al. Vitamin B12 among vegetarians: status, assessment and supplementation. Nutrients. 2016;8:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Leary F, Samman S.. Vitamin B12 in health and disease. Nutrients. 2010;2:299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wee AKH. COVID-19’s toll on the elderly and those with diabetes mellitus – is vitamin B12 deficiency an accomplice? Med Hypotheses. 2021;146:110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Young V, Garza C. Institute of Medicine (US). Vitamin B12. In: Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press (US); 1998:306–356. Available at: https://www.ncbi.nlm.nih.gov/books/NBK114302/. Accessed August 20, 2021. [PubMed] [Google Scholar]
- 36. Antony AC. Vegetarianism and vitamin B-12 (cobalamin) deficiency. Am J Clin Nutr. 2003;78:3–6. [DOI] [PubMed] [Google Scholar]
- 37. Bito T, Tanioka Y, Watanabe F.. Characterization of vitamin B12 compounds from marine foods. Fish Sci. 2018;84:747–755. [Google Scholar]
- 38. Madry E, Lisowska A, Grebowiec P, et al. The impact of vegan diet on B-12 status in healthy omnivores: five-year prospective study. Acta Sci Pol Technol Aliment. 2012;11:209–212. [PubMed] [Google Scholar]
- 39. Watanabe F, Yabuta Y, Tanioka Y, et al. Biologically active vitamin B12 compounds in foods for preventing deficiency among vegetarians and elderly subjects. J Agric Food Chem. 2013;61:6769–6775. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe F, Yabuta Y, Bito T, et al. Vitamin B12-containing plant food sources for vegetarians. Nutrients. 2014;6:1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bito T, Okumura E, Fujishima M, et al. Potential of Chlorella as a dietary supplement to promote human health. Nutrients. 2020;12:2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Madhubalaji CK, Rashmi V, Chauhan VS, et al. Improvement of vitamin B12 status with Spirulina supplementation in Wistar rats validated through functional and circulatory markers. J Food Biochem. 2019;43:e13038. [DOI] [PubMed] [Google Scholar]
- 43. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. [DOI] [PubMed] [Google Scholar]
- 44. Romain M, Sviri S, Linton DM, et al. The role of vitamin B12 in the critically ill - a review. Anaesth Intensive Care. 2016;44:447–452. [DOI] [PubMed] [Google Scholar]
- 45. Rizzo G, Laganà AS.. A review of vitamin B12. In: Patel VB, ed. Molecular Nutrition: Vitamins. London, United Kingdom: Academic Press; 2019:105–129. doi: 10.1016/B978-0-12-811907-5.00005-1. [DOI] [Google Scholar]
- 46. Green R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood. 2017;129:2603–2611. [DOI] [PubMed] [Google Scholar]
- 47. Lurz E. Vitamin B12; absorption, metabolism, and deficiency. In: Kuipers EJ, ed. Encyclopedia of Gastroenterology. 2nd ed. London, United Kingdom: Academic Press; 2020:727–733. doi: 10.1016/B978-0-12-801238-3.11357-1 [DOI] [Google Scholar]
- 48. Manzanares W, Hardy G.. Vitamin B12: the forgotten micronutrient for critical care. Curr Opin Clin Nutr Metab Care. 2010;13:662–668. [DOI] [PubMed] [Google Scholar]
- 49. Mikkelsen K, Stojanovska L, Prakash M, et al. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas. 2017;96:58–71. [DOI] [PubMed] [Google Scholar]
- 50. Herrmann W, Obeid R.. Cobalamin deficiency. In: Stanger OH, ed. Water Soluble Vitamins. Subcellular Biochemistry. vol 56. Dordrecht, Netherlands: Springer; 2012:301–322. [DOI] [PubMed] [Google Scholar]
- 51. Hughes CF, McNulty H.. Assessing biomarker status of vitamin B12 in the laboratory: no simple solution. Ann Clin Biochem. 2018;55:188–189. [DOI] [PubMed] [Google Scholar]
- 52. Bates CJ, Schneede J, Mishra G, et al. Relationship between methylmalonic acid, homocysteine, vitamins B12 intake and status and socio-economic indices, in a subset of participants in the British National Diet and Nutrition Survey of people aged 65y and over. Eur J Clin Nutr. 2003;57:349–357. [DOI] [PubMed] [Google Scholar]
- 53. Herrmann W. Vitamin B12 deficiency in vegetarians. In: Mariotti F, ed. Vegetarian and Plant-Based Diets in Health and Disease Prevention. London, United Kingdom: Academic Press; 2017:791–808. doi:10.1016/B978-0-12-803968-7.00043-5. [Google Scholar]
- 54. Herrmann W, Obeid R, Schorr H, et al. Enhanced bone metabolism in vegetarians - the role of vitamin B12 deficiency. Clin Chem Lab Med. 2009;47:1381–1387. [DOI] [PubMed] [Google Scholar]
- 55. Pawlak R, Lester SE, Babatunde T.. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J Clin Nutr. 2014;68:541–548. [DOI] [PubMed] [Google Scholar]
- 56. Allegra A, Tonacci A, Pioggia G, et al. Vitamin deficiency as risk factor for SARS-CoV-2 infection: correlation with susceptibility and prognosis. Eur Rev Med Pharmacol Sci. 2020;24:972–9738. [DOI] [PubMed] [Google Scholar]
- 57. Tal S, Shavit Y, Stern F, et al. Association between vitamin B12 levels and mortality in hospitalized older adults. J Am Geriatr Soc. 2010;58:523–526. [DOI] [PubMed] [Google Scholar]
- 58. Murthy VL, Koupenova M, Shah RV.. ACEing COVID-19 a role for angiotensin axis inhibition in SARS-CoV-2 infection? Circ Res. 2020;126:1682–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Green R, Datta Mitra A.. Megaloblastic anemias: nutritional and other causes. Med Clin North Am. 2017;101:297–317. [DOI] [PubMed] [Google Scholar]
- 60. Grangé S, Bekri S, Artaud-Macari E, et al. Adult-onset renal thrombotic microangiopathy and pulmonary arterial hypertension in cobalamin C deficiency. Lancet. 2015;386:1011–1012. [DOI] [PubMed] [Google Scholar]
- 61. Sabry W, Elemary M, Burnouf T, et al. Vitamin B12 deficiency and metabolism-mediated thrombotic microangiopathy (MM-TMA). Transfus Apher Sci. 2020;59:102717. [DOI] [PubMed] [Google Scholar]
- 62. Sender R, Fuchs S, Milo R.. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. [DOI] [PubMed] [Google Scholar]
- 63. Lurz E, Horne RG, Määttänen P, et al. Vitamin B12 deficiency alters the gut microbiota in a murine model of colitis. Front Nutr. 2020;7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Allen JM, Mailing LJ, Niemiro GM, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50:747–757. [DOI] [PubMed] [Google Scholar]
- 65. Turnbaugh P, Backhed F, Fulton L, et al. Marked alterations in the distal gut microbiome linked to diet-induced obesity. Cell Host Microbe. 2008;3:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. [DOI] [PubMed] [Google Scholar]
- 67. Lustgarten MS. The role of the gut microbiome on skeletal muscle mass and physical function: 2019 update. Front Physiol. 2019;10:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ticinesi A, Lauretani F, Milani C, et al. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: is there a gut–muscle axis? Nutrients. 2017;9:1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roth JR, Lawrence JG, Bobik TA.. Cobalamin (coenzyme B12): synthesis and biological significance. Annu Rev Microbiol. 1996;50:137–181. [DOI] [PubMed] [Google Scholar]
- 70. Troen AM. Folate and vitamin B12: function and importance in cognitive development. Nestle Nutr Inst Workshop Ser. 2012;70:161–171. [DOI] [PubMed] [Google Scholar]
- 71. Majumder A, Behera J, Jeremic N, et al. Hypermethylation: causes and consequences in skeletal muscle myopathy. J Cell Biochem. 2017;118:2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tamaddonfard E, Tamaddonfard S, Cheraghiyan S.. Effects of intracerebroventricular injection of vitamin B12 on formalin-induced muscle pain in rats: role of cyclooxygenase pathway and opioid receptors. Vet Res Forum. 2018;9:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy—a review. Nutrients. 2016;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Alfawaz H, Al-Onazi M, Bukhari SI, et al. The independent and combined effects of omega-3 and vitamin B12 in ameliorating propionic acid induced biochemical features in juvenile rats as rodent model of autism. J Mol Neurosci. 2018;66:403–413. [DOI] [PubMed] [Google Scholar]
- 75. Remacha AF, Montagud M, Cadafalch J, et al. Vitamin B12 transport proteins in patients with HIV-1 infection and AIDS. Haematologica. 1993;78:84–88. [PubMed] [Google Scholar]
- 76. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Muscogiuri G, Barrea L, Savastano S, et al. Nutritional recommendations for COVID-19 quarantine. Eur J Clin Nutr. 2020;74:850–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Martineau AR, Jolliffe DA, Greenberg L, et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019;23:1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Iwarson S, Lindberg J.. Coenzyme-B12 therapy in acute viral hepatitis. Scand J Infect Dis. 1977;9:157–158. [DOI] [PubMed] [Google Scholar]
- 80. Baldewicz TT, Goodkin K, Blaney NT, et al. Cobalamin level is related to self-reported and clinically rated mood and to syndromal depression in bereaved HIV-1+ and HIV-1- homosexual men. J Psychosom Res. 2000;48:177–185. [DOI] [PubMed] [Google Scholar]
- 81. Semeere AS, Nakanjako D, Ddungu H, et al. Sub-optimal vitamin B-12 levels among ART-naïve HIV-positive individuals in an urban cohort in Uganda. PLoS One. 2012;7:e40072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rocco A, Compare D, Coccoli P, et al. Vitamin B12 supplementation improves rates of sustained viral response in patients chronically infected with hepatitis C virus. Gut. 2013;62:766–773. [DOI] [PubMed] [Google Scholar]
- 83. Balfour L, Spaans JN, Fergusson D, et al. Micronutrient deficiency and treatment adherence in a randomized controlled trial of micronutrient supplementation in ART-naïve persons with HIV. PLoS One. 2014;9:e85607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sugihara T, Koda M, Okamoto T, et al. Falsely elevated serum vitamin B12 levels were associated with the severity and prognosis of chronic viral liver disease. Yonago Acta Med. 2017;60:31–39. [PMC free article] [PubMed] [Google Scholar]
- 85. Tenforde MW, Yadav A, Dowdy DW, et al. ; NWCS319 and ACTG 5175 study team. Vitamin A and D deficiencies associated with incident tuberculosis in HIV-infected patients initiating antiretroviral therapy in multinational case-cohort study. J Acquir Immune Defic Syndr. 2017;75:e71–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shivakoti R, Ewald ER, Gupte N, et al. ; NWCS 319 and ACTG PEARLS Study Team. Effect of baseline micronutrient and inflammation status on CD4 recovery post-cART initiation in the multinational PEARLS trial. Clin Nutr. 2019;38:1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Williams AM, Ladva CN, Leon JS, et al. Changes in micronutrient and inflammation serum biomarker concentrations after a norovirus human challenge. Am J Clin Nutr. 2019;110:1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jutzeler CR, Bourguignon L, Weis CV, et al. Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;37:101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Figliozzi S, Masci PG, Ahmadi N, et al. Predictors of adverse prognosis in COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020;50:e13362. [DOI] [PubMed] [Google Scholar]
- 90. Mahumud RA, Kamara JK, Renzaho AMN.. The epidemiological burden and overall distribution of chronic comorbidities in coronavirus disease-2019 among 202,005 infected patients: evidence from a systematic review and meta-analysis. Infection. 2020;48:813–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang P, He Z, Yu G, et al. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin Nutr. 2021;40:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Abu-Rumeileh S, Abdelhak A, Foschi M, et al. Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2021;268:1133–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Andalib S, Biller J, Di Napoli M, et al. Peripheral nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep. 2021;21:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Raahimi MM, Kane A, Moore CE, et al. Late onset of Guillain-Barré syndrome following SARS-CoV-2 infection: part of “long COVID-19 syndrome.” BMJ Case Rep. 2021;14:E240178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Havervall S, Rosell A, Phillipson M, et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325:2015–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kamal M, Abo Omirah M, Hussein A, et al. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. 2021;75:e13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ortelli P, Ferrazzoli D, Sebastianelli L, et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: insights into a challenging symptom. J Neurol Sci. 2021;420:117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Letizia Mauro G, Martorana U, Cataldo P, et al. Vitamin B12 in low back pain: a randomised, double-blind, placebo-controlled study. Eur Rev Med Pharmacol Sci. 2000;4:53–58. [PubMed] [Google Scholar]
- 100. Sun Y, Lai M-S, Lu C-J.. Effectiveness of vitamin B12 on diabetic neuropathy: systematic review of clinical controlled trials. Acta Neurol Taiwan. 2005;14:48–54. [PubMed] [Google Scholar]
- 101. Vidal-Alaball J, Butler C, Cannings-John R, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst Rev. 2005;CD004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Butler CC, Vidal-Alaball J, Cannings-John R, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. Fam Pract. 2006;23:279–285. [DOI] [PubMed] [Google Scholar]
- 103. Talaei A, Siavash M, Majidi H, et al. Vitamin B12 may be more effective than nortriptyline in improving painful diabetic neuropathy. Int J Food Sci Nutr. 2009;60:71–76. [DOI] [PubMed] [Google Scholar]
- 104. Volkov I, Rudoy I, Freud T, et al. Effectiveness of vitamin B12 in treating recurrent aphthous stomatitis: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Med. 2009;22:9–16. [DOI] [PubMed] [Google Scholar]
- 105. Syed EU, Wasay M, Awan S.. Vitamin B12 supplementation in treating major depressive disorder: a randomized controlled trial. Open Neurol J. 2013;7:44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Taneja S, Strand TA, Kumar T, et al. Folic acid and vitamin B-12 supplementation and common infections in 6-30-mo-old children in India: a randomized placebo-controlled trial. Am J Clin Nutr. 2013;98:731–737. [DOI] [PubMed] [Google Scholar]
- 107. Almeida OP, Ford AH, Flicker L.. Systematic review and meta-analysis of randomized placebo-controlled trials of folate and vitamin B12 for depression. Int Psychogeriatr. 2015;27:727–737. [DOI] [PubMed] [Google Scholar]
- 108. Scholten AM, Vermeulen E, Dhonukshe-Rutten RAM, et al. Surplus vitamin B12 use does not reduce fatigue in patients with irritable bowel syndrome or inflammatory bowel disease: a randomized double-blind placebo-controlled trial. Clin Nutr ESPEN. 2018;23:48–53. [DOI] [PubMed] [Google Scholar]
- 109. Wang JY, Wu YH, Liu SJ, et al. Vitamin B12 for herpetic neuralgia: a meta-analysis of randomised controlled trials. Complement Ther Med. 2018;41:277–282. [DOI] [PubMed] [Google Scholar]
- 110. Didangelos T, Karlafti E, Kotzakioulafi E, et al. Vitamin B12 supplementation in diabetic neuropathy: a 1-year, randomized, double-blind, placebo-controlled trial. Nutrients. 2021;13:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Markun S, Gravestock I, Jäger L, et al. Effects of vitamin B12 supplementation on cognitive function, depressive symptoms, and fatigue: a systematic review, meta-analysis, and meta-regression. Nutrients. 2021;13:923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stein J, Geisel J, Obeid R.. Association between neuropathy and B-vitamins: a systematic review and meta-analysis. Eur J Neurol. 2021;28:2054–2064. [DOI] [PubMed] [Google Scholar]
- 113. Paul C, Brady DM.. Comparative bioavailability and utilization of particular forms of B12 supplements with potential to mitigate B12-related genetic polymorphisms. Integr Med (Encinitas). 2017;16:42–49. [PMC free article] [PubMed] [Google Scholar]
- 114. Obeid R, Fedosov SN, Nexo E.. Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency. Mol Nutr Food Res. 2015;59:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Huemer M, Diodato D, Schwahn B, et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J Inherit Metab Dis. 2017;40:21–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fettelschoss V, Burda P, Sagné C, et al. Clinical or ATPase domain mutations in ABCD4 disrupt the interaction between the vitamin B12-trafficking proteins ABCD4 and LMBD1. J Biol Chem. 2017;292:11980–11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ponti G, Maccaferri M, Ruini C, et al. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Naseri M, Sarvari G-R, Esmaeeli M, et al. High doses of oral folate and sublingual vitamin B12 in dialysis patients with hyperhomocysteinemia. J Renal Inj Prev. 2016;5:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Guest J, Bilgin A, Hokin B, et al. Novel relationships between B12, folate and markers of inflammation, oxidative stress and NAD(H) levels, systemically and in the CNS of a healthy human cohort. Nutr Neurosci. 2015;18:355–364. [DOI] [PubMed] [Google Scholar]
- 120. Jang WS, Kim J, Baek J, et al. Clinical course of COVID-19 patients treated with ECMO: a multicenter study in Daegu, South Korea. Hear Lung. 2021;50:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. van de Lagemaat EE, de Groot L, van den Heuvel EGHM.. Vitamin B12 in relation to oxidative stress: a systematic review. Nutrients. 2019;11:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Siddiqua TJ, Ahmad SM, Ahsan KB, et al. Vitamin B12 supplementation during pregnancy and postpartum improves B12 status of both mothers and infants but vaccine response in mothers only: a randomized clinical trial in Bangladesh. Eur J Nutr. 2016;55:281–293. [DOI] [PubMed] [Google Scholar]
- 123. McMillan DC, Maguire D, Talwar D.. Relationship between nutritional status and the systemic inflammatory response: micronutrients. Proc Nutr Soc. 2019;78:56–67. [DOI] [PubMed] [Google Scholar]
- 124. Dai W, Zhang B, Jiang XM, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Narayanan N, Nair DT.. Vitamin B12 may inhibit RNA-dependent-RNA polymerase activity of nsp12 from the SARS-CoV-2 virus. IUBMB Life. 2020;72:2112–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Narayanan N, Nair DT.. Ritonavir may inhibit exoribonuclease activity of nsp14 from the SARS-CoV-2 virus and potentiate the activity of chain terminating drugs. Int J Biol Macromol. 2021;168:272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kaur H, Sarma P, Bhattacharyya A, et al. Folic acid as placebo in controlled clinical trials of hydroxychloroquine prophylaxis in COVID-19: is it scientifically justifiable? Med Hypotheses. 2021;149:110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li T, Yu B, Liu Z, et al. Homocysteine directly interacts and activates the angiotensin II type I receptor to aggravate vascular injury. Nat Commun. 2018;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Singh Y, Gupta G, Kazmi I, et al. SARS CoV-2 aggravates cellular metabolism mediated complications in COVID-19 infection. Dermatol Ther. 2020;33:11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wolffenbuttel BHR, Heiner-Fokkema MR, Green R, et al. Relationship between serum B12 concentrations and mortality: experience in NHANES. BMC Med. 2020;18:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ersöz A, Yılmaz TE.. The association between micronutrient and hemogram values and prognostic factors in COVID-19 patients: a single-center experience from Turkey. Int J Clin Pract. 2021;75:e14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sviri S, Khalaila R, Daher S, et al. Increased vitamin B12 levels are associated with mortality in critically ill medical patients. Clin Nutr. 2012;31:53–59. [DOI] [PubMed] [Google Scholar]
- 133. Cappello S, Cereda E, Rondanelli M, et al. Elevated plasma vitamin B12 concentrations are independent predictors of in-hospital mortality in adult patients at nutritional risk. Nutrients. 2016;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Flores-Guerrero JL, Minović I, Groothof D, et al. Association of plasma concentration of vitamin B12 with all-cause mortality in the general population in the Netherlands. JAMA Netw Open. 2020;3:e1919274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Andrès E, Serraj K, Zhu J, et al. The pathophysiology of elevated vitamin B12 in clinical practice. QJM. 2013;106:505–515. [DOI] [PubMed] [Google Scholar]
- 136. Hinkel J, Schmitt J, Wurm M, et al. Elevated plasma vitamin B12 in patients with hepatic glycogen storage diseases. J Clin Med. 2020;9:2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Arendt JFH, Sørensen HT, Horsfall LJ, et al. Elevated vitamin B12 levels and cancer risk in UK primary care: a thin database cohort study. Cancer Epidemiol Biomarkers Prev. 2019;28:814–821. [DOI] [PubMed] [Google Scholar]
- 138. Dalbeni A, Bevilacqua M, Teani I, et al. Excessive vitamin B12 and poor outcome in COVID-19 pneumonia. Nutr Metab Cardiovasc Dis. 2021;31:774–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tinelli CD, Pino A, Ficulle E, et al. Hyperhomocysteinemia as a risk factor and potential nutraceutical target for certain pathologies. Front Nutr. 2019;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nehme J, Borghesan M, Mackedenski S, et al. Cellular senescence as a potential mediator of COVID-19 severity in the elderly. Aging Cell. 2020;19:e13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wheatley C. A scarlet pimpernel for the resolution of inflammation? The role of supra-therapeutic doses of cobalamin, in the treatment of systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic or traumatic shock. Med Hypotheses. 2006;67:124–142. [DOI] [PubMed] [Google Scholar]
- 142. Surendran S, Adaikalakoteswari A, Saravanan P, et al. An update on vitamin B12-related gene polymorphisms and B12 status. Genes Nutr. 2018;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zafer MM, El-Mahallawy HA, Ashour HM.. Severe COVID-19 and sepsis: immune pathogenesis and laboratory markers. Microorganisms. 2021;9:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Chen R, Huang Y, Quan J, et al. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon 2020;6:e05672. [DOI] [PMC free article] [PubMed] [Google Scholar]