ABSTRACT
Background
Vitamin D may have a role in immune responses to viral infections. However, data on the association between vitamin D and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and coronavirus disease 2019 (COVID-19) severity have been limited and inconsistent.
Objective
We examined the associations of predicted vitamin D status and intake with risk of SARS-CoV-2 infection and COVID-19 severity.
Methods
We used data from periodic surveys (May 2020 to March 2021) within the Nurses’ Health Study II. Among 39,315 participants, 1768 reported a positive test for SARS-CoV-2 infection. Usual vitamin D intake from foods and supplements were measured using a semiquantitative, pre-pandemic food-frequency questionnaire in 2015. Predicted 25-hydroxyvitamin D [25(OH)D] concentration were calculated based on a previously validated model including dietary and supplementary vitamin D intake, UV-B, and other behavioral predictors of vitamin D status.
Results
Higher predicted 25(OH)D concentrations, but not vitamin D intake, were associated with a lower risk of SARS-CoV-2 infection. Comparing participants in the highest quintile of predicted 25(OH)D concentrations with the lowest, the multivariable-adjusted OR was 0.76 (95% CI: 0.58, 0.99; P-trend = 0.04). Participants in the highest quartile of UV-B (OR: 0.76; 95% CI: 0.66, 0.87; P-trend = 0.002) and UV-A (OR: 0.76; 95% CI: 0.66, 0.88; P-trend < 0.001) also had a lower risk of SARS-CoV-2 infection compared with the lowest. High intake of vitamin D from supplements (≥400 IU/d) was associated with a lower risk of hospitalization (OR: 0.51; 95% CI: 0.29, 0.91; P-trend = 0.04).
Conclusions
Our study provides suggestive evidence on the association between higher predicted circulating 25(OH)D concentrations and a lower risk of SARS-CoV-2 infection. Greater intake of vitamin D supplements was associated with a lower risk of hospitalization. Our data also support an association between exposure to UV-B or UV-A, independently of vitamin D and SARS-CoV-2 infection, so results for predicted 25(OH)D need to be interpreted cautiously.
Keywords: vitamin D, SARS-CoV-2, COVID-19, diet, supplement, infection, severity, solar UV-B, UV-A, Robertson-Berger meter
See corresponding editorial on page 987.
Introduction
Infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 2019 (COVID-19) illnesses have spread globally, with more than 221 million confirmed cases and 4.5 million deaths as of 8 September 2021 (1). Identifying modifiable risk factors for SARS-CoV-2 infection and severity of COVID-19 remains vital. Given that vitamin D is important for immune function and has been shown to modulate host inflammatory responses to infection (2), it is plausible that vitamin D may play a protective role in the prevention and treatment of COVID-19 (3, 4). Risk factors or medical conditions associated with vitamin D deficiency, such as older age, Black race or Hispanic ethnicity, obesity, vascular comorbidities, and cancer, are also linked to increased risk of COVID-19 outcomes (5–7).
Recent data on the associations between vitamin D and the susceptibility to SARS-CoV-2 infection and COVID-19 severity have been limited and inconsistent. As a robust and reliable marker for vitamin D status, circulating 25-hydroxyvitamin D [25(OH)D] has been associated with SARS-CoV-2 infection and COVID-19 severity in some (8–14), but not all (15, 16), observational studies. For example, in a retrospective case-control study, hospitalized patients with COVID-19 had lower 25(OH)D concentrations compared with population-based controls, but no significant association between vitamin D deficiency and a composite outcome of COVID-19 severity (admission to the intensive care unit, requirements for mechanical ventilation, or mortality) was observed (15). Furthermore, studies evaluating the association between vitamin D supplementation and SARS-CoV-2 infection and COVID-19 severity have yielded mixed results (17–24). To our knowledge, very few studies have examined the potential impact of habitual vitamin D intake from foods and supplements. In a recent review, the UK National Institute for Health and Care Excellence concluded that sufficient evidence was still lacking to support vitamin D supplementation with the aim of preventing or treating COVID-19 and called for more research on the topic (25, 26). In light of these gaps, we examined associations between predicted vitamin D status and habitual vitamin D intake (e.g., from diet and supplements) in relation to risk of SARS-CoV-2 infection and COVID-19 severity within a large US cohort of female nurses.
Methods
Study population
For the current analysis, we used data from periodic surveys from May 2020 to March 2021 on participants’ experiences during the COVID-19 pandemic within the large, longitudinal cohort of registered nurses, Nurses’ Health Study II (NHS II) (27). The NHS II is an ongoing prospective cohort of 116,429 female nurses aged 25 to 42 y at study enrollment in 1989. Participants received questionnaires biennially in the mail that captured information on demographics, medical conditions, and lifestyle factors. Diet was assessed every 4 y. The follow-up rate was greater than 90% in each cycle. The study was approved as Protocol 2020P001020 of the Institutional Review Board of Brigham and Women's Hospital in Boston, Massachusetts, with voluntary survey completion representing participant consent.
In May 2020, we invited participants who returned the most recent main cohort questionnaire to complete a supplementary COVID-19 online survey. Of 55,925 invited participants, 39,564 (70.7%) completed the baseline COVID-19 survey (Supplemental Figure 1). They were subsequently administered monthly surveys while additional weekly surveys were administered to those who identified as frontline health care workers. Since August 2020, the scheduling of the surveys was changed to quarterly for all participants and monthly for frontline health care workers who physically worked or volunteered at a worksite providing clinical care. The end of follow-up for the current analysis was 23 March 2021.
Assessment of predicted 25(OH)D concentrations and vitamin D intake
Beginning in 1991 and every 4 y thereafter, participants completed a 131-item semiquantitative food-frequency questionnaire (28, 29). Women were asked to record how often they consumed a single serving of each food listed during the previous year, with possible response options ranging from less than once per month to 6 or more times per day. In addition, they reported the brand, formula, and dose of vitamin supplement use. Intake of vitamin D was calculated by multiplying the consumption frequency of each food or supplement by its nutrient content derived from composition values from USDA sources supplemented with other data, summing across all items. For the current analyses, we used diet data collected from the 2015 questionnaire.
We assessed predicted 25(OH)D concentrations by using a previously published and validated model (30–32). Briefly, the prediction model was developed in 1562 NHS II women who had directly measured plasma 25(OH)D concentrations. We built a linear regression model by including information on significant predictors for plasma 25(OH)D concentration. These include dietary and supplementary vitamin D intake, race, average annual UV-B radiation intensity based on state of residency (Supplemental Figure 2), postmenopausal hormone use, BMI, physical activity, and alcohol intake. We adjusted for age, season of blood draw, and laboratory batch in the model, although they were not included in the derivation of predicted 25(OH)D status. The prediction model was validated in an independent sample of 445 women in the NHS II and has been associated with multiple disease outcomes in other populations (33–35). For women in the lowest decile of predicted 25(OH)D, the actual plasma concentration of 25(OH)D was 18 ng/mL and in those in the highest decile of predicted 25(OH)D the actual plasma concentration of 25(OH)D was 30 ng/mL (31).
Ascertainment of COVID-19 outcomes
Our primary outcomes were SARS-CoV-2 infection (positive test for infection or antibody) and COVID-19 severity. For severity, we developed a modified WHO clinical progression scale (36) where participants received a score of 0 if asymptomatic and not infected (including those who did not report having a test), 1 for tested positive and asymptomatic, 2 for tested positive and symptomatic (persistent cough, score throat, loss of taste, loss of smell, shortness of breath or difficulty breathing, fever, muscle aches, digestive symptoms, or other COVID-19–related symptoms), and 3 if hospitalized and tested positive.
Statistical analysis
Of the 39,564 participants who completed the baseline COVID-19 survey, we excluded 162 women who had missing information on predicted 25(OH)D or vitamin D intake and 87 who reported hospitalization but had not tested positive for SARS-CoV-2 infection, leaving 39,315 participants in the analysis. We used multivariable logistic regression models to examine the risk of COVID-19 outcomes by categories of predicted 25(OH)D concentrations, total vitamin D intake, as well as dietary and supplementary vitamin D intake separately. We adjusted for potential confounding factors in sequential models: model 1 included age, race, smoking pack-years, and the Alternate Healthy Eating Index-2010 as an overall metric of diet quality; model 2 was further adjusted for BMI, physical activity, and alcohol intake, which were components of the predicted vitamin D model; model 3 was further adjusted for being a frontline health care worker, chronic comorbidities (hypertension, hypercholesterolemia, diabetes, heart disease, cancer, and asthma) and 2010 Census tract median income as an indicator for socioeconomic status. In addition, dietary and supplementary vitamin D intakes were mutually adjusted for each other. Duration of vitamin D supplement use was evaluated in a secondary analysis.
Because UV-B radiation is the major source of vitamin D (37), in secondary analyses we evaluated the association between UV-B exposure and SARS-CoV-2 infection. UV-A has also been associated with COVID-19 deaths previously; thus, we further analyzed the association between SARS-CoV-2 infection and county-level UV-A during winter times (averaged from 1 January to 30 April), which were less influenced by UV-B–induced vitamin D synthesis (38). We examined the possibly nonlinear relation nonparametrically with restricted cubic splines (39) and tested the nonlinearity using likelihood ratio test comparing the model with only the linear term to the model with the linear and the cubic spline terms. We conducted subgroup analyses by major risk factors for COVID-19, including age, race, BMI, being a frontline health care worker, and comorbidities as well as median level of UV-B and UV-A. We included cross-product terms of predicted 25(OH)D quintiles and these factors and assessed statistical significance of an interaction by using the likelihood ratio test where we compared the model with and without interaction terms.
In sensitivity analyses, we restricted the study population to those who had received a test for COVID-19. We also used inverse probability weighting (IPW) as a function of age, race, being a frontline health care worker, COVID-19–related symptoms, 2010 Census tract median income, and corresponding vitamin D–related variables to account for the likelihood of receiving a test with stabilized weight. Analyses were conducted using SAS version 9.4 (SAS Institute); P values < 0.05 were considered statistically significant.
Results
Descriptive characteristics of the 39,315 participants according to categories of predicted 25(OH)D concentrations, vitamin D intake from foods, and vitamin D intake from supplements are shown in Table 1. Women in the highest quintile of predicted 25(OH)D concentrations were more likely to be White, use postmenopausal hormones, reside in a state in the South and with higher UV-B exposure, be physically active, and have higher intake of alcohol and dietary and supplementary vitamin D. They also had a lower BMI and a lower likelihood of being a current smoker and having chronic comorbidities. In contrast, intakes of vitamin D from foods or supplements were not related to other clinical and lifestyle characteristics. However, when comparing those who did not take vitamin D supplements with those with supplementary vitamin D intake ≥2000 IU/d, supplement users had a higher prevalence of comorbidities such as hypercholesterolemia and asthma.
TABLE 1.
Characteristics of participants in the COVID-19 online survey within the Nurses’ Health Study II1
| Quintiles of predicted 25(OH)D concentrations (ng/mL) | |||||
|---|---|---|---|---|---|
| 1 (n = 7823) | 2 (n = 7836) | 3 (n = 7880) | 4 (n = 7883) | 5 (n = 7893) | |
| Median, ng/mL | 25.2 | 28.7 | 30.8 | 32.6 | 34.7 |
| Age, y | 60.8 (4.5) | 60.8 (4.6) | 60.8 (4.5) | 60.5 (4.5) | 60.5 (4.5) |
| White, % | 85.7 | 95.4 | 97.0 | 97.0 | 96.2 |
| Current postmenopausal hormone use, % | 5.7 | 10.5 | 12.7 | 19.1 | 35.9 |
| UV-B of state of residence | 126 (25.5) | 125 (25.1) | 125 (25.1) | 127 (25.3) | 133 (26.8) |
| Region, % | |||||
| West | 14.6 | 15.2 | 15.0 | 18.0 | 25.6 |
| South | 19.0 | 18.6 | 18.3 | 19.5 | 24.3 |
| Midwest | 34.2 | 33.5 | 32.3 | 31.0 | 27.0 |
| Northeast | 32.0 | 32.4 | 34.2 | 31.2 | 22.9 |
| Other | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 |
| 2010 Census tract median income, US dollars | 76,664 (28,327) | 79,995 (29,567) | 83,594 (31,418) | 86,482 (33,562) | 91,774 (36,163) |
| BMI, kg/m2 | 35.6 (6.5) | 29.7 (4.5) | 26.3 (3.5) | 24.3 (3) | 22.4 (2.5) |
| Physical activity, MET-h/wk | 13.9 (20.7) | 20.8 (24.1) | 26.9 (25.3) | 34.2 (31) | 47.6 (40.3) |
| Alcohol intake, g/d | 3.1 (8.1) | 5.2 (9.8) | 7.3 (11.7) | 9.3 (11.8) | 12.8 (12.4) |
| Total vitamin D intake, IU/d | 1107 (970) | 1180 (960) | 1219 (946) | 1249 (924) | 1406 (871) |
| Dietary vitamin D intake, IU/d | 170 (101) | 183 (108) | 190 (108) | 208 (116) | 228 (116) |
| Vitamin D intake from supplements, IU/d | 902 (910) | 963 (908) | 998 (896) | 1012 (870) | 1162 (8418) |
| Alternate Healthy Eating Index | 57.5 (12.0) | 59.9 (11.8) | 62.2 (11.7) | 63.9 (11.8) | 66.4 (11.3) |
| Past smoker, % | 30.0 | 30.2 | 30.9 | 32.0 | 32.9 |
| Current smoker, % | 3.9 | 4.3 | 3.9 | 3.4 | 2.2 |
| Smoking, pack-years | 5.6 (11.3) | 5.3 (11.0) | 4.9 (10.1) | 4.6 (9.5) | 4.0 (8.3) |
| Hypertension, % | 38.7 | 27.3 | 21.9 | 17.5 | 14.3 |
| Hypercholesterolemia, % | 32.9 | 31.5 | 27.7 | 24.5 | 20.9 |
| Diabetes, % | 13.1 | 6.3 | 3.0 | 2.2 | 1.2 |
| Heart disease, % | 0.6 | 0.4 | 0.2 | 0.3 | 0.2 |
| Cancer, % | 4.3 | 3.7 | 3.7 | 3.4 | 3.0 |
| Asthma, % | 20.2 | 17.4 | 14.3 | 11.9 | 10.9 |
| Frontline health care worker, % | 29.2 | 31.0 | 29.9 | 31.0 | 29.4 |
| Vitamin D intake from foods (IU/d) | |||||
| 0–99.9 (n = 6741) | 100–199.9 (n = 17,141) | 200–299.9 (n = 9723) | 300–399.9 (n = 3560) | ≥400 (n = 2150) | |
| Median, IU/d | 75.8 | 149 | 238 | 339 | 470 |
| Age, y | 60.6 (4.5) | 60.6 (4.5) | 60.7 (4.5) | 60.8 (4.6) | 61.0 (4.5) |
| White, % | 93.4 | 94.2 | 94.8 | 95.0 | 93.8 |
| Current postmenopausal hormone use, % | 16.6 | 16.8 | 17.3 | 15.7 | 17.3 |
| UV-B of state of residence | 129 (26.5) | 128 (25.8) | 127 (25.4) | 126 (25.2) | 125 (25.2) |
| Region, % | |||||
| West | 19.2 | 17.7 | 17.0 | 17.5 | 16.3 |
| South | 21.5 | 20.5 | 19.7 | 16.9 | 16.8 |
| Midwest | 30.8 | 31.3 | 31.2 | 34.1 | 34.8 |
| Northeast | 28.2 | 30.4 | 31.8 | 31.3 | 31.8 |
| Other | 0.3 | 0.2 | 0.3 | 0.3 | 0.4 |
| 2010 Census tract median income, US dollars | 82,867 (31,874) | 83,254 (32,041) | 84,928 (32,923) | 84,534 (33,353) | 83,072 (32,073) |
| BMI, kg/m2 | 27.6 (6.3) | 27.8 (6.3) | 27.7 (6.2) | 27.4 (6.1) | 27.3 (6.4) |
| Physical activity, MET-h/wk | 27.6 (30.4) | 28.5 (31.1) | 29.5 (31.7) | 29.9 (32.2) | 28.5 (33.1) |
| Alcohol intake, g/d | 9.0 (14.6) | 8.3 (11.5) | 6.9 (9.9) | 5.4 (8.2) | 4.1 (7.0) |
| Total vitamin D intake, IU/d | 1149 (971) | 1180 (927) | 1255 (919) | 1380 (932) | 1572 (943) |
| Dietary vitamin D intake, IU/d | 71.6 (20.9) | 149 (28.2) | 242 (27.9) | 343 (28.8) | 503 (106) |
| Vitamin D intake from supplements, IU/d | 1034 (912) | 1008 (891) | 982 (873) | 1010 (892) | 1032 (872) |
| Alternate Healthy Eating Index | 61.3 (13.2) | 62.2 (12.0) | 62.5 (11.8) | 61.7 (11.7) | 60.6 (11.8) |
| Past smoker, % | 32.2 | 31.6 | 31.0 | 29.4 | 29.1 |
| Current smoker, % | 4.7 | 3.6 | 3.1 | 2.9 | 2.4 |
| Smoking, pack-years | 5.7 (11.4) | 4.9 (10.0) | 4.6 (9.7) | 4.3 (9.5) | 4.1 (8.9) |
| Hypertension, % | 23.8 | 24.9 | 23.4 | 22.5 | 21.1 |
| Hypercholesterolemia, % | 26.6 | 28.0 | 27.4 | 27.1 | 26.9 |
| Diabetes, % | 5.0 | 5.3 | 5.2 | 4.9 | 5.0 |
| Heart disease, % | 0.4 | 0.3 | 0.3 | 0.4 | 0.5 |
| Cancer, % | 3.5 | 3.5 | 3.7 | 4.2 | 3.6 |
| Asthma, % | 16.0 | 14.6 | 15.0 | 13.9 | 15.9 |
| Frontline health care worker, % | 28.9 | 30.1 | 31.0 | 30.1 | 29.6 |
| Vitamin D intake from supplements (IU/d) | |||||
| 0 (n = 4398) | 0.1–399.9 (n = 8312) | 400–999.9 (n = 8666) | 1000–1999.9 (n = 10,044) | ≥2000 (n = 7895) | |
| Median, IU/d | 0 | 38 | 607 | 1400 | 2235 |
| Age, y | 59.9 (4.5) | 60.1 (4.6) | 60.9 (4.5) | 61.1 (4.5) | 60.9 (4.4) |
| White, % | 93.8 | 93.4 | 94.1 | 94.5 | 95.3 |
| Current postmenopausal hormone use, % | 13.7 | 14.7 | 16.7 | 16.7 | 20.9 |
| UV-B of state of residence | 128 (25.3) | 127 (25.6) | 127 (25.9) | 127 (25.4) | 128 (26.5) |
| Region, % | |||||
| West | 18.0 | 18.5 | 17.1 | 16.0 | 19.5 |
| South | 20.1 | 19.0 | 19.6 | 20.3 | 20.7 |
| Midwest | 31.4 | 31.9 | 32.3 | 31.5 | 30.9 |
| Northeast | 30.1 | 30.2 | 30.8 | 32.0 | 28.9 |
| Other | 0.4 | 0.4 | 0.2 | 0.2 | 0.1 |
| 2010 Census tract median income, US dollars | 82,913 (33,103) | 83,291 (32,379) | 82,951 (32,032) | 84,163 (32,396) | 84,773 (32,128) |
| BMI, kg/m2 | 27.9 (6.4) | 27.8 (6.3) | 27.5 (6.2) | 27.4 (6.2) | 27.9 (6.4) |
| Physical activity, MET-h/wk | 27.1 (30.4) | 28 (32.9) | 29.4 (31.3) | 29.6 (31.0) | 28.5 (30.6) |
| Alcohol intake, g/d | 7.8 (11.5) | 7.8 (11.7) | 7.5 (11.3) | 7.5 (11.2) | 7.2 (11.1) |
| Total vitamin D intake, IU/d | 184 (113) | 310 (180) | 906 (253) | 1646 (433) | 2621 (529) |
| Dietary vitamin D intake, IU/d | 188 (113) | 196 (107) | 200 (113) | 197 (114) | 191 (113) |
| Vitamin D intake from supplements, IU/d | 0 (0) | 114 (134) | 686 (205) | 1407 (368) | 2357 (395) |
| Alternate Healthy Eating Index | 59.7 (12.6) | 60.2 (12.2) | 62.1 (12.9) | 63.0 (11.8) | 63.8 (11.9) |
| Past smoker, % | 32.0 | 32.1 | 31.3 | 30.0 | 31.4 |
| Current smoker, % | 5.2 | 4.6 | 3.3 | 3.0 | 2.6 |
| Smoking, pack-years | 5.6 (11.2) | 5.3 (10.6) | 4.8 (10.1) | 4.5 (9.6) | 4.7 (9.7) |
| Hypertension, % | 22.0 | 22.9 | 25.7 | 24.0 | 24.1 |
| Hypercholesterolemia, % | 22.3 | 25.7 | 28.2 | 29.1 | 29.5 |
| Diabetes, % | 4.4 | 5.2 | 5.5 | 5.2 | 5.3 |
| Heart disease, % | 0.4 | 0.5 | 0.4 | 0.3 | 0.2 |
| Cancer, % | 3.2 | 3.3 | 3.7 | 3.9 | 3.7 |
| Asthma, % | 13.0 | 13.0 | 14.3 | 15.3 | 18.4 |
| Frontline healthcare worker, % | 31.0 | 31.1 | 29.4 | 29.5 | 29.8 |
Values are means (SD) or percentages standardized to the distribution of age, with the exception of age itself. COVID-19, coronavirus disease 2019; MET, metabolic equivalent of task; 25(OH)D, 25-hydroxyvitamin.
Vitamin D and SARS-CoV-2 infection
A total of 17,860 women reported having a test for COVID-19 and, among them, 1768 (9.9%) reported a positive test. Higher predicted 25(OH)D concentrations were associated with a lower risk of SARS-CoV-2 infection (Table 2). Compared with participants in the lowest quintile of predicted 25(OH)D concentrations, the multivariable-adjusted ORs and 95% CIs were 0.81 (0.68, 0.97), 0.80 (0.65, 0.99), 0.77 (0.61, 0.98), and 0.76 (0.58, 0.99) for those in the second to the highest quintile, respectively. The associations were slightly attenuated after further adjustment for residential region, with corresponding ORs (95% CIs) of 0.81 (0.68, 0.97), 0.80 (0.65, 0.99), 0.78 (0.61, 0.99), and 0.79 (0.60, 1.03) (data not shown).
TABLE 2.
Associations between predicted 25(OH)D concentrations, vitamin D intake from foods and supplements, and risk of SARS-CoV-2 infection1
| Predicted 25(OH)D concentrations, quintiles (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P-trend | |
| Median, ng/mL | 25.2 | 28.7 | 30.8 | 32.6 | 34.7 | |
| Cases/non-cases | 435/7388 | 368/7468 | 351/7529 | 321/7562 | 293/7600 | |
| Unadjusted | 1 (ref) | 0.84 (0.73, 0.96) | 0.79 (0.69, 0.91) | 0.72 (0.62, 0.84) | 0.65 (0.56, 0.76) | <0.001 |
| MV model 1 | 1 (ref) | 0.86 (0.74, 0.99) | 0.83 (0.71, 0.96) | 0.75 (0.65, 0.88) | 0.70 (0.60, 0.82) | <0.001 |
| MV model 2 | 1 (ref) | 0.81 (0.68, 0.97) | 0.80 (0.65, 0.98) | 0.77 (0.61, 0.97) | 0.75 (0.58, 0.98) | 0.04 |
| MV model 3 | 1 (ref) | 0.81 (0.68, 0.97) | 0.80 (0.65, 0.99) | 0.77 (0.61, 0.98) | 0.76 (0.58, 0.99) | 0.04 |
| Total vitamin D intake, quintiles (IU/d) | ||||||
| 1 | 2 | 3 | 4 | 5 | ||
| Median, IU/d | 154 | 520 | 1084 | 1725 | 2576 | |
| Cases/non-cases | 365/7497 | 365/7499 | 352/7504 | 329/7549 | 357/7498 | |
| Unadjusted | 1 (ref) | 1.00 (0.86, 1.16) | 0.96 (0.83, 1.12) | 0.90 (0.77, 1.04) | 0.98 (0.84, 1.14) | 0.44 |
| MV model 1 | 1 (ref) | 1.03 (0.89, 1.19) | 1.04 (0.89, 1.20) | 0.97 (0.83, 1.13) | 1.06 (0.91, 1.23) | 0.75 |
| MV model 2 | 1 (ref) | 1.04 (0.89, 1.20) | 1.05 (0.90, 1.22) | 0.97 (0.84, 1.14) | 1.05 (0.90, 1.22) | 0.87 |
| MV model 3 | 1 (ref) | 1.04 (0.90, 1.21) | 1.05 (0.90, 1.22) | 0.98 (0.84, 1.14) | 1.04 (0.90, 1.22) | 0.91 |
| Vitamin D intake from foods (IU/d) | ||||||
| 0–99.9 | 100–199.9 | 200–299.9 | 300–399.9 | ≥400 | ||
| Median, IU/d | 75.8 | 149 | 238 | 339 | 470 | |
| Cases/non-cases | 282/6459 | 787/16,354 | 458/9265 | 148/3412 | 93/2057 | |
| Unadjusted | 1 (ref) | 1.10 (0.96, 1.27) | 1.13 (0.97, 1.32) | 0.99 (0.81, 1.22) | 1.04 (0.81, 1.32) | 0.98 |
| MV model 1 | 1 (ref) | 1.11 (0.97, 1.28) | 1.15 (0.99, 1.34) | 1.01 (0.82, 1.23) | 1.04 (0.82, 1.33) | 0.93 |
| MV model 2 | 1 (ref) | 1.12 (0.97, 1.28) | 1.16 (0.99, 1.35) | 1.00 (0.82, 1.23) | 1.04 (0.81, 1.32) | 0.98 |
| MV model 3 | 1 (ref) | 1.11 (0.97, 1.28) | 1.15 (0.99, 1.34) | 1.01 (0.82, 1.24) | 1.04 (0.82, 1.32) | 0.95 |
| Vitamin D intake from supplements (IU/d) | ||||||
| 0 | 0.1–399.9 | 400–999.9 | 1000–1999.9 | ≥2000 | ||
| Median, IU/d | 0 | 38 | 607 | 1400 | 2235 | |
| Cases/non-cases | 213/4185 | 389/7923 | 380/8286 | 436/9608 | 350/7545 | |
| Unadjusted | 1 (ref) | 0.96 (0.81, 1.14) | 0.90 (0.76, 1.07) | 0.89 (0.75, 1.05) | 0.91 (0.77, 1.09) | 0.26 |
| MV model 1 | 1 (ref) | 0.97 (0.82, 1.15) | 0.96 (0.81, 1.14) | 0.96 (0.81, 1.14) | 0.99 (0.83, 1.18) | 0.98 |
| MV model 2 | 1 (ref) | 0.97 (0.82, 1.16) | 0.97 (0.81, 1.15) | 0.97 (0.82, 1.15) | 0.98 (0.82, 1.17) | 0.96 |
| MV model 3 | 1 (ref) | 0.98 (0.82, 1.16) | 0.97 (0.81, 1.15) | 0.97 (0.82, 1.15) | 0.98 (0.82, 1.17) | 0.93 |
Logistic regression models were used in the analysis. The number of participants included in the analysis was 39,315, and the number of participants who reported a positive SARS-CoV-2 infection was 1768. Model 1 was adjusted for age, White race, smoking pack-years (0, 0.1–10.0, 10.1–20.0, >20.0), and the Alternate Healthy Eating Index (quintiles). Vitamin D intakes from foods and supplements were mutually adjusted. Model 2 was further adjusted for BMI (kg/m2; <22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30–34.9, ≥35.0), physical activity (quintiles), and alcohol intake (0, 0.1–5.0, 5.1–10.0, >10 g/d). Model 3 was further adjusted for being a frontline health care worker; chronic comorbidities including hypertension, hypercholesterolemia, diabetes, heart disease, cancer, and asthma; and 2010 Census tract median income (quintiles). P-trend was evaluated using the median value in each category as a continuous variable. MV, multivariable; ref, reference; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; 25(OH)D, 25-hydroxyvitamin D.
Intakes of total vitamin D or vitamin D from foods were not associated with a risk of SARS-CoV-2 infection. Compared with participants in the lowest quintile, the multivariable OR (95% CI) for those in the highest quintile was 1.04 (0.90, 1.22; P-trend = 0.91) for total vitamin D intake and 1.04 (0.82, 1.32; P-trend = 0.95) for vitamin D intake from foods. Likewise, vitamin D intake from supplements was not significantly associated with SARS-CoV-2 infection (OR: 0.98 comparing supplement intake ≥2000 IU/d to non-use; 95% CI: 0.82, 1.17; P-trend = 0.93).
Findings for predicted 25(OH)D were slightly attenuated in sensitivity analyses. For example, compared with participants in the lowest quintile of predicted 25(OH)D, the multivariable-adjusted OR (95% CI) for those in the highest quintile was 0.78 (0.58, 1.04; P-trend = 0.13) when we used IPW to account for the possibility of receiving a test for COVID-19 based on information on age, race, being a frontline health care worker, onset of COVID-19 symptoms, 2010 Census tract median income, and corresponding vitamin D–related variables (Supplemental Table 1). Likewise, the extreme-quintile OR (95% CI) was 0.79 (0.60, 1.04; P-trend = 0.12) when restricting the study population to participants who had received a test for COVID-19 (Supplemental Table 2). Findings for vitamin D intake from foods or supplements remained similar in sensitivity analyses.
Vitamin D and COVID-19 severity
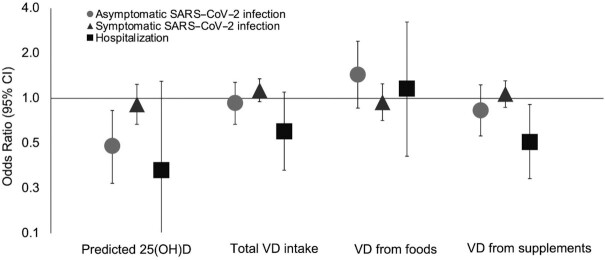
In crude analyses, higher predicted 25(OH)D concentrations were associated with a lower risk of COVID-19 severity, with the OR comparing the highest quintile with the lowest quintile of 0.64 (95% CI: 0.46, 0.88; P-trend = 0.004) for asymptomatic SARS-CoV-2 infection, 0.73 (95% CI: 0.61, 0.87; P-trend<0.001) for symptomatic SARS-CoV-2 infection, and 0.12 (95% CI: 0.05, 0.31; P-trend < 0.001; Supplemental Table 3) for COVID-19–related hospitalization. However, after we controlled for lifestyle factors, the associations with symptomatic SARS-CoV-2 infection (OR: 0.91; 95% CI: 0.67, 1.24; P-trend = 0.52) and hospitalization (OR: 0.33; 95% CI: 0.08, 1.30; P-trend = 0.15) were attenuated, and only the association with asymptomatic SARS-CoV-2 infection remained robust (OR: 0.48; 95% CI: 0.27, 0.83; P-trend = 0.01; Figure 1).
FIGURE 1.
Associations between predicted 25(OH)D concentrations, vitamin D intake from foods and supplements, and risk of COVID-19 severity. Logistic regression models were used in the analysis. The number of participants was 37,251 for those who were asymptomatic and did not test positive; 358 for asymptomatic and tested positive; 1321 for symptomatic and tested positive; and 89 for hospitalized and tested positive. Values represented the ORs of each severity outcome comparing the highest quintile with the lowest quintile of predicted 25(OH)D concentrations or total vitamin D intake, vitamin D intake from foods ≥400 vs. <100 IU/d, and vitamin D intake from supplements ≥2000 IU/d vs. non-use, respectively. Values for vitamin D from supplements and hospitalization are shown for ≥400 IU/d vs. non-use due to limited hospitalized cases. Models were adjusted for age (continuous, years); race (White/non-White); smoking pack-years (0, 0.1–10.0, 10.1–20.0, >20.0); BMI (in kg/m2; <22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30–34.9, ≥35.0); physical activity (quintiles); alcohol intake (0, 0.1–5.0, 5.1–10.0, >10 g/d); the Alternate Healthy Eating Index (quintiles); being a frontline health care worker; history of hypertension, hypercholesterolemia, diabetes, heart disease, cancer, and asthma (all yes/no); and 2010 Census tract median income (quintiles). COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VD, vitamin D; 25(OH)D, 25-hydroxyvitamin D.
Vitamin D intake from foods or supplements was not significantly associated with symptomatic SARS-CoV-2 infection. However, we observed that a greater intake of vitamin D from supplements was associated with a lower risk of hospitalization. The multivariable OR (95% CI) was 0.51 (0.29, 0.91; P-trend = 0.04) for participants consuming vitamin D from supplements ≥400 IU/d compared with non-use.
Secondary/subgroup analyses
We found that higher UV-B exposure was associated with a lower risk of SARS-CoV-2 infection (Table 3). Compared with participants living in low UV-B regions (range in the lowest quartile: 93–105), the multivariable OR for those living in regions with UV-B exposure in the ranges of 145–196 was 0.76 (95% CI: 0.66, 0.87; P-trend = 0.002). Participants in the highest quartile of UV-A also had a lower risk of SARS-CoV-2 infection compared with those in the lowest quartile (OR: 0.76; 95% CI: 0.66, 0.88; P-trend < 0.001). We further found that the relations between UV-B, UV-A, and SARS-CoV-2 infection might be not linear (P-nonlinearity = 0.002 and < 0.001, respectively; Supplemental Figure 3).
TABLE 3.
Associations between regional UV exposure and risk of SARS-CoV-2 infection1
| Quartiles | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | P-trend | |
| UV-B | |||||
| Median | 104 | 113 | 124 | 164 | |
| Cases/non-cases | 495/9142 | 434/10,150 | 428/7862 | 411/10,393 | |
| Unadjusted | 1 (ref) | 0.79 (0.69, 0.90) | 1.01 (0.88, 1.15) | 0.73 (0.64, 0.83) | <0.001 |
| MV model 1 | 1 (ref) | 0.79 (0.70, 0.91) | 0.97 (0.85, 1.11) | 0.76 (0.66, 0.87) | 0.001 |
| MV model 2 | 1 (ref) | 0.79 (0.69, 0.90) | 0.96 (0.84, 1.10) | 0.76 (0.66, 0.87) | 0.002 |
| MV model 3 | 1 (ref) | 0.79 (0.69, 0.90) | 0.96 (0.84, 1.10) | 0.76 (0.66, 0.87) | 0.002 |
| UV-A | |||||
| Median | 583 | 606 | 674 | 855 | |
| Cases/non-cases | 436/9079 | 503/9397 | 484/9139 | 325/9325 | |
| Unadjusted | 1 (ref) | 1.11 (0.98, 1.27) | 1.10 (0.97, 1.26) | 0.73 (0.63, 0.84) | <0.001 |
| MV model 1 | 1 (ref) | 1.12 (0.98, 1.27) | 1.08 (0.95, 1.24) | 0.76 (0.65, 0.88) | <0.001 |
| MV model 2 | 1 (ref) | 1.12 (0.98, 1.27) | 1.07 (0.94, 1.23) | 0.76 (0.65, 0.88) | <0.001 |
| MV model 3 | 1 (ref) | 1.10 (0.96, 1.26) | 1.07 (0.94, 1.22) | 0.76 (0.66, 0.88) | <0.001 |
Logistic regression models were used in the analysis. The number of participants included in the analysis was 39,315, and the number of participants who reported a positive SARS-CoV-2 infection was 1768. Model 1 was adjusted for age, White race, smoking pack-years (0, 0.1–10.0, 10.1–20.0, >20.0), and the Alternate Healthy Eating Index (quintiles). Model 2 was further adjusted for BMI (kg/m2; <22.5, 22.5–24.9, 25.0–27.4, 27. 5–29.9, 30–34.9, ≥35.0), physical activity (quintiles), and alcohol intake (0, 0.1–5.0, 5.1–10.0, >10 g/d). Model 3 was further adjusted for being a frontline health care worker; chronic comorbidities including hypertension, hypercholesterolemia, diabetes, heart disease, cancer, and asthma; and 2010 Census tract median income (quintiles). P-trend was evaluated using the median value in each category as a continuous variable. MV, multivariable; ref, reference; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; 25(OH)D, 25-hydroxyvitamin D.
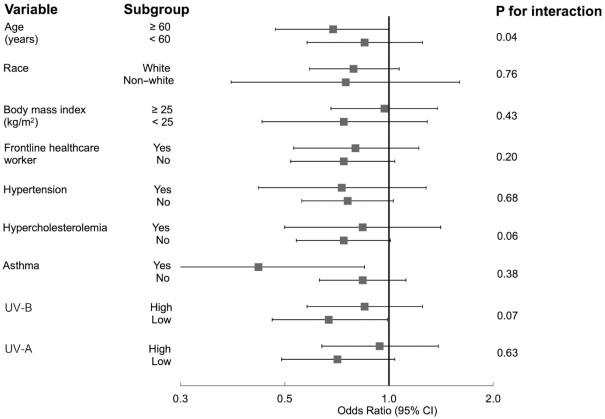
The inverse association between predicted 25(OH)D and risk of SARS-CoV-2 infection was slightly stronger among participants aged ≥ 60 y (OR comparing the highest quintile with the lowest: 0.69; 95% CI: 0.47, 1.00; P-trend = 0.07), compared with younger individuals (OR: 0.85; 95% CI: 0.58, 1.25; P-trend = 0.28, P-interaction = 0.04; Figure 2). Associations between predicted 25(OH)D and SARS-CoV-2 infection were not significantly modified by race; BMI; being a frontline health care worker; the presence of various comorbid conditions such as hypertension, hypercholesterolemia, and asthma; UV-A; or UV-B, although the inverse association between predicted 25(OH)D concentrations and risk of SARS-CoV-2 infection appeared stronger in participants residing in regions with low UV-B or UV-A.
FIGURE 2.
Associations between predicted 25(OH)D concentrations and risk of SARS-CoV-2 infection in subgroup analyses. Logistic regression models were used in the analysis. The number of participants/positive for SARS-CoV-2 infection in each subgroup was 23,867/934 for age ≥60 y and 15,448/834 for age <60 y; 37,062/1651 for White and 2253/117 for non-White; 23,557/1158 for BMI (kg/m2) ≥25.0 and 15,758/610 for BMI <25.0; 11,832/717 for frontline health care worker and 27,483/1051 for non–frontline health care workers; 9409/433 for hypertension and 29,906/1335 for non-hypertension; 10,801/497 for hypercholesterolemia and 28,514/1271 for non-hypercholesterolemia; 5867/272 for asthma and 33,448/1496 for non-asthma; 19,094/839 for UV-B above the median and 20,221/929 for UV-B below the median; 19,273/809 for UV-A above the median and 19,415/939 for UV-A below the median. Values represent ORs of SARS-CoV-2 infection comparing the highest quintile with the lowest quintile of predicted 25(OH)D concentrations. Models were adjusted for age (continuous, years); race (White/non-White); smoking pack-years (0, 0.1–10.0, 10.1–20.0, >20.0); BMI (<22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30–34.9, ≥35.0); physical activity (quintiles); alcohol intake (0, 0.1–5.0, 5.1–10.0, >10 g/d); the Alternate Healthy Eating Index (quintiles); being a frontline health care worker; history of hypertension, hypercholesterolemia, diabetes, heart disease, cancer, and asthma (all yes/no); and 2010 Census tract median income (quintiles). COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; 25(OH)D, 25-hydroxyvitamin D.
We also examined the duration of vitamin D supplement use in relation to the risk of COVID-19 severity (Supplemental Table 4). Current use of vitamin D supplements, regardless of the duration of use (<10 y or >10 y of use) was not significantly associated with symptomatic SARS-CoV-2 infection or hospitalization.
Discussion
Using data from periodic surveys administered from May 2020 to March 2021 within a cohort of 39,315 registered nurses, we found that higher pre-pandemic predicted 25(OH)D concentrations were associated with lower risk of SARS-CoV-2 infection. This association was independent of lifestyle determinants of circulating 25(OH)D concentrations and was possibly driven by UV-B exposure. On the other hand, predicted 25(OH)D concentrations were not significantly associated with symptomatic SARS-CoV-2 infection or hospitalization when conditioned on clinical and lifestyle factors associated with the severity of the disease. We found a protective association between vitamin D intake from supplements and risk of hospitalization due to COVID-19.
Prior reports on circulating 25(OH)D and SARS-CoV-2 infection and COVID-19 severity have yielded mixed results. While several studies suggested that low 25(OH)D concentrations were associated with higher risk of SARS-CoV-2 and severe COVID-19 outcomes such as hospitalization or mortality (8–12, 14, 40, 41), other studies were not able to demonstrate a consistent, beneficial association (15, 16). Several of these studies used historical blood measurements (8–11, 13, 14) or measured 25(OH)D concentration after the disease onset at the time of hospital admission (12, 15, 40, 41). Given that circulating vitamin D may serve as a negative acute-phase reactant in the presence of systemic inflammation (42, 43), differences in the temporality and the resulting reverse causation or differences in the lag time might partly explain the inconsistencies. Further, as low vitamin D concentrations share many risk factors with COVID-19 outcomes, such as race, BMI, and comorbidities, results might have been confounded by these factors (9, 12, 40, 41). In our study, interestingly, higher predicted 25(OH)D concentrations were associated with reduced risk of asymptomatic infection but not with symptomatic disease or hospitalization after lifestyle factors and comorbidities such as BMI were adequately controlled for. Our data support the role of vitamin D in the earlier phase of the disease by preventing virus replication and the cytokine storm (3, 44). On the other hand, we cannot exclude the possibility that the associations might not be causal.
We observed a strong, inverse association between UV-B exposure and SARS-CoV-2 infection. UV-B radiation exposure is a major source of vitamin D via promoting endogenous synthesis of previtamin D3 in the skin. Although not a direct measure of individual exposure to sunlight, average UV-B flux based on residential address has been associated with melanoma risk (45), suggesting that it is a reasonable proxy. Further, it is less subjective to misclassification and recall bias of time spent outdoors. Meanwhile, in our study, greater UV-A was also associated with a lower risk of SARS-CoV-2 infection. Our finding is consistent with recent reports that showed that increasing UV radiation was associated with lower rates of COVID-19 cases and deaths (38, 46, 47). These lines of evidence collectively support the protective role of higher UV exposure in the SARS-CoV-2 infection. While it is biologically plausible that the UV-B effect is mediated by circulating vitamin D concentrations, we acknowledge that we may not be able to rule out the possibility of reduced transmission due to direct inactivation of the virus by UV (48), as well as mediation or confounding via other environmental factors (38, 49, 50), such as UV-A, temperature, humidity, and air pollution, or individual factors, such as time spent indoors or social-distancing behaviors. It should also be noted that geographical differences in the epidemic of COVID-19 including community prevalence or different SARS-CoV-2 variants as well as differences in screening/testing practices may have partly contributed to the inverse association between UV and infection we observed.
Our results support a benefit of vitamin D intake from supplements for lowering the risk of COVID-19–related hospitalization. With randomized controlled trials underway (51), to the best of our knowledge, only very few small studies have evaluated the impact of vitamin D intake on COVID-19 outcomes (17–21, 24). In our app-based COVID Symptom Study, self-reported use of multivitamin or vitamin D after the pandemic was associated with a modest, lower risk of SARS-CoV-2 infection in women, but not in men (18). In a randomized controlled trial, a single high dose of vitamin D3 (200,000 IU/d) did not reduce hospital length of stay among hospitalized patients with COVID-19 (21). However, the supplementation did not start until 10 d after the onset of symptoms, and the time window for vitamin D to prevent virus replication and the cytokine storm may have already passed. In our current study, we collected the information to calculate habitual vitamin D supplement intake by incorporating the brand, formula, and dose of supplement use, facilitating a more detailed estimation of long-term use. However, we should be cautious about the finding given the limited number of hospitalized cases.
There are several strengths of the study including the large sample size and the availability of comprehensive information of lifestyle and dietary intake. Our study also has limitations. First, although the predicted equation for vitamin D status has shown good performance in validation studies, we are limited by the lack of actual measurements of circulating vitamin D concentrations. In addition, predicted 25(OH)D concentrations and vitamin D intake were based on data from 2015, 5 y prior to the pandemic. They may have changed over time, especially with increasing supplemental vitamin D intake among the US population (52). Second, despite adjustment for several potential confounders, including lifestyle factors and comorbidities, the possibility of residual confounding cannot be eliminated as in other observational studies. Third, with the majority of COVID-19 cases in our study being mild in severity, and especially our inability to ascertain deaths rapidly, the impact of vitamin D on more severe COVID-19 risk remains to be investigated. Finally, the predominance of White nurses may compromise the generalizability of our findings to other racial/ethnic populations.
In conclusion, we found that predicted 25(OH)D concentrations were associated with a lower risk of SARS-CoV-2 infection. This association was observed specifically for asymptomatic, but not symptomatic, infection. Vitamin D supplementation was associated with a lower risk of COVID-19–related hospitalization. These data also support an association between exposure to UV-B or UV-A, independently of vitamin D, and SARS-CoV-2 infection; thus, results for predicted 25(OH)D need to be interpreted cautiously. Additional research is needed, particularly high-quality randomized controlled trials, to guide the use of vitamin D supplementation in the prevention and treatment of COVID-19.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—WM, SNB, and ATC: designed the research; WM: conducted the data analysis; WM, SNB, and ATC: wrote the manuscript; ATC: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Supported by grants U01 CA176726 and U01 HL145386 from the National Institutes of Health, Harvard T.H. Chan School of Public Health Dean's Fund for Scientific Advancement: Acceleration Award, Massachusetts Consortium on Pathogen Readiness (MassCPR-003), Stuart and Suzanne Steele MGH Research Scholar Award, and American Gastroenterological Association (AGA 2021-13-01 and AGA 2021-5102). The funders had no role in the study design, data collection and analysis, interpretation of data, writing of the report, or decision to submit the paper for publication.
Supplemental Figures 1–3 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article at https://academic.oup.com/ajcn/.
SNB and ATC contributed equally to this work.
Abbreviations used: COVID-19, coronavirus disease 2019; IPW, inverse probability weighting; NHS II, Nurses’ Health Study II; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Wenjie Ma, Clinical and Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Long H Nguyen, Clinical and Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Yiyang Yue, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Ming Ding, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
David A Drew, Clinical and Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Kai Wang, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Jordi Merino, Diabetes Unit and Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA; Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA.
Janet W Rich-Edwards, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Division of Women's Health, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Qi Sun, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Division of Women's Health, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Carlos A Camargo, Jr., Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Emergency Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Edward Giovannucci, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Walter Willett, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
JoAnn E Manson, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Mingyang Song, Clinical and Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Shilpa N Bhupathiraju, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Andrew T Chan, Clinical and Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Conflict of interest
ATC previously served as a consultant for Pfizer Inc., Bayer Pharma AG, Boehringer Ingelheim for work unrelated to the topic. The remaining authors have no conflicts of interests to disclose.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval. Further information including the procedures to obtain and access data is described at https://www.nurseshealthstudy.org/researchers.
References
- 1. WHO . WHO coronavirus (COVID-19) dashboard. [Internet]. [Accessed 2021 Sep 8]. Available from: https://covid19.who.int/.
- 2. Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manson JE, Bassuk SS. Commentary. Eliminating vitamin D deficiency during the COVID-19 pandemic: a call to action. Metabolism. 2020;112:154322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martineau AR, Forouhi NG. Vitamin D for COVID-19: a case to answer?. Lancet Diabetes Endocrinol. 2020;8(9):735–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CDC . COVID-19 information for specific groups of people. [Internet]. [Accessed 2021 May 20]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html.
- 6. Lee KA, Ma W, Sikavi DR, Drew DA, Nguyen LH, Bowyer RCE, Cardoso MJ, Fall T, Freidin MB, Gomez Met al. Cancer and risk of COVID-19 through a general community survey. Oncologist. 2021;26(1):e182–e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lo CH, Nguyen LH, Drew DA, Warner ET, Joshi AD, Graham MS, Anyane-Yeboa A, Shebl FM, Astley CM, Figueiredo JCet al. Race, ethnicity, community-level socioeconomic factors, and risk of COVID-19 in the United States and the United Kingdom. EClinicalMedicine. 2021;38:101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green I, Frenkel-Morgenstern M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287:3693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15(9):e0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3(9):e2019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo X, Liao Q, Shen Y, Li H, Cheng L. Vitamin D deficiency is inversely associated with COVID-19 incidence and disease severity in Chinese people. J Nutr. 2021;151(1):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bennouar S, Cherif AB, Kessira A, Bennouar DE, Abdi S.. Vitamin D deficiency and low serum calcium as predictors of poor prognosis in patients with severe COVID-19. J Am Coll Nutr. 2021;40(2):104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cozier YC, Castro-Webb N, Hochberg NS, Rosenberg L, Albert MA, Palmer JR. Lower serum 25(OH)D levels associated with higher risk of COVID-19 infection in U.S. Black women. PLoS One. 2021;16(7):e0255132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jude EB, Ling SF, Allcock R, Yeap BXY, Pappachan JM. Vitamin D deficiency is associated with higher hospitalisation risk from COVID-19: a retrospective case-control study. J Clin Endocrinol Metab. 2021;106(11):e4708–e4715.. doi: 10.1210/clinem/dgab439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hernandez JL, Nan D, Fernandez-Ayala M, Garcia-Unzueta M, Hernandez-Hernandez MA, Lopez-Hoyos M, Munoz-Cacho P, Olmos JM, Gutierrez-Cuadra M, Ruiz-Cubillan JJet al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab. 2021;106(3):e1343–e1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora VM, Solway J. Association of vitamin D levels, race/ethnicity, and clinical characteristics with COVID-19 test results. JAMA Netw Open. 2021;4(3):e214117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Annweiler G, Corvaisier M, Gautier J, Dubee V, Legrand E, Sacco G, Annweiler C. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 Patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12(11):3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Louca P, Murray B, Klaser K, Graham MS, Mazidi M, Leeming ER, Thompson E, Bowyer R, Drew DA, Nguyen LHet al. Modest effects of dietary supplements during the COVID-19 pandemic: insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr Prev Health. 2021;4(1):149–57.. doi: 10.1136/bmjnph-2021-000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcala Diaz JF, Lopez Miranda J, Bouillon R, Quesada Gomez JM. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma H, Zhou T, Heianza Y, Qi L. Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: a prospective study in UK Biobank. Am J Clin Nutr. 2021;113:1275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, Silva CBR, Franco AS, Macedo MB, Dalmolin HHHet al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325(11):1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sabico S, Enani MA, Sheshah E, Aljohani NJ, Aldisi DA, Alotaibi NH, Alshingetti N, Alomar SY, Alnaami AM, Amer OEet al. Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate Covid-19: a randomized clinical trial. Nutrients. 2021;13(7):2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oristrell J, Oliva JC, Casado E, Subirana I, Dominguez D, Toloba A, Balado A, Grau M.. Vitamin D supplementation and COVID-19 risk: a population-based, cohort study. J Endocrinol Invest. 2021;July 17:1–13.. doi: 10.1007/s40618-021-01639-9. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pal R, Banerjee M, Bhadada SK, Shetty AJ, Singh B, Vyas A.. Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis. J Endocrinol Invest. 2021;June 24:1–16.. doi: 10.1007/s40618-021-01614-4. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centre for Guidelines Methods and Economics Team (UK) . Evidence reviews for the use of vitamin D supplementation as prevention and treatment of COVID-19: vitamin D for COVID-19: evidence review A. London: National Institute for Health and Care Excellence (UK); 2020. [PubMed] [Google Scholar]
- 26. Wise J. Covid-19: evidence is lacking to support vitamin D's role in treatment and prevention. BMJ. 2020;371:m4912. [DOI] [PubMed] [Google Scholar]
- 27. Rich-Edwards JW, Ding M, Rocheleau CM, Boiano JM, Kang JH, Becene I, Nguyen LH, Chan AT, Hart JE, Chavarro JEet al. American frontline healthcare personnel's access to and use of personal protective equipment early in the COVID-19 pandemic. J Occup Environ Med. 2021;63(11):913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC.. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26.; discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 29. Bertone-Johnson ER, Hankinson SE, Bendich A, Johnson SR, Willett WC, Manson JE. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165(11):1246–52. [DOI] [PubMed] [Google Scholar]
- 30. Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–9. [DOI] [PubMed] [Google Scholar]
- 31. Bertrand KA, Giovannucci E, Liu Y, Malspeis S, Eliassen AH, Wu K, Holmes MD, Laden F, Feskanich D. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three US cohorts. Br J Nutr. 2012;108(10):1889–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bertrand KA, Chang ET, Abel GA, Zhang SM, Spiegelman D, Qureshi AA, Laden F. Sunlight exposure, vitamin D, and risk of non-Hodgkin lymphoma in the Nurses' Health Study. Cancer Causes Control. 2011;22(12):1731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fuchs MA, Yuan C, Sato K, Niedzwiecki D, Ye X, Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel Aet al. Predicted vitamin D status and colon cancer recurrence and mortality in CALGB 89803 (Alliance). Ann Oncol. 2017;28(6):1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, Richter JM, Fuchs CS, Chan AT. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology. 2012;142(3):482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ng K, Wolpin BM, Meyerhardt JA, Wu K, Chan AT, Hollis BW, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer. 2009;101(6):916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6):1678S–1688S. [DOI] [PubMed] [Google Scholar]
- 38. Cherrie M, Clemens T, Colandrea C, Feng Z, Webb DJ, Weller RB, Dibben C. Ultraviolet A radiation and COVID-19 deaths in the USA with replication studies in England and Italy. Br J Dermatol. 2021;185(2):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. [DOI] [PubMed] [Google Scholar]
- 40. De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am J Clin Pathol. 2021;155(3):381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Panagiotou G, Tee SA, Ihsan Y, Athar W, Marchitelli G, Kelly D, Boot CS, Stock N, Macfarlane J, Martineau ARet al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin Endocrinol (Oxf). 2020;93(4):508–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waldron JL, Ashby HL, Cornes MP, Bechervaise J, Razavi C, Thomas OL, Chugh S, Deshpande S, Ford C, Gama R. Vitamin D: a negative acute phase reactant. J Clin Pathol. 2013;66(7):620–2. [DOI] [PubMed] [Google Scholar]
- 43. Smolders J, van den Ouweland J, Geven C, Pickkers P, Kox M. Vitamin D deficiency in COVID-19: mixing up cause and consequence [letter]. Metabolism. 2021;115:154434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020;13(10):1373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fears TR, Bird CC, Guerry Dt, Sagebiel RW, Gail MH, Elder DE, Halpern A, Holly EA, Hartge P, Tucker MA. Average midrange ultraviolet radiation flux and time outdoors predict melanoma risk. Cancer Res. 2002;62(14):3992–6. [PubMed] [Google Scholar]
- 46. Moozhipurath RK, Kraft L, Skiera B. Evidence of protective role of ultraviolet-B (UVB) radiation in reducing COVID-19 deaths. Sci Rep. 2020;10(1):17705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carleton T, Cornetet J, Huybers P, Meng KC, Proctor J. Global evidence for ultraviolet radiation decreasing COVID-19 growth rates. Proc Natl Acad Sci. 2021;118(1):e2012370118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ratnesar-Shumate S, Williams G, Green B, Krause M, Holland B, Wood S, Bohannon J, Boydston J, Freeburger D, Hooper Iet al. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J Infect Dis. 2020;222(2):214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bowe B, Xie Y, Gibson AK, Cai M, van Donkelaar A, Martin RV, Burnett R, Al-Aly Z. Ambient fine particulate matter air pollution and the risk of hospitalization among COVID-19 positive individuals: cohort study. Environ Int. 2021;154:106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Choi YW, Tuel A, Eltahir EAB. On the environmental determinants of COVID-19 seasonality. Geohealth. 2021;5(6):e2021GH000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang R, DeGruttola V, Lei Q, Mayer KH, Redline S, Hazra A, Mora S, Willett WC, Ganmaa D, Manson JE. The Vitamin D for COVID-19 (VIVID) trial: a pragmatic cluster-randomized design. Contemp Clin Trials. 2021;100:106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rooney MR, Harnack L, Michos ED, Ogilvie RP, Sempos CT, Lutsey PL. Trends in use of high-dose vitamin D supplements exceeding 1000 or 4000 international units daily, 1999–2014. JAMA. 2017;317(23):2448–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval. Further information including the procedures to obtain and access data is described at https://www.nurseshealthstudy.org/researchers.