Abstract
Retinal photoreceptors are neurons that convert dynamically changing patterns of light into electrical signals that are processed by retinal interneurons and ultimately transmitted to vision centers in the brain. They represent the essential first step in seeing without which the remainder of the visual system is rendered moot. To support this role, the major functions of photoreceptors are segregated into three main specialized compartments – the outer segment, the inner segment and the pre-synaptic terminal. This compartmentalization is crucial for photoreceptor function – disruption leads to devastating blinding diseases for which therapies remain elusive. In this review we examine the current understanding of the molecular and physical mechanisms underlying photoreceptor functional compartmentalization and highlight areas where significant knowledge gaps remain.
Keywords: Photoreceptor, trafficking, membrane proteins, cilia, rhodopsin, arrestin
Introduction
Photoreceptor cells are subdivided into three main functional compartments (Fig. 1). The outer segment compartments are modified primary cilia containing hundreds to thousands of specialized membrane structures, discs in rods and lamellar folds in cones, that house the phototransduction cascade (reviewed in [171]). The OS plasma membrane supports an inward flux of cations through channels that close in response to light. Outer segments are separated from the inner segment compartments by the connecting cilium, which serves as the sole cytoplasmic and membrane conduit between these compartments.
Fig. 1. Schematic of rod and cone photoreceptors.
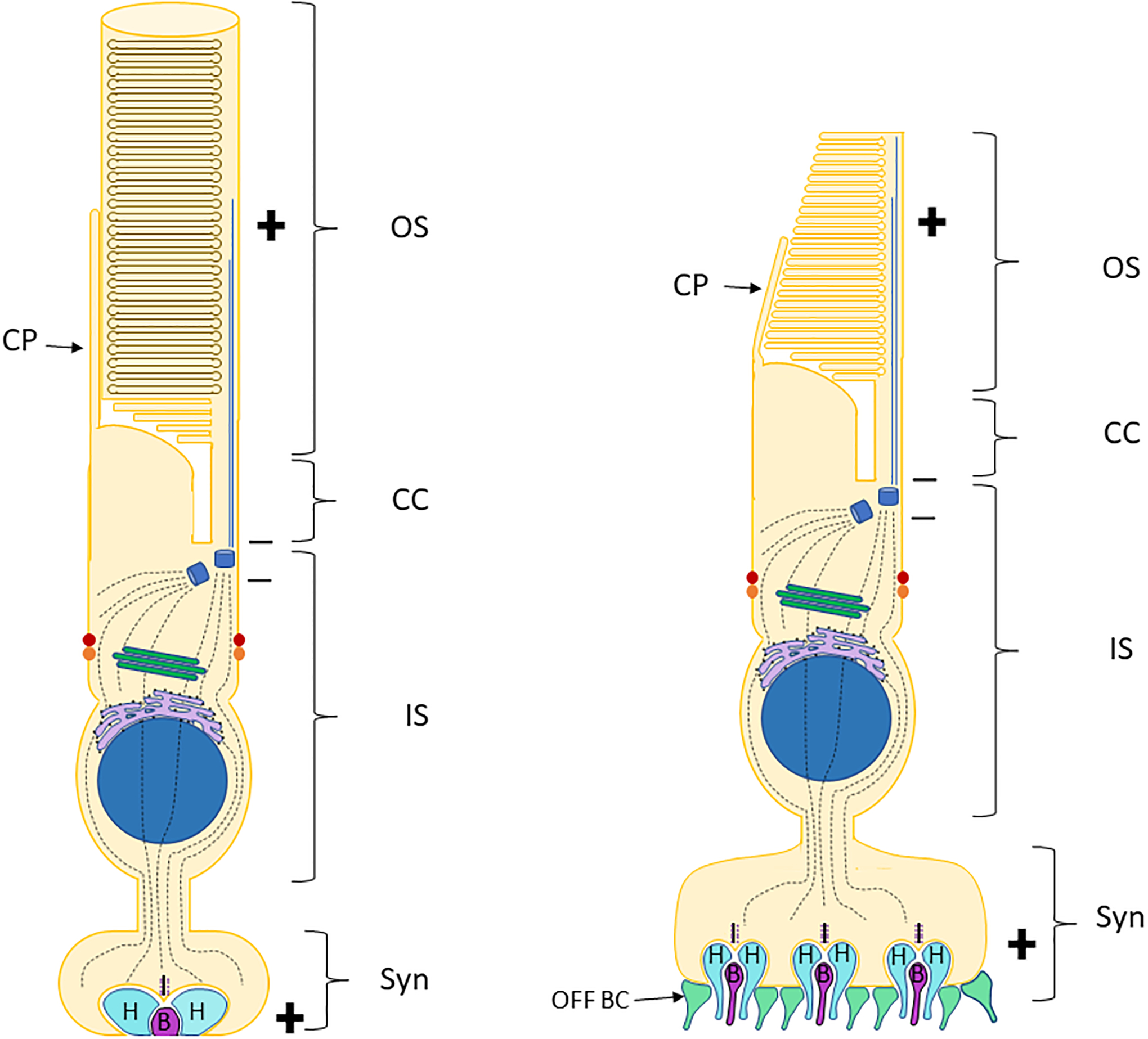
Simplified schematics of a rod photoreceptor (left) and a cone photoreceptor (right), indicating the functional compartments. Plus and minus indicate polarity of the microtubules. Axoneme microtubules are solid blue lines, cellular microtubules are grey broken lines. Orange circles represent the adherens junctions/outer limiting membrane. Red circles indicate subapical region (SAR). CP=Calyceal process, OS = Outer segment, IS = inner segment, CC = connecting cilium, Syn = synapse. H = horizontal cell process, B = ON bipolar cell process, OFF BC = OFF bipolar cell process.
The inner segment possesses an ellipsoid region close to the apical membrane that is packed with mitochondria, a myoid region that contains ER and Golgi membrane systems where proteins are synthesized, post-translationally processed and packaged into transport vesicles, and the nucleus. Microtubule tracks emanate from the basal body centrioles located at the periphery of the apical membrane: a 9+0 microtubule axoneme from the mother centriole forms the outer segment ciliary structure and cellular microtubules project from the basal body throughout the inner segment and into the pre-synaptic terminal. Like polarized epithelial cells, photoreceptor inner segments form adherens junction-like structures with the end feet of Muller glial cells called the outer limiting membrane (OLM). The inner segment membrane supports an outward cation current that opposes the inward current in the outer segment.
The pre-synaptic spherule (rods) or pedicle (cones) contain the transmitter release and vesicle reuptake machinery. It is separated from the inner segment by an axon that varies in dimension depending on species. In most amphibians and reptiles the axon is on the order of 1–5 μm and a micron in diameter. In mammals the axon may extend to tens of micrometers and is a fraction of a micron in diameter. There is no apparent physical barrier between the inner segment and the synapse, in either cytoplasm or membrane, other than the axon’s physical geometry. The inward cation current in the outer segment plasma membrane and the outward cation current in the inner segment plasma membrane set the photoreceptor membrane potential, which is depolarized in dark-adapted cells and hyperpolarizes as outer segment channels close in response to light. The membrane potential changes in a graded manner in response to changes in the light intensity falling on the photoreceptors, leading to graded modulation of the release of neurotransmitter at the synapse.
In this review we examine the mechanisms that drive partitioning of molecules that subserve the fundamental functions of photoreceptors into the appropriate subcellular compartment. Compartmentalization of proteins into the outer segment can be broken down to three fundamental problems: compartmentalization of intrinsic membrane proteins, peripheral membrane proteins and soluble proteins. We discuss each of these protein types independently. We have structured the review to examine what is known about these mechanisms within each of the main photoreceptor compartments. Areas where major knowledge gaps limit understanding are highlighted.
Protein enrichment in the photoreceptor outer segments
Photoreceptors bear a single cilium adapted for the high-fidelity capture and sensation of photons. To meet this demanding function, they have evolved specialized ciliary membrane structures, discs in rods and lamellar folds in cones, that support efficient photon capture. Together the ciliary transition zone, known as the connecting cilium in the photoreceptor field, the discs and plasma membrane form the rod outer segment. The cone outer segment consists of the connecting cilium and the lamellar membrane folds. In darkness, the outer segments support a constant influx of sodium and calcium ions across the outer segment plasma membrane through a single class of cGMP gated channel. The cGMP-gated channels (CNGα1/β1) close in response to light due to the activation of the phototransduction cascade, consisting of the light receptor, rhodopsin (Rho, rods) and cone opsin, the G protein, transducin (T), and the effector enzyme, cGMP phosphodiesterase (PDE6, rods; cPDE6, cones). A Na+/Ca2+,K+ ion exchanger (NCKX) drives [Ca2+]OS down during the light response. Photoresponses are terminated when activated opsins are phosphorylated by opsin kinases (GRK1, rods; GRK7, cones) and arrestin binds to halt transducin activation (Arr1, rods; Arr1/Arr4, cones). Guanylate cyclases (RetGC1/2) restore cGMP levels. Opsins are seven pass, CNGα/β are 6 pass each, NckX are 11 pass and RetGCs are single pass, intrinsic membrane proteins. The remaining proteins, with the exception of the arrestins, are peripheral membrane proteins. Transducin and the arrestins translocate between the inner and outer segments depending on the ambient light levels. A wide variety of mechanisms are thought to drive outer segment compartmentalization of these proteins, yet many questions remain. For a recent review on how photoreceptor outer segment compartmentalization is simar and differs from protein compartmentalization in other primary cilia, see [12].
Intrinsic membrane protein enrichment in the outer segment
The process of outer segment compartmentalization of membrane proteins in vertebrate photoreceptors is highly demanding (Fig. 2). Each day approximately 10% of rod outer segment tips are shed and replaced with new disc formation at the outer segment base [17, 234–236]. Outer segment turnover is especially demanding in lower vertebrates. With a diameter of up to 8 μm and a repeat distance of ~30 nm, the 200 discs replaced in each rod represents greater than 20,000 μm2 of new membrane added at the outer segment base, and digested by the RPE, each day. With the disc membranes, 300 million rhodopsin molecules and ~ 35 million other molecules essential to phototransduction are replaced. To make things more challenging, 75% of the turnover occurs within 8 hours of dawn [17]. This means that, at peak, disc membranes are being generated at the blistering pace of ~0.6 μm2s−1. This is equivalent to generating 7 primary cilia per minute. Mammalian photoreceptors have a diameter sevenfold smaller and are ~50% shorter, reducing the daily demand for new discs 100-fold. How photoreceptors manage the remarkable delivery of membranes and proteins to the outer segment has been the subject of intense study but remains largely unsolved.
Fig. 2. Intrinsic membrane protein compartmentalization within the OS.
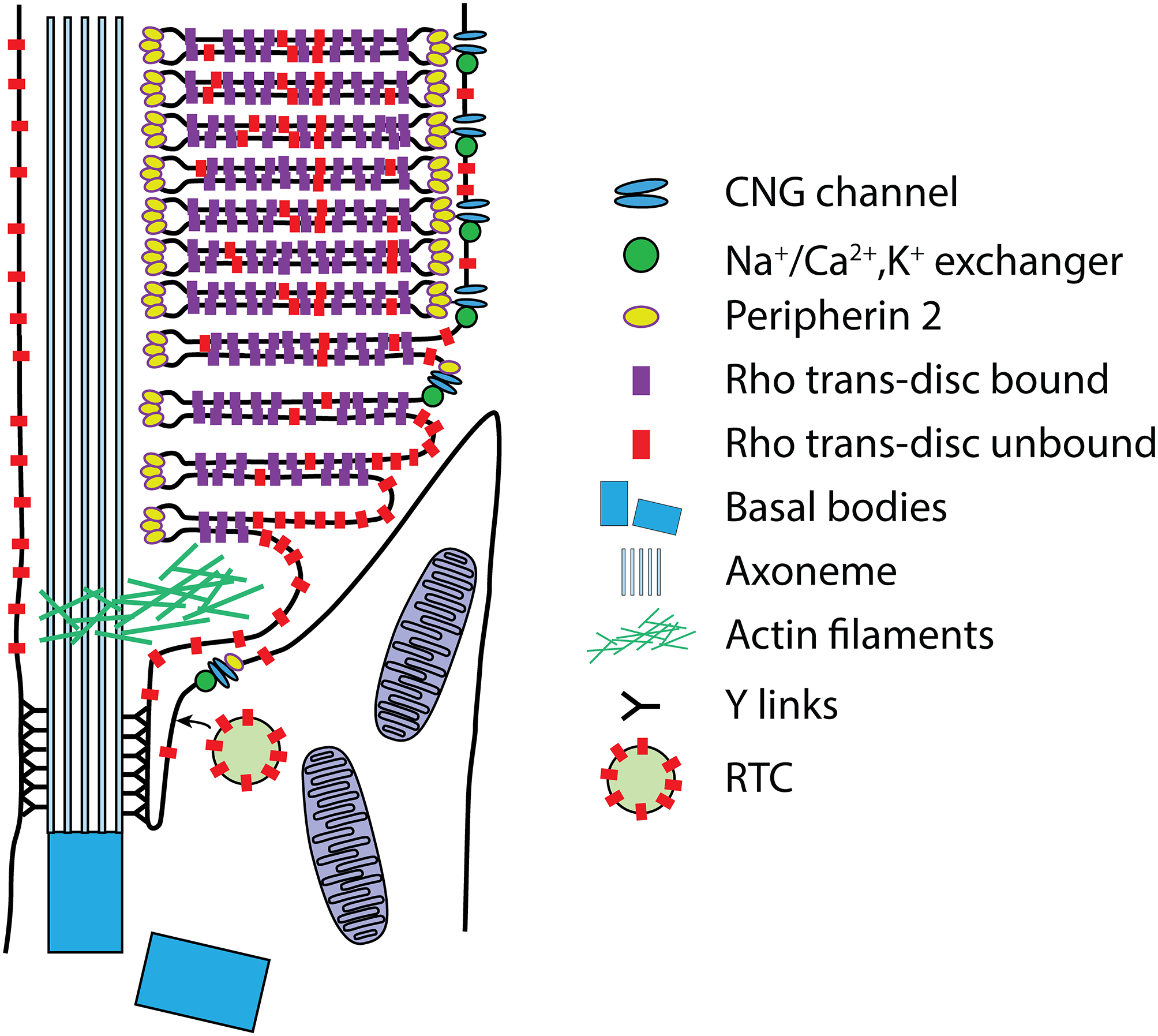
Schematic of a mammalian rod in the region of the CC. Intrinsic membrane proteins are thought to be delivered to the apical/periciliary membrane on rhodopsin transport carrier (RTC) vesicles, where they fuse (see text for details). Membrane proteins then enter the CC/transition zone, possibly mediated by the BBSome. Proteins destined for disc and plasma membrane transport to the lamellar membranes most likely by diffusion. Eventually the nascent disc membranes are enclosed by the plasma membrane through a mechanism that separates plasma membrane proteins, the CNG gated channel-Na+/Ca2+,K+ exchanger complex, from the disc proteins, rhodopsin and peripherin 2, which likely occurs during peripherin 2-Rom1-mediated disc rim expansion (see text). Note that this schematic is not meant to be an exhaustive representation of all OS membrane protein transport, or of disc morphogenesis.
Intrinsic membrane protein transport to the connecting cilium
Conventional and unconventional secretory pathways underlie intrinsic membrane protein transport to the outer segment. In the conventional pathway proteins are transported from the ER to the Golgi where they bud off into transport vesicles. Proteins delivered through the unconventional pathway bypass the Golgi, exiting from the ER. The majority of proteins traveling to outer segments, including Rhodopsin, which constitutes ~90% of the total protein in the OS [148, 150], Rom1, the cyclic nucleotide-gated channel subunit CNGα1/CNGβ1 complex, PRCD, and RetGC1, are delivered via the conventional pathway. Rhodopsin acts as a transport chaperone of sorts for PRCD and RetGC1, as shown by reduced OS delivery and overall expression in the Rho−/− mouse [162, 197].
Rhodopsin transport to the outer segment is thought to require the C-terminal VxPx sequence, an outer segment localization signal that mediates binding with the small GTPase Arf4 [44, 131]. More recent studies, however, show that VxPx may not be essential for rhodopsin delivery to the outer segment. A secondary signal within amino acids 322–336 appears to be responsible for its mislocalization in the absence of VxPx [123]. When both the primary and secondary signals are removed, rhodopsin again localizes to the outer segment, providing support for the idea that the outer segment is the default destination for most membrane proteins [9]. Trafficking of rhodopsin from the Golgi to the periciliary membrane has been well studied [46, 47, 217]. When activated by the GEF GBF1, Arf4, a member of the Arf family of small GTPases [53], binds to the rhodopsin VxPx motif [219]. ASAP1, the Arf GTPase accelerating protein (GAP), then binds to rhodopsin-Arf4 and recruits Rab11a and FIP3, which drive hydrolysis of the GTP bound to Arf4, leading to its inactivation and dissociation from the complex. Rabin8 is recruited to the developing secretory vesicle, called rhodopsin transport carriers (RTCs) [43, 217], forming the Rab11-Rabin8-Rab8 module, which recruits the SNARE, VAMP7, making the vesicles competent for fusion with the plasma membrane near the base of the cilium containing the partner SNAREs syntaxin 3 and SNAP25 [102].
Rhodopsin’s transport in RTCs from the Golgi to the base of the cilium is thought to require IFT20 which moves along microtubules [105], likely via KIFC1 (Kinesin family member C1), which has been shown to interact with ASAP1, or possibly via dynein motors [113]. Indeed, in cultured primary ciliary cells knockdown of Kifc1 resulted in a lack of cilia formation and accumulation of ASAP1 and receptors Smo and SSTR3 at the Golgi [118]. IFT20 differs from other components of the canonical anterograde IFT-B complex in that it is not solely localized to the cilium – it is also found associated with the Golgi. IFT20 is proposed to be an adaptor that binds rhodopsin and serves to transport the RTCs from the TGN to the base of the periciliary membrane [105]. Cultured cells where Ift20 is knocked out fail to develop cilia [58, 203], suggesting that IFT20’s main role is regulating ciliogenesis, maintenance of the cilium, and trafficking of ciliary components to the basal body.
Recent studies, however, have called the inner segment rhodopsin transport pathway into question. While the Arf4-based rhodopsin trafficking pathway has been extensively characterized in vitro and in amphibians [42, 44, 131, 141, 216, 218, 219], conditional knockout of Arf4 in mouse photoreceptors did not impact rhodopsin outer segment localization [163]. Deletion of Rab8a and Rab11a in mouse retina, individually or concurrently, also had no effect on outer segment protein localization or on responses to light measured by ERG [233]. It has, thus, been proposed that because the membrane and protein trafficking requirements in mice are less demanding than in lower vertebrates, another Arf may compensate [219], or another, Rab8a and Rab11a independent Arf pathway, perhaps utilizing Arf5, is operating [233]. Further in vivo studies will be necessary to define inner segment rhodopsin transport mechanisms.
However, careful consideration should be given to the use of mouse in studies of photoreceptor structure and compartmentalization. It has previously been demonstrated that some mouse models do not faithfully recapitulate the retinal disease phenotype of known pathogenic mutations. Such is the case for the Usher Syndrome USH1 mouse models, none of which display the retinal degeneration that is observed in USH1 patients [224]. Studies aimed to determine the localization of USH1 proteins in mouse versus primate photoreceptors have shed light on this discrepancy. It was found that USH1 proteins associate with calyceal processes in macaque photoreceptors. In contrast, USH1 proteins in mouse photoreceptors lack the discrete localization found in human and macaque. This may be due to the fact that, unlike humans and non-human primates, mice only possess a single, short “vestigial calyceal process” [180]. Moreover, calyceal processes have been implicated in regulation of disc morphogenesis and structural stability of outer segments in animal models that possess them [184]. Thus, while genetic mouse models can be extremely powerful tools to investigate the mechanisms underlying the pathology of human disease, they may not be suitable in all cases or may require complementary studies in additional animal models, perhaps by employing recent advances in gene editing.
Intrinsic membrane protein transport within the connecting cilium
The exact mode of membrane protein transport along the connecting cilium has yet to be determined. There are two main competing theories: The first is that RTCs themselves are transported through the connecting cilium via motor driven transport and fuse with nascent discs within the enclosed outer segment plasma membrane. This idea is supported by some electron microscopy-based studies that appear to show vesicular structures within the lumen of the connecting cilium [35, 65]. However, it is known that harsh treatment of retinal tissue, fixation, and processing can induce artifacts. Recently, these artifacts were addressed comprehensively leading to the conclusion that vesicles are not found within the connecting cilium [25, 50, 213]. Moreover, the connecting cilium is 300nm in diameter, with a 150nm central lumen surrounded by MTs and another 50nm cytoplasmic gap between the MTs and the ciliary PM [178]. RTCs average 250–300nm [41, 45], making them far too large to enter the connecting cilium. Finally, immunogold labeling experiments have shown rhodopsin to be located in the ciliary membrane of mouse photoreceptors with little present in the cilium lumen [25, 33, 225]. Thus, delivery of rhodopsin to new discs via vesicular transport through the connecting cilium is highly unlikely.
The second main theory proposes that RTCs fuse with membranes at the periciliary ridge complex, releasing their contents into the apical inner segment plasma membrane where they then enter the connecting cilium and are transported by IFT to nascent discs [159]. In this model, rhodopsin and other membrane proteins destined for the outer segment pass through the ciliary membrane diffusion barrier to enter the cilium by a poorly understood mechanism. However, the notion that rhodopsin is transported by IFT along the plasma membrane of the connecting cilium toward the site of disc formation [18, 112] is difficult to imagine considering the sheer mass of rhodopsin that is transported daily. Based on the total outer segment rhodopsin content of 3×109 molecules (amphibian) and 1×108 molecules (mammalian) [171], the rate of rhodopsin transport through the connecting cilium required to support 10% daily turnover is on the order of 3500 molecules s−1 (amphibian), and 100 molecules s−1 (mammals). To make matters more challenging, disc morphogenesis and rhodopsin transport undergo bursts of activity where 70% of new discs are formed within the first 8 hours of daylight (amphibian) [17]. Somewhat smaller variation in delivery has been observed upon onset of darkness in diurnal mammals [213]. Thus, at peak, 7300 rhodopsin molecules per second are delivered in amphibians and 100–200 molecules per second in mammals. Such high rates of delivery would require high frequency IFT transport within the connecting cilium, and significant recycling of IFT complexes. As of now, despite detailed analysis of IFT transport rates, IFT train capacity, and the number of IFT trains in flagella of Chlamydomonas (reviewed in [12]) we don’t know the frequency or capacity of IFT trains in photoreceptor or primary cilia, but the mass of rhodopsin transport suggests that IFT, alone, is not likely a viable mechanism.
Moreover, evidence for rhodopsin active transport within the connecting cilium is controversial. Conditional knock out of the kinesin motor subunit, KIF3A, in mouse photoreceptors resulted in the mislocalization of opsin to the inner segment, leading to the conclusion that IFT is essential for connecting cilium rhodopsin transport [99, 129]. In contrast, transport appeared normal for 2 to 4 weeks in a rod-specific Kif3A knockout mouse [6]. Similarly, rhodopsin localization was normal for two weeks after retina-specific tamoxifen-inducible deletion of KIF3A and IFT88 in adult mice using the six3 Cre driver. Embryonic deletion of KIF3A and IFT88 resulted in failure of connecting cilium assembly and lack of outer segments [98]. Therefore, IFT transport does not appear to be essential for connecting cilium rhodopsin transport and opsin mislocalization is likely an indirect result of improper formation and maintenance of the outer segment cilium. Importantly, knockout of the only other known anterograde IFT motor, Kif17, in conjunction with Kif3A does not prevent rhodopsin trafficking to the ROS, showing that outer segment transport is not the result of compensation by other motors [97].
Interestingly, cone specific Kif3A knockout resulted in major mislocalization of phototransduction components in cones [6] and expression of a dominant negative KIF3B mutant in Zebrafish results in accumulation of large vesicles in cone IS and disruption of OS lamellar morphogenesis [93]. Finally, mutations in Bardet-Biedl complex (BBSome) proteins cause mislocalization of cone opsin and phototransduction proteins [1, 11]. Together, these results suggest that IFT and the BBSome are required for protein transport in cones, but not in rods, possibly because cones do not internalize discs and thus separate them from the plasma membrane.
The majority of evidence points to an IFT-independent rhodopsin transport mechanism within rod connecting cilia. We propose that diffusion along the ciliary membrane is the primary mode of rhodopsin transport. We have shown that transport of rhodopsin heterologously expressed in the primary cilia of IMCD3 cells is exclusively by diffusion [117]. Other rhodopsin-like GPCRs, including SSTR3 and Smo receptors, also move mostly by diffusion in primary cilia [117, 231]. The average diffusion coefficient of 0.23 μm2s−1 [117] means a rhodopsin molecule could travel through a 1 μm long connecting cilium in ~ 2 seconds, easily accounting for the rate of rhodopsin delivery to nascent discs. These studies strongly challenge the dogma that GPCR transport within primary or photoreceptor cilia is exclusively IFT-dependent. This does not rule out IFT operating in conjunction with diffusion, however. Indeed recent work shows that in primary cilia the SSTR3 receptor couples to IFT upon stimulation with ligand or agonist [232], thus showing these mechanisms are not mutually exclusive.
However, this raises the question of the driving force behind concentrating rhodopsin within the discs if IFT is not involved? Biochemical experiments that separated outer segment discs from plasma membrane showed that the density of rhodopsin is ~ twofold higher in the disc membranes than in the outer segment plasma membrane [138], which is contiguous with the connecting cilium membrane. More recent studies using immunogold labeling of rhodopsin in rod EMs suggest rhodopsin in discs may be even more dense, perhaps approaching fivefold over the plasma membrane [33]. One enticing possibility is that rhodopsin is drawn to the nascent discs by a binding sink created during disc elaboration. Rhodopsins are thought to form transient dimers between adjacent nascent disc membranes [57, 87, 144], interacting at their extracellular faces, which may enhance close juxtaposition of extracellular membranes by “Velcroing” them together (Fig. 3). The binding between rhodopsin extracellular domains is likely weak, but the sheer number of rhodopsins stochastically interacting may result in a large fraction bound together at any given time, thus operating as “stochastic Velcro”. The propensity for rhodopsins to form cis dimers [167, 245] within the same disc membrane may further drive concentration by recruiting rhodopsins into higher order oligomers, structures which have been observed in isolated discs by atomic force microscopy [59, 60, 186, 187] and cryo-EM tomography [73].
Fig. 3. The Stochastic Velcro model of rhodopsin enrichment in disc membranes.
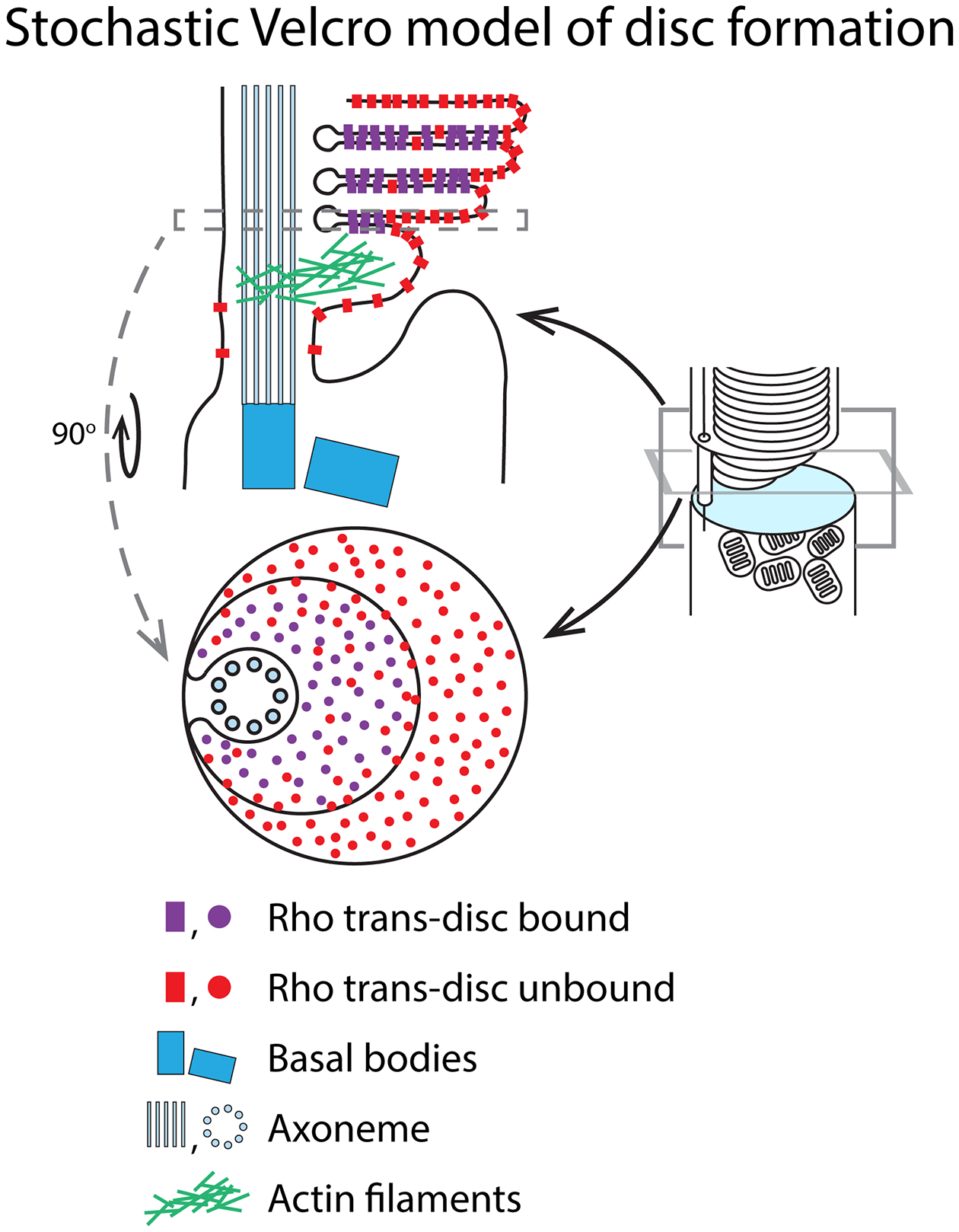
Rhodopsin density in the disc membranes is twice that in the plasma membrane, indicating that it is not efficiently separated into disc membranes. One possible explanation for this asymmetry is that rhodopsin self-associates at is extracellular N-termini. This may result in a Velcro-like coupling of the nascent disc membranes and producing a self-binding sink driving disc enrichment. Affinity would not be expected to be high for this interaction since it only produces a twofold difference in disc vs plasma membrane density. This interaction may also help drive disc morphogenesis. For simplicity, rhodopsin cis dimers (in the same membrane) are not depicted in this schematic.
Roles of the unconventional secretory pathway
Peripherin 2, a tetraspanin protein that is localized to the rims of discs (Fig. 2) and is required for proper OS formation, appears to be transported through the unconventional pathway. Ciliary targeting of peripherin 2 is dependent on COPII-mediated exit from the ER [207] and requires a C-terminal sequence including Valine 332 [37, 140, 181, 182, 204]. Peripherin 2 may traffic to the outer segment through the late endosome [156] and also requires interactions with SNARE machinery [252]. However, in mice, peripherin 2 appears to take the conventional route approximately 30% of the time, likely via hetero-oligomerization with rom-1 [38]. Other proteins that traffic to the outer segment independently of rhodopsin, like R9AP [161], may transport via the unconventional pathway. Exploring new ways to decipher what pathways proteins take from the ER/Golgi to the outer segment will be useful for further understanding the signals that dictate which route a protein will take after posttranslational processing.
Roles of phosphoinositides in protein enrichment in the photoreceptor cilium
Phosphoinositide phospholipids are essential for photoreceptor function [221]. They are involved in generation of specialized membrane compartments, recruiting proteins to membranes, trafficking of proteins, and second messenger signaling. PI4P and PI(4,5)P2 are the two most abundant phosphoinositides in photoreceptors [56]. PI4P is enriched in the Golgi via dephosphorylation of PI(4,5)P2 and PI(3,4)P2 or phosphorylation of PtdIns [40, 121] and is thought to be important in trafficking through the Golgi as well as vesicle budding from the Golgi and vesicular trafficking to the plasma membrane [66, 119]. The Golgi, also contains a small pool of PI(4,5)P2.
Ezrin and moesin, members of the ezrin/radixin/moesin (ERM) protein family, interact with transmembrane proteins, PDZ-containing proteins, the cytoskeleton, and bind membranes via PI(4,5)P2 [21]. Both ezrin and moesin were shown to be present on RTCs, particularly on those found at the site of vesicle docking near the IS/OS junction [43]. Altering biosynthesis of acidic phospholipids, which reduced the content of PI(4,5)P2, reduced association of ezrin and moesin with RTCs and interfered with RTC docking and fusion at the base of the connecting cilium [43].
The tubby mouse (Jackson Laboratories) has a spontaneous mutation leading to obesity [36]. The tubby gene product is expressed in the brain and retina, and tubby mice have retinal and cochlear degeneration [153] and reduced fertility [154]. Other tubby-like proteins (TULPs) have since been identified which bind to plasma membrane PI(4,5)P2 and are released from the membrane when Gq activated PLC-β hydrolyzes PI(4,5)P2 [183]. Tubby-like protein 1 (Tulp1) is exclusively found in photoreceptors and tulp1 mice have retinal degeneration but lack cochlear defects and the obesity phenotype [90]. Mutations in TULP1 account for approximately 5% of total RP cases [72] and tulp1 mice accumulate vesicles in the interphotoreceptor matrix [75] similar to those seen in the retinal degeneration slow (rds) and the Purkinje cell degeneration (pcd) mice. TULP1 interacts with F-actin [228] and dynamin [229] in photoreceptors, suggesting that it may play a role in vesicular trafficking. Similar functions have been identified for TULP3 in primary cilia. TULP3 knockdown decreased trafficking of some GPCRs to cilia through an IFT-A and phosphoinositide-dependent mechanism [8]. Knockdown of the inositol polyphosphate 5’ phosphatase, INPP5E, which plays a role in the enrichment of PI4P in the ciliary membrane via dephosphorylation of PI(4,5)P2, leads to an accumulation of TULP3-dependent GPCR cargo in primary cilia [8].
INPP5E is present in photoreceptors where it is concentrated near the Golgi and proximal IS, but not in the outer segment [19, 76]. This leads to the question of how higher concentration of PI(4)P is maintained in the outer segment [56]. One possibility is that other phosphatases hydrolyze PI(4,5)P2 in the outer segment. Interestingly, Lowe and Dent syndromes result from mutations in the PI 5-phosphatase ORCL (Oculocerebrorenal syndrome), which localizes to the outer segments of zebrafish photoreceptors [126].
Targeting of outer segment plasma membrane proteins
Two key proteins, the CNG channel and the Na+/Ca2+,K+ exchanger are localized to the outer segment plasma membrane as a complex [139] (Fig. 2). The CNG channel is a heterotetramer consisting of three α1 and one β1 subunits [220, 246, 247]. A glutamic acid rich GARP domain on the β1 subunit binds to the disc rim localized peripherin 2 [168], stabilizing the plasma membrane and tethering the CNG channels along the outer segment [149]. Interaction between CNG and peripherin 2 begins in the inner segment [177] and CNGβ1 is not found in subretinal space vesicles of the rds mouse [198], which lacks peripherin 2, leading to the speculation that trafficking of the CNG channel complex relies on peripherin 2. CNG channels are mislocalized to the inner segment in mouse rods lacking the endocytic adapter proteins Numb and Numb-like, which may implicate endosomes in CNGα1 trafficking [174].
The CNG channel-Na+/Ca2+,K+ exchanger complex must undergo an additional sorting between the disc membranes and the plasma membrane through an undefined mechanism. It has been speculated that the mechanism of disc enclosure within the plasma membrane and sorting of the CNG channel-Na+/Ca2+,K+ exchanger complex are linked [199], possibly by a peripherin 2-Rom1-dependent mechanism [38]. However, direct evidence remains to be found for such a mechanism.
Transport across the ciliary diffusion barrier
A major question for all membrane proteins is the mechanism by which they pass through the diffusion barrier that separates the outer segment and inner segment compartments. Failure of the G protein coupled receptors SSTR3 and MCHR1 to enrich in primary cilia of hippocampal neurons of BBS2−/− and BBS4−/− mice implicated the BBSome in ciliary enrichment of GPCRs [15]. Accumulation of dopamine 1 receptor (DP1) and reduced agonist-induced transport of DP1 out of central nervous system cilia in BBS2−/−, BBS4−/− or BBS7−/− mice also implicated the BBSome in removal of membrane proteins from cilia [52] [243].
Rhodopsin content within the ciliary outer segment does not, however, strongly rely on the BBSome. While knockout of several BBSome proteins results in mislocalization of rhodopsin, it is important to note that this mislocalization is either incomplete, where the majority of rhodopsin properly localizes to the OS [1, 96, 170], or is accompanied by major structural disruption, or complete absence of the outer segment [1, 151, 190]. In the case where there is loss of the outer segment structure, it is impossible to ascertain if the mislocalization is due to rhodopsin transport deficits or from lack of the ciliary destination. In the case where there is slight mislocalization with the majority of rhodopsin properly localized to the ciliary outer segment, it is hard to make a case that the BBSome is playing a major role.
One possibility is that the BBSome tilts the equilibrium of rhodopsin diffusion between the connecting cilium and inner segment plasma membrane toward the connecting cilium. Deletion of this function allows rhodopsin to more uniformly equilibrate between connecting cilium and the inner segment plasma membrane, but the stochastic Velcro mechanism in the nascent discs provides a binding sink that drives outer segment enrichment. This equilibrium tilting could work in both directions, where the BBSome tilts the diffusion equilibrium of proteins that are not normally found in the outer segment toward the inner segment. Indeed, recent studies have identified the role of the BBSome in removal of non-ciliary proteins from the outer segment. Quantitative proteomic analysis of photoreceptor OSs of WT and BBS17 mutant mice showed enrichment of 139 proteins in the outer segment of the mutant, including a 3-fold increase in Stx3 and Munc18–1/Stxbp, inner segment proteins involved in rhodopsin transport carrier vesicle fusion with periciliary membranes [39]. Eight proteins normally localized to the OS showed reduced OS localization, including Arl13b and GNAT2. Although rhodopsin levels were unchanged in the BBS17 mutant mouse OS, the mass of mislocalized rhodopsin in other BBS mutant mice is minor compared to that in the outer segment, indicating the subtle impact of the BBSome on rhodopsin in rods. Accumulation of Stx3 in the OS was later confirmed in BBS8−/−, BBS4−/− and BBS1−/− mice as well [48, 84]. These results show that the BBSome may operate to tilt equilibrium diffusion both into and out of the photoreceptor outer segment.
Peripheral membrane protein enrichment in outer segments
Peripheral membrane proteins reversibly interact with membranes, establishing equilibria between soluble and membrane-bound states. Membrane affinity is set by a combination of hydrophobic, electrostatic, protein-protein and other binding interactions. The majority of phototransduction components, including transducin, PDE6, GRK1, recoverin (Rec), members of the transducin GTPase accelerating complex RGS9 and Gβ5L, and RetGC1, are peripheral membrane proteins that are mostly localized to the ciliary outer segment. Some of these proteins undergo light-dependent redistribution from the OS to the IS/cell body [27]. Owing to the relative impermanence of membrane association, compartmentalization requires mechanisms in addition to the secretory pathways already discussed.
Roles of lipid binding chaperone proteins in outer segment enrichment of peripheral membrane proteins
Several lipid binding chaperone proteins are expressed in photoreceptors, including the prenyl binding protein, PrBPδ [152], and the uncoordinated proteins, Unc119a/b [120, 241]. PrBPδ is a prenyl binding protein with similar structural features to other lipid binding proteins, including Unc119 and RhoGDI, although overall sequence identity is limited [240]. Immunohistochemistry shows that it is distributed throughout the photoreceptor, with labeling highest in the inner segment and synapse [240]. PrBPδ is thought to drive solubilization of prenylated proteins by binding them upon membrane dissociation, rather than extracting them from the membrane [172], and it is required for outer segment localization of farnesylated and geranylgeranylated PDE6α and PDEβ [5] and the stability of farnesylated GRK1 expression [92, 240].
Unc119a is an acyl binding protein found in photoreceptor inner segments and synaptic spherules, with a small amount in the OS. It has been shown to bind both rod and cone Tα [241] and has been implicated in transport of Tα to the outer segments [241]. Unc119a is not required for light-dependent Tα transport out of the outer segment, however. Unc119a is expressed in a molar ratio of 1:4 with respect to Tα [191]. Interestingly, Unc119a expression is reduced 2-fold in the GNAT1−/− (Tα−/−) mouse [191], perhaps showing that Unc119a expression level scales with acylated protein load. Efficient binding of Unc119a to Tα requires N-terminal acylation and the GAGASAEEKH peptide sequence adjacent to the lipidation site [241]. Unc119a also interacts with a number of nonlipidated proteins, including the SH2 and SH3 domains of some Src tyrosine kinases [32], Arl2 [109], Arl3 [212], CaBP4 [74], and the synaptic protein RIBEYE [2], thus showing that it is not solely an acyl binding protein.
AIPL1 is another putative lipid binding chaperone protein expressed in rods [195], mutations in which are linked to Leber congenital amaurosis (LCA) [193]. A yeast two hybrid screen showed that AIPL1 specifically bound farnesylated proteins [173]. AIPL1−/− mouse showed that AIPL1 is essential for the assembly of PDE6 [110] likely by cooperating with the inhibitory gamma subunit of PDE6 (PDE6γ) to catalyze proper folding [230]. Thus, although not directly involved in transport, AIPL1 is key for PDE6 function. Interestingly AIPL1 is expressed in developing primate rods and cones and adult rods but appears to be absent in adult cones [81], suggesting it is not required for cone PDE6C maintenance.
Peripheral membrane proteins that associate with lipid binding chaperones require small ADP ribosylation factor-like GTPases, Arl2 and Arl3, that act as cargo displacement factors, releasing them to destination membranes [76, 226]. Arl2 and Alr3 can displace cargo from PrBPδ, but only Arl3 can displace cargo from Unc119a [94]. Cargo release occurs when Arl2 and Arl3 in the GTP bound state. The guanine nucleotide exchange factor (GEF), Arl13b, catalyzes GTP binding to Arl3 [69, 244]. Upon cargo release, the GTPase accelerating protein (GAP) Rp2 (retinitis pigmentosa protein 2) accelerates hydrolysis of GTP to GDP [55, 212]. The GEF and GAP for Arl2 have not yet been identified.
Arl3 is found throughout the photoreceptor cell body and possibly in the connecting cilium, but is absent from the outer segment [71, 76, 227]. Myristoylated Rp2 is enriched on the basal bodies, perinuclear region, synapse and the periciliary membrane of photoreceptors [55, 83]. Arl3 KO in both retina and rod showed that Arl3 is important for ciliogenesis and ciliary maintenance as well as efficient localization of lipidated proteins such as PDE6, GRK1, Tα and Tβγ to the outer segment [76].
Arl13b is enriched in the proximal region of the outer segment [49, 77], associated with membrane via double N-terminal palmitoylation [179]. Mutations or deletions of Arl13b cause Joubert syndrome [29] with a more severe phenotype than mutations in Arl3 [76]. Retina specific Arl13b−/− mice fail to form photoreceptor OSs and have improperly localized basal bodies [49]. Depletion of Arl13b in adult mouse rods causes accumulation of IFT88 at the proximal end of the connecting cilia and Rhodopsin mislocalization. Interestingly, PDE6, GRK1, and transducin localization are not affected, suggesting their transport to the OS is Arl13b-independent.
Electrostatic interactions and peripheral membrane protein compartmentalization
In our recent study we showed the differential compartmentalization of peripheral membrane proteins may be encoded by the lipid moiety post-translationally attached and surface charge of the protein proximal to the lipid, alone [130]. To show this we engineered peripheral membrane protein probes, consisting of EGFP or PAGFP fused to linkers containing variable amino acid sequences that resulted in different net charge and terminated with lipidation motifs specific for N-terminal acylation or C-terminal prenylation (farnesyl or geranylgeranyl). Strikingly, we found that prenylated proteins with positive charge neighboring the lipid moiety are depleted from the outer segment and enriched in the synaptic spherule, while myristoylated (acylated) proteins are enriched in the outer segment (Fig. 4). The probes did not associate with lipid binding chaperone proteins, PrBPδ or Unc119a/b, as shown by pulldowns followed by western blot and mass spectrometry. Assessing the diffusivities of the probes in the different compartments showed that membrane binding strength varied with charge and lipid moiety, where negatively charged probes had lowest membrane affinity, as expected based on the calculated membrane surface charge. Myristoylated and geranylgeranylated proteins had the highest membrane affinity. Farnesylated proteins with neutral charge neighboring the lipid, which is the arrangement of several key outer segment enriched phototransduction proteins, including GRK1 and Tβγ, have outer segment diffusion coefficients only twofold different from soluble E/PAGFP, and thus have weak membrane affinity. These results show that compartmentalization of peripheral membrane proteins can be achieved by simple diffusion and weak local electrostatic binding interactions that allow for rapid exchange of proteins within and between compartments, in the absence of interactions with lipid binding chaperone proteins.
Fig. 4. Role of electrostatic interactions in the compartmentalization of peripheral membrane proteins.
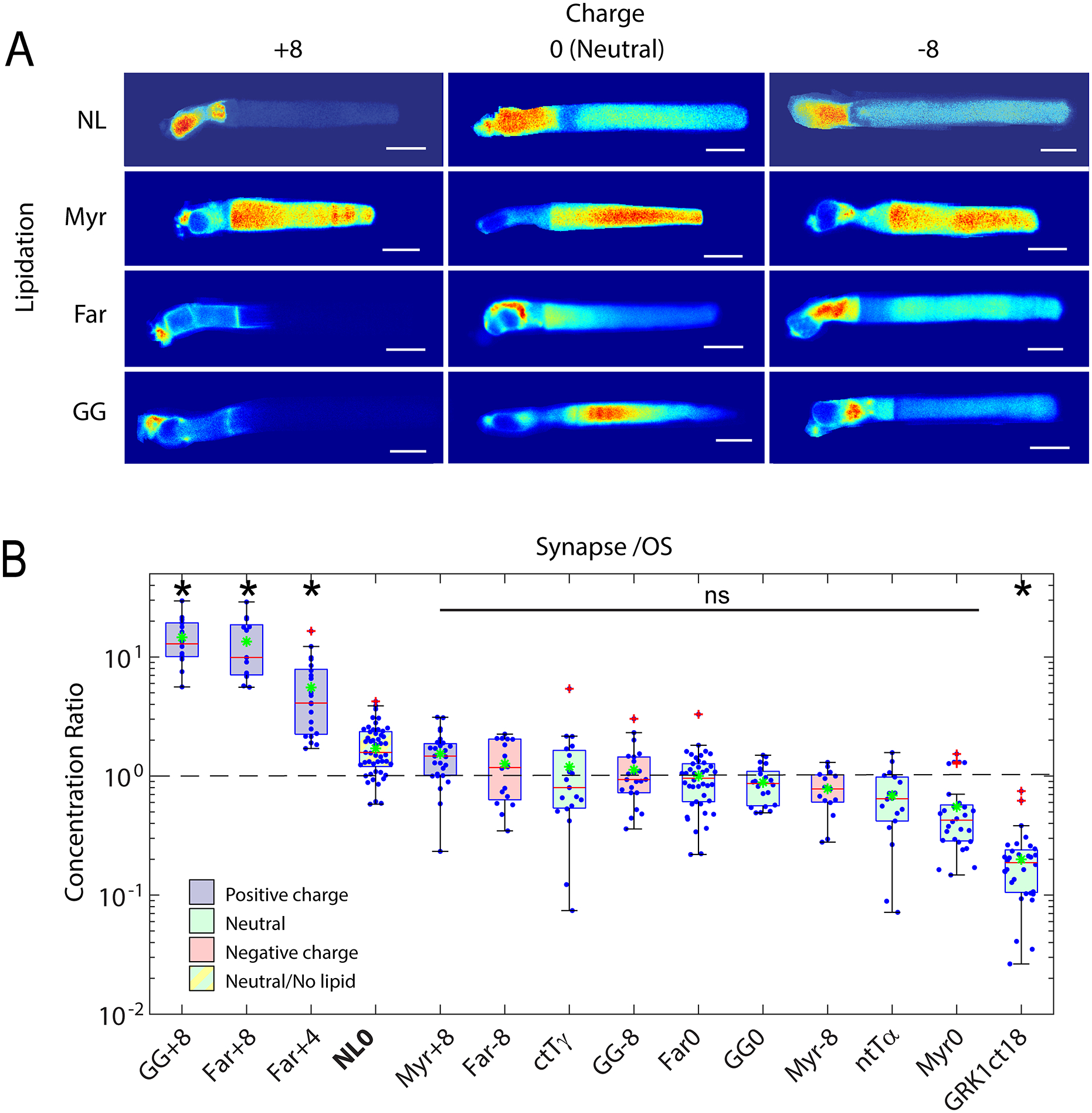
A. Montage of confocal images of living Xenopus rods expressing EGFP probes with indicated surface charge and lipidation motif. Note that none of the probes possess binding motifs for the lipid binding chaperone proteins, PrBPδ and Unc119. Significant OS localization of most probes shows that lipid binding chaperone proteins are not required for OS access and enrichment of peripheral membrane proteins. B. Box-whisker plots of average fluorescence in the pre-synapse divided by average fluorescence in the OS shows that positively charged probes with prenyl lipids are depleted from the OS and enriched in the pre-synapse, while probes containing myristoylation and neutral or negative charge equally distributed between compartments. A probe consisting of EGFP fused to the myristoylation motif containing N-terminal 16 amino acids of Tα, which binds to Unc119, was not significantly more OS enriched than the Myr0 probe, which does not bind Unc119, suggesting that Unc119 association alone is not sufficient for OS enrichment. The probe containing the farnesylated C-terminus of GRK1, which does bind to PrBPδ, is more strongly OS localized, thus, PrBP delta tilted the equilibrium toward OS enrichment. However, presence of the Far0 probe in the OS shows that PrBPδ is not required for OS entry. Modified from [130].
An alternative hypothesis for the roles of lipid binding chaperone proteins in peripheral membrane protein compartmentalization
The current model for peripheral membrane protein enrichment within the photoreceptor outer segment assumes that the lipid binding chaperones association is required for ciliary entry and that tight membrane binding is required for outer segment retention. Our recent work in Xenopus rods shows that this model is incorrect or incomplete [130]. Using live cell 2-photon and confocal microscopy we showed that peripheral membrane proteins enter the outer segment and may be enriched there without associating with lipid binding chaperone proteins (Fig. 4). We also showed that strong affinity of peripheral membrane proteins to outer segment membranes is not required for compartment enrichment. Mass spectrometry analysis of pulldowns with lipidated-GFP probes did not identify obvious alternative lipid binding protein candidates [130]. Thus, the basic distribution of peripheral membrane proteins in photoreceptors may be governed by diffusion and local binding with membranes, or membrane protein components, where compartment-specific variation in membrane surface charge, membrane surface area, protein content, ions, as well as the lipid moiety and surface charge of the protein itself, set differential compartment enrichment.
We also showed that the lipid binding chaperone protein, PrBPδ, drives strong outer segment enrichment of a probe consisting of GFP fused to the farnesylated C-terminal 18 amino acids of GRK1, even though the probe has low membrane affinity [130]. A similar probe that did not bind to PrBPδ was not enriched in the outer segment (Fig. 4B, compare Far0 to GRK1ct18). Our results show that the role of PrBPδ and relevant cargo release factors is to tilt the equilibrium distribution of peripheral membrane proteins toward the outer segment, rather than to deliver them to a terminal membrane destination. This mechanism allows peripheral membrane proteins within the OS, like GRK1, to equilibrate along the length of the outer segment, leading to equal numbers on each disc and uniform light responses throughout the ciliary outer segment [14]. Moreover, the localization to the OS is achieved without a nonspecific diffusion barrier blockade of the connecting cilium. The requirement of Unc119 acceleration of transducin α transport to the outer segment, but not for its light-dependent transport to the inner segment, further supports the model that lipid binding chaperone proteins act as equilibrium tilting factors rather than transport chaperones.
Lipid switch proteins
Some lipidated proteins rely on sequestration of lipid modifications within the proteins themselves to govern membrane binding affinity. For example, the gamma subunit of the constitutive transducin βγ dimer (Tβγ) is farnesylated, lending increased membrane affinity [61, 114]. While farnesylation of Tγ and association with PrBPδ is required for outer segment localization of Tβγ [23, 240], PrBPδ does not appear to be involved in light-dependent transport of Tβγ transport to the inner segment. Instead, phosducin, a phosphoprotein in rods that undergoes light-dependent dephosphorylation [194], associates with Tβγ [116] and drives sequestration of the Tγ farnesyl moiety between the β propeller blades 6 and 7 of Tβ [63, 124], increasing solubility of the complex. Phosducin phosphorylation by casein kinase 2 (CK2) in darkness [88] and dephosphorylated by protein phosphatase 2A in light [24], thus, appears to modulate transport of Tβγ in a light dependent manner. However, as of now there is no evidence phosducin-mediated Tβγ transport modulates vision [111, 194].
Light-dependent GRK1 activity is mediated by the calcium binding protein, recoverin [26, 34, 103, 104, 108, 128]. Recoverin is N-terminally acylated [51] and, in the absence of Ca2+ the acyl moiety folds into a hydrophobic cleft on recoverin [206]. The acyl lipid extends when recoverin is Ca2+-bound [4], through a mechanism known as the calcium-myristoyl switch [251]. Interestingly, the myristoyl moiety induces cooperativity in calcium regulation of GRK1 [3, 26]. Recoverin undergoes a modest light-dependent redistribution in mouse rods [201], however Unc119a does not bind to recoverin [241], suggesting that the light-dependent redistribution operates through the myristoyl switch mechanism and diffusion.
Soluble protein compartmentalization in photoreceptors
The most abundant soluble protein in rod photoreceptors, arrestin-1 (Arr1), is nearly equimolar to rhodopsin [196, 202]. Arr1 is found distributed in the inner segments, cell bodies and synaptic spherules of a dark-adapted rods, where it is nearly absent from the outer segments [22, 146, 165, 166, 202, 223]. In light adapted rods the distribution of arrestin nearly quantitatively shifts to the outer segments, effectively reversing the distribution pattern. Arr1 binds to light-activated, phosphorylated rhodopsin, preventing further activation of the phototransduction cascade, thus the outer segment localization may primarily be due to tight phospho-rhodopsin binding. However, Arr1 outer segment translocation in response to light was partially maintained in GRK1 knockout mice and mice where rhodopsin phosphorylation sites were mutated [133, 239], which was interpreted as evidence for motor-dependent translocation – a conclusion later supported by the finding that Arr1 transport in Xenopus was energy dependent and possibly triggered by an ATP-dependent PLC activated mechanism [155]. These conclusions are controversial, however. Considering that ~2 billion (amphibian) or ~75 million (mammalian) Arr1 molecules move to rod outer segment in response to bright light with a halftime of 2–10 minutes, depending on species [54, 146, 165, 202], makes transport entirely dependent on motor proteins unlikely [27]. Additionally, others showed that at more moderate light intensities Arr1 transport to the outer segment was reduced in the GRK1−/− and rhodopsin phosphorylation mutant mice [146], suggesting that Arr1 binding to bleached, unphosphorylated rhodopsin might be strong enough to drive transport. This notion is supported by theory. Our diffusion/active-transport/binding model [130] predicts that the distribution of Arr1 in rods with OS binding sites corresponding to the affinity of Arr1 for bleached, non-phosphorylated rhodopsin (Kd ~ 150 μM) [249] would be nearly indistinguishable from that of rods with OS binding sites corresponding to the affinity of Arr1 for bleached, phosphorylated rhodopsin (Kd ~ 50 nM) (Fig. 6). This remarkable result, despite the three orders of magnitude difference in Kd, is due to the fact that Arr1 and rhodopsin are nearly 6 mM in the disc-excluded rod outer segment cytoplasm, significantly above either Kd.
Figure 6. DBT model predicts the distribution of Arr1 in rods possessing bleached-unphosphorylated and bleached-phosphorylated rhodopsin to be nearly indistinguishable.
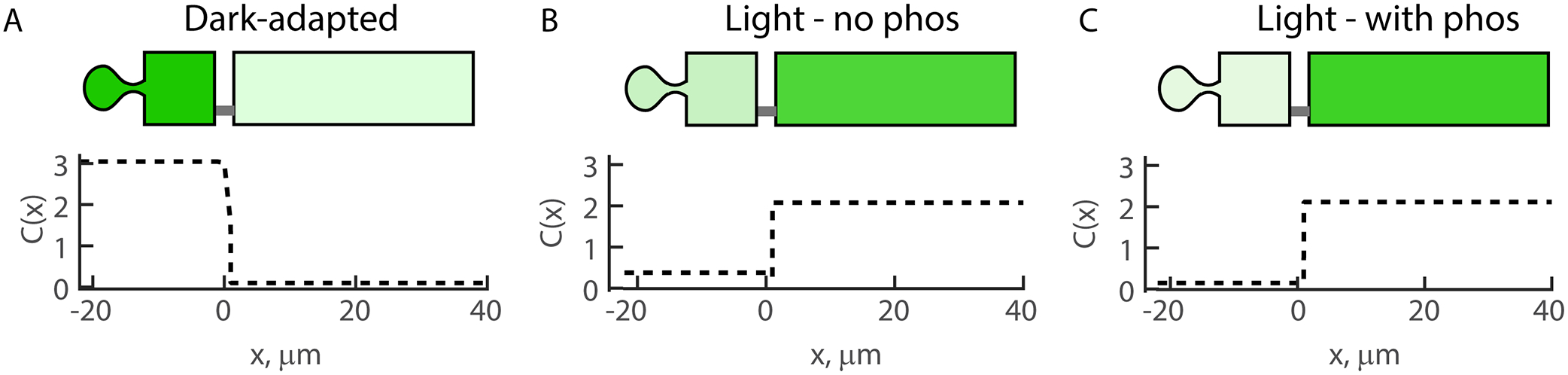
We calculated the distribution of Arr1 concentration in rod photoreceptors using our diffusion, binding active transport (DBT) model [130]. We used our published values for compartment specific soluble protein diffusion coefficients [28] and the dissociation constants for Arr1 binding with bleached-unphosphorylated rhodopsin or bleached-phosphorylated rhodopsin [249]. The concentration of rhodopsin in the outer segment was assumed to be 6 mM [171], and that of Arr1 to be 4.7 mM [202], relative to the disc excluded outer segment volume. Despite three orders of magnitude differences in Kd, the distribution of Arr1 was strongly outer segment biased in both bleached rods. This can be explained by the high concentration of Arr1 and rhodopsin relative to the Kds for respective bleached rhodopsins and the lack of binding sites in the inner segment near the concentration of Arr1. Despite the strong outer segment enrichment of Arr1 in the case of bleached-unphosphorylated rhodopsin, the outer segment mobility remained reasonably high due to higher off rate. The distribution of Arr1 in the dark-adapted rod assumed inner segment partitioning via the steric volume exclusion mechanism [147].
Another aspect of light dependent Arr1 transport was revealed by Strissel et al. who observed that light stimulated Arr1 transport occurs at a threshold that activated ~3% of rhodopsins [202], a light level at which tenfold more Arr1 molecules transported to the outer segment than the number of light activated rhodopsins. The mechanisms underlying this “super-stoichiometric” Arr1 transport are not understood except that it appears to require phototransduction. One intriguing possibility is that the ATP-dependent PLC activated mechanism of Arr1 transport proposed by Orisme, et al. [155] operates at these low rhodopsin bleach levels.
The mechanisms by which Arr1 is strongly enriched in the inner segment of dark-adapted rods is another area of controversy. Proposed mechanisms include a diffusion barrier at the base of the connecting cilium and Arr1 inner segment binding partners, including NSF, enolase-1, and tubulin [86, 145, 192]. However, our studies of soluble EGFP and PAGFP diffusion in living rods show that diffusion of soluble proteins up to ~ 80 kDa through the connecting cilium is unimpeded [28, 147], and the putative Arr1 inner segment binding partners lack sufficient concentration and distribution to account for dark-adapted rod Arr1 distribution. We, thus, explored alternative mechanisms. We reasoned that the distribution of soluble proteins within cells simply will be governed by the available aqueous cytoplasmic spaces [164]. We proposed that the available aqueous spaces in cells are distributed among a heterogeneous patchwork of cytoplasmic structures with varying density that lead to partitioning of solutes into different cytoplasmic domains based on their size and the geometry of the structures [95, 125, 136, 137, 248, 250].
The large size of amphibian rods and the unique structure of photoreceptor outer segments, with highly uniform spacing and close juxtaposition of the discs (~12nm inter-disc spacing), allowed us to quantify the distributions of soluble molecules of different sizes between the outer segment and the much less structurally constrained inner segment/cell body (Fig. 5A) [147]. We found the ratio of molecules falls steeply, from ~0.85 to ~0.15, as the size of molecules increase from 600 Da to ~81 kDa (Fig. 5B) and that the volume within the outer segment accessible to the center of mass of proteins, which ultimately dictates their concentration, declines much more steeply than the accessible volume in the inner segment, due to steric interactions between the molecules and cell surfaces (Fig. 5C).
Fig. 5. Steric volume exclusion and the compartmentalization of soluble proteins in photoreceptors.
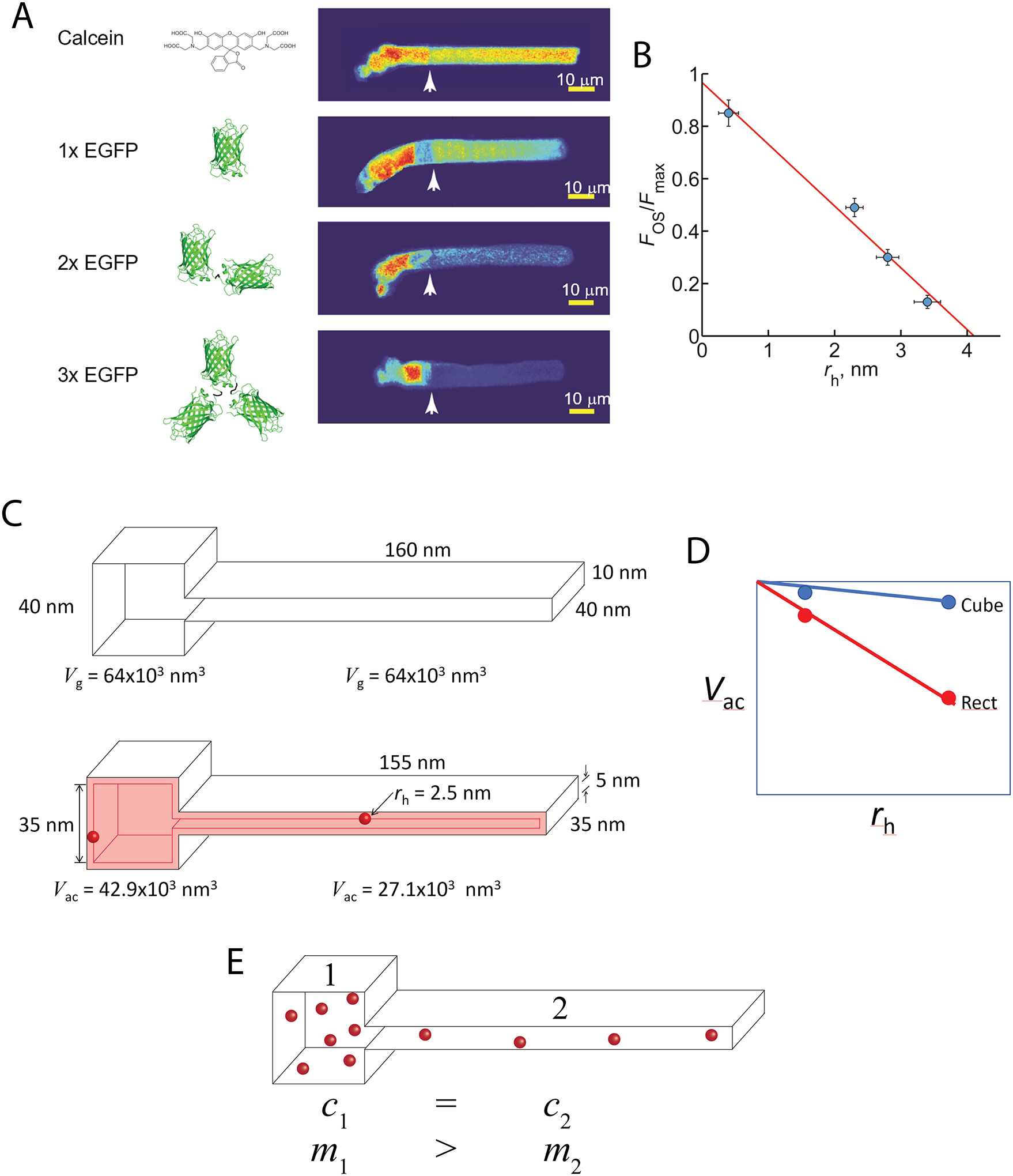
A. The distribution of soluble molecules in Xenopus rods depends on the size of the molecule. Note that the conformation of the EGFP dimers and tetramer shown are only one of many possible. B. The relationship of the ratio of the OS fluorescence to the maximum IS fluorescence scaled inversely and linearly with the estimated average hydrated radius of the molecules. This phenomenon can be explained by the asymmetrical reduction in the available aqueous volume of the differently shaped compartments caused by steric volume exclusion (i.e. loss of volume available to the center of mass of the molecule). C. For example, two interconnected boxes have the same geometric volume (Vg), but vastly different shapes. Introducing a spherical molecule reduces the geometry of both compartments, and thus the volumes accessible (Vac) to their centers of mass of the molecule. This reduction is larger for the rectangular compartment. D. As the size of the molecules increase, the reduction in Vac falls more steeply for the rectangular compartment. E. Since soluble molecules will equilibrate to equalize their concentrations (c) everywhere, the shape asymmetry will cause partitioning of the soluble molecules into the cubical compartment, where the total mass (m) will be higher. Panels A and B modified from [147].
To illustrate how steric volume exclusion can lead to partitioning of soluble proteins among cell compartments with different geometries, consider two interconnected cuboid compartments with identical geometrical volumes (Fig. 5C). The volume accessible to the center of mass of a spherical protein is lower within the rectangular compartment than it is within the cuboid compartment. The accessible volume falls more steeply in the rectangular compartment as the size of the molecules increases (Fig. 5D). This means that the effective concentration of a given number of molecules in these two compartments will be higher in the rectangular compartment, driving movement the molecules into the cube shaped compartment in order for their concentrations to equilibrate. Thus, although the effective concentration of the soluble molecules will be the same in both compartments, the number of molecules will be lower in the rectangular compartment. In many scenarios it is the number of molecules that is most important. In photoreceptors, the number of soluble Arr1 molecules in the inter-discal spaces dictate how quickly a light activated rhodopsin molecule can be silenced.
The distribution by accessible volumes model predicts that Arr1 will be fivefold enriched in the inner segment relative to the outer segment [147], somewhat less than the ~ 13-fold enrichment observed in dark-adapted rods. However, in vitro studies have shown that Arr1 can form dimers and tetramers with weak affinity [70, 78, 79, 82, 91, 106, 185, 189]. The millimolar concentration of Arr1 in rods predicts the majority of Arr1 is in the dimer (~96 kDa) or tetramer (~192 kDa) forms. If the physiological form of Arr1 in dark-adapted rods is indeed dimer or tetramer, the dark-adapted distribution of Arr1 may be explained by simple partitioning into the inner segment. This mechanism is appealing for a number of reasons. First, it explains why Arr1 is found approximately uniformly filling the entire non-outer segment compartments of dark-adapted rods, from the myoid region to the presynaptic spherule. Second, it overcomes the problem that the proposed non-rhodopsin Arr1 binding partners are not expressed to levels approaching that of Arr1. Third, although it has been proposed that Arr1 binds to microtubules [146, 155] and the BBSome protein BBS5 [155], the sheer mass of Arr1 binding would appear to substantially inhibit other vital microtubule and BBS5 functions, including delivery of tubulin and other cargoes by IFT. Finally, distribution by steric volume exclusion offers the possibility of straightforward regulation of Arr1 distribution, either through regulation of self-association or disc spacing in the outer segments, which appear to elongate in response to light in the intact eye [242].
Inner segment protein enrichment
The inner segment of photoreceptors houses vital functions, including protein synthesis and degradation, post-translational protein modification, transport via ER/Golgi secretory pathways, energy production and control of gene expression. Essential to photoreceptor physiology, ionic conductances that support the circulating current, which ultimately sets the electrical membrane potential, are present in the inner segment membranes.
To date six channels have been identified that carry K currents in the inner segment membranes: The primary voltage gated potassium current is carried by Kv8.2, Kv2.1 and Kv2.2 channels, in various combinations depending on photoreceptor type and species. Calcium-activated potassium channels identified are KCa1.1 (BK) and KCa3.1 (IK). Additionally, HCN1, inwardly rectified currents activated by hyperpolarization, are present in the inner segment membranes. For a recent review on photoreceptor channels see [211]. Inner segment membranes also contain ion transporters, including NCX1, a Na+/Ca2+ exchanger found in the inner segment of rods and cones [100, 135], and NKAα3, the ubiquitous Na+/K+-ATPase found in most excitable cells [10, 115]. Finally, the inner segment membranes contain a glucose transporter (GLUT1) [68].
Few studies have examined the targeting of membrane proteins to the inner segment compartment. Recent studies have identified important regulatory sequences in HCN1 and NKAα3 which are proposed to serve as IS compartment signals. The Baker group identified a di-arginine ER retention sequence present in the intrinsically disordered C-terminus of HCN1 which is predicted to serve as a negative regulator of its surface expression [157]. It is speculated that this signal may be masked by the proper assembly of HCN1 channel subunits and serve as a quality control mechanism for proper channel assembly in the ER. In addition to the ER retention signal, a leucine-based ER export signal was identified in HCN1’s N-terminus [157]. Together, these signals appear to function as regulators of HCN1 inner segment plasma membrane delivery. Additionally, the 20 N-terminal amino acids of HCN1 redirected an OS localized probe to the inner segment plasma membrane [157], suggesting that it may serve as an OS rejection signal.
NKAα3 is also mostly absent from the photoreceptor outer segments. Immunohistochemistry shows it to be located throughout the photoreceptor inner segment and outer plexiform layer, with faint staining in the outer segment and/or connecting cilium [115, 222]. In epithelial cells it has been proposed that NKA is localized to the basolateral membranes in an ankyrin-dependent manner [200]. In photoreceptors, ankyrin-B (AnkB) is required for expression of NKAα3, suggesting they interact [107]. However, pull downs of NKAα3 failed to identify AnkB and the expression pattern of AnkB and NKAα3 in isolated photoreceptors did not exactly match, casting doubt on such a mechanism [115]. Expression of the sperm flagella NKAα4 in photoreceptors was exclusively outer segment localized, and dependent on a N-terminal VxP motif that bears resemblance to the C-terminal VxPx motif that drives outer segment localization of several photoreceptor membrane proteins. This observation led to the alternative hypothesis that localization of NKAα3, which possesses VxT in the analogous N-terminal domain, to the inner segment plasma membrane was the result of an incomplete VxP OS localization signal. However, mutation of VxT to VxP in NKAα3 did not result in OS localization, suggesting that VxP, alone, is an incomplete OS enrichment signal or that other signals on NKAα3 may override OS localization [115], perhaps through BBSome mediated checkpoint within the connecting cilium.
In polarized epithelial cells, asymmetrical membrane protein trafficking is achieved by targeting secretory vesicles to the apical or basolateral membranes based on the eight protein exocyst complex (reviewed in [169, 238]). This raises the question of whether photoreceptors employ polarized trafficking to partition inner segment proteins from the outer segment or pre-synaptic terminal. Epithelial cell polarization begins with cell-cell contacts and the formation of adherens junctions (AJ) that impose a diffusion barrier belt between the apical and basolateral membranes. Photoreceptors contain a specialized adherens junctions called the outer limiting membrane (OLM). The OLM is formed by contact between photoreceptor inner segment membranes, at a position just apical to the outer nuclear layer, and Muller glial cell end feet, and consists of N-cadherin, β-catenin, α-catenin and the zonula occludens proteins, ZO1–3. A subapical region, which lies just apical to the AJ/OLM, is formed by the crumbs complex, which consists of Crumbs proteins 1–3 (CRB1–3), MPP5, PATJ, MUPP1, aPKC, EPB41L5, Cdc-41, veli-3, F-Actin [67, 208]. The location of the Crumbs complex just apical to the AJ is thought to be involved in targeting apical membrane destined proteins via the exocyst complex.
Directional trafficking to cilia has been suggested to be mediated by exocyst complexes and plays an important role in ciliogenesis [7, 253]. Similarly, photoreceptor specific Exo5−/− mice showed severely compromised outer segments at 4 weeks of age followed by retinal degeneration; in zebrafish global knockout of Exoc5 resulted in complete loss of photoreceptors. These results indicate a role for polarized trafficking in photoreceptor ciliogenesis and maintenance [122]. The exocyst complex has been shown to be involved in Rho transport to rhabdomeres in drosophila [16]. Exocyst complex proteins, sec6/8, have been shown to be present in the apical inner segment and perinuclear membranes in Xenopus rods and co-localize with syntaxin 3 and Rab8 near the connecting cilium, possibly implicating them in RTC vesicle fusion and, thus, rhodopsin transport to the outer segment [132].
However, significant evidence suggests that polarized trafficking is not operating in the inner segment. First, the OLM/AJ does not appear to serve as a diffusion barrier for inner segment proteins. Proteins found in the inner segment plasma membrane, like HCN1 and NKAα3, are generally distributed throughout the inner segment and outer plexiform layer [115, 157, 158]. Moreover, mutations that lead to rhodopsin mislocalization to the inner segment membrane show it to be approximately uniformly distributed throughout, including into the pre-synaptic terminal in the outer plexiform layer. For example, photoreceptor-specific syntaxin 3 knockouts in mouse lead to rhodopsin mislocalization throughout the inner segment outer nuclear layer and outer plexiform layer and loss of the outer segment [101] and the retinitis pigmentosa causing rhodopsin mutant, Q334Ter, expressed in transgenic Xenopus rods, where the outer segments remained intact, is found in the outer segment and evenly distributed throughout the inner segment and pre-synaptic terminal membranes [205]. Moreover, the presence of crumbs proteins at the photoreceptor pre-synaptic terminal [135, 209] as well as at the OLM [67, 208] suggests polarized transport, in its traditional sense, is not involved in partitioning inner segment and pre-synaptic proteins.
Protein enrichment in the photoreceptor pre-synaptic terminal
The third essential compartment of photoreceptor neurons is the pre-synaptic terminal. Rods and cones communicate with bipolar and horizontal cells through elaborate triad ribbon synapses consisting of bipolar and horizontal cell dendrites closely juxtaposed within invaginations of the photoreceptor terminal, as well as bipolar and horizontal cell dendrite contacts on the cone pedicle surface neighboring invaginations (Fig. 1). Additionally, rod and cone presynaptic terminals are electrically coupled through gap junctions (reviewed in [20]). The photoreceptor pre-synaptic terminals are highly dynamic structures. In darkness, rods and cones steadily release the neurotransmitter, glutamate, through synaptic vesicle fusion at rates of 100s (rods) −1000s (cones) per second [80, 188]. This enormous release is countered by endocytic vesicle re-uptake – necessary for recycling of vesicle release machinery and maintaining synaptic membrane homeostasis [142, 214, 215]. Synaptic vesicles are organized above the active release zone by ribbon structures consisting of the core ribbon protein Ribeye. The ribbons and associated synaptic vesicles are anchored to the active zone through Bassoon and Piccolo, that, in turn, associate with CAST, Rim2, vesicle associated Rab3a, Munc13, plasma membrane associated Syntaxin/SNAP25 and the voltage-activated calcium channel that mediates vesicle release, CaV1.4. CaV1.4 is anchored to the active zone by filamentous actin, retinoschisin and a CaBP4-Unc119-RibeyeB complex [62, 142] (Fig. 7).
Fig. 7. Interactions of photoreceptor ribbon synapse proteins.
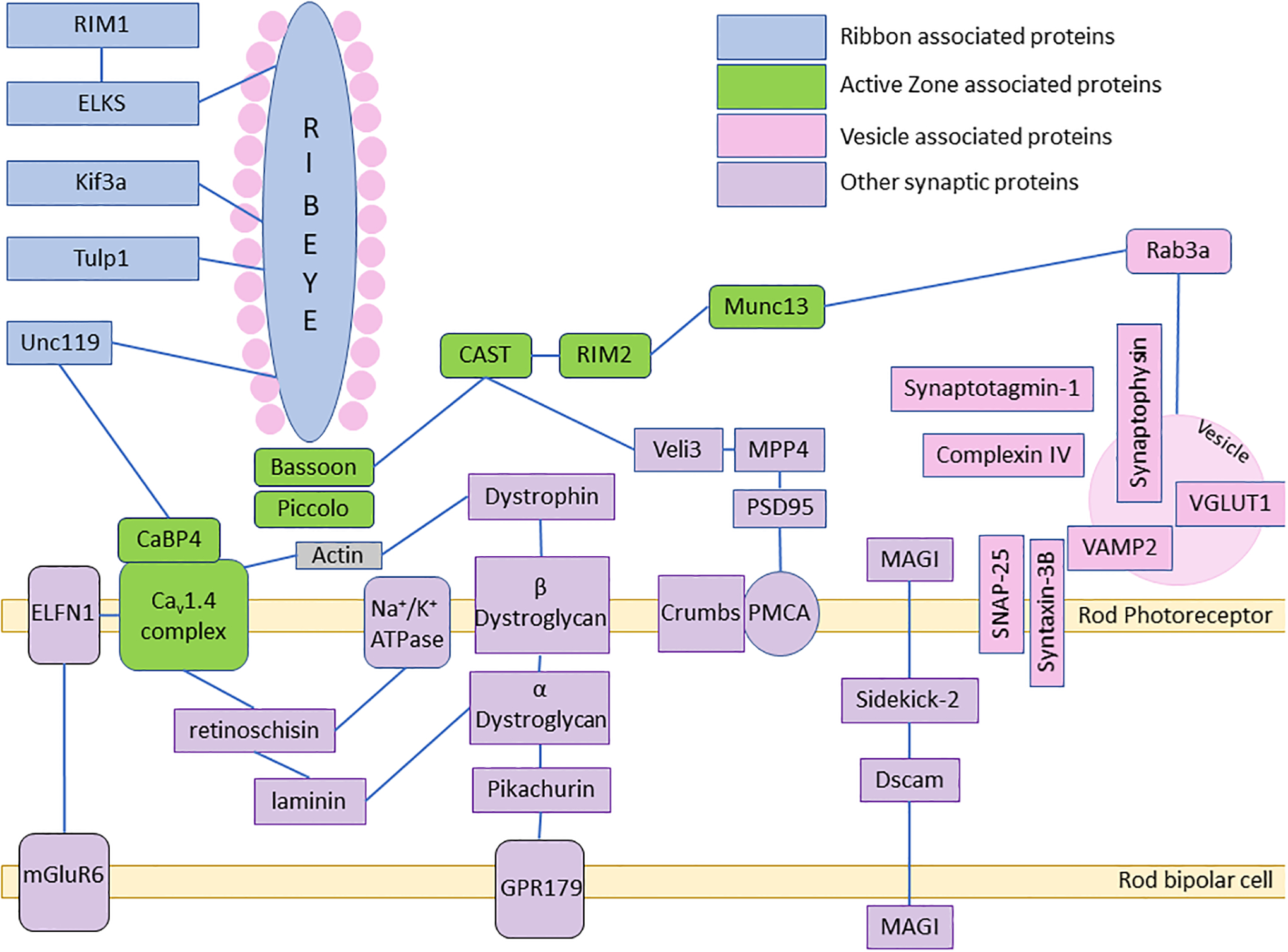
Schematic of the ribbon synapse protein interactions. Schematic based on [62, 135, 237], among others.
The large intracellular ribbon complex is anchored to extracellular matrix components and to post-synaptic proteins (Fig. 6). F-actin forms a complex with the trans membrane protein β dystroglycan, which is bound to α dystroglycan in the extracellular space between the pre-synaptic and post-synaptic membranes. α dystroglycan complexes with Pikachurin, Laminin, and retinoschisin [135]. Laminin was shown to mediate links between pre-and post-synaptic laminin receptors, α dystroglycan and pikachurin and to maintain proper transsynaptic alignment [89]. Retinoschisin complexes with CaV1.4α and NKAα3. In rods, the trans-membrane protein ELFN1 associates with CaV1.4 channels in the rod spherule and with mGluR6 in the on rod bipolar cell dendrite [30]. Interestingly, a recently discovered protein in cones, ELFN2, appears to play a similar role, associating with the post-synaptic mGluR6 in cone on bipolar cell dendrites [31]. The interactions of ELFN1 were proposed to drive selective rod synapse formation in the retina. However, deletion of ELFN2, which resulted in upregulation of ELFN1 in cones, did not disrupt cone to cone on bipolar cell synapse formation. Moreover ELFN1/ELFN2 double knockouts disrupted cone synaptic transmission, but the cone synapse appeared structurally intact, suggesting other factors are at play [31].
CaV1.4 is essential for ribbon docking to the active zone. Knockout of CaV1.4 results in formation of ectopic synapses in the ONL, presence of floating spherical ribbons, lack of terminal invaginations and abnormal distribution of Veli3 and PSD95 in rods [13, 175, 237]. Until recently it was not known if this disruption was due to a structural impact of CaV1.4, or to the channel activity. Genome editing to introduce the G369i mutation in CaV1.4, which disrupts Ca2+ conductance but not cell surface trafficking or voltage sensor movements, into mice showed that the CaV1.4 protein was required for proper assembly of the pre-synaptic scaffold, but that Ca2+ conductance was needed to assemble the proper invaginating structure [127].
Together, these results show that the synaptic transmission machinery in photoreceptors is a massive, interconnected complex between pre- and post-synaptic molecules and the extracellular matrix (Fig. 7). This massive complex might lead one to view the synaptic machinery as a semi-permanent structure that would require specialized mechanisms for turnover. However, several lines of evidence show that proteins in the synaptic machinery are highly dynamic. Single molecule tracking experiments show that CaV1.4 channels at rod and cone synapses are not fixed, but move within a membrane domain equal to or slightly larger than the area beneath the synaptic ridge at the base of the ribbon [134]. Pharmacological disruption of the cytoskeleton or depletion of membrane cholesterol significantly increased the dimensions of the confinement domain of CaV1.4 channels and synaptic vesicle release, suggesting that macromolecular scaffolds and lipid rafts are important for organizing the synapse. Moreover, ankyrin-B, a component of the membrane cytoskeleton that is highly enriched in the photoreceptor pre-synaptic terminal, is also found sparsely distributed along the entire inner segment membrane [115], suggesting localization may be mediated by a diffusion to binding sink mechanism.
Machinery involved in vesicle reuptake is found in the peri-active zone (reviewed in [142]). Clearance of the fused synaptic vesicles and associated vesicle proteins may involve interplay between exocytic and endocytic proteins [214]. Individual vesicles are thought to be retrieved by a relatively slow (minutes), clathrin-mediated endocytic process which appears to operate under slightly depolarizing conditions. This slow process requires clathrin, dynamin and dynamin interacting proteins [214]. TULP1, a photoreceptor exclusive protein which interacts with plasma membrane via PI(4,5)P2, might also contribute to membrane endocytosis at the pre-synaptic terminal. It has been shown that TULP1 colocalizes with dynamin-1/2, clathrin heavy chain and F-actin. TULP1−/− mice failed to recruit endocytic proteins to the synapse resulting in severely reduced endocytosis from the peri-active zone at the synapse [215, 228, 229]. A fast retrieval mechanism, where large infoldings of membrane are retrieved at more depolarized conditions (dark-adapted), operates through a poorly delineated dynamin and GTPase independent mechanism [210]. Tight control of synaptic vesicle fusion and membrane reuptake at the synapse is crucial to maintain synaptic function. A mutation in polyphosphoinositide phosphatase, Synaptojanin 1, which plays a role in endocytosis, caused mislocalization of RIBEYE and Vamp2 to the IS of cones. Prolonged dark adaptation resulted in accumulation of large vesicles in the inner segment of these photoreceptors. Aberrant staining of Rab7 and LC3 suggested that it may be due to disruption of endolysosomal trafficking, which exchanges proteins and membrane between pre-synaptic termini and the Golgi [64].
Cellular microtubules extend from the basal bodies, through the myoid, past the nucleus and into the pre-synaptic terminal (Fig. 1). This organization results in microtubule plus ends terminating in the pre-synaptic structure, leading to the question of whether motor proteins transport cargoes to the synapse. Immunohistochemistry has identified intraflagellar proteins IFT88/57/52/20 in the outer plexiform layer of bovine retina [160]. Kif3a is found at the PR synapse, directly associated with the ribbon [143]. Immunohistochemistry shows BBS4 is enriched in the outer plexiform layer [1]. However, it is unclear what the function of these IFT components is in the pre-synaptic terminal. Recently, congenital BBS mutations were shown to cause mislocalized ribbon synapses in the outer nuclear layer of mice where horizontal cell processes aberrantly projected [85]. However, rod-specific BBSome mutations generated under control of the Rho-Cre failed to reproduce the phenotype, suggesting either that the effect of loss of BBSome activity was due to downstream neurons or that loss of activity in both rods and horizontal cells is responsible for the faulty synapse formation. Thus, while there are intriguing hints that IFT and BBSome mechanisms may be at play in the synapse, their roles are far from clear.
One interesting possibility is that active transport is involved in the development of the ribbon synapse. In a study using transmission electron and STED super resolution microscopy, Regus-Leidig, et al. [176] showed that synaptic ribbon precursor spheres containing Bassoon, Piccolo, RIBEYE, and RIM1 appear to transport as a unit to the nascent synapse where they dock and await the arrival of other synaptic components, including Munc13, CAST1, RIM2, and plasma membrane spanning CaV1.4. Thus, the formation of the photoreceptor ribbon synapse compartment could be envisioned as resulting from a combination of active transport of the precursor sphere and diffusion of the remaining components to a developing binding sink. Future studies will be needed to elucidate the mechanisms that underlie the formation and maintenance of the highly dynamic photoreceptor synapse.
Conclusions
Altogether, the seemingly varied results reviewed in this article suggest that functional compartmentalization of retinal photoreceptors may be governed by relatively simple physical principles. We propose that compartmentalization relies heavily on random diffusion of proteins to sites where specific interactions dictate compartment enrichment. In this scheme, the inner segment plays a central role. Membrane proteins are synthesized, post-translationally modified and packaged into transport vesicles that arrive at the IS plasma membrane. Vesicle fusion releases proteins into the plasma membrane, where they diffuse. Proteins that are destined to remain in the inner segment plasma membrane simply diffuse randomly to uniformity. Proteins destined to be enriched in the pre-synaptic terminal, like the CaV1.4 channel, diffuse until they encounter a binding partner in that structure that serves as a binding sink, such as the retinoschisin-laminin-α-dystroglycan complex. Proteins destined for the outer segment diffuse until they encounter the BBSome complex or some other gatekeeper at the base of the connecting cilium. The BBSome checks the ID of the protein and if they have the right credentials, they are let in – if not, they are kicked out. Once they are through the gate, they are free to diffuse once again toward binding partners that determine their location in the outer segment. In the case of rhodopsin, that binding partner is itself, where extracellular domains on a newly forming disc membrane interact with extracellular domains on the previously assembled membrane lamella, holding the membranes together like stochastic Velcro.
Peripheral membrane proteins also diffuse throughout the cytoplasm, hopping on and off membranes as they go. Some encounter binding partners that tether them, resulting in local enrichment once again through a binding sink. Others find chaperone proteins, like PrBPδ or Unc119, that increase their solubility, perhaps helping them avoid the checkpoint at the base of the connecting cilium to enter the outer segment. There, they are released from the chaperone and free to diffuse once again, perhaps to find a binding partner. However, results from [130] show that a binding partner is not necessary to maintain outer segment enrichment of a loosely bound peripheral membrane protein that interacts with PrBPδ. This result implies that some additional force is required to maintain the outer segment enrichment.
Soluble proteins avoid the checkpoint at the connecting cilium altogether. They are free to roam and fill in all the cytoplasmic spaces, equilibrating to uniform concentration everywhere. But the concentration is dictated by how big they are and the size of the spaces they enter. The bigger the protein, the smaller the effective space and thus the volume. In this way, variations in protein size and the density of cell structures can generate a size-dependent partitioning of proteins based on effective local concentrations. Changes in the size of proteins or in the geometry of the cytoplasmic spaces changes the math for the distribution [147].
This simple physical model of photoreceptor compartmentalization may explain many unsolved mysteries in photoreceptor pathobiology. For instance, it may explain why rhodopsins are only partially mislocalized when the BBSome is dysfunctional. In the case of rhodopsin, the BBSome may operate as a weak selective gate of sorts that tilts the diffusive equilibrium of rhodopsin toward the cilium membrane, a tilting that is enhanced by the stochastic Velcro binding sink created by the nascent disc membranes. This mechanism predicts that rhodopsin would be found in the inner segment, connecting cilium and disc membranes in progressively higher concentrations, a prediction that is supported in a recent study quantifying rhodopsin in these membranes by immunogold EM (Chadha et al., 2019). Disabling the gate, or making it less effective, removes the tilt, slightly shifting the equilibrium back toward the inner segment membranes allowing some of the rhodopsins being packaged into nascent discs in rods or the lamellae in cones, to leak out of the outer segment. The many other proteins that are more heavily influenced by the BBSome would become mislocalized to a greater extent. Similarly, peripheral membrane proteins, like transducin, may have a more inner segment-tilted equilibrium in the absence of Unc119.
Much is left to do to determine the mechanisms of photoreceptor functional compartmentalization and to test the veracity of the transport, diffusion, binding and equilibrium tilting hypotheses outlined here. Sophisticated, high resolution, quantitative live-cell imaging will lead the way to understanding the functional compartmentalization of these elegant cells.
Acknowledgements:
Our work is supported by grants from the National Institutes of Health - National Eye Institute, R01EY018421 (PDC) and R01 EY028303 (PDC). PDC is recipient of a Stein Innovation Award from Research to Prevent Blindness Inc. The Department of Ophthalmology and Visual Sciences is supported by an unrestricted grant from Research to Prevent Blindness Inc.
Funding:
Our work is supported by grants from the National Eye Institute, R01EY018421 (PDC) and R01 EY028303 (PDC). PDC is recipient of a Stein Innovation Award from Research to Prevent Blindness Inc. The Department of Ophthalmology and Visual Sciences is supported by an unrestricted grant from Research to Prevent Blindness Inc.
Footnotes
Conflicts of interest/Competing interests: The author declare no conflicts of interest
Consent for publication: All authors consent to publish
References
- 1.Abd-El-Barr MM, et al. , Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet–Biedl syndrome. Vision Research, 2007. 47(27): p. 3394–3407. DOI: 10.1016/j.visres.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpadi K, et al. , RIBEYE recruits Munc119, a mammalian ortholog of the Caenorhabditis elegans protein unc119, to synaptic ribbons of photoreceptor synapses. J Biol Chem, 2008. 283(39): p. 26461–7. DOI: 10.1074/jbc.M801625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ames JB, et al. , Amino-terminal myristoylation induces cooperative calcium binding to recoverin. J Biol Chem, 1995. 270(9): p. 4526–33. [DOI] [PubMed] [Google Scholar]
- 4.Ames JB, et al. , Nuclear magnetic resonance evidence for Ca(2+)-induced extrusion of the myristoyl group of recoverin. J Biol Chem, 1995. 270(52): p. 30909–13. [DOI] [PubMed] [Google Scholar]
- 5.Anant JS, et al. , In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem, 1992. 267(2): p. 687–90. [PubMed] [Google Scholar]
- 6.Avasthi P, et al. , Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J Neurosci, 2009. 29(45): p. 14287–98. DOI: 10.1523/jneurosci.3976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babbey CM, Bacallao RL, and Dunn KW, Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. American Journal of Physiology-Renal Physiology, 2010. 299(3): p. F495–F506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badgandi HB, et al. , Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. Journal of Cell Biology, 2017. 216(3): p. 743–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker SA, et al. , The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol, 2008. 183(3): p. 485–98. DOI: 10.1083/jcb.200806009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker SA and Kerov V, Photoreceptor inner and outer segments. Curr Top Membr, 2013. 72: p. 231–65. DOI: 10.1016/B978-0-12-417027-8.00007-6. [DOI] [PubMed] [Google Scholar]
- 11.Bales KL, et al. , BBSome Component BBS5 Is Required for Cone Photoreceptor Protein Trafficking and Outer Segment Maintenance. Invest Ophthalmol Vis Sci, 2020. 61(10): p. 17. DOI: 10.1167/iovs.61.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes CL, Malhotra H, and Calvert PD, Compartmentalization of Photoreceptor Sensory Cilia. Frontiers in Cell and Developmental Biology, 2021. 9(68). DOI: 10.3389/fcell.2021.636737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayley PR and Morgans CW, Rod bipolar cells and horizontal cells form displaced synaptic contacts with rods in the outer nuclear layer of the nob2 retina. J Comp Neurol, 2007. 500(2): p. 286–98. DOI: 10.1002/cne.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baylor DA, Lamb TD, and Yau KW, The membrane current of single rod outer segments. J Physiol, 1979. 288: p. 589–611. [PMC free article] [PubMed] [Google Scholar]
- 15.Berbari NF, et al. , Bardet–Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proceedings of the National Academy of Sciences, 2008. 105(11): p. 4242–4246. DOI: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beronja S, et al. , Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. The Journal of cell biology, 2005. 169(4): p. 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besharse JC, Hollyfield JG, and Rayborn ME, Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. The Journal of Cell Biology, 1977. 75(2): p. 507–527. DOI: 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhowmick R, et al. , Photoreceptor IFT complexes containing chaperones, guanylyl cyclase 1 and rhodopsin. Traffic, 2009. 10(6): p. 648–63. DOI: 10.1111/j.1600-0854.2009.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bielas SL, et al. , Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet, 2009. 41(9): p. 1032–6. DOI: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloomfield SA and Volgyi B, The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci, 2009. 10(7): p. 495–506. DOI: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bretscher A, Edwards K, and Fehon RG, ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol, 2002. 3(8): p. 586–99. DOI: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 22.Broekhuyse RM, et al. , Light induced shift and binding of S-antigen in retinal rods. Curr Eye Res, 1985. 4(5): p. 613–8. DOI: 10.3109/02713688508999993. [DOI] [PubMed] [Google Scholar]
- 23.Brooks C, et al. , Farnesylation of the Transducin G Protein Gamma Subunit Is a Prerequisite for Its Ciliary Targeting in Rod Photoreceptors. Front Mol Neurosci, 2018. 11: p. 16. DOI: 10.3389/fnmol.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown BM, et al. , Light-driven translocation of the protein phosphatase 2A complex regulates light/dark dephosphorylation of phosducin and rhodopsin. Biochemistry, 2002. 41(46): p. 13526–38. [DOI] [PubMed] [Google Scholar]
- 25.Burgoyne T, et al. , Rod disc renewal occurs by evagination of the ciliary plasma membrane that makes cadherin-based contacts with the inner segment. Proc Natl Acad Sci U S A, 2015. 112(52): p. 15922–7. DOI: 10.1073/pnas.1509285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calvert PD, Klenchin VA, and Bownds MD, Rhodopsin kinase inhibition by recoverin. Function of recoverin myristoylation. J Biol Chem, 1995. 270(41): p. 24127–9. [DOI] [PubMed] [Google Scholar]
- 27.Calvert PD, et al. , Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol, 2006. 16(11): p. 560–8. DOI: S0962–8924(06)00237–6 [pii] 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Calvert PD, Schiesser WE, and Pugh EN Jr., Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J Gen Physiol, 2010. 135(3): p. 173–96. DOI: jgp.200910322 [pii] 10.1085/jgp.200910322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantagrel V, et al. , Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet, 2008. 83(2): p. 170–9. DOI: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Y, et al. , Mechanism for Selective Synaptic Wiring of Rod Photoreceptors into the Retinal Circuitry and Its Role in Vision. Neuron, 2015. 87(6): p. 1248–1260. DOI: 10.1016/j.neuron.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Y, et al. , Interplay between cell-adhesion molecules governs synaptic wiring of cone photoreceptors. Proc Natl Acad Sci U S A, 2020. 117(38): p. 23914–23924. DOI: 10.1073/pnas.2009940117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cen O, et al. , Identification of UNC119 as a novel activator of SRC-type tyrosine kinases. J Biol Chem, 2003. 278(10): p. 8837–45. DOI: 10.1074/jbc.M208261200. [DOI] [PubMed] [Google Scholar]
- 33.Chadha A, et al. , The route of the visual receptor rhodopsin along the cilium. J Cell Sci, 2019. 132(10). DOI: 10.1242/jcs.229526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CK, et al. , Ca(2+)-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem, 1995. 270(30): p. 18060–6. [DOI] [PubMed] [Google Scholar]
- 35.Chuang J-Z, Zhao Y, and Sung C-H, SARA-Regulated Vesicular Targeting Underlies Formation of the Light-Sensing Organelle in Mammalian Rods. Cell, 2007. 130(3): p. 535–547. DOI: 10.1016/j.cell.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coleman DL and Eicher EM, Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered, 1990. 81(6): p. 424–7. DOI: 10.1093/oxfordjournals.jhered.a111019. [DOI] [PubMed] [Google Scholar]
- 37.Conley SM, Al-Ubaidi MR, and Naash MI, The Role of the Prph2 C-Terminus in Outer Segment Morphogenesis. Adv Exp Med Biol, 2019. 1185: p. 495–499. DOI: 10.1007/978-3-030-27378-1_81. [DOI] [PubMed] [Google Scholar]
- 38.Conley SM, et al. , Prph2 initiates outer segment morphogenesis but maturation requires Prph2/Rom1 oligomerization. Hum Mol Genet, 2019. 28(3): p. 459–475. DOI: 10.1093/hmg/ddy359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Datta P, et al. , Accumulation of non-outer segment proteins in the outer segment underlies photoreceptor degeneration in Bardet–Biedl syndrome. Proceedings of the National Academy of Sciences, 2015. 112(32): p. E4400–E4409. DOI: 10.1073/pnas.1510111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Matteis M, Godi A, and Corda D, Phosphoinositides and the golgi complex. Curr Opin Cell Biol, 2002. 14(4): p. 434–47. DOI: 10.1016/s0955-0674(02)00357-5. [DOI] [PubMed] [Google Scholar]
- 41.Deretic D and Papermaster DS, Polarized sorting of rhodopsin on post-Golgi membranes in frog retinal photoreceptor cells. J Cell Biol, 1991. 113(6): p. 1281–93. DOI: 10.1083/jcb.113.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deretic D, et al. , Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc Natl Acad Sci U S A, 1998. 95(18): p. 10620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deretic D, et al. , Phosphoinositides, ezrin/moesin, and rac1 regulate fusion of rhodopsin transport carriers in retinal photoreceptors. Mol Biol Cell, 2004. 15(1): p. 359–70. DOI: 10.1091/mbc.e03-04-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deretic D, et al. , Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proceedings of the National Academy of Sciences, 2005. 102(9): p. 3301–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deretic D and Mazelova J, Assay for in vitro budding of ciliary-targeted rhodopsin transport carriers. Methods Cell Biol, 2009. 94: p. 241–57. DOI: 10.1016/s0091-679x(08)94012-7. [DOI] [PubMed] [Google Scholar]
- 46.Deretic D and Wang J, Molecular assemblies that control rhodopsin transport to the cilia. Vision Res, 2012. 75: p. 5–10. DOI: 10.1016/j.visres.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deretic D, Lorentzen E, and Fresquez T, The ins and outs of the Arf4-based ciliary membrane-targeting complex. Small GTPases, 2019: p. 1–12. DOI: 10.1080/21541248.2019.1616355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dilan TL, et al. , Bardet-Biedl syndrome-8 (BBS8) protein is crucial for the development of outer segments in photoreceptor neurons. Hum Mol Genet, 2018. 27(2): p. 283–294. DOI: 10.1093/hmg/ddx399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dilan TL, et al. , ARL13B, a Joubert Syndrome-Associated Protein, Is Critical for Retinogenesis and Elaboration of Mouse Photoreceptor Outer Segments. J Neurosci, 2019. 39(8): p. 1347–1364. DOI: 10.1523/JNEUROSCI.1761-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding J-D, Salinas RY, and Arshavsky VY, Discs of mammalian rod photoreceptors form through the membrane evagination mechanism. The Journal of Cell Biology, 2015. 211(3): p. 495–502. DOI: 10.1083/jcb.201508093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dizhoor AM, et al. , The NH2 terminus of retinal recoverin is acylated by a small family of fatty acids. J Biol Chem, 1992. 267(23): p. 16033–6. [PubMed] [Google Scholar]
- 52.Domire JS, et al. , Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cellular and Molecular Life Sciences, 2011. 68(17): p. 2951–2960. DOI: 10.1007/s00018-010-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donaldson JG, Arfs, phosphoinositides and membrane traffic. Biochem Soc Trans, 2005. 33(Pt 6): p. 1276–8. DOI: 10.1042/BST20051276. [DOI] [PubMed] [Google Scholar]
- 54.Elias RV, et al. , Temporal kinetics of the light/dark translocation and compartmentation of arrestin and alpha-transducin in mouse photoreceptor cells. Mol Vis, 2004. 10: p. 672–81. [PubMed] [Google Scholar]
- 55.Evans RJ, et al. , The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum Mol Genet, 2010. 19(7): p. 1358–67. DOI: 10.1093/hmg/ddq012. [DOI] [PubMed] [Google Scholar]
- 56.Finkelstein S, et al. , Phosphoinositide Profile of the Mouse Retina. Cells, 2020. 9(6). DOI: 10.3390/cells9061417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fliesler SJ, Rayborn ME, and Hollyfield JG, Membrane morphogenesis in retinal rod outer segments: inhibition by tunicamycin. J Cell Biol, 1985. 100(2): p. 574–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Follit JA, et al. , The Intraflagellar Transport Protein IFT20 Is Associated with the Golgi Complex and Is Required for Cilia Assembly. Molecular Biology of the Cell, 2006. 17(9): p. 3781–3792. DOI: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fotiadis D, et al. , The G protein-coupled receptor rhodopsin in the native membrane. FEBS Lett, 2004. 564(3): p. 281–8. DOI: 10.1016/S0014-5793(04)00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fotiadis D, et al. , Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors. Curr Opin Struct Biol, 2006. 16(2): p. 252–259. DOI: 10.1016/j.sbi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Fukada Y, et al. , Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature, 1990. 346(6285): p. 658–60. [DOI] [PubMed] [Google Scholar]
- 62.Furukawa T, Ueno A, and Omori Y, Molecular mechanisms underlying selective synapse formation of vertebrate retinal photoreceptor cells. Cell Mol Life Sci, 2020. 77(7): p. 1251–1266. DOI: 10.1007/s00018-019-03324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaudet R, Bohm A, and Sigler PB, Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell, 1996. 87(3): p. 577–88. [DOI] [PubMed] [Google Scholar]
- 64.George AA, et al. , Synaptojanin 1 is required for endolysosomal trafficking of synaptic proteins in cone photoreceptor inner segments. PloS one, 2014. 9(1): p. e84394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilliam JC, et al. , Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell, 2012. 151(5): p. 1029–41. DOI: 10.1016/j.cell.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Godi A, et al. , FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol, 2004. 6(5): p. 393–404. DOI: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- 67.Gosens I, et al. , Composition and function of the Crumbs protein complex in the mammalian retina. Exp Eye Res, 2008. 86(5): p. 713–26. DOI: 10.1016/j.exer.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Gospe SM 3rd, Baker SA, and Arshavsky VY, Facilitative glucose transporter Glut1 is actively excluded from rod outer segments. J Cell Sci, 2010. 123(Pt 21): p. 3639–44. DOI: 10.1242/jcs.072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gotthardt K, et al. , A G-protein activation cascade from Arl13B to Arl3 and implications for ciliary targeting of lipidated proteins. eLife, 2015. 4: p. e11859. DOI: 10.7554/eLife.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Granzin J, et al. , X-ray crystal structure of arrestin from bovine rod outer segments. Nature, 1998. 391(6670): p. 918–21. DOI: 10.1038/36147. [DOI] [PubMed] [Google Scholar]
- 71.Grayson C, et al. , Localization in the human retina of the X-linked retinitis pigmentosa protein RP2, its homologue cofactor C and the RP2 interacting protein Arl3. Hum Mol Genet, 2002. 11(24): p. 3065–74. DOI: 10.1093/hmg/11.24.3065. [DOI] [PubMed] [Google Scholar]
- 72.Gu S, et al. , Tubby-like protein-1 mutations in autosomal recessive retinitis pigmentosa. Lancet, 1998. 351(9109): p. 1103–4. DOI: 10.1016/s0140-6736(05)79384-3. [DOI] [PubMed] [Google Scholar]
- 73.Gunkel M, et al. , Higher-order architecture of rhodopsin in intact photoreceptors and its implication for phototransduction kinetics. Structure, 2015. 23(4): p. 628–38. DOI: 10.1016/j.str.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 74.Haeseleer F, Interaction and colocalization of CaBP4 and Unc119 (MRG4) in photoreceptors. Invest Ophthalmol Vis Sci, 2008. 49(6): p. 2366–75. DOI: 10.1167/iovs.07-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hagstrom SA, et al. , Retinal Degeneration in tulp1 −/− Mice: Vesicular Accumulation in the Interphotoreceptor Matrix. Investigative Ophthalmology & Visual Science, 1999. 40(12): p. 2795–2802. [PubMed] [Google Scholar]
- 76.Hanke-Gogokhia C, et al. , Arf-like protein 3 (ARL3) regulates protein trafficking and ciliogenesis in mouse photoreceptors. Journal of Biological Chemistry, 2016. DOI: 10.1074/jbc.M115.710954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanke-Gogokhia C, et al. , The guanine nucleotide exchange factor Arf-like protein 13b is essential for assembly of the mouse photoreceptor transition zone and outer segment. J Biol Chem, 2017. 292(52): p. 21442–21456. DOI: 10.1074/jbc.RA117.000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hanson SM, et al. , Structure and function of the visual arrestin oligomer. EMBO J, 2007. 26(6): p. 1726–36. DOI: 10.1038/sj.emboj.7601614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanson SM, et al. , A model for the solution structure of the rod arrestin tetramer. Structure, 2008. 16(6): p. 924–34. DOI: 10.1016/j.str.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heidelberger R, Thoreson WB, and Witkovsky P, Synaptic transmission at retinal ribbon synapses. Progress in retinal and eye research, 2005. 24(6): p. 682–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hendrickson A, et al. , Rod photoreceptor differentiation in fetal and infant human retina. Exp Eye Res, 2008. 87(5): p. 415–26. DOI: 10.1016/j.exer.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirsch JA, et al. , The 2.8 A crystal structure of visual arrestin: a model for arrestin’s regulation. Cell, 1999. 97(2): p. 257–69. DOI: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 83.Holopainen JM, et al. , Interaction and localization of the retinitis pigmentosa protein RP2 and NSF in retinal photoreceptor cells. Biochemistry, 2010. 49(35): p. 7439–47. DOI: 10.1021/bi1005249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsu Y, et al. , BBSome function is required for both the morphogenesis and maintenance of the photoreceptor outer segment. PLoS Genet, 2017. 13(10): p. e1007057. DOI: 10.1371/journal.pgen.1007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsu Y, et al. , the absence of BBSome function decreases synaptogenesis and causes ectopic synapse formation in the retina. Scientific Reports, 2020. 10(1): p. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang SP, Brown BM, and Craft CM, Visual Arrestin 1 acts as a modulator for N-ethylmaleimide-sensitive factor in the photoreceptor synapse. J Neurosci, 2010. 30(28): p. 9381–91. DOI: 10.1523/JNEUROSCI.1207-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hubbell WL, et al. , Rhodopsin structure, dynamics, and activation: A perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking, in Advances in Protein Chemistry. 2003, Academic Press. p. 243–290. [DOI] [PubMed] [Google Scholar]
- 88.Humrich J, et al. , Regulation of phosducin-like protein by casein kinase 2 and N-terminal splicing. J Biol Chem, 2003. 278(7): p. 4474–81. DOI: 10.1074/jbc.M206347200. [DOI] [PubMed] [Google Scholar]
- 89.Hunter DD, et al. , CNS synapses are stabilized trans-synaptically by laminins and laminin-interacting proteins. J Comp Neurol, 2017. DOI: 10.1002/cne.24338. [DOI] [PubMed] [Google Scholar]
- 90.Ikeda S, et al. , Retinal degeneration but not obesity is observed in null mutants of the tubby-like protein 1 gene. Hum Mol Genet, 2000. 9(2): p. 155–63. DOI: 10.1093/hmg/9.2.155. [DOI] [PubMed] [Google Scholar]
- 91.Imamoto Y, et al. , Concentration-dependent tetramerization of bovine visual arrestin. Biophys J, 2003. 85(2): p. 1186–95. DOI: 10.1016/S0006-3495(03)74554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inglese J, et al. , Isoprenylation of a protein kinase. Requirement of farnesylation/alpha-carboxyl methylation for full enzymatic activity of rhodopsin kinase. J Biol Chem, 1992. 267(3): p. 1422–5. [PubMed] [Google Scholar]
- 93.Insinna C, et al. , Different roles for KIF17 and kinesin II in photoreceptor development and maintenance. Dev Dyn, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ismail SA, et al. , Structural basis for Arl3-specific release of myristoylated ciliary cargo from UNC119. EMBO J, 2012. 31(20): p. 4085–94. DOI: 10.1038/emboj.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Janson LW, Ragsdale K, and Luby-Phelps K, Mechanism and size cutoff for steric exclusion from actin-rich cytoplasmic domains. Biophys J, 1996. 71(3): p. 1228–34. DOI: S0006–3495(96)79367–0 [pii] 10.1016/S0006-3495(96)79367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang J, et al. , Depletion of BBS Protein LZTFL1 Affects Growth and Causes Retinal Degeneration in Mice. J Genet Genomics, 2016. 43(6): p. 381–91. DOI: 10.1016/j.jgg.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang L, et al. , Kinesin family 17 (osmotic avoidance abnormal‐3) is dispensable for photoreceptor morphology and function. The FASEB Journal, 2015. 29(12): p. 4866–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang L, et al. , Heterotrimeric kinesin-2 (KIF3) mediates transition zone and axoneme formation of mouse photoreceptors. Journal of Biological Chemistry, 2015. 290(20): p. 12765–12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jimeno D, et al. , Analysis of kinesin-2 function in photoreceptor cells using synchronous Cre-loxP knockout of Kif3a with RHO-Cre. Invest Ophthalmol Vis Sci, 2006. 47(11): p. 5039–46. DOI: 10.1167/iovs.06-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnson JE Jr., et al. , Spatiotemporal regulation of ATP and Ca2+ dynamics in vertebrate rod and cone ribbon synapses. Mol Vis, 2007. 13: p. 887–919. [PMC free article] [PubMed] [Google Scholar]
- 101.Kakakhel M, et al. , Syntaxin 3 is essential for photoreceptor outer segment protein trafficking and survival. Proceedings of the National Academy of Sciences, 2020. 117(34): p. 20615–20624. DOI: 10.1073/pnas.2010751117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kandachar V, et al. , An interaction network between the SNARE VAMP7 and Rab GTPases within a ciliary membrane-targeting complex. J Cell Sci, 2018. 131(24). DOI: 10.1242/jcs.222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kawamura S, Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature, 1993. 362(6423): p. 855–7. [DOI] [PubMed] [Google Scholar]
- 104.Kawamura S, et al. , Recoverin has S-modulin activity in frog rods. J Biol Chem, 1993. 268(20): p. 14579–82. [PubMed] [Google Scholar]
- 105.Keady BT, Le YZ, and Pazour GJ, IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol Biol Cell, 2011. 22(7): p. 921–30. DOI: 10.1091/mbc.E10-09-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim M, et al. , Robust self-association is a common feature of mammalian visual arrestin-1. Biochemistry, 2011. 50(12): p. 2235–42. DOI: 10.1021/bi1018607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kizhatil K, et al. , Ankyrin-B is required for coordinated expression of beta-2-spectrin, the Na/K-ATPase and the Na/Ca exchanger in the inner segment of rod photoreceptors. Exp Eye Res, 2009. 88(1): p. 57–64. DOI: 10.1016/j.exer.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 108.Klenchin VA, Calvert PD, and Bownds MD, Inhibition of rhodopsin kinase by recoverin. Further evidence for a negative feedback system in phototransduction. J Biol Chem, 1995. 270(27): p. 16147–52. [DOI] [PubMed] [Google Scholar]
- 109.Kobayashi A, et al. , Photoreceptor synaptic protein HRG4 (UNC119) interacts with ARL2 via a putative conserved domain. FEBS Lett, 2003. 534(1–3): p. 26–32. DOI: 10.1016/s0014-5793(02)03766-3. [DOI] [PubMed] [Google Scholar]
- 110.Kolandaivelu S, et al. , AIPL1, a protein associated with childhood blindness, interacts with alpha-subunit of rod phosphodiesterase (PDE6) and is essential for its proper assembly. J Biol Chem, 2009. 284(45): p. 30853–61. DOI: 10.1074/jbc.M109.036780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krispel CM, et al. , Phosducin regulates the expression of transducin betagamma subunits in rod photoreceptors and does not contribute to phototransduction adaptation. J Gen Physiol, 2007. 130(3): p. 303–12. DOI: 10.1085/jgp.200709812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krock BL and Perkins BD, The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle–kinesin-II dissociation in vertebrate photoreceptors. Journal of cell science, 2008. 121(11): p. 1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Krock BL, Mills-Henry I, and Perkins BD, Retrograde intraflagellar transport by cytoplasmic dynein-2 is required for outer segment extension in vertebrate photoreceptors but not arrestin translocation. Investigative ophthalmology & visual science, 2009. 50(11): p. 5463–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lai RK, et al. , The gamma subunit of transducin is farnesylated. Proc Natl Acad Sci U S A, 1990. 87(19): p. 7673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Laird JG, et al. , Identification of a VxP Targeting Signal in the Flagellar Na(+) /K(+) -ATPase. Traffic, 2015. 16(12): p. 1239–53. DOI: 10.1111/tra.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee RH, Lieberman BS, and Lolley RN, A novel complex from bovine visual cells of a 33,000-dalton phosphoprotein with beta- and gamma-transducin: purification and subunit structure. Biochemistry, 1987. 26(13): p. 3983–90. DOI: 10.1021/bi00387a036. [DOI] [PubMed] [Google Scholar]
- 117.Lee S, et al. , Actin filaments partition primary cilia membranes into distinct fluid corrals. J Cell Biol, 2018. 217(8): p. 2831–2849. DOI: 10.1083/jcb.201711104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee SH, et al. , Export of membrane proteins from the Golgi complex to the primary cilium requires the kinesin motor, KIFC1. Faseb j, 2018. 32(2): p. 957–968. DOI: 10.1096/fj.201700563R. [DOI] [PubMed] [Google Scholar]
- 119.Lenoir M and Overduin M, PtdIns(4)P signalling and recognition systems. Adv Exp Med Biol, 2013. 991: p. 59–83. DOI: 10.1007/978-94-007-6331-9_5. [DOI] [PubMed] [Google Scholar]
- 120.Liu Q, et al. , The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics, 2007. 6(8): p. 1299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu Y and Bankaitis VA, Phosphoinositide phosphatases in cell biology and disease. Prog Lipid Res, 2010. 49(3): p. 201–17. DOI: 10.1016/j.plipres.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lobo GP, et al. , The exocyst is required for photoreceptor ciliogenesis and retinal development. J Biol Chem, 2017. 292(36): p. 14814–14826. DOI: 10.1074/jbc.M117.795674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lodowski KH, et al. , Signals governing the trafficking and mistrafficking of a ciliary GPCR, rhodopsin. J Neurosci, 2013. 33(34): p. 13621–38. DOI: 10.1523/jneurosci.1520-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Loew A, et al. , Phosducin induces a structural change in transducin beta gamma. Structure, 1998. 6(8): p. 1007–19. DOI: 10.1016/s0969-2126(98)00102-6. [DOI] [PubMed] [Google Scholar]
- 125.Luby-Phelps K, Taylor DL, and Lanni F, Probing the structure of cytoplasm. J Cell Biol, 1986. 102(6): p. 2015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luo N, et al. , OCRL localizes to the primary cilium: a new role for cilia in Lowe syndrome. Hum Mol Genet, 2012. 21(15): p. 3333–44. DOI: 10.1093/hmg/dds163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maddox JW, et al. , A dual role for Cav1.4 Ca(2+) channels in the molecular and structural organization of the rod photoreceptor synapse. Elife, 2020. 9. DOI: 10.7554/eLife.62184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Makino CL, et al. , Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J Gen Physiol, 2004. 123(6): p. 729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marszalek JR, et al. , Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell, 2000. 102(2): p. 175–87. DOI: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 130.Maza NA, Schiesser WE, and Calvert PD, An intrinsic compartmentalization code for peripheral membrane proteins in photoreceptor neurons. The Journal of Cell Biology, 2019. 218(11): p. 3753–3772. DOI: 10.1083/jcb.201906024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mazelova J, et al. , Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J, 2009. 28(3): p. 183–92. DOI: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mazelova J, et al. , Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci, 2009. 122(Pt 12): p. 2003–13. DOI: 10.1242/jcs.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mendez A, et al. , Light-dependent translocation of arrestin in the absence of rhodopsin phosphorylation and transducin signaling. J Neurosci, 2003. 23(8): p. 3124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mercer AJ, Chen M, and Thoreson WB, Lateral mobility of presynaptic L-type calcium channels at photoreceptor ribbon synapses. Journal of Neuroscience, 2011. 31(12): p. 4397–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mercer AJ and Thoreson WB, The dynamic architecture of photoreceptor ribbon synapses: cytoskeletal, extracellular matrix, and intramembrane proteins. Vis Neurosci, 2011. 28(6): p. 453–71. DOI: 10.1017/S0952523811000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Minton AP, Confinement as a determinant of macromolecular structure and reactivity. Biophys J, 1992. 63(4): p. 1090–100. DOI: S0006–3495(92)81663–6 [pii] 10.1016/S0006-3495(92)81663-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Minton AP, Influence of excluded volume upon macromolecular structure and associations in ‘crowded’ media. Curr Opin Biotechnol, 1997. 8(1): p. 65–9. [DOI] [PubMed] [Google Scholar]
- 138.Molday RS and Molday LL, Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin-gold-dextran density perturbation method. J Cell Biol, 1987. 105(6 Pt 1): p. 2589–601. DOI: 10.1083/jcb.105.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Molday RS and Molday LL, Molecular properties of the cGMP-gated channel of rod photoreceptors. Vision Res, 1998. 38(10): p. 1315–23. DOI: 10.1016/s0042-6989(97)00409-4. [DOI] [PubMed] [Google Scholar]
- 140.Molday RS and Goldberg AFX, Peripherin diverts ciliary ectosome release to photoreceptor disc morphogenesis. J Cell Biol, 2017. 216(5): p. 1227–1229. DOI: 10.1083/jcb.201703020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moritz OL, et al. , Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell, 2001. 12(8): p. 2341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moser T, Grabner CP, and Schmitz F, Sensory processing at ribbon synapses in the retina and the cochlea. Physiological reviews, 2020. 100(1): p. 103–144. [DOI] [PubMed] [Google Scholar]
- 143.Muresan V, Lyass A, and Schnapp BJ, The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. Journal of Neuroscience, 1999. 19(3): p. 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Murray AR, Fliesler SJ, and Al-Ubaidi MR, Rhodopsin: The Functional Significance of Asn-Linked Glycosylation and Other Post-Translational Modifications. Ophthalmic Genetics, 2009. 30(3): p. 109–120. DOI: 10.1080/13816810902962405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nair KS, et al. , Direct binding of visual arrestin to microtubules determines the differential subcellular localization of its splice variants in rod photoreceptors. J Biol Chem, 2004. 279(39): p. 41240–8. DOI: 10.1074/jbc.M406768200. [DOI] [PubMed] [Google Scholar]
- 146.Nair KS, et al. , Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron, 2005. 46(4): p. 555–67. DOI: S0896–6273(05)00279–5 [pii] 10.1016/j.neuron.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Najafi M, Maza NA, and Calvert PD, Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc Natl Acad Sci U S A, 2012. 109(1): p. 203–8. DOI: 10.1073/pnas.1115109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nathans J, Rhodopsin: structure, function, and genetics. Biochemistry, 1992. 31(21): p. 4923–31. DOI: 10.1021/bi00136a001. [DOI] [PubMed] [Google Scholar]
- 149.Nemet I, Tian G, and Imanishi Y, Submembrane assembly and renewal of rod photoreceptor cGMP-gated channel: insight into the actin-dependent process of outer segment morphogenesis. J Neurosci, 2014. 34(24): p. 8164–74. DOI: 10.1523/JNEUROSCI.1282-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nickell S, et al. , Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol, 2007. 177(5): p. 917–25. DOI: jcb.200612010 [pii] 10.1083/jcb.200612010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nishimura DY, et al. , Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proceedings of the National Academy of Sciences, 2004. 101(47): p. 16588–16593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Norton AW, et al. , Evaluation of the 17-kDa prenyl-binding protein as a regulatory protein for phototransduction in retinal photoreceptors. J Biol Chem, 2005. 280(2): p. 1248–56. DOI: 10.1074/jbc.M410475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ohlemiller KK, et al. , Cochlear and retinal degeneration in the tubby mouse. Neuroreport, 1995. 6(6): p. 845–9. DOI: 10.1097/00001756-199504190-00005. [DOI] [PubMed] [Google Scholar]
- 154.Ohlemiller KK, et al. , The murine tub (rd5) mutation is not associated with a primary axonemal defect. Cell Tissue Res, 1998. 291(3): p. 489–95. DOI: 10.1007/s004410051018. [DOI] [PubMed] [Google Scholar]
- 155.Orisme W, et al. , Light-dependent translocation of arrestin in rod photoreceptors is signaled through a phospholipase C cascade and requires ATP. Cellular Signalling, 2010. 22(3): p. 447–456. DOI: 10.1016/j.cellsig.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Otsu W, et al. , The Late Endosomal Pathway Regulates the Ciliary Targeting of Tetraspanin Protein Peripherin 2. J Neurosci, 2019. 39(18): p. 3376–3393. DOI: 10.1523/jneurosci.2811-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pan Y, et al. , A di-arginine ER retention signal regulates trafficking of HCN1 channels from the early secretory pathway to the plasma membrane. Cell Mol Life Sci, 2015. 72(4): p. 833–43. DOI: 10.1007/s00018-014-1705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pan Y, et al. , An N-terminal ER export signal facilitates the plasma membrane targeting of HCN1 channels in photoreceptors. Investigative ophthalmology & visual science, 2015. 56(6): p. 3514–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Papermaster DS, et al. , Biosynthesis and vectorial transport of opsin on vesicles in retinal rod photoreceptors. Journal of Histochemistry & Cytochemistry, 1986. 34(1): p. 5–16. [DOI] [PubMed] [Google Scholar]
- 160.Pazour GJ, et al. , The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol, 2002. 157(1): p. 103–13. DOI: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pearring JN, et al. , R9AP targeting to rod outer segments is independent of rhodopsin and is guided by the SNARE homology domain. Mol Biol Cell, 2014. 25(17): p. 2644–9. DOI: 10.1091/mbc.E14-02-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pearring JN, et al. , Guanylate cyclase 1 relies on rhodopsin for intracellular stability and ciliary trafficking. eLife, 2015. 4: p. e12058. DOI: 10.7554/eLife.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pearring JN, et al. , Loss of Arf4 causes severe degeneration of the exocrine pancreas but not cystic kidney disease or retinal degeneration. PLoS Genet, 2017. 13(4): p. e1006740. DOI: 10.1371/journal.pgen.1006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Peet JA, et al. , Quantification of the cytoplasmic spaces of living cells with EGFP reveals arrestin-EGFP to be in disequilibrium in dark adapted rod photoreceptors. J Cell Sci, 2004. 117(Pt 14): p. 3049–59. DOI: 10.1242/jcs.01167 117/14/3049 [pii]. [DOI] [PubMed] [Google Scholar]
- 165.Peterson JJ, et al. , Arrestin migrates in photoreceptors in response to light: a study of arrestin localization using an arrestin-GFP fusion protein in transgenic frogs. Exp Eye Res, 2003. 76(5): p. 553–63. [DOI] [PubMed] [Google Scholar]
- 166.Peterson JJ, et al. , A role for cytoskeletal elements in the light-driven translocation of proteins in rod photoreceptors. Invest Ophthalmol Vis Sci, 2005. 46(11): p. 3988–98. DOI: 10.1167/iovs.05-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ploier B, et al. , Dimerization deficiency of enigmatic retinitis pigmentosa-linked rhodopsin mutants. Nature Communications, 2016. 7: p. 12832. DOI: 10.1038/ncomms1283210.1038/ncomms12832http://dharmasastra.live.cf.private.springer.com/articles/ncomms12832#supplementary-informationhttp://dharmasastra.live.cf.private.springer.com/articles/ncomms12832#supplementary-information . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Poetsch A, Molday LL, and Molday RS, The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J Biol Chem, 2001. 276(51): p. 48009–16. [DOI] [PubMed] [Google Scholar]
- 169.Polgar N and Fogelgren B, Regulation of Cell Polarity by Exocyst-Mediated Trafficking. Cold Spring Harb Perspect Biol, 2018. 10(3). DOI: 10.1101/cshperspect.a031401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pretorius PR, et al. , Identification and functional analysis of the vision-specific BBS3 (ARL6) long isoform. PLoS Genet, 2010. 6(3): p. e1000884. DOI: 10.1371/journal.pgen.1000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pugh EN Jr. and Lamb TD, Phototransduction in Vertebrate Rods and Cones: Molecular Mechanisms of Amplification, Recovery and Light Adaptation, in Hanbook of Biological Physics, Stavenga DG, de Grip WJ, and Pugh EN Jr., Editors. 2000, Elsevier Science B. V. p. 183–255. [Google Scholar]
- 172.Qureshi BM, et al. , Mechanistic insights into the role of prenyl-binding protein PrBP/delta in membrane dissociation of phosphodiesterase 6. Nat Commun, 2018. 9(1): p. 90. DOI: 10.1038/s41467-017-02569-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ramamurthy V, et al. , AIPL1, a protein implicated in Leber’s congenital amaurosis, interacts with and aids in processing of farnesylated proteins. Proc Natl Acad Sci U S A, 2003. 100(22): p. 12630–5. DOI: 10.1073/pnas.2134194100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ramamurthy V, et al. , Numb regulates the polarized delivery of cyclic nucleotide-gated ion channels in rod photoreceptor cilia. J Neurosci, 2014. 34(42): p. 13976–87. DOI: 10.1523/jneurosci.1938-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Raven MA, et al. , Early afferent signaling in the outer plexiform layer regulates development of horizontal cell morphology. J Comp Neurol, 2008. 506(5): p. 745–58. DOI: 10.1002/cne.21526. [DOI] [PubMed] [Google Scholar]
- 176.Regus‐Leidig H, et al. , Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. Journal of Comparative Neurology, 2009. 512(6): p. 814–824. [DOI] [PubMed] [Google Scholar]
- 177.Ritter LM, et al. , In situ visualization of protein interactions in sensory neurons: glutamic acid-rich proteins (GARPs) play differential roles for photoreceptor outer segment scaffolding. J Neurosci, 2011. 31(31): p. 11231–43. DOI: 10.1523/JNEUROSCI.2875-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Robichaux MA, et al. , Defining the layers of a sensory cilium with STORM and cryoelectron nanoscopy. Proc Natl Acad Sci U S A, 2019. 116(47): p. 23562–23572. DOI: 10.1073/pnas.1902003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Roy K, et al. , Palmitoylation of the ciliary GTPase ARL13b is necessary for its stability and its role in cilia formation. J Biol Chem, 2017. 292(43): p. 17703–17717. DOI: 10.1074/jbc.M117.792937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sahly I, et al. , Localization of Usher 1 proteins to the photoreceptor calyceal processes, which are absent from mice. J Cell Biol, 2012. 199(2): p. 381–99. DOI: 10.1083/jcb.201202012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Salinas RY, et al. , A single valine residue plays an essential role in peripherin/rds targeting to photoreceptor outer segments. PLoS One, 2013. 8(1): p. e54292. DOI: 10.1371/journal.pone.0054292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Salinas RY, et al. , Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. The Journal of Cell Biology, 2017. 216(5): p. 1489–1499. DOI: 10.1083/jcb.201608081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Santagata S, et al. , G-protein signaling through tubby proteins. Science, 2001. 292(5524): p. 2041–50. DOI: 10.1126/science.1061233. [DOI] [PubMed] [Google Scholar]
- 184.Schietroma C, et al. , Usher syndrome type 1-associated cadherins shape the photoreceptor outer segment. J Cell Biol, 2017. 216(6): p. 1849–1864. DOI: 10.1083/jcb.201612030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schubert C, et al. , Visual arrestin activity may be regulated by self-association. J Biol Chem, 1999. 274(30): p. 21186–90. DOI: 10.1074/jbc.274.30.21186. [DOI] [PubMed] [Google Scholar]
- 186.Senapati S, et al. , Effect of dietary docosahexaenoic acid on rhodopsin content and packing in photoreceptor cell membranes. Biochim Biophys Acta Biomembr, 2018. 1860(6): p. 1403–1413. DOI: 10.1016/j.bbamem.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Senapati S and Park PS, Investigating the Nanodomain Organization of Rhodopsin in Native Membranes by Atomic Force Microscopy. Methods Mol Biol, 2019. 1886: p. 61–74. DOI: 10.1007/978-1-4939-8894-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sheng Z, et al. , Synaptic Ca2+ in darkness is lower in rods than cones, causing slower tonic release of vesicles. J Neurosci, 2007. 27(19): p. 5033–42. DOI: 10.1523/jneurosci.5386-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shilton BH, et al. , The solution structure and activation of visual arrestin studied by small-angle X-ray scattering. Eur J Biochem, 2002. 269(15): p. 3801–9. DOI: 10.1046/j.1432-1033.2002.03071.x. [DOI] [PubMed] [Google Scholar]
- 190.Simons DL, et al. , Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proc Natl Acad Sci U S A, 2011. 108(15): p. 6276–81. DOI: 10.1073/pnas.1019222108. [DOI] [PMC free article] [PubMed] [Google Scholar]; financial interest in the use of adeno-associated virus therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work.
- 191.Sinha S, et al. , Expression and subcellular distribution of UNC119a, a protein partner of transducin alpha subunit in rod photoreceptors. Cell Signal, 2013. 25(1): p. 341–8. DOI: 10.1016/j.cellsig.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Smith WC, et al. , Interaction of arrestin with enolase1 in photoreceptors. Invest Ophthalmol Vis Sci, 2011. 52(3): p. 1832–40. DOI: 10.1167/iovs.10-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sohocki MM, et al. , Prevalence of AIPL1 mutations in inherited retinal degenerative disease. Mol Genet Metab, 2000. 70(2): p. 142–50. DOI: 10.1006/mgme.2000.3001. [DOI] [PubMed] [Google Scholar]
- 194.Sokolov M, et al. , Phosducin facilitates light-driven transducin translocation in rod photoreceptors. Evidence from the phosducin knockout mouse. J Biol Chem, 2004. 279(18): p. 19149–56. DOI: 10.1074/jbc.M311058200 M311058200 [pii]. [DOI] [PubMed] [Google Scholar]
- 195.Sokolov M, et al. , Chaperones and retinal disorders. Adv Protein Chem Struct Biol, 2019. 114: p. 85–117. DOI: 10.1016/bs.apcsb.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 196.Song X, et al. , Arrestin-1 expression level in rods: balancing functional performance and photoreceptor health. Neuroscience, 2011. 174: p. 37–49. DOI: 10.1016/j.neuroscience.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Spencer WJ, et al. , Progressive Rod-Cone Degeneration (PRCD) Protein Requires N-Terminal S-Acylation and Rhodopsin Binding for Photoreceptor Outer Segment Localization and Maintaining Intracellular Stability. Biochemistry, 2016. 55(36): p. 5028–37. DOI: 10.1021/acs.biochem.6b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Spencer WJ, et al. , Photoreceptor disc membranes are formed through an Arp2/3-dependent lamellipodium-like mechanism. Proc Natl Acad Sci U S A, 2019. DOI: 10.1073/pnas.1913518117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Spencer WJ, et al. , Photoreceptor Discs: Built Like Ectosomes. Trends Cell Biol, 2020. 30(11): p. 904–915. DOI: 10.1016/j.tcb.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stabach PR, et al. , Ankyrin facilitates intracellular trafficking of alpha1-Na+-K+-ATPase in polarized cells. Am J Physiol Cell Physiol, 2008. 295(5): p. C1202–14. DOI: 10.1152/ajpcell.00273.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Strissel KJ, et al. , Recoverin undergoes light-dependent intracellular translocation in rod photoreceptors. J Biol Chem, 2005. 280(32): p. 29250–5. [DOI] [PubMed] [Google Scholar]
- 202.Strissel KJ, et al. , Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J Neurosci, 2006. 26(4): p. 1146–53. DOI: 26/4/1146 [pii] 10.1523/JNEUROSCI.4289-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Takei R, Katoh Y, and Nakayama K, Robust interaction of IFT70 with IFT52–IFT88 in the IFT-B complex is required for ciliogenesis. Biology open, 2018. 7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tam BM, Moritz OL, and Papermaster DS, The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol Biol Cell, 2004. 15(4): p. 2027–37. DOI: 10.1091/mbc.E03-09-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tam BM, et al. , Mislocalized rhodopsin does not require activation to cause retinal degeneration and neurite outgrowth in Xenopus laevis. J Neurosci, 2006. 26(1): p. 203–9. DOI: 10.1523/JNEUROSCI.3849-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tanaka T, et al. , Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature, 1995. 376(6539): p. 444–7. [DOI] [PubMed] [Google Scholar]
- 207.Tian G, et al. , An unconventional secretory pathway mediates the cilia targeting of peripherin/rds. J Neurosci, 2014. 34(3): p. 992–1006. DOI: 10.1523/jneurosci.3437-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.van de Pavert SA, Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. Journal of Cell Science, 2004. 117(18): p. 4169–4177. DOI: 10.1242/jcs.01301. [DOI] [PubMed] [Google Scholar]
- 209.van de Pavert SA, et al. , Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J Cell Sci, 2004. 117(Pt 18): p. 4169–77. DOI: 10.1242/jcs.01301. [DOI] [PubMed] [Google Scholar]
- 210.Van Hook MJ and Thoreson WB, Rapid synaptic vesicle endocytosis in cone photoreceptors of salamander retina. Journal of Neuroscience, 2012. 32(50): p. 18112–18123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Van Hook MJ, Nawy S, and Thoreson WB, Voltage-and calcium-gated ion channels of neurons in the vertebrate retina. Progress in retinal and eye research, 2019. 72: p. 100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Veltel S, et al. , Specificity of Arl2/Arl3 signaling is mediated by a ternary Arl3-effector-GAP complex. FEBS Lett, 2008. 582(17): p. 2501–7. DOI: 10.1016/j.febslet.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 213.Volland S, et al. , Three-dimensional organization of nascent rod outer segment disk membranes. Proc Natl Acad Sci U S A, 2015. 112(48): p. 14870–5. DOI: 10.1073/pnas.1516309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wahl S, Katiyar R, and Schmitz F, A local, periactive zone endocytic machinery at photoreceptor synapses in close vicinity to synaptic ribbons. Journal of Neuroscience, 2013. 33(25): p. 10278–10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wahl S, et al. , The Disease Protein Tulp1 Is Essential for Periactive Zone Endocytosis in Photoreceptor Ribbon Synapses. J Neurosci, 2016. 36(8): p. 2473–93. DOI: 10.1523/JNEUROSCI.2275-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang J, et al. , The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J, 2012. 31(20): p. 4057–71. DOI: 10.1038/emboj.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang J and Deretic D, Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res, 2014. 38: p. 1–19. DOI: 10.1016/j.preteyeres.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang J and Deretic D, The Arf and Rab11 effector FIP3 acts synergistically with ASAP1 to direct Rabin8 in ciliary receptor targeting. J Cell Sci, 2015. 128(7): p. 1375–85. DOI: 10.1242/jcs.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang J, et al. , The Arf GEF GBF1 and Arf4 synergize with the sensory receptor cargo, rhodopsin, to regulate ciliary membrane trafficking. J Cell Sci, 2017. 130(23): p. 3975–3987. DOI: 10.1242/jcs.205492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Weitz D, et al. , Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron, 2002. 36(5): p. 881–9. DOI: 10.1016/s0896-6273(02)01098-x. [DOI] [PubMed] [Google Scholar]
- 221.Wensel TG, Phosphoinositides in Retinal Function and Disease. Cells, 2020. 9(4). DOI: 10.3390/cells9040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wetzel RK, Arystarkhova E, and Sweadner KJ, Cellular and subcellular specification of Na,K-ATPase alpha and beta isoforms in the postnatal development of mouse retina. J Neurosci, 1999. 19(22): p. 9878–89. DOI: 10.1523/jneurosci.19-22-09878.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Whelan JP and McGinnis JF, Light-dependent subcellular movement of photoreceptor proteins. J Neurosci Res, 1988. 20(2): p. 263–70. [DOI] [PubMed] [Google Scholar]
- 224.Williams DS, Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res, 2008. 48(3): p. 433–41. DOI: 10.1016/j.visres.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wolfrum U and Schmitt A, Rhodopsin transport in the membrane of the connecting cilium of mammalian photoreceptor cells. Cell Motility and the Cytoskeleton, 2000. 46(2): p. 95–107. DOI: . [DOI] [PubMed] [Google Scholar]
- 226.Wright KJ, et al. , An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev, 2011. 25(22): p. 2347–60. DOI: 10.1101/gad.173443.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wright ZC, et al. , ARL3 regulates trafficking of prenylated phototransduction proteins to the rod outer segment. Hum Mol Genet, 2016. 25(10): p. 2031–2044. DOI: 10.1093/hmg/ddw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Xi Q, et al. , Tubby-like protein 1 (TULP1) interacts with F-actin in photoreceptor cells. Invest Ophthalmol Vis Sci, 2005. 46(12): p. 4754–61. DOI: 10.1167/iovs.05-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Xi Q, et al. , Interaction between the photoreceptor-specific tubby-like protein 1 and the neuronal-specific GTPase dynamin-1. Invest Ophthalmol Vis Sci, 2007. 48(6): p. 2837–44. DOI: 10.1167/iovs.06-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yadav RP, et al. , Interaction of the tetratricopeptide repeat domain of aryl hydrocarbon receptor-interacting protein-like 1 with the regulatory Pγ subunit of phosphodiesterase 6. J Biol Chem, 2019. 294(43): p. 15795–15807. DOI: 10.1074/jbc.RA119.010666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ye F, et al. , Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. eLife, 2013. 2. DOI: 10.7554/eLife.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ye F, Nager AR, and Nachury MV, BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J Cell Biol, 2018. DOI: 10.1083/jcb.201709041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ying G, et al. , Small GTPases Rab8a and Rab11a Are Dispensable for Rhodopsin Transport in Mouse Photoreceptors. PLoS One, 2016. 11(8): p. e0161236. DOI: 10.1371/journal.pone.0161236. [DOI] [PMC free article] [PubMed] [Google Scholar]; and the University of Florida own equity in the company AGTC that might in the future commercialize some aspect of this work. This does not alter the authors’ adherence to PLOS ONE policies on sharing data and materials.
- 234.Young RW, The renewal of photoreceptor cell outer segments. J Cell Biol, 1967. 33(1): p. 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Young RW and Droz B, The renewal of protein in retinal rods and cones. J Cell Biol, 1968. 39(1): p. 169–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Young RW and Bok D, Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol, 1969. 42(2): p. 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zabouri N and Haverkamp S, Calcium channel-dependent molecular maturation of photoreceptor synapses. PLoS One, 2013. 8(5): p. e63853. DOI: 10.1371/journal.pone.0063853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zeng J, et al. , Polarized exocytosis. Cold Spring Harbor Perspectives in Biology, 2017. 9(12): p. a027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang H, et al. , Light-dependent redistribution of visual arrestins and transducin subunits in mice with defective phototransduction. Mol Vis, 2003. 9: p. 231–7. [PubMed] [Google Scholar]
- 240.Zhang H, et al. , Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc Natl Acad Sci U S A, 2007. 104(21): p. 8857–62. DOI: 10.1073/pnas.0701681104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang H, et al. , UNC119 is required for G protein trafficking in sensory neurons. Nat Neurosci, 2011. 14(7): p. 874–80. DOI: 10.1038/nn.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhang P, et al. , In vivo optophysiology reveals that G-protein activation triggers osmotic swelling and increased light scattering of rod photoreceptors. Proceedings of the National Academy of Sciences, 2017. 114(14): p. E2937–E2946. DOI: 10.1073/pnas.1620572114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang Q, et al. , BBS7 is required for BBSome formation and its absence in mice results in Bardet-Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking. Journal of cell science, 2013. 126(11): p. 2372–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang Q, et al. , GTP-binding of ARL-3 is activated by ARL-13 as a GEF and stabilized by UNC119. Sci Rep, 2016. 6: p. 24534. DOI: 10.1038/srep24534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang T, et al. , Dimerization of visual pigments in vivo. Proc Natl Acad Sci U S A, 2016. 113(32): p. 9093–8. DOI: 10.1073/pnas.1609018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zheng J, Trudeau MC, and Zagotta WN, Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron, 2002. 36(5): p. 891–6. DOI: 10.1016/s0896-6273(02)01099-1. [DOI] [PubMed] [Google Scholar]
- 247.Zhong H, et al. , The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature, 2002. 420(6912): p. 193–8. DOI: 10.1038/nature01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhou HX, Rivas G, and Minton AP, Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys, 2008. 37: p. 375–97. DOI: 10.1146/annurev.biophys.37.032807.125817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhuang T, et al. , Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin. Proc Natl Acad Sci U S A, 2013. 110(3): p. 942–7. DOI: 10.1073/pnas.1215176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zimmerman SB and Minton AP, Macromolecular crowding: biochemical, biophysical, and physiological consequences. Annu Rev Biophys Biomol Struct, 1993. 22: p. 27–65. DOI: 10.1146/annurev.bb.22.060193.000331. [DOI] [PubMed] [Google Scholar]
- 251.Zozulya S and Stryer L, Calcium-myristoyl protein switch. Proc Natl Acad Sci U S A, 1992. 89(23): p. 11569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zulliger R, et al. , SNAREs Interact with Retinal Degeneration Slow and Rod Outer Segment Membrane Protein-1 during Conventional and Unconventional Outer Segment Targeting. PLoS One, 2015. 10(9): p. e0138508. DOI: 10.1371/journal.pone.0138508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zuo X, Guo W, and Lipschutz JH, The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Molecular biology of the cell, 2009. 20(10): p. 2522–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]


