Abstract
Objective:
Cognitive difficulties are a common complaint among patients with breast cancer and may adversely affect psychological well-being. In particular, problems with executive functioning (EF) may interfere with coping, which is known to influence depressive symptoms. The current study was designed to examine correlations between EF, coping, and depressive symptoms in breast cancer survivors and to longitudinally test the hypothesis that coping mediates the relationship between EF and depressive symptoms.
Methods:
Participants included 171 women with early-stage breast cancer assessed at the end of primary treatment with surgery, radiation, and/or chemotherapy and at 6 months, 1 year, and 2 years after treatment follow-ups as part of the Mind-Body Study. Participants completed questionnaires to assess subjective EF, approach and avoidant coping, and depressive symptoms, and neuropsychological testing was conducted to assess objective EF. Bivariate correlations were used to examine associations between EF, coping, and depressive symptoms. Mediation analyses were conducted using a bootstrapping approach (PROCESS).
Results:
At 1 year after treatment, objective and subjective EFs were correlated with avoidant coping (r = −0.172 [p = .024] and r = 0.297 [p < .001], respectively). In longitudinal analyses, use of the avoidant strategy behavioral disengagement at 1 year mediated the association between objective (95% bootstrap confidence interval = −0.282 to −0.042) and subjective (95% bootstrap confidence interval = 0.020 to 0.254) EFs at 6 months and depressive symptoms at 2 years.
Conclusions:
This study highlights how problems with EF during survivorship are associated with avoidant coping and depressive symptoms. Thus, these findings identify potential cognitive and affective targets for depression intervention in this population.
Keywords: executive functioning, coping, depression, breast cancer, cognitive dysfunction, emotion regulation
INTRODUCTION
Cognitive dysfunction is a commonly feared consequence of breast cancer treatment. Women with breast cancer report more cognitive difficulties than age-matched controls, with prevalence estimates ranging 21% to 90% (1,2). Difficulties with executive function (EF) are particularly common in breast cancer survivors who report cognitive problems (3). These include the higher-order cognitive processes of shifting (between tasks or mental sets), inhibition (ability to deliberately inhibit automatic responses), and updating (maintaining and manipulating information in working memory) (4). Disruptions in these cognitive processes are distressing and can affect quality of life (5). Indeed, survivors report that distress and reduced self-efficacy due to cognitive problems lead them to withdraw from work and avoid more demanding tasks (6).
Problems with EF may also negatively affect quality of life through interfering with women’s ability to cope with stressors and regulate their emotions, further exacerbating distress. Coping refers to cognitive, behavioral, and emotional efforts to manage specific external and/or internal demands that are appraised as taxing or exceeding one’s resources (7). Many coping strategies are explicitly or implicitly designed to manage one’s emotional state (8). Therefore, psychological adjustment is typically conceptualized and measured as an outcome of the coping process (9). Certain coping strategies are associated with better mental health outcomes within the cancer survivorship context. In particular, approach-oriented strategies, which are effortful attempts to engage with a stressor and the emotions it elicits, are associated with beneficial outcomes in cancer survivors, including lower depressive symptoms (10). Approach-oriented strategies include problem solving, emotional processing and expression, acceptance, and cognitive reappraisal (9). In contrast, avoidance-oriented coping, which is characterized by disengaging from a stressor and includes denial and behavioral and mental disengagement, is a consistent predictor of higher depressive symptoms in cancer survivors (10,11). Given the differences in cognitive resources required to engage versus disengage from a stressor and its associated emotions, approach and avoidant coping likely differentially rely on effortful cognitive processes such as EF.
Despite the potential relevance of EF for coping, only a handful of studies have evaluated the associations between EF and approach and avoidant coping strategies. We identified one study that evaluated these processes in a cancer sample: in a study of adult survivors of pediatric acute lymphocytic leukemia, better EF performance measured via objective neuropsychological (NP) testing was associated with the use of more approach-oriented coping strategies and less avoidant coping (12). Similar associations between EF and coping have also been detected across a spectrum of cognitive functioning—from healthy adults with intact cognitive functioning to those with frank impairment due to a traumatic brain injury (TBI). For example, in a study of middle-aged adults, women with better objective EF performance reported greater use of approach-oriented coping strategies (13). Similarly, in a sample of TBI patients, better objective EF correlated with greater use of approach coping strategies, whereas poorer objective EF correlated with greater use of avoidance-oriented coping strategies (14). In another sample of TBI patients, subjective EF difficulties correlated with a greater use of avoidance-oriented coping strategies (15). Of note, the use of avoidant strategies was found to mediate the relationship between EF problems and depressive symptoms in this study. Finally, in one study of breast cancer survivors evaluating the associations between coping and objective impairment in other cognitive domains (memory, verbal fluency), avoidant coping strategies mediated the associations between stress and NP performance (16). These data were collected cross-sectionally; therefore, it is difficult to interpret directionality. However, these results provide further evidence for the potential relations between cognitive problems and coping within the breast cancer context. Indeed, it has been proposed that self-regulatory processes such as coping may have a bidirectional association with cancer-related cognitive problems (17).
Although extant empirical evidence supporting the association between EF and coping is limited, there is a closely related and rich literature on the cognitive basis of emotion regulation. Emotion regulation is the process by which individuals influence their emotional experience—including what emotions they have and how they experience and express them (18)—and overlaps with coping in its focus on management of emotion (19). Certain emotion regulation strategies, such as cognitive reappraisal, are considered to be more cognitively involved and have been deemed more difficult and effortful than other strategies such as acceptance (20). Cognitive reappraisal involves rethinking the meaning of affectively charged stimuli or events in terms that alter their emotional impact (21). Effectively executing this regulation strategy requires shifting between perspectives while inhibiting automatic emotional responses and updating representations of the emotional stimulus in working memory (22).
Thus, EF is thought to underlie cognitive reappraisal and other cognitively demanding emotion regulation strategies (23). Indeed, brain imaging studies support an overlap in neural networks activated during tasks that engage EF and these cognitive emotion regulation strategies (24,25). Cognitive and affective scientists have linked individual differences in EF performance with emotion regulation ability and have shown that manipulating EF (through cognitive load or ego depletion) affects emotion regulation success (26). In addition, problems with EF have been reliably observed in patients with major depressive disorder, which is characterized by problems with emotion regulation (27,28). Given that cognitive complaints, and problems with EF in particular, are observed in breast cancer survivors and that breast cancer survivors are at increased risk for depressive symptoms (29), evaluating links between EF and depressive symptoms in this population is clinically important. Moreover, given the well-established relationship between coping and depressive outcomes in this population specifically (11), understanding how EF, coping, and depressive symptoms are associated during survivorship might illuminate cognitive and affective targets for intervention.
Therefore, the goal of the current study was to examine associations between EF, coping, and depressive symptoms in a longitudinal study of breast cancer survivors (Mind-Body Study, or MBS). The MBS involved an intensive assessment of cognitive and psychological function after primary breast cancer treatment and at 6 months, 1 year, and 2 years of follow-ups (30). Drawing from work on EF and emotion regulation/coping in other contexts (26), we hypothesized that greater problems with EF would be associated with lower use of approach-oriented coping and greater use of avoidance-oriented coping in cross-sectional analyses at 1 year after treatment. In addition, we hypothesized that coping would mediate the association between EF and depressive symptoms in longitudinal analyses.
METHODS
Participants
We used data from the MBS at the University of California, Los Angeles, a longitudinal study of breast cancer survivors aimed at characterizing the effects of endocrine therapy on cognitive functioning (30–33). Enrollment in the study began in May 2007, and data collection ended in July 2014. Inclusion criteria for the MBS were as follows: a) age 21 to 65 years; b) diagnosed with stage 0, I, II, or IIIA breast cancer; c) completed primary breast cancer treatments (surgery, radiation, and/or chemotherapy) within the past 3 months; d) had not started endocrine therapy; and e) proficient in the English language. Exclusion criteria were a) evidence of current or past disorder/disease of the central nervous system or any medical condition that might be expected to affect cognitive functioning (e.g., multiple sclerosis and thyroid dysfunction); b) history of head trauma with loss of consciousness greater than 30 minutes; c) epilepsy, dementia, or severe learning disability; d) current psychotic-spectrum disorder (e.g., schizophrenia), major affective disorder, or substance abuse or dependence; e) history of whole-brain irradiation or surgery; f) history of past cancer treatment with chemotherapy; g) active diagnosis of autoimmune and/or inflammatory disorder or disorders that may influence inflammatory processes; h) chronic use of oral steroid medication; and i) hormone therapy (estrogen, progestin compounds) other than vaginal estrogen. Current major affective disorder was assessed by asking if women had depressed mood and/or anhedonia nearly every day for 2 weeks; women were considered ineligible if they said yes to this question and were not under the care of a physician. For complete information on recruitment, screening, and exclusion criteria, please see Ganz et al. 2013 (32).
Procedures
Initial (baseline) study assessments were conducted within 3 months after the completion of primary treatment (surgery, radiation, and/or chemotherapy) and before the onset of endocrine therapy, if indicated. Follow-up assessments were conducted at 6 months and 1 year after treatment, with annual follow-up thereafter (up to 6 years after treatment). NP testing was conducted at baseline, 6-month, and 1-year assessments, and questionnaires were administered at each assessment point. Here, we focus on the 1-year assessment, when the coping measures were obtained, and the assessments that preceded (6 month) and followed (2 years) this time point for longitudinal analyses. A total of 191 women were enrolled, 175 completed the 6-month assessment, 175 completed the 1-year assessment (171 with NP data), and 152 completed the 2-year assessment.
Measures
Demographic and Clinical Characteristics
Demographic and clinical characteristics were obtained from both self-report and medical records at baseline. IQ was ascertained from the Wechsler Test of Adult Reading administered at baseline (34).
Objective Measures of Executive Functioning
Participants completed a full NP test battery (33,35); however, only tasks used to assess EF were included in the current analyses. Shifting was assessed using the Trails B task completion time from the Halstead-Reitan NP battery, where longer time indicates worse performance (36). Inhibition was assessed using the Stroop Interference trial completion time from the Halstead-Reitan NP battery, where longer time indicates worse performance (37). Updating was assessed using the Letter-Number Sequencing task raw score from the Wechsler Adult Intelligence Scale-III, where a higher score indicates better performance (34). Standardized scores for each task were computed based on published normative data from healthy individuals, consistent with standard NP practice, and a summary EF score was computed by averaging the standardized scores for each task (higher scores indicate better performance; see Supplementary Data, http://links.lww.com/PSYMED/A725, from Ganz et al., 2013 (32) for detailed methods and normative data used).
Subjective Measure of Executive Functioning
Subjective measures of EF can illuminate dysfunction that is below the clinical threshold for impairment, thus detecting problems that may not be reflected in objective NP measures (38). The Patient’s Assessment of Own Functioning Inventory (PAOFI) was used to assess subjective experience of cognitive impairment (39). Factor analysis of the PAOFI in the MBS cohort at baseline identified five subscales; we focused on the Higher-Level Cognitive Complaints Subscale (PAOFI-HLC) to specifically assess subjective EF difficulty (40). This 12-item subscale assesses the degree to which participants recently have experienced problems with higher-level cognitive processes on a scale from 1 to 6, with higher scores indicating more complaints. Of note, we confirmed the consistency of this factor structure in PAOFI data at the 1-year assessment. Reverse scoring was used so that higher scores represented greater cognitive difficulties.
Coping
A modified version of the COPE, a widely used and validated self-report measure of coping, was used to assess how women were coping with their experience of cancer at 1 year after treatment, including their current physical or emotional concerns related to their cancer experience (41). Items are endorsed on a Likert-type scale from 1 (“I do not do this at all”) to 4 (“I do this a lot”). Based on previous literature, approach-oriented coping was assessed with the following subscales from the COPE: positive reappraisal (e.g., “I learn something from the experience”), seeking social support (e.g., “I try to get advice from someone about what to do”), acceptance (e.g., “I accept the reality of the fact that it happened”), planning (e.g., “I try to come up with a strategy about what to do”), and active coping (e.g., “I take action to try to make the situation better”) (8,42). In addition, emotional approach subscales “emotional processing” (e.g., “I take time to figure out what I’m feeling”) and “emotional expression” (e.g., “I allow myself to express my emotions”) were added to the COPE and included in the approach-oriented composite score (43). Subscales for avoidant coping strategies included behavioral disengagement (e.g., “I just give up trying to deal with it”), mental disengagement (e.g., “I do something to think about it less, such as going to movies or watching TV”), and denial (e.g., “I pretend that it has not really happened”) (44). Composite measures of approach and avoidance-oriented coping were computed by averaging scores for each subscale, following previous research (10,44,45). Cronbach’s α for internal consistency of subscales within the approach composite was high (α = .75). Importantly, Cronbach’s α for internal consistency of subscales within the avoidance composite was relatively low (α = .44); thus, we performed exploratory analyses of each subscale of the composite.
Depressive Symptoms
Depressive symptoms were assessed using the Beck Depression Inventory (BDI-II), a valid and reliable measure of depression (46). The BDI-II assesses depressive symptoms over the past 2 weeks and comprises 21 questions assessing somatic, cognitive, and affective dimensions of depression. Clinical cutoffs for this version of the BDI are 0–13, minimal depression; 14–19, mild depression; 20–28, moderate depression; and 29–63, severe depression.
Data Analysis
Bivariate correlations between objective and subjective measures of EF, coping subscales, and depressive symptoms at the 1-year assessment were assessed. For continuous variables, Pearson correlation was used, and for ordinal variables, Spearman correlation was used. Multiple regression analyses were used to assess whether bivariate associations were robust to the inclusion of a priori–determined covariates that have been linked with cognitive performance and/or coping strategies in previous research: age, race, IQ (Wechsler Test of Adult Reading), cancer stage (0/1 or 2/3), receipt of chemotherapy, and receipt of endocrine therapy at the time of the 1-year study assessment. These covariates were specifically included given that age, IQ, and race have been associated with cognitive performance (47,48) and coping strategies (49–51); receipt of chemotherapy has been explicitly linked with EF (3); and endocrine therapy has been shown to influence cognitive functioning (33).
We then examined longitudinal relations between EF at 6 months, coping at 1 year, and depressive symptoms at 2 years within a mediation framework to examine whether problems with EF lead to problems with coping, which then result in greater depressive symptoms. We used the PROCESS macro for SPSS Statistics to derive point estimates, standard errors, and 95% bootstrap confidence intervals (CIs; 5000 random samples) to determine the indirect effect of EF at 6 months on depressive symptoms at 2 years through coping at 1 year. This analysis uses ordinary least squares regression to establish associations between the independent variable (IV; subjective or objective EF), the dependent variable (DV; depressive symptoms), and the mediator (coping). The analysis results in five coefficients: a) the total effect (c path) of the IVon the DV, b) the direct effect (c′ path) of the IVon the DV, c) the effect of the IVon the mediator (a path), d) the effect of the mediator on the DV accounting for the IV (b path), and e) the indirect effect of the mediator on the relationship between the IV and DV (ab path). Significant mediation was concluded if the upper and lower bounds of the CIs for the ab path did not contain 0 (52). Analyses were conducted with and without the aforementioned covariates.
To correct for positive skewness and normalize data, log transformations of the COPE avoidance subscale and square root transformations of the COPE approach subscale and of the BDI-II were conducted. There was one outlier (three standard deviations above the mean) for the COPE behavioral disengagement subscale; therefore, we conducted bivariate associations with and without the outlier to ensure that they did not account for any of the observed relationships (53). All statistical analyses were performed in IBM SPSS Statistics for Macintosh, Version 25.0, using two-tailed significance testing at α = .05 (IBM Corp. Released 2013; IBM Corp., Armonk, New York).
RESULTS
Sample Characteristics and Descriptives
Demographics and clinical characteristics of the participants are reported in Table 1. On average, women were 53 years old, were non-Hispanic White, were partnered, had received education beyond college, were employed, and had an annual household income of at least $100,000. Most participants were diagnosed with stage 1 or 2 breast cancer and had undergone a lumpectomy. Half the sample had received chemotherapy, 74% had received radiation, and 68% were taking endocrine therapy at the 1-year assessment.
TABLE 1.
Descriptive Statistics of Clinical, Demographic, and Study Variables (n = 171)
| Age, mean (SD), y | 53 (8) |
| Race/ethnicity | |
| White | 134 (78.4%) |
| Other | 37 (21.6%) |
| Partner status | |
| Partner | 113 (66.1%) |
| Not partnered | 57 (33.3%) |
| Education status | |
| Less than college | 32 (18.7%) |
| College degree | 51 (29.8%) |
| Postgraduate degree | 88 (51.5%) |
| Employment status | |
| Employed full- or part-time | 110 (64.3%) |
| Not employed | 60 (35.1%) |
| Annual household income | |
| <$100,000 | 63 (36.8%) |
| ≥$100,000 | 104 (60.8%) |
| Surgery type | |
| Lumpectomy | 114 (66.7%) |
| Mastectomy | 57 (33.3%) |
| Cancer stage at diagnosis | |
| 0 | 25 (14.6%) |
| 1 | 77 (45%) |
| 2+ | 69 (40.4%) |
| Received chemotherapy | 87 (50.9%) |
| Received radiation | 127 (74.3%) |
| Endocrine therapy at 1 y | 116 (67.8%) |
| Objective EF summary score, mean (SD) | 0.65 (0.74) |
| PAOFI-HLC, mean (SD) | 1.63 (0.74) |
| Modified COPE—approach composite, mean (SD) | 2.97 (0.50) |
| Modified COPE—avoidance composite, mean (SD) | 1.59 (0.34) |
| BDI-II, mean (SD) | 8.79 (6.98) |
There were missing observations for certain variables. Percentages are based on the number with complete data.
SD = standard deviation; EF = executive functioning; PAOFI-HLC = Patient’s Assessment of Own Functioning–Higher Level Cognitive Complains Subscale; BDI = Beck Depression Inventory.
Descriptive statistics for primary variables at the 1-year assessment are presented in Table 1. On average, the objective EF summary scores were positive, indicating performance better than healthy adults. However, there was substantial variability in EF performance and 14.6% had an EF summary score lower than 0, indicating performance worse than controls. Subjective EF complaints within this sample were on average low, although 16% of women complained of experiencing EF problems “once in a while” to “fairly often.” Coping strategies used within this sample varied, but on average, women endorsed using approach-oriented strategies between a “medium amount” and “a lot” and avoidant coping strategies “a little bit” to “not at all,” similar to other studies of coping in breast cancer survivors (10). Regarding depressive symptoms, on average (SD), women scored an 8.79 (6.98) on the BDI-II with a range of 0 to 32; 76% of women reported minimal symptoms, and 24% reported either mild or moderate levels of symptoms based on BDI-II clinical cutoffs (46). These levels are within the range of those detected in other studies of depressive symptoms in breast cancer survivors (11); however, they are on the lower end because of the exclusion criteria of current affective disorder in the MBS sample.
Bivariate Correlations Between EF, Coping, and Depressive Symptoms
Bivariate correlations between study variables are presented in Table 2. As hypothesized, poorer objective (r = −0.172, p = .024) and subjective EFs (r = 0.297, p < .001) significantly correlated with greater use of avoidant coping strategies. To further interrogate this effect, we conducted follow-up analyses with the three individual subscales that comprise the avoidant coping composite. These analyses indicated that poorer objective and subjective EFs correlated with greater endorsement of behavioral disengagement (r = −0.258, p = .001 for objective EF; r = 0.321, p < .001 for subjective EF). Objective and subjective EFs also correlated with greater endorsement of mental disengagement (r = −0.163, p = .033 for objective EF; r = 0.218, p = .004 for subjective EF) but not with denial (p values > 0.30). Contrary to hypotheses, neither objective nor subjective EF correlated significantly with the approach-oriented coping composite (p values > .25). Analyses conducted with a priori covariates yielded similar results (Table S1, Supplemental Digital Content, http://links.lww.com/PSYMED/A725).
TABLE 2.
Bivariate Correlations at 1-Year Visit Between Study Variables and Covariates
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Objective EF | ||||||||||
| 2. Subjective EF | −0.341** | |||||||||
| 3. Approach coping | 0.038 | 0.082 | ||||||||
| 4. Avoidance coping | −0.172* | 0.297** | 0.125 | |||||||
| 5. Depressive symptoms | −0.172* | 0.563** | 0.028 | 0.273** | ||||||
| 6. Age | −0.094 | 0.043 | −0.081 | −0.086 | −0.029 | |||||
| 7. Race | −0.161* | 0.127 | 0.109 | 0.057 | 0.077 | −0.335** | ||||
| 8. IQ | 0.450** | −0.213** | 0.080 | 0.021 | −0.066 | 0.034 | −0.178* | |||
| 9. Cancer stage | 0.014 | −0.028 | −0.078 | −0.040 | 0.054 | −0.028 | −0.056 | 0.060 | ||
| 10. History of chemotherapy | −0.081 | 0.133 | 0.070 | −0.059 | 0.180* | −0.155* | 0.090 | −0.082 | 0.617** | |
| 11. Endocrine therapy | 0.075 | 0.094 | 0.025 | −0.090 | 0.104 | 0.090 | −0.094 | 0.108 | 0.056 | 0.075 |
Pearson correlations are used for continuous variables, and Spearman correlations are used for categorical variables.
EF = executive functioning.
p < .05.
p < .01.
With respect to depressive symptoms, poorer objective (r = −0.172, p = .024) and subjective (r = 563, p < .001) measures of EF were correlated with higher depressive symptoms. Avoidant coping as a composite was also positively correlated with depressive symptoms (r = 0.273, p < .001) and specifically the behavioral (r = 0.281, p < .001) and mental disengagement (r = 0.273, p < .001). In contrast, the approach-oriented coping composite score was not significantly correlated with depressive symptoms (p = .71). All results remained significant in multiple regression analyses including covariates, except for the association between objective EF and depressive symptoms, which trended toward significance (p = .063; Table S2, Supplemental Digital Content, http://links.lww.com/PSYMED/A725).
Mediation Analysis
Given that the relationship between measures of EF and coping was significant only for behavioral and mental disengagement, we tested whether these disengagement-based strategies mediated the relationship between EF and depressive symptoms. These mediation analyses were tested using longitudinal data to examine temporal precedence, focusing on EF performance and complaints at the 6-month assessment, behavioral and mental disengagement at the 1-year assessment, and depressive symptoms at the 2-year assessment. Mediation models and corresponding pathways are presented in Figures 1 and 2.
FIGURE 1.
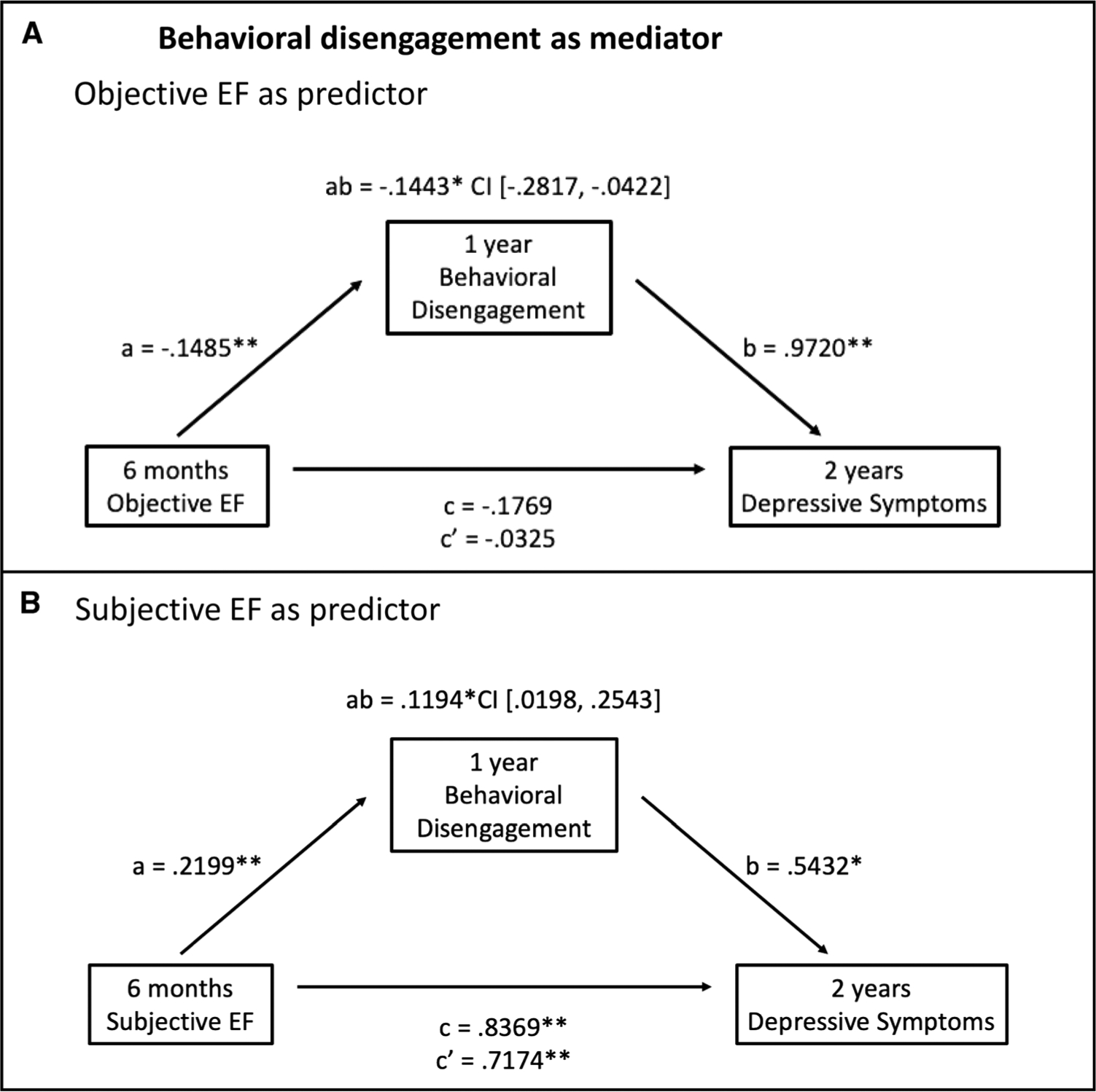
Mediation models of the associations between objective (A) and subjective (B) EFs at 6 months and depressive symptoms at 2 years mediated by behavioral disengagement at 1 year among breast cancer survivors. Unstandardized regression coefficients for all paths and the 95% CI for the indirect path are reported. a, direct effect of EF on behavioral disengagement; b, direct effect of behavioral disengagement on depressive symptoms after adjusting for EF; c, total effect of EF on depressive symptoms; c′, direct effect of EF on depressive symptoms after adjusting for behavioral disengagement; ab, indirect effect of EF on depressive symptoms mediated by behavioral disengagement. EF = executive functioning; CI = confidence interval. * p < .05, ** p < .01.
FIGURE 2.
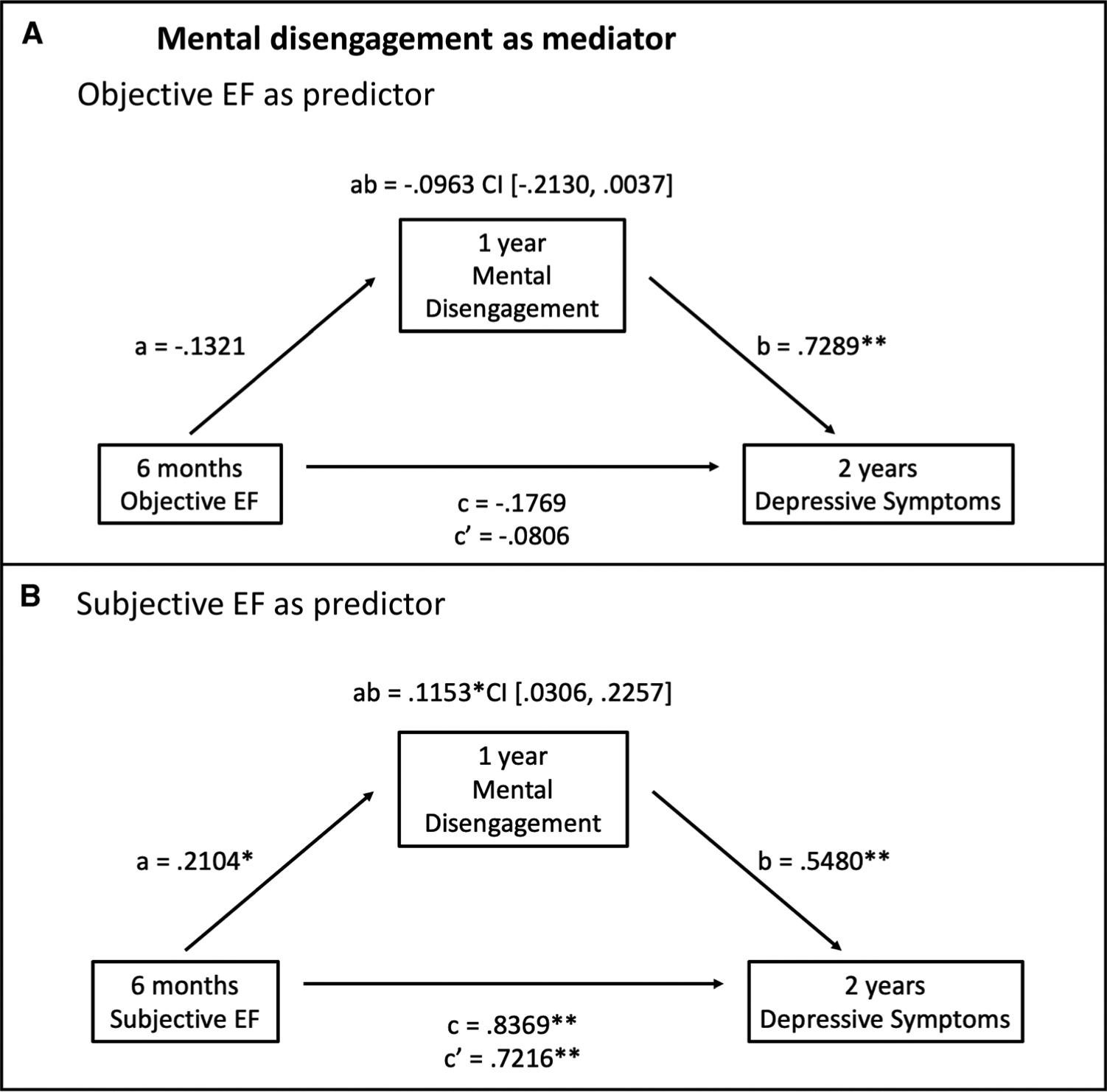
Mediation models of the associations between objective (A) and subjective (B) EFs at 6 months and depressive symptoms at 2 years mediated by mental disengagement at 1 year among breast cancer survivors. Unstandardized regression coefficients for all paths and the 95% CI for the indirect path are reported. a, direct effect of EF on mental disengagement; b, direct effect of mental disengagement on depressive symptoms after adjusting for EF; c, total effect of EF on depressive symptoms; c′, direct effect of EF on depressive symptoms after adjusting for mental disengagement; ab, indirect effect of EF on depressive symptoms mediated by mental disengagement. EF = executive functioning; CI = confidence interval. * p < .05, ** p < .01.
Behavioral Disengagement
Mediation analyses for behavioral disengagement are presented in Figure 1. With objective EF as the predictor, there was not a significant total (c path) or direct (c′ path) effect of EF on depressive symptoms. However, there was a significant negative indirect effect (ab path) of EF on depressive symptoms through behavioral disengagement (ab = −0.1443, SE = 0.0609, 95% CI = −0.2817 to −0.0422; Figure 1A). Therefore, there was an indirect effect of objective EF performance at 6 months on depressive symptoms at 2 years, mediated by behavioral disengagement at 1 year. For subjective EF, there were significant total and direct effects of EF on depressive symptoms (c = 0.8369, SE = 0.1432, 95% CI = 0.5537 to 1.1200; c′ = 0.7174, SE = 0.1522, 95% CI = 0.4166 to 1.0183). There was also a significant positive indirect effect of EF complaints on depressive symptoms through behavioral disengagement (ab = 0.1194, SE = 0.0589, 95% CI = 0.0198 to 0.2543; Figure 1B). Therefore, behavioral disengagement at 1 year mediated the relationship between subjective problems with EF at 6 months and depressive symptoms at 2 years. Both models and their corresponding pathways remained significant when IQ, race, age, cancer stage, receipt of chemotherapy, and endocrine therapy were included in the analyses (Figure S1, Supplemental Digital Content, http://links.lww.com/PSYMED/A725). Of note, when we examined EF mediator models using cross-sectional data from the 1-year time point, both objective and subjective EFs had a significant indirect effect on depressive symptoms through behavioral disengagement, consistent with the longitudinal results (data not shown).
Mental Disengagement
Mediation analyses for mental disengagement are presented in Figure 2. With objective EF as the predictor, there was not a significant total (c path) or direct (c′ path) effect of EF on depressive symptoms. There was also not a significant negative indirect effect (ab path) of EF on depressive symptoms through mental disengagement (Figure 2A). For subjective EF, there were significant total and direct effects of EF on depressive symptoms (c = 0.8369, SE = 0.1432, 95% CI = 0.5537 to 1.1200; c′ = 0.7216, SE = 0.1424, 95% CI = 0.4402 to 1.0030). There was also a significant positive indirect effect of EF complaints on depressive symptoms through mental disengagement (ab = 0.1153, SE = 0.0498, 95% CI = 0.0306 to 0.2257; Figure 2B). Therefore, mental disengagement at 1 year mediated the relationship between subjective problems with EF at 6 months and depressive symptoms at 2 years. When IQ, race, age, cancer stage, receipt of chemotherapy, and endocrine therapy were included in the analyses, the indirect effect of subjective EF on depressive symptoms through mental disengagement remained significant, and the indirect effect of objective EF on depressive symptoms through mental disengagement reached significance (95% CI = −0.2753 to −0.0274; Figure S2, Supplemental Digital Content, http://links.lww.com/PSYMED/A725). Of note, when we examined EF mediator models using cross-sectional data from the 1-year time point, subjective EF had a significant indirect effect on depressive symptoms through mental disengagement, whereas objective EF did not, consistent with the unadjusted longitudinal analyses (data not shown).
DISCUSSION
This study identified links between objective and subjective measures of EF and coping strategies and illuminated novel pathways to depressive symptoms during breast cancer survivorship. Consistent with previous analyses of this cohort, the majority of women performed well on objective EF tasks and reported low levels of cognitive complaints (54); however, there was considerable variability in EF, and a subset of women performed poorly and endorsed difficulties with EF. As hypothesized, the women who performed more poorly on objective EF tasks and reported more EF problems endorsed greater use of avoidant strategies, particularly behavioral and mental disengagement, to cope with their cancer experience. The association between EF and these avoidant coping strategies remained significant in analyses, adjusting for demographic and clinical variables known to influence cognitive processes, coping, and depressive symptoms. In addition, both EF and avoidant coping were correlated with depressive symptoms, and in longitudinal analyses, behavioral disengagement mediated the relationship between objective and subjective EFs and depressive symptoms; mental disengagement mediated the relationship between subjective EF and depressive symptoms. Overall, these findings suggest a specific relationship between problems with EF and coping through behavioral and mental disengagement in breast cancer survivors. This is consistent with the limited literature linking EF and avoidant coping in other samples, including healthy adults (13), adult survivors of pediatric leukemia (12), and TBI patients (14,15).
Although problems with EF correlated with the avoidance coping composite, when we examined individual avoidant strategies, EF was associated with behavioral and mental disengagement but not with denial. Denial involves deliberate attempts to avoid engaging with emotional/stressful stimuli and thus requires cognitive resources to suppress and monitor the thought one is attempting to avoid (55,56). Distinct from denial, mental disengagement does not involve deliberate attempts to inhibit or suppress a thought; rather, if successful, it results in not thinking about the stressful event or aversive thought at all (57). Overall, this pattern of findings is consistent with the cognitive affective neuroscience literature, which posits that problems with EF impede use of more cognitively demanding emotion regulation strategies (58).
Contrary to hypotheses, we did not observe any significant associations between objective and subjective EFs and approach-oriented coping. The handful of previous studies assessing links between EF and coping have generally supported an association between better objective EF and more approach-oriented coping (12–14), although null findings have also been reported (15). Our results suggest that approach- and avoidance-based coping are distinct constructs that may have independent associations with EF in the context of cancer survivorship. Indeed, approach and avoidance are typically not correlated or modestly negatively intercorrelated (41,43,59), and approach and avoidance coping composites were not significantly correlated in our study (r(169) = 0.125, p = .103). We also considered whether this null result was due to the heterogeneity of strategies that comprise the approach composite, which could result in variability in cognitive effort involved in the approach-oriented strategies (e.g., acceptance versus cognitive reappraisal). However, when we tested associations between objective and subjective EFs and individual approach-oriented strategies, we did not detect any statistically significant relationships.
In addition to highlighting links between EF and avoidant coping, our results demonstrate the relevance of this association for mental health in breast cancer survivorship. In particular, mediation analyses showed that behavioral disengagement at 1 year after treatment mediated the association between objective and subjective problems with EF at 6 months and depressive symptoms at 2 years after treatment, and mental disengagement mediated the association between subjective problems with EF and depressive symptoms. These findings are consistent with studies demonstrating an association between avoidant coping and depressive symptoms in cancer survivorship (10,11,60), and identify problems with EF as a potential upstream driver of disengagement coping and subsequent increases in depression in the aftermath of cancer. The association between EF and depression is complex, and studies of cognitive dysfunction in cancer patients and survivors have typically considered depression as a covariate that may influence cognitive function rather than as an outcome of cognitive difficulties (33). Indeed, current affective disorder was an exclusion criterion for participation in the MBS. Our findings paint a more complex picture and suggest that cognitive problems and depressive symptoms are likely influencing one another across the cancer trajectory. Given that cognitive theories of depression posit that cognitive problems precede depression (61), future studies are needed to assess the temporal dynamics between problems with EF and depressive symptoms in this population over time. For example, Jim and colleagues have assessed temporal dynamics between chemotherapy, sleep, mood, and fatigue during chemotherapy (62). This intensive repeated sampling and lagged analytic approach would provide a more fine-grained assessment of links between EF, coping, and depression.
Although the findings from this study support relations between EF, coping, and depressive symptoms, limitations also merit consideration. The study participants comprised a relatively homogeneous, high functioning sample of women with early-stage disease. Studying these associations within a homogenous sample is helpful in that it reduces impacts from other variables (e.g., meta-static disease and comorbidities) but also reduces generalizability and may underestimate effects among women who are struggling with more severe EF problems. In addition, the MBS excluded women with current major affective disorder, which reduced the variability of depressive symptoms in this sample, particularly at the higher end. Furthermore, because this was an observational study, causal links cannot be determined. The longitudinal mediation models testing the indirect effect of problems with EF at 6 months on depressive symptoms at 2 years through disengagement coping at 1 year suggest a temporal relationship; however, experimental studies are needed to verify causal effects. Finally, although we used validated, widely used measures of EF and coping, these measures may not capture the full range of cognitive function or emotion regulation/coping ability. Other assessment approaches using novel neurocognitive measures and/or emotion regulation tasks would enhance our understanding of the associations between cancer-related executive dysfunction and emotion regulation (for examples of such tasks, see Refs. (63,64)).
To the best of our knowledge, this is the first study to show associations between EF, coping, and depressive symptoms in breast cancer survivorship. Results identify behavioral and mental disengagement coping as a novel pathway through which problems with EF may influence depression. These findings may help to identify individuals at risk for persistent depression and also suggest new targets for depression intervention that are potentially more accessible for survivors with cancer-related cognitive problems, who may have difficulty with cognitive behavioral therapy. Cognitive rehabilitation interventions have been designed to remediate cognitive dysfunction in breast cancer survivors (65) and have demonstrated beneficial effects on subjective and objective cognitive functions (66). These interventions might also have promise for alleviating EF-related depressive symptoms, particularly if augmented with use of emotional stimuli. This approach has been shown to improve emotion regulation in noncancer samples (64,67) and could be tested as a novel method for reducing disengagement coping and potentially improving depression in cancer survivors. Indeed, executive functioning training is currently being explored as a way to augment depression treatment in noncancer samples (see Ref. (68) for a review). Overall, the current findings highlight the importance of cancer-related cognitive dysfunction as both an outcome of the cancer experience and a potential driver of longer-term mental health and well-being during breast cancer survivorship.
Supplementary Material
Source of Funding and Conflicts of Interest:
This study was supported by funding from National Institutes of Health/National Cancer Institute R01 CA 109650 and the Breast Cancer Research Foundation, and by National Institute of Mental Health T32 MH015750 to A.R.
Glossary
- BDI
Beck Depression Inventory
- CI
confidence interval
- EF
executive functioning
- MBS
Mind-Body Study
- PAOFI-HLC
Patient’s Assessment of Own Functioning–Higher Level Cognitive Complains Subscale
- TBI
traumatic brain injury
REFERENCES
- 1.Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, Mohile SG, Magnuson A, Kleckner IR, Guido JJ, Young KL, Conlin AK, Weiselberg LR, Mitchell JW, Ambrosone CA, Ahles TA, Morrow GR. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol 2017;35:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pullens MJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology 2010;19:1127–38. [DOI] [PubMed] [Google Scholar]
- 3.Yao C, Bernstein LJ, Rich JB. Executive functioning impairment in women treated with chemotherapy for breast cancer: a systematic review. Breast Cancer Res Treat 2017;166:15–28. [DOI] [PubMed] [Google Scholar]
- 4.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol 2000;41:49–100. [DOI] [PubMed] [Google Scholar]
- 5.Von Ah D, Habermann B, Carpenter JS, Schneider BL. Impact of perceived cognitive impairment in breast cancer survivors. Eur J Oncol Nurs 2013;17: 236–41. [DOI] [PubMed] [Google Scholar]
- 6.Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. New York: Spring Publishing Company; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Spring Publishing Company; 1984:445. [Google Scholar]
- 8.Austenfeld JL, Stanton AL. Coping through emotional approach: a new look at emotion, coping, and health-related outcomes. J Pers 2004;72:1335–64. [DOI] [PubMed] [Google Scholar]
- 9.Marroquín B, Tennen H, Stanton AL. Coping, emotion regulation, and well-being: intrapersonal and interpersonal processes. In: Robinson M, Eid M, editors. The Happy Mind: Cognitive Contributions to Well-Being. Springer, Cham; 2017. 10.1007/978-3-319-58763-9_14. [DOI] [Google Scholar]
- 10.Stanton A, Wiley JF, Krull JL, Crespi CM. Cancer-related coping processes as predictors of depressive symptoms, trajectories, and episodes. J Consult Clin Psychol 2018;86:820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanton AL, Bower JE. Psychological adjustment in breast cancer survivors. Adv Exp Med Biol 2015;862:231–42. [DOI] [PubMed] [Google Scholar]
- 12.Campbell LK, Scaduto M, Van Slyke D, Niarhos F, Whitlock JA, Compas BE. Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. J Pediatr Psychol 2008;34:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez Villegas AL, Salvador Cruz J. Executive functioning and adaptive coping in healthy adults. Appl Neuropsychol Adult 2015;22:124–31. [DOI] [PubMed] [Google Scholar]
- 14.Krpan KM, Levine B, Stuss DT, Dawson DR. Executive function and coping at one-year post traumatic brain injury. J Clin Exp Neuropsychol 2007;29:36–46. [DOI] [PubMed] [Google Scholar]
- 15.Wolters Gregório G, Ponds RW, Smeets SM, Jonker F, Pouwels CG, Verhey FR, van Heugten CM. Associations between executive functioning, coping, and psychosocial functioning after acquired brain injury. Br J Clin Psychol 2015; 54:291–306. [DOI] [PubMed] [Google Scholar]
- 16.Reid-Arndt SA, Cox CR. Stress, coping and cognitive deficits in women after surgery for breast cancer. J Clin Psychol Med Settings 2012;19:127–37. [DOI] [PubMed] [Google Scholar]
- 17.Arndt J, Das E, Schagen SB, Reid-Arndt SA, Cameron LD, Ahles TA. Broadening the cancer and cognition landscape: the role of self-regulatory challenges. Psychooncology 2014;23:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross JJ. The emerging field of emotion regulation: an integrative review. Rev Gen Psychol 1998;2:271–99. [Google Scholar]
- 19.Compas BE, Jaser SS, Bettis AH, Watson KH, Gruhn MA, Dunbar JP, Williams E, Thigpen JC. Coping, emotion regulation, and psychopathology in childhood and adolescence: a meta-analysis and narrative review. Psychol Bull 2017;143: 939–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troy AS, Shallcross AJ, Brunner A, Friedman R, Jones MC. Cognitive reappraisal and acceptance: effects on emotion, physiology, and perceived cognitive costs. Emotion 2018;18:58–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci 2008;17:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giesbrecht G, Müller U, Miller M. Psychological distancing in the development of executive function and emotion regulation. In: Sokol B, Muller U, Carpendale J, Young A, Iarocci G, editors. Self and Social Regulation: Social Interaction and the Development of Social Understanding and Executive Functions; 2010:337–57. [Google Scholar]
- 23.Zelazo PD, Cunningham WA. Executive function: mechanisms underlying emotion regulation. In: Gross J, editor. Handbook of Emotion Regulation. The Guildford Press; 2007:135–58. [Google Scholar]
- 24.Duncan J The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 2010;14:172–9. [DOI] [PubMed] [Google Scholar]
- 25.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuro-imaging studies. Cereb Cortex 2014;24:2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmeichel BJ, Tang D. Individual differences in executive functioning and their relationship to emotional processes and responses. Curr Dir Psychol Sci 2015;24:93–8. [Google Scholar]
- 27.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull 2013;139:81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joormann J, Vanderlind WM. Emotion regulation in depression: the role of biased cognition and reduced cognitive control. Clin Psychol Sci 2014;2:402–21. [Google Scholar]
- 29.Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, Meader N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haemato-logical, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 2011;12:160–74. [DOI] [PubMed] [Google Scholar]
- 30.Ganz PA, Kwan L, Castellon SA, Oppenheim A, Bower JE, Silverman DH, Cole SW, Irwin MR, Ancoli-Israel S, Belin TR. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst 2013;105:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganz PA, Petersen L, Bower JE, Crespi CM. Impact of adjuvant endocrine therapy on quality of life and symptoms: observational data over 12 months from the Mind-Body Study. J Clin Oncol 2016;34:816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, Geist C, Breen EC, Irwin MR, Cole SW. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun 2013;30: S99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganz PA, Petersen L, Castellon SA, Bower JE, Silverman DH, Cole SW, Irwin MR, Belin TR. Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: an observational cohort study. J Clin Oncol 2014; 32:3559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan JJ, Lopez SJ. Wechsler Adult Intelligence Scale-III. In: Dorfman WI, Hersen M, editors. Understanding Psychological Assessment. Boston, MA: Springer; 2001. [Google Scholar]
- 35.Van Dyk K, Petersen L, Ganz PA. Comparison of neurocognitive function after anthracycline-based chemotherapy vs nonanthracycline-based chemotherapy. JAMA Oncol 2016;2:964–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004;19:203–14. [DOI] [PubMed] [Google Scholar]
- 37.Demick J, Harkins DA. Role of cognitive style in the driving skills of young, middle-aged, and older drivers. 1997.
- 38.Savard J, Ganz PA. Subjective or objective measures of cognitive functioning—what’s more important? JAMA Oncol 2016;2:1263–4. [DOI] [PubMed] [Google Scholar]
- 39.Chelune GJ, Heaton RK, Lehman RAW. Neuropsychological and personality correlates of patients’ complaints of disability. In: Goldstein G, Tarter RE, editors. Advances in Clinical Neuropsychology, volume 3. Boston, MA: Springer; 1986:95–126. [Google Scholar]
- 40.Van Dyk K, Ganz PA, Ercoli L, Petersen L, Crespi CM. Measuring cognitive complaints in breast cancer survivors: psychometric properties of the patient’s assessment of own functioning inventory. Support Care Cancer 2016;24:4939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol 1989;56:267–83. [DOI] [PubMed] [Google Scholar]
- 42.Bauer MR, Harris LN, Wiley JF, Crespi CM, Krull JL, Weihs KL, Stanton AL. Dispositional and situational avoidance and approach as predictors of physical symptom bother following breast cancer diagnosis. Ann Behav Med 2016;50: 370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanton AL, Kirk SB, Cameron CL, Danoff-Burg S. Coping through emotional approach: scale construction and validation. J Pers Soc Psychol 2000;78: 1150–69. [DOI] [PubMed] [Google Scholar]
- 44.Stanton AL, Danoff-burg S, Huggins ME. The first year after breast cancer diagnosis: hope and coping strategies as predictors of adjustment. Psychooncology 2002;11:93–102. [DOI] [PubMed] [Google Scholar]
- 45.Hoyt MA, Marin-Chollom AM, Bower JE, Thomas KS, Irwin MR, Stanton AL. Approach and avoidance coping: diurnal cortisol rhythm in prostate cancer survivors. Psychoneuroendocrinology 2014;49:182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beck Aaron T, Steer Robert A, Brown GK. Beck Depression Inventory-II. In: Mental Measurements Yearbook. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 47.Leckliter IN, Matarazzo JD. The influence of age, education, IQ, gender, and alcohol abuse on Halstead-Reitan neuropsychological test battery performance. J Clin Psychol 1989;45:484–512. [DOI] [PubMed] [Google Scholar]
- 48.Heaton RK, Ryan L, Grant I, Matthews CG. Demographic influences on neuropsychological test performance. Neuropsychol Assess Neuropsychiatr Disord 1996;2:141–63. [Google Scholar]
- 49.Folkman S, Lazarus RS, Pimley S, Novacek J. Age differences in stress and coping processes. Psychol Aging 1987;2:171–84. [DOI] [PubMed] [Google Scholar]
- 50.Matud MP. Gender differences in stress and coping styles. Pers Individ Dif 2004; 37:1401–15. [Google Scholar]
- 51.Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: comparing african americans, Hispanics and non-Hispanic whites. Psychooncology 2002;11:495–504. [DOI] [PubMed] [Google Scholar]
- 52.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis, Second Edition: A Regression-Based Approach. 2nd ed. New York: The Guilford Press; 2017. [Google Scholar]
- 53.Parke C Module 5: identifying and addressing outliers. In: Parke CS, editor. Essential First Steps to Data Analysis: Scenario-Based Examples Using SPSS. Thousand Oaks, CA: SAGE Publications, Inc.; 2013:81–102. [Google Scholar]
- 54.Van Dyk K, Hunter AM, Ercoli L, Petersen L, Leuchter AF, Ganz PA. Evaluating cognitive complaints in breast cancer survivors with the FACT-Cog and quantitative electroencephalography. Breast Cancer Res Treat 2017;166:157–66. [DOI] [PubMed] [Google Scholar]
- 55.Muraven M, Tice DM, Baumeister RF. Self-control as limited resource: regulatory depletion patterns. J Pers Soc Psychol 1998;74:774–89. [DOI] [PubMed] [Google Scholar]
- 56.Wegner DM, Schneider DJ, Carter SR, White TL. Paradoxical effects of thought suppression. J Pers Soc Psychol 1987;53:5–13. [DOI] [PubMed] [Google Scholar]
- 57.Compas BE, Connor J, Osowiecki D, Welch A. Effortful and involuntary responses to stress. In: Gottlieb BH, editor. Coping With Chronic Stress. Boston, MA: Springer US; 1997:105–30. [Google Scholar]
- 58.Schmeichel BJ, Tang D. The relationship between individual differences in executive functioning and emotion regulation: a comprehensive review. In: Forgas J, E H-J, editors. The Control Within: Motivation and Its Regulation. New York: Psychology Press; 2014:151–70. [Google Scholar]
- 59.Smith JA, Lumley MA, Longo DJ. Contrasting emotional approach coping with passive coping for chronic myofascial pain. Ann Behav Med 2002;24:326–35. [DOI] [PubMed] [Google Scholar]
- 60.Williamson TJ, Stanton AL. Adjustment to life as a cancer survivor. In: Feuerstein M, Nekhlyudov L, editors. Handbook of Cancer Survivorship. Cham: Springer International Publishing; 2018:29–48. 10.1007/978-3-319-77432-9_3. [DOI] [Google Scholar]
- 61.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol 2010;6:285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jim HS, Jacobsen PB, Phillips KM, Wenham RM, Roberts W, Small BJ. Lagged relationships among sleep disturbance, fatigue, and depressed mood during chemotherapy. Health Psychol 2013;32:768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quinn ME, Stanton CH, Slavich GM, Joormann J. Executive control, cytokine reactivity to social stress, and depressive symptoms: testing the social signal transduction theory of depression. Stress 2020;23:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schweizer S, Grahn J, Hampshire A, Mobbs D, Dalgleish T. Training the emotional brain: improving affective control through emotional working memory training. J Neurosci 2013;33:5301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vance DE, Frank JS, Bail J, Triebel KL, Niccolai LM, Gerstenecker A, Meneses K. Interventions for cognitive deficits in breast cancer survivors treated with chemotherapy. Cancer Nurs 2017;40:E11–27. [DOI] [PubMed] [Google Scholar]
- 66.Ercoli LM, Petersen L, Hunter AM, Castellon SA, Kwan L, Kahn-Mills BA, Embree LM, Cernin PA, Leuchter AF, Ganz PA. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial. Psychooncology 2015;24:1360–7. [DOI] [PubMed] [Google Scholar]
- 67.Schweizer S, Hampshire A, Dalgleish T. Extending brain-training to the affective domain: increasing cognitive and affective executive control through emotional working memory training. PLoS One 2011;6:e24372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van den Bergh N, Hoorelbeke K, De Raedt R, Koster EHW. Remediation of depression-related cognitive impairment: cognitive control training as treatment augmentation. Expert Rev Neurother 2018;18:907–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


