Abstract
Lipoxins (LXs) are autacoids; specialized proresolving lipid mediators (SPMs) acting locally in a paracrine or autocrine fashion. They belong to a complex superfamily of dietary small polyunsaturated fatty acid (PUFA)- metabolites, which direct potent cellular responses to resolve inflammation and restore tissue homeostasis. Together, these SPM activities have been intensely studied in systemic inflammation and acute injury or infection, but less is known about LX signaling and activities in the central nervous system. LXs are derived from arachidonic acid (AA), an omega-6 PUFA. In addition to well established roles in systemic inflammation resolution, they have increasingly become implicated in regulating neuroinflammatory and neurodegenerative processes. In particular, chronic inflammation plays a central role in Alzheimer’s disease (AD) etiology, and dysregulated LX production and activities have been reported in a variety of AD rodent models and clinical tissue samples, yet with complex and sometimes conflicting results. In addition, we recently reported reduced LX production following retinal injury, and demonstrated an intriguing direct neuronal activity promoting survival and homeostasis in retinal and cortical neurons. Here we review and clarify this growing literature and suggest new research directions to further elaborate the role of lipoxins in neurodegeneration.
Keywords: lipoxins, LXA4, LXB4, ATL, arachidonic acid, poly-unsaturated fatty acids, neurodegeneration, neuroinflammation, Altzheimer’s disease, glaucoma
Graphical Abstract
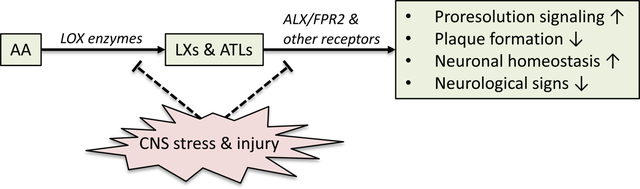
Lipoxins (LXs) are lipid mediators derived from arachidonic acid (AA), an omega-6 PUFA, and are potent signals to resolve inflammation and restore tissue homeostasis. LXs are increasingly implicated in neuroinflammation and neurodegeneration through dysregulated lipoxygenase (LOX) activity and receptor signaling, as well as directly affecting neuronal survival. We synthesize and clarify this growing literature.
INTRODUCTION
Lipoxins (LXs) are autacoids; specialized proresolving lipid mediators (SPMs) acting locally in a paracrine or autocrine fashion and metabolized rapidly via dehydrogenization [1–3]. They belong to a complex superfamily of omega-6 and omega-3 polyunsaturated fatty acid (PUFA)-derived metabolites, which direct potent cellular responses, particularly to resolve inflammation and restore homeostasis[1, 4, 5]. Most studies on SPM functions have therefore focused on immune and endothelial cells. However, SPM activities have been increasingly implicated in the central nervous system, particularly with regards to chronic injury and neurodegenerative diseases. For example, SPMs derived from the omega-3 PUFA docosahexaenoic acid (DHA) have been a focus of extensive investigations for their roles in neuroinflammation [6–8]. Recent studies have also implicated the structurally distinct LXs, derived from arachidonic acid (AA), in regulating neuroinflammation and neurodegeneration. These studies suggest that LX production and function becomes dysregulated in chronic neurodegenerative diseases, yet with complex and sometimes conflicting results. Additionally, we have described an intriguing new context in which lipoxins can act directly on central nervous system (CNS) neurons in a protective and homeostatic capacity. Here we review and clarify this growing literature and suggest new directions to further elaborate the role of lipoxins in CNS injury and repair.
The LX precursor AA, is itself a dietary omega 6 conditionally-essential PUFA that can also be derived from abundant sources of linoleic acid [9]. Both AA and linoleic acid are commercially available through supplements, or are rich in foods such as vegetable oils, nuts, seeds and meats. Literature regarding nutritional influence specifically on production of LX remains scarce. However, changes in AA or linoleic acid dietary intake can alter formation of other proinflammatory metabolites, such as leukotrienes and prostaglandins [10–13]. We have also recently shown that dietary changes in omega 6 and omega 3 PUFA intake can alter enzymatic LX production in the contexts of parenteral nutrition[14] and ocular inflammation[15].
Two naturally occurring LX family members are enzymatically generated from AA; lipoxin A4 (LXA4) and lipoxin B4 (LXB4), which are produced in multiple tissues including the CNS [16, 17]. Their production involves release of AA from membrane phospholipids via cytosolic and secretory phospholipases A2. Free AA is oxidized by the coordinated and sequential interactions of 5- and 12/15-lipoxygenases (LOXs) to generate hydroperoxy-eicosatetraenoic acid intermediates (H(p)ETEs), that are further catalyzed to generate each of the LXs; LXA4 (5S,6R,15S-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoic acid), or LXB4 (5S,14R,15S-trihydroxy-6,10,12-trans-8-cis-eicosatetraenoic acid) [18]. Currently, there are three canonical mechanisms of endogenous LX synthesis: 1) In a two-step reaction, 15-LOX generates the intermediate 15-H(p)ETE, which is a substrate for 5-LOX [9] to generate LX. 2) A similar two-step reaction starts with the oxygenation of AA via 5-LOX to generate the epoxide intermediate leukotriene A, which is converted by either 12-LOX or 15-LOX to LX [19]. 3) Interestingly, an aspirin-mediated mechanism was identified as a third route of LX formation. Aspirin acetylates cyclooxygenase-2 (COX-2) to inhibit formation of the prostaglandins from AA [20]. Acetylation only partially inhibits COX-2 activity and leads the generation of 15R-HETE, a positional isomer of the LOX generated 15S-HETE. 5-LOX converts the aspirin-triggered 15R-HETE to LX isomers, 15-epi-LXA4 (ATLXA4) and 15-epi-LXB4 (ATLXB4)[20] (Figure 1). These aspirin-triggered LX isomers are more resistant to enzymatic inactivation and have longer half-lives relative to LXs [21].
Fig 1. Overview of LX biosynthesis.
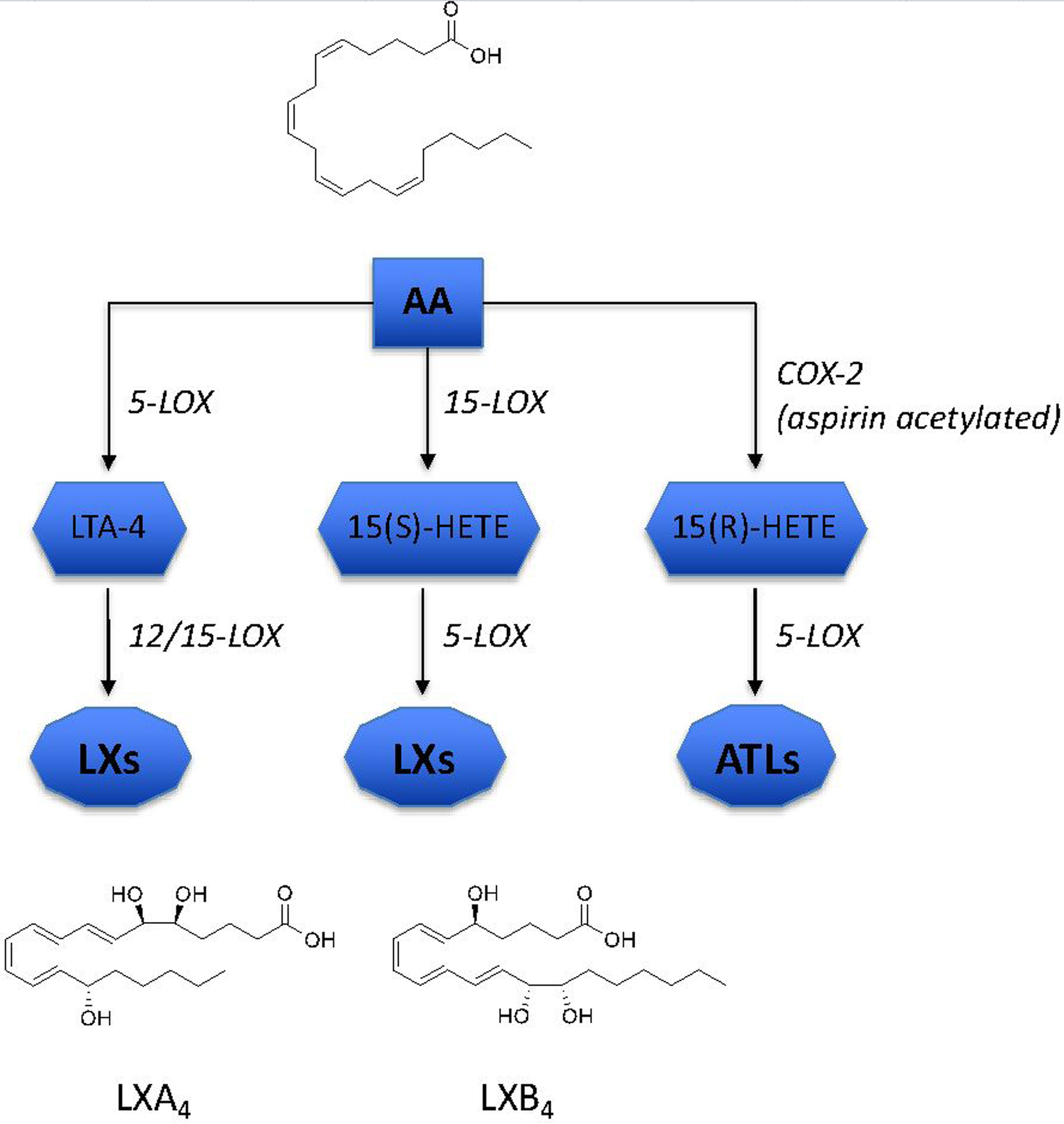
LXA4 and LXB4 are synthesized from arachidonic acid (AA) through sequential oxygenation by actions of 5-LOX and 12/15-LOX enzymes, or alternatively via COX-2 to form ATLs.
LXs were the first identified SPMs and drive the resolution of acute inflammation by inhibiting neutrophil infiltration and activation, recruiting nonphlogistic macrophages to drive efferocytosis, inhibiting and counter-regulating pro-inflammatory cytokines/chemokines and decreasing vascular permeability, all of which restore tissue homeostasis [5, 22–27]. LXA4 primarily exerts its activities through binding to the G-protein coupled formyl peptide receptor 2 (ALX/FPR2) [28] and GPR32 in humans, and ALX/FPR2 in rodents. However, LXA4 or ATLXA4 have also been reported to bind to the cysteinyl leukotriene receptor 1 (CysLT1) as an antagonist or inverse agonist [23, 29], act as allosteric modulators of the cannabinoid receptor CB1 [30], and a partial agonist for the PPARγ nuclear receptor [31], which elicit pro-resolution or anti-inflammatory responses. Thus, LXA4 mediates its actions via complex and cell-specific signal pathways that remain to be fully defined. For example, LXA4 via ALX/FPR2 activates a downstream cytokine signaling suppressor (SOCS-2), which is required to drive migration and cytokine production of dendritic cells in response to infection [32]. In comparison, although LXB4 shows potent bioactivities, it currently has no known receptor, making functional investigations challenging. Consequently, the vast majority of LX studies focus on LXA4 and ALX/FPR2 signaling. Yet, the activities of LXB4 are distinct from LXA4, and include potent effects on macrophages and nonphlogistic monocyte activation [26, 27, 33]. Together, these LX effects have been intensely studied in systemic inflammation and acute injury or infection models, but less is known about LX signaling and activities in the central nervous system and their roles in neurodegeneration.
LIPOXIN DYSREGULATION IN NEURODEGENERATION AND NEUROINFLAMMATION
Neuroinflammation can be initiated by a variety of stressors, such as infection, toxins, metabolic stress, invasive injury and autoimmunity. In the CNS, glial cells, such as astrocytes, oligodendrocytes, Müller cells, and microglia, play critical homeostatic roles by maintaining extracellular ion and neurotransmitter balance, phagocytosing cell debris, and providing nutrients [34–38]. However, dysfunction or chronic activation of these cells can also trigger detrimental effects, resulting in neuroinflammatory cascades that induce or exacerbate pathological mechanisms leading to neurodegeneration [39–44]. In the chronically stressed CNS, glial dysregulation of the LX synthetic circuit has been increasingly implicated in promoting this neuroinflammatory cycle and, subsequently contributing to neurodegenerative mechanisms.
A majority of studies regarding lipid mediators in neurodegeneration focus on the context of Alzheimer’s disease (AD). A contemporary view of AD pathogenesis integrates chronic neuroinflammatory events with accumulations of extracellular amyloid β (Aβ) aggregates and neurofibrillary tangles (NFT) of hyperphosphorylated tau protein [43, 45–47]. The limited clinical success, to date, of direct plaque targeting strategies has led to increased interest in targeting the neuroinflammatory components of the disease and reduce chronic activation of microglia and astrocytes [43, 48]. Strategies to modulate these processes have suggested therapeutic benefits to this approach [49–52]. In this regard proresolution strategies may compliment other anti-inflammatory approaches.
Levels of several SPM are reduced in AD cortex and have been demonstrated to reduce parainflammatory parameters when restored in animal models [53]. In cerebrospinal fluid and post-mortem hippocampus samples from AD patients levels of LXA4 were significantly lower compared to healthy control samples [45], and these levels correlated with the increased accumulation of tau in same brain tissues. Interestingly, these reduced levels also correlated with mini-mental state examination (MMSE) scores [45]. However, a follow up study showed no significant differences in LXA4 or LXB4 in post-mortem entorhinal cortex [51]. Reduced LXA4 levels were also reported in an AD mouse model harboring a pathogenic double mutation in the Aβ precursor protein (APP) gene [54, 55]. Corresponding rescue with ATLXA4 treatment reduced both Aβ and phosphorylated tau levels [54, 56]. In one study twice daily ATLXA4 administration to AD mice also demonstrated reduced levels of TNF-α, interleukin 1B (IL-1B), interferon-γ and IL-6, which correlate with AD progression [56]. The study also showed that ATLXA4 increased anti-inflammatory interleukin 10 (IL-10) and transforming growth factor-β (TGF- β), resulting in the recruitment of a non-classical, nonphlogistic microglia subtype, which reduced Aβ levels. Additionally, ATLXA4 is reported to attenuate proinflammatory cytokine production from microglia through NADPH oxidase inhibition [57], and supplemented LXA4 levels promote antioxidant thioredoxin production [58, 59]. Downstream of LXA4 signaling, expression of the cytokine signaling inhibitor (SOCS-2) is increased [32], which is also observed in AD patients [60]. Mice deficient in SOCS-2 have elevated production of pro-inflammatory cytokines and increased mortality [32]. Therefore, LXA4 treatment drives proresolving effects in a variety of AD-relevant contexts. (These findings and additional studies relevant to LX activity and production in AD are summarized in Table 1).
Table 1.
LX and LOX studies in AD degeneration
| Study Design | Tissue/Study Type | Findings | Citations |
|---|---|---|---|
| Human Pathology | |||
| AD brain, CSF | Post-mortem, biopsy | LXA4 ↓ in hippocampus & CSF, 15-LOX ↑ Reduced levels correlate with cognition |
Wang et al, 2015 |
| AD brain | Post-mortem | No significant change in LXA4, LXB4 in ENT | Zhu et al, 2016 |
| AD brain | Post-mortem | 5LOX protein ↑ | Firuzi et al, 2003 |
| AD brain | Post-mortem | 5LOX protein ↑ | Ikonomovic et al, 2008 |
| AD brain | Post-mortem | 12HETE & 15HETE ↑ | Pratico et al, 2004 |
| AD brain | Post-mortem | 15-LOX ↓ | Lukiw et al, 2005 |
| Animal Models | |||
| Tg2576 mice | In vivo | 5-LOX mRNA, protein ↑ | Firuzi et al, 2003 |
| Tg2576 mice overexpressing 12/15LO |
In vivo | 12/15-LOX protein ↑ BACE1 mRNA & protein ↑ Memory retention ↓ Aβ40 & Aβ42 ↑ Aβ plaques ↑ |
Chu et al, 2012 |
| Tg2576 mice overexpressing 12/15LO | In vivo | p-Tau cdk5, p35, & p25 ↑ PSD-95 & synaptophysin ↓ |
Giannopoulos et al, 2013 |
| Tg2576 mice overexpressing 5LO | In vivo | P-tau ↑ PSD-95 & synaptophysin & MAP2 ↓ P35 & p25 ↑ |
Chu et al, 2013 |
| Tg2576 × 12/15LO−/− mice | In vivo | Aβ40, Aβ42, Aβ plaques ↓ CTF-β, & BACE-1 ↓ Memory retention ↑ |
Yang et al, 2010 |
| Tg2576 × 5LO−/− mice | In vivo | Aβ42 ↓, γ-secretase ↓ | Firuzi et al, 2003 |
| Tg2576 mice treated with ATLA4 | In vivo | Cognitive impairment ↓ Aβ40 & Aβ42 ↓, Aβ42 plaques ↓ |
Medeiros et al, 2013 |
| 3xTg mice overexpressing 5-LO | In vivo | Aβ40 & Aβ42 ↑, p25 ↑ Fear response ↓ |
Chu et al, 2012 |
| 3xTg mice treated with ATLA4 | In vivo | Aβ40 & Aβ42 ↓, Aβ size & plaques ↓ Cognition ↑ |
Dunn et al, 2015 |
| 3xTg x 5LO−/− mice | In vivo | Aβ40 & Aβ42 ↓, Aβ plaques ↓ cdk5, p35, p25 ↓ PSD-95 & synaptophysin ↑ Long-term potentiation ↑ Working memory & retention ↑ |
Giannopoulos et al. 2014 |
| 12/15LO−/− 3-NP stressed mice | In vivo | Striatal lesion number ↑ Lesion incidence rate ↑ |
He et al, 2017 |
| 5xFAD mice | In vivo | LXA4 levels ↓, Aβ40 plaques ↓ IL-1b & IL-10 ↓ |
Kantarci et al, 2017 |
| Cellular Models | |||
| HEK293-APPC99 cells incubated with 5-HPETE | In vitro | γ-secretase ↑ | Firuzi et al, 2003 |
| APP x 5LO MEF cells treated with A23187 | In vitro | Aβ42 & γ-secretase ↑ | Firuzi et al, 2003 |
| N2A-APPswe x 12/15LO cells | In vitro | p-Tau↑, cdk5, p35 & p25 ↑ | Giannopoulos et al, 2013 |
| N2A-APPswe cells incubated with 12/15-HETE | In vitro | CTF-β, BACE-1 & sAPPβ ↓ BACE-1 mRNA ↓ |
Yang et al, 2010 |
| N2A-APPswe x 12/15LO overexpression | In vitro | BACE1 mRNA & protein ↑ | Chu et al, 2012 |
| N2A-APPswe x 5LO overexpression | In vitro | Tau & p-tau ↑ PSD-95, & synaptophysin ↓ |
Chu et al, 2012 |
| SHSY5Y cells treated with LXA4 & staurosporine | In vitro | Cell viability ↑ | Zhu et al, 2016 |
| Cortical neurons & HT22 cells treated with LXA4 and LXB4 | In vitro | Cell viability, neurites, mitochondria ↑ | Livne-bar et al, 2017 |
While LXA4 demonstrates a proresolving activity, its primary target receptor ALX/FPR2 binds also binds other ligands, such as N-Formylmethionine peptides and annexin 1, that can induce specific and sometimes opposing bioactions [61]. Interestingly, Aβ can also bind to ALX/FPR2 with antagonistic effects to LXA4. Le and colleagues reported Aβ1–42 inducing chemotactic activity in human leucocytes through ALX/FRP2 activation [62]. Other studies demonstrated similar chemotactic activity, as well as superoxide production in mouse neutrophils and stimulation of murine microglial cells in vitro [63, 64]. Furthermore, expression of the ALX/FPR2 receptor is elevated in microglia and astrocytes of AD hippocampal samples [45]. This counterintuitive result was attributed to increased receptor availability to transduce resolution signals. However, the underlying mechanism remains unclear. The increased ALX/FPR2 receptor relative to reduced LXA4 in the AD brain could indicate a compensatory mechanism to upregulate homeostatic and protective LX signaling.
LOX ENZYME DYSREGULATION IN AD
AD is also associated with dysregulated levels of the two key LOX enzymes and intermediates involved in LX production. Post-mortem analysis of AD patients revealed upregulation of 12/15 LOX and increased levels of the LX precursors 12-HETE and 15-HETE in the frontal and temporal lobes [65]. These findings are corroborated by another study which showed increase of the same precursors in cerebrospinal fluid of AD patients, as well as at-risk patients with mild cognitive impairment [66], suggesting 12/15 LOX may be involved in the initiation of AD [67]. However, another study has also demonstrated strongly reduced expression and activity of 15-LOX in the hippocampus of AD patients compared to the hippocampus of matched postmortem controls[68].
In genetic models, 12/15-LOX deficient mice show reduced production and deposition of Aβ [50], while overexpression of the 12/15-LOX gene (12/15-LO) in Tg2576 APP mutant mice activated astrocytes and microglial cells [69], and resulted in increased tau phosphorylation[70]. It is important to note that the mouse 12/15-LOX is the closest homolog to human 15-LOX, however, unlike the human enzyme the mouse 12/15-LOX primarily functions as a 12-LOX enzyme generating 12-HETE as its primary metabolite. Hence, data from mice need to be interpreted with caution. In addition, a body of work has demonstrated that 15-LOX metabolites in mouse, rat and rabbit models of neuro-inflammation and degeneration are protective in the retina and brain[71]. AD is similarly associated with increases in 5-LOX. For example, 5-LOX protein [72, 73] and mRNA levels [73] were increased in the hippocampus and frontal cortex of human AD patients and in pathogenic APP transgenic mice. Injections of inflammatory lipopolysaccharide (LPS), which have been proposed to play a role in AD pathogenesis [74], also induces expression of the 5-LOX gene (5-LO) in mouse hippocampus [75]. Increased 5-LOX has also been associated with activation of astrocytes and microglia [76], as well as increased Aβ levels through a γ-secretase dependent mechanism [72, 77], whereas absence of 5-LOX resulted in reduced NFTs and Aβ through γ-secretase inhibition [72, 76], and improved memory and synaptic integrity [78]. However, the role of 5-LOX is not clear as the enzyme can generate four leukotrienes that amplify or initiate inflammation, but is also required for the formation of a large number of protective and anti-inflammatory SPM. In a mouse model of allergic encephalomyelitis both 5-LOX and 12/15-LOX KO mice demonstrated significantly worse clinical scores compared to wild type controls [79]. In addition, relevant leukotriene formation in the brain or retina has not been clearly established and clinically the 5-LOX inhibitor zileuton has shown limited or no efficacy in human inflammatory diseases. Hence, further research is required to elucidate the role and function of 5-LOX in the brain.
Given the positive effects of LXA4 and ATLXA4 on neuroinflammation, it may appear paradoxical that reduction in LOXs, which catalyze LX production, would also reduce Aβ, phosphorylated tau, and reactive glia. However, LOX enzymes can use both omega-3 and omega-6 PUFA as substrates, including AA, DHA and eicosapentaenoic acid (EPA), and LOX intermediates. Therefore, LXs represent only one of several distinct classes of LOX-derived lipid mediators. For example, proinflammatory leukotrienes and HETES are eicosanoids that can be generated by leukocytes. Ultimately, LOX enzymes have cell and tissue specific expression and generate a large number of structurally distinct lipid mediators with diverse bioactions. Therefore, additional research is required to better understand the contributions of LOX products to this neuroinflammatory milieu.
THE LX CIRCUIT IN OTHER NEURODEGENERATIVE CONTEXTS
In addition to AD, levels of LX and other PUFA derived lipid mediators have recently been reported in a growing literature of other neurodegenerative contexts. In a study of Multiple Sclerosis patients lipid mediator levels were compared in serum and cerebrospinal fluid between highly active and less active MS patients [80]. While levels of the LXA4 precursor, 15-HETE, was increased in the active MS group, LXA4 itself was not significantly elevated in patients with active disease compared to inactive disease. In a rodent stroke model, studies have shown ALX/FPR2 mediated LXA4 effects on infarct size and cognition, suggesting a potential dysregulation of the LX pathway [81]. In clinical results the 12/15LOX enzyme is increased in both ischemic stroke patients and in animal models [82]. LX dysregulation may also affect neuronal health as a consequence of systemic inflammatory conditions that may impact neural tissues, such as diabetes and metabolic syndrome [83–85]. However, it remains unclear how LX actions may impact damage to these attendant neural tissues.
LIPOXINS CAN MEDIATE DIRECT NEURONAL ACTIONS
Studies assessing LXs in the CNS have generally focused on their proresolution roles. However, recent findings from our laboratory and others have also intriguingly uncovered a direct neuronal activity [51, 86, 87]. Previously, neuroprotective effects have been described for the structurally distinct DHA product NPD1 [88–90]. But, such activity had not been identified for LXs. Zhu and colleagues observed LXA4 protective effects on immortalized SH-SY5Y neuroblastoma cells [51]. Our studies focused on astrocyte-neuron interactions in retinal injury models, relevant to the neurodegeneration of retinal ganglion cells (RGCs) in the chronic blinding disease glaucoma.
As for the brain, LX and LOX activities in the eye have been primarily studied in the context of ocular inflammation, wound repair and angiogenesis [91–93]. However, we reported that LXA4 and LXB4 are produced by retinal astrocytes and are regulated under stress. Both LXs are detected in mouse retina and optic nerve, but their levels and 5-LOX expression decrease following injury. Intriguingly, either LXA4 or LXB4 were sufficient to protect primary RGCs and cortical neurons in a dose dependent manner, but related SPMs had no activity. In contrast, inhibition of LX synthetic and signaling pathway components exacerbated RGC loss following metabolic injury. Finally, therapeutic LXB4 treatment starting after eight weeks of increased intraocular pressure preserved RGC function and survival in a chronic rodent glaucoma model.
Surprisingly, although LXA4 signaling has been much more extensively investigated, LXB4 was substantially and consistently more potent in protecting RGC survival in vitro and in vivo. Additionally, only LXB4 treatment stabilized neuronal mitochondrial membrane potential. As described in the introduction, relatively little is known about LXB4 signaling, and its receptor has yet to be identified. However, the evidence that LXs operate through divergent pathways is suggested by reports indicating they also induce distinct proresolving pathways. For example, monocyte interaction with LXA4, but not LXB4, increases intracellular calcium in leukocytes [33] Also, treatment with LXB4, but not LXA4 conferred radioprotection of hematopoetic stem cells in mice [94]. These findings, combined with our own in neurons, suggest that the LXB4 receptor and signaling cascade is distinct from LXA4 (Figure 2). More research is needed to further describe the LXB4 signaling pathway in neuronal and non-neuronal contexts.
Fig 2. LX’s promote neuronal homeostasis.
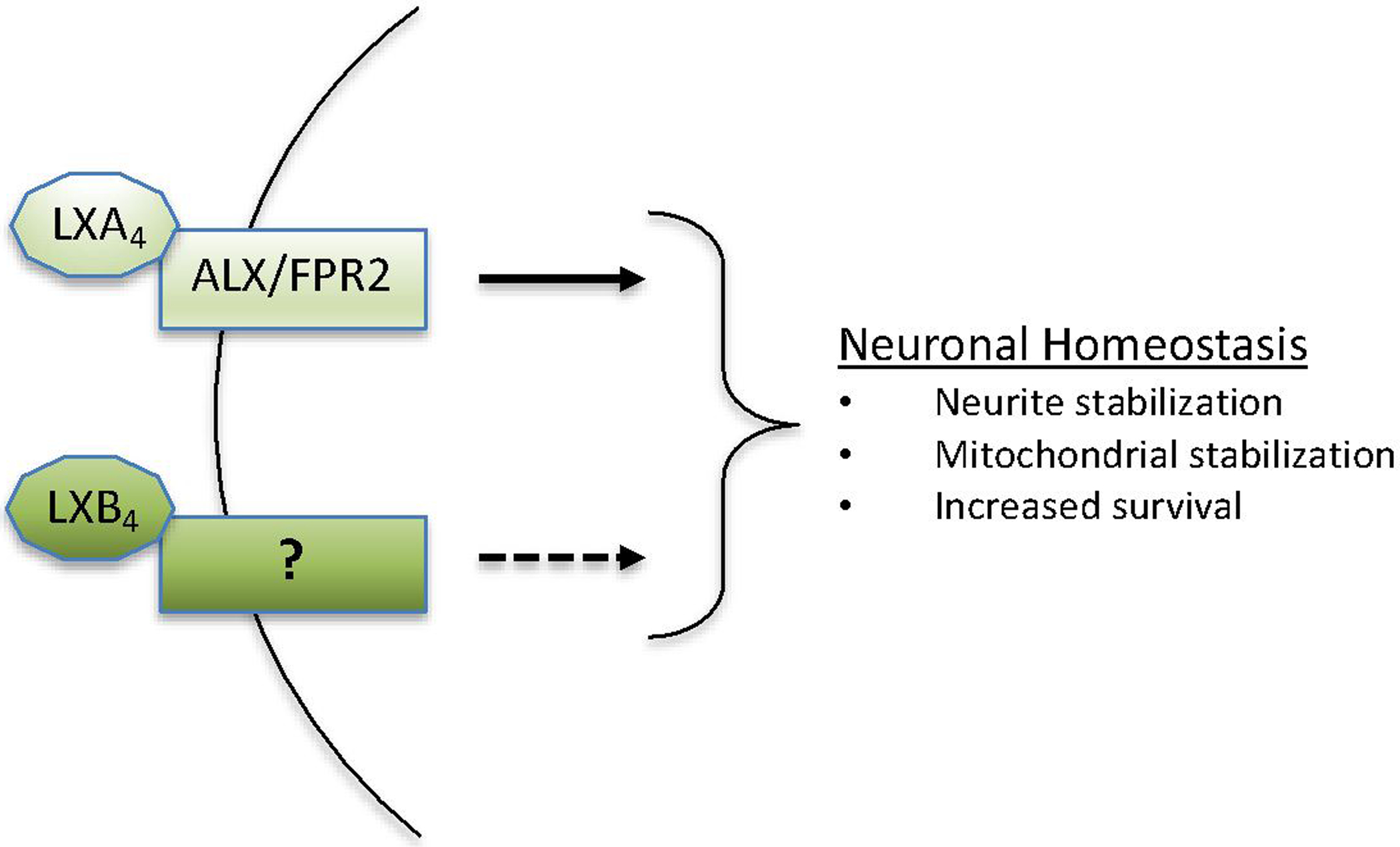
Proposed LX signaling to promote neuroprotective outcomes. LXA4 signaling through ALX/FPR2 is defined, but the more potent LXB4 signaling is unknown.
CONCLUSIONS
LXs have well established proresolution mechanisms in several types of activated immune cells, and have been increasingly implicated in neurodegenerative contexts. Accumulating evidence describes consistent dysregulation of LX synthesis and LOX function, as well as ALX/FPR2 signaling in the chronically injured CNS. Consequently, restoration or supplementation of LX signaling may also present promising therapeutic potential, as they induce a proresolving effect in models of neuroinflammation, while also exerting direct neuroprotective activity. Recent interesting evidence suggests that dietary supplementation of omega-3 PUFA can sustain the levels of SPMs in AD patients [95] and amplifies LXA4 levels in several tissues in mice [15].
However, more research is needed to understand the signaling induced by each LX in specific diseases. There is particular relevance for these effects in AD pathology, as LXA4 and ATLXA4 have been shown to reduce Aβ aggregates and neurodegeneration in transgenic mice expressing pathogenic APP. These beneficial effects have been attributed to their inflammation-resolution activity but a direct neuroprotective effect has not been ruled out. Our studies have also demonstrated that LXs exert direct neuroprotective effect on retinal and cortical neurons. Moreover, in vivo protection of retinal neurons was observed following acute injury which is independent of a classical inflammatory response. In this neuroprotective role, both in vivo and in vitro data surprisingly demonstrate that LXB4 is more potent than LXA4. These observations indicate that further investigations into LXB4 signaling will provide insights into novel neuroprotective strategies.
ACKNOWLEDGEMENTS
Support for this work was provided through the Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), and Glaucoma Research Society of Canada (GRSC). JS holds the Toronto General and Western Hospital Glaucoma Research Chair, and CK is the recipient of a VSRP fellowship. KG was supported by NIH/NEI grant (EY026082).
Common Abbreviations:
- 15-LOX
15 lipoxygenase
- 5-LOX
5 lipoxygenase
- Aβ
amyloid β
- AA
arachidonic acid
- AD
Alzheimer’s disease
- ALX/FPR2
LXA4 receptor/formyl peptide receptor 2
- APP
amyloid β precursor protein
- ATLXA4
aspirin-triggered LXA4
- ATLXB4
aspirin-triggered LXB4
- CNS
central nervous system
- DHA
docosahexaenoic acid
- H(p)ETE
hydroperoxy-eicosatetraenoic acid
- LX
lipoxin
- LXA4
lipoxin A4
- LXB4
lipoxin B4
- MMSE
mini-mental state examination
- NFT
neurofibrillary tangles
- PUFA
polyunsaturated fatty acid
- SOCS-2
suppressor of cytokine signaling
- SPM
specialized proresolving lipid mediator
REFERENCES
- [1].Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bennett M, Gilroy DW, Lipid Mediators in Inflammation. Microbiol Spectr 2016, 4. [DOI] [PubMed] [Google Scholar]
- [3].Serhan CN, Chiang N, Van Dyke TE, Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008, 8, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gronert K, Lipid autacoids in inflammation and injury responses: a matter of privilege. Mol Interv 2008, 8, 28–35. [DOI] [PubMed] [Google Scholar]
- [5].Romano M, Cianci E, Simiele F, Recchiuti A, Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur J Pharmacol 2015, 760, 49–63. [DOI] [PubMed] [Google Scholar]
- [6].Gordon WC, Bazan NG, Mediator lipidomics in ophthalmology: targets for modulation in inflammation, neuroprotection and nerve regeneration. Curr Eye Res 2013, 38, 995–1005. [DOI] [PubMed] [Google Scholar]
- [7].Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N, Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochimica et biophysica acta 2015, 1851, 397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grant R, Guest J, Role of Omega-3 PUFAs in Neurobiological Health. Adv Neurobiol 2016, 12, 247–274. [DOI] [PubMed] [Google Scholar]
- [9].Serhan CN, Hamberg M, Samuelsson B, Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A 1984, 81, 5335–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carlson SE, Colombo J, Docosahexaenoic Acid and Arachidonic Acid Nutrition in Early Development. Adv Pediatr 2016, 63, 453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Innes JK, Calder PC, Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids 2018, 132, 41–48. [DOI] [PubMed] [Google Scholar]
- [12].Kawashima H, Intake of arachidonic acid-containing lipids in adult humans: dietary surveys and clinical trials. Lipids Health Dis 2019, 18, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lien EL, Richard C, Hoffman DR, DHA and ARA addition to infant formula: Current status and future research directions. Prostaglandins Leukot Essent Fatty Acids 2018, 128, 26–40. [DOI] [PubMed] [Google Scholar]
- [14].Kalish BT, Le HD, Fitzgerald JM, Wang S, Seamon K, Gura KM, Gronert K, Puder M, Intravenous fish oil lipid emulsion promotes a shift toward anti-inflammatory proresolving lipid mediators. Am J Physiol Gastrointest Liver Physiol 2013, 305, G818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gao Y, Su J, Zhang Y, Chan A, Sin JH, Wu D, Min K, Gronert K, Dietary DHA amplifies LXA4 circuits in tissues and lymph node PMN and is protective in immune-driven dry eye disease. Mucosal Immunol 2018, 11, 1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Leo LM, Almeida-Correa S, Canetti CA, Amaral OB, Bozza FA, Pamplona FA, Age-dependent relevance of endogenous 5-lipoxygenase derivatives in anxiety-like behavior in mice. PLoS One 2014, 9, e85009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tassoni D, Kaur G, Weisinger RS, Sinclair AJ, The role of eicosanoids in the brain. Asia Pac J Clin Nutr 2008, 17 Suppl 1, 220–228. [PubMed] [Google Scholar]
- [18].Ryan A, Godson C, Lipoxins: regulators of resolution. Curr Opin Pharmacol 2010, 10, 166–172. [DOI] [PubMed] [Google Scholar]
- [19].Serhan CN, Sheppard KA, Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. The Journal of clinical investigation 1990, 85, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Claria J, Serhan CN, Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A 1995, 92, 9475–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Clish CB, O’Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN, Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci U S A 1999, 96, 8247–8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fierro IM, Colgan SP, Bernasconi G, Petasis NA, Clish CB, Arita M, Serhan CN, Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. Journal of immunology 2003, 170, 2688–2694. [DOI] [PubMed] [Google Scholar]
- [23].Gronert K, Martinsson-Niskanen T, Ravasi S, Chiang N, Serhan CN, Selectivity of recombinant human leukotriene D(4), leukotriene B(4), and lipoxin A(4) receptors with aspirin-triggered 15-epi-LXA(4) and regulation of vascular and inflammatory responses. Am J Pathol 2001, 158, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee TH, Horton CE, Kyan-Aung U, Haskard D, Crea AE, Spur BW, Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin Sci (Lond) 1989, 77, 195–203. [DOI] [PubMed] [Google Scholar]
- [25].Maddox JF, Colgan SP, Clish CB, Petasis NA, Fokin VV, Serhan CN, Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: design of stable lipoxin B4 analogs with increased biologic activity. FASEB J 1998, 12, 487–494. [DOI] [PubMed] [Google Scholar]
- [26].Maddox JF, Serhan CN, Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med 1996, 183, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Takano T, Clish CB, Gronert K, Petasis N, Serhan CN, Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. The Journal of clinical investigation 1998, 101, 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fiore S, Maddox JF, Perez HD, Serhan CN, Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med 1994, 180, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maderna P, Godson C, Lipoxins: resolutionary road. Br J Pharmacol 2009, 158, 947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pamplona FA, Ferreira J, Menezes de Lima O Jr., Duarte FS, Bento AF, Forner S, Villarinho JG, Bellocchio L, Wotjak CT, Lerner R, Monory K, Lutz B, Canetti C, Matias I, Calixto JB, Marsicano G, Guimaraes MZ, Takahashi RN, Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci U S A 2012, 109, 21134–21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernandez-Lopez D, Pradillo JM, Caso JR, Vivancos J, Nombela F, Serena J, Lizasoain I, Moro MA, Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci 2009, 29, 3875–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, Aliberti J, Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med 2006, 12, 330–334. [DOI] [PubMed] [Google Scholar]
- [33].Romano M, Maddox JF, Serhan CN, Activation of human monocytes and the acute monocytic leukemia cell line (THP-1) by lipoxins involves unique signaling pathways for lipoxin A4 versus lipoxin B4: evidence for differential Ca2+ mobilization. Journal of immunology 1996, 157, 2149–2154. [PubMed] [Google Scholar]
- [34].Colonna M, Butovsky O, Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu Rev Immunol 2017, 35, 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sivak JM, The aging eye: common degenerative mechanisms between the Alzheimer’s brain and retinal disease. Invest Ophthalmol Vis Sci 2013, 54, 871–880. [DOI] [PubMed] [Google Scholar]
- [36].Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr., Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A, Glial cells in (patho)physiology. J Neurochem 2012, 121, 4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zuchero JB, Barres BA, Glia in mammalian development and disease. Development 2015, 142, 3805–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nahirnyj A, Livne-Bar I, Guo X, Sivak JM, ROS Detoxification and Proinflammatory Cytokines Are Linked by p38 MAPK Signaling in a Model of Mature Astrocyte Activation. PLoS One 2013, 8, e83049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Guo X, Jiang Q, Tuccitto A, Chan D, Alqawlaq S, Won GJ, Sivak JM, The AMPK-PGC-1alpha signaling axis regulates the astrocyte glutathione system to protect against oxidative and metabolic injury. Neurobiol Dis 2018, 113, 59–69. [DOI] [PubMed] [Google Scholar]
- [40].Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA, Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Livne-Bar I, Lam S, Chan D, Guo X, Askar I, Nahirnyj A, Flanagan JG, Sivak JM, Pharmacologic inhibition of reactive gliosis blocks TNF-alpha-mediated neuronal apoptosis. Cell death & disease 2016, 7, e2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pekny M, Pekna M, Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiological reviews 2014, 94, 1077–1098. [DOI] [PubMed] [Google Scholar]
- [43].Bronzuoli MR, Iacomino A, Steardo L, Scuderi C, Targeting neuroinflammation in Alzheimer’s disease. J Inflamm Res 2016, 9, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Exler RE, Guo X, Chan D, Livne-Bar I, Vicic N, Flanagan JG, Sivak JM, Biomechanical insult switches PEA-15 activity to uncouple its anti-apoptotic function and promote erk mediated tissue remodeling. Exp Cell Res 2016, 340, 283–294. [DOI] [PubMed] [Google Scholar]
- [45].Wang X, Zhu M, Hjorth E, Cortes-Toro V, Eyjolfsdottir H, Graff C, Nennesmo I, Palmblad J, Eriksdotter M, Sambamurti K, Fitzgerald JM, Serhan CN, Granholm AC, Schultzberg M, Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement 2015, 11, 40–50 e41-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pimplikar SW, Neuroinflammation in Alzheimer’s disease: from pathogenesis to a therapeutic target. J Clin Immunol 2014, 34 Suppl 1, S64–69. [DOI] [PubMed] [Google Scholar]
- [47].Minter MR, Taylor JM, Crack PJ, The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J Neurochem 2016, 136, 457–474. [DOI] [PubMed] [Google Scholar]
- [48].Osborn LM, Kamphuis W, Wadman WJ, Hol EM, Astrogliosis: An integral player in the pathogenesis of Alzheimer’s disease. Prog Neurobiol 2016, 144, 121–141. [DOI] [PubMed] [Google Scholar]
- [49].Sudduth TL, Greenstein A, Wilcock DM, Intracranial injection of Gammagard, a human IVIg, modulates the inflammatory response of the brain and lowers Abeta in APP/PS1 mice along a different time course than anti-Abeta antibodies. J Neurosci 2013, 33, 9684–9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yang H, Zhuo JM, Chu J, Chinnici C, Pratico D, Amelioration of the Alzheimer’s disease phenotype by absence of 12/15-lipoxygenase. Biol Psychiatry 2010, 68, 922–929. [DOI] [PubMed] [Google Scholar]
- [51].Zhu M, Wang X, Hjorth E, Colas RA, Schroeder L, Granholm AC, Serhan CN, Schultzberg M, Pro-Resolving Lipid Mediators Improve Neuronal Survival and Increase Abeta42 Phagocytosis. Mol Neurobiol 2016, 53, 2733–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Koenigsknecht J, Landreth G, Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci 2004, 24, 9838–9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Whittington RA, Planel E, Terrando N, Impaired Resolution of Inflammation in Alzheimer’s Disease: A Review. Front Immunol 2017, 8, 1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dunn HC, Ager RR, Baglietto-Vargas D, Cheng D, Kitazawa M, Cribbs DH, Medeiros R, Restoration of lipoxin A4 signaling reduces Alzheimer’s disease-like pathology in the 3xTg-AD mouse model. Journal of Alzheimer’s disease : JAD 2015, 43, 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kantarci A, Aytan N, Palaska I, Stephens D, Crabtree L, Benincasa C, Jenkins BG, Carreras I, Dedeoglu A, Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Exp Neurol 2018, 300, 111–120. [DOI] [PubMed] [Google Scholar]
- [56].Medeiros R, Kitazawa M, Passos GF, Baglietto-Vargas D, Cheng D, Cribbs DH, LaFerla FM, Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces Alzheimer disease-like pathology in mice. Am J Pathol 2013, 182, 1780–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wu Y, Zhai H, Wang Y, Li L, Wu J, Wang F, Sun S, Yao S, Shang Y, Aspirin-triggered lipoxin A(4) attenuates lipopolysaccharide-induced intracellular ROS in BV2 microglia cells by inhibiting the function of NADPH oxidase. Neurochem Res 2012, 37, 1690–1696. [DOI] [PubMed] [Google Scholar]
- [58].Trovato A, Siracusa R, Di Paola R, Scuto M, Fronte V, Koverech G, Luca M, Serra A, Toscano MA, Petralia A, Cuzzocrea S, Calabrese V, Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: Relevance to Alzheimer’s disease pathogenesis. Neurotoxicology 2016, 53, 350–358. [DOI] [PubMed] [Google Scholar]
- [59].Trovato A, Siracusa R, Di Paola R, Scuto M, Ontario ML, Bua O, Di Mauro P, Toscano MA, Petralia CCT, Maiolino L, Serra A, Cuzzocrea S, Calabrese V, Redox modulation of cellular stress response and lipoxin A4 expression by Hericium Erinaceus in rat brain: relevance to Alzheimer’s disease pathogenesis. Immun Ageing 2016, 13, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Walker DG, Whetzel AM, Lue LF, Expression of suppressor of cytokine signaling genes in human elderly and Alzheimer’s disease brains and human microglia. Neuroscience 2015, 302, 121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cattaneo F, Parisi M, Ammendola R, Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists. Int J Mol Sci 2013, 14, 7193–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Le Y, Gong W, Tiffany HL, Tumanov A, Nedospasov S, Shen W, Dunlop NM, Gao JL, Murphy PM, Oppenheim JJ, Wang JM, Amyloid (beta) 42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J Neurosci 2001, 21, RC123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Martini AC, Forner S, Bento AF, Rae GA, Neuroprotective effects of lipoxin A4 in central nervous system pathologies. Biomed Res Int 2014, 2014, 316204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tiffany HL, Lavigne MC, Cui YH, Wang JM, Leto TL, Gao JL, Murphy PM, Amyloid-beta induces chemotaxis and oxidant stress by acting at formylpeptide receptor 2, a G protein-coupled receptor expressed in phagocytes and brain. J Biol Chem 2001, 276, 23645–23652. [DOI] [PubMed] [Google Scholar]
- [65].Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM, 12/15-lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am J Pathol 2004, 164, 1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yao Y, Clark CM, Trojanowski JQ, Lee VM, Pratico D, Elevation of 12/15 lipoxygenase products in AD and mild cognitive impairment. Annals of neurology 2005, 58, 623–626. [DOI] [PubMed] [Google Scholar]
- [67].Czapski GA, Czubowicz K, Strosznajder JB, Strosznajder RP, The Lipoxygenases: Their Regulation and Implication in Alzheimer’s Disease. Neurochem Res 2016, 41, 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG, A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. The Journal of clinical investigation 2005, 115, 2774–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chu J, Zhuo JM, Pratico D, Transcriptional regulation of beta-secretase-1 by 12/15-lipoxygenase results in enhanced amyloidogenesis and cognitive impairments. Annals of neurology 2012, 71, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [70].Giannopoulos PF, Joshi YB, Chu J, Pratico D, The 12–15-lipoxygenase is a modulator of Alzheimer’s-related tau pathology in vivo. Aging cell 2013, 12, 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Calandria JM, Marcheselli VL, Mukherjee PK, Uddin J, Winkler JW, Petasis NA, Bazan NG, Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J Biol Chem 2009, 284, 17877–17882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Pratico D, 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer’s disease. FASEB J 2008, 22, 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ikonomovic MD, Abrahamson EE, Uz T, Manev H, Dekosky ST, Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer’s disease. J Histochem Cytochem 2008, 56, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhan X, Stamova B, Sharp FR, Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Front Aging Neurosci 2018, 10, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Czapski GA, Gajkowska B, Strosznajder JB, Systemic administration of lipopolysaccharide induces molecular and morphological alterations in the hippocampus. Brain Res 2010, 1356, 85–94. [DOI] [PubMed] [Google Scholar]
- [76].Chu J, Li JG, Ceballos-Diaz C, Golde T, Pratico D, The influence of 5-lipoxygenase on Alzheimer’s disease-related tau pathology: in vivo and in vitro evidence. Biol Psychiatry 2013, 74, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde TE, Pratico D, 5-Lipoxygenase gene transfer worsens memory, amyloid, and tau brain pathologies in a mouse model of Alzheimer disease. Annals of neurology 2012, 72, 442–454. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [78].Giannopoulos PF, Chu J, Joshi YB, Sperow M, Li JG, Kirby LG, Pratico D, Gene knockout of 5-lipoxygenase rescues synaptic dysfunction and improves memory in the triple-transgenic model of Alzheimer’s disease. Mol Psychiatry 2014, 19, 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [79].Emerson MR, LeVine SM, Experimental allergic encephalomyelitis is exacerbated in mice deficient for 12/15-lipoxygenase or 5-lipoxygenase. Brain Res 2004, 1021, 140–145. [DOI] [PubMed] [Google Scholar]
- [80].Pruss H, Rosche B, Sullivan AB, Brommer B, Wengert O, Gronert K, Schwab JM, Proresolution lipid mediators in multiple sclerosis - differential, disease severity-dependent synthesis - a clinical pilot trial. PLoS One 2013, 8, e55859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Vital SA, Becker F, Holloway PM, Russell J, Perretti M, Granger DN, Gavins FN, Formyl-Peptide Receptor 2/3/Lipoxin A4 Receptor Regulates Neutrophil-Platelet Aggregation and Attenuates Cerebral Inflammation: Impact for Therapy in Cardiovascular Disease. Circulation 2016, 133, 2169–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N, Smirnova N, Gazaryan I, Ratan RR, Witztum JL, Montaner J, Holman TR, Lo EH, van Leyen K, Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Annals of neurology 2013, 73, 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Goicoechea M, Sanchez-Nino MD, Ortiz A, Garcia de Vinuesa S, Quiroga B, Bernis C, Morales E, Fernandez-Juarez G, de Sequera P, Verdalles U, Verde E, Luno J, Low dose aspirin increases 15-epi-lipoxin A4 levels in diabetic chronic kidney disease patients. Prostaglandins Leukot Essent Fatty Acids 2017, 125, 8–13. [DOI] [PubMed] [Google Scholar]
- [84].Schwartzman ML, Iserovich P, Gotlinger K, Bellner L, Dunn MW, Sartore M, Grazia Pertile M, Leonardi A, Sathe S, Beaton A, Trieu L, Sack R, Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes 2010, 59, 1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yu D, Xu Z, Yin X, Zheng F, Lin X, Pan Q, Li H, Inverse Relationship between Serum Lipoxin A4 Level and the Risk of Metabolic Syndrome in a Middle-Aged Chinese Population. PLoS One 2015, 10, e0142848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Alqawlaq S, Flanagan JG, Sivak JM, All roads lead to glaucoma: Induced retinal injury cascades contribute to a common neurodegenerative outcome. Exp Eye Res 2018. [DOI] [PubMed] [Google Scholar]
- [87].Livne-Bar I, Wei J, Liu HH, Alqawlaq S, Won GJ, Tuccitto A, Gronert K, Flanagan JG, Sivak JM, Astrocyte-derived lipoxins A4 and B4 promote neuroprotection from acute and chronic injury. The Journal of clinical investigation 2017, 127, 4403–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bazan NG, Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol 2005, 15, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Calandria JM, Asatryan A, Balaszczuk V, Knott EJ, Jun BK, Mukherjee PK, Belayev L, Bazan NG, NPD1-mediated stereoselective regulation of BIRC3 expression through cREL is decisive for neural cell survival. Cell Death Differ 2015, 22, 1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Calandria JM, Sharp MW, Bazan NG, The Docosanoid Neuroprotectin D1 Induces TH-Positive Neuronal Survival in a Cellular Model of Parkinson’s Disease. Cell Mol Neurobiol 2015, 35, 1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wei J, Gronert K, The role of pro-resolving lipid mediators in ocular diseases. Molecular aspects of medicine 2017, 58, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Biteman B, Hassan IR, Walker E, Leedom AJ, Dunn M, Seta F, Laniado-Schwartzman M, Gronert K, Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J 2007, 21, 2257–2266. [DOI] [PubMed] [Google Scholar]
- [93].Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, Carughi A, Perelman D, Kanaoka Y, Sangiovanni JP, Gronert K, Smith LE, 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med 2011, 3, 69ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Walden TL Jr., Radioprotection of mouse hematopoietic stem cells by leukotriene A4 and lipoxin B4. J Radiat Res 1988, 29, 255–260. [DOI] [PubMed] [Google Scholar]
- [95].Wang X, Hjorth E, Vedin I, Eriksdotter M, Freund-Levi Y, Wahlund LO, Cederholm T, Palmblad J, Schultzberg M, Effects of n-3 FA supplementation on the release of proresolving lipid mediators by blood mononuclear cells: the OmegAD study. J Lipid Res 2015, 56, 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]


