Supplemental Digital Content is available in the text.
Keywords: advanced melanoma, immune checkpoint inhibitors, pooled analysis, subgroup analyses, treatment-free survival
Abstract
Patients with advanced melanoma treated with immune checkpoint inhibitors can experience ongoing disease control after treatment discontinuation without subsequent systemic anticancer therapy. We previously defined a novel outcome, treatment-free survival (TFS), as the time between protocol therapy cessation and subsequent therapy initiation/death. We assessed the effect of established prognostic variables [lactate dehydrogenase (LDH), programmed death ligand 1 status, BRAF mutation status, performance status, and sex] on TFS in different treatment scenarios: treatment until toxicity/progression with frequent early cessation (nivolumab plus ipilimumab), treatment until toxicity/progression with a well-tolerated regimen (nivolumab), and treatment for a short fixed duration (ipilimumab). Data were pooled from 1077 patients with advanced melanoma treated in the CheckMate 069 and 067 trials. TFS was defined as the area between the Kaplan–Meier curves for time to therapy cessation and time to subsequent therapy initiation/death. TFS was estimated by restricted mean (r-mean) survival time at 36 months since randomization. Clinically meaningful TFS (r-mean TFS 3.7–12.7 months) was observed across all patient subgroups. TFS was longest in patients treated with nivolumab plus ipilimumab. The largest differences in r-mean TFS were observed with LDH in the nivolumab plus ipilimumab and ipilimumab treatment groups (TFS difference 4.7 and 4.9 months, respectively). In the nivolumab group, there was little difference in TFS across subgroups (r-mean TFS 3.7–5.5 months). TFS was sensitive to prognostic subgroup differences; however, duration of treatment affected the sensitivity of TFS. These results provide further support for TFS as a clinical outcome measure.
Introduction
Patients with advanced melanoma treated with immune checkpoint inhibitors (ICIs) can experience disease control following treatment discontinuation without ongoing toxicity and without the need for subsequent systemic anticancer therapy [1–6]. This unique effect of treatment with ICIs has not been captured in traditional outcome measurements such as progression-free survival or overall survival (OS). To characterize the period in which patients survive free of any anticancer treatment, treatment-free survival (TFS) has been proposed as a novel outcome measure and is defined as the time between ICI therapy cessation and subsequent therapy initiation or death [7]. TFS is part of an integrated analysis that comprehensively describes how patients spend OS time in different health states: on and off treatment, with and without treatment-related toxicity and on subsequent therapy [7].
In a pooled analysis of the phase 2 CheckMate 069 (NCT01927419) and phase 3 CheckMate 067 (NCT01844505) clinical trials, patients with advanced melanoma in the nivolumab plus ipilimumab, nivolumab and ipilimumab groups had spent 11.1, 4.6 and 8.7 months of the 36-month period, respectively, alive and treatment-free [7]. To determine the relevance of TFS as a novel outcome measure and clinically meaningful endpoint, it is important to understand how clinical prognostic factors and different treatment regimens affect TFS. Several prognostic factors have been identified in patients with advanced melanoma [8–16]. Elevated serum lactate dehydrogenase (LDH) is strongly and independently associated with decreased OS [8–12]. Programmed death-ligand 1 (PD-L1) expression is an imperfect marker that may correlate with response rate but has not been shown to correlate independently with OS in patients treated with ICIs in melanoma [13]. BRAF mutations were associated with more aggressive disease and a poorer prognosis prior to the development of targeted therapies and ICIs [14–16]. Patient characteristics such as lower performance status and male sex are also adverse prognostic markers associated with decreased OS [9–11].
To better understand the performance, interpretation and relevance of TFS as a novel outcome measure, we assessed the sensitivity of TFS to established prognostic variables and biomarkers in three different treatment scenarios: (1) treatment until toxicity or progression with frequent early cessation (nivolumab plus ipilimumab), (2) treatment until toxicity or progression with a relatively well-tolerated regimen not requiring frequent dose interruptions or early cessation (nivolumab) and (3) treatment for a short fixed duration of time (ipilimumab).
Methods
Patients and study design
Data were pooled from the randomized double-blind, placebo-controlled phase 2 CheckMate 069 trial [1] (nivolumab plus ipilimumab vs. ipilimumab monotherapy) and the phase 3 CheckMate 067 trial [2] (nivolumab plus ipilimumab or nivolumab monotherapy vs. ipilimumab monotherapy) in previously untreated patients with advanced melanoma. In these trials, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg was administered every 3 weeks for four doses, followed by nivolumab 3 mg/kg every 2 weeks; nivolumab 3 mg/kg was administered every 2 weeks; and ipilimumab 3 mg/kg was administered every 3 weeks for four doses. The monotherapy groups had matched placebo, which was not considered in these analyses. In all three groups, blinded treatment was continued until disease progression, unacceptable toxicity or patient decision. To maintain consistent follow-up in this analysis, data were pooled from the 36-month follow-up of both trials [1,2] (results of a 5-year follow-up have been published for CheckMate 067 [17] but not for CheckMate 069). Patients were grouped by protocol treatment and further divided into subgroups based on prognostic variables and biomarkers. The subgroups assessed in this analysis were based on LDH status [normal vs. elevated (greater than the upper limit of normal)], tumor PD-L1 status [PD-L1–positive (≥5% PD-L1 expression) vs. PD-L1–negative (<5% PD-L1 expression)], BRAF mutation status (mutant vs. wild-type), Eastern Cooperative Oncology Group (ECOG) performance status (0 vs. 1–2) and sex (male vs. female).
The original studies (CheckMate 069 and CheckMate 067) were conducted in accordance with the provisions of the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practice. All the patients provided written informed consent before enrollment.
Statistical considerations
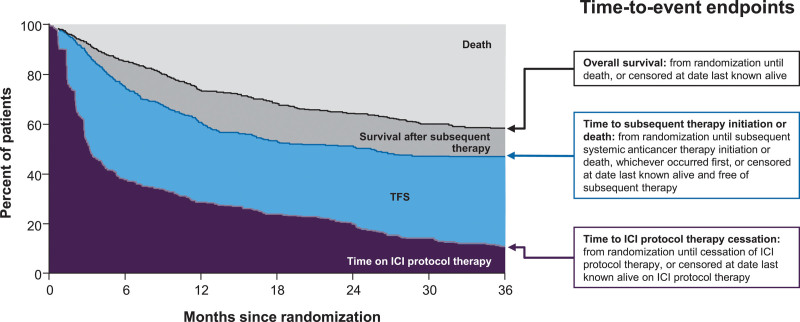
The analysis population included all 1077 patients who initiated protocol therapy in the two studies. To assess how patients spent OS time over the 36-month period since randomization, the following were calculated: Kaplan–Meier estimates of time-to-event endpoints, areas under each Kaplan–Meier curve as 36-month restricted mean (r-mean) survival times, differences in areas (i.e. differences in r-means) to quantify health state regions and percentages of the 36-month period spent alive and in each health state region (Fig. 1). Our primary outcome measure was TFS, which was defined as the area between the Kaplan–Meier curves for time to ICI protocol therapy cessation and time to subsequent therapy initiation or death. Time to ICI protocol therapy cessation was calculated as the time from randomization until the cessation of therapy or the censoring date, when the patient was last known to be alive on therapy. Time to subsequent therapy initiation was calculated as the time from randomization until initiation of subsequent systemic anticancer therapy or death, whichever came first, or the censoring date, when the patient was last known to be alive and free of subsequent therapy. Differences in r-mean TFS between subgroups of patients were calculated with bootstrapped 95% confidence intervals (CIs).
Fig. 1.
Schematic illustration of the end points that partition the area under the OS curve into TFS and other resulting health states. ICI, immune checkpoint inhibitor; OS, overall survival; TFS, treatment-free survival. Adapted from J Clin Oncol [7] under Creative Commons License 4.0 [CC BY 4.0].
Results
Patient population
Among 1077 patients who initiated ICI protocol therapy in CheckMate 069 and 067, 407 received nivolumab plus ipilimumab, 313 received nivolumab and 357 received ipilimumab. Within the treatment groups, 63–66% of patients had a normal LDH level, 23–26% had tumor PD-L1 expression ≥5%, 29–31% had mutant BRAF tumors, 72–76% had a baseline ECOG performance status of 0 and 64–66% were male (Tables 1–3). Overall, the r-mean TFS ranged from 3.7 to 12.7 months of the 36-month follow-up period across all treatment regimens and subgroups.
Table 1.
Health states according to subgroups in patients treated with nivolumab plus ipilimumab
| Subgroup | n (%) | 36-month r-mean time (months) | |||
|---|---|---|---|---|---|
| Time on ICI protocol therapy | TFS | TFS difference (95% CI) | Survival after subsequent therapy | ||
| Overall | 407 (100) | 10.3 | 11.1 | NA | 4.3 |
| LDH statusa | 4.7 (2.1–7.3) | ||||
| Normal | 268 (66) | 11.3 | 12.7 | 4.7 | |
| Elevated | 138 (34) | 8.0 | 8.0 | 3.8 | |
| PD-L1 5% statusb | 2.2 (−1.8 to 6.4) | ||||
| Positive | 92 (23) | 10.4 | 12.7 | 3.6 | |
| Negative | 264 (65) | 10.1 | 10.5 | 4.6 | |
| BRAF status | −3.8 (−5.8 to −1.7) | ||||
| Mutant | 124 (30) | 12.5 | 8.5 | 6.1 | |
| Wild-type | 283 (70) | 9.3 | 12.3 | 3.5 | |
| ECOG performance status | 2.5 (0.4–4.8) | ||||
| 0 | 308 (76) | 10.9 | 11.7 | 4.9 | |
| 1–2 | 99 (24) | 8.4 | 9.2 | 2.6 | |
| Sex | 1.3 (−1.5 to 4.2) | ||||
| Male | 268 (66) | 11.3 | 11.6 | 3.7 | |
| Female | 139 (34) | 8.2 | 10.3 | 5.7 | |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; NA, not applicable; PD-L1, programmed death ligand 1; TFS, treatment-free survival.
Unknown, n=1.
Unknown, n=51.
Table 3.
Health states according to subgroups in patients treated with ipilimumab
| Subgroup | n (%) | 36-month r-mean time (months) | |||
|---|---|---|---|---|---|
| Time on ICI protocol therapy | TFS | TFS difference (95% CI) | Survival after subsequent therapy | ||
| Overall | 357 (100) | 2.6 | 8.7 | NA | 10.1 |
| LDH statusa | 4.9 (3.1–6.7) | ||||
| Normal | 230 (64) | 2.7 | 10.3 | 11.7 | |
| Elevated | 125 (35) | 2.4 | 5.4 | 7.4 | |
| PD-L1 5% status | 2.1 (−1.2 to 5.4) | ||||
| Positive | 85 (24) | 2.6 | 10.2 | 12.0 | |
| Negative | 225 (63) | 2.5 | 8.1 | 9.8 | |
| BRAF status | −1.1 (−3.1 to 0.9) | ||||
| Mutant | 105 (29) | 2.5 | 7.9 | 12.6 | |
| Wild-type | 252 (71) | 2.6 | 9.0 | 9.1 | |
| ECOG performance status | 1.7 (−1.1 to 4.6) | ||||
| 0 | 257 (72) | 2.7 | 9.1 | 11.6 | |
| 1–2 | 100 (28) | 2.3 | 7.5 | 6.2 | |
| Sex | −0.8 (−3.2 to 1.6) | ||||
| Male | 229 (64) | 2.5 | 8.4 | 10.1 | |
| Female | 128 (36) | 2.6 | 9.2 | 10.3 | |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; NA, not applicable; PD-L1, programmed death ligand 1; TFS, treatment-free survival.
Unknown, n=2.
Unknown, n=47.
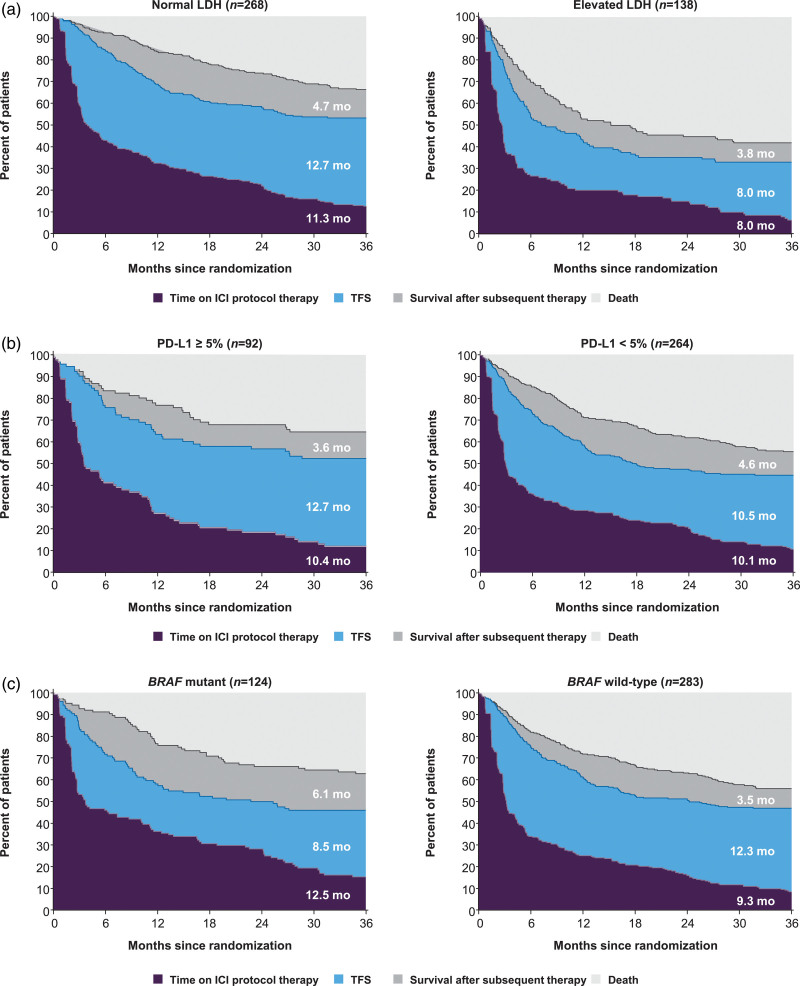
Subgroup analyses of health states in the nivolumab plus ipilimumab group
Patients who received treatment with nivolumab plus ipilimumab followed by nivolumab until unacceptable toxicity or progression were on protocol therapy for an r-mean time of 10.3 months. The overall r-mean TFS was 11.1 months, which varied from 8.0 to 12.7 months across the subgroups (Table 1). The largest difference in TFS was observed between the LDH subgroups: r-mean TFS was 12.7 months in patients with normal LDH and 8.0 months in those with elevated LDH (difference 4.7 months, 95% CI, 2.1–7.3) (Fig. 2a). TFS was 12.7 months in patients with PD-L1–positive tumors compared with 10.5 months in patients with PD-L1–negative tumors (difference 2.2 months, 95% CI, −1.8 to 6.4) (Fig. 2b). TFS was shorter in patients with mutant BRAF tumors than those with wild-type BRAF tumors, with r-mean TFS times of 8.5 and 12.3 months, respectively (difference −3.8 months, 95% CI, −5.8 to −1.7) (Fig. 2c). Additionally, patients with mutant BRAF tumors spent 3.2 months longer on ICI protocol therapy and survived 2.6 months longer after subsequent therapy initiation compared with those with wild-type tumors. In patients with ECOG performance status 0, ICI protocol therapy was 2.5 months longer, TFS was 2.5 months longer and survival was 2.3 months longer after subsequent therapy than in those with ECOG performance status 1/2 (online Supplementary Figure S1A, Supplemental digital content 1, http://links.lww.com/MR/A277). TFS was similar in male and female patients; however, males spent a longer time on ICI protocol therapy (r-mean time of 11.3 vs. 8.2 months) and survived for a shorter time after subsequent therapy (r-mean time of 3.7 vs. 5.7 months) (online Supplementary Figure S1B, Supplemental digital content 1, http://links.lww.com/MR/A277).
Fig. 2.
Estimates of TFS and other health states over the 36-month period in patients treated with nivolumab plus ipilimumab according to patient subgroups based on (a) LDH status, (b) PD-L1 status and (c) BRAF mutation status; r-mean times (months) are annotated on the health state areas. ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; PD-L1, programmed death 1; TFS, treatment-free survival.
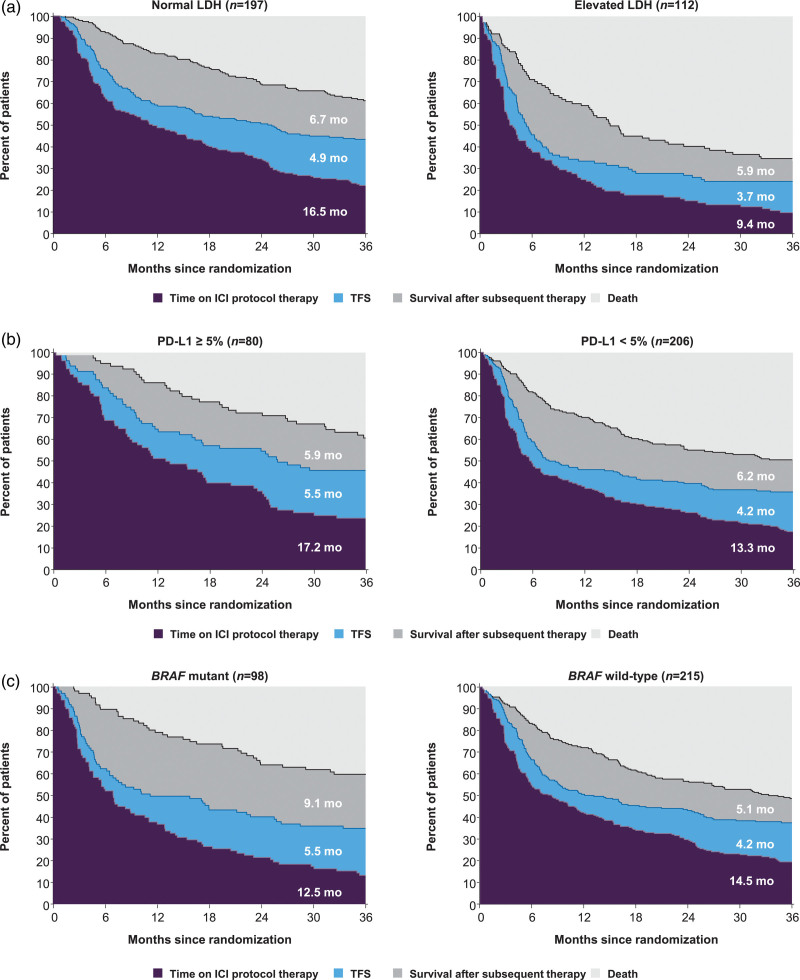
Subgroup analyses of health states in the nivolumab group
Among the three treatment groups, r-mean time on protocol therapy was longest (13.9 months) and r-mean TFS was shortest (4.6 months) in patients who received nivolumab until unacceptable toxicity or progression (Table 2). As TFS was short in these patients (varying from 3.7 to 5.5 months), differences in TFS were minimal across the subgroups. In patients with normal LDH, ICI protocol therapy was longer than in those with elevated LDH (r-mean time 16.5 vs. 9.4 months); however, r-mean TFS times were similar between the LDH subgroups (4.9 vs. 3.7 months; difference 1.2, 95% CI, –0.6 to 3.1) (Fig. 3a). Patients with PD-L1–positive tumors spent more time on ICI protocol therapy than those with PD-L1–negative tumors (r-mean time 17.2 vs. 13.3 months), but TFS times were similar between the PD-L1 subgroups (5.5 vs. 4.2 months) (Fig. 3b). In patients with mutant BRAF tumors, ICI protocol therapy was 2 months shorter, TFS was 1.3 months longer and survival was 4 months longer after initiation of subsequent therapy than in those with wild-type BRAF tumors (Fig. 3c). In patients with ECOG performance status 0, ICI protocol therapy was 5.2 months longer, TFS was 1 month longer and survival was 2.6 months longer after initiation of subsequent therapy than in those with ECOG performance status 1/2 (online Supplementary Figure S2A, Supplemental digital content 1, http://links.lww.com/MR/A277). In male patients, ICI protocol therapy was 3.9 months longer, TFS was 1.2 months longer and survival was 3 months shorter after initiation of subsequent therapy than in female patients (online Supplementary Figure S2B, Supplemental digital content 1, http://links.lww.com/MR/A277).
Table 2.
Health states according to subgroups in patients treated with nivolumab
| Subgroup | n (%) | 36-month r-mean time (months) | |||
|---|---|---|---|---|---|
| Time on ICI protocol therapy | TFS | TFS difference (95% CI) | Survival after subsequent therapy | ||
| Overall | 313 (100) | 13.9 | 4.6 | NA | 6.4 |
| LDH statusa | 1.2 (−0.6 to 3.1) | ||||
| Normal | 197 (63) | 16.5 | 4.9 | 6.7 | |
| Elevated | 112 (36) | 9.4 | 3.7 | 5.9 | |
| PD-L1 5% statusb | 1.3 (−0.7 to 3.4) | ||||
| Positive | 80 (26) | 17.2 | 5.5 | 5.9 | |
| Negative | 206 (66) | 13.3 | 4.2 | 6.2 | |
| BRAF status | 1.3 (−1.1 to 3.7) | ||||
| Mutant | 98 (31) | 12.5 | 5.5 | 9.1 | |
| Wild-type | 215 (69) | 14.5 | 4.2 | 5.1 | |
| ECOG performance status | 1.0 (−0.3 to 2.3) | ||||
| 0 | 234 (75) | 15.2 | 4.9 | 7.0 | |
| 1–2 | 79 (25) | 10.0 | 3.9 | 4.4 | |
| Sex | 1.2 (−0.1 to 2.6) | ||||
| Male | 200 (64) | 15.3 | 5.1 | 5.3 | |
| Female | 113 (36) | 11.4 | 3.8 | 8.3 | |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; NA, not applicable; PD-L1, programmed death ligand 1; TFS, treatment-free survival.
Unknown, n=4.
Unknown, n=27.
Fig. 3.
Estimates of TFS and other health states over the 36-month period in patients treated with nivolumab according to patient subgroups based on (a) LDH status, (b) PD-L1 status and (c) BRAF mutation status; r-mean times (months) are annotated on the health state areas. ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; PD-L1, programmed death 1; TFS, treatment-free survival.
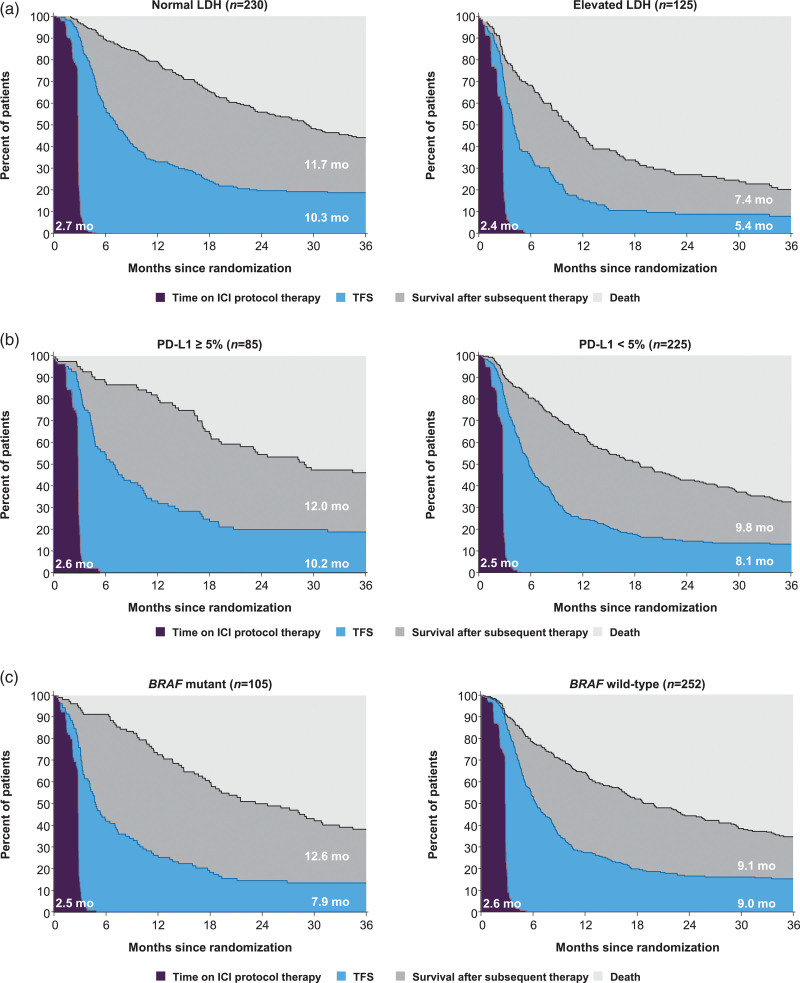
Subgroup analyses of health states in the ipilimumab group
In patients who received ipilimumab for a short fixed duration of time, the overall r-mean TFS was 8.7 months, which varied from 5.4 to 10.3 months across the subgroups (Table 3). The largest difference in TFS was observed between the LDH subgroups: r-mean TFS was 10.3 months in patients with normal LDH and 5.4 months in those with elevated LDH (difference 4.9 months, 95% CI, 3.1–6.7) (Fig. 4a). TFS was similar in patients with PD-L1–positive tumors than in those with PD-L1–negative tumors, with r-mean TFS times of 10.2 and 8.1 months, respectively (difference 2.1 months, 95% CI, −1.2 to 5.4) (Fig. 4b). In patients with mutant BRAF tumors, TFS was 1.1 months shorter and survival was 3.5 months longer after initiation of subsequent therapy than in those with wild-type BRAF (Fig. 4c). In patients with ECOG performance status 0, TFS was 1.7 months longer and survival was 5.4 months longer after initiation of subsequent therapy than in those with ECOG performance status 1/2 (online Supplementary Figure S3A, Supplemental digital content 1, http://links.lww.com/MR/A277). TFS was similar in male and female patients, with r-mean TFS times of 8.4 and 9.2 months, respectively; survival after initiation of subsequent therapy was also similar, with r-mean times of 10.1 and 10.3 months in the respective subgroups (online Supplementary Figure S3B, Supplemental digital content 1, http://links.lww.com/MR/A277).
Fig. 4.
Estimates of TFS and other health states over the 36-month period in patients treated with ipilimumab according to patient subgroups based on (a) LDH status, (b) PD-L1 status and (c) BRAF mutation status; r-mean times (months) are annotated on the health state areas; r-mean times (months) are annotated on the health state areas. ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; PD-L1, programmed death 1; TFS, treatment-free survival.
Discussion
Treatment with ICIs has improved OS in patients with advanced melanoma and provided some patients the ability to stop treatment and continue to survive with disease control for extended periods. In an earlier pooled analysis of the CheckMate 069 and 067 trials, patients in the nivolumab plus ipilimumab, nivolumab and ipilimumab groups had spent 11.1, 4.6 and 8.7 months of the 36-month period, respectively, alive and treatment-free [7]. In the current analysis, clinically meaningful TFS was observed with all three treatment regimens across various patient subgroups, including those with poor prognostic factors such as elevated LDH. TFS was sensitive to prognostic subgroup differences; however, the protocol-dictated duration of therapy affected the sensitivity of TFS.
Across all of the patient subgroups, r-mean TFS time was longer with nivolumab plus ipilimumab (8.0–12.7 months) than with nivolumab (3.7–5.5 months) or ipilimumab (5.4–10.3 months) monotherapy. Differences in TFS within the prognostic subgroups were more apparent in the nivolumab plus ipilimumab and ipilimumab monotherapy treatment groups. This may be partially attributed to the shorter time on nivolumab plus ipilimumab treatment with frequent early cessation or to the short fixed duration of ipilimumab treatment, which resulted in long TFS. Additionally, ipilimumab treatment itself may have contributed to the ongoing disease control noted after therapy cessation in the ipilimumab-containing treatment groups. Although nivolumab plus ipilimumab treatment is frequently stopped early due to toxicity, little toxicity was reported in the TFS period [7], which indicates that the toxicities requiring treatment cessation are often reversible and short-lived. In contrast, r-mean TFS was shorter in patients treated with nivolumab, which resulted in smaller differences in TFS between the subgroups. As nivolumab is an often well-tolerated therapy, patients were less likely to have stopped therapy due to toxicity and were more likely to stop treatment only after progression leading to shorter TFS. As a result, differences in TFS were negligible, whereas differences in the time on ICI protocol therapy and survival after subsequent therapy were observed between the subgroups. Future trials should investigate whether nivolumab monotherapy can be stopped earlier to allow longer TFS as has been suggested in prior studies [18,19].
The largest difference in TFS was observed between patients with normal and elevated LDH, which is the most established prognostic marker in melanoma. Previous studies have shown that patients with elevated LDH have inferior OS [8–12]. Elevated serum LDH has been shown to correlate with rapidly growing malignancies, resulting in a poor prognosis [12,20]. In the current analysis, patients with elevated LDH, which is more likely to be associated with more aggressive disease, were still able to experience long TFS with nivolumab plus ipilimumab (r-mean TFS 8.0 months); however, TFS was longer in patients with normal LDH (r-mean TFS 12.7 months). Similarly, TFS was 4.9 months shorter in patients with elevated LDH in the ipilimumab group and 1.2 months shorter in the nivolumab group than those with normal LDH. In the nivolumab group, patients with elevated LDH spent significantly less time on ICI protocol therapy (7.1 months), likely due to more aggressive and less treatment-responsive disease in this patient population.
Across the treatment groups, r-mean TFS was slightly longer in patients with positive PD-L1 status than in those with negative PD-L1 status, although clinically meaningful TFS was observed in both PD-L1 subgroups. This is consistent with the fact that PD-L1 is a limited biomarker that has been shown to correlate with response rate, but not with OS, in patients with melanoma treated with ICIs [13]. In the nivolumab plus ipilimumab and ipilimumab monotherapy groups, r-mean TFS was slightly longer in patients with wild-type BRAF tumors, which is consistent with the expectation that the BRAF mutation is associated with a poorer prognosis in patients with malignant melanoma [21]. However, survival after initiation of subsequent therapy was longer in patients with mutant BRAF tumors across all treatment groups, which may be attributed to the availability of a highly effective subsequent therapeutic approach in this patient population.
Poor performance status has been known to be an adverse prognostic marker in patients with advanced melanoma [9–11]. Consistent with previously published studies, the current analysis showed that TFS was shorter in patients with ECOG performance status 1/2 than in those with ECOG performance status 0 across all three treatment groups. In some studies, male sex has been associated with lower OS [9–11]. However, the current analysis showed that the duration of TFS was not affected by sex because TFS was similar between male and female patients across all treatment groups (differences in r-mean TFS varied from −0.8 to 1.3 months).
The analyses presented here have several limitations. Evaluation of TFS after different treatment regimens would have been ideal if all patients were required to stop treatment by a specific time. However, this analysis was constrained by protocol-specified differences in treatment administration: treatment until toxicity or progression for nivolumab plus ipilimumab and nivolumab monotherapy and treatment for a fixed period for ipilimumab monotherapy (four doses). Another limitation was that patient numbers were small in some of the subgroups. As this analysis focused on the sensitivity of TFS as a measure of prognostic differences, TFS with and without toxicity was not analyzed, although this is an important consideration for these regimens.
Conclusion
The current analysis showed that TFS reflected differences in established prognostic subgroups in the CheckMate 069 and 067 clinical trials, which provide further support for the use of TFS as a clinical outcome measure. Information on how patients spend survival time and how this time is impacted by various prognostic factors and choice of treatment regimen can help guide treatment choices. TFS as an outcome measure should be evaluated in clinical trials in which all patients stop treatment at a set time point. We believe that incorporating TFS into the design of future clinical trials with ICI therapy can add clinically meaningful information that is not provided by traditional endpoints such as progression-free survival and OS. The clinically significant TFS observed in patients with advanced melanoma following treatment with ICIs in this study demonstrates that patients can maintain disease control for an extended period of time after treatment discontinuation.
Acknowledgements
The authors would like to thank the patients and families who made these studies possible and the clinical study teams who participated in the studies. Editorial assistance was provided by Kakoli Parai, Mark Palangio, and Michele Salernitano of Ashfield MedComms, an Ashfield Health company, funded by Bristol Myers Squibb.
The original studies (CheckMate 069 and CheckMate 067) were conducted in accordance with the provisions of the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practice. All the patients provided written informed consent before enrollment.
Bristol Myers Squibb’s policy on data sharing is available at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
This work was supported by Bristol Myers Squibb (no grant number is applicable).
C.M.M.: contributed to the study conception or design of the study, contributed to data analysis and interpretation, prepared original draft, reviewed and approved the final version. L.W.: contributed to data acquisition and analysis, reviewed and approved the final version. B.S.: contributed to data interpretation, reviewed and approved the final version. C.R.: contributed to the study conception or design of the study; contributed to data interpretation, reviewed and approved the final version. A.A.T., M.B.A. and D.F.M.: contributed to the study conception or design of the study, contributed to data interpretation, reviewed and approved the final version. M.M.R.: contributed to the study conception or design of the study, contributed to data acquisition, analysis and interpretation, reviewed and approved the final version.
Conflicts of interest
L.W. has worked as a consultant to Bristol Myers Squibb. B.S. is an employee of and stockholder in Bristol Myers Squibb. C.R. is an employee of Bristol Myers Squibb. A.A.T. has received institutional research grants from Bristol Myers Squibb, Genentech/Roche, Merck, and OncoSec; and served as a consultant/advisor for Array Biopharma, BioNTech, Bristol Myers Squibb, EMD Serono, Genentech/Roche, Immunocore, Merck, NewLink Genetics, Novartis, Partner Therapeutics, Pfizer, and Sanofi-Genzyme/Regeneron. M.B.A. has served as an advisor for Arrowhead, Aveo, Bristol Myers Squibb, Eisai, Elpis, Genetech/Roche, Leads, Merck, Novartis, Pfizer, Pneuma, Pyxis Oncology, Werewolf; and as a consultant to Adagene, Agenus, Apexigen, Exelixis, Idera, ImmunoCore, Iovance, and Neoleuken. D.F.M. has served as a consultant to Alkermes, Bristol Myers Squibb, Eisai, Eli Lilly and Company, EMD Serono, Iovance, Merck, and Pfizer; received research support from Alkermes, Bristol Myers Squibb, Exelixis, Genentech, Merck, Pfizer, and X4 Pharma. M.M.R. has served as a consultant to Ipsen/Debiopharm; received research support from Bayer and Bristol Myers Squibb; received institutional research support from Bristol Myers Squibb, Ipsen, Ferring, Merck, Novartis, Pfizer, Pierre Fabre, Roche, and TerSera; received personal fees from Bristol Myers Squibb and Tolmar Pharmaceuticals; and received nonfinancial support from Bristol Myers Squibb. For the remaining author, there is no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.melanomaresearch.com.
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016; 17:1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol 2018; 36:1668–1674. [DOI] [PubMed] [Google Scholar]
- 4.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019; 30:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019; 20:1239–1251. [DOI] [PubMed] [Google Scholar]
- 6.Jansen YJL, Rozeman EA, Mason R, Goldinger SM, Geukes Foppen MH, Hoejberg L, et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol 2019; 30:1154–1161. [DOI] [PubMed] [Google Scholar]
- 7.Regan MM, Werner L, Rao S, Gupte-Singh K, Hodi FS, Kirkwood JM, et al. Treatment-free survival: a novel outcome measure of the effects of immune checkpoint inhibition-A pooled analysis of patients with advanced melanoma. J Clin Oncol 2019; 37:3350–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27:6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol 2000; 18:3782–3793. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura Y, Kitano S, Takahashi A, Tsutsumida A, Namikawa K, Tanese K, et al. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget 2016; 7:77404–77415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirott MN, Bajorin DF, Wong GY, Tao Y, Chapman PB, Templeton MA, Houghton AN. Prognostic factors in patients with metastatic malignant melanoma. A multivariate analysis. Cancer 1993; 72:3091–3098. [DOI] [PubMed] [Google Scholar]
- 12.Agarwala SS, Keilholz U, Gilles E, Bedikian AY, Wu J, Kay R, et al. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951). Eur J Cancer 2009; 45:1807–1814. [DOI] [PubMed] [Google Scholar]
- 13.Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018; 19:1480–1492. [DOI] [PubMed] [Google Scholar]
- 14.Kumar R, Angelini S, Czene K, Sauroja I, Hahka-Kemppinen M, Pyrhönen S, Hemminki K. BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res 2003; 9:3362–3368. [PubMed] [Google Scholar]
- 15.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 2011; 29:1239–1246. [DOI] [PubMed] [Google Scholar]
- 16.von Moos R, Seifert B, Simcock M, Goldinger SM, Gillessen S, Ochsenbein A, et al. Swiss Group for Clinical Cancer Research (SAKK). First-line temozolomide combined with bevacizumab in metastatic melanoma: a multicentre phase II trial (SAKK 50/07). Ann Oncol 2012; 23:531–536. [DOI] [PubMed] [Google Scholar]
- 17.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019; 381:1535–1546. [DOI] [PubMed] [Google Scholar]
- 18.Christiansen SA, Swoboda D, Gardner K, et al. Off treatment survival (OTS) in patients (pts) with advanced melanoma after anti-PD1 therapy. J Clin Oncol 2018; 36 (15 Suppl):9554. Abstract 9554. [Google Scholar]
- 19.Tan AC, Emmett L, Lo S, Liu V, Kapoor R, Carlino MS, et al. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann Oncol 2018; 29:2115–2120. [DOI] [PubMed] [Google Scholar]
- 20.Palmer SR, Erickson LA, Ichetovkin I, Knauer DJ, Markovic SN. Circulating serologic and molecular biomarkers in malignant melanoma. Mayo Clin Proc 2011; 86:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ny L, Hernberg M, Nyakas M, Koivunen J, Oddershede L, Yoon M, et al. BRAF mutational status as a prognostic marker for survival in malignant melanoma: a systematic review and meta-analysis. Acta Oncol 2020; 59:833–844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.