Abstract
We have used line HS-2 of Drosophila melanogaster, carrying a silenced transgene in the pericentric heterochromatin, to investigate in detail the chromatin structure imposed by this environment. Digestion of the chromatin with micrococcal nuclease (MNase) shows a nucleosome array with extensive long-range order, indicating regular spacing, and with well-defined MNase cleavage fragments, indicating a smaller MNase target in the linker region. The repeating unit is ca. 10 bp larger than that observed for bulk Drosophila chromatin. The silenced transgene shows both a loss of DNase I-hypersensitive sites and decreased sensitivity to DNase I digestion within an array of nucleosomes lacking such sites; within such an array, sensitivity to digestion by MNase is unchanged. The ordered nucleosome array extends across the regulatory region of the transgene, a shift that could explain the loss of transgene expression in heterochromatin. Highly regular nucleosome arrays are observed over several endogenous heterochromatic sequences, indicating that this is a general feature of heterochromatin. However, genes normally active within heterochromatin (rolled and light) do not show this pattern, suggesting that the altered chromatin structure observed is associated with regions that are silent, rather than being a property of the domain as a whole. The results indicate that long-range nucleosomal ordering is linked with the heterochromatic packaging that imposes gene silencing.
In eukaryotes, the very large genome size demands extensive packaging of the DNA, starting with association with histones in a nucleosome array and extending to the highly condensed chromosomes observed at mitosis. Cytological observations indicate that the genome can be subdivided into heterochromatin and euchromatin, heterochromatin being defined as that portion of the genome remaining heteropycnotic (deeply stained) as the chromosomes decondense during the passage from metaphase to interphase (31). In many species, large blocks of heterochromatin flank the centromeres and telomeres. Little gene expression is associated with this constitutive heterochromatin, which is primarily (but not exclusively) made up of repetitive sequences. Facultative heterochromatin is observed when one of the two homologues is silenced, for example, one of the X chromosomes in the somatic cells of female mammals. The inactive X chromosome is heteropycnotic, suggesting that the chromatin in such silent regions is relatively condensed. The commonly accepted model of heterochromatin is of a folded higher-order structure that might preclude access for RNA polymerase and/or other components of the transcriptional machinery. However, analyses using three-dimensional microscopy indicate that the inactive and active X chromosomes occupy the same amount of space in the mammalian nucleus, a contradiction of the simplest model (21).
A mechanism dependent on chromatin folding has also been inferred to explain the silencing of large domains in a mitotically heritable pattern, commonly seen as part of the developmental program. Well-studied examples of such epigenetic regulation include silencing of the mating type loci in Saccharomyces cerevisiae (29); Polycomb group (Pc-G)-dependent silencing of homeotic genes in Drosophila (48); and parent-specific allelic silencing of the imprinted genes in mammals (57). A similar, heritable silencing is observed when a gene normally within euchromatin is placed within or near heterochromatin by transposition or chromosomal rearrangement; expression is extinguished in many of the cells in which the gene is normally expressed. This position effect variegation (PEV) has been observed in a range of organisms (29, 46) but has been studied in the most detail in Drosophila melanogaster (54, 63).
The apparent use of alternative packaging to regulate gene expression, particularly to maintain patterns of gene silencing during development, raises interesting questions concerning the mechanism involved. Virtually all of the DNA in a eukaryotic nucleus is found in association with the histones in a nucleosome array, detected by digestion with micrococcal nuclease (MNase), with ca. 200 bp of DNA per repeating subunit (39). Such packaging alters the fundamental paradigms of gene regulation. The presence of a nucleosome will block access to a promoter and must be countered by specific multiprotein machines to activate transcription (39, 55). The 5′ regulatory regions of active genes are observed to be nucleosome free, sensitive to a variety of nucleases; such sites are referred to as DNase I-hypersensitive sites (DH sites) (26, 28). Early studies showed consistently that active genes are more susceptible to digestion by DNase I (but not MNase) than inactive genes (3, 65). Analysis using a variety of restriction enzymes has shown reduced access across the silent domain of the S. cerevisiae mating type locus (41). Transgenes inserted into heterochromatic domains in Drosophila show a loss of accessibility in the 5′ regulatory region normally established as a DH site (61). Does the resistance to digestion indicate an altered nucleosome array, with a concomitant loss of DH sites? Or might the original nucleosome array be present but the DH sites obscured by some higher-order folding of the chromatin or localization of the gene in an inaccessible compartment? If an altered nucleosome array is indicated in heterochromatin, how does it differ from that seen in euchromatin? There are some genes that are found within heterochromatin; how are these active genes packaged in comparison to the silenced transgenes?
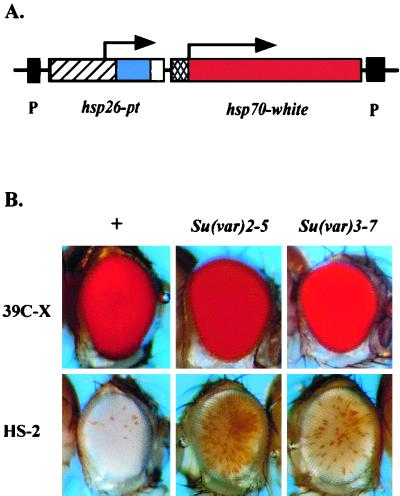
To address these questions in a systematic fashion, we have utilized a P element carrying two reporter genes: an hsp70-driven white gene, providing a readily scored visual phenotype, and a tagged copy of hsp26 (fused at position 490 with a fragment of barley cDNA, pt, followed by a termination sequence), providing a unique gene with a well-characterized regulatory region for chromatin structure analysis. When this P element is present in a euchromatic domain, the fly has a full red eye (at 25°C). Insertion into a heterochromatic domain (pericentric heterochromatin, telomeric regions, or a subset of sites within the small fourth chromosome) results in a variegating phenotype (61). To optimize comparison between the active transgene in a euchromatic site and the silenced transgene in a heterochromatic site, a screen was conducted to recover lines showing strong PEV (extensive silencing) at 28°C (18). (The higher temperature both favors expression from the hsp70 promoter and suppresses PEV [54], allowing expression of a transgene that otherwise would be silent and thus allowing us to identify that fly in a screen.) At 25°C, one line recovered (HS-2) exhibits a white eye, indicating extensive silencing of the transgene at that temperature and suggesting conversion to a fully heterochromatic form (Fig. 1). In situ hybridization has shown that the transgene in this line is inserted into the pericentric heterochromatin of chromosome arm 3L (18).
FIG. 1.
Silencing of the hsp70-white transgene in line HS-2 is suppressed by known Su(var) mutations. (A) Map of the P-element construct used. The 3′ and 5′ P-element ends (black boxes), hsp26 gene fragment (hatched box), pt (barley gene) fragment (blue box), termination signal (white box), hsp70 promoter region (cross-hatched box), and white gene fragment (red box) are diagrammed. (B) Control line 39C-X carries the P element in a euchromatic site, while HS-2 has the P element inserted into pericentric heterochromatin. The HS-2 transgene shows loss of silencing in the presence of the Su(var)2-502 and Su(var)3-7 mutations, while expression of the 39C-X transgene is unaffected.
Here we investigate the chromatin structure of the silenced transgene in line HS-2 using MNase, DNase I, and restriction enzymes. The most striking feature is the extensive ordered array of nucleosomes. A distinct 10-nucleosome-size DNA fragment can be observed in the MNase digestion pattern from the silenced transgene, while a five- or six-nucleosome-size DNA fragment is the largest distinguishable fragment observed (by ethidum bromide staining) in the pattern of digestion products for the genome as a whole. The repeating subunit is ca. 10 bp larger than the average size in Drosophila. As anticipated, reduced accessibility to restriction sites and loss of DNase I hypersensitivity are associated with the heterochromatic state. In addition, sensitivity to DNase I is decreased in regions that do not include DH sites in the endogenous gene, even though the rate of digestion by MNase does not appear to be significantly altered. Mapping shows that positioned nucleosomes block access to the promoter. This suggests that the high degree of silencing observed reflects a change in the nucleosomal array, with a concomitant loss of DH sites. In contrast to the HS-2 transgene, light and rolled, two active genes normally found within heterochromatin, have nucleosome arrays similar to that of the euchromatic transgene. The differences observed in the heterochromatin nucleosome array—regular spacing, a smaller MNase target, and general resistance to DNase I—are compatible with a stable higher-order structure. These structural features may be common characteristics of noncoding sequences in heterochromatin and may be a major factor in the silencing observed.
MATERIALS AND METHODS
Drosophila culture and genetic analysis.
Fly stocks were raised in a standard cornmeal sucrose-based medium (51) at 25°C. To test the effects of known modifiers of PEV, homozygous female flies from HS-2 were crossed with male flies carrying a Su(var) mutation; female progeny carrying both the transgene and the modifier were compared with hemizygous females in a y w background carrying the transgene. (Siblings containing the balancer chromosome were not used for comparison, because the balancers frequently have accumulated mutations, including some that modify PEV.) The following stocks were used: (i) y w67c23; Su(var)2–502/Cy; (ii) y w1118; Su(var)3–7/T(2;3)apXa; and (iii) y w67c23 (control). Female progeny (4 to 5 days postemergence) carrying both the transgene and the modifier mutation were photographed. To measure eye pigmentation, five flies were homogenized in 0.5 ml of 0.01 M HCl in ethanol. The homogenate was placed at 4°C overnight and then warmed at 50°C for 5 min. After centrifugation in a microcentrifuge for 10 min, the supernatant was recovered, and the optical density at 480 nm was recorded (37). Female y w67c23 flies were used to establish background values. Four to six independent samples (five flies each) were analyzed for each cross, and the mean was reported.
Chromatin structure analysis.
Chromatin structure was assessed by determining the pattern of digestion products obtained using MNase and DNase I, sensitivity to digestion by MNase and DNase I, and accessibility to digestion by restriction enzymes. Nuclei were prepared either from 6- to 18-h-old embryos (12) harvested from population cages (51) or from third instar larvae reared in bottles (44); all were maintained at 25°C.
Digestions of nuclei using MNase (catalog no. 107921; Boehringer) or DNase I (catalog no. 776785; Boehringer) were performed at 25°C for 3 min. Reactions (225 μl) were stopped by adding 5 μl of 0.4 M EDTA and 6.5 μl of 20% sodium dodecyl sulfate. Restriction enzyme digestion was performed by the method of Cartwright et al. (12). In all cases, DNA was purified using proteinase K digestion overnight followed by phenol-chloroform extraction. Samples for mapping experiments using the indirect end labeling technique were cleaved to completion with the appropriate restriction enzyme. Unless otherwise specified, the purified DNA samples were size separated on a 1.2 or 1.3% agarose gel, transferred to a positively charged nylon membrane (catalog no. 77419; Schleicher & Schuell), and hybridized using 32P-labeled probes. Autoradiograms were scanned and analyzed using Bio-Rad Quantity One software. See Lu et al. (43) or Cartwright et al. (12) for a detailed protocol.
Because DNase I and MNase show sequence preferences in cutting DNA, mapping experiments must take into account the pattern of digestion on naked (purified) DNA. Ten micrograms of genomic DNA from control line 39C-X or silenced line HS-2 was mixed with either DNase I digestion buffer or MNase digestion buffer in a final volume of 100 μl. DNase I was used at 0, 0.001, 0.002, and 0.004 U/μl. MNase was used at 0, 0.002, 0.004, and 0.006 U/μl. The reactions were performed at room temperature for 2 min and stopped with 2.5 μl of 0.4 M EDTA. The naked DNA samples used as controls for nucleosome mapping experiments were digested with SalI before treatment with MNase or DNase I.
RESULTS
Silencing of the hsp70-white gene in line HS-2 is a consequence of PEV.
Line HS-2 was recovered on the basis of an extreme variegating phenotype at 28°C; most of the flies in the population show a completely white eye at 25°C. To characterize the nature and extent of silencing, levels of eye pigmentation were used to provide a quantitative measurement. Responses to two known suppressors of PEV were assessed: Su(var)2-502, a point mutation in the chromo domain of heterochromatin protein 1 (HP1) (49), and Su(var)3-7, a mutation in a protein similarly associated with pericentric heterochromatin (15). The eye pigmentation level in HS-2 at 25°C is ca. 5% of that observed for euchromatic control line 39C-X. (The P element in line 39C-X, inserted into euchromatic region 2D on the X chromosome, gives a full red eye phenotype [61].) A significant loss of silencing is observed in the presence of Su(var)2-502 or Su(var)3-7 (Fig. 1). Eye pigmentation for HS-2 increased by a factor of 1.8 in the presence of Su(var)2-502 and by a factor of 1.3 in the presence of Su(var)3-7. The results confirm that the transgene in HS-2 is indeed subject to the PEV-silencing mechanism associated with heterochromatin packaging.
The low levels of white expression (only a few facets of red in the eyes of some flies in the population) in the absence of Su(var) mutations indicate that the test genes in line HS-2 are off in most cells most of the time at 25°C. Previous studies of the developmental regulation of heterochromatin-mediated gene silencing in Drosophila have found that silencing is initiated at the onset of gastrulation (2.5 h of embryogenesis), approximately 1 h after heterochromatin is first visible cytologically. Where it has been examined, expression of variegating genes in mitotically active embryonic and larval cells is generally less than that observed in differentiated cells. In particular, the red pigment scored in the adult eye appears to reflect a relaxation in silencing that occurs during differentiation of the eye imaginal disc in late third instar larvae (42). Thus, the very low levels of expression of the transgenes in line HS-2, observed as low levels of pigment in the adult eye, indicate that this line is a suitable substrate for analysis of heterochromatin packaging. In the discussion that follows, we will refer to line HS-2 as having a heterochromatic transgene and the control line 39C-X as having a euchromatic transgene.
Long-range nucleosomal ordering and an altered repeat size are observed for the HS-2 heterochromatic transgene.
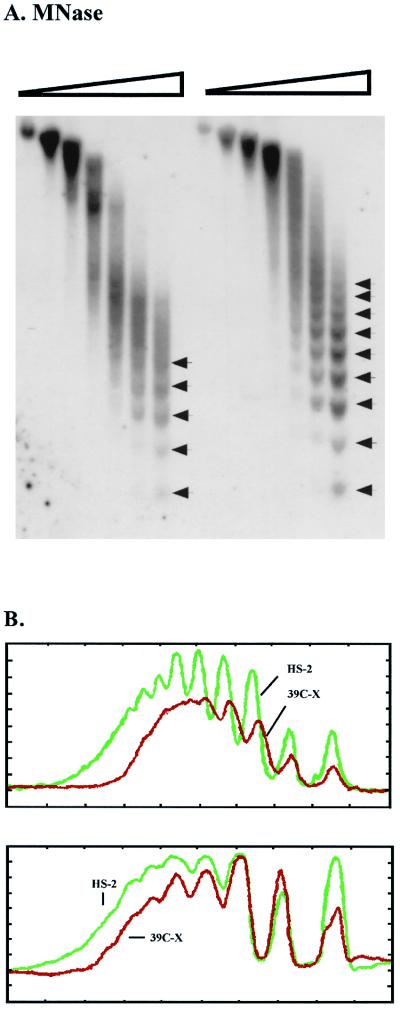
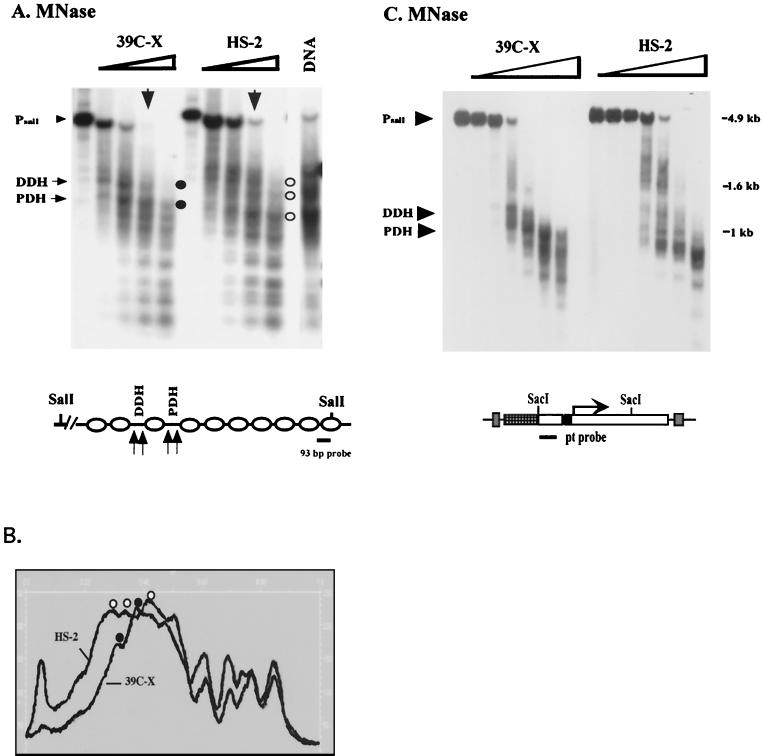
To examine the nucleosome array, nuclei isolated from 6- to 18-h-old embryos of lines 39C-X and HS-2 were treated with increasing amounts of MNase. The nucleosome array observed for the heterochromatic transgene in HS-2 is distinct and highly ordered; up to 9 or 10 nucleosomes can be clearly observed (Fig. 2A). The array for the euchromatic transgene in 39C-X shows only five or six nucleosomes before the pattern becomes blurred, presumably because of irregular spacing and the presence of DH sites (Fig. 2A). Ethidium bromide staining of the products of MNase digestion of D. melanogaster chromatin generally shows an array of five to seven nucleosomes, indicating that the pattern observed for the euchromatic transgene holds for the major portion of the genome (data not shown).
FIG. 2.
MNase digestion reveals long-range nucleosomal ordering, with an altered repeat size, at the silenced transgene. (A) Nuclei isolated from 6- to 18-h-old embryos from lines 39C-X and HS-2 were treated with increasing amounts of MNase (concentrations of 0, 0.01, 0.02, 0.04, 0.08, 0.12, and 0.24 U/μl), and the DNA was purified and run in a 1.5% agarose gel. The DNA was transferred to a positively charged nylon membrane, and the membrane was hybridized with α-32P-labeled pt fragment. Linker sites cleaved by MNase are indicated by arrows. (B) Densitometric scans from the last lane of each sample set are compared (top to bottom of each lane is left to right along the x axis), aligned at the position of the mononucleosome (top panel). The same blot used in panel A was stripped and rehybridized with a 0.8-kb PvuII-SacII fragment from the 3′ coding region of the endogenous hsp26 gene as a control panel. Densitometric scans from the last lane of this sample set are shown (bottom (data not shown)). (C) The DNA samples used for the last two lanes of each set in panel A were run in parallel on a 1.8% agarose gel. The blot was hybridized with the 0.8-kb PvuII-SacII fragment from the endogenous hsp26 coding region (left), stripped, and rehybridized with the pt DNA fragment (center) or with a fragment from the 5′ end of the P element (right). The positions of molecular size markers are indicated to the right of the gels. The map below the gels indicates the probes used for the center and right panels.
To assess the nucleosomal pattern of the transgene in lines 39C-X and HS-2, the autoradiogram (final lanes) was scanned. In addition to showing the longer distinct array for the heterochromatic transgene, confirming the suggestion of a more uniform spacing between the nucleosomes, the scan clearly shows a pattern with more pronounced peaks and valleys for line HS-2 (Fig. 2B, top). This suggests that the MNase target is more highly defined in the heterochromatic array, i.e., the portion of the linker DNA available for cleavage by MNase is smaller. However, there does not appear to be any significant change in sensitivity to digestion with increasing amounts of enzyme (see also Fig. 4C and D). As a control for uniformity of chromatin digestion, the Southern blot used in Fig. 2A was stripped and rehybridized using a 0.8-kb DNA fragment unique to the endogenous hsp26 coding region (see the map in Fig. 4B). A scan of the resulting autoradiogram (final lanes) shows that the nucleosomal patterns of the hsp26 endogenous gene in the two lines are almost identical (Fig. 2B, bottom). This confirms that the differences observed reflect the different chromosomal environments occupied by the test transgenes in lines HS-2 and 39C-X.
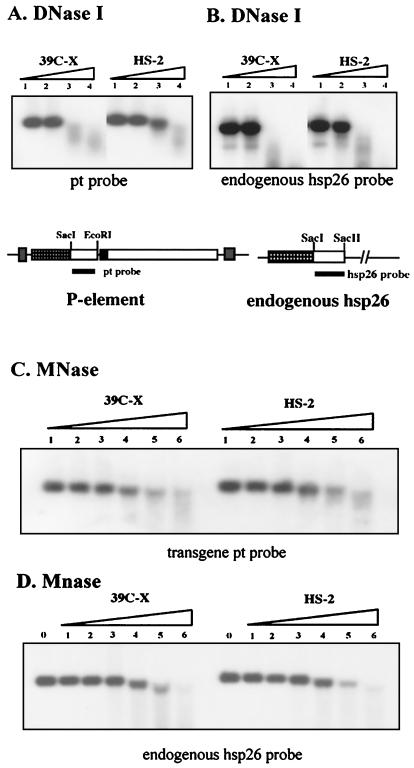
FIG. 4.
A nucleosome array in heterochromatin is resistant to digestion with DNase I, but not with MNase. (A) Nuclei from 6- to 18-h-old non-heat-shocked embryos (lines 39C-X and HS-2) were treated with DNase I at concentrations of 0.016, 0.032, 0.064, or 0.128 U/μl. Purified DNA was then cut with SacI and EcoRI, and the presence of the 1-kb restriction fragment was monitored using Southern blotting with the pt DNA fragment as a probe. (B) In the control experiment, the digested DNA was cut with SacI and SacII, and a 0.8-kb DNA fragment from the 3′ portion of the endogenous hsp26 gene was used as a probe. (C) An analogous experiment used MNase at concentrations of 0, 0.01, 0.02, 0.04, 0.08, 0.12, and 0.24 U/μl to digest the chromatin in nuclei. The pt DNA fragment was used as a probe. (D) The control experiment performed as described above for panel B to monitor disappearance of the SacI-SacII fragment of the endogenous hsp26 gene shows that digestion of the chromatin from the two lines occurred at the same rate.
The results in Fig. 2B also suggest an increase in average nucleosome repeat size in the heterochromatic transgene in the HS-2 line relative to the euchromatic transgene in the 39C-X line. The average nucleosome repeat size in Drosophila (assessed for the genome as a whole) is 190 bp (11). Samples of DNA from the MNase-treated HS-2 and 39C-X nuclei were run in parallel in a 1.8% agarose gel. The results show the nucleosomal repeat at the transgene in HS-2 to be ca. 10 bp larger than that at the transgene in 39C-X (Fig. 2C, center and right panels). The Southern blot was also analyzed using the DNA fragment from the endogenous hsp26 gene as a control (Fig. 2C, left). A common nucleosome repeat size is observed in the control, confirming that the difference found in repeat size for the test transgene reflects the heterochromatic environment.
Restriction site accessibility and DNase I hypersensitivity in the 5′ regulatory region are severely reduced in the heterochromatic transgene.
The presence of a long regular array of nucleosomes implies the loss of local perturbations, such as DH sites. The upstream regulatory region of endogenous hsp26 is characterized by two DH sites which encompass the heat shock regulatory elements, allowing rapid activation of the gene (11, 60). Generation of these DH sites is dependent upon GAGA factor binding sites [(CT)n elements] (44). A molecule of RNA polymerase II, already engaged but paused at positions +30 to +50, also contributes to this chromatin configuration (27). Fortuitously, each of the regulatory heat shock elements contains one or two XbaI restriction sites (see the map in Fig. 3). Digestion of the chromatin with excess XbaI enzyme allows a quantitative assessment of chromatin accessibility. Accessibility of the proximal XbaI site is correlated with the inducible transcriptional activity of the hsp26 gene. Alterations in the DNA sequence that result in a loss of XbaI accessibility lead to a loss of heat shock-inducible activity, apparently because of changes in the local chromatin structure that reduce access for the heat shock factor or other regulatory proteins (44, 45). Loss of accessibility to restriction sites within the regulatory region of the hsp26-pt transgene in heterochromatin has been previously reported, with the loss in accessibility roughly paralleling the loss of gene expression, monitored either by eye phenotype or heat shock-inducible transcription of hsp26-pt (56, 61). We have confirmed that this change in restriction enzyme accessibility has also occurred for the HS-2 transgene; accessibility of the XbaI proximal site in line HS-2 is ca. 5% that in the euchromatic control line 39C-X (data not shown). To generalize this result, the accessibility of two EcoRI sites, one at position +7 in the hsp26-pt transgene and one immediately upstream of the hsp70-white promoter, were also determined. Accessibility to these sites, both close to 5′ regulatory regions, was also severely reduced to 5% in the former case and 4% in the latter case relative to the 39C-X control (data not shown).
FIG. 3.
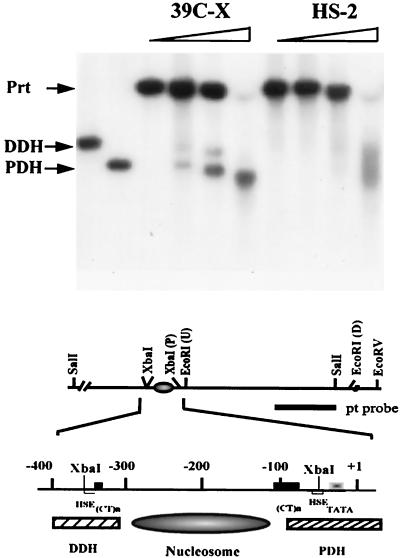
Configuration of DH sites in the heterochromatic transgene. Nuclei isolated from third instar larvae were incubated with DNase I at concentrations of 0, 0.03, 0.06, and 0.12 U/μl. Purified DNA was cleaved with SalI, and the fragments were analyzed by Southern blotting using the pt DNA fragment as the hybridization probe. Proximal (PDH) and distal (DDH) DH sites are indicated; the parental band, which is created by SalI digestion of the DNA not cleaved in nuclei by DNase I, is indicated (Prt). A partial restriction map of the hsp26 transgene is shown below: the (CT)n regions (black box), heat shock elements (HSE) (white box), the TATA box (gradient box), the PDH site (hatched box), the DDH site (hatched box), and the positioned nucleosome between the PDH and the DDH are diagrammed.
The indirect end labeling approach was used to examine the pattern of DNase I sensitivity. Nuclei isolated from third instar larvae were treated with increasing amounts of DNase I, and the purified DNA was further digested with SalI to generate a parental DNA fragment. As anticipated, two distinct DH sites are observed in control line 39C-X, confirming that the chromatin structure of the hsp26-pt transgene in a euchromatic site mimics that of the endogenous hsp26 gene (11). In line HS-2, however, no distinct DH sites are observed; a smeared signal is apparent on digestion with high concentrations of DNase I, indicating that some DNase I sensitivity remains (Fig. 3), likely due to the small fraction of cells in which silencing has been lost. Control samples of naked DNA indicate some cleavage in this region, but it does not resemble either the distinct DH sites of the euchromatic transgene or the faint smeared signal of the heterochromatic transgene. The data from these experiments indicate a loss of organized DH sites in the heterochromatic transgene.
DNase I sensitivity of a nucleosomal region differs for the heterochromatic and euchromatic transgenes, while MNase sensitivity is not significantly altered.
The loss of organized DH sites raises the question of whether the previously reported loss of general DNase I sensitivity in inactive regions might be due simply to the loss of such hypersensitive sites. To investigate, we examined changes in susceptibility of the nucleosome array, looking at the disappearance of a DNA fragment entirely packaged into nucleosomes (having no DH sites) as a function of increasing levels of nuclease. The barley cDNA fragment that is the 3′ transcribed portion of the hsp26-pt transgene was used. Disappearance of this SacI-EcoRI fragment in this assay reflects the first cleavage event in this region of the chromatin. The SacI-EcoRI DNA fragment in line HS-2 is significantly more resistant to digestion than that in line 39C-X (Fig. 4A). To ensure uniformity in digestion, samples were tested for disappearance of the SacI-SacII fragment that is unique to the endogenous, euchromatic hsp26 gene; this gene shows equivalent susceptibility to digestion in both lines, as expected (Fig. 4B). The results indicate that the packaging of the transgene in heterochromatin is altered in a manner that inhibits overall DNase I sensitivity within the nucleosome array. However, no major differences are observed when nuclei from lines 39C-X and HS-2 are treated with MNase (compare Fig. 4C and control D), indicating that the heterochromatin structure does not generate a barrier to digestion by this enzyme. As MNase preferentially cuts the linker region between nucleosomes, the results indicate that the increased inaccessibility to nucleases that is characteristic of a heterochromatic structure is not the consequence of a major change in linker accessibility in general. Thus, the shift in sensitivity to DNase I digestion suggests a change in higher-order packaging.
Nucleosome positions are altered over the heterochromatic transgene in line HS-2.
The observation of long-range nucleosomal ordering suggests that changes in nucleosome positioning might be involved in the loss of DNase I hypersensitivity and restriction enzyme accessibility in the 5′ regulatory region of the heterochromatic transgene. We therefore mapped the nucleosome positions across the hsp26-pt transgene in 6- to 18-h-old embryos using the indirect end labeling technique with a 93-bp SalI-XhoI fragment (abutting the SalI site) from the barley cDNA fragment as a probe. A comparison of nucleosome positions in 39C-X and HS-2 (based on MNase cleavage products) shows a loss of hypersensitivity in the 5′ region and a shift in nucleosome positions (Fig. 5A). The DNA sequences in the proximal and distal DH sites detected in line 39C-X are incorporated into nucleosomes in line HS-2 (see the comparative scan in Fig. 5B). A similar shift in the nucleosome pattern across the hsp26-pt transgene was observed when the experiment was repeated using nuclei from third instar larvae (data not shown). We also compared the nucleosome array across the hsp70-white transgene in lines 39C-X and HS-2 using nuclei isolated from 6- to 18-h-old embryos as described above. The distinct nuclease hypersensitivity observed in the 5′ regulatory region of the hsp70-white transgene in line 39C-X is lost in line HS-2, and the positions of the nucleosomes appear altered (Fig. 5C).
FIG. 5.
Mapping nucleosome positions in the heterochromatic transgenes using the indirect end labeling technique. (A) Nuclei isolated from 6- to 18-h-old embryos of lines 39C-X and HS-2 were treated with MNase (concentrations 0, 0.04, 0.08, 0.12, and 0.24 U/μl). The purified DNA was cut with SalI, size separated by gel electrophoresis, and transferred to a positively charged nylon membrane, and the membrane was hybridized with a 93-bp fragment from the barley cDNA to map the hsp26-pt transgene. The positions of the proximal and distal hypersensitive sites (PDH and DDH, respectively) in the 39C-X samples are indicated. Control digestion of purified DNA was performed as described in Materials and Methods. A map of the hsp26-pt transgene, indicating the position of the probe, is shown below the gel. (B) A scan of the MNase (0.12 U/μl) digest samples, showing the shift in pattern at the 5′ regulatory region of the gene. Accessible sites in 39C-X (black circles) and HS-2 (white circles) are indicated. (C) DNA samples from a MNase digest done as described above were cut with SacI and SacII, and the DNA samples were size separated by gel electrophoresis, transferred to a nylon filter, and hybridized with the pt DNA fragment to map the hsp70-white transgene. The parental DNA fragment (Prt) and the 5′ DH sites are indicated. The SacI and SacII restriction sites in the P element are shown on the map below.
Other heterochromatic sequences show increased nucleosomal ordering.
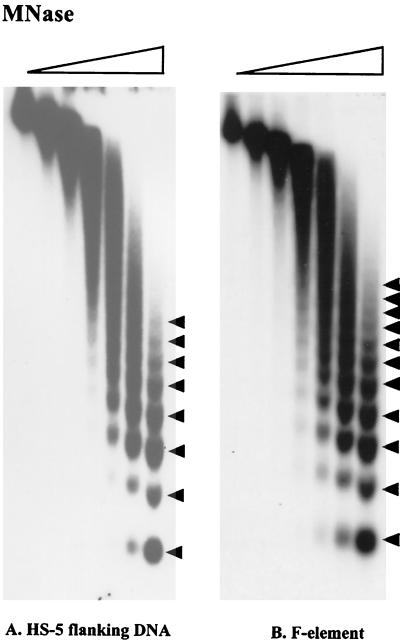
Having observed the difference in the nucleosome arrays of heterochromatic and euchromatic transgenes (Fig. 2), we examined the nucleosome arrays of endogenous heterochromatic sequences, both noncoding and coding (63). The DNA immediately flanking the heterochromatic transgene in line HS-5 (inserted in the 2L pericentric heterochromatin, showing a variegating phenotype) has been cloned by inverse PCR and shown to be unique (18). This DNA was used as a probe to hybridize the filter from Fig. 2. The nucleosome pattern of this heterochromatic region (in the absence of the transgene) shows a shift toward long-range nucleosomal order, giving a regular array with eight nucleosomes evident (Fig. 6A). When the filter was stripped and rehybridized with a fragment from the F element, a transposable element shown to be localized primarily in the pericentric heterochromatin (estimated 71% [47]), an extremely uniform array is observed; a distinct fragment encompassing 11 nucleosomes is seen (Fig. 6B). Evidence of a shift toward long-range order was also obtained using fragments from other transposable elements (including gypsy, I, and copia) as the probe sequence (data not shown). gypsy is estimated to be 88% heterochromatic, I is estimated to be 73% heterochromatic, and copia is estimated to be 82% heterochromatic (47). The results suggest that a more uniform nucleosome array may be a common characteristic of pericentric heterochromatin.
FIG. 6.
Nucleosome arrays associated with heterochromatic DNA sequences. Blots containing DNA samples from nuclei digested with MNase as described in the legend to Fig. 2 were hybridized with a 1-kb DNA fragment that flanks the HS-5 insertion site (A) or a 4.5-kb F-element fragment (B). Linker sites cleaved by MNase are indicated by arrowheads.
Endogenous genes active in heterochromatin show a euchromatic nucleosome pattern.
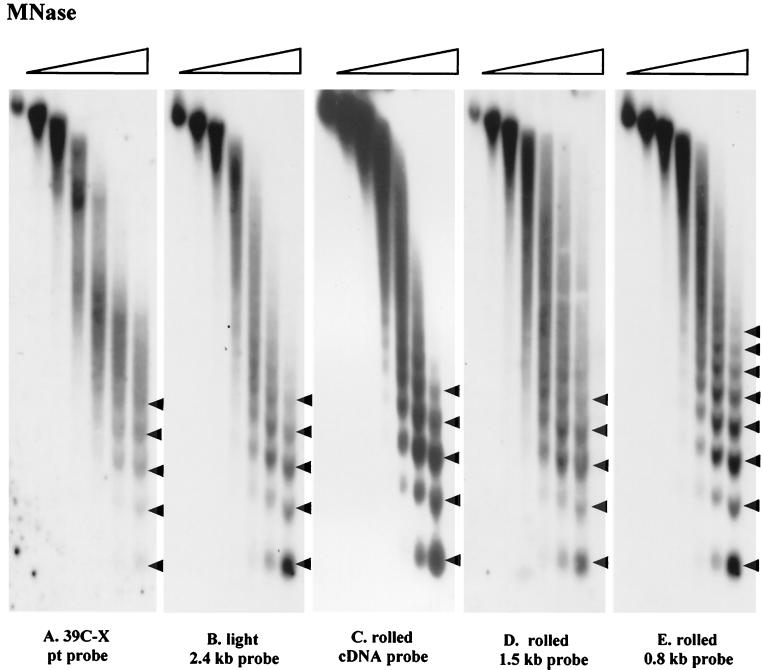
While a euchromatic gene juxtaposed or transposed into heterochromatin is silenced, there are genes present in heterochromatin that are normally expressed in that environment and are silenced if transposed to euchromatic regions (35). To determine whether this type of gene has a nucleosomal array similar to that of the nontranscribed heterochromatin, DNA from known heterochromatic genes was used to assess the products of MNase digestion. rolled, located in the 2R pericentric heterochromatin, encodes a mitogen-activated protein kinase (7, 22); the rolled gene is widely expressed in all stages of development (4, 6). light, located in the 2L pericentric heterochromatin, encodes an essential gene with homology to VPS41, an S. cerevisiae gene involved in cellular-protein trafficking; it is also widely expressed (20, 62, 64). The rolled cDNA, a 1.4-kb fragment at the 5′ end of rolled, and a 2.4-kb fragment including the first exon and ca. 1 kb upstream of light were used as probes to hybridize to filters prepared for Fig. 2. In these cases, five or six nucleosomes can be clearly distinguished, a pattern similar to the nucleosome array observed for the hsp26-pt transgene in euchromatin (line 39C-X) (Fig. 7A to D). In contrast, results obtained using a 0.8-kb fragment 14 kb upstream of the rolled gene showed an extensive nucleosome array, clearly indicating long-range order (Fig. 7E). Computer analysis of the 14-kb interval shows the presence of repetitious elements, including sequences homologous to the F element. Thus, these active heterochromatic genes show a nucleosome array characteristic of euchromatin while (in the case studied) being flanked by regions that are packaged in the characteristic heterochromatic fashion, with long-range order. The data support the conclusion that the highly ordered nucleosome array correlates with the inactive state, rather than simply correlating with the chromosomal location (within pericentric heterochromatin), and suggest that there is a mechanism to protect active genes normally found within heterochromatin from being incorporated into the highly ordered form.
FIG. 7.
Nucleosome arrays associated with genes active in heterochromatin compared to the euchromatic transgene. Blots containing DNA samples from nuclei digested with MNase as described in the legend to Fig. 2 were hybridized with a 2.4-kb BamHI-PstI fragment encompassing most of the first exon and ca. 1 kb of upstream DNA at light (20) (B), a 1.4-kb rolled gene cDNA (C), a 1,463-bp fragment starting at position +106 and extending upstream, encompassing the putative 5′ regulatory region of rolled (D), and a 813-bp fragment located 14038 bp upstream of the rolled gene start site (E). The fragments used for panels D and E were prepared by PCR from NCBI clone AE003090. Panel A shows the nucleosome array of the transgene in a euchromatic site (line 39C-X) for reference.
DISCUSSION
Long-range order in nucleosome arrays as a characteristic of heterochromatin.
We have used a P element carrying two reporter genes, hsp26-pt and hsp70-white, to characterize the changes in chromatin structure that occur when a euchromatic gene is placed within a heterochromatic environment by P-element-mediated transposition. Such transgenes are subject to silencing by the same mechanism responsible for PEV, as shown by genetic analysis (Fig. 1); several lines of evidence indicate that the silenced transgene has been packaged in a heterochromatic form (63). The silenced transgene in pericentric heterochromatin (line HS-2) shows a very uniform nucleosome array on digestion with MNase, indicating long-range order with a constant repeat length. The pattern of DNA fragments generated, observed using Southern analysis, is characterized by relatively sharp bands and by the ability to detect an extensive nucleosome ladder, up to 9- or 10-mers. This stands in contrast to the pattern obtained for the euchromatic transgene (line 39C-X), which shows broader bands, and a distinct pattern only up to 5- or 6-mers (Fig. 2). The latter pattern is characteristic of the genome as a whole on digestion of chromatin with MNase and visualization of the DNA fragments on a gel using ethidium bromide. The heterochromatic pattern suggests a distinctive nucleosome array with better-defined MNase cleavage sites and a more uniform spacing of nucleosomes. These characteristics are also observed for the endogenous heterochromatic sequences tested, the unique DNA identified as flanking the HS-5 transgene insertion site (Fig. 6A), the F element (Fig. 6B), and other transposable elements found in heterochromatin (data not shown). The nucleosome array of the D. melanogaster 1.688-g/cm3 satellite DNA, a major constituent of pericentric heterochromatin, shows a ladder with such regularity that 20-mers can be detected (10). The data argue that these characteristics of the nucleosome array are general for noncoding pericentric heterochromatin in Drosophila. Similar characteristics have been observed for telomeric domains of Drosophila that induce silencing of transgenes (19).
Could this organized structure be a default pattern, generated in the absence of perturbations in the nucleosome array, such as DH sites? Previous chromatin structure studies suggest that this is unlikely. Mutations in the 5′ regulatory region of hsp26 can alter the immediate chromatin structure, but they have not been observed to impact the overall nucleosome array of transgenes in a euchromatic domain (44, 45). To suggest that the limited genomic pattern in which five or six nucleosomes are observed is a consequence of DH sites is to argue that DH sites occur every 1 to 2 kb throughout the genome. Given that DH sites are generally observed in conjunction with active regulatory regions and other signal sequences (26, 28), this seems unlikely, even in a compact genome such as that of D. melanogaster. Two assembly systems derived from Drosophila, ACF and CHRAC, have been found to generate very uniform nucleosome arrays on a variety of templates in vitro (38). Such systems could generate the chromatin structure observed for the HS-2 transgene. Whether some assembly systems intrinsically generate a more uniform array than others is unknown, as the choice of template DNA, presence of appropriate DNA-binding proteins, etc., may be critical.
The nucleosome repeat length in the silenced heterochromatic transgene studied here (HS-2) is larger than that of the euchromatic transgene (39C-X) (Fig. 2C). While some of the other heterochromatic nucleosome arrays also have a larger MNase unit size, there is no consensus; in fact, the array of the 1.688-g/cm3 satellite DNA has a relatively short MNase unit size at 180 bp (10), presumably reflecting the underlying 359-bp DNA sequence repeat (two nucleosomes per repeat). In addition to the differences reflecting the DNA sequence, one would expect that repeat length might vary depending on the group of associated nonhistone chromosomal proteins in a given heterochromatic domain. Pericentric heterochromatin is a mosaic, with blocks of satellite DNA and transposable element sequences (40, 47). One can also anticipate a mosaic of protein complexes, analogous to those found at genes regulated by Pc-G proteins, where different members of the Pc-G associate at different DNA sites to achieve silencing (48). Thus, it is not surprising that different regions of heterochromatin appear to use a different repeating unit size in packaging DNA into nucleosome arrays; what is notable is that in all cases tested to date, we have seen evidence of increased long-term order.
A striking feature is the sharper bands generated by MNase cleavage of heterochromatin in comparison to the bands obtained from euchromatin. A change in DNA topology has been associated with silencing at the S. cerevisiae mating type loci, implying a change in nucleosomal structure in that domain (5, 13). The altered topological state can be maintained until replication; maintenance of this state throughout the cell cycle requires the cis-acting silencing sites E and I (14, 33). The change in topology suggests a shift either in the extent of DNA association with the histone core or in the path of the linker or both. The change in the MNase digestion pattern observed here, showing sharper bands, suggests a reduction in the target size in the linker DNA. This could be achieved by having a larger percentage of the DNA in close association with the histone core, a change that should also result in the altered topology observed in S. cerevisiae.
Nuclease accessibility in Drosophila heterochromatin.
In cases where the hsp70-white gene shows a variegating phenotype, analysis of the chromatin structure of the hsp26-pt gene shows a significant reduction in restriction enzyme accessibility in the 5′ regulatory region; the level of accessibility is correlated with the observed degree of gene silencing (56, 61). Consistent with this quantitative shift, a loss of distinct DH sites is also observed in the extreme case shown here, line HS-2 (Fig. 3). The loss of DH sites is reflected in the shift in the nucleosome array seen in Fig. 5. There have been previous reports that active and/or inducible genes are more readily digested by DNase I than inactive regions of the genome (3, 65). One can infer that some of the difference can be attributed to the lack of DH sites in inactive regions, including heterochromatic regions.
However, the change in chromatin accessibility in 5′ regulatory regions is not the only change observed. The reduced sensitivity to digestion by DNase I, monitored using a fragment that is entirely nucleosomal and contains no potential DH sites, in the comparison of heterochromatic (HS-2) and euchromatic (39C-X) transgenes, indicates a change in packaging in addition to the loss of DH sites (Fig. 4). A consistent reduction in accessibility as monitored by restriction enzyme digestion has been reported along the entire silenced domains at the HML and HMR loci in S. cerevisiae and across the pericentric heterochromatin in Schizosaccharomyces pombe (1, 23, 41, 53). The consistent loss in general accessibility across broad nucleosomal regions implies that heterochromatic domains in different organisms may share a common form of altered packaging. Whether this can be accounted for by the changes in the nucleosome array or reflects some higher-order packaging has not yet been resolved.
Interestingly, no major difference in sensitivity to digestion by MNase was observed for the nucleosomal chromatin fragment, comparing the heterochromatic transgene (HS-2) and euchromatic transgene (39C-X) (Fig. 4). MNase preferentially cleaves the nucleosomal linker region. Clearly, packaging of the transgene into heterochromatin does not impede access for this enzyme to the DNA, implying that any higher-order folding that occurs does not structurally block such a small protein (16,800 Da) and that the association of heterochromatic proteins does not obscure the linker DNA in general. However, mapping of the nucleosome array using MNase (Fig. 5) has allowed us to answer our initial question: the nucleosome array in heterochromatin is altered. Changes in accessibility and gene expression do not simply reflect the imposition of some higher-order structure or sequestration of the original chromatin fiber, packaged at the nucleosome level in the form used in euchromatin, but reflect an altered nucleosome array with altered positioning of nucleosomes.
Genes endogenous to heterochromatin.
Over 30 genetic functions reside within D. melanogaster heterochromatin; those characterized require a heterochromatic environment for their proper expression, exhibiting a variegating phenotype or reduced expression when rearrangements place them adjacent to a breakpoint in euchromatin (63, 64). Particularly striking is the observation that expression of the heterochromatic genes rolled and light in their endogenous heterochromatic position is reduced in larvae mutant for HP1, suggesting that proper maintenance of heterochromatin structure is required for expression of these genes (43). Thus, we were somewhat surprised to observe that light and rolled have nucleosome arrays similar to that observed for the euchromatic transgene, rather than the heterochromatic transgene (Fig. 7). This suggests that the regulation of these heterochromatic genes by HP1 may not be based on the impact of HP1 on heterochromatin structure in general (which is correlated with silencing of transgenes such as hsp70-white) but may be the consequence of a context-dependent (positive or negative) activity, similar to that displayed by RAP1 in S. cerevisiae. Alternatively, the impact of HP1 on a heterochromatic gene may reflect packaging of the surrounding area, rather than the transcribed region (25). A more detailed analysis of the chromatin structure encompassing these genes and their regulatory regions will be required to resolve this question.
Mechanisms of heterochromatin-associated gene silencing.
A number of models have been proposed to describe the formation of heterochromatin and explain PEV-associated gene silencing (32, 63). PEV was initially observed as a result of chromosomal rearrangements (inversions and translocations), generally a product of X-irradiation, which place a euchromatic gene close to a heterochromatic breakpoint. The resulting inactivation suggested a cis-acting silencing activity of heterochromatin acting across the rearrangement breakpoint. This has been suggested to take the form of continuing assembly of a heterochromatic structure (59) or a coalescence biased toward repetitious sequences (58), extending to encompass the formerly euchromatic gene. Such a model is supported by the observation that proteins such as HP1, visibly associated with the pericentric heterochromatin, have a dosage-dependent impact on the effectiveness of silencing (24).
In contrast, numerous studies have suggested that a gene might be silenced by its transfer to a nuclear compartment that is inimicable to gene expression (16). In its simplest form, such a model does not require any change in chromatin structure. Nuclear compartmentalization suggests that the silencing of a euchromatic gene in heterochromatin could be due to the exclusion of critical transcription factors or chromatin remodeling complexes from the heterochromatic environment. Conversely, one could suggest that association with a heterochromatic mass could prevent a gene from gaining access to RNA polymerase factories localized in the nucleoplasm (17). Studies such as those with the Ikaros transcription factor have implicated a change in nuclear position as a mechanism to achieve silencing (9).
Regardless of the overall mechanism that drives heterochromatin formation, the results reported here indicate that a distinctive nucleosome array is generated and that packaging in this form is associated with gene silencing in heterochromatin. We find that heterochromatic sequences (excluding heterochromatic genes) have a nucleosome array distinct from the general pattern and this array appears to be imposed on the transgene resident in heterochromatin. The differences observed compared to the transgene in a euchromatic site (including incorporation of the hsp26-pt promoter region into a regular nucleosome array, with loss of the DH site) would certainly contribute to a loss of gene expression. The presence of a nucleosome will significantly reduce restriction enzyme accessibility (2, 50) and prevent binding of TFIID (38, 39). Hence, such a loss of accessibility in the regulatory regions of the transgene would be expected to result in a loss of inducible gene expression and is likely to constitute an important aspect of the mechanism of gene silencing by heterochromatin formation. Such a model is supported by the recent analysis of transgenes silenced within telomeric heterochromatin of Drosophila, where DNase I footprinting showed a loss of GAGA factor and TFIID association, while potassium permanganate experiments showed a loss of RNA polymerase II association (19).
What might drive the formation of the alternative heterochromatic structure is unknown. In theory, either the DNA sequences or the associated proteins could play a determinative role in the formation of constitutive heterochromatin. While no DNA sequence has been identified as nucleating heterochromatin formation in normal assembly, it has been suggested that tandem arrays of repetitious sequences might trigger this event (18, 32). Differences in the local concentrations of nuclear proteins, such as HP1 (36), might drive formation of one packaging mode over the other. The final model describing formation of the alternative chromatin structure found in heterochromatin is likely to depend both on the underlying DNA sequence and on the prevalent population of chromosomal proteins, perhaps defined by time of replication or by nuclear compartment—parameters that may be closely related. The results shown here (Fig. 7) indicate that the mechanism of that drives assembly of the heterochromatic nucleosome array must be sufficiently sensitive to recognize the genes within heterochromatin (such as rolled) that are maintained in the active state, as these show characteristics of euchromatic nucleosome arrays.
Some of the differences in heterochromatin structure might be explained by a shift in histone acetylation. The core histones in heterochromatin are generally hypoacetylated; specific patterns have been suggested to be important in maintaining silencing (8). Studies of the β-globin genes of chickens have shown that activation involves a shift in histone acetylation, covering a large domain, that comaps with a change in general DNase I sensitivity (30). Histone acetylation is frequently involved in the process of altering chromatin structure to create an accessible site (a DH site) for initiation of transcription in the 5′ regulatory region of a gene (34, 38). Thus, a change in the chromatin environment that blocked histone acetylation and/or promoted histone deacetylation might account both for the loss of accessibility to the 5′ regulatory regions of the test transgenes and for the shift in general DNase I sensitivity observed here.
In contrast to other regions of the genome, where the activity state can vary with the demands of development and environmental response, the silent state in constitutive heterochromatin must be stably maintained. To be packaged in heterochromatin is to be removed from possible conversion by NURF or other chromatin remodeling machines to the chromatin structure of a transcriptionally active gene. Most intriguing is the possibility that the altered nucleosome array reflects packaging into a specific higher-order structure, for example, a stable solenoid. Heterochromatic silencing is dependent on HP1 (Fig. 1), known to impact accessibility of the transgene as measured by XbaI digestion (18). HP1 and other heterochromatic proteins might play a role in stabilizing that structure, protecting the region from conversion, much as the Pc-G complex has been reported to protect nucleosome arrays from remodeling (52). A final model of heterochromatin will need to encompass both the structural features delineated here and the functional attributes that dictate gene silencing.
ACKNOWLEDGMENTS
We thank Lori L. Wallrath (University of Iowa) for important contributions during the initial stages of this work and Lori Wallrath, Joel Eissenberg, and members of the Elgin lab for review and discussion of the manuscript. We thank S. Lawrence Zipursky (University of California, Los Angeles) for providing the rolled cDNA probe, Barbara Wakimoto (University of Washington) for providing the light DNA probe, and Sergio Pimpinelli (Rome, Italy) for providing the probes for endogenous transposable elements.
This work was supported by Public Health Service grant HD23844 from the National Institute of Child Health and Human Development to S.C.R.E.
REFERENCES
- 1.Allshire R C, Javerzat J-P, Redhead N J, Cranston G. Position effect variegation at the fission yeast centromere. Cell. 1994;76:157–169. doi: 10.1016/0092-8674(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 2.Almer A, Horz W. Nuclease hypersensitive regions with adjacent positioned nucleosomes mark the gene boundaries of the PHO5/PHO3 locus in yeast. EMBO J. 1986;5:268–287. doi: 10.1002/j.1460-2075.1986.tb04551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellard M, Gannon F, Chambon P. Nucleosome structure III: the structure and transcriptional activity of the chromatin containing the ovalbumin and globin genes in chick oviduct nuclei. Cold Spring Harbor Symp Quant Biol. 1978;2:779–791. doi: 10.1101/sqb.1978.042.01.078. [DOI] [PubMed] [Google Scholar]
- 4.Berghella L, Dimitri P. The heterochromatic rolled gene of Drosophila melanogaster is extensively polytenized and transcriptionally active in the salivary gland chromocenter. Genetics. 1996;144:117–125. doi: 10.1093/genetics/144.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi X, Broach J R. DNA in transcriptionally silent chromatin assumes a distinct topology that is sensitive to cell cycle progression. Mol Cell Biol. 1997;17:7077–7087. doi: 10.1128/mcb.17.12.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biggs W H, III, Zipursky S L. Primary structure, expression, and signal-dependent tyrosine phosphorylation of a Drosophila homolog of extracellular signal-regulated kinase. Proc Natl Acad Sci USA. 1992;89:6295–6299. doi: 10.1073/pnas.89.14.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biggs W H, III, Zavitz K H, Dickson B, van der Straten A, Brunner D, Hafen E, Zipursky S L. The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J. 1994;13:1628–1635. doi: 10.1002/j.1460-2075.1994.tb06426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braunstein M, Sobel R E, Allis C D, Turner B M, Broach J R. Efficient transcriptional silencing in Saccharomyces cerevisiae requires a heterochromatin histone acetylation pattern. Mol Cell Biol. 1996;16:4349–4356. doi: 10.1128/mcb.16.8.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown K E, Guest S S, Smale S T, Hahm K, Merkenschlager M, Fisher A G. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell. 1997;91:845–854. doi: 10.1016/s0092-8674(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 10.Cartwright I L, Hertzberg R P, Dervan P B, Elgin S C R. Cleavage of chromatin with methidiumpropyl-EDTA · iron(II) Proc Natl Acad Sci USA. 1983;80:3213–3217. doi: 10.1073/pnas.80.11.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartwright I L, Elgin S C R. Nucleosomal instability and induction of new upstream protein-DNA associations accompany activation of four small heat shock protein genes in Drosophila melanogaster. Mol Cell Biol. 1986;6:779–791. doi: 10.1128/mcb.6.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartwright I L, Cryderman D E, Gilmour D S, Pile L A, Wallrath L L, Weber J A, Elgin S C R. Analysis of Drosophila chromatin structure in vivo. Methods Enzymol. 1999;304:462–496. doi: 10.1016/s0076-6879(99)04028-8. [DOI] [PubMed] [Google Scholar]
- 13.Cheng T H, Li Y C, Gartenberg M R. Persistence of an alternate chromatin structure at silenced loci in the absence of silencers. Proc Natl Acad Sci USA. 1998;95:5521–5526. doi: 10.1073/pnas.95.10.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng T H, Gartenberg M R. Yeast heterochromatin is a dynamic structure that requires silencers continuously. Genes Dev. 2000;14:452–463. [PMC free article] [PubMed] [Google Scholar]
- 15.Cleard F, Delattre M, Spierer P. SU(VAR)3–7, a Drosophila heterochromatin-associated protein and companion of HP1 in the genomic silencing of position-effect variegation. EMBO J. 1997;16:5280–5288. doi: 10.1093/emboj/16.17.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cockell M, Gasser S M. Nuclear compartments and gene regulation. Curr Opin Genet Dev. 1999;9:199–205. doi: 10.1016/S0959-437X(99)80030-6. [DOI] [PubMed] [Google Scholar]
- 17.Cook P R. The organization of replication and transcription. Science. 1999;284:1790–1795. doi: 10.1126/science.284.5421.1790. [DOI] [PubMed] [Google Scholar]
- 18.Cryderman D E, Cuaycong M H, Elgin S C R, Wallrath L L. Characterization of sequences associated with position-effect variegation at pericentric sites in Drosophila heterochromatin. Chromosoma. 1998;107:277–285. doi: 10.1007/s004120050309. [DOI] [PubMed] [Google Scholar]
- 19.Cryderman D E, Tang H, Bell C, Gilmour D S, Wallrath L L. Heterochromatic silencing of Drosophila heat shock genes acts at the level of promoter potentiation. Nucleic Acids Res. 1999;27:3364–3370. doi: 10.1093/nar/27.16.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devlin R H, Bingham B, Wakimoto B T. The organization and expression of the light gene, a heterochromatic gene of Drosophila melanogaster. Genetics. 1990;125:129–140. doi: 10.1093/genetics/125.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietzel S, Schiebel K, Little G, Edelmann P, Rappold G A, Eils R, Cremer C, Cremer T. The 3D positioning of ANT2 and ANT3 genes within female X chromosome territories correlates with gene activity. Exp Cell Res. 1999;252:363–375. doi: 10.1006/excr.1999.4635. [DOI] [PubMed] [Google Scholar]
- 22.Dimitri P. Cytogenetic analysis of the second chromosome heterochromatin of Drosophila melanogaster. Genetics. 1991;127:553–564. doi: 10.1093/genetics/127.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donze D, Adam C R, Rine J, Kamakaka R T. The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev. 1999;13:698–708. doi: 10.1101/gad.13.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eissenberg J C, Morris G D, Reuter G, Hartnett T. The heterochromatin-associated protein HP1 is an essential protein in Drosophila with dosage-dependent effects on position-effect variegation. Genetics. 1992;131:345–352. doi: 10.1093/genetics/131.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eissenberg J C, Elgin S C R. The HP1 protein family: getting a grip on chromatin. Curr Opin Genet Dev. 2000;10:204–210. doi: 10.1016/s0959-437x(00)00058-7. [DOI] [PubMed] [Google Scholar]
- 26.Elgin S C R. The formation and function of DNase I hypersensitive sites in the process of gene activation. J Biol Chem. 1988;263:19259–19262. [PubMed] [Google Scholar]
- 27.Giardina C, Perez-Riba M, Lis J T. Promoter melting and TFIID complexes on Drosophila genes in vivo. Genes Dev. 1992;6:2190–2200. doi: 10.1101/gad.6.11.2190. [DOI] [PubMed] [Google Scholar]
- 28.Gross D S, Garrard W T. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- 29.Grunstein M. Yeast heterochromatin: regulation of its assembly and inheritance by histones. Cell. 1998;93:325–328. doi: 10.1016/s0092-8674(00)81160-5. [DOI] [PubMed] [Google Scholar]
- 30.Hebbes T R, Clayton A L, Thorne A W, Crane-Robinson C. Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken β-globin chromosomal domain. EMBO J. 1994;13:1823–1830. doi: 10.1002/j.1460-2075.1994.tb06451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heitz E. Das heterochromatin der moose. Jahrb Wiss Bot. 1928;69:726–818. [Google Scholar]
- 32.Henikoff S. Heterochromatin function in complex genomes. Biochim Biophys Acta. 2000;1470:1–8. doi: 10.1016/s0304-419x(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 33.Holmes S G, Broach J R. Silencers are required for inheritance of the repressed state in yeast. Genes Dev. 1996;10:1021–1032. doi: 10.1101/gad.10.8.1021. [DOI] [PubMed] [Google Scholar]
- 34.Howe L, Brown C E, Lechner T, Workman J L. Histone acetyltransferase complexes and their link to transcription. Crit Rev Eukaryot Gene Exp. 1999;9:231–243. doi: 10.1615/critreveukargeneexpr.v9.i3-4.80. [DOI] [PubMed] [Google Scholar]
- 35.Howe M, Dimitri P, Berloco M, Wakimoto B T. Cis-effects of heterochromatin on heterochromatic and euchromatic gene activity in Drosophila melanogaster. Genetics. 1995;140:1033–1045. doi: 10.1093/genetics/140.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James T C, Eissenberg J E, Craig C, Dietrich V, Hobson A, Elgin S C R. Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur J Cell Biol. 1989;50:170–180. [PubMed] [Google Scholar]
- 37.Khesin R B, Leibovitch B A. Influence of deficiency of the histone gene-containing 38B-40 region on X-chromosome template activity and the white gene position effect variegation in Drosophila melanogaster. Mol Gen Genet. 1978;162:323–328. doi: 10.1007/BF00268858. [DOI] [PubMed] [Google Scholar]
- 38.Kingston R E, Narlikar G J. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 1999;13:2339–2352. doi: 10.1101/gad.13.18.2339. [DOI] [PubMed] [Google Scholar]
- 39.Kornberg R D, Lorch Y T. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- 40.Le M H, Duricka D, Karpen G H. Islands of complex DNA are widespread in Drosophila centric heterochromatin. Genetics. 1995;141:283–303. doi: 10.1093/genetics/141.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loo S, Rine J. Silencers and domains of generalized repression. Science. 1994;164:1768–1771. doi: 10.1126/science.8209257. [DOI] [PubMed] [Google Scholar]
- 42.Lu B Y, Eissenberg J C. Developmental regulation of heterochromatin-mediated gene silencing in Drosophila. Development. 1998;125:2223–2234. doi: 10.1242/dev.125.12.2223. [DOI] [PubMed] [Google Scholar]
- 43.Lu B Y, Emtage P C R, Duyf B J, Hilliker A J, Eissenberg J C. Heterochromatin Protein 1 is required for the normal expression of two heterochromatin genes in Drosophila. Genetics. 2000;155:699–708. doi: 10.1093/genetics/155.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Q, Wallrath L L, Granok H, Elgin S C R. (CT)n · (GA)n repeats and heat shock elements have distinct roles in chromatin structure and transcriptional activation of the Drosophila hsp26 gene. Mol Cell Biol. 1993;13:2802–2814. doi: 10.1128/mcb.13.5.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Q, Wallrath L L, Elgin S C R. The role of a positioned nucleosome at the Drosophila melanogaster hsp26 promoter. EMBO J. 1995;14:4738–4746. doi: 10.1002/j.1460-2075.1995.tb00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matzke A J, Matzke M A. Position effects and epigenetic silencing of plant transgenes. Curr Opin Plant Biol. 1998;1:142–148. doi: 10.1016/s1369-5266(98)80016-2. [DOI] [PubMed] [Google Scholar]
- 47.Pimpinelli S, Berloco M, Fanti L, Dimitri P, Bonaccorsi S, Marchetti E, Caizzi R, Caggese C, Gatti M. Transposable elements are stable structural components of Drosophila melanogaster heterochromatin. Proc Natl Acad Sci USA. 1995;92:3804–3808. doi: 10.1073/pnas.92.9.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pirrotta V. Polycombing the genome: PcG, trxG, and chromatin silencing. Cell. 1998;93:333–336. doi: 10.1016/s0092-8674(00)81162-9. [DOI] [PubMed] [Google Scholar]
- 49.Platero J S, Hartnett T, Eissenberg J C. Functional analysis of the chromo domain of HP1. EMBO J. 1995;14:3977–3986. doi: 10.1002/j.1460-2075.1995.tb00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polach K J, Widom J. Mechanism of protein access to specific DNA sequences in chromatin: a dynamic equilibrium model for gene regulation. J Mol Biol. 1995;254:130–149. doi: 10.1006/jmbi.1995.0606. [DOI] [PubMed] [Google Scholar]
- 51.Shaffer C D, Wuller J M, Elgin S C R. Raising large quantities of Drosophila for biochemical experiments. Methods Cell Biol. 1994;44:99–108. doi: 10.1016/s0091-679x(08)60908-5. [DOI] [PubMed] [Google Scholar]
- 52.Shao Z, Raible F, Mollaaghababa R, Guyon J R, Wu C T, Bender W, Kingston R E. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell. 1999;98:37–46. doi: 10.1016/S0092-8674(00)80604-2. [DOI] [PubMed] [Google Scholar]
- 53.Singh J, Klar A J. Active genes in budding yeast display enhanced in vivo accessibility to foreign DNA methylases: a novel in vivo probe for chromatin structure of yeast. Genes Dev. 1992;6:186–196. doi: 10.1101/gad.6.2.186. [DOI] [PubMed] [Google Scholar]
- 54.Spofford J B. Position-effect variegation in Drosophila. In: Ashburner M, Novitski E, editors. The genetics and biology of Drosophila. 1c. New York, N.Y: Academic Press; 1976. pp. 955–1018. [Google Scholar]
- 55.Struhl K. Fundamentally different logic of gene regulation in eukaryotes and prokaryotes. Cell. 1999;9:1–4. doi: 10.1016/S0092-8674(00)80599-1. [DOI] [PubMed] [Google Scholar]
- 56.Sun F L, Cuaycong H M, Craig C A, Wallrath L L, Locke J, Elgin S C R. The fourth chromosome of Drosophila melanogaster: interspersed euchromatic and heterochromatic domains. Proc Natl Acad Sci USA. 2000;97:5340–5345. doi: 10.1073/pnas.090530797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Surani M A. Imprinting and the initiation of gene silencing in the germ line. Cell. 1998;93:309–312. doi: 10.1016/s0092-8674(00)81156-3. [DOI] [PubMed] [Google Scholar]
- 58.Talbert P B, Henikoff S. A re-examination of spreading of position-effect variegation in the white-roughest region of Drosophila melanogaster. Genetics. 2000;154:259–272. doi: 10.1093/genetics/154.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tartof K D, Hobbs C, Jones M. A structural basis for variegating position effects. Cell. 1984;37:869–878. doi: 10.1016/0092-8674(84)90422-7. [DOI] [PubMed] [Google Scholar]
- 60.Thomas G H, Elgin S C R. Protein/DNA architecture of the DNase I hypersensitive region of the Drosophila hsp26 promoter. EMBO J. 1988;7:2191–2201. doi: 10.1002/j.1460-2075.1988.tb03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallrath L L, Elgin S C R. Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev. 1995;9:1263–1277. doi: 10.1101/gad.9.10.1263. [DOI] [PubMed] [Google Scholar]
- 62.Warner T S, Sinclair D A, Fitzpatrick K A, Singh M, Devlin R H, Honda B M. The light gene of Drosophila melanogaster encodes a homologue of VPS41, a yeast gene involved in cellular-protein trafficking. Genome. 1998;41:236–243. [PubMed] [Google Scholar]
- 63.Weiler K S, Wakimoto B T. Heterochromatin and gene expression in Drosophila. Annu Rev Genet. 1995;29:577–605. doi: 10.1146/annurev.ge.29.120195.003045. [DOI] [PubMed] [Google Scholar]
- 64.Weiler K S, Wakimoto B T. Chromosome rearrangements induce both variegated and reduced, uniform expression of heterochromatic genes in a development-specific manner. Genetics. 1998;149:1451–1464. doi: 10.1093/genetics/149.3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weintraub H, Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976;193:848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]