Abstract
E4F is a ubiquitously expressed GLI-Krüppel-related transcription factor which has been identified for its capacity to regulate transcription of the adenovirus E4 gene in response to E1A. However, cellular genes regulated by E4F are still unknown. Some of these genes are likely to be involved in cell cycle progression since ectopic p120E4F expression induces cell cycle arrest in G1. Although p21WAF1 stabilization was proposed to mediate E4F-dependent cell cycle arrest, we found that p120E4F can induce a G1 block in p21−/− cells, suggesting that other proteins are essential for the p120E4F-dependent block in G1. We show here that cyclin A promoter activity can be repressed by p120E4F and that this repression correlates with p120E4F binding to the cyclic AMP-responsive element site of the cyclin A promoter. In addition, enforced expression of cyclin A releases p120E4F-arrested cells from the G1 block. These data identify the cyclin A gene as a cellular target for p120E4F and suggest a mechanism for p120E4F-dependent cell cycle regulation.
E4F is a ubiquitously expressed GLI-Krüppel-related mammalian transcription factor which was first identified as a cellular factor binding to regulatory regions of the adenovirus E4 promoter and responsible for E4 regulation of expression in the course of adenovirus infection (13, 35, 36). The adenovirus E4 promoter contains two ATF binding sites which are also targets for E4F, as observed in extracts from adenovirus-infected cells. E4F DNA binding specificity differs from that of CREB-ATF protein family members, as only subsets of ATF sites are recognized by E4F. For example, E4F binds to two out of the four ATF binding sites found in the E4 promoter but recognizes none of the ATF sites found in the E2 or E3 promoter (37). Divergence in binding specificities between E4F and ATF proteins was further demonstrated by point mutagenesis of their DNA recognition sequence and by methylation interference assays (37). E4F is synthesized as a 120-kDa protein (p120E4F) that upon proteolytical cleavage gives rise to p50E4F, a 50-kDa amino-terminal fragment (11). Interestingly the murine homolog of E4F, termed φAP3, was independently identified as the cellular factor responsible for the repression of the E1A promoter in mouse fibroblasts (13). Although p50E4F and p120E4F recognize the same DNA motifs in vitro, they differentially regulate gene expression in vivo. p50E4F transactivates expression of the adenoviral E4 gene in a E1A-dependent fashion (34, 35). p120E4F, on the other hand, is likely to play a key role in mammalian cell cycle control. Indeed, overexpression of p120E4F in NIH 3T3 fibroblasts inhibits progression from G1 to S phase (12). Moreover, it was recently reported that p120E4F interacts directly with the key cell cycle regulators pRB (retinoblastoma tumor suppressor protein) and p53 (10, 39). One proposed mechanism to explain the cell cycle arrest mediated by p120E4F is the stabilization of p21WAF1 through a post transcriptional mechanism (12). However, as we observed that p120E4F is still able to block cell cycle in the absence of p21WAF1, the question remained as to whether p120E4F could also exert a direct transcriptional control on genes whose products are involved in cell cycle progression.
Based on this rationale, we identified a putative E4F binding site in the 5′ regulatory region of both human and mouse cyclin A genes. Cyclin A, as a regulatory component of cyclin-dependent kinase 2 (CDK2) plays an essential role in the progression through S phase (reviewed in references 18, 40, and 42). Cyclin A mRNA and protein accumulations at the end of G1 are required for progression into S phase and DNA replication (15, 29, 45). We have previously analyzed the expression of cyclin A in human and rodent cells (1, 2, 30–33) and identified functional DNA sequences present in the mouse cyclin A promoter (2, 21). In vivo genomic dimethyl sulfate footprinting revealed the presence of cell cycle-regulated protein binding elements close to the major transcription initiation sites. One of these elements, termed the cell cycle-responsive element (CCRE) (21, 31, 33) or cell cycle-dependent element (46), is periodically occupied in G0/early G1 when transcription of cyclin A is off. The CCRE constitutes, with a directly adjacent motif (cell cycle gene homology region [CHR]) which is shared by several other cell cycle-regulated genes, a bipartite cell cycle-dependent transcriptional regulatory module. Upstream from this element, a cyclic AMP-responsive element (CRE) site is occupied throughout the cell cycle. The cyclin A CRE site, which is conserved between the human and murine promoters, is required for full transcriptional activation of cyclin A transcription (21).
In this study, we identify the cyclin A gene as a cellular target for the transcription factor p120E4F. E4F binds to the CRE site of both human and murine cyclin A genes with a binding specificity distinct from that of CREB and ATF proteins. Expression of p120E4F leads to transcriptional inhibition of the cyclin A gene which correlates with cell cycle arrest in G1. Finally, ectopic expression of cyclin A, but not that of cyclin E, releases p120E4F arrested cells from the G1 block. Altogether, the data presented here identify cyclin A as the first functional cellular target for p120E4F which could provide a mechanism for p120E4F-dependent cell cycle regulation.
MATERIALS AND METHODS
Plasmids and oligonucleotides.
Details of all constructs are available upon request. pCycA-Luc is a pGL2-basic vector containing murine cyclin A promoter sequences (−177 to +100 relative to the most 3′ transcription initiation site). The strategy used previously to generate cyclin A promoter mutants (21) was used for CRE mutants with oligonucleotides 5′-TCTGCTCGAGTCACGGACTCCGGA-3′ and 5′-GTGACTCGAGCAGAAGCGCCGGTC-3′. A GAL4 recognition site (5′-CAGGTCGGAGTACTGTCCTCCGACTGCGA-3′) was introduced into the XhoI site of the mutant cyclin A CRE to generate the gal4-cycA promoter. Plasmid pcycA-nucYFP was constructed as follows. An oligonucleotide containing the nuclear localization signal (NLS) of simian virus 40 T antigen (5′-CCTCGAGCCCGGGAAGCTTTCTAGAATGGCTCCAAAAAAGAGAAAGGTACCGG-3′) together with a multiple cloning site (SV-NLS) was inserted between the SphI and HindIII sites of pCH110 (Pharmacia), giving vector pL-NLS-lacZ. The HindIII-Asp718 mouse cyclin A promoter fragment of pCycA-luc (21) was inserted between sites SmaI and HindIII of pL-NLS-lacZ, giving pCycA-NLS-lacZ vector. A pCycA-NLS-lacZ XhoI-Asp718 fragment was inserted into the same sites of pd2EYFP-N1 vector (Clontech) whose cytomegalovirus (CMV) promoter had been deleted (AseI-BglII deletion). Plasmid pd2EYFP-N1 encodes yellow fluorescent protein (YFP), a destabilized yellow-green variant of enhanced green fluorescent protein (Clontech). Plasmid pmCycA-nucYFP contains a cyclin A promoter mutated on the CCRE in place of the wild-type (WT) promoter. The mutant cyclin A promoter was generated using a splice overlap extension PCR technique to mutate the CCRE site of the HindIII-Asp718 minimal cyclin A promoter (30) inserted into pBS-SK+. The pcDNA3-derived p120E4F expression vector and plasmid pGEX-p120E4F, encoding glutathione S-transferase (GST)–p120E4F fusion protein, are described elsewhere (10). The N-terminal deletion (ΔN) and DNA binding domain deletion (ΔDBD) mutants of pcDNA-p120E4F were obtained by replacing the EcoRI-SfiI fragment by a fragment generated by PCR and recut with EcoRI and SfiI. The two subfragments were obtained by amplification with the common 3′ oligonucleotide 5′-CGCCACAGCGGAAGCGGCGCTCAC-3′ used in combination with 5′-GTGGAATTCCTGGTGAACAAGGAT-3′ (ΔN) or 5′-ATCGAATTCCACCGGCGGCACACG-3′ (ΔDBD).
The expression vector pTISP-p50E4F, used for preparation of the inducible TTN5-p50E4F cell line, was constructed as follows. A SacI/EcoRI restriction fragment from plasmid pcDNA-p120E4F, which contains the first 358 amino acids (aa) of p120E4F and includes the E4F DNA binding domain, was inserted into the EcoRI-EcoRV sites of the pTISP-POLYvector (4). The RcCMV expression plasmids encoding cyclin E and cyclin A were previously described (19).
The pRBΔp34 expression plasmid (16) is a pECE-based pRB expression vector corresponding to a dominant form of pRB with all phosphorylation sites contained within p34 kinase consensus sequence mutated.
DNA probes used in gel shift experiments were obtained by annealing the oligonucleotides 5′-GGCGCTTCTGGTGACGTCACGGACTCCGGA-3′ plus 5′-GCGTCCGGAGTCCGTGACGTCACCAGAAGCG-3′ (mCREwt), 5′-GGCGCTTCTGGTGACGTCTCGGACTCCGGA-3′ plus 5′-GCGTCCGGAGTCCGAGACGTCACCAGAAGCG-3′ (mCREpm4), and 5′-ATCCGAATTCTGACGTAACAGATCCACTAG-3′ plus 5′-CTAGTGGATCTGTTACGTCAGAATTCGGAT-3′ (E4-ATF). Primers used for reverse transcription (RT)-PCR amplification were 5′-CCTGTCCAGGAAGTTGACAGCCAA-3′ plus 5′-CC ATGCCCAGTCAGAGGAAGCAAC-3′ (cyclin A) and 5′-GCTCACTGGCA TGGCCTTCCGTGT-3′ plus 5′-GGAAGAGTGGGAGTTGCTGTTGA-3′ (GAPDH [glyceraldehyde-3-phosphate dehydrogenase]). Primers used for PCR amplification of DNA obtained by chromatin immunoprecipitation were 5′-AAGATTCCCGTCGGGCCTTCGCTCG-3′ plus 5′-CAGGAGCCGCGAGCTGCGCG-3′ (CRE-E4F locus) and 5′-CTCTGGGATTAAAGGTATGTACCAC-3′ plus 5′-GGTTGTGACATCAGACCATGAAGTTCC-3′ (upstream locus).
Electrophoretic mobility shift assays (EMSAs), Western blots, and antibodies.
GST-p120E4F fusion protein (10) was preincubated for 15 min at room temperature with 50 ng of poly(dI-dC)-poly(dI-dC) in binding buffer (20 mM HEPES [pH 7.9], 50 mM KCl, 1 mM MgCl2, 0.1 mM EDTA, 5 mM dithiothreitol [DTT], 4% glycerol). 32P-labeled DNA probes were added to the reaction and incubated for 20 min at room temperature. Protein-DNA complexes were separated by electrophoresis in 0.5× Tris-borate-EDTA buffer through a 5% polyacrylamide gel containing 2.5% glycerol. Supershift experiments were performed by adding the rabbit E4F polyclonal antibody 88.2 to the preincubation mix prior to the DNA probe. For the preparation of nuclear extracts, subconfluent cultures of CCL39/p50E4F fibroblasts, which express p50E4F under the transcriptional control of the tetracycline repressor, were extensively washed with phosphate-buffered saline (PBS) and further grown for 24 h in 10% fetal bovine serum (FBS) in the presence (1 μg/ml) or absence of tetracycline. The cells were scraped from the dishes in 1 ml of hypotonic buffer (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride). Cells were lysed by vigorous vortexing after addition of NP-40 (final concentration, 0.1%). Crude extracts were centrifuged for 15 s at 16,000 × g at 4°C. Nuclear pellets were resuspended in 100 μl of high-salt buffer (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride, 10% glycerol), left on ice for 20 min, and clarified by centrifugation (5 min at 16,000 × g at 4°C).
For the p50E4F Western blot experiments, nuclear extracts (10 μg) prepared from TTN-p50E4F cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The blot was incubated with rabbit E4F polyclonal antibody 88.2 (generated using a N-terminal peptide of p120E4F [EEDEDDVHRCGRCQA; aa 50 to 64]) as an antigen and affinity purified on an agarose-peptide column. Western blot experiments aimed at checking expression of transfected cyclin A and cyclin E were performed on 50 μg of U2OS whole-cell extracts probed with anti-cyclin A and anti-cyclin E antibodies (Santa Cruz Biotechnology).
Cell lines, cell culture, and transfections.
p21−/− mouse embryonic fibroblasts (MEFs) (gift from T. Jacks) (3), p53−/− MEFs (gift from L. Donehower) (8), pRB−/− MEFs (gift from D. Cobrinik), WT MEFs, NIH 3T3 cells, CCL39 Chinese hamster lung fibroblasts, and U2OS osteosarcoma cells (19) were grown in Dulbecco modified Eagle medium supplemented with 10% FBS. For luciferase experiments, 105 cells per 3.5-cm-diameter petri dishes were transfected with 4 μg of DNA (1 μg of pCycA-Luc, 1 ng of pCMV-RLuc, and 3 μg of p120E4F expression vector or control empty vector). Cells were transfected for 10 h by the calcium phosphate procedure and further grown for 14 h prior to luciferase activity measurement (Promega). p21−/−, p53−/−, pRB−/−, and WT MEFs as well as U2OS osteosarcoma cells and NIH 3T3 cells were transfected using the Lipofectamine Plus reagent (Gibco Life Technologies). The TTN5-p50E4F cell line was generated as follows. TTN5 cells, a CCL39-derived cell line expressing the tetracycline repressor (4), was transfected with the expression vector pTISP-p50E4F. Expression of p50E4F is directed by the CMV promoter and under the control of the tetO repressor, which represses expression of p50E4F in the presence of tetracycline. Stably transfected cells were selected in the continuous presence of puromycin (10 μg/ml) and tetracycline (1 μg/ml) for 10 days.
Immunofluorescence and flow cytometry.
For immunofluorescence, p21−/−, p53−/−, pRB−/−, and WT MEFs as well as U2OS and NIH 3T3 cells were grown on coverslips and transfected with 0.25 to 1 μg of the indicated plasmids. For bromodeoxyuridine (BrdU) staining, cells were incubated for 8 h with BrdU starting 16 h after transfection. After formalin fixation for 5 min followed by a 5-min methanol permeabilization, cells were treated with 1.5 N HCl for 10 min at room temperature and incubated with a rabbit anti-E4F polyclonal antibody (AD1; raised against a GST-E4F protein [aa 358 to 783]) (47), anti-BrdU monoclonal antibody (mouse; DAKO), or anti-cyclin A monoclonal antibody (Sigma). Immunofluorescence was monitored by incubation with a Texas red-conjugated anti-rabbit immunoglobulin G and fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G. To analyze cell cycle distribution by fluorescence-activated cell sorting, cells were transfected with the pcDNA3-derived plasmid p120E4F alone or in combination with vectors encoding either cyclin A or cyclin E. At 36 h after transfection, cells were analyzed by flow cytometry (27), and the cell cycle distribution of transfected cells was determined using ModFit software (Becton Dickinson).
RT-PCR.
NIH 3T3 cells were transfected with 5 μg of either pcDNA3 or pcDNA-p120E4F in 10-cm-diameter petri dishes. Twenty-four hours after transfection, RNA was prepared (Qiagen RNAeasy miniprep) and RT was performed using avian myeloblastosis virus reverse transcriptase. Twenty five cycles of PCR were performed in 20 μl with 1 μl of RT reaction mixture, 10 pmol of each cyclin A primer, 3 pmol of each GAPDH primer, and 0.2 μl of Taq DNA polymerase (Sigma).
Chromatin immunoprecipitation.
We performed chromatin immunoprecipitation with p120E4F-transfected NIH 3T3 cells. Three 10-cm-diameter petri dishes (approximately 2 × 106 cells per dish) were used per chromatin immunoprecipitation reaction. Each plate was transfected with 6 μg of either pcDNA3 or pcDNA-p120E4F. Twenty-four hours after transfection, the transfection efficiency was checked by immunofluorescence on a coverslip initially put in the 10-cm-diameter dish, and the chromatin immunoprecipitation reaction was carried out on the remaining cells of the dish. (Transfection efficiency ranged from 45 to 55%.) Cross-linking was performed by direct addition of formaldehyde (final concentration, 1%) to the dish and proceeded for 10 min at room temperature before addition of glycine (final concentration, 125 mM). Cells were washed three times with ice-cold PBS and scraped into 1 ml of PBS. Cells were collected by centrifugation, resuspended in 800 μl of chIP lysis buffer (50 mM HEPES [pH 7.5], 140 mM NaCl, 1% Triton, protease inhibitors) per each set of three dishes, and rocked at 4°C for 30 min. Sonication was performed four times for 1 min each at 60% amplitude. Samples were centrifuged at 4°C for 10 min at 14,000 rpm, and the supernatant was sonicated again four times for 1 min each at 60% amplitude. (Such sonication conditions yielded DNA fragments with an average length of 300 bp, as confirmed on an agarose gel after reversion of the cross-linking and DNA purification.) Extracts were again spun for 10 min at 14,000 rpm at 4°C. Chromatin was precleared at 4°C for 1 h with protein A-Sepharose previously blocked with salmon sperm DNA (1 mg/ml) and bovine serum albumin (1 mg/ml). Immunoprecipitation was carried out with a mixture of rabbit anti-E4F polyclonal antibodies AD1 and 88.2 for 1 h at 4°C, followed by another hour of incubation with 20 μl of a 50% slurry of blocked protein A-Sepharose. Immunoprecipitates were washed two times with 1 ml of each of the following buffers: chIP lysis buffer, high-salt chIP lysis buffer (50 mM HEPES [pH 7.5], 500 mM NaCl, 1% Triton), chIP wash (10 mM Tris [pH 8], 250 mM LiCl, 0.5% NP-40), and Tris-EDTA. Protein A-Sepharose pellets were resuspended in 100 μl of Tris-EDTA and incubated for 3 h at 55°C with 10 μg of RNase A and 20 μg of proteinase K. Cross-linking was reversed by incubation at 65°C during 4 h to overnight. DNA was purified on resin (Wizard protocol; Promega) and eluted in 50 μl of H2O. An aliquot of chromatin DNA prepared from E4F-transfected cells was taken prior to immunoprecipitation and further treated and purified as the immunoprecipitated DNAs. This DNA corresponded to the total DNA sample. Immunoprecipitated and total DNAs were assayed by PCR. Forty cycles of PCR were performed in 12.5 μl with 1 μl of immunoprecipitated DNA, 10 pmol of each primer, 0.5 U of Taq DNA polymerase (Perkin-Elmer), and 0.11 μCi of [α-32P]dCTP. PCR products were analyzed by electrophoresis on a 6% denaturing polyacrylamide gel in parallel with a Maxam-Gilbert DNA sequence used as a migration standard.
RESULTS
p120E4F-dependent cell cycle arrest is maintained in p21WAF1−/− MEFs.
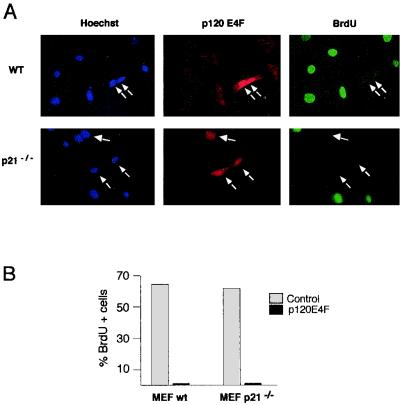
p120E4F-arrested cells contain elevated levels of p21WAF1 protein, which results from a p53-independent posttranscriptional stabilization of p21WAF1 (12). To explore whether this was the unique mechanism for p120E4F-dependent cell cycle arrest, we tested the capacity of p120E4F to block DNA synthesis in both WT and p21-deficient MEFs. As expected, WT MEFs expressing p120E4F were not able to enter S phase, as demonstrated by the absence of BrdU incorporation (Fig. 1A). Surprisingly, expression of p120E4F in p21WAF1−/− primary fibroblasts led to similar effects (Fig. 1B and C). These data suggested that besides p21WAF1, p120E4F might target other cellular genes encoding proteins essential for G1-to-S phase transition.
FIG. 1.
p120E4F -dependent cell cycle arrest is maintained in p21WAF1−/− MEFs. WT or p21WAF1−/− MEFs were transfected with a p120E4F expression vector and BrdU labeled. The panels show Hoechst staining, E4F immunodetection in E4F-transfected cells, and BrdU staining of cells undergoing DNA synthesis (A). Quantitation of the BrdU-labeled cells that either overexpressed p120E4F (p120E4F) or did not (control) is schematized for both WT and p21WAF1−/− MEFs (B).
Cyclin A can bypass p120E4F-induced cell cycle arrest.
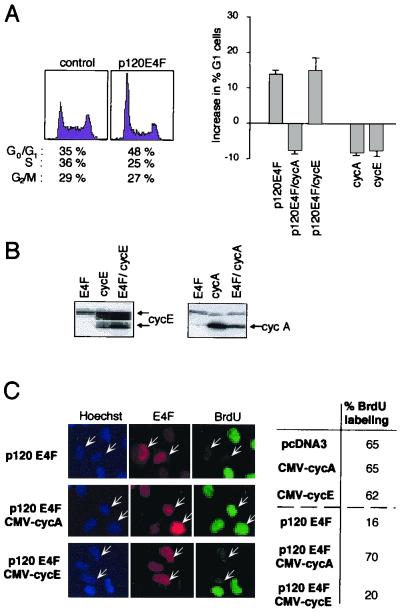
In a search for E4F targets, we focused on genes encoding cyclins, such as cyclin A or cyclin E, which are essential for entry and progression into S phase. We reasoned that if these cyclins are important targets for p120E4F, one would expect coexpression of cyclin A or of cyclin E to rescue cells from the cell cycle arrest induced by p120E4F. We first analyzed the changes in cell cycle profiles in response to ectopic expression of p120E4F in human U2OS osteosarcoma cells. As previously described for NIH 3T3 mouse fibroblasts (12), introduction of p120E4F into U2OS cells resulted in accumulation of cells in the G0/G1 phase of the cell cycle, as assessed by fluorescence-activated cell sorting analysis (Fig. 2A, left). We then analyzed the effect of coexpression of either cyclin A or cyclin E on this E4F-induced G1 arrest. Cotransfection with a cyclin A expression plasmid completely reverted the E4F-induced cell cycle arrest, whereas coexpression of cyclin E had no significant effect (Fig. 2A, right).
FIG. 2.
Enforced expression of cyclin A, but not of cyclin E, releases cells from the G1 block induced by overexpressed p120E4F. (A) p120E4F induces a G1 arrest which is released by coexpression of cyclin A. (Left) U2OS cells were transfected with plasmids expressing p120E4F (10 μg) and the CD20 marker (1μg). Cells were harvested 36 h after transfection, and DNA profiles of CD20-positive cells were obtained using bivariate flow cytometry. Percentage of cells present in the different cell cycle phases is indicated below each plot. (Right) U2OS cells were transfected with plasmids expressing p120E4F and the CD20 marker alone or in combination with a cyclin A (0.1 μg) or cyclin E (0.1 μg) expression vector. As a control, U2OS cells were also transfected with CD20 expression plasmid in combination with either cyclin A or cyclin E expression plasmid. Absolute increase in percentage of U2OS cells in the G0/G1 phase upon expression of the indicated proteins is plotted on the y axis, the baseline representing the percentage of G0/G1 cells in mock-transfected cells. Data are representative of at least three independent experiments. (B) Total cellular extracts from CD20-positive cells were submitted to Western blot analysis using an antibody directed at cyclin E (left) or cyclin A (right). Arrows point to specific bands. (C) U2OS cells overexpressing both p120E4F and cyclin A resume DNA synthesis. U2OS cells were cotransfected with plasmids expressing p120E4F in combination with either cyclin A or cyclin E. The panels show Hoechst staining of nuclei, E4F immunodetection in E4F-transfected cells (indicated by arrows), and BrdU staining of cells undergoing DNA synthesis. The percentage of BrdU-labeled cells (85 counted cells) is indicated for cells expressing p120E4F alone or in combination with either cyclin A (p120E4F/CMV-cycA) or cyclin E (p120E4F/CMV-cycE). As a control, the index of BrdU labeling was measured for cells transfected with either cyclin A or cyclin E expression plasmids.
The role of cyclin A in E4F-mediated cell cycle arrest was further confirmed by immunofluorescence analysis. U2OS cells expressing p120E4F were tested for the ability to synthesize DNA as monitored by BrdU incorporation. Whereas mock transfection resulted in 65% BrdU-labeled cells, this proportion fell to 16% for p120E4F-transfected cells (Fig. 2C). Coexpression of cyclin A was able to reverse this block, whereas no effect was seen upon cyclin E overexpression, even though the protein was expressed at high levels in transfected cells (Fig. 2B). Altogether, these data show that the p120E4F-dependent block in G1 can be alleviated by expression of cyclin A, but not of cyclin E, and therefore suggest that cyclin A gene might be a critical target for p120E4F, whose repression contributes to E4F-dependent cell cycle arrest.
p120E4F represses the expression of cyclin A.
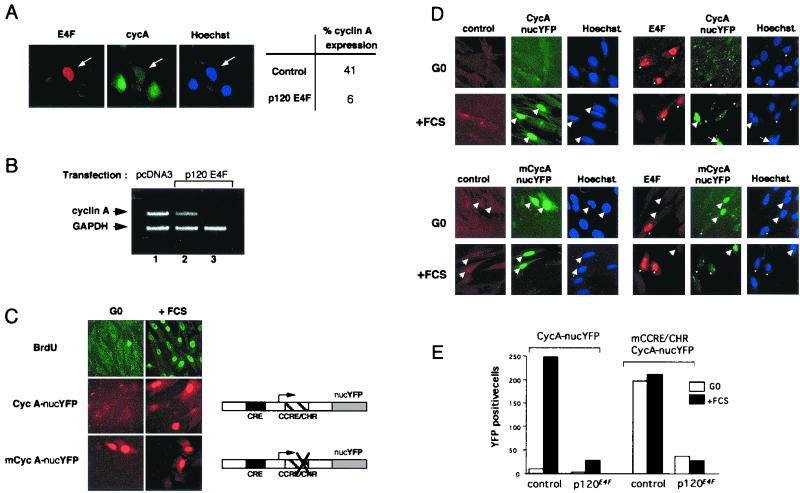
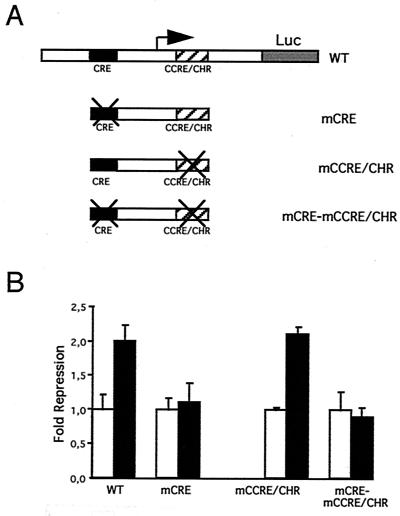
Examining the status of endogenous cyclin A in p120E4F-transfected cells, we found a decrease of cyclin A expression at both the protein (Fig. 3A) and RNA (Fig. 3B) levels. In the latter case, the observed decrease in cyclin A mRNA, as assayed by RT-PCR, was in good agreement with the estimated percentage of transfected cells (roughly 30%). We further investigated whether the control by p120E4F of cyclin A cellular concentration occurred at the transcriptional level. For that purpose, we prepared a reporter construct in which the gene encoding YFP fused to an NLS is under the transcriptional control of the mouse cyclin A promoter (Fig. 3C). We first checked that this reporter reproduced the transcriptional regulation of the endogenous cyclin A gene during cell cycle. As expected for endogenous cyclin A, YFP expression was not observed in G0-arrested cells and resumed in serum-refed cells. This induced expression correlated with entry into S phase, as determined by BrdU labeling of the cells (Fig. 3C). This negative regulation of cyclin A expression in G0/early G1 cells was previously shown to depend on the integrity of a CCRE/CHR site present in the cyclin A promoter (21). To further characterize our cyclin A reporter system, we checked the expression of another construct mutated at the CCRE/CHR site. Expression of YFP was observed in both G0-arrested and serum-refed cells, which corresponded to the expected cell cycle expression pattern of a deregulated cyclin A promoter and fully validated our in vivo cyclin A reporter system (Fig. 3C). We therefore used the cycA-nucYFP reporter construct to test the transcriptional effect of p120E4F in vivo and found that most cells coexpressing p120E4F lost YFP expression (Fig. 3D, upper panels). To further demonstrate that cyclin A repression was a direct effect of the expression of p120E4F and not an indirect consequence of E4F-mediated G1 arrest, we also tested the cyclin A promoter construct mutated at the CCRE/CHR site. Similarly to the wild-type construct, we observed a repression of the cyclin A promoter activity in response to p120E4F when cells were serum refed. Moreover, the repression was also visible in quiescent cells (Fig. 3D, lower panels). These data showed that the expression of both cyclin A reporter constructs was strongly inhibited when p120E4F was cotransfected whether cells were quiescent or proliferating (Fig. 3E); they suggested that the observed decrease in cyclin A cellular concentration upon p120E4F expression was due to an E4F-dependent transcriptional regulation of the cyclin A gene. To confirm the role of p120E4F as a transcriptional repressor of cyclin A promoter activity, we used various cyclin A promoter-luciferase constructs to test the effect of p120E4F on cyclin A transcription in transient transfection assays (Fig. 4). Cotransfection of CCL39 fibroblasts with a p120E4F expression vector led to a twofold repression of the WT cyclin A promoter-dependent luciferase activity (Fig. 4B). This repression was not observed with a cyclin A promoter mutated at the CRE site. Altogether, the data from immunofluorescence analysis and luciferase assays show that expression of p120E4F leads to a decrease of cyclin A cellular concentration and that this effect can be accounted for by a p120E4F-dependent control of cyclin A transcription that depends on the integrity of the CRE site.
FIG. 3.
p120E4F represses expression of cyclin A. (A) p120E4F overexpression decreases cyclin A cellular concentrations. U2OS cells were transfected with p120E4F expression plasmid and immunostained for overexpressed p120E4F and endogenous cyclin A. The panels show immunodetection of E4F and endogenous cyclin A as well as Hoechst staining. The percentage of cyclin A-expressing cells, depending on whether they overexpress p120E4F (p120E4F) or not (control), is indicated (50 cells counted). (B) p120E4F overexpression decreases cyclin A mRNA concentrations. NIH 3T3 cells were transfected with p120E4F expression plasmid (lanes 2 and 3) or with pcDNA3 control vector (lane 1). mRNA was amplified by RT-PCR using, in the same PCR, primers to amplify both the cyclin A and the control GAPDH loci (lanes 1 and 2). In lane 3, cyclin A primers were omitted from the PCR in order to further confirm the identification of the cylin A amplified DNA fragment. (C) The YFP gene fused to the cyclin A promoter recapitulates cyclin A transcriptional regulation during the cell cycle. Mutation of the CCRE/CHR module leads to expression of the reporter in both quiescent (G0) and stimulated (+FCS) cells. Plasmid pCycA-nucYFP, encoding YFP tagged with an NLS and placed under the transcriptional control of the murine cyclin A promoter, was used in transient transfections. After transfection, cells were split in half; each half was submitted to serum starvation (24 h), followed by either serum refeeding (16 h; +FCS) or further starvation (16 h; -FCS). In parallel, cells were transfected with the expression plasmid pmCycA-nucYFP, where the WT cyclin A promoter was replaced by a cyclin A promoter mutated at the CCRE/CHR site. The panels show BrdU staining as well as expression of YEP in the nuclei. (D) p120E4F overexpression represses both WT (upper panels) and mutated (lower panels) cyclin A promoters. CCL39 cells were cotransfected with p120E4F and either pCycA-nucYFP or pmCycA-nucYFP expression vector. The panels show E4F immunodetection, YFP expression, and Hoechst staining in E4F-transfected serum-starved (G0) or serum-restimulated (+FCS) cells. Arrows and asterisks point to YFP-and E4F-expressing cells, respectively. (E) Number of YFP-expressing cells, plotted for a representative experiment, in serum-starved (G0) or serum-restimulated (+FCS) cells. Cells were cotransfected with pCycA-nucYFP or pmCycA-nucYFP together with respectively an empty (control) or a p120E4F -expressing (p120E4F) vector.
FIG. 4.
p120E4F overexpression represses cyclin A transcription. (A) Luciferase reporter gene under the transcriptional regulation of the murine cyclin A promoter. The various cyclin A promoter constructs tested, which include the WT promoter as well as the promoter mutated at the CRE (mCRE), at the CCRE/CHR (mCCRE/CHR) or at both (mCRE-mCCRE/CHR), are schematized. (B) Luciferase activities of the various cyclin A promoter constructs were measured in CCL39 cells cotransfected with either a p120E4F-expressing vector (filled bars) or an empty vector (empty bars). The fold repression of cyclin A promoter activity in response to overexpression of p120E4F is shown. Standard deviations are indicated for experiments done in triplicate.
p120E4F specifically binds to a DNA motif encompassing the CRE site of the cyclin A promoter.
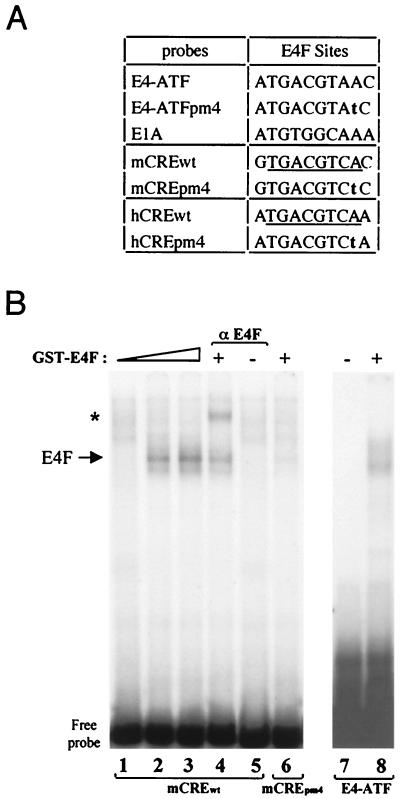
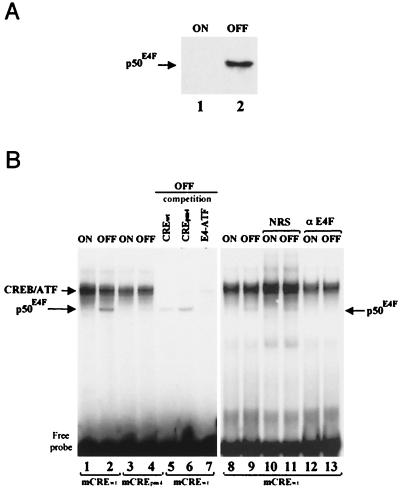
We found sequence homologies between the E4-ATF sites from the adenoviral E4 promoter (35) and the CRE sites found in the promoters of both the human and murine cyclin A genes (Fig. 5A). We used an affinity-purified GST-p120E4F fusion protein (10) to test by EMSA whether E4F could bind to the cyclin A CRE site in vitro. The p120E4F protein gave rise to a protein-DNA complex in a dose-dependent fashion, which was supershifted by the addition of anti-E4F antibodies and was not seen with the CREpm4 point mutant previously shown to prevent E4F binding (Fig. 5B, lanes 1 to 6) (23, 37). Likewise, specific E4F DNA binding activity was observed with the human cyclin A CRE site (data not shown). These data showed that a purified p120E4F protein can specifically bind to the cyclin A CRE site in vitro. However, because the cyclin A CRE site is also the target for CREB and ATF factors (21), we checked the binding of E4F in nuclear extracts containing also these factors. For that purpose, we constructed a cell line that enabled inducible expression of E4F in a tetracycline-dependent fashion (see Materials and Methods). Because the full-length p120E4F and CREB-ATF DNA-protein complexes comigrated in our gel shift experiments and made it difficult to distinguish the p120E4F specific band (data not shown), we decided to overexpress p50E4F, a truncated form of p120E4F previously characterized to retain the E4F-specific DNA binding activity (11). The stable E4F transfectants were selected for the inducible expression of p50E4F, as shown in nuclear extracts from cells grown under repressing or inducing conditions (Fig. 6A). When the same extracts were tested by EMSA on the murine cyclin A CRE probe, we specifically observed a DNA-protein complex with extracts from the E4F-induced cells (Fig. 6B, lane 2). This band was not observed with the CREpm4 point mutant (lane 4) and was competed by an E4-ATF oligonucleotide (lane 7) but not by the CREpm4 oligonucleotide (lane 6). In addition, the p50E4F DNA-protein complex was recognized by anti-E4F antibodies but not by normal rabbit serum (NRS) (lanes 8 to 13). Similar data were obtained with the human cyclin A CRE (data not shown). Altogether, these data show that E4F expressed in cells can specifically bind to the CRE site found in the promoter of the cyclin A gene and that this binding occurs independently of CREB and ATF proteins which target this same site.
FIG. 5.
The CRE of the cyclin A promoter is a p120E4F binding site. (A) Sequences of E4F binding sites found in E4 and E1A promoters. The E4-ATFpm4 point mutation abolishes E4F binding to DNA. The CRE sites found in the murine (mCREwt) and human (hCREwt) cyclin A promoters are indicated. The corresponding pm4 mutations are indicated in bold characters; the consensus CRE is underlined. (B) Binding of the purified p120E4F protein to cyclin A CRE. Purified GST-p120E4F was incubated with either the WT (lanes 1 to 5) or pm4 mutant (lane 6) murine cyclin A CRE probe. As a control, GST-E4F binding was tested on the E4-ATF site (lanes 7 and 8). DNA-protein complexes were analyzed by EMSA. The E4F complex is indicated, as is the E4F-containing complex (∗) supershifted with anti-p120E4F antibodies.
FIG. 6.
E4F binds to the CRE-E4F site of the cyclin A promoter independently of CREB and ATF proteins. (A) Expression of p50E4F in tetracycline-inducible cell lines. Cultures of TTN5-p50E4F cells were grown for 24 h in the presence (ON) or absence (OFF) of tetracycline. The blotted membrane was probed with anti-E4F antibodies, and antibody-antigen complexes were visualized by enhanced chemiluminescence. The Western blot shows the specific expression of p50E4F upon removal of tetracycline (OFF), whereas basal levels of E4F in the inducible TTN5-p50E4F cell line in the presence of tetracycline (ON) were undetectable. (B) Evidence that E4F present in nuclear extracts can bind to the CRE-E4F site of the cyclin A promoter. Nuclear extracts (1 μg) from TTN5-p50E4F cells grown in the presence (ON) or absence (OFF) of tetracycline were incubated with mCREwt (lanes 1, 2, and 5 to 13) or mCREpm4 (lanes 5 to 7) and subjected to EMSA. Competition was done with a 500-fold molar excess of unlabeled oligonucleotides corresponding to the WT murine cyclin A CRE site (lane 5), to the pm4 mutant site (lane 6), or to the E4-ATF site previously characterized (lane 7). Gel shift reactions were done in the presence of 1 μl of NRS (lanes 10 and 11) or rabbit anti-E4F antibodies (αE4F; lanes 12 and 13). Positions of migration of the CREB-ATF and p50E4F DNA-protein complexes and of the free probe are indicated.
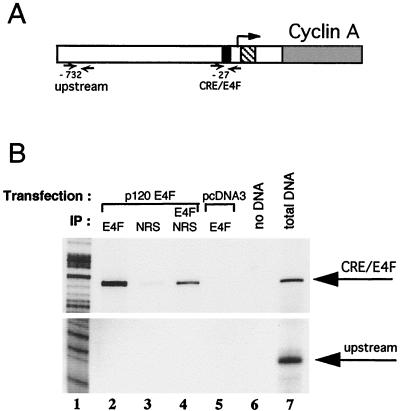
To further demonstrate that p120E4F could compete in vivo with CREB and ATF proteins for binding to the CRE-E4F site of the endogenous cyclin A promoter, we performed chromatin immunoprecipitation experiments using anti-E4F antibodies (Fig. 7). PCR amplification at the CRE-E4F locus of the cyclin A promoter obtained with chromatin purified from p120E4F-transfected cells gave a strong signal (Fig. 7B, lane 2), whereas the control immunoprecipitation with NRS gave only a background amplification signal (lane 3) even though p120E4F bound DNA was present in that chromatin extract, as demonstrated by the signal obtained with the secondary E4F immunoprecipitation (lane 4). The PCR signal obtained with anti-E4F antibodies was specific of E4F, as no PCR product was obtained with chromatin purified from mock-transfected NIH 3T3 cells (lane 5). When instead of the CRE-E4F site of the cyclin A promoter, an upstream site was used, no PCR amplification signal was obtained (Fig. 7B, lower panel, lanes 2 and 4) even though that specific locus could be PCR amplified from the DNA mixture used as starting material for the E4F immunoprecipitation (lower panel, lane 7). These data correlate with the apparent average size of 300 bp of the sonicated chromatin DNA (see Materials and Methods). They reinforce the observation that p120E4F recognizes in vivo the CRE-E4F site at position −27 of the endogenous murine cyclin A promoter even in the presence of endogenous CREB or ATF. Because these experiments were performed with overexpressed p120E4F protein which has been characterized to recognize CRE-like sites, we decided to further check that p120E4F binding to the cyclin A promoter was specific of the cyclin A CRE site and not general for any CRE promoter site. For that purpose, we analyzed a CRE site from the c-fos gene which binds CREB and ATF proteins with apparent affinities similar to that of the cyclin A CRE site. Again, despite the fact that the c-fos CRE locus could be amplified from the DNA mixture used as starting material for the E4F immunoprecipitation, no amplification was seen in the E4F immunoprecipitates (data not shown), demonstrating that the observed p120E4F binding was not general to all CRE promoter sites. Altogether, these data show that p120E4F can compete in vivo with CREB and ATF proteins to bind to the endogenous cyclin A CRE-E4F locus.
FIG. 7.
p120E4F binds to the CRE-E4F site of the cyclin A promoter in vivo. (A) Chromatin immunoprecipitation experiments were performed with NIH 3T3 cells that were transfected either with p120E4F expression plasmid or with the empty pcDNA3 vector. Immunoprecipitations were then carried out with either anti-E4F rabbit antibodies or NRS. Two loci of the murine cyclin A promoter were checked by PCR amplification of the immunoprecipitated chromatin. The CRE-E4F site (black box) is positioned 27 bp upstream of the most 3′ transcription initiation site (arrow); a presumably irrelevant locus was chosen 732 bp upstream of this same transcription initiation site (upstream) as a control for immunoprecipitation specificity. The CCRE/CHR site is represented by a hatched box, while cyclin A coding sequence is in grey. (B) PCR amplification products of E4F chromatin immunoprecipitations were analyzed on a 6% denaturing gel along with a sequence ladder. (Top) amplification at the CRE-E4F locus; (bottom) results obtained with primers at the upstream locus. PCRs in lanes 2, 3, 4, and 7 were obtained with chromatin DNA purified from E4F-transfected cells, while the reaction in lane 5 was obtained from DNA of mock-transfected NIH 3T3 cells. Antibodies used for the immunoprecipitations (IP) are indicated. In lane 4, the supernatant of the immunoprecipitation done with NRS (lane 3) was further immunoprecipitated with anti-E4F antibodies. The PCR in lane 7 (total DNA) was performed with an aliquot of the DNA obtained from E4F-transfected cells taken prior to immunoprecipitation. The sequence ladder is shown in lane 1.
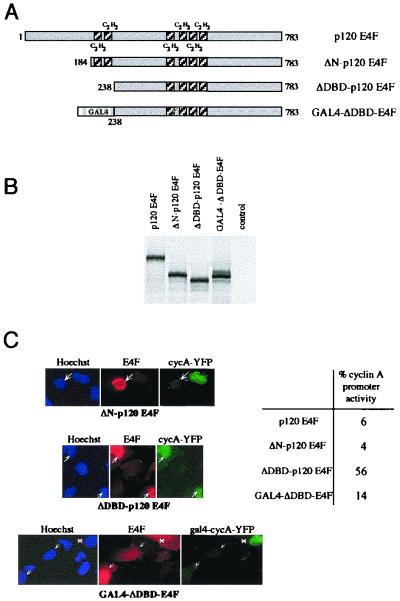
p120E4F DNA binding is required for E4F-dependent cyclin A transcriptional repression.
To check whether the DNA binding capacity of p120E4F is required for cyclin A transcriptional regulation, we tested two E4F deletion mutants, ΔN-p120E4F and ΔDBD-p120E4F (Fig. 8). These polypeptides were designed on the basis of previous data showing that truncation of the first 198 aa of p50E4F still enabled specific DNA binding while further truncation up to aa 214, which eliminates the two zinc finger domains of p50E4F, prevented DNA binding (38). Because p120E4F contains four additional zinc finger domains which could provide DNA binding activity on their own, we checked whether a GST fusion protein encompassing these residues could bind DNA. Contrary to GST-p120E4F (full length) or GST-p50E4F (aa 1 to 358), a polypeptide encompassing the four C-terminal zinc finger domains of p120E4F (aa 358 to 783) was unable to bind DNA (data not shown). We then tested the effect of ΔN-p120E4F or ΔDBD-p120E4F expression on the pcycA-nucYFP reporter. Whereas ΔN-p120E4F repressed cyclin A promoter activity as did the full-length p120E4F, ΔDBD-p120E4F appeared to be inactive (Fig. 8C). These results showed the specific requirement of the two amino-terminal zinc finger domains of p120E4F for the E4F-dependent cyclin A promoter transcriptional repression. The requirement of direct E4F-DNA binding to the cyclin A promoter for its transcriptional repression was further confirmed by the use of a GAL4-ΔDBD-E4F fusion protein, which expresses the GAL4 DNA binding domain. This protein restored repression of a cyclin A promoter containing a GAL4 recognition site in place of the CRE-E4F site, and repression correlated with the nuclear localization of the GAL4-ΔDBD-E4F fusion protein (Fig. 8C).
FIG. 8.
p120E4F DNA binding activity is required for cyclin A transcriptional repression. (A) Amino-terminal deletion mutants of p120E4F. Deletion of the first 183 aa of p120E4F (ΔN-p120E4F mutant) preserves the integrity of the six zinc finger domains. The ΔDBD-p120E4F mutant corresponds to a polypeptide (aa 238 to 783) which lacks the two p120E4F amino-terminal zinc finger motifs. The C2H2 zinc finger motifs are shown as shaded boxes. The GAL4-ΔDBD-E4F fusion protein contains the GAL4 DNA binding domain (aa 1 to 147) fused N terminally to ΔDBD-p120E4F. (B) Expression of the mutants in vitro. Full-length p120E4F as well as the mutants ΔN-p120E4F, ΔDBD-p120E4F, and GAL4-ΔDBD-E4F were in vitro translated and analyzed by SDS-PAGE along with unprogrammed reticulocyte lysates (control). (C) The two p120E4F N-terminal zinc finger domains are required for repression of cyclin A transcription. U2OS cells were cotransfected with expression vectors for either pCycA-nucYFP and p120E4F, ΔN-p120E4F, or ΔDBD-p120E4F or for gal4-cycA-YFP and GAL4-ΔDBD-E4F. The panels show E4F immunodetection, YFP expression, and Hoechst staining. Arrows indicate E4F-transfected cells. A transfected cell with a cytosolic localization of GAL4-ΔDBD-E4F fusion protein is indicated by the asterisk. The percentage of cells that contain an active cyclin A promoter, as estimated by expression of YFP, is indicated (about 100 counted cells).
p120E4F-dependent transcriptional repression of the cyclin A gene can be enhanced by pRB.
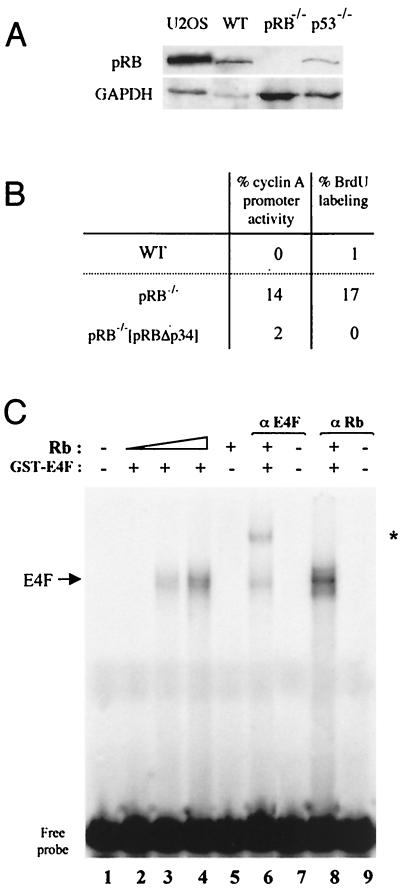
Because pRB and p53 tumor suppressors have been involved in p120E4F-mediated growth arrest (10, 39), we investigated the potential role of these proteins for p120E4F-dependent transcriptional repression of the cyclin A gene. For that purpose, we used MEFs not expressing pRB and MEFs not expressing p53. Expression of p120E4F in p53−/− MEFs led to a repression of cyclin A expression similar to that seen in WT MEFs, and this repression correlated with inhibition of DNA synthesis, indicating that these effects were independent of p53 (Fig. 9). We took a similar approach, using pRB−/− MEFs, to check the effect of pRB on p120E4F-dependent cyclin A downregulation. We found that these MEFs showed a reduced efficiency in cyclin A transcriptional repression, while repression rates similar to those observed in WT MEFs were restored upon cotransfection in pRB−/− MEFs of a plasmid encoding pRBΔp34, a constitutively active pRB (16) (Fig. 10B). At the molecular level, and as already observed for p120E4F binding to the ATF site of the E4 gene promoter (10), p120E4F binding to the cyclin A CRE site appeared to be enhanced by pRB that, again, was absent from the DNA-protein complex (Fig. 10C). These data suggest that although not intrinsincally required for p120E4F-dependent cyclin A downregulation, pRB appears to increase the efficiency of this process (10), possibly by favoring p120E4F-DNA binding to the cyclin A promoter.
FIG. 9.

p120E4F overexpression represses cyclin A transcription independently of p53. (A) Absence of UV-induced p53 expression in p53−/− MEFs. Cells were submitted to UV irradiation (20 J/cm2) and further grown for 8 h before fixation. The panels show p53 immunostaining in WT or p53−/− MEFs. (B) p53−/− or WT MEFs were cotransfected with p120E4F and pCycA-nucYFP expression vectors. The panels show E4F immunodetection in E4F-transfected cells, YFP expression under the control of the murine cyclin A promoter, BrdU labeling, and Hoechst staining. (C) The percentage of mock- or E4F-transfected cells that contain an active cyclin A promoter as determined by YFP expression or that are BrdU labeled is indicated for both WT and p53−/− MEFs. Three independent transfection experiments were analyzed.
FIG. 10.
p120E4F -dependent transcriptional repression of the cyclin A gene is enhanced by pRB. (A) pRB Western blot of cellular extracts from U2OS or either WT, pRB−/−, or p53−/− MEFs. The blot was probed with anti-pRB antibodies and reprobed with anti-GAPDH antibodies for normalization. (B) Absence of pRB results in loss of efficiency for E4F-dependent repression of cyclin A transcription. pRB−/− or WT MEFs were cotransfected with p120E4F and pCycA-nucYFP expression vectors. The percentage of E4F-transfected cells that contain an active cyclin A promoter or that are BrdU labeled is indicated for WT and pRB−/− MEFs as well as for pRB−/− MEFs cotransfected with pRBΔp34 expression plasmid. (C) Binding of purified p120E4F protein to the cyclin A CRE site is enhanced by pRB. Purified GST-p120E4F was incubated with the WT murine cyclin A CRE probe and with increasing concentrations of purified baculovirus-expressed pRB protein (lanes 2 to 4). The DNA-protein complex obtained with both p120E4F and pRB (lane 4) was incubated with antibodies directed against either E4F (lane 6) or pRB (lane 8). DNA-protein complexes were analyzed by EMSA. The E4F-containing complex supershifted with anti-p120E4F antibodies is indicated (∗).
DISCUSSION
Up to now, the mechanism by which p120E4F regulates cell cycle progression has remained elusive. Previous observations showed that the p120E4F-dependent cell cycle arrest was associated to p21WAF1 stabilization and therefore suggested that p21WAF1 could be a mediator of this arrest (12). However, we show here that p21WAF1−/− primary fibroblasts are still efficiently blocked in G1 upon ectopic expression of p120E4F, suggesting that stabilization of p21 by E4F could be a consequence rather than the primary cause of the G1 arrest. Seeking other cell cycle regulators whose expression could be regulated by p120E4F, we found that expression of p120E4F leads to cyclin A downregulation. Consistent with this, endogenous cyclin A was not expressed in p120E4F-expressing cells. This repression was also observed with a reporter containing the cyclin A promoter and required direct binding of p120E4F to a CRE-E4F site found in it. Interestingly, p120E4F alleviated the activity of a cyclin A promoter construct mutated on the CCRE when expressed in quiescent cells. These data identify cyclin A as the first established cellular target gene for p120E4F. In addition, the fact that the p120E4F-dependent G1 block can be specifically released by overexpression of cyclin A identifies cyclin A as a potential mediator of p120E4F-induced cell cycle arrest.
Cyclin A expression is repressed in E4F-expressing cells.
Our data clearly show that p120E4F directly binds to the CRE site of the cyclin A promoter and, as a result, represses its activity. These results contrast with previous data indicating that cyclin A mRNA concentrations remained unchanged in CMV-driven p120E4F-overexpressing cell lines (12). However, it is worth mentioning that contrary to Fernandes et al. (12), we managed to generate p120E4F cell lines only under conditions of tetracycline-dependent repression of E4F expression, suggesting that p120E4F overexpression operated a counter selection of E4F-positive clones, which could be easily explained by the role of p120E4F in cell cycle arrest.
Interestingly, we also observed transcriptional repression of the cyclin A gene and cell cycle arrest with a p50E4F-like protein which contains the first 358 amino acids of E4F (unpublished results), which include E4F-DNA binding and dimerization domains (38). Indeed, a processed form of E4F, with a molecular mass of 50 kDa, that is generated by proteolytic cleavage of p120E4F has been characterized (11). However, the cellular stimuli that may regulate this process as well as the exact residues corresponding to the E4F proteolytic product have not been determined. Therefore, although our data clearly show that both DNA binding and transcriptional repression are supported by the amino-terminal domain of p120E4F (aa 1 to 358), determination of their biological relevance awaits further characterization of E4F proteolytical process.
Cyclin A is a mediator of p120E4F-dependent cell cycle arrest in G1.
Interestingly, although both cyclin E and cyclin A control the activity of CDK2 and are rate limiting for progression from G1 to S phase (20, 26), only cyclin A appeared to mediate E4F-dependent G1 arrest. This contrasts with the p21-dependent block of DNA replication initiation in Xenopus extracts which could be overcome by either cyclin (43) and thus supports our conclusion that stabilization of p21 is not the primary cause of the E4F-dependent G1 arrest.
Because p53 was recently reported to associate with p120E4F and be involved in p120E4F-induced growth arrest (39), we tested the p120E4F-dependent cyclin A transcriptional regulation in p53-deficient cells. Interestingly, we found similar rates of cyclin A transcriptional repression in p53−/− and WT MEFs. The apparent discrepancy between our results and those of Sandy et al. (39) could be partly strain dependent, as we used strains with distinct genetic backgrounds (8, 17). In addition, the previous finding that the inhibition of p120E4F-dependent colony formation was independent of p53 (12) is in agreement with our own data. Alternatively, p53 may be specifically required for p120E4F-dependent cell cycle arrest under conditions that normally induce p53 (14, 28).
The CRE site of the cyclin A promoter is the target of distinct signaling pathways.
The core of the cyclin A CRE-E4F site is also recognized by CREB and ATF family members (2, 7, 21), which raises the question of how access of p120E4F to this site and subsequent transcriptional regulation are controlled. Previous reports suggested that E4F could bind its DNA recognition site with higher stability than ATF factors, thus favoring E4F DNA binding (37). Other mechanisms such as posttranslational modification of the proteins or association with unidentified factors could also favor DNA binding. Phosphorylation of CREB and ATF proteins at serines 133 and 63, respectively, has been shown to enhance DNA binding and induce stable interaction with CREB binding protein in response to stimuli as diverse as stress, treatment with growth factors, or induction of differentiation (22, 24, 25, 41).
As previously reported (11), we observed that both GST-p120E4F fusion protein and p120E4F cellular extracts displayed a reduced apparent DNA binding affinity compared to the N-terminally truncated protein (aa 1 to 358) (data not shown). This induced DNA binding upon partial E4F proteolysis is reminiscent of the Ets-1 mechanism of activation (6) and suggests that posttranslational modifications of p120E4F, such as phosphorylation, could release E4F DNA binding from autoinhibition. This hypothesis is supported by the fact that p120E4F binding to DNA is phosphatase sensitive (11), that E4F phosphorylation is induced in E1A-expressing cells (11, 13, 34), and that φAP3, the murine homolog of p120E4F, exhibited variation in its rate of phosphorylation upon exposure to various cellular stimuli (13). However, the specific stimuli that can promote E4F phosphorylation and therefore cyclin A downregulation in non infected cells remain to be identified.
Auxiliary proteins like HMI(Y) or basic leucine zipper enhancing factor have been shown to enhance DNA binding of basic leucine zipper containing factors, i.e. CREB and ATF, that like E4F form dimeric protein-DNA (2:1) complexes (9, 38, 44). Such factors, which increase the stability of dimers versus monomers and thus favor kinetics of DNA binding of the dimer, can be envisioned as molecular chaperones (5). Such could also be the role of pRB, as suggested by the reported association between pRB and E4F (10) and the enhanced p120E4F-DNA binding on the cyclin A CRE-E4F site observed in the presence of pRB, as shown here.
In summary, this work establishes cyclin A as a mediator of p120E4F-dependent cell cycle regulation. Our future goals will be to determine the signaling cascades converging on the CRE-E4F site of the cyclin A promoter and to identify other genes that may also be regulated by E4F and contribute to its major role in cell cycle control.
ACKNOWLEDGMENTS
We thank R. Hipskind, A. Le Cam, and V. Coulon for critical comments on the manuscript.
This work was funded by grants from CNRS (ATIPE 3), l'Association pour le Recherche contre le Cancer, La Ligue Contre le Cancer, and the Human Frontier Science Program. L.F. was supported by a EEC/TMR postdoctoral fellowship.
REFERENCES
- 1.Barlat I, Fesquet D, Brechot C, Henglein B, Dupuy d'Angeac A, Vie A, Blanchard J M. Loss of the G1-S control of cyclin A expression during tumoral progression of Chinese hamster lung fibroblasts. Cell Growth Differ. 1993;4:105–113. [PubMed] [Google Scholar]
- 2.Barlat I, Henglein B, Plet A, Lamb N, Fernandez A, McKenzie F, Pouyssegur J, Vie A, Blanchard J M. TGF-beta 1 and cAMP attenuate cyclin A gene transcription via a cAMP responsive element through independent pathways. Oncogene. 1995;11:1309–1318. [PubMed] [Google Scholar]
- 3.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 4.Chambard J C, Pognonec P. A reliable way of obtaining stable inducible clones. Nucleic Acids Res. 1998;26:3443–3444. doi: 10.1093/nar/26.14.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin J W, Kohler J J, Schneider T L, Schepartz A. Protein escorts to the transcription ball. Curr Biol. 1999;9:R929–R932. doi: 10.1016/s0960-9822(00)80107-4. [DOI] [PubMed] [Google Scholar]
- 6.Cowley D O, Graves B J. Phosphorylation represses ets-1 DNA binding by reinforcing autoinhibition. Genes Dev. 2000;14:366–376. [PMC free article] [PubMed] [Google Scholar]
- 7.Desdouets C, Matesic G, Molina C A, Foulkes N S, Sassone-Corsi P, Brechot C, Sobczak-Thepot J. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol Cell Biol. 1995;15:3301–3309. doi: 10.1128/mcb.15.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 9.Du W, Maniatis T. The high mobility group protein HMG I(Y) can stimulate or inhibit DNA binding of distinct transcription factor ATF-2 isoforms. Proc Natl Acad Sci USA. 1994;91:11318–11322. doi: 10.1073/pnas.91.24.11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fajas L, Paul C, Zugasti O, Le Cam L, Polanowska J, Fabbrizio E, Medema R, Vignais M L, Sardet C. pRB binds to and modulates the transrepressing activity of the E1A-regulated transcription factor p120E4F. Proc Natl Acad Sci USA. 2000;97:7738–7743. doi: 10.1073/pnas.130198397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes E R, Rooney R J. The adenovirus E1A-regulated transcription factor E4F is generated from the human homolog of nuclear factor φAP3. Mol Cell Biol. 1997;17:1890–1903. doi: 10.1128/mcb.17.4.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes E R, Zhang J Y, Rooney R J. Adenovirus E1A-regulated transcription factor p120E4F inhibits cell growth and induces the stabilization of the cdk inhibitor p21WAF1. Mol Cell Biol. 1998;18:459–467. doi: 10.1128/mcb.18.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fognani C, Della Valle G, Babiss L E. Repression of adenovirus E1A enhancer activity by a novel zinc finger-containing DNA-binding protein related to the GLI-Kruppel protein. EMBO J. 1993;12:4985–4992. doi: 10.1002/j.1460-2075.1993.tb06192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giaccia A J, Kastan M B. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–83. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 15.Girard F, Strausfeld U, Fernandez A, Lamb N J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 16.Hamel P A, Gill R M, Phillips R A, Gallie B L. Transcriptional repression of the E2-containing promoters EIIaE, c-myc, and RB1 by the product of the RB1 gene. Mol Cell Biol. 1992;12:3431–3438. doi: 10.1128/mcb.12.8.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey D M, Levine A J. p53 alternation is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 1991;5:2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- 18.Heichman K A, Roberts J M. Rules to replicate by. Cell. 1994;79:557–562. doi: 10.1016/0092-8674(94)90541-x. [DOI] [PubMed] [Google Scholar]
- 19.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 20.Hua X H, Yan H, Newport J. A role for Cdk2 kinase in negatively regulating DNA replication during S phase of the cell cycle. J Cell Biol. 1997;137:183–192. doi: 10.1083/jcb.137.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huet X, Rech J, Plet A, Vie A, Blanchard J M. Cyclin A expression is under negative transcriptional control during the cell cycle. Mol Cell Biol. 1996;16:3789–3798. doi: 10.1128/mcb.16.7.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iordanov M, Bender K, Ade T, Schmid W, Sachsenmaier C, Engel K, Gaestel M, Rahmsdorf H J, Herrlich P. CREB is activated by UVC through a p38/HOG-1-dependent protein kinase. EMBO J. 1997;16:1009–1022. doi: 10.1093/emboj/16.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones C, Lee K A. E1A-mediated activation of the adenovirus E4 promoter can occur independently of the cellular transcription factor E4F. Mol Cell Biol. 1991;11:4297–4305. doi: 10.1128/mcb.11.9.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kingsley-Kallesen M L, Kelly D, Rizzino A. Transcriptional regulation of the transforming growth factor-beta2 promoter by cAMP-responsive element-binding protein (CREB) and activating transcription factor-1 (ATF-1) is modulated by protein kinases and the coactivators p300 and CREB-binding protein. J Biol Chem. 1999;274:34020–34028. doi: 10.1074/jbc.274.48.34020. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Shimomura A, Hagiwara M, Kawakami K. Phosphorylation of ATF-1 enhances its DNA binding and transcription of the Na,K-ATPase alpha 1 subunit gene promoter. Nucleic Acids Res. 1997;25:877–882. doi: 10.1093/nar/25.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krude T, Jackman M, Pines J, Laskey R A. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell. 1997;88:109–119. doi: 10.1016/s0092-8674(00)81863-2. [DOI] [PubMed] [Google Scholar]
- 27.Medema R H, Herrera R E, Lam F, Weinberg R A. Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc Natl Acad Sci USA. 1995;92:6289–6293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oren M. Regulation of the p53 tumor suppressor protein. J Biol Chem. 1999;274:36031–36034. doi: 10.1074/jbc.274.51.36031. [DOI] [PubMed] [Google Scholar]
- 29.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philips A, Chambeyron S, Lamb N, Vie A, Blanchard J M. CHF: a novel factor binding to cyclin A CHR corepressor element. Oncogene. 1999;18:6222–6232. doi: 10.1038/sj.onc.1203017. [DOI] [PubMed] [Google Scholar]
- 31.Philips A, Huet X, Plet A, Le Cam L, Vie A, Blanchard J M. The retinoblastoma protein is essential for cyclin A repression in quiescent cells. Oncogene. 1998;16:1373–1381. doi: 10.1038/sj.onc.1201655. [DOI] [PubMed] [Google Scholar]
- 32.Philips A, Huet X, Plet A, Rech J, Vie A, Blanchard J M. Anchorage-dependent expression of cyclin A in primary cells requires a negative DNA regulatory element and a functional Rb. Oncogene. 1999;18:1819–1825. doi: 10.1038/sj.onc.1202530. [DOI] [PubMed] [Google Scholar]
- 33.Plet A, Huet X, Algarte M, Rech J, Imbert J, Philips A, Blanchard J M. Relief of cyclin A gene transcriptional inhibition during activation of human primary T lymphocytes via CD2 and CD28 adhesion molecules. Oncogene. 1997;14:2575–2583. doi: 10.1038/sj.onc.1201103. [DOI] [PubMed] [Google Scholar]
- 34.Raychaudhuri P, Bagchi S, Nevins J R. DNA-binding activity of the adenovirus-induced E4F transcription factor is regulated by phosphorylation. Genes Dev. 1989;3:620–627. doi: 10.1101/gad.3.5.620. [DOI] [PubMed] [Google Scholar]
- 35.Raychaudhuri P, Rooney R, Nevins J R. Identification of an E1A-inducible cellular factor that interacts with regulatory sequences within the adenovirus E4 promoter. EMBO J. 1987;6:4073–4081. doi: 10.1002/j.1460-2075.1987.tb02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rooney R J, Daniels R R, Jenkins N A, Gilbert D J, Rothammer K, Morris S W, Higgs D R, Copeland N G. Chromosomal location and tissue expression of the gene encoding the adenovirus E1A-regulated transcription factor E4F in humans and mice. Mamm Genome. 1998;9:320–323. doi: 10.1007/s003359900758. [DOI] [PubMed] [Google Scholar]
- 37.Rooney R J, Raychaudhuri P, Nevins J R. E4F and ATF, two transcription factors that recognize the same site, can be distinguished both physically and functionally: a role for E4F in E1A trans activation. Mol Cell Biol. 1990;10:5138–5149. doi: 10.1128/mcb.10.10.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rooney R J, Rothammer K, Fernandes E R. Mutational analysis of p50E4F suggests that DNA binding activity is mediated through an alternative structure in a zinc finger domain that is regulated by phosphorylation. Nucleic Acids Res. 1998;26:1681–1688. doi: 10.1093/nar/26.7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandy P, Gostissa M, Fogal V, Cecco L D, Szalay K, Rooney R J, Schneider C, Sal G D. p53 is involved in the p120E4F-mediated growth arrest. Oncogene. 2000;19:188–199. doi: 10.1038/sj.onc.1203250. [DOI] [PubMed] [Google Scholar]
- 40.Sardet C, LeCam L, Fabrizio E, Vidal M. E2Fs and the retinoblastoma protein family. In: Ghysdael J, Yaniv M, editors. Progress in gene expression/oncogenes as transcriptional regulators. 2. Cell cycle and chromosomal translocation. Basel, Switzerland: Birkhäuser Verlag; 1997. pp. 1–62. [Google Scholar]
- 41.Shaywitz A J, Greenberg M E. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 42.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 43.Strausfeld U P, Howell M, Rempel R, Maller J L, Hunt T, Blow J J. Cip1 blocks the initiation of DNA replication in Xenopus extracts by inhibition of cyclin-dependent kinases. Curr Biol. 1994;4:876–883. doi: 10.1016/s0960-9822(00)00196-2. [DOI] [PubMed] [Google Scholar]
- 44.Virbasius C M, Wagner S, Green M R. A human nuclear-localized chaperone that regulates dimerization, DNA binding, and transcriptional activity of bZIP proteins. Mol Cell. 1999;4:219–228. doi: 10.1016/s1097-2765(00)80369-x. [DOI] [PubMed] [Google Scholar]
- 45.Zindy F, Lamas E, Chenivesse X, Sobczak J, Wang J, Fesquet D, Henglein B, Brechot C. Cyclin A is required in S phase in normal epithelial cells. Biochem Biophys Res Commun. 1992;182:1144–1154. doi: 10.1016/0006-291x(92)91851-g. [DOI] [PubMed] [Google Scholar]
- 46.Zwicker J, Lucibello F C, Wolfraim L A, Gross C, Truss M, Engeland K, Muller R. Cell cycle regulation of the cyclin A, cdc25C and cdc2 genes is based on a common mechanism of transcriptional repression. EMBO J. 1995;14:4514–4522. doi: 10.1002/j.1460-2075.1995.tb00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]