Abstract
Background: Alzheimer's disease (AD) is a progressive and multifactorial neurodegenerative disease accounting for 80% of dementia worldwide.
Objective: To assess the influence of probiotics on cognitive function in patients with mild cognitive impairment (MCI) and AD.
Methods: PubMed, Embase, and Cochrane Library databases were searched for relevant studies.
Results: Six randomized controlled trials involving 462 patients with MCI and AD were included in this meta-analysis. The probiotic administration had favorable effects on homeostasis model assessment–insulin resistance [HOMA-IR; Weighted mean difference (WMD) = −0.34, 95% confidence intervals (95% CI): −0.44 to 0.24, P < 0.001, I2 = 0%], very low–density lipoprotein levels (VLDL; WMD = −3.71, 95% CI: −6.11 to −1.32, P=0.002, I2 = 57.7%), quantitative insulin sensitivity check index (QUICKI; WMD = 0.01, 95% CI: 0.00–0.01, P = 0.003, I2 = 51%), and triglyceride levels (WMD = −15.65, 95% CI: −27.48 to −3.83, P = 0.009, I2 = 63.4%) in patients with AD. However, after Hartung-Knapp adjustment, all effects were non-significant except for HOMA-IR (MD = −0.34, 95%CI = −0.58 to −0.11). The changes in the Mini-Mental State Examination, repeatable battery for the assessment of neuropsychological status, and other biomarkers of oxidative stress, inflammation, and lipid profiles (high-sensitivity C-reactive protein, malondialdehyde, and total cholesterol) were negligible.
Conclusion: The findings suggested that the consumption of probiotics had favorable effects on the HOMA-IR in patients with AD. However, the probiotic treatment did not affect cognitive function, other biomarkers of oxidative stress, and other lipid profiles.
Keywords: Alzheimer's disease, biomarker, meta-analysis, mild cognitive impairment, probiotic, efficacy
Introduction
Alzheimer's disease (AD) is a progressive and multifactorial neurodegenerative disease that accounts for 80% of dementia globally, especially in people over 60 years of age. Clinically, AD is characterized by severe impairments in memory, cognition, and motor function, resulting in decreased mental, behavioral, and functional activities that affect the quality of daily life (1). Contrary to AD where other cognitive skills and the ability to live independently are affected, mild cognitive impairment (MCI) is characterized by deficits in memory that do not significantly impact daily functioning (2). According to the Epidemiological Survey of the Global Burden of Disease Study 2016, ~43.8 million people worldwide had AD in 2016 (3). According to the prediction of the world Alzheimer's report 2015, the total number of patients with AD will reach 74.7 million by 2030 and 131.5 million by 2050, posing a global health challenge (4).
Intestinal microbiota is a group of microorganisms found in the gastrointestinal tract, which plays a key role in anatomy, physiology, and immune host function (5, 6). During the aging process, the composition and function of intestinal microbiota change significantly, which may affect health and cause age-related diseases (7, 8). Recently, the concept of gut–brain axis has emerged, which refers to the two-way relationship between gut and brain, linking gut microbiota with age-related neurodegenerative diseases such as AD (9–11).ssss The interaction between gut and brain involves a complex network of endocrine, immune, and neurotransmitters, which is considered a key target for treating brain health and neurodegenerative diseases (12–14).
In recent years, several studies have evaluated the effect of probiotics in MCI and AD (15–20). Although some researchers have reported that probiotic supplementation can improve cognitive function, the relevant data are still rare, and the results of various studies are inconsistent. Therefore, the purpose of the present meta-analysis was to provide quantitative results of the effect of probiotics on the cognitive function in patients with MCI and AD.
Materials and Methods
Literature Search
This meta-analysis was prepared and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines (21). PubMed, Embase, and Cochrane Library databases were used to browse and search for reports on the effect of probiotics in patients with MCI or AD. The search focused on studies involving human participants and published in English before February 14, 2021. The references of the identified research and review papers were manually explored to avoid missing any relevant studies. The following keywords and medical terms were used alone or in combination: (probiotics OR probiotic) AND (mild cognitive impairment OR MCI) AND (Alzheimer's disease OR AD). A detailed search string for PubMed is presented in Supplementary Table 1.
Selection Criteria
Two independent authors reviewed all relevant studies. Any disagreements were resolved by consensus with a third investigator. The inclusion criteria were as follows: (bib1) randomized controlled trials (RCTs) including patients with MCI and AD; (2) patients in the trial group treated by probiotics and patients in the control group using no probiotic interventions; (3) the mean and standard deviation provided for at least one outcome; and (4) the study with the largest sample size selected for inclusion if more than one study was published with the same patients. The exclusion criteria were as follows: (1) letters, reviews, abstracts and case reports; (2) non-randomized trials; (3) experimental studies of animal models or cell lines; (4) studies published in languages other than English; and (5) lack of accurate data.
Data Extraction and Quality Assessment
The following data were extracted from the selected studies: study author, year of publication, study design, country in which the study was conducted, sex, mean age, intervention, duration of probiotic therapy, and outcome of the evaluation. The authors used the Cochrane Collaboration tool (22) to independently assess the risk of bias as “low,” “high,” or “unclear” in terms of performance bias, selection bias, detection bias, reporting bias, consumption bias, and other biases. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (23) to assess the certainty of the evidence. Data extraction and quality assessment were performed by two independent authors. Any disagreements were resolved by consensus with a third investigator.
Statistical Analyses
All statistical analyses were performed using Stata 16 software (Stata Corporation, TX, USA). Weighted mean difference (WMD) was used to measure data. Heterogeneity was calculated using Higgins (I2) test statistics. I2 values 0–25% indicated no heterogeneity, 26–50% indicated low heterogeneity, 51–75% indicated medium heterogeneity, and more than 75% indicated high heterogeneity (24). In case of significant heterogeneity (P < 0.10 or I2 > 50%), a random-effects model was used. Otherwise, the fixed-effects model was used. Since the number of included studies was small and the heterogeneity was high, Hartung-Knapp adjustment was used to produce more robust estimates of variance (25). In addition, funnel plots and Begg's and Egger's tests were used to assess the publication bias (26). A P < 0.05 indicated a significant difference.
Results
Literature Search and Study Characteristics
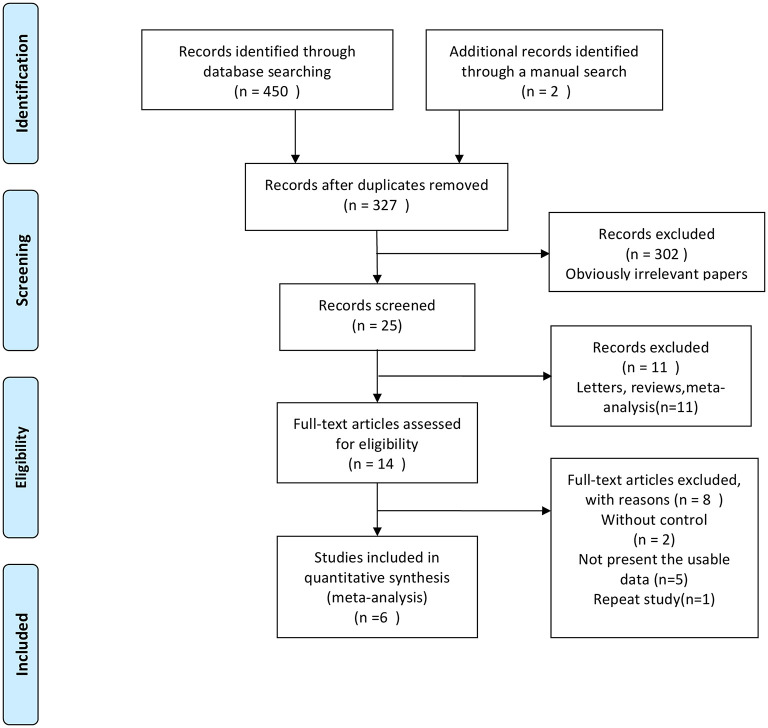
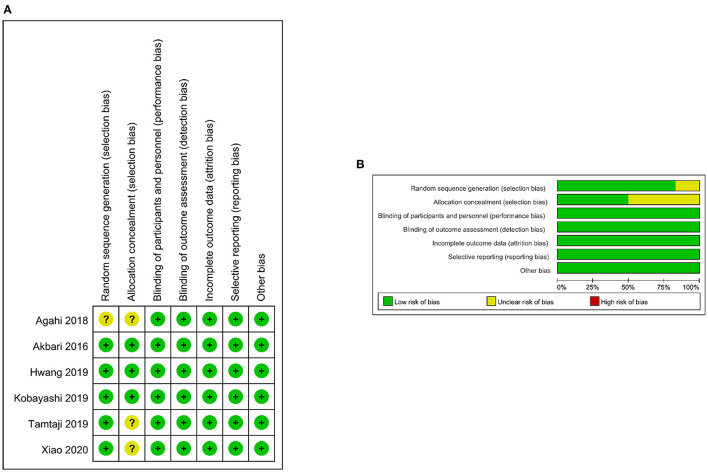
A flowchart describing the study selection process is shown in Figure 1. Six studies (15–20) were included in the present meta-analysis. The eligible studies were published between 2016 and 2020, with a total sample of 462. The average age of the participants ranged from 60.9 to 82 years. The main features of the included studies are shown in Table 1. All six studies were randomized, with most (n = 5) describing randomization methods. Half of the studies (n = 3) did not describe the method of allocation concealment in enough detail. In general, all studies were of high quality (Figure 2). The GRADE assessment of the certainty of the evidence showed that almost all endpoints estimates were derived from moderate quality evidence, and only one had low quality evidence (Table 2).
Figure 1.
Flow diagram of the study selection process.
Table 1.
Characteristics of the studies included in this meta-analysis.
| References | Country | Sex (Male%) | Mean age | Intervention | Duration of probiotic therapy | Study design | Outcomes assessed | |
|---|---|---|---|---|---|---|---|---|
| Exp | Con | |||||||
| Akbari et al. (16) | Iran | 20% | Exp: 77.67 ± 2.62 Con: 82 ± 1.69 |
Milk containing a mixture of probiotics (N = 30) |
Milk (N = 30) | 12 w | RCT | High-sensitivity C-reactive protein, homeostasis model assessment–insulin resistance, MMSE, plasma malondialdehyde, plasma triglycerides, quantitative insulin sensitivity check index, total cholesterol, and very low–density lipoprotein |
| Agahi et al. (15) | Iran | 35.4% | Exp: 79.7 ± 1.72 Con: 80.57 ± 1.79 |
A mixture of probiotic bacteria (N = 25) |
Placebo (N = 23) | 12 w | RCT | Plasma malondialdehyde |
| Hwang et al. (17) | Korea | 34% | Exp: 68 ± 5.12 Con: 69.2 ± 7 |
DW2009 (N = 50) |
Placebo (N = 50) | 12 w | RCT | Total cholesterol |
| Kobayashi et al. (18) | Japan | 49.6% | Exp: 61.5 ± 6.83 Con: 61.6 ± 6.37 |
Bifidobacterium breve A1 (N = 61) |
Placebo (N = 60) | 12 w | RCT | High-sensitivity C-reactive protein, MMSE, plasma triglycerides, RBANS, and total cholesterol |
| Tamtaji et al. (19) | Iran | NA | Exp: 76.2 ± 8.1 Con: 78.8 ± 10.2 |
Probiotic plus selenium (N = 27) |
Selenium (N = 26) | 12 w | RCT | High-sensitivity C-reactive protein, homeostasis model assessment–insulin resistance, MMSE, plasma malondialdehyde, plasma triglycerides, quantitative insulin sensitivity check index, total cholesterol, and very low–density lipoprotein |
| Xiao et al. (20) | Japan | 48.7% | Exp: 61.3 ± 7.7 Con: 60.9 ± 6.9 |
Probiotic (N = 40) |
Placebo (N = 40) | 16 w | RCT | RBANS |
Con, Controls; Exp, Experimental; MMSE, Mini-Mental State Examination; NA, not available; RBANS, repeatable battery for the assessment of neuropsychological status; RCT, randomized controlled trial; w, weeks.
Figure 2.
Risk-of-bias assessment for the randomized trials included in the meta-analysis. (A) Risk-of-bias summary. (B) Risk-of-bias graph. Symbols: (+), low risk of bias; (?), unclear risk of bias; (−), high risk of bias.
Table 2.
Summary of findings table.
| Effect of probiotic supplementation on cognitive function and metabolic status in MCI and AD | ||||||
|---|---|---|---|---|---|---|
|
Patient or population: patients with MCI and AD Settings: outpatient Intervention: probiotic supplementation Comparison: no probiotic interventions | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) |
No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No probiotic interventions | Probiotic supplementation | |||||
|
Mini-Mental State Examination Follow-up: 0–12 weeks |
The mean mini-mental state examination in the control groups was 0.6 |
The mean mini-mental state examination in the intervention groups was 1.08 higher (0.34 lower to 2.5 higher) |
230 (3 studies) |
⊕⊕⊕⊖ moderatea |
||
|
Repeatable battery for the assessment of neuropsychological status Follow-up: 0–15 weeks |
The mean repeatable battery for the assessment of neuropsychological status in the control groups was 5.3 |
The mean repeatable battery for the assessment of neuropsychological status in the intervention groups was 6.01 higher (4.85 lower to 16.86 higher) |
196 (2 studies) |
⊕⊕⊕⊖ moderatea |
||
|
High-sensitivity C-reactive protein Follow-up: 0–12 weeks |
The mean high-sensitivity c-reactive protein in the control groups was 0.4 |
The mean high-sensitivity c-reactive protein in the intervention groups was 1.31 lower (3.31 lower to 0.69 higher) |
234 (3 studies) |
⊕⊕⊕⊖ moderatea |
||
|
Homeostasis model assessment–insulin resistance Follow-up: 0–12 weeks |
The mean homeostasis model assessment–insulin resistance in the control groups was 0.2 |
The mean homeostasis model assessment–insulin resistance in the intervention groups was 0.34 lower (0.44 lower to 0.24 higher) |
113 (2 studies) |
⊕⊕⊕⊖ moderatea |
||
|
Malondialdehyde Follow-up: 0–12 weeks |
The mean malondialdehyde in the control groups was −0.07 |
The mean malondialdehyde in the intervention groups was 0.5 lower (1.1 lower to 0.1 higher) |
161 (3 studies) |
⊕⊕⊖⊖ lowa, b |
||
|
Triglycerides Follow-up: 0–12 weeks |
The mean triglycerides in the control groups was −2.5 |
The mean triglycerides in the intervention groups was 15.65 lower (27.48 to 3.83 lower) |
234 (3 studies) |
⊕⊕⊕⊖ moderatea |
||
|
Total cholesterol Follow-up: 0–12 weeks |
The mean total cholesterol in the control groups was −4.8 |
The mean total cholesterol in the intervention groups was 0.05 higher (0.29 lower to 0.39 higher) |
326 (4 studies) |
⊕⊕⊕⊖ moderatea |
||
|
Very low–density lipoprotein Follow-up: mean 0–12 weeks |
The mean very low–density lipoprotein in the control groups was −0.6 |
The mean very low–density lipoprotein in the intervention groups was 3.71 lower (6.11 to 1.32 lower) |
113 (2 studies) |
⊕⊕⊕⊖ moderatea |
||
|
Quantitative insulin sensitivity check index Follow-up: 0–12 weeks |
The mean quantitative insulin sensitivity check index in the control groups was −0.0075 |
The mean quantitative insulin sensitivity check index in the intervention groups was 0.01 higher (0 to 0.01 higher) |
113 (2 studies) |
⊕⊕⊕⊖ moderatea |
||
The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval.
GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate.
Unclear allocation concealment.
Unclear random sequence generation method.
Quantitative Synthesis
Mini-Mental State Examination
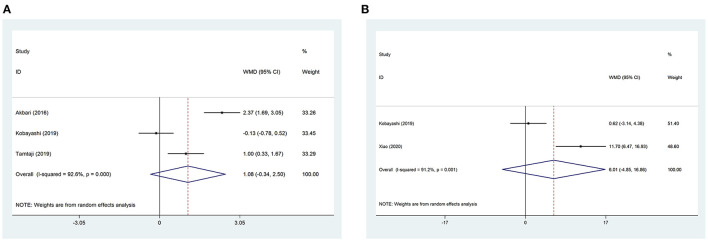
Data regarding Mini-Mental State Examination (MMSE) were reported in three studies. No significant differences were found between the trial and the control group (WMD = 1.08, 95% CI: −0.34 to 2.50, P = 0.137, I2 = 92.6%) (Figure 3A). After Hartung-Knapp adjustment due to few studies included in the analysis, the results were still non-significant (MD = 1.08, 95%CI = −2.03 to 4.19).
Figure 3.
Forest plots showing the effect of probiotic administration on cognitive function. (A) MMSE; (B) RBANS.
Repeatable Battery for the Assessment of Neuropsychological Status
Data regarding repeatable battery for the assessment of neuropsychological status (RBANS) were reported in two studies. No significant differences were found between the trial and the control group (WMD = 6.01, 95% CI: −4.85 to 16.86, P = 0.278, I2 = 91.2%) (Figure 3B). After Hartung-Knapp adjustment, non-significant differences were still shown (MD = 6.01, 95% CI = −64.36 to 76.37).
High-Sensitivity C-Reactive Protein
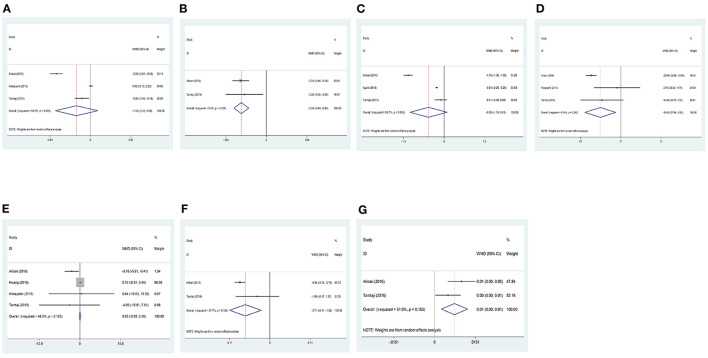
Data regarding high-sensitivity C-reactive protein (hs-CRP) were reported in three studies. No significant differences were found between the trial and the control group before Hartung-Knapp adjustment (WMD = −1.31, 95% CI: −3.31 to 0.69, P = 0.198, I2 = 98.2%) (Figure 4A), and after Hartung-Knapp adjustment (MD = −1.31, 95%CI = −5.53 to 2.91).
Figure 4.
Forest plots showing the effect of probiotic administration on the level of clinical biomarkers. (A) hs-CRP; (B) HOMA-IR; (C) MDA; (D) triglycerides; (E) total cholesterol; (F) VLDL; and (G) QUICKI.
Homeostasis Model Assessment–Insulin Resistance
Data regarding HOMA-IR were reported in two studies. The results showed that homeostasis model assessment–insulin resistance (HOMA-IR) were significantly reduced in the trial group compared with the control group (WMD = −0.34, 95% CI: −0.44 to 0.24, P < 0.001, I2 = 0%) (Figure 4B). However, after Hartung-Knapp adjustments, no significant differences were seen between the two groups (MD = −0.34, 95%CI = −0.58 to −0.11).
Malondialdehyde
Data regarding malondialdehyde (MDA) were reported in three studies. No significant differences were found between the trial and the control group before Hartung-Knapp adjustment (WMD = −0.50, 95% CI: −1.10 to 0.10, P = 0.101, I2 = 98.7%) (Figure 4C), and after Hartung-Knapp adjustment (MD = −0.5, 95%CI = −1.92 to 0.92).
Triglycerides
Data regarding triglycerides were reported in two studies. The results showed that triglyceride levels were significantly reduced in the trial group compared with the control group (WMD = −15.65, 95% CI: −27.48 to −3.83, P = 0.009, I2 = 63.4%) (Figure 4D). However, after Hartung-Knapp adjustment, the results became non-significant (MD = −15.73, 95%CI = −40.31 to 8.84).
Total Cholesterol
Data regarding total cholesterol were reported in three studies. No significant differences were found between the trial and the control group before Hartung-Knapp adjustment (WMD = 0.05, 95% CI: −0.29 to 0.39, P = 0.788, I2 = 48%) (Figure 4E), and after Hartung-Knapp adjustment (MD = −1.22, 95%CI = −4.3 to 1.86).
Very Low–Density Lipoprotein (VLDL)
Data regarding VLDL were reported in two studies. The results showed that VLDL levels were significantly reduced in the trial group compared with the control group (WMD = −3.71, 95% CI: −6.11 to −1.32, P = 0.002, I2 = 57.7%) (Figure 4F). However, non-significant differences were observed between the two groups after Hartung-Knapp adjustment (MD = −3.71, 95%CI = −19.26 to 11.83).
Quantitative Insulin Sensitivity Check Index
Data regarding quantitative insulin sensitivity check index (QUICKI) were reported in two studies. QUICKI was significantly increased in the trial group compared with the control group (WMD = 0.01, 95% CI: 0.00–0.01, P = 0.003, I2 = 51%) (Figure 4G), but no significant differences were seen after Hartung-Knapp adjustment (MD = −0.01, 95%CI = −0.02 to 0.04).
Publication BiasN
Publication bias across the included studies was assessed using the funnel plot (Figure 5) and Begg's and Egger's tests. The results for total cholesterol (Begg's test P = 1.000, Egger's test P = 0.342) showed no evidence of publication bias.
Figure 5.
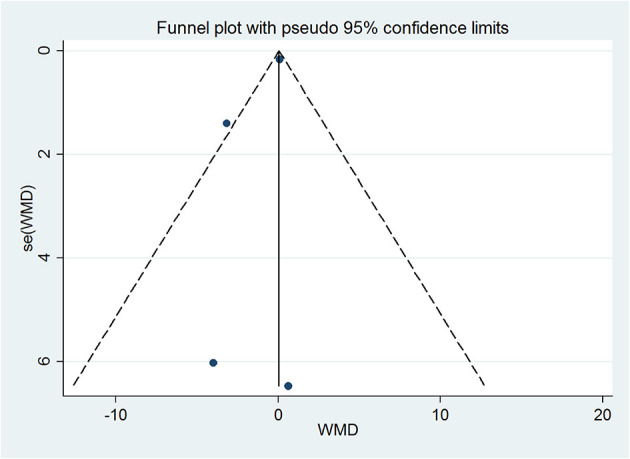
Funnel plot for publication bias test of total cholesterol. Each point represents a separate study for the indicated association.
Discussion
All included studies were RCTs, which further improved the preciseness and credibility of this meta-analysis. The present study revealed that the probiotic administration had favorable effects on HOMA-IR, VLDL, QUICKI, and triglyceride levels in the patients with AD without Hartung-Knapp adjustment. However, the changes in MMSE, RBANS, and other biomarkers of oxidative stress, inflammation, and lipid profiles (hs-CRP, MDA, and total cholesterol) were negligible.
Recently, Tamtaji et al. (27) conducted a systematic review and meta-analysis to evaluate the effects of probiotic supplementation on metabolic status in patients with neurological disorders (including AD, parkinson's disease, multiple sclerosis, and migraine). Tamtaji et al. (27) demonstrated beneficial effects of probiotics on CRP, MDA, insulin, HOMA-IR, triglyceride, VLDL cholesterol, and HDL cholesterol levels in patients with neurological diseases. Compared with the study by Tamtaji et al. (27), we only focused on patients with MCI and AD. The strengths of our meta-analysis included a comprehensive search strategy, supplemented by manual search, bias risk assessment and GRADE certainty of evidence assessment for each outcome. In addition, we used Hartung-Knapp adjustment to provide conservative summary estimates with wide confidence intervals.
The intestinal microbiota is an ecosystem formed by a broad range of symbiotic communities of non-pathogenic microorganisms that exist in the distal end of the human intestine. Gut flora plays an important role in the normal physiological functions of organisms (28). The imbalance of intestinal microbiota, that is, intestinal disorders, is directly related to the origin of various acute and chronic dysfunction processes in the host (29). Therefore, intervention in the gut microbiota is becoming a possible strategy for the treatment of various diseases such as heart failure (HF) (30). Recent preclinical and clinical studies have emphasized the key role of the gut microbiota in cardiovascular health, especially HF prognosis (31). Some probiotic strains (particularly Lactobacillus, Bifidobacterium, and Saccharomyces boulardii) can be used as adjuvants for conventional treatment of HF (30).
Dementia, especially AD, is a chronic and progressive syndrome that takes more than 20 years to manifest into cognitive impairment (32). Therefore, finding safe drugs to prevent dementia has become a subject of increasing interest. Previous studies showed that probiotic supplementation had beneficial effects on the cognitive function of patients with AD (16), so it might be developed as a dietary supplement for MCI. Dietary habits and regular consumption of functional foods prior to the onset of AD may be beneficial to health and reduce the risk of the disease. The present study showed no significant difference in cognitive function between the trial group and the control group. The results were consistent with those of the study by Krüger et al. (33). Moreover, Ticinesi et al. (34) found that although several observational and interventional studies in animal models of Alzheimer's disease supported the concept of gut–brain regulation of cognitive symptoms, the lack of human data prevented any clinical recommendations on this topic. The effect of probiotics on MCI and moderate AD needs further exploration.
Patients with AD are prone to a variety of complications, such as increased inflammatory markers and oxidative damage, mortality (35), microvascular disease, dyslipidemia, and insulin resistance (36). Current research showed that probiotics could significantly improve HOMA-IR, QUICKI, and triglyceride and VLDL levels in patients with AD, but had no effect on hs-CRP, MDA, and total cholesterol levels. New evidence suggests that brain insulin resistance, as a key mediator of prediabetes and diabetes, might play a role in AD (37). Insulin resistance is important in the development of cognitive dysfunction in elderly patients with essential hypertension (38). On the contrary, the role of lipids in the pathogenesis and progression of AD remains unclear. In a meta-analysis conducted by Kasińska et al. (38), probiotics significantly reduced the HbA1c levels and HOMA-IR in patients with type 2 diabetes, but had no significant effect on fasting glucose, insulin, and lipid profiles. Insulin simulation and lipid-lowering effects of probiotic supplements might be mediated by reducing oxidative stress and pro-inflammatory markers (39), and increasing B-oxidation of long-chain fatty acids in liver and muscle tissue (40). Probiotics can regulate serum lipoproteins by increasing the activities of cholesterol 7α-hydroxylase (CYP7α1), liver X receptor α and CYP7α1 enzyme. This may be the mechanism by which they reduce total cholesterol and triglycerides, increase the production of short-chain fatty acids, and regulate the expression of lipid and sugar genes of glucose-6-polyphosphatase and glucose transporter (40, 41).
This meta-analysis had some limitations that might have affected the interpretation of the results. First, as a protocol has not been pre-registered for this meta-analysis, it may introduce potential bias. Second, the heterogeneity in this study was significant. Due to the small number of studies, subgroup and meta-regression analyses were not possible to be performed to explore the sources of heterogeneity. Third, the number of human participants was limited. Some data were used for two trials, which might lead to bias. Finally, the evaluation of cognitive function lacked consistency and objectivity, and more accurate methods were needed for evaluation.
Conclusion
In conclusion, the present study demonstrated that the consumption of probiotics had favorable effects on the HOMA-IR in patients with AD. However, the probiotic treatment did not affect cognitive function, other biomarkers of oxidative stress, and other lipid profiles. More randomized controlled trials are warranted to produce robust results and definite conclusions.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
XL, CL, and JS contributed to conception and design of the study. XL organized the database. CL performed the statistical analysis. JS wrote the first draft of the manuscript. XL, CL, JS, and JL wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by Key Science and Technology Research Program for Medical Science Research in Hebei Province (20190215).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank all study participants who were enrolled in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.757673/full#supplementary-material
References
- 1.Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer's disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomed. (2019) 14:5541. 10.2147/IJN.S200490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busse A, Angermeyer MC, Riedel-Heller SG. Progression of mild cognitive impairment to dementia: a challenge to current thinking. Br J Psychiatry. (2006) 189:399–404. 10.1192/bjp.bp.105.014779 [DOI] [PubMed] [Google Scholar]
- 3.Nichols E, Szoeke CE, Vollset SE. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:88–106. 10.1016/S1474-4422(18)30403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesika P, Suganthy N, Sivamaruthi BS, Chaiyasut C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer's disease. Life Sci. (2021) 264:118627. 10.1016/j.lfs.2020.118627 [DOI] [PubMed] [Google Scholar]
- 5.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. (2003) 361:512–9. 10.1016/S0140-6736(03)12489-0 [DOI] [PubMed] [Google Scholar]
- 6.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. (2016) 16:341–52. 10.1038/nri.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Toole PW, Jeffery IB. Gut microbiota and aging. Science. (2015) 350:1214–5. 10.1126/science.aac8469 [DOI] [PubMed] [Google Scholar]
- 8.Vaiserman AM, Koliada AK, Marotta F. Gut microbiota: a player in aging and a target for anti-aging intervention. Ageing Res Rev. (2017) 35:36–45. 10.1016/j.arr.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 9.Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res. (2017) 179:223–44. 10.1016/j.trsl.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 10.Seo D-O, Holtzman DM. Gut microbiota: from the forgotten organ to a potential key player in the pathology of Alzheimer's disease. J Gerontol Ser A. (2020) 75:1232–41. 10.1093/gerona/glz262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. (2016) 167:915–32. 10.1016/j.cell.2016.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin. (2017) 46:77–89. 10.1016/j.gtc.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 13.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. (2014) 817:115–33. 10.1007/978-1-4939-0897-4_5 [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Baptista LC, Roberts LM, Jumbo-Lucioni P, McMahon LL, Buford TW, et al. The gut microbiome as a therapeutic target for cognitive impairment. J Gerontol Ser A Biol Sci Med Sci. (2020) 75:1242–50. 10.1093/gerona/glz281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agahi A, Hamidi GA, Daneshvar R, Hamdieh M, Soheili M, Alinaghipour A, et al. Does severity of Alzheimer's disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front Neurol. (2018) 9:662. 10.3389/fneur.2018.00662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. (2016) 8:256. 10.3389/fnagi.2016.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang YH, Park S, Paik JW, Chae SW, Kim DH, Jeong DG, et al. Efficacy and safety of Lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: a 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients. (2019) 11:305. 10.3390/nu11020305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi Y, Kuhara T, Oki M, Xiao JZ. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benef Microbes. (2019) 10:511–20. 10.3920/BM2018.0170 [DOI] [PubMed] [Google Scholar]
- 19.Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: a randomized, double-blind, controlled trial. Clin Nutr. (2019) 38:2569–75. 10.1016/j.clnu.2018.11.034 [DOI] [PubMed] [Google Scholar]
- 20.Xiao J, Katsumata N, Bernier F, Ohno K, Yamauchi Y, Odamaki T, et al. Probiotic Bifidobacterium breve in improving cognitive functions of older adults with suspected mild cognitive impairment: a randomized, double-blind, placebo-controlled trial. J Alzheimer's Dis JAD. (2020) 77:139–47. 10.3233/JAD-200488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. (2001) 20:3875–89. 10.1002/sim.1009 [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamtaji OR, Milajerdi A, Reiner Ž, Asemi Z, Dadgostar E, Heidari-Soureshjani R, et al. A systematic review and meta-analysis: the effects of probiotic supplementation on metabolic profile in patients with neurological disorders. Complement Ther Med. (2020) 53:102507. 10.1016/j.ctim.2020.102507 [DOI] [PubMed] [Google Scholar]
- 28.Rinaldi E, Consonni A, Guidesi E, Elli M, Mantegazza R, Baggi F. Gut microbiota and probiotics: novel immune system modulators in myasthenia gravis? Ann N Y Acad Sci. (2018) 1413:49–58. 10.1111/nyas.13567 [DOI] [PubMed] [Google Scholar]
- 29.Vasquez EC, Pereira T, Peotta VA, Baldo MP, Campos-Toimil M. Probiotics as beneficial dietary supplements to prevent and treat cardiovascular diseases: uncovering their impact on oxidative stress. Oxid Med Cell Longev. (2019) 2019:3086270. 10.1155/2019/3086270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cicero AF, Colletti A, von Haehling S, Vinereanu D, Bielecka-Dabrowa A, Sahebkar A, et al. Nutraceutical support in heart failure: a position paper of the International Lipid Expert Panel (ILEP). Nutr Res Rev. (2020) 33:155–79. 10.1017/S0954422420000049 [DOI] [PubMed] [Google Scholar]
- 31.Nagatomo Y, Tang WW. Intersections between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail. (2015) 21:973–80. 10.1016/j.cardfail.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack CR., Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. (2010) 9:119–28. 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krüger JF, Hillesheim E, Pereira A, Camargo CQ, Rabito EI. Probiotics for dementia: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. (2021) 79:160–70. 10.1093/nutrit/nuaa037 [DOI] [PubMed] [Google Scholar]
- 34.Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. (2018) 13:1497–511. 10.2147/CIA.S139163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schelke MW, Hackett K, Chen JL, Shih C, Shum J, Montgomery ME, et al. Nutritional interventions for Alzheimer's prevention: a clinical precision medicine approach. Ann N Y Acad Sci. (2016) 1367:50–6. 10.1111/nyas.13070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sridhar GR, Lakshmi G, Nagamani G. Emerging links between type 2 diabetes and Alzheimer's disease. World J Diabetes. (2015) 6:744–51. 10.4239/wjd.v6.i5.744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cervellati C, Wood PL, Romani A, Valacchi G, Squerzanti M, Sanz JM, et al. Oxidative challenge in Alzheimer's disease: state of knowledge and future needs. J Investig Med. (2016) 64:21–32. 10.1136/jim-2015-000017 [DOI] [PubMed] [Google Scholar]
- 38.Kasińska MA, Drzewoski J. Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol Arch Med Wewn. (2015) 125:803–13. 10.20452/pamw.3156 [DOI] [PubMed] [Google Scholar]
- 39.Hsieh FC, Lee CL, Chai CY, Chen WT, Lu YC, Wu CS. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr Metab. (2013) 10:35. 10.1186/1743-7075-10-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Z, Mu C, Chen Y, Zhu Z, Chen C, Lan L, et al. Effects of dietary probiotic supplementation on LXRα and CYP7α1 gene expression, liver enzyme activities and fat metabolism in ducks. Br Poult Sci. (2015) 56:218–24. 10.1080/00071668.2014.1000821 [DOI] [PubMed] [Google Scholar]
- 41.Miraghajani M, Dehsoukhteh SS, Rafie N, Hamedani SG, Sabihi S, Ghiasvand R. Potential mechanisms linking probiotics to diabetes: a narrative review of the literature. São Paulo Medical J. (2017) 135:169–78. 10.1590/1516-3180.2016.0311271216 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.