Abstract
Shikonin is one of the major bioactive components of Lithospermum erythrorhizon. It has a good killing effect in a variety of tumor cells. Its antitumor effect involves multiple targets and pathways and has received extensive attention and study in recent years. In this review, we systematically review recent progress in determining the antitumor mechanism of shikonin and its derivatives, specifically their induction of reactive oxygen species production, inhibition of EGFR and PI3K/AKT signaling pathway activation, inhibition of angiogenesis and induction of apoptosis and necroptosis. We also discuss the application of nanoparticles loaded with shikonin in the targeted therapy of various cancers. Finally, we suggest new strategies for the clinical application of shikonin and its derivatives.
Keywords: shikonin, antitumor, apoptosis, reactive oxygen species, nanoparticles
Introduction
Cancer is one of the major killers in the world, and its high morbidity and mortality seriously threaten human health. Because of the infinite proliferation and metastasis of cancer cells, cancer treatment is difficult [1, 2]. Currently, the main methods of cancer treatment are surgery and chemotherapy, but they have certain limitations. Surgery is suitable for tumors that are easy to remove. For tumors that are difficult to remove, chemotherapy and immunotherapy are alternative treatments [3]. Although high-dose chemotherapy drugs are sufficient to kill tumor cells, the side effects of chemotherapy drugs and drug resistance have limited the application of chemotherapy drugs [4–7]. The latest immunotherapy is not suitable for all patients because of its specificity, and the success rate is very low [8]. Therefore, there is an urgent need to design and discover new drugs with better pharmacological properties for cancer treatment. Currently, many anticancer drugs are extracted from botanicals and structurally modified to be used in the clinical treatment of cancer [9, 10].
Lithospermum erythrorhizon is a perennial herbaceous plant, also known as “Zicao”, and is a component in traditional Chinese medicine. It is mainly distributed in Inner Mongolia, Xinjiang, Gansu, Western Tibet, and northeast China [11].. Shikonin is the main bioactive component of L. erythrorhizon extracts. Studies have shown that shikonin promotes wound healing and has antibacterial, anti-inflammatory and antitumor activity [1, 12–14]. In particular, the antitumor effect has attracted extensive attention from researchers. Research in the past 30 years has shown that shikonin is a potential anticancer drug. Numerous studies have confirmed that its antitumor mechanism involves multiple targets. These targets include inducing apoptosis, regulating signaling pathways and inducing the production of reactive oxygen species (ROS) and antitumor vascular regeneration (Figure 1). Therefore, shikonin is currently one of the research hotspots of antitumor therapy in traditional Chinese medicine [15–17].
Figure 1.
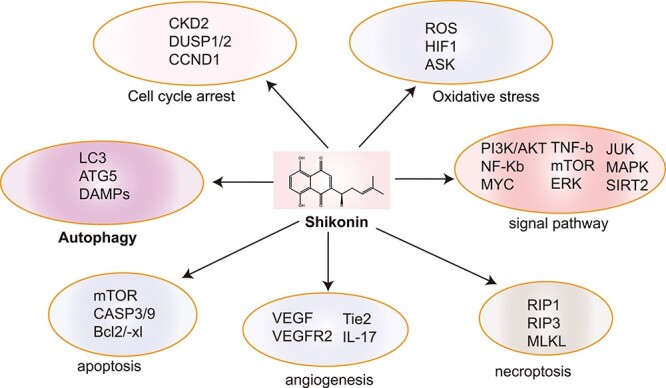
Multiple targets involved in the anti-tumor mechanism of shikonin.
Structural Analysis of Shikonin
The main components of L. erythrorhizon are naphthoquinones, alkaloids, quinones, phenolic acids, polysaccharides and other chemical components, with fat-soluble naphthoquinones and water-soluble polysaccharides being the main antitumor substances [18, 19]. Among these components, naphthoquinone compounds, including shikonin, isobutyrylshikonin, deoxyshikonin and acetylshikonin, have been the most extensively studied [20]. The antitumor activity of naphthoquinone compounds is mainly related to the structure of the α-1,4 naphthoquinone core. The naphthoquinone scaffold can induce the generation of ROS and indirectly affect oxidative stress in cells (Figure 2A) [21]. The semiquinone group of the naphthoquinone ring is reduced by single electron transfer and then oxidized back to quinone under the action of oxygen molecules. In this redox process, a large amount of ROS is simultaneously produced to affect the membrane potential of cells and mitochondria (Figure 2B) [22, 23].
Figure 2.
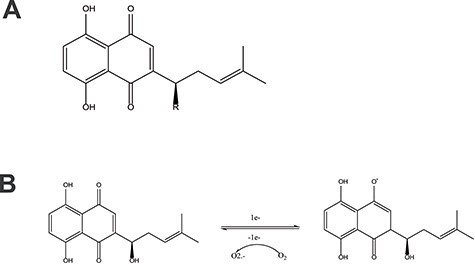
A. Parent nuclear structure of shikonin compounds. B. Mechanism of shikonin inducing reactive oxygen species (ROS) production.
Antitumor Mechanism of Shikonin
Induction of ROS production
Many studies have shown that ROS can induce cell death of different types of cancer cells after treatment with anticancer drugs [24]. DCF-DA staining is used to detect the generation of ROS, and ROS were thus found to be produced by shikonin and its derivatives, leading to the increased consumption of reduced glutathione (GSH) and increased Ca2+ concentration in the cells and destroying the mitochondrial membrane potential. The accumulation of ROS causes oxidative stress in cells, leading to the death of gastric cancer (GC) cells [25–27]. Treatment with shikonin for 6 h resulted in necroptosis due to changes in mitochondrial permeability, but the effect was reversed by Nec-1. However, after 24 h of treatment with shikonin, ERK 1/2 and AKT activities were significantly inhibited, and p38 activity was upregulated, which ultimately led to pro-caspase-3 cleavage and triggered the apoptosis of GC cells. This process was suppressed by NAC, but not by Nec-1 [28–30].
Similarly, shikonin-induced apoptosis of hepatoma cells is associated with increased levels of ROS. The production of ROS in Huh 7 and BEL 7402 cells increased in a time-dependent manner after shikonin treatment. When hepatoma cells were pretreated with the ROS scavengers NAC and GSH before treatment with shikonin, the production of ROS was significantly inhibited, the cytotoxicity of shikonin was attenuated, and cell viability was rescued [31]. In glioma cells, RIP1 binds to NADPH oxidase-1 (NOX1) and the small GTPase RAC1 to induce ROS production. RIP3 upregulates ROS levels through its physical interaction and subsequent activation with GLUD1. MLKL, the functional substrate of RIP3, can also cause excessive production of mitochondrial superoxide dismutase by binding to mitochondrial proteins, thus participating in shikonin-induced DNA double-strand breaks (DSBs). It was also shown that shikonin can promote the production of ROS, reduce the concentration of glutathione and destroy the mitochondrial membrane potential, thus leading to apoptosis of glioma cells [32].
Inhibition of the epidermal growth factor receptor (EGFR) signaling pathway
EGFR is a transmembrane glycoprotein in the receptor tyrosine kinase (RTk) superfamily and contains an extracellular ligand binding domain, a single transmembrane domain and an intracellular domain with an ATP-binding site [33, 34]. DEGFR is a mutant of EGFR in glioma that plays a key role in glioma cell migration, invasion, and drug resistance due to the lack of an extracellular binding domain [35, 36]. Studies have found that the combined use of shikonin and erlotinib to treat glioma cells. These two compounds compete for the ATP-binding site in the phosphorylated TK domain of EGFR, inhibiting the EGFR signaling pathway. In DEGFR U87MG cells, shikonin and erlotinib synergistically inhibited the phosphorylation of DEGFR and regulated the phosphorylation level of downstream molecules such as AKT, P44/42, MAPK and PLCγ1 to kill glioma cells. Studies have shown that shikonin blocks EGFR signaling and reduces ERK activity, thereby reducing the proliferation of epidermoid cancer cells [37–39].
Inhibition of the PI3K/AKT signaling pathway
Shikonin can inhibit the phosphorylation of AKT by inhibiting the PI3K/AKT signaling pathway to prevent activated AKT from expressing its downstream target proteins Bad and caspase and to upregulate the level of Bax protein [40, 41]. Shikonin can also prevent the activation of NF-κB by AKT and then downregulate the expression of Bcl-xl, a member of the Bcl-2 family, and plays a role in promoting apoptosis in endometrial carcinoma [42, 43]. The protein encoded by the PTEN gene in cervical cancer cells has phosphatase activity and plays a key negative regulatory role in the PI3K/AKT signaling pathway. It can reduce PIP3 activation of AKT and its downstream molecules, inhibit cell growth and promote apoptosis [44, 45]. H1650/R and H1975/R non-small cell lung cancer (NSCLC) cells are resistant to afatinib. Shikonin negatively regulates the PI3K/AKT signaling pathway, increases caspase-3 cleavage and reduces Bax expression, thereby inducing apoptosis in these cells [46].
Antitumor angiogenesis
Tumor blood vessels can provide the necessary nutrition and oxygen for tumor development. Tumor cells have a function in inducing angiogenesis in vitro. Vascular endothelial growth factor (VEGF), as the most important factor for promoting angiogenesis, plays a key role in tumor angiogenesis, leading to tumor cell survival and migration [47–50]. It has been found that shikonin not only directly promotes the death of tumor cells but also inhibits the regeneration of tumor blood vessels and reduces the supply of nutrients to tumor cells [51]. Shikonin and b-HIVS can inhibit angiogenesis by inhibiting the phosphorylation of vascular endothelial growth factor receptor 2 (VEGFR2) and Tie2, thereby reducing the expression of VEGFR2 and Tie2 [52]. Other studies have shown that shikonin can inhibit the expression of VEGF mediated by IL-17, and its mechanism may be related to the JAK2 and STAT3 pathways [53, 54].
Induced apoptosis
Apoptosis is the type of programmed cell death critical for cell clearance in normal tissue. It is a basic life process in multicellular organisms, and it depends on caspases, with the cleavage of PARP and pro-caspase-3 being two important events in apoptosis [29, 55–57]. Shikonin has been found to induce apoptosis through a caspase-dependent pathway in HL-60 human promyelocytic leukemia, A375-S2 melanoma cells, HeLa human cervical cancer cells, and Colo-250 and human colorectal cancer cells [58–60] (Table 1). PARP cleavage and caspase-3 expression increased when AGS cells were treated with shikonin. Studies have shown that, in addition to the effective induction of the cleavage of caspase-8, caspase-9 and PARP and the inhibition of PI3K/AKT and NF-κB signaling pathways, shikonin increases intracellular ROS production, thereby inducing the apoptosis of liver cancer cells [26, 40]. It was also found that shikonin can reduce the expression of Bcl-2 protein and increase the level of Bax protein in A549 cells. Shikonin can also reduce the mitochondrial membrane potential of A549 cells and destroy the integrity of the mitochondrial membrane. Then, shikonin promotes the release of proapoptotic proteins and thereby activates downstream signaling pathways (Figure 3) [61–63]. Recently, it was reported that shikonin induces apoptosis and attenuates epithelial-mesenchymal transition in human colon carcinoma [64].
Table 1.
Shikonin induces tumor cell apoptosis
| Cancer types | Cell types | Molecular mechanism | References |
|---|---|---|---|
| Glioma | U251 and U87MG cells | Downregulation of CD147 | [87] |
| Non-small cell lung cancer | A549 cells | Inhibited the STAT3 and AKT pathways, downregulated the expression of Bcl-2 | [88] |
| Breast cancer | 4 T1 and MDA-MB-231 cells | Regulated p38 signaling pathways | [15] |
| Hpatocellular cancer | huh7 and BEL7402 cells | Induced reactive oxygen species (ROS), downregulation of AKT and RIP1/NF-κB activity | [24] |
| Gastric cancer | AGS、AZ521 and SCM-1 cells | Upregulated p38 activities,inhibited ERK1/2 and AKT activities | [17] |
| Glioma | GBM、U87MG、DK-MG cells | Inhibited the epidermal growth factor receptor signaling pathway | [52] |
| Melanoma | A357 cells | Activated ROS-mediated ER stress and p38 pathways | [74, 89] |
| Non-small cell lung cancer | A549 and NCI-H1437 cells | Activated FOXO3a/EGR1/SIRT1 signaling | [90] |
| Gastric carcinoma | AGS cells | Regulated p53 and Nrf2 signaling pathways | [91] |
| Thyroid carcinoma | TT cells | Through the mitochondrial signaling pathway | [16] |
| Gastric cancer | NCI-N87 cells | Inhibition of PI3K/AKT signal pathway | [40] |
| Leukemia | K562 cells | Increased the PTEN level and inactivated the PI3K/AKT signaling pathway | [44] |
Figure 3.
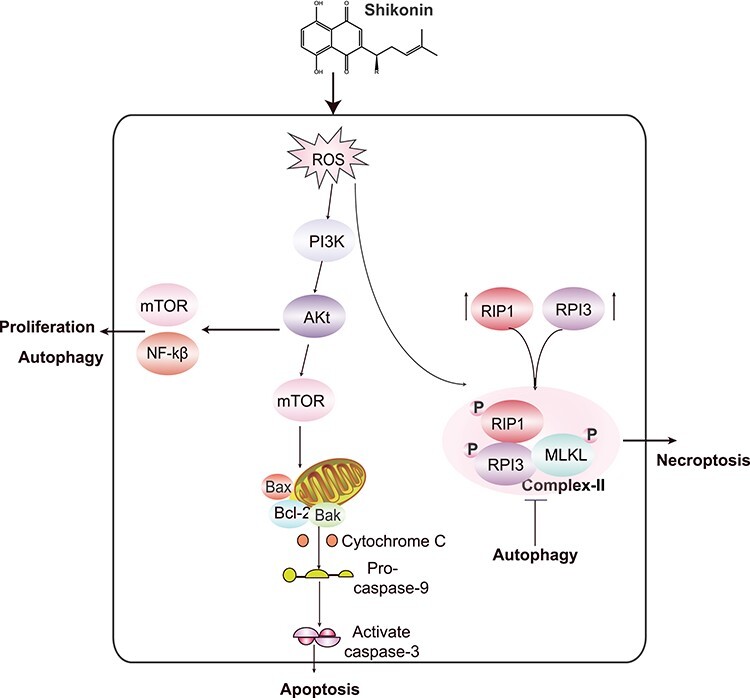
Cell death pathways involved in the anti-cancer mechanism of Shikonin.
Induction of necroptosis
Necroptosis is a type of programmed cell death that is independent of caspase activation. Necrotic cells generally have two significant characteristics. One is that they are morphologically different from apoptotic cells. The other is that they are dependent on the formation of necrotic bodies mainly composed of RIP1 and RIP3 [65, 66]. Coimmunoprecipitation of RIP1 or RIP3 revealed that shikonin can induce the aggregation of necrosis factors, such as RIP1 and RIP3, which are involved in the regulation of shikonin-induced ROS production [67, 68]. It has been shown that the shikonin-induced DNA DSBs in SHG-44 and U87 glioma cells are caused by ROS, and ROS are involved in necrotic body formation and the upregulation of RIP-1 and RIP-3. On the one hand, RIP-1 and RIP-3 complexes directly increase intracellular ROS levels and are involved in shikonin-induced DNA DSBs. On the other hand, RIP1 and RIP3 complexes phosphorylate MLKL to activate MLKL, which interferes with mitochondrial function, increases ROS levels and promotes glioma cell necroptosis by promoting nuclear translocation of AIF and the formation of γ-H2AX [69–71]. The RIP1 inhibitor Nec-1 and the RIP3 inhibitor GSK 872 significantly block cell death induced by shikonin but also block ROS production [69, 72]. Similarly, shikonin induced RIP1- and RIP3-dependent necrosis in the mouse osteosarcoma cell lines K7, K12 and K7 M3 (Table 2) [73]. These results indicate that ROS promote the formation of necrotic cells by enhancing the interaction between RIP1 and RIP3 and are involved in the killing effect of shikonin on glioma cells (Figure 3).
Table 2.
Shikonin induces tumor cell necroptosis
| Cancer types | Cell types | Molecular mechanism | References |
|---|---|---|---|
| Glioma | SHG-44、U87、U251 cells | Upregulation of RIP1 and RIP3, induced DNA DSBs | [67] |
| Multiple myeloma | K7、K12 and K7M3 cells KMS-12-PE and U266 cells |
Upregulation of RIP1 and RIP3 | [68, 92] |
| Gastric cancer | AGS、AZ521 and SCM-1 cells | Induced reactive oxygen species (ROS) | [17, 93] |
| Non-small cell lung cancer and glioma | A549 cells Rat C6 glioma cells |
Increased the levels of the RIP1 | [62, 94] |
| Histiocytic lymphoma | U937 cells | Up-regulation of TNF expression | [95] |
| Glioma | SHG-44 and U251 cells | Inducesd MLKL activation and chromatinolysis | [96] |
| Nasopharynx cancer | 5-8F cells | Increased ROS production and the upregulation of RIPK1/RIPK3/MLKL expression | [97] |
| Prostate Cancer | PC3, DU145, LNCaP, and 22Rv1 | cell cycle arrest | [98] |
Induction of autophagy and cell cycle arrest
Shikonin can also induce autophagy in several types of cancer cells through different mechanisms (Table 3). In melanoma cells, shikonin induces apoptosis and autophagy via ROS-mediated ER stress and p38 pathways [74]. Shikonin can also induce autophagy in colorectal carcinoma cells by targeting galectin-1/JNK signaling pathway [15]. In non-small cell lung cancer cells, shikonin-induced necroptosis is enhanced by the inhibition of autophagy [75].
Table 3.
Shikonin induces autophagy and inhibit cell proliferation
| Cancer types | Cell types | Molecular mechanism | References |
|---|---|---|---|
| Pncreatic cancer | BXPC-3, A375 | Asociated with the PI3K/Akt signaling pathway, p38 pathways | [74, 99] |
| Hpatocellular carcinoma | BEL7402 and Huh7 | Ativation of ERK | [100] |
| Colon cancer | HCT116 and SW620 | Inhibiting es-associated protein, targeting galectin-1/JNK signaling axis | [15, 101] |
| Non-small cell lung cancer | A549 | Regulated necroptosis | [75] |
| Skin Carcinogenesis | JB6 Cl-41 | Suppressed the ATF2,and Cdk4, | [76] |
| Esophageal cancer | EC109 and EC9706 | Decreased EGFR, PI3K, p-AKT, HIF1α and PKM2 | [78] |
| Esophageal cancer | KYSE150 | inhibited cell proliferation by suppressing PKM2 | [102] |
| Lung cancer | A549 and H446, | downregulating PFKFB2 expression | [103] |
Shikonin can also induce cell cycle arrest and proliferation in different types of cancer cells (Table 3). In human skin cancers, shikonin suppresses the ATF2 pathway in skin carcinogenesis. Furthermore, inhibition of ATF2 expression also decreased the expression levels of Cdk4 and Fra-1 [76]. RNA-seq transcriptome analysis indicated that shikonin induces the expression of dual specificity phosphatase (DUSP)-1 and DUSP2 and causes cell cycle arrest and apoptosis in breast cancer cells [77]. In esophageal cancer, shikonin decreased EGFR, PI3K, p-AKT, HIF1α and PKM2 expression by regulating HIF1α/PKM2 signal pathway [78].
Nanoparticles composed of shikonin
Studies have shown that shikonin is a potential anti-glioma drug. However, due to the limitations caused by its poor water solubility, it has a short half-life and nonselective biological distribution. Therefore, an effective route of administration is urgently needed to improve shikonin bioavailability and safety [79–81]. Nanoparticles have valuable pharmacokinetic properties, a large surface mass ratio, high drug solubility and adjustable controlled release of cargo [82, 83]. The lactoferrin receptor is highly expressed in glioma cells, and lactoferrin functionalized shikonin PEG-PLGA nanoparticles can be effectively routed upon administration. Because of their unique functions in targeting glioma cells and crossing the blood–brain barrier, these nanoparticles have important application prospects for the targeted therapy of glioma [84, 85].
Studies have shown that shikonin-loaded polylactic acid (PLGA) biodegradable nanoparticles killed only epithelial ovarian cancer cells in the treatment of ovarian cancer but did not induce strong cytotoxicity in normal ovarian cells, endothelial MS1 cells or lymphocytes. Therefore, these nanoparticles can be used as new drug treatments targeted to solid tumors [9, 86].
Conclusion
Although great progress has been made in the antitumor research of shikonin, there are still many questions to be solved. Firstly, shikonin and its derivatives have certain effects on many kinds of tumor cells. However, there are some problems such as selectivity and cytotoxicity. Secondly, the anti-tumor effect of shikonin and its derivatives has a wide range of mechanisms, involving many targets, such as inducing apoptosis, necrosis, anti-tumor angiogenesis, regulating signal pathway and so on. The interaction between them needs to be further determined. Finally, the naphthoquinone core structure of shikonin and its derivatives can produce superoxide radicals and potential bioalkylation. Although it has significant antitumor activity, this structure is also a source of side effects.
In general, the anti-tumor effect of shikonin and its derivatives has attracted wide attention, but its anti-tumor mechanism research is not deep enough. At present, the therapeutic effects of shikonin and its derivatives are based on cells and animal studies, but clinical trials are rarely reported. Therefore, more clinical trials must be carried out to verify the anti-tumor potential of shikonin. In addition, we can further improve the structure and functional group modification of shikonin from the perspective of chemical modification. We can also screen out derivatives with high activity, high selectivity and low cytotoxicity to minimize side effects. In addition, improving the way of administration is the focus of future research.
Author contributions
Qiang Wang, Xiaoli Ju and Jiayou Wang wrote the manuscript, Jing Wang and Heng Zhang revised the manuscript. All authors read and approved this manuscript.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81502621 and 81502088), Nanjing Medical Science and Technology Development Project (YKK19136) and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_3323).
Contributor Information
Qiang Wang, School of Life Sciences, Jiangsu University, Zhenjiang, Jiangsu, 212013, PR China.
Jing Wang, School of Life Sciences, Jiangsu University, Zhenjiang, Jiangsu, 212013, PR China.
Jiayou Wang, School of Life Sciences, Jiangsu University, Zhenjiang, Jiangsu, 212013, PR China.
Xiaoli Ju, School of Medicine, Jiangsu University, Zhenjiang, Jiangsu, 212013, China.
Heng Zhang, Department of General Surgery, Nanjing Lishui District People’s Hospital, Zhongda Hospital Lishui Branch, Southeast University, Nanjing, China.
Declaration of conflict of interest
None.
References
- 1. Guo C, He J, Song X et al. Pharmacological properties and derivatives of shikonin-a review in recent years. Pharmacol Res 2019;149:104463. [DOI] [PubMed] [Google Scholar]
- 2. Ricke J, Seidensticker M, Mohnike K. Noninvasive diagnosis of hepatocellular carcinoma in cirrhotic liver: current guidelines and future prospects for radiological imaging. Liver Cancer 2012;1:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med 2007;13:353–61. [DOI] [PubMed] [Google Scholar]
- 4. Olaussen KA, Postel-Vinay S. Predictors of chemotherapy efficacy in non-small-cell lung cancer: a challenging landscape. Ann Oncol 2016;27:2004–16. [DOI] [PubMed] [Google Scholar]
- 5. Efferth T, Saeed MEM, Kadioglu O et al. Collateral sensitivity of natural products in drug-resistant cancer cells. Biotechnol Adv 2020;38:107342. [DOI] [PubMed] [Google Scholar]
- 6. Yang CJ, Hung JY, Tsai MJ et al. The salvage therapy in lung adenocarcinoma initially harbored susceptible EGFR mutation and acquired resistance occurred to the first-line gefitinib and second-line cytotoxic chemotherapy. BMC Pharmacol Toxicol 2017;18:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rocconi RP, Sullivan P, Long B et al. Treatment of chemotherapy-induced anemia in ovarian cancer patients: does the use of erythropoiesis-stimulating agents worsen survival? Int J Gynecol Cancer 2012;22:786–91. [DOI] [PubMed] [Google Scholar]
- 8. Reck M, Rodriguez-Abreu D, Robinson AG et al. Investigators, K.-. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 9. Chen X, Yang L, Oppenheim JJ, Howard MZ. Cellular pharmacology studies of shikonin derivatives. Phytother Res 2002;16:199–209. [DOI] [PubMed] [Google Scholar]
- 10. Andujar I, Rios JL, Giner RM, Recio MC. Pharmacological properties of shikonin - a review of literature since 2002. Planta Med 2013;79:1685–97. [DOI] [PubMed] [Google Scholar]
- 11. Zhou W, Jiang Hda G, Peng Y, Li SS. Comparative study on enantiomeric excess of main akannin/shikonin derivatives isolated from the roots of three endemic Boraginaceae plants in China. Biomed Chromatogr 2011;25:1067–75. [DOI] [PubMed] [Google Scholar]
- 12. Tian YQ, Ding P, Yan XH, Hu WJ. Discussion on quality control of preparations with cortex moutan in volume I pharmacopoeia of People's Republic of China (2005 edition). Zhongguo Zhong Yao Za Zhi 2008;33:339–41. [PubMed] [Google Scholar]
- 13. Papageorgiou VP, Assimopoulou AN, Couladouros EA et al. The chemistry and biology of Alkannin, Shikonin, and related Naphthazarin natural products. Angew Chem Int Ed Engl 1999;38:270–301. [DOI] [PubMed] [Google Scholar]
- 14. Wang W, Dai M, Zhu C et al. Synthesis and biological activity of novel shikonin analogues. Bioorg Med Chem Lett 2009;19:735–7. [DOI] [PubMed] [Google Scholar]
- 15. Zhang N, Peng F, Wang Y et al. Shikonin induces colorectal carcinoma cells apoptosis and autophagy by targeting galectin-1/JNK signaling axis. Int J Biol Sci 2020;16:147–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang XX, Zhang C, Wei JZ et al. Apoptosis is induced by shikonin through the mitochondrial signaling pathway. Mol Med Rep 2016;13:3668–74. [DOI] [PubMed] [Google Scholar]
- 17. Lee MJ, Kao SH, Hunag JE et al. Shikonin time-dependently induced necrosis or apoptosis in gastric cancer cells via generation of reactive oxygen species. Chem Biol Interact 2014;211:44–53. [DOI] [PubMed] [Google Scholar]
- 18. Zhan ZL, Hu J, Liu T et al. Advances in studies on chemical compositions and pharmacological activities of Arnebiae radix. Zhongguo Zhong Yao Za Zhi 2015;40:4127–35. [PubMed] [Google Scholar]
- 19. Akula SK, McCullough KB, Weichselbaum C et al. The trajectory of gait development in mice. Brain Behav 2020;e01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lian Q. Advances in the study of chemical constituents and pharmacological actions of Cnidium monnieri (L.) Cusson. Zhong Yao Cai 2003;26:141–4. [PubMed] [Google Scholar]
- 21. Huang G, Zhao HR, Zhou W et al. 6-substituted 1,4-naphthoquinone oxime derivatives (I): synthesis and evaluation of their cytotoxic activity. Monatshefte Fur Chemie 2017;148:1011–23. [Google Scholar]
- 22. Zhang X, Cui JH, Zhou W, Li SS. Design, synthesis and anticancer activity of Shikonin and Alkannin derivatives with different substituents on the Naphthazarin scaffold. Chem Res Chin Univ 2015;31:394–400. [Google Scholar]
- 23. Esmaeilzadeh E, Gardaneh M, Gharib E, Sabouni F. Shikonin protects dopaminergic cell line PC12 against 6-hydroxydopamine-mediated neurotoxicity via both glutathione-dependent and independent pathways and by inhibiting apoptosis. Neurochem Res 2013;38:1590–604. [DOI] [PubMed] [Google Scholar]
- 24. Gong K, Li W. Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: a potential new treatment for hepatocellular carcinoma. Free Radic Biol Med 2011;51:2259–71. [DOI] [PubMed] [Google Scholar]
- 25. Chang IC, Huang YJ, Chiang TI et al. Shikonin induces apoptosis through reactive oxygen species/extracellular signal-regulated kinase pathway in osteosarcoma cells. Biol Pharm Bull 2010;33:816–24. [DOI] [PubMed] [Google Scholar]
- 26. Lee MJ, Kao SH, Hunag JE et al. Shikonin time-dependently induced necrosis or apoptosis in gastric cancer cells via generation of reactive oxygen species. Chem Biol Interact 2014;211:44–53. [DOI] [PubMed] [Google Scholar]
- 27. Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc) 2005;70:231–9. [DOI] [PubMed] [Google Scholar]
- 28. Han W, Xie J, Li L et al. Necrostatin-1 reverts shikonin-induced necroptosis to apoptosis. Apoptosis 2009;14:674–86. [DOI] [PubMed] [Google Scholar]
- 29. Konopleva M, Zhao S, Xie Z et al. Apoptosis. Molecules and mechanisms. Adv Exp Med Biol 1999;457:217–36. [PubMed] [Google Scholar]
- 30. Han W, Xie J, Fang Y et al. Nec-1 enhances shikonin-induced apoptosis in leukemia cells by inhibition of RIP-1 and ERK1/2. Int J Mol Sci 2012;13:7212–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gong K, Li WH. Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: a potential new treatment for hepatocellular carcinoma. Free Radic Biol Med 2011;51:2259–71. [DOI] [PubMed] [Google Scholar]
- 32. Chen HM, Wang PH, Aravindaram K et al. Shikonin enhances efficacy of a gene-based cancer vaccine via induction of RANTES. J Biomed Sci 2012;19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun WX, Han HW, Yang MK et al. Design, synthesis and biological evaluation of benzoylacrylic acid shikonin ester derivatives as irreversible dual inhibitors of tubulin and EGFR. Bioorg Med Chem 2019;27. [DOI] [PubMed] [Google Scholar]
- 34. Taylor TE, Furnari FB, Cavenee WK. Targeting EGFR for treatment of glioblastoma: molecular basis to overcome resistance. Curr Cancer Drug Targets 2012;12:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu M, Yang Y, Wang C et al. The effect of epidermal growth factor receptor variant III on glioma cell migration by stimulating ERK phosphorylation through the focal adhesion kinase signaling pathway. Arch Biochem Biophys 2010;502:89–95. [DOI] [PubMed] [Google Scholar]
- 36. Furnari FB, Fenton T, Bachoo RM et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 2007;21:2683–710. [DOI] [PubMed] [Google Scholar]
- 37. Brown PD, Krishnan S, Sarkaria JN et al. North central cancer treatment group study, N., phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: north central cancer treatment group study N0177. J Clin Oncol 2008;26:5603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Calonghi N, Pagnotta E, Parolin C et al. A new EGFR inhibitor induces apoptosis in colon cancer cells. Biochem Biophys Res Commun 2007;354:409–13. [DOI] [PubMed] [Google Scholar]
- 39. Singh F, Gao D, Lebwohl MG, Wei H. Shikonin modulates cell proliferation by inhibiting epidermal growth factor receptor signaling in human epidermoid carcinoma cells. Cancer Lett 2003;200:115–21. [DOI] [PubMed] [Google Scholar]
- 40. Jia L, Zhu Z, Li H, Li Y. Shikonin inhibits proliferation, migration, invasion and promotes apoptosis in NCI-N87 cells via inhibition of PI3K/AKT signal pathway. Artif Cells Nanomed Biotechnol 2019;47:2662–9. [DOI] [PubMed] [Google Scholar]
- 41. Lu H, Zhang S, Wu J et al. Molecular targeted therapies elicit concurrent apoptotic and GSDME-dependent Pyroptotic tumor cell death. Clin Cancer Res 2018;24:6066–77. [DOI] [PubMed] [Google Scholar]
- 42. Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res 2005;65:10669–73. [DOI] [PubMed] [Google Scholar]
- 43. Xin MG, Deng XM. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem 2005;280:10781–9. [DOI] [PubMed] [Google Scholar]
- 44. Chen Y, Wang T, Du J et al. The critical role of PTEN/PI3K/AKT Signaling pathway in Shikonin-induced apoptosis and proliferation inhibition of chronic myeloid Leukemia. Cell Physiol Biochem 2018;47:981–93. [DOI] [PubMed] [Google Scholar]
- 45. He W, Xu ZJ, Song DL et al. Antitumor effects of rafoxanide in diffuse large B cell lymphoma via the PTEN/PI3K/Akt and JNK/c-Jun pathways. Life Sci 2020;243. [DOI] [PubMed] [Google Scholar]
- 46. Li X, Fan XX, Jiang ZB et al. Shikonin inhibits gefitinib-resistant non-small cell lung cancer by inhibiting TrxR and activating the EGFR proteasomal degradation pathway. Pharmacol Res 2017;115:45–55. [DOI] [PubMed] [Google Scholar]
- 47. Lee HJ, Lee HJ, Magesh V et al. Shikonin, Acetylshikonin, and Isobutyroylshikonin inhibit VEGF-induced angiogenesis and suppress tumor growth in Lewis lung carcinoma-bearing mice. Yakugaku Zasshi-Journal of the Pharmaceutical Society of Japan 2008;128:1681–8. [DOI] [PubMed] [Google Scholar]
- 48. Xia HM, Tang CY, Gui H et al. Preparation, cellular uptake and angiogenic suppression of shikonin-containing liposomes in vitro and in vivo. Biosci Rep 2013;33:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Folkman J. Seminars in medicine of the Beth Israel hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 1995;333:1757–63. [DOI] [PubMed] [Google Scholar]
- 50. Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med 1971;133:275–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res 2005;65:10669–73. [DOI] [PubMed] [Google Scholar]
- 52. Zhao Q, Kretschmer N, Bauer R, Efferth T. Shikonin and its derivatives inhibit the epidermal growth factor receptor signaling and synergistically kill glioblastoma cells in combination with erlotinib. Int J Cancer 2015;137:1446–56. [DOI] [PubMed] [Google Scholar]
- 53. Xu Y, Xu X, Gao X et al. Shikonin suppresses IL-17-induced VEGF expression via blockage of JAK2/STAT3 pathway. Int Immunopharmacol 2014;19:327–33. [DOI] [PubMed] [Google Scholar]
- 54. Komi Y, Suzuki Y, Shimamura M et al. Mechanism of inhibition of tumor angiogenesis by beta-hydroxyisovalerylshikonin. Cancer Sci 2009;100:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin 2005;55:178–94. [DOI] [PubMed] [Google Scholar]
- 56. Budihardjo I, Oliver H, Lutter M et al. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 1999;15:269–90. [DOI] [PubMed] [Google Scholar]
- 57. Communal C, Sumandea M, de Tombe P et al. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci U S A 2002;99:6252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yoon Y, Kim YO, Lim NY et al. Shikonin, an ingredient of Lithospermum erythrorhizon induced apoptosis in HL60 human premyelocytic leukemia cell line. Planta Med 1999;65:532–5. [DOI] [PubMed] [Google Scholar]
- 59. Hsu PC, Huang YT, Tsai ML et al. Induction of apoptosis by shikonin through coordinative modulation of the Bcl-2 family, p27, and p53, release of cytochrome c, and sequential activation of caspases in human colorectal carcinoma cells. J Agric Food Chem 2004;52:6330–7. [DOI] [PubMed] [Google Scholar]
- 60. Thangapazham RL, Singh AK, Seth P et al. Shikonin analogue (SA) 93/637 induces apoptosis by activation of caspase-3 in U937 cells. Front Biosci 2008;13:561–8. [DOI] [PubMed] [Google Scholar]
- 61. Larrayoz IM, Huang JD, Lee JW et al. 7-Ketocholesterol-induced inflammation: involvement of multiple kinase Signaling pathways via NF kappa B but independently of reactive oxygen species formation. Invest Ophthalmol Vis Sci 2010;51:4942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim HJ, Hwang KE, Park DS et al. Shikonin-induced necroptosis is enhanced by the inhibition of autophagy in non-small cell lung cancer cells. J Transl Med 2017;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yeh YC, Liu TJ, Lai HC. Shikonin induces apoptosis, necrosis, and premature senescence of human A549 lung cancer cells through upregulation of p53 expression. Evid Based Complement Alternat Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shilnikova K, Piao MJ, Kang KA et al. Natural compound Shikonin induces apoptosis and attenuates epithelial to mesenchymal transition in radiation-resistant human colon cancer cells. Biomol Ther (Seoul) 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xuan YY, Hu X. Naturally-occurring shikonin analogues - a class of necroptotic inducers that circumvent cancer drug resistance. Cancer Lett 2009;274:233–42. [DOI] [PubMed] [Google Scholar]
- 66. Han WD, Li L, Qiu S et al. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther 2007;6:1641–9. [DOI] [PubMed] [Google Scholar]
- 67. Zhou ZJ, Lu B, Wang C et al. RIP1 and RIP3 contribute to shikonin-induced DNA double-strand breaks in glioma cells via increase of intracellular reactive oxygen species. Cancer Lett 2017;390:77–90. [DOI] [PubMed] [Google Scholar]
- 68. Fu ZZ, Deng BY, Liao YX et al. The anti-tumor effect of shikonin on osteosarcoma by inducing RIP1 and RIP3 dependent necroptosis. BMC Cancer 2013;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cai, Z. Y.; Jitkaew, S.; Zhao, J.; Chiang, H. C.; Choksi, S.; Liu, J.; Ward, Y.; Wu, L. G.; Liu, Z. G., Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 2014, 16 , 55−+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Artus C, Boujrad H, Bouharrour A et al. AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX. EMBO J 2010;29:1585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sun L, Wang H, Wang Z et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012;148:213–27. [DOI] [PubMed] [Google Scholar]
- 72. O'Driscoll M, Jeggo PA. The role of double-strand break repair - insights from human genetics. Nat Rev Genet 2006;7:45–54. [DOI] [PubMed] [Google Scholar]
- 73. Fu Z, Deng B, Liao Y et al. The anti-tumor effect of shikonin on osteosarcoma by inducing RIP1 and RIP3 dependent necroptosis. BMC Cancer 2013;13:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu Y, Kang X, Niu G et al. Shikonin induces apoptosis and prosurvival autophagy in human melanoma A375 cells via ROS-mediated ER stress and p38 pathways. Artif Cells Nanomed Biotechnol 2019;47:626–35. [DOI] [PubMed] [Google Scholar]
- 75. Kim HJ, Hwang KE, Park DS et al. Shikonin-induced necroptosis is enhanced by the inhibition of autophagy in non-small cell lung cancer cells. J Transl Med 2017;15:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li W, Zhang C, Ren A et al. Shikonin suppresses skin carcinogenesis via inhibiting cell proliferation. PLoS One 2015;10:e0126459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lin KH, Huang MY, Cheng WC et al. RNA-seq transcriptome analysis of breast cancer cell lines under shikonin treatment. Sci Rep 2018;8:2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tang JC, Zhao J, Long F et al. Efficacy of Shikonin against Esophageal cancer cells and its possible mechanisms in vitro and in vivo. J Cancer 2018;9:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen J, Xie J, Jiang Z et al. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 2011;30:4297–306. [DOI] [PubMed] [Google Scholar]
- 80. Matthaiou EI, Barar J, Sandaltzopoulos R et al. Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int J Nanomedicine 2014;9:1855–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bhowmik A, Khan R, Ghosh MK. Blood brain barrier: a challenge for effectual therapy of brain Tumors. Biomed Res Int 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 2012;64:206–12. [DOI] [PubMed] [Google Scholar]
- 83. Chen J, Xie J, Jiang Z et al. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 2011;30:4297–306. [DOI] [PubMed] [Google Scholar]
- 84. Elfinger M, Maucksch C, Rudolph C. Characterization of lactoferrin as a targeting ligand for nonviral gene delivery to airway epithelial cells. Biomaterials 2007;28:3448–55. [DOI] [PubMed] [Google Scholar]
- 85. Suzuki YA, Lopez V, Lonnerdal B. Mammalian lactoferrin receptors: structure and function. Cell Mol Life Sci 2005;62:2560–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vitorino P, Meyer T. Modular control of endothelial sheet migration. Genes Dev 2008;22:3268–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guo N, Miao R, Gao X et al. Shikonin inhibits proliferation and induces apoptosis in glioma cells via downregulation of CD147. Mol Med Rep 2019;19:4335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guo ZL, Li JZ, Ma YY et al. Shikonin sensitizes A549 cells to TRAIL-induced apoptosis through the JNK, STAT3 and AKT pathways. BMC Cell Biol 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lee JH, Han SH, Kim YM et al. Shikonin inhibits proliferation of melanoma cells by MAPK pathway-mediated induction of apoptosis. Biosci Rep 2021;41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jeung YJ, Kim HG, Ahn J et al. Shikonin induces apoptosis of lung cancer cells via activation of FOXO3a/EGR1/SIRT1 signaling antagonized by p300. Biochim Biophys Acta 2016;1863:2584–93. [DOI] [PubMed] [Google Scholar]
- 91. Ko H, Kim SJ, Shim SH et al. Shikonin induces apoptotic cell death via regulation of p53 and Nrf2 in AGS human stomach carcinoma cells. Biomol Ther (Seoul) 2016;24:501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wada N, Kawano Y, Fujiwara S et al. Shikonin, dually functions as a proteasome inhibitor and a necroptosis inducer in multiple myeloma cells. Int J Oncol 2015;46:963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shahsavari Z, Karami-Tehrani F, Salami S. Shikonin induced necroptosis via reactive oxygen species in the T-47D breast cancer cell line. Asian Pac J Cancer Prev 2015;16:7261–6. [DOI] [PubMed] [Google Scholar]
- 94. Huang CJ, Luo YA, Zhao JW et al. Shikonin kills glioma cells through necroptosis mediated by RIP-1. PLoS One 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Piao JL, Cui ZG, Furusawa Y et al. The molecular mechanisms and gene expression profiling for shikonin-induced apoptotic and necroptotic cell death in U937 cells. Chem Biol Interact 2013;205:119–27. [DOI] [PubMed] [Google Scholar]
- 96. Ding Y, He C, Lu S et al. MLKL contributes to shikonin-induced glioma cell necroptosis via promotion of chromatinolysis. Cancer Lett 2019;467:58–71. [DOI] [PubMed] [Google Scholar]
- 97. Liu T, Sun X, Cao Z. Shikonin-induced necroptosis in nasopharyngeal carcinoma cells via ROS overproduction and upregulation of RIPK1/RIPK3/MLKL expression. Onco Targets Ther 2019;12:2605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Markowitsch SD, Juetter KM, Schupp P et al. Shikonin reduces growth of docetaxel-resistant prostate cancer cells mainly through necroptosis. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shi S, Cao H. Shikonin promotes autophagy in BXPC-3 human pancreatic cancer cells through the PI3K/Akt signaling pathway. Oncol Lett 2014;8:1087–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gong K, Zhang Z, Chen Y et al. Extracellular signal-regulated kinase, receptor interacting protein, and reactive oxygen species regulate shikonin-induced autophagy in human hepatocellular carcinoma. Eur J Pharmacol 2014;738:142–52. [DOI] [PubMed] [Google Scholar]
- 101. Zhu J, Zhao L, Luo B, Sheng W. Shikonin regulates invasion and autophagy of cultured colon cancer cells by inhibiting yes-associated protein. Oncol Lett 2019;18:6117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang Q, Liu Q, Zheng S et al. Shikonin inhibits tumor growth of ESCC by suppressing PKM2 mediated aerobic glycolysis and STAT3 phosphorylation. J Cancer 2021;12:4830–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sha L, Lv Z, Liu Y et al. Shikonin inhibits the Warburg effect, cell proliferation, invasion and migration by downregulating PFKFB2 expression in lung cancer. Mol Med Rep 2021;24. [DOI] [PMC free article] [PubMed] [Google Scholar]


