Abstract
The nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) is a ligand-regulated nuclear receptor superfamily member. Liganded PPARγ exerts diverse biological effects, promoting adipocyte differentiation, inhibiting tumor cellular proliferation, and regulating monocyte/macrophage and anti-inflammatory activities in vitro. In vivo studies with PPARγ ligands showed enhancement of tumor growth, raising the possibility that reduced immune function and tumor surveillance may outweigh the direct inhibitory effects of PPARγ ligands on cellular proliferation. Recent findings that PPARγ ligands convey PPARγ-independent activities through IκB kinase (IKK) raises important questions about the specific mechanisms through which PPARγ ligands inhibit cellular proliferation. We investigated the mechanisms regulating the antiproliferative effect of PPARγ. Herein PPARγ, liganded by either natural (15d-PGJ2 and PGD2) or synthetic ligands (BRL49653 and troglitazone), selectively inhibited expression of the cyclin D1 gene. The inhibition of S-phase entry and activity of the cyclin D1-dependent serine-threonine kinase (Cdk) by 15d-PGJ2 was not observed in PPARγ-deficient cells. Cyclin D1 overexpression reversed the S-phase inhibition by 15d-PGJ2. Cyclin D1 repression was independent of IKK, as prostaglandins (PGs) which bound PPARγ but lacked the IKK interactive cyclopentone ring carbonyl group repressed cyclin D1. Cyclin D1 repression by PPARγ involved competition for limiting abundance of p300, directed through a c-Fos binding site of the cyclin D1 promoter. 15d-PGJ2 enhanced recruitment of p300 to PPARγ but reduced binding to c-Fos. The identification of distinct pathways through which eicosanoids regulate anti-inflammatory and antiproliferative effects may improve the utility of COX2 inhibitors.
The peroxisome proliferator-activated receptor γ (PPARγ) is a member of the nuclear receptor superfamily that mediates adipocyte differentiation (61), exerts anti-inflammatory effects in monocyte/macrophages (29, 50), modulates insulin sensitivity, and inhibits cellular proliferation (5). PPARγ exhibits a modular structure with a central DNA-binding domain, an amino-terminal activation domain (AF-1), a carboxyl-terminal ligand-binding domain (LBD), and a ligand-dependent activation domain (AF-2). The natural ligands for PPARγ include a series of fatty acids such as linoleic acid, eicosanoid derivatives, and synthetic ligands called thiazolidinediones (TZDs) (22–34). The eicosanoid 15-deoxy-Δ12,14 prostaglandin J2 (15d-PGJ2) is a naturally occurring and potent PPARγ ligand, binding and activating PPARγ activity at micromolar concentrations. The TZDs were the first identified synthetic PPARγ ligands and bound with high affinity (Kd of 40 nM). A serine residue within the N-terminal AF-1 domain (Ser 82 in PPARγ1 and Ser 112 in PPARγ2) is phosphorylated in vitro by mitogen-activated protein kinase (MAPK) (1, 28, 57). Mutation of this MAPK phosphorylation site negatively regulated the transcriptional and biological functions of PPARγ in some (1, 28, 57) but not all (40, 66) studies, suggesting cell type-specific activities.
The regulation of gene transcription by ligand-bound PPARγ involves DNA binding and recruitment of coactivator proteins including p300 (also known as CBP), the SRC-1 class of coactivators and DRIP205 (also known as TRAP220) (46, 60, 61, 68, 71; reviewed in reference 17). Cocrystallization of the PPARγ LBD with one of the steroid receptor coactivator 1 (SRC-1) binding domains showed that the two LXXLL motifs of a single SRC-1 molecule interacts separately with the AF-2 helix of each receptor molecule as a dimer (46). The response to different PPARγ ligands requires distinct residues C terminal to the core LXXLL motif (44), and different ligands differentially recruit distinct coactivators (35, 68), suggesting the capacity for important specificity in the biological effects of PPARγ. p300 contains LXXLL motifs that interact with nuclear receptors, including PPARγ (56). In a ligand-dependent manner, p300 contacts the AF-2 region and, in a ligand-independent manner, contacts AF-1 (24). The mechanisms governing PPARγ-dependent transcriptional repression have been studied in some detail for the promoter of the inducible nitric oxide synthase (iNOS) gene promoter (40, 50). It is the gamma interferon (IFN-γ) and lipopolysaccharide-induced expression of the iNOS gene that is inhibited by liganded PPARγ (40, 50). PPARγ forms relatively weak interactions with corepressor proteins such as NCoR and SMRT (26). The efficacy of specific mutants within helix 12 of PPARγ to inhibit ligand-induced PPARγ signaling through corepressor release suggests an important role for corepressors in select PPARγ functions (26).
In addition to expression in adipose tissue and mammary epithelium, PPARγ is also expressed in monocytes (51). In monocytes and monocyte-derived macrophages, PPARγ activation inhibits the expression of interleukin-6, iNOS, gelatinase B (also known as matrix metalloproteinase-9), and the CD36 scavenger receptor (14, 29, 42, 50). The anti-inflammatory effects, such as inhibition of IFN-γ-induced inducible protein 10 activity, interleukin-2 promoter activity, or iNOS activity, are observed for both the natural 15d-PGJ2 and synthetic TZD ligands (29, 42). An additional complexity has arisen from the findings that the PPARγ ligand 15d-PGJ2 can also exert anti-inflammatory activity through a PPARγ-independent mechanism (13, 14, 52). 15d-PGJ2 is a cell type-specific regulator of intracellular kinases, including IκB kinase (IKK) (13, 52, 59). The serine-threonine IKK phosphorylates IκB, leading to the nuclear translocation of NF-κB and thereby induction of gene transcription (30, 33). The inhibition of IKK and NF-κB activity is selective for A- and J-type cyclopentanone prostaglandins (cyPGs) as they contain a cyclopentane ring with a reactive α,β unsaturated carbonyl group. This structure renders the molecule able to form Michael adducts with cellular nucleophilics and covalently modify specific proteins (52). These findings have necessitated a high-level analysis of the molecular mechanisms governing the effects of 15d-PGJ2.
In contrast with the anti-inflammatory effects, the molecular mechanisms governing the antiproliferative effects of PPARγ in breast and colon cancer cells are relatively poorly understood. PPARγ agonists inhibit the growth of human colorectal cancer cells (8, 55) but promote intestinal tumorigenesis in the Min mouse (39, 53). These studies raised the possibility that tumor growth may have been enhanced in vivo through the anti-inflammatory function of these agents, reducing tumor surveillance (39, 53). PPARγ is expressed at significant levels in primary and metastatic human breast adenocarcinomas. The antiproliferative action of 15d-PGJ2 has been linked to its role as a high-affinity ligand for PPARγ; however, a direct relationship and the molecular mechanisms remain to be formally established (51). The ability to selectively inhibit breast tumor cellular proliferation using nontoxic ligands has provided the impetus to assess the efficacy of other PPARγ ligands in the treatment of other tumors and to investigate the molecular mechanisms governing the antiproliferative activity of cyPGs.
Orderly progression through the cell cycle is coordinated by sequential phosphorylation of target substrates, including the retinoblastoma protein (pRB) and by serine-threonine kinase cyclin-dependent kinases (Cdks), including the cyclin D1-Cdk4 and cyclin E-Cdk2 complexes (47). Both cyclin D1 and cyclin E may independently contribute to progression into the S phase of the cell cycle (47, 49). Immunoneutralization and antisense experiments have shown that the abundance of the regulatory subunit, cyclin D1, determines the rate of progression through the G1 phase in mammary epithelial cells in response to mitogenic and oncogenic signals (37, 41). Mouse embryo fibroblasts, derived from animals homozygously deleted of the cyclin D1 gene, have reduced rates of DNA synthesis at subconfluence and increased rates of apoptosis, suggesting an important role for cyclin D1 in cellular proliferation and survival (3, 10). The induction of cyclin D1 gene expression by oncogenic and mitogenic stimuli involves several distinct pathways, including STATs (signal transducers and activators of transcription) (9, 43), NF-κB (27, 31), and members of the AP-1 family (e.g., c-Fos and c-Jun) (4, 10). Cyclin D1 expression is induced by c-Fos (4, 10). In c-fos−/− fosB−/−-derived mouse embryo fibrolasts, reduced DNA synthesis rates in response to serum were associated with a selective reduction in cyclin D1 abundance (10). The introduction of one c-fos allele restored both cyclin D1 expression and DNA synthesis, suggesting a pivotal role for c-Fos and cyclin D1 in proliferative signaling.
The identification of distinct DNA sequences within the cyclin D1 promoter involved in regulation by select signaling pathways establishes the cyclin D1 promoter as a powerful molecular probe of signaling pathways governing cellular proliferation. Prostaglandins (PGs) and PPARγ ligands convey both anti-inflammatory activity and antiproliferative effects. The anti-inflammatory effects of PGs involve PPARγ-dependent and -independent mechanisms. We assessed the molecular mechanisms by which 15d-PGJ2 regulates cellular proliferation. In MCF-7 cells, 15d-PGJ2 selectively inhibited S-phase entry and both the abundance and kinase activity of cyclin D1. Cyclin D1 mRNA and promoter activity were repressed by 15d-PGJ2, while cyclin D1 overexpression reversed 15d-PGJ2-mediated S-phase inhibition. Several lines of evidence support the conclusion that cyclin D1 repression by PPARγ ligands is distinct from the anti-inflammatory effects mediated by inhibition of IKK activity. First, the cyclin D1 promoter repression by PGs was observed with PGD2, which is incapable of binding IKK. Second, 15d-PGJ2 did not inhibit NFκB signaling at concentrations that repressed cyclin D1. Third, repression of the cyclin D1 promoter by 15d-PGJ2 required PPARγ and involved the cyclin D1 AP-1–cyclic AMP response element (CRE) site. p300 and c-Fos were identified within the AP-1 site; p300 overcame 15d-PGJ2-mediated cyclin D1 repression, and 15d-PGJ2 induced selective association of p300 with PPARγ and reduced association between p300 and c-Fos. Together, these studies demonstrate that 15d-PGJ2-mediated repression of cyclin D1 involves competition between PPARγ and c-Fos for limiting abundance of p300 and is mechanistically distinct from the anti-inflammatory action of PGs.
MATERIALS AND METHODS
Reporter genes and expression vectors.
The cyclin D1 promoter luciferase (LUC) reporter constructions (4), c-jun LUC (16), human iNOS (hiNOS LUC (2), murine iNOS (miNOS LUC (50), the 3xRel LUC reporter (2), cyclin E LUC (3), c-fos LUC (65), junB LUC, and c-myc LUC (c-myc P1-P2 promoters) were previously described. The expression vectors for pCMX-PPARγ, pCMV-PPARγS112A, pCMV-PPARγS112D (57), pcDNA3-PPARγL468A/E471A (26), (AOX)3 LUC (acyl-coenzyme A oxidase triple PPAR response element [PPRE]), pCMV-HA-p300 pCMV-HA-p300Δ1737-1809, pCMV-HA-p300Δ1737-1809 (3), CD20 (62), and pCMV-IKKαSS/EE (36) were previously described.
Cell culture, DNA transfection, and luciferase assays.
Cell culture, DNA transfection, and luciferase assays were performed as previously described (21, 63). MCF-7 and HeLa cells were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum, 1% penicillin, and 1% streptomycin. The culture conditions for T47D, MDA-MB-453, MDA-MB-231, T89G (21, 37), and RAW264.7 (50) were described elsewhere. Cells were transfected by Superfect Transfection reagent (Qiagen, Valencia, Calif.). The medium was changed after 5 h, cells were treated with ligand or vehicle as indicated in the figure legends, and luciferase activity was determined after 24 h. Rosiglitazone was a gift from P. G. Treagust (Smithkline Beecham, West Sussex, United Kingdom) and troglitazone was from Sanky Co., Ltd., (Tokyo, Japan). At least two different plasmid preparations of each construct were used. In cotransfection experiments, a dose-response curve was determined in each experiment with 20 ng of expression vector and the promoter reporter plasmids (1 μg). Luciferase activity was normalized for transfection with β-galactosidase reporters as an internal control. Luciferase assays were performed at room temperature with an Autolumat LB 953 (EG&G Berthold) (63). The fold effect was determined by comparison to the empty expression vector cassette, and statistical analyses were performed using the Mann Whitney U test.
Western blots, immunoprecipitation-Western blotting, immune-complex kinase assays, and flow cytometric analysis.
The antibodies used in Western blot analysis were polyclonal cyclin D1 antibody Ab3 DCS-11 (for immunoprecipitation [IP] kinase assays [NeoMarkers Lab Vision Corporation, Fremont, Calif.]), guanine nucleotide dissociation inhibitor (GDI) antibody (37) used as an internal control for protein abundance, and antibodies to cyclin E (M20), Cdk4 (C22), and phospho-specific pRB (serine 780) (Ab 169). IP-Western blotting was performed as previously described (7). Saturating amounts of antibodies to either c-Fos (C4; Santa Cruz Biotechnology, Santa Cruz, Calif.) or to PPARγ (E8; Santa Cruz Biotechnology) were compared using equal amounts of control immunoglobulin G (IgG) and the same amount of cellular extracts determined by total protein determination. Western blotting of the IP was performed with antibodies to either p300 (C20; Santa Cruz Biotechnology), PPARγ, or c-Fos as indicated in the figure legend. For detection of proteins, the membrane was incubated with horseradish peroxidase-conjugated second antibody (Santa Cruz Biotechnology) and washed three times with 0.05% Tween 20–phosphate-buffered saline. Proteins were visualized by the enhanced chemiluminescence system (Amersham, Arlington Heights, Ill.). The abundance of immunoreactive protein was quantified by phosphorimaging with a molecular Dynamics Computing densitometer (Image Quant, version 1.11; Sunnyvale, Calif.). Flow cytometric analyses were carried out with a fluorescence-activated cell sorter (FACS) (FACStar Plus; Becton Dickinson) with a 360-5 nM argon-iron laser (3). Selection of transfected cells with CD4 (7) or CD20 (62) as a marker was performed as previously described.
Cyclin D1-IP kinase assays were performed essentially as previously described (65) using saturating amounts of the cyclin D1 antibody, DCS-11 (NeoMarkers). The pRB substrate was prepared by transforming Escherichia coli with the vector pGEX-RB (65). IKK immune complex assays were performed as previously described (52). The phosphorylation of glutathione S-transferase (GST) pRb or IκB substrates was quantified by densitometry after exposure to autoradiographic film (Labscientific, Inc., Livingston, N.J.) using ImageQuant software, version 1.11, and a Molecular Dynamics Computing densitometer.
Electrophoretic mobility gel shift assays.
Complementary oligodeoxyribonucleotide strands of the AP-1 site of the cyclin D1 promoter, the wild type AP-1 (D1AP-1wt) site and a mutant AP-1 (D1AP-1mt) site were used for electrophoretic mobility gel shift assays (EMSA) as previously described (64, 65). γ32P-labeled oligonucleotides (50 fmol; 50,000 cpm) were added to 10 μg of nuclear extracts in binding buffer containing 20 mM HEPES (pH 7.4), 40 mM KCl, 1 mM MgCl2, 0.1 mM EDTA, and 0.1% NP-40 to which probe and 1 μg of dI-dC were added. Supershift analyses were performed using c-Jun (also known as KM-1), c-Fos, JunB, and JunD antibodies from Santa Cruz Biotechnology, and the polyclonal p300 antibody (3). The reaction products were separated on 5% polyacrylamide gels run in 0.5× Tris-borate-EDTA at room temperature at 200 V for 3 h. The gels were dried and exposed to XAR5 (Kodak, Rochester, N.Y.) radiographic film or to the phosphorImager system (Storm; Molecular Dynamics densitometer).
RESULTS
15d-PGJ2 inhibits G1 cell cycle transition and selectively inhibits cyclin D1 in a PPARγ-dependent manner.
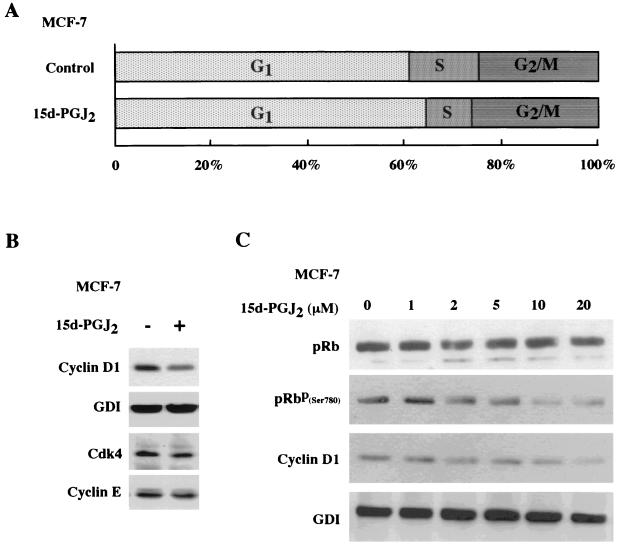
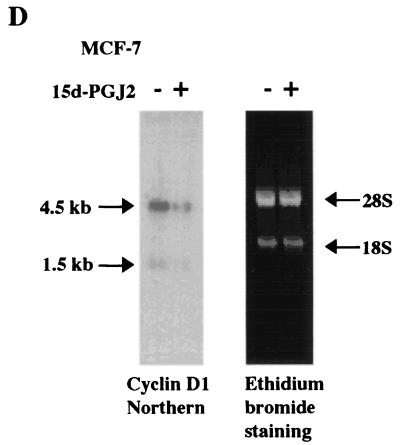
Previous studies indicated that MCF-7 cells express PPARγ and that addition of the PPARγ synthetic ligand troglitazone inhibited clonal growth of MCF-7 cells (20). As recent studies have suggested that PPARγ ligands may function through receptor-independent mechanisms (14), we investigated the molecular mechanism by which 15d-PGJ2 regulated the cell cycle in MCF-7 cells. Cells treated with 10 μM 15d-PGJ2 subjected to FACS analysis demonstrated a 40% reduction in the proportion of cells in S phase, suggesting an inhibition of the G1-S transition (Fig. 1A). Western blot analysis of 15d-PGJ2-treated MCF-7 cells demonstrated a 50% reduction in cyclin D1 protein levels when normalized to the internal control, GDI. The abundance of Cdk4 and cyclin E was unchanged (Fig. 1B). Western blot analysis was performed with MCF-7 cells treated with increasing concentrations of 15d-PGJ2. An antibody to the site of cyclin D1-Cdk phsophorylation of pRB (serine 780) was used. The phospho-specific pRB band was reduced 50 to 60% in a dose-dependent manner by 15d-PGJ2, commensurate with a reduction in cyclin D1 protein levels (Fig. 1C). As the abundance of cyclin D1 is rate limiting in G1-S transition in MCF-7 cells in response to diverse mitogenic stimuli (41), the mechanism by which 15d-PGJ2 repressed cyclin D1 was further assessed. Cyclin D1 mRNA levels were reduced in MCF-7 cells treated with 15d-PGJ2 for 6 h (Fig. 1D).
FIG. 1.
15d-PGJ2 inhibition of S-phase and cyclin D1 expression. (A) Cell cycle analysis of MCF-7 cells treated with 10 μM 15d-PGJ2 for 48 h. (B) Western blot analysis of MCF-7 cells treated with 10 μM 15d-PGJ2 for 16 h, showing cyclin D1, GDI, Cdk4, and cyclin E expression. Similar selective reduction in cyclin D1 protein levels were observed at 6 and 12 h (data not shown). (C) MCF-7 cells treated with 15d-PGJ2 for 24 h at increasing doses are shown, with Western blotting using either total pRB or an antibody specific for pRB Ser 780. (D) Northern blot analysis using the cyclin D1 cDNA probe of mRNA from MCF-7 cells treated with 10 μM 15d-PGJ2 for 6 h shows reduction in the 4.5-kb cyclin D1-specific mRNA.
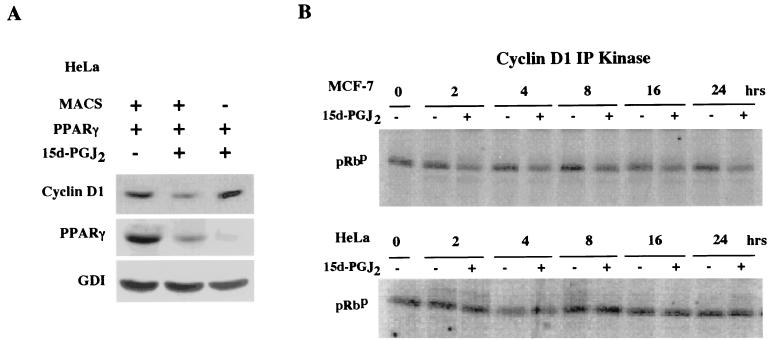
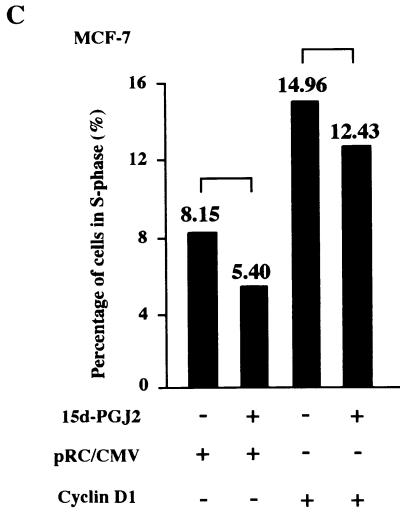
To determine whether the regulation of cyclin D1 by 15d-PGJ2 was PPARγ receptor dependent, the regulation of cyclin D1 was assessed in PPARγ-deficient HeLa cells. Cells were transfected with the PPARγ expression vector or control vector, selected by magnetic cell sorting (7), and treated with 15d-PGJ2 or vehicle. Western blotting for cyclin D1, normalized to the internal control (GDI), showed a 70% reduction in cyclin D1 abundance in the cells transfected with PPARγ and treated with 15d-PGJ2 (Fig. 2A, lanes 1 versus 2). Inhibition of cyclin D1 protein levels required the presence of PPARγ and its ligand and did not occur with ligand alone. The effect of 15d-PGJ2 on the holoenzyme kinase activity of cyclin D1 was assessed with cyclin D1 IP kinase assays with GST-pRB as a substrate (70). A comparison was made between MCF-7 cells and HeLa cells. 15d-PGJ2 inhibited cyclin D1 kinase activity by 40% within 2 h and by 70% at 24 h in MCF-7 cells (Fig. 2B). There was no significant change in cyclin D1 kinase activity in the PPARγ-negative HeLa cells treated with 15d-PGJ2. To determine whether the reduction in cyclin D1 levels was necessary for the inhibition of S phase by 15d-PGJ2, MCF-7 cells were transfected with an expression vector for cyclin D1 or the control empty expression vector cassette (pRC/CMV) and treated with either 15d-PGJ2 or vehicle for 24 h. 15d-PGJ2 inhibited S phase; however, the overexpression of cyclin D1 abolished the inhibition of S phase (mean S phase, 5.4 versus 12.4%) (Fig. 2C).
FIG. 2.
Repression of cyclin D1 expression and kinase activity by 15d-PGJ2 requires PPARγ. (A) Western blot analysis for cyclin D1 and GDI of HeLa cells selected by magnetic cell sorting after transfection with PPARγ and treated with 15d-PGJ2 (10 μM) for 24 h. (B) Activity of the cyclin D1-dependent serine-threonine kinase was assessed by IP kinase assays using GST-pRB as substrate in MCF-7 (top) or HeLa (bottom) cells. Cells were treated with 15d-PGJ2 (10 μM) for the time points indicated. (C) Cell cycle analysis was performed with MCF-7 cells transfected with either pRC-CMV–cyclin D1 or the control empty vector cassette, followed by treatment with 10 μM 15d-PGJ2 for 24 h. FACS analysis was performed to compare the effect of 15d-PGJ2 or vehicle on S phase. Values represent the percentage of cells in S phase. Cyclin D1 overexpression abrogates the inhibition of S phase by 15d-PGJ2 (5.4 versus 12.4%).
The cyclin D1 promoter is repressed by prostaglandin ligands that fail to inhibit IKK activity.
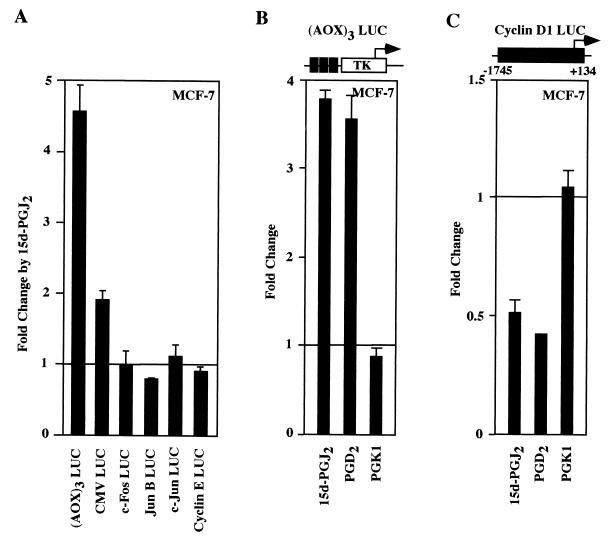
As cyclin D1 mRNA and protein levels were inhibited by 15d-PGJ2, we examined the possibility that 15d-PGJ2 may directly repress activity of the human cyclin D1 promoter in MCF-7 cells. The PPRE from the acyl-CoA oxidase (AOX)3 LUC, used as a positive control, was induced sixfold by 15d-PGJ2 (Fig. 3A and B). In contrast, the cyclin D1 promoter was repressed by 50% (Fig. 3C). In order to examine further the specificity of 15d-PGJ2-dependent transcriptional repression of cyclin D1 in MCF-7 cells, the effect of 15d-PGJ2 on both synthetic promoters (cytomegalovirus [CMV] LUC) and several natural promoters (c-fos, junB, c-jun, and cyclin E LUC) was determined (Fig. 3A). While the CMV promoter reporter was induced less than twofold, there was no significant repression of the c-fos c-jun, or junB promoters. The cyclin E promoter, which like the cyclin D1 promoter is induced in a cell cycle-dependent manner during G1-S phase progression, was not repressed by 15d-PGJ2. These studies suggest that the cyclin D1 promoter is selectively inhibited by 15d-PGJ2. Several different PPARγ ligands were next assessed to compare regulation of (AOX)3 LUC and the cyclin D1 promoter. The addition of PGD2, a PPARγ ligand that lacks the α,β unsaturated carbonyl group in the cyclopentone ring required for inhibition of IKK (52), induced (AOX)3 LUC 3.5-fold and repressed cyclin D1 promoter activity 50 to 60% (Fig. 3B and C). PGK1, which does not activate PPARγ (69), did not activate (AOX)3 LUC or repress the cyclin D1 promoter (Fig. 3B). Arachadonic acid, which is metabolized by COX to PGs and activates PPARγ (17), repressed the cyclin D1 promoter by 50% (data not shown). These findings suggest that a subset of specific natural high-affinity PPARγ ligands repress the cyclin D1 promoter.
FIG. 3.
Selective transcriptional repression of cyclin D1 by both J- and D-class prostaglandins. (A) Luciferase reporter constructions were analyzed in MCF-7 cells for the effect of 15d-PGJ2 on their activity. Cells were transfected with either (AOX)3 LUC (B) or the human cyclin D1 promoter linked to luciferase reporter genes (C), and the effect of specific PGs (10 μM) was assessed. PGD2 is a ligand for PPARγ but does not contain the α,β unsaturated carbonyl group in the cyclopentone ring required for inhibition of IKK activity (52). The data are shown as the mean ± standard deviation (SD) of at least six separate experiments.
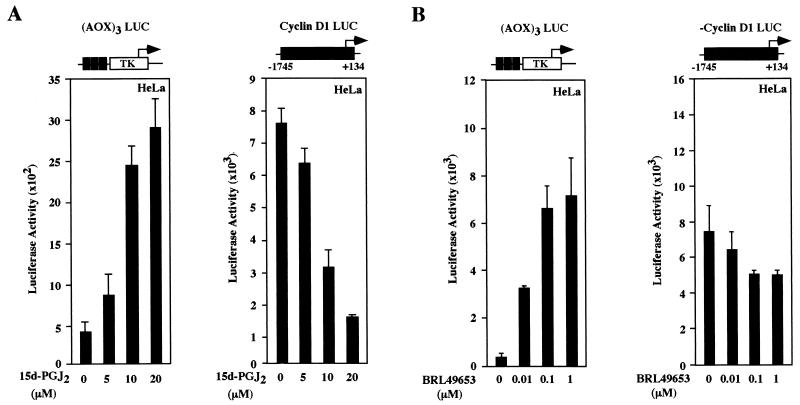
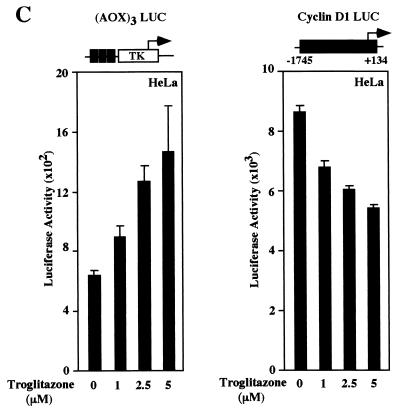
Detailed dose-response curves were conducted to compare the effects of 15d-PGJ2 with the synthetic TZD PPARγ ligands. (AOX)3 LUC was induced in the presence of coexpressed PPARγ plasmid six- to eightfold by the addition of 15d-PGJ2 (Fig. 4A). The activity of cyclin D1 promoter was reduced 50 to 80% in the presence of 15d-PGJ2 in HeLa cells in cells co transfected with the PPARγ receptor (Fig. 4A) but not in the cells transfected with control vector (data not shown). The decrease in cyclin D1 promoter activity was dose dependent with a T50 for 15d-PGJ2 of approximately 7.5 μM (within the range of the Kd of 15d-PGJ2 for PPARγ of 2 to 50 μM). The TZD BRL49653 and troglitazone induced dose-dependent induction of (AOX)3 LUC and repression of the cyclin D1 promoter (Fig. 4B and C). Together, these studies indicate that the cyclin D1 promoter is inhibited in a PPARγ-dependent manner by both natural and synthetic ligands.
FIG. 4.
Natural and synthetic PPARγ ligands repress cyclin D1 through PPARγ. HeLa cells were transfected with either (AOX)3 LUC or the human cyclin D1 promoter, together with expression plasmids for PPARγ. Cells were treated for 24 h with 15d-PGJ2 (A), BRL49653 (B), or troglitazone (C) at the indicated doses for 24 h, and luciferase activity was determined. The data are shown as the mean ± SD of at least six separate transfections.
Repression of cyclin D1 by 15d-PGJ2 is independent of the PPARγ MAPK phosphorylation site.
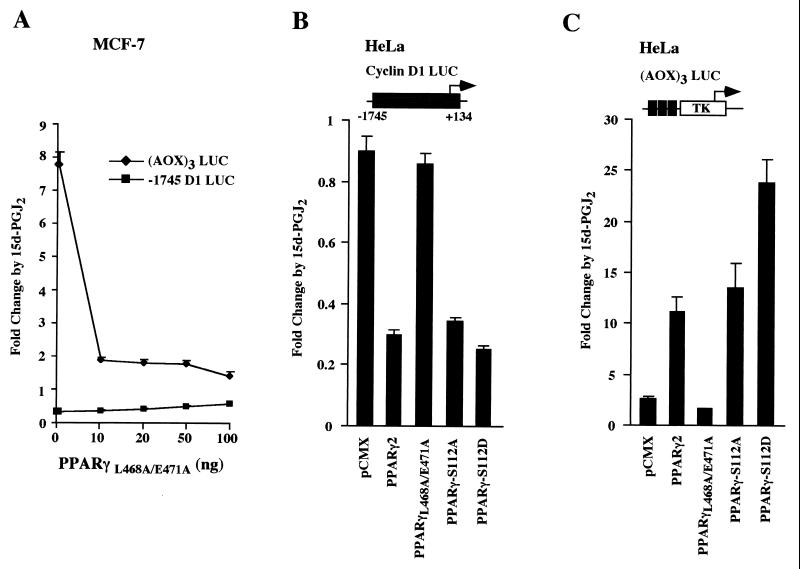
Members of the steroid hormone receptor superfamily can negatively regulate gene expression by interfering with transcription factor protein-protein interactions, by competing for DNA binding, or by competing for limiting coactivators (32, 48). PPARs bind p300 through AF-2 (24) in the presence of the ligand 15d-PGJ2 (35). To determine the mechanisms by which 15d-PGJ2 inhibited cyclin D1 expression, we assessed the activity of several mutant PPARγ expression plasmids. A dominant-negative mutant of PPARγ (PPARγL468A/E471A), which binds ligand and DNA but fails to interact with several coactivators (26), was used. This mutant functions as a dominant negative for ligand-dependent activation through binding DNA and constitutive recruitment of corepressors (26). As previously shown with the TZD BRL49653 ligand in 293EBNA cells, PPARγL468A/E471A repressed 15d-PGJ2-dependent activity induced by endogenous PPAR in MCF-7 cells (Fig. 5A) but was without effect in the absence of PPARγ in HeLa cells (Fig. 5C). In contrast, the cyclin D1 promoter was not repressed by PPARγL468A/E471A in either MCF-7 or HeLa cells (Fig. 5A and B).
FIG. 5.
PPARγ repression of cyclin D1 is MAPK function independent. (A) MCF-7 cells transfected with either the cyclin D1 promoter or the (AOX)3 LUC reporter were treated with 15d-PGJ2, and the effect of the PPARγ dominant-negative mutant PPARγL468A/E471A was assessed. (B) HeLa cells transfected with the cyclin D1 promoter and PPARγ mutants were treated with 15d-PGJ2. The PPARγL468A/E471A mutant failed to repress the cyclin D1 promoter; however, both MAPK phosphorylation site mutants of PPARγ (PPARγS112A and PPARγS112D) repressed cyclin D1. (C) The (AOX)3 LUC reporter was transfected with PPARγ mutants and treated with 15d-PGJ2.
Induction of intracellular MAPK activity by extracellular signals leads to phosphorylation of PPARγ at serine 112, inhibiting PPARγ function (1, 12, 28). Since the cyclin D1 promoter is induced by MAPK activation (4, 64, 65), we determined whether PPARγ serine 112 phosphorylation was required for cyclin D1 repression. We used point mutants defective in MAPK phosphorylation (Fig. 5B) and found that cyclin D1 promoter repression by 15d-PGJ2 was preserved with both the PPARγS112A and the PPARγS112D mutants, indicating that MAPK function was not required for liganded PPARγ inhibition of cyclin D1 promoter activity.
The cyclin D1 promoter 15d-PGJ2 DNA response element contains AP-1–p300 protein complexes.
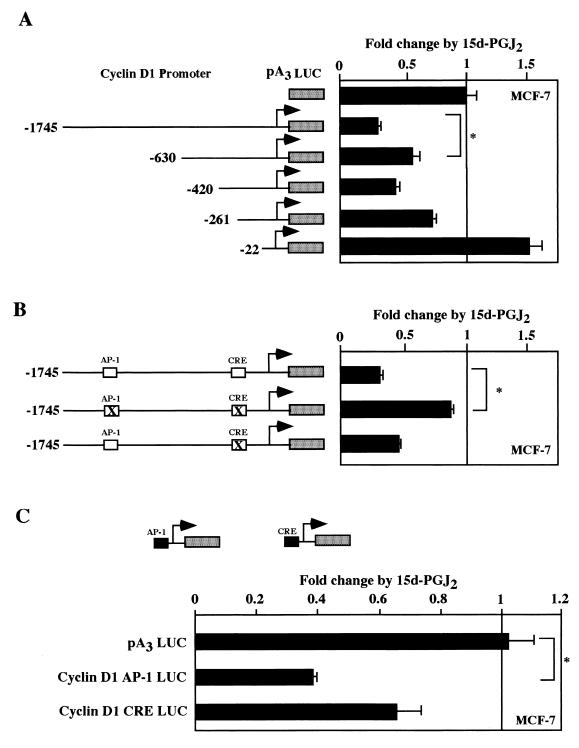
In order to identify the DNA sequences involved in the transcriptional repression of the cyclin D1 promoter by 15d-PGJ2, a series of cyclin D1 5′ promoter deletion constructs was employed (Fig. 6A). The inhibition of the cyclin D1 promoter by 15d-PGJ2 was reduced from 75 to 50% repression upon deletion from positions −1,745 to −630 (n = 6; P < 0.05). Deletion of the promoter from −261 to −22 abolished inhibition by 15d-PGJ2. DNA sequences within the −1,745 to −630 region include an AP-1 binding site (4, 65), and the −261 to −22 region contains DNA sequences capable of binding CREB–ATF-2 (64), Sp1 (63), E2F (63), and NF-κB (31). Point mutation of the CRE site in the context of the −1,745 D1 LUC construction reduced repression twofold (Fig. 4B). Additional mutation of the AP-1 site abrogated repression indicating that both the CRE and the AP-1 sites are required for full repression (Fig. 6B) (n = 6; P < 0.01). There was no significant effect upon mutation of the NF-κB site (data not shown). When the cyclin D1 AP-1 or CRE sites were linked to minimal promoters, the AP-1 site was repressed 60%, although either element was sufficient for repression by 15d-PGJ2 (Fig. 6C).
FIG. 6.
15d-PGJ2 repression of the cyclin D1 promoter through AP-1–CREB. (A) Cyclin D1 promoter 5′ deletion constructions were transfected into MCF-7 cells, the cells were treated with 15d-PGJ2 for 24 h, and luciferase activity was determined. Point mutants of the −1,745-bp cyclin D1 promoter (B) or heterologous reporters containing the cyclin D1 AP-1 site or the CRE site linked to minimal promoters (C) were assessed for regulation by 15d-PGJ2. ∗, significant differences (P < 0.05).
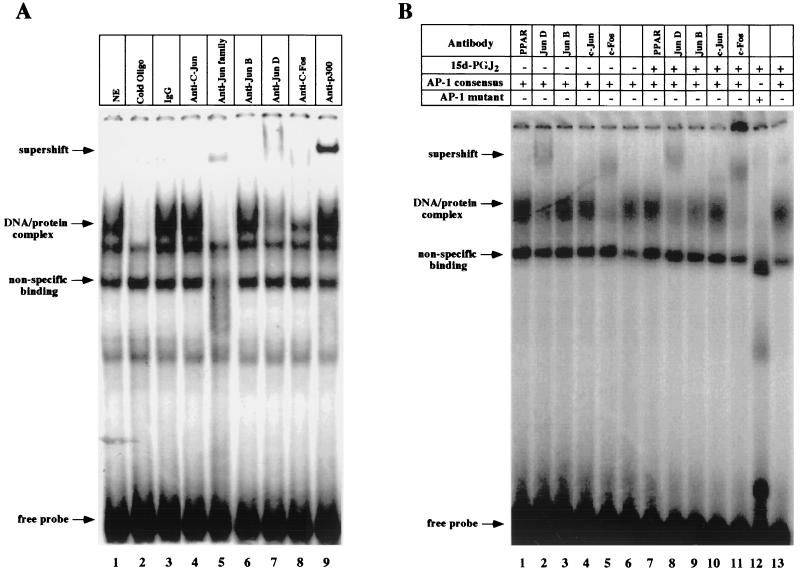
As mutation of the cyclin D1 AP-1 site abrogated the 15d-PGJ2-mediated repression of the cyclin D1 promoter in MCF-7 cells, EMSA were performed with the cyclin D1 AP-1 site. The complex formed with extracts from MCF-7 cells was competed selectively by 100-fold molar excess of cold cognate probe and was supershifted with antibodies to JUN family proteins (Fig. 7A, lane 5), JunD, c-Fos, and p300 (Fig. 7A, lanes 7 to 9). The formation of this complex was not affected by treatment with 15d-PGJ2 (Fig. 7B, lane 6 versus 13), and a PPARγ supershifting antibody did not affect the complex.
FIG. 7.
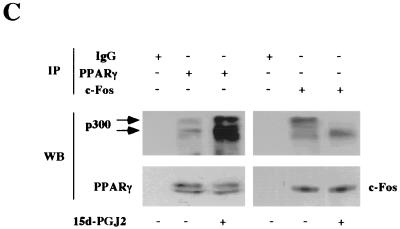
EMSA characterization of nuclear protein binding of the cyclin D1 promoter by 15d-PGJ2. (A) MCF-7 cell nuclear extracts were analyzed for binding to the γ-32P-labeled cyclin D1 AP-1 site probe. EMSA were performed with the addition of either cold cognate excess oligonucleotide or supershifting antibodies, as indicated. (B) EMSA were conducted with nuclear extracts from MCF-7 cells treated with 15d-PGJ2 or vehicle for 24 h. (C) IP-Western blot analysis was performed with cells treated with either 15d-PGJ2 or vehicle for 24 h. The IP analysis with PPARγ or c-Fos-specific antibodies or equal amounts of IgG control was subjected to Western blotting for PPARγ, c-Fos, or p300, as indicated. 15d-PGJ2 enhanced binding of p300 to PPARγ and reduced binding to c-Fos.
p300 is recruited to PPARγ by 15d-PGJ2 and rescues 15d-PGJ2-mediated repression of cyclin D1.
Because p300 functions as a coactivator for both AP-1 proteins (6) and PPARγ (24), we examined the possibility that the limiting abundance of p300 (32) may constitute a component of the transcriptional repression by 15d-PGJ2. The dominant protein binding the cyclin D1 AP-1 site in MCF-7 cells is c-Fos, which is a positive regulator of cyclin D1 promoter activity and gene expression (4, 10). We examined the possibility that 15d-PGJ2 may enhance binding of p300 to PPARγ and reduce binding to c-Fos, thereby contributing to reduced activation of AP-1 at the cyclin D1 promoter. Cells were treated with either 15d-PGJ2 or vehicle and analyzed by Western blotting or IP-Western blot analysis. PPARγ levels were unchanged by Western blotting (not shown). IP-Western blotting was performed with cells using saturating amounts of the PPARγ antibody or IgG control with sequential Western blotting for p300 or PPARγ (Fig. 7C). Equal amounts of PPARγ were detected by Western blotting in the PPARγ IP assay but not in the control lane. p300 was detected in the PPARγ IP assay, consistent with previous studies showing ligand enhanced binding of p300 to PPARγ in vitro (35). p300 runs as a doublet in randomly cycling MCF-7 cells, corresponding to differentially phosphorylated forms (67). The c-Fos IP assay contained equal amounts of c-Fos protein by Western blotting but reduced binding to p300, particularly to the hyperphosphorylated form of p300 (Fig. 7C).
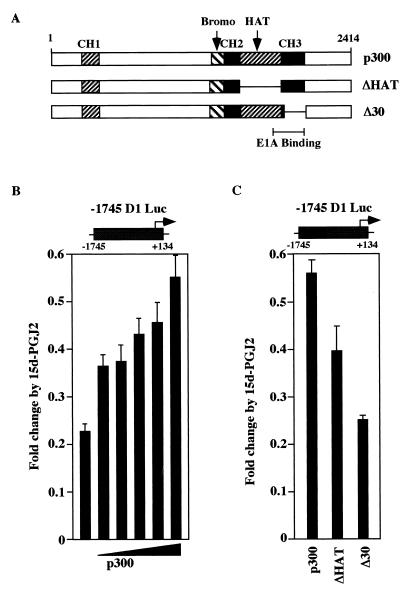
Overexpression of p300 reduced 15d-PGJ2 repression of cyclin D1 by threefold in a dose-dependent manner compared to vector control in MCF-7 cells, (Fig. 8B) as well as in HeLa cells (data not shown). Expression plasmids encoding mutants of p300 were assessed for their ability to rescue 15d-PGJ2 repression of the cyclin D1 promoter. The rescue function of p300 was abrogated by deletion of the CH3 region and partially reduced by deletion of the intrinsic histone acetylase (HAT) domain (Fig. 8C).
FIG. 8.
p300 rescue of cyclin D1 repression by 15d-PGJ2 involves the p300 HAT and CH3 domains. (A) Schematic representation of p300 expression plasmids. (B) MCF-7 cells were transfected with the −1,745-bp D1 LUC reporter and increasing amounts of the p300 expression vector or equal amounts of empty control expression vector (pCMV5). Repression by 15d-PGJ2 is shown compared with empty vector in the presence of 15d-PGJ2. (C) p300 mutants were examined for rescue of cyclin D1 repression by 15d-PGJ2. Data are shown as levels of luciferase activity (mean ± SD from six separate transfections).
15d-PGJ2 repression of cyclin D1 is independent of IKK and is distinct from mechanisms repressing iNOS.
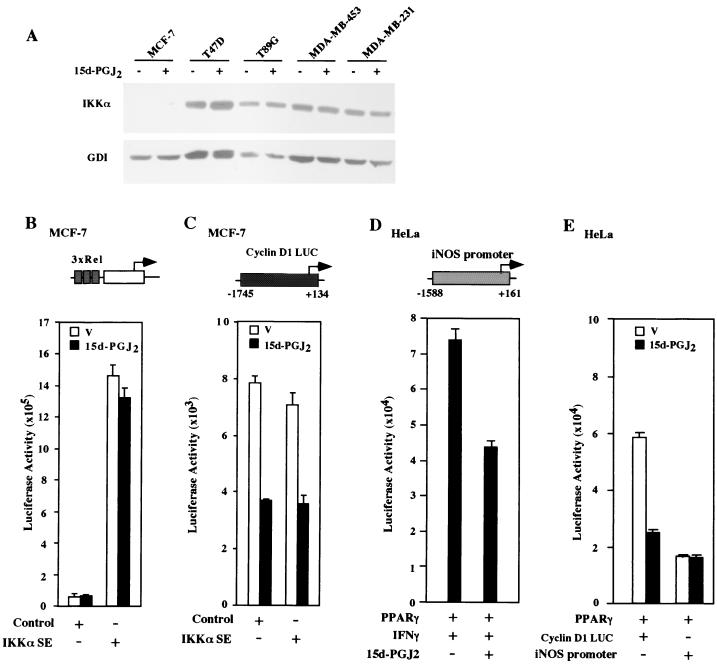
15d-PGJ2 can regulate PPARγ-independent signaling pathways and inhibit NF-κB signaling (14, 15, 52, 59). The cyclin D1 gene is induced by NF-κB (27, 31), and although the NFκB site was not required for repression of the cyclin D1 promoter, further studies were performed to exclude a possible role for NF-κB in 15d-PGJ2 repression of cyclin D1. Western blotting for IKKα was performed to determine IKKα abundance in MCF-7 cells (Fig. 9A). While IKKα was readily detected in several mammary epithelial cell lines, it was undetectable in MCF-7 cells. To determine whether 15d-PGJ2 regulated NF-κB activity in MCF-7 cells, a sensitive NF-κB reporter assay system (3xRel LUC) was used. 15d-PGJ2 did not regulate NF-κB reporter activity (Fig. 9B; mean activity of vehicle, 6.29 × 104; mean activity of treated cells, 6.27 × 104 ALU/s) at the same concentrations that repressed cyclin D1 expression and promoter activity. To assess whether IKKα was capable of regulating NF-κB activity in MCF-7 cells, activating mutants of IKK were used. The T loops of IKKα and IKKβ contain two conserved serines whose conversion to glutamates generates a constitutively active kinase (18). An activating mutation of IKKαSS/EE (36) induced the canonical NF-κB reporter 14-fold in MCF-7 cells (Fig. 9B), and this induction was not affected by the addition of 10 μM 15d-PGJ2. In contrast, the cyclin D1 promoter was inhibited by 15d-PGJ2 and was not induced by IKKαS177E (Fig. 9C). The IFN-γ-induced activity of the miNOS promoter was inhibited by 10 μM 15d-PGJ2 (Fig. 9D) as previously described (40). However, in contrast with the repression of the basal level of cyclin D1 promoter activity by 15d-PGJ2, the basal level activity of neither the miNOS (data not shown) nor the hiNOS promoter (Fig. 9E) was inhibited by 15d-PGJ2. These studies suggest that 15d-PGJ2 inhibits basal cyclin D1 promoter activity in an IKK-independent manner and through mechanisms that are distinguishable from the iNOS promoter.
FIG. 9.
15d-PGJ2 repression of cyclin D1 is independent of IKK. (A) IKKα abundance, normalized for GDI, was assessed by Western blotting in MCF-7 and several other mammary epithelial cell lines. MCF-7 cells were transfected with either the NF-κB response element reporter (3xRel LUC) (B) or the cyclin D1 promoter reporter (C). Cotransfection was conducted with activating IKKαSS/EE mutants (36) or control vector and treated with either 15d-PGJ2 or vehicle. 15d-PGJ2 does not affect basal 3xRel LUC but inhibits the cyclin D1 promoter. (D) The IFN-γ-induced activity of the miNOS promoter was inhibited by 15d-PGJ2; however, basal activity of either miNOS (not shown) or hiNOS (E) was not inhibited by 15d-PGJ2. Data are shown as levels of luciferase activity (mean ± SD from six separate transfections).
DISCUSSION
Ligands of the PPARγ nuclear receptor inhibit cellular proliferation and induce differentiation in exponentially growing fibroblasts and human breast cancer cells (5, 45). In vivo, however, these ligands enhance tumor growth (39, 53). It was hypothesized that, as PPARγ ligands also convey anti-inflammatory activity, the enhanced tumor growth may have been secondary to reduced tumor surveillance (39, 53). In recent studies, natural PPARγ ligands were shown to directly inhibit IKK activity independently of their PPARγ binding (52), and several anti-inflammatory functions of both synthetic and natural ligands have been shown to occur through PPARγ-independent means (14). We show that PPARγ ligands inhibit cellular proliferation and cyclin D1 expression through mechanisms that are distinct from these previously described anti-inflammatory effects. The inhibition of DNA synthesis by 15d-PGJ2 was associated with a selective reduction in cyclin D1 abundance, with cyclin E and Cdk4 levels being unaffected. 15d-PGJ2 inhibition of DNA synthesis was rescued through cyclin D1 overexpression. Repression of the cyclin D1 promoter by both natural (15d-PGJ2) and synthetic (BRL49653 and rosiglitazone) PPARγ ligands required PPARγ. Cyclin D1 was repressed by PGD2, which is a ligand for PPARγ that does not inhibit IKK (Fig. 3C). PGD2 does not contain the α,β unsaturated carbonyl group in the cyclopentone ring, which is essential for IKK inhibition (52). Liganded PPARγ repressed cyclin D1 through AP-1–CRE sequences, and repression was rescued through p300. Both cyclin D1 (11) and PPARγ are overexpressed in a significant proportion of human breast cancers (45), and the abundance of cyclin D1 is a rate-limiting component in human breast cancer epithelial-cell proliferation (41). The identification of the cyclin D1 gene as a direct downstream target of PPARγ provides important insight into the antiproliferative effect of PPARγ ligands.
From the present study, several lines of evidence suggest that PPARγ ligands repress cyclin D1 through mechanisms that are distinguishable from those regulating anti-inflammation through IKK which are PPARγ independent. First, at the concentration of 15d-PGJ2 that inhibited cell cycle progression and cyclin D1 expression in MCF-7 cells (Fig. 1), the basal and IKK-induced activity of a heterologous NF-κB-responsive reporter gene (3xRel LUC) (Fig. 9B) and IKK activity (data not shown) were unaffected. Second, in the present study, the effects of natural and synthetic ligands on antiproliferation were PPARγ nuclear receptor dependent. Thus, in HeLa cells, which lack PPARγ, 15d-PGJ2 inhibited IKK activity with a 50% inhibitory concentration of 5 μM (reference 52 and data not shown), without affecting cyclin D1 kinase activity, even at 10 μM (Fig. 2B). Third, ligands that do not affect IKK activity directly repressed cyclin D1 promoter activity. PGD2, which does not inhibit IKK activity (52) but binds PPARγ, repressed cyclin D1. Fourth, inhibition of cyclin D1 protein levels and promoter activity by 15d-PGJ2 was dependent upon the presence of PPARγ, whereas inhibition of IKK by 15d-PGJ2 occurs through direct covalent modification of cysteine residues within the IKK activation loop (13, 52). Finally, in the present study, synthetic PPARγ ligands (BRL49653 and troglitazone) which are not known to inhibit IKK repressed cyclin D1 and induced (AOX)3 LUC activity in a dose- and nuclear receptor-dependent manner (Fig. 4). Together these findings suggest that the inhibition of cyclin D1 by PGs is distinct from the anti-inflammatory effect mediated through inhibition of IKK.
As further evidence that the anti-inflammatory and cell cycle effects of PPARγ ligands occur through distinct mechanisms, we used the cyclin D1 promoter sequences as a molecular probe of these pathways. First, 15d-PGJ2 inhibited cyclin D1 promoter activity in MCF-7 cells (Fig. 3C) but did not inhibit NF-κB reporter activity (Fig. 9B), suggesting that inhibition of cyclin D1 by 15d-PGJ2 does not involve NF-κB. Second, the induction of NF-κB activity by an activating IKKα mutant induced the heterologous NF-κB binding site from the human immunodeficiency virus long terminal repeat but did not induce the cyclin D1 promoter (Fig. 9B), further suggesting that the cyclin D1 gene is not a target of IKKα in MCF-7 cells. Third, the DNA sequences of the cyclin D1 promoter required for repression by 15d-PGJ2 did not include the previously described NF-κB site (27, 31) but rather involved an AP-1 site that bound c-Fos–JUN–p300 complexes. Finally, the basal activity of the iNOS promoter, which is a specific target of the anti-inflammatory effects of 15d-PGJ2 (40, 50, 59), was not repressed by 15d-PGJ2 in the absence of cytokines (Fig. 9E and data not shown), unlike the cyclin D1 promoter which was selectively repressed in a dose-dependent manner.
In the present study, the use of defined PPARγ mutants, defective in MAPK phosphorylation or coactivator binding, revealed important differences between the mechanism by which PPARγ regulates the cyclin D1 gene and several other genes identified as targets of cytokines and/or tumor necrosis factor alpha (28). As noted above, mutation of the PPARγ MAPK phosphorylation site negatively regulated the transcriptional and biological functions of PPARγ in some (1, 28, 57) but not all (40, 66) studies, suggesting cell type-specific functions. Thus, PPARγS112A enhanced PPARγ activity in one study (28) but not another (40). In the present study, the MAPK phosphorylation site mutants conveyed modestly enhanced activation of a synthetic PPRE (Fig. 5B). In contrast, cyclin D1 regulation by wild-type and MAPK site mutants was identical, suggesting cyclin D1 repression is independent of MAPK signaling. The repression of cyclin D1 may therefore be mechanistically distinct from the PPARγ-mediated inhibition of lipoprotein lipase expression, which was abrogated by mutation of the MAPK phosphorylation site (28). Furthermore, these results indicate that negative regulation of cyclin D1 by PPARγ in the presence of 15d-PGJ2 is mechanistically distinct from the properties described for PPARγL468A/E471A (26) and does not involve the recruitment of corepressors. Conversely, repression does involve an active AF-2 and ligand-dependent coactivator binding (26). The MAPK independence of cyclin D1 regulation by PPARγ through natural eicosanoid ligands is consistent with prior clinical findings and may have important therapeutic implications. MAPK activity is induced in many tumors, including breast cancer (58), and is associated with resistance to the antiproliferative effect of TZDs (45). Cyclin D1 abundance in breast epithelial cells is induced by MAPK activation (64, 65) and is repressed by MAPK inhibitors (37, 38). The addition of MAPK inhibitors enhanced the antiproliferative effect of TZDs (45). The present study suggests that MAPK inhibitors may function independently of PPARγ phosphorylation in their cytostatic function through collaborative inhibition at the level of cyclin D1.
In the present study, liganded PPARγ repression of cyclin D1 was substantially reversed by p300, requiring the CH3 and intrinsic HAT domains. p300, which binds PPARγ through several domains (24), was identified within the complex binding the cyclin D1 promoter AP-1 site. The selective repression of cyclin D1 by 15d-PGJ2 and rescue by p300 are consistent with in vitro findings that 15d-PGJ2 recruits PPARγ to p300 (35). The abundance of p300 is rate limiting in transcriptional coregulation between members of the AP-1 family and several other nuclear receptors (32). In our studies, 15d-PGJ2 recruited PPARγ to p300 in living cells (Fig. 7C) and reduced binding of p300 to c-Fos. As c-Fos activity is induced by p300 (reviewed in reference 25) and c-Fos is an important activator of cyclin D1 (4, 10), the ligand-regulated reduction in binding of p300 to c-Fos may contribute to 15d-PGJ2-mediated repression of cyclin D1. PPARγ forms multisubunit coactivator complexes (DRIPs or TRAPs) and binds coactivators (SRC-1, TIF2, AIB-1 [ACTR], TRAP220 [DRIP205]) in a ligand-dependent manner through the C-terminal α-helix 12 in the LBD (19, 68). Binding of specific ligands induces distinct conformational changes in the receptor, suggesting an important capacity of PPARγ to discriminate subtle signaling events by forming distinct complexes that may in turn coordinate binding to other transcription factors, including AP-1 proteins.
The antiproliferative effect of cyPGs (54) suggests an important potential therapeutic application of PPARγ ligands. PPARγ is overexpressed in human primary and metastatic breast cancers. The present study links the antiproliferative effects of eicosanoid-liganded PPARγ to the repression of cyclin D1. Cyclin D1 is an attractive therapeutic target, as it is induced by several oncogenic signals implicated in breast and colon cancers. The identification of distinguishable mechanisms by which PPARγ regulates anti-inflammatory and antiproliferative events is of relevance to tailoring cancer therapeutics. COX2 synthesis is under NF-κB control, suggesting that inhibition of IKK by cyPGs may contribute to the inhibition of inflammation by blocking an autoregulatory activation loop. As the role of the inflammatory response in tumor therapy remains an area of controversy, the identification of distinguishable pathways by which 15d-PGJ2 regulates antiproliferative and anti-inflammatory effects may contribute to the identification of more selective anticancer therapeutics.
ACKNOWLEDGMENTS
We thank D. Baltimore, R. Evans, R. Gaynor, and C. Glass for reagents and helpful discussion.
This work was supported by grants R01CA70897, RO1CA75503, and RO1CA77552; the Komen Foundation; Breast Cancer Alliance, Inc.; and Cancer Center Core National Institute of Health grant 5-P30-CA13330-26 (to R.G.P.).
REFERENCES
- 1.Adams M, Reginato M J, Shao D, Lazar M A, Chatterjee V K. Transcriptional activation by PPARγ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 2.Akama K T, Albanese C, Pestell R G, Van Eldik L J. Amyloid β-peptide stimulates nitric oxide production in astrocytes through an NFκB-dependent mechanism. Proc Natl Acad Sci USA. 1998;95:5795–5800. doi: 10.1073/pnas.95.10.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albanese C, D'Amico M, Reutens A T, Fu M, Watanabe G, Lee R J, Kitsis R N, Henglein B, Avantaggiati M, Somasundaram K, Thimmapaya B, Pestell R G. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J Biol Chem. 1999;274:34186–34195. doi: 10.1074/jbc.274.48.34186. [DOI] [PubMed] [Google Scholar]
- 4.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 5.Altiok S, Xu M, Spiegelman B M. PPARgamma induces cell cycle withdrawal: inhibition of E2F/DP DNA-binding activity via down-regulation of PP2A. Genes Dev. 1997;11:1987–1998. doi: 10.1101/gad.11.15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias J, Alberts A S, Brindle P, Claret F X, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- 7.Ashton A W, Watanabe G, Albanese C, Harrington E O, Ware J A, Pestell R G. Protein kinase Cδ inhibition of S-phase transition in capillary endothelial cells involves the cyclin dependent kinase inhibitor p27Kip1. J Biol Chem. 1999;274:20805–20811. doi: 10.1074/jbc.274.30.20805. [DOI] [PubMed] [Google Scholar]
- 8.Brockman J A, Gupta R A, Dubois R N. Activation of PPARγ leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology. 1998;115:1049–1055. doi: 10.1016/s0016-5085(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 9.Bromberg J F, Wrzeszczynska M H, Devgan G, Zhao Y, Pestell R G, Albanese C, Darnell J E. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 10.Brown J R, Nigh E, Lee R J, Ye H, Thompson M A, Saudou F, Pestell R G, Greenberg M E. Fos family members induce cell cycle entry by activating cyclin D1. Mol Cell Biol. 1998;18:5609–5619. doi: 10.1128/mcb.18.9.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckley M F, Sweeney K J, Hamilton J A, Sini R L, Manning D L, Nicholson R I, deFazio A, Watts C K, Musgrove E A, Sutherland R L. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993;8:2127–2133. [PubMed] [Google Scholar]
- 12.Camp H S, Tafuri S R. Regulation of peroxisome proliferator-activated receptor γ activity by mitogen-activated protein kinase. J Biol Chem. 1997;272:13452–13457. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 13.Castrillo A, Díaz-Guerra M J M, Hortelano S, Martín-Sanz P, Boscá L. Inhibition of IκB kinase and IκB phosphorylation by 15-deoxy-Δ12,14-prostaglandin J2 in activated murine macrophages. Mol Cell Biol. 2000;20:1692–1698. doi: 10.1128/mcb.20.5.1692-1698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawla A, Barak Y, Nagy L, Lio D, Tontonoz P, Evans R M. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism. Nat Med. 2001;1:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 15.Chinetti G, Griglio S, Antonucci M, Torra I P, Delerive P, Majd Z, Fruchart J C, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 16.Clarke N, Arenzana N, Hai T, Minden A, Prywes R. Epidermal growth factor induction of the c-jun promoter by a Rac pathway. Mol Cell Biol. 1998;18:1065–1073. doi: 10.1128/mcb.18.2.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corton J C, Anderson S P, Stauber A. Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annu Rev Pharmacol Toxicol. 2000;40:491–518. doi: 10.1146/annurev.pharmtox.40.1.491. [DOI] [PubMed] [Google Scholar]
- 18.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 19.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 20.Elstner E, Muller C, Koshizuka K, Williamson E A, Park D, Asou H, Shintaku P, Said J W, Heber D, Koeffler H P. Ligands for peroxisome proliferator-activated receptor and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci USA. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan S, Wang J-A, Yuan R, Ma Y, Meng Q, Erdos M R, Pestell R G, Yuan F, Auborn K J, Goldberg I D, Rosen E M. BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science. 1999;284:1354–1356. doi: 10.1126/science.284.5418.1354. [DOI] [PubMed] [Google Scholar]
- 22.Forman B M, Chen J, Evans R M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. 15-Deoxy-delta12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 24.Gelman L, Zhou G, Fajas L, Raspe E, Fruchart J C, Auwerx J. p300 interacts with the N- and C-terminal part of PPARγ2 in a ligand-independent and -dependent manner, respectively. J Biol Chem. 1999;274:7681–7688. doi: 10.1074/jbc.274.12.7681. [DOI] [PubMed] [Google Scholar]
- 25.Giordano A, Avantaggiati M L. p300 and CBP: partners for life and death. J Cell Physiol. 1999;181:218–230. doi: 10.1002/(SICI)1097-4652(199911)181:2<218::AID-JCP4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Gurnell M, Wentworth J M, Agostini M, Adams M, Collingwood T N, Provenzano C, Browne P O, Rajanayagam O, Burris T P, Schwabe J W, Lazar M A, Chatterjee V K. A dominant-negative peroxisome proliferator-activated receptor γ (PPARγ) mutant is a constitutive repressor and inhibits PPARγ-mediated adipogenesis. J Biol Chem. 2000;275:5754–5759. doi: 10.1074/jbc.275.8.5754. [DOI] [PubMed] [Google Scholar]
- 27.Guttridge D C, Albanese C, Reuther J Y, Pestell R G, Baldwin A S. NF-κB controls cell growth and differentiation through the transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu E, Kim J B, Sarraf P, Spiegelman B M. Inhibition of adipogenesis through MAP kinase-mediate phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 29.Jiang C, Ting A T, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 30.Joyce, D., C. Albanese, and R. G. Pestell. NF-κB and cell-cycle regulation: the cyclin connection. Cytokine growth factor rev., in press. [DOI] [PubMed]
- 31.Joyce D, Bouzahzah B, Fu M, Albanese C, D'Amico M, Steer J, Klein J U, Lee R J, Segall J E, Westwick J K, Der C J, Pestell R G. Integration of Rac-dependent regulation of cyclin D1 transcription through an NF-κB-dependent pathway. J Biol Chem. 1999;274:25245–25249. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- 32.Kamei Y, Xu L, Heinzel T, Torchia J, Kurakawa R, Gloss B, Lin S-C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 33.Karin M, Delhase M. The IκB kinase (IKK) and NF-κB: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 34.Kliewer S A, Lenhard J M, Wilson T M, Patel I, Morris D C, Lehmann J M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 35.Kodera Y, Takeyama K-I, Murayama A, Suzawa M, Masuhiro Y, Kato S. Ligand-type specific interactions of peroxisome proliferator-activated receptor γ with transcriptional coactivators. J Biol Chem. 2000;275:33201–33204. doi: 10.1074/jbc.C000517200. [DOI] [PubMed] [Google Scholar]
- 36.Kwak Y T, Guo J, Shen J, Gaynor R B. Analysis of domains in the IKKα and IKKβ proteins that regulate their kinase activity. J Biol Chem. 2000;275:14752–14759. doi: 10.1074/jbc.m001039200. [DOI] [PubMed] [Google Scholar]
- 37.Lee R J, Albanese C, Fu M, D'Amico M, Lin B, Watanabe G, Haines G K I, Siegel P M, Hung M C, Yarden Y, Horowitz J M, Muller W J, Pestell R G. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol. 2000;20:672–683. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee R J, Albanese C, Stenger R J, Watanabe G, Inghirami G, Haines G K I, Webster M, Muller W J, Brugge J S, Davis R J, Pestell R G. pp60v-src induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways: a role for cAMP response element-binding protein and activating transcription factor-2 in pp60v-src signaling in breast cancer cells. J Biol Chem. 1999;274:7341–7350. doi: 10.1074/jbc.274.11.7341. [DOI] [PubMed] [Google Scholar]
- 39.Lefebvre A M, Chen I, Desreumaux P, Najib J, Fruchart J C, Geboes K, Briggs M, Heyman R, Auwerx J. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med. 1998;4:1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Pascual G, Glass C K. Peroxisome proliferator-activated receptor γ-dependent represion of the inducible nitric oxide synthase gene. Mol Cell Biol. 2000;20:4699–4707. doi: 10.1128/mcb.20.13.4699-4707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D–cyclin-dependent kinase–pRb-controlled G1 checkpoint. Mol Cell Biol. 1996;16:6917–6925. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marx N, Mach F, Sauty A, Leung J H, Sarafi M N, Ransohoff R M, Libby P, Plutzky J, Luster A D. Peroxisome proliferator-activated receptor-γ activators inhibit IFN-γ-induced expression of the T cell-active CXC chemokines IP10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000;164:6503–6508. doi: 10.4049/jimmunol.164.12.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumura I, Kitamura T, Wakao H, Tanaka H, Hashimoto K, Albanese C, Downward J, Pestell R G, Kanakura Y. Transcriptional regulation of cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J. 1999;18:1367–1377. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McInerney E M, Rose D W, Flynn S E, Westin S, Mullen T M, Krones A, Inostroza J, Torchia J, Nolte R T, Assa-Munt N, Milburn M V, Glass C K, Rosenfeld M G. Determinants of coactivator LXXLL motif specificity in nuclear receptor transcriptional activation. Genes Dev. 1998;12:3357–3368. doi: 10.1101/gad.12.21.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mueller E, Sarraf P, Tontonoz P, Evans R M, Martin K J, Zhang M, Fletcher C, Singer S, Spiegelman B M. Terminal differentiation of human breast cancer through PPARγ. Mol Cell. 1998;1:465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 46.Nolte R T, Wisely G B, Westin S, Cobb J E, Lambert M H, Kurokawa R, Rosenfeld M G, Willson T M, Glass C K, Milburn M V. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ. Nature. 1998;395:137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 47.Pestell R G, Albanese C, Reutens A T, Segall J E, Lee R J, Arnold A. The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr Rev. 1999;20:501–534. doi: 10.1210/edrv.20.4.0373. [DOI] [PubMed] [Google Scholar]
- 48.Pestell R G, Jameson J L. Bruce Weintraub (ed.), Molecular endocrinology: basic concepts and clinical correlations. 1995. Hormone action II: transcriptional regulation of endocrine genes by second messenger signalling pathways; pp. 59–76. [Google Scholar]
- 49.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ricote M, Li A C, Wilson T M, Kelly C J, Glass C K. The peroxisome proliferator-activated receptor γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 51.Rosen E D, Walkey C J, Puigserver P, Spiegelman B M. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 52.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karrin M, Santoro M G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 53.Saez E, Tontonoz P, Nelson M C, Alvarez J G, Ming U T, Baird S M, Thomazy V A, Evans R M. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nat Med. 1998;4:1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 54.Santoro M G, Garaci E, Amici C. Prostaglandins with antiproliferative activity induce the synthesis of a heat shock protein in human cells. Proc Natl Acad Sci USA. 1989;86:8407–8411. doi: 10.1073/pnas.86.21.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarraf P, Mueller E, Jones D, King F J, DeAngelo D J, Partridge J B, Holden S A, Chen L B, Singer S, Fletcher C, Spiegelman B M. Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nat Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 56.Schulman I G, Shao G, Heyman R A. Transactivation by retinoid X receptor-peroxisome proliferator-activated receptor γ (PPARγ) heterodimers: intermolecular synergy requires only the PPARγ hormone-dependent activation function. Mol Cell Biol. 1998;18:3483–3494. doi: 10.1128/mcb.18.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao D, Rangwala S M, Bailey S T, Krakow S L, Reginato M J, Lazar M A. Interdomain communication regulating ligand binding by PPAR-γ. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 58.Sivamaran V S, Wang H, Nuovo G J, Malbon C C. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Investig. 1997;99:1478–1483. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Straus D S, Pascual G, Li M, Welch J S, Ricote M, Hsiang C H, Sengchanthalangsy L L, Ghosh G, Glass C K. 15-deoxy-Δ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc Natl Acad Sci USA. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tontonoz P, Hu E, Graves R A, Budavari A I, Spiegelman B M. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 61.Tontonoz P, Hu E, Spiegelman B M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 62.van den Heuvel S, Harlow E. Distinct role for cyclin dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe G, Albanese C, Lee R J, Reutens A, Vairo G, Henglein B, Pestell R G. Inhibition of cyclin D1 kinase activity is associated with E2F-mediated inhibition of cyclin D1 promoter activity through E2F and Sp1. Mol Cell Biol. 1998;18:3212–3222. doi: 10.1128/mcb.18.6.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe G, Howe A, Lee R J, Albanese C, Shu I-W, Karnezis A N, Zon L, Kyriakis J, Rundell K, Pestell R G. Induction of cyclin D1 by simian virus 40 small tumor antigen. Proc Natl Acad Sci USA. 1996;93:12861–12866. doi: 10.1073/pnas.93.23.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watanabe G, Lee R J, Albanese C, Rainey W E, Batlle D, Pestell R G. Angiotensin II activation of cyclin D1-dependent kinase activity. J Biol Chem. 1996;271:22570–22577. doi: 10.1074/jbc.271.37.22570. [DOI] [PubMed] [Google Scholar]
- 66.Werman A, Hoolenberg A, Solanes G, Bjorbaek C, Vidal-Puig A J, Flier J S. Ligand-independent activation domain in the N-terminus of peroxisome proliferator-activated receptor γ (PPARγ) J Biol Chem. 1997;272:20230–20235. doi: 10.1074/jbc.272.32.20230. [DOI] [PubMed] [Google Scholar]
- 67.Yaciuk P, Moran E. Analysis with specific polyclonal antiserum indicates that the E1A- associated 300-kDa product is a stable nuclear phosphoprotein that undergoes cell cycle phase-specific modification. Mol Cell Biol. 1991;11:5389–5397. doi: 10.1128/mcb.11.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang W, Rachez C, Freedman L P. Discrete roles for peroxisome proliferator-activated receptor γ and retinoid X receptor in recruiting nuclear receptor coactivators. Mol Cell Biol. 2000;20:8008–8017. doi: 10.1128/mcb.20.21.8008-8017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu K, Bayona W, Kallen C B, Harding H P, Ravera C P, McMahon G, Brown M, Lazar M A. Differential activation of peroxisome-proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270:23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 70.Zafonte B, Amanatullah D, Sage D, Augenlicht L H, Pestell R G. Ras regulation of cyclin dependent immunoprecipitation kinase assays. Regulators and effectors of small GTPases. Methods Enzymol. 2001;333:127–138. doi: 10.1016/s0076-6879(01)33051-3. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Y, Qi C, Jain S, Rao M S, Reddy J K. Isolation and characterization of PBP, a protein that interacts with peroxisome proliferator-activated receptor. J Biol Chem. 1997;272:25500–25506. doi: 10.1074/jbc.272.41.25500. [DOI] [PubMed] [Google Scholar]