Abstract
Since the Cambrian, animals diversified from a few body forms or bauplans, into many extinct and all extant species. A characteristic neural architecture serves each bauplan. How the connectome of each animal differs from that of closely related species or whether it converged into an optimal architecture shared with more distant ones is unknown. Recent technological innovations in molecular biology, microscopy, digital data storage and processing, and computational neuroscience have lowered the barriers for whole-brain connectomics. Comparative connectomics of suitable, relatively small, representative species across the phylogenetic tree can infer the archetypal neural architecture of each bauplan and identify any circuits that possibly converged onto a shared and potentially optimal, structure.
Highlights
-
•
Technological innovations have lowered the barriers for whole-brain connectomics.
-
•
Comparative connectomics (CC) across suitable representative species is now feasible.
-
•
CC infers the common neural architecture across closely related animal species.
-
•
CC reveals the neural circuit details that make animals different from each other.
-
•
CC identifies structurally convergent circuits in distantly related species.
Introduction
Five hundred and fifty million years of evolution have generated an astounding diversity of animal forms with unique neural architectures. Grouped by common descent, each animal clade presents a characteristic, identifiable bauplan, served by an idiosyncratic neural architecture. While similarities are more evident among recently diverged groups, deep homologies among distant clades have been suggested by gene expression [1], developmental patterns [2], and neural organization [3]. In vertebrates, the division of the pallium into four neurogenetic regions has been reported in clades as distant as fishes and mammals [4]. In invertebrates, neuroblasts and their progeny of neuronal cell types are conserved not only across insect orders [5,6] but also within outgroups, such as crustaceans [7] and tardigrades [8]. With insects [9], fishes [10], and cephalopods [11] dating from the Cambrian, their nervous system architectures have remained stable over hundreds of millions of years while simultaneously specializing in a myriad of ways under evolutionary pressures. An approach to analyze neural architectures is comparative connectomics, consisting of first mapping the synaptic wiring diagram or connectome, of the whole or a part of a nervous system, and then analyzing its structure relative to another connectome, either of another species or of a different genotype or life stage [12∗∗, 13∗∗, 14∗∗]. A comparative connectomics approach across representative species (Figure 1) would overcome inter- and intraspecies variability to identify the fundamental neural circuit architecture of each bauplan as well as any shared yet nonhomologous circuit, a product of convergent evolution.
Figure 1.
Phylogenetic tree of possible candidate reference species for comparative connectomics plus a few others for reference such as humans. See also Table 1.
Existing connectomes
Today, connectomics research focuses primarily on the mouse [15, 16, 17∗∗], fruit fly [18, 19∗, 20, 21∗, 22∗], nematode Caenorhabditis elegans [14,23, 24, 25], and zebrafish [26], with additional contributions from polychaete worms (Platynereis sp.; [27]), chordates (Ciona intestinalis, [28]), and others. This contrasts with the origin of neuroscience as a discipline, where more and diverse species were used [29,30], leading to seminal discoveries such as action potentials in the giant axon of the squid [31], synaptic function in Aplysia [32], and in the relationship between neural circuit structure and function in crayfish [33,34].
For the four species that dominate neuroscience research, there is a complete connectome for one (C. elegans [14,23, 24, 25]) as well as complete electron microscopy (EM) volumes with partial connectomes for two (Drosophila [18, 19∗, 20, 21∗, 22∗] and Danio rerio [26]) and a proposal to map one (Mus musculus [35]). Broadening our reach beyond these few species will open up the opportunity to discover fundamental neural circuit architectures [30,36].
Techniques
Step improvements in electron microscopy (EM) automation, namely reliable focused ion beam scanning electron microscopy (FIB-SEM) for isotropic small volumes [37] and GridTape transmission electron microscopy (TEM) for much larger volumes [38], have expanded the set of feasible species (Table 1) to larger animals. Such larger samples require reliable, high-performance image registration methods to assemble continuous EM volumes from millions of image tiles, overcoming nonlinear deformations and artifacts [39,40]. In turn, larger EM volumes have shifted the focus from manual methods for neuronal arbor reconstruction and synapse annotation [41,42,43] to automated ones that target precision (accuracy) at the expense of recall (completeness) [21,44, 45, 46], guided by studies on redundancy in synaptic connectivity [42,47]. Reconstructed neurons are then matched across image modalities by registration and morphological similarity (e.g. with neuron BLAST (NBLAST); [48]), enriching connectomes with functional information [15, 16, 17∗∗, 18] or neurotransmitter signatures [18], with the latter also inferred directly from EM [49]. Comparing the resulting connectomes across left-right symmetric brain hemispheres or across individuals or species requires matching graph nodes — where a node is a neuron or a group of neurons in a coarsened graph — by either exploiting known shared molecular information, location, and morphology (such as insect neuroblasts and their progeny of neurons [5,6] or cortical neurons [50]) to seed the alignment of at least some nodes across graphs [51] or from connectivity only with spectral graph analysis [24,42]. With improvements across the board, we now have the opportunity to traverse the whole phylogenetic tree to sample representative species in the light of comparative connectomics.
Table 1.
List of brain volumes and estimated imaging time with GridTape TEM [38], using the formula Timeimaging = (Volumebrain∗151/0.25), with 151 being the number of days necessary to acquire a volume of 1×0.5 × 0.5 mm according to Graham et al. (2019). Asterisk, volumes that can be acquired in less than or up to about a year of 24/7 imaging. TEM, transmission electron microscopy.
Suitable representative species
An ideal data set includes whole-brain connectomes of both closely related and widely divergent species. Presently, only EM of densely labeled samples can deliver the complete, nanometer-resolution volumes necessary for mapping every neuronal arbor and synapse. A number of practical constraints reduce the pool of possible species (Table 1).
First, sample preparation for densely labeled, whole-brain connectomics is lengthy and costly, as evidenced by work in Drosophila [52] and the mouse [53]. Small brains available in large numbers ease the development of sample preparation protocols, which favor animals with fast life cycles and abundant progeny.
Second, in practice, sample dimensions are constrained to ∼1 mm3 by the combination of resolution requirements, imaging speed, data costs, and funding cycles.
Third, free-living, nonparasitic small animals retaining a full complement of ancestral body parts and brain structures are best suited for both comparisons of individual brain modules and whole-brain architectural relationships. As follows, the comparison of a blind fish, for example, with an anosmic snake would be limited to brain structures besides olfaction and limb-based locomotion. This constraint favors a small lizard [54, 55] over a small soil-dwelling anosmic snake that presents poor vision [56] and, likewise, favors a small nonblind fish, such as Danionella sp. [57]. Exceptionally, species that lost body parts while retaining the corresponding neural modules would serve as models for how a neural architecture takes on new functions (e.g. visual inputs dominate the mushroom bodies of anosmic beetles [58]), a situation inducible experimentally [59, 60, 61∗∗].
To overcome most constraints, an option is to consider juveniles. Typically, some animal groups present juveniles that closely resemble adults, such as coleoid cephalopods, reptiles, and some fishes, among many others. This approach works best when juveniles live independently of parental care, indicating that all aspects of their brains are already functional, except for those related to sexual maturity. An example, if unconstrained by dimensions would be the juvenile of some crocodiles that have been shown to present approximately the same number of neurons and presumably the same overall neural architecture, as the adults, differing primarily in neuronal cell size, not number [62]. Within the dimensional constraints, we find free-living lizard hatchlings, such as the chameleon Brookesia sp. [54] and the gecko Sphaerodactylus sp. [55, 63∗], and cephalopod hatchlings, such as Idiosepius sp. [64]. A comparative connectomics approach targeting free-living juveniles would save time and resources (animal length ∼ volume3) while meeting the above constraints and compromising only on circuits associated with sexual maturity.
Case studies
Evolution of a brain structure: the cortical microcircuit
The apparent uniformity of the mammalian neocortex [65] suggested the existence of a basic microcircuit repeated throughout all cortical areas [66]. On the basis of sparsely sampled neuronal anatomy and electrophysiology of the cat and monkey visual cortex, a diagram for the basic cortical microcircuit was proposed, limited to excitatory neurons [67]. The addition of inhibitory connections led to the reformulation of the diagram as a canonical microcircuit that captured commonly observed motifs across multiple areas and species and which suggested fundamental features of cortical processing [68]. Mainly, the inseparability of excitation and inhibition, and the primacy of intracortical excitation over thalamic drive. Synaptic weights were later estimated from further sparse anatomical reconstructions [69].
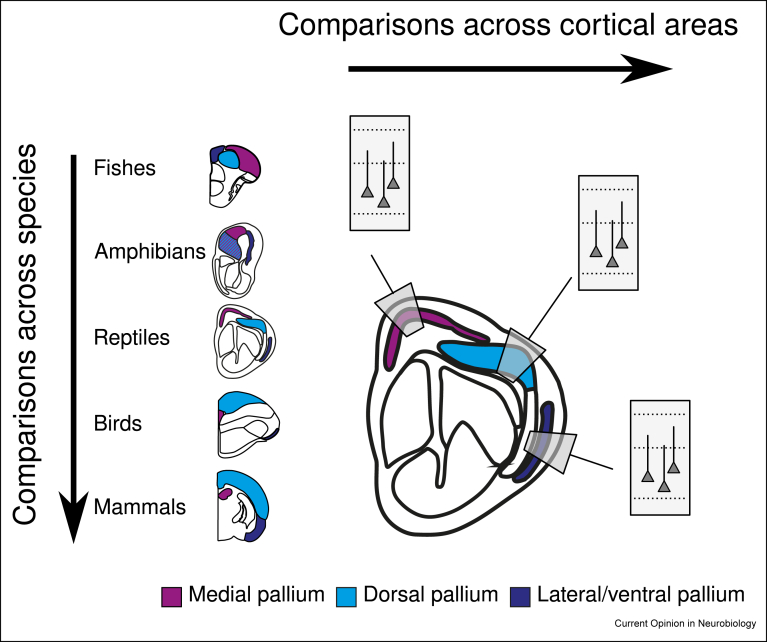
The hypothesis of a repeated unit of computation throughout the cortex is attractive for its reductionist properties: the study of the immense cortical sheet becomes the study of its building block and interblock relationships. Dense reconstructions of volumes of cortex at synaptic resolution possibly containing a complete canonical microcircuit have only recently become possible for limited subregions of the mouse brain [15, 16, 17∗∗]. While the many similarities in microcircuit structure across cortical areas grant enormous support to the canonical microcircuit hypothesis, differences exist across areas and species [70, 71, 72, 73, 74, 75]. The reconstruction of multiple instances of the cortical microcircuit in multiple cortical areas of various vertebrates will identify commonalities and differences in the cortical microcircuit of each brain area and species (Figure 2).
Figure 2.
Schematic representation of the comparison of cortical microcircuits across brain areas and species, on the basis of known genetic and developmental correspondences within the vertebrates. Cartoons show the right brain hemisphere; adapted from Naumann et al., 2015, with permission.
All amniotes — mammals, birds, and the polyphyletic reptiles — present a layered cortical sheet suitable for the study of the homogeneity or heterogeneity of the canonical microcircuit [76,77]. The architecture of the vertebrate forebrain is thought to be conserved across all vertebrates, including the lamprey [78, 79].
Extant reptiles offer a useful model of vertebrate cortical architectures [50]. Juvenile lizards, including Brookesia sp. [54] and Sphaerodactylus sp. [55] [63], are free-living predators with a complete tetrapod bauplan and brain volumes of ∼1 cubic millimeter.
Comparative connectomics of cortical columns from the same homologous brain regions across species, such as lizards and rodents, will highlight a possibly conserved cortical microcircuit and which circuit motifs are unique to mammals. Across the whole brain, such comparisons will further identify large-scale common circuits interrelating different cortical areas and whether such patterns are already present outside amniotes. In summary, mapping the cortical architectures of multiple small vertebrates opens the opportunity to infer the archetypal cerebral circuit architecture.
Evolution of a substrate for computation: circuits for pursuit predation
Vision-driven behaviors, such as pursuit predation (the tracking and interception of prey), are present in coleoid cephalopods, vertebrates, and insects. Successful predation requires the integration of the prey motion vector with self-motion to intercept the prey [80]. Supporting these abilities is a visual system capable of distinguishing prey from background, tracking prey relative motion, and anticipating future prey position. Although coleoid cephalopods, vertebrates, and insects contain vastly diverse nervous systems, all comprise species that engage in pursuit predation. The possibility exists that some aspects of the neural circuit architectures for visually guided predation have converged throughout evolution into a common, optimal configuration in animals with shared ecological niches such as fishes and squids.
Pursuit predation consists of three major components: visual tracking of prey; computation of speed and direction vectors; and motor planning toward interception.
Vertebrates and coleoid cephalopods present camera eyes of superficial similarities but deep developmental and structural differences [81], whereas insects present compound eyes (Figure 3). Despite divergent eye morphology, strong parallels have been found in the circuits for motion detection in mammals and insects [82]. The coleoid cephalopod's visual circuits are mostly unknown but present a single-layer retina and an associated optic lobe [83]. Visual signals in all three animal groups arrive at the brain already processed: in mammals by the multilayered retina; and in insects and cephalopods by the optic lobes. The early visual circuits of the retina or associated optic lobes compute direction of motion of objects in the visual field, in insects and mammals [82]. Presumably, the visual circuits of coleoid cephalopods, like the fly's, also implement an equivalent to the Hassenstein-Reichardt motion detector [84]. The mammalian and insect retinas have been studied in depth with connectomics [84, 85, 86, 87]. Comparative connectomics of the visual circuits of species that share a bauplan (e.g., squids and octopus) will establish a baseline against which any similarities with species of other bauplans (e.g., insects and mice) could be interpreted as potentially optimal, products of convergent evolution.
Figure 3.
Phylogenetic tree illustrating differences and similarities in eye structure among insects, coleoid cephalopods, and vertebrates, with the insect presenting a compound eye and the other two a camera eye. For full comparisons with the multilayered circuits of the vertebrate retina, additional brain structures of the insect and cephalopod must be considered such as their corresponding optic lobes. Blue, lens or crystalline cone; red, photoreceptors. Original hand drawings by Ana Correia.
In pursuit predation, in addition to tracking prey motion, the predator must compute an intersection trajectory that not only compensates for prey motion but also accounts for its own head and body motion. In mammals, circuits in the midbrain including the superior colliculus represent motor space [88]. In insects, circuits in the central brain including the central complex encode body direction [89]. The geometric computations of the multiple direction vectors are implemented in neural circuits whose architecture can be compared across species. Whether the circuits for spatial orientation share an overall architecture across species can be studied by whole-brain comparative connectomics of suitably small species.
All foraging animals, regardless of limb presence and overall body morphology, are endowed with different locomotion modes; therefore, the neural circuits for coordinated movement postsynaptic to command neurons will be idiosyncratic for each. Upstream, in neural circuits for decision making, surprising conservation has been observed. One remarkable example of conserved intermediate circuits connected to different, specialized motor modules is the Mooncrawler/Moonwalker neuron, which controls backward locomotion for both the limbless Drosophila larva and the legged adult [90]. In analyzing the connectomes of vertebrates, insects, and coleoid cephalopods, we expect extreme diversity of neural circuits for locomotion but potentially shared neural architectures for optimally computing direction vectors and behavior selection.
The connectomes of Drosophila, the zebrafish, and the mouse retina are either complete or imminent, whereas no studies have yet mapped the visual circuits of a cephalopod. Meeting all of the constraints, the pygmy squid Idiosepius sp. and the pygmy octopus Octopus joubini both present free-living juveniles with brain volumes within a cubic millimeter. Mapping the cephalopod connectome from an EM volume of the whole body, as is now possible for Idiosepius juveniles, will address further questions central to this phylum, including camouflage control [91] and soft limb coordination throughout multiple styles of locomotion and tool manipulation.
Analysis of diversification: the insect brain
Insects are likely the most speciose group of animals on Earth, with the Coleoptera (beetles; particularly the Phytophaga clade [92]), Hymenoptera (wasps, bees, ants, and sawflies; particularly parasitoid wasps [93]), and Diptera (flies, midgets, and mosquitoes; particularly the Cecidomyiidae family [94]) being extraordinarily rich. The impact of insects on human life is immense, either as pests or vectors of deadly diseases (mosquitoes; [95,96]) or for their vital ecosystem services, such as the pollination of crops and pest control [97]. An approach to pest control that harms pollinators would result in a net loss, a predicament human societies are currently facing. Comparative connectomics would reveal the commonalities and particularities of each insect group and enable the design of targeted pest control, for example, by molecularly targeting circuits for human-seeking behavior (e.g. CO2 plume tracking [98]) while avoiding interference with beneficial services such as pollination (e.g. sensing flower-specific odors [99]).
Beyond the use of insects as experimental subjects for understanding cognition [100,101], these tiny animals pack mighty abilities, rivaling computer vision systems many orders of magnitude their size with extremely small energy requirements, and have been a continuous source of inspiration in engineering (e.g. [102, 103∗, 104∗]) and machine learning (e.g. [104∗, 105∗]). The reduced dimensions and numerically reduced nervous systems of insects offer unmatched experimental tractability.
The connectomes of the adult and larval Drosophila brain are almost fully mapped [18, 19∗, 20,22]. Among the Hymenoptera, species as large as bumblebees [106] and as small as fairy wasps [107] are currently under study. Meeting our criteria of small, complete, free-living, accessible species, we find the adult Drosophila melanogaster, the beetle Tribolium castaneum, the honeybee Apis mellifera, and the mosquito Anopheles gambiae, and all four of them are already laboratory animals and realistic targets for whole-brain connectomics today. The brains and nerve cords of all of these species share a recognizable architecture, with genetically identified neuroblasts [5,6] and individual neurons morphologically recognizable across species. Comparative connectomics across these species would produce an approximated insect brain neural circuit archetype, alongside species-specific brain modules and circuit motifs that confer each insect group with unique properties. With these animals, we now have the opportunity to understand in what way each different insect species has specialized its brain to better fit its ecological niche, in a process of divergent evolution, and how, in a process of convergent evolution, some of their brain structures — for example, the olfactory system [108,109], the visual system [82], and the learning and memory system [110] — have converged with those of distantly related animals.
Conclusion
The study of neural circuit architectures with synaptic resolution, or connectomics, has until now focused on a few species, primarily a nematode, a fly, a fish, and a mouse. Concentrating resources on few species generated synergies from the sharing of tools, databases, and understanding, which carried the neuroscience field forward. Now, technological improvements across the board open the opportunity to explore the diversity of nervous systems across the tree of life. With comparative connectomics, the search for neural circuit architectures common across species or independently converged into an optimal layout is now possible.
Conflict of interest statement
Nothing declared.
Acknowledgements
The authors thank Laura Lungu for posing as a model for the illustration of a human in Figure 3. The authors thank Nadine Randel and Marc Corrales for discussions on evolution. A. Cardona thanks the Wellcome Trust Investigator Award 205038/Z/16/Z and the MRC LMB for funding. We thank G. Laurent for helpful comments.
This review comes from a themed issue on Evolution of Brains and Computation
Edited by Catherine Carr, Fred Wolf and Gilles Laurent
References
- 1.Hirth F., Reichert H. Conserved genetic programs in insect and mammalian brain development. Bioessays. 1999;8:677–684. doi: 10.1002/(SICI)1521-1878(199908)21:8<677::AID-BIES7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Puelles L., Sandoval J.E., Ayad A., del Corral R., Alonso A., Ferran J.L., Martínez-de-la Torre M. The pallium in reptiles and birds in the light of the updated tetrapartite pallium model. Evol Nerv Syst. 2017;1:519–555. [Google Scholar]
- 3.Strausfeld Nicholas J., Hirth Frank. Deep homology of arthropod central complex and vertebrate basal ganglia. Science. 2013;340:157–161. doi: 10.1126/science.1231828. [DOI] [PubMed] [Google Scholar]
- 4.Mueller Thomas, Dong Zhiqiang, Berberoglu Michael A., Guo Su. The dorsal pallium in zebrafish, Danio rerio (cyprinidae, teleostei) Brain Res. 2011;1381:95–105. doi: 10.1016/j.brainres.2010.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witten J.L., Truman J.W. Distribution of GABA-like immunoreactive neurons in insects suggests lineage homology. J Comp Neurol. 1998;398:515–528. doi: 10.1002/(sici)1096-9861(19980907)398:4<515::aid-cne4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Schmid A., Chiba A., Doe C.Q. Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development. 1999;126:4653–4689. doi: 10.1242/dev.126.21.4653. [DOI] [PubMed] [Google Scholar]
- 7.Strausfeld Nicholas J. Crustacean–insect relationships: the use of brain characters to derive phylogeny amongst segmented invertebrates. Brain Behav Evol. 1998;52:186–206. doi: 10.1159/000006563. [DOI] [PubMed] [Google Scholar]
- 8.Smith Frank W., Bartels Paul J., Goldstein Bob. A hypothesis for the composition of the tardigrade brain and its implications for panarthropod brain evolution. Integr Comp Biol. 2017;57:546–559. doi: 10.1093/icb/icx081. [DOI] [PubMed] [Google Scholar]
- 9.Ma Xiaoya, Hou Xianguang, Edgecombe Gregory D., Strausfeld Nicholas J. Complex brain and optic lobes in an early Cambrian arthropod. Nature. 2012;490:258–261. doi: 10.1038/nature11495. [DOI] [PubMed] [Google Scholar]
- 10.Simon Conway Morris and Jean-Bernard Caron A primitive fish from the Cambrian of north America. Nature. 2014;512:419–422. doi: 10.1038/nature13414. [DOI] [PubMed] [Google Scholar]
- 11.Smith Martin R., Caron Jean-Bernard. Primitive soft-bodied cephalopods from the Cambrian. Nature. 2010;465:469–472. doi: 10.1038/nature09068. [DOI] [PubMed] [Google Scholar]
- Gerhard S., Andrade I., Fetter R.D., Cardona A., Schneider-Mizell C. eLife; 2017. Conserved neural circuit structure across Drosophila larval development revealed by comparative connectomics. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comparative connectomics approach to studying neural circuit development in Drosophila larvae revealed how neuronal arbors grow in size and synaptic input number while the circuit structure remains constant.
- Valdes-Aleman Javier, Fetter Richard D., Emily C Sales, Chris Q Doe, Landgraf Matthias, Cardona Albert, Zlatic Marta. bioRxiv; 2019. Synaptic specificity is collectively determined by partner identity, location and activity; p. 697763. [Google Scholar]; A comparison of the circuit wiring diagrams between wild-type and various mutants in Drosophila identified a structural change that explained the altered behavior of the mutants.
- Witvliet Daniel, Mulcahy Ben, Mitchell James K., Meirovitch Yaron, Berger Daniel R., Wu Yuelong, Liu Yufang, Wan Xian Koh, Parvathala Rajeev, Douglas Holmyard, Schalek Richard L., Shavit Nir, Chisholm Andrew D., Lichtman Jeff W., Samuel Aravinthan D.T., Zhen Mei. Connectomes across development reveal principles of brain maturation. Nature. 2021:1–5. doi: 10.1038/s41586-021-03778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comparative connectomics approach to studying neural circuit development in C. elegans revealed how a circuit grows in neuronal number and remodels itself while remaining functional.
- 15.Bock D.D., Lee W.C., Kerlin A.M., Andermann M.L., Hood G., Wetzel A.W., Yurgenson S., Soucy E.R., Kim H.S., Reid R.C. Network anatomy and in vivo physiology of visual cortical neurons. Nature. 2011;47:177–182. doi: 10.1038/nature09802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen Lee Wei-Chung, Bonin Vincent, Reed Michael, Graham Brett J., Hood Greg, Glattfelder Katie, Reid R Clay. Anatomy and function of an excitatory network in the visual cortex. Nature. 2016;532:370–374. doi: 10.1038/nature17192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICrONS Consortium. Alexander Bae J., Baptiste Mahaly, Bodor Agnes L., Brittain Derrick, Buchanan JoAnn, Bumbarger Daniel J., Castro Manuel A., Celii Brendan, Cobos Erick, Collman Forrest, Maçarico da Costa Nuno, Dorkenwald Sven, Elabbady Leila, Fahey Paul G., Tim Fliss, Froudakis Emmanouil, Gager Jay, Gamlin Clare, Halageri Akhilesh, James Hebditch, Jia Zhen, Jordan Chris, Kapner Daniel, Kemnitz Nico, Sam Kinn, Koolman Selden, Kuehner Kai, Lee Kisuk, Li Kai, Lu Ran, Macrina Thomas, Mahalingam Gayathri, McReynolds Sarah, Miranda Elanine, Mitchell Eric, Mondal Shanka Subhra, Moore Merlin, Shang Mu, Muhammad Taliah, Nehoran Barak, Ogedengbe Oluwaseun, Papadopoulos Christos, Papadopoulos Stelios, Patel Saumil, Pitkow Xaq, Popovych Sergiy, Ramos Anthony, Clay Reid R., Reimer Jacob, Casey M., Schneider-Mizell, Sebastian Seung H., Silverman Ben, Silversmith William, Amy Sterling, Sinz Fabian H., Smith Cameron L., Suckow Shelby, Tan Zheng H., Tolias Andreas S., Torres Russel, Turner Nicholas L., Walker Edgar Y., Wang Tianyu, Williams Grace, Williams Sarah, Kyle Willie, Ryan Willie, Wong William, Wu Jingpeng, Xu Chris, Yang Runzhe, Yatsenko Dimitri, Ye Fei, Yin Wenjing, Yu Szi-chieh. bioRxiv; 2021. Functional connectomics spanning multiple areas of mouse visual cortex. [Google Scholar]; A large-scale study captured the activity of 75,000 neurons from the mouse visual cortex and then reconstructed their synaptic connectivity from volume EM.
- 18.Ohyama Tomoko, Schneider-Mizell Casey M., Fetter Richard D., Valdes Aleman Javier, Franconville Romain, Rivera-Alba Marta, Mensh Brett D., Branson Kristin M., Simpson Julie H., James W Truman, Cardona Albert, Zlatic Marta. A multilevel multimodal circuit enhances action selection in Drosophila. Nature. 2015;520:633–639. doi: 10.1038/nature14297. [DOI] [PubMed] [Google Scholar]
- Winding Michael, Benjamin Pedigo, Barnes Chris L., Patsolic Heather G., Park Youngser, Kazimiers Tom, Fushiki Akira, Andrade Ingrid V., Li Feng, Aleman Javier Valdes, Khandelwal Avinash, Herren Laura, Randel Nadine, Barsotti Elizabeth, Correia Ana, Fetter Richard D., Hartenstein Volker, Priebe Carey E., Vogelstein Joshua, Zlatic Marta, Cardona Albert. 2021. The wiring diagram of the Drosophila larval brain. in preparation. [Google Scholar]; The complete connectome of the Drosophila larval brain, comprising over 3000 fully mapped neurons, reveals the complexity of even numerically reduced nervous systems and their modular organization.
- 20.Zheng Zhihao, Lauritzen J Scott, Perlman Eric, Robinson Camenzind G., Nichols Matthew, Milkie Daniel, Omar Torrens, Price John, Fisher Corey B., Sharifi Nadiya, Steven A., Calle-Schuler, Kmecova Lucia, Ali Iqbal J., Karsh Bill, Trautman Eric T., Bogovic John, Hanslovsky Philipp, Gregory S., Jefferis X.E., Kazhdan Michael, Khairy Khaled, Saalfeld Stephan, Fetter Richard D., Bock Davi D. BioRxiv; 2017. A complete electron microscopy volume of the brain of adult Drosophila melanogaster; p. 140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Peter H., Lindsey Larry F., Januszewski Michał, Tyka Mike, Maitin-Shepard Jeremy, Tim Blakely, Jain Viren. Automated reconstruction of a serial-section em Drosophila brain with flood-filling networks and local realignment. Microsc Microanal. 2019;25:1364–1365. [Google Scholar]; A novel computer vision method to map neuronal arbors successfully reconstructed large fragments of a serial section volume EM of the whole Drosophila brain.
- Scheffer L.K., Xu C.S., Januszewski M., Lu Z., Takemura S.Y., Hayworth K.J., Huang G.B., Shinomiya K., Maitlin-Shepard J., Berg S., Clements J. A connectome and analysis of the adult drosophila central brain. Elife. 2020;9 doi: 10.7554/eLife.57443. [DOI] [PMC free article] [PubMed] [Google Scholar]; The partial connectome of the Drosophila central brain, mapped from volume EM, serves as a reference for follow up studies in neural circuit architecture and function.
- 23.White J.G., Southgate E., Thomson J.N., Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans Roy Soc Lond B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 24.Varshney Lav R., Chen Beth L., Paniagua Eric, Hall David H., Chklovskii Dmitri B. Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput Biol. 2011;7 doi: 10.1371/journal.pcbi.1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarrell Travis A., Wang Yi, Bloniarz Adam E., Brittin Christopher A., Xu Meng, Thomson J Nichol, Albertson Donna G., Hall David H., Emmons Scott W. The connectome of a decision-making neural network. Science. 2012;337:437–444. doi: 10.1126/science.1221762. [DOI] [PubMed] [Google Scholar]
- 26.Hildebrand David Grant Colburn, Cicconet Marcelo, Torres Russel Miguel, Choi Woohyuk, Quan Tran Minh, Moon Jungmin, Wetzel Arthur Willis, Champion Andrew Scott, Graham Brett Jesse, Owen Randlett, Plummer George Scott, Portugues Ruben, Bianco Isaac Henry, Saalfeld Stephan, Baden Alex, Lillaney Kunal, Burns Randal, Vogelstein Joshua Tzvi, Schier Alexander Franz, Allen Lee Wei-Chung, Jeong Won-Ki, Lichtman Jeff William, Engert Florian. Whole-brain serial-section electron microscopy in larval zebrafish. Nature. 2017;545:345–349. doi: 10.1038/nature22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verasztó C., Jasek S., Gühmann M., Shahidi R., Ueda N., Beard J.D., Mendes S., Heinz K., Bezares-Calderón L.A., Williams E., Jékely G. bioRxiv; 2020. Whole-animal connectome and cell-type complement of the three-segmented Platynereis dumerilii larva. [Google Scholar]; The complete connectome of the larval marine annelid Platynereis, comprising over 1500 neurons controlling four different effector systems.
- 28.Ryan Kerrianne, Lu Zhiyuan, Meinertzhagen Ian A. The CNS connectome of a tadpole larva of Ciona intestinalis (l.) highlights sidedness in the brain of a chordate sibling. eLife. 2016;5 doi: 10.7554/eLife.16962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marder Eve. Searching for insight: using invertebrate nervous systems to illuminate fundamental principles in neuroscience. Invertebr Neurobiol. 2007 [Google Scholar]
- Laurent Gilles. On the value of model diversity in neuroscience. Nat Rev Neurosci. 2020;21:395–396. doi: 10.1038/s41583-020-0323-1. [DOI] [PubMed] [Google Scholar]; A compelling essay arguing the case for studying the brain of as many animal species as possible to better understand the many properties of brains.
- 31.Hodgkin Alan L., Huxley Andrew F. Action potentials recorded from inside a nerve fibre. Nature. 1939;144:710–711. [Google Scholar]
- 32.Vincent Castellucci, Pinsker Harold, Kupfermann Irving, Kandel Eric R. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in aplysia. Science. 1970;167:1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- 33.Prinz Astrid A., Bucher Dirk, Marder Eve. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- 34.Bucher Dirk, Prinz Astrid A., Marder Eve. Animal-to-animal variability in motor pattern production in adults and during growth. J Neurosci. 2005;25:1611–1619. doi: 10.1523/JNEUROSCI.3679-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott L.F., Bock D.D., Callaway E.M., Denk W., Dulac C., Fairhall A.L., Fiete I., Harris K.M., Helmstaedter M., Jain V., Kasthuri N. The mind of a mouse. Cell. 2020;182:1372–1376. doi: 10.1016/j.cell.2020.08.010. [DOI] [PubMed] [Google Scholar]; Inspired by early work in C. elegans and more recent in Drosophila and in small volumes of the mouse brain, a consortium of labs makes the case for mapping the connectome of the whole mouse brain.
- Laurent Gilles. Connectomics: a need for comparative studies. e-Neuroforum. 2016;7:54–55. [Google Scholar]; A brilliant essay arguing the case for diversifying the animals under study towards identifying fundamental neural circuit architectures, either shared among closely related species or arrived to by convergence evolution.
- Xu C Shan, Hayworth Kenneth J., Lu Zhiyuan, Grob Patricia, Hassan Ahmed M., García-Cerdán José G., Niyogi Krishna K., Nogales Eva, Weinberg Richard J., Hess Harald F. Enhanced fib-sem systems for large-volume 3d imaging. Elife. 2017;6 doi: 10.7554/eLife.25916. [DOI] [PMC free article] [PubMed] [Google Scholar]; Customization and automatic close-loop monitoring of an off-the-shelf FIBSEM microscope with the ability to image 0.03 mm3 per year opened access to larger than ever brain volumes at an isotropic nanometer resolution sufficient to resolve synaptic and cytoplasmic detail.
- Graham Brett J., Hildebrand David Grant Colburn, Kuan Aaron T., Maniates-Selvin Jasper T., Thomas Logan A., Shanny Brendan L., Allen Lee Wei-Chung. bioRxiv; 2019. High-throughput transmission electron microscopy with automated serial sectioning. [Google Scholar]; The development of a new reel-based serial section sample holder delivers high-throughput volume EM at unprecedented reliability and low cost.
- 39.Saalfeld Stephan, Fetter Richard, Cardona Albert, Tomancak Pavel. Elastic volume reconstruction from series of ultra-thin microscopy sections. Nat Methods. 2012;9:717–720. doi: 10.1038/nmeth.2072. [DOI] [PubMed] [Google Scholar]
- 40.Lee Kisuk, Turner Nicholas, Macrina Thomas, Wu Jingpeng, Lu Ran, Seung H Sebastian. Convolutional nets for reconstructing neural circuits from brain images acquired by serial section electron microscopy. Curr Opin Neurobiol. 2019;55:188–198. doi: 10.1016/j.conb.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helmstaedter Moritz, Kevin Briggman, Denk Winfried. High-accuracy neurite reconstruction for high-throughput neuroanatomy. Nat Neurosci. 2011;14:1081–1088. doi: 10.1038/nn.2868. [DOI] [PubMed] [Google Scholar]
- 42.Schneider-Mizell C.M., Gerhard S., Longair M., Kazimiers T., Li F., Zwart M.F., Champion A., Midgley F.M., Fetter R.D., Saalfeld S., Cardona A. eLife; 2016. Quantitative neuroanatomy for connectomics in Drosophila. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardona A., Saalfeld S., Schindelin J., Arganda-Carreras I., Preibisch S., Longair M., Tomancak P., Hartenstein V., Douglas R.J. TrakEM2 software for neural circuit reconstruction. PLoS ONE. 2012:e38011. doi: 10.1371/journal.pone.0038011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheridan Arlo, Nguyen Tri, Deb Diptodip, Lee Wei-Chung Allen, Saalfeld Stephan, Turaga Srini, Manor Uri, Funke Jan. bioRxiv; 2021. Local shape descriptors for neuron segmentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buhmann J., Sheridan A., Malin-Mayor C., Schlegel P., Gerhard S., Kazimiers T., Krause R., Nguyen T.M., Heinrich L., Lee W.C., Wilson R. Automatic detection of synaptic partners in a whole-brain Drosophila electron microscopy data set. Nat Methods. 2021:1–4. doi: 10.1038/s41592-021-01183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macrina Thomas, Lee Kisuk, Lu Ran, Turner Nicholas L., Wu Jingpeng, Popovych Sergiy, William Silversmith William, Kemnitz Nico, Alexander Bae J., Castro Manuel A., Dorkenwald Sven, Halageri Akhilesh, Jia Zhen, Jordan Chris, Li Kai, Mitchell Eric, Mondal Shanka Subhra, Shang Mu, Nehoran Barak, Wong William, Yu Szi-chieh, Bodor Agnes L., Brittain Derrick, Buchanan JoAnn, Bumbarger Daniel J., Cobos Erick, Collman Forrest, Elabbady Leila, Fahey Paul G., Froudarakis Emmanouil, Kapner Daniel, Sam Kinn, Mahalingam Gayathri, Papadopoulos Stelios, Patel Saumil, Casey M., Schneider-Mizell, Sinz Fabian H., Takeno Marc, Torres Russel, Yin Wenjing, Pitkow Xaq, Reimer Jacob, Tolias Andreas S., Clay Reid R., Maçarico da Costa Nuno, Sebastian Seung H. bioRxiv; 2021. Petascale neural circuit reconstruction: automated methods. [Google Scholar]
- Shapson-Coe Alexander, Januszewski Michał, Berger Daniel R., Pope Art, Wu Yuelong, Tim Blakely, Schalek Richard L., Li Peter, Wang Shuohong, Maitin-Shepard Jeremy, Karlupia Neha, Dorkenwald Sven, Sjostedt Evelina, Leavitt Laramie, Lee Dongil, Bailey Luke, Fitzmaurice Angerica, Kar Rohin, Field Benjamin, Wu Hank, Wagner-Carena Julian, Aley David, Lau Joanna, Lin Zudi, Wei Donglai, Pfister Hanspeter, Peleg Adi, Jain Viren, Lichtman Jeff W. bioRxiv; 2021. A connectomic study of a petascale fragment of human cerebral cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]; A partial connectomic reconstruction of a small fragment of human cerebral cortex revealed that, as in Drosophila, some neurons contribute far more synaptic input onto target neurons that others; these numerically stronger connections are likely to dominate functionally and can be more reliably mapped from volume EM without perfect recall of all synapses.
- 48.Costa Marta, Manton James D., Ostrovsky Aaron D., Prohaska Steffen, Jefferis Gregory SXE. NBLAST: rapid, sensitive comparison of neuronal structure and construction of neuron family databases. Neuron. 2016;91:293–311. doi: 10.1016/j.neuron.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eckstein Nils, Bates Alexander S., Du Michelle, Hartenstein Volker, Jefferis Gregory SXE., Funke Jan. bioRxiv; 2020. Neurotransmitter classification from electron microscopy images at synaptic sites in Drosophila. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonietta Tosches Maria, Yamawaki Tracy M., Naumann Robert K., Jacobi Ariel A., Tushev Georgi, Laurent Gilles. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science. 2018;360:881–888. doi: 10.1126/science.aar4237. [DOI] [PubMed] [Google Scholar]; A comparative RNAseq analysis of hippocampal neurons in reptiles and mammals demonstrates close homology of GABAergic neuron types and suggests mammalian glutamatergic types are evolutionarily novel.
- 51.Lyzinski V., Fishkind D.E., Priebe C.E. Seeded graph matching for correlated Erdös-Rényi graphs. J Mach Learn Res. 2014;1:3513–3540. [Google Scholar]
- Lu Zhiyuan, Xu C Shan, Hayworth Kenneth J., Rivlin Patricia, Plaza Stephen M., Scheffer Louis, Rubin Gerald M., Hess Harald F., Meinertzhagen Ian A. bioRxiv; 2019. En bloc preparation of Drosophila brains enables high-throughput fib-sem connectomics; p. 855130. [DOI] [PMC free article] [PubMed] [Google Scholar]; A sample preparation method for the Drosophila brain greatly speeds up FIBSEM imaging, and can be applied broadly to insects and beyond.
- 53.Mikula Shawn, Denk Winfried. High-resolution whole-brain staining for electron microscopic circuit reconstruction. Nat Methods. 2015;12:541–546. doi: 10.1038/nmeth.3361. [DOI] [PubMed] [Google Scholar]
- 54.Frank Glaw, Köhler Jörn, Townsend Ted M., Vences Miguel. Rivaling the world's smallest reptiles: discovery of miniaturized and microendemic new species of leaf chameleons (Brookesia) from northern Madagascar. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blair Hedges S., Thomas Richard. At the lower size limit in amniote vertebrates: a new diminutive lizard from the West Indies. Caribb J Sci. 2001;37:168–173. [Google Scholar]
- 56.Blair Hedges S. At the lower size limit in snakes: two new species of threadsnakes (squamata: Leptotyphlopidae: Leptotyphlops) from the lesser antilles. Zootaxa. 2008;1841:1–30. [Google Scholar]
- 57.Schulze L., Henninger J., Kadobianskyi M., Chaigne T., Faustino A.I., Hakiy N., Albadri S., Schuelke M., Maler L., Del Bene F., Judkewitz B. Transparent Danionella translucida as a genetically tractable vertebrate brain model. Nat Methods. 2018;15:977–983. doi: 10.1038/s41592-018-0144-6. [DOI] [PubMed] [Google Scholar]
- 58.Lin Chan, Strausfeld Nicholas J. Visual inputs to the mushroom body calyces of the whirligig beetle dineutus sublineatus: modality switching in an insect. J Comp Neurol. 2012;520:2562–2574. doi: 10.1002/cne.23158. [DOI] [PubMed] [Google Scholar]
- 59.Roe Anna W., Pallas Sarah L., Hahm Jong-On, Sur Mriganka. A map of visual space induced in primary auditory cortex. Science. 1990;250:818–820. doi: 10.1126/science.2237432. [DOI] [PubMed] [Google Scholar]
- 60.Sen Sonia, Cao Deshou, Choudhary Ramveer, Biagini Silvia, Wang Jing W., Reichert Heinrich, VijayRaghavan K. Genetic transformation of structural and functional circuitry rewires the drosophila brain. Elife. 2014;3 doi: 10.7554/eLife.04407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Godino Lucia L., Silbering Ana F., Khallaf Mohammed A., Cruchet Steeve, Bojkowska Karolina, Pradervand Sylvain, Hansson Bill S., Knaden Markus, Benton Richard. Functional integration of “undead” neurons in the olfactory system. Sci Adv. 2020;6:eaaz7238. doi: 10.1126/sciadv.aaz7238. [DOI] [PMC free article] [PubMed] [Google Scholar]; By interfering with programmed cell death, the authors enable neurons to develop that would otherwise not, and observe how their integration into brain circuits brings in new functionality.
- 62.Ngwenya Ayanda, Patzke Nina, Manger Paul R., Herculano-Houzel Suzana. Continued growth of the central nervous system without mandatory addition of neurons in the nile crocodile (Crocodylus niloticus) Brain Behav Evol. 2016;87:19–38. doi: 10.1159/000443201. [DOI] [PubMed] [Google Scholar]
- Perez-Martinez and Manuel Leal Christian A. Contribution to the special issue on reptile cognition: lizards as models to explore the ecological and neuroanatomical correlates of miniaturization. Behaviour. 2021;1:1–48. [Google Scholar]; An analysis of miniaturization in lizards highlights the proportionally larger telecephalon and smaller olfactory bulbs of smaller species.
- 64.Yamamoto Masamichi, Shimazaki Yumiko, Shigeno Shuichi. Atlas of the embryonic brain in the pygmy squid, Idiosepius paradoxus. Zool Sci. 2003;20:163–179. doi: 10.2108/zsj.20.163. [DOI] [PubMed] [Google Scholar]
- 65.Hubel David H., Wiesel Torsten N. Uniformity of monkey striate cortex: a parallel relationship between field size, scatter, and magnification factor. J Comp Neurol. 1974;158:295–305. doi: 10.1002/cne.901580305. [DOI] [PubMed] [Google Scholar]
- 66.Otto D Creutzfeldt. Generality of the functional structure of the neocortex. Naturwissenschaften. 1977;64:507–517. doi: 10.1007/BF00483547. [DOI] [PubMed] [Google Scholar]
- 67.Gilbert Charles D. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- 68.Douglas Rodney J., Martin Kevan AC., Whitteridge David. A canonical microcircuit for neocortex. Neural Comput. 1989;1:480–488. [Google Scholar]
- 69.Tom Binzegger, Douglas Rodney J., Martin Kevan AC. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rudolf Nieuwenhuys. The neocortex. Anat Embryol. 1994;190:307–337. doi: 10.1007/BF00187291. [DOI] [PubMed] [Google Scholar]
- 71.Elson Guy N., DeFelipe Javier. Spine distribution in cortical pyramidal cells: a common organizational principle across species. Prog Brain Res. 2002;136:109–133. doi: 10.1016/s0079-6123(02)36012-6. [DOI] [PubMed] [Google Scholar]
- 72.Douglas Rodney J., Martin Kevan AC. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 73.DeFelipe Javier, Jones Edward G. Neocortical microcircuits. Handbook Brain Microcircuits. 2010:5–14. [Google Scholar]
- 74.Harris Kenneth D., Shepherd Gordon MG. The neocortical circuit: themes and variations. Nat Neurosci. 2015;18:170. doi: 10.1038/nn.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bopp Rita, Holler-Rickauer Simone, Martin Kevan AC., Schuhknecht Gregor FP. An ultrastructural study of the thalamic input to layer 4 of primary motor and primary somatosensory cortex in the mouse. J Neurosci. 2017;37:2435–2448. doi: 10.1523/JNEUROSCI.2557-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Yuan, Brzozowska-Prechtl Agnieszka, Karten Harvey J. Laminar and columnar auditory cortex in avian brain. Proc Natl Acad Sci Unit States Am. 2010;107:12676–12681. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fournier Julien, Müller Christian M., Laurent Gilles. Looking for the roots of cortical sensory computation in three-layered cortices. Curr Opin Neurobiol. 2014;31:119–126. doi: 10.1016/j.conb.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stephenson-Jones Marcus, Ericsson Jesper, Robertson Brita, Grillner Sten. Evolution of the basal ganglia: dual-output pathways conserved throughout vertebrate phylogeny. J Comp Neurol. 2012;520:2957–2973. doi: 10.1002/cne.23087. [DOI] [PubMed] [Google Scholar]
- 79.Suryanarayana S.M., Robertson B., Wallén P., Grillner S. The lamprey pallium provides a blueprint of the mammalian layered cortex. Current Biology. 2017;27(21):3264–3277. doi: 10.1016/j.cub.2017.09.034. [DOI] [PubMed] [Google Scholar]
- 80.Mischiati M., Lin H.T., Herold P., Imler E., Olberg R., Leonardo A. Internal models direct dragonfly interception steering. Nature. 2015;517:333–338. doi: 10.1038/nature14045. [DOI] [PubMed] [Google Scholar]
- 81.Packard A. Cephalopods and fish: the limits of convergence. Biol Rev. 1972:241–307. [Google Scholar]
- Borst Alexander, Helmstaedter Moritz. Common circuit design in fly and mammalian motion vision. Nat Neurosci. 2015;18:1067–1076. doi: 10.1038/nn.4050. [DOI] [PubMed] [Google Scholar]; A comparison of the motion-sensitive neurons in the fly and mouse visual system revealed a common, likely evolutionarily convergent circuit architecture for computing direction of visual motion.
- 83.Young John Zachary. The central nervous system of Loligo i. the optic lobe. Philos Trans R Soc Lond B Biol Sci. 1974;267:263–302. doi: 10.1098/rstb.1974.0002. [DOI] [PubMed] [Google Scholar]
- 84.Takemura S.Y., Bharioke A., Lu Z., Nern A., Vitaladevuni S., Rivlin P.K., Katz W.T., Olbris D.J., Plaza S.M., Winston P., Zhao T. A visual motion detection circuit suggested by Drosophila connectomics. Nature. 2013;500:175–181. doi: 10.1038/nature12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Helmstaedter Moritz, Briggman Kevin L., Turaga Srinivas C., Jain Viren, Seung H Sebastian, Denk Winfried. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature. 2013;500:168–174. doi: 10.1038/nature12346. [DOI] [PubMed] [Google Scholar]
- 86.Kim Jinseop S., Greene Matthew J., Zlateski Aleksandar, Lee Kisuk, Richardson Mark, Turaga Srinivas C., Purcaro Michael, Balkam Matthew, Robinson Amy, Behabadi Bardia F., Campos Michael, Denk Winfried, Sebastian Seung H., The EyeWirers Space-time wiring specificity supports direction selectivity in the retina. Nature. May 2014;509:331–336. doi: 10.1038/nature13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meinertzhagen I.A., O'Neil S.D. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol. 1991;305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- 88.Wilson Jonathan J., Alexandre Nicolas, Trentin Caterina, Tripodi Marco. Three-dimensional representation of motor space in the mouse superior colliculus. Curr Biol. 2018;28:1744–1755. doi: 10.1016/j.cub.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johannes D Seelig, Jayaraman Vivek. Neural dynamics for landmark orientation and angular path integration. Nature. 2015;521:186–191. doi: 10.1038/nature14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira-Rosario Arnaldo, Zarin Aref Arzan, Clark Matthew Q., Manning Laurina, Fetter Richard D., Cardona Albert, Chris Q Doe. eLife; 2018. Mdn brain descending neurons coordinately activate backward and inhibit forward locomotion. [DOI] [PMC free article] [PubMed] [Google Scholar]; Genetic markers revealed that a command neuron for backwards crawling in Drosophila larva survives metamorphosis to become a command neuron for backwards walking in the adult, indicating that while sensory and motor systems match body morphology, high-order neurons and circuits can be shared.
- 91.Reiter S., Hülsdunk P., Woo T., Lauterbach M.A., Eberle J.S., Akay L.A., Longo A., Meier-Credo J., Kretschmer F., Langer J.D. Elucidating the control and development of skin patterning in cuttlefish. Nature. 2018;562:361–366. doi: 10.1038/s41586-018-0591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farrell Brian D. ”inordinate fondness” explained: why are there so many beetles? Science. 1998;281:555–559. doi: 10.1126/science.281.5376.555. [DOI] [PubMed] [Google Scholar]
- 93.Forbes Andrew A., Bagley Robin K., Beer Marc A., Hippee Alaine C., Widmayer Heather A. Quantifying the unquantifiable: why hymenoptera, not coleoptera, is the most speciose animal order. BMC Ecol. 2018;18:1–11. doi: 10.1186/s12898-018-0176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hebert Paul DN., Ratnasingham Sujeevan, Zakharov Evgeny V., C Telfer Angela, Levesque-Beaudin Valerie, Milton Megan A., Pedersen Stephanie, Paul Jannetta, R DeWaard Jeremy. Counting animal species with DNA barcodes: Canadian insects. Phil Trans Biol Sci. 2016;371:20150333. doi: 10.1098/rstb.2015.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.World Health Organization . 2020. World malaria report 2020: 20 years of global progress and challenges. [Google Scholar]
- 96.Bourtzis K., Dobson S.L., Xi Z., Rasgon J.L., Calvitti M., Moreira L.A., Bossin H.C., Moretti R., Baton L.A., Hughes G.L., Mavingui P. Harnessing mosquito–wolbachia symbiosis for vector and disease control. Acta Trop. 2014;132:S150–S163. doi: 10.1016/j.actatropica.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 97.Brock Ryan E., Cini Alessandro, Sumner Seirian. Ecosystem services provided by aculeate wasps. Biol Rev. 2021 doi: 10.1111/brv.12719. [DOI] [PubMed] [Google Scholar]
- 98.McMeniman Conor J., Corfas Román A., Matthews Benjamin J., Ritchie Scott A., Vosshall Leslie B. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 2014;156:1060–1071. doi: 10.1016/j.cell.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lahondère Chloé, Vinauger Clément, Okubo Ryo P., Wolff Gabriella H., Chan Jeremy K., Akbari Omar S., Riffell Jeffrey A. The olfactory basis of orchid pollination by mosquitoes. Proc Natl Acad Sci Unit States Am. 2020;117:708–716. doi: 10.1073/pnas.1910589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Webb Barbara. Cognition in insects. Phil Trans Biol Sci. 2012;367:2715–2722. doi: 10.1098/rstb.2012.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simons Meagan, Tibbetts Elizabeth. Insects as models for studying the evolution of animal cognition. Curr Opin Insect Sci. 2019;34:117–122. doi: 10.1016/j.cois.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 102.Helmstaedter Moritz. The mutual inspirations of machine learning and neuroscience. Neuron. 2015;86:25–28. doi: 10.1016/j.neuron.2015.03.031. [DOI] [PubMed] [Google Scholar]
- Jürgensen Anna-Maria, Khalili Afshin, Chicca Elisabetta, Indiveri Giacomo, Paul Nawrot Martin. bioRxiv; 2021. A neuromorphic model of olfactory processing and sparse coding in the Drosophila larva brain. [Google Scholar]; A neuromorphic hardware and software simulation models of the EM-mapped connectome of the Drosophila larval olfactory and learning and memory circuits achieve parallel processing and efficient encoding of sensory input.
- Hong Jinyung, Pavlic Theodore P. arXiv preprint arXiv; 2021. An insect-inspired randomly, weighted neural network with random fourier features for neuro-symbolic relational learning. 2109.06663. [Google Scholar]; The expansion-contraction architecture of the insect's circuits for learning and memory inspired the design of an efficient artificial neural network for machine learning applications.
- Delahunt Charles B., Kutz J Nathan. Putting a bug in ml: the moth olfactory network learns to read MNIST. Neural Network. 2019;118:54–64. doi: 10.1016/j.neunet.2019.05.012. [DOI] [PubMed] [Google Scholar]; The layout of the moth brain's olfactory circuits inspired an artificial neural network to efficiently identify hand-written digits.
- 106.Sayre Marcel E., Templin Rachel, Chavez Johanna, Kempenaers Julian, Heinze Stanley. eLife; 2021. A projectome of the bumblebee central complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Polilov Alexey A. The smallest insects evolve anucleate neurons. Arthropod Struct Dev. 2012;41:29–34. doi: 10.1016/j.asd.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 108.Strausfeld N.J., Hildebrand J.G. Olfactory systems: common design, uncommon origins? Curr Opin Neurobiol. 1999;9:634–639. doi: 10.1016/S0959-4388(99)00019-7. [DOI] [PubMed] [Google Scholar]
- Wang Peter Y., Sun Yi, Axel Richard, Abbott L.F., Yang Guangyu Robert. bioRxiv; 2021. Evolving the olfactory system with machine learning. [DOI] [PubMed] [Google Scholar]; Upon training 3-layer artificial neural networks to successfully perform the in silico equivalent of an olfactory task, the network connectivity was found to parallel the organization of both the insect and the mammal olfactory circuits, indicating that the nature of the task suffices to drive the evolution of a neural architecture towards an optimal configuration, illustrating the process of convergent evolution.
- 110.Farris Sarah M. Are mushroom bodies cerebellum-like structures? Arthropod Struct Dev. 2011;40:368–379. doi: 10.1016/j.asd.2011.02.004. [DOI] [PubMed] [Google Scholar]